Abstract
If foreign genes are ubiquitously expressed in mice using a viral vector, expression is abrogated by CD8+ cells in 2 to 4 weeks. However, if the expression of the genes is confined to skeletal muscle cells, the CD8+ T-cell response is much weaker and expression is maintained for more than 6 weeks. These data show that restricting the expression of foreign genes to skeletal muscle cells and presumably to other cells that are inefficient at antigen presentation can prolong the expression of a foreign gene product.
A major limitation in gene therapy is the rapid loss of cells expressing the foreign gene. This loss of cells is mediated by CD8+ T cells (TCD8+) specific for vector proteins or the transgene product (31, 35, 36). Although vectors have been developed that produce little or no vector gene products (4, 15, 24), the transgene product itself is often highly immunogenic, if it is absent from the host. For example, there are, in patients with Duchenne's or Becker's muscular dystrophy, large deletions in the coding region of the dystrophin gene that cause frameshifts (13). In such patients, if the normal protein is expressed using gene therapy, it may be recognized as foreign and provoke the immune system. Immune responses to factor VIII have been a major problem in patients undergoing replacement therapy for hemophilia (1, 5, 38). Similar problems would be encountered in treating Tay-Sachs disease, Sandhoff disease, certain types of cystic fibrosis, and other genetic diseases caused by mutations that abrogate gene expression (14, 25, 34). For gene therapy to be successful in situations like these in the absence of immunosuppressive drugs, it is vital to develop the means to deliver foreign proteins that do not provoke the recipient's immune system. In the present study we have examined the persistence of the expression of genes delivered to mice by recombinant avian retroviruses. Our findings point to a strategy that prolongs the expression of foreign genes by avoiding a powerful immune response.
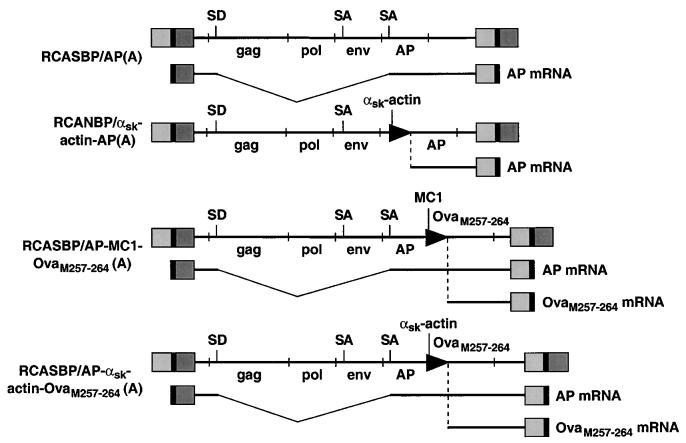
We used the avian sarcoma leukosis virus (ASLV)-derived vector RCASBP(A) to express the alkaline phosphatase (AP) gene or a strongly immunogenic peptide SIINFEKL, corresponding to residues 257 to 264 of chicken ovalbumin, which is recognized in association with H-2Kb by TCD8+ (7). The DNA constructs used to express the vectors and the corresponding mRNAs encoding AP and OvaM257–264 (the Met is needed for initiation and may be removed by Met-aminopeptidase) the vectors give rise to are diagrammed in Fig. 1. The expression of the mRNAs for AP and OvaM257–264 are controlled either by the retroviral long terminal repeat (LTR) or the MC1 or chicken α-skeletal-muscle (αsk)-actin internal promoters. The MC1 promoter is active in virtually all cell types (30); the chicken αsk-actin promoter is expressed primarily in striated muscle cells (26). The mice used for the avian retrovirus-mediated gene transfer are transgenic and express the gene for the receptor for subgroup A ASLV (tva) under the control of the ubiquitously expressed β-actin promoter (βAKE transgenic mice) (9). The subgroup A receptor is expressed in essentially all cells and/or tissues in these mice, and the virus can infect any dividing cell (12, 19, 33) it comes in contact with following intramuscular (i.m.) injection. The ability of the virus to infect the striated muscle cells of newborn mice drops precipitously after day 5 (8), when the myocytes cease rapid division. Consequently, all i.m. injections were performed on 1-day-old βAKE neonates.
FIG. 1.
