Abstract
Background
People with HIV (PWH) have subclinical coronary artery disease (CAD) despite low traditional atherosclerotic cardiovascular disease (ASCVD) risk scores. Coronary plaque in PWH presents as a unique phenotype, but little is known about the contributions of specific inflammatory pathways to plaque phenotypes in PWH.
Methods
The REPRIEVE Mechanistic Substudy enrolled PWH on ART without known cardiovascular disease. We used a targeted discovery proteomics approach to evaluate 246 unique proteins representing cardiovascular, inflammatory, and immune pathways. Proteomic signatures were determined for presence of coronary artery calcium (CAC > 0) and presence of coronary plaque.
Results
Data were available for 662 participants (aged 51 [SD 6] years, ASCVD risk score 4.9% [SD 3.1%]). Among 12 proteins associated with both CAC and presence of coronary plaque, independent of ASCVD risk score, the odds ratios were highest for NRP1: 5.1 (95% confidence interval [CI], 2.3–11.4) for CAC and 2.9 (95% CI, 1.4–6.1) for presence of plaque. Proteins uniquely related to presence of plaque were CST3, LTBR, MEPE, PLC, SERPINA5, and TNFSF13B; in contrast, DCN, IL-6RA, OSMR, ST2, and VCAM1 were only related to CAC.
Conclusions
Distinct immune and inflammatory pathways are differentially associated with subclinical CAD phenotypes among PWH. This comprehensive set of targets should be further investigated to reduce atherosclerosis and ASCVD in PWH.
Clinical Trials Registration
Keywords: proteomics, HIV, coronary artery disease, plaque, CTA
Subclinical coronary artery disease is common among people with HIV, despite relatively low traditional cardiovascular disease risk. We performed a detailed proteomic analysis, demonstrating unique patterns of novel proteins in inflammatory and immune pathways, relating to different plaque phenotypes common.
A significant proportion of antiretroviral therapy (ART) treated people with human immunodeficiency virus (PWH) have subclinical coronary artery disease (CAD) despite relatively low traditional atherosclerotic cardiovascular disease (ASCVD) risk scores and effective ART [1]. This population demonstrates a unique phenotype of subclinical atherosclerosis, with relatively high prevalence of plaque but more modest degrees of coronary artery calcium (CAC) [1]. Prior studies suggest that inflammation and immune activation are associated with cardiac events in PWH [2, 3], but a comprehensive assessment of these factors using a targeted discovery proteomic approach in relation to this plaque phenotype has not been performed.
The Randomized Trial to Prevent Vascular Events (REPRIEVE) is a global primary cardiovascular disease (CVD) prevention trial enrolling ART-treated PWH with low-to-moderate traditional cardiovascular risk and without known history of CVD [4]. Embedded within REPRIEVE is a mechanistic substudy including performance of coronary computed tomography angiography (CTA) [5]. Baseline data demonstrated the presence of CAD in almost 50% and CAC in 35% [1]. In analyses utilizing enzyme-linked immunosorbent assay (ELISA) assays, we demonstrated that markers of arterial inflammation and immune activation, including lipoprotein-associated phospholipase A2 (Lp-PLA2) and interleukin 6 (IL-6), were significantly associated with coronary plaque.
To further our understanding of the potential mechanism of subclinical CAD in PWH with low-to-moderate traditional risk, we now perform a targeted discovery proteomic assessment among participants in the mechanistic substudy of REPRIEVE focusing on proteins with known associations with CVD, inflammation, cardiometabolism, and immunology with the following objectives: (1) identify an association between individual proteins and specific phenotypes of subclinical CAD, (2) assess for potential knowledge-based interactions between proteins to provide mechanistic insight into subclinical CAD in PWH, (3) evaluate whether the proteins are potential mediators of CAD independent of traditional ASCVD risk, and (4) determine the relationship of these proteins to standard measures of immune status.
METHODS
Study Population
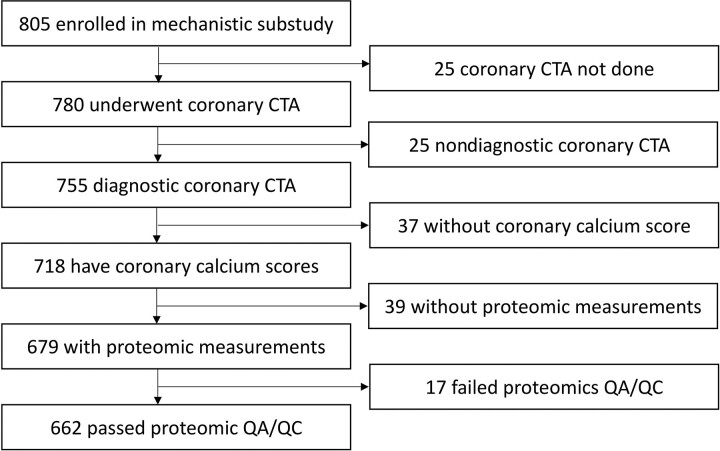
ART-treated PWH aged between 40 and 75 years with low-to-moderate cardiovascular risk and low-density lipoprotein levels, without known CVD or statin therapy, were recruited into REPRIEVE (NCT02344290) [4, 6]. We assessed ASCVD risk prospectively using the 2013 American College of Cardiology/American Heart Association pooled-cohort equation [6] including terms for age, sex, race, cholesterol (high-density lipoprotein and total), diabetes, blood pressure, and smoking. Individuals enrolled into the mechanistic substudy from 1 of 31 participating US REPRIEVE sites, mostly from the AIDS Clinical Trials Group (ACTG) Network. Detailed inclusion and exclusion criteria have been published [4, 5]. The Mass General Brigham Human Research Committee and local institutional review boards approved the study protocol, and participants provided written informed consent. Race, ethnicity, and natal sex were self-reported in accordance with ACTG guidelines. We analyzed data from individuals with diagnostic-quality CTA imaging and paired baseline proteomic measurements. Data from individuals with proteomic measurements not meeting quality assurance criteria were excluded (Figure 1). Immune parameters were obtained prior to the performance of CTA [1].
Figure 1.
Study flow chart. Overall, 662 PWH were analyzed enrolling in the REPRIEVE mechanistic substudy. Abbreviations: CTA, computed tomography angiography; HIV, human immunodeficiency virus; PWH, people with HIV; QA/QC, quality assurance and quality control.
Coronary CTA Acquisition and Analysis
Detailed coronary CTA acquisition and analysis protocols have been previously described [1, 5]. In brief, electrocardiogram (ECG)-synchronized coronary CTA were performed on 64 or more slice computed tomography (CT) scanners at baseline using a standardized study protocol [1, 5]. CAC was evaluated using noncontrast CT images using a modified Agatston score [7]. Coronary arteries were evaluated on CTA images for the presence and composition of atherosclerotic plaque and the degree of stenosis. All CTA images were evaluated at a core laboratory facility in a standardized fashion [1, 5]. To assess proteomic signatures of subclinical CAD, CAC > 0 on noncontrast CT and the presence of any coronary artery plaque (including both noncalcified and calcified plaques) were chosen as our primary outcomes given their relatively high prevalence in our population and the known relationship of CAC and plaque to major adverse cardiovascular events (MACE) in the general population [8]. An exploratory analysis was performed for the smaller subset with vulnerable plaques [9], defined as the presence of positive remodeling (dilatation of the coronary artery outer wall with remodeling index, >1.1), low-density plaque (CT attenuation <30 Hounsfield units), or napkin-ring sign (low central attenuation with ring-like peripheral high attenuation) [1].
Proteomic Measurements and Quality Control
For proteomic analysis, fasting plasma samples were drawn prior to study drug initiation, and stored at −80°C. Three commercially available multiplex immunoassays were used (Olink Target 96 Cardiovascular III, Immuno-oncology, and Cardiometabolic) based on prior studies in smaller cohorts [10] to quantify 275 unique proteins. Definitions, reproducibility, and validation information regarding the proteins can be found at: https://www.olink.com. Data for individuals in whom all measurements on 1 of the protein panels were flagged with warnings were excluded (n = 17; Figure 1). Consistent with the evaluation methodology of other CVD cohorts with this proteomic technology [11], data for 29 proteins were excluded as ≥50% of study samples had values below the limit of detection; 246 proteins met quality control requirements and were analyzed using Normalized Protein eXpression values provided by Olink. A list of proteins included and excluded in the analysis can be found in Supplementary Tables 1 and 2.
