Abstract
Human immunodeficiency virus (HIV) infection associated with weakened immune system due to decreased CD4 T cell count favors development of tuberculosis. Effector immune responses are also associated with micronutrient status due to their prominent role in maintaining immune functions. Micronutrient deficiencies are quite common among HIV patients that further result into compromised immunity thus making the conditions even more favorable for mycobacteria to establish disease. So, current study was designed to assess association of different micronutrients with development of TB in HIV patients. Micronutrient levels were measured in asymptomatic HIV patients who were monitored for the development of TB during follow up period (incident TB) within one month to one year and also in symptomatic microbiologically confirmed HIV-TB patients. Among various micronutrients assessed, levels of ferritin were found to be significantly increased (p < 0.05) with significant decreased zinc (p < 0.05) and selenium (p < 0.05) levels in incident TB group as well as in HIV-TB subjects compared to asymptomatic HIV patients who did not develop TB in the follow up period. Importantly, increased levels of ferritin and decreased levels of selenium were significantly associated with development of tuberculosis in HIV patients.
Keywords: Ferritin, Selenium, Zinc, Incident TB
Introduction
Tuberculosis (TB) is one of the leading infectious diseases, which in spite of being treatable accounts for significant number of deaths annually. Large number of people infected with the disease remains latently infected, and only few of them develop active disease. The presence of weakened immune system is a key factor in progression of latent TB infection to active TB disease. In HIV patients, due to immune-compromised state, risk of active TB is much higher and is known to be primary cause of early mortality [1]. Besides, malnutrition is also quite common in people living with HIV, which further adds to the risk of acquiring opportunistic infections including active tuberculosis [2].
Malnutrition may refer to deficient protein energy intake or scarcity of various micronutrients, both affect the functionality of immune system. The levels of various micronutrients also affect immune-pathogenesis of TB as well as clinical outcome of disease [3]. Deficiencies in some of the micronutrients viz. vitamin A, carotenoids, vitamin D, vitamin E, selenium and folic acid are commonly seen in TB patients [4]. Similar kind of nutrient deficiencies have also been noticed in HIV patients and few studies have shown their relationship with TB development [1, 5]. Tenforde et al. [1] reported that vitamin A and vitamin D deficiencies were associated with increased risk of incident TB in HIV patients, thus suggesting these vitamins as important modifiable risk factors for TB in HIV-infected patients, especially in resource limited TB-endemic settings. However, role of many micronutrients as a trigger for active TB disease in HIV patients is still not clear. Therefore, to see if there is any association between baseline micronutrients levels and development of TB in HIV patients, present study was planned to monitor the levels of various trace elements and vitamins in stored sera collected from asymptomatic HIV patients, recruited over a period of 2 years and followed for the development of TB.
Materials and Methods
Study Subjects
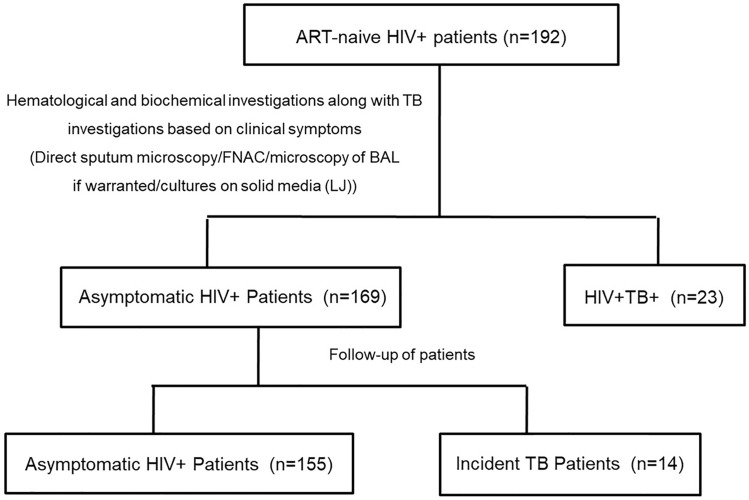
The patients in current study were recruited from the HIV clinic at PGIMER as a part of another study during 2012–2014 which was approved by institutional ethics committee (IEC no. PGI/IEC/2011/154-155 dated 20/09/2011). Patients who were screened for HIV-infection by ELISA and were confirmed for HIV infection by two rapid tests provided by National AIDS Control Organization (NACO) were included in the current study prior to initiation of antiretroviral therapy (ART). The patients who were HIV negative or those already on ART were excluded from current study. Based on these inclusion and exclusion criteria, 192 ART naive HIV+ patients were recruited. Besides, various laboratory