Abstract
The auditory system transforms auditory stimuli from the external environment into perceptual auditory objects. Recent studies have focused on the contribution of the auditory cortex to this transformation. Other sets of studies have yielded important insights into the contributions of neural activity in the auditory cortex to cognition and decision-making. However, despite this important work, the relationship between auditory-cortex activity and behavior/perception has not been fully elucidated. Two of the more important gaps in our understanding are (1) the specific and differential contributions of different fields of the auditory cortex to auditory perception and behavior and (2) the way networks of auditory neurons impact and facilitate auditory information processing. Here, we focus on recent work from non-human-primate models of hearing and review work related to these gaps and put forth challenges to further our understanding of how single-unit activity and network activity in different cortical fields contribution to behavior and perception.
Introduction
A fundamental goal of the auditory system is to parse an unlabeled mixture of environmental auditory stimuli into coherent perceptual units (Bregman, 1990). The auditory system accomplishes this task, in part, by computations that group together stimuli with similar spectral and temporal features, while simultaneously segregating stimuli with different spectral and temporal features into different groups. Groupings with similar spectral and temporal features lead to the generation of coherent auditory perceptual units, which are also known as auditory objects (Bizley and Cohen, 2013; Griffiths and Warren, 2004).
Our understanding of these stimulus-to-perception computations has been facilitated by elegant anatomical, psychophysical, electrophysiological, and imaging studies in humans and in non-human animal models. However, there are still substantial gaps in our understanding of the relationship between neural activity (in particular, spiking activity) in the auditory cortex, perception, and behavior (Banno et al., 2020; Cohen, 2012). Here, we discuss and review two gaps in our understanding: (1) the specific and differential contributions of different fields of the auditory cortex to auditory perception and behavior and (2) the way network activity impact and facilitate auditory information processing. In particular, we emphasize recent work that has identified underlying computational principles in the cortex of non-human primates and when appropriate we note studies from other animal models of hearing.
In the 1990s, Hackett, Romanski, Kaas, Rauschecker, and others (Hackett et al., 1998, 1999; Kaas and Hackett, 1998, 1999; Romanski and Goldman-Rakic, 2002; Romanski et al., 1999; Romanski et al., 2000) revolutionized our understanding of cortical anatomical connectivity as it relates to auditory processing. First, Hackett and Kaas demonstrated a new organizational principle for the auditory cortex; namely, a “core” that is surrounded by a “belt”, which, in turn, is surrounded by a “parabelt”. From those initial anatomical studies, two cortical processing streams were identified: the so-called “dorsal” and “ventral” pathways. Because these pathways were hypothesized to be analogous to the visual dorsal and ventral pathways (Ungerleider and Mishkin, 1982), it was thought that they contribute to auditory perception and spatial/audiomotor behaviors, respectively.
However, despite the elegance of these studies, we still do not have a coherent theory of how auditory and related cognitive information is processed within and across defined auditory fields. For example, what information is preferentially processed in the primary auditory cortex and how is this processing different from processing that occurs in other core auditory fields? Further, we do not have a good understanding of how information is transformed and represented across different cortical fields (e.g., from the core to the belt and ultimately to the prefrontal cortex). Finally, at a higher hierarchical level of processing, the unique functional role(s) of the auditory dorsal and ventral pathways still remains an active area of research (Rauschecker and Scott, 2009).
Although single-unit recordings have shed considerable light on the functional properties of the auditory cortex and the representation of stimulus-, task-, and cognitive-related variables in individual auditory fields (Cohen, 2012; Fritz et al., 2013; King et al., 2018; Recanzone, 2018; Recanzone and Sutter, 2008; Shamma et al., 2013; Shamma et al., 2011; Tsunada and Cohen, 2014; Wang and Walker, 2012), the cortex is not just composed of billions of individual neurons, but instead, it is composed of networks of interconnected neurons (Cohen and Kohn, 2011; Kohn et al., 2016; Semedo et al., 2020). These cortical networks operate over different computational scales: network ensembles exist within a single cortical field (e.g., the primary auditory cortex), across cortical fields (e.g., primary and non-primary auditory cortex), and across brain systems (e.g., the dorsal and ventral auditory pathways or the auditory and visual pathways) (Atencio and Schreiner, 2016; Bastos et al., 2012; Fries, 2009, 2015; See et al., 2018; See et al., 2021). These spatial scales include not only feedforward patterns of connectivity but also complex patterns of feedback connectivity (Banno et al., 2020). Indeed, the richness and extent of auditory feedback connectivity distinguishes it from other sensory systems, like the visual system (Brugge, 1992; Felleman and Van Essen, 1991; Kaas and Hackett, 2000). Across these spatial scales, we can also consider different temporal scales of neural activity, which range from single-neuron spiking activity to local-field potentials to neural oscillations. Unfortunately, our understanding of the contribution of network activity to auditory information processing is relatively nascent.
Functional and hierarchical organization of auditory cortex
The auditory cortex is made up of the core, belt, and parabelt (Fig. 1). These cortical areas are based on architectonics and corticocortical connectivity (Hackett, 2011; Rolls et al., 2022; Romanski and Averbeck, 2009). Anatomically, each cortical field is connected most strongly to and reciprocally with its immediate neighbors in all directions (Hackett et al., 1998). For example, the core and belt auditory cortices are highly connected with one another. This is consistent with a general cortical principle that stronger connectivity exists between immediately adjacent neighboring fields (Averbeck and Seo, 2008). On the other hand, the core and parabelt auditory cortices have minimal connectivity.
Figure 1: Anatomical locations and organization of auditory cortical fields.

A schematic side view (anterior is to the left and dorsal is toward the top) of a rhesus brain. The auditory cortex is located on the supratemporal plane and in the lateral sulcus (LS). In the cut-out, which is a top-down view of the temporal lobe, we schematize the relative organization of the core auditory cortex (dark blue), belt auditory cortex (lighter blue), and parabelt auditory cortex (0range). The solid red line indicates the border of the lateral sulcus. Superior temporal sulcus (STS), rostral superior temporal gyrus (STGr), rostral parabelt (RPB), caudal parabelt (CPB), primary auditory cortex (A1), rostral (R), rostral temporal (RT), caudal medial (CM), middle medial (MM), rostral medial (RM), medial rostral temporal (RTM), caudal-lateral (CL), middle lateral (ML), rostral-lateral (RL), lateral rostral temporal (RTL), rostrotemporal polar (RTp), and temporoparietal (TPT).
Beyond cortical-cortical connectivity, these auditory fields are also distinguished by their thalamic inputs. All the fields in the core auditory cortex (A1, R, and RT) receive direct input from the ventral division of the medial geniculate nucleus; A1 receives the strongest projections, whereas RT receives the weakest projection (Jasmin et al., 2019). Because of this connectivity, each is considered to be a “primary” (A1) or “primary-like” (R and RT) auditory field. We do not know fully the computational advantage of multiple such cortical fields (Jasmin et al., 2019), unlike a single primary field that is ubiquitous in the primate and mammalian visual systems. Thalamic input to the belt and parabelt auditory cortices arises mostly from the anterodorsal and posterodorsal divisions of the medial geniculate nucleus (Hackett, 2011). The medial division of the medial geniculate projects widely to all fields in the auditory cortex.
Both the core and belt auditory cortex lie on the dorsal surface of the temporal lobe, which is buried in the lateral sulcus. The belt auditory cortex surrounds the core and has multiple divisions such as the middle-lateral, caudolateral, and rostrolateral belt regions. The parabelt auditory cortex lies lateral to the belt on the superior temporal gyrus. The belt is divided into the caudal, middle, anterior, and rostral auditory fields, as well as medial and lateral auditory fields. The parabelt auditory cortex includes caudal and rostral components. Caudal and rostral auditory fields are also anatomically connected with regions of the prefrontal cortex: the caudal auditory fields project to dorsolateral prefrontal cortex (dorsal area 46) and the rostral fields project to ventral lateral prefrontal cortex (ventral area 46 and area 12) (Romanski et al., 1999).
Although the architectonic divisions and corresponding connectivity of the auditory cortex are well defined, much less is known about its functional properties and organization (Brewer and Barton, 2016; Escabí and Read, 2003; Gerstein and Kiang, 1964; Hackett, 2011; Linden and Schreiner, 2003; Miller et al., 2002). Historically, single neurons in the auditory cortex have been probed to identify how they represent fundamental features of acoustic stimuli, such as frequency tuning and bandwidth. However, it is becoming increasingly clear that the computations occurring in the auditory cortex are not simply restricted to spectral decompositions of auditory stimuli. Indeed, the frequency tuning of an auditory-cortex site is not hard wired to a specific value but is dependent, to a degree, on the demands of an ongoing behavior task (David et al., 2012; Fritz et al., 2013; Kilgard, 2012; Yin et al., 2014). Moreover, this frequency tuning may not even represent the physical features of a stimulus but may be more closely tied with its perceptual qualities (e.g., its pitch) (Bendor and Wang, 2006; Kikuchi et al., 2019). Further and consistent with a role of the auditory cortex in perception, several studies have demonstrated a role for the core and belt auditory cortices in segregating and organizing the external auditory environment into perceptual units. This notion is supported by studies that have examined the contributions of these cortical fields to auditory streaming and prediction (Christison-Lagay and Cohen, 2018; Kikuchi et al., 2018; Selezneva et al., 2018) as well as those that examine how listeners extract an auditory figure from a noisy background (Christison-Lagay et al., 2017; Schneider et al., 2021; Schneider et al., 2018; Teki et al., 2016).
