Abstract
The rabies virus P protein is involved in viral transcription and replication but its precise function is not clear. We investigated the role of P (CVS strain) by searching for cellular partners by using a two-hybrid screening of a PC12 cDNA library. We isolated a cDNA encoding a 10-kDa dynein light chain (LC8). LC8 is a component of cytoplasmic dynein involved in the minus end-directed movement of organelles along microtubules. We confirmed that this molecule interacts with P by coimmunoprecipitation in infected cells and in cells transfected with a plasmid encoding P protein. LC8 was also detected in virus particles. Series of deletions from the N- and C-terminal ends of P protein were used to map the LC8-binding domain to the central part of P (residues 138 to 172). These results are relevant to speculate that dynein may be involved in the axonal transport of rabies virus along microtubules through neuron cells.
Rhabdoviruses have a single-stranded negative-sense RNA genome (11 to 15 kb) that is tightly encapsidated by the viral nucleoprotein (N) to form a ribonucleoprotein (RNP). This RNP serves as the template for viral transcription and replication. During transcription, a positive-strand leader RNA and five mRNAs are synthesized. The replication process yields nucleocapsids containing full-length antisense genome RNA which in turn serves as a template for the synthesis of sense genome RNA. The active virus-encoded RNA polymerase complex consists of the large protein (L) and its cofactor, the phosphoprotein (P) (13). The L protein is a multifunctional enzyme and acts as the RNA-dependent RNA polymerase. This polymerase complex carries out all the enzymatic steps of transcription, including the initiation and elongation of transcripts, and cotranscriptional modifications of RNAs, such as capping and polyadenylation (2). The functions of the P protein are not clear. Studies with vesicular stomatitis virus (VSV) have shown that the P protein works as a noncatalytic cofactor of the viral RNA polymerase and as a molecular chaperone helping the viral N protein to bind specifically and correctly to the nascent RNA chain during genome replication. VSV P protein has different phosphorylation states that are believed to bind to the RNP with different affinities and to have different transcription activities (3, 4, 17). The VSV P protein has also been shown to form oligomers, and oligomerization seems to be necessary for binding both to the L protein and to the template (16).
By analogy with the VSV P protein, rabies virus P protein is also thought to act as a chaperone and to be a noncatalytic subunit of the viral RNA polymerase. In vitro and in vivo studies have shown that rabies virus P protein forms specific complexes with N and L proteins (10, 11, 14). We have previously demonstrated the existence of two N protein-binding sites on the P protein: one located between amino acids 69 and 138 and the other in the carboxy-terminal region comprising amino acids 268 to 297 (10). We have shown that the major L binding site resides within the first 19 residues of P (11). It has recently been shown that rabies virus P protein is phosphorylated by two kinases, one unique cellular protein kinase, rabies virus protein kinase, and the other, protein kinase C (21). Both kinases phosphorylate specific sites on the P protein, resulting in the formation of different phosphorylated forms of P protein with different mobilities in sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (21). In addition, four other N-terminal-truncated products translated from P mRNA have been found in purified virus, in infected cells, and in cells transfected with a plasmid encoding the complete P protein. For these proteins, translation is initiated from internal in-frame AUG initiation codons by a leaky scanning mechanism (9). Their potential role in the virus cycle remains to be determined.