Schematic representation of the ASLV proviral DNAs and mRNAs coding for the reporter genes, AP and OVAM257–264. The viral genes gag, pol, and env are shown (not to scale), as are the genes AP and OVAM257–264. Internal promoters are shown as arrowheads. The positions of splice donors (SD) and splice acceptors (SA) are also shown. The retroviral vectors RCASBP(A) and RCANBP(A) and the Cla12 adapter plasmid have been described elsewhere (18, 27), as has RCASBP/AP(A) (10). The αsk-actin promoter was cloned into the ClaI-EcoRI site of the Cla12 adapter plasmid, and the AP cDNA was cloned into the EcoRI-SalI site of the same adapter plasmid. The ClaI fragment containing the αsk-actin AP fragment was excised from the Cla12 adapter and cloned into the ClaI site of RCANBP(A) to produce RCANBP/αsk-actin AP(A). The MC1 promoter, kindly provided by Mario Capecchi, contains a polyomavirus enhancer and a minimal TK promoter. The two oligonucleotides 5′-CCCGCCTCTAGACTCGAGCAGTGTGGTTTTCAAGAGG-3′ and 5′-CCCGCCGTCGACTCAGAGCTTCTCGAAGTTGATGATCGACATGGTTGCAGGGTCGCTCGG-3′ were used to produce the MC1 OvaM257–264 PCR product. This product was cloned into the XbaI-SalI site of Cla12 that contained AP in the EcoRI site. The ClaI fragment that contained the AP-MC1-OvaM257–264 was excised from the Cla12 adapter and cloned into the ClaI site of RCASBP(A) to produce RCASBP/AP-MC1-OvaM257–264(A). The αsk-actin promoter was cloned into the SmaI-EcoRI site of pBluescript SK(+). The two oligonucleotides 5′-AATTCACCATGTCGATCATCAACTTCGAGAAGCTCTGAG-3′ and 5′-TCGACTCAGAGCTTCTCGAAGTTGATGATCGACATGGTG-3′ that code for the peptide were cloned into the EcoRI-SalI site of pBluescript SK(+) that contained the αsk-actin promoter. The XbaI-SalI fragment that contained the αsk-actin promoter linked to OvaM257–264 was cloned into the XbaI-Sa1I site of Cla12 that contained AP in the EcoRI site. The ClaI fragment that contained the AP–αsk-actin–OvaM257–264 segment was excised from the Cla12 adapter and cloned into the ClaI site of RCASBP(A) to produce RCASBP/AP–αsk-actin–OvaM257–264(A).
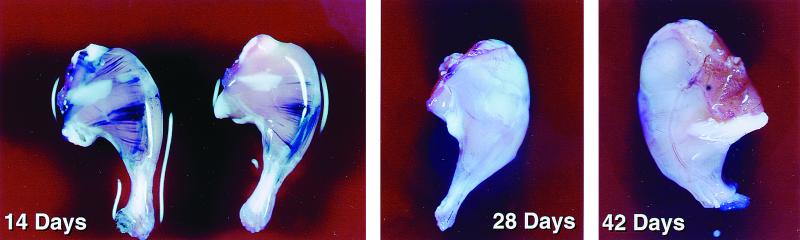
In the initial experiments, DF-1 tissue culture cells producing RCASBP/AP (A) were injected i.m. into the hind legs of βAKE neonates. DF-1 cells (16, 29) were grown in Dulbecco's modified Eagle medium (Life Technologies, Rockville, Md.) supplemented with 10% tryptose phosphate broth (Life Technologies), 5% fetal bovine serum (HyClone), 5% newborn calf serum (Advanced Biotechnologies, Columbia, Md.), 100 U of penicillin per ml, and 100 μg of streptomycin per ml. Virus production was initiated by transfection of plasmid DNA that contained the retroviral vector in proviral form, using the calcium phosphate precipitation method. DF-1 producer cells were harvested from four confluent 100-mm plates by trypsin, collected by centrifugation, and resuspended in 1 ml of cell supernatant. Each day-old neonatal mouse received an i.m. injection of 50 μl of this suspension. Hind legs were analyzed 14, 28, and 42 days later for the presence of AP (Fig. 2); four pups were used for each time point.
