Abstract
Background.
Trauma-induced coagulopathy (TIC) has been the subject of intense study for greater than a century and it is associated with high morbidity and mortality. The Trans-Agency Consortium for Trauma-Induced Coagulopathy (TACTIC), funded by the National Health Heart, Lung and Blood Institute, was tasked with developing a clinical TIC score, distinguishing between injury-induced bleeding from persistent bleeding due to TIC. We hypothesized that the TACTIC clinical TIC score would correlate with laboratory measures of coagulation, transfusion requirements, and mortality.
Methods.
Trauma activation patients requiring a surgical procedure for hemostasis were scored in the operating room (OR) and in the first ICU day by the attending trauma surgeon. Conventional and viscoelastic (thrombelastography, TEG) coagulation assays, transfusion requirements, and mortality were correlated to the coagulation scores using the Cochran-Armitage trend test or linear regression for numerical variables.
Results.
Increased OR TIC scores were significantly associated with abnormal conventional and viscoelastic measurements, including hyperfibrinolysis incidence, as well as with higher mortality and more frequent requirement for massive transfusion (p<0.0001 for all trends). Patients with OR TIC score greater than 3, were over 31 times more likely to have an ICU TIC score greater than 3 (Relative risk: 31.6; 95% Confidence interval: 12.7–78.3; p<0.0001).
Conclusions.
A clinically defined TIC score obtained in the OR reflected the requirement for massive transfusion and mortality in severely injured trauma patients, and also correlated with abnormal coagulation assays. The OR TIC score should be validated in multicenter studies.
Level of Evidence:
Prognostic and Epidemiological Level II
Keywords: Trauma-Induced Coagulopathy, OR TIC Score
Background
Dysfunctional coagulation following severe injury has been the subject of scientific investigation for more than a century (1), but defining the responsible mechanisms to guide precision-management for distinct phenotypes remains an open scientific pursuit. In 2010, the National Institute of Health (NIH), recognizing the ongoing knowledge gaps in the diagnosis and management of coagulopathy associated with severe injury, organized a workshop and arrived at a consensus to name this phenomenon trauma-induced coagulopathy (TIC) (2). However, investigative efforts have been limited by the lack of 1) a standardized clinical scoring system for coagulopathy and 2) criteria for determining the extent that coagulopathy impacts postinjury mortality (3). Although clinical scoring systems for disseminated intravascular coagulopathy in diseases such as sepsis exist (4), to date, no consensus regarding the definition and quantification of TIC has emerged. Standardized scoring systems for key clinical definitions, which can be validated, are critical to progress in challenging clinical entities.
In recognition of the significance of TIC as a clinical problem, the NIH funded the Trans-Agency Consortium for Trauma-Induced Coagulopathy (TACTIC) through the National Heart, Lung, and Blood Institute (NHLBI) (2), a collaborative effort between the NIH and the Department of Defense (DOD). A major task of TACTIC was to propose a TIC clinical coagulation score (5). In keeping with this task, we developed a quantitative scoring system for TIC on a Likert scale, which differentiated 1) injuries requiring hemostasis but not complicated by a coagulopathy (mechanical bleeding) versus 2) bleeding injuries compounded by TIC, which stratified trauma patients by their degree of clinically observable TIC (6). Through this system, we attempted to distinguish between bleeding severity resulting from injury alone (e.g., controllable with pressure or suturing/stapling versus bleeding which persists due to a coagulopathy). We hypothesize that this clinical TIC score in this prospective study will correlate with laboratory measures of coagulation, transfusion requirements, and mortality in adult patients (>18 years) who required a surgical intervention for hemostasis in the first 24 hours postinjury were included.
METHODS
Inclusion Criteria.