Due to the small size of their genomes, which encode only a limited number of proteins, rhabdoviruses may depend on cellular helper functions. The regulatory products of these viruses are multifunctional, and thus, P proteins may need to interact with specific cellular products to achieve their functions. In order to better understand the role of P during rabies virus replication, we looked for interacting partners by using the two-hybrid system. The P protein of the CVS strain fused to the DNA-binding domain (DNA BD) of LexA is used as a bait to screen a nerve growth factor-induced PC12 cell (rat adrenal pheochromocytoma cell line) cDNA library in which each DNA was fused to the sequence encoding the GAL4 activation domain (AD). The yeast L40 strain containing the two LexA-responsive reporter genes, HIS3 and lacZ, was first transformed with the bait plasmid pLex-P by using a lithium acetate protocol (19, 22). pLex-P-expressing L40 cells selected and grown in Trp-deficient medium were then transformed with plasmid DNA from the PC12 cDNA library. Double transformants were grown on plates containing medium lacking Trp and Leu (Trp− Leu−) to select for the presence of both the bait and library plasmids and deprived of His (Trp− Leu− His−) to select for protein-protein interaction. Positive clones were then assayed for β-galactosidase activity. Six hundred of the 4 × 106 independent transformants were isolated on the basis of their ability to activate the transcription of both HIS3 and lacZ reporter genes. These clones conferred on L40 the ability to grow in the absence of histidine and to produce β-galactosidase activity in the presence of the LexA BD-P hybrid but not with LexA BD alone or with LexA BD-lamin. One quarter of the clones were analyzed further after elimination of false positives, and 130 clones were partially sequenced. Homology searches were performed at the National Center for Biotechnology Information (NCBI) using GAPPED BLAST and PSI-BLAST (1). We found that 75% of positive clones contained, fused to the AD, a 270-bp open reading frame preceded by an in-frame stop codon, encoding an 89-amino-acid protein designated PIN for protein inhibitor of neuronal nitric oxide synthase (nNOS) (23). PIN from rat has 100% amino acid sequence identity to the human 10-kDa dynein light chain (LC8, previously called DLC1), a component of cytosolic and flagellar dynein (25). LC8 is highly conserved throughout evolution and is ubiquitously expressed in various cell types (25). It has been shown to form dimers (6, 27). The insertion of an untranslated sequence between the AD and the LC8 coding sequence has been also reported in cDNAs selected by two-hybrid screening with either the nNOS or IκBα as bait (12, 23). This could be a regulation translation process used by yeast cells to reduce the toxicity of dynein LC8.
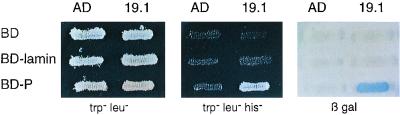
The yeast L40 strain was cotransformed with the DNA of one positive clone (19-1) and either the BD-P-encoding plasmid or the BD-lamin-encoding plasmid. Both the HIS3 and lacZ reporter genes were activated when both P and LC8 were coexpressed (Fig. 1). Under conditions in which no P-LC8 interaction could take place (coexpression of BD and AD, or BD-lamin and AD-LC8, or BD-P and AD), the reporter genes were not induced, resulting in no growth of yeasts in medium lacking histidine or white colonies in the presence of X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside). The latter control indicated that viral protein P could not on its own activate the promoter driving the expression of the reporter gene, thereby demonstrating the specificity of the interaction.
FIG. 1.
Interaction of P with LC8. L40 yeast cells expressing the indicated bait and prey pairs were streaked onto plates containing minimal medium lacking tryptophan and leucine (trp− leu−) for double transformants or lacking tryptophan, leucine, and histidine (trp− leu− his−) to assay the activation of the HIS3 reporter gene. The induction of the lacZ reporter gene was assayed by the appearance of blue colonies as follows: an X-Gal mixture containing 0.5% agar, 0.1% SDS, 6% dimethylformamide, and 0.04% X-Gal was overlaid on fresh transformants grown on Trp− Leu− dishes, and blue clones were detected after 60 min to 18 h at 30°C. Note that 19.1 corresponds to the AD-LC8 clone.
