Abstract
Background
Tumour-infiltrating lymphocytes (TILs) represent a robust biological prognostic biomarker in triple-negative breast cancer (TNBC); however, the contribution of different subsets of immune cells is unclear. We investigated the prognostic value of immune markers, including stromal TILs (sTILs), CD8+T and FOPX3+T cells, PD-1 and PD-L1 in non-metastatic TNBC.
Methods
In total, 259 patients with Stage I–III TNBC were reviewed. The density of sTILs along with the presence of total (t), stromal (s), and intratumoral (i) CD8+T cells and FOPX3+T cells were evaluated by haematoxylin and eosin and immunohistochemical staining. Immunohistochemical staining of PD-1, PD-L1 was also conducted.
Results
All immune markers were positively correlated with each other (P < 0.05). In the multivariate analysis, sTILs (P = 0.046), tCD8+T cells (P = 0.024), iCD8+T cells (P = 0.050) and PD-1 (P = 0.039) were identified as independent prognostic factors for disease-free survival (DFS). Further analysis showed that tCD8+T cells (P = 0.026), iCD8+T cells (P = 0.017) and PD-1 (P = 0.037) increased the prognostic value for DFS beyond that of the classic clinicopathological factors and sTILs.
Conclusions
In addition to sTILs, inclusion of tCD8+T, iCD8+T cells, or PD-1 may further refine the prognostic model for non-metastatic TNBC beyond that including classical factors alone.
Subject terms: Prognostic markers, Breast cancer
Background
Tumour-infiltrating lymphocytes (TILs) are an important indicator of the function of the tumour microenvironment and have been reported to be associated with the prognosis of patients with malignant tumours, including breast cancer [1]. Previous studies have shown that triple-negative breast cancer (TNBC) has a higher density of TILs than hormone receptor positive (HR+) tumours [2, 3]. Furthermore, the total density of TILs is associated with pathologic response to neoadjuvant therapy as well as disease-free survival (DFS) after adjuvant chemotherapy in patients with TNBC [2–8]. However, accumulating evidence indicates that increased TILs density is an adverse prognostic factor for survival in HR+ breast cancer [3, 9, 10]. It has been speculated that the differences between HR+ breast cancer and TNBC may be due to the contribution of different subsets of immune cells since TILs provide only imprecise information about immune activation [3, 11]. As TILs counting is not currently used in clinical practice, these issues need to be further explored.
In breast cancer, TILs are composed mainly of T cells including cytotoxic T lymphocytes (CD8+T) and forkhead box P3 (FOXP3) regulatory T lymphocytes (FOXP3+T). Tumour-infiltrating CD8+T cells play important roles in antitumor immunity, while tumour-infiltrating FOXP3+T cells play a crucial role in immune escape, indicating that the presence of different subsets of immune cells infiltrating the breast cancer microenvironment may have different prognostic implications [12]. Moreover, when programmed cell death-1 (PD-1), which is mainly expressed on the surface of the T cells, binds with its ligand programmed cell death-ligand-1 (PD-L1), T-cell apoptosis is induced and T-cell differentiation toward the FOXP3+T lineage is promoted [13]. Recent studies have demonstrated the benefit of immune checkpoint blockade treatments targeting PD-1/PD-L1 axis in patients with TNBC. These studies have demonstrated the predictive value of PD-L1 in patients with unresectable advanced or metastatic TNBC as first-line treatment [14, 15], but not in patients with early-stage TNBC as neoadjuvant treatment [16, 17]. Indeed, the correlation and influence of these markers in TNBC are not fully understood. Therefore, we conducted a retrospective analysis to investigate the prognostic value of different subsets of immune cells (CD8+T and FOXP3+T), and the expression of PD-1 and PD-L1 beyond established clinicopathological prognostic factors and sTILs in patients with non-metastatic TNBC.
Methods
Patients and study design
A total of 259 women with Stage I–III TNBC treated between August 2010 and December 2013 at Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College (Beijing, China), were retrospectively reviewed. All patients met the following inclusion criteria: (1) underwent modified radical mastectomy (MRM) or breast-conserving surgery (BCS) with sentinel lymph node biopsy or axillary lymph node dissection; (2) did not receive neoadjuvant systemic therapy; (3) received adjuvant chemotherapy and (4) received radiotherapy after BCS. All treatment decisions were made by physicians based on guidelines and consensus from the European Society for Medical Oncology or other Societies at the time. The study was approved by the Institutional Review Board of Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College (approval number 15-057/984). The requirement for informed consent was waived. The reporting recommendations for tumour marker prognostic studies (REMARK) were followed in the preparation of this article [18].
Clinicopathological data and clinical outcomes were retrieved from medical records. Locoregional recurrence (LRR) was defined as a recurrence in the ipsilateral breast/chest wall or in the axillary, internal mammary, or supra-/infraclavicular nodes. Distant metastasis (DM) was defined as the first recurrence in any distant site. DFS was defined as the time from the date of the definitive surgery to death or the first breast cancer recurrence. Overall survival (OS) was defined as the time from the date of the definitive surgery until death from any cause. The sample size was determined by the availability of tumour samples and cost.
Tissue samples
Breast cancer tissues were obtained from the Department of Pathology of Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, originally collected from surgical specimens before chemotherapy or radiotherapy, preserved in formalin-fixed, paraffin-embedded (FFPE) blocks, and stored at room temperature prior to processing. All tissue samples were evaluated by histopathology prior to further processing.
Stromal TIL assessment
Whole sections of hematoxylin and eosin (H&E) stained slides were used to evaluate stromal TILs (sTILs) in strict accordance with the criteria proposed by the International TIL Working Group [1]. Briefly, the percentage of all mononuclear cells (including lymphocytes and plasma cells) in the stromal compartment within the border of the invasive tumour was evaluated visually.
Immunohistochemistry
Tissue sections (4-µm thickness) were prepared using FFPE samples, then deparaffinized and rehydrated in graded ethanol. The immunohistochemistry (IHC) analysis was performed by staining the sections with primary antibodies against CD8 (EP334; dilution 1:100; ZSGB-BIO, Beijing, China), FOXP3 (236A/E7; dilution 1:100; Abcam, Burlingame, CA, USA), PD-1 (UMAB199; dilution 1:100; ZSGB-BIO, Beijing, China) or PD-L1 (SP142; dilution 1:100; Ventana Medical System Inc., Tucson, AZ, USA) on a Ventana BenchMark ULTRA autostainer. The secondary antibody was used in the OptiView amplification kit (Ventana Medical Systems, Inc.) with the OptiView DAB IHC detection kit (Ventana Medical Systems, Inc.) according to the manufacturer’s instructions. In addition, IHC for PD-L1 was repeated using PD-L1 (22C3; dilution 1:100; Dako, Glostrup, Denmark) with the PD-L1 IHC 22C3 pharmDx kit (Dako, Glostrup, Denmark) on the Dako Auto Stainer Link 48, according to the manufacturer’s instructions.
CD8+T and FOXP3+T cell assessments
All cells that stained positive for CD8 and FOXP3 in the stromal compartment and tumour nests were evaluated. CD8+T cells were reported as a percentage of the total cells and classified as stromal CD8+T (sCD8+T, the percentage of the area of CD8+T infiltration within the tumour stroma if the cells were within the stromal compartment but not in direct contact with tumour cells), intratumoral CD8+T (iCD8+T, the percentage of the area of CD8+T infiltration within the tumour area if the cells were in direct contact with tumour cells), and total CD8+ T (tCD8+T, the total percentage of sCD8+T and iCD8+T lymphocytes) [19]. Using this approach, 5% increments were recorded in the areas corresponding to sCD8+T, iCD8+T, and tCD8+T cells on matched H&E stained sections [19]. FOXP3+T cells were also classified as stromal FOXP3+T (sFOXP3+T), intratumoral FOXP3+T (iFOXP3+T) and total FOXP3+T (tFOXP3+T) cells according to similar criteria. Due to the low prevalence of FOXP3+T cells in the preliminary experiments, the number of sFOXP3+T, iFOXP3+T, and tFOXP3+T cells in each section was counted manually in five high-power fields (HPF; magnification, ×400) and reported as the mean value of these five HPFs [20].