Statistical Analysis
Continuous variables are presented as means and standard deviations, or medians and interquartile ranges, while categorical parameters are presented as counts and percentages. To assess associations between proteins and CAD phenotypes, logistic regression was used to estimate the odds ratios (OR) for CAD outcomes per a doubling of each protein. Multiple comparisons were adjusted for using the false discovery rate (FDR) method by Benjamini and Hochberg [12]. To assess whether these associations are independent of traditional risk, we ran multivariate logistic regression models adjusting for ASCVD risk score. Proteins showing an association with any CAD outcome at an FDR level <0.1 were further analyzed. We calculated the interpair Pearson correlation between these proteins and used the 1-correlation value as a distance metric for hierarchical clustering to assess whether there are any apparent protein clusters among significant proteins.
We used the Search Tool for the Retrieval of Interacting Genes/Proteins (STRING) knowledge-based protein-protein interaction database to further elucidate potential connections between the significant proteins [13]. Enrichment analysis was not done due to the lack of sufficient background proteins.
We conducted mediation analysis to determine whether key proteins mediated the direct association of traditional ASCVD risk to CAD outcomes. We simulated mediation using 1000 Monte Carlo draws for quasi-Bayesian approximation using the mediation R package (version 4.5.0) [14]. Analyses were done using the ggstatsplot (version 0.9.0) R package.
Protein expression levels were compared by CD4, nadir CD4, viral load, and ART regimen (Mann-Whitney or Kruskal-Wallis ANOVA as appropriate) with post hoc comparisons (Dunn-test with Holm’s P value correction).
An FDR corrected P value of <.1 was considered significant in assessing the relationship of proteins to plaque outcomes in our primary analysis. We used a nominal P value threshold of ≤.01 in exploratory analyses. For comparisons between those with and without plaque or CAC, and for our mediation analysis, a 2-sided P value smaller than .05 was considered statistically significant. All statistical analyses were done using R (version 4.0.2) [15].
RESULTS
Patient Characteristics
Overall, full data were available and analyzed for 662 individuals (Figure 1) (aged 50.8 [SD 5.8] years; male sex, 83%, 547/662; ASCVD risk score, 4.9% [SD 3.1%]). Age and demographics were similar in the analysis cohort and the full cohort recruited into the substudy (Supplementary Table 3). All participants were taking ART (duration 11.6 [SD 6.5] years) and 88% had undetectable viral load. Among the 662 PWH, 321 (48%) had evidence of coronary plaque, of whom 231 (72%, 231/321) had CAC > 0 and 145 (45%, 145/321) demonstrated vulnerable plaque. Of those with CAC, the majority, 163/231 had CAC values between 1 and 100. Smaller subsets, 56/231 and 12/231 demonstrated CAC between 101 and 400 and greater than 400, respectively. Most individuals with plaque had nonobstructive CAD, with only 22 individuals having stenoses greater than 50%. Demographic, risk factor, and HIV-associated parameter information can be found in Table 1, stratified by presence of any CAC and any plaque.
Table 1.
Patient Characteristics
| Variable | Overall (n = 662) | Presence of Coronary Calcium | Presence of Coronary Plaque | ||
|---|---|---|---|---|---|
| No (n = 431) | Yes (n = 231) | No (n = 341) | Yes (n = 321) | ||
| Demographic and behavioral characteristics | |||||
| Age, y | 50.8 ± 5.8 | 49.8 ± 5.5 | 52.6 ± 5.8 | 49.6 ± 5.5 | 52.0 ± 5.8 |
| Natal sex | |||||
| Female | 115 (17) | 88 (20) | 27 (12) | 78 (23) | 37 (12) |
| Male | 547 (83) | 343 (80) | 204 (88) | 263 (77) | 284 (88) |
| Race | |||||
| Asian | 8 (1.2) | 5 (1.2) | 3 (1.3) | 5 (1.5) | 3 (0.9) |
| Black or African American | 233 (35) | 166 (39) | 67 (29) | 141 (41) | 92 (29) |
| White | 354 (53) | 214 (50) | 140 (61) | 159 (47) | 195 (61) |
| Other | 67 (10) | 46 (11) | 21 (9.1) | 36 (11) | 31 (9.7) |
| Ethnicity (n = 653) | |||||
| Hispanic or Latino | 163 (25) | 117 (28) | 46 (20) | 85 (25) | 78 (25) |
| Not Hispanic or Latino | 490 (75) | 307 (72) | 183 (80) | 251 (75) | 239 (75) |
| Smoking status (n = 661) | |||||
| Current | 158 (24) | 100 (23) | 58 (25) | 81 (24) | 77 (24) |
| Former | 211 (32) | 131 (30) | 80 (35) | 99 (29) | 112 (35) |
| Never | 292 (44) | 200 (46) | 92 (40) | 161 (47) | 131 (41) |
| Cardiovascular and metabolic characteristics | |||||
| ASCVD, % | 4.9 ± 3.1 | 4.4 ± 2.9 | 5.8 ± 3.3 | 4.3 ± 2.9 | 5.6 ± 3.2 |
| ASCVD risk group | |||||
| 0–2.5 | 156 (24) | 120 (28) | 36 (16) | 107 (31) | 49 (15) |
| 2.5–5 | 224 (34) | 154 (36) | 70 (30) | 116 (34) | 108 (34) |
| 5–7.5 | 154 (23) | 91 (21) | 63 (27) | 70 (21) | 84 (26) |
| 7.5–10 | 88 (13) | 47 (11) | 41 (18) | 32 (9.4) | 56 (17) |
| >10 | 40 (6.0) | 19 (4.4) | 21 (9.1) | 16 (4.7) | 24 (7.5) |
| Family history of premature CVD | 145 (23) | 90 (21) | 55 (25) | 65 (20) | 80 (26) |
| Hypertension | 213 (32) | 127 (29) | 86 (37) | 100 (29) | 113 (35) |
| Diabetes | 3 (0.5) | 1 (0.2) | 2 (0.9) | 0 (0) | 3 (0.9) |
| BMI, kg/m2 | 27.3 ± 4.3 | 27.4 ± 4.5 | 27.3 ± 4.1 | 27.4 ± 4.5 | 27.3 ± 4.2 |
| Fasting glucose, mg/dL | 92.9 ± 12.5 | 92.3 ± 12.1 | 94.0 ± 13.2 | 91.7 ± 11.0 | 94.1 ± 13.8 |
| eGFR, mL/min/1.73m2 | 88.4 ± 16.3 | 88.7 ± 16.5 | 87.6 ± 15.8 | 89.3 ± 16.5 | 87.3 ± 15.9 |
| LDL-C, mg/mL | 108.3 ± 30.5 | 106.8 ± 30.3 | 111.0 ± 30.8 | 104.1 ± 29.4 | 112.6 ± 31.1 |
| HDL-C, mg/dL | 50.9 ± 18.7 | 50.6 ± 18.5 | 51.3 ± 19.0 | 51.7 ± 19.3 | 49.9 ± 17.9 |
| Triglycerides, mg/dL | 133.6 ± 85.2 | 135.0 ± 86.0 | 131.2 ± 83.7 | 133.1 ± 88.9 | 134.2 ± 81.1 |
| Prior statin use | 53 (8.0) | 28 (6.5) | 25 (11) | 17 (5.0) | 36 (11) |
| Prior antihypertensive medication | 133 (20) | 76 (18) | 57 (25) | 61 (18) | 72 (22) |
| HIV-related health history | |||||
| Total ART use, y | 11.6 ± 6.5 | 11.1 ± 6.4 | 12.6 ± 6.7 | 11.0 ± 6.4 | 12.2 ± 6.6 |
| Entry ART regimen | |||||
| NRTI + INSTI | 293 (44) | 187 (43) | 106 (46) | 147 (43) | 146 (45) |
| NRTI + NNRTI | 167 (25) | 111 (26) | 56 (24) | 90 (26) | 77 (24) |
| NRTI + PI | 118 (18) | 80 (19) | 38 (16) | 64 (19) | 54 (17) |
| NRTI-sparing | 20 (3.0) | 11 (2.6) | 9 (3.9) | 9 (2.6) | 11 (3.4) |
| Other NRTI-containing | 64 (9.7) | 42 (9.7) | 22 (9.5) | 31 (9.1) | 33 (10) |
| HIV RNA, copies/mL, (n = 654) | |||||
| <LLQ | 573 (88) | 374 (88) | 199 (88) | 291 (86) | 282 (89) |
| LLQ–400 | 65 (9.9) | 39 (9.1) | 26 (11) | 33 (9.8) | 32 (10) |
| 400+ | 16 (2.4) | 14 (3.3) | 2 (0.9) | 13 (3.9) | 3 (0.9) |
| CD4 category, cells/mm3 | 624.2 ± 275.1 | 642.4 ± 274.4 | 590.3 ± 273.8 | 635.2 ± 266.1 | 612.6 ± 284.3 |
| Nadir CD4 category, cells/mm3 (n = 646) | |||||
| <50 | 146 (23) | 84 (20) | 62 (27) | 64 (19) | 82 (26) |
| 50–199 | 192 (30) | 127 (30) | 65 (29) | 102 (31) | 90 (28) |
| 200–349 | 180 (28) | 116 (28) | 64 (28) | 95 (29) | 85 (27) |
| 350+ | 128 (20) | 92 (22) | 36 (16) | 69 (21) | 59 (19) |
Data are presented as mean ± standard deviations, or as frequencies (percentages). Percentages are presented considering the proportion of available data presented in parenthesis for parameters with missing values.