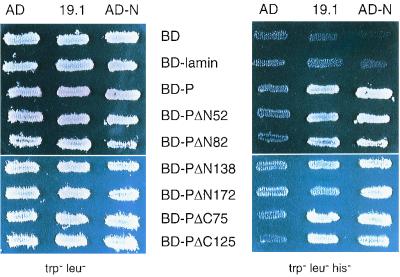
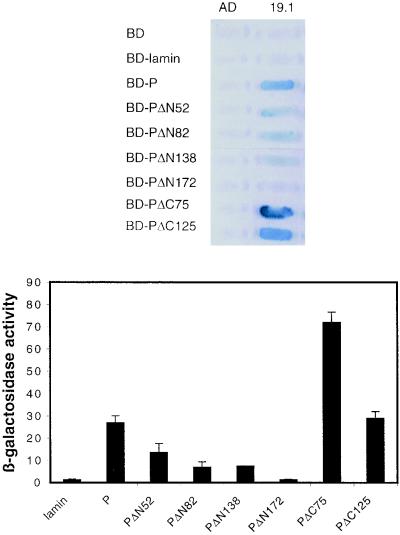
To identify the portion of P that mediates binding to LC8, DNA sequences encoding N-terminal-truncated and C-terminal-truncated P protein coding sequences were fused to the coding sequence of LexA BD (Fig. 2). We first checked that the deletion mutants of P were produced in yeast extracts by Western blotting with a polyclonal anti-P antibody (29) (data not shown). We then assessed the interaction of these proteins with the GAL4 AD-LC8 in yeast cells. Qualitative and quantitative results were obtained by assessing the ability of the yeast to grow in the absence of histidine, by the appearance of blue colonies in the presence of X-Gal and by assaying the β-galactosidase activity of yeast grown in liquid medium (Fig. 3 and 4). The interaction of P with the nucleoprotein N was used as a positive control by analyzing the induction of the HIS3 gene (Fig. 3). The fusion proteins containing PΔC75 and PΔC125 very efficiently activated the transcription of the HIS3 and lacZ genes and then interacted with LC8. The amino-terminal-truncated P proteins (PΔN52, PΔN82, and PΔN138) also bound LC8. A more-extended amino-terminal deletion of 172 amino acids impaired binding to LC8 since yeast producing both LC8 and PΔN172 did not grow in the absence of histidine and did not activate lacZ transcription. However, PΔN172 interacted efficiently with the N protein (Fig. 3), indicating that this small protein was correctly folded in the yeast expression system. These results suggest that the central part of P, from residues 139 to 172, is necessary for binding to LC8. This region contains a large hydrophilic domain that is poorly conserved within the Lyssavirus genus (7). This is consistent with previous suggestions that functions of P depend on structural rather than sequence similarity (7). P-N interaction analysis provided results consistent with the coimmunoprecipitation data we previously obtained for infected cells and for cells cotransfected with both genes (10). The fact that all the truncated P proteins conferred the ability to grow in the absence of histidine and interacted with N is consistent with there being two N-binding sites on P (10).
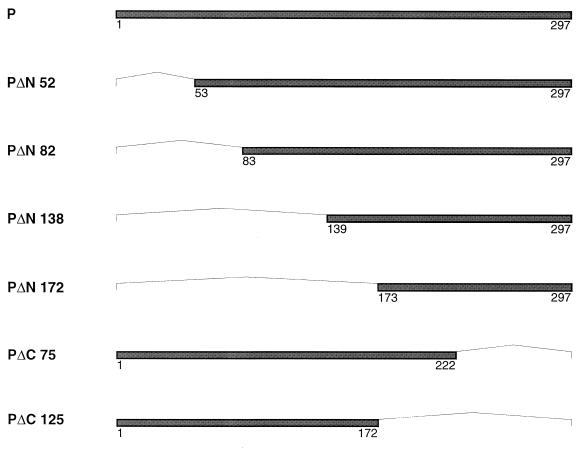
FIG. 2.
Schematic diagram of the truncated P proteins fused to the LexA BD. Dark bars represent the protein product of each deleted P gene, with amino acid positions indicated. Each deleted region is indicated by a thin line. The constructs pLex-PΔN52, pLex-PΔN82, pLex-PΔN138, and pLex-PΔN172 differed from pLex-P by deletion at the 5′ terminus of the P gene of 186, 276, 444, and 546 bp, respectively. The constructs pLex-PΔC75 and pLex-PΔC125 differed from the wild-type P gene by deletion of 225 and 375 nucleotides from the 3′ terminus of the P gene, respectively. These deletions were created by PCR amplification of the wild-type P protein using specific oligonucleotides.
