Abstract
Background
Enhancing the response rate of immunotherapy will aid in the success of cancer treatment. Here, we aimed to explore the combined effect of immunogenic radiotherapy with anti-PD-L1 treatment in immunotherapy-resistant HNSCC mouse models.
Methods
The SCC7 and 4MOSC2 cell lines were irradiated in vitro. SCC7-bearing mice were treated with hypofractionated or single-dose radiotherapy followed by anti-PD-L1 therapy. The myeloid-derived suppressive cells (MDSCs) were depleted using an anti-Gr-1 antibody. Human samples were collected to evaluate the immune cell populations and ICD markers.
Results
Irradiation increased the release of immunogenic cell death (ICD) markers (calreticulin, HMGB1 and ATP) in SCC7 and 4MOSC2 in a dose-dependent manner. The supernatant from irradiated cells upregulated the expression of PD-L1 in MDSCs. Mice treated with hypofractionated but not single-dose radiotherapy were resistant to tumour rechallenge by triggering ICD, when combined with anti-PD-L1 treatment. The therapeutic efficacy of combination treatment partially relies on MDSCs. The high expression of ICD markers was associated with activation of adaptive immune responses and a positive prognosis in HNSCC patients.
Conclusion
These results present a translatable method to substantially improve the antitumor immune response by combining PD-L1 blockade with immunogenic hypofractionated radiotherapy in HNSCC.
Subject terms: Immunosurveillance, Oral cancer, Radiotherapy
Background
Advances in immunotherapy have led to a new-generation cancer treatment by amplifying the priming of effector T cells [1]. However, only a fraction of patients with certain cancer types exhibits durable responses to a single-agent immune checkpoint inhibitor [2–4]. For a certain proportion of patients, primary or acquired resistance often occurs due to low immunogenicity, absence of tumour-specific antigens, dysfunction in the antigen-presenting process, or infiltration of immunosuppressive cells in the tumour microenvironment (TME) [5]. Head and neck squamous cell carcinoma (HNSCC) is one of the most common types of malignancies with poor prognosis and is considered an immunologically cold tumour of human papillomavirus (HPV)-negative type [6, 7]. The efficacy of anti-programmed death-1 (anti-PD-1) treatment in recurrent/metastatic HNSCC is limited, with response rates ranging from 13 to 20% [8–11]. To deliver the clinical benefit of immunotherapy to the majority of patients with HNSCC, a variety of combinatorial strategies are being investigated, such as radioimmunotherapy or chemoimmunotherapy [12–14].
Radiotherapy (RT) is a part of the standard-of-care treatment for patients with HNSCC, and has significant effects on immune responses in several cancer types [15–17]. Briefly, cytosolic DNA generated by RT leads to dendritic cell (DC) activation and T‑cell priming by type I interferon (IFN), which is produced in a cGAS-STING signalling-dependent manner [18]. RT-based immunogenic cell death (ICD) of tumours displays potential immunogenicity through the exposure/release of damage-associated molecular patterns, including calreticulin (CRT), adenosine triphosphate (ATP), and high-mobility group box 1 (HMGB1), to promote DC activation and enhance tumour antigen presentation [17]. Conversely, as a result of local RT, the production of cytokines and chemokines, such as TGF-β1 and CXCL2, may upregulate the substantial intratumoral infiltration of T regulatory cells (Tregs) and myeloid-derived suppressive cells (MDSCs), which contribute to immunosuppression [19–22]. In addition, RT can also induce constitutive programmed death-ligand 1 (PD-L1) expression in the TME as feedback to IFNs [23–25], which indicates the potential synergistic effects of anti-PD-1/PD-L1 immunotherapy. Although several preclinical studies have shown optimistic outcomes of radioimmunotherapy [22, 26–28], recent Phase 3 clinical data suggested that concurrent anti-PD-L1 with cisplatin and standard fractionation RT (70 Gy in 35 fractions) did not prolong the progression-free survival of patients with locally advanced HNSCC, and treatment-related adverse events commonly occurred [14]. Therefore, these paradoxical results led the researchers to clarify: (i) whether different fractionation and radiation doses orchestrate the balance between antitumor and immunosuppressive responses, and (ii) whether the differences in the sequencing of RT and immunotherapy alter the immunogenicity and dynamics of TME.
In this study, we used murine SCC7 and 4MOSC2 cells as the poorly immunogenic models of HPV-negative HNSCC to test the following hypothesis: that high-dose or hypofractionated RT, instead of standard fractionation RT, could act as ICD inducers to overcome the resistance to anti-PD-L1 treatment. Here, we studied the therapeutic efficacy and immune profile of the combination treatment of radioimmunotherapy. We also investigated the impact of MDSC depletion by anti-Gr-1 on anti-tumour immune responses induced by the combination treatment.
Methods
Mice and cell lines
C3H/HeN and C57BL/6 female mice (6–8 weeks old) were purchased from Beijing Vital River Laboratory Animal Technology Co. Ltd and housed in pathogen-free Animal Biosafety Level 3 (ABSL-III) Laboratory of Wuhan University or Wuhan Myhalic Biotechnological Co. Ltd, according to the National Institute of Health guidelines for the use of laboratory animals.
The murine squamous carcinoma cell line SCC7 was kindly provided by Prof. Qian-Ming Chen at Sichuan University, P. R. China. The cells were cultured in RPMI1640 medium supplemented with 10% foetal bovine serum (FBS) and 1% penicillin/streptomycin at 37 °C and 5% CO2.
4MOSC2 cell line was kindly gifted by Prof. Silvio J. Gutkind at the University of California San Diego. 4MOSC2 cells were cultured in Defined Keratinocyte-SFM medium (Gibco-BRL,) supplemented with Cholera Toxin (50 pM), EGF Recombinant Mouse Protein (5 ng/ml) and 1% penicillin/streptomycin at 37 °C and 5% CO2 [29].
Human samples
The patients’ samples were reviewed and approved by the Institutional Medical Ethics Committee of School and Hospital of Stomatology, Wuhan University (2012LUNSHENZI46, 2018LUNSHENZIA28), and informed consent was obtained from all patients.
Study cohort 1
Fresh tumour tissues were obtained from 15 patients with primary HNSCC admitted in the Hospital of Stomatology, Wuhan University and were used to isolate tumour-infiltrating T cells (TILs). All patients with HNSCC underwent primary surgery (without preoperative adjuvant chemotherapy or RT) from October 2017 to March 2019. The HPV statue was detected by p16 staining. One patient with tumour located in the base of tongue and soft palate was diagnosed with HPV infection, and the other 14 patients were confirmed to be negative for HPV infection.
Study cohort 2
FFPE samples of HNSCC were provided by 103 patients with HNSCC from the Hospital of Stomatology, Wuhan University, which were enrolled between 2012 and 2015. These HNSCC tissues were used to generate human HNSCC tissue microarrays for survival analysis.
The pathological diagnosis was confirmed by the Department of Oral Pathology, Wuhan University, in accordance with the guidelines of the World Health Organization Classification of Head and Neck Tumours (4th, 2017) [30].
Irradiation
Irradiation was delivered to cell lines in vitro or in a C3H mouse model in vivo using two different irradiators. For in vitro RT, the cell lines were seeded at 1 × 105 cells per well in 12-well plates. After 24 h, the culture medium was removed and irradiation was performed at 160 kVp and 25 mA with reflector by Rad Source 2000 X-ray irradiator (Rad Source Technologies Inc., Buford, GA) at the Core Facility of Medical Research Institute at Wuhan University. After irradiation, the cell lines were cultured in Opti-MEM (Thermo-Scientific, Waltham, MA). For the in vivo RT, tumour-bearing C3H mice were anaesthetised with isoflurane. The mice were placed in the prone orientation, and the tumour sites were located by fluoroscopy. Irradiation was performed at 225 kVp and 13 mA with appropriate copper filters using an image-guided PXi-225Cx irradiator (Precision X-Ray Inc., Branford, CT) at Zhongnan Hospital of Wuhan University.
Colony-formation assay
The colony-formation assay was performed as described previously [31], and is detailed in the Supplementary Methods.