These representations of frequency, pitch, the auditory scene, etc. are also modulated by cognitive- and task-related variables (Fig. 2). For example, several studies have identified neural signatures of auditory working memory in the early auditory cortex (Bigelow et al., 2014; Huang et al., 2016; Plakke et al., 2013; Poremba et al., 2013), which can also be seen in the prefrontal cortex (Plakke et al., 2015). Other studies have shown that A1 neurons are modulated by visual signals that cue the start of an auditory task, by motor movements associated with the task (i.e., the grasping and release of a touch-sensitive bar), and the delivery of juice rewards (Brosch et al., 2005; Huang and Brosch, 2020; Huang et al., 2019; Knyazeva et al., 2020; Werner-Reiss et al., 2003; Wikman et al., 2019).
Figure 2: Extra-auditory influences on the auditory cortex.

Schematic of the auditory cortex showing the core (central ellipse) and surrounding belt auditory cortex (grey ellipse). Classic work focused primarily on the (1) tonotopic maps in each core field (A1, R, and Rt), which is schematized by changes in color (see color bar) and (2) increased tuning sensitivity for vocalizations, spatial location, etc. between the core and belt, which is schematized by differently colored broad Gaussian “tuning curves” in the core auditory cortex and narrower Gaussians in the belt auditory cortex. More recent studies have highlighted that auditory-cortex responsivity is subject to multiple influences including extrasensory inputs, attention, choice, reward, memory, motor etc. (as schematized by arrows). The challenge is to identify how, when, and why these inputs modulate auditory perception and ongoing behavior.
There is also evidence for hierarchical information processing in the auditory cortex. For example, neurons in rostral fields tend to be more sensitive to stimulus identity (e.g., different monkey vocalizations) than caudal-field neurons (Fukushima et al., 2014; Kikuchi et al., 2018; Rauschecker, 1998; Rauschecker and Tian, 2000; Rauschecker et al., 1995). These auditory-cortex representations of vocalizations are further refined in those regions of the prefrontal/frontal cortex that receive input from the auditory cortex (Diehl et al., 2022; Jovanovic et al., 2022). Computational and imaging studies also suggest hierarchical processing of object (perceptual) information in the human auditory cortex (Kell et al., 2018). Spatial information is also hierarchically organized: single neurons in the caudal belt auditory cortex, including CL and CM, tend to encode more information about the spatial location of an auditory stimulus than those in the core auditory cortex. The spatial tuning of these caudal-belt neurons is sharp enough to support a listener’s spatial acuity (Miller and Recanzone, 2009).
As just discussed, and as alluded to above, there is both anatomical organization and physiological response properties in support of a “what versus where” distinction in dorsal and ventral auditory-cortex circuits. We caution, however, that, with the exception of a single study in cats (Lomber and Malhotra, 2008), there is not any direct causal evidence, to our knowledge, favoring a dissociation between object- (“what”) and spatial- (“where”) related processing in the ventral and dorsal pathways, respectively, in primate models of hearing. Indeed, in non-human primates, neural sensitivity to spatial and non-spatial processing is seen in both pathways, with almost equal degrees of tuning sensitivity (Cohen et al., 2004; Gifford III and Cohen, 2005). Similarly, other studies have also found that neurons in the dorsal pathway are modulated by both non-spatial and spatial auditory information (Belin and Zatorre, 2000; Cusack, 2005; Engel et al., 2009; Lewis et al., 2005; Pizzamiglio et al., 2005; Rauschecker, 2011; Recanzone, 2008; Walker et al., 2011; Warren et al., 2005; Zatorre et al., 2002). Although it is not a primate study, it is worth noting that a recent ferret study also failed to identify a clean disassociation between auditory object and spatial behavior in these two pathways (Town et al., 2022).
We would like to offer that this parcellation of “what” and “where (perceptual and non-perceptual) information into parallel pathways may be simplified and does not account for the complexities of audition. Spatial information, like non-spatial information (see above), is critical to the parsing of the auditory scene into distinct perceptual objects: two stimuli that are far apart are more likely to be heard as two distinct auditory objects (sounds) versus two stimuli that are close together. This suggests that spatial information in the dorsal pathway could be used for parsing the auditory scene into perceptual objects (Cusack, 2005). Could the dorsal pathway have a privileged role in those situations in which “where” information is needed to parse the auditory scene? A non-exclusive alternative possibility is that, in these situations, there may be enhanced functional connectivity between the dorsal and ventral pathways.
In addition to processing different types of auditory information, the auditory cortex also has pervasive connections with other sensory cortices, which may contribute, in part, to multisensory behavior and perception (Fig. 2) (Caruso et al., 2021; Ghazanfar and Schroeder, 2006; Khandhadia et al., 2021; Raposo et al., 2012; Schmehl and Groh, 2021). For example, the belt auditory cortex (mainly the caudal fields) has considerable connections with the secondary visual cortex (Falchier et al., 2009) and the secondary somatosensory cortex (Cappe and Barone, 2005; Smiley et al., 2007). In contrast, the core auditory cortex receives sparse input from these extrasensory fields (Cappe and Barone, 2005; Falchier et al., 2009). Consistent with these patterns of connectivity, belt neurons have more robust multisensory responses than core neurons (Bizley and Dai, 2020; Bizley et al., 2006; Ghazanfar et al., 2005; Morrill and Hasenstaub, 2018). However, in general, the differential contribution of the core and belt auditory cortex to multisensory processing is still not fully understood (Ghazanfar et al., 2005; Lehmann et al., 2006; Merrikhi et al., 2023).
These extrasensory signals may also serve to facilitate and enhance auditory processing. For example, when somatosensory and auditory stimuli are presented simultaneously, somatosensory signals arrive at A1 faster than the auditory responses. Because these somatosensory signals reset ongoing neural oscillations in the auditory cortex, when the auditory signals reach A1, they are coupled to an optimal oscillation phase. This coupling, in turn, facilitates auditory processing (Lakatos et al., 2007). This facilitation is largest when the auditory response is the weakest. Similar coupling and enhanced processing have also been found using audiovisual stimuli (Kayser et al., 2008; Mégevand et al., 2020).
Representation of variables related to decision making in the auditory cortex
Neural correlates of decision-making have been found in early auditory fields (Fig. 2). For example, in a spatial-delayed match-to-sample task, there was a strong representation of several decision and cognitive variables in A1 (Napoli et al., 2021). In that study, neurons were recorded in A1 and dorsolateral prefrontal cortex (dlPFC; caudal, dorsal area 46 and adjacent area 8) while monkeys carried out the spatial-delayed match-to-sample task. Cue-specific activity was found in A1, which preceded similar cue-related activity in the dlPFC. There was also robust delay- period activity in both A1 and dlPFC. Interestingly, decision-related activity was also found in A1 and dlPFC but earlier in A1 than in dlPFC. The A1 decision-related activity also shifted with reaction times. This choice-related activity was earlier when reaction times were shorter but later when reaction times were longer. Furthermore, the choice-related activity on error trials represented the actual choice of the animal in A1 but not dlPFC. Thus, this study found substantial choice-related activity in A1, which appeared to precede similar signals in dlPFC. The finding of choice-related activity in A1 is consistent with other studies that identified choice-related neural modulation in A1 (Bizley et al., 2013; Ceballo et al., 2019; Christison-Lagay and Cohen, 2018; Kilian-Hütten et al., 2011; Mohn et al., 2021; Niwa et al., 2012, 2013).
These results may seem surprising, given findings that early visual fields have little activity related to decision making (Freedman and Assad, 2016; Jasper et al., 2019; Krishna et al., 2021). Indeed, the finding of choice-related activity in the early auditory fields is not universal. For example, in an auditory flutter discrimination task, Lemus et al. did not find choice-related activity in the auditory cortex (Lemus et al., 2009a). Similarly, Tsunada et al. did not identify choice-related signals in the middle-lateral belt of the auditory cortex during a categorization task nor did they identify choice activity during a frequency-discrimination task (Tsunada et al., 2011; Tsunada et al., 2016). Using a combination of modeling, electrophysiological recordings, and computational modeling, Tsunada et al. argued that activity in the anterolateral belt causally contributes to the current auditory decision (Tsunada et al., 2016). Lemus et al. and Tsunada et al. did, however, identify choice-related activity in regions downstream from the auditory cortex (Lemus et al., 2009b; Tsunada et al., 2019). Interestingly, because the PFC choice activity that was identified by Tsunada et al. related to the next trial, it most likely reflected an evaluative process of the previous trial and/or the biasing of subsequent trials.
How can we reconcile these different sets of findings? We do not have a definitive answer, but there are several non-exclusive possibilities. One possibility may relate to the different perceptual and cognitive demands of the different auditory tasks/stimuli and how each task/stimulus engages different auditory fields. Another possibility relates to the analysis of the choice activity itself, including clearly differentiating between choice and stimulus signals and differentiating between causal feedforward signals that relate to the ongoing decision versus attention-related feedback signals (Nienborg et al., 2012; Nienborg and Cumming, 2009; Tsunada et al., 2016). Finally, neural selection bias (e.g., only analyzing neurons with high stimulus sensitivity or other response properties) may limit how well we can extrapolate from the properties of a specific recorded population to a general statement on the contribution of an auditory field to decision-making.
