Abstract
Microphysiological systems (MPS) are 2D or 3D multicellular constructs able to mimic tissue microenvironments. The latest models encompass a range of techniques, including co-culturing of various cell types, utilization of scaffolds and extracellular matrix materials, perfusion systems, 3D culture methods, 3D bioprinting, organ-on-a-chip technology, and examination of tissue structures. Several human brain 3D cultures or brain MPS (BMPS) have emerged in the last decade. These organoids or spheroids are 3D culture systems derived from induced pluripotent cells or embryonic stem cells that contain neuronal and glial populations and recapitulate structural and physiological aspects of the human brain. BMPS have been introduced recently in the study and modeling of neuroinfectious diseases and have proven to be useful in establishing neurotropism of viral infections, cell-pathogen interactions needed for infection, assessing cytopathological effects, genomic and proteomic profiles, and screening therapeutic compounds. Here we review the different methodologies of organoids used in neuroinfectious diseases including spheroids, guided and unguided protocols as well as microglia and blood-brain barrier containing models, their specific applications, and limitations. The review provides an overview of the models existing for specific infections including Zika, Dengue, JC virus, Japanese encephalitis, measles, herpes, SARS-CoV2, and influenza viruses among others, and provide useful concepts in the modeling of disease and antiviral agent screening.
Keywords: Brain organoid, Infection model, Brain spheroid, Microphysiological system, iPSC, in-vitro infection
INTRODUCTION
Neurological infections leading to encephalitis, meningitis, and myelitis are frequently severe and prolonged neurological disorders that result in significant long-term disability. Neurological infections are produced by neurotropic pathogens, viruses, bacteria, or fungi, that can penetrate the nervous system by direct infection (e.g., trans neural or axonal transport) or hematogenous spreading. Although the laboratory diagnosis of neurological infections has improved in the past few years with the introduction of novel next-generation molecular and immunological diagnostic assays, there are still limited options for treatment. There is an urgent need for suitable models of nervous system infection that may increase our understanding of the pathogenesis of neurological infections and high-throughput screening testing of therapeutic approaches.
Microphysiological systems are 2D or 3D multicellular constructs able to mimic tissue microenvironments. This term encompasses organotypic cultures, on-a-chip technologies, scaffolds, and other 3D cultures. In this review, we will focus on 3D human brain microphysiological systems (BMPS). 3D BMPS are in-vitro culture systems, usually derived from reprogrammed somatic cells into induced pluripotent stem cells (iPSCs) or embryonic stem cells (ESC), that are later differentiated into human neuronal and glial populations. While some BMPS self-assemble to recapitulate aspects of the normal physiology of the human brain (Organoid) or can resemble the human cortex or specific regions of the brain (Zhang, D. Y., Song and Ming., 2021, Hopkins, Traverse and Barr., 2021, Depla et al., 2022, Anderson et al., 2021), others represent more homogeneous models without specific regional organization(Kim, J. J. et al., 2021, Leite et al., 2019, Song, L. et al., 2019). Various studies have demonstrated BMPS can form neuronal networks, neuronal-glial interactions, electrical activity, and a cellular architecture similar to that of the human brain (Li, X., Shopit and Wang., 2022, Pașca., 2018, Pamies, David et al., 2017). These 3D biological systems have been used to study central nervous system (CNS) development, neurotoxicity, neoplasms, neurodegenerative diseases, and more recently neurological infections (Ho, Pek and Soh., 2018, Barreras et al., 2022, Qian et al., 2016, Sundar et al., 2022, Lancaster et al., 2013, Pamies, David, Zurich and Hartung., 2020, Plummer et al., 2019, Zhong et al., 2020, Pamies, David et al., 2022).
The BMPS models offer advantages over immortalized cell lines, single-layer cultures of human cells, and animal models to study neuroinfectious disease as they bypass the inter-species difference in cellular identity, signaling, and interactions, and have a closer genetic and transcriptomic profile to the human brain which can facilitate a better understanding of pathogen-cell interactions, tropism, and infection responses. BMPS allow studying interactions between various CNS cell types including neurons, astrocytes, oligodendrocytes, and in some ependymal cells (Depla et al., 2022) in co-culture in a 3D structure which closely resemble the CNS environment where the infection would take place and avoid the ethical issues of using animals in experimentation (Fan et al., 2022). BMPS have been used to study the viral tropism of various pathogens, including Zika virus (ZIKV) and severe acute respiratory syndrome coronavirus 2 (SARS-CoV2) during the recent epidemic and pandemic (Qian et al., 2016, Song, E. et al., 2021, Abreu et al., 2018, Bullen et al., 2020), and have been used to assess the effect of the infection in function, structure, genetic expression and innate immune response in the CNS as well as to discover potential therapeutic targets and test antiviral therapies (Depla et al., 2022, Hopkins, Traverse and Barr., 2021). These novel in-vitro systems have then the potential to contribute to the advancement of our understanding of neurological infections. In this review, we summarize the existing technology of BMPS used to study neurological infections, their advantages over other models, and their limitations.
METHODOLOGY AND TYPES OF BRAIN MICROPHYSIOLOGICAL SYSTEMS USED TO STUDY NEUROINFECTIOUS DISEASES
Three-dimensional BMPSs have been developed through different protocols. Most protocols start with iPSCs or ESC that are cultured in suspension into embryonic bodies (small spherical cell aggregates that mimic features of the developing embryo) which are later induced with specific neural induction media (usually containing factors that inhibit BMP/TGF-beta signaling pathways) to form neural stem cells that can later generate glial and neuronal populations. In addition, modulation of Wnt or Shh pathways may help embryonic bodies to be modeled into distinct cortical subregions with neural stem cells showing specific regional identities like dorsal or ventral telencephalic identities (Fan et al., 2022, Hopkins, Traverse and Barr., 2021).
The type of cells used for the generation of BMPS and their quality are important factors when performing neurological infection studies. iPSCs can be derived from adult tissues such as skin fibroblasts or epithelial cells or from blood monocytes which can be from healthy donors or patients with specific conditions of interest. ESC are derived from the inner cell mass of early preimplantation embryonic tissue, neural progenitor cells can be obtained from cerebral fetal tissue (Dos Reis et al., 2020). Quality control approaches need to be implemented to confirm stem cell pluripotency, genetics, and a normal karyotype (Pamies, D. et al., 2022). Particular attention should be paid to the vectors used in line generation to avoid the use of integrating vectors that can alter the host genome.
Beyond the analysis of specific neurotropism, BMPS with diverse glial populations allow the study of glial-neuronal interactions during CNS infection. Astrocytes play key roles in the metabolic support of neurons, synaptic and neurotransmitter homeostasis as well as interactions with other glial cells such as oligodendrocytes and limiting the spread of neurotropic pathogens, functions that can be disrupted in infections like HIV and Toxoplasma gondii infection(Drogemuller et al., 2008, Pandey and Seth., 2019). Astroglia and microglia mediate inflammatory responses to infection with direct consequences for neurons. For example, microglia serve as a reservoir for CNS infection as seen in HIV and activated microglia contribute to loss of inhibitory synapses in Toxoplasma gondii infection(Carrillo et al., 2020). Similarly, astrocyte dysfunction can lead to altered glutamate metabolism and neuronal disturbances contributing to HIV neuropathogenesis (dos Reis, Sant and Ayyavoo., 2022, Pandey and Seth., 2019). In Japanese Encephalitis Virus (JEV) infection, microglia was found to be the source of neurotoxic mediators that contributed to neuronal death(Chen et al., 2010). A similar observation had been found in West Nile Virus (WNV) infection(Van Marle et al., 2007). Some of these glial-neuronal interactions have been studied for specific infections in BMPS such as in flavivirus infection models (Abreu et al., 2018, Dos Reis et al., 2020). Oligodendrocytes contribute to myelination and axonal protection which is relevant in some infections such as JCV which was studied in a brain spheroid model (Barreras et al., 2022).
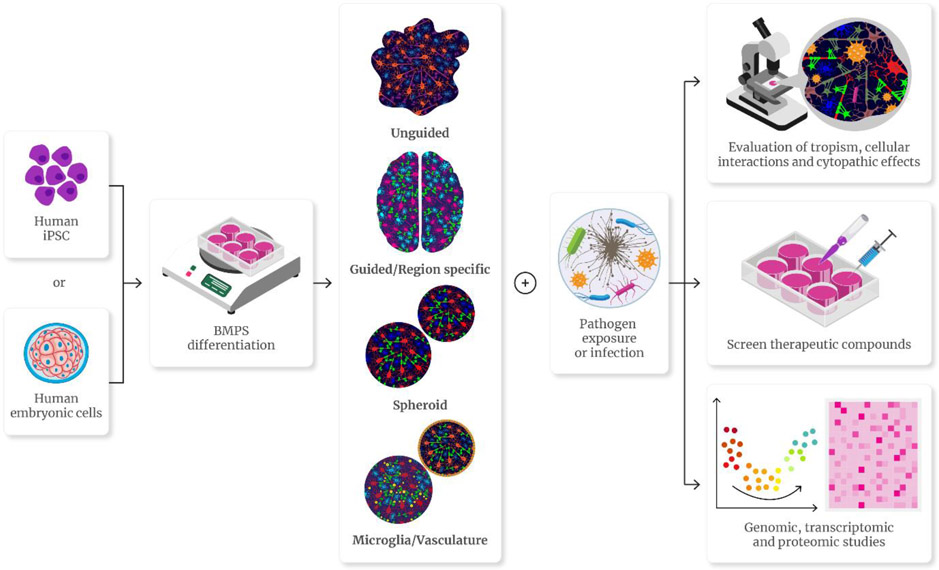
Most BMPS protocols are comprised of the same basic steps: Induction, differentiation, and maturation. In induction, embryonic bodies are produced from the original cell line, cultured in suspension, and directed towards a neuroectoderm lineage to be cultured in a differentiation medium to generate neuronal and glial populations which are cultured until reaching the desired mature phenotypes. Most of these protocols use a scaffold or basement membrane such as Matrigel to support morphogenesis and nutrient absorption during differentiation. Matrigel can enhance the formation of neural rosettes within the 3D structure (Fan et al., 2022, Lancaster et al., 2013, Tanaka et al., 2020). The production and in-vitro development periods of various protocols vary in length from 4 weeks to 2 months, although most organoids can be cultured beyond that period. The longer the development time the closer the phenotype is to the mature CNS environment, although problems of increasing size and nutrient depletion in the center of the organoids may deteriorate the quality of the system (Gordon et al., 2021, Lancaster et al., 2013). Depending on the protocol used to achieve maturation, the final BMPS can be considered spheroids, unguided, or guided/region-specific organoids (Figure 1).
