Abstract
Background
Efficacy of endocrine therapy in HR+/HER2− metastatic breast cancer could differ depending on the presence of BRCA1/2 germline mutation.
Methods
The ESME metastatic breast cancer platform (NCT03275311) is a French real world database. Multivariable models including a time-varying approach and landmark analyses assessed the association between time-dependent gBRCA status (categorised as gBRCAm, gBRCAwt (wild type), and untested), overall survival (OS), and first-line progression-free survival (PFS1).
Results
A total of 170 patients were gBRCAm carriers, 676 gBRCAwt, and 12,930 were untested at baseline. In the multivariable analysis, gBRCAm carriers overall had a lower OS compared to gBRCAwt (adjusted HR [95% CI] 1.26 [1.03–1.55]). gBRCAm patients treated with front-line endocrine therapy had lower adjusted OS (adjusted HR [95% CI] = 1.54 [1.03–2.32]) and PFS1 (adjusted HR [95% CI] 1.58 [1.17–2.12]) compared to gBRCAwt patients. However, for patients who received frontline chemotherapy, neither OS nor PFS1 differed between gBRCAm carriers and the other groups (HR versus gBRCAwt for OS: 1.12 [0.88–1.41], p = 0.350; PFS1: 1.09 [0.90–1.31], p = 0.379).
Conclusion
In this large cohort of HR+/HER2− MBC patients treated in a pre-CDK4/6 inhibitors era, gBRCAm status was associated with a lower OS and lower PFS following first-line endocrine therapy, but not following first-line chemotherapy.
Subject terms: Breast cancer, Cancer genetics
Introduction
Approximately 20 and 77% of breast cancers occurring in germline BRCA1 and BRCA2 mutation carriers, respectively, are hormone receptor (HR)-positive [1]. HR-positive BC among BRCA1/2 carriers is frequently characterised by a more aggressive phenotype with higher tumour grade and higher proliferation assessed by KI67 level [1, 2]. In addition, higher recurrent score as assessed by Oncotype DX test are noted as compared to BRCAwt patients, with >80% of patient having intermediate of high-risk disease [3]. Conflicting results have been reported on the prognostic impact of BRCA1/2 mutation in HR-positive breast cancer and data in the metastatic setting are very scarce [4, 5].
Specific therapeutic options for these patients have emerged with PARP inhibitors thanks to two pivotal randomised trials demonstrating the benefit of olaparib or talazoparib, compared with chemotherapy in the first- to third-line metastatic setting. The magnitude of the benefit was similar between triple-negative and HR-positive breast cancer subtypes [6, 7]. However, front-line endocrine-based treatment remains the preferred option in the guidelines for treating patients with HR-positive/HER2-negative metastatic breast cancer, regardless of gBRCA status [8]. This treatment include now CDK4/6 inhibitors in combination with endocrine therapy.
The real efficacy of endocrine-based therapy among gBRCA carriers with HR-positive/HER2-negative MBC has not been well documented. Outcomes from combined endocrine and CDK4/6 inhibitor therapy may be less favourable among gBRCA carriers compared with their wild-type counterparts, although conflicting results have been reported [9–12]. Given the lack of evidence regarding outcome and first-line endocrine treatment efficacy in patients with HR-positive/HER2-negative MBC harbouring a gBRCA1/2 mutation, we collected and analysed this data from a large, real-world database (ESME).
Methods
Study design
This non-interventional, retrospective, comparative study was performed to determine the outcome of selected MBC patients from the ESME MBC database (NCT03275311). This database is an ongoing unique national cohort that gathers real-world individual, retrospective data from all consenting consecutive patients, male or female, ≥18 years, having started a first-line anti-cancer treatment for MBC in any of the 18 cancer centres that participate in the ESME Research Programme. We included for the present study patients initiating a treatment from January 01, 2008 to December 31, 2016. Patient-, hospitalisation-, and pharmacy-related data are collected, including patient demographic characteristics, pathology, and outcomes. Treatment protocols are recorded, which include chemotherapy, targeted agents, endocrine therapy (ET), radiotherapy (RT), or other local treatments, as well as supportive therapy. In the present study, we specifically selected MBC patients with HR+/HER2− status and the available information on germline BRCA1/2 status. The data were compiled until the cut-off date (October 15, 2018), death, or date of last contact (if lost to follow-up). The analysis was approved by an independent ethics committee (Comité De Protection Des Personnes Sud-Est II- 2015-79). No formal dedicated informed consent was required; however, all patients had approved the re-use of their electronically recorded data. In compliance with French regulations, the ESME MBC database was authorised by the French data protection authority (Registration ID 1704113 and authorisation N°DE-2013.-117). Moreover, in compliance with the applicable European regulations, a complementary authorisation was obtained on October 14, 2019 regarding the ESME Research Data Warehouse.
Objectives and endpoints
The primary objective of the present study was to compare the OS of gBRCAm HR+/HER2− MBC patients to that of gBRCAwt and untested patients. The secondary objectives were to compare first-line PFS (PFS1) between these groups of HR+/HER2− MBC patients and to compare OS and PFS1 between these groups in patients treated with first-line endocrine-based (in the absence of chemotherapy) or chemotherapy-based (+/− ET) treatment.
OS was the primary endpoint and was defined as the time between the starting date of first-line treatment (baseline) and the date of death from any cause or the date of last contact (censored data). PFS1 was defined as the time between the starting date of first-line treatment and the date of first disease progression or date of death. Patients that were still alive and without progression at the time of the analysis were censored at their last follow-up. A treatment line was defined as a given therapeutic strategy set up until disease progression or death; therefore, it may involve multiple treatments including chemotherapy, targeted agents, or ET. De novo metastatic disease was defined as the presence of metastasis at the time or within six months (180 days) from primary tumour diagnosis.
Tumour subtype assessment and evaluation
HER2 and hormone receptor status were performed locally and derived from the existing results of metastatic tissue sampling if available, or from the last sampling of early disease if not available. Breast cancer was classified as hormone receptor-positive if the estrogen receptor or progesterone receptor expression was ≥10% as determined by immunohistochemistry following the European guidelines. Subtypes (and the use of metastatic versus primary sample data) have been uniformly defined for the ESME database and were described previously [13].
Germline BRCA testing
gBRCA1/2 status was used to define three groups of patients: gBRCAm (presence of a demonstrated germline deleterious alteration of either BRCA1 or BRCA2), BRCAwt (patient was tested and no gBRCA alteration was identified, although the patient could carry another germline alteration), and untested. Only pathogenic BRCA alterations (i.e., variant class 4 or 5) were considered to define gBRCAm. The mutational status was defined, according to different analyses, either at baseline, or at any time during the disease course using a time-varying approach and at different time points using a landmark approach: within the first three months or within the first six months of treatment initiation.