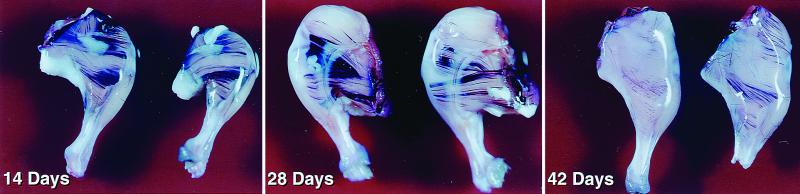
FIG. 2.
βAKE mice infected with RCASBP/AP(A). The mice were sacrificed on days 14, 28, and 42, and their legs were stained for AP (see the text). Muscle fibers that express AP can be seen as purple streaks.
The mouse legs were stained for AP according to the method described by Fields-Berry et al. (10) with minor modifications (8). Whole-leg mounts were fixed in 4% paraformaldehyde in phosphate-buffered saline (PBS) overnight at 4°C. Tissues were washed three times in PBS for 1 h for each wash and were heat treated (in PBS) at 65°C for 45 min to inactivate the endogenous AP activity. They were then washed twice for 10 min in AP detection buffer (100 mM Tris-Cl, pH 9.5; 100 mM NaCl; 50 mM MgCl2) and exposed to the AP chromogenic substrate nitroblue tetrolium and 5-bromo-4-chloro-3-indolylphosphate (Life Technologies). Enzymatically active AP produces an insoluble purple precipitate.
As expected, the virus successfully infected the striated muscle, as judged by clearly positive AP staining of individual muscle fibers of all four mice tested on day 14. However, AP was not detected in any in any of the pups tested 2 or 4 weeks later. One possible explanation for these results is that the infected tissues were destroyed by TCD8+ specific for either viral gene products or AP. Technical issues prevented us from directly measuring TCD8+ responses to either the viral proteins or AP. To monitor TCD8+ responses, we injected βAKE neonates with DF-1 cells producing RCASBP/AP-MC1 OvaM257–264(A). In this virus (see Fig. 1), OvaM257–264 is produced under the control of the ubiquitously active MC1 promoter. In a parallel set of control experiments, a recombinant vaccinia virus (rVV) was used to express OvaM257–264 in βAKE mice (2).
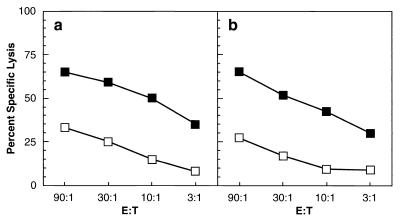
Splenocytes from mice injected with either OVAM257–264VV21 or RCASBP/MC1-OVAM257–264(A) virus-producing DF-1 cells were restimulated with 0.1 μM Ova257–264 (SIINFEKL) for 60 min at 37°C, washed, resuspended in 20 ml of Iscove modified Dulbecco's medium IMDM (Life Technologies) and cultured for 6 days. Subsequently, the effector cytotoxic T lymphocytes (CTLs) were centrifuged over Ficoll to remove dead cells. The live cells were harvested from the medium-Ficoll interface, washed, resuspended in IMDM, and plated in 96-well, round-bottom microtiter plates according to the effector/target (E:T) ratios indicated in the figures. Target cells (RMA) expressing H-2Kb were incubated with 0.1 μM SIINFEKL for 60 min at 37°C, washed, and labeled with Na51CrO4 (10 μCi) for 60 min at 37°C. The RMA target cells were then extensively washed and resuspended in IMDM, and incubated for an additional 15 min to allow the release of loosely incorporated 51Cr. Cells were collected by centrifugation and resuspended at 105/ml in IMDM, and 100 μl was plated into each well containing CTLs. RMA cells were also incubated without CTLs for the spontaneous release control or with cetrimide for the total release control. After a 4-h incubation, 100 μl of the supernatant was removed from each well, and the amount of 51Cr released was determined by gamma counting. Percent specific release was measured as follows: % specific release = (total release − spontaneous release)/total release × 100. Mice infected as day-old neonates with retrovirus-encoded OvaM257–264 had an easily detected TCD8+ response (Fig. 3).
