Abstract
Background:
Cryoballoon ablation (CBA) is an established approach for rhythm management of atrial fibrillation (AF).
Objective:
We sought to assess balloon temperature (BT) parameters as predictors of pulmonary vein (PV) reconnection and AF recurrence following CBA.
Methods:
BT was monitored in 119 AF patients undergoing CBA. PVs were assessed for reconnection during the procedure and patients were followed for arrhythmia recurrence.
Results:
PV reconnection was identified in 39 (8.3%) of 471 PVs. BT was significantly colder in the absence of PV reconnection (30s: −33.5°C vs −29.5°C, p=0.001; 60s: −41°C vs −36.5°C, p<0.001; nadir: −47°C vs −41.5°C, p<0.001). PV reconnection was associated with significantly longer time to reach −15°C and −40°C (14.5s vs 12s, p=0.023; and 75s vs 46s, p=0.005) and shorter rewarming time (5.75s vs 7s, p=0.012). ROC analysis of these procedural parameters had an AUC=0.71 in predicting PV reconnection. During an average follow-up period of 559 days, AF recurrence occurred in 51 (42.8%) patients. Kaplan Meier analysis showed better arrhythmia free survival for patients in whom BT decreased below −40°C in all PVs and patients who had no early PV reconnections, compared to patients in whom BT below −40°C was not achieved in at least one PV (Log Rank=6.3, p=0.012) and patients who had PV reconnections (Log Rank=4.1, p=0.043).
Conclusion:
Slower BT decline, warmer BT nadir, and faster rewarming time predict early PV reconnection. Absence of early PV reconnections and BT dropping below −40°C in all PVs during CBA are associated with lower rates of AF recurrence.
Keywords: atrial fibrillation, cryoballoon ablation, pulmonary vein reconnection, arrhythmia recurrence, balloon temperature
Introduction
Pulmonary vein isolation by catheter ablation is the current recommendation for symptomatic atrial fibrillation (AF)1. The success rate of catheter ablation in maintaining sinus rhythm is universally superior to that of antiarrhythmic drugs2–5. Radiofrequency ablation (RFA) is the most widely used method, applied in a point-by-point mode, whereas cryoballoon ablation (CBA) applies cryogenic energy with a balloon catheter in a single application.
Lesion formation in CBA occurs via convective cooling. The cryorefrigerant delivered absorbs the heat of tissue surrounding the catheter, in contrast to RFA, in which lesion formation is due to necrosis by tissue heating.
CBA is as effective as RFA for treatment of paroxysmal AF6 and offers several advantages. First, CBA is associated with freeze-mediated catheter adhesion to the target tissue, which is beneficial for technically challenging regions7. Second, CBA lesions are associated with minimal endothelial surface disruption compared to RFA, significantly lowering the incidence of overlying thrombosis8,9. Third, CBA results in dense, homogenous, and clearly demarcated lesions that are speculated to (1) be less arrhythmogenic than indistinct RFA lesions10 and (2) preserve ultrastructural tissue integrity, thereby reducing risk of myocardial perforation, esophageal injury, and pulmonary vein stenosis7. CBA is widely used today for the reasons above; however, arrhythmia recurrence continues to occur, partly due to pulmonary vein reconnection from recovered conduction11.
We sought to assess balloon temperature parameters as predictors of intraprocedural PV reconnection and AF recurrence following CBA.
Methods
Study design
This was an observational study of 119 patients presenting for cryoballoon catheter ablation for paroxysmal and persistent AF at the University of Washington Medical Center. Access to patient information was approved by the Institutional Review Board (IRB) of the University of Washington and all participants provided verbal consent (HSD#6058) for use of their anonymized clinical data for research purposes. The REDCap system hosted at the University of Washington was used to collect and manage study data11,12. Heart Rhythm Society consensus criteria were used to determined persistent AF status13.
Catheter ablation
All catheter ablation procedures were performed under general anesthesia. A single trans-septal approach was used for left atrial access. CARTO (Biosense Webster, Inc.) or Precision (Abbott, Inc) systems were used for electro-anatomical mapping to create a virtual geometry of the left atrium prior to CBA.
All patients were maintained on uninterrupted anticoagulation for at least 3 weeks prior to the procedure which was continued afterwards. Heparin was administered before transseptal puncture and throughout the procedure to maintain an activated clotting time (ACT) between 350–450 seconds (s).