Abbreviations: ART, antiretroviral therapy; ASCVD, atherosclerotic cardiovascular disease; BMI, body mass index; CVD, cardiovascular disease; eGFR, estimated glomerular filtration rate; HDL-C, high-density lipoprotein cholesterol; HIV, human immunodeficiency virus; INSTI, integrase strand transfer inhibitor; LDL-C, low-density lipoprotein cholesterol; LLQ, lower limit of quantification; NRTI, nucleoside reverse transcriptase inhibitor; PI, protease inhibitor.
Associations Between Proteins and Plaque Phenotypes
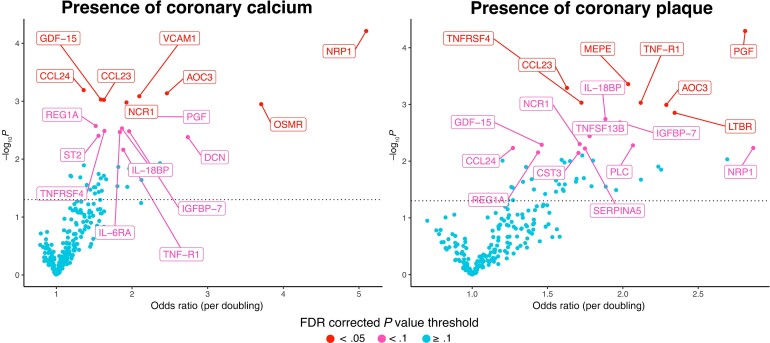
Proteins significantly associated with CAD outcomes before adjustment for ASCVD are shown in Supplementary Figure 1, while results after adjustment are shown in Figure 2. The known biological function and relationship to ASCVD of individually significant proteins are shown in Table 2. Results for all proteins are presented in Supplementary Table 2.
Figure 2.
Volcano plots of ASCVD risk adjusted odds ratios between proteins and CAD outcomes. Each point represents a single protein. The position of each point represents the odds ratio and the P value for the association between the protein and the given CAD outcome corrected for ASCVD risk. The y axis shows the −log10 of the P values, therefore the higher it is the more significant the association. The dotted horizontal line indicates P = .05. The proteins are colored according to FDR corrected P values, where P values >.1 are blue, P values between .05 and .1 are pink, and P < .05 are red. All proteins with FDR corrected P < .1 are labeled on the plots. Abbreviations: ASCVD, atherosclerotic cardiovascular disease; CAD: coronary artery disease; FDR, false discovery rate; OR, odds ratio. Protein name abbreviations can be found in Supplementary Material.
Table 2.
Known Biological Function and Relationship to ASCVD in Significant Proteins
| Protein | Biological Function, From STRING | Clinically Relevant Function, From the Literature | Related to CAC | Related to Presence of Plaque |
|---|---|---|---|---|
| AOC3 | Amine oxidase, copper containing 3; membrane primary amine oxidase Cell adhesion protein that participates in lymphocyte extravasation and recirculation by mediating the binding of lymphocytes to peripheral lymph node vascular endothelial cells in an L-selectin-independent fashion Has semicarbazide-sensitive (SSAO) monoamine oxidase activity May play a role in adipogenesis |
|
X | X |
| CCL23 | C-C motif chemokine ligand 23 |
|
X | X |
| CCL24 | C-C motif chemokine 24 Chemotactic for resting T-lymphocytes, and eosinophils Has lower chemotactic activity for neutrophils but none for monocytes and activated lymphocytes Is a strong suppressor of colony formation by a multipotential hematopoietic progenitor cell line Binds to CCR3 Belongs to the intercrine β (chemokine CC) family |
|
X | X |
| CST3 | Cystatin-C As an inhibitor of cysteine proteinases, this protein is thought to serve an important physiological role as a local regulator of this enzyme activity Belongs to the cystatin family |
|
X | |
| DCN | Decorin May affect the rate of fibrils formation Small leucine rich repeat proteoglycans |
|
X | |
| GDF-15 | Growth differentiation factor 15 Belongs to the TGF-β family |
|
X | X |
| IGFBP-7 | Insulin-like growth factor-binding protein 7 Binds IGF-I and IGF-II with a relatively low affinity Stimulates prostacyclin (PGI2) production Stimulates cell adhesion I-set domain containing |
|
X | X |
| IL-18BP | Interleukin-18-binding protein Isoform A binds to IL-18 and inhibits its activity Functions as an inhibitor of the early Th1 cytokine response Immunoglobulin-like domain containing |
|
X | X |
| IL-6RA | Interleukin-6 receptor subunit α Part of the receptor for interleukin 6 Binds to IL-6 with low affinity, but does not transduce a signal Signal activation necessitate an association with IL6ST Activation may lead to the regulation of the immune response, acute-phase reactions, and hematopoiesis CD molecules |
X | ||
| LTBR | Tumor necrosis factor receptor superfamily member 3 Receptor for the heterotrimeric lymphotoxin containing LTA and LTB, and for TNFS14/LIGHT Promotes apoptosis via TRAF3 and TRAF5 May play a role in the development of lymphoid organs Tumor necrosis factor receptor superfamily |
|
X | |
| MEPE | Matrix extracellular phosphoglycoprotein Promotes renal phosphate excretion and modulates mineralization SIBLING family |
|
X | |
| NCR1 | Natural cytotoxicity triggering receptor 1 Cytotoxicity-activating receptor that may contribute to the increased efficiency of activated natural killer (NK) cells to mediate tumor cell lysis |
X | X | |
| NRP1 | Neuropilin-1 The membrane-bound isoform 1 is a receptor involved in the development of the cardiovascular system, in angiogenesis, in the formation of certain neuronal circuits, and in organogenesis outside the nervous system It mediates the chemorepulsant activity of semaphorins It binds to semaphorin 3A, PLGF-2 isoform of PGF, VEGF165 isoform of VEGFA, and VEGFB Coexpression with KDR results in increased VEGF165 binding to KDR as well as increased chemotaxis Regulate VEGF-induced angiogenesis |
|
X | X |
| OSMR | Oncostatin-M-specific receptor subunit β Associates with IL31RA to form the IL-31 receptor Binds IL-31 to activate STAT3 and possibly STAT1 and STAT5 Capable of transducing OSM-specific signaling events Fibronectin type III domain containing |
|
X | |
| PGF | Placental growth factor; placenta growth factor Growth factor active in angiogenesis and endothelial cell growth, stimulating their proliferation and migration It binds to the receptor FLT1/VEGFR-1 Isoform PlGF-2 binds NRP1/neuropilin-1 and NRP2/neuropilin-2 in a heparin-dependent manner Also promotes cell tumor growth |
|
X | X |
| PLC | Basement membrane-specific heparan sulfate proteoglycan core protein Integral component of basement membranes Component of the glomerular basement membrane, responsible for the fixed negative electrostatic membrane charge, and provides a barrier which is both size- and charge-selective It serves as an attachment substrate for cells Plays essential roles in vascularization Critical for normal heart development and for regulating the vascular response to injury Also required for avascular cartilage development I-set domain containing |