FIG. 3.
Analysis of P-N and P-LC8 interactions by induction of the HIS3 gene. L40 yeast cells expressing the indicated bait and prey pairs were streaked onto Trp− Leu− plates for double transformants and Trp− Leu− His− plates for assaying the activation of the HIS3 reporter gene. The interaction between P and N was used as a positive control.
FIG. 4.
Qualitative and quantitative analyses of P-LC8 interactions by induction of the lacZ reporter gene. L40 yeast cells were cotransformed with the indicated combinations of plasmids. The interaction was assessed by the appearance of blue colonies in the presence of X-Gal (top) and by assaying the β-galactosidase activity of yeast grown in liquid medium (bottom). Quantitative results were obtained from three independent yeast cotransformants assayed with o-nitrophenyl-β-d-galactopyranoside (ONPG) as substrate (20). β-galactosidase activity was expressed in units and calculated using the following formula: (A420 · 1,000)/A600 · T · V), where A420 is the absorbance of the reaction mixture, A600 is the cell density of the culture, T is the reaction time (in minutes), and V is the volume (in milliliters) used for the assay. Error bars indicate the standard deviation.
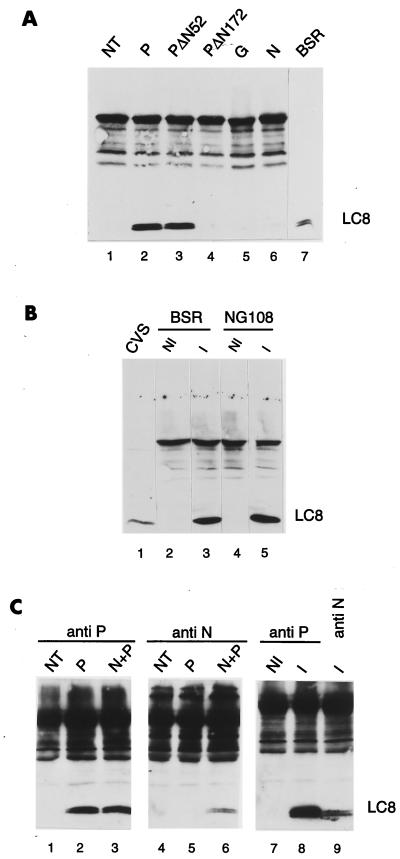
To demonstrate that P protein associates with LC8 in vivo independently of the yeast genetic assay, the gene encoding P protein was expressed transiently in BSR cells by using the vaccinia virus T7 system, as previously described (10, 15, 31). P was immunoprecipitated from cell extracts by using a polyclonal anti-P antibody (29). Protein extraction and immunoprecipitations were carried out under nondenaturing conditions. The proteins present in the immune complexes were then detected on a Western blot with a rabbit polyclonal anti-LC8 antibody (R4058) (26). The LC8 protein was detected in immunoprecipitates from cells producing the P protein (Fig. 5A, lane 2). The interaction between P and LC8 seems to be specific, as LC8 was absent from immunoprecipitates of untransfected cells (lane 1). In addition, LC8 was not coprecipitated with N protein or G protein in extracts of cells producing N or G (lanes 5 and 6). Cells were also transfected with two plasmids encoding amino-terminally truncated P proteins, PΔN52 and PΔN172 (10). We assessed the binding of these proteins to LC8. The PΔN52 protein interacted with LC8, whereas PΔN172 did not (lanes 3 and 4). These results from transfection experiments demonstrate that there is a specific interaction between P and LC8, consistent with the data obtained using the two-hybrid system. However, it was important to confirm this association in the context of viral infection. We infected BSR cells or neuroblastoma cells (NG108) with rabies virus (CVS strain), and cell extracts were prepared and treated as described above. The LC8 protein was present in the immunoprecipitates of both types of infected cells (Fig. 5B, lanes 3 and 5), indicating that P and LC8 interact even if a complex set of regulatory and structural viral proteins are coexpressed with P and may have an effect on one of the partners in infected cells. Indeed, P protein associated with the N protein in the P-N complex and detected with an anti-N antibody in cotransfected or infected cells was able to bind LC8 (Fig. 5C, lanes 6 and 9) as efficiently as the P detected with an anti-P antibody (Fig. 5C, lanes 3 and 8). These results show that P-LC8 was not affected by P-N and P-L interactions during viral infection. This is in accordance with our finding that the LC8 binding site is different from the N and L binding domains described previously (10, 11) and suggests that P can interact simultaneously with its three viral partners (N, L, and LC8). This should enable it to perform other as-yet-unknown functions in addition to its function in viral transcription and replication. We also investigated whether this P-LC8 interaction could mediate the uptake of LC8 protein into virus particles by testing LC8 in purified virus by Western blotting. We found that LC8 was incorporated into the virus particle purified as described previously (18) (Fig. 5B, lane 1). The incorporation of LC8 may be mediated by contacts with P protein. In addition to nonspecific trapping of cell membrane proteins in viral envelopes, a number of cytosolic proteins (enzymes, cytoskeletal components, molecular chaperones) appear to be present in virions due to specific protein-protein interactions. Rabies virus particles have been reported to contain proteins, such as Hsp70 (32), actin and actin-binding proteins (34), and a CD99-related transmembrane protein (33). This work provides another example of the entrapment of a cellular protein within virus particles.
FIG. 5.
Detection of P-LC8 complex in transfected cells and infected cells. (A) BSR cells were infected with vTF7-3 (lanes 1 to 6) and transfected with plasmids encoding P (lane 2), PΔN52 (lane 3), PΔN172 (lane 4), G (lane 5), or N (lane 6). These plasmids have been described elsewhere (10). Twenty hours after infection, samples of cytoplasmic cell extracts were immunoprecipitated with the murine polyclonal anti-P antibody (lanes 1 to 4), a monoclonal anti-G antibody (lane 5), or a monoclonal anti-N antibody (lane 6). Immunoprecipitated proteins were analyzed by Western blotting (SDS–15% PAGE). The blot was immunostained with a rabbit polyclonal anti-LC8 antibody (26) and with an anti-rabbit peroxidase-labeled antibody. An aliquot of uninfected BSR cell extract was used to indicate the position of LC8 in the gel (lane 7). (B) BSR (lanes 2 and 3) and NG108 (lanes 4 and 5) cells were uninfected (lanes 2 and 4) or infected with CVS rabies virus (lanes 2 and 4). Twenty hours after infection, cell extracts were immunoprecipitated with the murine polyclonal anti-P antibody and then treated as in panel A. Lane 1, proteins from purified virus. (C) BSR cells were infected with vTF7-3 (lanes 1 to 6) and transfected with plasmids encoding P (lanes 2 and 5) or cotransfected with plasmids encoding P and N (lanes 3 and 6). Cells were also infected with rabies virus. At 20 h after infection, identical samples of cytoplasmic cell extracts were immunoprecipitated with the anti-P antibody (lanes 1 to 3, 7, and 8) or anti-N antibody (lanes 4 to 6 and 9) and then treated as in panel A.
The functional significance of the P-LC8 interaction remains a matter for speculation. In addition to interacting with nNOS and dynein, LC8 interacts with myosin V and IκBα, suggesting that it may have multiple regulatory roles (12). LC8 regulates NO levels by inhibiting nNOS (23). NO is a major messenger molecule in the cardiovascular, immune, and nervous systems. In the brain, nNO is required for NMDA receptor-mediated neurotoxicity and 3′,5′-cyclic GMP (cGMP) elevation, and it may be involved in apoptosis, synaptic transmission, and neuronal development. NO has also been reported to be an efficient inhibitor of viral replication, resulting in lower viral yields and more efficient host clearance of the infection. Inhibition by NO has been reported for both DNA and RNA viruses, whether or not they are enveloped or encapsidated: several picornaviruses, Japanese encephalitis virus, mouse hepatitis virus, Friend murine leukemia virus, herpes simplex virus type 1 (HSV1), vaccinia virus, and VSV (30). Further experiments are required to analyze the role of the P-LC8 interaction in pathogenesis of rabies virus infection.