PD-1 and PD-L1 assessments
PD-1 and PD-L1 positivity was scored according to methods that were previously reported in the literature [14, 15, 21], and confirmed by a pathologist as tumour-infiltrating immune cells (ICs) by H&E staining. Immunostaining of PD-1 was scored as high level if ≥1% of the ICs displayed PD-1 staining [21]. PD-L1 (SP142) expression by ICs was quantified as per the IMpassion130 study [14]. PD-L1 (22C3) expression based on combined positive score (CPS) was quantified as per the KEYNOTE-355 study [15]. The presence of discernible PD-L1 (SP142) staining of any intensity covering ≥1% of the tumour area was assigned a high score [14], and high PD-L1 (22C3) level was defined by CPS ≥ 10 [15].
Statistical analyses
Statistical analysis was performed using SPSS for Windows, version 23.0 (IBM Corp., Armonk, NY, USA) and R 4.1.2 (https://www.r-project.org/). The general characteristics of the subjects were expressed as frequencies and percentages. We stratified sTILs, CD8+T and FOXP3+T cells into high and low-expression groups according to the calculated cut-off values and predicted DFS by using maximally selected rank statistics from the MaxStat package in R [22]. Statistical associations between clinicopathological characteristics and TILs were assessed using the Fisher exact or χ2 test. Correlations were determined using Spearman’s correlation test. Survival rates were calculated using the Kaplan–Meier method and compared by log-rank test. The association of survival outcomes with potential prognostic factors was evaluated by univariate Cox regression analysis. Multivariate Cox regression was performed to evaluate the effect of sTILs density on DFS, after adjusting for clinicopathological parameters including age, pathological stage, lymphovascular invasion (LVI) and histological grade. The likelihood ratio test was used to compare the different prognostic models. A P value of <0.05 was considered to indicate statistical significance.
Results
Patient characteristics
The distribution of clinicopathological characteristics and immune markers is shown in Table 1. The median age was 49 years (range, 28–75 years). All cases received adjuvant chemotherapy with a median of six cycles (range, 1–8). In most cases (98.8%), the patients received 4–8 cycles of adjuvant chemotherapy. In three cases (1.2%), only up to two cycles of adjuvant chemotherapy were administered because the patients refused to undergo further treatment. A total of 97 of 259 cases (37.1%) received adjuvant radiotherapy (46 after BCS and 51 after MRM). Among 213 cases who underwent MRM, 0 of 59 (0%) with Stage I disease, 25 of 122 (20.5%) with Stage II and 26/32 (81.3%) with Stage III received adjuvant radiotherapy.
Table 1.
Baseline characteristics of the entire patient cohort.
| N (%) | |
|---|---|
| Age (years) | |
| ≤40 | 86 (33.2) |
| >40 | 173 (66.8) |
| Site | |
| Left | 131 (50.6) |
| Right | 128 (49.4) |
| Quadrant | |
| Inner | 57 (22.0) |
| Others | 202 (78.0) |
| Histology | |
| Ductal carcinoma | 248 (95.8) |
| Others | 11 (4.2) |
| T stage | |
| T1 | 120 (46.3) |
| T2 | 136 (52.5) |
| T3 | 3 (1.2) |
| N stage | |
| N0 | 160 (61.8) |
| N1 | 63 (24.3) |
| N2 | 17 (6.6) |
| N3 | 19 (7.3) |
| Pathological stage | |
| 1 | 84 (32.4) |
| 2 | 139 (53.7) |
| 3 | 36 (13.9) |
| LVI | |
| No | 240 (92.7) |
| Yes | 19 (7.3) |
| Histological grade | |
| 1–2 | 70 (27.0) |
| 3 | 177 (68.3) |
| Unknown | 12 (4.6) |
| Surgery | |
| MRM | 213 (82.2) |
| BCS | 46 (17.8) |
| Chemotherapy regimen | |
| Taxane | 221 (85.3) |
| Others | 18 (6.9) |
| Unknown | 20 (7.7) |
| Radiotherapy | |
| No | 162 (62.5) |
| Yes | 97 (37.5) |
| sTILs | |
| Low (<60%) | 202 (78.0) |
| High (≥60%) | 57 (22.0) |
| tCD8 + T | |
| Low (≤7%) | 192 (75.9) |
| High (>7%) | 61 (24.1) |
| sCD8 + T | |
| Low (≤10%) | 181 (71.5) |
| High (>10%) | 72 (28.8) |
| iCD8 + T | |
| Low (≤5%) | 206 (81.4) |
| High (>5%) | 47 (18.6) |
| tFOXP3 + T | |
| Low (≤6/HPF) | 190 (73.4) |
| High (>6/HPF) | 69 (26.6) |
| sFOXP3 + T | |
| Low (≤3/HPF) | 187 (72.2) |
| High (>3/HPF) | 72 (27.8) |
| iFOXP3 + T | |
| Low (≤14/HPF) | 205 (79.2) |
| High (>14/HPF) | 54 (20.8) |
| PD-1 | |
| Low (<1%) | 170 (68.5) |
| High (≥1%) | 78 (31.5) |
| PD-L1 (SP142) | |
| Low (<1%) | 150 (57.9) |
| High (≥1%) | 109 (42.1) |
| PD-L1 (22C3) | |
| Low (<10) | 204 (78.8) |
| High (≥10) | 55 (21.2) |
LVI lymphovascular invasion, MRM modified radical mastectomy, BCS breast-conserving surgery, HPF high-power fields.
In the entire cohort, the median percentage of sTILs, tCD8+T cells, sCD8+T cells, and iCD8+T cells was 20% (interquartile range [IQR], 5%–55%), 3% (IQR, 1%–7%), 10% (IQR, 5%–15%) and 1% (IQR 0%–5%), respectively (Fig. 1 and Supplementary Figs. S1 and 2). The median number of tFOXP3+T cells, sFOXP3+T cells, and iFOXP3+T cells was 0/HPF (IQR, 0–8.6/HPF), 0/HPF (IQR, 0–5.2/HPF) and 0/HPF (IQR, 0–9.2/HPF), respectively (Fig. 1 and Supplementary Figs. S1 and 2).
Fig. 1. Representative microphotographs (×200) of the stroma and tumour nest.
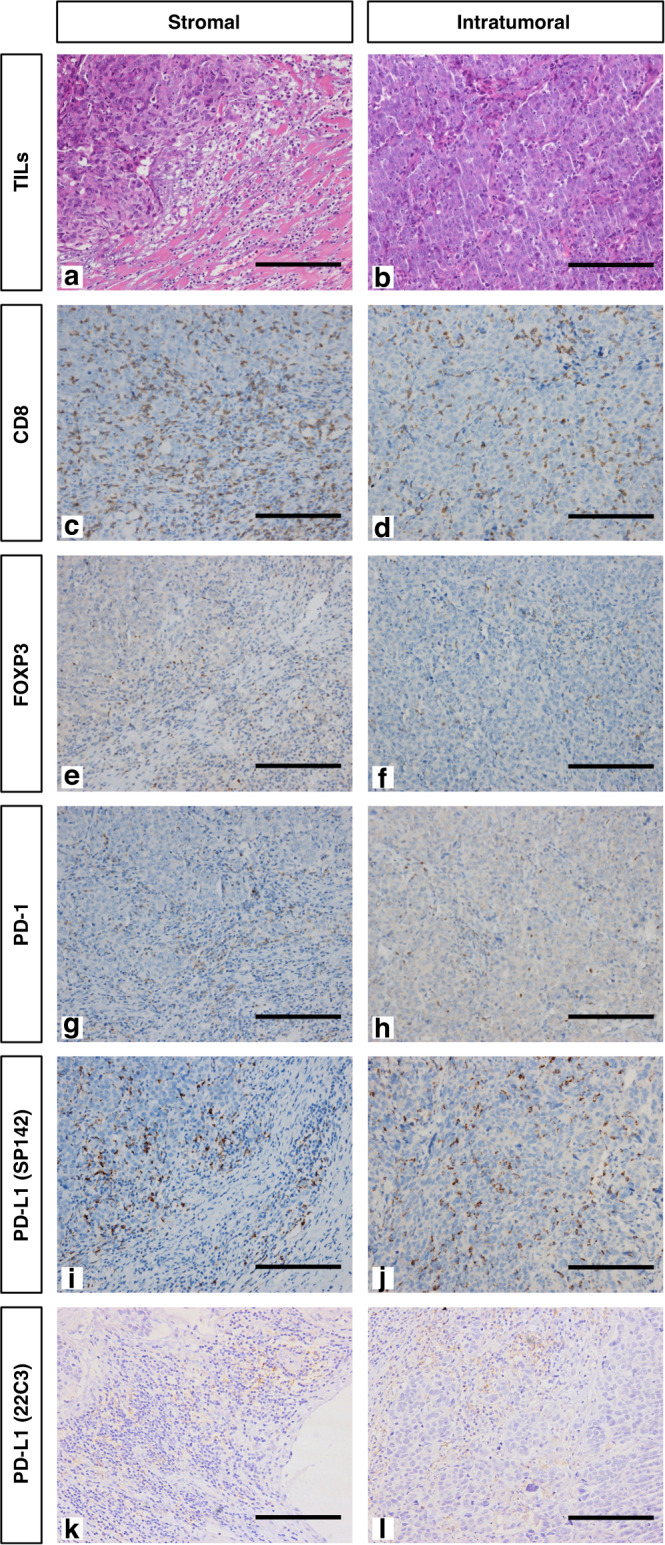
Serial sections of triple-negative breast cancer were stained with hematoxylin and eosin (a, b) and with antibodies against CD8 (c, d), FOXP3 (e, f), PD-1 (g, h) and PD-L1 (i and j for SP142; k and l for 22C3). Each row represents a different antibody. Scale bar = 200 μm.