FIGURE 1.
Types of 3D human brain microphysiological systems (BMPS) and their use in neuroinfectious disease. Human BMPS are 2D or 3D multicellular constructs able to mimic tissue microenvironments. Normally derived from human induced pluripotent cells (iPSC) or human embryonic cells, various 3D protocols have been used using direct aggregation of various cell types without specific organizational structure (spheroids), using self-aggregation protocols resulting in the formation of various cell types with complex structures throughout the organoid (unguided), using patterning molecules to model specific regions of the human brain (guided) and some models incorporating microglia or blood-brain barrier-like structures. When exposed to infectious agents, these models can be used to study neurotropism, cytopathogenic effects, transcriptomic changes, and screen therapeutic compounds.
Unguided organoids:
These methods take advantage of the properties of iPSC to spontaneously signal and differentiate into cerebral organoids mimicking the early stages of neurodevelopment without the need for patterning factors. The spontaneous differentiation results in different cell types and potentially different brain regions' cellular identities like midbrain or dorsal forebrain (Fan et al., 2022). This type of organoid results in a heterogeneous and diverse population of neurons and glial cells in clusters within the organoid which potentially represent different regions of the developing brain. This spontaneous differentiation can lead to unpredictable proportions of such populations and inter-organoid variability which may impact study results (Hopkins, Traverse and Barr., 2021, Qian et al., 2016, Qian, Song and Ming., 2019). The first unguided human brain organoid protocol was developed by Preynat-Seauve, followed by Lancaster, using ESCs or iPSCs-derived embryonic bodies (Lancaster et al., 2013, Preynat-Seauve et al., 2009). Transcriptomic analyses have demonstrated diversity, maturity, and spontaneous neural networks in unguided BMPS (Tanaka et al., 2020). This type of model has been used for multiple studies of viral infections including Dengue virus (DENV), ZIKV, SARS-CoV2, measles, cytomegalovirus (CMV), and herpes simplex virus-1 (HSV-1) (Brown et al., 2019, D'Aiuto et al., 2019, Dang et al., 2016, Song, E. et al., 2021, Li, Y. et al., 2017).
Guided organoids:
The protocol for the production of this type of BMPS uses media with specific patterning and growth factors to induce specific cell lineages or regional identities within the brain organoid which results in the formation of a region-specific organoid with multilayered structural organization and cellular composition resembling specific areas of interest such as areas of the cerebral cortex, thalamus, midbrain or hippocampus (Li, X., Shopit and Wang., 2022, Qian et al., 2016, Xu, J. and Wen., 2021, Jo et al., 2016, Kim, H. et al., 2019). Eiraku et al. described a guided protocol from mouse ESCs (Eiraku et al., 2008), which was later adapted for one of the first guided human BMPS protocols, using a TGFβ inhibitor and a Wnt inhibitor, to promote telencephalic differentiation from ESC (Kadoshima et al., 2013). Since then, various protocols have used different patterning cocktails to produce different regional identities (Fan et al., 2022, Jacob et al., 2021, Jo et al., 2016, Qian, Song and Ming., 2019, Xiang et al., 2019), although most of these have paucity in oligodendrocyte lineage relative to the neuronal and astrocytic cells for which some models add specific oligodendrocyte growth factors to overcome such limitation (Li, X., Shopit and Wang., 2022, Madhavan et al., 2018). The main advantage of guided brain organoids is the ability to study a specific region of interest that may be relevant to the physiopathology of the infection to study. Multiple viral studies on guided brain organoids have been performed, including studies of SARS-CoV2 and ZIKV infections, with several ZIKV studies reproducing microcephaly (Garcez et al., 2016), including one study showing the effects of ZIKV on structural organization and post-infection thinning of the ventricular zone layer (Xu, Y. et al., 2019).
Spheroid BMPS:
This type of BMPS consists of 3D cell-aggregate culture grown in a scaffold-free environment. These BMPS can be from a single cell type and lineage (endo, meso- or ectodermal lineage) or multiple cell types using guided differentiation of iPSC and grown without a basal membrane or scaffold such as Matrigel. Spheroid BMPS usually lack the structural organization of specific regions of the human brain. Overall, several scaffold-free protocols result in spheroids that while lacking region-specific structures, do recapitulate important aspects of the physiological environment of the brain including mature neuronal types and neuronal-glial interactions suitable to be used in the study of neurological infections with advantages that include fewer inter-organoid variability in size and complexity, and a lesser amount of necrosis over the more structured organoids. Some spheroid BMPS have sizable oligodendrocyte populations (Chesnut, Paschoud et al., 2021, Chesnut, Hartung et al., 2021), which can be further expanded (Romero et al., 2023). In addition, they are very reproducible compared with more complex models such as organoids (Pamies, David et al., 2017). Spheroid BMPS have been used successfully to model viral infections, including ZIKV, DENV, SARS-CoV2, and John Cunningham virus (JCV) (Dingle et al., 2015, Barreras et al., 2022, Pamies, David et al., 2017, Abreu et al., 2018, Kang et al., 2021, Bullen et al., 2020). An example of a spheroid BMPS is shown in Figure 2.
FIGURE 2.
Example of spheroid BMPS and a model for JCV infection. Panels A, B, and C show human brain spheroids immunostained for neurons (MAP-2) (A), astrocytes (GFAP) (B), and myelin basic protein (MBP) (C). The panel D demonstrates a close-up confocal image of a spheroid BMPS system used to model JCV infection showing triple immunostaining for demonstration of JC virus-infected nuclei (green channel, arrows) in NOGO+ oligodendroglia (red channel), nuclei identified with DAPI (blue channel).
BMPS with microglia:
One of the main limitations of most models in the study of neurological infections is the lack of cells of the innate immune system embedded in the model microenvironment. Microglia, the only neuroglia cell derived from the mesoderm and representative of innate immune cells within the CNS, is lacking in most BMPS models. Microglia have critical roles in the neuronal-neuroglia interaction during normal and pathological stages, brain development, injury repair, pruning of redundant synapses, and phagocytosis of dead cells; and are a source of inflammatory cytokines during infection and other pathologies in the brain (Colonna and Butovsky., 2017). The integration of microglia into BMPS is a promising tool to produce a more physiological CNS environment to study infections and study microglia pathophysiology (Xu, R. et al., 2021). Various protocols have shown it is possible to integrate mature microglia into BMPS (Abud et al., 2017, Ormel et al., 2018, Xu, R. et al., 2021). Microglia have also been co-cultured with iPSC-derived spheroid BMPS which was used to evaluate inflammatory responses to LPS and flavivirus infection demonstrating increased expression of cytokines IL-6, IL-1, and TNF-alpha in response to infection with ZIKV and DENV (Abreu et al., 2018). Other protocols have used macrophage progenitors in co-culture with iPSC-derived neural progenitor cells resulting in a brain region-specific BMPS with a well-controlled number of microglia with phagocytic activity and inflammatory responses to ZIKV infection (Xu, R. et al., 2021). A microglia containing BMPS was used recently to study HIV infection (Dos Reis et al., 2020). Interestingly, most recent studies have integrated CD4+T cells into BMPS co-culture systems which may be a future avenue to study infections more robustly (Zhou, Q. et al., 2021).
Brain blood barrier and choroid plexus BMPS:
Most of the BMPS systems lack vasculature and the equivalent of the blood-brain barrier (BBB). The BBB is a critical factor in the penetrance of medications to the CNS and protection against infection (Villabona-Rueda et al., 2019). The choroid plexus, a structure formed by epithelial that surround capillaries forming a blood-cerebrospinal fluid barrier, plays important role in the production of CSF and preventing free-passage of molecules and microorganisms from the systemic circulations into the CSF (Lun, Monuki and Lehtinen., 2015). The absence of BBB and choroid plexus in BMPS is a limiting factor for comprehensive modeling of the pathogenesis of CNS infections and testing of potential therapeutic agents. Recently, a guided organoid of the choroid plexus was developed to model SARS-CoV2 infection in the CNS, demonstrating the efficiency of SARS-CoV2 infection of the choroid plexus (Pellegrini et al., 2020). Organ-on-a-chip models have been applied to model the BBB in the study of viral infection and therapeutic compound effectiveness (Boghdeh et al., 2022).
APPLICATIONS OF HUMAN BRAIN ORGANOIDS IN NEUROLOGICAL INFECTIONS
Most neurological infections studied using BMPS have used viruses with only a few reports exploring other organisms. The most common application of BMPS focused on the ability of different viruses to produce infection in specific cell populations of the 3D cultures and assess neurotropism. Other uses have included evaluation of specific viral receptor expression or factors required to sustain the infection, changes in gene expression profiles to the infection, the effects of the infection in organoid size, cellular morphology or organization, innate immune responses in models with microglia and few, testing of antiviral compounds (Depla et al., 2022).