Apoptosis assays
After irradiation, the cell lines were incubated with Opti-MEM (Thermo-Scientific, Waltham, MA). Twenty-four hours later, the culture medium was collected. The cell lines were digested with 0.25% trypsin without ethylenediaminetetraacetic acid (EDTA) (Gibco, Carlsbad, CA; 15050065) and centrifuged with culture medium. Annexin V-FITC/PI staining was performed according to the manufacturer’s protocol (BD Biosciences, La Jolla, CA) and flow cytometry was performed using a CytoFLEX LX Flow Cytometer (Beckman Coulter, Brea, CA).
In vitro detection for CRT exposure and the release of HMGB1 and ATP
The surface expression of CRT was detected by flow cytometry and immunofluorescence. Intracellular HMGB1 staining was detected by immunofluorescence. The quantitative release of HMGB1 was detected using enzyme-linked immunoassay (ELISA). The extracellular levels of ATP were assessed using a luciferin-related ATP Assay Kit (Merck, Darmstadt, Germany; 119107) according to the manufacturer’s protocol. The detection details are described in the Supplementary Methods.
Isolation of MDSCs
Isolation of MDSCs was performed as described previously [32], and is detailed in the Supplementary Methods.
Ectopic implanted tumour model and in vivo treatments
The in vivo studies were approved by the Institutional Animal Care and Use Committee (IACUC) of Wuhan University (2019038, 2019108, 2019161, 20220283).
The SCC7 cells were injected subcutaneously into the shaved right inguinal region of syngeneic C3H mice. The tumours were measured using a micrometre calliper, and tumour volumes were calculated using the following equation: tumour volume = (long length × short length2)/2 (mm3). When exhibiting evidence of morbidity according to the guidelines of the IACUC, the mice were euthanized immediately. In vivo treatment strategies are detailed in Supplementary Methods.
Isolation of TILs
Isolation of TILs was performed as described previously [32], and is detailed in the Supplementary Methods.
Flow cytometry
The single-cell suspension of splenocytes, lymph node cells and TILs from mice were first pre-incubated with purified anti-mouse CD16/CD32 (eBioscience, San Diego CA; #14-0161-82) for 20 min at 4 °C. The cells were centrifuged and resuspended in staining buffer containing membrane antibodies and incubated at 4 °C for 30 min.
For mouse nuclear transcription factor staining, the splenocytes and TILs were initially stained with surface marker antibodies. The cells were then fixed with fixation/permeabilization buffer (eBioscience, San Diego, CA; #00-5521-00), and stained with nuclear antibodies in 1× permeabilization buffer (eBioscience, San Diego, CA; #00-8333-56).
For intracellular cytokine staining, the TILs were collected and stimulated with Cell Activation Cocktail with Brefeldin A (BioLegend, San Diego, CA; #423303) in vitro for 6–8 h. The cells were stained with surface marker antibodies, fixed with fixation buffer (BioLegend, San Diego, CA; #420801) and permeabilized with 1× intracellular staining perm wash buffer (BioLegend, San Diego, CA; #421002). The fixed cells were then stained with cytokine antibodies.
Flow cytometry was performed using CytoFLEX LX Flow Cytometer (Beckman Coulter, Brea, CA), and data were analysed using the CytoExpert software (Beckman Coulter, Brea, CA) and FlowJo V10 software (FlowJo™, Ashland, OR). The dead cells were stained with Fixable Viability Dye-eFluor 506 (eBioscience, San Diego, CA; #65-0866-14). A List of antibodies for flow cytometry is shown in Supplementary Table 2.
Immunohistochemistry
The tumour tissues of patients or mice were harvested and formalin-fixed for paraffin embedding. After deparaffinization, rehydration, and antigen retrieval, the sections were stained with the specific primary antibody overnight at 4 °C. The primary antibodies used included HMGB1 (1:100; Abcam, Cambridge, UK; ab18256), CRT (1:400; Cell Signaling Technology, Danvers, MA; #12238), α-SMA (1:640; Cell Signaling Technology, Danvers, MA; #19245), CD3 (1:150; Cell Signaling Technology, Danvers, MA; #78588), and Foxp3 (1:200; Cell Signaling Technology, Danvers, MA; #12653) for mouse tissues; HMGB1 (1:100; Cell Signaling Technology, Danvers, MA; #6893) and CRT (1:500; Abcam, Cambridge, UK; ab92516) for human tissues. On the second day, the sections were incubated with secondary antibodies and reacted with ABC kits (Vector, Burlingame, CA). The slides were scanned using a Pannoramic® Midi (3DHISTECH, Budapest, Hungary) and retrieved using CaseViewer software (3DHISTECH, Budapest, Hungary). For the quantification of α-SMA and CD3, each image was randomly chosen from ten equal-size square frames to calculate the positive staining counts. The mean value indicates the expression levels in the sections. For the quantification of HMGB1 and CRT in Study cohort 1, 10 random areas were selected in the sections and analysed using Pannoramic® Midi (3DHISTECH, Budapest, Hungary) to calculate the Histoscore. For the quantification of HMGB1 and CRT in Study cohort 2, the area of interest was selected in the scanned tissue microarrays by CaseViewer software. The Histoscore was calculated by the Pannoramic® Midi.
Statistical analysis
Statistical analyses were conducted using GraphPad Prism 7.0 for Windows (GraphPad Software Inc., La Jolla, CA). The unpaired t test was used for two-group comparisons, while a two-way analysis of variance (ANOVA) was used for multiple-group comparisons. For survival analysis of mice, the Kaplan–Meier method followed by the long-rank (Mantel–Cox) test was used. The hazard ratios were estimated for pairs of groups. All values were presented as mean ± SEM. P < 0.05 considered statistical significance.
Results
SCC7 and 4MOSC2 cells resistance against the anti-PD-L1 treatment
Tumours were generated by injecting 3 × 105 SCC7 cells to syngeneic C3H/HeN female mice or 1 × 106 4MOSC2 cells to the tongue of C57BL/6 female mice. The mice were administered with PD-L1 mAb at an early stage when the tumours were palpable, with an average diameter of 5 mm (day 8 after inoculation) in SCC7-bearing mice or an average volume of 10 mm3 (day 6 after inoculation) in 4MOSC2-bearing mice. Results indicated that the SCC7 cells and 4MOSC2 cells were resistant to anti-PD-L1 treatment (Supplementary Fig. 1a, d). We found that the anti-PD-L1 treatment failed to increase the number of CD3+ cells in the peripheral immune organs and TME (Supplementary Fig. 1b, e). In addition, no significant differences were observed in the contents of tumour-infiltrating total DCs and CCR7+CD103+ DCs (Supplementary Fig. 1c), which were reported with signatures of antigen transfer and T-cell repriming [33], between the two groups. These results indicated that SCC7 and 4MOSC2 cells were resistant to anti-PD-L1 monotherapy because of the inefficiency of the adaptive immune response.