|
X | |
| REG1A | Lithostathine-1-α Might act as an inhibitor of spontaneous calcium carbonate precipitation May be associated with neuronal sprouting in brain, and with brain and pancreas regeneration C-type lectin domain containing |
X | X | |
| SERPINA5 | Plasma serine protease inhibitor Heparin-dependent serine protease inhibitor acting in body fluids and secretions Inactivates serine proteases by binding irreversibly to their serine activation site Involved in the regulation of intravascular and extravascular proteolytic activities Plays hemostatic roles in the blood plasma Acts as a procoagulant and proinflammatory factor by inhibiting the anticoagulant activated protein C factor as well as the generation of activated protein C factor by the thrombin/thrombomodulin complex |
X | ||
| ST2 | Interleukin-1 receptor-like 1 Receptor for IL-33 Signaling requires association of the coreceptor IL1RAP Its stimulation recruits MYD88, IRAK1, IRAK4, and TRAF6, followed by phosphorylation of MAPK3/ERK1 and/or MAPK1/ERK2, MAPK14, and MAPK8 Possibly involved in helper T-cell function I-set domain containing |
|
X | |
| TNF-R1 | Tumor necrosis factor receptor superfamily member 1A Receptor for TNFSF2/TNF-αand homotrimeric TNFSF1/lymphotoxin-α The adapter molecule FADD recruits caspase-8 to the activated receptor The resulting death-inducing signaling complex (DISC) performs caspase-8 proteolytic activation which initiates the subsequent cascade of caspases (aspartate- specific cysteine proteases) mediating apoptosis Contributes to the induction of noncytocidal TNF effects including antiviral state and activation of the acid sphingomyelinase CD molecules |
|
X | X |
| TNFRSF4 | Tumor necrosis factor receptor superfamily member 4 Receptor for TNFSF4/OX40L/GP34 A costimulatory molecule implicated in long-term T-cell immunity CD molecules |
|
X | X |
| TNFSF13B | Tumor necrosis factor ligand superfamily member 13B Cytokine that binds to TNFRSF13B/TACI and TNFRSF17/BCMA TNFSF13/APRIL binds to the same 2 receptors Together, they form a 2 ligands-2 receptors pathway involved in the stimulation of B- and T-cell function and the regulation of humoral immunity A third B-cell specific BAFF-receptor (BAFFR/BR3) promotes the survival of mature B cells and the B-cell response CD molecules |
X | ||
| VCAM1 | Vascular cell adhesion protein 1 Important in cell-cell recognition Appears to function in leukocyte-endothelial cell adhesion Interacts with integrin α-4/β-1 (ITGA4/ITGB1) on leukocytes, and mediates both adhesion and signal transduction The VCAM1/ITGA4/ITGB1 interaction may play a pathophysiologic role both in immune responses and in leukocyte emigration to sites of inflammation C2-set domain containing |
|
X |
Abbreviations: ASCVD, atherosclerotic cardiovascular disease; CAC, coronary artery calcium; CAD, coronary artery disease; CVD, cardiovascular disease; HIV, human immunodeficiency virus; IL, interleukin; MACE, major adverse cardiovascular events; NF-kB, nuclear factor-κB; STRING, Search Tool for the Retrieval of Interacting Genes/Proteins.
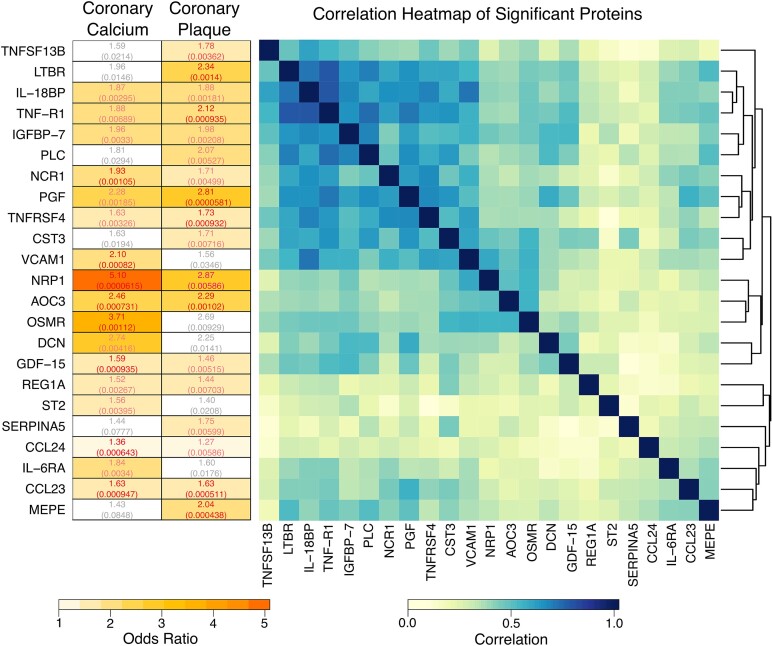
Overall, 23 proteins were significant for at least 1 of our 2 CAD outcomes in models adjusting for ASCVD risk (Figure 3). Of these 23 proteins, 12 were associated with both CAC > 0 and presence of plaque. Of these, the ORs were highest for NRP1: 5.1 (95% confidence interval [CI], 2.3–11.4) for CAC and 2.9 (95% CI, 1.4–6.1) for plaque. CST3, LTBR, MEPE, PLC, SERPINA5, and TNFSF13B were uniquely related to presence of plaque (see Supplementary Table 2 for protein functions and adjusted ORs for plaque phenotypes). In contrast, DCN, IL-6RA, OSMR, ST2, and VCAM1 were associated with the presence of CAC. Hierarchical clustering showed 1 or 2 clusters of moderately interrelated proteins among those significantly related to either plaque or CAC. For the 23 proteins, adjusted ORs and nominal P values for both outcomes can be found in Figure 3.
Figure 3.
Odds ratios and correlation heatmap of significant proteins. Left, odds ratios and the corresponding nominal P values in parenthesis are shown for proteins that had a significant association with at least 1 of the CAD outcomes after correcting for ASCVD risk score. The numbers are colored according to the FDR corrected values, where grey is P > .1, pink is P between .05 and .1, and red indicates P < .05. The boxes are colored according to the magnitude of the odds ratio, where nonsignificant associations are white. Right, Pearson correlation heatmap of the proteins with the corresponding hierarchical clustering dendrogram is shown. Abbreviations: ASCVD, atherosclerotic cardiovascular disease; CAD, coronary artery disease; FDR, false discovery rate. Protein name abbreviations can be found in Supplementary Material.
In exploratory analyses, NRP1, AOC3, MEPE, and PGF were most strongly related to the presence of vulnerable plaque adjusting for ASCVD risk, with NRP1 having the highest OR, 3.3 (95% CI, 1.4–7.9) (Supplementary Table 2 and Supplementary Figure 2).
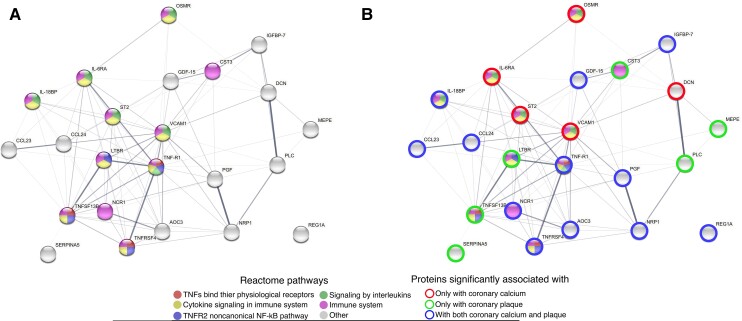
Knowledge-Based Interactions Between Significant Proteins Using the STRING Database
To provide biologic insight, we annotated the 23 proteins described above using the STRING database and found multiple connections. The most common reactome pathways associated with our proteins were (pathway name [number of proteins involved]): immune system (n = 11), cytokine signaling in immune system (n = 9), signaling by interleukins (n = 6), TNFR2 noncanonical NF-κB pathway (n = 4), and TNFs binding to physiological receptors (n = 3). The resulting protein-protein interaction network is presented in Figure 4A. Patterns of proteins uniquely associated with either CAC, coronary plaque, or both are shown in Figure 4B. STRING characteristics on homology, coexpression, experimentally determined interaction, and text mining scores are shown in Supplementary Table 4.