Assessment of neurotropism and infectibility in BMPS:
Evaluating the capacity of a virus to infect specific neural cells is the most common use for BMPS in neuroinfectious disease (Depla et al., 2022). An important factor to evaluate neurotropic infective pathogens is the time of infection and the stage of in-vitro development as neurons and neuroglial cells that comprise a different type of BMPS present different stages of maturity and development that influence their susceptibility to infective pathogens as determined by the age-associated expression of specific viral receptors or cellular co-factors needed for infection. The age of the BMPS at the time of infection varies greatly across studies ranging from starting the infection at the iPSC stage to several months of in vitro development (Brown et al., 2019, Depla et al., 2022).
Taking into consideration the age of the BMPS and the stage of differentiation are important steps for infection modeling. In the process of differentiating from iPSCs to mature phenotypes, BMPS maturation over time mirrors fetal and early postnatal transitions, a process in which gene and metabolic profiles reflect such stages of maturation. Therefore, knowing the biology of virus receptor expression, virus-receptor interactions, and the timeframe of infection susceptibility are critical factors in the modeling of infections in BMPS. For example, a study using 10-day-old BMPS for ZIKV and HSV infections considered an early stage in development given the expression of neuroectodermal markers Nestin and Pax, found that these early stages of BMPS were highly susceptible to infection by those viruses but not for CMV, a finding that was attributed to lack of intermediate progenitor cells at that stage, a population that was described as the target of CMV infection in 45 days old BMPS (Sun et al., 2020). In another study, 10-day-old BMPS exposed to ZIKV appeared to have a microcephaly phenotype that was dependent on Toll-like receptor 3 (TLR3) overactivation (Dang et al., 2016). Such observation was not confirmed in a second study that used forebrain dorsal organoids in which the cell population was immature and comprised almost purely of NPCs (Liu et al., 2019). Other experimental observations showed that human cortical organoids with relatively long periods of in-vitro culture (250-300 days) but not during in early stages, expressed genes relevant to adult-onset pathology and aging (such as APOE and PRK1) (Gordon et al., 2021), a finding that support the view organoid models are subject to an aging period which may influence their use in infection models.
The use of BMPS became relevant in the recent epidemic of ZIKV and the SARS-CoV2 pandemic, situations in which those viruses were linked to neurological symptoms but the ability of such viruses to infect the brain was unclear and there were no reliable models available for their study. A good demonstration of the use of BMPS in modeling viral infection was the use of a human iPSC-derived forebrain organoid to study the neurotropism of ZIKV, a model which demonstrated the preferential infection of neural progenitor cells (Qian et al., 2016). The use of BMPS has also been relevant for studying viruses that do not infect animal tissue such as JCV, a polyomavirus and causative agent of progressive multifocal leukoencephalopathy (PML), an infectious demyelinating disease of the brain. A spheroid BMPS model containing neurons and neuroglial cells including oligodendrocytes was used to model JCV infection, demonstrating productive infection in oligodendrocytes and astrocytes but not neurons (Barreras et al., 2022).
Assessing the effects of the infection at the cellular and structural level:
Given that most BMPS recapitulate early stages of neurodevelopment, some studies have been used to evaluate the effects of infection during neurodevelopment or stages relevant to the pregnancy of in-utero infections such as ZIKV and CMV. A model of ZIKV infection resulted in a reduction in progenitor cell and cortical layers and reduced size of the BMPS which resembled microcephaly. Similarly, a model of CMV infection led to reduced BMPS size (Brown et al., 2019, Qian et al., 2016).
Evaluating changes in gene expression:
Mechanisms of pathogenesis and specific signaling pathways may be altered during the infection of the CNS. Genomic, proteomic, and metabolomic studies can be important approaches to assess the effects of infection on CNS models like BMPS. Comparative single-cell transcriptomic studies of various BMPS and fetal brains identified differences determined by the type of BMPS production protocols such as more divergent cell compositions in non-guided protocols, variable amounts of oligodendrocyte and interneuron gene makers as well as different genes expressed during neuronal differentiation trajectory when using different protocols (Tanaka et al., 2020). In transcriptomic analyses of neuroinfectious disease BMPS models, a SARS-CoV2 BMPS infection model demonstrated the expression of angiotensin converting enzyme (ACE2) in the model and its functional requirement for infection, as well as gene expression, changes in the metabolic profile of infected neurons (Song, E. et al., 2021). In a BMPS model comparing ZIKV and HSV-1 infection, distinct transcriptomic profiles were identified in both infections, with HSV-1 activating non-neuronal developmental programs and disrupting the expression of N-Cadherin and SOX1 in neural progenitor cells in the model and both viruses failing to induce the type I interferon system (Krenn et al., 2021). Another potential application of genomic and transcriptomic studies is the possibility of studying the infection effect from genetically different BMPS derived from patients with a genetic background of interest. This approach has been done in the context of neurodegenerative disease where iPSC derived from patients with Parkinson's disease-generated BMPS was proven to have different gene expressions compared to those derived from normal individuals (Schultz et al., 2021).
Evaluating factors mediating infection:
BMPS can be used to assess factors that influence pathogen-cell interaction, receptor usage, or cellular properties needed to achieve productive infection. Studies to assess if ACE2 expression is required for SARS-CoV2 infection of BMPS neurons (Song, E. et al., 2021) or the role of EGFR and PDGFRα as mediators of CMV infection in human iPSC-derived BMPS models, where BMPS treated with EGFR- or PDGFRα-specific siRNA exhibited lower levels of infection, are good examples of the use of BMPS to test factors needed for infection (Sun et al., 2020).
Therapeutic drug-testing screening:
Although testing of potential anti-infective therapies in BMPS remains underutilized to date, the potential to offer a more physiologically relevant system and high-throughput screening of therapeutic compounds is one of their most promising applications. Most clinical trials in neurology fail to translate promising findings derived from animal studies, in part due to the interspecies differences in genetics and diversity of cellular phenotypes. BMPS has been used to study drug efficacy including testing of antiviral drugs in ZIKV (Ho, Pek, and Soh., 2018, Li, X., Shopit and Wang., 2022, Xu, M. et al., 2016, Zhou, T. et al., 2017). Specifically, BMPS was used in high-throughput compound screening for anti-ZIKV infection compounds that identified hippeastrine hydrobromide and amodiaquine dihydrochloride as potential inhibitors of ZIKV infection (Zhou, T. et al., 2017). Similarly, a human iPSC-derived BMPS model found that sofosbuvir, an anti-hepatitis C drug, could rescue cell death and impair synaptogenesis induced by SARS-CoV2 infection (Mesci et al., 2022). In addition, BMPS platforms have also been used to explore the neurotoxicity of antiviral treatments in HIV (Fan et al., 2022).
ADVANTAGES OF HUMAN BRAIN MICROPHYSIOLOGICAL SYSTEMS OVER OTHER MODELS FOR STUDYING NEUROLOGICAL INFECTIONS
Given the lack of readily accessible human cerebral tissue, particularly in vivo, to study neurological infections, most of the knowledge about pathogenesis and pathophysiology is derived from animal models or autopsy studies. Some basic studies are performed in 2D cultures of neurons or glial cells. Using a BMPS model better reflects human gene expression and cellular identity and allows for the co-culture and interaction between different cell types. There are important differences between human and mouse cells and their susceptibility to infective pathogens as some viruses are human-specific and do not infect mouse cells efficiently due to a lack of required receptors or other cellular or cellular-virus interaction properties (Fan et al., 2022). Notably, differences in gene expression have been identified in 3D versus 2D models which can influence the effect of infection and biological outcomes (Tekin et al., 2018). Two-dimensional cultures lack the complexity of the microenvironment of the human brain which may limit conclusions about neurological infection physiopathology.
A significant advantage of using BMPS is the ability to produce models with human-specific genetic backgrounds originating from cells of healthy donors but also from specific patients or donors with mutations of interest that may influence susceptibility to infection. A good example of such approach was demonstrated by the “cell villages” model introduced by Wells et. al., where neuronal progenitor cells from 44 different human donors were cultured in a shared environment, to demonstrate that genome-wide SNPs could account for most inter-donor variation in NPC ZIKA viral susceptibility (Wells et al., 2023). A study of Chikungunya virus infection in BMPS compared infection in healthy BMPS to BMPS derived from a Parkinson’s disease patient and found that Parkinson’s derived BMPS had a higher loss of volume and different antiviral responses in response to the infection (Schultz et al., 2021). Using BMPS while understanding the genetic background of the cell line used to generate them could be useful to better understand how genetic factors interact with infectious pathogens in CNS infection,
BMPS are also able to be mass-produced (Pamies, David et al., 2017, Decembrini et al., 2020, Dossena et al., 2020). The ability to produce BMPS in large numbers relatively quickly has been proven useful in the recent epidemic of ZIKV and COVID-19 pandemic where there was a pressing need to investigate neurotropism due to the limited access to human-infected tissue (Abreu et al., 2018, Bullen et al., 2020). Finally, human-derived BMPS models bypass the ethical issues of animal experimentation as they can originate from tissues readily available for biopsy (e.g., skin).
LIMITATIONS
While BMPS are promising tools in the field of neuroinfectious diseases, they have important limitations. One major limitation is the isolation of the brain system from other organ systems as opposed to animal models, a factor that may limit the understanding of the neurological effects that other organ infections or systemic infections may have. Most BMPS models are limited to neural cell types, neurons, and some glial cells, but lack an immune system with only a few recently incorporating microglia to overcome this limitation (Abreu et al., 2018). The lack of an immune system limits the understanding of the inflammatory response to infection in the brain which is a key aspect in many common infections such as HSV-1 and HIV.