Irradiation inducing immune phenotypes in murine tumour cell
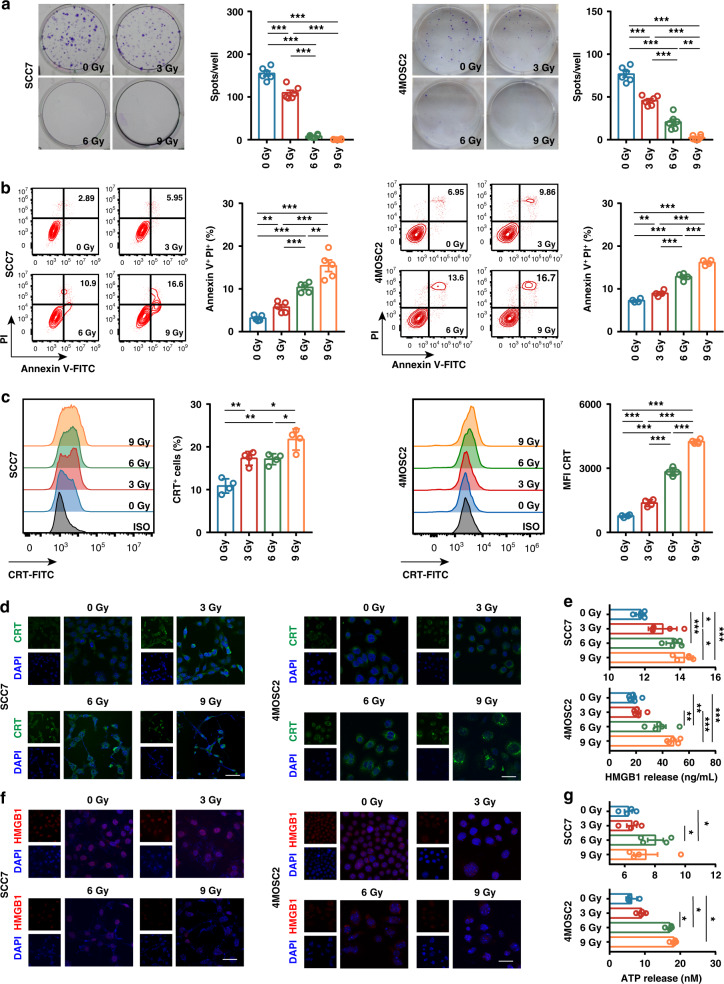
RT is thought to induce a type of immunogenic tumour cell death to increase lymphocyte infiltration and proinflammatory response in the TME [15, 34]. To determine whether irradiation can invert SCC7 and 4MOSC2 cells into an immunogenic phenotype, we explored the effect of RT on the murine tumour cells in vitro. When the cells were exposed to irradiation at 0, 3, 6 and 9 Gy in one fraction, a dose-dependent inhibition of the cell proliferation was observed on colony-formation assay (Fig. 1a). Colony formation in SCC7 cells was almost completely suppressed after administering 6 Gy and 9 Gy dose of RT. We also observed a dose-dependent increase in the apoptosis of SCC7 and 4MOSC2 cells 24 h after irradiation (Fig. 1b). However, the apoptotic ratio was only 15% even exposed at 9 Gy dose of RT. To study the changes in immunogenic phenotypes triggered by RT, we examined three classic components of immunogenic cell death, including CRT cell surface exposure, ATP release, and HMGB1 protein liberation. Flow cytometry analysis was performed on irradiated tumour cells to characterise the surface expression of CRT in the population of PI-negative cells. The percentage of CRT-positive cells significantly increased after irradiation (Fig. 1c). Confocal microscopy also confirmed that CRT was indeed exposed to the plasma membrane of tumour cells after RT (Fig. 1d). Subsequently, we tested the nuclear release of HMGB1 using ELISA and immunofluorescence, and detected the secretion of ATP by luminescence. A dose-dependent increase was also observed in the liberation of HMGB1 in irradiated cells (Fig. 1e, f). The ATP content in the cell supernatant increased at RT dose of 6 Gy, but was slightly decreased at the dose of 9 Gy in SCC7 cells (Fig. 1g). These results suggest that irradiation induced the ICD in murine tumour cells in a dose-dependent manner.
Fig. 1. Irradiation-induced immunogenic cell death (ICD) in SCC7 and 4MOSC2 tumour cells in a dose-dependent manner.
a Representative images of anchor-dependent colony formation and quantitative analysis of SCC7 and 4MOSC2 tumour cells exposed to irradiation in vitro. b Representative contour plots and quantitative analysis of annexin V+ PI+ apoptotic SCC7 tumour cells exposed to irradiation in vitro. c Representative histogram and quantitative analysis of calreticulin (CRT) surface expression in SCC7 and 4MOSC2 tumour cells exposed to irradiation in vitro. d Representative IF images of CRT (green) staining of SCC7 and 4MOSC2 tumour cells exposed to irradiation in vitro. The nuclei were stained with DAPI (blue). Scale bar = 20 μm. e Quantitation of HMGB1 released from SCC7 and 4MOSC2 tumour cells exposed to irradiation in vitro. f Representative IF images of HMGB1 (red) staining of SCC7 and 4MOSC2 tumour cells exposed to irradiation in vitro. The nuclei were stained with DAPI (blue). Scale bar = 20 μm. g Quantitation of ATP released from SCC7 and 4MOSC2 tumour cells exposed to irradiation in vitro. P < 0.05 was considered statistically significant. *P < 0.05; **P < 0.005; ***P < 0.0005. Experiments were repeated twice. ISO isotype.
Several studies have indicated the lack of PD-L1 expression in SCC7 and 4MOSC2 cells [29, 35]. To examine whether irradiation could induce PD-L1 expression of SCC7 and 4MOSC2, the murine tumour cells were treated in vitro with 0, 3, 6, and 9 Gy of RT delivered in one fraction and the PD-L1 expression was analysed by flow cytometry. An upregulation in the expression of PD-L1 was observed on tumour cells after irradiation compared with the expression levels of non-irradiated tumour cells (Fig. 2a, b). MDSCs are reported to augment resistance to immunotherapy in a direct or indirect manner [36]. In addition, recent studies have indicated that RT induces the rapid recruitment of myeloid cell infiltration [37]. To investigate the possible changes in the phenotypes of myeloid cell subsets after an irradiation treatment, we focused on the MDSCs. MDSCs from tumour-bearing mice had elevated PD-L1 expression levels compared with the MDSCs from non-tumour-bearing mice (Fig. 2c, d). After coculturing with the supernatants of irradiated tumour cells, the PD-L1 expression in M-MDSCs was significantly higher than that in M-MDSCs co-cultured with the supernatant of non-irradiated tumour cells (Fig. 2c, d). However, the PMN-MDSCs had moderate baseline PD-L1 expression levels, while the supernatants of irradiated SCC7 cells did not enhance the PD-L1 expression in PMN-MDSCs (Fig. 2c, d). These results raised the possibility that irradiation might invert the SCC7 and 4MOSC2 cells into an immunogenic phenotype and enhance the response rate to PD-L1 blockade.
Fig. 2. Irradiation upregulated PD-L1 expression on tumour cells and MDSCs.
a Representative histogram and quantitative analysis of PD-L1 expression on SCC7 tumour cells with irradiation exposed in vitro. b Representative histogram and quantitative analysis of PD-L1 expression on 4MOSC2 tumour cells with irradiation exposed in vitro. c Representative histogram and quantitative analysis of PD-L1 expression in MDSCs incubated with culture medium from irradiation-treated SCC7 tumour cells. d Representative histogram and quantitative analysis of PD-L1 expression in MDSCs incubated with culture medium from irradiation-treated 4MOSC2 tumour cells. P < 0.05 was considered statistically significant. *P < 0.05; ***P < 0.0005; ns, not significant. Experiments were repeated twice. NT non-tumour-bearing, TB tumour-bearing, ISO isotype, IR irradiation.
Irradiation enhancing the efficacy of anti-PD-L1 treatment
To investigate whether radiation-induced ICD and PD-L1 expression could increase the sensitivity of murine tumour cells to anti-PD-L1 treatment, we utilised two separate RT strategies in the mouse model. Since the 4MOSC2 cells were transplanted into the tongue tissue, precise RT could not be achieved, so we only used the C3H/HeN mouse model for the in vivo experiments. For preventive experiments, the tumour-bearing mice were exposed to a single high dose of 20 Gy or a hypofractionated irradiation of 27 Gy delivered in three fractions concurrently with anti-PD-L1 mAb injection at an early stage. Treatment with a combination of hypofractionated or single high-dose RT anti-PD-L1 effectively controlled the tumour growth compared with anti-PD-L1 treatment alone (Fig. 3a and Supplementary Fig. 2a). Flow cytometry data indicated that the number of CCR7+CD103+ DCs was significantly increased in the TME of mice that received combination treatment compared with those that received anti-PD-L1 treatment alone (Fig. 3b). However, only hypofractionated RT plus anti-PD-L1 treatment induced a remarkable decrease in the number of TOX+PD-1+ exhausted CD8+ T cells and a significant increase in the number of IFN-γ+TNF-α+ effector CD8+ T cells in the TME compared with anti-PD-L1 treatment alone or a combination of anti-PD-L1 treatment and a single high-dose irradiation (Fig. 3b and Supplementary Fig. 3a). In addition, hypofractionated RT plus anti-PD-L1 treatment also enhanced the percentage of central memory (TCM) CD8+ T cells in tumour-draining lymph nodes (Fig. 3c and Supplementary Fig. 3a). These results indicate that hypofractionated RT may display a more durable response to anti-PD-L1 treatment than single-dose irradiation. To test this hypothesis, a rechallenge experiment was performed by reinoculating SCC7 cells on the contralateral side of combination treatment mice on day 34. The hypofractionated RT plus anti-PD-L1 group elicited secondary antitumor responses against tumour rechallenge and prolonged the survival rate in the mouse model (Fig. 3d and Supplementary Fig. 2b, c). When examined by IHC, significantly more CD3+ T cells were detectable in the rechallenge lesions from the hypofractionated RT group than the single high-dose treatment group (Fig. 3e). Although a certain number of CD3+ cells infiltrated the primary lesions from the single high-dose treatment group, it was almost undetectable in the rechallenge lesions (Fig. 3e). In summary, RT induces a strong local antitumor response to anti-PD-L1 treatment and only hypofractionated RT provides a durable and memory response.
