Abstract
After 25 years of varicella vaccination in the United States, classic varicella and its complications have become an uncommon occurrence. The clinical manifestation of varicella among vaccinated persons is usually modified, with fewer skin lesions, mostly maculopapular, and milder presentation. However, the potential for severe manifestations from varicella still exists among both vaccinated and unvaccinated persons, and thus healthcare providers should keep varicella in the differential diagnosis of a maculopapular or vesicular rash. The prompt recognition and diagnosis of varicella is important because when confirmed, clinical and public health measures need to be taken swiftly.
Keywords: breakthrough, chickenpox, maculopapular rash, varicella vaccine, varicella-zoster virus
Varicella (chickenpox) is the result of primary infection with the varicella-zoster virus (VZV). After varicella, VZV becomes latent in neurons and can reactivate years or decades later to cause herpes zoster (HZ). Before 1995, varicella was a ubiquitous childhood disease, with approximately 95% of people in the United States becoming infected with VZV before adulthood [1]. Therefore, in the past, most adults had encountered and remembered the characteristic itchy vesicles of the varicella rash. However, in the 25 years since the introduction of the varicella vaccine in the United States, varicella incidence has declined more than 97% [2], making the disease an uncommon occurrence. Medical professionals and trainees who are now in their twenties were likely vaccinated as children and may not have encountered varicella in their personal lives or professional training. In this paper, we aim to aid the recognition of varicella and its clinical importance by describing the classic rash presentation and complications. In addition, we describe the nonclassic presentation of varicella in vaccinated persons and emphasize the need to maintain varicella in the differential diagnosis of skin rashes.
VARICELLA IN UNVACCINATED PERSONS
Classic Rash of Varicella
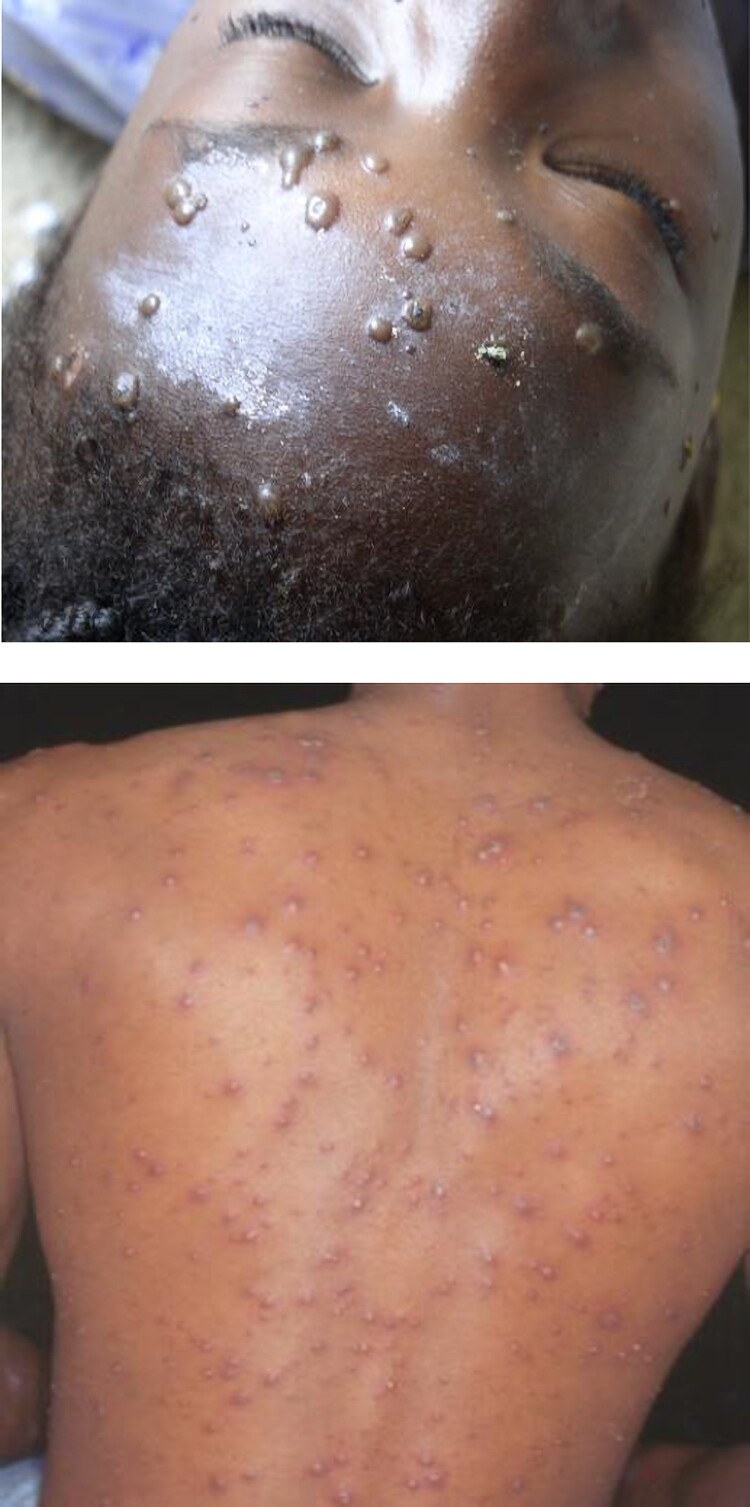
Primary infection with wild-type VZV is primarily acquired through the airborne route. The skin vesicles of patients with varicella or HZ are full of highly infectious virions. Aerosolized VZV virions from these lesions infect new hosts via the respiratory tract, almost exclusively in persons who have not had varicella or been vaccinated against the disease. Exposure has most commonly occurred from other persons with varicella but can also be from persons with herpes zoster rash. A primary viremia develops 4–6 days later, and approximately 14–16 days (range 10–21 days) after exposure, the onset of rash occurs. The infection manifests as a generalized, pruritic, maculopapular and vesicular rash, typically consisting of 250 to 500 skin lesions surrounded by an erythematous base [3, 4]. Lesions are superficial (located in the epidermis), appearing as “dew drops on a rose petal”, and of various sizes. The lesions develop in successive waves or “crops” for 3–7 days and therefore are in varying stages of development (macules, papules, vesicles) and resolution (pustular, crusting) when examined in the days after rash onset (Figure 1). As they dry, vesicles can appear umbilicated. Ultimately, scabs form and fall off, signaling the end of illness. Simultaneous existence of lesions at varying stages of development is pathognomonic of varicella.
Figure 1.
Classic varicella. Reproduced with permission from Red Book Online, Copyright © 2022 by the AAP.
Skin lesions usually start on the trunk or face and spread peripherally to the scalp and extremities, with occasional involvement of the palms and soles. The greatest concentration of lesions is usually on the trunk and proximally on the extremities. Vesicles can also develop on the mucosa (oropharynx, conjunctiva, trachea, vagina, and rectum) but rapidly rupture to form shallow ulcers that heal without forming scabs.
Other Signs and Symptoms
The rash can be preceded by a 1- to 2-day prodrome of fever and malaise and occasionally abdominal pain. The prodrome is more pronounced in adolescents and adults than in children, who can have no prodrome. Fever often accompanies the rash, the severity of which often mirrors the severity of the rash. Other common symptoms include headache, malaise, and anorexia. Involvement of other organ systems is rare; elevation of hepatic transaminases (frequently asymptomatic) has been reported.
Complications of Varicella
Complications of varicella include bacterial superinfection of lesions, usually with Streptococcal (including group A) and Staphylococcal infection, resulting in a local infection of skin and soft tissue or systemic infection, at times with sepsis [4, 5]. Neurologic complications include cerebellar ataxia (1 per 4000 cases of varicella in unvaccinated children), meningitis, and encephalitis (1 per 33 000–50 000 cases of varicella in unvaccinated children) [6–8]. Reye's syndrome can follow varicella, although this outcome has become very rare with the recommendation not to use salicylate-containing compounds (eg, aspirin, bismuth-subsalicylate) for children with varicella [9]. Thrombocytopenia and rarer complications such as glomerulonephritis, arthritis, and hepatitis can occur. Primary viral pneumonia is uncommon among immunocompetent children but is the most common complication among adults and other high-risk populations. Dehydration due to poor oral intake because of oral lesions is occasionally severe enough to require hospitalization. Rarely, varicella can lead to death. Complications from varicella led to 10 500–13 500 hospitalizations (4–5 per 100 000 population) and approximately 145 deaths (0.6 per 1 million population) per year before vaccine introduction in the United States [5, 10, 11].