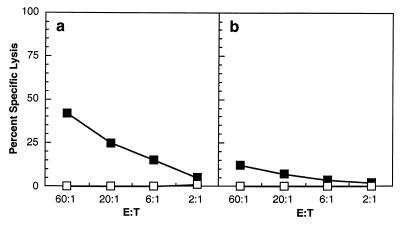
FIG. 3.
Splenocytes from adult C57BL/6 mice infected with OVAM257–264 VV (a) or 2-week-old βAKE mice injected on day 1 with RCASBP/AP-MC1 OVAM257–264(A) (b) virus-producing DF-1 cells were restimulated in vitro with 0.1 μM SIINFEKL and subsequently assayed for cytolytic activity against control (open boxes) or peptide-pulsed (closed boxes) targets at various E:T ratios (see the text).
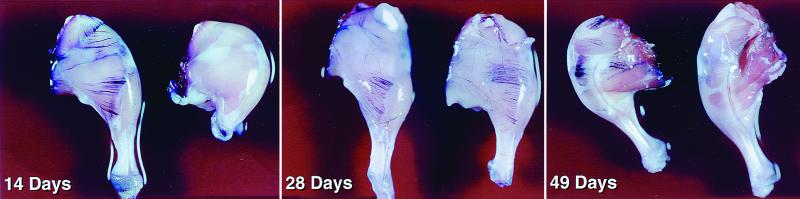
This finding is consistent with the idea that TCD8+ are involved in the time-dependent loss of AP expression following retrovirus infection. We tested this idea by generating mice that lack the transporter associated with antigen processing (TAP1) and that express the Tva receptor under the control of the β-actin promoter (TAP1−/−/βAKE mice). To produce TAP1−/− mice carrying the Tva receptor, the TAP1−/− mice were mated with the βAKE mice. The F1 progeny were intercrossed and their progeny were analyzed first for the TAP1−/− knockout by PCR as described by van Kaer et al. (32). Subsequently, the mice that had the TAP1−/− genotype were analyzed for the presence of the receptor as described by Federspiel et al. (9). TAP1 is resident in the endoplasmic reticulum and delivers cytosolic peptides to nascent class I molecules (6). TAP1−/− mice are doubly deficient in TCD8+-mediated immunosurveillance (32): they have a greatly reduced TCD8+ repertoire due to limited expression of self peptides with class I molecules in the thymus, and their antigen-presenting cells (APCs) demonstrate a greatly diminished capacity to present peptides derived from the cytosolic substrates. Six-day-old TAP1−/−/βAKE mice were injected with DF-1 cells producing RCASBP/AP(A); AP expression persisted for at least 49 days (Fig. 4). There was no AP expression in TAP1−/− mice that do not carry the tva receptor gene. This implies that the TCD8+ response is required for the elimination of transgene expression in the infected mice.
FIG. 4.
TAP1−/− mice carrying the Tva receptor infected with RCASBP/AP(A). The mice were sacrificed on days 14, 28, and 49, and their legs were stained for AP (see the text). Two of the legs (14 and 49 days) are negative for AP staining. These legs are from mice that do not have the subgroup A receptor.
Ideally, gene therapy should not require immunosuppression. Since muscle cells are poor APCs (11, 17, 20, 22), we examined the consequences of placing AP and OvaM257–264 under the control of the chicken αsk-actin promoter, which we have already shown is largely specific for striated muscle in transgenic mice (26). βAKE neonates were injected with DF-1 cells producing either RCASBP/AP-MC1 OvaM257–264(A) virus or RCASBP/AP–αsk-actin OvaM257–264(A). Spleens analyzed 14 days later for an OvaM257–264-specific TCD8+ response. As shown in Fig. 5, use of the αsk-actin promoter to express MSIINFEKL greatly reduced OvaM257–264-specific responses.
FIG. 5.
Splenocytes from 2-week-old βAKE mice injected on day 1 with RCASBP/AP-MC1 OVAM257–264(A) virus-producing DF-1 cells (a) or 2-week-old βAKE mice injected on day 1 with RCASBP/AP-αsk OVAM257–264(A) virus-producing DF-1 cells (b) were restimulated in vitro with 0.1 μM SIINFEKL and subsequently assayed for cytolytic activity against control (open boxes) or peptide-pulsed (closed boxes) targets at various E:T ratios (see the text).