investigations as a routine workup by the clinician, at the time of recruitment all HIV+ patients were screened for TB based on clinical symptoms (fever/cough/weight loss etc.), radiological evidence (Chest X-ray/CECT) and various microbiological investigations including direct sputum microscopy/ fine needle aspirate cytology (FNAC), if feasible/microscopy of broncho-alveolar lavage (BAL) if warranted/ cultures on solid media (LJ). Thus, finally there were two groups of patients, symptomatic microbiologically confirmed HIV+TB+ patients (n = 23); and asymptomatic HIV+ patients with no radiological and microbiological evidence of TB (n = 169). 169 asymptomatic HIV patients were then followed for development of TB during their follow up visits to HIV clinic and 14 patients developed TB within one month to 13 months of recruitment on basis of radiological/microbiological/ in-house molecular test [6] and these patients were termed as incident TB cases (Fig. 1). 5 ml blood sample was withdrawn from each of the recruited subjects after taking written consent; serum was separated and stored at −80 °C. Present study was carried out with these stored sera samples after fresh ethical approval from IEC PGIMER (IEC no. INT/IEC/2018/000449).
Fig. 1.
Flowchart showing patient recruitment and grouping
Analysis of Trace Elements
For analysis of trace elements, Inductively Coupled Plasma-Mass Spectrometry (ICP-MS) technique was carried out using ICP-MS instrument (Agilent Technologies, Santa Clara, CA, USA). Briefly, for pre analytical processing, digestion vessels were first prepared by rinsing in 2–3% nitric acid (HNO3) overnight, followed by rinsing with milliQ water after which vessels were dried at 80 °C in hot air oven. Thereafter, 100 μL of serum samples were transferred into microwave vessels followed by addition of 100 μL gold solution and 1 mL ultrapure HNO3. The microwave digestion was performed at pre-defined conditions in auto mode with sample type selection as human blood. After cooling, the desired dilution (1:100) was made with ICP grade water and solution was transferred to 15 mL falcon tube for further analysis using ICP-MS. Instrument was operated with high-purity argon (99.999%) at radiofrequency of 1100 W and data was acquired in counts per second (CPS).
Assessment of Vitamin Levels
Vitamin B12, Ferritin and Folate were estimated using commercially available immunoassay kits (Elecsys Vitamin B12 II/ ferritin and folate assay) based on chemiluminescence on ECL Cobas 6000 Chemistry Analyzer (Roche, Basel, Switzerland).
Statistical Analysis
All the statistical analysis was performed using SPSS software (version 20.0). Baseline characteristics and values of micronutrients in asymptomatic HIV, incident TB and HIV-TB cases were compared using Kruskal–Wallis test with Dunn’s multiple comparison for continuous variable and Chi square for discrete variables. Fisher’s exact test was used to analyze association between variables. The p-value < 0.05 was considered statistically significant.
Results
Samples from total of 23 confirmed HIV-TB cases and 155 asymptomatic HIV and 14 incident TB patients were analyzed in present study as illustrated in Fig. 1. Overall in study population, 60.42% of cases were males. In category wise analysis, proportion of males was higher in HIV-TB (91.3%) and incident TB (64.29%) patients than the asymptomatic HIV patients (55.48%) as shown in Table 1. The pooled median age of the patients was 32 years (IQR:27–40), 34.5 years (IQR:27–38) and 35.5 years (IQR:32.8–38) for asymptomatic HIV patients, Incident TB patients and HIV TB patients respectively, thus indicating no significant difference in median age in all the three groups. Amongst various hematological parameters, there was no significant difference in total leukocyte count and ESR of the patients from three groups whereas the hemoglobin (Hb) levels were significantly lesser in HIV-TB (p < 0.05) patients as compared to asymptomatic HIV. CD4 count was significantly lower in both incident TB (p < 0.05) as well as HIV-TB (p < 0.001) patients as compared to asymptomatic HIV patients who did not develop TB as shown in Table 1.