Population codes matter in the AC
The combined activity of auditory-cortex neurons –that is, its network or ensemble-level properties (Eggermont, 2007)– is a better predictor of behavior than single-unit activity alone (Bathellier et al., 2012; Christison-Lagay et al., 2017; Engineer et al., 2008; Ince et al., 2013; Miller and Recanzone, 2009; Pachitariu et al., 2015), consistent with models of population coding (Averbeck and Romanski, 2006; Bartolo and Averbeck, 2020). But how do populations of neurons in the auditory cortex encode stimuli? One popular approach is to test the noise correlations between pairs of auditory-cortex neurons (Cohen and Kohn, 2011; Downer et al., 2021; Gourévitch and Eggermont, 2010; See et al., 2018); noise correlations reflect the functional connectivity of the underlying network (Aertsen et al., 1989; Cohen and Kohn, 2011; Greschner et al., 2011). More specifically in the auditory cortex, neurons with similar frequency-tuning profiles tend to be more strongly correlated than those with dissimilar frequency tuning and have more synchronous activity (Fukushima et al., 2012). Because networks of correlated neurons are stable over time and stimulus conditions, it is possible that such networks reflect fundamental units of information processing in the auditory cortex (See et al., 2018). Indeed, neurons with similar frequency-tuning profiles tend to be more strongly correlated than those with dissimilar frequency tuning and have more synchronous activity (Fukushima et al., 2012).
These noise correlations are specifically relevant in population coding because they affect the amount of information that can be encoded by the population, which, in turn, impacts behavioral performance (Abbott and Dayan, 1999; Averbeck et al., 2006; Downer et al., 2021; Panzeri et al., 2022). Despite the existence of large noise correlations in the auditory cortex (Rothschild et al., 2010), which are often associated with information limiting neural encoding (Bartolo and Averbeck, 2020; Kafashan et al., 2021; Panzeri et al., 2022), certain groups of neurons exhibiting strong noise correlations were also shown to improve auditory neural encoding. For instance, neural ensembles constructed from neurons that have coincident firing have higher information capacity than individual neurons and random groupings of neurons (See et al., 2018) These findings can possibly be reconciled by considering the scale of the neuronal populations studied. Information limiting correlations in large neuronal population (>100 neurons) tend to be detrimental to neural encoding (Abbott and Dayan, 1999; Bartolo and Averbeck, 2020; Kafashan et al., 2021; Zohary et al., 1994), whereas the information limiting effects are minimal in smaller populations (Averbeck et al., 2006; Averbeck and Lee, 2006). However, effects in populations do not tend to reverse, such that positive effects in small populations become negative effects in large populations. Additionally, it is always possible to find some pairs that show positive effects of noise correlations, but at the population level the effects of noise correlations have only been found to be negative.
Despite the ubiquitous existence of noise correlations in the auditory cortex (Downer et al., 2015; Rothschild et al., 2010; Winkowski and Kanold, 2013), the role and the effect of noise correlations on auditory encoding are still unclear. For example, motivated by findings in the monkey visual cortex showing that attention seems to facilitate behavioral performance by decreasing noise correlations (Cohen and Maunsell, 2009), several studies have investigated the relationship between task engagement and auditory-cortex noise correlations (Downer et al., 2015, 2017a; Issa and Wang, 2013). In one study, Downer et al. measured the noise correlations between neurons in the core auditory cortex while monkeys listened passively to amplitude-modulated tone bursts or selectively attended to these tone bursts (Downer et al., 2017a). This study found that the noise correlations were lower during active listening relative to the passive-listening condition. Although these findings point to a straightforward inverse relationship between attention and auditory-cortex noise correlations, the story is more complicated: in the belt, task engagement increased the noise correlations (Downer et al., 2017a). The relationship between engagement and noise correlations is further nuanced by the finding that changes in neural-correlation structure depend not only on the neural sensitivity to the attended stimulus feature but also on the properties of non-attended stimulus features (Downer et al., 2017b). To further complicate this issue, noise correlations in the auditory cortex increase with age (Shilling-Scrivo et al., 2021; Shilling-Scrivo et al., 2022) and after parturition (Rothschild et al., 2013), at least in rodent species. Thus, the relationship between noise correlations, behavior, and neural representations in the auditory cortex is complex and requires further investigation.
Information in the form of “temporal regularities” are particularly important in audition as a means to parse the auditory scene (Bregman, 1990; Darwin, 1997). Stimuli with similar temporal regularities tend to group together and are heard as a single auditory object. For example, a series of inharmonic tone bursts with simultaneous (synchronous) temporal onsets are heard as one sound that is distinct (i.e., perceptually segregated) from other tone bursts in the environment (Christison-Lagay and Cohen, 2014; Elhilali et al., 2009; Krishnan et al., 2014; Lu et al., 2017; O’Sullivan et al., 2015; Shamma et al., 2013; Teki et al., 2016; Teki et al., 2013; Teki et al., 2011; Thakur et al., 2015).
This temporal information may, in part, be encoded by neural correlations over various lengths of time windows (Panzeri et al., 2022). Longer time windows could filter out faster neural fluctuations that are uncorrelated across neurons, while simultaneously emphasizing the slower, correlated variability (Averbeck and Lee, 2003). In sensory areas, the relevant time windows vary greatly: from less than a millisecond to several hundreds of seconds (Panzeri et al., 2010). This variability in encoding-window length may be a mechanism by which neural ensembles can encode different information at different timescales (Norman-Haignere et al., 2022; Runyan et al., 2017). In particular, longer time windows may facilitate the processing of natural (ethological) auditory stimuli, which are characterized by low rates of temporal modulation(Cohen et al., 2007; DiTullio et al., 2022; Singh and Theunissen, 2003).
This process of encoding different information on different time scales is sometimes referred to as temporal multiplexing (Panzeri et al., 2010). It has been observed in multiple sensory areas, including the auditory system in which neurons in the core and belt auditory cortex encode formants earlier than pitch (Caruso et al., 2018; Panzeri et al., 2010; Walker et al., 2011). Interestingly, the neurons that multiplex information at different time scales are not limited to the cortex: fluctuations in feature encoding are coordinated across neurons in the inferior colliculus. This multiplexing of information at different time scales may be a means by which the brain efficiently codes information.
Beyond the correlation structure that can be gleaned from the spiking activity of groups of neurons, there is also evidence for population coding at larger spatiotemporal scales. This coding may be particularly relevant to multisensory processing. For example, the simultaneous presentation of auditory and non-auditory stimuli increases oscillatory power at different hierarchical levels of the auditory cortex (Karthik et al., 2021; Keil et al., 2013; Schroeder et al., 2008) (Kuroki et al., 2018; Maier et al., 2008; Wang et al., 2019). This cross-areal phase/power synchronization is correlated with improvements in multisensory behavior and appears to play a causal role in multisensory behavior (Hipp et al., 2011; Mercier et al., 2015). Indeed, experimentally induced increases in gamma-band synchronization slows multisensory response times (Misselhorn et al., 2019).
Conclusion and open questions
As discussed throughout, although substantial progress has been made, several open and fundamental questions remain regarding the relationship between neural activity, perception, and decision-making in the auditory cortex. For example, although the contribution of corticofugal activity to audition has received recent attention (Blackwell et al., 2020; Clayton et al., 2020; King et al., 2018; Williamson and Polley, 2019; Yin et al., 2020), we still know relatively little about how feedback activity in the auditory cortex supports hearing. Relatedly, we need to differentiate whether early choice-related activity reflects causal contributions to the current decision or whether it reflects bias- or evaluative-related activity that occurs after the outcome of a decision (Fig. 3). Similarly, what is the relationship between a stimulus, a task, and the contribution of a particular auditory field to decision-making and behavior? Do simple choices engage earlier auditory fields, whereas more complex choices engage later auditory fields? The contribution (if any) of the dorsal auditory pathway to perception need further study. Finally, in addition to the study of pairwise correlations, further work is needed to identify how larger networks of neurons contribute to perception (Francis et al., 2018) and whether these larger networks can best be described by lower-dimensional subspaces (Fitzgerald et al., 2013; Ganguli et al., 2008; Gao et al., 2017; Ni et al., 2018).
Figure 3: Schematic of how and when neural activity can be modulated by behavioral choice.