Varicella During Pregnancy
Varicella during pregnancy can lead to serious maternal and fetal diseases [12]. Varicella can cause severe infection in pregnant women, often complicated by viral pneumonia, mainly if the infection occurs in the third trimester of pregnancy. The consequences for the fetus/infant depend on the time of infection. Maternal primary infection with VZV in the first or early second trimester can result in fetal anomalies known as congenital varicella syndrome (0.4%–2% of maternal infections through 20 weeks’ gestation) [13]. The most common anomalies involve the skin and skeleton, resulting in dermatomal cicatricial skin lesions, contractures, and limb hypoplasia [14, 15]. The central nervous system (microcephaly, paralysis, convulsions, encephalitis, intellectual delay) and eyes (microphthalmia, chorioretinitis, cataract, Horner syndrome) are involved in almost half of cases. Congenital varicella syndrome frequently results in fetal death or death in the neonatal period. Varicella infection in the third trimester can result in HZ in infancy or childhood. Perinatal varicella (if mothers develop varicella from 5 days before delivery to 2 days after birth) can result in disseminated neonatal varicella, which is life threatening, because infants are exposed to a large dose of VZV without sufficient maternal anti-VZV antibody to lessen the severity of disease [15]. Although rare, the devastating effects of congenital varicella syndrome and neonatal varicella emphasize the importance of protection against varicella for women of reproductive age.
Factors Affecting Varicella Severity
Risk factors for severe varicella include age, immunocompromised status, and pregnancy. Although the incidence of varicella is highest among children, the risk for severe disease, both hospitalizations and deaths, is significantly increased during infancy and adulthood [5, 11, 16]. During 1990–1995, in the 5 years before vaccine introduction in the United States, adults aged ≥20 years and infants aged ≤1 year had a 25 times and 4 times greater risk of dying, respectively, from varicella than did children 1–4 years old [11]. Varicella in immunocompromised patients can become very serious. The severity of varicella among immunocompromised children was the impetus for developing the varicella vaccine in Japan and studying it in the United States [17, 18]. Patients receiving chemotherapy, radiotherapy, high doses of steroids (transplantation, severe asthma), and those with congenital deficits of cell-mediated immunity are at greatest risk for developing severe varicella. By the early 1970s, 30% of children with leukemia who developed varicella manifested visceral dissemination, with 7% mortality [19]. Cases or contacts of a case who are pregnant, immunocompromised, or in the perinatal period should be evaluated for receipt of antiviral therapy (cases) or postexposure prophylaxis (contacts). In the era of antiviral therapy and improved supportive care, the prognosis has improved with treatment administered early in the course of illness, but deaths have continued to occur among the unvaccinated.