Figure 4.
A and B, Protein-protein interaction network of significant proteins. Protein-protein interaction network of proteins that had a significant association with 1 of the CAD outcomes after correcting for ASCVD risk score. The edges between the protein nodes are proportional to the interaction score between the proteins from the STRING database considering all types of evidence. Only edges with interaction scores >0.15 are shown. The color of the node indicates the reactome pathway with which the protein is associated. The 5 most enriched reactome pathways are displayed. Summary of the function of the proteins and the interactions between them are provided in Supplementary Material. Image was generated using: https://string-db.org/. Abbreviations: ASCVD, atherosclerotic cardiovascular disease; CAD, coronary artery disease.
Mediation Analysis on the Effects of ASCVD on CAD Outcomes
We further assessed whether these 23 proteins mediated the associations between ASCVD risk score and plaque phenotypes. ASCVD risk score was highly related to both CAD outcomes. Several proteins were shown to have a significant individual effect to mediate the association between ASCVD risk score and CAC, including IL-18BP, TNF-R1, IGFBP-7, PLC, NCR1, PGF, CST3, DCN, GDF-15, and ST2. However, the absolute value of the proportion of the effect of ASCVD mediated through these proteins was very low (range, 4.6%–12.6%; Table 3). Results in the mediation analysis for any plaque were generally similar, with the addition of MEPE as having a significant effect to mediate the association of ASCVD risk score. Again, however, the absolute proportion of the effect of ASCVD mediated through these proteins was very low (range, 3.9%–10.3%; Table 3).
Table 3.
Mediation Analysis Results Considering the Presence of Coronary Calcium and Coronary Plaque
| Proteins | Direct Effect of ASCVD | Indirect Effect of ASCVD | |||||
|---|---|---|---|---|---|---|---|
| OR | (95% CI) | P | OR | (95% CI) | P | Proportion Mediated, % | |
| Presence of coronary calcium | |||||||
| TNFSF13B | 1.024 | (1.017–1.030) | <.0001 | 1.001 | (1.000–1.002) | .084 | 2.600 |
| LTBR | 1.024 | (1.017–1.030) | <.0001 | 1.001 | (1.000–1.002) | .088 | 2.740 |
| IL-18BP | 1.024 | (1.017–1.029) | <.0001 | 1.001 | (1.000–1.003) | .016 | 4.650 |
| TNF-R1 | 1.023 | (1.016–1.029) | <.0001 | 1.001 | (1.000–1.003) | .002 | 5.711 |
| IGFBP-7 | 1.022 | (1.015–1.028) | <.0001 | 1.002 | (1.001–1.005) | .002 | 9.396 |
| PLC | 1.023 | (1.017–1.029) | <.0001 | 1.001 | (1.000–1.003) | .024 | 4.844 |
| NCR1 | 1.026 | (1.020–1.032) | <.0001 | 0.999 | (.997–1.000) | .010 | −5.476 |
| PGF | 1.023 | (1.016–1.029) | <.0001 | 1.002 | (1.001–1.004) | .004 | 8.225 |
| TNFRSF4 | 1.025 | (1.018–1.030) | <.0001 | 1.000 | (.999–1.001) | .694 | −0.639 |
| CST3 | 1.023 | (1.015–1.029) | <.0001 | 1.002 | (1.000–1.004) | .012 | 7.501 |
| VCAM1 | 1.025 | (1.018–1.031) | <.0001 | 1.000 | (.998–1.001) | .642 | −0.993 |
| NRP1 | 1.024 | (1.017–1.029) | <.0001 | 1.001 | (1.000–1.003) | .128 | 4.381 |
| AOC3 | 1.025 | (1.018–1.031) | <.0001 | 0.999 | (.998–1.001) | .376 | −1.876 |
| OSMR | 1.025 | (1.018–1.030) | <.0001 | 1.000 | (.999–1.001) | .782 | 0.597 |
| DCN | 1.023 | (1.016–1.029) | <.0001 | 1.001 | (1.000–1.003) | .016 | 5.527 |
| GDF-15 | 1.021 | (1.014–1.027) | <.0001 | 1.003 | (1.001–1.006) | .002 | 12.578 |
| REG1A | 1.024 | (1.018–1.030) | <.0001 | 1.001 | (.999–1.002) | .290 | 2.112 |
| ST2 | 1.023 | (1.016–1.029) | <.0001 | 1.001 | (1.000–1.003) | .018 | 5.224 |
| SERPINA5 | 1.024 | (1.018–1.030) | <.0001 | 1.000 | (1.000–1.001) | .358 | 1.102 |
| CCL24 | 1.025 | (1.019–1.030) | <.0001 | 1.000 | (.998–1.001) | .778 | −0.656 |
| IL-6RA | 1.026 | (1.019–1.032) | <.0001 | 0.999 | (.998–1.000) | .094 | −3.559 |
| CCL23 | 1.025 | (1.018–1.031) | <.0001 | 1.000 | (.999–1.001) | .968 | −0.084 |
| MEPE | 1.025 | (1.019–1.031) | <.0001 | 0.999 | (.998–1.000) | .098 | −2.786 |
| Presence of coronary plaque | |||||||
| TNFSF13B | 1.031 | (1.021–1.040) | <.0001 | 1.001 | (1.000–1.003) | .064 | 3.305 |
| LTBR | 1.032 | (1.021–1.041) | <.0001 | 1.001 | (1.000–1.003) | .082 | 3.576 |
| IL-18BP | 1.031 | (1.021–1.040) | <.0001 | 1.002 | (1.000–1.004) | .016 | 4.685 |
| TNF-R1 | 1.030 | (1.020–1.040) | <.0001 | 1.002 | (1.001–1.004) | .002 | 6.763 |
| IGFBP-7 | 1.029 | (1.018–1.039) | <.0001 | 1.003 | (1.001–1.006) | <.0001 | 9.519 |
| PLC | 1.031 | (1.021–1.040) | <.0001 | 1.002 | (1.001–1.004) | <.0001 | 5.944 |
| NCR1 | 1.035 | (1.024–1.043) | <.0001 | 0.998 | (.997–1.000) | .010 | −4.416 |
| PGF | 1.029 | (1.019–1.038) | <.0001 | 1.003 | (1.001–1.006) | <.0001 | 10.291 |
| TNFRSF4 | 1.033 | (1.023–1.042) | <.0001 | 1.000 | (.998–1.001) | .694 | −0.724 |
| CST3 | 1.030 | (1.019–1.039) | <.0001 | 1.003 | (1.001–1.005) | <.0001 | 8.152 |
| VCAM1 | 1.033 | (1.022–1.042) | <.0001 | 1.000 | (.999–1.001) | .674 | −0.512 |
| NRP1 | 1.032 | (1.021–1.041) | <.0001 | 1.001 | (1.000–1.003) | .144 | 2.777 |
| AOC3 | 1.033 | (1.022–1.043) | <.0001 | 0.999 | (.998–1.001) | .376 | −1.704 |
| OSMR | 1.033 | (1.022–1.042) | <.0001 | 1.000 | (.999–1.001) | .786 | 0.370 |
| DCN | 1.031 | (1.021–1.040) | <.0001 | 1.002 | (1.000–1.003) | .028 | 4.473 |
| GDF-15 | 1.029 | (1.018–1.038) | <.0001 | 1.003 | (1.001–1.006) | .008 | 10.315 |
| REG1A | 1.032 | (1.022–1.041) | <.0001 | 1.001 | (.999–1.002) | .296 | 1.777 |
| ST2 | 1.031 | (1.020–1.041) | <.0001 | 1.001 | (1.000–1.003) | .030 | 3.917 |
| SERPINA5 | 1.032 | (1.022–1.041) | <.0001 | 1.001 | (.999–1.002) | .314 | 1.896 |
| CCL24 | 1.033 | (1.023–1.041) | <.0001 | 1.000 | (.998–1.001) | .782 | −0.454 |
| IL-6RA | 1.034 | (1.023–1.043) | <.0001 | 0.999 | (.997–1.000) | .100 | −2.679 |
| CCL23 | 1.033 | (1.023–1.042) | <.0001 | 1.000 | (.998–1.002) | .968 | −0.085 |
| MEPE | 1.035 | (1.025–1.043) | <.0001 | 0.998 | (.996–1.000) | .014 | −5.565 |
Proteins that significantly mediate the effects of ASCVD risk are shown in bold.