Cut-off values
To determine whether there was a correlation between immune markers and DFS, maximally selected rank statistics were employed with the maxstat R package [22]. The cut-off values for sTILs, tCD8+T, sCD8+T, iCD8+T, tFOXP3+T, sFOXP3+T and iFOXP3+T cells were calculated as 60%, 7%, 10%, 5%, 6/HPF, 3/HPF and 14/HPF, respectively. Based on the calculated cut-off values, all patients were divided into high- and low-expression groups for different immune markers (Table 1).
Association of immune makers with clinicopathologic characteristics and correlation between biomarkers
Table 2 shows the relationship between the expression of various immune markers and clinicopathological features. Patients with high histological grade had higher levels of sTILs (P < 0.001), tCD8+T cells (P = 0.024), sCD8+T cells (P = 0.010), iCD8+T cells (P = 0.020), tFOXP3+T cells (P = 0.009), sFOXP3+T cells (P < 0.001), iFOXP3+T cells (P < 0.001), PD-L1 (SP142) (P < 0.001) and PD-L1 (22C3) (P < 0.001). Patients with low T stage had lower levels of PD-1 (P = 0.020), while those with low N stage had higher levels of tCD8+T cells, iCD8+T cells and PD-L1 (SP142) (P = 0.007, P = 0.004 and P = 0.019, respectively). Patients with low pathological stage had higher levels of sCD8+T cells and PD-L1 (SP142) (P = 0.015 and P = 0.017, respectively). In addition, those with LVI-negative disease had higher levels of tCD8+T, iCD8+T and sFOXP3+T cells and PD-L1 (SP142) (P = 0.009, P = 0.029, P = 030 and P = 0.027, respectively). Age was not related to the levels of TILs (all P > 0.05).
Table 2.
Association of sTILs, CD8+T, FOXP3+T, PD-1 and PD-L1 with clinicopathologic characteristics.
| sTILs | tCD8+T* | sCD8+T* | iCD8+T* | tFOXP3+T | sFOXP3+T | iFOXP3+T | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Low n (%) | High n (%) | P | Low n (%) | High n (%) | P | Low n (%) | High n (%) | P | Low n (%) | High n (%) | P | Low n (%) | High n (%) | P | Low n (%) | High n (%) | P | Low n (%) | High n (%) | P | |
| Age (year) | 0.308 | 0.553 | 0.710 | 0.520 | 0.567 | 0.059 | 0.406 | ||||||||||||||
| ≤40 | 30 (14.9) | 12 (21.1) | 34 (17.7) | 8 (13.1) | 29 (16.0) | 13 (18.1) | 36 (17.5) | 6 (12.8) | 29 (15.3) | 13 (18.8) | 25 (13.4) | 17 (23.6) | 31 (15.1) | 11 (20.4) | |||||||
| >40 | 172 (85.1) | 45 (78.9) | 158 (82.3) | 53 (86.9) | 152 (84.0) | 59 (81.9) | 170 (82.5) | 41 (87.2) | 161 (84.7) | 56 (81.2) | 162 (86.6) | 55 (76.4) | 174 (84.9) | 43 (79.6) | |||||||
| T stage | 0.455 | 0.239 | 0.095 | 0.419 | 1.00 | 1.00 | 0.762 | ||||||||||||||
| T1 | 91 (45.0) | 29 (50.9) | 86 (44.8) | 33 (54.1) | 79 (43.6) | 40 (55.6) | 94 (45.6) | 25 (53.2) | 88 (46.3) | 32 (46.4) | 87 (46.5) | 33 (45.8) | 94 (45.9) | 26 (48.1) | |||||||
| T2–3 | 111 (55.0) | 28 (49.1) | 106 (55.2) | 28 (45.9) | 102 (56.4) | 32 (44.4) | 112 (54.4) | 22 (46.8) | 102 (53.7) | 37 (53.6) | 100 (53.5) | 39 (54.2) | 111 (54.1) | 28 (51.9) | |||||||
| N stage | 0.166 | .007 | 0.087 | 0.004 | 1.00 | 0.253 | 0.876 | ||||||||||||||
| N0 | 120 (59.4) | 40 (70.2) | 111 (57.8) | 47 (77.0) | 107 (59.1) | 51 (70.8) | 120 (58.3) | 38 (80.9) | 117 (61.6) | 43 (62.3) | 111 (59.4) | 49 (68.1) | 126 (61.5) | 34 (63.0) | |||||||
| N1–3 | 82 (40.6) | 17 (29.8) | 81 (42.2) | 14 (23.0) | 74 (40.9) | 21 (29.2) | 86 (41.7) | 9 (19.1) | 73 (38.4) | 26 (37.7) | 76 (40.6) | 23 (31.9) | 79 (38.5) | 20 (37.0) | |||||||
| Pathological stage | 0.074 | 0.201 | 0.015 | 0.349 | 0.685 | 0.159 | 1.00 | ||||||||||||||
| I–II | 169 (83.7) | 54 (94.7) | 162 (84.4) | 56 (91.8) | 150 (82.9) | 68 (94.4) | 175 (85.0) | 43 (91.5) | 162 (85.3) | 61 (88.4) | 157 (84.0) | 66 (91.7) | 176 (85.9) | 47 (87.0) | |||||||
| III | 33 (16.3) | 3 (5.3) | 30 (15.6) | 5 (8.2) | 31 (17.1) | 4 (5.6) | 31 (15.0) | 4 (8.5) | 28 (14.7) | 8 (11.6) | 30 (16.0) | 6 (8.3) | 29 (14.1) | 7 (13.0) | |||||||
| LVI | 0.773 | 0.009 | 0.017 | 0.029 | 0.113 | 0.030 | 0.380 | ||||||||||||||
| No | 186 (92.1) | 54 (94.7) | 173 (90.1) | 61 (100) | 163 (90.1) | 71 (98.6) | 187 (90.8) | 47 (100) | 173 (91.1) | 67 (97.1) | 169 (90.4) | 71 (98.6) | 188 (91.7) | 52 (96.3) | |||||||
| Yes | 16 (7.9) | 3 (5.3) | 19 (9.9) | 0 (0) | 18 (9.9) | 1 (1.4) | 19 (9.2) | 0 (0) | 17 (8.9) | 0 (0) | 18 (9.6) | 1 (1.4) | 17 (8.3) | 2 (3.7) | |||||||
| Histological grade | <0.001 | 0.024 | 0.001 | 0.020 | 0.009 | <0.001 | <0.001 | ||||||||||||||
| 1–2 | 71 (35.1) | 6 (10.5) | 64 (33.3) | 11 (18.0) | 64 (35.4) | 11 (15.3) | 68 (33.0) | 7 (14.9) | 65 (34.2) | 12 (17.4) | 72 (35.1) | 5 (9.3) | 69 (36.9) | 8 (11.1) | |||||||
| 3 | 131 (64.9) | 51 (89.5) | 128 (66.7) | 50 (82.0) | 117 (64.6) | 61 (84.7) | 138 (67.0) | 40 (85.1) | 125 (65.8) | 57 (82.6) | 133 (64.9) | 49 (90.7) | 118 (63.1) | 64 (88.9) | |||||||
| PD-1† | PD-L1 (SP142) | PD-L1 (22C3) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Low n (%) | High n (%) | P | Low n (%) | High n (%) | P | Low n (%) | High n (%) | P | |
| Age (year) | 0.855 | 0.395 | 0.219 | ||||||
| ≤40 | 29 (17.1) | 12 (15.4) | 22 (14.5) | 20 (18.7) | 30 (14.7) | 12 (21.8) | |||
| >40 | 141 (82.9) | 66 (84.6) | 130 (85.5) | 87 (81.3) | 174 (85.3) | 43 (78.2) | |||
| T stage | 0.020 | 0.899 | 0.761 | ||||||
| T1 | 70 (41.2) | 45 (57.7) | 70 (46.1) | 50 (46.7) | 96 (47.1) | 24 (43.6) | |||
| T2–3 | 100 (58.8) | 33 (42.3) | 82 (53.9) | 56 (52.3) | 108 (52.9) | 31 (56.4) | |||
| N stage | 1.00 | 0.019 | 0.274 | ||||||
| N0 | 108 (63.5) | 47 (60.3) | 85 (55.9) | 75 (70.1) | 122 (59.8) | 38 (69.1) | |||
| N1–3 | 62 (36.5) | 29 (37.2) | 67 (44.1) | 31 (29.2) | 82 (40.2) | 17 (30.9) | |||
| Pathological stage | 0.845 | 0.017 | 0.281 | ||||||
| I–II | 146 (85.9) | 68 (87.2) | 124 (81.6) | 98 (92.5) | 173 (84.8) | 50 (90.9) | |||
| III | 24 (14.1) | 10 (12.8) | 28 (18.4) | 8 (7.5) | 31 (15.2) | 5 (9.1) | |||
| LVI | 0.586 | 0.027 | 0.086 | ||||||
| No | 160 (94.1) | 72 (92.3) | 136 (89.5) | 104 (97.2) | 186 (91.2) | 54 (98.2) | |||
| Yes | 10 (5.9) | 6 (7.7) | 16 (10.5) | 3 (2.8) | 18 (8.8) | 1 (1.8) | |||
| Histological grade | 0.881 | <0.001 | <0.001 | ||||||
| 1–2 | 50 (29.4) | 22 (28.2) | 63 (41.4) | 14 (13.1) | 73 (35.8) | 4 (7.3) | |||
| 3 | 120 (70.6) | 56 (71.8) | 89 (58.6) | 93 (86.9) | 131 (64.2) | 51 (92.7) | |||
LVI lymphovascular invasion.