A 2nd-generation 28-mm catheter (Arctic Front Advance, Medtronic) was used in CBA to target each pulmonary vein antrum. The standard approach was to deliver two consecutive 180 seconds freeze applications to each pulmonary vein antrum after optimizing the balloon location and seal using a contrast injection. If entrance block was observed within 60 s then the second freeze was shortened to 120 s. The freeze application was discontinued if the balloon temperature dropped to below −55 °C. Entrance and exit block were confirmed post ablation. Early PV reconnection was assessed after an observation period of 30 minutes following isolation of all PVs. If a given PV was reconnected, additional cryo-applications were performed until PV isolation was achieved. RFA was used to complete the pulmonary vein isolation it this was not achieved after additional freeze applications failed to achieve isolation.
The phrenic nerve was monitored during the ablation of the right pulmonary veins by pacing the nerve from the superior vena cava. Phrenic nerve capture was monitored via contractions of the right hemidiaphragm by manual palpation of the abdomen.
Ablation related parameters
Balloon temperature (BT) recorded by the console during CBA targeting each of the four pulmonary veins was analyzed offline to determine time needed to reach −15°C and −40°C from the start of the freeze. BT at 30 s, 60 s and the nadir point were also recorded. Rewarming, depicted by thaw time from the nadir point to 0 °C, was also determined.
Post ablation follow-up
Anti-arrhythmic drugs were typically stopped after the 3-month blanking period. Continuous ambulatory monitoring was performed for 7 days at 3, 6, and 12 months after ablation and annually thereafter. Additional 12 lead ECGs or ambulatory monitors were performed as needed based on reported symptoms. Any atrial arrhythmia episode lasting more than 30 s and recorded on a surface ECG or ambulatory monitor, after an initial 90 day post procedure blanking period, was considered a recurrence.
Statistical analysis
Categorical variables are expressed as percentages. Continuous variables were assessed for normality of distribution using the Shapiro-Wilk test and were reported as mean ± SD if normally distributed, or median and interquartile range if not. For continuous variables, differences between two groups were compared using the Student’s t-test (for parametric variables) and the Mann-Whitney U test (for non-parametric variables). Categorical variables were compared using the chi-square test or Fischer’s exact test. A logistic regression model with the procedural parameters was created to predict early PV reconnection and a receiver operating characteristic (ROC) curve analysis was used to determine the predictive performance of these variables for early PV reconnection. AF recurrence-free survival was then estimated by the Kaplan-Meier method and compared using a log-rank test between the study groups. All tests were 2-sided and a p<0.05 was considered statistically significant. Statistical analysis was performed using SPSS Statistics (version 26.0, International Business Machines Inc) and R Statistical Software version 4.1.1 (R Foundation for Statistical Computing).
Results
Baseline characteristics
A total of 119 patients were included in this study. 471 PVs were analyzed. The average age was 65.1±11.9 years (66% male). Body mass index was 30.45 ±7.29 kg/m2. The majority of patients had paroxysmal AF (61.9%). 15 patients were lost to follow up after the post ablation blanking period and 104 patients were followed for arrhythmia recurrence.
Predictors of PV reconnection
Reconnection occurred in 39 (8.3%) of 471 PVs. BT at 30 s, 60 s and the nadir were significantly lower in the absence of PV reconnection (−33.5°C vs −29.5°C, p=0.001; −41°C vs −36.5°C, p<0.001; −47°C vs −41.5°C, p<0.001 respectively). Time to reach −15°C and −40°C were also significantly shorter in the absence of PV reconnection (12 s vs 14.5 s, p=0.023; and 46 s vs 75 s, p=0.005), whereas rewarming time was longer (7s vs 5.75s, p=0.012). Freezing time was not different between the 2 groups (180 [172.37, 180] vs 180 [170, 180], p=0.91), as well as the BT at the beginning of the freeze (31.5 [30.5, 33] vs 31.67 [30.5, 33], p=0.518). Table 1 outlines the different procedural parameters and observed differences between PVs that showed reconnection and those that did not. ROC analysis of these procedural parameters had an AUC=0.71, p<0.001 in predicting PV reconnection (Figure 2).