Fig. 3. Radiotherapy (RT) enhanced the efficacy of anti-PD-L1 treatment in the mouse model.
a Schematic illustration of the preventive experimental protocol (top). Tumour growth analysis of SCC7 tumour-bearing mice in each group (bottom). The mice received 20 Gy RT in one fraction on day 8 or 27 Gy RT in three fractions on days 8, 10 and 12. Anti-PD-L1 treatment was initiated on day 9 and was delivered three times per week for 2 weeks. The mice were randomly grouped. n = 4 per group. b Quantitation of SCC7 tumour-infiltrating CCR7+CD103+ DCs (left), TOX+PD-1+ CD8 T cells (middle) and IFN-γ+TNF-α+ CD8+ T cells (right). c Pie chart analysis of CD8+ T-cell subsets with naive, central memory (TCM), effector memory (TEM), and effector (eff) phenotype in draining lymph nodes. Quantitation of CD8+ TCM in draining lymph nodes (right). d Schematic illustration of rechallenge experimental protocol (top). Tumour growth analysis of SCC7 tumour-bearing mice in each group (middle). Survival curves in each group (bottom). The mice were randomly grouped. The mice received 20 Gy RT in one fraction on day 8 or 27 Gy RT in three fractions on days 8, 10 and 12. Anti-PD-L1 treatment was started on day 9 and were delivered three times per week for 2 weeks. Mice with irradiation treatment were rechallenged with SCC7 tumour on day 34. n = 4 per group. e Representative IHC images of staining for CD3 in the primary and rechallenge lesions of each group (top), with quantification of the number of CD3+ cells per view (bottom). Scale bar = 100 μm. P < 0.05 was considered statistically significant. *P < 0.05; **P < 0.005; ***P < 0.0005. Experiments were repeated twice. Con control, PL primary lesions, RL rechallenge lesions.
Hypofractionated radiotherapy inducing ICD and enriching the PD-L1+ MDSCs
To further confirm the synergistic effect of hypofractionated RT and anti-PD-L1 treatment, the tumour-bearing mice received 9 Gy × 3 RT, anti-PD-L1 alone, or a combination of RT plus anti-PD-L1 at the later stage of tumour progression. In the absence of anti-PD-L1, hypofractionated RT alone showed a moderate effect in controlling tumour growth, but almost complete regression of the irradiated tumour was achieved by the addition of anti-PD-L1 (Fig. 4a, b and Supplementary Fig. 4a). Changes in vascular structure enhanced the extravasation of chemokines, which results in the migration and infiltration of immune cells [38, 39]. To investigate whether the changes in the microenvironment might be induced by hypofractionated RT, vascular normalisation and MDSCs were evaluated. Significantly elevated levels of α-SMA were observed in the tumour sections from the RT and combination treatment groups, indicating that vascular remodelling occurred after irradiation (Fig. 4c). The flow cytometry data demonstrated that combination treatment induced an increase in tumour-infiltrating M-MDSCs and a decrease in tumour-infiltrating PMN-MDSCs at day 16 (Fig. 4d and Supplementary Fig. 4b). Similar to the in vitro results, irradiation also significantly increased the expression level of PD-L1 in M-MDSCs and PMN-MDSCs in vivo (Fig. 4d). However, the expression level of PD-L1 in MDSCs was downregulated after anti-PD-L1 treatment (Fig. 4d). Furthermore, unlike anti-PD-L1 treatment alone, hypofractionated RT significantly increased the CRT expression and nuclear HMGB1 staining in the tumour niche, which was mirrored by our in vitro studies (Fig. 4e). In addition, the application of anti-PD-L1 did not enhance the exposure of ICD markers (Fig. 4e). In summary, these results demonstrated that hypofractionated RT induced the ICD and enriched the PD-L1+ MDSCs to enhance the efficacy of anti-PD-L1.
Fig. 4. Fractionated radiotherapy (RT) induced immunogenic cell death (ICD) and promoted anti-PD-L1 treatment in a therapeutic study.
a Schematic illustration of therapeutic experimental protocol. The mice were treated with isotype control or anti-PD-L1 antibody, alone or in combination with 27 Gy RT in three fractions on days 11, 13 and 15. Anti-PD-L1 or isotype control antibody treatment was initiated on day 12 and was delivered three times in one week. The mice were randomly grouped. n = 6 per group. b Tumour growth analysis of SCC7-bearing mice in each group. c Representative IHC images of staining for α-SMA in the tumour lesions of each group (top), with quantification of the number of α-SMA+ cells per view (bottom). Scale bar = 100 μm. d Quantitation of the number of tumour-infiltrating M-MDSCs and PMN-MDSCs in CD45+ cells (left). Quantitation of the PD-L1 expression based on the MFI in tumour-infiltrating M-MDSCs and PMN-MDSCs (right). e Representative IHC images of staining for calreticulin (CRT) and HMGB1 in the tumour lesions of each group (left), with quantification of the CRT and HMGB1 expression score (right). Scale bar = 100 μm. *P < 0.05; **P < 0.005; ***P < 0.0005. Experiments were repeated twice. Con control, IR irradiation.
Anti-PD-L1-based radioimmunotherapy is more effective than cisplatin-based chemoradiotherapy
Cisplatin-based chemoradiotherapy acted as the standard-of-care therapy for locally advanced HNSCC [40]. To further compare the therapeutic effect of cisplatin-based chemoradiotherapy and anti-PD-L1-based radioimmunotherapy, the tumour-bearing mice received 9 Gy × 3 RT alone, a combination of RT plus cisplatin, or a combination of RT plus anti-PD-L1. The results indicated that treatment with the combination of hypofractionated RT with anti-PD-L1 effectively controlled the tumour growth compared with cisplatin-based chemoradiotherapy (Fig. 5a and Supplementary Fig. 5a). Flow cytometry data indicated that the number of CD107a+ T cells, CCR7+CD103+ DCs in the TME and CD80+CD86+ DCs in DLN was significantly increased in the mice that received radioimmunotherapy compared with those that received anti-PD-L1 treatment alone (Fig. 5b). In addition, cisplatin-based chemoradiotherapy and anti-PD-L1-based radioimmunotherapy both induced a remarkable decrease in the number of TOX+PD-1+ exhausted CD8+ T cells in the TME compared with the mice received radiotherapy alone (Fig. 5b).
Fig. 5. The therapeutic efficacy of fractionated radiotherapy in combination with anti-PD-L1 or cisplatin.
a Schematic illustration of the experimental protocol (left). Tumour growth analysis of SCC7-bearing mice in each group (right). The mice were treated with 27 Gy in three fractions (9 Gy × 3), cisplatin + 27 Gy in three fractions (9 Gy × 3), or anti-PD-L1 + 27 Gy in three fractions (9 Gy × 3). Three fractions of 27 Gy RT were delivered on days 13, 15 and 17. Anti-PD-L1 treatment was initiated on day 14 and was administered three times per week. Cisplatin treatment was initiated on day 14 and was administered twice per week. The mice were randomly grouped. n = 4 per group. b Quantitation of CD107a+ CD3 T cells in spleens (left), tumour-infiltrating CCR7+CD103+ DCs and TOX+PD-1+ CD8 T cells (middle), and CD80+CD86+ DCs in draining lymph nodes (right). *P < 0.05; **P < 0.005. IR irradiation.