Abbreviations: ASCVD, atherosclerotic cardiovascular disease; CI, confidence interval; OR, odds ratio.
Association With Immune Function Indices
Lower nadir CD4 counts were associated with higher GDF-15 levels and lower SERPINA5 levels. Lower CD4 counts were associated with higher CST3, IL-18BP, IL-6RA, NCR1, PLC, TNF-R1, and TNFRSF4 levels (Supplementary Table 5 and Supplementary Figure 3). Minimal differences were seen comparing by detectable viral load and by ART regimen, without a consistent pattern (Supplementary Figure 4).
DISCUSSION
In this study, we demonstrate a novel proteomic signature of subclinical CAD in PWH, with different sets of proteins relating to the presence of CAC and any plaque, after controlling for traditional ASCVD risk. Key findings include discovery of proteins representing inflammatory and immune pathways not previously associated with CAD in PWH, including NRP1, involved in cell migration and endothelial function [16]. Clear relationships of proteins to known pathways of NF-κB, cytokine response and TNF-related inflammation are seen. These findings inform us of novel biological pathways beyond traditional risk factors that may contribute to subclinical CAD and may be targets for specific immune modulation strategies.
We identified a rich set of proteins associated with presence of subclinical CAD, controlling for traditional ASCVD risk in PWH. Among the proteins most strongly associated were some that have been shown in general-population studies to be highly relevant to CAD including: TNF-R1, associated with CV mortality in those with chronic coronary heart disease (CHD) [17]; Il-18BP, increased in acute coronary patients [18]; IGFBP-7, a recently discovered marker of CAD [19]; PGF, thought to represent a chronic source of vascular inflammation, and a marker of overall mortality after non-ST-segment acute coronary syndrome [20]; CCL23, a chemokine that may contribute to vascular inflammation and a useful marker to detect atherosclerosis [21]; AOC3, associated with cardiovascular risk factors and related to preclinical atherosclerosis [22, 23]; TNFRSF4, postulated to influence atherosclerosis [24]; MEPE, associated with ischemic stroke [25]; PLC, which regulates inflammation and angiogenesis [26]; CCL24, which acts on CCR3-bearing cells including T-helper cells type 2, found to be a strong marker of cardiac ageing [27]; and CST3, which encodes cystatin 3, a potent cysteine protease inhibitor, thought to play a role in human vascular pathophysiology, relating strongly to future CHD and ischemic stroke in humans [28]. Significant interest has been focused on GDF-15, which was recently associated with incident all-cause mortality and CVD death among a population of patients without HIV infection [29], as well as death and MACE in the Framingham Heart Study. GDF-15 expression is thought to increase in relation to cardiovascular inflammation and tissue injury, potentially in a counter-regulatory protective role [30].
GDF-15, CCL24, PGF, and DCN related to CAD in a prior proteomics study of patients in the general population with chest pain [31]. Activation of such pathways in an asymptomatic younger population with HIV and lower traditional ASCVD risk provides insight into the biology of the development of subclinical atherosclerotic changes in PWH. Other proteins found to be significant in the general population did not relate to CAD in our cohort of PWH, suggesting unique pathways may also be associated with CAD among PWH [31].
PWH have been shown to have a CAD pattern enriched with noncalcified plaque [1, 32]. Indeed, we saw a relatively low percentage with CAC > 0 (35%) and a higher percentage with any plaque (48%) among PWH. In contrast, among an older, general population of European participants enrolled in the SCAPIS study without known CVD, plaque was seen in 42%, but the vast majority also had CAC > 0 [33]. While several proteins related to both CAC and plaque in our study, there are differences in the protein patterns, suggesting that different pathways may contribute more specifically to early calcification compared to a more mixed plaque pattern. Among the strongest related proteins to both CAC and presence of plaque in our study was NRP1, a neuropilin, that has roles in signaling and angiogenesis [34]. NRPs are thought to be involved in cell migration and promotion of endothelial cell proliferation, motility, and apoptosis [16]. Recent data in animal models suggests that increased NRP1 expression on T cells is atherogenic, results in increased IFN secretion, and facilitates T-cell migration to the aorta [35]. Increased expression of NRP1 may mark inflammatory T cells, which expand to facilitate atherosclerosis [35]. Moreover, NRP1 mediates vascular disease in the context of PDGF and TGFB signaling [36]. In contrast, unique proteins more associated with CAC in our study included VCAM1, a marker of endothelial function associated with plaque and carotid intima–media thickness (CIMT) [37]; OSMR, an anti-inflammatory protein thought to decrease IL-8–mediated recruitment of neutrophils [38]; DCN, decorin, with antifibrotic and anti-inflammatory properties [39]; and IL-6RA. Differential upregulation of pro- and anti-inflammatory pathways may contribute to the development of specific plaque phenotypes.
We have previously shown a relatively high proportion of asymptomatic PWH to have vulnerable plaque [1], which has been linked to increased MACE in the general population [40]. In exploratory analyses, we now demonstrate that NRP1, AOC3, MEPE, and PGF are most strongly associated with the presence of vulnerable plaque in our study population. Demonstration of overlapping proteins, including NRP1 and PGF, associated with multiple plaque phenotypes assessed in this analysis reinforces the potential role of these pathways in subclinical CAD among PWH. Indeed, NRP1 was the protein most strongly and consistently associated with all 3 plaque phenotypes examined in this study. Further work is needed to understand the unique pathways contributing to these subclinical plaque phenotypes, relating specific proteins to progression of disease and assessing the effects of modulating these pathways on CAD in PWH.
We demonstrated interrelationships among the proteins associated with CAD, after adjustment for ASCVD risk score. A critical pathway in atherogenesis involves activation of NF-κB, with subsequent activation of the NLRP3 inflammasome as well as IL-1β. Analysis of reactome pathways, showed 4 key proteins involved in the NF-κB signaling pathways: LTBR, TNF-R1, TNFSF13B, and TNFRSF4. Multiple studies have demonstrated that activation of the inflammasome is involved in atherosclerosis [41]. Recently, activation of caspase-1, a critical component of the inflammasome pathway, was associated with atherosclerosis among PWH [42]. Additional pathways associated with CAD in this study include proteins related to immune function as well as TNF, cytokine, and interleukin signaling. Among these proteins, TNFSF13B (survival of mature B-cells), LTBR (tumor necrosis factor superfamily), NCR1 (activates NK cells), and TNFRSF4 (involved in long-term T-cell immunity) have key immune regulatory functions which may link persistent immune activation to subclinical atherogenesis among PWH.
Among PWH, ART reduces viremia and increases CD4 count, but indices of inflammation and immune activation persist [2]. Drivers of this inflammatory milieu are thought to include persistent low-level viral replication, coinfections, and increased intestinal permeability. Prior work has demonstrated key proteins in these domains to be upregulated in PWH compared to individuals without HIV infection [10]. This study advances the field by now linking proteins previously found to be upregulated within these domains among PWH, including CST3, GDF-15, IGFBP-7, IL-18BP, REG1A, SERPINA5, TNFSF13B, and VCAM1 [10], to distinct subclinical CAD phenotypes in a well-defined low–traditional-risk population and further builds on work in smaller pilot studies initially linking protein pathways to CAD [43].