Most systems are devoid of a BBB, a factor that limits the study of the BBB components and interactions with other CNS cells in the viral entry mechanisms to the CNS. The recently described BBB MPS have complex production protocols which have not been widely used in the study of neurological infections. In addition, BMPS lack elements of a vascular system, which makes access to nutrients and oxygen to the center of the cultures limited, especially on the large organoids, which can result in necrosis in the center of the model and impact transcriptomic and cytokine analysis studies (Kiaee et al., 2021). Another limiting factor is the presence of a population of immature or partially differentiated cells in most BMPS models, which can limit the extrapolation of conclusions to adult human brains.
Guided region-specific organoids are very useful to study specific brain regions, but those models may limit the understanding of the disease process in the brain as a whole. The guided BMPS protocols are still technically complex and expensive to produce. On the other hand, unguided BMPS production protocols can have significant organoid-to-organoid variability. In the case of the BMPS that have been able to up-scale to large production, even while the ability to produce BMPS in high numbers per experiment is an advantage, these cells all share the same genetic material and donor-specific characteristics which can bias the experiment results (Hopkins, Traverse and Barr., 2021). An additional limitation is the potentially unknown effects of some of the chemicals or patterning factors use in some differentiation protocols, such as Wnt inhibitors or retinoic acid, in neuronal and glial populations and their interaction with pathogens of interest (Wang, Y. et al., 2022, Miura et al., 2020, Watanabe et al., 2017).
Some groups have developed new approaches to generate BMPS that overcome some of these limitations, like the use of single self-organized neural rosettes to derive organoid models without using agonists/antagonists of brain morphogenesis, as opposed to the traditional generation of organoids or spheroids with multiple neural rosettes, which resulted in a telencephalic BMPS with more predictable organization and cytoarchitecture (Wang, Y. et al., 2022). Other groups have suggested cutting large cerebral organoids into various pieces and then continuing to culture the cut pieces or slicing the organoids and growing them as a slice as a way to limit the central necrosis that has been observed in the more complex models (Choe et al., 2021, Qian et al., 2020). These approaches could yield more homogenous results in the study of neurological infections.
HUMAN BRAIN MICROPHYSIOLOGICAL SYSTEMS MODELS FOR SPECIFIC INFECTIONS
Most of the studies of BMPS and infection have focused on viruses with only a few on bacterial or parasitic infections. Considering the recent ZIKV and SARS-CoV2 epidemic and pandemic, most infection studies in BMPS have focused on such viruses as well as in models of HSV-1 and CMV infection. Other viruses that have been modeled include DENV, Japanese encephalitis virus measles, JCV, Chikungunya virus, and adenovirus among others (Depla et al., 2022). A summary of the main findings of BMPS models of infection is outlined in Table 1. Please notice that an extended review of in vitro and in silico models to study flavivirus can be found elsewhere (Chesnut et al., 2019).
Table 1.
Studies using human brain microphysiological systems in infectious diseases
| Infection | Outcomes | BMPS used | Reference |
|---|---|---|---|
| ZIKV |
|
Forebrain, Unguided cerebral, Spheroid | (Abreu et al., 2018, Dang et al., 2016, Krenn et al., 2021, Garcez et al., 2016, Qian et al., 2016) |
| SARS-CoV2 |
|
Unguided cerebral, Choroid plexus, Dorsal forebrain, Spheroid | (Song, E. et al., 2021, Pellegrini et al., 2020, Bullen et al., 2020, Ramani et al., 2020, Wang, C. et al., 2021) |
| Dengue Virus |
|
BMPS + Microglia, Forebrain, Cortical | (Abreu et al., 2018, Yoon et al., 2017, Li, Y. et al., 2017) |
| Chikungunya Virus |
|
Unguided cerebral | (Schultz et al., 2021) |
| Japanese Encephalitis Virus |
|
Telencephalon | (Zhang, B. et al., 2018) |
| VEEV |
|
BBB model | (Boghdeh et al., 2022) |
| HSV-1 |
|
Unguided cerebral, Dorsal root ganglia | (D'Aiuto et al., 2019, Krenn et al., 2021, Mazzara et al., 2022) |
| CMV |
|
Unguided cerebral; Cortical | (Brown et al., 2019, Sison et al., 2019, Sun et al., 2020) |
| HIV |
|
Microglia containing BMPS | (Dos Reis et al., 2020, dos Reis, Sant and Ayyavoo., 2022) |
| JC virus |
|
Spheroid | (Barreras et al., 2022) |
| Enterovirus |
|
Unguided cerebral | (Sridhar et al., 2022) |
| Measles |
|
Unguided cerebral | (Mathieu et al., 2021) |
| LACV |
|
Guided cortical | (Winkler et al., 2019, Ojha et al., 2021) |
| Influenza |
|
Unguided cerebral | (Zhang, X. et al., 2022) |
Zika virus
Guided forebrain-specific BMPS was used to assess the infectivity of African and Asian strains of ZIKV, demonstrating that both strains preferentially infected neural progenitors, leading to increased cell death and decreased neuronal cell layer volume which resembled a microcephaly-like phenotype (Qian et al., 2016). The specific tropism for neural progenitor cells was confirmed in a BMPS model that showed productive infection in NPC resulting in a reduced organoid size (Krenn et al., 2021). Similar findings were documented in other studies (Garcez et al., 2016) including the demonstration that ZIKV decreased the number of progenitor cells through activation of the TLR3 receptor which dysregulated genes involved in neurogenesis, axon guidance, and apoptosis (Dang et al., 2016). More recently BMPS containing microglia were used to demonstrate the infectivity of ZIKV and the inflammatory responses triggered by the infection (Abreu et al., 2018).
SARS-CoV2
The ability of SARS-CoV2 to infect cells within the CNS has been questioned as the virus appears to be absent in the CSF of infected patients (Garcia et al., 2021) and rarely seen in the brain of autopsied patients that die during the acute phase of COVID-19 (Thakur et al., 2021). Multiple studies used BMPS to assess the potential neurotropism of SARS-CoV2. The expression of ACE2, the putative receptor of SARS-CoV2, was shown in an organoid model in which exposure to the virus for 6 hours resulted in infection of a fraction of the neural cells and the presence of replicating virus in the neuronal cell body and neurites (Bullen et al., 2020). In-vitro infection by SARS-CoV2 of neurons and to a lesser degree neural stem cells can be prevented by blocking the ACE2 receptor (Song, E. et al., 2021). Interestingly, other models of SARS-CoV2 infection in BMPS showed that neurons were infected at higher rates when co-cultured with astrocytes (Wang, C. et al., 2021). Changes in SARS-CoV2-infected neurons appear to be associated with altered distribution of Tau from axons to soma, hyperphosphorylation, and neuronal death (Ramani et al., 2020). A choroid plexus BMPS model showed that required entry factors for the virus were expressed in choroid plexus cells and that those cells were more susceptible to infection than neurons (Pellegrini et al., 2020).
Dengue virus
Most studies of DENV infection in BMPS were done in the context of ZIKV studies where DENV was used as a control flavivirus with the assumption that such virus was not known to be particularly neuroinvasive or to cause microcephaly. A comparison of ZIKV and DENV infection in a BMPS spheroid model with microglia revealed DENV was capable to produce infection although to a lesser degree than ZIKV. In that model, DENV seemed to preferentially infect microglia over neurons or astrocytes (Abreu et al., 2018). A comparative study of ZIKV and DENV in human forebrain BMPS showed that ZIKV infection but not DENV infection resulted in reduced radial glial cell proliferation and degradation of adherens junction complex proteins (Yoon et al., 2017). In comparison to ZIKV, DENV did not trigger severe apoptosis or cortical folding defects in a cortical BMPS model (Li, Y. et al., 2017).
Chikungunya
Chikungunya virus (CHIKV) is an alphavirus that caused outbreaks of febrile illness in the Americas and India and has been associated with neurological complications including encephalitis, myelitis, and Guillain-Barre syndrome (Mehta et al., 2018). Human BMPS derived from a Parkinson’s patient and a healthy individual were infected with CHIKV and compared. Although both conditions had dysregulation of IL-1, IL-10, and IL-6 and increased expression of CXCL10 after infection, the Parkinson’s derived BMPS had a reduction in mass and different gene expression and antiviral responses as compared to the healthy BMPS (Schultz et al., 2021). One of the limitations of experimental studies and manipulation of CHIKV is the need for BSL3 facilities.
Japanese encephalitis virus
Japanese encephalitis virus (JEV) is one of the most important causes of encephalitis in Asia affecting mainly children and leading to serious neurological sequelae. Telencephalon BMPS exposed to JEV were susceptible to infection in astrocytes and neural progenitor cells leading to increased cell death and decreased cell proliferation resulting in smaller BMPS size. In that model, interferon signaling pathways were activated in older BMPS but not in early ones (Zhang, B. et al., 2018).
Venezuelan Equine Encephalitis virus
Venezuelan Equine Encephalitis Virus (VEEV) is an alphavirus that can lead to fatal encephalitis in children and adults. VEEV infection was modeled using a neurovascular unit model of the BBB which found the therapeutic effectiveness of omaveloxolone, a compound that preserved BBB integrity and decreased viral and inflammatory load (Boghdeh et al., 2022).
