Abstract
Mechanisms of fetal immune system development in utero remain incompletely elucidated. Protective immunity, the arm of reproductive immunology concerned with the progressive education of the fetal immune system as pregnancy advances, allows for programming of the immune system and immune maturation in utero and provides a responsive system to respond to rapid microbial and other antigenic exposure ex utero. Challenges in studying fetal tissues, immune system development, and the contributions of various endogenous and exogenous factors to this process are difficult to study as a progressive sampling of fetal biological samples is impractical during pregnancy, and animal models are limited. This review provides a summary of mechanisms of protective immunity and how it has been shaped, from transplacental transfer of immunoglobulins, cytokines, metabolites, as well as antigenic microchimeric cells to perhaps more controversial notions of materno-fetal transfer of bacteria that subsequently organize into microbiomes within the fetal tissues. This review will also provide a quick overview of future direction in the area of research on fetal immune system development and discusses methods to visualize fetal immune populations and determine fetal immune functions, as well as a quick look into appropriate models for studying fetal immunity.
Keywords: fetal immunity, protective immunity, immune training, ontogeny, pregnancy, immunology
Graphical Abstract.
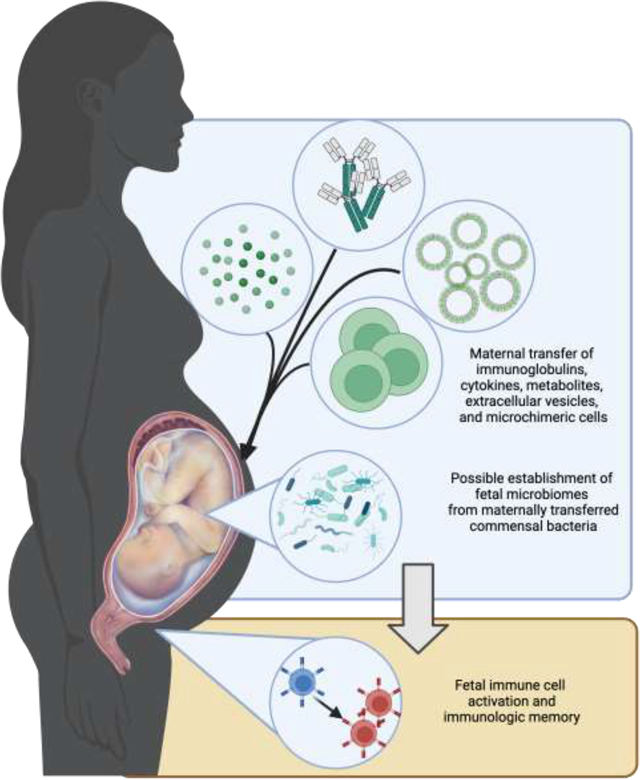
Several maternal-derived factors, such as soluble mediators, microchimeric cells, and extracellular vesicles may cross transplacentally to help train fetal immunity. The establishment of fetal microbiomes from maternal commensal bacteria is also a proposed mechanism of fetal immune education.
1. The ex-utero immunity requirement for neonates
In general, naïve immunity function is broadly relegated to two categories – tolerogenic immunity and protective immunity. In the first few weeks of conception, implantation, and early growth, the fetus needs to be accepted into the maternal niche as an isolated system that is permissively allowed by the maternal immune system. This type of immunity, called tolerogenic immunity, refers to the accommodation of the maternal tissue towards the semi-allogeneic fetal tissues to broach the maternal environment to establish vascular networks supporting fetal growth (Jørgensen et al., 2019). This allows for the establishment and achievement of a successful pregnancy, as it produces an immune-privileged environment where the fetus can grow.
However, the mere presence of a tolerogenic environment does not guarantee entirely the survival of the fetus once it is out of the sterile womb. As the fetus exits during labor, its immune system must be able to respond to a massive load of possible antigenic stimuli that it would encounter, starting from the vaginal canal up to the external environment. Various exposures result in rapid colonization by microbes in the newborn baby (Dominguez-Bello et al., 2010). Therefore, there is a need for a working preemptive immune response that would be beneficial upon exposure to a magnitude of environmental antigens once it is out of the protective feto-maternal environment post-partum. This type of immunity, called protective immunity, refers to the gradual priming of the fetal immune system for adaptive memory development in utero (Rackaityte and Halkias, 2020).
There are certain key tissues and organ systems in which protective immunity is highly necessary. For instance, the respiratory tracts and the alveolar linings would necessitate protection from tissue-resident immune cells as the neonate breathes and swallows the air from the environment. The gastrointestinal tracts also need robust responses toward breastmilk-associated microbes and native antigens. In this light, infiltrating fetal monocytes in the respiratory tracts are stimulated to differentiate into alveolar macrophages, while conventional dendritic cells and Tregs mediate phagocytic abilities and Th2-skewed responses (Torow et al., 2017). In the gastrointestinal lining soluble immunoglobulin A (IgA), either endogenously from commensal bacteria or exogenously from the breastmilk is necessary for protection against enteric pathogens (Harris et al., 2006; Kramer and Cebra, 1995; Langel et al., 2020). Additionally, environmental allergens produce a Th2-skewed response, but the distinctions between the Th1/Th2 dichotomy are blurred and are suggested to be stimulus-dependent instead (Apostol et al., 2022; Prescott et al., 1998; Semmes et al., 2021).
Overall, environmental exposure and bacterial colonization result in an apparent inflammatory response post-delivery. Interestingly, the neonatal immune system is also trained to quench any excessive inflammation via a host of immunosuppressive and immunoregulatory responses (Elahi et al., 2013; Gibbons et al., 2014). This comprehensive lack of an overwhelming and disadvantageous fetal immune response connotes the presence of a trained immunity sufficient to allow tolerance to these immunogenic inputs (Apostol et al., 2022; Semmes et al., 2021). These mechanisms remain incompletely elucidated, but years of study have provided us with some hypotheses as to how protective immunity is generated. In this review, we will briefly discuss established mechanisms of immune ontology, gloss over methods utilized to visualize development, and enumerate several biological models employed in these studies.
1.1. Maternal-fetal transfer of immunoglobulins: a central player in fetal immunity development
For years, the most-studied model of the influence of the maternal immune system on fetal and neonatal immunity is the transfer of maternally derived immunoglobulin G (IgG) to the offspring, or passive immunity (den Braber et al., 2012). This model was discussed more than half a century ago when IgG fragments were observed to traverse the maternal circulation and partially end up in the fetal circulation (Brambell et al., 1960). Eventually, it was found that circulating IgG is taken up via endocytosis in areas of contact with syncytiotrophoblasts. It subsequently binds to the neonatal receptor for the fragment crystallizable (Fc) region of IgG (FcRn) in endosomes and is transported to the basolateral side (Kristoffersen, 1996; Ober et al., 2004; Roopenian and Akilesh, 2007). Total maternal IgG concentrations have been shown to affect the fetal concentration of IgG (Palmeira et al., 2012. However, higher concentrations of IgG in maternal circulation would not normally translate to higher transplacental transfer of the immunoglobulin; the limitation lies in the saturability of the FcRn receptor (Kim et al., 2007). Any unbound IgG is lysosomally degraded, which limits cord sera IgG concentrations when maternal IgG reaches around 15 g/L (Michaux et al., 1966; Wilcox et al., 2017). Nonetheless, much of fetal immunity is postulated to occur via this method, as seen in maternal vaccinations against several viral (Albrecht et al., 2022) and bacterial pathogens (Altman et al., 2017; Mawa et al., 2017; Post et al., 2019; Voer et al., 2009).