To further address potential relationships with HIV-specific factors, we investigated if these proteins were associated with current CD4 (determined at time of CT acquisition) and nadir CD4 count, as surrogates of immune function. We found some significant relationships, which were generally consistent in their directionality, eg higher inflammatory protein levels were associated with lower CD4 count and lower nadir CD4 count. We demonstrated a novel relationship between GDF-15 and nadir CD4 count. Nonetheless, differences in protein levels between CD4 groups, although statistically significant, were relatively small in absolute terms, suggesting that standard measures of immune function among those on long-term ART may not robustly predict inflammatory pathways contributing to subclinical CAD. Minimal differences were seen comparing by detectable viral load, although a majority of participants were on ART.
In prior studies, we have shown statin effects on LTBR and CCL24 in PWH [43]. Through the longitudinal assessment of statin effects in REPRIEVE, we will determine if effects on specific proteins mediate reductions in plaque volume. Our data suggest that strategies to modulate the NF-κB pathways with canakinumab, a monoclonal antibody targeting IL-1β, may be useful as seen in the CANTOS trial [44] and in a small pilot study of PWH [45] to reduce arterial inflammation among PWH. However, IL-1β may increase infections, suggesting potential limitations for PWH [44]. Other strategies, including strategies to reduce IL-6 [46], may be useful, given our prior findings of IL-6 association with plaque in this cohort and the new finding of IL-6RA in the current analysis [1].
This study has strengths and limitations. The data are cross-sectional and represent participants from the United States only. We compared our data to a similar analysis in patients without HIV, but future studies will need to validate these findings in longitudinal cohorts, which we will do in relationship to events over time in REPRIEVE. Nonetheless, this study is the first comprehensive proteomic assessment of pathways associated with subclinical CAD among PWH. We adjusted for ASCVD risk and assessed relationships to different plaque phenotypes, further performing a mediation analysis to assess independent effects of these proteins. This study population is representative of the large number of PWH with only low-to-moderate traditional risk factors, on ART with restored CD4 count, but demonstrating CAD, and thus at risk for future events. We assessed CAD in a standardized fashion to ensure generalizability. Moreover, the proximity extension assay allows for sensitive and precise measurement of proteins and has been used to create predictive models of CAD in people without HIV [31]. Given that higher-risk features were less prevalent, we focused primarily on the proteomic signature of more common phenotypes of plaque presence and CAC, to assess potential mechanisms of subclinical disease but performed exploratory analyses for higher-risk phenotypes.
Our data extend a growing body of work identifying related patterns of elevated plasma biomarkers and cellular gene expression profiles related to CVD in PWH [47–49]. These data provide a road map for future investigation of the mechanisms of subclinical CAD in HIV, highlighting key interrelated immune and inflammatory pathways significantly associated with coronary phenotypes in PWH. These data support a construct in which upregulated inflammatory and immune pathways further contribute independently to CAD beyond traditional risk factors. Critically, the field needs to assess whether these abnormal protein signatures will identify PWH at greater risk for increased plaque and cardiovascular events over time, to optimize preventive care.
Supplementary Material
Contributor Information
Márton Kolossváry, Cardiovascular Imaging Research Center, Massachusetts General Hospital, Boston, Massachusetts, USA.
Chris deFilippi, Inova Heart and Vascular Institute, Falls Church, Virginia, USA.
Michael T Lu, Cardiovascular Imaging Research Center, Massachusetts General Hospital, Boston, Massachusetts, USA.
Markella V Zanni, Metabolism Unit, Massachusetts General Hospital, Boston, Massachusetts, USA.
Evelynne S Fulda, Metabolism Unit, Massachusetts General Hospital, Boston, Massachusetts, USA.
Borek Foldyna, Cardiovascular Imaging Research Center, Massachusetts General Hospital, Boston, Massachusetts, USA.
Heather Ribaudo, Center for Biostatistics in AIDS Research, Harvard T. H. Chan School of Public Health, Boston Massachusetts, USA.
Thomas Mayrhofer, Cardiovascular Imaging Research Center, Massachusetts General Hospital, Boston, Massachusetts, USA; School of Business Studies, Stralsund University of Applied Sciences, Stralsund, Germany.
Ann C Collier, Department of Medicine, University of Washington, Seattle, Washington, USA.
Gerald S Bloomfield, Duke Clinical Research Institute, Duke University School of Medicine, Durham, North Carolina, USA.
Carl Fichtenbaum, Department of Medicine for Translational Research, University of Cincinnati, Cincinnati, Ohio, USA.
Edgar T Overton, Division of Infectious Diseases, University of Alabama at Birmingham, Birmingham, Alabama, USA.
Judith A Aberg, Division of Infectious Diseases, Department of Medicine, Icahn School of Medicine at Mount Sinai, New York, New York, USA.
Judith Currier, Division of Infectious Diseases, University of California at Los Angeles, Los Angeles, California, USA.
Kathleen V Fitch, Metabolism Unit, Massachusetts General Hospital, Boston, Massachusetts, USA.
Pamela S Douglas, Duke Clinical Research Institute, Duke University School of Medicine, Durham, North Carolina, USA.
Steven K Grinspoon, Metabolism Unit, Massachusetts General Hospital, Boston, Massachusetts, USA.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. The study investigators thank the study participants, site staff, and study-associated personnel for their ongoing participation in the trial. In addition, we thank the following: the AIDS Clinical Trials Group (ACTG) for clinical site support; ACTG Clinical Trials Specialists for regulatory support; the Data Management Center, Frontier Science Foundation, for data support; and the Center for Biostatistics in AIDS Research for statistical support.
Disclaimer . The views expressed in this article are those of the authors and do not necessarily represent the views of the National Heart Lung and Blood Institute (NHLBI), or the National Institute of Allergy and Infectious Diseases (NIAID), the National Institutes of Health (NIH), or the Department of Health and Human Services.
Financial support. This work was supported by the National Institutes of Health (grant numbers U01HL123336 to the Clinical Coordinating Center, and U01HL123339 to the Data Coordinating Center); Kowa Pharmaceuticals; Gilead Sciences; ViiV Pharmaceuticals; and the National Institute of Allergy and Infectious Diseases (grant numbers UM1 AI068636 to the AIDS Clinical Trials Group Leadership and Operations Center, and UM1 AI106701 to the ACTG Laboratory Center).
Data sharing . Data will be shared in accordance with National Institutes of Health policy.
References
- 1. Hoffmann U, Lu MT, Foldyna B, et al. Assessment of coronary artery disease with computed tomography angiography and inflammatory and immune activation biomarkers among adults with HIV eligible for primary cardiovascular prevention. JAMA Netw Open 2021; 4:e2114923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Subramanian S, Tawakol A, Burdo TH, et al. Arterial inflammation in patients with HIV. JAMA 2012; 308:379–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Triant VA, Regan S, Lee H, Sax PE, Meigs JB, Grinspoon SK. Association of immunologic and virologic factors with myocardial infarction rates in a US healthcare system. J Acquir Immune Defic Syndr 2010; 55:615–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Grinspoon SK, Fitch KV, Overton ET, et al. Rationale and design of the Randomized Trial to Prevent Vascular Events in HIV (REPRIEVE). Am Heart J 2019; 212:23–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hoffmann U, Lu MT, Olalere D, et al. Rationale and design of the mechanistic substudy of the Randomized Trial to Prevent Vascular Events in HIV (REPRIEVE): effects of pitavastatin on coronary artery disease and inflammatory biomarkers. Am Heart J 2019; 212:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Goff DC J, Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014; 129(25 Suppl 2):S49–73. [DOI] [PubMed] [Google Scholar]
- 7. Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol 1990; 15:827–32. [DOI] [PubMed] [Google Scholar]
- 8. Kolossvary M, Szilveszter B, Merkely B, Maurovich-Horvat P. Plaque imaging with CT-a comprehensive review on coronary CT angiography based risk assessment. Cardiovasc Diagn Ther 2017; 7:489–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ferencik M, Mayrhofer T, Bittner DO, et al. Use of high-risk coronary atherosclerotic plaque detection for risk stratification of patients with stable chest pain: a secondary analysis of the PROMISE randomized clinical trial. JAMA Cardiol 2018; 3:144–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. deFilippi C, Toribio M, Wong LP, et al. Differential plasma protein regulation and statin effects in human immunodeficiency virus (HIV)-infected and non-HIV-infected patients utilizing a proteomics approach. J Infect Dis 2020; 222:929–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wijk S S-v, Tromp J, Beussink-Nelson L, et al. Proteomic evaluation of the comorbidity-inflammation paradigm in heart failure with preserved ejection fraction: results from the PROMIS-HFpEF study. Circulation 2020; 142:2029–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Roy Stat Soc B Met 1995; 57:289–300. [Google Scholar]
- 13. Szklarczyk D, Gable AL, Lyon D, et al. STRING v11: protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res 2019; 47:D607–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tingley D, Yamamoto T, Hirose K, Keele L, Imai K. mediation: R package for causal mediation analysis. J Stat Softw 2014; 59:1–38.26917999 [Google Scholar]
- 15.R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, 2021. https://www.R-project.org/. Accessed 10 May 2021.