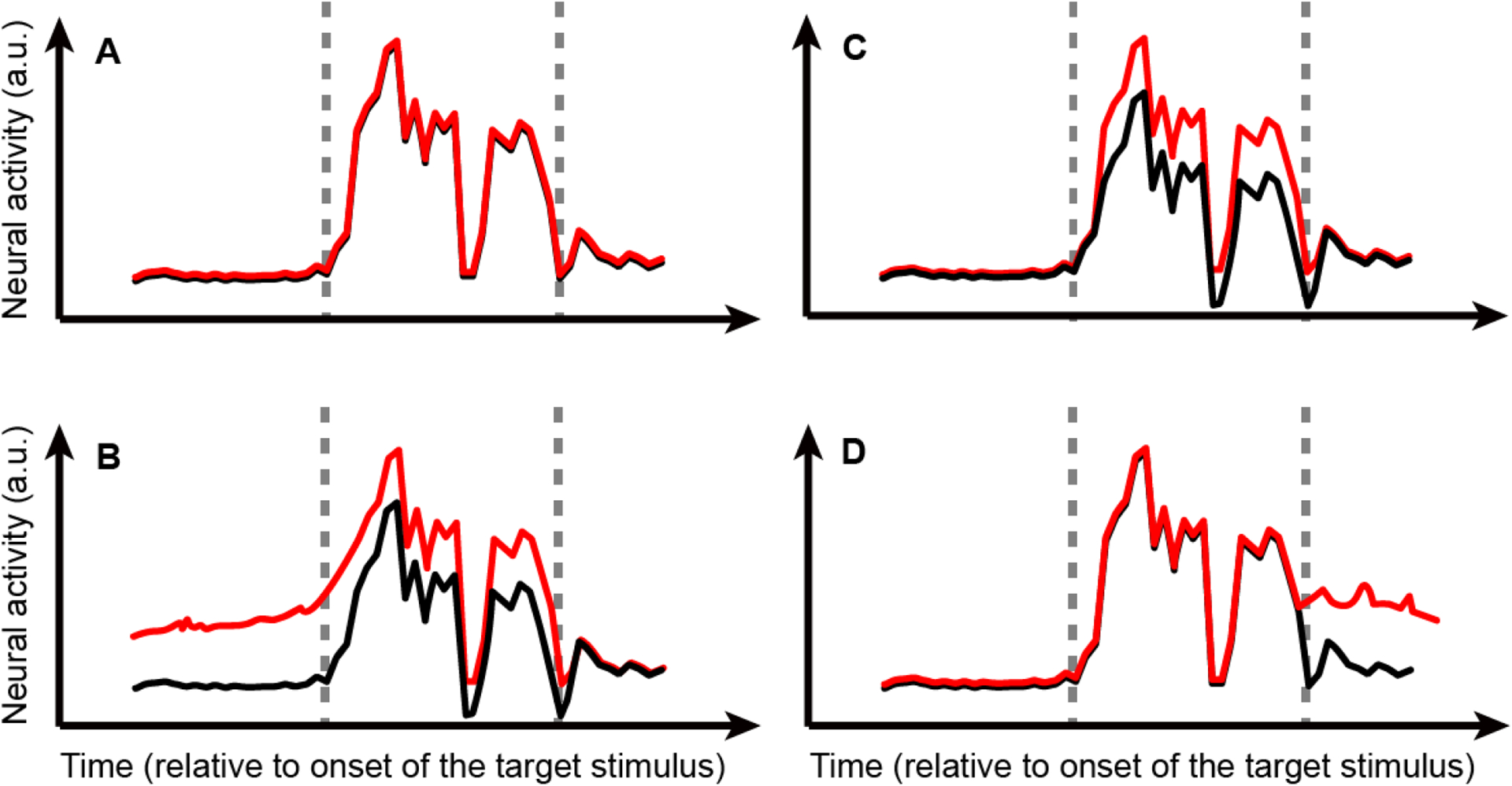
In each schematic, neural activity is plotted (arbitrary units [a.u.]) relative to the onset of a target stimulus. The first vertical dotted line indicates target onset, whereas the second vertical line indicates target offset. The red and black activity traces in each panel represent possible outcomes for a listener’s different choices (e.g., choosing “high frequency” versus choosing “low frequency”, respectively) in response to the same target stimulus. A: Choice does not modulate activity (black and red traces overlap). B: A particular choice (red curve [e.g., high-frequency choice]) modulates neural activity prior to stimulus onset and throughout target presentation, which is suggestive of some form of expectation or attention. C: Different choices (red and black curves) modulate neural activity only during target-stimulus presentation. D: A particular choice (red curve) modulates neural activity after stimulus onset and could reflect motor processing and/or post-hoc processing of the previous trial and evaluation of the subsequent trial.
Highlights.
We review the current understanding of the functional organization of auditory cortex.
While much work has been done in auditory cortex, basic question remain unanswered, including why are there multiple primary auditory fields? What is their differential contribution to auditory perception?
How do neural ensembles within and across auditory areas contribute to auditory perception?
Acknowledgments:
This work was supported by the Intramural Research Program of the National Institute of Mental Health (ZIA MH002928) and grants from the NIDCD and DoD ARL.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbott LF, Dayan P, 1999. The effect of correlated variability on the accuracy of a population code. Neural computation 11(1), 91–101. 10.1162/089976699300016827. [DOI] [PubMed] [Google Scholar]
- Aertsen AM, Gerstein GL, Habib MK, Palm G, 1989. Dynamics of neuronal firing correlation: modulation of “effective connectivity”. Journal of neurophysiology 61(5), 900–917. 10.1152/jn.1989.61.5.900. [DOI] [PubMed] [Google Scholar]
- Atencio CA, Schreiner CE, 2016. Functional congruity in local auditory cortical microcircuits. Neuroscience 316, 402–419. 10.1016/j.neuroscience.2015.12.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Averbeck BB, Latham PE, Pouget A, 2006. Neural correlations, population coding and computation. Nature reviews. Neuroscience 7(5), 358–366. 10.1038/nrn1888. [DOI] [PubMed] [Google Scholar]
- Averbeck BB, Lee D, 2003. Neural noise and movement-related codes in the macaque supplementary motor area. The Journal of neuroscience : the official journal of the Society for Neuroscience 23, 7630–7641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Averbeck BB, Lee D, 2006. Effects of noise correlations on information encoding and decoding. Journal of neurophysiology 95(6), 3633–3644. 10.1152/jn.00919.2005. [DOI] [PubMed] [Google Scholar]
- Averbeck BB, Romanski LM, 2006. Probabilistic encoding of vocalizations in macaque ventral lateral prefrontal cortex. The Journal of neuroscience : the official journal of the Society for Neuroscience 26(43), 11023–11033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Averbeck BB, Seo M, 2008. The statistical neuroanatomy of frontal networks in the macaque. PLoS computational biology 4(4), e1000050. 10.1371/journal.pcbi.1000050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banno T, Lestang JH, Cohen YE, 2020. Computational and neurophysiological principles underlying auditory perceptual decisions. Curr Opin Physiol 18, 20–24. 10.1016/j.cophys.2020.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartolo R, Averbeck BB, 2020. Prefrontal Cortex Predicts State Switches during Reversal Learning. Neuron 106(6), 1044–1054.e1044. 10.1016/j.neuron.2020.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastos AM, Usrey WM, Adams RA, Mangun GR, Fries P, Friston KJ, 2012. Canonical microcircuits for predictive coding. Neuron 76(4), 695–711. 10.1016/j.neuron.2012.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bathellier B, Ushakova L, Rumpel S, 2012. Discrete neocortical dynamics predict behavioral categorization of sounds. Neuron 76(2), 435–449. 10.1016/j.neuron.2012.07.008. [DOI] [PubMed] [Google Scholar]
- Belin P, Zatorre RJ, 2000. ‘What,’ ‘where’ and ‘how’ in auditory cortex. Nature neuroscience 3(10), 965–966. [DOI] [PubMed] [Google Scholar]
- Bendor D, Wang X, 2006. Cortical representations of pitch in monkeys and humans. Current opinion in neurobiology 16(4), 391–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigelow J, Rossi B, Poremba A, 2014. Neural correlates of short-term memory in primate auditory cortex. Front Neurosci 8, 250. 10.3389/fnins.2014.00250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bizley JK, Cohen YE, 2013. The what, where, and how of auditory-object perception. Nat Reviews Neuroscience 14, 693–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bizley JK, Dai Y, 2020. Non-auditory processing in the central auditory pathway. Current Opinion in Physiology 18, 100–105. https://doi.org/ 10.1016/j.cophys.2020.09.003. [DOI] [Google Scholar]
- Bizley JK, Nodal FR, Bajo VM, Nelken I, King AJ, 2006. Physiological and Anatomical Evidence for Multisensory Interactions in Auditory Cortex. Cerebral cortex 17(9), 2172–2189. 10.1093/cercor/bhl128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bizley JK, Walker KM, Nodal FR, King AJ, Schnupp JW, 2013. Auditory cortex represents both pitch judgments and the corresponding acoustic cues. Current biology : CB 23(7), 620–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwell JM, Lesicko AM, Rao W, De Biasi M, Geffen MN, 2020. Auditory cortex shapes sound responses in the inferior colliculus. eLife 9. 10.7554/eLife.51890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bregman AS, 1990. Auditory Scene Analysis. MIT Press, Boston, MA. [Google Scholar]
- Brewer AA, Barton B, 2016. Maps of the Auditory Cortex. Annual review of neuroscience 39, 385–407. 10.1146/annurev-neuro-070815-014045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosch M, Selezneva E, Scheich H, 2005. Nonauditory events of a behavioral procedure activate auditory cortex of highly trained monkeys. The Journal of neuroscience : the official journal of the Society for Neuroscience 25(29), 6797–6806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brugge JF, 1992. An Overview of Central Auditory Processing, in: Popper AN, Fay RR (Eds.), The Mammalian Auditory Pathway: Neurophysiology. Springer New York, New York, NY, pp. 1–33. [Google Scholar]
- Cappe C, Barone P, 2005. Heteromodal connections supporting multisensory integration at low levels of cortical processing in the monkey. European Journal of Neuroscience 22(11), 2886–2902. https://doi.org/ 10.1111/j.1460-9568.2005.04462.x. [DOI] [PubMed] [Google Scholar]
- Caruso VC, Mohl JT, Glynn C, Lee J, Willett SM, Zaman A, Ebihara AF, Estrada R, Freiwald WA, Tokdar ST, Groh JM, 2018. Single neurons may encode simultaneous stimuli by switching between activity patterns. Nature communications 9(1), 2715. 10.1038/s41467-018-05121-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caruso VC, Pages DS, Sommer MA, Groh JM, 2021. Compensating for a shifting world: evolving reference frames of visual and auditory signals across three multimodal brain areas. Journal of neurophysiology 126(1), 82–94. 10.1152/jn.00385.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceballo S, Piwkowska Z, Bourg J, Daret A, Bathellier B, 2019. Targeted Cortical Manipulation of Auditory Perception. Neuron 104(6), 1168–1179.e1165. 10.1016/j.neuron.2019.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christison-Lagay KL, Bennur S, Cohen YE, 2017. Contribution of spiking activity in the primary auditory cortex to detection in noise. J Neurophysiolology 118, 3118–3131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christison-Lagay KL, Cohen YE, 2014. Behavioral correlates of auditory streaming in rhesus macaques. Hearing research 309, 17–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christison-Lagay KL, Cohen YE, 2018. The contribution of primary auditory cortex to auditory categorization in behaving monkeys. Front Neurosci 21, 601. 10.3389/fnins.2018.00601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton KK, Williamson RS, Hancock KE, Tasaka GI, Mizrahi A, Hackett TA, Polley DB, 2020. Auditory Corticothalamic Neurons Are Recruited by Motor Preparatory Inputs. Current biology : CB. 10.1016/j.cub.2020.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MR, Kohn A, 2011. Measuring and interpreting neuronal correlations. Nature neuroscience 14(7), 811–819. 10.1038/nn.2842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MR, Maunsell JH, 2009. Attention improves performance primarily by reducing interneuronal correlations. Nature neuroscience 12(12), 1594–1600. 10.1038/nn.2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen YE, 2012. Auditory Cognition: The Integration of Psychophysics with Neurophysiology, in: Cohen YE, Popper AN, Fay RR (Eds.), Neural Correlates of Auditory Cognition. Springer-Verlag, New York, pp. 1–6. [Google Scholar]
