Abstract
Background
Pancreatic ductal adenocarcinoma (PDAC) is highly malignant with a very poor prognosis due to its silent development and metastatic profile with a 5-year survival rate below 10%. PDAC is characterised by an abundant desmoplastic stroma modulation that influences cancer development by extracellular matrix/cell interactions. Elastin is a key element of the extracellular matrix. Elastin degradation products (EDPs) regulate numerous biological processes such as cell proliferation, migration and invasion. The aim of the present study was to characterise for the first time the effect of two EDPs with consensus sequences “GxxPG” and “GxPGxGxG” (VG-6 and AG-9) on PDAC development. The ribosomal protein SA (RPSA) has been discovered recently, acting as a new receptor of EDPs on the surface of tumour cells, contributing to poor prognosis.
Methods
Six week-old female Swiss nude nu/nu (Nu(Ico)-Foxn1nu) mice were subcutaneously injected with human PDAC MIA PaCa-2/eGFP-FLuc+ cells, transduced with a purpose-made lentiviral vector, encoding green fluorescent protein (GFP) and Photinus pyralis (firefly) luciferase (FLuc). Animals were treated three times per week with AG-9 (n = 4), VG-6 (n = 5) or PBS (n = 5). The influence of EDP on PDAC was examined by multimodal imaging (bioluminescence imaging (BLI), fluorescence imaging (FLI) and magnetic resonance imaging (MRI). Tumour volumes were also measured using a caliper. Finally, immunohistology was performed at the end of the in vivo study.
Results
After in vitro validation of MIA PaCa-2 cells by optical imaging, we demonstrated that EDPs exacerbate tumour growth in the PDAC mouse model. While VG-6 stimulated tumour growth to some extent, AG-9 had greater impact on tumour growth. We showed that the expression of the RPSA correlates with a possible effect of EDPs in the PDAC model. Multimodal imaging allowed for longitudinal in vivo follow-up of tumour development. In all groups, we showed mature vessels ending in close vicinity of the tumour, except for the AG-9 group where mature vessels are penetrating the tumour reflecting an increase of vascularisation.
Conclusions
Our results suggest that AG-9 strongly increases PDAC progression through an increase in tumour vascularisation.
Subject terms: Cancer microenvironment, Cancer imaging
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is the most frequent pancreatic malignancy (90% of pancreatic cancers). It is expected to become the second leading cause of cancer-related death by 2030 [1]. The poor prognosis is mainly due to late diagnosis and high invasiveness with dissemination of metastases in the advanced stage of the disease. Gold standard treatments consist of surgical resection; chemotherapy or combined radio- and chemotherapy (e.g. gemcitabine and 5-fluorouracile). However, all therapies have a limited responsiveness [2]. The 5-year survival rate remains below 10% [3]. Consequently, alternative therapeutic approaches are of critical need, with the identification of biomarkers allowing early detection of PDAC, together with the development of new tools for clinical imaging. The microenvironment of PDAC is complex and is composed of two entities: the PDAC cells themselves and the stroma composed of cancer-associated fibroblasts, pancreatic stellate cells, endothelial cells, immune cells, various growth factors/cytokines and an abundant extracellular matrix (ECM) [4].
PDAC is histologically characterised by the abundance of extracellular matrix (ECM) components, also referred to as desmoplasia. ECM components have been highlighted as a major contributor to PDAC progression and to resistance to therapy [5, 6]. ECM components include collagens, elastin, proteoglycans as well as ECM remodelling enzymes involved in degradation or crosslinking of the ECM components. Elastin is a fibrous protein of the ECM, responsible for the structural integrity and function of tissues. This protein is extremely stable, resistant and its half-life is estimated to be ~70 years [7]. During physiological [8, 9] or pathological conditions [10, 11], elastin is degraded by elastases that release elastin-derived peptides (EDPs). Pancreatic elastase, which has been discovered by Baló and Banga, accumulates near the degradation site [12]. Other proteases have been described, such as serine proteases, cysteine proteases and matrix metalloproteinases (MMPs) [8]. Also called matrikines, the released EDPs with a consensus sequence “GXXPG” or “GxPGxGxG” have biological activities [13]. In cancer, EDPs, especially VGVAPG (VG-6) and AGVPGLGVG (AG-9) peptides, favour tumour progression and metastasis formation by increasing MMP-2, MMP-9 MT1-MMP, uPA and Hsp90 secretion [14–16]. VG-6 and AG-9 peptides are located within exons 24 and 26 of human tropoelastin, respectively. RPSA, a 37–67 kDa protein, interacts with several ECM proteins such as laminin-1 and EDPs [17–19], including AG-9 peptide. RPSA regulates biological processes such as cell differentiation, adhesion and migration through induction of signalling transduction pathways [20]. This is also a membrane receptor for pathogens, prions and growth factors. Moreover, RPSA is involved in the assembly and/or stability of the 40S ribosomal subunit. Recently, Lefebvre et al. suggested that the transient receptor potential melastatin-related 7 (TRPM7) channel/RPSA complex could regulate human pancreatic cancer cell migration [21].
It is our hypothesis that EDPs which accumulate in PDAC stroma, especially AG-9 and VG-6 peptides, may promote tumour growth. We first decided to assess the overexpression of RPSA in PDAC tissue based on literature data. We then selected a subcutaneous PDAC model to study AG-9 and VG-6 effects on tumour growth using different methodologies (caliper, MRI, BLI and FLI). This model presents several advantages, which include technical feasibility and tumour accessibility (direct measurement of the subcutaneous tumour), allowing longitudinal follow-up. In the context of translational research, different non-invasive imaging techniques have been tested and further developed allowing diagnosis, staging and longitudinal evaluation for preclinical and clinical purposes. Currently, PDAC diagnosis mainly relies on tissue sample analysis and medical imaging, namely computed tomography (CT), magnetic resonance imaging (MRI) and endoscopic ultrasound guided fine-needle aspiration [22–26]. From a preclinical point of view, optical imaging is another widely used method. It includes bioluminescence imaging (BLI) and fluorescence imaging (FLI). BLI is based on the detection of photons resulting in D-luciferin degradation by luciferase (e.g. the Photinus pyralis (firefly) luciferase (FLuc)), which is expressed by genetically modified cells. FLI is based on the detection of fluorescent proteins, allowing longitudinal non-invasive follow-up of tumour growth in individual animals [27, 28]. These techniques provide valuable information on cell viability, cell density and expression of targeted genes with longitudinal profiles on temporal and spatial distribution. However, they are restricted to preclinical use due to limitations (e.g. poor depth penetration of light, need for cell engineering to express the imaging genes, poor resolution, etc.), preventing its direct translation to clinical use. MRI is a preclinical and clinical imaging technique with high spatial resolution and good soft tissue contrast enabling determination of tumour location and size. It provides an excellent soft tissue contrast with multiparametric, quantitative read-outs (T1- and T2-weighted, proton density, water diffusion, perfusion, metabolism, function and vascularisation) with no need of ionising radiation as opposed to CT [29, 30]. Multimodal imaging allows to maximise the amount of molecular, functional, anatomical and metabolic information on the same subject over time. It is a powerful approach to improve the understanding of tumour growth and to accelerate the discovery of new therapeutic strategies for cancer.