Duration of pregnancy also impacts the transplacental transfer of IgG. In preterm newborns, IgG class 1 and class 3 were both demonstrated to be lower than term newborns (Costa-Carvalho et al., 1996). It was observed that extremely preterm children have comparable IgG as term children, but there are lower absolute concentrations and shorter half-lives (Pou et al., 2019). This phenomenon occurs across various exposures, including certain bacterial- and viral-specific IgG (Berg et al., 2014, 2010; Lessa et al., 2011). It is hypothesized that the cytotrophoblast layer, which does not express FcRn and may prevent IgG translocation, is gradually reduced in thickness as gestation proceeds, allowing the eventual active transport of IgG (Lozano et al., 2018). Although work remains to be done regarding mechanisms that lead to these differences, it has been suggested that a decrease in FcRn expression in preterm placentas may contribute to the decreased fetal concentration of IgG in such patients (Lozano et al., 2018).
More recently, it has been described that maternal immunoglobulin E (IgE) is transferred transplacentally secondary to IgE-IgG complex-mediated transcytosis. The immunoglobulin can prime fetal mast cells to allow the detection of allergens postnatally (Keith and Kabashima, 2021; Msallam et al., 2020). The maternal transfer of complexed IgE is hypothesized to occur via FcRn interaction similar to IgG, challenging decades-long notions that only the latter can be transferred to the fetus (Bundhoo et al., 2015). However, it appears that mast cell sensitization from IgE is not completed prenatally in normal conditions due to its relative immaturity in the skin within the first few days of birth (Honda et al., 2019), suggesting that this immaturity may be advantageous instead in preventing massive IgE-induced degranulation that may harm the neonate (Honda et al., 2019).
1.2. Other mechanisms of fetal immune development in the perinatal period
Transplacental transfer of immunoglobulins is well described in the literature, but evidence of other mechanisms of fetal immunity training have been also been proposed over the previous years. Classical exposure to maternal antigens may also be contributory to the education of the fetal immune system. For instance, maternal allergens have been observed to cross the placenta in an ex vivo perfusion model with some allergens necessitating the co-presence of Ig to allow transfer (Szépfalusi et al., 2000). Other antigenic mediators, such as short-chain fatty acids, acetate, and other metabolites can also influence the regulation of lymphocyte development (Hu et al., 2018; Thorburn et al., 2015).
Maternal inflammation has also been thought to contribute to fetal immune ontogeny (Apostol et al., 2022; Espin-Palazon et al., 2017; Lim et al., 2020; Mariani et al., 2019). In several animal models of bacterial and viral infections during pregnancy, there is an observable increase in fetal inflammatory cytokines such as interferon-gamma, tumor necrosis factor (TNF)-alpha, and other interleukins that parallels maternal response even in the absence of pathogenic DNA within the fetal compartment (Cardenas et al., 2010; Waldorf et al., 2011). Transplacental transfer of maternal cytokines across the human placenta may be contributory, although it has been demonstrated that only interleukin (IL)-6 has been shown to be bidirectionally transported across the feto-maternal compartments (Aaltonen et al., 2005; Zaretsky et al., 2004). In this context, the fetal inflammatory response secondary to maternal inflammation becomes a more important contributor to immune development. The fetal inflammatory response is a double-edged sword, since excessive fetal inflammation compounded with decreased antiinflammatory IL-10 in the placental trophoblast cells (Mor, 2022) and in fetal membrane cells (Noda-Nicolau et al., 2016) may induce adverse pregnancy outcomes. However, there is evidence that fetal inflammation may also be beneficial for immune priming. Strong neonatal inflammatory responses have been demonstrated in mice models of antenatal exposures to various inflammatory stimuli (Gleditsch et al., 2014; Mukherjee et al., 2012). In utero, gene expression profiles relating to activation of innate immunity and T cell function may also occur upon maternal inflammation (Weitkamp et al., 2016).
Extracellular vesicles (EVs) coming from various maternal tissues and cells may propagate these maternal inflammatory signals to the fetal compartments. In response to triggers such as oxidative stress and active inflammation, exosomes from decidual and myometrial cells have been demonstrated to induce the production of pro-inflammatory mediators from the amniochorion (Shepherd et al., 2021). Maternal macrophages are also able to induce the production of placental pro-inflammatory cytokines (Holder et al., 2016). Therefore, even without any transplacental passage of maternal antigens or cytokines, inflammatory signals coming from nearby tissues secondary to adverse stimuli may be indirectly transmitted to the fetus via these nanoparticles.
As EVs have been demonstrated to transport various cellular products, it is also possible that these EVs can potentially induce immunoregulation via anti-inflammatory effects. In early pregnancy, EVs have been shown to be able to carry FasL and TRAIL2, which are constitutive proteins expressed by the placenta that can induce pro-apoptosis in T cells and peripheral blood mononuclear cells (Stenqvist et al., 2013). Aside from performing allogenic protection from activated maternal immune cells, it is possible that these exosomes can also cross the placenta, interact with immune cells, and induce apoptosis to provide negative selection in amenable naïve cells. Alternatively, since any excessive inflammation may be detrimental to the fetus, these exosomes can also possibly dampen inflammation to a level permissible for immune development in utero, as demonstrated by Treg differentiation and M2 polarization upon exposure to placental exosomes (Bai et al., 2022; DEGENNE et al., 1986; Holder et al., 2016; Pallinger et al., 2018; Thibault et al., 1991).
Non-classical antigens may also provide stimuli for immune development. Although limited in number, maternal alloantigenic cells - microchimeric cells - are transported across the placenta into the fetal circulation. These cells were first observed in cord blood, either in the mononuclear fraction or the lymphocyte fraction upon enrichment. They have been initially proposed to be contaminants during the processing of the sample (Hall et al., 1995). However, it has been demonstrated that these microchimeric cells can engraft themselves within fetal tissues, typically starting in the second trimester (49) and that these alloreactive cells are immunologically competent (Frascoli et al., 2017). Lymph nodes are one of the targets of microchimeric cells, wherein they induce the development of Tregs that contribute to antimaternal immunity (Mold et al., 2008). More recently, vertical transmission of microchimeric cells has been shown to promote monocyte differentiation, possibly mediated via epigenetic changes in the hematopoietic stem cell genome, and reduce the severity and incidence of viral infections in a mouse model (Stelzer et al., 2021). These microchimeric cells may serve as cellular messengers of immune priming, carrying cytokines and growth factors that may boost immune development (Stelzer et al., 2021). Since persistence of microchimeric cells has been demonstrated, and lower infections are linked to higher numbers of these cells, it can be surmised that a priori, microchimeric cells may also regulate immune memory development.