Adult trauma activations from 2014–2019 in an urban Level I trauma center enrolled in our ongoing Trauma Activation Patient (TAP) registry under an IRB waiver of consent and who required a surgical intervention for hemostasis in the first 24 hours postinjury were included. Inclusion Criteria: age >18 years presenting to the Ernest E Moore Shock Trauma Center at Denver Health for whom trauma activation was triggered, including all “Code 10” trauma transports by the Denver Health Emergency Medical Services (EMS). Trauma activation criteria were any of the following: 1) Glasgow Coma Scale (GCS) <8 with presumed thoracic, abdominal or pelvic injury; 2) respiratory compromise, obstruction and/or intubation with presumed thoracic, abdominal or pelvic injury; 3) blunt trauma with: SBP<90 mmHg; 4) mechanically unstable pelvic injury (open or obvious by physical exam); 5) penetrating injuries with: a) injury to neck or torso with systolic blood pressure (SBP) <90mmHg or b) gunshot wounds penetrating the neck or torso or c) stab wounds to the neck or torso that required endotracheal intubation; 6) amputation proximal to the ankle or wrist; 7) the Emergency Medicine attending or chief surgical resident suspected the patient was likely to require urgent operative intervention. Injured patients were only enrolled in the prospective database only if they had a rapid thrombelastogram (rTEG) within one hour of injury, and all research TEGs and samples were drawn at the same time as the R-TEG prior to the administration of tranexamic acid (TxA) or any blood products. All prospective clinical were collected in accord with the EQUATOR guideline and please see the STARD checklist that is included as supplemental digital data.
Exclusion criteria.
Exclusion criteria for this study were: 1) patients for whom the initial blood sample was not collected within one hour of injury, 2) referrals from external hospitals, 3) documented chronic liver disease (admission total bilirubin >2.0 mg/dL) or advanced cirrhosis discovered on laparotomy, 4) known or subsequently discovered inherited defects of coagulation function (e.g. hemophilia or Von Willebrand’s disease), 5) subsequent downgrade from Trauma Activation to Trauma Alert or to non-trauma status in the emergency department (ED). We did not exclude patients taking anti-coagulants prior to injury, as this information is often not known upon admission time.
Coagulation Score.
The data was collected prospectively, and the scoring done in relatively real time, within 24 hours for scoring. The initial aim was to have the attending surgeon (there are 8 Trauma surgeons) score the coagulopathy as soon as mechanical control of bleeding was obtained; however, this proved to be impractical in the dynamic, busy environment of trauma resuscitation, and although some scores were completed at this time, many were done as soon as possible following the achievement of mechanical hemostatic control and all were done prior to the ICU coagulation scores so none were done simultaneously. The attending trauma surgeon was blinded to all research TEG results but had access to clinical rapid TEGs (rTEGs) if those were requested as part of the resuscitation. The TACTIC-developed OR TIC score ranged from 1 to 5 and are described in Table 1. Attending trauma surgeons attempted to score the coagulopathy, OR TIC score, as soon as mechanical control of the bleeding was achieved, which was not always practical, but no more than 24 hours after the end of the surgical hemostasis to minimize recall bias. The scoring was repeated in the first 24 hours of ICU admission, at least 4 hours post OR.
Table 1:
TACTIC Trauma-induced coagulopathy (TIC) score
| Score | Description |
|---|---|
| 1 | Normal hemostasis (negative) |
| 2 | Mild coagulopathy, no intervention required except direct pressure or temporary gauze tamponade (equivocal) |
| 3 | Coagulopathy refractory to direct pressure, requiring multiple routine hemostasis techniques (e. g. electrocautery, topic hemostatic agents, staples, or suturing) (possible positive) |
| 4 | Coagulopathy requiring adjunctive blood component therapy or systemic therapeutics in response to continued bleeding despite above surgical hemostatic maneuvers (positive) |
| 5 | Diffuse persistent bleeding from multiple sites remote from injury; e.g. endotracheal tube, intravenous catheter, chest tubes, etc. (definitive positive) |
Measurement of Coagulation.