We did detect a weak response to OvaM257–264 following infection with RCASBP/AP-αsk-actin OvaM257–264(A). This response might have been induced by infection of nonmuscle cells that could have expressed low amounts of OvaM257–264 due to leakiness of the αsk-actin promoter. Due to its preprocessed nature, the efficiency of generating major histocompatibility complexes with the OvaM257–264 peptide is at least 10-fold higher on a molar basis than from ovalbumin itself (28), which increases the probability of such complexes being formed and provoking the immune system. The fact that OvaM257–264 is preprocessed could also be a critical factor for an alternative, if less likely, explanation, i.e., that the response is due to presentation by the infected skeletal muscle cells. Even though muscle cells are believed to be relatively poor APCs, the preprocessed OvaM257–264 can be presented more efficiently than peptides derived from an intact protein. There is also the possibility that the OvaM257–264-specific response involves cross-priming, a phenomenon in which peptides or proteins expressed by nonprofessional APCs are acquired and presented by professional APCs (3).
Consistent with the weak CD8+ response to OvaM257–264 in these experiments, six mice were injected with the virus that expresses AP from the αsk-actin promoter. Expression of AP persisted for at least 42 days (Fig. 6). Since retroviral proteins could be expressed from the promoter in the viral LTR, the persistence of AP expression suggests either that the ASLV proteins are poorly expressed in nonmuscle cells or that they are poorly immunogenic under these conditions (37). Whichever explanation is correct, the results indicate that limiting the expression of AP and MSIINFEKL to skeletal muscle increases the persistence of AP expression and provides a strategy for enhancing gene therapy in humans.
FIG. 6.
βAKE mice infected with RCANBP/αskAP(A). The mice were sacrificed on days 14, 28, and 42, and their legs were stained for AP.
The data presented here provide a simple explanation for our prior findings with mice expressing the Tva receptor under the control of the αsk-actin promoter (8). In these mice, infection is confined to the skeletal muscle cells and foreign gene expression persists for more than 12 weeks. We now attribute the persistence of the expression of foreign gene products in this system to the lack of a strong immune response and the lack of an immune response to the fact that expression was confined to the muscle cells.
The present findings are consistent with what is now known about the induction of TCD8+ responses following immunization with DNA vaccines (37). Such vaccines usually involve the cytomegalovirus promoter, which is ubiquitously expressed. The ability to successfully transfect professional APCs has been implicated in the induction of a TCD8+ response to DNA vaccines in some studies. In other studies, the TCD8+ response seems to stem from cross-priming. Cross-priming of TCD8+ responses has been shown to occur following introduction of foreign cells, most recently for Ova expressed in the proximal tubules in kidneys of transgenic mice (23).
With the possible exception of MSIINFEKL, there does not seem to be significant cross-priming in our system. An obvious difference between retroviruses targeted to myocytes and DNA vaccines is that the latter should direct gene expression in any or all of the numerous nonprofessional APCs present in muscles, such as endothelial cells or fibroblasts, which may be better APCs than the myocytes and/or better sources of antigens for cross-priming. There are three major differences between our system and Ova-expressing transgenic mice. First, Ova-expressing mice were studied using adoptively transferred Ova-specific TCD8+ derived from T-cell receptor transgenic mice, and it is possible that these cells were more easily triggered than the (lower number of) naive normal Ova-specific TCD8+ in our study. Second, there may be differences between myocytes and kidney cells either in terms of their ability to release antigen or the ability of professional APCs to acquire antigens from these different cell types. Third, different antigens were studied, Ova versus AP/OvaM257–264 and, perhaps, retrovirus proteins. Cross-priming may be dependent on specific properties of the antigen and on the quantities of antigens synthesized by cells. The latter possibility points to the need to determine the extent to which our findings depend on the nature of the transgene and its level of expression.
Immunologically privileged sites such as the eye and the central nervous system that do not constitutively express class I molecules have traditionally been considered the best locales for transgenic therapies. For technical reasons, these tissues are generally poor targets for the current generation of vectors. Skeletal muscle offers several advantages as a site for transgene expression; skeletal muscle is readily accessible, abundant and, best of all, redundant and replaceable should immune responses be accidentally triggered. Although the system we have used here provides a useful model, the fact that simple retroviruses cannot successfully infect nondividing cells would limit the usefulness of the current version of the RCAS vectors for use in gene therapy. However, other viral vectors could be used. It was previously shown that adenovirus-associated virus is capable of inducing prolonged expression of foreign genes in skeletal muscle due to the absence of TCD8+ priming (21).