Table 1:
Procedural parameters in the prediction of early PV reconnection (BT: balloon temperature, PV: pulmonary vein, s: second, °C: degree Celsius).
| All PVs (471) | No PV reconnection (432) | PV reconnection (39) | p value | |
|---|---|---|---|---|
| Freeze time (s) | 180 [172.3, 180] | 180 [172.37, 180] | 180 [170, 180] | 0.91 |
| BT nadir (°C) | −47 [−52, −42.3] | −47 [−52, −43] | −41.5 [−47, −38] | <0.001 |
| Starting BT | 31.5 [30.5, 33] | 31.5 [30.5, 33] | 31.67 [30.5, 33] | 0.518 |
| BT at 30s (°C) | −33 [−36, −29.5] | −33.5 [−36, −30] | −29.5 [−35, −25.5] | 0.001 |
| BT at 60s (°C) | −40.75 [−44, −37] | −41 [−44, −37] | −36.5 [−42, −33.5] | <0.001 |
| Time to reach −15°C (s) | 12 [10, 15.5] | 12 [10, 15.5] | 14.5 [11.5, 18.5] | 0.023 |
| Time to reach −40°C (s) | 46.75 [37, 71] | 46 [37, 66.7] | 75 [40,95.5] | 0.005 |
| Time to reach 0°C (s) | 7 [5.5, 9] | 7 [6,9] | 5.75 [4.75, 8.5] | 0.012 |
Figure 2:
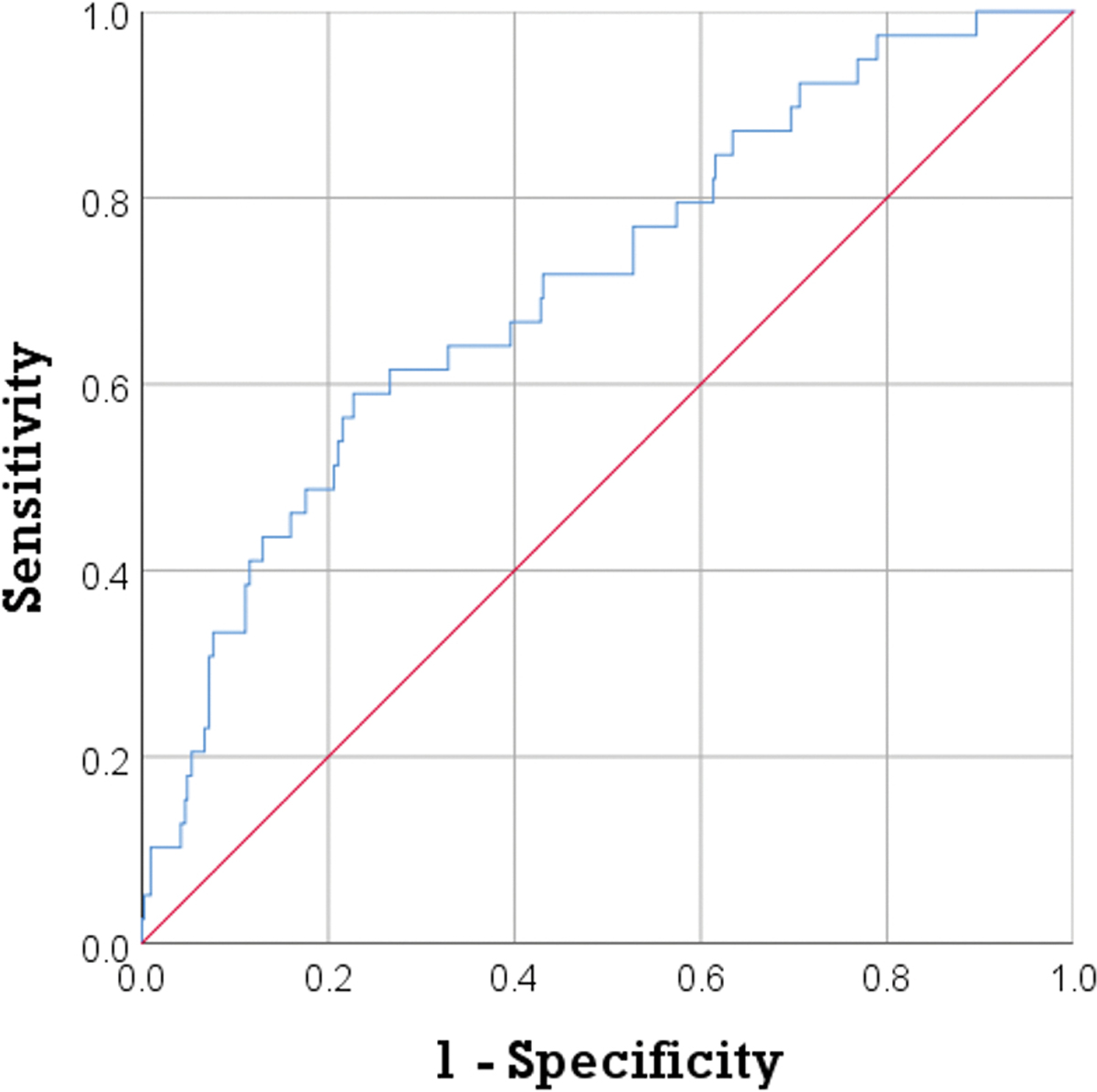
ROC analysis of procedural parameters during cryoballoon ablation showed an AUC=0.71, p<0.001 in predicting early PV reconnection. AUC: area under the curve, PV: pulmonary vein, ROC: receiver operating characteristic.