- Cohen YE, Russ BE, Gifford GW 3rd, Kiringoda R, MacLean KA, 2004. Selectivity for the spatial and nonspatial attributes of auditory stimuli in the ventrolateral prefrontal cortex. Journal Neuroscience 24(50), 11307–11316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen YE, Theunissen F, Russ BE, Gill P, 2007. Acoustic features of rhesus vocalizations and their representation in the ventrolateral prefrontal cortex. Journal of neurophysiology 97(2), 1470–1484. [DOI] [PubMed] [Google Scholar]
- Cusack R, 2005. The Intraparietal Sulcus and Perceptual Organization. Journal of cognitive neuroscience 17, 641–651. [DOI] [PubMed] [Google Scholar]
- Darwin CJ, 1997. Auditory Grouping. Trends in cognitive sciences 1, 327–333. [DOI] [PubMed] [Google Scholar]
- David SV, Fritz JB, Shamma SA, 2012. Task reward structure shapes rapid receptive field plasticity in auditory cortex. Proceedings of the National Academy of Sciences of the United States of America 109(6), 2144–2149. 10.1073/pnas.1117717109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diehl MM, Plakke BA, Albuquerque ER, Romanski LM, 2022. Representation of Expression and Identity by Ventral Prefrontal Neurons. Neuroscience 496, 243–260. 10.1016/j.neuroscience.2022.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiTullio RW, Piasini E, Chaudhari P, Balasubramanian V, Cohen YE, 2022. Time as a supervisor: temporal regularity and auditory object learning. bioRxiv, 2022.2011.2010.515986. 10.1101/2022.11.10.515986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downer JD, Niwa M, Sutter ML, 2015. Task engagement selectively modulates neural correlations in primary auditory cortex. Journal Neuroscience 35(19), 7565–7574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downer JD, Niwa M, Sutter ML, 2017a. Hierarchical Differences in Population Coding Within Auditory Cortex. J Neurophysiolology, jn.00899.02016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downer JD, Rapone B, Verhein J, O’Connor KN, Sutter ML, 2017b. Feature-Selective Attention Adaptively Shifts Noise Correlations in Primary Auditory Cortex. The Journal of neuroscience : the official journal of the Society for Neuroscience 37(21), 5378–5392. 10.1523/jneurosci.3169-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downer JD, Verhein JR, Rapone BC, O’Connor KN, Sutter ML, 2021. An Emergent Population Code in Primary Auditory Cortex Supports Selective Attention to Spectral and Temporal Sound Features. The Journal of neuroscience : the official journal of the Society for Neuroscience 41(36), 7561–7577. 10.1523/jneurosci.0693-20.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggermont JJ, 2007. Correlated neural activity as the driving force for functional changes in auditory cortex. Hearing research 229(1–2), 69–80. [DOI] [PubMed] [Google Scholar]
- Elhilali M, Ma L, Micheyl C, Oxenham AJ, Shamma SA, 2009. Temporal coherence in the perceptual organization and cortical representation of auditory scenes. Neuron 61(2), 317–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel LR, Frum C, Puce A, Walker NA, Lewis JW, 2009. Different categories of living and non-living sound-sources activate distinct cortical networks. Neuroimage 47, 1778–1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engineer CT, Perez CA, Chen YH, Carraway RS, Reed AC, Shetake JA, Jakkamsetti V, Chang KQ, Kilgard MP, 2008. Cortical activity patterns predict speech discrimination ability. Nature neuroscience 11(5), 603–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escabí MA, Read HL, 2003. Representation of spectrotemporal sound information in the ascending auditory pathway. Biol Cybern 89, 350–362. [DOI] [PubMed] [Google Scholar]
- Falchier A, Schroeder CE, Hackett TA, Lakatos P, Nascimento-Silva S, Ulbert I, Karmos G, Smiley JF, 2009. Projection from Visual Areas V2 and Prostriata to Caudal Auditory Cortex in the Monkey. Cerebral cortex 20(7), 1529–1538. 10.1093/cercor/bhp213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felleman DJ, Van Essen DC, 1991. Distributed hierarchical processing in the primate cerebral cortex. Cerebral cortex (New York, N.Y. : 1991) 1(1), 1–47. [DOI] [PubMed] [Google Scholar]
- Fitzgerald JK, Freedman DJ, Fanini A, Bennur S, Gold JI, Assad JA, 2013. Biased associative representations in parietal cortex. Neuron 77(1), 180–191. 10.1016/j.neuron.2012.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis NA, Winkowski DE, Sheikhattar A, Armengol K, Babadi B, Kanold PO, 2018. Small Networks Encode Decision-Making in Primary Auditory Cortex. Neuron 97(4), 885–897.e886. 10.1016/j.neuron.2018.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman DJ, Assad JA, 2016. Neuronal Mechanisms of Visual Categorization: An Abstract View on Decision Making. Annual review of neuroscience 39, 129–147. 10.1146/annurev-neuro-071714-033919. [DOI] [PubMed] [Google Scholar]
- Fries P, 2009. Neuronal gamma-band synchronization as a fundamental process in cortical computation. Annual review of neuroscience 32, 209–224. 10.1146/annurev.neuro.051508.135603. [DOI] [PubMed] [Google Scholar]
- Fries P, 2015. Rhythms for Cognition: Communication through Coherence. Neuron 88(1), 220–235. 10.1016/j.neuron.2015.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritz JB, David SV, Shamma S, 2013. Attention and Dynamic, Task-Related Receptive Field Plasticity in Adult Auditory Cortex in: Cohen YE, Popper AN, Fay RR (Eds.), Neural Correlates of Auditory Cognition. Springer-Verlag, New York. [Google Scholar]
- Fukushima M, Saunders Richard C., Leopold David A., Mishkin M, Averbeck Bruno B., 2012. Spontaneous High-Gamma Band Activity Reflects Functional Organization of Auditory Cortex in the Awake Macaque. Neuron 74(5), 899–910. https://doi.org/ 10.1016/j.neuron.2012.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukushima M, Saunders RC, Leopold DA, Mishkin M, Averbeck BB, 2014. Differential coding of conspecific vocalizations in the ventral auditory cortical stream. The Journal of neuroscience : the official journal of the Society for Neuroscience 34(13), 4665–4676. 10.1523/jneurosci.3969-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganguli S, Bisley JW, Roitman JD, Shadlen MN, Goldberg ME, Miller KD, 2008. One-dimensional dynamics of attention and decision making in LIP. Neuron 58(1), 15–25. 10.1016/j.neuron.2008.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao P, Trautmann E, Yu B, Santhanam G, Ryu S, Shenoy K, Ganguli S, 2017. A theory of multineuronal dimensionality, dynamics and measurement. bioRxiv, 214262. 10.1101/214262. [DOI] [Google Scholar]
- Gerstein GL, Kiang NY, 1964. RESPONSES OF SINGLE UNITS IN THE AUDITORY CORTEX. Experimental neurology 10, 1–18. 10.1016/0014-4886(64)90083-4. [DOI] [PubMed] [Google Scholar]
- Ghazanfar AA, Maier JX, Hoffman KL, Logothetis NK, 2005. Multisensory Integration of Dynamic Faces and Voices in Rhesus Monkey Auditory Cortex. The Journal of Neuroscience 25(20), 5004–5012. 10.1523/jneurosci.0799-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghazanfar AA, Schroeder CE, 2006. Is neocortex essentially multisensory? Trends in cognitive sciences 10(6), 278–285. https://doi.org/S1364-6613(06)00104-5 [pii] 10.1016/j.tics.2006.04.008. [DOI] [PubMed] [Google Scholar]
- Gifford III GW, Cohen YE, 2005. Spatial and non-spatial auditory processing in the lateral intraparietal area. Experimental brain research 162, 509–512. [DOI] [PubMed] [Google Scholar]
- Gourévitch B, Eggermont JJ, 2010. Maximum decoding abilities of temporal patterns and synchronized firings: application to auditory neurons responding to click trains and amplitude modulated white noise. J Comput Neurosci 29(1–2), 253–277. 10.1007/s10827-009-0149-3. [DOI] [PubMed] [Google Scholar]
- Greschner M, Shlens J, Bakolitsa C, Field GD, Gauthier JL, Jepson LH, Sher A, Litke AM, Chichilnisky EJ, 2011. Correlated firing among major ganglion cell types in primate retina. The Journal of physiology 589(Pt 1), 75–86. 10.1113/jphysiol.2010.193888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths TD, Warren JD, 2004. What is an auditory object? Nature reviews. Neuroscience 5, 887–892. [DOI] [PubMed] [Google Scholar]
- Hackett TA, 2011. Information flow in the auditory cortical network. Hearing research 271, 133–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackett TA, Stepniewska I, Kaas JH, 1998. Subdivisions of auditory cortex and ipsilateral cortical connections of the parabelt auditory cortex in macaque monkeys. The Journal of comparative neurology 394(4), 475–495. [DOI] [PubMed] [Google Scholar]
- Hackett TA, Stepniewska I, Kaas JH, 1999. Prefrontal connections of the parabelt auditory cortex in macaque monkeys. Brain research 817(1–2), 45–58. [DOI] [PubMed] [Google Scholar]
- Hipp JF, Engel AK, Siegel M, 2011. Oscillatory Synchronization in Large-Scale Cortical Networks Predicts Perception. Neuron 69(2), 387–396. https://doi.org/ 10.1016/j.neuron.2010.12.027. [DOI] [PubMed] [Google Scholar]
- Huang Y, Brosch M, 2020. Associations between sounds and actions in primate prefrontal cortex. Brain research 1738, 146775. 10.1016/j.brainres.2020.146775. [DOI] [PubMed] [Google Scholar]