Acknowledgments
We thank Lori Sewell, Mary Beth Hilton, and Barbara Shankle for help in generating, maintaining, and injecting the transgenic mouse lines; Connie Cepko for the RCASBP/AP(A) vector; Luc Van Kaer for permission to use the TAP1−/− mice and help with the PCR analysis of the TAP1−/− phenotype; Bethany Buschling for the recombinant vaccinia virus vector; and Hilda Marusiodis for preparation of the manuscript.
This research was sponsored by the National Cancer Institute, DHHS, under contract with ABL.
REFERENCES
- 1.Arai M, Scandella D, Hoyer L W. Molecular basis of factor VIII inhibition by human antibodies: antibodies that bind to factor VIII light chain prevent the interaction of factor VIII with phospholipid. J Clin Investig. 1989;83:1978–1984. doi: 10.1172/JCI114107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bacik I, Cox J H, Anderson R, Yewdell J W, Bennink J R. Tap-independent presentation of endogenously synthesized peptides is enhanced by endocytoplasmic reticulum insertion sequences located at the amino but not carboxy terminus of the peptide. J Immunol. 1994;152:381–338. [PubMed] [Google Scholar]
- 3.Bevan M J. Cross-priming for a secondary cytotoxic response to minor H antigens with H-2 congenic cells which do not cross-react in the cytotoxic assay. J Exp Med. 1976;143:1283–1288. doi: 10.1084/jem.143.5.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen H-H, Mack L M, Kelly R, Ontell M, Kochanek S, Clemens P R. Persistence in muscle of an adenoviral vector that lacks viral genes. Proc Natl Acad Sci USA. 1997;94:1645–1650. doi: 10.1073/pnas.94.5.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ehrenforth S, Kreuz W, Scharrer I, Linde R, Funk M, Gungor T, Krackhardt B, Kornhuber B. Incidence of development of factor VIII and factor IX inhibitors in haemophiliacs. Lancet. 1992;339:594–598. doi: 10.1016/0140-6736(92)90874-3. [DOI] [PubMed] [Google Scholar]
- 6.Elliot T. Transporter associated with antigen processing. Adv Immunol. 1997;65:47–109. [PubMed] [Google Scholar]
- 7.Falk K, Rotzschke O, Derse K, Metzger J, Jung G, Rammensee H G. Identification of naturally processed viral nonapeptides allows their quantification in infected cells and suggests an allele-specific T cell epitope forecast. J Exp Med. 1991;174:425–434. doi: 10.1084/jem.174.2.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Federspiel M J, Bates P, Young J A T, Varmus H E, Hughes S H. Expression of transduced genes in mice generated by infecting blastocysts with avian leukosis virus-based retroviral vectors. Proc Natl Acad Sci USA. 1994;91:11241–11245. doi: 10.1073/pnas.93.10.4931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Federspiel M J, Swing D A, Eagleson B, Reid S W, Hughes S H. Expression of transduced genes in mice generated by infecting blastocysts with avian leukosis virus-based retroviral vectors. Proc Natl Acad Sci USA. 1996;93:4931–4936. doi: 10.1073/pnas.93.10.4931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fields-Berry S C, Halliday A L, Cepko C L. A recombinant retrovirus encoding alkaline phosphatase confirms clonal boundary assignment in lineage analysis of murine retina. Proc Natl Acad Sci USA. 1992;89:693–697. doi: 10.1073/pnas.89.2.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fisher K J, Joos K, Alston J, Yang Y, Haecker S E, High K, Pathak R, Raper S E, Wilson J M. Recombinant adeno-associated virus for muscle directed gene therapy. Nat Med. 1997;3:306–312. doi: 10.1038/nm0397-306. [DOI] [PubMed] [Google Scholar]