Fist-line systemic treatment
Systemic therapies were classified into the following four groups: ET, chemotherapy, immunotherapy, and targeted therapy (CDK4/6 Inhibitors, mTOR inhibitors, PARP inhibitors). Patients who received ET as maintenance after chemotherapy were included with the chemotherapy group.
Statistical analyses
Quantitative variables are presented as the median and range (minimum–maximum), whereas qualitative variables are summarised by frequency and percentage. Comparisons between groups were assessed using the Kruskal–Wallis test for quantitative variables and the Chi-squared or Fisher exact test for qualitative variables. The Kaplan–Meier method and log-rank test were used to estimate and compare time-to-event endpoints (PFS1 and OS). Hazard ratios and 95% confidence intervals (95% CI) were calculated using the Cox proportional hazards model. Multivariable Cox proportional hazard models were performed to evaluate the association between time-to-event endpoint and BRCA status adjusted on the main known prognostic factors such age at metastatic diagnosis, metastatic free interval (<6 months versus [6–24] months versus >24 months), number of metastatic sites (<3 versus ≥3), presence of visceral metastases (yes versus no).
The establishment of a gBRCA group was done according to gBRCA status, which was defined at baseline (first-line initiation). Associations between gBRCA status and time-to-event endpoints were assessed using a Cox proportional hazards model including gBRCA status as a time-dependent variable to avoid immortal-time biases related to the time between treatment initiation and knowledge of gBRCAm or gBRCAwt status, which may occur after baseline. Thus, gBRCA status can change over time. A patient may be switched from untested status to gBRCAm or gBRCAwt status. In addition, sensitivity analyses were performed by defining gBRCA status at different time points to prevent immortal-time biases: at first-line initiation (baseline) or using a landmark approach at three or six months after first-line initiation. For each of these sensitivity analyses, gBRCA status was defined on the basis of genetic testing, which was performed before the time point, and for patients who died, progressed, or were lost of follow-up before the time point, were excluded. All statistical tests were two-sided, and a p-value <0.05 was considered statistically significant. Statistical analyses were carried out using STATA software version 16 (StataCorp LLC, College Station, TX).
Results
Patients, tumour characteristics, and initial treatments according to gBRCA mutational status
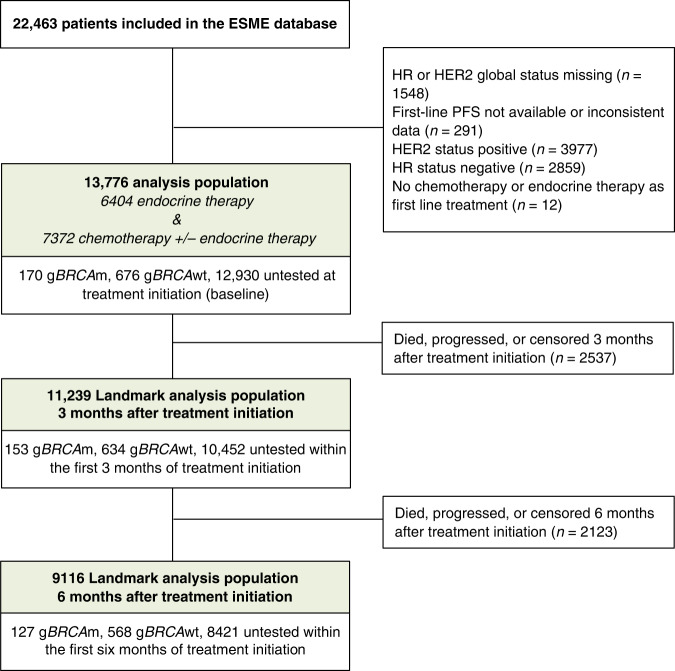
A total of 13,776 patients in the ESME database matched the inclusion criteria. The flow chart is shown in Fig. 1. The gBRCA status at baseline was identified as “mutated” (presence of a deleterious alteration of gBRCAm), negative (gBRCAwt) or untested for 170 (1.2%), 676 (4.9%), and 12,930 (93.9%) patients, respectively. The median time (range) from diagnosis of metastatic disease to BRCA testing (months) (n = 226) was −16.4 (−171.5: 67.7) months. Indeed, 75% (170/226) of BRCA carriers were known to bear a mutation prior to be diagnosed with mBC. Patient characteristics according to baseline gBRCA status are shown in Table 1. Out of 170 baseline gBRCAm patients, 51 (30.0%) harboured a BRCA1 alteration, 117 (68.8%) a BRCA2 alteration, and two (1.2%) had both. Characteristics of patients with BRCA1 versus BRCA2 alterations are shown on Supplementary Table 1. As expected, gBRCAm patients were young, with a median age of 47 years (range 26–82), primary histological tumour grade of 2 or 3 (95.1%), and de novo metastases accounted for 5.9% of the cases. Visceral metastases were more frequent at initial presentation in gBRCAm patients (67.6%) compared with the gBRCAwt population (56.7%). Bone-only metastases were present in 20% of the gBRCAm patients, which was significantly less frequent, compared with the remaining patients. Regarding systemic front-line treatment, gBRCAm patients more frequently received first-line chemotherapy (72.9%) compared with gBRCAwt (60.7%) and non-tested patients (52.9%) (p < 0.0001). Characteristics of patients who received frontline endocrine therapy versus chemotherapy are shown on Supplementary Table 2. Only ten patients from the entire cohort received PARP inhibitors alone or in combination as front-line treatment, including seven with gBRCAm.
Fig. 1.
Flow Chart.
Table 1.
Patient characteristics and treatment according to gBRCA1/2 mutation status defined at baseline (treatment initiation).