Combination treatment partially relying on Gr-1+ myeloid cells
Next, we investigated the specific role of MDSCs in the combination treatment of hypofractionated RT and anti-PD-L1. According to our previous studies [32], to completely eliminate the Gr-1+ MDSCs in the mouse model, each C3H/HeN mouse was injected with 250 μg of anti-Gr-1 antibody (clone RB6-8C5) on day −1 and days 4, 9 and 14 (Fig. 6a). Results showed that the anti-Gr-1 only slightly inhibited the tumour growth in the anti-PD-L1 treatment or control groups (Fig. 6b and Supplementary Fig. 6b). In addition, the anti-Gr-1 treatment partially abolished the antitumor efficacy of hypofractionated RT or combination treatment and shortened the survival time of tumour-bearing mice (Fig. 6b, c). These unexpected results indicated that although anti-Gr-1 successfully deleted MDSCs, it did not aid in tumour control by combination treatment. To investigate what factors might be related with the tumour relapse after anti-Gr-1 injection, we quantified the Foxp3+ Tregs in the tumours by IHC. The results showed that anti-Gr-1 injection significantly enhanced Foxp3+ Tregs in the tumours compared with isotype groups (Fig. 6d). These results indicate that the combination treatment may partially rely on Gr-1+ myeloid cells and eradication of MDSCs may impair the hypofractionated RT-induced anti-tumour effect.
Fig. 6. The therapeutic efficacy of anti-PD-L1 in combination with fractionated radiotherapy (RT) is dependent on the Gr-1+ myeloid cells.
a Schematic illustration of the experimental protocol. Gr-1 mAb or isotype control was pre-injected to the C3H mice at 250 μg the day before the SCC7 cells were implanted, with other three injections administered on days 4, 9 (1 day after radiotherapy), and 14 (6 days after radiotherapy). The mice were treated with isotype control or anti-PD-L1 antibody, alone or in combination with 27 Gy RT in three fractions on days 8, 10 and 12. Anti-PD-L1 or isotype control antibody treatment was initiated on day 9 and was delivered and administered three times for 3 weeks. The mice were randomly grouped. n = 5 per group. b Tumour growth analysis of SCC7-bearing mice in each group. c Survival curves of SCC7-bearing mice in each group. d Representative IHC images of staining for Foxp3 in the tumour lesions of each group (top), with quantification of the number of Foxp3+ cells per view (bottom). Scale bar = 100 μm. *P < 0.05; **P < 0.005; ***P < 0.0005. Experiments were repeated twice. Con control, IR irradiation.
ICD markers were associated with immune response and patient survival in HNSCC
To clarify the correlation between ICD markers and immune response, 15 fresh tumour samples were collected from patients with HNSCC (cohort 1), and the ICD markers (CRT and HMGB1) and immune cell infiltration were analysed; 103 formalin-fixed paraffin-embedded (FFPE) tumour samples were collected (cohort 2), and the overall survival (OS) were analysed. Heterogeneity was observed in the ICD marker expression in the HNSCC tissues. Therefore, the patients were divided into four groups according to the expression levels of CRT and HMGB1 (Fig. 7a). The tumour-infiltrating immune cells were also evaluated with a focus on CD3+, CD4+, CD8+ T lymphocytes, subsets of memory T cells, conventional dendritic cells (cDCs), plasmacytoid dendritic cells (pDCs) and subsets of MDSCs in cohort 1 (Fig. 7a and Supplementary Fig. 8). We found a significantly higher percentage of CD3+, CD4+, and CD8+ T lymphocytes in the TILs from dual CRThigh and HMGB1high patients than in CRTlow or HMGB1low patients (Fig. 7b). In addition, based only on the expression level of one marker in HMGB1 or CRT for classification, no statistical difference was observed in the changes in immune cells (Fig. 7b). Compared with CRTlow or HMGB1low tumours, dual CRThigh and HMGB1high tumours were significantly more infiltrated by CD8+ effector memory T cells (CD45RA−/CCR7−), whereas the percentage of CD8+ naive T cells (CD45RA+/CCR7+) was significantly decreased (Fig. 7c). However, no difference was seen for the subsets of CD4+ memory T cells (Supplementary Fig. 8a, b). We also observed a significant increase in the expression level of PD-L1 in the M-MDSCs and a significantly higher percentage of cDCs in CRThigh patients or dual CRThigh and HMGB1high patients (Fig. 7d, e). Meanwhile, no difference was observed for PD-L1+ PMN-MDSCs and pDCs (Supplementary Fig. 8c, d). Furthermore, we acquired and analysed the publicly available transcriptomic data from The Cancer Genome Atlas (TCGA) data portal [41] to show the immune status among HNSCC patients with various ICD signatures. An ICD marker-based classification of HNSCC was developed according to the CALR (encoding calreticulin)and HMGB1 expression. The results indicated tumours in type D with CALR hiHMGB1hi gene type were highly expressed the immunostimulatory related genes and MHC-related genes (Supplementary Fig. 9a). In cohort 2, the baseline characteristics, including gender, age, smoking status, alcohol consumption, T stage, N stage and grade, were examined to evaluate the relationship between ICD markers and patient outcomes (Supplementary Table 1). Cohort 2 contained 103 samples, with 73 samples exhibiting positive CRT expression, 50 samples exhibiting positive HMGB1 expression and 44 samples exhibiting dual positive expression of CRT and HMGB1. High expression of CRT or HMGB1 was not significantly associated with positive OS [CRT: HR = 0.586; 95% confidence interval (CI), 0.2967–1.156, P = 0.0779 by the log-rank test; HMGB1: HR = 0.825; 95% CI, 0.4598–1.48, P = 0.514 by the log-rank test; Supplementary Fig. 8e, f]. Only dual positive expression of CRT and HMGB1 was significantly associated with positive OS, compared with CRT– or HMGB1– subset (HR = 0.527; 95% CI, 0.294–0.946, P = 0.0368 by the log-rank test; Fig. 7f). In addition, univariate and multivariate Cox regression analyses of cohort 2 demonstrated that dual positive expression of CRT and HMGB1 was an independent predictor of HNSCC for predicting clinical outcomes (HR = 0.439; 95% CI, 0.229–0.839, multivariate Cox regression, P = 0.013; Supplementary Table 1). Overall, these data suggest that the elevated expression of ICD markers in tumour is correlated with the increased immune response and positive OS in patients with HNSCC.
Fig. 7. Phenotypic analysis of the tumour-infiltrating immune cells according to the expression of immunogenic cell death (ICD) markers among the prospective cohort of HNSCC patients.
a IHC on serial sections of four individual human patient tissue with HNSCC for staining with calreticulin (CRT) and HMGB1 (top). Scale bar = 50 μm. Representative contour plots of CD3+ T cells of four individual human patients with HNSCC (bottom). b Quantitation of the number of tumour-infiltrating CD3+ T cells (left), CD4+ T cells (middle) and CD8+ (right) T cells in each group according to the expression of CRT and HMGB1. c Pie chart analysis of CD8+ and CD4+ T-cell subsets with naive, central memory (TCM), effector memory (TEM), and terminally differentiated effector memory (TEMRA) phenotype (left). Quantitation of the number of tumour-infiltrating CD8+ naive and TEM T cells in each group (right). d Quantitation of the PD-L1 expression based on the MFI in tumour-infiltrating M-MDSCs in each group. e Quantitation of the number of tumour-infiltrating conventional dendritic (cDCs) cells in each group. f Kaplan–Meier survival curves for overall survival for patients with HNSCC according to the expression of CRT and HMGB1. *P < 0.05; **P < 0.005; ns not significant.
Discussion
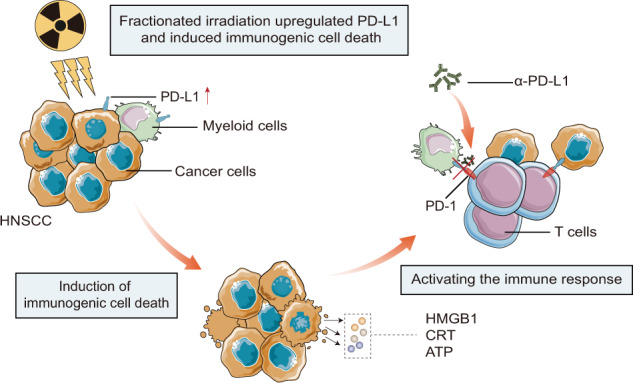
HPV-negative HNSCC exhibits immunologically cold in the TME [6], and the method of improving the response rate of these patients to current immunotherapies remains to be investigated further. Although a number of studies have reported the optimistic effects of radioimmunotherapy in several preclinical models, such as prostate cancer [21, 42], pancreatic ductal adenocarcinoma (PDAC) [43] and HNSCC [22, 26], our understanding of the optimal dose, fractionation and time schedule of radioimmunotherapy is still limited. In this study, we compared the effects of hypofractionated or single high-dose RT followed by anti-PD-L1 treatment in the immunotherapy-resistant mouse models; we established that hypofractionated RT induced both ICD and PD-L1+ MDSCs infiltration and enhanced the response rate of anti-PD-L1 therapy in MDSC-dependent manner.