- 12.Fritsch E, Temin H M. Inhibition of viral DNA synthesis in stationary chicken embryo fibroblasts infected with avian retroviruses. J Virol. 1977;24:461–469. doi: 10.1128/jvi.24.2.461-469.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gillard E J, Chamberlain J S, Murphy E G, Duff C L, Smith B, Burghes A H M, Thompson M W, Sutherland J, Oss I, Bodrug S E, Klamut H J, Ray P N, Worton R G. Molecular and phenotypic analysis of patients with deletions within the deletion-rich region of Duchenne muscular dystrophy (DMD) gene. Am J Hum Genet. 1989;45:507–520. [PMC free article] [PubMed] [Google Scholar]
- 14.Gravel R A, Clarke J T R, Kaback M M, Mahuran D, Sandhoff K, Suzuki K. The Gm2 gangliosides. In: Scriver C V, Beaudet A L, Sly W S, Valle D, editors. The metabolic basis of inherited disease. Vol. 2. New York, N.Y: McGraw-Hill; 1995. pp. 2839–2879. [Google Scholar]
- 15.Haecker S E, Stedman H H, Balice-Gordon R J, Smith D B J, Greelish J P, Mitchell M A, Wells A, Sweeney H L, Wilson J M. In vivo expression of full-length human dystrophin from adenoviral vectors deleted of all viral genes. Hum Gene Ther. 1996;7:1907–1914. doi: 10.1089/hum.1996.7.15-1907. [DOI] [PubMed] [Google Scholar]
- 16.Himly M, Foster D N, Bottoli I, Iacovoni J S, Vogt P K. The DF-1 chicken fibroblast cell line: transformation induced by diverse oncogenes and cell death resulting from infection by avian leukosis viruses. Virology. 1998;248:295–304. doi: 10.1006/viro.1998.9290. [DOI] [PubMed] [Google Scholar]
- 17.Hohlfeld K, Engel A G. The immunology of muscle. Immunol Today. 1994;15:269–274. doi: 10.1016/0167-5699(94)90006-X. [DOI] [PubMed] [Google Scholar]
- 18.Hughes S H, Greenhouse J J, Petropoulos C J, Sutrave P. Adaptor plasmids simplify the insertion of foreign DNA into helper-independent retroviral vectors. J Virol. 1987;61:3004–3012. doi: 10.1128/jvi.61.10.3004-3012.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Humphries E H, Glover C, Reichmann M E. Rous sarcoma virus infection of synchronized cells establishes proviral integration during S-phase DNA synthesis prior to cellular division. Proc Natl Acad Sci USA. 1981;78:2601–2605. doi: 10.1073/pnas.78.4.2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iwasaki A, Niclas Stiernholm B J, Chan A K, Berinstein N L, Barber B H. Enhanced CTL responses mediated by plasmid DNA immunogens encoding costimulatory molecules and cytokines. J Immunol. 1997;158:4591–4601. [PubMed] [Google Scholar]
- 21.Jooss K, Yang Y, Fisher K J, Wilson J M. Transduction of dendritic cells by DNA viral vectors directs the immune response to transgene products in muscle fibers. J Virol. 1998;72:4212–4223. doi: 10.1128/jvi.72.5.4212-4223.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim J J, Bagarazzi M L, Trivedi N, Hu Y, Kazahaya K, Wilson D M, Ciccarelli R, Chattergoon M A, Dang K, Mahalingam S, Chalian A A, Agadjanyan M G, Boyer J D, Wang B, Weiner D B. Engineering of in vivo immune responses to DNA immunization via codelivery of costimulatory molecule genes. Nat Biotechnol. 1997;15:641–646. doi: 10.1038/nbt0797-641. [DOI] [PubMed] [Google Scholar]
- 23.Kurts C, Kosaka H, Carbone F R, Miller J F, Heath W R. Class I restricted cross-presentation of exogenous self-antigens leads to deletion of autoreactive CD8+ T cells. J Exp Med. 1997;186:239–245. doi: 10.1084/jem.186.2.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lieber A, He C-Y, Kirillova I, Kay M A. Recombinant adenoviruses with large deletions generated by Cre-mediated excision exhibit different biological properties compared with first-generation vectors in vitro and in vivo. J Virol. 1996;70:8944–8960. doi: 10.1128/jvi.70.12.8944-8960.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mahuran D J. The biochemistry of HEXA and HEXB gene mutations causing Gm2 gangliosidosis. Biochim Biophys Acta. 1991;1096:87–94. doi: 10.1016/0925-4439(91)90044-a. [DOI] [PubMed] [Google Scholar]