1.3. In utero bacterial presence and potential contributions to immunity development
One of the more controversial notions is the presence of bacteria within the supposedly sterile feto-maternal compartment. The identification of a low biomass microbiome from fetal gestational tissues brings about the hypothesis of early colonization in utero (Senn et al., 2020). It was initially observed that when pregnant mice were orally inoculated with labeled E. fecium, DNA is readily detectable in the amniotic fluid (Jiménez et al., 2005) and meconium (Jiménez et al., 2008) upon abdominal delivery of pups. It is hypothesized that bacteria can colonize the amniotic fluid, and the ingestion of this fluid facilitates the entry of microbes into the fetal lungs, gut, and, eventually, the meconium (Alam et al., 2020; Ardissone et al., 2014; Collado et al., 2016; Lal et al., 2016; Mshvildadze et al., 2010)
In line with this hypothesis, a human fetal lung microbiome has been proposed recently that is temporally dynamic with pregnancy progression (Alam et al., 2020; Lal et al., 2016). Additionally, a viable fetal gut microbiome has also been described recently, which is strictly limited to the intestinal environment (Collado et al., 2016; Rackaityte et al., 2020). The presence of such culturable bacteria allows for in utero priming (Mishra et al., 2021). The latter is crucial because it was demonstrated that isolated fetal dendritic cells from the gut could initiate fetal memory T cell expansion post-exposure to fetally isolated bacteria (Mishra et al., 2021).
It is imaginable that the primary path as to which these bacteria enter the fetal compartment is via the placental labyrinths, wherein the maternal blood that admixes with the fetal villi possibly brings with it a host of maternally derived bacteria. Interestingly, the placenta has been reported to harbor its microbiome, with its purported origins coming from a possible oral source (Aagaard et al., 2014; Zakis et al., 2021). It has been demonstrated that tagged bacteria from the oral environment can cross transplacentally, albeit by using a sheep model only (Yu et al., 2021). However, this placental microbiome theory has been controversial since (1) the presence of a live culturable microbiome in the placenta is yet to be established unequivocally, and (2) the demonstration of actual transplacental transport of live bacteria in a human placenta has not been yet observed, and (3) the sterility of the methods as to how the placenta has been processed for sequencing is not entirely invulnerable to environmental contamination. Indeed, the latter hypothesis has been demonstrated by multiple other researchers, who purport that the bacterial DNA signals from the supposed “microbiomes” are more likely environmental contaminants, except in cases of active feto-maternal infection such as chorioamnionitis (Doyle et al., 2017; Goffau et al., 2019, 2018; Leon et al., 2018).
Indeed, it would be unimaginable to think that a viable bacterial community is being harbored inside a supposedly sterile environment. The mechanisms of bacteria breaching the tight placental barrier in the absence of clinical infection remain a mystery. As an alternative, shuttling of bacteria via dendritic cell absorption may also occur but is less feasible (Funkhouser and Bordenstein, 2013; Rescigno et al., 2001). The ascending infection model is also a possible means of bacterial transport, originating from the cervicovaginal compartments and passing through the amniochorion barrier (Kim et al., 2009); however, the active immune system within the cervix and amniochorion themselves make this a highly improbable event. Besides, such an ascent of microbes contributing to the fetal immune response is reported only during infection-associated preterm birth when the cervical and membrane barrier functions are compromised. Nonetheless, proving the presence of these microbiomes in utero still is challenging since there is a heavy burden to prove that low signals from these bacterial communities are stringently and consistently above background noise (Goffau et al., 2018; Jervis-Bardy et al., 2015; Salter et al., 2014).
Nonetheless, it remains to be seen whether the studies challenging the “sterile womb” paradigm and the theory of the existence of a living microbiome in utero are replicable. Unless more robust evidence is available, the notion of an active fetal microbiome is still debatable.
2. Visualizing immune development
Flow cytometry is primarily used for the differential identification of immune cells. In one classic example, fetal antigen-presenting cells (APCs) have been profiled using a combination of flow cytometry and gene array analysis. With knowledge of the original organs from where the immune cells have been isolated, localization, migration, and targeting are feasible (McGovern et al., 2017). Fetal immune cells from the gut have also been isolated via conventional methods. These cells have been profiled similarly, and flow sorting of specific subpopulations of interest is also possible for close interrogation for correlation with exposure to certain bacterial populations (Rackaityte et al., 2020). Functional markers of immune cell function, such as cytokines and other protein products important for immune system actions, can also be visualized via flow cytometry (Rackaityte et al., 2020).
Insights toward immune cell-specific function can also be visualized via transcriptomics. For instance, McGovern et al. performed microarray and CyTOF analyses in multiple immune cell subpopulations and showed that despite comparable levels of gene expression of lineage-specific transcription factors between fetal and adult circulating dendritic cells, there remains a differential regulation of immune training pathways between fetal and adult antigen-presenting cells depending on the location of the dendritic cells (McGovern et al., 2017). Interestingly, by looking at the expression of various antibodies in specific dendritic cell subsets across different tissues and trimesters, tracking temporal development of these cells may be possible (McGovern et al., 2017).
Human fetal liver hematopoiesis, as well as immune cell development in the yolk sac, fetal skin, and fetal kidney, has also been mapped in terms of heterogeneity and dynamic temporality using single-cell RNA sequencing (scRNA-seq) (Popescu et al., 2019). In their analysis, they utilized pseudotime analysis, partition-based graph abstraction (PAGA), and diffusion maps in order to determine dynamically changing genes across lymphoid, erythroid, mast cell, and megakaryocyte lineages. The combination of these bioinformatic tools take scRNA-seq data to allow categorization of differentially expressed genes into context-specific time periods, e.g., „early‟, „mid‟, and „late‟ categories. Overall trajectory analyses reveals a hematopoietic stem cell/multipotent progenitor that differentiates into pre-/pro-B cells, neutrophil-myeloid progenitors, and megakaryocyte-erythroid-mast cell progenitors; it was demonstrated that there is an apparent shift from erythroid lineages to myeloid lineages in the liver going into the second trimester.
With the banking of datasets in the public database, secondary data analysis is also possible via clustering analysis of published scRNA-seq data. For instance, an analysis of the immune cell landscape in fetal skin by Xu et al. not only provided data for scRNA-seq from their own fetal skin samples but also validated their dataset using the previously published dataset on human fetal liver hematopoiesis (Popescu et al., 2019; Xu et al., 2021). In this study, Xu et al. combined fetal skin scRNA-seq dataset and analyzed skin immune cells that are present in both studies. The eliminated cell types present in the earlier weeks of gestation confirms the temporal difference in fetal skin immune populations. They also were able to confirm that the remaining cell types were closely matched in the two studies, reinforcing the finding that lymphoid precursors, mono-macrophages, and tissue-resident macrophages dominate the fetal skin immune population at around second trimester.
In summary, the combination of flow cytometry, transcriptomics, and bioinformatics is crucial to providing a scoping view of the phenotypes, functions, and even temporal development of fetal immune cells. External validation of findings can also be done by capitalizing on published scRNA-seq datasets and using these to confirm observations and trends in the immune cell populations of interest.