The application of RT as a method for triggering the bona fide ICD of tumour cells represents a promising advancement in the field of radiation oncology [34, 44–46]. Several studies conducted in mouse models indicated that ICD induction by RT was accompanied by CRT exposure [47], HMGB1 release [48], ATP secretion [34, 49], and activation of type I IFN signalling [18]. Although RT is one of the standard-of-care treatments for HNSCC, there is currently a lack of direct evidence showing the ability of RT to induce ICD in HNSCC. In this study, we demonstrated that RT could induce signals for these components of ICD in SCC7 cells in vitro in a fractionation-dependent manner. In vivo studies also highlighted that the expression of CRT and HMGB1 in the TME could be enhanced by hypofractionated RT. The expression of PD-L1 can be upregulated by IFN signalling, which has emerged as a negative feedback mechanism to reduce effector T-cell functions [50]. Our studies also confirmed that the expression of PD-L1 in tumour cells and M-MDSCs could be upregulated by irradiation in a fractionation-dependent manner. These results suggested that radiotherapy could transform the immunotherapy-resistant tumour into an immunogenic phenotype, which is sensitive to anti-PD-L1 therapy, and this is the primary reason for the application of combination therapy. In addition, high expression levels of CRT and HMGB1 in HNSCC patients were correlated with the high infiltration of effector memory T cells, cDCs and PD-L1+ M-MDSCs, which indicated the possibility that immune reactions in tumours with bona fide ICD may be activated in antitumor immunosurveillance, while that of CRTlow or HMGB1low tumours would be dysfunctional.
The optimal RT fractionation and dose to induce an immune response remain unclear. In clinical practice, RT is usually delivered in multiple daily fractions to control tumour progression and avoid normal tissue damage concurrently, but severe lymphopenia or neutropenia often occurs in the peripheral circulation [13, 14, 51]. Currently, extremely hypofractionated or single high-dose RT can be delivered precisely using stereotactic body radiotherapy [52]. Several studies have suggested that hypofractionated RT could synergise with anti-PD-1/PD-L1 therapy [19, 21, 26, 44]. However, whether hypofractionated RT delivered in multiple fractions or a single high-dose fraction could display an advantage in activating the immune response remains controversial. In this study, we compared the efficacy of single high-dose and hypofractionated RT in the less immunogenic model, instead of standard fractionation RT of 1.8–2.0 Gy daily, due to the negative result reported in the JAVELIN Head and Neck 100 clinical trial [14]. Our data suggested that a single high dose of 20 Gy was as effective as the hypofractionated regimen of 27 Gy delivered in three fractions to enhance the efficacy of anti-PD-L1. However, the mice administered with an RT dose of 20 Gy in one fraction with anti-PD-L1 did not show a significant improvement in response to tumour rechallenge; by contrast, a significant improvement in the control of both primary and rechallenged tumours with a higher infiltration of CD3+ T cells was observed when the mice were treated with hypofractionated regimens of 27 Gy delivered in three fractions and anti-PD-L1. In addition, hypofractionated radioimmunotherapy dramatically reshaped the TME by increasing the infiltration of central memory T cells and decreasing the TOX+PD-1+ exhausted T cells, thus suggesting the indispensable role of hypofractionated RT in increasing tumour immunogenicity.
RT may regulate MDSC-related immunity by affecting repolarization and recruitment [19, 21, 53, 54]. However, the precise effects may react in a variable and spatiotemporal manner, which may be dependent on the type of cancer, dose and the fraction of irradiation, and MDSC phenotypes. For example, in HPV-positive HNSCC mouse models, TGF-β1 can transform MDSCs into functional myeloid cells when combined with RT [20]. However, in HPV-negative mouse models, MDSCs were dominant drivers of resistance to RT during Treg depletion [19]. Moreover, increased PMN-MDSC infiltration and proliferation in response to RT were observed in murine and human PDAC samples [54]. However, 8 Gy × 2 but not 2 Gy × 10 RT resulted in the reduction of PMN-MDSCs in MOC1 and MC38-CEA tumours, whereas the accumulation of M-MDSCs was consistently increased in both the TME and periphery of MC38-CEA tumour-bearing mice [55]. In this study, we found that hypofractionated RT increased the levels of PD-L1 expression in both M-MDSCs and PMN-MDSCs, but only M-MDSCs were enriched in the TME. The different infiltration profiles of MDSC subsets may be due to the different cytokine release patterns and reconstruction of the vasculature caused by RT [56]. However, when combined with anti-PD-L1 therapy, the upregulation of PD-L1 expression in MDSCs, which was caused by RT, resulted in reactive downregulation. This is perhaps the reason for the off-target effect of anti-PD-L1 treatment alone in this mouse model. To determine the exact role of MDSCs in this combination treatment, a Gr-1-depletion antibody was used to eliminate systemic and intratumoral MDSCs. Surprisingly, knockout of MDSCs with Gr-1 mAb did not enhance the efficacy of the combination therapy, but partially abolished the efficacy on tumour growth. These results indicate that Gr-1+ myeloid cells may play a decisive role in combination therapy. A recent study revealed that depletion of MDSCs induced a compensatory increase in Tregs and resulted in the revitalisation of Treg-mediated immunosuppressive responses [21]. We also confirmed that Foxp3+ cells were infiltrated when Gr-1+ myeloid cells were eliminated in the mouse model. These results suggest that the change in the immunosuppressive microenvironment caused by the clearance of Gr-1+ myeloid cells may be the reason for the failure of the combination therapy. Therefore, we hypothesised that PD-L1+ M-MDSCs may act as biomarkers of the response to radioimmunotherapy in HNSCC. The mechanism of the differential infiltration of MDSC subsets and the specific mode of crosstalk between Tregs and MDSCs require further investigation.
In summary, hypofractionated RT induces immunogenic effects on the immunotherapy-resistant HNSCC model and sensitises the anti-PD-L1 treatment in MDSC-dependent manner. Anti-PD-L1 treatment in combination with immunogenic hypofractionated RT may be a potential therapeutic strategy for HNSCC.
Supplementary information
Acknowledgements
We want to thank Shuyan Liang and Zhixin Qiu from Wuhan Biobank Co. Ltd., Hubei, China for their excellent technical assistance on flow cytometry and cells isolation. And we also want to thank Ji Chen, Yan-Peng Ding and Yan Qi from Zhongnan Hospital of Wuhan University and Ying Liu and Jin Jing from the Core Facility of Medical Research Institute at Wuhan University for their kind guidance on radiotherapy experiments.
Author contributions
The authors contributed in the following way: designed and performed the research: LM; wrote the manuscripts: LM, HY and ZS; data analysis: LM, JZ, YX, QY, SY, SW, ZW and HX; performed experiments: LM, JZ, YX, QY, SY, SW, ZW and HX; supervised the research: HY and ZS.
Funding
This work was supported by the National Natural Science Foundation of China (NFSC): 82103333, 82072996, 81874131 and the Fundamental Research Funds for the Central Universities (2042021kf0174, 2042021kf0216).
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
All animal experiments in this study were approved by the Institutional Animal Care and Use Committee (IACUC) of Wuhan University (2019038, 2019108, 2019161, 20220283). All experiments performed using the patients’ samples were reviewed and approved by the Institutional Medical Ethics Committee of School and Hospital of Stomatology, Wuhan University (2012LUNSHENZI46, 2018LUNSHENZIA28), and informed consent was obtained from all patients. All studies were conducted in accordance with the Declaration of Helsinki.
Consent for publication
Not applicable.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Hai-Jun Yu, Email: haijunyu@whu.edu.cn.
Zhi-Jun Sun, Email: sunzj@whu.edu.cn.
Supplementary information
The online version contains supplementary material available at 10.1038/s41416-023-02230-0.