Materials and methods
Bioinformatics and clinical data mining
The pattern of RPSA expression and staining in human clinical samples was characterised using the Human Protein Atlas web portal (available from www.proteinatlas.org). Semi-quantitative analysis was performed from 10 pancreatic cancer cases for classification of immunohistochemical (IHC) outcome (staining intensity and fraction of stained cells).
Elastin-derived peptides
The elastin-derived nonapeptide AG-9 (AGVPGLGVG), hexapeptide VG-6 (VGVAPG), TAMRA-AG-9 and TAMRA-VG-6 were synthesised and purified by ProteoGenix.
Cell culture
The human ductal pancreatic adenocarcinoma MIA PaCa-2/WT cell line was obtained from the American Type Culture Collection (ATCC® CRL-1420). The cells were cultured in DMEM (Gibco) containing 4.5 g/L glucose supplemented with 10% FBS (Sigma–Aldrich) and 1% penicillin-streptomycin antibiotics (Gibco). Flasks were kept at +37 °C in an incubator with controlled 5% CO2. The absence of mycoplasma was controlled.
Cell transduction
An HIV-based viral vector (pCH-EF1a-eGFP-T2A-FLuc-IRES-PuroR) was generated by the Leuven Viral Vector Core (KU Leuven) as described before [31]. MIA PaCa-2/WT cells were seeded on plates and were transduced with lentiviral vectors (10 vectors per cell) during 24 h incubation. MIA PaCa-2-transduced cells (MIA PaCa-2/eGFP-FLuc+) were selected by supplementing the growth medium with 10 µg/mL puromycin (Merck Millipore) over a time period of 2 weeks.
Immunocytochemistry
Cells were seeded on glass coverslips and incubated in DMEM supplemented by 10% FBS for 24 h at a temperature of +37 °C in the presence or absence of EDPs, TAMRA-AG-9 and TAMRA-VG-6. After washing, cells were incubated with an anti-RPSA antibody (Abcam, ab137338) in culture medium supplemented with 1% (BSA), washed and incubated with the AlexaFluor™ 405 goat anti-rabbit secondary antibody (Thermo Fisher Scientific, A31556). Cells were fixed with 4% paraformaldehyde (PFA, Electron Microscopy Sciences) solution. Coverslips were mounted and fixed on the slide with a mounting solution containing DAPI (Thermo Fisher Scientific). Immunofluorescence-labelled cell preparations were imaged using a Zeiss LSM710® NLO confocal laser scanning microscope (Carl ZEISS SAS) with the ×63 oil-immersion objective (ON1.4) coupled with a CHAMELEON femtosecond Titanium-Sapphire Laser (Coherent). GFP and TAMRA were sequentially excited at 488nm of Argon laser and diode laser 561nm. Emitted signals were respectively collected with 493–560 nm and 570–700 bandpass filters. Alexa405 was collected by using 740 nm excitation wavelength with 420–440 bandpass filters. Image acquisitions were performed with ZEN Software (Carl ZEISS SAS). All acquisition settings were constant between specimens.
Western blotting analysis
Western blot analyses were performed as previously described (Brassart et al., 2019). The mouse anti-GFP (MA5–15256), rabbit anti-FLuc (ab21176) and horseradish peroxidase-conjugated secondary anti-IgG antibody (goat anti-mouse IgG (H + L) and goat anti-rabbit IgG (H + L) (NA931V and NA934V)) were purchased from Thermo Fisher Scientific, Abcam and GE Healthcare, respectively.
RNA isolation and real-time PCR analysis
Total RNA was isolated from MIA PaCa-2/WT and MIA PaCa-2/eGFP-FLuc+ cells using the RNeasy Plus Mini kit (Qiagen) following the manufacturer’s instruction. The amount and integrity of isolated RNA were analysed using the Bioanalyzer RNA 6000 nano assay (Agilent Technologies). Single-stranded cDNA was synthesised using a maxima first strand cDNA synthesis kit for RT-qPCR (Thermo Scientific). Real-time PCR was performed using the maxima SYBR Green/ROX qPCR Master Mix (Thermo Scientific) according to the manufacturer’s instructions, the thermocycler Agilent MX300P device and the MxPro software (Agilent Technologies). Relative expression of different gene transcripts was calculated using the ΔCt method. The Ct of the gene of interest was normalised to the Ct of the normaliser (EEF1A1).
RT-qPCR primers were designed according to the sequence of RPSA (NM_002295). The forward primer for RPSA was 5’-CCA-TTG-AAA-ACC-CTG-CTG-AT-3’ and the reverse primer was 5’-CTG-CCT-GGA-TCT-GGT-TAG-TGA-3’ with a 144 bp product. The forward primer for EEF1a1 was 5’-CTG-GAG-CCA-AGT-GCT-AAC-ATG-CC-3’ and the reverse primer was 5’-CCG-GGT-TTG-AGA-ACA-CCA-GTC-3’ with a 221 bp product. All primers were synthesised by Eurofins.
In vivo experiments
The in vivo experiments were conducted according to European regulations on the protection of animals used for scientific purposes (Directive 2010/63/EU) and their Belgian (Royal Decree of 29 May 2013) and Flemish implementations (Decision of the Flemish Government to adapt the Royal Decree of 29 May 2013, 17 February 2017) on principles for laboratory animal care. The experimental in vivo procedures were approved by the Animal Ethics Committee of KU Leuven (ECD p259/2015). Based on previous data of an in vivo melanoma model [16], power calculations for the required animal numbers were made resulting in a sample size of n = 5 to demonstrate a significant effect of EDPs. As shown in Fig. S1, all groups started with five animals. Unfortunately, no tumour development was observed in one mouse of the AG-9 group and two mice died (control and VG-6 groups) during the last imaging session. Six-week-old female Swiss Nude nu/nu (Nu(Ico)-Foxn1nu) mice (average body weight, 20–24 g) were purchased from Charles River Laboratories. Animals were housed at the animal facility of KU Leuven in standard, individually ventilated cages under a 12 h light/dark cycle under controlled environmental conditions of humidity (50–70%) and temperature (22±2 °C) with food and water supplied ad libitum. Animal cages were kept in a room with constant temperature and humidity. All mice were acclimatised to our laboratory conditions for one week before starting the experiments. For induction of the tumour model and imaging, mice were anaesthetised using 2% isoflurane (IsoVET, 100 mg/g, Eurovet, Piramal, Healthcare) in 100% oxygen. Mice were sacrificed at the end of the experiments.