3. Elucidating fetal immune ontogeny using biological models
Regardless of all this evidence, much more work is needed to understand the in utero training of fetal immune system. We are limited to animal models, especially mice, to closely observe fetal immune changes that happen within the entire pregnancy due to restrictions regarding the utilization of human fetal tissues from earlier stages of pregnancy. Utilization of early trimester fetal tissues for experimental protocols can potentially answer immune system development, the type of immune system, and its immunophenotype that can substantially save fetal lives occurring due to immune intolerance associated with fetal demises, fetal inflammatory response, and neonatal immune behavior based on fetal immune priming and development of tolerance to several maternal antigenic exposures.
Mouse models rely highly on a lifelong thymic supply of naive T cells. Their T-cell ontogeny development starts from skin-destined thymocytes that become peripherally distributed and continue to be replaced until the first week of life (den Braber et al., 2012; Friedberg and Weissman, 1974). This is quite different from human lymphocyte development, in which lymphocytes exit earlier and remain in the fetal periphery in utero longer than those in mice (den Braber et al., 2012). There is less reliance on thymic output for later lymphopoiesis (Mancebo et al., 2008). Human fetal immune cells may be isolated and primed as previously demonstrated (McGovern et al., 2017; Mishra et al., 2021) and provide us with a more directed experimental approach to cell-specific responses; however, in vitro conditions may limit and preclude us from considering the natural microenvironment that these immune cells reside in during pregnancy. Immune chip models may provide the symphony between in vitro and in vivo models; however, the relative transience of the lifespan of primary immune cells and even of immortalized immune cells remains a huge obstacle for microfluidic models (Morsink et al., 2020). Nonetheless, the active research discourse in this field remains a steadfast mover in providing us a greater understanding of fetal immunity priming and how it influences diseases related to pregnancy and early-life development.
4. Conclusion
Although much work on perinatal reproductive immunology has focused on the tolerogenic mechanisms that allow the maintenance of pregnancy, protective mechanisms such as early training and education of the fetal immune system are still of interest and are areas of active research. Animal models have been the standard for observing these mechanisms, but the promise of in vitro setups using isolated fetal immune cells and integrated organ-on-chip microfluidic methods may provide more convenient and perhaps more powerful models for manipulation and experimentation. There is a need to study the exact mechanisms of conventional paradigms of fetal immune development; however, elucidating new and alternative immune activation and priming hypotheses would give us a more robust view of how fetal ontogeny is mediated. Hopefully, the following years will bring more answers than questions for us, as knowledge from this research would allow the development of new strategies in protecting neonates against deadly acute microbial infections, as well as reducing the burden of the development of chronic inflammatory diseases.
Highlights:
Protective immunity – progressive in utero education of immunity– is understudied.
This is a short review of existing and emerging mechanisms in fetal immune ontogeny.
We briefly discuss methods to visualize fetal immune populations and functions.
We also provide a glance on available biological models for these studies.
Acknowledgments:
This study was supported via the NIH/NICHD Grant to R Menon: 5 R01 HD100729-03. We would like to thank Nina Truong for lending her talent in helping illustrate a part of the summary figure for this article. Other elements of the summary figure were created using BioRender.
MSVJ is is an MD-PhD in Molecular Medicine Program trainee supported by Department of Science and Technology - Philippine Council for Health Research and Development (DOST-PCHRD)
Footnotes
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Aagaard K, Ma J, Antony KM, Ganu R, Petrosino J, Versalovic J, 2014. The Placenta Harbors a Unique Microbiome. Sci Transl Med 6, 237ra65. 10.1126/scitranslmed.3008599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aaltonen R, Heikkinen T, Hakala K, Laine K, Alanen A, 2005. Transfer of Proinflammatory Cytokines Across Term Placenta. Obstetrics Gynecol 106, 802–807. 10.1097/01.aog.0000178750.84837.ed [DOI] [PubMed] [Google Scholar]
- Alam DA, Danopoulos S, Grubbs B, Ali NABM, MacAogain M, Chotirmall SH, Warburton D, Gaggar A, Ambalavanan N, Lal CV, 2020. Human Fetal Lungs Harbor a Microbiome Signature. Am J Resp Crit Care 201, 1002–1006. 10.1164/rccm.201911-2127le [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albrecht M, Pagenkemper M, Wiessner C, Spohn M, Lütgehetmann M, Jacobsen H, Gabriel G, Zazara DE, Haertel C, Hecher K, Diemert A, Arck PC, 2022. Infant immunity against viral infections is advanced by the placenta-dependent vertical transfer of maternal antibodies. Vaccine 40, 1563–1571. 10.1016/j.vaccine.2020.12.049 [DOI] [PubMed] [Google Scholar]
- Altman SPN, Tino-De-Franco M, Carbonare CB, Palmeira P, Carbonare SB, 2017. Placental and colostral transfer of antibodies reactive with enteropathogenic Escherichia coli intimins α, β, or γ. J Pediat 93, 568–575. 10.1016/j.jped.2016.12.005 [DOI] [PubMed] [Google Scholar]