| gBRCAm | gBRCAwt | Not Tested | ||
|---|---|---|---|---|
| (N = 170) | (N = 676) | (N = 12,930) | p-value | |
| Age at metastatic disease diagnosis (years) | <0.0001 | |||
| Median | 47.0 | 51.0 | 62.0 | |
| (Range) | (26.0;82.0) | (26.0;88.0) | (22.0;103.0) | |
| Age at metastatic disease diagnosis | <0.0001 | |||
| <50 years | 101 (59.4%) | 314 (46.4%) | 2358 (18.2%) | |
| 50–70 years | 57 (33.5%) | 306 (45.3%) | 6947 (53.7%) | |
| >70 years | 12 (7.1%) | 56 (8.3%) | 3625 (28.0%) | |
| Gender | 0.0163 | |||
| Female | 166 (97.6%) | 663 (98.1%) | 12,801 (99.0%) | |
| Male | 4 (2.4%) | 13 (1.9%) | 129 (1.0%) | |
| Germline BRCA mutation — no. (%) | ||||
| BRCA1 | 51 (30.0%) | NR | NR | |
| BRCA2 | 117 (68.8%) | NR | NR | |
| BRCA1 and BRCA2 | 2 (1.2%) | NR | NR | |
| Histological grade of primary tumour | <0.0001 | |||
| Grade I | 7 (4.9%) | 74 (13.1%) | 1541 (14.4%) | |
| Grade II | 74 (51.4%) | 319 (56.6%) | 6532 (60.9%) | |
| Grade III | 63 (43.8%) | 171 (30.3%) | 2661 (24.8%) | |
| Missing | 26 | 112 | 2196 | |
| Histological type of primary tumour | <0.0001 | |||
| Invasive ductal carcinoma | 134 (79.3%) | 505 (75.3%) | 9146 (72.2%) | |
| Invasive lobular carcinoma | 13 (7.7%) | 76 (11.3%) | 2177 (17.2%) | |
| Other | 22 (13.0%) | 90 (13.4%) | 1351 (10.7%) | |
| Missing | 1 | 5 | 256 | |
| Primary tumour surgery | <0.0001 | |||
| No | 18 (10.7%) | 55 (8.1%) | 3214 (25.3%) | |
| Yes | 151 (89.3%) | 620 (91.9%) | 9484 (74.7%) | |
| Missing | 1 | 1 | 232 | |
| Metastasis-free interval (months) | <0.0001 | |||
| <6 months | 10 (5.9%) | 26 (3.8%) | 3904 (30.3%) | |
| [6–24] months | 21 (12.4%) | 72 (10.7%) | 1002 (7.8%) | |
| >24 months | 139 (81.8%) | 578 (85.5%) | 7991 (62.0%) | |
| Missing | 0 | 0 | 33 | |
| De novo metastatic disease | <0.0001 | |||
| No | 160 (94.1%) | 650 (96.2%) | 8997 (69.8%) | |
| Yes | 10 (5.9%) | 26 (3.8%) | 3900 (30.2%) | |
| Missing | 0 | 0 | 33 | |
| Number of metastatic sites at MBC diagnosis | 0.0034 | |||
| 1 site | 78 (45.9%) | 366 (54.1%) | 7252 (56.1%) | |
| 2 sites | 37 (21.8%) | 165 (24.4%) | 3036 (23.5%) | |
| ≥3 sites | 55 (32.4%) | 145 (21.4%) | 2642 (20.4%) | |
| Type of Metastases at MBC diagnosis | 0.0003 | |||
| Visceral | 115 (67.6%) | 383 (56.7%) | 6910 (53.4%) | |
| Non-visceral | 55 (32.4%) | 293 (43.3%) | 6020 (46.6%) | |
| Bone metastases only | <0.0001 | |||
| No | 136 (80.0%) | 507 (75.0%) | 8898 (68.8%) | |
| Yes | 34 (20.0%) | 169 (25.0%) | 4032 (31.2%) | |
| Adjuvant endocrine therapy | <0.0001 | |||
| No | 40 (23.0%) | 115 (17%) | 5356 (41.5%) | |
| Yes | 130 (76.5%) | 561 (83.0%) | 7543 (58.5%) | |
| Aromatase inhibitor | 56 | 240 | 4343 | |
| Tamoxifen | 100 | 434 | 4006 | |
| LHRH analogues | 11 | 89 | 369 | |
| Others | 1 | 2 | 63 | |
| Missing | 3 | 11 | 628 | |
| Chemotherapy or Endocrine therapy within first treatment line | <0.0001 | |||
| Chemotherapy alone | 55 (32.4%) | 153 (22.6%) | 2463 (19.0%) | |
| Endocrine therapy alone | 46 (27.1%) | 266 (39.3%) | 6092 (47.1%) | |
| Chemotherapy + Endocrine therapy | 69 (40.6%) | 257 (38.0%) | 4375 (33.8%) | |
| HR status derived from a metastasis biopsy by subgroup of treatment | ||||
| Endocrine therapy alone (subgroup n = 6404) | ||||
| Yes | 24 (52.2%) | 125 (47.0%) | 2111 (34.7%) | |
| No | 22 (47.8%) | 141 (53.0%) | 3981 (65.3%) | |
| Chemotherapy +/− Endocrine therapy (subgroup n = 7372) | ||||
| Yes | 27 (21.8%) | 135 (32.9%) | 2010 (29.4%) | |
| No | 97 (78.2%) | 275 (67.1%) | 4828 (70.6%) | |
| CDK4-6 inhibitors within first treatment line | ||||
| No | 170 (100.0%) | 667 (98.7%) | 12,808 (99.1%) | 0.2858 |
| Yes | 0 (0.0%) | 9 (1.3%) | 122 (0.9%) | |
| PARP inhibitor within first treatment line | ||||
| No | 163 (95.9%) | 676 (100.0%) | 12,927 (100.0%) | |
| Yes | 7 (4.1%) | 0 (0.0%) | 3 (0.0%) | |
Statistically significant p-values are in bold.
Outcomes of gBRCAm patients versus others (Table 2 and Fig. 2)
Table 2.
Survival analysis for the overall population and according to first-line therapy (endocrine therapy or chemotherapy).
| Overall population | Patients treated with first line endocrine therapy | Patients treated with 1st line chemotherapy (+/− endocrine therapy) | ||||
|---|---|---|---|---|---|---|
| Adjusted HR [95% CI] | p | Adjusted HR [95% CI] | p | Adjusted HR [95% CI] | p | |
| Overall survival | ||||||
| gBRCAwt | 1 | 1 | 1 | |||
| gBRCAm | 1.26 [1.03–1.55] | 0.024 | 1.54 [1.03–2.32] | 0.037 | 1.12 [0.88–1.41] | 0.350 |
| gBRCA untested | 1.13 [1.03–1.25] | 0.010 | 1.25 [1.05–1.49] | 0.012 | 1.09 [0.98–1.23] | 0.125 |
| 1st line Progression-free survival | ||||||
| gBRCAwt | 1 | 1 | 1 | |||
| gBRCAm | 1.21 [1.03–1.41] | 0.017 | 1.58 [1.17–2.12] | 0.003 | 1.09 [0.90–1.31] | 0.379 |
| gBRCA untested | 1.02 [0.95–1.10] | 0.551 | 1.09 [0.97–1.24] | 0.149 | 0.98 [0.90–1.07] | 0.698 |
Statistically significant p-values are in bold.
The adjusted Hazard Ratios were estimated using a multivariable Cox model including gBRCA status as a time-varying variable.