Murine xenograft model
For the subcutaneous (s.c.) PDAC model, 3.5 106 MIA PaCa-2/eGFP-FLuc+ cells suspended in 100 µL of sterile DPBS were implanted subcutaneously into the right flank of randomised groups of Swiss Nude nu/nu (Nu(Ico)-Foxn1nu) mice (Charles River Laboratories). MIA PaCa-2/eGFP-FLuc+ cells were preincubated for 30 min in sterile PBS (control; n = 5; Gibco), AG-9 (n = 5) or VG-6 (n = 5) before the initial injection. Elastin-derived peptides were solubilized extemporaneously in sterile PBS (Gibco) to reach a final concentration of 10 mg/kg (mouse weight) for intraperitoneal injection. Intraperitoneal administrations of AG-9, VG-6 or control were performed three times per week during all experiments starting from tumour cell inoculation. Tumour volumes were determined using a caliper according to the following formulae: v = (A × B2)/2, where A is the largest dimension of the tumour and B is the smallest dimension. Tumour measurements were performed three times per week. Animals were scanned at weeks 2, 3 and 4 using optical imaging to study cell viability by using BLI (FLuc) and the expression of eGFP in cell line by using FLI. At weeks 2 and 4, animals underwent MRI scans to study three-dimensional (3D) tumour volumes, tumour tissue heterogeneity and vascularisation, using two-dimensional (2D) and 3D T2-weighted MR images, MR angiography (MRA) and parametric maps (T2-relaxation and apparent diffusion coefficients (ADC)). At the end of the study, mice were sacrificed, and tumours were surgically extracted, weighted, analysed by ex vivo bioluminescence and then fixed in 4% PFA for histology studies (Fig. S1).
Bioluminescence imaging
In vitro, in vivo and ex vivo BLI were performed using an IVIS Spectrum imaging system (Perkin Elmer). BLI was performed for estimating the number of viable, FLuc expressing cells. For in vitro experiments, MIA PaCa-2/WT and MIA PaCa-2/eGFP-FLuc+ cells were seeded in quadruplicate on 48-well plate before imaging. An equal volume of D-luciferin solution (Luciferin-EF) was added. The signal was correlated with the number of cells present in each well by calculating the total photon flux (p/sec/cm²/sr). Regions of interest (ROIs) were placed over each well for quantification of the total photon flux using the Living Image software, version 4.2 (Perkin Elmer). For in vivo, experiments, mice were anaesthetised with 2.5% isoflurane in 100% O2 (2 L/min) and placed in the imaging chamber of the IVIS Spectrum. A D-luciferin solution was injected intraperitoneally at a dose of 126 mg/kg body weight 10 min before imaging. For ex vivo imaging, a D-luciferin solution as for in vivo imaging was injected 10min before sacrifice. Then, the tumours were excised and placed in a D-luciferin solution. The BLI signal was acquired using the following settings: exposure time of 1second (for in vivo and ex vivo) or 10seconds (for in vitro) per image, F/stop of 1, a subject height of 1.5 cm, field-of-view of 22.8 cm and medium binning. The maximal radiance (p/s/cm²/sr) was then reported. Individual and identical ellipsoid ROIs of 5.8 cm² were placed over each tumour for quantification of the total photon flux.
Fluorescence Imaging
In vitro and in vivo FLI was performed using an IVIS Spectrum imaging system (Perkin Elmer). FLI was performed to estimate the protein transcription level of eGFP. In vitro experiments were performed on the same sample that was used for in vitro BLI. The FLI signal was acquired using the following settings: exposure time of 1second (for in vivo) or 4 s (for in vitro) per image, F/stop of 1, a subject height of 1.5 cm, field-of-view of 22.8 cm and medium binning. The maximal radiance (p/s/cm²/sr) of the tumours was measured in vitro and in vivo. Individual and identical ellipsoid ROIs of 5.8 cm² were placed over each tumour for quantification of the total photon flux.
Small-animal magnetic resonance imaging
MRI measurements were acquired using a 7T Bruker Biospec small-animal MR scanner (Bruker Biospin) with a horizontal bore of 30 cm and equipped with actively shielded gradients (200 mT.m−1). In vivo images were acquired using a quadrature radio-frequency resonator (transmit/receive; inner diameter 8.6 cm, Bruker Biospin). Body temperature and breathing rate of the animals were monitored using a physiological monitoring system (Small Animal Instruments Inc., Stony Brook) and maintained at 37±2 °C and 60–80 min−1, respectively. Animals were anaesthetised and kept under anaesthesia using 2.5% of isoflurane in 100% O2. After obtaining initial localiser images, the protocol consisted of the acquisition of T2-weighted anatomical reference images using a high-resolution 2D Turbo Rapid acquisition with relaxation enhancement (Turbo-RARE) sequence followed by a 3D T2-weighted MR image using respiration gated acquisitions. To visualise if mature vessels are present within the tumour, we used a 3D MR angiography (time of flight) protocol. Tissue integrity (water content and cellular density) was investigated by acquisition of parametric T2 maps (multi-slice multi-echo sequence) and apparent diffusion coefficient maps (diffusion MRI with EPI readout). MRI sequences and scan parameters are represented in Table S1.
Histology
Harvested tumours were collected and fixed in PFA and stored in 0.1% sodium azide in PBS (Acros Organics) until further processing. Samples were transferred into a 15% sucrose solution in PBS at +4 °C overnight and subsequently into a 30% sucrose solution in PBS before embedded with optimum cutting temperature compound (OCT, Thermo Fisher Scientific). Ten-micrometre sections were made using a cryostat-sectioning apparatus (NX70 Cryostat, Thermo Fisher Scientific). Histological analyses were performed on hematoxylin and eosin (HE) sections that were prepared by using routine histological methods. For IHC, sections were thawed, fixed in acetic acid, and dried at RT. Serum blocking was carried out with nonspecific binding immunoglobulin in 2% FBS, 10% donkey serum in PBS-Tween-20 0.5% (Sigma–Aldrich). Sections were incubated overnight with rabbit anti-RPSA antibody (Abcam, ab99484) and Firefly Luciferase (Abcam, ab21176). After washing with PBS, secondary the antibody was added for 90min at RT with the appropriated Alexa Fluor®647 conjugated donkey antibody (Abcam, ab150075) against rabbit IgG. After washing with PBS, the sections were counterstained with DAPI-mounting medium (Thermo Fisher Scientific). All images were obtained with a Zeiss Axioscan Z.1 slide scanner microscope (Carl Zeiss) equipped with a Hamamatsu Orca Flash 4.0 V3 camera in combination with a Plan Apo ×20 lens with a numerical aperture (NA) of 0.80. Alexa Fluor®647, GFP and DAPI were excited with red (640 nm), green (561 nm) and blue (488 nm) diodes (100mW) and detected with BP538–562/BS570/BP570–640 (dsRed), BP450–490/BS495/BP500–550 (GFP) and BP335–383/BS395/BP420–470 (DAPI) emission filters, respectively. Images were then digitised using ZEN black (ZEN 3.2 version, Carl Zeiss Microscopy GmbH).
Statistical analysis
A stringent non-parametric statistical test, which does not preclude to have a Gaussian distribution of the data was performed. GraphPad Prism 8.2 software (GraphPad) was used for all statistical analyses. Data is expressed as the mean±SEM, SD or Min to Max. A Mann–Whitney U test was used for experiments. For all tests, statistical significance was assumed when p < 0.05 (*, $ and #) and p < 0.01 (**).