Herpes simplex virus 1
HSV-1 is one of the most important causes of infectious encephalitis worldwide. Multiple BMPS has been used to study HSV-1 infection. In a recent model, HSV-1 was able to infect iPSC-derived neurons, with the virus spreading from the outer layers of the organoid and transported to the interior lamina demonstrating the virus trafficking capability. In addition, the BMPS was able to support HSV-1 reactivation (D'Aiuto et al., 2019). Another study that compared ZIKV and HSV-1 infection in a human BMPS showed that HSV-1 led to increased cell death and decreased cortical layer formation, as well as disruption of expression of N-Cadherin and SOX1 in the neuronal progenitor cells (Krenn et al., 2021). One study used human stem-cell-derived dorsal root ganglia organoids to be infected with HSV-1 as part of a Neurosensory–Epithelial Circuitry on a Chip to Model, as a model of viral latency and reactivation which demonstrated receptors for the virus were present in the sensory neurons (Mazzara et al., 2022).
Cytomegalovirus
CMV infection during pregnancy can cause serious birth defects including microcephaly, chorioretinitis, and hydrocephalus. The understanding of CMV neuropathogenesis is limited as CMV does not efficiently infect animal models. Modeling CMV in human BMPS demonstrated infection resulting in reduced organoid growth, impaired formation of the cortical layer, and abnormal calcium signaling. Such infection effects could be prevented by neutralizing antibodies against the CMV pentamer complex (Sun et al., 2020). Another model of CMV-infected cortical BMPS showed infection in neurons and astrocytes, impaired calcium signaling, and disruption of the BMPS structure. The antiviral maribavir partially restored the structural features and effects of the infection (Sison et al., 2019). Interestingly, an unguided cerebral BMPS model showed that CMV-infected iPSC can be differentiated into BMPS and express viral proteins, and exhibited pathological changes such as necrosis, vacuoles, and cysts similar to what is observed in-utero infections (Brown et al., 2019).
HIV
HIV infection of the CNS may result in neurological complications such as dementia, an important cause of disability in HIV-infected populations. The main target of HIV in the CNS is microglia. BMPS co-cultured with HIV-infected microglia demonstrated HIV-productive viral infection and increased inflammatory responses including elevation of tumor necrosis factor (TNF-α) and interleukin-1 (IL-1β) (Dos Reis et al., 2020, dos Reis, Sant and Ayyavoo., 2022).
JCV
JC virus is a polyomavirus that can infect oligodendrocytes and astrocytes, causing brain inflammation and demyelination leading to PML. A good understanding of the pathophysiology of PML has been limited by the lack of an animal model given JCV only infects efficiently human cells. A human iPSC-derived BMPS containing neurons, astrocytes, and oligodendrocytes to model JCV infection demonstrated the presence of productive infection as documented by PCR, immunohistochemistry, and electron microscopy (Barreras et al., 2022).
Enterovirus
Enterovirus D68 has been linked to the recent outbreaks of acute flaccid myelitis, a polio-like illness that has affected children in the last decade. The pathogenesis of acute flaccid myelitis is still unclear highlighting the need for physiologically relevant models. Various strains of enterovirus D68 (B2/2042, B2/947, and A1/1348) were used to test the ability of these viruses to infect human BMPS. There was a productive infection in the model in which the 3 strains were tested. This model was used to investigate the mechanistic role of heparan sulfate proteoglycans in infection and found no additional role for heparan sulfate binding in neurotropism (Sridhar et al., 2022).
Measles
Recent outbreaks of measles, especially among vaccine-hesitant populations have raised interest in the re-emergence of this viral illness which may have neurological effects including measles inclusion body encephalitis and subacute sclerosing panencephalitis, a severe neurological complication of measles. Measles virus isolated from patients with CNS infection containing a single amino acid mutation in the fusion protein were used to infect human BMPS, finding that the mutation enhanced the spread of the virus which could be blocked by fusion protein inhibitors, a finding that confirmed the role of the fusion protein in CNS viral spread (Mathieu et al., 2021).
La Crosse encephalitis virus
LACV is an orthobunyavirus that causes encephalitis in children. LACV produced infection in cortical BMPS in both neural progenitor cells and neurons, but committed neurons were more susceptible to LACV-induced apoptosis which seemed mediated by reduced expression of interferon signaling in response to the infection (Winkler et al., 2019). Treatment with rottlerin inhibited LACV replication (Ojha et al., 2021).
Influenza
The influenza virus primarily causes respiratory illness, but cases of neurological involvement have been reported including cases of encephalitis. Exposure to influenza virus (H1N1-WSN and H3N2-HKT68) to human iPSC-derived BMPS resulted in decreased size, with the WSN strain preferentially infecting neurons over neural stem cells and astrocytes, and increasing the release of inflammatory cytokines. In this study, a potential antiviral drug candidate PYC-12 decreased viral replication (Zhang, X. et al., 2022).
Adenovirus
Adenovirus was used in BMPS as a vector in gene therapy (Choudhury et al., 2017), as a virus-based vector for the transduction of astrocytes, and as a vehicle for delivery of specific genes but not as a model of CNS disease (Depla et al., 2020, Kunze et al., 2018).
Non-viral organisms
While most neuroinfectious disease studies in human brain organoids have focused on viral pathogens, there are a few reports using other organisms. Human BMPS to study cerebral malaria showed hemolysis byproducts that resulted in injury changes in the model and increased expression of BDNF, CXCR3, and CXCL-10. This model also showed neuroprotective effects when treating the BMPS with neuregulin-1 (Harbuzariu et al., 2019). Another study of bacterial infection in BMPS used Staphylococcus aureus, a model that demonstrated such infection upregulated Aβ-protein load in human tonsil organoids and BMPS (Lim et al., 2022).
Conclusion
3D human BMPS are powerful and versatile tools for the investigation of neuroinfectious diseases, allowing observation of infection in a physiologically relevant CNS microenvironment with neural and glial human cells in co-culture, allowing for analysis not only of viral tropism but of pathophysiological mechanisms and treatment responses.
Disclosures
PB has received support from the Foundation for Sarcoidosis Research
DP and TH are named inventors on a patent by Johns Hopkins University on the production of brain organoids, which is licensed to AxoSim, New Orleans, LA, USA. TH also consults to AxoSim. CP received funding support from NIH (R01 NS110122 AND 1R01NS123712)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abreu CM, Gama L, Krasemann S, Chesnut M, Odwin-Dacosta S, Hogberg HT, Hartung T, Pamies D, 2018. Microglia Increase Inflammatory Responses in iPSC-Derived Human BrainSpheres. Frontiers in Microbiology 9, 2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abud EM, Ramirez RN, Martinez ES, Healy LM, Nguyen CHH, Newman SA, Yeromin AV, Scarfone VM, Marsh SE, Fimbres C, Caraway CA, Fote GM, Madany AM, Agrawal A, Kayed R, Gylys KH, Cahalan MD, Cummings BJ, Antel JP, Mortazavi A, Carson MJ, Poon WW, Blurton-Jones M, 2017. iPSC-Derived Human Microglia-like Cells to Study Neurological Diseases. Neuron (Cambridge, Mass.) 94, 278–293.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson WA, Bosak A, Hogberg HT, Hartung T, Moore MJ, 2021. Advances in 3D neuronal microphysiological systems: towards a functional nervous system on a chip. In Vitro Cell. Dev. Biol. -Animal 57, 191–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barreras P, Pamies D, Monaco MC, Muñoz LS, Zhong X, Major EO, Hogberg HT, Hartung T, Pardo CA, 2022. A human-derived 3D brain organoid model to study JC virus infection. J. Neurovirol 28, 17–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boghdeh NA, Risner KH, Barrera MD, Britt CM, Schaffer DK, Alem F, Brown JA, Wikswo JP, Narayanan A, 2022. Application of a Human Blood Brain Barrier Organ-on-a-Chip Model to Evaluate Small Molecule Effectiveness against Venezuelan Equine Encephalitis Virus. Viruses 14, 2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RM, Rana, Pranav SJB, Jaeger HK, O'Dowd JM, Balemba OB, Fortunato EA, 2019. Human Cytomegalovirus Compromises Development of Cerebral Organoids. Journal of virology 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullen CK, Hogberg HT, Bahadirli-TaIbott A, Bishai WR, Hartung T, Keuthan C, Looney MM, Pekosz A, Romero JC, Sillé FCM, Um P, Smirnova L, 2020. Infectability of Human BrainSphere Neurons Suggests Neurotropism of SARS-CoV-2. ALTEX, alternatives to animal experimentation 37, 665–671. [DOI] [PubMed] [Google Scholar]
