Abstract
Background
Optimal penetration of anti-infectives in the female genital tract (FGT) is paramount in the treatment and prevention of infectious diseases. While exposure of anti-infectives in lower FGT tissues (e.g. cervix, vagina) has been described, little data exist on upper genital tissues (e.g. ovary, uterus).
Methods
Autopsies were performed and post-mortem tissues were collected within 24 h of death for female participants with advanced HIV in Uganda (n = 27). Tenofovir, lamivudine, efavirenz and fluconazole concentrations were measured using LC-MS/MS in plasma, ovarian, uterine, cervical and vaginal tissues. Tissue penetration was calculated as tissue-to-plasma concentration ratios (TPRs).
Results
TPRs of tenofovir, lamivudine and fluconazole were highest in vaginal tissue (medians 1.86, 1.83 and 0.94, respectively), while the TPR of efavirenz was highest in ovarian tissue (median 0.65). With cervix as a reference compartment, vaginal TPRs were significantly higher than cervical for all four drugs; TPRs of efavirenz in uterine and ovarian compartments were also significantly higher than cervical. Most of the post-mortem FGT samples had a TPR of greater than 1 for tenofovir and lamivudine, while less than 50% had a TPR of greater than 1 for both efavirenz and fluconazole.
Conclusions
Penetration of anti-infectives was not homogeneous among the FGT compartments. Approximately 70% of FGT tissues had a TPR of greater than 1 for tenofovir and lamivudine, favouring the prevention of local HIV replication and transmission in the FGT.
Introduction
The female genital tract (FGT) is a significant anatomical site of pathogen persistence and sexual transmission of infectious diseases. Optimal drug exposure in the FGT is paramount for prevention and treatment of FGT-involved infectious diseases, such as HIV and vaginal candidiasis. Although much of the focus on mucosal HIV transmission in women has been the lower FGT, HIV infection can occur throughout the entire FGT, as demonstrated in the rhesus macaque vaginal transmission model of simian immunodeficiency virus.1 Candidiasis, while more often defined as vulvovaginal compared with other FGT sites, can, in rare cases, occur in the upper FGT as well.2 Despite this, our knowledge of FGT tissue exposure to antiretrovirals and antifungals has predominantly been obtained through sampling of the lower FGT, mostly via cervicovaginal tissue and cervicovaginal fluid.3–9 Studies related to FGT drug exposure in upper tissue tracts (i.e. ovarian and uterine tissue) in non-pregnant women are scarce due to lack of easiness of sampling.
In order to fill the knowledge gap surrounding exposure of anti-infectives in solid tissues of the upper and lower FGT, we explored tissue penetration of two commonly prescribed NRTIs, tenofovir and lamivudine, and an NNRTI, efavirenz, in addition to an antifungal, fluconazole, in post-mortem FGT tissues (i.e. ovarian, uterine, cervical and vaginal) from a population of Ugandan women living with HIV/AIDs prior to the time of death.
Methods
Study participants and sample collection
All study participants were hospitalized patients living with HIV/AIDs who passed away at Mulago National Referral Hospital in Kampala, Uganda from 2017 to 2020. Written informed consent was obtained from the next of kin. ART history, including recent medication adherence, was extracted from medical charts and from interviews with caretakers. Autopsies were performed shortly after consent and time of death (typically within 24 h). During autopsies, approximately 1–2 g tissue sections from various organs and anatomical sites (including vagina, cervix, uterus and ovary) were collected and immediately snap-frozen in liquid nitrogen or dry ice/ethanol bath. Whole blood from the femoral vein was collected into EDTA vacutainers and spun at 2400–3000 rpm at 4°C for 10 min to separate plasma. Tissue and plasma specimens were transferred via liquid nitrogen to –80°C freezers prior to analysis. The study protocol was approved by the Research Ethics Review Committee at Mulago National Referral Hospital.
Sample processing and quantification of anti-infective concentrations
Plasma and tissue concentrations of tenofovir, lamivudine and fluconazole were quantified simultaneously, while efavirenz was quantified separately from the other three drugs, according to the methods previously described.10 Briefly, 200 μL of plasma was spiked with 20 μL of a mixed internal standard of tenofovir-d6, lamivudine-13C1, d2, efavirenz-d4 and fluconazole-d4. Protein was then precipitated by adding 800 μL of ice-cold acetonitrile and removed after centrifugation at 15 000 g for 15 min. The supernatant was then condensed under a nitrogen flow, following reconstitution with mobile phase and injection for LC-MS/MS assay. The method for FGT tissue processing and LC-MS/MS quantification was adapted from the aforementioned plasma method, with some modification. Briefly, for each female genital specimen (i.e. ovarian, uterine, cervical and vaginal tissue), 0.3 g of tissue was weighed, cut into small pieces, and homogenized in 0.6 mL of PBS with a portable rotor stator (Omni TH). Two hundred microlitres of supernatant were collected after centrifuging the homogenate at 4696 g for 15 min, then spiked with 20 μL of the mixed internal standard followed by the same process as plasma.