Results
Justification for the choice of pancreatic tumour model
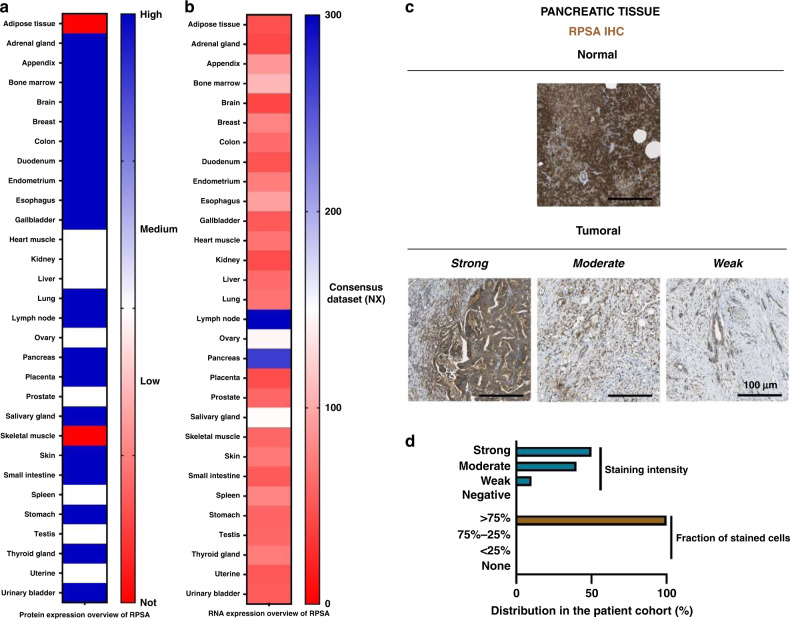
The Human Protein Atlas presents an overview of protein and RNA expression in human tissue. The RPSA is present in most human organs except for skeletal muscle and adipose tissue (Fig. 1a). In the pancreas, RPSA is highly expressed. RPSA RNA is found in all human tissue (Fig. 1b). However, in lymph nodes and in the pancreas, the RPSA RNA level is higher than in other organs with 300.2 and 246.7 NX, respectively. RPSA immunohistochemistry (IHC) shows a strong staining in healthy pancreas tissue and differential protein expression ranging from weak to strong within pancreatic tumour tissue (Fig. 1c, d). In most cases, more than 75% of tumour cells are RPSA positive (Fig. 1d). During PDAC development, elastases release EDPs that were previously reported to favour tumour growth when they interact with RPSA (Da Silva et al, 2018). The high expression of RPSA in pancreatic tissue may contribute during the initial phase and/or to progression and aggressiveness of pancreatic cancer [32]. Therefore, we decided to study the EDP effect in vivo in the PDAC model.
Fig. 1. RPSA expression in healthy and pancreatic tissue.
a Protein expression of RPSA in human tissues from the Human Protein Atlas cohort. b RNA expression of RPSA in human tissues. Consensus Normalised Expression (NX) levels by combining the data from different transcriptomics datasets (HPA, GTEx and FANTOM5) using the internal normalisation pipeline of the Human Protein Atlas. c Microphotographs of RPSA IHC in normal and pancreatic carcinoma tissue microarrays from the Human Protein Atlas dataset. Anti-RPSA pAb (sc-20979, Santa Cruz Biotechnology) has been used at a 1:100 dilution for IHC staining. d Distribution of pancreatic cancer cases among four levels of RPSA staining intensity (Blue) and fraction of cells stained (Brown) from the Human Protein Atlas dataset.
In vitro validation of engineered MIA PaCa-2 cells for optical imaging
The MIA PaCa-2 pancreatic cell line was transduced to enable tumour monitoring by using optical imaging as described before [31]. For this purpose, a lentiviral vector, encoding fluorescent eGFP and bioluminescent FLuc proteins, was used. The transduced MIA PaCa-2 cells were named MIA PaCa-2/eGFP-FLuc+ (Fig. 2a). Successful insertion of the FLuc gene into cells was confirmed by in vitro BLI, as shown in Fig. 2b, c. Hereby, different cell numbers (1.25 × 104–1.00 × 105) were analysed. The in vitro BLI signal increased significantly in a cell number-dependent manner (*p < 0.0286). No signal was observed in the parental MIA PaCa-2 cells. In addition, successful transduction of the eGFP gene into cells was confirmed by in vitro FLI, as shown in Fig. 2d, e. The FLI signal in MIA PaCa-2/eGFP-FLuc+ cells increased proportionally with increasing numbers of cells (*p < 0.0286). Furthermore, transduction of the FLuc and eGFP genes into cells was confirmed by western blotting on total protein extracts (Fig. 2f). The gene expression of RPSA was studied by RT-qPCR and revealed that in both cell lines the expression of RPSA was the same (Fig. 2g). Finally, colocalization of RPSA with TAMRA-AG-9 and TAMRA-VG-6 was evidenced in both cell lines (Fig. 2h). These results confirm that the new MIA PaCa-2/eGFP-FLuc+ cell line is suitable for fluorescent and bioluminescent analysis. The expression of RPSA and the fixation of EDPs are not impacted by cell transduction.
Fig. 2. Generation of stable reporter gene expression in the pancreatic cancer cell line for optical imaging.
a Schematic representation of the eGFP-FLuc+ lentiviral vector used for MIA PaCa-2/WT transduction. b Representative in vitro BLI of WT and transduced MIA PaCa-2 cells. c Box plot and whiskers quantification of total photon flux (p/sec) of BLI signal intensities in vitro (Min to Max, n = 4 per group). d Representative in vitro FLI of WT and transduced MIA PaCa-2 cells. e Box plot and whiskers quantification of total photon flux (p/sec) of FLI signal intensities in vitro (Min to Max, n = 4 per group). f Western blot analysis on total proteins extracted from WT and transduced MIA PaCa-2 cells of FLuc and GFP proteins in WT and transduced MIA PaCa-2 cells. The actin antibody was used as internal control. g Total RNAs were purified from WT and transduced MIA PaCa-2 cells. The mRNA level of RPSA was assessed by qPCR. EFa1 mRNA was used for the quantification. h RPSA and TAMRA-EDPs (AG-9 and VG-6) distribution on MIA PaCa-2/eGFP-FLuc+ cells were studied by confocal microscopy. Colocalizations were studied using the ‘Colocalization’ plugin of ImageJ. Scale bar: 10 µm. Statistical analyses were performed using a Mann–Whitney test. The results were considered statistically significant *p < 0.05.