*Only 253 cases that could evaluate the results of CD8 immunohistochemistry were included.
†Only 248 cases that could evaluate the results of PD-1 immunohistochemistry were included.
As shown in Supplementary Table S1, all immune markers were significantly positively correlated with each other (P < 0.05). sTILs levels were strongly correlated with sCD8+T cell levels and PD-L1 (SP142) expression (ρ = 0.735 and ρ = 0.737, respectively), and moderately correlated with tCD8+T cell levels and PD-L1 (22C3) expression (ρ = 0.654 and ρ = 0.607). Both sTILs and sCD8+T cells were moderately correlated with iCD8+T cells (ρ = 0.577 and ρ = 0.667, respectively). PD-L1 (SP142) expression was also moderately correlated with levels of tCD8+T, sCD8+T and iCD8+T cells (ρ = 0.568, ρ = 0.631 and ρ = 0.617, respectively).
Association of immune makers and clinical outcomes
The median follow-up period was 80 months (range, 9–111 months). Locoregional recurrence occurred in 34 patients (13.1%), distant metastases in 32 patients (12.4%), and 28 patients (10.8%) died. The 5-year LRR, DM, DFS, and OS rates for the entire cohort were 12.4%, 11.7%, 86.8% and 92.3%, respectively.
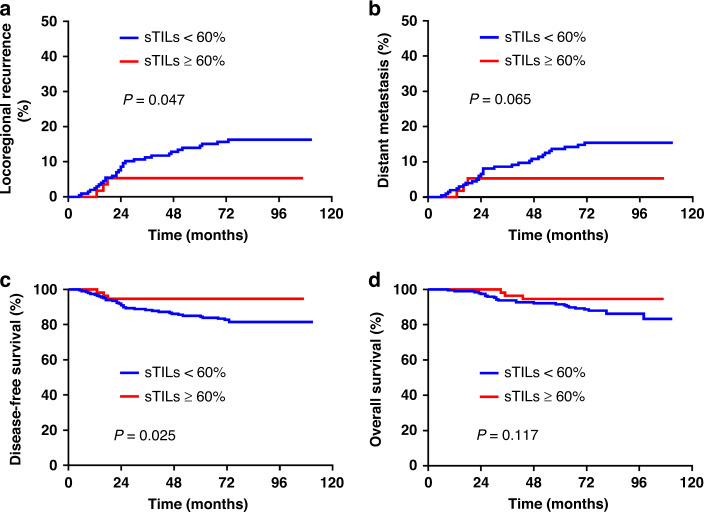
The results of the univariate analysis are shown in Supplementary Table S2. High levels of sTILs were significantly associated with a lower risk of LRR (P = 0.047) and improved DFS (P = 0.025) (Fig. 2). High levels of tCD8+T was significantly associated with a lower risk of LRR (P = 0.015) and DM (P = 0.020), and improved DFS (P = 0.012) and OS (P = 0.040) (Supplementary Fig. S3). High levels of sCD8+T cells were significantly associated with lower risk of LRR (P = 0.022) and DM (P = 0.015) and improved DFS (P = 0.031) (Supplementary Fig. S4). High levels of iCD8+T and sFOXP3+T cells were significantly associated with lower risk of LRR (P = 0.012 and P = 0.001, respectively) and DM (P = 0019 and P = 0.043, respectively), and improved DFS (P = 0.007 and P = 0.034, respectively) and OS (P = 0.026 and P = 0.039, respectively) (Supplementary Figs. S5 and S7). The levels of tFOXP3+T and iFOXP3+T cells were not associated with clinical outcomes (Supplementary Figs. S6 and S8). High expression of PD-1 was significantly associated with improved DFS (P = 0.044) (Supplementary Fig. S9). High expression of PD-L1 (SP142) was significantly associated with lower risk of LRR (P = 0.019) and DM (P = 0.013), and improved DFS (P = 0.015) and OS (P = 0.035) (Supplementary Fig. S10). However, the expression of PD-L1 (22C3) was not associated with survival (Supplementary Fig. S11).
Fig. 2. Kaplan–Meier curves stratified by sTILs expression.
The locoregional recurrence (a), distant metastasis (b), disease-free survival (c) and overall survival (d) in the low sTILs expression and high sTILs expression groups.
In the multivariate analysis (Table 3), sTILs (hazard ratio [HR] = 0.23, 95% confidence interval [CI]: 0.05–0.97; P = 0.046) were identified as an independent prognostic factor for DFS. To further demonstrate the prognostic power of other immune markers reported in the present study, we examined the impact of incorporating their effects into the DFS analysis with a panel of classical clinicopathological factors (age, pathological stage, LVI and histological grade) based on the results of the univariate analysis and previous literature. As shown in Table 4, we confirmed that the sTILs levels provide significant additional prognostic information beyond that provided by the classical factors. Furthermore, inclusion of tCD8+T (LR χ2 = 4.93, P = 0.026) or iCD8+T cell levels (LR χ2 = 5.73, P = 0.017), or PD-1 expression (LRχ2 = 4.33, P = 0.037) increased the prognostic value for DFS beyond that determined using the classical clinicopathological factors and sTILs. However, neither PD-L1 (SP142) nor PD-L1 (22C3) expression provided additional prognostic value under the same settings as above.
Table 3.