Table1.
Demographic details of study groups at the time of recruitment in the study
| Characteristics | Asymptomatic HIV | Incident TB | TB-HIV | p |
|---|---|---|---|---|
| Age in yrs (IQR) | 32 (27–40) (n = 155) | 34.50 (27–38) (n = 14) | 35.50 (32.75–38) (n = 23) | 0.37 (NS) |
| Males (%), n | 86 (55.48) (n = 155) | 9 (64.29) (n = 14) | 21 (91.3) (n = 23) | 0.004^^ |
| Hb in g/dL (IQR) | 11.20 (8.60–13.00) (n = 109)a | 11.20 (9.80- 13.30) (n = 9) a | 9.70 (8.93–10.68) (n = 18) a | 0.046*, ns, < 0.05$ |
| TLC per cubic mm (IQR) | 6550 (5525–7400) (n = 107) a | 6400 (5050–8100) (n = 8) a | 6150 (4575–8325) (n = 16) a | 0.919 (NS) |
| ESR in mm/hour (IQR) | 25 (12–40) (n = 95)a | 27 (20–49) (n = 6) a | 37.5 (25.5–42.5) (n = 14) a | 0.097 (NS) |
| CD4 cells per cubic mm (IQR) | 351 (243.5–563) (n = 137)a | 194 (90–298) (n = 11) a | 134 (60–286) (n = 19) a | < 0.001***, < 0.05#, < 0.001$$$ |
Data represents the median and IQR (inter-quartile range), ^^p < 0.01 using Chi square. *p < 0.05, ***p < 0.001: Kruskal wallis p values. Dunn’s multiple comparison p values: ns (non-significant) and #p < 0.05: for incident TB vs. Asymptomatic HIV. $p ≤ 0.05 & $$$p < 0.001 for TB-HIV vs. Asymptomatic HIV. n: number, a: number of patients included for comparison in each category are lower than the actual number of patients in each category as the data was not available for all the patients. Hb haemoglobin; TLC total leukocyte count, NS non-significant w. r.t. all categories
Comparative Micronutrient Levels in Study Subjects
The median levels of ferritin (p < 0.001), zinc (p < 0.01), selenium (p < 0.001) and manganese (p < 0.01) were significantly different among asymptomatic HIV patients, incident TB and HIV-TB group, however, no significant difference was observed in levels of other micronutrients as shown in Table 2.
Table 2.
Comparative of baseline concentration of micronutrients in the sera of asymptomatic HIV, incident TB and HIV-TB patients
| Micronutrients conc. | Asymptomatic HIV Median (IQR) n = 155 | Incident TB Median (IQR) n = 14 | HIV-TB Median (IQR) n = 23 | p-Value |
|---|---|---|---|---|
| Folate (ng/mL) | 9.21 (5.40–16.25) | 11.41 (4.77- 17.78) | 7.88 (5.91–16.77) | 0.99 (NS) |
| Vit. B12 (pg/mL) | 275.00 (168.45–448.75) | 250.10 (138.95–360.58) | 304.5 (176.90–1084.55) | 0.54 (NS) |
| Ferritin (ng/mL) | 81.57 (34.20–198.75) | 489.81 (106.82–824.3) | 442.3 (236.55–1546.00) | ***,#,$$$,ns |
| Mg (mg/dL) | 2.15 (1.97–2.40) | 2.23 (2.12–2.34) | 2.27 (2.11–2.48) | 0.37 (NS) |
| Cr (µg/L) | 11.20 (1.06 -51.70) | 2.59 (0.00–57.36) | 7.72 (2.53–11.89) | 0.51 (NS) |
| Mn (µg/L) | 20.67 (12.03- 35.51) | 34.99 (18.56–43.74) | 9.22 (4.85–31.35) | **,Ns,$, + + |
| Fe (µg/dL) | 416.51 (246.11–714.32) | 331.57 (193.70–615.40) | 390.43 (272.27–672.66) | 0.67 (NS) |
| Co (µg/dL) | 0.07 (0.04–0.11) | 0.06 (0.03–0.09) | 0.09 (0.05–0.11) | 0.31 (NS) |