AF recurrence post ablation
After an average follow-up period of 559 days, AF recurrence occurred in 51 (49%) patients. The two groups of patients showed no differences in relation to comorbidities, except a higher rate of persistent AF in those with recurrent arrhythmia (52.9% vs 26.4%, p=0.006). There were no significant differences between other baseline characteristics for patients with and without AF recurrence; these variables are summarized in Table 2. Kaplan Meier analysis for recurrence free survival showed that patients in whom BT decreased below −40°C in all PVs had better arrhythmia free survival compared to patients in whom BT below −@°C was not achieved in at least one PV (Log Rank = 6.3, p=0.012). Similarly arrhythmia free survival was better in patients who had no PV reconnections compared to those who had PV reconnections (Log Rank = 4.1, p=0.043) (Figure 3).
Table 2:
Baseline characteristics for patients with and without AF recurrence following ablation (AF: atrial fibrillation, BMI: body mass index).
| No AF recurrence (53) | AF recurrence (51) | p value | |
|---|---|---|---|
| Age (years) | 63.9 ± 11.52 | 66.1 ± 12.67 | 0.354 |
| BMI (kg/m2) | 30.45 ± 7.41 | 30.22 ± 7.55 | 0.879 |
| Sex, male | 35 (66%) | 35 (68.6%) | 0.778 |
| Hypertension | 28 (52.8%) | 31 (60.8%) | 0.413 |
| Coronary artery disease | 8 (15.1%) | 13 (25.5%) | 0.187 |
| Congestive heart failure | 6 (11.3%) | 11 (21.6%) | 0.158 |
| Obstructive sleep apnea | 17 (32.1%) | 24 (47.1%) | 0.118 |
| Stroke | 5 (9.4%) | 5 (9.8%) | 1 |
| Hyperlipidemia | 25 (47.2%) | 18 (35.3%) | 0.219 |
| Diabetes mellitus | 10 (18.9%) | 10 (19.6%) | 0.924 |
| Non-paroxysmal AF | 14 (26.4%) | 27 (52.9%) | 0.006 |
Figure 3:
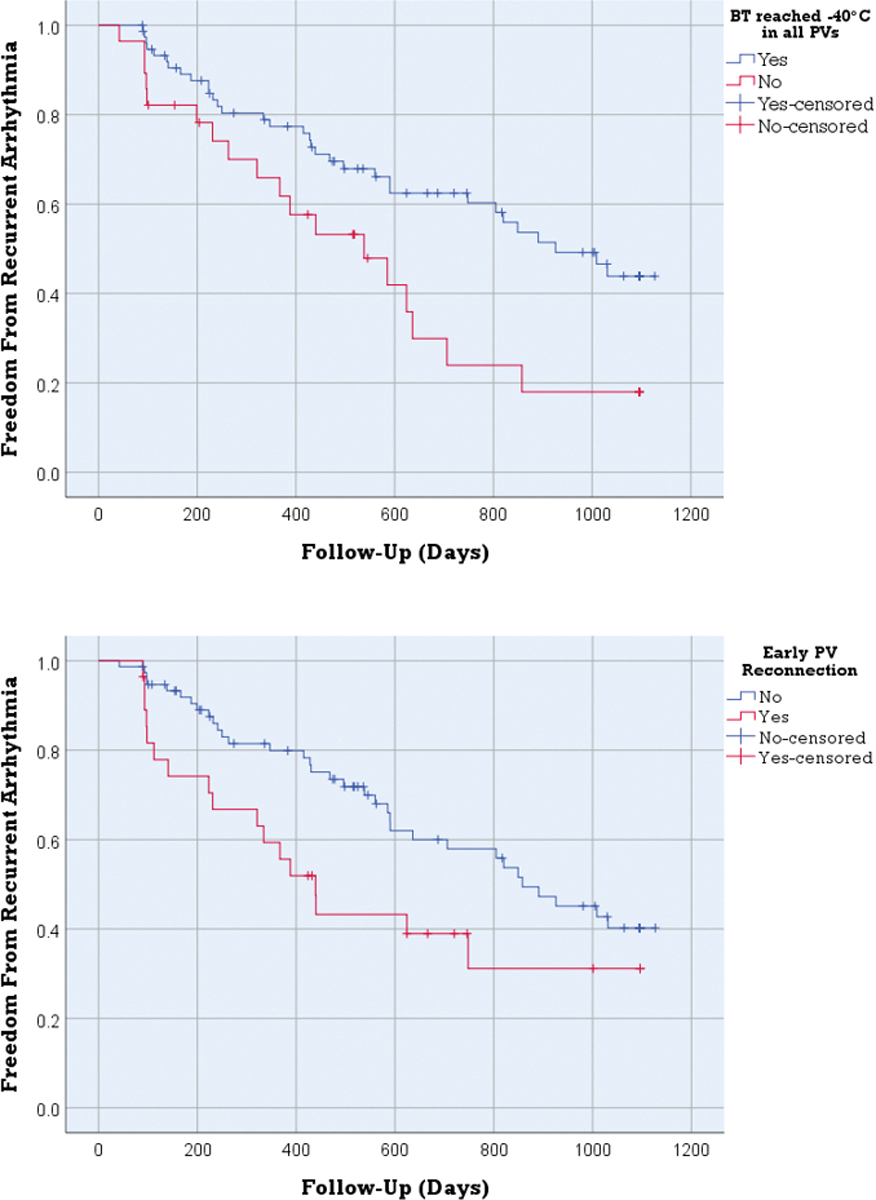
Better arrhythmia free survival was detected in patients where BT decreased below −40°C in all PVs (A) and patients who had no early PV reconnections (B), compared to patients where BT below −40°C was not achieved in at least one PV (Log Rank = 6.3, p=0.012) and patients who had PV reconnections (Log Rank = 4.1, p=0.043). BT: balloon temperature, PV: pulmonary vein.
Discussion
This study aimed to assess the balloon temperature indicators of PV reconnection and AF recurrence in patients undergoing CBA. The main findings of our study are: first, PV reconnection can be predicted by a slower decline in BT, a warmer nadir BT and a shorter rewarming time. Second, absence of early PV reconnections and achieving a BT below −40°C in all PVs were both associated with a lower rate of AF recurrence.