The simultaneous detection and quantification of tenofovir, lamivudine and fluconazole in FGTs were performed using UPLC (Thermo Scientific) coupled with a TSQ Quantum triple-stage quadrupole mass spectrometer (Thermo Electron, San Jose, CA, USA) (LC-MS/MS). The chromatographic separation was performed with an ACQUITY UPLC HSS T3 (2.1 × 50 mm), reversed-phase column with a 1.8 μm particle size. The mobile phase used for the gradient elution consisted of (A) 0.1% formic acid in deionized water and (B) 0.1% formic acid in acetonitrile. The chromatographic conditions were isocratic from 0 to 1.50 min at 0% B, followed by a linear gradient at 1.5 to 2.75 min of 0%–45% B and then returning to the starting conditions (at 3.0 min) with a flow rate of 0.3 mL/min, for a total run time of 6 min. The column temperature was maintained at 30°C. The detector settings of the mass spectrometer were: ESI with the stainless-steel spray needle, positive polarity ionization and multiple reaction monitoring (MRM) mode. The ion transitions (m/z) were as follows: 288 to 176 for tenofovir, 230 to 112 for lamivudine, and 307 to 238 for fluconazole.
Chromatographic separation of efavirenz was performed with an ACQUITY UPLC BEH C18 (2.1 × 50 mm), reversed-phase column with a 1.7 μm particle size (Waters, Milford, MA, USA), on the same LC-MS/MS system mentioned above. The mobile phase used was a mixture of 10 mM ammonium acetate in water, pH 6.8 (30%), and acetonitrile (70%) with an isocratic elution flow rate of 0.25 mL/min and a total run time of 2 min. The column temperature was maintained at 30°C. The detector settings of the mass spectrometer were: ESI with the stainless-steel spray needle, negative polarity ionization and selective reaction monitoring (SRM) mode. The ion transition (m/z) was 314 to 244 for efavirenz.
Efficiency (or recovery) of extraction from tissue homogenate was estimated by repeating the step of homogenizing the sample with PBS three additional times for four randomly chosen samples. Supernatant from each homogenate was analysed by the above assay, and an average extraction efficiency factor of 0.7 was derived by fitting exponential regression curves for the remaining amount of tenofovir, lamivudine and fluconazole in the same samples.
Statistical analysis
The penetration metric was defined as the ratio between tissue and plasma concentration (tissue-to-plasma ratio; TPR). TPR was used rather than raw concentration as there was expected variability in concentrations due to the various times since the last dose and, in the case of fluconazole, variable dosages. Concentrations and TPRs of tenofovir, lamivudine, efavirenz and fluconazole in each tissue compartment were expressed as median (IQR). Friedman test (non-parametric analysis for repeated measures) was used for the global comparisons of difference among the four FGT compartments and the paired Wilcoxon test was used for comparisons between other FGT compartments and the cervical compartment, as it was the most common tissue compartment measured and has been used to broadly refer to FGT in previous studies.3–5,7 Pearson correlation coefficients (r) and the corresponding P values were used to show the correlation between plasma concentration and tissue concentration. Linear regression was used to analyse the relationship between TPR and post-mortem interval.
Results
Participant demographics
Post-mortem tissues were sampled from 27 female Ugandan participants living with HIV/AIDs at the time of death. Demographic characteristics of deceased participants can be seen in Table 1. The majority of study participants were young to middle-aged. Twenty-two participants were receiving ART at the time of death and 13 were receiving fluconazole for the treatment of cryptococcal meningitis. Of the 21 participants with available dosing history, all had received ART for at least 7 days prior to the time of death and 67% were said to be adherent to their ART. The most common ART regimen prescribed was tenofovir disoproxil fumarate/lamivudine/efavirenz (44.4%). The most common causes of death were cryptococcal meningitis and TB. Other causes included various types of cancer, anaemia, respiratory failure, heart failure, etc. Tissue concentrations were only reported for those with detectable corresponding plasma concentrations.
Table 1.