All included patients had a research, rapid TEG drawn within one-hour (one hour + 11 minutes) postinjury as part of our TAP registry performed in our research lab by 24/7 trained professional research assistants. All samples for research TEGs and other assays were completed prior to the administration of tranexamic acid (TXA) or blood products. Abnormal TEG values were determined using previously published cutoff values: activated clotting time (ACT) >128 sec, angle <65 degrees, maximum amplitude (MA) <55mm and percent clot lysis at 30 minutes after maximum clot strength (LY30) >3.0% (7).
Massive Transfusion (MT).
Massive transfusion was defined as greater than 4 red blood cell (RBC) units or death (after receiving at least one RBC unit) in the first hour postinjury (8). Our institution’s MT protocol is activated if arrival SBP<90mmHg in patients with torso penetrating wounds, unstable pelvic fracture, or positive FAST in >1 area.
Statistical Analysis.
The correlations of the coagulation score with abnormal TEGs, massive transfusion and mortality were examined by the Cochran-Armitage trend test. Linear regression was used to assess the association of increasing OR TIC scores with normally distributed numerical variables (PT-INR, PTT, Fibrinogen, ionized-Calcium). Numerical data are presented as median with interquartile range, while categorical variables are presented as N and percent. All tests were two-tailed with significance set at p<0.05. All analyses were conducted in SAS vs 9.4 (SAS Institute, Cary, NC).
RESULTS
Patient Population.
Overall, 556 patients were included, and the patient characteristics are depicted in Supplemental Table 1. These were mostly young males, with severe injuries and the time from injury begins as the “time of call to Emergency medical Services”. Blunt mechanism was the cause of injuries in about half of these patients. About one third arrived with hypotension (<90mmHg) and had a positive FAST. Mortality was 15.3%, and 10.4% required massive transfusion.
Operating Room (OR) TIC Scores.
Higher OR TIC scores were significantly associated with increasing injury severity as measured by the new injury severity score (NISS), anemia, physiologic derangement (systolic blood pressure, base excess, lactate, ionized -calcium), and coagulation abnormalities as measured by conventional coagulation assays prothrombin time-international normalized ratio (PT-INR), activated partial thromboplastin time (PTT), fibrinogen, platelet count and TEG values, including both fibrinolysis shutdown and hyperfibrinolysis.
Massive Transfusion (MT) and Mortality correlations.
The OR TIC score was significantly associated with trends toward more frequent requirement of MT and higher mortality (Cochran-Armitage Trend Test p<0.0001 for both outcomes) (Figure 1). Patients with a OR TIC score greater than 3 were more likely to die in this hospitalization (Relative Risk, RR: 7.10; 95% Confidence Intervals, 95% CI: 4.88–10.32, p<0.0001) and 4 times more likely to require a massive transfusion (RR: 4.12; 95%CI: 2.58–6.57, p<0.0001). Stratification by TBI status showed significant associations in both strata (With TBI=RR for death: 3.32; 95%CI: 2.12– 5.21; RR for MT: 2.66; 95%CI: 1.14– 6.20; Without TBI: RR for death: 13.27; 95%CI: 7.16– 24.59; RR for MT: 4.97; 95%CI: 2.84–8.69).
Figure 1. The % of patients, based on OR TIC scores, with mortality and increased massive transfusion requirements.