- Apostol AC, López DA, Lebish EJ, Valencia CH, Romero-Mulero MC, Pavlovich P, Hernandez GE, Forsberg EC, Cabezas-Wallscheid N, Beaudin AE, 2022. Prenatal inflammation perturbs fetal hematopoietic development and causes persistent changes to postnatal immunity. Biorxiv 2022.05.08.491095. 10.1101/2022.05.08.491095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardissone AN, Cruz D.M. de la, Davis-Richardson AG, Rechcigl KT, Li N, Drew JC, Murgas-Torrazza R, Sharma R, Hudak ML, Triplett EW, Neu, 2014. Meconium Microbiome Analysis Identifies Bacteria Correlated with Premature Birth. Plos One 9, e90784. 10.1371/journal.pone.0090784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai K, Lee C-L, Liu X, Li J, Cao D, Zhang L, Hu D, Li H, Hou Y, Xu Y, Kan ASY, Cheung K-W, Ng EHY, Yeung WSB, Chiu PCN, 2022. Human placental exosomes induce maternal systemic immune tolerance by reprogramming circulating monocytes. J Nanobiotechnol 20, 86. 10.1186/s12951-022-01283-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg J.P. van den, Westerbeek EAM, Berbers GAM, Gageldonk P.G.M. van, Klis F.R.M. van der, Elburg R.M. van, 2010. Transplacental Transport of IgG Antibodies Specific for Pertussis, Diphtheria, Tetanus, Haemophilus influenzae Type b, and Neisseria meningitidis Serogroup C Is Lower in Preterm Compared With Term Infants. Pediatric Infect Dis J 29, 801–805. 10.1097/inf.0b013e3181dc4f77 [DOI] [PubMed] [Google Scholar]
- Berg J.P. van den, Westerbeek EAM, Smits GP, Klis F.R.M. van der, Berbers GAM, Elburg R.M. van, 2014. Lower Transplacental Antibody Transport for Measles, Mumps, Rubella and Varicella Zoster in Very Preterm Infants. Plos One 9, e94714. 10.1371/journal.pone.0094714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brambell FWR, Hemmings WA, Oakley CL, Porter RR, 1960. The relative transmission of the fractions of papain hydrolyzed homologous γ-globulin from the uterine cavity to the foetal circulation in the rabbit. Proc Royal Soc Lond Ser B Biological Sci 151, 478–482. 10.1098/rspb.1960.0011 [DOI] [PubMed] [Google Scholar]
- Bundhoo A, Paveglio S, Rafti E, Dhongade A, Blumberg RS, Matson AP, 2015. Evidence that FcRn mediates the transplacental passage of maternal IgE in the form of IgG anti-IgE/IgE immune complexes. Clin Exp Allergy 45, 1085–1098. 10.1111/cea.12508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardenas I, Means RE, Aldo P, Koga K, Lang SM, Booth CJ, Booth C, Manzur A, Oyarzun E, Romero R, Mor G, 2010. Viral infection of the placenta leads to fetal inflammation and sensitization to bacterial products predisposing to preterm labor. J Immunol Baltim Md 1950 185, 1248–57. 10.4049/jimmunol.1000289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collado MC, Rautava S, Aakko J, Isolauri E, Salminen S, 2016. Human gut colonisation may be initiated in utero by distinct microbial communities in the placenta and amniotic fluid. Sci Rep-uk 6, 23129. 10.1038/srep23129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa-Carvalho BT, Vieria HM, Dimantas RB, Arslanian C, Naspitz CK, Solé D, Carneiro-Sampaio MM, 1996. Transfer of IgG subclasses across placenta in term and preterm newborns. Braz J Medical Biological Res Revista Brasileira De Pesquisas Medicas E Biologicas 29, 201–4. [PubMed] [Google Scholar]
- DEGENNE D, KHALFOUN B, BARDOS P, 1986. In Vitro Inhibitory Effect of Human Syncytiotrophoblast Plasma Membranes on the Cytolytic Activities of CTL and NK Cells. Am J Reprod Im Mic 12, 106–110. 10.1111/j.1600-0897.1986.tb00074.x [DOI] [PubMed] [Google Scholar]
- den Braber I, Mugwagwa T, Vrisekoop N, Westera L, Mögling R, Bregje de Boer A, Willems N, Schrijver EHR, Spierenburg G, Gaiser K, Mul E, Otto SA, Ruiter AFC, Ackermans MT, Miedema F, Borghans JAM, de Boer RJ, Tesselaar K, 2012. Maintenance of Peripheral Naive T Cells Is Sustained by Thymus Output in Mice but Not Humans. Immunity 36, 288–297. 10.1016/j.immuni.2012.02.006 [DOI] [PubMed] [Google Scholar]
- Dominguez-Bello MG, Costello EK, Contreras M, Magris M, Hidalgo G, Fierer N, Knight R, 2010. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc National Acad Sci 107, 11971–11975. 10.1073/pnas.1002601107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle RM, Harris K, Kamiza S, Harjunmaa U, Ashorn U, Nkhoma M, Dewey KG, Maleta K, Ashorn P, Klein N, 2017. Bacterial communities found in placental tissues are associated with severe chorioamnionitis and adverse birth outcomes. Plos One 12, e0180167. 10.1371/journal.pone.0180167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elahi S, Ertelt JM, Kinder JM, Jiang TT, Zhang X, Xin L, Chaturvedi V, Strong BS, Qualls JE, Steinbrecher KA, Kalfa TA, Shaaban AF, Way SS, 2013. Immunosuppressive CD71+ erythroid cells compromise neonatal host defence against infection. Nature 504, 158–162. 10.1038/nature12675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espin-Palazon R, Weijts B, Mulero V, Traver D, 2017. Proinflammatory Signals as Fuel for the Fire of Hematopoietic Stem Cell Emergence. Trends Cell Biol 28, 58–66. 10.1016/j.tcb.2017.08.003 [DOI] [PubMed] [Google Scholar]
- Frascoli M, Coniglio L, Witt R, Jeanty C, Fleck-Derderian S, Myers DE, Lee T-H, Keating S, Busch MP, Norris PJ, Tang Q, Cruz G, Barcellos LF, Gomez-Lopez N, Romero R, MacKenzie TC, 2017. Alloreactive fetal T cells promote uterine contractility in preterm labor via IFN-γ and TNF-α. Sci Transl Med 10. 10.1126/scitranslmed.aan2263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedberg SH, Weissman IL, 1974. Lymphoid tissue architecture. II. Ontogeny of peripheral T and B cells in mice: evidence against Peyer’s patches as the site of generation of B cells. J Immunol Baltim Md 1950 113, 1477–92. [PubMed] [Google Scholar]
- Funkhouser LJ, Bordenstein SR, 2013. Mom Knows Best: The Universality of Maternal Microbial Transmission. Plos Biol 11, e1001631. 10.1371/journal.pbio.1001631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons D, Fleming P, Virasami A, Michel M-L, Sebire NJ, Costeloe K, Carr R, Klein N, Hayday A, 2014. Interleukin-8 (CXCL8) production is a signatory T cell effector function of human newborn infants. Nat Med 20, 1206–1210. 10.1038/nm.3670 [DOI] [PubMed] [Google Scholar]
- Gleditsch DD, Shornick LP, Steenwinckel JV, Gressens P, Weisert RP, Koenig JM, 2014. Maternal inflammation modulates infant immune response patterns to viral lung challenge in a murine model. Pediatr Res 76, 33–40. 10.1038/pr.2014.57 [DOI] [PubMed] [Google Scholar]
- Goffau M.C. de, Lager S, Salter SJ, Wagner J, Kronbichler A, Charnock-Jones DS, Peacock SJ, Smith GCS, Parkhill J, 2018. Recognizing the reagent microbiome. Nat Microbiol 3, 851–853. 10.1038/s41564-018-0202-y [DOI] [PubMed] [Google Scholar]