| Ni (µg/dL) | 14.15 (10.04–25.54) | 10.27 (6.99–17.54) | 13.01 (9.37–18.25) | 0.13 (NS) |
| Cu (µg/dL) | 132.17 (102.37–174.69) | 150.18 (115.38–172.05) | 127.6 (110.13–164.42) | 0.83 (NS) |
| Zn (µg/dL) | 154.32 (120.21–209.41) | 93.67 (88.34–177.05) | 107 (79.09–186.22) | **,#,$,ns |
| As (µg/dL) | 0.11 (0.07–0.17) | 0.10 (0.05–0.15) | 0.11 (0.06–0.15) | 0.72 (NS) |
| Se (µg/dL) | 10.82 (7.36–14.42) | 6.19 (3.72–9.62) | 2.96 (1.26–4.62) | ***,#,$$$,ns |
| Cd (µg/dL) | 0.06 (0.03–0.16) | 0.04 (0.03–0.18) | 0.04 (0.03–0.08) | 0.33 (NS) |
| Hg (µg/dL) | 0.29 (0.04–0.69) | 0.17 (0.01–0.32) | 0.23 (0.11–0.46) | 0.27 (NS) |
| Pb (µg/dL) | 0.26 (0.01–0.67) | 0.10 (0.01–0.29) | 0.08 (0.00–0.32) | 0.17 (NS) |
Kruskal wallis p-values, **p < 0.01 &***p < 0.001; Dunn’s multiple comparison p values: #p < 0.05 for incident TB vs. Asymptomatic HIV, $p < 0.05, $$$p < 0.001 for HIV-TB vs. asymptomatic HIV; + + p < 0.01 for incident TB vs. HIV-TB; NS non-significant w.r.t all categories, Ns non-significant for incident TB vs. Asymptomatic HIV, ns non-significant for incident TB vs. HIV-TB; IQR (interquartile range) Conc concentration
Comparative Micronutrient Status Among Asymptomatic HIV and Incident TB Patients
On comparing incident TB group with asymptomatic HIV, median levels of ferritin, zinc and selenium were found to be significantly different. The median levels of ferritin in the incident TB group (489.81 ng/mL) were significantly higher (p < 0.05) than asymptomatic HIV patients (81.57 ng/mL). However, median levels of zinc and selenium were significantly lower (p < 0.05) in incident TB group (93.67 µg/dL and 6.19 µg/dL respectively) than asymptomatic HIV patients (154.32 µg/dL and 10.82 µg/dL) as given in Table 2. There was no significant difference in median values of other micronutrients.
Comparative Micronutrient Status in Asymptomatic HIV and HIV-TB Cases
Similar to Incident TB, HIV-TB patients also had significantly higher (p < 0.001) ferritin concentration (442.3 ng/mL) than asymptomatic HIV (81.57 ng/mL) and significantly lower zinc (p < 0.05) and selenium (p < 0.001) concentration (107 µg/dL & 2.96 µg/dL) as compared to asymptomatic HIV group. The median levels of manganese were also significantly lower (p < 0.05) in HIV-TB patients (9.22 µg/L) in comparison to asymptomatic HIV patients (20.67 µg/L) as shown in Table 2.
Association of Micronutrients with the TB Development in HIV Infected Patients
Further analysis of association of significantly altered baseline micronutrient levels with the development of TB in asymptomatic HIV patients, only elevation in serum ferritin (p < 0.01) and deficiency in serum selenium (p < 0.01) were found to be significantly associated with the incident TB in asymptomatic HIV patients. However, the zinc deficiency (p = 0.195) was not associated with the development of TB in these patients.