VARICELLA IN VACCINATED PERSONS
Nonclassic Varicella Presentation
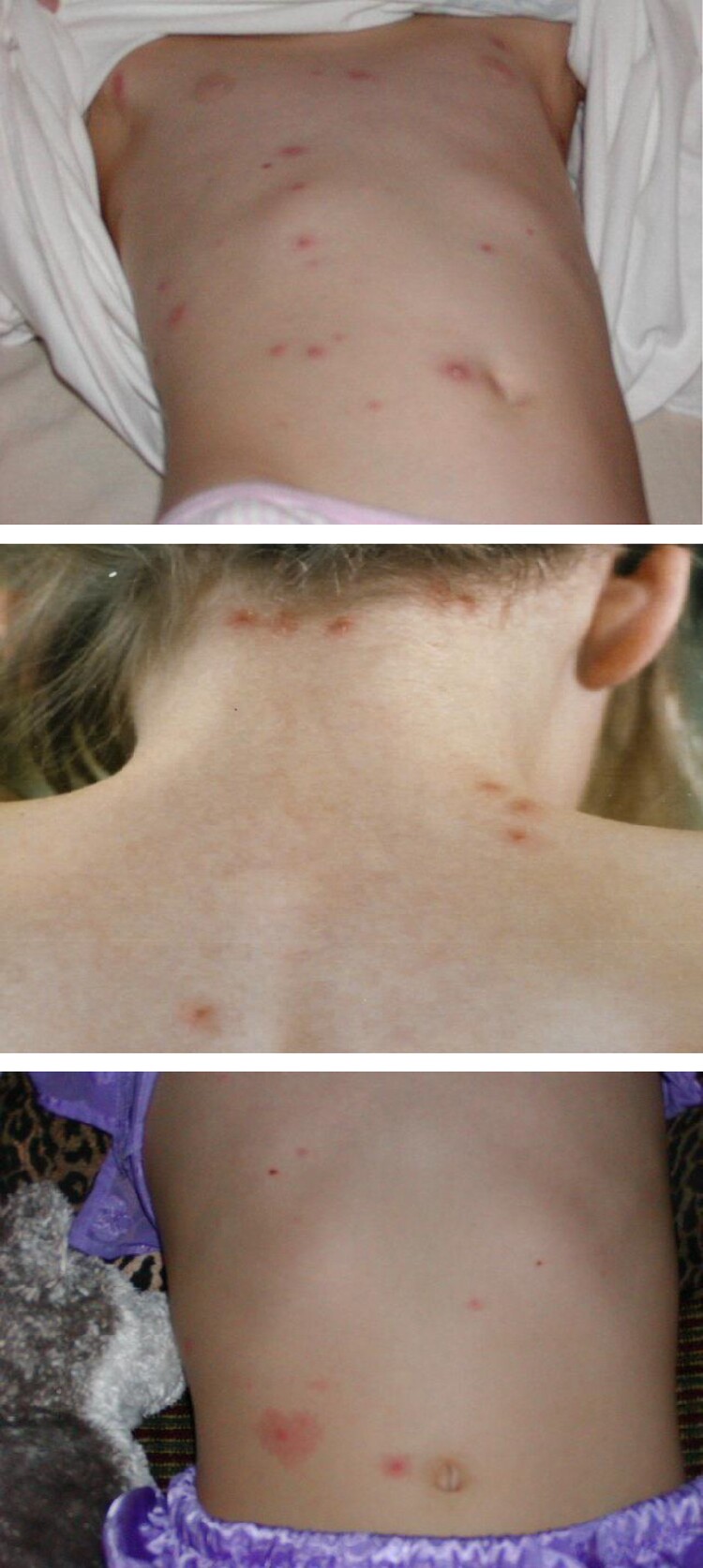
Although rare, infection with wild-type VZV can occur in vaccinated persons (termed breakthrough varicella). Breakthrough varicella cases have been reported among both 1- and 2-dose vaccine recipients although with greater frequency among 1-dose recipients compared to 2-dose recipients [20]. Protection against varicella of any severity is 92% vs 82%, after 2 and 1 dose of vaccine, respectively [21]. Breakthrough varicella rashes tend to be modified, predominantly maculopapular with no or a small proportion of vesicular lesions compared to a classic rash in an unvaccinated person (Figure 2). Most reports of varicella in children vaccinated with 1 dose describe the rash consisting of fewer than 50 lesions, less than one third of patients experiencing fever (usually low grade), and fewer days of illness compared to varicella in unvaccinated patients [22, 23]. Data suggest that breakthrough varicella might be further attenuated among 2-dose vaccine recipients [24, 25].
Figure 2.
Breakthrough varicella.