LC8 is also a light chain component of cytosolic and flagellar dynein, which forms the microtubule (MT)-associated motor protein complexes involved in the minus end-directed movement of chromosomes and particles along MT (8, 28). This light chain is essential for dynein heavy chain localization and nuclear migration in Aspergillus and for retrograde intraflagellar transport in Chlamydomonas (5, 28). Transport over long distances is particularly critical for viruses that infect neurons in which the site of entry can be located far from the cell body. The retrograde transport of two incoming alphaherpesviruses, HSV1 and pseudorabies virus, and of adenoviruses occurs rapidly and efficiently via an MT-mediated mechanism (24, 35, 36). Ye et al. have recently shown that the binding of U(L)34 to a cytoplasmic dynein intermediate chain may be involved in the nuclear targeting of HSV1 (37). As the plus ends of MT are located toward the synapse and the minus ends are anchored at the MT organization center in the cell body, incoming rabies virus capsids may bind to MT and use dynein for their transport through the neuron cells, and this process may be mediated by the interaction of P with LC8.
Acknowledgments
We thank Jacques Camonis for help in setting up the yeast two-hybrid system and for providing the yeast strain L40 and the yeast plasmids pLex10, pGAD, and pLex-lamin, Simon Halegoua for the PC12 cDNA library, and Stephen King for sending the anti-LC8 antibody. We are grateful to Yves Gaudin and Christine Tuffereau for helpful discussions and for careful reading of the manuscript.
This work was supported by CNRS UPR 9053.
ADDENDUM
Similar results were obtained independently by screening a human brain cDNA library with the P protein of Mokola virus by Jacob et al. (22a).
REFERENCES
- 1.Altschul S F, Madden T L, Schaffer A A, Zhang J, Zhang W, Miller W, Lipman D J. A new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Banerjee A K. Transcription and replication of rhabdoviruses. Microbiol Rev. 1987;51:66–87. doi: 10.1128/mr.51.1.66-87.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barik S, Banerjee A K. Phosphorylation by cellular casein kinase II is essential for the transcriptional activity of vesicular stomatitis virus phosphoprotein P. Proc Natl Acad Sci USA. 1992;89:6570–6574. doi: 10.1073/pnas.89.14.6570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barik S, Banerjee A K. Sequential phosphorylation of the phosphoprotein of vesicular stomatitis virus by cellular and viral protein kinases is essential for transcription activation. J Virol. 1992;66:1109–1118. doi: 10.1128/jvi.66.2.1109-1118.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beckwith S M, Roghi C H, Liu B, Morris N R. The “8kD” cytoplasmic dynein light chain is required for nuclear migration and for dynein heavy chain localization. J Cell Biol. 1998;143:1239–1247. doi: 10.1083/jcb.143.5.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benashski S E, Harrison R S, Patel-King R S, King S M. Dimerisation of the highly conserved light chain shared by dynein and myosin V. J Biol Chem. 1997;272:20929–20935. doi: 10.1074/jbc.272.33.20929. [DOI] [PubMed] [Google Scholar]
- 7.Bourhy H, Kissi B, Tordo N. Molecular diversity of the Lyssavirus genus. Virology. 1993;194:70–81. doi: 10.1006/viro.1993.1236. [DOI] [PubMed] [Google Scholar]
- 8.Bowman A B, Patel-King R S, Benashski S E, McCaffery J M, Goldstein L S, King S M. Drosophila roadblock and Chlamidomonas LC7: A conserved family of dynein-associated proteins involved in axonal transport, flagellar motility and mitosis. J Cell Biol. 1999;146:165–179. [PMC free article] [PubMed] [Google Scholar]