Discussion
TB has become leading killer in HIV infected patients due to weakened immunity. Along with HIV, nutrition is also an important factor which can affect the immune system, making it vulnerable for attack by certain bacteria and viruses. Not only macronutrients, but micronutrients also hold a prominent role in the maintenance of immune system and their deficiencies or accumulation have been shown to be associated with tuberculosis [1, 7]. However, only few studies have seen association between micronutrient levels and risk of TB development in HIV infected patients, though it is already known that HIV patients have alterations in levels of various micronutrients. Therefore, current study was designed to assess levels of various trace elements and vitamins in the sera from asymptomatic HIV patients recruited over a period of 2 years and followed for the development of TB, to see, if there is any relationship between baseline micronutrients level and development of TB in HIV patients. Through our study we have found a significant difference only in the level of three micronutrients, ferritin, selenium and zinc between the three groups of patients and among them only ferritin and selenium were significantly associated with development of TB in HIV patients.
Among asymptomatic HIV patients, 14 patients developed TB over a follow up period of two years and were referred to as incident TB group. More number of males (60.42%) in comparison to females developed TB among HIV asymptomatic patients. Lower CD4 count as observed in incident TB and TB-HIV group has been previously found to be associated with increased risk of TB [8]. Further, lower levels of hemoglobin in HIV-TB co-infected patient as compared to patients with single infection either by virus or bacteria have also been reported earlier [9]. However, lack of any information regarding the baseline general nutritional status of the study subjects is limitation of this work.
Amongst various micronutrients tested, the median ferritin levels were found to be significantly higher in the incident TB and HIV-TB group as compared to asymptomatic HIV patients (Table 2). Ferritin is an acute phase protein and its increased levels are associated with several inflammatory conditions. HIV patients also have higher ferritin levels as compared to normal [10]. In the current study, when compared among HIV patients, incident TB patients had a comparatively higher ferritin levels as compared to asymptomatic HIV patients. Increased ferritin levels in sera from TB patients have also been reported earlier [7, 11]. Mycobacteria require iron for its growth, but excess of free iron can be toxic to bacteria, which, it stores with the help of iron storage proteins like ferritin [7, 11]. So, the presence of increased ferritin levels in incident TB group as well as HIV TB group suggests its role in progression of TB disease.
Some trace elements have important role in maintaining the immune system, hence their deficiency can make person more vulnerable to infection by different pathogens. Among all the trace elements studied in the current study, levels of selenium and zinc were found to be significantly lower in the incident TB group, along with manganese in HIV-TB group in comparison to the asymptomatic HIV patients. Lower levels of selenium are reported in both TB and HIV-TB co-infected patients [12]. Inflammatory response and oxidative stress are well known to be associated with HIV pathogenesis as well as tuberculosis. It has been reported that prolonged inflammation can deplete selenium levels from body due to increased oxidative stress as selenium is a key factor of antioxidant system [12]. Depletion of selenium in body may further affect the functioning of immune system paving a favorable situation for TB infection as functions of T and B cells as well as of macrophages are affected by selenium deficiency [13]. Further, on assessing the association between micronutrient status and TB development in asymptomatic HIV patients, ferritin and selenium were found to be associated with development of TB among HIV asymptomatic patients [1]. Earlier, Tenforde et al. have also found selenium as a predictor of TB in HIV population [1]. Results of present study further adds to the current knowledge that in addition to selenium, ferritin is also associated with the incidence of TB in HIV population.
Similar to selenium, zinc deficiency has earlier been implicated in the development of TB disease [14]. Zinc helps in proper functioning of both innate and adaptive immune system [15]. Besides the various cell types, macrophages, one of the prominent antigen presenting cells playing a role in anti-tuberculosis immunity also require zinc for effective action. Zinc deficiency leads to impaired phagocytosis and intracellular killing by macrophages as well as impaired release of pro-inflammatory cytokines from these cells [15]. Besides, Zn deficiency also affects the functioning of T cells by altering Th1 and Th2 cytokine balance favoring it towards Th2 type [15] response involved in the progression of TB [16]. Based on all these facts, we may say that deficiency of zinc acts as a promoting factor for TB infection as seen in the current study, thus relating with decreased levels of zinc in incident TB and HIV- TB group.