- Carrillo GL, Ballard VA, Glausen T, Boone Z, Teamer J, Hinkson CL, Wohlfert EA, Blader IJ, Fox MA, 2020. Toxoplasma infection induces microglia-neuron contact and the loss of perisomatic inhibitory synapses. Glia 68, 1968–1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CJ, Ou YC, Lin SY, Raung SL, Liao SL, Lai CY, Chen SY, Chen JH, 2010. Glial activation involvement in neuronal death by Japanese encephalitis virus infection. Journal of General Virology 91, 1028–1037. [DOI] [PubMed] [Google Scholar]
- Chesnut M, Hartung T, Hogberg H, Pamies D, 2021. Human Oligodendrocytes and Myelin In Vitro to Evaluate Developmental Neurotoxicity. International journal of molecular sciences 22, 7929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesnut M, Muñoz LS, Harris G, Freeman D, Gama L, Pardo CA, Pamies D, 2019. In vitro and in silico Models to Study Mosquito-Borne Flavivirus Neuropathogenesis, Prevention, and Treatment. Frontiers in Cellular and Infection Microbiology 9, 223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesnut M, Paschoud H, Repond C, Smirnova L, Hartung T, Zurich M, Hogberg HT, Pamies D, 2021. Human IPSC-Derived Model to Study Myelin Disruption. International journal of molecular sciences 22, 9473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choe MS, Kim SJ, Oh ST, Bae CM, Choi W, Baek KM, Kim JS, Lee MY, 2021. A simple method to improve the quality and yield of human pluripotent stem cell-derived cerebral organoids. Heliyon 7, e07350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhury SR, Hudry E, Maguire CA, Sena-Esteves M, Breakefield XO, Grandi P, 2017. Viral vectors for therapy of neurologic diseases. Neuropharmacology 120, 63–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colonna M, Butovsky O, 2017. Microglia Function in the Central Nervous System During Health and Neurodegeneration. Annual review of immunology 35, 441–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Aiuto L, Bloom DC, Naciri JN, Smith A, Edwards TG, McClain L, Callio JA, Jessup M, Wood J, Chowdari K, Demers M, Abrahamson EE, Ikonomovic MD, Viggiano L, De Zio R, Watkins S, Kinchington PR, Nimgaonkar VL, 2019. Modeling Herpes Simplex Virus 1 Infections in Human Central Nervous System Neuronal Cells Using Two- and Three-Dimensional Cultures Derived from Induced Pluripotent Stem Cells. Journal of virology 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang J, Tiwari SK, Lichinchi G, Qin Y, Patil V, Eroshkin A, Rana T, 2016. Zika Virus Depletes Neural Progenitors in Human Cerebral Organoids through Activation of the Innate Immune Receptor TLR3. Cell stem cell 19, 258–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decembrini S, Hoehnel S, Brandenberg N, Arsenijevic Y, Lutolf MP, 2020. Hydrogel-based milliwell arrays for standardized and scalable retinal organoid cultures. Scientific reports 10, 10275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depla JA, Mulder LA, de Sá RV, Wartel M, Sridhar A, Evers MM, Wolthers KC, Pajkrt D, 2022. Human Brain Organoids as Models for Central Nervous System Viral Infection. Viruses 14, 634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depla JA, Sogorb-Gonzalez M, Mulder LA, Heine VM, Konstantinova P, van Deventer SJ, Wolthers KC, Pajkrt D, Sridhar A, Evers MM, 2020. Cerebral Organoids: A Human Model for AAV Capsid Selection and Therapeutic Transgene Efficacy in the Brain. Molecular therapy. Methods & clinical development 18, 167–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingle YL, Boutin ME, Chirila AM, Livi LL, Labriola NR, Jakubek LM, Morgan JR, Darling EM, Kauer JA, Hoffman-Kim D, 2015. Three-Dimensional Neural Spheroid Culture: An In Vitro Model for Cortical Studies. Tissue engineering. Part C, Methods 21, 1274–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dos Reis RS, Sant S, Keeney H, Wagner MCE, Ayyavoo V, 2020. Modeling HIV-1 neuropathogenesis using three-dimensional human brain organoids (hBORGs) with HIV-1 infected microglia. Scientific reports 10, 15209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- dos Reis RS, Sant S, Ayyavoo V, 2022. Three-Dimensional Human Brain Organoids to Model HIV-1 Neuropathogenesis. In: Anonymous Methods in Molecular Biology (Clifton, N.J.). Springer US, New York, NY, pp. 167–178. [DOI] [PubMed] [Google Scholar]
- Dossena M, Piras R, Cherubini A, Barilani M, Dugnani E, Salanitro F, Moreth T, Pampaloni F, Piemonti L, Lazzari L, 2020. Standardized GMP-compliant scalable production of human pancreas organoids. Stem Cell Research & Therapy 11, 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drogemuller K, Helmuth U, Brunn A, Sakowicz-Burkiewicz M, Gutmann DH, Mueller W, Deckert M, Schluter D, 2008. Astrocyte gp130 Expression Is Critical for the Control of Toxoplasma Encephalitis. The Journal of immunology (1950) 181, 2683–2693. [DOI] [PubMed] [Google Scholar]
- Eiraku M, Watanabe K, Matsuo-Takasaki M, Kawada M, Yonemura S, Matsumura M, Wataya T, Nishiyama A, Muguruma K, Sasai Y, 2008. Self-Organized Formation of Polarized Cortical Tissues from ESCs and Its Active Manipulation by Extrinsic Signals. Cell Stem Cell 3, 519–532. [DOI] [PubMed] [Google Scholar]
- Fan W, Christian KM, Song H, Ming G, 2022. Applications of Brain Organoids for Infectious Diseases. Journal of molecular biology 434, 167243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcez PP, Loiola EC, Costa R.M.d., Higa LM, Trindade P, Delvecchio R, Nascimento JM, Brindeiro R, Tanuri A, Rehen SK, 2016. Zika virus impairs growth in human neurospheres and brain organoids. Science (American Association for the Advancement of Science) 352, 816–818. [DOI] [PubMed] [Google Scholar]
- Garcia MA, Barreras PV, Lewis A, Pinilla G, Sokoll LJ, Kickler T, Mostafa H, Caturegli M, Moghekar A, Fitzgerald KC, Pardo CA, 2021. Cerebrospinal fluid in COVID-19 neurological complications: Neuroaxonal damage, anti-SARS-Cov2 antibodies but no evidence of cytokine storm. Journal of the neurological sciences 427, 117517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon A, Yoon S, Tran SS, Makinson CD, Park JY, Andersen J, Valencia AM, Horvath S, Xiao X, Huguenard JR, Pasca SP, Geschwind DH, 2021. Long-term maturation of human cortical organoids matches key early postnatal transitions. Nature neuroscience 24, 331–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harbuzariu A, Pitts S, Cespedes JC, Harp KO, Nti A, Shaw AP, Liu M, Stiles JK, 2019. Modelling heme-mediated brain injury associated with cerebral malaria in human brain cortical organoids. Scientific Reports 9, 19162–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho BX, Pek NMQ, Soh B, 2018. Disease Modeling Using 3D Organoids Derived from Human Induced Pluripotent Stem Cells. International Journal of Molecular Sciences 19, 936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins HK, Traverse EM, Barr KL, 2021. Methodologies for Generating Brain Organoids to Model Viral Pathogenesis in the CNS. Pathogens (Basel) 10, 1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob F, Schnoll JG, Song H, Ming G, 2021. Building the brain from scratch: engineering region-specific brain organoids from human stem cells to study neural development and disease. Current topics in developmental biology 142, 477–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo J, Xiao Y, Sun A, Cukuroglu E, Tran H, Göke J, Tan Z, Saw T, Tan C, Lokman H, Lee Y, Kim D, Ko H, Kim S, Park J, Cho N, Hyde T, Kleinman J, Shin J, Weinberger D, Tan E, Je H, Ng H, 2016. Midbrain-like Organoids from Human Pluripotent Stem Cells Contain Functional Dopaminergic and Neuromelanin-Producing Neurons. Cell stem cell 19, 248–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadoshima T, Sakaguchi H, Nakano T, Soen M, Ando S, Eiraku M, Sasai Y, 2013. Self-organization of axial polarity, inside-out layer pattern, and species-specific progenitor dynamics in human ES cell-derived neocortex. Proceedings of the National Academy of Sciences - PNAS 110, 20284–20289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang S, Kim D, Lee J, Takayama S, Park JY, 2021. Engineered Microsystems for Spheroid and Organoid Studies. Advanced healthcare materials 10, e2001284-n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiaee K, Jodat YA, Bassous NJ, Matharu N, Shin SR, 2021. Transcriptomic Mapping of Neural Diversity, Differentiation and Functional Trajectory in iPSC-Derived 3D Brain Organoid Models. Cells (Basel, Switzerland) 10, 3422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Xu R, Padmashri R, Dunaevsky A, Liu Y, Dreyfus CF, Jiang P, 2019. Pluripotent Stem Cell-Derived Cerebral Organoids Reveal Human Oligodendrogenesis with Dorsal and Ventral Origins. Stem Cell Reports 12, 890–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JJ, Jorfi M, Tanzi RE, Kim DY, Doyle PS, Irimia D, 2021. Patterning of interconnected human brain spheroids. Lab on a chip 21, 3532–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krenn V, Bosone C, Burkard TR, Spanier J, Kalinke U, Calistri A, Salata C, Rilo Christoff R, Pestana Garcez P, Mirazimi A, Knoblich JA, 2021. Organoid modeling of Zika and herpes simplex virus 1 infections reveals virus-specific responses leading to microcephaly. Cell stem cell 28, 1362–1379.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunze C, Börner K, Kienle E, Orschmann T, Rusha E, Schneider M, Radivojkov-Blagojevic M, Drukker M, Desbordes S, Grimm D, Brack-Werner R, 2018. Synthetic AAV/CRISPR vectors for blocking HIV-1 expression in persistently infected astrocytes. Glia 66, 413–427. [DOI] [PubMed] [Google Scholar]