- Goffau M.C. de, Lager S, Sovio U, Gaccioli F, Cook E, Peacock SJ, Parkhill J, Charnock-Jones DS, Smith GCS, 2019. Human placenta has no microbiome but can contain potential pathogens. Nature 572, 329–334. 10.1038/s41586-019-1451-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall J, Lingenfelter P, Adams S, Lasser D, Hansen J, Bean M, 1995. Detection of maternal cells in human umbilical cord blood using fluorescence in situ hybridization. Blood 86, 2829–2832. 10.1182/blood.v86.7.2829.2829 [DOI] [PubMed] [Google Scholar]
- Harris NL, Spoerri I, Schopfer JF, Nembrini C, Merky P, Massacand J, Urban JF, Lamarre A, Burki K, Odermatt B, Zinkernagel RM, Macpherson AJ, 2006. Mechanisms of Neonatal Mucosal Antibody Protection. J Immunol 177, 6256–6262. 10.4049/jimmunol.177.9.6256 [DOI] [PubMed] [Google Scholar]
- Holder B, Jones T, Shimizu VS, Rice TF, Donaldson B, Bouqueau M, Forbes K, Kampmann B, 2016. Macrophage Exosomes Induce Placental Inflammatory Cytokines: A Novel Mode of Maternal–Placental Messaging. Traffic 17, 168–178. 10.1111/tra.12352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda Y, Ono S, Honda T, Kataoka TR, Egawa G, Kitoh A, Otsuka A, Nakajima S, Nomura T, Dainichi T, Kabashima K, 2019. Murine neonatal skin mast cells are phenotypically immature and minimally sensitized with transplacentally transferred IgE. J Allergy Clin Immun 144, 617–620.e5. 10.1016/j.jaci.2019.05.011 [DOI] [PubMed] [Google Scholar]
- Hu M, Eviston D, Hsu P, Mariño E, Chidgey A, Santner-Nanan B, Wong K, Richards JL, Yap YA, Collier F, Quinton A, Joung S, Peek M, Benzie R, Macia L, Wilson D, Ponsonby A-L, Tang MLK, O’Hely M, Daly NL, Mackay CR, Dahlstrom JE, Saffery R, Allen KJ, Ranganathan S, Burgner D, Harrison LC, Sly P, Dwyer T, Vuillermin P, Nanan R, 2018. Decreased maternal serum acetate and impaired fetal thymic and regulatory T cell development in preeclampsia. Nat Commun 10, 3031. 10.1038/s41467-019-10703-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jervis-Bardy J, Leong LEX, Marri S, Smith RJ, Choo JM, Smith-Vaughan HC, Nosworthy E, Morris PS, O’Leary S, Rogers GB, Marsh RL, 2015. Deriving accurate microbiota profiles from human samples with low bacterial content through post-sequencing processing of Illumina MiSeq data. Microbiome 3, 19. 10.1186/s40168-015-0083-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiménez E, Fernández L, Marín ML, Martín R, Odriozola JM, Nueno-Palop C, Narbad A, Olivares M, Xaus J, Rodríguez JM, 2005. Isolation of Commensal Bacteria from Umbilical Cord Blood of Healthy Neonates Born by Cesarean Section. Curr Microbiol 51, 270–274. 10.1007/s00284-005-0020-3 [DOI] [PubMed] [Google Scholar]
- Jiménez E, Marín ML, Martín R, Odriozola JM, Olivares M, Xaus J, Fernández L, Rodríguez JM, 2008. Is meconium from healthy newborns actually sterile? Res Microbiol 159, 187–193. 10.1016/j.resmic.2007.12.007 [DOI] [PubMed] [Google Scholar]
- Jørgensen N, Persson G, Hviid TVF, 2019. The Tolerogenic Function of Regulatory T Cells in Pregnancy and Cancer. Front Immunol 10, 911. 10.3389/fimmu.2019.00911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keith YH, Kabashima K, 2021. Maternal IgE is transferred to fetuses with IgG and minimally sensitizes fetal/neonatal skin mast cells. J Allergy Clin Immun 148, 903–904. 10.1016/j.jaci.2021.05.040 [DOI] [PubMed] [Google Scholar]
- Kim J, Hayton WL, Robinson JM, Anderson CL, 2007. Kinetics of FcRn-mediated recycling of IgG and albumin in human: Pathophysiology and therapeutic implications using a simplified mechanism-based model. Clin Immunol 122, 146–155. 10.1016/j.clim.2006.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MJ, Romero R, Gervasi MT, Kim J-S, Yoo W, Lee D-C, Mittal P, Erez O, Kusanovic JP, Hassan SS, Kim CJ, 2009. Widespread microbial invasion of the chorioamniotic membranes is a consequence and not a cause of intra-amniotic infection. Lab Invest 89, 924–936. 10.1038/labinvest.2009.49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer DR, Cebra JJ, 1995. Early appearance of “natural” mucosal IgA responses and germinal centers in suckling mice developing in the absence of maternal antibodies. J Immunol Baltim Md 1950 154, 2051–62. [PubMed] [Google Scholar]
- Kristoffersen EK, 1996. Human placental Fcγ-binding proteins in the maternofetal transfer of IgG. Apmis 104, 5–36. 10.1111/j.1600-0463.1996.tb05583.x [DOI] [PubMed] [Google Scholar]
- Lal CV, Travers C, Aghai ZH, Eipers P, Jilling T, Halloran B, Carlo WA, Keeley J, Rezonzew G, Kumar R, Morrow C, Bhandari V, Ambalavanan N, 2016. The Airway Microbiome at Birth. Sci Rep-uk 6, 31023. 10.1038/srep31023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langel SN, Otero CE, Martinez DR, Permar SR, 2020. Maternal gatekeepers: How maternal antibody Fc characteristics influence passive transfer and infant protection. Plos Pathog 16, e1008303. 10.1371/journal.ppat.1008303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leon LJ, Doyle R, Diez-Benavente E, Clark TG, Klein N, Stanier P, Moore GE, 2018. Enrichment of Clinically Relevant Organisms in Spontaneous Preterm-Delivered Placentas and Reagent Contamination across All Clinical Groups in a Large Pregnancy Cohort in the United Kingdom. Appl Environ Microb 84, e00483–18. 10.1128/aem.00483-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lessa ALS, Krebs VLJ, Brasil TB, Pontes GN, Carneiro-Sampaio M, Palmeira P, 2011. Preterm and term neonates transplacentally acquire IgG antibodies specific to LPS from Klebsiella pneumoniae, Escherichia coli and Pseudomonas aeruginosa. Fems Immunol Medical Microbiol 62, 236–243. 10.1111/j.1574-695x.2011.00807.x [DOI] [PubMed] [Google Scholar]
- Lim AI, McFadden T, Link VM, Han S-J, Karlsson R-M, Stacy A, Farley TK, Lima-Junior DS, Harrison OJ, Desai JV, Lionakis MS, Shih H-Y, Cameron HA, Belkaid Y, 2020. Prenatal maternal infection promotes tissue-specific immunity and inflammation in offspring. Sci New York N Y 373. 10.1126/science.abf3002 [DOI] [PubMed] [Google Scholar]