Elastin-derived peptides boost tumour growth in a human pancreatic cancer mouse model
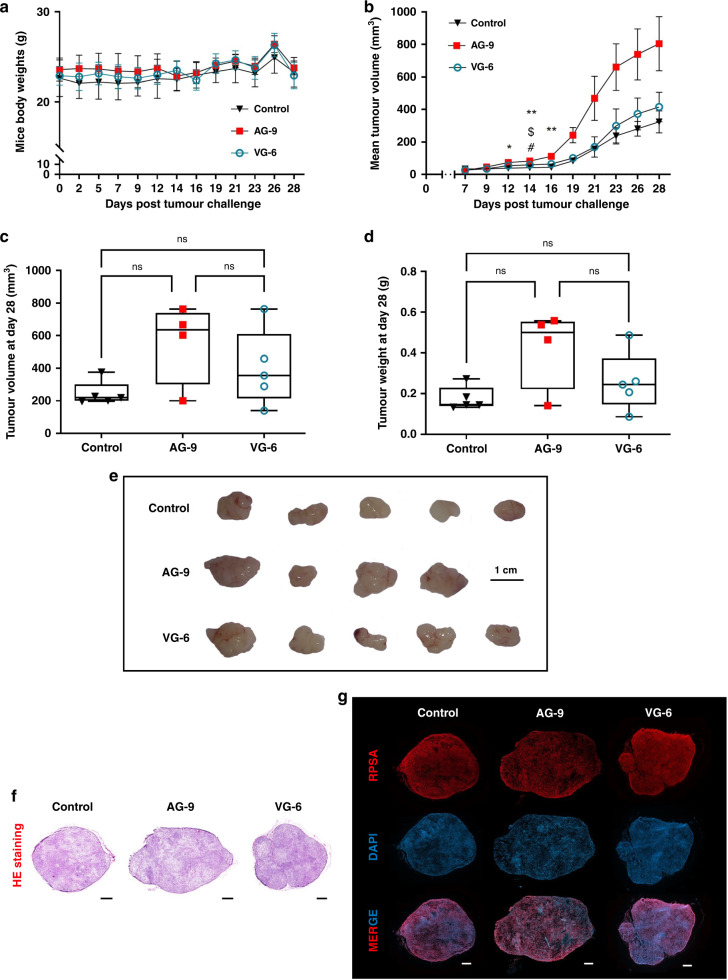
We tested the effect of EDP on tumour growth in the xenograft pancreatic cancer model described in the Materials and Methods section. First, intraperitoneal administrations of EDP did not exhibit any other clinical sign or weight loss (Fig. 3a). For follow-up of tumour growth, tumours were measured every 2–3 days until day 28 (sacrifice). MIA PaCa-2/eGFP-FLuc+ s.c. xenografts treated by AG-9 significantly accelerated tumour growth from day 14 to day 28 (Fig. 3b). At days 14 (*p < 0.0159), 16 (**p < 0.0079), 19 (**p < 0.0079), 21 (ns p < 0.2063) and 28 (ns p < 0.0.0794), tumour growth increased by two-fold compared to the control group. Moreover, MIA PaCa-2/eGFP-FLuc+ s.c. xenografts treated by VG-6 show less tumour growth than AG-9 from day 16 (#p < 0.0397) to day 28. Ex vivo evaluation after dissections shows that AG-9 treatment increased the tumour size by 2.3-fold and VG-6 treatment by 1.8-fold compared to controls. Weighting of surgically removed tumours confirmed this increase (2.41-fold) (Fig. 3c–e). Interestingly, this data also shows differences between VG-6 and the control group in terms of tumour size (increase by 1.64-fold) and weight (increase by 1.45-fold), suggesting a difference in tumour cell density. The same trend was observed between both EDPs when we compared AG-9 to VG-6 with 1.39-fold and 1.65-fold increases for tumour size and weight, respectively. To study further the impact of EDP on PDAC tumours, tumour sections were stained with HE (Fig. 3f). Tumours were homogeneous and solid masses of cells without necrotic areas. As tumours were reactive to the AG-9 peptide, the presence of its receptor has been investigated. Histological analysis shows the presence of RPSA in tumours (Fig. 3g). RPSA is also largely involved in tumour development.
Fig. 3. Evaluation of the elastin-derived peptide on tumour growth in a pancreatic mouse model.
a Mice body weights (BW) post tumour challenge (mean ± standard error of the mean (SEM), Control n = 5, AG-9 n = 4 and VG-6 n = 5). b Tumour volumes post tumour challenge (mm³) (mean ± SD). c Box plot and whiskers representation of individual tumour volumes on day 28 (mm³) (Min to Max, Control n = 5, AG-9 n = 4 and VG-6 n = 5). d Box plot and whiskers representation of individual tumour weights on day 28 (Min to Max, Control n = 5, AG-9 n = 4 and VG-6 n = 5). e Representative photographs of MIA PaCa-2/eGFP-FLuc+ xenografts after tumour excision (scale bar: 1 cm). f S.c. xenograft whole sections stained with HE. g IHC analyses of s.c. PDAC xenograft whole sections allowing visualisation of RPSA within tumours. All acquisitions were performed with a ×20 magnification. Scale bar: 150 mm. Statistical analyses were performed using a Mann–Whitney test. The results were considered statistically significant when we compared control to AG-9; *p < 0.05, **p < 0.01, AG-9 to VG-6 EDPs; $ p < 0.05 and control to VG-6; # p < 0.05.
Optical imaging confirmed the impact of Elastin-derived peptides on tumour cells
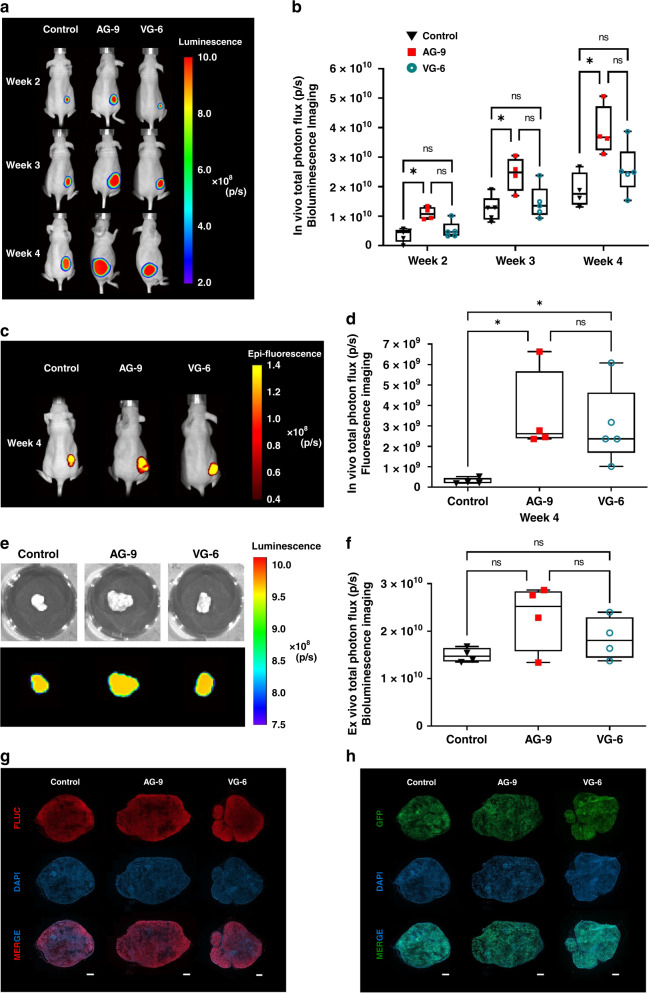
To further evaluate the effects of EDPs, we performed in vivo longitudinal BLI scans to assess the amount of viable cancer cells within the tumour tissue (Fig. 4a, b).