Reports of serious varicella-related complications in vaccinated persons are rare and have included meningitis, pneumonia, acute transverse myelitis, encephalitis, hepatitis, and sepsis [23, 26]. A systematic review through 2016 reported 6 cases of fatal breakthrough varicella, all in 1-dose vaccine recipients, with 5 of the 6 cases occurring in immunocompromised persons due to either medical conditions or medications [26]. Of reported breakthrough cases, only approximately 1% met the regulatory definition of “serious” in a postmarketing safety surveillance, and a significant proportion occurred in patients who developed an immunocompromising condition [27]. In the United States, severe varicella outcomes have become rare (1390 average annual hospitalizations in 2018–2019, 0.4 per 100 000 population; 29 average annual deaths in 2017–2019, 0.08 per 1 million population) [28]; moreover, deaths among persons aged <20 years born during the varicella vaccination program are virtually eliminated.
The nonclassic rash presentation of varicella in vaccinated persons can make diagnosis challenging. Mild herpes zoster, in particular, can be difficult to distinguish from breakthrough varicella because both present with a vesicular rash, although zoster tends to be more painful than varicella and vesicles tend to cluster in a single dermatome. In addition, impetigo, insect bites, herpes simplex or eczema herpeticum, hand, foot and mouth disease, molluscum contagiosum, monkeypox, vaccine-related varicella, as well as breakthrough varicella, should be considered in the differential diagnosis of a limited vesicular rash in a varicella-vaccinated person. This atypical presentation highlights the need for laboratory confirmation, including genotyping to differentiate vaccine-related from wild-type strains (see below) [29].
The diagnosis of breakthrough varicella is important because these cases are infectious. Overall, breakthrough cases are less infectious than infections in unvaccinated persons. However, contagiousness varies with the number of skin lesions: typical breakthrough cases (<50 lesions) are approximately one third as contagious as disease in unvaccinated persons, whereas breakthrough cases with ≥50 lesions are as contagious as cases in unvaccinated persons [30].
Laboratory Testing for Varicella
Given the modified presentation of varicella in vaccinated persons and unfamiliarity of many providers and the public with the presentations of varicella, laboratory diagnosis is becoming increasingly necessary [23, 29]. State public health and commercial laboratories can perform diagnostic tests for laboratory confirmation of varicella. Vesicular swabs and scabs from crusted lesions are the preferred specimens, and polymerase chain reaction (PCR) assays are the diagnostic method of choice for both unvaccinated and vaccinated patients [29, 31]. In the absence of vesicles or scabs, scrapings of maculopapular lesions can be collected for testing (https://www.cdc.gov/chickenpox/lab-testing/collecting-specimens.html). PCR is highly sensitive and specific in confirming modified disease if adequate samples are provided. In the differential diagnosis, providers should consider possible varicella-related complications when biologically plausible, even when varicella vaccine was received. It is also possible to distinguish vaccine-type VZV from wild type by examination of the viral deoxyribonucleic acid through specialized PCR testing [29, 31].
CONCLUSIONS
Widespread adoption of varicella vaccine in the United States has rendered varicella disease largely forgotten, but, unfortunately, it is not gone. Healthcare providers should keep varicella in the differential diagnosis of a maculopapular or vesicular rash, especially if there is local transmission, contact with a person with HZ, or recent travel outside the United States (varicella vaccine is not included in the World Health Organization list of routine vaccines and VZV circulates widely in many regions). Providers should be aware that the presentation of varicella is most commonly atypical and milder in a vaccinated person. The prompt recognition and diagnosis of varicella is important because clinical and public health measures need to be taken swiftly. For patients at higher risk of developing serious illness, varicella-zoster immune globulin after an exposure or antiviral treatment for disease may be indicated and should be administered as soon as possible after diagnosis [32]. From a public health point of view, varicella is a reportable disease in most states and, therefore, should be reported to local public health officials to inform disease surveillance and evaluate vaccine performance. In addition, household and school contacts should be protected, and if the person with varicella was seen in a medical facility, infection control personnel should be notified. The success of the varicella vaccine program in the United States calls for celebration but also continued vigilance.