- 9.Chenik M, Chebli K, Blondel D. Translation initiation at alternate in-frame AUG codons in the rabies virus phosphoprotein mRNA is mediated by a ribosomal leaky scanning mechanism. J Virol. 1995;69:707–712. doi: 10.1128/jvi.69.2.707-712.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chenik M, Chebli K, Gaudin Y, Blondel D. In vivo interaction of rabies virus phosphoprotein (P) and nucleoprotein (N), existence of two N binding sites on P protein. J Gen Virol. 1994;75:2889–2896. doi: 10.1099/0022-1317-75-11-2889. [DOI] [PubMed] [Google Scholar]
- 11.Chenik M, Schnell M, Conzelmann K K, Blondel D. Mapping the interacting domains between the rabies virus polymerase and phosphoprotein. J Virol. 1998;72:1925–1930. doi: 10.1128/jvi.72.3.1925-1930.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crepieux P, Kwon H, Leclerc N, Spencer W, Richard S, Lin R, Hiscott J. I kappa B alpha physically interacts with a cytoskeleton-associated protein through its signal response domain. Mol Cell Biol. 1997;17:7375–7385. doi: 10.1128/mcb.17.12.7375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Emerson S U, Wagner R R. Dissociation and reconstitution of the transcriptase and template activities of vesicular stomatitis B and T virions. J Virol. 1972;10:1348–1356. doi: 10.1128/jvi.10.2.297-309.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fu Z F, Zheng Y, Wunner W H, Koprowski H, Dietzschold B. Both the N- and the C-terminal domains of the nominal phosphoprotein of rabies virus are involved in binding to the nucleoprotein. Virology. 1994;200:590–597. doi: 10.1006/viro.1994.1222. [DOI] [PubMed] [Google Scholar]
- 15.Fuerst R T, Niles E G, Studier F W, Moss B. Eukaryotic transient-expression system based on recombinant vaccinia virus that synthesizes bacteriophage T7 RNA polymerase. Proc Natl Acad Sci USA. 1986;83:8122–8126. doi: 10.1073/pnas.83.21.8122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gao Y, Greenfield J, Cleverley D Z, Lenard J. The transcriptional form of the phosphoprotein of vesicular stomatitis virus is a trimer: structure and stability. Biochemistry. 1996;35:14569–14573. doi: 10.1021/bi9613133. [DOI] [PubMed] [Google Scholar]
- 17.Gao Y, Lenard J. Multimerization and transcriptional activation of the phosphoprotein (P) of vesicular stomatitis virus by casein kinase-II. EMBO J. 1995;14:1240–1247. doi: 10.1002/j.1460-2075.1995.tb07107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gaudin Y, Ruigrok R, Tuffereau C, Knossow M, Flamand A. Rabies virus glycoprotein is a trimer. Virology. 1992;187:627–632. doi: 10.1016/0042-6822(92)90465-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gietz D, Jean A S, Woods R A, Schiestl R H. Improved method for high efficiency transformation of intact yeast cells. Nucleic Acids Res. 1992;20:1425. doi: 10.1093/nar/20.6.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guarante L. Strategies for the identification of interacting proteins. Proc Natl Acad Sci USA. 1993;90:1639–1641. doi: 10.1073/pnas.90.5.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gupta A, Blondel D, Choudhary S, Banerjee A. Phosphoprotein (P) of rabies virus is phosphorylated by a unique cellular protein kinase and specific isomers of protein kinase C. J Virol. 2000;74:91–98. doi: 10.1128/jvi.74.1.91-98.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ito H, Fukuda Y, Murata K, Kimura A. Transformation of intact yeast cells treated with alkali cations. J Bacteriol. 1983;153:163–168. doi: 10.1128/jb.153.1.163-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22a.Jacob Y, Badrane H, Ceccaldi P-E, Tordo N. Cytoplasmic dynein LC8 interacts with lyssavirus phosphoprotein. J Virol. 2000;74:10217–10222. doi: 10.1128/jvi.74.21.10217-10222.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jaffrey S R, Snyder S H. PIN: an associated protein inhibitor of neuronal nitric oxyde synthase. Science. 1996;274:774–777. doi: 10.1126/science.274.5288.774. [DOI] [PubMed] [Google Scholar]