Cold-induced cellular injury is mainly attributed to the formation of ice crystals in the extracellular (at −15°C) and intracellular spaces (at −40°C), in addition to ischemic cell death caused by microcirculatory failure14. The extracellular ice crystals create a hypertonic milieu which induces an osmotic followed by a diffusion gradient leading to cell shrinkage, cellular protein damage, enzyme impairment and eventually cell death. Intracellular ice crystals are associated with mechanical cellular disruption leading to membrane lysis and irreversible cellular damage. Tissue freezing results in vasoconstriction, hypoperfusion and ischemic necrosis7. Our findings showed that a shorter time to reach −15°C and −40°C, and thus longer time spent below these temperatures, was associated with absence of early PV reconnection. Although BT is not an accurate measure of absolute tissue temperature, it provides an approximate measure of tissue temperature while in contact with the PV antral region.
Subsequent tissue rewarming at the end of the freeze produces a hyperemic response with increased vascular permeability and edema formation. We found that a longer rewarming time was associated with absence of early PV reconnection. Ice recrystallization occurs during the thawing phase. Intra- and extracellular ice crystals rewarm during this phase, and while they transition back to water their size and structure dynamically change further injuring the tissue. Therefore, a longer rewarming time may not only be a marker of an effective freeze but it can also cause the ablation to be more effective. The final phase of lesion formation in CBA is characterized by reactive inflammation followed by tissue repair and fibrosis formation7.
There is limited data on procedural parameters during cryoablation and early PV reconnection. In a recent study with a relatively small sample size (n=50), a nadir temperature lower than −51°C and a rewarming time longer than 28 s predicted the absence of spontaneous and adenosine induced early PV reconnections15. In another study, only the freezing temperature slope, which was also predictive of the temperature nadir, was correlated to acute PV reconnection; in this case, the authors suggested that temperature slope would be useful in deciding on pull-down maneuvers or aborting the cryoablation early on16. Keçe et al showed that a warmer nadir temperature, a higher number of unsuccessful freezes and a longer time to isolation were independently associated with early reconnection, arguing that the use of these parameters during cryoballoon ablation can help avoid the 30-minute waiting period and adenosine testing to check for early PV reconnection17. On the other hand, durable PV isolation and absence of late PV reconnection were associated with a time to isolation ≤ 60 s and an interval thaw time ≥ 10 s18.