Contributor Information
Kathleen Dooling, Division of Viral Diseases, National Center for Immunization and Respiratory Diseases, Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
Mona Marin, Division of Viral Diseases, National Center for Immunization and Respiratory Diseases, Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
Anne A Gershon, Columbia University College of Physicians and Surgeons, New York, New York, USA.
Notes
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Financial support. No financial support was received for this work.
Supplement sponsorship. This supplement is sponsored by the Centers for Disease Control and Prevention, Atlanta, GA, USA.
References
- 1. Kilgore PE, Kruszon-Moran D, Seward JF, et al. Varicella in Americans from NHANES III: implications for control through routine immunization. J Med Virol 2003; 70(Suppl 1):S111–8. [DOI] [PubMed] [Google Scholar]
- 2. Marin M, Leung J, Anderson TC, Lopez AS. Monitoring varicella vaccine impact on varicella incidence in the United States: surveillance challenges and changing epidemiology, 1995–2019. J Infect Dis 2022; 226(Suppl 4):S392–9. [DOI] [PubMed] [Google Scholar]
- 3. LaRussa P. Clinical manifestations of varicella. In: Arvin AM, Gershon AA (eds), Varicella-Zoster Virus: Virology and Clinical Management. Cambridge, UK: Cambridge University Press, 2000: pp 206–19. [Google Scholar]
- 4. LaRussa PS, Marin M, Gershon AA. Varicella-Zoster virus. In: Kliegman RM, St. Geme JW (eds), Nelson's Textbook of Pediatrics. 21th ed. Philadelphia: Elsevier, 2020: pp 374–81. [Google Scholar]
- 5. Galil K, Brown C, Lin F, Seward J. Hospitalizations for varicella in the United States, 1988 to 1999. Pediatr Infect Dis J 2002; 21:931–5. [DOI] [PubMed] [Google Scholar]
- 6. Guess HA, Broughton DD, Melton LJ 3rd, Kurland LT. Population-based studies of varicella complications. Pediatrics 1986; 78:723–7. [PubMed] [Google Scholar]
- 7. Preblud SR. Age-specific risks of varicella complications. Pediatrics 1981; 68:14–7. [PubMed] [Google Scholar]
- 8. Bozzola E, Tozzi AE, Bozzola M, et al. Neurological complications of varicella in childhood: case series and a systematic review of the literature. Vaccine 2012; 30:5785–90. [DOI] [PubMed] [Google Scholar]
- 9. Belay ED, Bresee JS, Holman RC, Khan AS, Shahriari A, Schonberger LB. Reye's syndrome in the United States from 1981 through 1997. N Engl J Med 1999; 340:1377–82. [DOI] [PubMed] [Google Scholar]
- 10. Davis MM, Patel MS, Gebremariam A. Decline in varicella-related hospitalizations and expenditures for children and adults after introduction of varicella vaccine in the United States. Pediatrics 2004; 114:786–92. [DOI] [PubMed] [Google Scholar]
- 11. Meyer PA, Seward JF, Jumaan AO, Wharton M. Varicella mortality: trends before vaccine licensure in the United States, 1970-1994. J Infect Dis 2000; 182:383–90. [DOI] [PubMed] [Google Scholar]
- 12. Gnann JW Jr. Varicella-zoster virus: prevention through vaccination. Clin Obstet Gynecol 2012; 55:560–70. [DOI] [PubMed] [Google Scholar]
- 13. Enders G, Miller E, Cradock-Watson J, Bolley I, Ridehalgh M. Consequences of varicella and herpes zoster in pregnancy: prospective study of 1739 cases. Lancet 1994; 343:1548–51. [DOI] [PubMed] [Google Scholar]
- 14. Birthistle K, Carrington D. Fetal varicella syndrome–a reappraisal of the literature. A review prepared for the UK Advisory Group on Chickenpox on behalf of the British Society for the Study of Infection. J Infect 1998; 36(Suppl 1):25–9. [DOI] [PubMed] [Google Scholar]