Demographic characteristics and medication use
| Characteristic | Median (IQR) unless otherwise noted |
|---|---|
| Age (years) | 34 (26–45) |
| Height (cm) | 161 (156–163) |
| Most recent CD4+ T cell count (cells/mm3)a | 23 (5–112) |
| eGFR (mL/min/1.73 m2) | 14 (9–32) |
| Time on current ART regimen (days) | 200 (42–558) |
| Time on fluconazole regimen (days) | 7.5 (3–15) |
| Most recent viral load (copies/mL)b | Undetectable–1 100 000 |
| Time since last antiretroviral dose prior death (h)c | 20.2 (13.6–42.4) |
| Time since last fluconazole dose prior to death (h) | 18.9 (10.9–24.8) |
| Post-mortem interval (h) | 7.5 (5.2–12.2) |
| Characteristic | N = 27 |
| Cryptococcal meningitis, n (%) | 12 (44.4) |
| TB, n (%) | 12 (44.4) |
| Antiretrovirals, n (%) | |
| Tenofovir disoproxil fumarate | 17 (63.0) |
| Lamivudine | 21 (77.8) |
| Efavirenz | 11 (40.7) |
| Dolutegravir | 7 (25.9) |
| Nevirapine | 3 (11.1) |
| Abacavir | 2 (7.4) |
| Zidovudine | 2 (7.4) |
| Fluconazole | 15 (55.6) |
| 200 mg, n | 3 |
| 400 mg, n | 1 |
| 800 mg, n | 2 |
| 1200 mg, n | 5 |
| Unknown, n | 4 |
Time interval between death and the most recent CD4+ T cell counts ranged from 2 days to 6.9 years.
Most recent viral load is shown as range for seven participants. Time interval between death and the most recent viral load ranged from 11 days to 783 days.
Time of last ART dose was provided by next of kin or taken from medical records.
NRTIs
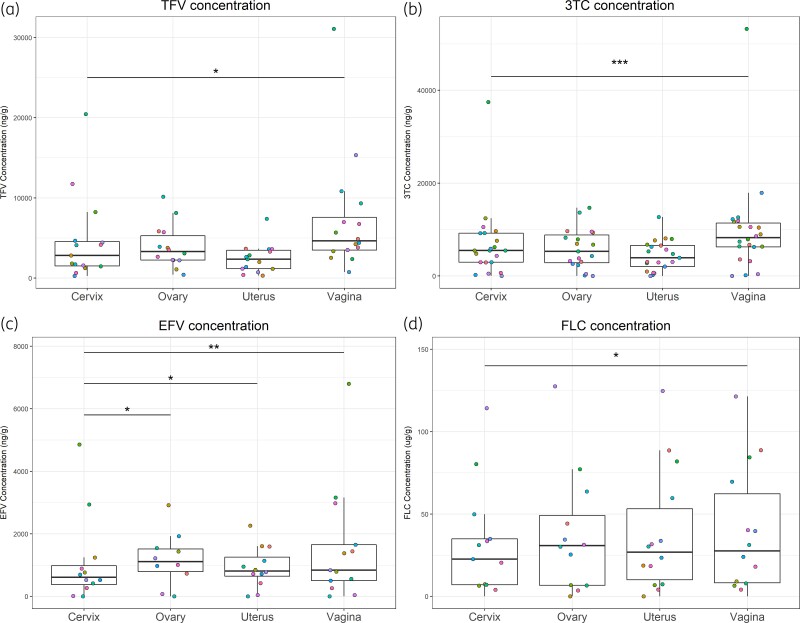
The median concentration of tenofovir was highest in vaginal tissue across the four FGT compartments (i.e. vaginal, cervical, uterine and ovarian) and median vaginal concentrations were 64% higher than cervical concentrations (P = 0.03) (Figure 1a). When normalizing for plasma, the TPR of tenofovir in vaginal tissue was greater than ovarian, uterine and cervical tissues as well (Table 2). More than 50% of participants attained TPR > 1 in all four FGT compartments, with the highest proportion of TPR > 1 being in vaginal tissue (Table 3).
Figure 1.
Concentrations of tenofovir (a), lamivudine (b), efavirenz (c) and fluconazole (d) in ovarian, uterine, cervical and vaginal tissue. In each plot, points with the same colour represent the same individual. *P < 0.05; **P < 0.01; ***P < 0.005. TFV, tenofovir; 3TC, lamivudine; EFV, efavirenz; FLC, fluconazole. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
Table 2.