- Lancaster MA, Renner M, Martin C, Wenzel D, Bicknell LS, Hurles ME, Homfray T, Penniger JM, Jackson AP, Knoblich JA, 2013. Cerebral organoids model human brain development and microcephaly. Nature 501, 373–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leite PEC, Pereira MR, Harris G, Pamies D, Dos Santos, Lisia Maria Gobbo, Granjeiro JM, Hogberg HT, Hartung T, Smirnova L, 2019. Suitability of 3D human brain spheroid models to distinguish toxic effects of gold and poly-lactic acid nanoparticles to assess biocompatibility for brain drug delivery. Particle and Fibre Toxicology 16, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Shopit A, Wang J, 2022. A Comprehensive Update of Cerebral Organoids between Applications and Challenges. Oxidative medicine and cellular longevity 2022, 7264649–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Muffat J, Omer A, Bosch I, Lancaster MA, Sur M, Gehrke L, Knoblich JA, Jaenisch R, 2017. Induction of Expansion and Folding in Human Cerebral Organoids. Cell stem cell 20, 385–396.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim JY, Lee JE, Kim HK, Park Y, Jeon JH, Park S, Lee N, Lee IH, Kim DH, Yang SH, Yoo J, Kim SW, 2022. Human Palatine Tonsils Are Linked to Alzheimer’s Disease through Function of Reservoir of Amyloid Beta Protein Associated with Bacterial Infection. Cells (Basel, Switzerland) 11, 2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Chen Z, Zhang X, Li S, Hui Y, Feng H, Du Y, Jin G, Zhou X, Zhang X, 2019. Protection of ZIKV infection-induced neuropathy by abrogation of acute antiviral response in human neural progenitors. Cell death and differentiation 26, 2607–2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lun MP, Monuki ES, Lehtinen MK, 2015. Development and functions of the choroid plexus–cerebrospinal fluid system. Nature reviews. Neuroscience 16, 445–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madhavan M, Nevin ZS, Shick HE, Garrison E, Clarkson-Paredes C, Karl M, Clayton BLL, Factor DC, Allan KC, Barbar L, Jain T, Douvaras P, Fossati V, Miller RH, Tesar PJ, 2018. Induction of myelinating oligodendrocytes in human cortical spheroids. Nature methods 15, 700–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathieu C, Bovier FT, Ferren M, Lieberman NAP, Predella C, Lalande A, Peddu V, Lin MJ, Addetia A, Patel A, Outlaw V, Corneo B, Dorrello NV, Briese T, Hardie D, Horvat B, Moscona A, Greninger AL, Porotto M, 2021. Molecular Features of the Measles Virus Viral Fusion Complex That Favor Infection and Spread in the Brain. mBio 12, e0079921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzara PG, Criscuolo E, Rasponi M, Massimino L, Muggeo S, Palma C, Castelli M, Clementi M, Burioni R, Mancini N, Broccoli V, Clementi N, 2022. A Human Stem Cell-Derived Neurosensory–Epithelial Circuitry on a Chip to Model Herpes Simplex Virus Reactivation. Biomedicines 10, 2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta R, Gerardin P, Brito CAA, Soares CN, Ferreira MLB, Solomon T, 2018. The neurological complications of chikungunya virus: A systematic review. Reviews in Medical Virology 28, e1978-n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesci P, de Souza JS, Martin-Sancho L, Macia A, Saleh A, Yin X, Snethlage C, Adams JW, Avansini SH, Herai RH, Almenar-Queralt A, Pu Y, Szeto RA, Goldberg G, Bruck PT, Papes F, Chanda SK, Muotri AR, 2022. SARS-CoV-2 infects human brain organoids causing cell death and loss of synapses that can be rescued by treatment with Sofosbuvir. PLoS Biology 20, e3001845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura Y, Li M, Birey F, Ikeda K, Revah O, Thete MV, Park J, Puno A, Lee SH, Porteus MH, Pasca SP, 2020. Generation of human striatal organoids and cortico-striatal assembloids from human pluripotent stem cells. Nature biotechnology 38, 1421–1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojha D, Winkler CW, Leung JM, Woods TA, Chen CZ, Nair V, Taylor K, Yeh CD, Tawa GJ, Larson CL, Zheng W, Haigh CL, Peterson KE, 2021. Rottlerin inhibits La Crosse virus-induced encephalitis in mice and blocks release of replicating virus from the Golgi body in neurons. Nature microbiology 6, 1398–1409. [DOI] [PubMed] [Google Scholar]
- Ormel PR, Vieira de Sá R, van Bodegraven EJ, Karst H, Harschnitz O, Sneeboer MAM, Johansen LE, van Dijk RE, Scheefhals N, Berdenis van Berlekom A, Ribes Martínez E, Kling S, MacGillavry HD, van den Berg, Leonard H, Kahn RS, Hoi EM, de Witte LD, Pasterkamp RJ, 2018. Microglia innately develop within cerebral organoids. Nature Communications 9, 4167–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pamies D, Leist M, Coecke S, Bowe G, Allen D, Gstraunthaler G, Vries R. B. M. de, Hartung T, Stacey G, 2022. Guidance Document on Good Cell and Tissue Culture Practice 2.0 (GCCP 2.0). ALTEX 39, 30–70. [DOI] [PubMed] [Google Scholar]
- Pamies D, Zurich M, Hartung T, 2020. Organotypic Models to Study Human Glioblastoma: Studying the Beast in Its Ecosystem. iScience 23, 101633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pamies D, Wiersma D, Katt ME, Zhao L, Burtscher J, Harris G, Smirnova L, Searson PC, Hartung T, Hogberg HT, 2022. Human IPSC 3D brain model as a tool to study chemical-induced dopaminergic neuronal toxicity. Neurobiology of disease 169, 105719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pamies D, Barreras P, Block K, Makri G, Kumar A, Wiersma D, Smirnova L, Zang C, Bressler J, Christian KM, Harris G, Ming G, Berlinicke CJ, Kyro K, Song H, Pardo CA, Hartung T, Hogberg HT, 2017. A human brain microphysiological system derived from induced pluripotent stem cells to study neurological diseases and toxicity. ALTEX 34, 362–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey HS, Seth P, 2019. Friends Turn Foe—Astrocytes Contribute to Neuronal Damage in NeuroAIDS. J Mol Neurosci 69, 286–297. [DOI] [PubMed] [Google Scholar]
- Pasca SP, 2018. The rise of three-dimensional human brain cultures. Nature (London) 553, 437–445. [DOI] [PubMed] [Google Scholar]
- Pellegrini L, Albecka A, Mallery DL, Kellner MJ, Paul D, Carter AP, James LC, Lancaster MA, 2020. SARS-CoV-2 Infects the Brain Choroid Plexus and Disrupts the Blood-CSF Barrier in Human Brain Organoids. Cell Stem Cell 27, 951–961.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plummer S, Wallace S, Ball G, Lloyd R, Schiapparelli P, Quiñones-Hinojosa A, Hartung T, Pamies D, 2019. A Human iPSC-derived 3D platform using primary brain cancer cells to study drug development and personalized medicine. Scientific Reports 9, 1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preynat-Seauve O, Suter DM, Tirefort D, Turchi L, Virolle T, Chneiweiss H, Foti M, Lobrinus J, Stoppini L, Feki A, Dubois-Dauphin M, Krause KH, 2009. Development of Human Nervous Tissue upon Differentiation of Embryonic Stem Cells in Three-Dimensional Culture. Stem cells (Dayton, Ohio) 27, 509–520. [DOI] [PubMed] [Google Scholar]
- Qian X, Song H, Ming G, 2019. Brain organoids: Advances, applications and challenges. Development (Cambridge) 146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian X, Su Y, Adam CD, Deutschmann AU, Pather SR, Goldberg EM, Su K, Li S, Lu L, Jacob F, Nguyen PTT, Huh S, Hoke A, Swinford-Jackson SE, Wen Z, Gu X, Pierce RC, Wu H, Briand LA, Chen HI, Wolf JA, Song H, Ming G, 2020. Sliced Human Cortical Organoids for Modeling Distinct Cortical Layer Formation. Cell stem cell 26, 766–781.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian X, Nguyen H, Song M, Hadiono C, Ogden S, Hammack C, Yao B, Hamersky G, Jacob F, Zhong C, Yoon K, Jeang W, Lin L, Li Y, Thakor J, Berg D, Zhang C, Kang E, Chickering M, Nauen D, Ho C, Wen Z, Christian K, Shi P, Maher B, Wu H, Jin P, Tang H, Song H, Ming G, 2016. Brain-Region-Specific Organoids Using Mini-bioreactors for Modeling ZIKV Exposure. Cell 165, 1238–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramani A, Müller L, Ostermann PN, Gabriel E, Abida-lslam P, Müller-Schiffmann A, Mariappan A, Goureau O, Gruell H, Walker A, Andrée M, Hauka S, Houwaart T, Dilthey A, Wohlgemuth K, Omran H, Klein F, Wieczorek D, Adams O, Timm J, Korth C, Schaal H, Gopalakrishnan J, 2020. SARS-CoV-2 targets neurons of 3D human brain organoids. The EMBO journal 39, e106230-n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero JC, Berlinicke C, Chow S, Duan Y, Wang Y, Chamling X, Smirnova L, 2023. Oligodendrogenesis and myelination tracing in a CRISPR/Cas9-engineered brain microphysiological system. Frontiers in cellular neuroscience 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz EM, Jones TJ, Xu S, Dean DD, Zechmann B, Barr KL, 2021. Cerebral Organoids Derived from a Parkinson’s Patient Exhibit Unique Pathogenesis from Chikungunya Virus Infection When Compared to a Non-Parkinson’s Patient. Pathogens (Basel) 10, 913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sison SL, O'Brien BS, Johnson AJ, Seminary ER, Terhune SS, Ebert AD, 2019. Human Cytomegalovirus Disruption of Calcium Signaling in Neural Progenitor Cells and Organoids. Journal of virology 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song E, Zhang C, Israelow B, Lu-Culligan