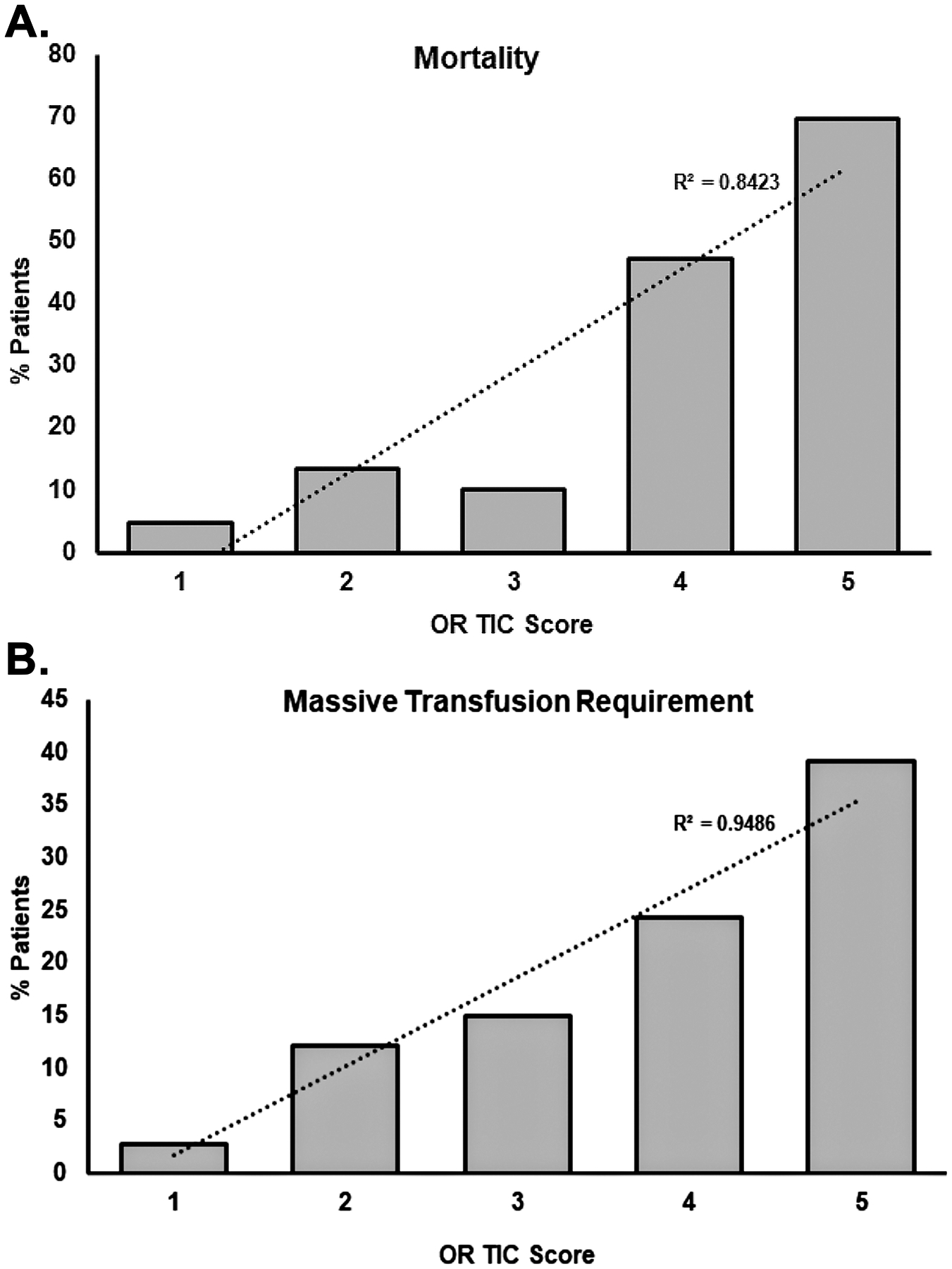
Increasing OR TIC scores caused a higher mortality (Panel A) and increased requirement of massive transfusion (Panel B). A Cochran-Armitage Trend Test was done for both panels (p<0.0001).
Coagulation:
OR TIC scores had a significant association with longer PT/INR and PTT times (p<0.0001 for both) (Supplemental Table 1). TIC scores were significantly associated with increased frequency of abnormal ACT, angle, and MA rapid TEG measurements (Figure 2, panels A, B and C, Cochran-Armitage Trend Test p<0.0001 for all) as well as with higher incidence of hyperfibrinolysis (Figure 2, panel D, Cochran-Armitage Trend Test p<0.0001). The OR TIC score was also correlated with lower platelet counts as well as lower fibrinogen and ionized-calcium levels (p<0.0001 for all) (Supplemental Table 1).
Figure 2. The % of patients, based on OR TIC scores, that had abnormal coagulation assays.