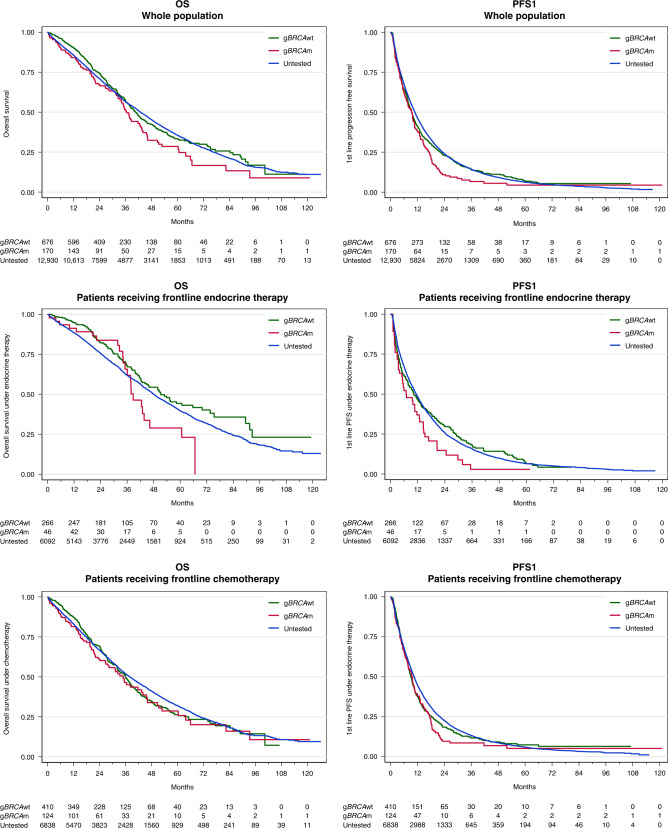
Fig. 2. Overall survival and first-line progression-free survival according to gBRCA groups at treatment initiation and type of first-line treatment.
gBRCAwt, gBRCAm and untested patients are pictures in green, red and blue respectively.
The median follow-up for the entire population was 50.6 months (95% CI: 49.7–51.7) and the median OS was 42.6 months (41.9–43.6). Survival analyses were performed for the three groups (gBRCAm/BRCAwt/untested) with respect to different time points: at treatment initiation (baseline), with landmark analysis at three and six months, or at any time (time-varying analysis). The median OS for the three groups was 36.5 (33.4–42.9), 40.6 (37.8–44.1), and 42.8 (42.0–44.0) months, respectively, considering baseline BRCA status (p = 0.1351; log-rank test) (Fig. 2). For the multivariable analysis using gBRCA status as a time-dependent variable and adjusted for age, visceral metastases, the number of metastatic sites, and metastasis-free interval, gBRCAm patients exhibited a lower OS compared with gBRCAwt patients (adjusted HR [95% CI] = 1.26 [1.03–1.55]). The sensitivity analyses defining gBRCA status at different time points to prevent immortal-time biases revealed a similar trend in terms of OS, which was, however, not statistically significant (Supplementary Table 3).
Outcomes of patients treated with first-line endocrine-based therapy by gBRCA status
A total of 6404 patients (46.5%) received front-line ET alone or in combination with targeted treatment (but no chemotherapy) (46, 266, and 6092 patients in the gBRCAm, gBRCAwt, and untested groups, respectively). These patients were treated between 2008 and 2016, thus only a few received a combination of ET plus targeted therapy (n = 381; 5.9%) including 201 patients with everolimus and 113 patients with a CDK4/6 inhibitor. The characteristics of these patients are shown in Supplementary Table 4. Briefly, gBRCAm patients were younger and had more visceral disease compared with gBRCAwt patients. The univariable analysis indicated that gBRCAm patients had a significantly lower PFS1 (median PFS1 [95% CI]: 6.9 [3.4–12.7], 10.3 [7.8–13.4], and 11.4 [10.8–11.9] months in patients with gBRCAm, gBRCAwt, and untested patients, respectively; log-rank test p = 0.0403) (Fig. 2). In a multivariable analysis using gBRCA status as a time-dependent variable and adjusted for age, visceral metastasis, number of metastatic sites, and metastasis-free interval, PFS1 remained significantly lower for gBRCAm patients who received first-line ET compared with the gBRCAwt group (adjusted HR [95% CI]: 1.58 [1.17–2.12]) (Table 2). Sensitivity analysis using the landmark approach confirmed this trend (Supplementary Table 3). Additional analyses adjusting for endocrine sensitivity or resistance revealed similar results (Supplementary Table 4). OS analyses revealed similar results: median OS was 38.7 (35.1–46.2), 51.3 (42.8–65.7), and 48.1 (46.7–49.5) months in the gBRCAm, BRCAwt, and untested groups at baseline, respectively (p = 0.0391; log-rank test). In a multivariable analysis (Cox model with time-dependent variable adjusted on age, visceral metastases, number of metastatic sites, and metastasis-free interval), gBRCA mutation remained a pejorative prognostic factor for OS (adjusted HR 1.54 [1.03–2.32]) compared with the gBRCAwt group. Sensitivity analyses using the landmark approach confirmed this trend (Supplementary Table 3). Descriptive analyses by type of BRCA alteration (BRCA1 versus BRCA2) showed a median PFS of 5.9 months (95% CI [2.9; 14.5]) and 6.9 months (95% CI [3.4; 12.9]) for BRCA1 and BRCA2, respectively. The median OS was 37.5 months (95% CI [9.9; NR]) and 38.7 months (95% CI [35.1; NR]) for BRCA1 and BRCA2, respectively.
Outcome of patients treated with first-line chemotherapy by gBRCA status
A total of 7372 patients (53.5%) received front-line chemotherapy as part of first-line MBC treatment (+/− ET and +/− targeted therapy) with 124, 410 and 6838 patients in the gBRCAm, gBRCAwt, and untested groups at baseline, respectively. Bevacizumab was associated with chemotherapy in 2194 (29.8%) patients. Maintenance ET was proposed for 69 (55.6%), 257 (62.7%), and 4375 (64%) patients in the gBRCAm, gBRCAwt, and untested groups, respectively. Anthracycline and/or taxane-based chemotherapy accounted for 83.5% of the chemotherapy regimens. The characteristics of the patients are listed in Supplementary Table 6. Median PFS1 and OS were 9.5 (7.6–10.7) and 34.9 (26.7–44.1) months, respectively, in the gBRCAm patients receiving front-line chemotherapy. In a multivariable analysis using gBRCA status as a time-dependent variable and adjusted for age, visceral metastasis, number of metastatic sites, and metastasis-free interval, outcomes were similar for gBRCAm patients who received first-line chemotherapy compared with the gBRCAwt group (OS: adjusted HR [95% CI]: 1.12 [0.88–1.41]) and (PFS1: adjusted HR [95% CI]: 1.09 [0.90–1.31]). (Table 2, Fig. 2, and Supplementary Table 3). Only descriptive analyses were performed for type of BRCA alteration (1 versus 2) given the small numbers of patients. The median PFS was 5.4 months (95% CI [3.8; 7.4]) and 10.7 months (95% CI [9.4; 14.3]) for BRCA1 and BRCA2 altered groups, respectively. The median OS was 21.2 months (95% CI [9.5; 34.9]) and 43.4 months (95% CI [31.4; 51.4]) for BRCA1 and BRCA2 altered groups, respectively.