- Huang Y, Heil P, Brosch M, 2019. Associations between sounds and actions in early auditory cortex of nonhuman primates. eLife 8. 10.7554/eLife.43281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Matysiak A, Heil P, König R, Brosch M, 2016. Persistent neural activity in auditory cortex is related to auditory working memory in humans and nonhuman primates. eLife 5. 10.7554/eLife.15441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ince RA, Panzeri S, Kayser C, 2013. Neural codes formed by small and temporally precise populations in auditory cortex. The Journal of neuroscience : the official journal of the Society for Neuroscience 33(46), 18277–18287. 10.1523/jneurosci.2631-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Issa EB, Wang X, 2013. Increased neural correlations in primate auditory cortex during slow-wave sleep. Journal of neurophysiology 109(11), 2732–2738. 10.1152/jn.00695.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasmin K, Lima CF, Scott SK, 2019. Understanding rostral-caudal auditory cortex contributions to auditory perception. Nature reviews. Neuroscience 20(7), 425–434. 10.1038/s41583-019-0160-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasper AI, Tanabe S, Kohn A, 2019. Predicting Perceptual Decisions Using Visual Cortical Population Responses and Choice History. The Journal of neuroscience : the official journal of the Society for Neuroscience 39(34), 6714–6727. 10.1523/jneurosci.0035-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic V, Fishbein AR, de la Mothe L, Lee KF, Miller CT, 2022. Behavioral context affects social signal representations within single primate prefrontal cortex neurons. Neuron 110(8), 1318–1326.e1314. 10.1016/j.neuron.2022.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaas JH, Hackett TA, 1998. Subdivisions of auditory cortex and levels of processing in primates. Audiol Neurootol 3(2–3), 73–85. [DOI] [PubMed] [Google Scholar]
- Kaas JH, Hackett TA, 1999. ‘What’ and ‘where’ processing in auditory cortex. Nature neuroscience 2(12), 1045–1047. [DOI] [PubMed] [Google Scholar]
- Kaas JH, Hackett TA, 2000. Subdivisions of auditory cortex and processing streams in primates. Proceedings of the National Academy of Sciences of the United States of America 97(22), 11793–11799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kafashan M, Jaffe AW, Chettih SN, Nogueira R, Arandia-Romero I, Harvey CD, Moreno-Bote R, Drugowitsch J, 2021. Scaling of sensory information in large neural populations shows signatures of information-limiting correlations. Nature communications 12(1), 473. 10.1038/s41467-020-20722-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karthik G, Plass J, Beltz AM, Liu Z, Grabowecky M, Suzuki S, Stacey WC, Wasade VS, Towle VL, Tao JX, Wu S, Issa NP, Brang D, 2021. Visual speech differentially modulates beta, theta, and high gamma bands in auditory cortex. European Journal of Neuroscience 54(9), 7301–7317. https://doi.org/ 10.1111/ejn.15482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayser C, Petkov CI, Logothetis NK, 2008. Visual Modulation of Neurons in Auditory Cortex. Cerebral cortex 18(7), 1560–1574. 10.1093/cercor/bhm187. [DOI] [PubMed] [Google Scholar]
- Keil J, Müller N, Hartmann T, Weisz N, 2013. Prestimulus Beta Power and Phase Synchrony Influence the Sound-Induced Flash Illusion. Cerebral cortex 24(5), 1278–1288. 10.1093/cercor/bhs409. [DOI] [PubMed] [Google Scholar]
- Kell AJE, Yamins DLK, Shook EN, Norman-Haignere SV, McDermott JH, 2018. A Task-Optimized Neural Network Replicates Human Auditory Behavior, Predicts Brain Responses, and Reveals a Cortical Processing Hierarchy. Neuron 98(3), 630–644.e616. 10.1016/j.neuron.2018.03.044. [DOI] [PubMed] [Google Scholar]
- Khandhadia AP, Murphy AP, Romanski LM, Bizley JK, Leopold DA, 2021. Audiovisual integration in macaque face patch neurons. Current biology : CB 31(9), 1826–1835.e1823. 10.1016/j.cub.2021.01.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi Y, Kumar S, Baumann S, Overath T, Gander PE, Sedley W, Patterson RD, Petkov CI, Griffiths TD, 2019. The distribution and nature of responses to broadband sounds associated with pitch in the macaque auditory cortex. Cortex 120, 340–352. 10.1016/j.cortex.2019.07.005. [DOI] [PubMed] [Google Scholar]
- Kikuchi Y, Sedley W, Griffiths TD, Petkov CI, 2018. Evolutionarily conserved neural signatures involved in sequencing predictions and their relevance for language. Curr Opin Behav Sci 21, 145–153. 10.1016/j.cobeha.2018.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilgard MP, 2012. Harnessing plasticity to understand learning and treat disease. Trends in neurosciences 35(12), 715–722. 10.1016/j.tins.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilian-Hütten N, Valente G, Vroomen J, Formisano E, 2011. Auditory cortex encodes the perceptual interpretation of ambiguous sound. The Journal of neuroscience : the official journal of the Society for Neuroscience 31(5), 1715–1720. 10.1523/jneurosci.4572-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AJ, Teki S, Willmore BDB, 2018. Recent advances in understanding the auditory cortex. F1000Research 7. 10.12688/f1000research.15580.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knyazeva S, Selezneva E, Gorkin A, Ohl FW, Brosch M, 2020. Representation of Auditory Task Components and of Their Relationships in Primate Auditory Cortex. Front Neurosci 14, 306. 10.3389/fnins.2020.00306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohn A, Coen-Cagli R, Kanitscheider I, Pouget A, 2016. Correlations and Neuronal Population Information. Annual review of neuroscience 39, 237–256. 10.1146/annurev-neuro-070815-013851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishna A, Tanabe S, Kohn A, 2021. Decision Signals in the Local Field Potentials of Early and Mid-Level Macaque Visual Cortex. Cerebral cortex (New York, N.Y. : 1991) 31(1), 169–183. 10.1093/cercor/bhaa218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan L, Elhilali M, Shamma S, 2014. Segregating complex sound sources through temporal coherence. PLoS computational biology 10(12), e1003985. 10.1371/journal.pcbi.1003985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroki S, Yoshida T, Tsutsui H, Iwama M, Ando R, Michikawa T, Miyawaki A, Ohshima T, Itohara S, 2018. Excitatory Neuronal Hubs Configure Multisensory Integration of Slow Waves in Association Cortex. Cell Reports 22(11), 2873–2885. https://doi.org/ 10.1016/j.celrep.2018.02.056. [DOI] [PubMed] [Google Scholar]
- Lakatos P, Chen C-M, O’Connell MN, Mills A, Schroeder CE, 2007. Neuronal Oscillations and Multisensory Interaction in Primary Auditory Cortex. Neuron 53(2), 279–292. https://doi.org/ 10.1016/j.neuron.2006.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann C, Herdener M, Esposito F, Hubl D, di Salle F, Scheffler K, Bach DR, Federspiel A, Kretz R, Dierks T, Seifritz E, 2006. Differential patterns of multisensory interactions in core and belt areas of human auditory cortex. NeuroImage 31(1), 294–300. https://doi.org/ 10.1016/j.neuroimage.2005.12.038. [DOI] [PubMed] [Google Scholar]
- Lemus L, Hernandez A, Romo R, 2009a. Neural codes for perceptual discrimination of acoustic flutter in the primate auditory cortex. Proceedings of the National Academy of Sciences of the United States of America 106, 9471–9476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemus L, Hernandez A, Romo R, 2009b. Neural encoding of auditory discrimination in ventral premotor cortex. Proceedings of the National Academy of Sciences of the United States of America 106(34), 14640–14645. 10.1073/pnas.0907505106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis JW, Brefczynski JA, Phinney RE, Janik JJ, DeYoe EA, 2005. Distinct cortical pathways for processing tool versus animal sounds. The Journal of neuroscience : the official journal of the Society for Neuroscience 25, 5148–5159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden JF, Schreiner CE, 2003. Columnar transformations in auditory cortex? A comparison to visual and somatosensory cortices. Cerebral cortex (New York, N.Y. : 1991) 13(1), 83–89. 10.1093/cercor/13.1.83. [DOI] [PubMed] [Google Scholar]
- Lomber SG, Malhotra S, 2008. Double dissociation of ‘what’ and ‘where’ processing in auditory cortex. Nature neuroscience 11(5), 609–616. [DOI] [PubMed] [Google Scholar]
- Lu K, Xu Y, Yin P, Oxenham AJ, Fritz JB, Shamma SA, 2017. Temporal coherence structure rapidly shapes neuronal interactions. Nature communications 8, 13900. 10.1038/ncomms13900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier JX, Chandrasekaran C, Ghazanfar AA, 2008. Integration of Bimodal Looming Signals through Neuronal Coherence in the Temporal Lobe. Current Biology 18(13), 963–968. https://doi.org/ 10.1016/j.cub.2008.05.043. [DOI] [PubMed] [Google Scholar]