Multivariate analysis of immune markers for LRR, DM, DFS and OS in entire cohort.
| LRR | DM | DFS | OS | |||||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | |
| sTILs | ||||||||
| Low | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| High | 0.25 (0.06–1.07) | 0.061 | 0.32 (0.07–1.37) | 0.123 | 0.23 (0.05–0.97) | 0.046 | 0.32 90.07–1.39) | 0.127 |
| tCD8+T | ||||||||
| Low | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| High | 0.10 (0.01–0.74) | 0.024 | 0.12 (0.02–0.86) | 0.035 | 0.19 (0.05–0.80) | 0.024 | 0.27 (0.06–1.15) | 0.077 |
| sCD8+T | ||||||||
| Low | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| High | 0.33 (0.10–1.12) | 0.075 | 0.25 (0.06–1.06) | 0.060 | 0.42 (0.14–1.22) | 0.109 | 0.40 (0.12–1.37) | 0.145 |
| iCD8+T | ||||||||
| Low | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| High | 0.15 (0.02–1.13) | 0.065 | 0.16 (0.02–1.23) | 0.078 | 0.13 (0.02–1.00) | 0.050 | 0.16 (0.02–1.19) | 0.074 |
| tFOXP3+T | ||||||||
| Low | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| High | 0.80 (0.32–1.98) | 0.623 | 1.15 (0.48–2.74) | 0.752 | 1.02 (0.45–2.28) | 0.968 | 1.10 (0.43–2.83) | 0.836 |
| sFOXP3+T | ||||||||
| Low | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| High | 0.32 (0.09–1.08) | 0.066 | 0.57 (0.19–1.68) | 0.308 | 0.53 (0.20–1.42) | 0.207 | 0.43 (0.12–1.48) | 0.181 |
| iFOXP3+T | ||||||||
| Low | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| High | 0.62 (0.21–1.81) | 0.386 | 0.94 (0.35–2.51) | 0.909 | 0.72 (0.27–1.87) | 0.496 | 0.84 (0.28–2.49) | 0.749 |
| PD-1 | ||||||||
| Low | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| High | 0.44 (0.18–1.09) | 0.076 | 0.40 (0.15–1.07) | 0.068 | 0.39 (0.62–0.95) | 0.039 | 0.37 (0.13–1.10) | 0.073 |
| PD-L1 (SP142) | ||||||||
| Low | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| High | 0.44 (0.18–1.05) | 0.063 | 0.40 (0.16–1.00) | 0.051 | 0.45 (0.20–1.01) | 0.052 | 0.41 (0.16–1.05) | 0.064 |
| PD-L1 (22C3) | ||||||||
| Low | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| High | 0.85 (0.31–2.31) | 0.742 | 0.94 (0.34–2.59) | 0.908 | 0.71 (0.27–1.91) | 0.499 | 0.95 (0.34–2.64) | 0.919 |
LRR locoregional recurrence, DM distant metastasis, DFS disease-free survival, OS overall survival, HR hazard ratio, CI confidence interval.
Analysis was adjusted for age, grade, stage and lymphovascular invasion.
Table 4.
Additional (DFS) value of immune markers to prognostic multivariable models.
| Model variables | Likelihood ratio χ2 | Likelihood ratio P value |
|---|---|---|
| CP + sTILs versus CP | 5.659 | 0.017 |
| CP + sTILs + tCD8+T versus CP + sTIL | 4.934 | 0.026 |
| CP + sTILs + sCD8+T versus CP + sTIL | 1.202 | 2.273 |
| CP + sTILs + iCD8+T versus CP + sTIL | 5.730 | 0.017 |
| CP + sTILs + tFOXP3+T versus CP + sTIL | 0.046 | 0.831 |
| CP + sTILs + sFOXP3+T versus CP + sTIL | 1.540 | 0.215 |
| CP + sTILs + iFOXP3+T versus CP + sTIL | 0.166 | 0.684 |
| CP + sTILs + PD-1 versus CP + sTIL | 4.334 | 0.037 |
| CP + sTILs + PD-L1 (SP142) versus CP + sTIL | 2.066 | 0.151 |
| CP + sTILs + PD-L1 (22C3) versus CP + sTIL | 0.016 | 0.900 |
CP clinicopathological factors (age, grade, stage, LVI).
Given that 30% has previously been validated as a cut-off for sTILs in early-stage TNBC [8, 23], we performed additional analyses on the prognostic value of sTILs using 30% as a cut-off. However, the results showed that dichotomising the sTILs levels according to the 30% cut-off did not have any prognostic significance in terms of DFS (Supplementary Fig. S12 and Supplementary Tables S3 and S4).
Discussion
In this study, we showed that the levels of TILs and their subsets correlated with pathological N stage, pathological stage, histological grade and LVI in non-metastatic TNBC. We confirmed that sTILs are an independent prognostic factor for DFS and that the inclusion of tCD8+T, iCD8+T levels, or PD-1 expression can provide further prognostic information and improve the prediction of patient prognosis beyond that determined using classical factors and sTILs.
All patients in this study received adjuvant chemotherapy without neoadjuvant chemotherapy and all stained sections were obtained from surgically resected gross tissue specimens, which can accurately reflect the tumour microenvironment of these patients. Although it is widely accepted that lymphocytes interacting directly with carcinoma cells are more biologically relevant [1], most current studies have found sTILs to be superior to iTILs and a more reproducible parameter in predicting response to therapy [1]. This is mainly due to the difficulty in recognising and scoring the iTILs embedded in the tumour compared to the sTILs. Therefore, in this study, we validated only the prognostic value of sTILs, while the influences of CD8+T and FOXP3+T cells on prognosis were explored in both the stroma and tumour nests. Since there are currently no accepted cut-off values for CD8+T and FOXP3+T cells, we adopted maximally selected rank statistics to calculate the optimal thresholds. This method has been widely used in other cancers [24, 25].
As an established prognostic factor for TNBC, sTILs are useful for predicting chemotherapeutic response or DFS (Supplementary Table S5) [2–8, 23, 26], and we confirmed the independent prognostic role of sTILs in TNBC. The median score for sTILs in our study was 20%, which was comparable to that reported in previous studies (Supplementary Table S5) [2–8, 23, 26]. The cut-off value of sTILs in this study was calculated as 60%, which was also consistent with the cut-off values used in other studies to define lymphocyte-predominant breast cancer in TNBC [3, 5, 6]. However, all other studies were conducted with the inclusion of patients with TNBC requiring neoadjuvant treatments. At present, explorations of the cut-off value of sTILs in the setting of without neoadjuvant treatments are rare, and sTILs were analysed as a continuous variable in most studies [2, 4, 7, 8, 23]. A pooled analysis showed that a cut-off value of 30% is optimal for sTILs in predicting the prognosis of node-negative TNBC [8]. This cut-off value was also confirmed in another study of patients with Stage I TNBC [23]. This indicates that a lower cut-off may be sufficient to predict prognosis in early-stage TNBC. Moreover, a follow-up analysis of the above-pooled analysis showed that patients with Stage II TNBC and high sTILs have a better outcome than patients with Stage I and low sTILs [27]. In our study, although 86.1% of the patients in the whole group had pathological Stage I–II disease, there was no significant correlation with prognosis when the cut-off value of sTILs was defined as 30%. Therefore, this suggests that further research should take the tumour stage into account when identifying the optimal cut-off value of sTILs for prognosis.
CD8+T cells, which comprise the majority of TILs, have been shown to be significantly related to the prognosis of patients with TNBC due to their cytotoxicity and antitumor immunity [28–31]. The observed moderate-strong correlation between sTILs and CD8+T cell density in our study was similar to that reported previously [31, 32]. Similar to the results for sTILs, higher tCD8+T cell density was also correlated with improved DFS (HR = 0.24) in the present study. Further, tCD8+T cells exhibited additional prognostic value for DFS (LRχ2 = 4.93, P = 0.026) based on the classical clinicopathological factors plus sTILs. This prognostic value was also observed for iCD8+T cells. These findings indicate that the prognostic value of tCD8+T cells was derived from iCD8+T cells rather than sCD8+T cells. Similarly, previous studies showed that a high iCD8+T cell density was independently associated with better DFS (HR = 0.48) or breast cancer specific-survival (HR = 0.72) [29, 33], and tCD8+T cell density can provide significant additional prognostic information beyond clinicopathological factors plus TILs for DFS (LRχ2 = 5.89, P = 0.015) [31, 34]. Interestingly, a further in-depth study showed that in addition to the quantity of TILs, the tissue-resident memory (TRM) gene signature can be used to discriminate between patients with high CD8 expression and was significantly associated with improved patient survival in early-stage TNBC [35]. Furthermore, CD8+ TRM cells, which expressed high levels of immune checkpoint molecules and effector proteins, may be the key mediator of improved clinical outcomes observed in breast cancers with a high level of TILs [35].