Discussion
This large unique real-world dataset enables a comprehensive analysis of the prognostic impact of gBRCA status in patients with hormone receptor-positive, HER2-negative MBC. We found that these patients have a median OS of 36.5 months (33.4–42.9), which is less than would have been expected in this setting, despite a pre-CDK4/6 inhibition era [14]. Of note, a recent study using the Flatiron Health electronic health record database identified 165 gBRCAm patients with HR-positive/HER2-negative metastatic disease [15]. Their median OS was 38 months (30.8–42.9), which appears similar to our population and confirms the representation of our data. Our data strongly suggest an independent detrimental effect of gBRCA mutations on both PFS1 and OS in the overall population, as well as among patients who received first-line endocrine treatment. However, this negative effect disappeared in patients who received front-line chemotherapy.
The prognostic impact of gBRCA status has been widely reported in early settings with discordant results with respect to the impact of gBRCAm [4, 16–23]. In the metastatic setting, data are very scarce, though we confirmed a more aggressive tumour phenotype for gBRCAm patients versus gBRCAwt patients with primarily grade 3 tumours, which reflects an over-representation of the luminal B-like HR+ breast cancer phenotype [24, 25]. However, the detrimental OS impact of gBRCA mutations in the present study remained after adjusting for different classical prognostic factors. In contrast, a recent study which included 2595 MBC patients with 129 gBRCAm, did not shown an effect of gBRCA mutation on patient outcome [26]. This small cohort, however, included both HR-positive and HR-negative MBC patients and no subgroup analysis was performed.
The inferior OS observed among gBRCAm patients is remarkable for patients receiving first-line ET, with a hazard ratio of 1.54 [1.03–2.32] compared with gBRCAwt patients in multivariable analyses. In addition, gBRCAm patients had a significantly lower PFS1 with front-line ET compared with gBRCAwt patients, with a median PFS1 of 6.9 months for the front-line ET group. Although this number appears very low, it is concordant with that of a small study showing a median PFS of 7.8 months in the same context [27]. Similar lower PFS in gBRCAm patients are reported with ET combined with CDK4/6 inhibitors which is now a standard front-line treatment for HR+/HER2− MBC [8]. The registration trials for CDK4/6 inhibitors lacked pre-specified subgroup analysis plans for gBRCA1/2 m carriers. However, in a pooled analysis of the MonaLEEsa two, three, and seven trials, ctDNA BRCA1/2 m patients had a median PFS of 7.06 months with ET alone, whereas median PFS was not reached with ribociclib (HR 0.3 95% CI [0.15–0.61]) [9]. In the PADA-1 trial, our group reported that gBRCAm or gPALB2 m patients tended to have a shorter PFS with first-line aromatase inhibitor plus palbociclib compared with that of gBRCAwt patients (14.3 m (CI95% [10.4; NR]) versus 26.7 m (95% CI [24.1; 29.4]); HR 0.58 (CI95%[0.2–1.02]), p = 0.056) [10]. This observation was supported by two recent studies [12, 28]. In a MSKCC patient cohort, gBRCA2 alterations were significantly associated with inferior PFS (HR 2.17, 95% CI 1.46–3.22, p < 0.001) for first-line treatment with CDK4/6 inhibitor plus ET [28]. In this study, the 24 gBRCA2m patients exhibited a median PFS of seven months [4.0–10.0] with front-line CDK4-6 inhibitor. The median PFS was very similar to that observed in our study population.
One potential explanation is that BRCA deficiency and the subsequent defect in homologous repair (HRD) remains a key driver for HR+ tumours arising in gBRCAm patients. PARP inhibitors are effective in the metastatic setting irrespective of HR status, which highlights the importance of HRD, even in the context of HR + MBC [6, 7]. Genomic analysis revealed a specific mutational signature (signature 3) for HR+/HER2− MBC harbouring HRD [29]. Most BRCA1m HR + tumours exhibit a BRCAness copy number profile and LOH, indicating that a loss of functional BRCA1 protein plays a central role in tumorigenesis [30]. Meanwhile, evidence of endocrine resistance in patients with HRD dominant HR+/HER2− is increasing. In a study based on the MSK-IMPACT cohort, patients with HRD HR+/HER2− MBC experienced shorter PFS on ET +/− CDK4/6i (7.6 m [5.3–12.3]; HR = 1.71 [1.2–2.5]; p = 0.006) suggesting that the genomic instability conferred by HRD results in early resistance [31]. Similarly, patients with tumours harbouring HRD-related genomic scars at the initiation of palbociclib had a significantly worse PFS in a recent study and, as expected, BRCA1/2 mutations were significantly associated with the HRD index [32]. The link between HRD and endocrine resistance is still to be investigated. The occurrence rate of ESR1 mutation under endocrine therapy was not more predominant in BRCA1/2 m compared to BRCAwt MBC patients in a sub study of the PADA1 trial but additional data are necessary [10].
These data suggest that HR + BC in gBRCAm carriers are unique at the molecular level and early introduction of a PARP inhibitor may be effective for HR + gBRCAm patients. Combination trials of ET, CDK4/6 inhibitors, and PARP inhibitors in gBRCAm patients are ongoing and may answer this important question. These findings may be relevant to determine the choice of adjuvant treatment in the context of high clinical risk disease in gBRCAm HR + patients. Indeed, there are currently two options that should be discussed in this setting: either one year of olaparib or 2 years of abemaciblib combined with ET.
In addition, we would point out that in gBRCAm patients, higher recurrent score as assessed by Oncotype DX test are noted as compared to BRCAwt patients [3]. This finding may reflect that the biology of gBRCAm HR + BC is biased toward a more aggressive behaviour. Moreover, higher RS is associated with increased sensitivity to chemotherapy. These findings may show that chemotherapy plays a more important role for gBRCAm HR + BC than endocrine treatment, and provide additional arguments to explain our results.