References
- 1.Galon J, Bruni D. Tumor immunology and tumor evolution: intertwined histories. Immunity. 2020;52:55–81. doi: 10.1016/j.immuni.2019.12.018. [DOI] [PubMed] [Google Scholar]
- 2.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–54. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372:2018–28. doi: 10.1056/NEJMoa1501824. [DOI] [PubMed] [Google Scholar]
- 4.Burtness B, Harrington KJ, Greil R, Soulieres D, Tahara M, de Castro G, Jr., et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet. 2019;394:1915–28. doi: 10.1016/S0140-6736(19)32591-7. [DOI] [PubMed] [Google Scholar]
- 5.Sharma P, Hu-Lieskovan S, Wargo JA, Ribas A. Primary, adaptive, and acquired resistance to cancer immunotherapy. Cell. 2017;168:707–23. doi: 10.1016/j.cell.2017.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Whiteside TL. Head and neck carcinoma immunotherapy: facts and hopes. Clin Cancer Res. 2018;24:6–13. doi: 10.1158/1078-0432.CCR-17-1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mandal R, Senbabaoglu Y, Desrichard A, Havel JJ, Dalin MG, Riaz N, et al. The head and neck cancer immune landscape and its immunotherapeutic implications. JCI Insight. 2016;1:e89829. doi: 10.1172/jci.insight.89829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferris RL, Blumenschein G, Jr, Fayette J, Guigay J, Colevas AD, Licitra L, et al. Nivolumab vs investigator’s choice in recurrent or metastatic squamous cell carcinoma of the head and neck: 2-year long-term survival update of CheckMate 141 with analyses by tumor PD-L1 expression. Oral Oncol. 2018;81:45–51. doi: 10.1016/j.oraloncology.2018.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferris RL, Blumenschein G, Jr, Fayette J, Guigay J, Colevas AD, Licitra L, et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med. 2016;375:1856–67. doi: 10.1056/NEJMoa1602252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chow LQM, Haddad R, Gupta S, Mahipal A, Mehra R, Tahara M, et al. Antitumor activity of pembrolizumab in biomarker-unselected patients with recurrent and/or metastatic head and neck squamous cell carcinoma: results from the phase Ib KEYNOTE-012 expansion cohort. J Clin Oncol. 2016;34:3838–45. doi: 10.1200/JCO.2016.68.1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cohen EEW, Soulieres D, Le Tourneau C, Dinis J, Licitra L, Ahn MJ, et al. Pembrolizumab versus methotrexate, docetaxel, or cetuximab for recurrent or metastatic head-and-neck squamous cell carcinoma (KEYNOTE-040): a randomised, open-label, phase 3 study. Lancet. 2019;393:156–67. doi: 10.1016/S0140-6736(18)31999-8. [DOI] [PubMed] [Google Scholar]
- 12.Powell SF, Gold KA, Gitau MM, Sumey CJ, Lohr MM, McGraw SC, et al. Safety and efficacy of pembrolizumab with chemoradiotherapy in locally advanced head and neck squamous cell carcinoma: a phase IB study. J Clin Oncol. 2020;38:2427–37. doi: 10.1200/JCO.19.03156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weiss J, Sheth S, Deal AM, Grilley Olson JE, Patel S, Hackman TG, et al. Concurrent definitive immunoradiotherapy for patients with stage III-IV head and neck cancer and cisplatin contraindication. Clin Cancer Res. 2020;26:4260–7. doi: 10.1158/1078-0432.CCR-20-0230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee NY, Ferris RL, Psyrri A, Haddad RI, Tahara M, Bourhis J, et al. Avelumab plus standard-of-care chemoradiotherapy versus chemoradiotherapy alone in patients with locally advanced squamous cell carcinoma of the head and neck: a randomised, double-blind, placebo-controlled, multicentre, phase 3 trial. Lancet Oncol. 2021;22:450–62. doi: 10.1016/S1470-2045(20)30737-3. [DOI] [PubMed] [Google Scholar]
- 15.Demaria S, Coleman CN, Formenti SC. Radiotherapy: changing the game in immunotherapy. Trends Cancer. 2016;2:286–94. doi: 10.1016/j.trecan.2016.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karam SD, Raben D. Radioimmunotherapy for the treatment of head and neck cancer. Lancet Oncol. 2019;20:e404–16. doi: 10.1016/S1470-2045(19)30306-7. [DOI] [PubMed] [Google Scholar]
- 17.Galluzzi L, Buque A, Kepp O, Zitvogel L, Kroemer G. Immunogenic cell death in cancer and infectious disease. Nat Rev Immunol. 2017;17:97–111. doi: 10.1038/nri.2016.107. [DOI] [PubMed] [Google Scholar]
- 18.Deng L, Liang H, Xu M, Yang X, Burnette B, Arina A, et al. STING-dependent cytosolic DNA sensing promotes radiation-induced type I interferon-dependent antitumor immunity in immunogenic tumors. Immunity. 2014;41:843–52. doi: 10.1016/j.immuni.2014.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oweida AJ, Darragh L, Phan A, Binder D, Bhatia S, Mueller A, et al. STAT3 modulation of regulatory T cells in response to radiation therapy in head and neck cancer. J Natl Cancer Inst. 2019;111:1339–49. doi: 10.1093/jnci/djz036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jayaraman P, Parikh F, Newton JM, Hanoteau A, Rivas C, Krupar R, et al. TGF-beta1 programmed myeloid-derived suppressor cells (MDSC) acquire immune-stimulating and tumor killing activity capable of rejecting established tumors in combination with radiotherapy. Oncoimmunology. 2018;7:e1490853. doi: 10.1080/2162402X.2018.1490853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin L, Kane N, Kobayashi N, Kono EA, Yamashiro JM, Nickols NG, et al. High-dose per fraction radiotherapy induces both antitumor immunity and immunosuppressive responses in prostate tumors. Clin Cancer Res. 2021;27:1505–15. doi: 10.1158/1078-0432.CCR-20-2293. [DOI] [PubMed] [Google Scholar]
- 22.Oweida A, Hararah MK, Phan A, Binder D, Bhatia S, Lennon S, et al. Resistance to radiotherapy and PD-L1 blockade is mediated by TIM-3 upregulation and regulatory T-cell infiltration. Clin Cancer Res. 2018;24:5368–80. doi: 10.1158/1078-0432.CCR-18-1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Deng L, Liang H, Burnette B, Beckett M, Darga T, Weichselbaum RR, et al. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J Clin Invest. 2014;124:687–95. doi: 10.1172/JCI67313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vanpouille-Box C, Diamond JM, Pilones KA, Zavadil J, Babb JS, Formenti SC, et al. TGFbeta is a master regulator of radiation therapy-induced antitumor immunity. Cancer Res. 2015;75:2232–42. doi: 10.1158/0008-5472.CAN-14-3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dovedi SJ, Adlard AL, Lipowska-Bhalla G, McKenna C, Jones S, Cheadle EJ, et al. Acquired resistance to fractionated radiotherapy can be overcome by concurrent PD-L1 blockade. Cancer Res. 2014;74:5458–68. doi: 10.1158/0008-5472.CAN-14-1258. [DOI] [PubMed] [Google Scholar]
- 26.Oweida A, Lennon S, Calame D, Korpela S, Bhatia S, Sharma J, et al. Ionizing radiation sensitizes tumors to PD-L1 immune checkpoint blockade in orthotopic murine head and neck squamous cell carcinoma. Oncoimmunology. 2017;6:e1356153. doi: 10.1080/2162402X.2017.1356153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Newton JM, Hanoteau A, Liu HC, Gaspero A, Parikh F, Gartrell-Corrado RD, et al. Immune microenvironment modulation unmasks therapeutic benefit of radiotherapy and checkpoint inhibition. J Immunother Cancer. 2019;7:216. doi: 10.1186/s40425-019-0698-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wennerberg E, Spada S, Rudqvist NP, Lhuillier C, Gruber S, Chen Q, et al. CD73 blockade promotes dendritic cell infiltration of irradiated tumors and tumor rejection. Cancer Immunol Res. 2020;8:465–78. doi: 10.1158/2326-6066.CIR-19-0449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang Z, Wu VH, Allevato MM, Gilardi M, He Y, Luis Callejas-Valera J, et al. Syngeneic animal models of tobacco-associated oral cancer reveal the activity of in situ anti-CTLA-4. Nat Commun. 2019;10:5546. doi: 10.1038/s41467-019-13471-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.El-Naggar AK, Chan JKC, Grandis JR, Takata T, Slootweg PJ. WHO classification of head and neck tumours. Lyon, Fracnce: International Agency for Research on Cancer (IARC); 2017.