- 26.Petropoulos C J, Rosenberg M P, Jenkins N A, Copeland N G, Hughes S H. The chicken skeletal muscle α-actin promoter is tissue specific in transgenic mice. Mol Cell Biol. 1989;9:3785–3792. doi: 10.1128/mcb.9.9.3785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Petropoulos C J, Hughes S H. Replication-competent retrovirus vectors for the transfer and expression of gene cassettes in avian cells. J Virol. 1991;65:3728–3737. doi: 10.1128/jvi.65.7.3728-3737.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Porgador A, Yewdell J W, Deng Y, Bennink J R, Germain R N. Localization, quantitation, and in situ detection of specific peptide-MHC class I complexes using a monoclonal antibody. Immunity. 1997;6:715–726. doi: 10.1016/s1074-7613(00)80447-1. [DOI] [PubMed] [Google Scholar]
- 29.Shaefer-Klein J, Givol I, Barsov E V, Whitcomb J M, VanBrocklin M, Foster D N, Federspiel M J, Hughes S H. The EV-O derived cell lined DF-1 supports the efficient replication of avian leukosis-sarcoma viruses and vectors. Virology. 1998;248:305–311. doi: 10.1006/viro.1998.9291. [DOI] [PubMed] [Google Scholar]
- 30.Thomas K R, Cappecchi M R. Site-directed mutagenesis by gene targeting in mouse embryo-derived stem cells. Cell. 1987;51:503–512. doi: 10.1016/0092-8674(87)90646-5. [DOI] [PubMed] [Google Scholar]
- 31.Tripathy S K, Black H B, Goldwasser E, Leiden J M. Immune responses to transgene-encoded proteins limit the stability of gene expression after injection of replication-defective adenovirus vectors. Nat Med. 1996;2:545–550. doi: 10.1038/nm0596-545. [DOI] [PubMed] [Google Scholar]
- 32.Van Kaer L, Ashton-Rickardt P G, Ploegh H L, Tonegawa S. TAP1 mutant mice are deficient in antigen presentation, surface class I molecules, and CD4-8+ T cells. Cell. 1992;71:1205–1214. doi: 10.1016/s0092-8674(05)80068-6. [DOI] [PubMed] [Google Scholar]
- 33.Varmus H E, Padgett T, Heasly S, Simon G, Bishop J M. Cellular functions are required for the synthesis and integration of avian sarcoma virus-specific DNA. Cell. 1977;11:307–319. doi: 10.1016/0092-8674(77)90047-2. [DOI] [PubMed] [Google Scholar]
- 34.Welsh M J, Tsui L-C, Boat T F, Beaudet A L. Cystic fibrositis. In: Scriver C V, Beaudet A L, Sly W S, Valle D, editors. The metabolic basis of inherited disease. Vol. 3. New York, N.Y: McGraw-Hill; 1995. pp. 3799–3876. [Google Scholar]
- 35.Yang Y, Ertl H C J, Wilson J M. MHC class I-restricted T lymphocytes to viral antigens destroy hepatocytes in mice infected with E1-deleted recombinant adenoviruses. Immunity. 1994;1:433–442. doi: 10.1016/1074-7613(94)90074-4. [DOI] [PubMed] [Google Scholar]
- 36.Yang Y, Li Q, Ertl H C J, Wilson J M. Cellular and humoral immune responses to viral antigens create barriers to lung-directed gene therapy with recombinant adenoviruses. J Virol. 1995;69:2004–2015. doi: 10.1128/jvi.69.4.2004-2015.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yewdell J W, Norbury C C, Bennink J R. Mechanism of exogenous antigen presentation by MHC class I molecules in vitro and in vivo: implications for generating CD8+ T cell responses to infectious agents, tumors, transplants, and vaccines. Adv Immunol. 1999;73:1–77. doi: 10.1016/s0065-2776(08)60785-3. [DOI] [PubMed] [Google Scholar]
- 38.Zhong D, Saenko E L, Shima M, Feich M, Scandella D. Some human inhibitor antibodies interfere with factor VIII binding to factor IX. Blood. 1998;92:136–142. [PubMed] [Google Scholar]