A, Prado AV, Skriabine S, Lu P, Weizman O, Liu F, Dai Y, Szigeti-Buck K, Yasumoto Y, Wang G, Castaldi C, Heltke J, Ng E, Wheeler J, Alfajaro MM, Levavasseur E, Fontes B, Ravindra NG, Van Dijk D, Mane S, Gunel M, Ring A, Kazmi SAJ, Zhang K, Wilen CB, Horvath TL, Plu I, Haik S, Thomas J, Louvi A, Farhadian SF, Huttner A, Seilhean D, Renier N, Bilguvar K, Iwasaki A, 2021. Neuroinvasion of SARS-CoV-2 in Human and Mouse Brain. The Journal of experimental medicine 218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song L, Yuan X, Jones Z, Vied C, Miao Y, Marzano M, Hua T, Sang QA, Guan J, Ma T, Zhou Y, Li Y, 2019. Functionalization of brain region-specific spheroids with isogenic microglia-like cells. Scientific Reports 9, 11055–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sridhar A, Depla JA, Mulder LA, Karelehto E, Brouwer L, Kruiswijk L, Vieira de Sá R, Meijer A, Evers MM, van Kuppeveld, Frank JM, Pajkrt D, Wolthers KC, 2022. Enterovirus D68 Infection in Human Primary Airway and Brain Organoids: No Additional Role for Heparan Sulfate Binding for Neurotropism. Microbiol Spectr 10, e0169422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun G, Chiuppesi F, Chen X, Wang C, Tian E, Nguyen J, Kha M, Trinh D, Zhang H, Marchetto MC, Song H, Ming G, Gage FH, Diamond DJ, Wussow F, Shi Y, 2020. Modeling Human Cytomegalovirus-Induced Microcephaly in Human iPSC-Derived Brain Organoids. Cell reports. Medicine 1, 100002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundar SJ, Shakya S, Barnett A, Wallace LC, Jeon H, Sloan A, Recinos V, Hubert CG, 2022. Three-dimensional organoid culture unveils resistance to clinical therapies in adult and pediatric glioblastoma. Translational oncology 15, 101251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka Y, Cakir B, Xiang Y, Sullivan GJ, Park I, 2020. Synthetic Analyses of Single-Cell Transcriptomes from Multiple Brain Organoids and Fetal Brain. Cell Reports 30, 1682–1689.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tekin H, Simmons S, Cummings B, Gao L, Adiconis X, Hession CC, Ghoshal A, Dionne D, Choudhury SR, Yesilyurt V, Sanjana NE, Shi X, Lu C, Heidenreich M, Pan JQ, Levin JZ, Zhang F, 2018. Effects of 3D culturing conditions on the transcriptomic profile of stem-cell-derived neurons. Nature biomedical engineering 2, 540–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakur KT, Miller EH, Glendinning MD, Al-Dalahmah O, Banu MA, Boehme AK, Boubour AL, Bruce SS, Chong AM, Claassen J, Faust PL, Hargus G, Hickman RA, Jambawalikar S, Khandji AG, Kim CY, Klein RS, Lignelli-Dipple A, Lin C, Liu Y, Miller ML, Moonis G, Nordvig AS, Overdevest JB, Prust ML, Przedborski S, Roth WH, Soung A, Tanji K, Teich AF, Agalliu D, Uhlemann A, Goldman JE, Canoll P, 2021. COVID-19 neuropathology at Columbia University Irving Medical Center/New York Presbyterian Hospital. Brain (London, England : 1878) 144, 2696–2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Marle G, Antony J, Power C, Ostermann H, Dunham C, Hunt T, Halliday W, Maingat F, Urbanowski MD, Hobman T, Peeling J, 2007. West Nile Virus-Induced Neuroinflammation: Glial Infection and Capsid Protein-Mediated Neurovirulence. Journal of Virology 81, 10933–10949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villabona-Rueda A, Erice C, Pardo CA, Stins MF, 2019. The Evolving Concept of the Blood Brain Barrier (BBB): From a Single Static Barrier to a Heterogeneous and Dynamic Relay Center. Frontiers in cellular neuroscience 13, 405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Zhang M, Garcia G, Tian E, Cui Q, Chen X, Sun G, Wang J, Arumugaswami V, Shi Y, 2021. ApoE-Isoform-Dependent SARS-CoV-2 Neurotropism and Cellular Response. Cell Stem Cell 28, 331–342.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Chiola S, Yang G, Russell C, Armstrong CJ, Wu Y, Spampanato J, Tarboton P, Arif Ullah HM, Edgar NU, Chang AN, Harmin DA, Bocchi VD, Vezzoli E, Besusso D, Cui J, Cattaneo E, Kubanek J, Shcheglovitov A, 2022. Modeling human telencephalic development and autism-associated SHANK3 deficiency using organoids generated from single neural rosettes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe M, Buth JE, Vishlaghi N, de la Torre-Ubieta L, Taxidis J, Khakh BS, Coppola G, Pearson CA, Yamauchi K, Gong D, Dai X, Damoiseaux R, Aliyari R, Liebscher S, Schenke-Layland K, Caneda C, Huang EJ, Zhang Y, Cheng G, Geschwind DH, Golshani P, Sun R, Novitch BG, 2017. Self-Organized Cerebral Organoids with Human-Specific Features Predict Effective Drugs to Combat Zika Virus Infection. Cell reports (Cambridge) 21, 517–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells MF, Nemesh J, Ghosh S, Mitchell JM, Salick MR, Mello CJ, Meyer D, Pietilainen O, Piccioni F, Guss EJ, Raghunathan K, Tegtmeyer M, Hawes D, Neumann A, Worringer KA, Ho D, Kommineni S, Chan K, Peterson BK, Raymond JJ, Gold JT, Siekmann MT, Zuccaro E, Nehme R, Kaykas A, Eggan K, McCarroll SA, 2023. Natural variation in gene expression and viral susceptibility revealed by neural progenitor cell villages. Cell stem cell 30, 312–332.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler CW, Woods TA, Groveman BR, Carmody AB, Speranza EE, Martens CA, Best SM, Haigh CL, Peterson KE, 2019. Neuronal maturation reduces the type I IFN response to orthobunyavirus infection and leads to increased apoptosis of human neurons. Journal of Neuroinflammation 16, 229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang Y, Tanaka Y, Cakir B, Patterson B, Kim K, Sun P, Kang Y, Zhong M, Liu X, Patra P, Lee S, Weissman SM, Park I, 2019. hESC-Derived Thalamic Organoids Form Reciprocal Projections When Fused with Cortical Organoids. Cell stem cell 24, 487–497.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Wen Z, 2021. Brain Organoids: Studying Human Brain Development and Diseases in a Dish. Stem cells international 2021, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, Lee EM, Wen Z, Cheng Y, Huang W, Qian X, Tcw J, Kouznetsova J, Ogden SC, Hammack C, Jacob F, Nguyen HN, Itkin M, Hanna C, Shinn P, Allen C, Michael SG, Simeonov A, Huang W, Christian KM, Goate A, Brennand KJ, Huang R, Xia M, Ming G, Zheng W, Song H, Tang H, 2016. Identification of small-molecule inhibitors of Zika virus infection and induced neural cell death via a drug repurposing screen. Nature medicine 22, 1101–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu R, Boreland AJ, Li X, Erickson C, Jin M, Atkins C, Pang ZP, Daniels BP, Jiang P, 2021. Developing human pluripotent stem cell-based cerebral organoids with a controllable microglia ratio for modeling brain development and pathology. Stem cell reports 16, 1923–1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Qiu Y, Zhang B, Chen G, Chen Q, Wang M, Mo F, Xu J, Wu J, Zhang R, Cheng M, Zhang N, Lyu B, Zhu W, Wu M, Ye Q, Zhang D, Man J, Li X, Cui J, Xu Z, Hu B, Zhou X, Qin C, 2019. Zika virus infection induces RNAi-mediated antiviral immunity in human neural progenitors and brain organoids. Cell research 29, 265–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon K, Song G, Qian X, Pan J, Xu D, Rho H, Kim N, Habela C, Zheng L, Jacob F, Zhang F, Lee EM, Huang W, Ringeling FR, Vissers C, Li C, Yuan L, Kang K, Kim S, Yeo J, Cheng Y, Liu S, Wen Z, Qin C, Wu Q, Christian KM, Tang H, Jin P, Xu Z, Qian J, Zhu H, Song H, Ming G, 2017. Zika-Virus-Encoded NS2A Disrupts Mammalian Cortical Neurogenesis by Degrading Adherens Junction Proteins. Cell stem cell 21, 349–358.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, He Y, Xu Y, Mo F, Mi T, Shen QS, Li C, Li Y, Liu J, Wu Y, Chen G, Zhu W, Qin C, Hu B, Zhou G, 2018. Differential antiviral immunity to Japanese encephalitis virus in developing cortical organoids. Cell Death and Disease 9, 719–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang DY, Song H, Ming G, 2021. Modeling neurological disorders using brain organoids. Seminars in cell & developmental biology 111, 4–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Lin H, Dong L, Xia Q, 2022. Recapitulating influenza virus infection and facilitating antiviral and neuroprotective screening in tractable brain organoids. Theranostics 12, 5317–5329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong X, Harris G, Smirnova L, Zufferey V, Sá, Rita de Cássia da Silveira E, Baldino Russo F, Baleeiro Beltrao Braga, Patricia Cristina, Chesnut M, Zurich M, Hogberg HT, Hartung T, Pamies D, 2020. Antidepressant Paroxetine Exerts Developmental Neurotoxicity in an iPSC-Derived 3D Human Brain Model. Frontiers in Cellular Neuroscience 14, 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Niño DF, Yamaguchi Y, Wang S, Fulton WB, Jia H, Lu P, Prindle J, Thomas, Pamies D, Morris M, Chen LL, Sodhi CP, Hackam DJ, 2021. Necrotizing Enterocolitis Induces T Lymphocyte–Mediated Injury in the Developing Mammalian Brain. Science translational medicine 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou T, Tan L, Cederquist GY, Fan Y, Hartley BJ, Mukherjee S, Tomishima M, Brennand KJ, Zhang Q, Schwartz RE, Evans T, Studer L, Chen S, 2017. High-Content Screening in hPSC-Neural Progenitors Identifies Drug Candidates that Inhibit Zika Virus Infection in Fetal-like Organoids and Adult Brain. Cell stem cell 21, 274–283.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]