TPRs in ovarian, uterine, cervical and vaginal tissue
| Cervix TPR | Ovary TPR | Uterus TPR | Vagina TPR | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Drug | Median (IQR) | n | P | Median (IQR) | n | P | Median (IQR) | n | P | Median (IQR) | n | P |
| Tenofovir | 1.22 (0.77–1.96) | 15 | ref | 1.21 (0.88–1.89) | 14 | 0.95 | 1.14 (0.65–2.91) | 15 | 0.41 | 1.86 (1.31–5.54) | 16 | 0.03 |
| Lamivudine | 1.24 (0.98–1.67) | 21 | ref | 1.34 (0.97–1.72) | 19 | 0.81 | 1.05 (0.94–1.63) | 21 | 0.36 | 1.83 (1.11–2.92) | 22 | 0.001 |
| Efavirenz | 0.51 (0.22–0.82) | 12 | ref | 0.65 (0.24–1.23) | 10 | 0.01 | 0.51 (0.35–0.88) | 12 | 0.04 | 0.59 (0.28–0.7) | 13 | 0.02 |
| Fluconazole | 0.92 (0.90–0.96) | 13 | ref | 0.85 (0.64–0.94) | 12 | 1 | 0.89 (0.86–0.94) | 14 | 0.69 | 0.94 (0.87–1.10) | 14 | 0.017 |
Values of n are the sample size and P values are from the Wilcoxon test between the other FGT tissues and the cervical tissue (indicated as ‘ref’).
Table 3.
The proportion of TPR greater than 1 by tissue and drug
| Tissue | Tenofovir | Lamivudine | Fluconazole | Efavirenz | ||||
|---|---|---|---|---|---|---|---|---|
| n | TPR > 1 (%) | n | TPR > 1 (%) | n | TPR > 1 (%) | n | TPR > 1 (%) | |
| Ovary | 14 | 71 | 19 | 68 | 12 | 17 | 10 | 40 |
| Uterus | 15 | 60 | 21 | 57 | 14 | 14 | 12 | 8 |
| Cervix | 15 | 60 | 21 | 71 | 13 | 15 | 12 | 25 |
| Vagina | 16 | 88 | 22 | 86 | 14 | 43 | 13 | 23 |
| % of TPR > 1 in all tested tissue samples | 70 | 71 | 23 | 23 | ||||
| % of participants with at least 1 tissue with TPR > 1 | 100 | 91 | 43 | 29 | ||||
Similarly, vaginal concentrations of lamivudine were higher than those of other FGT compartments and median vaginal concentrations were 50% higher than the cervical concentrations (P = 0.0003) (Figure 1b). TPRs of lamivudine were greater in vaginal tissue when compared with the other three tissue compartments (Table 2). More than 50% of participants attained TPR > 1 in all four FGT compartments, with vaginal tissue again having the highest proportion of participants with TPR > 1 (Table 3).
NNRTI
Median concentration of efavirenz was highest in ovarian tissue, and concentrations in ovarian, vaginal and uterine tissues were all significantly higher than in cervical tissue by 82% (P = 0.01), 37% (P < 0.01) and 33% (P = 0.03), respectively (Figure 1c). TPRs of efavirenz demonstrated the same relationships among the four FGTs as concentration (Table 2). All four FGT compartments had less than 50% of participant samples attaining TPR > 1. Ovarian tissue had the highest TPR > 1 proportion compared with the other FGT tissues (Table 3).
Antifungal
While the median concentration of fluconazole was highest in the ovarian tissue, only vaginal concentrations were statistically higher (22%) than cervical concentrations (P = 0.02) (Figure 1d). TPRs of fluconazole in the four FGT compartments were comparable, with the largest median in vaginal tissue (Table 2). Vaginal TPRs were significantly larger than cervical TPRs (P = 0.02). The proportion of participants attaining TPR > 1 was less than 50% in all of the four FGT compartments, with vaginal tissue having the highest proportion (Table 3).
Correlation between plasma and tissue concentration
Figure S1, available as Supplementary data at JAC Online, shows the correlation between plasma and tissue concentration. Tenofovir concentrations in plasma were not correlated with any FGT compartment. Plasma concentrations of lamivudine and efavirenz were only significantly correlated with uterine concentration (r = 0.68, P < 0.001 and r = 0.6, P = 0.4, respectively). Fluconazole plasma concentrations were significantly correlated with fluconazole in all tissue compartments (ovarian: r = 0.9, P < 0.001; uterine: r = 0.93, P < 0.001; cervical: r = 0.91, P = 0.03; vaginal: r = 0.91, P < 0.001).
Impact of post-mortem redistribution
To examine the impact of post-mortem redistribution, we explored the relationship between TPR and post-mortem intervals (Figure S2). Post-mortem interval was not significant as a predictor for TPR in any of the models for tenofovir, lamivudine, efavirenz or fluconazole in any of the FGT tissue compartments (P > 0.05), apart from lamivudine in uterine tissue, where longer time to post-mortem was associated with increased TPR (P = 0.036, slope coefficient = 0.066, suggesting an 0.066 increase in TPR every hour post-mortem).