- 16. Lyu Z, Jin H, Yan Z, et al. Effects of NRP1 on angiogenesis and vascular maturity in endothelial cells are dependent on the expression of SEMA4D. Int J Mol Med 2020; 46:1321–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wallentin L, Eriksson N, Olszowka M, et al. Plasma proteins associated with cardiovascular death in patients with chronic coronary heart disease: A retrospective study. PLoS Med 2021; 18:e1003513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ji Q, Zeng Q, Huang Y, et al. Elevated plasma IL-37, IL-18, and IL-18BP concentrations in patients with acute coronary syndrome. Mediators Inflamm 2014; 2014:165742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lisowska A, Swiecki P, Knapp M, et al. Insulin-like growth factor-binding protein 7 (IGFBP 7) as a new biomarker in coronary heart disease. Adv Med Sci 2019; 64:195–201. [DOI] [PubMed] [Google Scholar]
- 20. Oemrawsingh RM, Lenderink T, Akkerhuis KM, et al. Multimarker risk model containing troponin-T, interleukin 10, myeloperoxidase and placental growth factor predicts long-term cardiovascular risk after non-ST-segment elevation acute coronary syndrome. Heart 2011; 97:1061–6. [DOI] [PubMed] [Google Scholar]
- 21. Castillo L, Rohatgi A, Ayers CR, et al. Associations of four circulating chemokines with multiple atherosclerosis phenotypes in a large population-based sample: results from the Dallas heart study. J Interferon Cytokine Res 2010; 30:339–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Aalto K, Maksimow M, Juonala M, et al. Soluble vascular adhesion protein-1 correlates with cardiovascular risk factors and early atherosclerotic manifestations. Arterioscler Thromb Vasc Biol 2012; 32:523–32. [DOI] [PubMed] [Google Scholar]
- 23. Chen DW, Jin Y, Zhao RM, et al. Age-, sex- and glucose-dependent correlation of plasma soluble vascular adhesion protein-1 concentration with cardiovascular risk factors and subclinical atherosclerosis. Eur Rev Med Pharmacol Sci 2016; 20:1544–58. [PubMed] [Google Scholar]
- 24. van Wanrooij EJ, van Puijvelde GH, de Vos P, Yagita H, van Berkel TJ, Kuiper J. Interruption of the Tnfrsf4/Tnfsf4 (OX40/OX40L) pathway attenuates atherogenesis in low-density lipoprotein receptor-deficient mice. Arterioscler Thromb Vasc Biol 2007; 27:204–10. [DOI] [PubMed] [Google Scholar]
- 25. Schurks M, Buring JE, Ridker PM, Chasman DI, Kurth T. Genetic determinants of cardiovascular events among women with migraine: a genome-wide association study. PLoS One 2011; 6:e22106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gubbiotti MA, Neill T, Iozzo RV. A current view of perlecan in physiology and pathology: a mosaic of functions. Matrix Biol 2017; 57–58:285–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ma Y, Chiao YA, Clark R, et al. Deriving a cardiac ageing signature to reveal MMP-9-dependent inflammatory signalling in senescence. Cardiovasc Res 2015; 106:421–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. van der Laan SW, Fall T, Soumare A, et al. Cystatin C and cardiovascular disease: a mendelian randomization study. J Am Coll Cardiol 2016; 68:934–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ho JE, Lyass A, Courchesne P, et al. Protein biomarkers of cardiovascular disease and mortality in the community. J Am Heart Assoc 2018; 7:e008108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wesseling M, de Poel JHC, de Jager SCA. Growth differentiation factor 15 in adverse cardiac remodelling: from biomarker to causal player. ESC Heart Fail 2020; 7:1488–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bom MJ, Levin E, Driessen RS, et al. Predictive value of targeted proteomics for coronary plaque morphology in patients with suspected coronary artery disease. EBioMedicine 2019; 39:109–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zanni MV, Abbara S, Lo J, et al. Increased coronary atherosclerotic plaque vulnerability by coronary computed tomography angiography in HIV-infected men. AIDS 2013; 27:1263–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bergstrom G, Persson M, Adiels M, et al. Prevalence of subclinical coronary artery atherosclerosis in the general population. Circulation 2021; 144:916–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zachary I. Neuropilins: role in signalling, angiogenesis and disease. Chem Immunol Allergy 2014; 99:37–70. [DOI] [PubMed] [Google Scholar]
- 35. Gaddis DE, Padgett LE, Wu R, Hedrick CC. Neuropilin-1 expression on CD4 T cells is atherogenic and facilitates T cell migration to the aorta in atherosclerosis. J Immunol 2019; 203:3237–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kofler N, Simons M. The expanding role of neuropilin: regulation of transforming growth factor-β and platelet-derived growth factor signaling in the vasculature. Curr Opin Hematol 2016; 23:260–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Dessein PH, Joffe BI, Singh S. Biomarkers of endothelial dysfunction, cardiovascular risk factors and atherosclerosis in rheumatoid arthritis. Arthritis Res Ther 2005; 7:R634–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wahl AF, Wallace PM. Oncostatin M in the anti-inflammatory response. Ann Rheum Dis 2001; 60(Suppl 3):iii75–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Vu TT, Marquez J, Le LT, Nguyen ATT, Kim HK, Han J. The role of decorin in cardiovascular diseases: more than just a decoration. Free Radic Res 2018; 52:1210–9. [DOI] [PubMed] [Google Scholar]
- 40. Motoyama S, Kondo T, Sarai M, et al. Multislice computed tomographic characteristics of coronary lesions in acute coronary syndromes. J Am Coll Cardiol 2007; 50:319–26. [DOI] [PubMed] [Google Scholar]
- 41. Grebe A, Hoss F, Latz E. NLRP3 Inflammasome and the IL-1 pathway in atherosclerosis. Circ Res 2018; 122:1722–40. [DOI] [PubMed] [Google Scholar]
- 42. Kearns AC, Liu F, Dai S, et al. Caspase-1 activation is related with HIV-associated atherosclerosis in an HIV transgenic mouse model and HIV patient cohort. Arterioscler Thromb Vasc Biol 2019; 39:1762–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. deFilippi C, Lo J, Christenson R, et al. Novel mediators of statin effects on plaque in HIV: a proteomics approach. AIDS 2018; 32:867–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ridker PM, Everett BM, Thuren T, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med 2017; 377:1119–31. [DOI] [PubMed] [Google Scholar]
- 45. Hsue PY, Li D, Ma Y, et al. IL-1beta inhibition reduces atherosclerotic inflammation in HIV infection. J Am Coll Cardiol 2018; 72:2809–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Tenorio AR, Zheng Y, Bosch RJ, et al. Soluble markers of inflammation and coagulation but not T-cell activation predict non-AIDS-defining morbid events during suppressive antiretroviral treatment. J Infect Dis 2014; 210:1248–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bowman ER, Cameron CM, Richardson B, et al. Macrophage maturation from blood monocytes is altered in people with HIV, and is linked to serum lipid profiles and activation indices: a model for studying atherogenic mechanisms. PLoS Pathogens 2020; 16:e1008869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. van der Heijden WA, Van de Wijer L, Keramati F, et al. Chronic HIV infection induces transcriptional and functional reprogramming of innate immune cells. JCI Insight 2021; 6:e145928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Cornwell A, Palli R, Singh MV, et al. Molecular characterization of atherosclerosis in HIV positive persons. Sci Rep 2021; 11:3232. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.