Our findings provide valuable information to operators who can use these procedural indicators to predict early PV reconnection, and perhaps adjust their ablation procedures. For example, operators can gently pull on the balloon catheter to improve contact with the ostium or abort the freeze early on and try to achieve better contact. Individualizing the cryoablation strategy has been shown to allow shorter AF ablation without affecting the procedural outcome19.
We also found that absence of early PV reconnection and achieving a BT below −40°C in all PVs was associated with a lower rate of AF recurrence. Although additional applications are performed if a PV shows reconnection, patients who had early reconnections were at a higher risk for AF recurrence in our study cohort. In an study by Efremidis et al, PV reconnection 30 minutes after PVI was associated with late AF recurrence in patients with paroxysmal AF, however the strategy of waiting 30 minutes and re-ablating did not appear to be superior to immediate termination of the procedure after initial PVI20. One possible explanation would be anatomical characteristics that can prevent maximal heart transfer from the tissue to the balloon. In a study by Knecht et al, multiple anatomical variables were shown to predict early and midterm cryoballoon PVI failure (a continuous sharp left lateral ridge between the left PVs and the left lateral appendage, a sharp carina between the left superior and left inferior PV and those with early branching of the right inferior PV with change in PV axis)21. Additionally, exposing all PVs to a BT below −40°C, will increase the likelihood of intracellular ice crystal formation in the tissue leading to a more robust and stable cryoinjury, thus a lower rate of AF recurrence.
Limitations:
Our study is a single center observational study with a relatively small sample size analyzing procedural indicators of early PV reconnection and AF recurrence. The results of this study need to be validated by larger cohort studies. In addition, our analysis was limited to intra-procedural reconnection, it would be interesting to study predictors of late reconnection and its relation to AF recurrence. Post ablation recurrence was denoted based on documented events; patients who had a recurrence of symptoms but without AF documentation were not listed as recurrences. Longer rhythm monitoring could have detected more recurrent AF episodes.
Conclusion
Slower BT decline, warmer BT nadir, and faster rewarming time predict early PV reconnection. A lower rate of AF recurrence was associated with absence of early PV reconnections and achieving a BT below −40 °C in all PVs.
Figure 1:
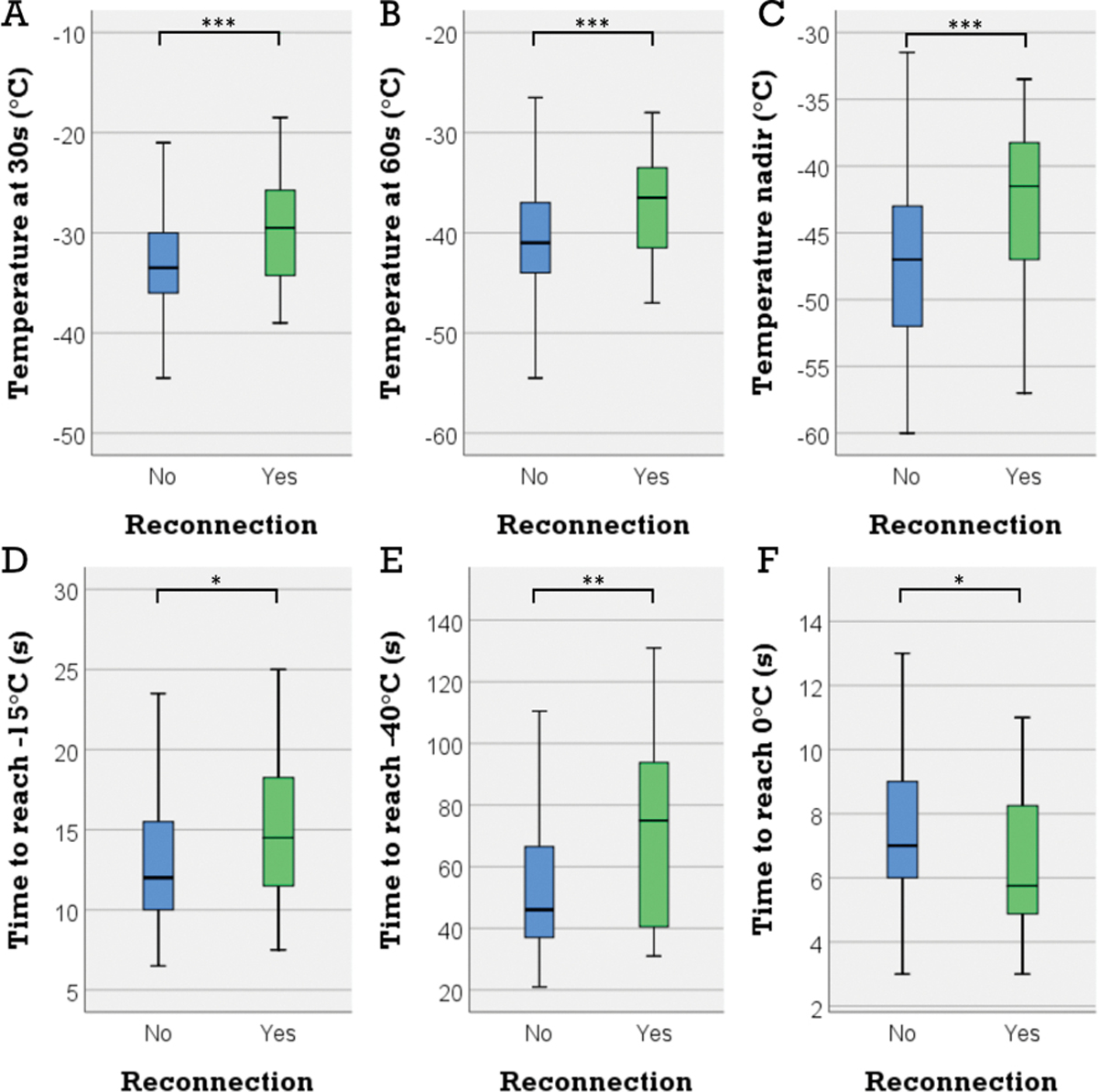