Our study also showed that PD-1 rather than PD-L1 offered additional prognostic value for DFS beyond the classical clinicopathological factors plus sTILs. In accordance with these findings, other studies showed that high PD-1, but not PD-L1, was a favourable prognostic factor for DFS in TNBC [36, 37]. Multiplex immunofluorescence studies showed that the density of cells expressing both CD8 and PD-1 (CD8+PD-1+), but not the density of CD8−PD-1+ immune infiltrates, was associated with improved DFS [34]. There are conflicting reports of the relationship between PD-L1 expression and prognosis. Some studies demonstrated that high PD-L1 was correlated with better DFS [31, 38–40] and provided additional prognostic value beyond classical clinicopathological factors plus TILs [31], while another study showed that PD-L1 expression was correlated with poor DFS among patients with high-level TIL levels [41]. The controversy regarding the prognostic value of PD-L1 might partly be explained by the variation in the criteria used for evaluation in these studies, such as the use of different PD-L1 antibodies and the measurement of PD-L1 expression in tumour and/or immune cells [42]. In the setting of neoadjuvant immune checkpoint therapy, PD-L1 did not exhibit a predictive value for survival or any treatment response benefit in the IMpassion031 and Keynote 522 studies [16, 17]. However, the NeoTRIP study showed that the presence of PD-L1 expression was the most significant factor influencing the pathological complete response rate only in the atezolizumab treatment arm; this may partly be the result of changes in PD-L1 expression induced by atezolizumab [43, 44]. In our study, we observed a strong correlation between sTILs and PD-L1 expression and a weak correlation between sTILs and PD-1 expression, supporting the notion that PD-1 and PD-L1 expression should be combined with the TILs for prognostic evaluation. A post-hoc analysis of the IMpassion130 study showed an increased clinical benefit of atezolizumab immunotherapy in TNBC patients with high CD8+T cells or sTILs who also had high PD-L1 expression [45]. Moreover, studies on atezolizumab and pembrolizumab immunotherapy in metastatic TNBC also showed that clinical activity was greatest with high levels of CD8+T cells and/or sTILs [46, 47].
In contrast to the prevalence of CD8+T cells, our study showed that FOXP3+T cells were under-represented by more than one order of magnitude and had only a weak correlation with sTILs and CD8+T cells. This relatively low prevalence of FOXP3+T was consistent with that reported in other studies (Supplementary Table S5) [20, 31, 32, 48–50]. Considering that FOXP3+T cells are usually immunosuppressive, it can be speculated that the low detection rate of FOXP3+T cells and the prevalence of CD8+T cells were due to host anti-tumour efforts. One study showed that a high CD8+T to FOXP3+T cell ratio indicated better OS of TNBC, although we found that FOXP3+T cell prevalence was not associated with prognosis. However, other studies showing a high prevalence of FOXP3+T cells was significantly associated with a better prognosis in TNBC, but worse prognosis in luminal breast cancer [48, 51, 52]. Some studies have revealed heterogeneity of FOXP3+ regulatory T (Treg) cell subpopulations. Effector Tregs play roles in blocking antitumor immune responses, while non-Tregs plays an active role in the antitumor immune response [53, 54], suggesting that the balance between different immune cell subsets, even different subpopulations, may influence the prognosis.
In this study, we have comprehensively evaluated the prognostic value of various immune markers and identified the additional prognostic value of tCD8+T, iCD8+T cell prevalence and PD-1 expression in combination with classical clinicopathological factors plus sTILs in non-metastatic TNBC without neoadjuvant chemotherapy. This is a unique data set which includes patients with Stage I disease that were excluded from IMpassion031 and Keynote 522 studies [16, 17]. It provides ideas for the inclusion of patients with Stage I disease in the design of adjuvant chemo/immunotherapy for TNBC in the future. However, our study has several limitations, such as the small sample size and retrospective design. In addition, since multiplexed immunofluorescence staining was not conducted, we were unable to assess the prognostic value of the co-expression of these markers. With the recent granting of accelerated approvals for the PD-1/PD-L1 targeting agents in TNBC, focus has now shifted to investigating the clinical utility of these immune markers in the setting of immune checkpoint agents [55]. However, our findings could not be applied to predicting the response to immune checkpoint inhibitors due to the lack of immune therapy intervention in this cohort.
In summary, in addition to sTILs, inclusion of tCD8+T, iCD8+T or PD-1 may further refine the prognostic model for non-metastatic TNBC beyond classic factors, although this finding requires further in-depth research to fully understand the critical role of tCD8+T, iCD8+T and PD-1 in T-cell function.
Supplementary information
Acknowledgements
We express our heartfelt gratitude to Ms. Xiu-Yun Liu, Ms. Bo Zheng and Ms. Lei Guo from the Department of Pathology, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College for their invaluable technical support throughout the course of this project. Their expertise and guidance were essential in making this work a reality.
Author contributions
G-YS: formal analysis, investigation, data collection, methodology and writing of the original draft. JZ: formal analysis, investigation, data collection, methodology and writing of the original draft. B-ZW: formal analysis, investigation, data collection, methodology and writing of the original draft. HJ: patient care and review and editing of the manuscript. HF: patient care and review and editing of the manuscript. Yu Tang: patient care and review and editing of the manuscript. Y-WS: patient care and review and editing of the manuscript. JJ: patient care and review and editing of the manuscript. Y-PL: patient care and review and editing of the manuscript. Yuan Tang: patient care and review and editing of the manuscript. S-NQ: patient care and review and editing of the manuscript. BC: patient care and review and editing of the manuscript. N-NL: patient care and review and editing of the manuscript. NL: patient care and review and editing of the manuscript. Y-XL: formal analysis and data collection, validation, statistical analysis guidance, and project administration, patient care, and writing and editing of the first draft of the manuscript. J-MY: formal analysis and data collection, validation, statistical analysis guidance, project administration, patient care and writing and editing of the first draft of the manuscript. S-LW: formal analysis and data collection, validation, statistical analysis guidance, project administration, patient care and writing and editing of the first draft of the manuscript.
Funding
This work was supported by the CAMS Innovation Fund for Medical Sciences (2020-I2M-C&T-B-075, 2021-I2M-1-014) and the National Natural Science Foundation of China (81972860).
Data availability
All data generated and analysed during this study are included in this article (and its supplementary information files).
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The study was approved by the Institutional Review Board of Cancer Hospital, the Chinese Academy of Medical Sciences and Peking Union Medical College (approval number 15-057/984) and was conducted according to the Declaration of Helsinki. The requirement of informed consent was waived.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Guang-Yi Sun, Jing Zhang, Bing-Zhi Wang.
Contributor Information
Ye-Xiong Li, Email: yexiong12@163.com.
Jian-Ming Ying, Email: jmying@hotmail.com.
Shu-Lian Wang, Email: wangsl@cicams.ac.cn.
Supplementary information
The online version contains supplementary material available at 10.1038/s41416-023-02218-w.