Fig. 4. Optical imaging approach enables in vivo assessment of the effect of elastin-derived peptide on tumour growth.
a Representative in vivo BLI of MIA PaCa-2/eGFP-FLuc+ nude mice 2, 3 and 4 weeks after s.c. injection. b Box plot and whiskers quantification of total photon flux (p/sec) of tumour BLI signals (Min to Max, for n values see Fig. S1c). c Representative in vivo FLI of MIA PaCa-2/eGFP-FLuc+ nude mice at four weeks after s.c. injection. d Box plot and whiskers quantification of total photon flux (p/sec) of tumour FLI signals (Min to Max, for n values see Fig. S1c). e Representative ex vivo BLI of MIA PaCa-2/eGFP-FLuc+ excised tumour. f Box plot and whiskers quantification of total photon flux (p/sec) signal from the tumour above mentioned in (e) (Min to Max, for n values see Fig. S1c). The size of the ROI for in vivo BLI and FLI was identical for all animals (5.8 cm²). g, h Histological analyses of s.c. PDAC xenograft whole sections allowing visualisation of FLuc (g), and GFP (h) within tumours. All acquisitions were performed with a ×20 magnification. Scale bar: 150 mm. Statistical analyses were performed using a Mann–Whitney test. The results were considered statistically significant *p < 0.05.
BLI results indicated a significant increase in signal intensity of tumours in the presence or after pre-incubation with AG-9 (increase of 3.0, 1.94 and 2.1-fold after week 2 (*p < 0.0159), 3 (*p < 0.0317) and 4 (*p < 0.0286), respectively). Concerning the impact of VG-6, the number of photons emitted from tumours did not increase significantly but a trend of increased signal intensity was observed at all experimental time points (on average by 1.4-fold).
In vivo FLI was used for quantification of the signal intensity from tumour cells expressing eGFP (Fig. 4c, d). As for BLI measurements, we observed a significant increase in signal intensity (11.9-fold) when tumours were treated with AG-9 compared to the control group (*p < 0.0286). A 10-fold increase was observed when tumours were treated with VG-6 (*p < 0.0159).
Ex vivo BLI analysis of isolated tumours showed an association between signal intensity (p/sec) and a high rank of tumour cell viability (Fig. 4e, f). The signal in AG-9 treated tumours at week 4 increased by 1.5-fold compared to control tumours. A similar trend was observed for VG-6 with a 1.24-fold increase compared to control tumours. Optical imaging and especially in vivo BLI showed that treatment with EDPs increased tumour development in terms of number of viable tumour cells. Like BLI, FLI shows differences in tumour growth by different signal intensities from the tumour regions. But, FLI appears less sensitive. Comparing both EDPs, AG-9 exerts a greater effect on pancreatic cancer development compared to VG-6. Histological analysis of FLuc and eGFP was performed to assess the presence of MIA PaCa-2/eGFP-FLuc+ cells. Presence of tumour cells was confirmed for lesions of all groups (Fig. 4g, h).
Magnetic resonance imaging based assessment of the impact of elastin-derived peptides on tumour growth
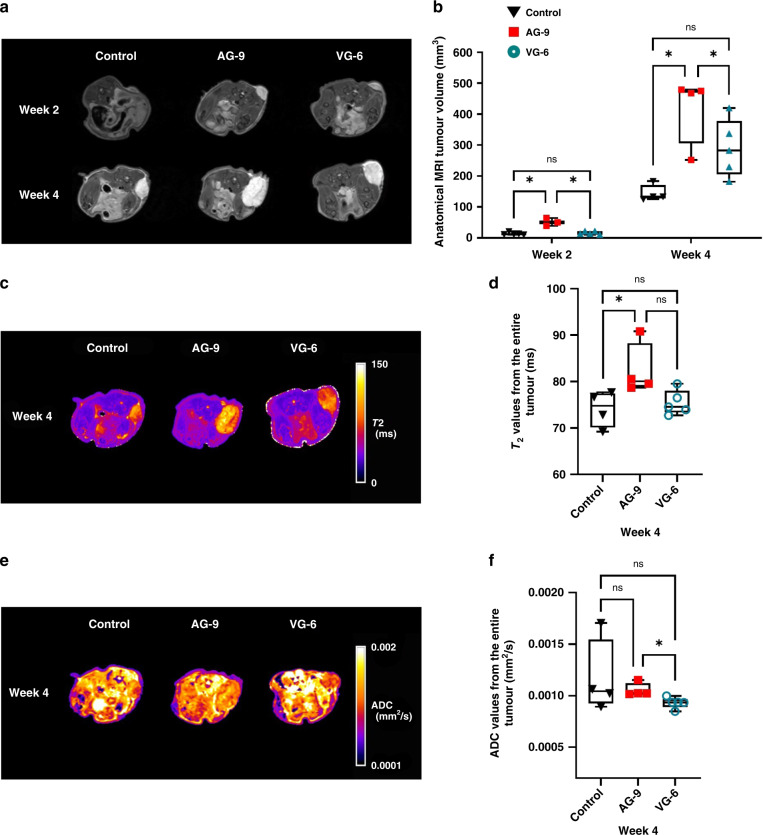
Similar, to what was found with optical imaging, tumour volumes determined by anatomical T2-weighted MRI) were higher in AG-9 (*p < 0.0357) compared to the control group (Fig. 5a, b) at week 2. At week 4, we showed the same effects on tumour volumes with AG-9 EDP (*p < 0.0286). Tumour volumes in the control group were lower than in the two other groups (201.4 ± 56.7, 517 ± 189 and 318.6 ± 118.2 mm3 for control, AG-9 and VG-6 respectively, Table S2). All tumours showed homogenous contrast on the 3D T2w-RARE MR images with no sign of fluid cavities. Only a small hypointense region is visible in all tumours (blood vessel network with low velocity (venules/veins)). For the AG-9 group, T2 values were lower at week 2 (Fig. S2), while they were significant higher at week 4 (*p < 0.0286), indicative of higher water content at week 4 (Fig. 5c, d). Furthermore, ADC values indicate a dense tumour mass (lower ADC values) at early time points (Fig. 5e, f, Fig. S2). Moreover, homogenous tumours (no macroscopically detectable necrotic regions) were seen in all groups. MRI shows more accurate information compared to optical imaging concerning tumour growth and tissue composition. EDPs directly influenced PDAC cell amount in tumours and AG-9 had the highest effect on tumour growth.
Fig. 5. Magnetic resonance imaging (MRI) based in vivo assessment of the effect of elastin-derived peptide on tumour growth.
a Representative in vivo MRI of 2D T2-weighted MRI scans of mice with pancreatic tumour xenografts 2 and 4 weeks after s.c. injection. b Box plot and whiskers of tumour volume measurements (mm³) based on anatomical MRI from (a) (Min to Max, for n values see Fig. S1c). c Representative in vivo MRI of parametric T2-maps of mice with pancreatic tumours 4 weeks after s.c. injection. d Box and whiskers representation of T2 values (ms) obtained from (c) (Min to Max, for n values see Fig. S1c) at week 4. e Representative in vivo MRI of parametric ADC maps of mice with pancreatic tumours 4 weeks after s.c. injection. f Box plot and whiskers representation of ADC values (mm²/s) obtained from (e) (Min to Max, for n values see Fig. S1c) at week 4. Statistical analyses were performed using a Mann–Whitney test. The results were considered statistically significant *p < 0.05.