- 15. Enders G, Miller E. Varicella and herpes zoster in pregnancy and the newborn. In: Arvin AM, Gershon AA (eds), Varicella-Zoster Virus: Virology and Clinical Management. Cambridge, UK: Cambridge University Press, 2000: pp 317–47. [Google Scholar]
- 16. Wharton M. The epidemiology of varicella-zoster virus infections. Infect Dis Clin North Am 1996; 10:571–81. [DOI] [PubMed] [Google Scholar]
- 17. Takahashi M, Otsuka T, Okuno Y, Asano Y, Yazaki T. Live vaccine used to prevent the spread of varicella in children in hospital. Lancet 1974; 304:1288–90. [DOI] [PubMed] [Google Scholar]
- 18. Gershon AA, Steinberg SP, Gelb L, et al. Live attenuated varicella vaccine. Efficacy for children with leukemia in remission. JAMA 1984; 252:355–62. [DOI] [PubMed] [Google Scholar]
- 19. Feldman S, Hughes WT, Daniel CB. Varicella in children with cancer: seventy-seven cases. Pediatrics 1975; 56:388–97. [PubMed] [Google Scholar]
- 20. Kuter B, Matthews H, Shinefield H, et al. Ten year follow-up of healthy children who received one or two injections of varicella vaccine. Pediatr Infect Dis J 2004; 23:132–7. [DOI] [PubMed] [Google Scholar]
- 21. Marin M, Marti M, Kambhampati A, Jeram SM, Seward JF. Global varicella vaccine effectiveness: a meta-analysis. Pediatrics 2016; 137:e20153741. [DOI] [PubMed] [Google Scholar]
- 22. Bernstein HH, Rothstein EP, Watson BM, et al. Clinical survey of natural varicella compared with breakthrough varicella after immunization with live attenuated Oka/Merck varicella vaccine. Pediatrics 1993; 92:833–7. [PubMed] [Google Scholar]
- 23. Chaves SS, Zhang J, Civen R, et al. Varicella disease among vaccinated persons: clinical and epidemiological characteristics, 1997-2005. J Infect Dis 2008; 197(Suppl 2):S127–31. [DOI] [PubMed] [Google Scholar]
- 24. Leung J, Lopez AS, Marin M. Changing epidemiology of varicella outbreaks in the United States during the varicella vaccination program, 1995–2019. J Infect Dis 2022; 226(Suppl 4):S400–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Thomas CA, Shwe T, Bixler D, et al. Two-dose varicella vaccine effectiveness and rash severity in outbreaks of varicella among public school students. Pediatr Infect Dis J 2014; 33:1164–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Leung J, Broder KR, Marin M. Severe varicella in persons vaccinated with varicella vaccine (breakthrough varicella): a systematic literature review. Expert Rev Vaccines 2017; 16:391–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Galea SA, Sweet A, Beninger P, et al. The safety profile of varicella vaccine: a 10-year review. J Infect Dis 2008; 197(Suppl 2):S165–9. [DOI] [PubMed] [Google Scholar]
- 28. Marin M, Lopez AS, Melgar M, Curns AT, Dooling K, Leung J. Decline in severe varicella disease during the United States varicella vaccination program: hospitalizations and deaths, 1990–2019. J Infect Dis 2022; 226(Suppl 4):S407–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dollard S, Chen MS, Lindstrom S, Marin M, Rota PA. Diagnostic and immunologic testing for varicella in the era of high-impact varicella vaccination: an evolving problem. J Infect Dis 2022; 226(Suppl 4):S450–5. [DOI] [PubMed] [Google Scholar]
- 30. Seward JF, Zhang JX, Maupin TJ, Mascola L, Jumaan AO. Contagiousness of varicella in vaccinated cases: a household contact study. JAMA 2004; 292:704–8. [DOI] [PubMed] [Google Scholar]
- 31. Lopez A, Leung J, Schmid S, Marin M. Manual for the surveillance of vaccine-preventable diseases. Chapter 17: varicella. Available at: https://www.cdc.gov/vaccines/pubs/surv-manual/chpt17-varicella.html#case. Accessed 5 July 2022.
- 32. Marin M, Guris D, Chaves SS, et al. Prevention of varicella: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2007; 56:1–40. [PubMed] [Google Scholar]