Manganese was seen to be deficient in only HIV-TB patients. Decreased level of manganese in TB patients has been reported earlier also [17]. Mycobacteria utilize manganese, an important cofactor for many enzymes like superoxide dismutase and stimulant for its replication in macrophages [17]. Thus, reduction in manganese levels as seen in HIV-TB patients may probably be due to increased utilization of manganese by bacteria. As deficiency was only observed in HIV-TB group but not in the incident TB group it could be speculated that as the bacterial numbers are increased during the active infection, so there is increased requirement of Mn by bacteria, thus leading to lower levels in HIV–TB group. Out of various vitamins and trace elements estimated in current study only few have shown significant variation in incident TB and active TB group but majority of them have not shown any significant change. Previous studies for vitamin B12 and folate have shown both kind of results, supporting [18, 19] and opposing our findings [19]. Similarly, for other trace elements, though few studies done earlier have shown their alterations in TB [7, 17] but majority of these studies have compared the levels in healthy controls whereas, in the current study we have compared their levels with asymptomatic HIV patients who are already having alterations in these trace metals [20]. Besides the study population and number of study subjects may also affect the overall results.
Conclusion
Overall, the present study lead to the conclusion that reduced ferritin and selenium levels are significantly associated with TB development in HIV asymptomatic patients. But lower number of subjects in the incident TB group is a limitation of current study and hence these observations need further validation in larger number of study subjects in a multi-centric study.
Acknowledgements
We acknowledge INDO-US grant (INDO-US/98/9/2010-ECD-II) provided by ICMR under which samples of current study were collected. We also thank Dr. Manisha Wadhwa and Mr. Gurpreet for their help in sample collection in INDO-US grant. Professor Ashutosh N. Aggarwal is also acknowledged for his help in statistical analysis.
Funding
No funding was received from any organization for conducting this study.
Declarations
Conflict of interest
The authors have no competing interests to declare that are relevant to the content of this article.
Consent to Participate
Written informed consent was obtained from all the participants at the time of sample collection where it was stated that leftover samples will be used for future studies.
Consent for Publication
All authors have given their consent for publication.
Ethics Approval
The current study was approved by Institute Ethics Committee vide IEC No. INT/IEC/2018/000449.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Tenforde MW, Yadav A, Dowdy DW, Gupte N, Shivakoti R, Yang WT, et al. Vitamin A and D deficiencies associated with incident tuberculosis in HIV-Infected Patients initiating antiretroviral trherapy in multinational case-cohort study. J Acquir Immune Defic Syndr. 2017;75(3):e71–e79. doi: 10.1097/QAI.0000000000001308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alebel A, Demant D, Petruck P, Sibbritt D. Effects of undernutrition on mortality and morbidity among adults living with HIV in sub-Saharan Africa: a systematic review and meta-analysis. BMC Infect Dis. 2021;21:1. doi: 10.1186/s12879-020-05706-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wheelwright M, Kim EW, Inkeles MS, De Leon A, Pellegrini M, Krutzik SR, et al. All-trans retinoic acid-triggered antimicrobial activity against Mycobacterium tuberculosis is dependent on NPC2. J Immunol. 2014;192(5):2280–2290. doi: 10.4049/jimmunol.1301686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cegielski JP, McMurray DN. The relationship between malnutrition and tuberculosis: evidence from studies in humans and experimental animals. Int J Tuberc Lung Dis. 2004;8(3):286–298. [PubMed] [Google Scholar]