- Lozano NA, Lozano A, Marini V, Saranz RJ, Blumberg RS, Baker K, Agresta MF, Ponzio MF, 2018. Expression of FcRn receptor in placental tissue and its relationship with IgG levels in term and preterm newborns. Am J Reprod Immunol 80, e12972. 10.1111/aji.12972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancebo E, Clemente J, Sanchez J, Ruiz-Contreras J, Pablos PD, Cortezon S, Romo E, Paz-Artal E, Allende LM, 2008. Longitudinal analysis of immune function in the first 3 years of life in thymectomized neonates during cardiac surgery. Clin Exp Immunol 154, 375–83. 10.1111/j.1365-2249.2008.03771.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariani SA, Li Z, Rice S, Krieg C, Fragkogianni S, Robinson M, Vink CS, Pollard JW, Dzierzak E, 2019. Pro-inflammatory Aorta-Associated Macrophages Are Involved in Embryonic Development of Hematopoietic Stem Cells. Immunity 50, 1439–1452.e5. 10.1016/j.immuni.2019.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mawa PA, Webb EL, Filali-Mouhim A, Nkurunungi G, Sekaly R-P, Lule SA, Prentice S, Nash S, Dockrell HM, Elliott AM, Cose S, 2017. Maternal BCG scar is associated with increased infant proinflammatory immune responses. Vaccine 35, 273–282. 10.1016/j.vaccine.2016.11.079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGovern N, Shin A, Low G, Low D, Duan K, Yao LJ, Msallam R, Low I, Shadan NB, Sumatoh HR, Soon E, Lum J, Mok E, Hubert S, See P, Kunxiang EH, Lee YH, Janela B, Choolani M, Mattar CNZ, Fan Y, Lim TKH, Chan DKH, Tan K-K, Tam JKC, Schuster C, Elbe-Bürger A, Wang X, Bigley V, Collin M, Haniffa M, Schlitzer A, Poidinger M, Albani S, Larbi A, Newell EW, Chan JKY, Ginhoux F, 2017. Human fetal dendritic cells promote prenatal T-cell immune suppression through arginase-2. Nature 546, 662–666. 10.1038/nature22795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaux JL, Heremans JF, Hitzig WH, 1966. Immunoglobulin levels in cord-blood serum of negroes and Caucasians. Trop Geogr Med 18, 10–4. [PubMed] [Google Scholar]
- Mishra A, Lai GC, Yao LJ, Aung TT, Shental N, Rotter-Maskowitz A, Shepherdson E, Singh GSN, Pai R, Shanti A, Wong RMM, Lee A, Khyriem C, Dutertre CA,Chakarov S, Srinivasan KG, Shadan NB, Zhang X-M, Khalilnezhad S, Cottier F, Tan ASM, Low G, Chen P, Fan Y, Hor PX, Lee AKM, Choolani M, Vermijlen D, Sharma A, Fuks G, Straussman R, Pavelka N, Malleret B, McGovern N, Albani S, Chan JKY, Ginhoux F, 2021. Microbial exposure during early human development primes fetal immune cells. Cell 184, 3394–3409.e20. 10.1016/j.cell.2021.04.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mold JE, Micha lsson J, Burt TD, Muench MO, Beckerman KP, Busch MP, Lee T-H, Nixon DF, McCune JM, 2008. Maternal Alloantigens Promote the Development of Tolerogenic Fetal Regulatory T Cells in Utero. Science 322, 1562–1565. 10.1126/science.1164511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mor G, 2022. Introduction to the immunology of pregnancy. Immunol Rev 308, 5–8. 10.1111/imr.13102 [DOI] [PubMed] [Google Scholar]
- Morsink MAJ, Willemen NGA, Leijten J, Bansal R, Shin SR, 2020. Immune Organs and Immune Cells on a Chip: An Overview of Biomedical Applications. Micromachinesbasel 11, 849. 10.3390/mi11090849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Msallam R, Balla J, Rathore APS, Kared H, Malleret B, Saron WAA, Liu Z, Hang JW, Dutertre CA, Larbi A, Chan JKY, John AL St., Ginhoux F, 2020. Fetal mast cells mediate postnatal allergic responses dependent on maternal IgE. Science 370, 941–950. 10.1126/science.aba0864 [DOI] [PubMed] [Google Scholar]
- Mshvildadze M, Neu J, Shuster J, Theriaque D, Li N, Mai V, 2010. Intestinal Microbial Ecology in Premature Infants Assessed with Non–Culture-Based Techniques. J Pediatrics 156, 20–25. 10.1016/j.jpeds.2009.06.063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee S, Allen RM, Lukacs NW, Kunkel SL, Carson WF, 2012. STAT3-Mediated IL-17 Production by Postseptic T Cells Exacerbates Viral Immunopathology of the Lung. Shock 38, 515–523. 10.1097/shk.0b013e31826f862c [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda-Nicolau NM, Polettini J, Peltier MR, Silva MG, Menon R, 2016. Combinations and loads of bacteria affect the cytokine production by fetal membranes: An in vitro study. Am J Reprod Immunol 76, 504–511. 10.1111/aji.12596 [DOI] [PubMed] [Google Scholar]
- Ober RJ, Martinez C, Lai X, Zhou J, Ward ES, 2004. Exocytosis of IgG as mediated by the receptor, FcRn: An analysis at the single-molecule level. Proc National Acad Sci 101, 11076–11081. 10.1073/pnas.0402970101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallinger E, Bognar Z, Bogdan A, Csabai T, Abraham H, Szekeres-Bartho J, 2018. PIBF+ extracellular vesicles from mouse embryos affect IL-10 production by CD8+ cells. Sci Rep-uk 8, 4662. 10.1038/s41598-018-23112-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popescu D-M, Botting RA, Stephenson E, Green K, Webb S, Jardine L, Calderbank EF, Polanski K, Goh I, Efremova M, Acres M, Maunder D, Vegh P, Gitton Y, Park J-E, Vento-Tormo R, Miao Z, Dixon D, Rowell R, McDonald D, Fletcher J, Poyner E, Reynolds G, Mather M, Moldovan C, Mamanova L, Greig F, Young MD, Meyer KB, Lisgo S, Bacardit J, Fuller A, Millar B, Innes B, Lindsay S, Stubbington MJT, Kowalczyk MS, Li B, Ashenberg O, Tabaka M, Dionne D, Tickle TL, Slyper M, Rozenblatt-Rosen O, Filby A, Carey P, Villani A-C, Roy A, Regev A, Chédotal A, Roberts I, Göttgens B, Behjati S, Laurenti E, Teichmann SA, Haniffa M, 2019. Decoding human fetal liver haematopoiesis. Nature 574, 365–371. 10.1038/s41586-019-1652-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Post AL, Li SH, Berry M, Itell H, Martinez DR, Xie G, Permar SR, Swamy GK, Fouda GG, 2019. Efficiency of placental transfer of vaccine-elicited antibodies relative to prenatal Tdap vaccination status. Vaccine 38, 4869–4876. 10.1016/j.vaccine.2020.05.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pou C, Nkulikiyimfura D, Henckel E, Olin A, Lakshmikanth T, Mikes J, Wang J, Chen Y, Bernhardsson AK, Gustafsson A, Bohlin K, Brodin P, 2019. The repertoire of maternal anti-viral antibodies in human newborns. Nat Med 25, 591–596. 10.1038/s41591-019-0392-8 [DOI] [PubMed] [Google Scholar]
- Prescott SL, Macaubas C, Holt BJ, Smallacombe TB, Loh R, Sly PD, Holt PG, 1998. Transplacental priming of the human immune system to environmental allergens: universal skewing of initial T cell responses toward the Th2 cytokine profile. J Immunol Baltim Md 1950 160, 4730–7. [PubMed] [Google Scholar]