References
- 1.Salgado R, Denkert C, Demaria S, Sirtaine N, Klauschen F, Pruneri G, et al. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group 2014. Ann Oncol. 2015;26:259–71. doi: 10.1093/annonc/mdu450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Loi S, Sirtaine N, Piette F, Salgado R, Viale G, Van Eenoo F, et al. Prognostic and predictive value of tumor-infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy: BIG 02-98. J Clin Oncol. 2013;31:860–7. doi: 10.1200/JCO.2011.41.0902. [DOI] [PubMed] [Google Scholar]
- 3.Denkert C, von Minckwitz G, Darb-Esfahani S, Lederer B, Heppner BI, Weber KE, et al. Tumour-infiltrating lymphocytes and prognosis in different subtypes of breast cancer: a pooled analysis of 3771 patients treated with neoadjuvant therapy. Lancet Oncol. 2018;19:40–50. doi: 10.1016/S1470-2045(17)30904-X. [DOI] [PubMed] [Google Scholar]
- 4.Adams S, Gray RJ, Demaria S, Goldstein L, Perez EA, Shulman LN, et al. Prognostic value of tumor-infiltrating lymphocytes in triple-negative breast cancers from two phase III randomized adjuvant breast cancer trials: ECOG 2197 and ECOG 1199. J Clin Oncol. 2014;32:2959–66. doi: 10.1200/JCO.2013.55.0491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Issa-Nummer Y, Darb-Esfahani S, Loibl S, Kunz G, Nekljudova V, Schrader I, et al. Prospective validation of immunological infiltrate for prediction of response to neoadjuvant chemotherapy in HER2-negative breast cancer–a substudy of the neoadjuvant GeparQuinto trial. PLoS ONE. 2013;8:e79775. doi: 10.1371/journal.pone.0079775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Denkert C, von Minckwitz G, Brase JC, Sinn BV, Gade S, Kronenwett R, et al. Tumor-infiltrating lymphocytes and response to neoadjuvant chemotherapy with or without carboplatin in human epidermal growth factor receptor 2-positive and triple-negative primary breast cancers. J Clin Oncol. 2015;33:983–91. doi: 10.1200/JCO.2014.58.1967. [DOI] [PubMed] [Google Scholar]
- 7.Loi S, Michiels S, Salgado R, Sirtaine N, Jose V, Fumagalli D, et al. Tumor infiltrating lymphocytes are prognostic in triple negative breast cancer and predictive for trastuzumab benefit in early breast cancer: results from the FinHER trial. Ann Oncol. 2014;25:1544–50. doi: 10.1093/annonc/mdu112. [DOI] [PubMed] [Google Scholar]
- 8.Loi S, Drubay D, Adams S, Pruneri G, Francis PA, Lacroix-Triki M, et al. Tumor-Infiltrating lymphocytes and prognosis: a pooled individual patient analysis of early-stage triple-negative breast cancers. J Clin Oncol. 2019;37:559–69. doi: 10.1200/JCO.18.01010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kurozumi S, Matsumoto H, Kurosumi M, Inoue K, Fujii T, Horiguchi J, et al. Prognostic significance of tumour-infiltrating lymphocytes for oestrogen receptor-negative breast cancer without lymph node metastasis. Oncol Lett. 2019;17:2647–56. doi: 10.3892/ol.2019.9938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gao Z-H, Li C-X, Liu M, Jiang J-Y. Predictive and prognostic role of tumour-infiltrating lymphocytes in breast cancer patients with different molecular subtypes: a meta-analysis. BMC Cancer. 2020;20:1150. doi: 10.1186/s12885-020-07654-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pellegrino B, Hlavata Z, Migali C, De Silva P, Aiello M, Willard-Gallo K, et al. Luminal breast cancer: risk of recurrence and tumor-associated immune suppression. Mol Diagn Ther. 2021;25:409–24. doi: 10.1007/s40291-021-00525-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baxevanis CN, Sofopoulos M, Fortis SP, Perez SA. The role of immune infiltrates as prognostic biomarkers in patients with breast cancer. Cancer Immunol Immunotherapy: CII. 2019;68:1671–80. doi: 10.1007/s00262-019-02327-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen J, Jiang CC, Jin L, Zhang XD. Regulation of PD-L1: a novel role of pro-survival signalling in cancer. Ann Oncol. 2016;27:409–16. doi: 10.1093/annonc/mdv615. [DOI] [PubMed] [Google Scholar]
- 14.Schmid P, Rugo HS, Adams S, Schneeweiss A, Barrios CH, Iwata H, et al. Atezolizumab plus nab-paclitaxel as first-line treatment for unresectable, locally advanced or metastatic triple-negative breast cancer (IMpassion130): updated efficacy results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2020;21:44–59. doi: 10.1016/S1470-2045(19)30689-8. [DOI] [PubMed] [Google Scholar]
- 15.Cortes J, Cescon DW, Rugo HS, Nowecki Z, Im SA, Yusof MM, et al. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer (KEYNOTE-355): a randomised, placebo-controlled, double-blind, phase 3 clinical trial. Lancet. 2020;396:1817–28. doi: 10.1016/S0140-6736(20)32531-9. [DOI] [PubMed] [Google Scholar]
- 16.Mittendorf EA, Zhang H, Barrios CH, Saji S, Jung KH, Hegg R, et al. Neoadjuvant atezolizumab in combination with sequential nab-paclitaxel and anthracycline-based chemotherapy versus placebo and chemotherapy in patients with early-stage triple-negative breast cancer (IMpassion031): a randomised, double-blind, phase 3 trial. Lancet. 2020;396:1090–1100. doi: 10.1016/S0140-6736(20)31953-X. [DOI] [PubMed] [Google Scholar]
- 17.Schmid P, Cortes J, Dent R, Pusztai L, McArthur H, Kümmel S, et al. Event-free survival with pembrolizumab in early triple-negative breast cancer. N Engl J Med. 2022;386:556–67. doi: 10.1056/NEJMoa2112651. [DOI] [PubMed] [Google Scholar]
- 18.Sauerbrei W, Taube SE, McShane LM, Cavenagh MM, Altman DG. Reporting recommendations for tumor marker prognostic studies (REMARK): an abridged explanation and elaboration. J Natl Cancer Inst. 2018;110:803–11. doi: 10.1093/jnci/djy088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang J, Abubakar M, Yuan P, Koka H, Guo L, Li X, et al. Prognostic significance of tumor-infiltrating lymphocytes in premenopausal, luminal breast cancer treated with adjuvant endocrine therapy. Am J Transl Res. 2021;13:12750–62. [PMC free article] [PubMed] [Google Scholar]
- 20.Goto R, Hirota Y, Aruga T, Horiguchi S, Miura S, Nakamura S, et al. The number of FoxP3-positive tumor-infiltrating lymphocytes in patients with synchronous bilateral breast cancer. Breast Cancer. 2020;27:586–93. doi: 10.1007/s12282-020-01049-4. [DOI] [PubMed] [Google Scholar]
- 21.Noske A, Mobus V, Weber K, Schmatloch S, Weichert W, Kohne CH, et al. Relevance of tumour-infiltrating lymphocytes, PD-1 and PD-L1 in patients with high-risk, nodal-metastasised breast cancer of the German Adjuvant Intergroup Node-positive study. Eur J Cancer. 2019;114:76–88. doi: 10.1016/j.ejca.2019.04.010. [DOI] [PubMed] [Google Scholar]
- 22.Hothorn T, Lausen B. On the exact distribution of maximally selected rank statistics. Comput Stat Data Anal. 2003;43:121–37. doi: 10.1016/S0167-9473(02)00225-6. [DOI] [Google Scholar]
- 23.Park JH, Jonas SF, Bataillon G, Criscitiello C, Salgado R, Loi S, et al. Prognostic value of tumor-infiltrating lymphocytes in patients with early-stage triple-negative breast cancers (TNBC) who did not receive adjuvant chemotherapy. Ann Oncol. 2019;30:1941–9. doi: 10.1093/annonc/mdz395. [DOI] [PubMed] [Google Scholar]
- 24.Wei ZW, Wu J, Huang WB, Li J, Lu XF, Yuan YJ, et al. Immune-infiltration based signature as a novel prognostic biomarker in gastrointestinal stromal tumour. EBioMedicine. 2020;57:102850. doi: 10.1016/j.ebiom.2020.102850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sperlich A, Balmert A, Doll D, Bauer S, Franke F, Keller G, et al. Genetic and immunological biomarkers predict metastatic disease recurrence in stage III colon cancer. BMC Cancer. 2018;18:998. doi: 10.1186/s12885-018-4940-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de Jong VMT, Wang Y, Ter Hoeve ND, Opdam M, Stathonikos N, Jóźwiak K, et al. Prognostic value of stromal tumor-infiltrating lymphocytes in young, node-negative, triple-negative breast cancer patients who did not receive (neo)adjuvant systemic therapy. J Clin Oncol. 2022;40:2361–74. doi: 10.1200/JCO.21.01536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Loi S, Salgado R, Adams S, Pruneri G, Francis PA, Lacroix-Triki M, et al. Tumor infiltrating lymphocyte stratification of prognostic staging of early-stage triple negative breast cancer. NPJ Breast Cancer. 2022;8:3. doi: 10.1038/s41523-021-00362-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mahmoud SM, Paish EC, Powe DG, Macmillan RD, Grainge MJ, Lee AH, et al. Tumor-infiltrating CD8+ lymphocytes predict clinical outcome in breast cancer. J Clin Oncol. 2011;29:1949–55. doi: 10.1200/JCO.2010.30.5037. [DOI] [PubMed] [Google Scholar]