Several limitations of our study should be considered. First, the study was retrospective, even though the data was collected with a clinical trial-like methodology. PFS assessment is a major challenge in RWD studies. In ESME, data are collected in a clinical trial-like fashion, through an independent assessment of patients’ files. This ensures that the strict definition of PFS we use is homogenously applied throughout the dataset. Second, the very poor OS observed in patients who received endocrine treatment first could be linked to biased baseline characteristics leading to this choice, such as a poorer general condition. Propensity score weighting could help clarify this issue but the limited number of patients precludes this approach. Third, the management of patients was done before CDK4/6 and PARP inhibitors became available era and chemotherapy was commonly used as first line in the early years of the cohort. This limits the interpretation of some data given the current importance of these drugs in current treatment protocols. Indeed, the combination of CDK4/6 inhibitors with ET should still be offered to every eligible patients as stated in the guidelines. Finally, a limited portion of the population was tested for gBRCA status, and indications were primarily based on non-theranostic approaches at that time. We could not completely rule out biases linked to the setting of gBRCA testing and its potential association with specific tumour features and behaviours, as well as survivors’ biases. This has been widely described in retrospective studies assessing the prognosis of localised breast cancer patients according to gBRCA status. In the present study, the setting was metastatic disease. Prognosis is assessed according to baseline status, which avoids most of these biases. Furthermore, time-varying analyses include both the onset of new mutant or wild type status. Several sensitivity analyses as well as landmark analyses were implemented to limit these potential biases.
Conclusion
In a large, real-world cohort of HR-positive/HER2-negative MBC patients treated in the pre-CDK4/6 inhibitor era, gBRCAm status was associated with a lower OS mostly driven by a lower progression-free and OS in patients who received first-line ET. However, eligible HR-positive/HER2-negative MBC patients with gBRCAm should receive first-line ET combined with CDK4-6 inhibitors as stated in guideline. Future clinical trials combining ET, CDK4-6 inhibitors, and new PARP inhibitors may significantly improve the management of this disease
Supplementary information
Acknowledgements
We thank the 18 French Comprehensive Cancer Centres for providing the data and each ESME local coordinator for managing the project at the local level. 18 Participating French Comprehensive Cancer Centres (FCCC):; I. Curie, Paris/ Saint-Cloud, G. Roussy, Villejuif, I. Cancérologie de l’Ouest, Angers/Nantes, C. F. Baclesse, Caen, ICM Montpellier, C. L. Bérard, Lyon, C. G-F Leclerc, Dijon, C. H. Becquerel, Rouen; I. C. Regaud, Toulouse; C. A. Lacassagne, Nice; Institut de Cancérologie de Lorraine, Nancy; C. E. Marquis, Rennes; I. Paoli-Calmettes, Marseille; C. J. Perrin, Clermont Ferrand; I. Bergonié, Bordeaux; C. P. Strauss, Strasbourg; I. J. Godinot, Reims; C. O. Lambret, Lille. Moreover, we thank the ESME Scientific Group, the Strategic Committee, and the ESME coordination team for their ongoing support. Prior presentations This work has been presented at the ESMO meeting as a poster in 2021.
Author contributions
Conceptualisation JSF, AL, SD, AMa, WJ, TDLM. Formal analysis JSF, AL, SD, AMa, WJ, TDLM. Project administration MC. Investigation JSF, AL, SD, JMF, TB, ID, CL, JCE, AG, AP, MAM, JCT, TP, LC, LU, MD, MC, WJ, TDLM. Writing original draught JSF, AL, SD, AMa, WJ, TDLM. Writing review and editing JSF, AL, SD, JMF, TB, ID, CL, JCE, AG, AP, MAM, JCT, TP, LC, LU, MD, MC, WJ, TDLM
Funding
The ESME MBC database receives financial support from an industrial consortium (Roche, Pfizer, AstraZeneca, MSD, Eisai, and Daiichi Sankyo). Data collection, analysis, and publication are managed entirely by UNICANCER independently of the industrial consortium.
Data availability
All data are contained in the ESME database, which is managed by Unicancer (http://www.unicancer.fr/). However, the ESME database is not publicly available for the following reason: in the ESME Research programme, public data sharing is not automatic in order to ensure that only trained users can analyse the ESME datasets. The analysis datasets will be made available only under data transfer and use agreements executed between Unicancer and the potential licensee. Interested parties should contact the corresponding author. Amélie Lusque had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Data supporting the findings of this study are available from UNICANCER but restrictions apply to the availability of these data, which was used under licence for this study and is therefore not publicly available. However, the data are available from the authors upon reasonable request and with permission from UNICANCER.
Code availability
Codes used to generate the data are available upon reasonable request.
Competing interests
JSF reports personal fees from Roche Genentech, personal fees and non-financial support from SeattleGenetics, personal fees and non-financial support from Novartis, personal fees and non-financial support from Pfizer, personal fees and non-financial support from Lilly, personal fees and non-financial support from Novartis, personal fees and non-financial support from GSK, personal fees and non-financial support from Clovis oncoloy, personal fees and non-financial support from Astra Zeneca, personal fees and non-financial support from Daiichi Sankyo, personal fees and non-financial support from Gilead, personal fees and non-financial support from MSD, personal fees and non-financial support from Pierre Fabre, personal fees and non-financial support from Amgen, outside the submitted work. AL has declared no conflict of interest. SD reports grants and non-financial support from Pfizer, grants from Novartis, grants and non-financial support from Astra Zeneca, grants and non-financial support from Roche Genentech, grants from Lilly, grants from Puma, grants from Myriad, grants from Orion, grants from Amgen, grants from Sanofi, grants from Genomic Health, grants from GE, grants from Servier, grants from MSD, grants from BMS, grants from Pierre Fabre, grants from Seagen, grants from Exact Sciences, grants from Rappta, grants from Besins, grants from European Commission, grants from French government grants, grants from Fondation ARC, outside the submitted work. JMF reports personal fees from Pfizer, Novartis, Pierre Fabre, Lilly. TB reports personal fees and non-financial support from Roche, grants, personal fees and non-financial support from Astra Zeneca, grants, personal fees and non-financial support from Pfizer, grants and personal fees from SeaGen, grants, personal fees and non-financial support from Novartis, outside the submitted work; ID has declared no conflict of interest. CL has received reports personal fees from Daiichi, Astra Zeneca, Lilly, MSD. JCE has declared no conflict of interest. AG has declared no conflict of interest. AP reports from Lilly, other from Daiichi-Sankyo, other from Pfizer, other from Pierre Fabre, outside the submitted work; MAM has declared no conflict of interest. JCT has received reports personal fees from PFIZER, personal fees from MSD, personal fees from ASTRAZENECA, non-financial support from NOVARTIS, outside the submitted work. TP eports personal fees and non-financial support from Pfizer, personal fees from Lilly, personal fees and non-financial support from Novartis, personal fees and non-financial support from Astra-Zeneca, personal fees from Seagen, personal fees and non-financial support from Daiichi Sankyo, personal fees from Pierre Fabre, outside the submitted work. LC has declared no conflict of interest. LU has declared no conflict of interest. MD has declared no conflict of interest. MC has declared no conflict of interest. AM has declared no conflict of interest. WJ reports personal fees and non-financial support from Novartis, grants, personal fees and non-financial support from Astra Zeneca, personal fees and non-financial support from Roche Genentech, personal fees and non-financial support from Lilly, non-financial support from Sanofi, personal fees from Daiichi Sankyo, personal fees from MSD, personal fees from Rain Pharmaceuticals, non-financial support from Pierre Fabre, personal fees from Seagen, personal fees from BMS, personal fees and non-financial support from Eisai, personal fees and non-financial support from Pfizer, non-financial support from Glaxo Smithkline, non-financial support from Chugai Pharma, outside the submitted work. TDLM reports grants from Novartis, grants, personal fees and non-financial support from Astra Zeneca, personal fees and non-financial support from Roche Genentech, grants and personal fees from MSD, grants from Seagen, personal fees from Eisai, grants, personal fees and non-financial support from Pfizer, personal fees and non-financial support from Glaxo Smithkline, personal fees and non-financial support from CLOVIS ONCOLOGY, outside the submitted work.