Discussion
In this study, we simultaneously measured the concentration of two commonly prescribed NRTIs, tenofovir and lamivudine, and an NNRTI, efavirenz, in addition to the antifungal fluconazole, in post-mortem tissues of the reproductive tracts of Ugandan women living with HIV/AIDs prior to death. We normalized these data to plasma to understand the relative penetration into the FGT. Our major findings were: (1) penetration ratios of the four drugs were different among the ovarian, uterine, cervical and vaginal tissues, thus, cervical tissue may not be an adequate representative for the entire FGT; and (2) penetration ratios of tenofovir and lamivudine in female genital tissues were >1 for most participants, but not for efavirenz and fluconazole.
Several studies have measured tenofovir exposure in cervical or vaginal biopsies from healthy, non-pregnant women without HIV infection, and results are very variable given different study designs.5,11–13 In a 2021 study conducted by Thurman and colleagues,11 at 24 h after a 14 day multiple dose period, tenofovir concentrations (medians 56 ng/mL for blood, 52.8 ng/g for cervical tissue and 63.1 ng/g for vaginal tissue) were more than 50-fold less than what we measured, but both were notably similar to plasma, as we reported. Hendrix et al.13 measured tenofovir concentration in vaginal biopsy of HIV-uninfected women who took oral tenofovir disoproxil fumarate daily for 6 weeks. However, tenofovir concentrations of half of the vaginal biopsy samples were lower than the lower limit of quantification of that particular assay, such that a measure of tissue penetration compared with blood was not accessible.13 There are other studies, including that conducted by Thurman et al.,11 which measured tenofovir in cervical/vaginal biopsies after a single oral dose of tenofovir disoproxil fumarate, but they are not discussed here or comparable with our results as our participants were considered to be at steady state.5,11,12 Data on lamivudine exposure in the FGT at steady state are even more scarce. Herrera and colleagues14 measured exposure of lamivudine in vaginal tissues of 18 healthy, non-HIV participants during and after they took the combination of lamivudine and raltegravir for 7 days, and the average lamivudine TPR was 8, which is higher than what we found (median 1.86).
So far, only two studies have measured tenofovir or lamivudine in upper reproductive tissues. Rahangdale et al.3 measured tenofovir in endometrial tissues of non-pregnant women living with HIV and reported values ∼1% (median 26 ng/g) of what we measured in the uterine tissues (median 2356.6 ng/g). In addition, Yeh et al.15 measured ART concentrations in amniotic fluid of women living with HIV during delivery; however, the sample size was small (n = 1 for tenofovir; n = 6 for lamivudine). The concentrations of tenofovir and lamivudine in amniotic fluid were one-third and one-quarter of the median tenofovir and lamivudine concentrations of uterine tissues in our study but comparisons are challenging given the small sample size in this group, and the different tissue compartment/matrix sampled.15
Before our current study, efavirenz concentration had only been measured in cervicovaginal fluid as representative of its exposure in the FGT by Kwara et al.7 and Dumond et al.4 Both studies measured efavirenz concentration in paired cervicovaginal fluid and blood plasma samples at steady state of women who were living with HIV.4,7 In the study by Kwara et al.,7 the mean cervicovaginal fluid-to-plasma ratio was 0.01 for both samples taken before and 3–4 h after administration. Dumond et al.4 had more intensive sampling and derived the penetration (i.e. cervicovaginal fluid-to-plasma ratio) with AUCs of efavirenz concentration in cervicovaginal fluid and blood plasma, and the median ratio was 0.004. Since a different biomatrix was used in our study for FGT, the results are not directly comparable, but the relationship of FGT penetration of efavirenz compared with the NRTIs is the same (i.e. penetration of efavirenz at FGT was less than that of tenofovir and lamivudine).4,7
Exposure of fluconazole in the lower FGT from two previous studies by Mikamo et al.8 and Houang et al.9 are lower than what we reported; however, these were both single-dose studies using a dose lower than any used in our cohort. The two studies investigated the pharmacokinetics of fluconazole after a single oral dose of 150 mg for treatment of vaginal candidiasis, where the peak concentrations in vaginal tissue were estimated as 3.88 and 2.4 μg/g,9 respectively. Meanwhile, Mikamo et al.8 is the only study published to date that has measured fluconazole exposure in the upper FGT (i.e. the uterus, endometrium, oviduct and ovary), with estimated Cmax of 4, 4.1, 4.5 and 3.9 μg/g, respectively, following a 150 mg dose. The exposure of fluconazole in the FGT was reported by Mikamo et al.8 to be similar to that in serum, which implies a TPR of about 1, similar to our findings. Most importantly, these exposures are considered optimal as MICs of fluconazole for Candida albicans are within 0.4–0.8 mg/L (or μg/mL).9