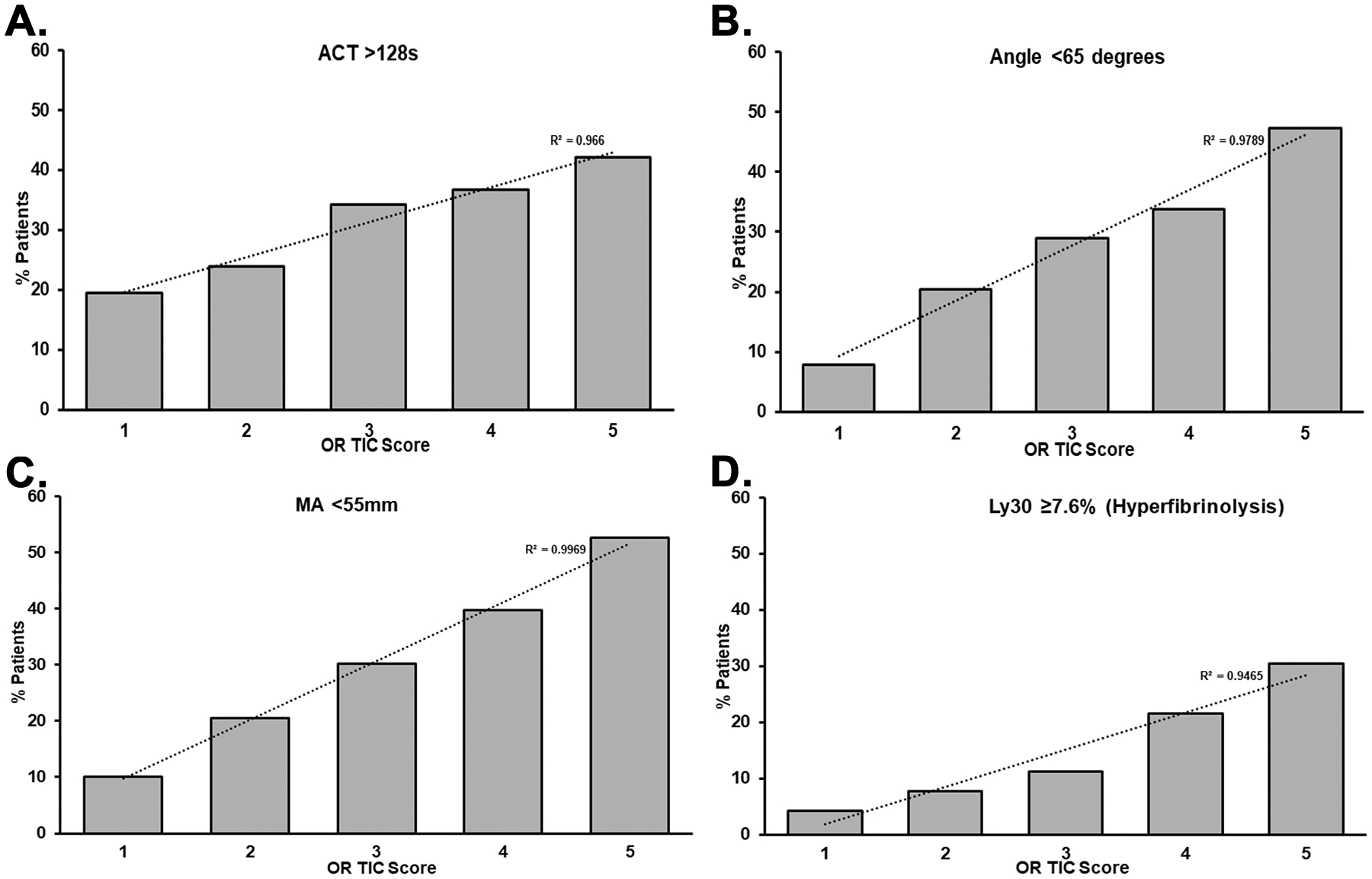
Thrombelastograms (TEGs) were done on the whole blood from trauma patients and, based on the % of total patients and OR TIC Scores, abnormal coagulation results were plotted. Panel A: Activated Clotting Time (ACT)>128s to measure the time to initial fibrin formation; Panel B: Angle<65 degrees for clot formation; Panel C: Maximum amplitude (MA)<55mm; and Panel D: Hyperfibrinolysis (TEG Ly30>=7.6%), the clot lysis time at 30 minutes, all p<0.0001.