Ethics approval and consent to participate
ESME MBC database was authorised by the French data protection authority (Registration ID 1704113 and authorisation N°DE-2013.-117).
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41416-023-02248-4.
References
- 1.Mavaddat N, Barrowdale D, Andrulis IL, Domchek SM, Eccles D, Nevanlinna H, et al. Pathology of breast and ovarian cancers among BRCA1 and BRCA2 mutation carriers: results from the consortium of investigators of modifiers of BRCA1/2 (CIMBA) Cancer Epidemiol Biomark Prev. 2012;21:134–47. doi: 10.1158/1055-9965.EPI-11-0775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aleskandarany M, Caracappa D, Nolan CC, Macmillan RD, Ellis IO, Rakha EA, et al. DNA damage response markers are differentially expressed in BRCA-mutated breast cancers. Breast Cancer Res Treat. 2015;150:81–90. doi: 10.1007/s10549-015-3306-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Halpern N, Sonnenblick A, Uziely B, Divinsky L, Goldberg Y, Hamburger T, et al. Oncotype Dx recurrence score among BRCA1/2 germline mutation carriers with hormone receptors positive breast cancer. Int J Cancer. 2017;140:2145–9. doi: 10.1002/ijc.30616. [DOI] [PubMed] [Google Scholar]
- 4.De Talhouet S, Peron J, Vuilleumier A, Friedlaender A, Viassolo V, Ayme A, et al. Clinical outcome of breast cancer in carriers of BRCA1 and BRCA2 mutations according to molecular subtypes. Sci Rep. 2020;10:7073. doi: 10.1038/s41598-020-63759-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Metcalfe K, Lynch HT, Foulkes WD, Tung N, Olopade OI, Eisen A, et al. Oestrogen receptor status and survival in women with BRCA2-associated breast cancer. Br J Cancer. 2019;120:398–403. doi: 10.1038/s41416-019-0376-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Litton JK, Rugo HS, Ettl J, Hurvitz SA, Goncalves A, Lee KH, et al. Talazoparib in patients with advanced breast cancer and a germline BRCA mutation. N. Engl J Med. 2018;379:753–63.. doi: 10.1056/NEJMoa1802905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Robson M, Im SA, Senkus E, Xu B, Domchek SM, Masuda N, et al. Olaparib for metastatic breast cancer in patients with a germline BRCA mutation. N. Engl J Med. 2017;377:523–33. [DOI] [PubMed]
- 8.Cardoso F, Paluch-Shimon S, Senkus E, Curigliano G, Aapro MS, Andre F, et al. 5th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 5). Ann Oncol: Off J Eur Soc Med Oncol / ESMO. 2020;31:1623–49. doi: 10.1016/j.annonc.2020.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Andre F, Su F, Solovieff N, Arteaga CL, Hortobagyi GN, Chia SKL, et al. Pooled ctDNA analysis of the MONALEESA (ML) phase III advanced breast cancer (ABC) trials. J Clin Oncol. 2020;38:1009. doi: 10.1200/JCO.2020.38.15_suppl.1009. [DOI] [PubMed] [Google Scholar]
- 10.Frenel JS, Dalenc F, Pistilli B, de La Motte Rouge T, Levy C, Mouret-Reynier MA, et al. 304P ESR1 mutations and outcomes in BRCA1/2 or PALB2 germline mutation carriers receiving first line aromatase inhibitor + palbociclib (AI+P) for metastatic breast cancer (MBC) in the PADA-1 trial. Ann Oncol. 2020;31:S364. doi: 10.1016/j.annonc.2020.08.406. [DOI] [Google Scholar]
- 11.Collins JM, Nordstrom BL, McLaurin KK, Dalvi TB, McCutcheon SC, Bennett JC, et al. A real-world evidence study of CDK4/6 inhibitor treatment patterns and outcomes in metastatic breast cancer by germline BRCA mutation status. Oncol Ther. 2021;9:575–89.. doi: 10.1007/s40487-021-00162-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bruno L, Ostinelli A, Waisberg F, Enrico D, Ponce C, Rivero S, et al. Cyclin-dependent kinase 4/6 inhibitor outcomes in patients with advanced breast cancer carrying germline pathogenic variants in DNA repair-related genes. JCO Precis Oncol. 2022;6:e2100140. doi: 10.1200/PO.21.00140. [DOI] [PubMed] [Google Scholar]
- 13.Gobbini E, Ezzalfani M, Dieras V, Bachelot T, Brain E, Debled M, et al. Time trends of overall survival among metastatic breast cancer patients in the real-life ESME cohort. Eur J Cancer. 2018;96:17–24. doi: 10.1016/j.ejca.2018.03.015. [DOI] [PubMed] [Google Scholar]
- 14.Deluche E, Antoine A, Bachelot T, Lardy-Cleaud A, Dieras V, Brain E, et al. Contemporary outcomes of metastatic breast cancer among 22,000 women from the multicentre ESME cohort 2008–2016. Eur J cancer. 2020;129:60–70. doi: 10.1016/j.ejca.2020.01.016. [DOI] [PubMed] [Google Scholar]
- 15.Quek RGW, Mardekian J. Clinical outcomes, treatment patterns, and health resource utilization among metastatic breast cancer patients with germline BRCA1/2 mutation: a real-world retrospective study. Adv Ther. 2019;36:708–20.. doi: 10.1007/s12325-018-0867-x. [DOI] [PubMed] [Google Scholar]
- 16.Baretta Z, Mocellin S, Goldin E, Olopade OI, Huo D. Effect of BRCA germline mutations on breast cancer prognosis: A systematic review and meta-analysis. Med (Baltim) 2016;95:e4975. doi: 10.1097/MD.0000000000004975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rennert G, Bisland-Naggan S, Barnett-Griness O, Bar-Joseph N, Zhang S, Rennert HS, et al. Clinical outcomes of breast cancer in carriers of BRCA1 and BRCA2 mutations. N Engl J Med. 2007;357:115–23. doi: 10.1056/NEJMoa070608. [DOI] [PubMed] [Google Scholar]
- 18.Verhoog LC, Brekelmans CT, Seynaeve C, van den Bosch LM, Dahmen G, van Geel AN, et al. Survival and tumour characteristics of breast-cancer patients with germline mutations of BRCA1. Lancet. 1998;351:316–21. doi: 10.1016/S0140-6736(97)07065-7. [DOI] [PubMed] [Google Scholar]