Aside from different biological sample matrices and varying study design, there are other possible explanations for the high drug concentrations in plasma and FGT tissues in our study. As all our participants were hospitalized and critically ill at the time of death, multi-organ dysfunction would not be unexpected. As a consequence, the pharmacokinetics of drugs may have been affected as a collective result of altered absorption, distribution, elimination and excretion under a critically ill status. For instance, change of gastrointestinal function and hypoperfusion of vital organs reduces absorption, and albumin escape increases the free drug portion that is subject to greater distribution and clearance. However, it is worth noting that tenofovir, lamivudine and fluconazole have relatively low protein binding, which implies that decreased albumin should only minimally affect their free drug portion available for elimination. Hepatic and renal failure usually result in reduced metabolic capacity and excretion.16 The majority of participants in our study had an estimated glomerular filtration rate (eGFR) less than 60 mL/min/1.73 m2, which indicates a degree of renal impairment and is presumably a contributing factor of the high concentrations of tenofovir, lamivudine and fluconazole, as all three are primarily renal excreted, whereas efavirenz concentrations in our study were more similar to those observed clinically. Moreover, tissue concentration after death may change due to post-mortem redistribution, under mechanisms including cell death, ongoing blood movement, passive diffusion along a concentration gradient and putrefaction,17 but the exact ‘necrokinetics’ are still unclear. Commonly proposed properties that make a drug subject to post-mortem redistribution include having a volume of distribution (Vd) greater than 3 L/kg and being lipophilic and basic.17,18 Tenofovir, lamivudine and fluconazole have a Vd of less than 3 L/kg and relatively small logP; however, lamivudine and fluconazole are weak bases, which may lead to post-mortem redistribution. On the contrary, efavirenz is lipophilic and has a rather high Vd. Nonetheless, we conducted most of our autopsy within 24 h post-death (median 7.5 h), and all but one of the TPRs were not significantly associated with post-mortem interval, thus, we believe post-mortem redistribution is not particularly worrisome in this case. Finally, there were some outliers in our cohort who had extremely high FGT concentrations. This could be a reflection of the nature of post-mortem sampling and unaccounted-for variables such as the high variability in patient deterioration in their hospital course before death, variable dosing before death, and variable post-mortem storage conditions.
Optimal penetration of antiretrovirals is important when used as prevention to protect women from new infection and viral replication, but it is also critical in women living with HIV to minimize viral shedding and help protect their sexual partners. Female genital shedding of HIV has been shown in previous studies to be correlated with plasma viral load and is reduced by ART.19–21 Furthermore, Kourtis et al.22 found that higher efavirenz concentration in plasma was associated with lower risk of HIV genital shedding (however, the association was not significant for efavirenz concentration in cervicovaginal fluid and genital shedding). Though the relationship between systemic or local exposure of other antiretrovirals and genital viral shedding remains unexplored, we would reasonably assume that an optimal systemic/local exposure will suppress viral shedding and viral reservoir at the transmission site. We would also generally assume that when antiretrovirals are used as pre-exposure prophylaxis (PrEP), an optimal exposure in female genital tissues is critical in prevention of HIV acquisition from sexual transmission, which is supported, for example, by the CAPRISA study, which suggested higher tenofovir concentration in the cervicovaginal fluid correlated with higher protection against HIV infection.23 Generally, for the NRTIs tenofovir and lamivudine, most of our study participants had tissue concentrations in the FGT close to or larger than the plasma concentrations, implying an optimal penetration for the antiviral effect; the highest penetration in vaginal tissue implies even better protection at the common inter-sex transmission site. In addition, the low penetration of efavirenz emphasizes the importance of combined therapy for HIV. However, efficacy of oral tenofovir-based PrEP for women reported by clinical trials is controversial, which is likely confounded by non-adherence.24 Therefore, further studies of the exposure–efficacy relationship are needed and so are more alternative PrEP options for women.
There are several limitations to our study. First, we only measured total drug concentrations in tissues, which are inevitably higher than the free drug concentrations. For the NRTIs tenofovir and lamivudine, attainment of expected therapeutic effect depends on the free drug portion that can diffuse into infected CD4+ T cells, transforming into the active metabolites tenofovir diphosphate and lamivudine triphosphate before suppressing the intracellular viral replication. As there are multiple binding species in tissues, such as phospholipids, interstitial albumin and lipoproteins,25 the free drug concentrations are lower than the total concentration we measured. Second, limited by the nature of post-mortem analysis, we were not able to sample over a period of time for calculation of AUC and derive penetration ratios based on AUCs, which may be a more relevant measure of relative penetration. Third, target concentrations or a TPR cut-off for these antiretrovirals have not been established to relate to efficacy of HIV suppression or prevention. Thus, the cut-off of 1 was chosen as it could be intuitively interpreted as the ‘penetrating ability’ relative to the concentration in the circulating system.