- 29.Ali HR, Provenzano E, Dawson SJ, Blows FM, Liu B, Shah M, et al. Association between CD8+ T-cell infiltration and breast cancer survival in 12,439 patients. Ann Oncol. 2014;25:1536–43. doi: 10.1093/annonc/mdu191. [DOI] [PubMed] [Google Scholar]
- 30.Vihervuori H, Autere TA, Repo H, Kurki S, Kallio L, Lintunen MM, et al. Tumor-infiltrating lymphocytes and CD8(+) T cells predict survival of triple-negative breast cancer. J Cancer Res Clin Oncol. 2019;145:3105–14. doi: 10.1007/s00432-019-03036-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dieci MV, Tsvetkova V, Griguolo G, Miglietta F, Tasca G, Giorgi CA, et al. Integration of tumour infiltrating lymphocytes, programmed cell-death ligand-1, CD8 and FOXP3 in prognostic models for triple-negative breast cancer: analysis of 244 stage I-III patients treated with standard therapy. Eur J Cancer. 2020;136:7–15. doi: 10.1016/j.ejca.2020.05.014. [DOI] [PubMed] [Google Scholar]
- 32.Koletsa T, Kotoula V, Koliou GA, Manousou K, Chrisafi S, Zagouri F, et al. Prognostic impact of stromal and intratumoral CD3, CD8 and FOXP3 in adjuvantly treated breast cancer: do they add information over stromal tumor-infiltrating lymphocyte density? Cancer Immunol Immunother: CII. 2020;69:1549–64. doi: 10.1007/s00262-020-02557-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Matsumoto H, Thike AA, Li H, Yeong J, Koo SL, Dent RA, et al. Increased CD4 and CD8-positive T cell infiltrate signifies good prognosis in a subset of triple-negative breast cancer. Breast Cancer Res Treat. 2016;156:237–47. doi: 10.1007/s10549-016-3743-x. [DOI] [PubMed] [Google Scholar]
- 34.Yeong J, Lim JCT, Lee B, Li H, Ong CCH, Thike AA, et al. Prognostic value of CD8 + PD-1+ immune infiltrates and PDCD1 gene expression in triple negative breast cancer. J Immunother Cancer. 2019;7:34. doi: 10.1186/s40425-019-0499-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Savas P, Virassamy B, Ye C, Salim A, Mintoff CP, Caramia F, et al. Single-cell profiling of breast cancer T cells reveals a tissue-resident memory subset associated with improved prognosis. Nat Med. 2018;24:986–93. doi: 10.1038/s41591-018-0078-7. [DOI] [PubMed] [Google Scholar]
- 36.Millar E, Browne L, Slapetova I, Shang F, Ren Y, Bradshaw R, et al. TILs immunophenotype in breast cancer predicts local failure and overall survival: analysis in a large radiotherapy trial with long-term follow-up. Cancers. 2020;12:2365. doi: 10.3390/cancers12092365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ren X, Wu H, Lu J, Zhang Y, Luo Y, Xu Q, et al. PD1 protein expression in tumor infiltrated lymphocytes rather than PDL1 in tumor cells predicts survival in triple-negative breast cancer. Cancer Biol Ther. 2018;19:373–80. doi: 10.1080/15384047.2018.1423919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee J, Kim DM, Lee A. Prognostic role and clinical association of tumor-infiltrating lymphocyte, programmed death ligand-1 expression with neutrophil-lymphocyte ratio in locally advanced triple-negative breast cancer. Cancer Res Treat. 2019;51:649–63. doi: 10.4143/crt.2018.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li X, Wetherilt CS, Krishnamurti U, Yang J, Ma Y, Styblo TM, et al. Stromal PD-L1 expression is associated with better disease-free survival in triple-negative breast cancer. Am J Clin Pathol. 2016;146:496–502. doi: 10.1093/ajcp/aqw134. [DOI] [PubMed] [Google Scholar]
- 40.Ahn SG, Kim SK, Shepherd JH, Cha YJ, Bae SJ, Kim C, et al. Clinical and genomic assessment of PD-L1 SP142 expression in triple-negative breast cancer. Breast Cancer Res Treat. 2021;188:165–78. doi: 10.1007/s10549-021-06193-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xiao B, Peng J, Wang Y, Deng Y, Ou Q, Wu X, et al. Prognostic value of tumor infiltrating lymphocytes combined with PD-L1 expression for patients with solitary colorectal cancer liver metastasis. Ann Transl Med. 2020;8:1221. doi: 10.21037/atm-20-2762a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Peg V, López-García M, Comerma L, Peiró G, García-Caballero T, López ÁC, et al. PD-L1 testing based on the SP142 antibody in metastatic triple-negative breast cancer: summary of an expert round-table discussion. Future Oncol. 2021;17:1209–18. doi: 10.2217/fon-2020-1100. [DOI] [PubMed] [Google Scholar]
- 43.Bianchini G, Huang C, Egle D, Bermejo B, Zamagni C, Thill M, et al. LBA13 Tumour infiltrating lymphocytes (TILs), PD-L1 expression and their dynamics in the NeoTRIPaPDL1 trial. Ann Oncol. 2020;31:S1145–S1146. doi: 10.1016/j.annonc.2020.08.2241. [DOI] [Google Scholar]
- 44.Gianni L, Huang CS, Egle D, Bermejo B, Zamagni C, Thill M, et al. Pathologic complete response (pCR) to neoadjuvant treatment with or without atezolizumab in triple-negative, early high-risk and locally advanced breast cancer: NeoTRIP Michelangelo randomized study. Ann Oncol. 2022;33:534–43. doi: 10.1016/j.annonc.2022.02.004. [DOI] [PubMed] [Google Scholar]
- 45.Emens LA, Molinero L, Loi S, Rugo HS, Schneeweiss A, Diéras V, et al. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer: biomarker evaluation of the IMpassion130 study. J Natl Cancer Inst. 2021;113:1005–16. doi: 10.1093/jnci/djab004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Emens LA, Cruz C, Eder JP, Braiteh F, Chung C, Tolaney SM, et al. Long-term clinical outcomes and biomarker analyses of atezolizumab therapy for patients with metastatic triple-negative breast cancer: a phase 1 study. JAMA Oncol. 2019;5:74–82. doi: 10.1001/jamaoncol.2018.4224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Loi S, Adams S, Schmid P, Cortés J, Cescon D, Winer E, et al. Relationship between tumor infiltrating lymphocyte (TIL) levels and response to pembrolizumab (pembro) in metastatic triple-negative breast cancer (mTNBC): results from KEYNOTE-086. Ann Oncol. 2017;28:v608. doi: 10.1093/annonc/mdx440.005. [DOI] [Google Scholar]
- 48.Liu S, Foulkes WD, Leung S, Gao D, Lau S, Kos Z, et al. Prognostic significance of FOXP3+ tumor-infiltrating lymphocytes in breast cancer depends on estrogen receptor and human epidermal growth factor receptor-2 expression status and concurrent cytotoxic T-cell infiltration. Breast Cancer Res: BCR. 2014;16:432. doi: 10.1186/s13058-014-0432-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ogiya R, Niikura N, Kumaki N, Bianchini G, Kitano S, Iwamoto T, et al. Comparison of tumor-infiltrating lymphocytes between primary and metastatic tumors in breast cancer patients. Cancer Sci. 2016;107:1730–5. doi: 10.1111/cas.13101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jørgensen N, Hviid TVF, Nielsen LB, Sønderstrup IMH, Eriksen JO, Ejlertsen B, et al. Tumour-infiltrating CD4-, CD8- and FOXP3-positive immune cells as predictive markers of mortality in BRCA1- and BRCA2-associated breast cancer. Br J Cancer. 2021;125:1388–98. doi: 10.1038/s41416-021-01514-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.West NR, Kost SE, Martin SD, Milne K, Deleeuw RJ, Nelson BH, et al. Tumour-infiltrating FOXP3(+) lymphocytes are associated with cytotoxic immune responses and good clinical outcome in oestrogen receptor-negative breast cancer. Br J Cancer. 2013;108:155–62. doi: 10.1038/bjc.2012.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Merlo A, Casalini P, Carcangiu ML, Malventano C, Triulzi T, Menard S, et al. FOXP3 expression and overall survival in breast cancer. J Clin Oncol. 2009;27:1746–52. doi: 10.1200/JCO.2008.17.9036. [DOI] [PubMed] [Google Scholar]
- 53.Wing JB, Tanaka A, Sakaguchi S. Human FOXP3(+) regulatory T cell heterogeneity and function in autoimmunity and cancer. Immunity. 2019;50:302–16. doi: 10.1016/j.immuni.2019.01.020. [DOI] [PubMed] [Google Scholar]
- 54.Sugiyama D, Nishikawa H, Maeda Y, Nishioka M, Tanemura A, Katayama I, et al. Anti-CCR4 mAb selectively depletes effector-type FoxP3+CD4+ regulatory T cells, evoking antitumor immune responses in humans. Proc Natl Acad Sci USA. 2013;110:17945–50. doi: 10.1073/pnas.1316796110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Loi S, Michiels S, Adams S, Loibl S, Budczies J, Denkert C, et al. The journey of tumor-infiltrating lymphocytes as a biomarker in breast cancer: clinical utility in an era of checkpoint inhibition. Ann Oncol. 2021;32:1236–44. doi: 10.1016/j.annonc.2021.07.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated and analysed during this study are included in this article (and its supplementary information files).