Correlation with ICU TIC score:
The ICU TIC score, assessed on the first ICU day, had the following distribution: 1: 76.2%; 2: 14.4%; 3: 2.2%; 4: 5.7%; and 5: 1.6%. The mortality increased steadily with higher ICU TIC scores, from 5.9% with ICU TIC score=1, to 19.2% with ICU TIC=2, 18.2% with ICU TIC=3, 44.8% with ICU TIC=4 and 75.0% with ICU TIC=5 (Cochran-Armitage Trend Test, p<0.0001). Patients with OR TIC score greater than 3, were >31 times more likely to have an ICU TIC score greater than 3 (Relative risk: 31.6; 95% Confidence interval: 12.7–78.3; p<0.0001).
DISCUSSION
We have presented a clinical, TACTIC-developed clinical OR TIC score. In the presented patient population 18% were truly coagulopathic and received large volumes of transfused blood products, and it is these patients to whom this proposed OR TIC score is directed. From these data the OR TIC score reflected the requirement for massive transfusion and increased mortality in severely injured trauma patients. Abnormal TEG values, including hyperfibrinolysis, prolonged conventional coagulation assays (PT-INR and PTT), and decreased plasma fibrinogen concentrations, platelet counts, and ionized calcium all correlated with the OR TIC score. Finally, the OR TIC score predicted the ICU TIC score.
There were several difficulties in developing this scoring system. First, quantification of impaired hemostasis and bleeding due to coagulation disturbance as opposed to surgical bleeding (uncontrolled arterial or venous disruption) is, at times, a challenging distinction, thus requiring a balanced scoring system. Second, any clinical definition is fundamentally a subjective assessment, therefore, rather than have the interpretation of TIC as definitive or dichotomous, we contend that it should be presented as an impression made with greater or lesser confidence and stratified using a five-point Likert scale, as is often done in the radiology literature (negative, equivocal, possible positive, positive, and definitive positive) (6).
Coagulation scores, such as the sequential organ failure assessment coagulation score (SOFA-CS) have been used to predict mortality in critically injured patients with acute kidney injury undergoing continuous renal replacement (9). In severely injured trauma patients, a disseminated intravascular coagulation score correlated with the development of multiple organ dysfunction syndrome (MODS) (10). With the incidence of multiple organ failure significantly decreasing, scoring systems have been developed to track the need for massive transfusion, which include the shock index, the TASH score, and the ABC score (11–14). Previously, tissue plasminogen activator (tPA) challenge TEGs identified those severely injured patients who required massive transfusion within 15 minutes, which is quicker than the shock index and the TASH score and has a higher positive predictive value than the ABC score and does not require a focused assessment with sonography (FAST); however, most Trauma Centers do not have specialized TEGs available (15). In addition, others have addressed the issue of coagulopathic bleeding versus abnormal coagulation laboratory values in a multi-center trial and confirmed that bleeding from non-injured sites or bleeding not controlled by sutures represented a rarer phenotype that had poor outcomes and impairment of both clotting factor and platelet hemostasis (16). Most importantly, Receiver Operating Characteristics (ROCs) have misleading results due to the relatively infrequent incidence of MT among trauma populations, which decreases the positive predictive value (11–17). Thus, with these previous scoring systems in mind the presented OR TIC score differentiated between mechanical bleeding versus bleeding injuries compounded by TIC and stratified trauma patients by their degree of clinically observable TIC. This scoring system also correlated with required MT, the increased mortality in these severely injured patients, and both standard and viscoelastic measured hemostasis. Importantly, our MT is defined by RBC units alone, which is guided by bleeding and not by laboratory signs of coagulopathy, while other blood products are given based on goal-directed, viscoelastic test-guided, hemostatic resuscitation (17). Previous investigations suggest adding platelet and cryoprecipitate do not improve correlation with outcome data and introduces intervention bias (17). Lastly, the stratified analysis by TBI status, which demonstrates that the TIC score performed well in both TBI and non-TBI strata. Our group has demonstrated that TBI’s TIC manifests as a unique phenotype, but it is not more frequent than in torso and/or extremities injuries, especially when associated with shock (18–20).