- Mégevand P, Mercier MR, Groppe DM, Zion Golumbic E, Mesgarani N, Beauchamp MS, Schroeder CE, Mehta AD, 2020. Crossmodal Phase Reset and Evoked Responses Provide Complementary Mechanisms for the Influence of Visual Speech in Auditory Cortex. The Journal of neuroscience : the official journal of the Society for Neuroscience 40(44), 8530–8542. 10.1523/jneurosci.0555-20.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercier MR, Molholm S, Fiebelkorn IC, Butler JS, Schwartz TH, Foxe JJ, 2015. Neuro-Oscillatory Phase Alignment Drives Speeded Multisensory Response Times: An Electro-Corticographic Investigation. The Journal of Neuroscience 35(22), 8546–8557. 10.1523/jneurosci.4527-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrikhi Y, Kok MA, Lomber SG, Meredith MA, 2023. A comparison of multisensory features of two auditory cortical areas: primary (A1) and higher-order dorsal zone (DZ). Cereb Cortex Commun 4(1), tgac049. 10.1093/texcom/tgac049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller LM, Escabí MA, Read HL, Schreiner CE, 2002. Spectrotemporal receptive fields in the lemniscal auditory thalamus and cortex. Journal of neurophysiology 87, 516–527. [DOI] [PubMed] [Google Scholar]
- Miller LM, Recanzone GH, 2009. Populations of auditory cortical neurons can accurately encode acoustic space across stimulus intensity. Proceedings of the National Academy of Sciences of the United States of America 106, 5931–5535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misselhorn J, Schwab BC, Schneider TR, Engel AK, 2019. Synchronization of Sensory Gamma Oscillations Promotes Multisensory Communication. eNeuro 6(5). 10.1523/eneuro.0101-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohn JL, Downer JD, O’Connor KN, Johnson JS, Sutter ML, 2021. Choice-related activity and neural encoding in primary auditory cortex and lateral belt during feature-selective attention. Journal of neurophysiology 125(5), 1920–1937. 10.1152/jn.00406.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrill RJ, Hasenstaub AR, 2018. Visual Information Present in Infragranular Layers of Mouse Auditory Cortex. The Journal of Neuroscience 38(11), 2854–2862. 10.1523/jneurosci.3102-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napoli JL, Camalier CR, Brown AL, Jacobs J, Mishkin MM, Averbeck BB, 2021. Correlates of Auditory Decision-Making in Prefrontal, Auditory, and Basal Lateral Amygdala Cortical Areas. The Journal of neuroscience : the official journal of the Society for Neuroscience 41(6), 1301–1316. 10.1523/jneurosci.2217-20.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni AM, Ruff DA, Alberts JJ, Symmonds J, Cohen MR, 2018. Learning and attention reveal a general relationship between population activity and behavior. Science (New York, N.Y.) 359(6374), 463–465. 10.1126/science.aao0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nienborg H, Cohen MR, Cumming BG, 2012. Decision-related activity in sensory neurons: correlations among neurons and with behavior. Annual review of neuroscience 35, 463–483. [DOI] [PubMed] [Google Scholar]
- Nienborg H, Cumming BG, 2009. Decision-Related Activity in Sensory Neurons Reflects More Than a Neuron’s Causal Effect. Nature 459, 89–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niwa M, Johnson JS, O’Connor KN, Sutter ML, 2012. Activity related to perceptual judgment and action in primary auditory cortex. Journal Neuroscience 32(9), 3193–3210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niwa M, Johnson JS, O’Connor KN, Sutter ML, 2013. Differences between Primary Auditory Cortex and Auditory Belt Related to Encoding and Choice for AM Sounds. Journal Neuroscience 33(19), 8378–8395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman-Haignere SV, Long LK, Devinsky O, Doyle W, Irobunda I, Merricks EM, Feldstein NA, McKhann GM, Schevon CA, Flinker A, Mesgarani N, 2022. Multiscale temporal integration organizes hierarchical computation in human auditory cortex. Nature Human Behaviour 6(3), 455–469. 10.1038/s41562-021-01261-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Sullivan JA, Shamma SA, Lalor EC, 2015. Evidence for Neural Computations of Temporal Coherence in an Auditory Scene and Their Enhancement during Active Listening. The Journal of neuroscience : the official journal of the Society for Neuroscience 35(18), 7256–7263. 10.1523/jneurosci.4973-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pachitariu M, Lyamzin DR, Sahani M, Lesica NA, 2015. State-dependent population coding in primary auditory cortex. The Journal of neuroscience : the official journal of the Society for Neuroscience 35(5), 2058–2073. 10.1523/jneurosci.3318-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panzeri S, Brunel N, Logothetis NK, Kayser C, 2010. Sensory neural codes using multiplexed temporal scales. Trends in neurosciences 33(3), 111–120. 10.1016/j.tins.2009.12.001. [DOI] [PubMed] [Google Scholar]
- Panzeri S, Moroni M, Safaai H, Harvey CD, 2022. The structures and functions of correlations in neural population codes. Nature reviews. Neuroscience 23(9), 551–567. 10.1038/s41583-022-00606-4. [DOI] [PubMed] [Google Scholar]
- Pizzamiglio L, Aprile T, Spitoni G, Pitzalis S, Bates E, D’Amico S, Di Russo F, 2005. Separate neural systems for processing action- or non-action-related sounds. Neuroimage 24, 852–861. [DOI] [PubMed] [Google Scholar]
- Plakke B, Hwang J, Romanski LM, 2015. Inactivation of Primate Prefrontal Cortex Impairs Auditory and Audiovisual Working Memory. The Journal of neuroscience : the official journal of the Society for Neuroscience 35(26), 9666–9675. 10.1523/jneurosci.1218-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plakke B, Ng CW, Poremba A, 2013. Neural correlates of auditory recognition memory in primate lateral prefrontal cortex. Neuroscience 244, 62–76. [DOI] [PubMed] [Google Scholar]
- Poremba A, Bigelow J, Rossi B, 2013. Processing of communication sounds: contributions of learning, memory, and experience. Hearing research 305, 31–44. 10.1016/j.heares.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raposo D, Sheppard JP, Schrater PR, Churchland AK, 2012. Multisensory decision-making in rats and humans. The Journal of neuroscience : the official journal of the Society for Neuroscience 32(11), 3726–3735. 10.1523/JNEUROSCI.4998-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauschecker JP, 1998. Cortical control of the thalamus: top-down processing and plasticity. Nature neuroscience 1(3), 179–180. 10.1038/625 625 [pii]. [DOI] [PubMed] [Google Scholar]
- Rauschecker JP, 2011. An expanded role for the dorsal auditory pathway in sensorimotor control and integration. Hearing research 271, 16–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauschecker JP, Scott SK, 2009. Maps and streams in the auditory cortex: nonhuman primates illuminate human speech processing. Nature neuroscience 12(6), 718–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauschecker JP, Tian B, 2000. Mechanisms and streams for processing of “what” and “where” in auditory cortex. Proceedings of the National Academy of Sciences of the United States of America 97(22), 11800–11806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauschecker JP, Tian B, Hauser M, 1995. Processing of complex sounds in the macaque nonprimary auditory cortex. Science (New York, N.Y.) 268(5207), 111–114. [DOI] [PubMed] [Google Scholar]
- Recanzone G, 2018. The effects of aging on auditory cortical function. Hearing research 366, 99–105. https://doi.org/ 10.1016/j.heares.2018.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recanzone GH, 2008. Representation of con-specific vocalizations in the core and belt areas of the auditory cortex in the alert macaque monkey. The Journal of neuroscience : the official journal of the Society for Neuroscience 28(49), 13184–13193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recanzone GH, Sutter ML, 2008. The biological basis of audition. Annual Review Psychology 59, 119–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls ET, Rauschecker JP, Deco G, Huang CC, Feng J, 2022. Auditory cortical connectivity in humans. Cerebral cortex (New York, N.Y. : 1991) 10.1093/cercor/bhac496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanski LM, Averbeck BB, 2009. The Primate Cortical Auditory System and Neural Representation of Conspecific Vocalizations. Annual Reviews Neuroscience 32, 315–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanski LM, Goldman-Rakic PS, 2002. An auditory domain in primate prefrontal cortex. Nature neuroscience 5(1), 15–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanski LM, Tian B, Fritz J, Mishkin M, Goldman-Rakic PS, Rauschecker JP, 1999. Dual streams of auditory afferents target multiple domains in the primate prefrontal cortex. Nature neuroscience 2(12), 1131–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanski LM, Tian B, Fritz JB, Mishkin M, Goldman-Rakic PS, Rauschecker JP, 2000. Reply to “What’, ‘where’ and ‘how’ in auditory cortex’. Nature neuroscience 3(10), 966. 10.1038/79892. [DOI] [PubMed] [Google Scholar]
- Rothschild G, Cohen L, Mizrahi A, Nelken I, 2013. Elevated correlations in neuronal ensembles of mouse auditory cortex following parturition. The Journal of neuroscience : the official journal of the Society for Neuroscience 33(31), 12851–12861. 10.1523/jneurosci.4656-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothschild G, Nelken I, Mizrahi A, 2010. Functional organization and population dynamics in the mouse primary auditory cortex. Nature neuroscience 13(3), 353–360. 10.1038/nn.2484. [DOI] [PubMed] [Google Scholar]