Tumour vascularisation and the impact of elastin-derived peptides on angiogenesis
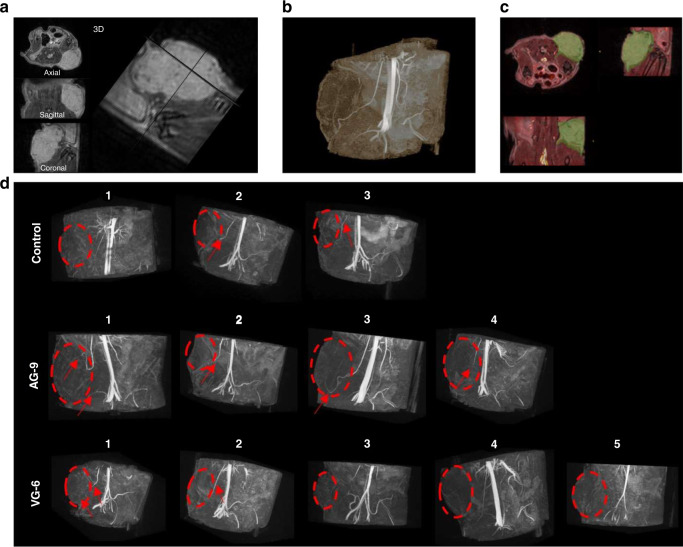
Tumour vascularisation was assessed by 3D MR angiography in vivo, showing penetrating patent vessel in tumours (Fig. 6a, c). From the MRA, we could identify mature vessels ending in close vicinity of the tumour in all groups. Only in the AG-9 group, these mature vessels are penetrating the tumour in three out of four animals. Interestingly, the only animal in which we could not detect penetrating mature vessels from MR angiography data is the one with the smallest tumour volume (244.6 mm3 compared to 533–646 mm3 for the tumours in the three other animals) (Fig. 6d).
Fig. 6. 3D MRI angiography for in vivo assessment of the effect of elastin-derived peptide on tumour vascularisation and tumour growth.
a 3D T2-weighted RARE of the tumour and b its corresponding 3D TOF MRA maximum intensity projection with the blue arrow pointing to a penetrating patent vessel. Both 3D volumes are spatially co-registered. c (Overlay of anatomical 3D in greyscale and 3D TOF MRA in hot colour) and tumour were segmented (green overlay). d 3D TOF MRA patent arteries (red arrows) reached the vicinity of the tumour (red dashed circle, based on T2-weighted anatomical MRI) and penetrate inside the tumour in some cases.
Discussion
For the last three decades, many publications have adressed the important role of different EDPs in influencing cell migration [14], proliferation, chemotaxis [33–35], survival, tumour progression [14, 16, 36–39], angiogenesis [40], aneurysm formation and atherogenesis [41]. VG-6 is, among them, the most frequently described [14, 15, 34, 38, 40, 42–51]. It modulates many physiological and pathological phenomena such as invasiveness ability to form metastases in fibrosarcoma, lung cancer and melanoma, aggressiveness in glioblastoma, angiogenesis, aneurysm formation and atherogenesis [14–16, 38, 40, 51]. We previously demonstrated that the xGxxPG consensus sequence stabilises a type VIII β-turn in several similar, but not identical peptides that maintain a sufficient conformation to be recognised by the elastin receptor complex at the fibroblast cell surface [49]. Da Silva et al compared AG-9 and VG-6 reference peptides using circular dichroism, nuclear magnetic resonance and Fourier-transform infrared spectroscopy, showing similar structural features [16]. They highlighted that xGxPGxGxG peptides, such as AG-9, modulate the same biological activities as xGxxPG but in a more efficient manner. Cell adhesion, proteinase secretion, cell migration, cell proliferation and angiogenesis were systematically more pronounced under AG-9 conditions compared to VG-6 conditions. The RPSA receptor was identified with AG-9 or VG-6 affinity chromatography [16]. AG-9 induced cancer cell blebbing and shedding of extracellular vesicles through RPSA binding [38]. A public database search was first performed in this study, demonstrating the presence of RPSA in almost all human tissue types. In pancreatic tissue, RPSA is present under normal and pathological conditions. In pancreatic cancer, RPSA is a marker for poor prognosis [21, 32]. Furthermore, pancreatic elastases promote the release of EDPs in pancreas [12]. EDPs interact directly with RPSA and initiate or further promote cancer progression. In case of EDP diffusion, this also occurs far from the pancreas, potentially promoting formation of metastases [52, 53]. In this study, we focused on a murine xenograft model of pancreatic cancer using MIA PaCa-2 cells, a PDAC cell line in which we already demonstrated the high expression of RPSA [21]. PDAC is characterised by an abundant and intense desmoplastic stroma, originating from a fibrotic response of healthy tissue to invasive carcinoma by an abnormal accumulation of ECM components and cell landscape which correspond to 90% with the tumour mass [54]. Desmoplastic stroma leads to modifications in the local immune system and vascularisation, hereby influencing prognosis [55]. In vivo PDAC models are mainly represented by genetically engineered mouse models of the KRAS (for Kristen RAS) proto-oncogene [56]. KRAS is a small GTPase of the RAS family and is involved in several signalling pathways leading to cell proliferation [57]. The proto-oncogene is found is more than 90% of PDACs. A correlation with poor prognosis has been reported [58]. Furthermore, an accumulation of hyaluronan was observed in tumour stroma, which leads to reduced elasticity of tumour tissue, a high interstitial fluid pressure blocking and reduced distribution of therapeutic agents [59]. To our knowledge, the role of EDPs in PDAC in vivo has not yet been described. The most frequently used in vivo PDAC model is the MIA PaCa-2 cell line xenograft [60]. We aimed at using multimodal imaging to better characterise our model longitudinally. We demonstrated that we efficiently transduced our cell line for the expression of eGFP and FLuc with preservation of the expression of RPSA, a pre-requisite in our study.
Our data clearly indicate that EDPs, especially AG-9, are associated with rapidly growing pancreatic allografts. MIA PaCa-2/eGFP-FLuc+ xenografts showed an increase of tumour growth under the influence of EDPs while preserving their relatively homogenous composition as determined by MRI. This finding is in line with results from other groups that have reported on the influence of EDP in other cancer types such as sarcoma and lung tumour [14, 15, 38]. Interestingly, VG-6 was previously tested as a reference peptide in terms of cancer progression [16]. In our study, VG-6 has only a small effect on pancreatic tumour growth, compared to AG-9, which doubles the size of tumours compared to the control group. Hereby, multimodal/multiparametric imaging provides not only information on tumour size changes as an effect of EDP on PDAC development in a mouse model but also other structural and functional information. This includes longitudinal information on cell viability, cell density, expression of target-genes by preclinical optical imaging, tumour tissue heterogeneity/homogeneity and tumour vascularisation using MRI (T2 and ADC maps). From our ex vivo studies of tumour volume and mass (Fig. 3), we estimated the tumour density which slightly decreased over time in both AG-9 and VG-6 groups. This is also confirmed by a non-significant decrease in ADC values from diffusion MRI and by optical imaging data. The quantitative MRI assessment (parametric T2 and ADC maps) show values similar to what was reported in the literature for this model [61, 62]. The only significant change we have observed was an increase of T2 values over time for the AG-9 group, which could reflect subtle structural remodelling such as an increase of vascularisation, which was also observed in the MR angiography experiments. We hypothesised that such changes in vascularisation could also influence tumour metabolism. In a recent publication small metabolic and topologic differences were reported between MIA PaCa-2 and Hs 766-T tumours, another model of pancreatic tumour [63]. Our results also confirm a previous hypothesis on hypervascularization of MIA PaCa-2 tumours [62, 64].