In conclusion, the current study measured concentrations and determined penetration ratios of tenofovir, lamivudine, efavirenz and fluconazole in post-mortem ovarian, uterine, cervical and vaginal tissues from a population of Ugandan women previously living with HIV/AIDs. The post-mortem analysis provides a unique opportunity to evaluate antiretroviral exposure in submucosal tissues. At steady state, tenofovir and lamivudine had better penetration at the solid tissue compartments of the FGT, while penetration of efavirenz was relatively poorer. Fluconazole exposure in FGT was consistently less than that in plasma but still expected to reach FGT tissues in concentrations adequate to treat target pathogens. In addition, although it is often used as an FGT surrogate, we found that cervical tissue may not be able to represent the entire FGT when measuring penetration of anti-infective drugs.
Supplementary data
Figures S1 and S2 are available as Supplementary data at JAC Online.
Supplementary Material
Acknowledgements
We thank the study participants and their next of kin, for making such generous contributions to close the knowledge gap and benefit the successors.
Contributor Information
Fan Wang, Department of Experimental and Clinical Pharmacology, University of Minnesota, Minneapolis, MN, USA.
Olivie C Namuju, Infectious Disease Institute, Kampala, Uganda.
Katelyn A Pastick, Department of Medicine, University of Minnesota, Minneapolis, MN, USA; Massachusetts General Hospital, Boston, MA, USA.
Kizito Abdusalaamu, Infectious Disease Institute, Kampala, Uganda.
Usha Mishra, Department of Experimental and Clinical Pharmacology, University of Minnesota, Minneapolis, MN, USA.
Lindsey Collins, Department of Experimental and Clinical Pharmacology, University of Minnesota, Minneapolis, MN, USA.
David R Boulware, Department of Medicine, University of Minnesota, Minneapolis, MN, USA.
Robert Lukande, Makerere University, Kampala, Uganda.
David B Meya, Department of Medicine, University of Minnesota, Minneapolis, MN, USA; Makerere University, Kampala, Uganda.
Melanie R Nicol, Department of Experimental and Clinical Pharmacology, University of Minnesota, Minneapolis, MN, USA.
Funding
This work was supported by the National Institute of Neurologic Diseases and Stroke (R21NS108344 and R01NS086312), the National Institute of Allergy and Infectious Diseases (K08AI134262) and the University of Minnesota’s NIH Clinical and Translational Science Award (UL1TR002494).
Transparency declarations
All authors: none to declare.
References
- 1. Stieh DJ, Maric D, Kelley ZLet al. Vaginal challenge with an SIV-based dual reporter system reveals that infection can occur throughout the upper and lower female reproductive tract. PLoS Pathog 2014; 10: e1004440. 10.1371/journal.ppat.1004440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Toy EC, Scerpella EG, Riggs JW. Tuboovarian abscess associated with Candida glabrata in a woman with an intrauterine device. A case report. J Reprod Med 1995; 40: 223–5. [PubMed] [Google Scholar]
- 3. Rahangdale L, De Paris K, Kashuba ADMet al. Immunologic, virologic, and pharmacologic characterization of the female upper genital tract in HIV-infected women. J Acquir Immune Defic Syndr 2015; 68: 420–4. 10.1097/QAI.0000000000000480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dumond JB, Yeh RF, Patterson KBet al. Antiretroviral drug exposure in the female genital tract: implications for oral pre- and post-exposure prophylaxis. AIDS 2007; 21: 1899–907. 10.1097/QAD.0b013e328270385a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Patterson KB, Prince HA, Kraft Eet al. Penetration of tenofovir and emtricitabine in mucosal tissues: implications for prevention of HIV-1 transmission. Sci Transl Med 2011; 3: 112re4. 10.1126/scitranslmed.3003174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dumond JB, Nicol MR, Kendrick RNet al. Pharmacokinetic modelling of efavirenz, atazanavir, lamivudine and tenofovir in the female genital tract of HIV-infected pre-menopausal women. Clin Pharmacokinet 2012; 51: 809–22. 10.1007/s40262-012-0012-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kwara A, DeLong A, Rezk Net al. Antiretroviral drug concentrations and HIV RNA in the genital tract of HIV-infected women receiving long-term highly active antiretroviral therapy. Clin Infect Dis 2008; 46: 719–25. 10.1086/527387 [DOI] [PubMed] [Google Scholar]