- 5.Aibana O, Huang CC, Aboud S, Arnedo PA, Becerra MC, Bellido-Blasco JB, et al. Vitamin D status and risk of incident tuberculosis disease: a nested case-control study, systematic review, and individual-participant data meta-analysis. PLoS Med. 2019;16(9):e1002907. doi: 10.1371/journal.pmed.1002907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sharma K, Gupta V, Bansal R, Sharma A, Sharma M, Gupta A. Novel multi-targeted polymerase chain reaction for diagnosis of presumed tubercular uveitis. J Ophthalmic Inflamm Infect. 2013;3(1):25. doi: 10.1186/1869-5760-3-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dai Y, Shan W, Yang Q, Guo J, Zhai R, Tang X, et al. Biomarkers of iron metabolism facilitate clinical diagnosis in mycobacterium tuberculosis infection. Thorax. 2019;74(12):1161. doi: 10.1136/thoraxjnl-2018-212557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Geremew D, Melku M, Endalamaw A, et al. Tuberculosis and its association with CD4+ T cell count among adult HIV positive patients in Ethiopian settings: a systematic review and meta-analysis. BMC Infect Dis. 2020;20(1):325. doi: 10.1186/s12879-020-05040-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abay F, Yalew A, Shibabaw A, Enawgaw B. Hematological abnormalities of pulmonary tuberculosis patients with and without HIV at the University of Gondar Hospital, Northwest Ethiopia: a comparative cross-sectional study. Tuberc Res Treat. 2018;5740951–5740951. [DOI] [PMC free article] [PubMed]
- 10.López-Calderón C, Palacios R, Cobo A, et al. Serum ferritin in HIV-positive patients is related to immune deficiency and inflammatory activity. Int J STD AIDS. 2015;26(6):393–397. doi: 10.1177/0956462414539669. [DOI] [PubMed] [Google Scholar]
- 11.D’Souza B, Sinha S, Manjrekar P, D’Souza V. Hyperferritinemia in pulmonary tuberculosis. Indian J Clin Bio chem. 2012;28(3):309–310. doi: 10.1007/s12291-012-0289-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Muzembo BA, Mbendi NC, Ngatu NR, Suzuki T, Wada K, Ikeda S. Serum selenium levels in tuberculosis patients: a systematic review and meta-analysis. J Trace Elem Med Biol. 2018;50:257–262. doi: 10.1016/j.jtemb.2018.07.008. [DOI] [PubMed] [Google Scholar]
- 13.Avery JC, Hoffmann PR. Selenium, selenoproteins, and immunity. Nutrients. 2018;10(9):1203. doi: 10.3390/nu10091203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barman N, Khan MM, Ghosh D, Towhid MI, Uddin MN, Paul D, et al. Serum zinc level and its association with multidrug-resistant tuberculosis. Int J Mycobacteriol. 2021;10:177–181. doi: 10.4103/ijmy.ijmy_67_21. [DOI] [PubMed] [Google Scholar]
- 15.Bonaventura P, Benedetti G, Albarède F, Miossec P. Zinc and its role in immunity and inflammation. Autoimmun Rev. 2015;14(4):277–285. doi: 10.1016/j.autrev.2014.11.008. [DOI] [PubMed] [Google Scholar]
- 16.Ashenafi S, Aderaye G, Bekele A, Zewdie M, Aseffa G, Hoang ATN, et al. Progression of clinical tuberculosis is associated with a Th2 immune response signature in combination with elevated levels of SOCS3. Clin Immunol. 2014;151(2):84–99. doi: 10.1016/j.clim.2014.01.010. [DOI] [PubMed] [Google Scholar]
- 17.Festus OO, Ekun V, Dada FL, Eidangbe G, Iweka FJ. Evaluation of some trace elements (zinc, chromium, cadmium and manganese) in patients with active tuberculosis attending central hospital Benin city, Edo state. Int J Basic Appl Innov Res. 2016;5(2):35–41. [Google Scholar]
- 18.Oh J, Choi R, Park H-D, Lee H, Jeong B-H, Park HY, et al. Evaluation of vitamin status in patients with pulmonary tuberculosis. J Infect. 2017;74(3):272–280. doi: 10.1016/j.jinf.2016.10.009. [DOI] [PubMed] [Google Scholar]
- 19.Adhikari PMR, Chowta MN, Ramapuram JT, Rao S, Udupa K, Acharya SD. Prevalence of Vitamin B (12) and folic acid deficiency in HIV-positive patients and its association with neuropsychiatric symptoms and immunological response. Indian J Sex Transm Dis AIDS. 2016;37(2):178–184. doi: 10.4103/0253-7184.192117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shah KK, Verma R, Oleske JM, Scolpino A, Bogden JD. Essential trace elements and progression and management of HIV infection. Nutr Res. 2019;71:21–29. doi: 10.1016/j.nutres.2019.08.001. [DOI] [PubMed] [Google Scholar]