- 31.Huang CF, Chen L, Li YC, Wu L, Yu GT, Zhang WF, et al. NLRP3 inflammasome activation promotes inflammation-induced carcinogenesis in head and neck squamous cell carcinoma. J Exp Clin Cancer Res. 2017;36:116. doi: 10.1186/s13046-017-0589-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu L, Mao L, Liu JF, Chen L, Yu GT, Yang LL, et al. Blockade of TIGIT/CD155 signaling reverses T-cell exhaustion and enhances antitumor capability in head and neck squamous cell carcinoma. Cancer Immunol Res. 2019;7:1700–13. doi: 10.1158/2326-6066.CIR-18-0725. [DOI] [PubMed] [Google Scholar]
- 33.Roberts EW, Broz ML, Binnewies M, Headley MB, Nelson AE, Wolf DM, et al. Critical role for CD103(+)/CD141(+) dendritic cells bearing CCR7 for tumor antigen trafficking and priming of T cell immunity in melanoma. Cancer Cell. 2016;30:324–36. doi: 10.1016/j.ccell.2016.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Golden EB, Frances D, Pellicciotta I, Demaria S, Helen Barcellos-Hoff M, Formenti SC. Radiation fosters dose-dependent and chemotherapy-induced immunogenic cell death. Oncoimmunology. 2014;3:e28518. doi: 10.4161/onci.28518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kondo Y, Ohno T, Nishii N, Harada K, Yagita H, Azuma M. Differential contribution of three immune checkpoint (VISTA, CTLA-4, PD-1) pathways to antitumor responses against squamous cell carcinoma. Oral Oncol. 2016;57:54–60. doi: 10.1016/j.oraloncology.2016.04.005. [DOI] [PubMed] [Google Scholar]
- 36.Veglia F, Perego M, Gabrilovich D. Myeloid-derived suppressor cells coming of age. Nat Immunol. 2018;19:108–19. doi: 10.1038/s41590-017-0022-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xu J, Escamilla J, Mok S, David J, Priceman S, West B, et al. CSF1R signaling blockade stanches tumor-infiltrating myeloid cells and improves the efficacy of radiotherapy in prostate cancer. Cancer Res. 2013;73:2782–94. doi: 10.1158/0008-5472.CAN-12-3981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Carmeliet P, Jain RK. Principles and mechanisms of vessel normalization for cancer and other angiogenic diseases. Nat Rev Drug Discov. 2011;10:417–27. doi: 10.1038/nrd3455. [DOI] [PubMed] [Google Scholar]
- 39.Sharabi AB, Lim M, DeWeese TL, Drake CG. Radiation and checkpoint blockade immunotherapy: radiosensitisation and potential mechanisms of synergy. Lancet Oncol. 2015;16:e498–509. doi: 10.1016/S1470-2045(15)00007-8. [DOI] [PubMed] [Google Scholar]
- 40.Pfister DG, Spencer S, Adelstein D, Adkins D, Anzai Y, Brizel DM, et al. Head and neck cancers, version 2.2020, NCCN clinical practice guidelines in oncology. J Natl Compr Cancer Netw. 2020;18:873–98. doi: 10.6004/jnccn.2020.0031. [DOI] [PubMed] [Google Scholar]
- 41.Thorsson V, Gibbs DL, Brown SD, Wolf D, Bortone DS, Ou Yang TH, et al. The immune landscape of cancer. Immunity. 2018;48:812–30.e814. doi: 10.1016/j.immuni.2018.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dudzinski SO, Cameron BD, Wang J, Rathmell JC, Giorgio TD, Kirschner AN. Combination immunotherapy and radiotherapy causes an abscopal treatment response in a mouse model of castration resistant prostate cancer. J Immunother Cancer. 2019;7:218. doi: 10.1186/s40425-019-0704-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Poty S, Carter LM, Mandleywala K, Membreno R, Abdel-Atti D, Ragupathi A, et al. Leveraging bioorthogonal click chemistry to improve (225)Ac-radioimmunotherapy of pancreatic ductal adenocarcinoma. Clin Cancer Res. 2019;25:868–80. doi: 10.1158/1078-0432.CCR-18-1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dewan MZ, Galloway AE, Kawashima N, Dewyngaert JK, Babb JS, Formenti SC, et al. Fractionated but not single-dose radiotherapy induces an immune-mediated abscopal effect when combined with anti-CTLA-4 antibody. Clin Cancer Res. 2009;15:5379–88. doi: 10.1158/1078-0432.CCR-09-0265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Galluzzi L, Kepp O, Kroemer G. Immunogenic cell death in radiation therapy. Oncoimmunology. 2013;2:e26536. doi: 10.4161/onci.26536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Weichselbaum RR, Liang H, Deng L, Fu YX. Radiotherapy and immunotherapy: a beneficial liaison? Nat Rev Clin Oncol. 2017;14:365–79. doi: 10.1038/nrclinonc.2016.211. [DOI] [PubMed] [Google Scholar]
- 47.Obeid M, Panaretakis T, Joza N, Tufi R, Tesniere A, van Endert P, et al. Calreticulin exposure is required for the immunogenicity of gamma-irradiation and UVC light-induced apoptosis. Cell Death Differ. 2007;14:1848–50. doi: 10.1038/sj.cdd.4402201. [DOI] [PubMed] [Google Scholar]
- 48.Werthmoller N, Frey B, Wunderlich R, Fietkau R, Gaipl US. Modulation of radiochemoimmunotherapy-induced B16 melanoma cell death by the pan-caspase inhibitor zVAD-fmk induces anti-tumor immunity in a HMGB1-, nucleotide- and T-cell-dependent manner. Cell Death Dis. 2015;6:e1761. doi: 10.1038/cddis.2015.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ko A, Kanehisa A, Martins I, Senovilla L, Chargari C, Dugue D, et al. Autophagy inhibition radiosensitizes in vitro, yet reduces radioresponses in vivo due to deficient immunogenic signalling. Cell Death Differ. 2014;21:92–9. doi: 10.1038/cdd.2013.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Taube JM, Anders RA, Young GD, Xu H, Sharma R, McMiller TL, et al. Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci Transl Med. 2012;4:127ra137. doi: 10.1126/scitranslmed.3003689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Campian JL, Sarai G, Ye X, Marur S, Grossman SA. Association between severe treatment-related lymphopenia and progression-free survival in patients with newly diagnosed squamous cell head and neck cancer. Head Neck. 2014;36:1747–53. doi: 10.1002/hed.23535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bernstein MB, Krishnan S, Hodge JW, Chang JY. Immunotherapy and stereotactic ablative radiotherapy (ISABR): a curative approach? Nat Rev Clin Oncol. 2016;13:516–24. doi: 10.1038/nrclinonc.2016.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yang X, Lu Y, Hang J, Zhang J, Zhang T, Huo Y, et al. Lactate-modulated immunosuppression of myeloid-derived suppressor cells contributes to the radioresistance of pancreatic cancer. Cancer Immunol Res. 2020;8:1440–51. doi: 10.1158/2326-6066.CIR-20-0111. [DOI] [PubMed] [Google Scholar]
- 54.Oweida AJ, Mueller AC, Piper M, Milner D, Van Court B, Bhatia S, et al. Response to radiotherapy in pancreatic ductal adenocarcinoma is enhanced by inhibition of myeloid-derived suppressor cells using STAT3 anti-sense oligonucleotide. Cancer Immunol Immunother. 2021;70:989–1000. doi: 10.1007/s00262-020-02701-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Morisada M, Clavijo PE, Moore E, Sun L, Chamberlin M, Van Waes C, et al. PD-1 blockade reverses adaptive immune resistance induced by high-dose hypofractionated but not low-dose daily fractionated radiation. Oncoimmunology. 2018;7:e1395996. doi: 10.1080/2162402X.2017.1395996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Barker HE, Paget JT, Khan AA, Harrington KJ. The tumour microenvironment after radiotherapy: mechanisms of resistance and recurrence. Nat Rev Cancer. 2015;15:409–25. doi: 10.1038/nrc3958. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.