BT was significantly colder in the absence of early PV reconnection (30 s: −33.5°C vs −29.5°C, p=0.001 (A); 60 s: −41°C vs –36.5°C, p<0.001 (B); nadir: −47°C vs –41.5°C, p<0.001 (C)). Early PV reconnection was associated with significantly longer time to reach −15°C and −40°C (14.5s vs 12s, p=0.023 (D); and 75s vs 46s, p=0.005 (E)) and shorter rewarming time (5.75s vs 7s, p=0.012). BT: balloon temperature, PV: pulmonary vein.
Acknowledgments
We acknowledge the tremendous support of the Division of Cardiology Section of Electrophysiology and the Department of Bioengineering at the University of Washington.
Funding:
This work was supported by the John Locke Charitable Trust to NA, and by NIH R01-HL158667 to PMB and NA
Footnotes
Conflict of interest: The authors declare that they have no competing interest
References:
- 1.Stabile G, Bertaglia E, Senatore G, et al. Catheter ablation treatment in patients with drug-refractory atrial fibrillation: a prospective, multi-centre, randomized, controlled study (Catheter Ablation For The Cure Of Atrial Fibrillation Study). Eur Heart J. 2006;27(2):216–221. doi: 10.1093/EURHEARTJ/EHI583 [DOI] [PubMed] [Google Scholar]
- 2.Packer DL, Kowal RC, Wheelan KR, et al. Cryoballoon ablation of pulmonary veins for paroxysmal atrial fibrillation: First results of the North American arctic front (STOP AF) pivotal trial. J Am Coll Cardiol. 2013;61(16):1713–1723. doi: 10.1016/j.jacc.2012.11.064 [DOI] [PubMed] [Google Scholar]
- 3.Wilber DJ, Pappone C, Neuzil P, et al. Comparison of antiarrhythmic drug therapy and radiofrequency catheter ablation in patients with paroxysmal atrial fibrillation: a randomized controlled trial. JAMA. 2010;303(4):333–340. doi: 10.1001/JAMA.2009.2029 [DOI] [PubMed] [Google Scholar]
- 4.Pappone C, Augello G, Sala S, et al. A randomized trial of circumferential pulmonary vein ablation versus antiarrhythmic drug therapy in paroxysmal atrial fibrillation: the APAF Study. J Am Coll Cardiol. 2006;48(11):2340–2347. doi: 10.1016/J.JACC.2006.08.037 [DOI] [PubMed] [Google Scholar]
- 5.Jaïs P, Cauchemez B, Macle L, et al. Catheter ablation versus antiarrhythmic drugs for atrial fibrillation: the A4 study. Circulation. 2008;118(24):2498–2505. doi: 10.1161/CIRCULATIONAHA.108.772582 [DOI] [PubMed] [Google Scholar]
- 6.Kuck KH, Brugada J, Fürnkranz A, et al. Cryoballoon or Radiofrequency Ablation for Paroxysmal Atrial Fibrillation. N Engl J Med. 2016;374(23):393–394. doi: 10.1056/NEJMOA1602014 [DOI] [PubMed] [Google Scholar]
- 7.Andrade JG, Khairy P, Dubuc M. Catheter cryoablation: biology and clinical uses. Circ Arrhythm Electrophysiol. 2013;6(1):218–227. doi: 10.1161/CIRCEP.112.973651 [DOI] [PubMed] [Google Scholar]
- 8.Khairy P, Dubuc M. Transcatheter cryoablation part I: preclinical experience. Pacing Clin Electrophysiol. 2008;31(1):112–120. doi: 10.1111/J.1540-8159.2007.00934.X [DOI] [PubMed] [Google Scholar]
- 9.Khairy P, Chauvet P, Lehmann J, et al. Lower incidence of thrombus formation with cryoenergy versus radiofrequency catheter ablation. Circulation. 2003;107(15):2045–2050. doi: 10.1161/01.CIR.0000058706.82623.A1 [DOI] [PubMed] [Google Scholar]