There are limitations to the described data. As the attending surgeon was given 24 hours to document the OR TIC score, the data may be subject to this individual’s recall of the specific case. In addition, although the surgeon was blinded to the research TEG results, they were aware of all the hospital laboratory requested coagulation tests but not to any research TEGs, which potentially influenced their determination. The OR TIC scores were not assessed independently, and the on call attending physician completed the scoring. However, in clinical practice physicians make decisions based upon all prior laboratory results and other clinical data, as in Bayes Theorem, and not in isolation. Unfortunately, we did not collect data on whether higher OR TIC scores correlated with 1) damage control surgery, 2) MT protocol activation, or 3) delayed intervention for hemostasis. Lastly, these data are from a single institution, thus with limited generalizability, and should be validated at other facilities in a multi-center trial.
In conclusion, we propose a quantitative scoring system for TIC to overcome a critical barrier to research progress in this lethal condition. The clinical scoring is immediately available unlike clinical laboratory testing and available to facilities not using or not savvy with FAST. The OR TIC scoring system may provide a common language for the grading of clinical coagulopathy and allow for enhanced communication and research in this critical area but must be validated at other institutions. Future use of this scoring system must include correlations with 1) damage control surgery, 2) MT protocol activation versus implementation, and 3) delayed intervention for hemostasis in severely injured patients. Furthermore, this clinical coagulation scoring system may be useful as an observable measure of coagulopathy relevant to ensuring hemostasis, which can inform interventional trials when used as an inclusion criterion or outcome and to evaluate novel laboratory measures of coagulation.
Supplementary Material
Funding
was received from grants T32- GM008315, P50- GM49222, and 1RM1GM131968 NIGMS, NIH, UM 1HL 120877 from NHLBI, NIH and W81XWH-12-2-0028 from the Department of Defense, USAMRAA.
Footnotes
Conflicts of interest: Haemonetics® provided reagents and devices to run viscoelastic assays but had no involvement with data analysis, interpretation or any contribution to this manuscript.
REFERENCES
- 1.Cannon WB F J, Coswell EM Nature and Treatment of Wound Shock and Allied Conditions. The preventitive Treatment of Wound Shock JAMA. 1918;70(70):618–21. [Google Scholar]
- 2.Mann KG, Freeman K. TACTIC: Trans-Agency Consortium for Trauma-Induced Coagulopathy. J Thromb Haemost. 2015;13 Suppl 1:S63–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morton AP, Moore EE, Wohlauer MV, Lo K, Silliman CC, Burlew CC, et al. Revisiting early postinjury mortality: are they bleeding because they are dying or dying because they are bleeding? J Surg Res. 2013;179(1):5–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bakhtiari K, Meijers JC, de Jonge E, Levi M. Prospective validation of the International Society of Thrombosis and Haemostasis scoring system for disseminated intravascular coagulation. Crit Care Med. 2004;32(12):2416–21. [DOI] [PubMed] [Google Scholar]
- 5.Neal MD, Moore HB, Moore EE, Freeman K, Cohen MJ, Sperry JL, et al. Clinical assessment of trauma-induced coagulopathy and its contribution to postinjury mortality: A TACTIC proposal. J Trauma Acute Care Surg. 2015;79(3):490–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Drinkwater BL. A comparison of the direction-of-perception technique with the Likert method in the measurement of attitudes. J Soc Psychol. 1965;67(2):189–96. [DOI] [PubMed] [Google Scholar]