- Rackaityte E, Halkias J, 2020. Mechanisms of Fetal T Cell Tolerance and Immune Regulation. Front Immunol 11, 588. 10.3389/fimmu.2020.00588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rackaityte E, Halkias J, Fukui E, Mendoza V, Hayzelden C, Crawford E, Fujimura K, Burt T, Lynch S, 2020. Viable bacterial colonization is highly limited in the human intestine in utero. Nat Med 26, 599–607. 10.1038/s41591-020-0761-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rescigno M, Urbano M, Valzasina B, Francolini M, Rotta G, Bonasio R, Granucci F, Kraehenbuhl J-P, Ricciardi-Castagnoli P, 2001. Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nat Immunol 2, 361–367. 10.1038/86373 [DOI] [PubMed] [Google Scholar]
- Roopenian DC, Akilesh S, 2007. FcRn: the neonatal Fc receptor comes of age. Nat Rev Immunol 7, 715–725. 10.1038/nri2155 [DOI] [PubMed] [Google Scholar]
- Salter SJ, Cox MJ, Turek EM, Calus ST, Cookson WO, Moffatt MF, Turner P, Parkhill J, Loman NJ, Walker AW, 2014. Reagent and laboratory contamination can critically impact sequence-based microbiome analyses. Bmc Biol 12, 87. 10.1186/s12915-014-0087-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semmes EC, Chen J-L, Goswami R, Burt TD, Permar SR, Fouda GG, 2021. Understanding Early-Life Adaptive Immunity to Guide Interventions for Pediatric Health. Front Immunol 11, 595297. 10.3389/fimmu.2020.595297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senn V, Bassler D, Choudhury R, Scholkmann F, Righini-Grunder F, Vuille-dit-Bille RN, Restin T, 2020. Microbial Colonization From the Fetus to Early Childhood—A Comprehensive Review. Front Cell Infect Mi 10, 573735. 10.3389/fcimb.2020.573735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd MC, Radnaa E, Tantengco OA, Kechichian T, Urrabaz-Garza R, Kammala AK, Sheller-Miller S, Menon R, 2021. Extracellular vesicles from maternal uterine cells exposed to risk factors cause fetal inflammatory response. Cell Commun Signal Ccs 19, 100. 10.1186/s12964-021-00782-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stelzer IA, Urbschat C, Schepanski S, Thiele K, Triviai I, Wieczorek A, Alawi M, Ohnezeit D, Kottlau J, Huang J, Fischer N, Mittrücker H-W, Solano ME, Fehse B, Diemert A, Stahl FR, Arck PC, 2021. Vertically transferred maternal immune cells promote neonatal immunity against early life infections. Nat Commun 12, 4706. 10.1038/s41467-021-24719-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenqvist A-C, Nagaeva O, Baranov V, Mincheva-Nilsson L, 2013. Exosomes Secreted by Human Placenta Carry Functional Fas Ligand and TRAIL Molecules and Convey Apoptosis in Activated Immune Cells, Suggesting Exosome-Mediated Immune Privilege of the Fetus. J Immunol 191, 5515–5523. 10.4049/jimmunol.1301885 [DOI] [PubMed] [Google Scholar]
- Szépfalusi Z, Loibichler C, Pichler J, Reisenberger K, Ebner C, Urbanek R, 2000. Direct Evidence for Transplacental Allergen Transfer. Pediatr Res 48, 404–407. 10.1203/00006450-200009000-00024 [DOI] [PubMed] [Google Scholar]
- Thibault G, Degenne D, Girard AC, Guillaumin JM, Lacord M, Bardos P, 1991. The inhibitory effect of human syncytiotrophoblast plasma membrane vesicles on in vitro lymphocyte proliferation is associated with reduced interleukin 2 receptor expression. Cell Immunol 138, 165–174. 10.1016/0008-8749(91)90141-w [DOI] [PubMed] [Google Scholar]
- Thorburn AN, McKenzie CI, Shen S, Stanley D, Macia L, Mason LJ, Roberts LK, Wong CHY, Shim R, Robert R, Chevalier N, Tan JK, Mariño E, Moore RJ, Wong L, McConville MJ, Tull DL, Wood LG, Murphy VE, Mattes J, Gibson PG, Mackay CR, 2015. Evidence that asthma is a developmental origin disease influenced by maternal diet and bacterial metabolites. Nat Commun 6, 7320. 10.1038/ncomms8320 [DOI] [PubMed] [Google Scholar]
- Torow N, Marsland BJ, Hornef MW, Gollwitzer ES, 2017. Neonatal mucosal immunology. Mucosal Immunol 10, 5–17. 10.1038/mi.2016.81 [DOI] [PubMed] [Google Scholar]
- Voer R.M. de, Klis F.R.M. van der, Nooitgedagt JE, Versteegh FGA, Huisseling J.C.M. van, Rooijen D.M. van, Sanders EAM, Berbers GAM, 2009. Seroprevalence and Placental Transportation of Maternal Antibodies Specific for Neisseria meningitidis Serogroup C, Haemophilus influenzae Type B, Diphtheria, Tetanus, and Pertussis. Clin Infect Dis 49, 58–64. 10.1086/599347 [DOI] [PubMed] [Google Scholar]
- Waldorf KMA, Gravett MG, McAdams RM, Paolella LJ, Gough GM, Carl DJ, Bansal A, Liggitt HD, Kapur RP, Reitz FB, Rubens CE, 2011. Choriodecidual group B streptococcal inoculation induces fetal lung injury without intra-amniotic infection and preterm labor in Macaca nemestrina. Plos One 6, e28972. 10.1371/journal.pone.0028972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weitkamp J-H, Guthrie SO, Wong HR, Moldawer LL, Baker HV, Wynn JL, 2016. Histological chorioamnionitis shapes the neonatal transcriptomic immune response. Early Hum Dev 98, 1–6. 10.1016/j.earlhumdev.2016.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox CR, Holder B, Jones CE, 2017. Factors Affecting the FcRn-Mediated Transplacental Transfer of Antibodies and Implications for Vaccination in Pregnancy. Front Immunol 8, 1294. 10.3389/fimmu.2017.01294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Zhang J, Hu Y, Li X, Sun L, Peng Y, Sun Y, Liu B, Bian Z, Rong Z, 2021. Single-cell transcriptome analysis reveals the dynamics of human immune cells during early fetal skin development. Cell Reports 36, 109524. 10.1016/j.celrep.2021.109524 [DOI] [PubMed] [Google Scholar]
- Yu K, Rodriguez M, Paul Z, Gordon E, Gu T, Rice K, Triplett EW, Keller-Wood M, Wood CE, 2021. Transfer of oral bacteria to the fetus during late gestation. Sci Rep-uk 11, 708. 10.1038/s41598-020-80653-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakis DR, Paulissen E, Kornete L, Kaan AM, Nicu EA, Zaura E, 2021. The evidence for placental microbiome and its composition in healthy pregnancies: a systematic review. J Reprod Immunol 149, 103455. 10.1016/j.jri.2021.103455 [DOI] [PubMed] [Google Scholar]
- Zaretsky MV, Alexander JM, Byrd W, Bawdon RE, 2004. Transfer of Inflammatory Cytokines Across the Placenta. Obstetrics Gynecol 103, 546–550. 10.1097/01.aog.0000114980.40445.83 [DOI] [PubMed] [Google Scholar]