- 19.Huzarski T, Byrski T, Gronwald J, Gorski B, Domagala P, Cybulski C, et al. Ten-year survival in patients with BRCA1-negative and BRCA1-positive breast cancer. J Clin Oncol: Off J Am Soc Clin Oncol. 2013;31:3191–6. doi: 10.1200/JCO.2012.45.3571. [DOI] [PubMed] [Google Scholar]
- 20.Bordeleau L, Panchal S, Goodwin P. Prognosis of BRCA-associated breast cancer: a summary of evidence. Breast Cancer Res Treat. 2010;119:13–24. doi: 10.1007/s10549-009-0566-z. [DOI] [PubMed] [Google Scholar]
- 21.van den Broek AJ, Schmidt MK, van ‘t Veer LJ, Tollenaar RA, van Leeuwen FE. Worse breast cancer prognosis of BRCA1/BRCA2 mutation carriers: what’s the evidence? A systematic review with meta-analysis. PloS One. 2015;10:e0120189. doi: 10.1371/journal.pone.0120189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schmidt MK, van den Broek AJ, Tollenaar RA, Smit VT, Westenend PJ, Brinkhuis M, et al. Breast cancer survival of BRCA1/BRCA2 mutation carriers in a hospital-based cohort of young women. J Natl Cancer Inst. 2017;109. 10.1093/jnci/djw329. [DOI] [PubMed]
- 23.Copson ER, Maishman TC, Tapper WJ, Cutress RI, Greville-Heygate S, Altman DG, et al. Germline BRCA mutation and outcome in young-onset breast cancer (POSH): a prospective cohort study. Lancet Oncol. 2018;19:169–80.. doi: 10.1016/S1470-2045(17)30891-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Atchley DP, Albarracin CT, Lopez A, Valero V, Amos CI, Gonzalez-Angulo AM, et al. Clinical and pathologic characteristics of patients with BRCA-positive and BRCA-negative breast cancer. J Clin Oncol: Off J Am Soc Clin Oncol. 2008;26:4282–8. doi: 10.1200/JCO.2008.16.6231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goodwin PJ, Phillips KA, West DW, Ennis M, Hopper JL, John EM, et al. Breast cancer prognosis in BRCA1 and BRCA2 mutation carriers: an international prospective breast cancer family registry population-based cohort study. J Clin Oncol: Off J Am Soc Clin Oncol. 2012;30:19–26. doi: 10.1200/JCO.2010.33.0068. [DOI] [PubMed] [Google Scholar]
- 26.Fasching PA, Yadav S, Hu C, Wunderle M, Haberle L, Hart SN, et al. Mutations in BRCA1/2 and other panel genes in patients with metastatic breast cancer -association with patient and disease characteristics and effect on prognosis. J Clin Oncol: Off J Am Soc Clin Oncol. 2021;39:1619–30.. doi: 10.1200/JCO.20.01200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Niyazov A, Quek RGW, Lewis K, Kemp J, Rider A. 161P - BRCA status, treatment patterns and outcomes in HER2- advanced breast cancer (ABC): A multi-country real-world study. Ann Oncol. 2019;30:iii51–iii2. doi: 10.1093/annonc/mdz100.012. [DOI] [Google Scholar]
- 28.Safonov A, Bandlamudi C, Tallon de Lara P, Ferraro E, Derakhshan F, Will M et al. Comprehensive genomic profiling of patients with breast cancer identifies germline-somatic interactions mediating therapy resistance. Presented at SABCS 2021; December 7–10, 2021; San Antonio, TX. Abstract GS4-08.
- 29.Bertucci F, Ng CKY, Patsouris A, Droin N, Piscuoglio S, Carbuccia N, et al. Genomic characterization of metastatic breast cancers. Nature. 2019;569:560–4. doi: 10.1038/s41586-019-1056-z. [DOI] [PubMed] [Google Scholar]
- 30.Lips EH, Debipersad RD, Scheerman CE, Mulder L, Sonke GS, van der Kolk LE, et al. BRCA1-mutated estrogen receptor-positive breast cancer shows BRCAness, suggesting sensitivity to drugs targeting homologous recombination deficiency. Clin Cancer Res: Off J Am Assoc Cancer Res. 2017;23:1236–41.. doi: 10.1158/1078-0432.CCR-16-0198. [DOI] [PubMed] [Google Scholar]
- 31.Marra A, Gazzo A, Gupta A, Selenica P, Da Silva EM, Pareja F, et al. 210O Mutational signature analysis reveals patterns of genomic instability linked to resistance to endocrine therapy (ET) +/− CDK 4/6 inhibition (CDK4/6i) in estrogen receptor-positive/HER2-negative (ER+/HER2-) metastatic breast cancer (MBC) Ann Oncol. 2022;33:S632. doi: 10.1016/j.annonc.2022.07.249. [DOI] [Google Scholar]
- 32.Park YH, Im S-A, Park K, Wen J, Min A, Bonato V, et al. Prospective longitudinal multi-omics study of palbociclib resistance in hormone receptor+/HER2- metastatic breast cancer. J Clin Oncol. 2021;39:1013. doi: 10.1200/JCO.2021.39.15_suppl.1013. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are contained in the ESME database, which is managed by Unicancer (http://www.unicancer.fr/). However, the ESME database is not publicly available for the following reason: in the ESME Research programme, public data sharing is not automatic in order to ensure that only trained users can analyse the ESME datasets. The analysis datasets will be made available only under data transfer and use agreements executed between Unicancer and the potential licensee. Interested parties should contact the corresponding author. Amélie Lusque had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Data supporting the findings of this study are available from UNICANCER but restrictions apply to the availability of these data, which was used under licence for this study and is therefore not publicly available. However, the data are available from the authors upon reasonable request and with permission from UNICANCER.
Codes used to generate the data are available upon reasonable request.