- 7.Stettler GR, Moore EE, Moore HB, Nunns GR, Silliman CC, Banerjee A, et al. Redefining postinjury fibrinolysis phenotypes using two viscoelastic assays. J Trauma Acute Care Surg. 2019;86(4):679–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nunns GR, Moore EE, Stettler GR, Moore HB, Ghasabyan A, Cohen M, et al. Empiric transfusion strategies during life-threatening hemorrhage. Surgery. 2018;vol. 164,2 (2018): 306–311. doi: 10.1016/j.surg.2018.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin J, Gallagher M, Bellomo R, Duan M, Trongtrakul K, Wang AY, et al. SOFA coagulation score and changes in platelet counts in severe acute kidney injury: Analysis from the randomized evaluation of normal versus augmented level (RENAL) study. Nephrology (Carlton). 2019;24(5):518–25. [DOI] [PubMed] [Google Scholar]
- 10.Gando S, Kameue T, Matsuda N, Hayakawa M, Ishitani T, Morimoto Y, et al. Combined activation of coagulation and inflammation has an important role in multiple organ dysfunction and poor outcome after severe trauma. Thromb Haemost. 2002;88(6):943–9. [PubMed] [Google Scholar]
- 11.Brockamp T, Nienaber U, Mutschler M, Wafaisade A, Peiniger S, Lefering R, et al. Predicting on-going hemorrhage and transfusion requirement after severe trauma: a validation of six scoring systems and algorithms on the TraumaRegister DGU. Crit Care. 2012;16(4):R129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Jong A, Deras P, Martinez O, Latry P, Jaber S, Capdevila X, et al. Relationship between Obesity and Massive Transfusion Needs in Trauma Patients, and Validation of TASH Score in Obese Population: A Retrospective Study on 910 Trauma Patients. PLoS One. 2016;11(3):e0152109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krumrei NJ, Park MS, Cotton BA, Zielinski MD. Comparison of massive blood transfusion predictive models in the rural setting. J Trauma Acute Care Surg. 2012;72(1):211–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wo CC, Shoemaker WC, Appel PL, Bishop MH, Kram HB, Hardin E. Unreliability of blood pressure and heart rate to evaluate cardiac output in emergency resuscitation and critical illness. Crit Care Med. 1993;21(2):218–23. [DOI] [PubMed] [Google Scholar]
- 15.Moore HB, Moore EE, Chapman MP, Huebner BR, Einersen PM, Oushy S, et al. Viscoelastic Tissue Plasminogen Activator Challenge Predicts Massive Transfusion in 15 Minutes. J Am Coll Surg. 2017;225(1):138–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chang R, Fox EE, Greene TJ, Swartz MD, DeSantis SM, Stein DM, et al. Abnormalities of laboratory coagulation tests versus clinically evident coagulopathic bleeding: results from the prehospital resuscitation on helicopters study (PROHS). Surgery. 2018;163(4):819–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nunns GR, Moore EE, Stettler GR, Moore HB, Ghasabyan A, Cohen M, et al. Empiric transfusion strategies during life-threatening hemorrhage. Surgery. 2018;164(2):306–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meizoso JP, Moore HB, Moore EE, Gilna GP, Ghasabyan A, Chandler J, et al. Traumatic brain injury provokes low fibrinolytic activity in severely injured patients. J Trauma Acute Care Surg. 2022;93(1):8–12. [DOI] [PubMed] [Google Scholar]
- 19.Moore EE, Moore HB, Kornblith LZ, Neal MD, Hoffman M, Mutch NJ, et al. Trauma-induced coagulopathy. Nat Rev Dis Primers. 2021;7(1):30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Samuels JM, Moore EE, Silliman CC, Banerjee A, Cohen MJ, Ghasabyan A, et al. Severe traumatic brain injury is associated with a unique coagulopathy phenotype. J Trauma Acute Care Surg. 2019;86(4):686–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