- Runyan CA, Piasini E, Panzeri S, Harvey CD, 2017. Distinct timescales of population coding across cortex. Nature 548(7665), 92–96. 10.1038/nature23020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmehl MN, Groh JM, 2021. Visual Signals in the Mammalian Auditory System. Annual review of vision science 7, 201–223. 10.1146/annurev-vision-091517-034003. [DOI] [PubMed] [Google Scholar]
- Schneider F, Balezeau F, Distler C, Kikuchi Y, van Kempen J, Gieselmann A, Petkov CI, Thiele A, Griffiths TD, 2021. Neuronal figure-ground responses in primate primary auditory cortex. Cell reports 35(11), 109242. 10.1016/j.celrep.2021.109242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider F, Dheerendra P, Balezeau F, Ortiz-Rios M, Kikuchi Y, Petkov CI, Thiele A, Griffiths TD, 2018. Auditory figure-ground analysis in rostral belt and parabelt of the macaque monkey. Scientific reports 8(1), 17948. 10.1038/s41598-018-36903-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder CE, Lakatos P, Kajikawa Y, Partan S, Puce A, 2008. Neuronal oscillations and visual amplification of speech. Trends in Cognitive Sciences 12(3), 106–113. https://doi.org/ 10.1016/j.tics.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- See JZ, Atencio CA, Sohal VS, Schreiner CE, 2018. Coordinated neuronal ensembles in primary auditory cortical columns. eLife 7. 10.7554/eLife.35587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- See JZ, Homma NY, Atencio CA, Sohal VS, Schreiner CE, 2021. Information diversity in individual auditory cortical neurons is associated with functionally distinct coordinated neuronal ensembles. Scientific reports 11(1), 4064. 10.1038/s41598-021-83565-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selezneva E, Gorkin A, Budinger E, Brosch M, 2018. Neuronal correlates of auditory streaming in the auditory cortex of behaving monkeys. The European journal of neuroscience 48(10), 3234–3245. 10.1111/ejn.14098. [DOI] [PubMed] [Google Scholar]
- Semedo JD, Gokcen E, Machens CK, Kohn A, Yu BM, 2020. Statistical methods for dissecting interactions between brain areas. Current opinion in neurobiology 65, 59–69. 10.1016/j.conb.2020.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamma S, Elhilali M, Ma L, Micheyl C, Oxenham AJ, Pressnitzer D, Yin P, Xu Y, 2013. Temporal coherence and streaming of complex sounds. Advances in experimental medicine and biology 787, 535–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamma S, Elhilali M, Micheyl C, 2011. Temporal coherence and attention in auditory scene analysis. Trends in neurosciences 34, 114–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shilling-Scrivo K, Mittelstadt J, Kanold PO, 2021. Altered Response Dynamics and Increased Population Correlation to Tonal Stimuli Embedded in Noise in Aging Auditory Cortex. The Journal of Neuroscience 41(46), 9650–9668. 10.1523/jneurosci.0839-21.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shilling-Scrivo K, Mittelstadt J, Kanold PO, 2022. Decreased modulation of population correlations in auditory cortex is associated with decreased auditory detection performance in old mice. The Journal of neuroscience : the official journal of the Society for Neuroscience. 10.1523/jneurosci.0955-22.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh NC, Theunissen FE, 2003. Modulation spectra of natural sounds and ethological theories of auditory processing. The Journal of the Acoustical Society of America 114, 3394–3411. [DOI] [PubMed] [Google Scholar]
- Smiley JF, Hackett TA, Ulbert I, Karmas G, Lakatos P, Javitt DC, Schroeder CE, 2007. Multisensory convergence in auditory cortex, I. Cortical connections of the caudal superior temporal plane in macaque monkeys. Journal of Comparative Neurology 502(6), 894–923. https://doi.org/ 10.1002/cne.21325. [DOI] [PubMed] [Google Scholar]
- Teki S, Barascud N, Picard S, Payne C, Griffiths TD, Chait M, 2016. Neural Correlates of Auditory Figure-Ground Segregation Based on Temporal Coherence. Cerebral cortex (New York, N.Y. : 1991) 10.1093/cercor/bhw173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teki S, Chait M, Kumar S, Shamma S, Griffiths TD, 2013. Segregation of complex acoustic scenes based on temporal coherence. eLife 2, e00699. 10.7554/eLife.00699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teki S, Chait M, Kumar S, von Kriegstein K, Griffiths TD, 2011. Brain bases for auditory stimulus-driven figure-ground segregation. The Journal of neuroscience : the official journal of the Society for Neuroscience 5, 164–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakur CS, Wang RM, Afshar S, Hamilton TJ, Tapson JC, Shamma SA, van Schaik A, 2015. Sound stream segregation: a neuromorphic approach to solve the “cocktail party problem” in real-time. Front Neurosci 9, 309. 10.3389/fnins.2015.00309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Town SM, Poole KC, Wood KC, Bizley JK, 2022. Reversible inactivation of ferret auditory cortex impairs spatial and non-spatial hearing. bioRxiv, 2021.2011.2016.468798. 10.1101/2021.11.16.468798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsunada J, Cohen Y, Gold JI, 2019. Post-decision processing in primate prefrontal cortex influences subsequent choices on an auditory decision-making task. eLife 8, e46770. 10.7554/eLife.46770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsunada J, Cohen YE, 2014. Neural mechanisms of auditory categorization:from across brain areas to within local microcircuits. Front Neuroscience 8(161). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsunada J, Lee JH, Cohen YE, 2011. Representation of speech categories in the primate auditory cortex. Journal of neurophysiology 105, 2634–2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsunada J, Liu AS, Gold JI, Cohen YE, 2016. Causal contribution of primate auditory cortex to auditory perceptual decision-making. Nature neuroscience 19(1), 135–142. 10.1038/nn.4195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungerleider LG, Mishkin M, 1982. Two Cortical Visual Systems, in: Ingle DJ, Goodale MA, Mansfield RJW (Eds.), Analysis of Visual Behavior. MIT Press, Boston. [Google Scholar]
- Walker KM, Bizley JK, King AJ, Schnupp JW, 2011. Multiplexed and robust representations of sound features in auditory cortex. The Journal of neuroscience : the official journal of the Society for Neuroscience 31(41), 14565–14576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P, Göschl F, Friese U, König P, Engel AK, 2019. Long-range functional coupling predicts performance: Oscillatory EEG networks in multisensory processing. NeuroImage 196, 114–125. https://doi.org/ 10.1016/j.neuroimage.2019.04.001. [DOI] [PubMed] [Google Scholar]
- Wang X, Walker KM, 2012. Neural mechanisms for the abstraction and use of pitch information in auditory cortex. The Journal of neuroscience : the official journal of the Society for Neuroscience 32(39), 13339–13342. 10.1523/jneurosci.3814-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren JE, Wise RJ, Warren JD, 2005. Sounds do-able: auditory-motor transformations and the posterior temporal plane. Trends in neurosciences 28(12), 636–643. [DOI] [PubMed] [Google Scholar]
- Werner-Reiss U, Kelly KA, Trause AS, Underhill AM, Groh JM, 2003. Eye position affects activity in primary auditory cortex of primates. Current biology : CB 13(7), 554–562. [DOI] [PubMed] [Google Scholar]
- Wikman P, Rinne T, Petkov CI, 2019. Reward cues readily direct monkeys’ auditory performance resulting in broad auditory cortex modulation and interaction with sites along cholinergic and dopaminergic pathways. Scientific reports 9(1), 3055. 10.1038/s41598-019-38833-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson RS, Polley DB, 2019. Parallel pathways for sound processing and functional connectivity among layer 5 and 6 auditory corticofugal neurons. eLife 8. 10.7554/eLife.42974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkowski DE, Kanold PO, 2013. Laminar transformation of frequency organization in auditory cortex. The Journal of neuroscience : the official journal of the Society for Neuroscience 33(4), 1498–1508. 10.1523/jneurosci.3101-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin P, Fritz JB, Shamma SA, 2014. Rapid spectrotemporal plasticity in primary auditory cortex during behavior. The Journal of neuroscience : the official journal of the Society for Neuroscience 34(12), 4396–4408. 10.1523/jneurosci.2799-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin P, Strait DL, Radtke-Schuller S, Fritz JB, Shamma SA, 2020. Dynamics and Hierarchical Encoding of Non-compact Acoustic Categories in Auditory and Frontal Cortex. Current biology : CB 30(9), 1649–1663.e1645. 10.1016/j.cub.2020.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zatorre RJ, Bouffard M, Ahad P, Belin P, 2002. Where is ‘where’ in the human auditory cortex? Nature neuroscience 5(9), 905–909. [DOI] [PubMed] [Google Scholar]
- Zohary E, Shadlen MN, Newsome WT, 1994. Correlated neuronal discharge rate and its implications for psychophysical performance [published erratum appears in Nature 1994 Sep 22;371(6495):358]. Nature 370(6485), 140–143. [DOI] [PubMed] [Google Scholar]