- 24.Kaelin K, Dezélée S, Masse M J, Bras F, Flamand A. The UL25 protein of pseudorabies virus associates with capsids and localizes to the nucleus and to microtubules. J Virol. 2000;74:474–482. doi: 10.1128/jvi.74.1.474-482.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.King S M, Barbarese E, Dillman J F, Patel-King R S, Carson J H, Pfister K K. Brain cytoplasmic and flagellar outer arm dyneins share a highly conserved Mr 8,000 light chain. J Biol Chem. 1996;271:19358–19366. doi: 10.1074/jbc.271.32.19358. [DOI] [PubMed] [Google Scholar]
- 26.King S M, Patel-King R S. The Mr=8,000 and 11,000 outer arm dynein light chains from Chlamydomonas flagella have cytoplasmic homologues. J Biol Chem. 1995;270:11445–11452. doi: 10.1074/jbc.270.19.11445. [DOI] [PubMed] [Google Scholar]
- 27.Liang J, Jaffrey S R, Guo W, Snyder S H, Clardy J. Structure of the PIN/LC8 dimer with a bound peptide. Nat Struct Biol. 1999;6:735–740. doi: 10.1038/11501. [DOI] [PubMed] [Google Scholar]
- 28.Pazour G J, Wilkerson C G, Witman G B. A dynein light chain is essential for the retrograde particle movement of intraflagellar transport (IFT) J Cell Biol. 1998;141:979–992. doi: 10.1083/jcb.141.4.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Raux H, Iseni F, Lafay F, Blondel D. Mapping of monoclonal antibody epitopes of the rabies virus P protein. J Gen Virol. 1997;78:119–124. doi: 10.1099/0022-1317-78-1-119. [DOI] [PubMed] [Google Scholar]
- 30.Reiss C S, Komatsu T. Does nitric oxide play a critical role in viral infection? J Virol. 1998;72:4547–4551. doi: 10.1128/jvi.72.6.4547-4551.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rose J K, Buonocore L, Whitt M A. A new cationic liposome reagent mediating nearly quantitative transfection of animal cells. BioTechniques. 1991;10:520–525. [PubMed] [Google Scholar]
- 32.Sagara J, Kawai A. Identification of heat shock protein 70 in the rabies virions. Virology. 1992;206:845–848. doi: 10.1016/0042-6822(92)90923-d. [DOI] [PubMed] [Google Scholar]
- 33.Sagara J, Tochikura T, Tanaka H, Baba Y, Tsukita S, Kawai A. The 21-kDa polypeptide (VAP21) in the rabies virion is a CD99-related host cell protein. Microbiol Immunol. 1998;42:289–297. doi: 10.1111/j.1348-0421.1998.tb02285.x. [DOI] [PubMed] [Google Scholar]
- 34.Sagara J, Tsukita S, Yonemura S, Tsukita S, Kawai A. Cellular actin-binding ezrin-radixin-moesin (ERM) family proteins are incorporated into the rabies virions and closely associated with viral envelope proteins in the cell. Virology. 1995;206:485–494. doi: 10.1016/s0042-6822(95)80064-6. [DOI] [PubMed] [Google Scholar]
- 35.Sodeik B, Ebersold M W, Helenius A. Microtubule-mediated transport of incoming Herpes simplex virus 1 capsids to the nucleus. J Cell Biol. 1997;136:1007–1021. doi: 10.1083/jcb.136.5.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Suomalainen M, Nakano M Y, Keller S, Boucke K, Stidwill R P, Greber U F. Microtubule-dependent plus- and minus end-directed motilities are competing processes for nuclear targeting of adenovirus. J Cell Biol. 1999;144:657–672. doi: 10.1083/jcb.144.4.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ye G J, Vaughan K T, Roizman B. The herpes simplex virus 1 U(L)34 protein interacts with a cytoplasmic dynein intermediate chain and targets nuclear membrane. J Virol. 2000;74:1355–1363. doi: 10.1128/jvi.74.3.1355-1363.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]