- 8. Mikamo H, Kawazoe K, Sato Yet al. Penetration of oral fluconazole into gynecological tissues. Antimicrob Agents Chemother 1999; 43: 148–51. 10.1128/AAC.43.1.148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Houang ET, Chappatte O, Byrne Det al. Fluconazole levels in plasma and vaginal secretions of patients after a 150-milligram single oral dose and rate of eradication of infection in vaginal candidiasis. Antimicrob Agents Chemother 1990; 34: 909–10. 10.1128/AAC.34.5.909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nicol MR, Pastick KA, Taylor Jet al. Cerebrospinal fluid and brain tissue penetration of tenofovir, lamivudine, and efavirenz in postmortem tissues with cryptococcal meningitis. Clin Transl Sci 2019; 12: 445–9. 10.1111/cts.12661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Thurman AR, Schwartz JL, Cottrell MLet al. Safety and pharmacokinetics of a tenofovir alafenamide fumarate-emtricitabine based oral antiretroviral regimen for prevention of HIV acquisition in women: a randomized controlled trial. EClinicalMedicine 2021; 36: 100893. 10.1016/j.eclinm.2021.100893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Louissaint NA, Cao YJ, Skipper PLet al. Single dose pharmacokinetics of oral tenofovir in plasma, peripheral blood mononuclear cells, colonic tissue, and vaginal tissue. AIDS Res Hum Retroviruses 2013; 29: 1443–50. 10.1089/aid.2013.0044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hendrix CW, Chen BA, Guddera Vet al. MTN-001: randomized pharmacokinetic cross-over study comparing tenofovir vaginal gel and oral tablets in vaginal tissue and other compartments. PLoS One 2013; 8: e55013. 10.1371/journal.pone.0055013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Herrera C, Lwanga J, Lee Met al. Pharmacokinetic/pharmacodynamic investigation of raltegravir with or without lamivudine in the context of HIV-1 pre-exposure prophylaxis (PrEP). J Antimicrob Chemother 2021; 76: 2129–36. 10.1093/jac/dkab136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yeh RF, Rezk NL, Kashuba ADMet al. Genital tract, cord blood, and amniotic fluid exposures of seven antiretroviral drugs during and after pregnancy in human immunodeficiency virus type 1-infected women. Antimicrob Agents Chemother 2009; 53: 2367–74. 10.1128/AAC.01523-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Owen EJ, Gibson GA, Buckman SA. Pharmacokinetics and pharmacodynamics of antimicrobials in critically ill patients. Surg Infect (Larchmt) 2018; 19: 155–62. 10.1089/sur.2017.262 [DOI] [PubMed] [Google Scholar]
- 17. Yarema MC, Becker CE. Key concepts in postmortem drug redistribution. Clin Toxicol (Phila) 2005; 43: 235–41. 10.1081/CLT-58950 [DOI] [PubMed] [Google Scholar]
- 18. Leikin JB, Watson WA. Post-mortem toxicology: what the dead can and cannot tell us. J Toxicol Clin Toxicol 2003; 41: 47–56. 10.1081/CLT-120018270 [DOI] [PubMed] [Google Scholar]
- 19. Vettore MV, Schechter M, Melo MFet al. Genital HIV-1 viral load is correlated with blood plasma HIV-1 viral load in Brazilian women and is reduced by antiretroviral therapy. J Infect 2006; 52: 290–3. 10.1016/j.jinf.2005.06.002 [DOI] [PubMed] [Google Scholar]
- 20. Nelson JAE, De Paris K, Ramirez Cet al. Female genital tract shedding of HIV-1 is rare in women with suppressed HIV-1 in plasma. AIDS 2020; 34: 39–46. 10.1097/QAD.0000000000002373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Launay O, Tod M, Tschope Iet al. Residual HIV-1 RNA and HIV-1 DNA production in the genital tract reservoir of women treated with HAART: the prospective ANRS EP24 GYNODYN study. Antivir Ther 2011; 16: 843–52. 10.3851/IMP1856 [DOI] [PubMed] [Google Scholar]
- 22. Kourtis AP, Wiener J, Hurst Set al. HIV shedding in the female genital tract of women on ART and progestin contraception: extended follow-up results of a randomized clinical trial. J Acquir Immune Defic Syndr 2019; 81: 163–5. 10.1097/QAI.0000000000002011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kashuba ADM, Gengiah TN, Werner Let al. Genital tenofovir concentrations correlate with protection against HIV infection in the CAPRISA 004 trial: importance of adherence for microbicide effectiveness. J Acquir Immune Defic Syndr 2015; 69: 264–9. 10.1097/QAI.0000000000000607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hodges-Mameletzis I, Fonner VA, Dalal Set al. Pre-exposure prophylaxis for HIV prevention in women: current status and future directions. Drugs 2019; 79: 1263–76. 10.1007/s40265-019-01143-8 [DOI] [PubMed] [Google Scholar]
- 25. Rodgers T, Rowland M. Mechanistic approaches to volume of distribution predictions: understanding the processes. Pharm Res 2007; 24: 918–33. 10.1007/s11095-006-9210-3 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.