- 10.Chun KRJ, Brugada J, Elvan A, et al. The impact of cryoballoon versus radiofrequency ablation for paroxysmal atrial fibrillation on healthcare utilization and costs: An economic analysis from the FIRE AND ICE trial. J Am Heart Assoc. 2017;6(8). doi: 10.1161/JAHA.117.006043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harris PA, Taylor R, Minor BL, et al. The REDCap consortium: Building an international community of software platform partners. J Biomed Inform. 2019;95. doi: 10.1016/J.JBI.2019.103208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. doi: 10.1016/J.JBI.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Calkins H, Hindricks G, Cappato R, et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Hear Rhythm. 2017;14(10):e275–e444. doi: 10.1016/J.HRTHM.2017.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Andrade JG, Wazni OM, Kuniss M, et al. Cryoballoon Ablation as Initial Treatment for Atrial Fibrillation: JACC State-of-the-Art Review. J Am Coll Cardiol. 2021;78(9):914–930. doi: 10.1016/J.JACC.2021.06.038 [DOI] [PubMed] [Google Scholar]
- 15.Ciconte G, Chierchia GB, De Asmundis C, et al. Spontaneous and adenosine-induced pulmonary vein reconnection after cryoballoon ablation with the second-generation device. J Cardiovasc Electrophysiol. 2014;25(8):845–851. doi: 10.1111/jce.12421 [DOI] [PubMed] [Google Scholar]
- 16.Deubner N, Greiss H, Akkaya E, et al. The slope of the initial temperature drop predicts acute pulmonary vein isolation using the second-generation cryoballoon. Europace. 2017;19(9):1470–1477. doi: 10.1093/EUROPACE/EUW192 [DOI] [PubMed] [Google Scholar]
- 17.Keçe F, M de Riva, RA Dehnavi, et al. Predicting early reconnection after cryoballoon ablation with procedural and biophysical parameters. Hear Rhythm O2. 2021;2(3):290–297. doi: 10.1016/J.HROO.2021.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aryana A, Mugnai G, Singh SM, et al. Procedural and biophysical indicators of durable pulmonary vein isolation during cryoballoon ablation of atrial fibrillation. Hear Rhythm. 2016;13(2):424–432. doi: 10.1016/J.HRTHM.2015.10.033 [DOI] [PubMed] [Google Scholar]
- 19.Chun KRJ, Stich M, Fürnkranz A, et al. Individualized cryoballoon energy pulmonary vein isolation guided by real-time pulmonary vein recordings, the randomized ICE-T trial. Hear Rhythm. 2017;14(4):495–500. doi: 10.1016/J.HRTHM.2016.12.014 [DOI] [PubMed] [Google Scholar]
- 20.Efremidis M, Letsas K, Giannopoulos G, et al. Early pulmonary vein reconnection as a predictor of left atrial ablation outcomes for paroxysmal atrial fibrillation. Europace. 2015;17(5):741–746. doi: 10.1093/EUROPACE/EUU216 [DOI] [PubMed] [Google Scholar]
- 21.Knecht S, Kühne M, Altmann D, et al. Anatomical predictors for acute and mid-term success of cryoballoon ablation of atrial fibrillation using the 28 mm balloon. J Cardiovasc Electrophysiol. 2013;24(2):132–138. doi: 10.1111/JCE.12003 [DOI] [PubMed] [Google Scholar]