Non-invasive longitudinal multimodal imaging enables the follow-up of the same animal during the disease progression, increasing the statistical power and giving more information with the same number of animals [65]. Preclinical MRI is a potentially multiparametric technique, which offers numerous outcome measures during a single MRI session and is in line with the 3R principle. In combination with other imaging modalities (i.e. PET or optical imaging) and complementing with clinical examinations and histological validation, a refinement of animals is performed and the reduction in animal numbers is accomplished. We designed the experiment with five mice per group, according to the G-power calculation and to the European guidelines (3R rule). AG-9 significantly increased tumour growth. Unfortunately, one graft did not grow in the AG-9 group and two mice died (control and VG-6 groups) just before the acquisitions at 28th day). Although, by increasing the sample size will probably resulted in a significant increase of tumour sizes for the VG-6 group, we demonstrated the more potent effect of AG-9 which was the main objective of this study.
For ex vivo experiments, histological validations were performed to confirm the expression and localisation of markers (GFP, FLuc and RPSA). To date, several technologies are available to analyse proteins in a tissue specimen. Along with or in addition to histology, mass spectroscopy offers many advantages. Matrix-assisted laser desorption/ionisation (MALDI) imaging mass spectrometry (IMS) analysis of tissue is a technology that can be used to assess the localisation of proteins [66]. However, gas-phase fragmentation efficiency of MALDI generated proteins presents significant challenges, making macromolecules identification directly from tissue difficult [67]. For future ex vivo experiments, MALDI-IMS will be considered to determine the spatial distributions of many peptides and proteins from tissue sections. This approach will allow us to study changes in expression levels and protein distributions associated with PDAC development.
To conclude, the present study highlights the AG-9 pro-tumour effect in vivo, which we have previously reported to stimulate migration in MIA PaCa-2 cells in vitro. Moreover, our findings are of interest for studies in pancreatic cancer, especially to test pro-oncogenic or anti-oncogenic molecules and/or drugs. Subcutaneous xenografts of human-derived cell lines in immunodeficient animals are the most frequently used models in preclinical cancer research. This model has several advantages, including technical feasibility and tumour accessibility (direct measurement of the subcutaneous tumour allowing longitudinal follow-up as well as easy BLI and FLI measurement). In term of stroma reaction, Bhardwaj et al, and Wegner et al, showed an ECM enrichment (collagen I, collagen IV and fibronectin) in orthotopic and subcutaneous MIA PaCa-2 models, respectively [68, 69]. However, recent studies on tumour stroma involvement in drug resistance, metastasis formation, immune escape and angiogenesis strongly suggests that orthotopic models should provide valuable information that one could not obtain from subcutaneous models, tumour development in its ‘natural’ environment. This represents the next step for testing the AG-9 peptide in vivo. We will also consider highly metastatic tumours with abundant elastin tissue such as lungs or liver carcinoma.
Supplementary information
In vivo Magnetic Resonance Imaging (MRI) assessment of the elastin-derived peptide on tumour growth
Overview of MRI sequences and scan parameters
Summary of MRI data for tumour volume (3D T2-weighted MRI) and tumour vascularisation (3D MRI angiography)
Acknowledgements
We gratefully acknowledge the VIB Bio Imaging Core of KU Leuven for their support and assistance in this work. We thank the PICT-IBiSA Platform of the University of Reims Champagne-Ardenne (France), Dr. Carla Rios Luci and Pr. Stefaan Soenen (NanoHealth and Optical Imaging Group, KU Leuven, Belgium), Dr. Bella Manshian (Translational Cell and Tissue Research, KU Leuven, Belgium) and Aurélie Dupont-Deshorgue (UMR7369) for their skilful technical assistance.
Author contributions
LN, WG, UH, CG, JW, SB and BB designed, performed and analysed all the experiments. LN wrote the paper with BB, WG, UH and SBP provided essential editorial oversight and critical review. BB and UH jointly supervised this work.
Funding
This work was supported by grants from the European Commission for the PANA project (H2020-NMP-2015-two-stage, grant 686009), the Centre National de la Recherche Scientifique (UMR7369), the University of Reims Champagne-Ardenne, the Region Champagne-Ardenne and the Department Imaging and Pathology of KU Leuven provided a FLOV mandate to partially finance LNs PhD scholarship.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Competing interests
The authors declare no competing interests.
Ethics approvals
This manuscript does not need any ethics approval.
Patient consent
This manuscript does not contain any individual person’s data.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Uwe Himmelreich, Bertrand Brassart.
Supplementary information
The online version contains supplementary material available at 10.1038/s41416-023-02242-w.
References
- 1.Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74:2913–21. doi: 10.1158/0008-5472.CAN-14-0155. [DOI] [PubMed] [Google Scholar]
- 2.Kamisawa T, Wood LD, Itoi T, Takaori K. Pancreatic cancer. Lancet (Lond, Engl) 2016;388:73–85. doi: 10.1016/S0140-6736(16)00141-0. [DOI] [PubMed] [Google Scholar]
- 3.Rawla P, Sunkara T, Gaduputi V. Epidemiology of pancreatic cancer: global trends, etiology and risk factors. World J Oncol. 2019;10:10–27. doi: 10.14740/wjon1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Procacci P, Moscheni C, Sartori P, Sommariva M, Gagliano N. Tumor−stroma cross-talk in human pancreatic ductal adenocarcinoma: a focus on the effect of the extracellular matrix on tumor cell phenotype and invasive potential. Cells. 2018;7:1–12. doi: 10.3390/cells7100158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feig C, Gopinathan A, Neesse A, Chan DS, Cook N, Tuveson DA. The pancreas cancer microenvironment. Clin Cancer Res. 2012;18:4266–76. doi: 10.1158/1078-0432.CCR-11-3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thomas D, Radhakrishnan P. Tumor-stromal crosstalk in pancreatic cancer and tissue fibrosis. Mol Cancer. 2019;18:14. doi: 10.1186/s12943-018-0927-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shapiro SD, Endicott SK, Province MA, Pierce JA, Campbell EJ. Marked longevity of human lung parenchymal elastic fibers deduced from prevalence of D-aspartate and nuclear weapons-related radiocarbon. J Clin Invest. 1991;87:1828–34. doi: 10.1172/JCI115204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Qin Z. Soluble elastin peptides in cardiovascular homeostasis: foe or ally. Peptides. 2015;67:64–73. doi: 10.1016/j.peptides.2015.03.006. [DOI] [PubMed] [Google Scholar]
- 9.Quan T, Wang F, Shao Y, Rittié L, Xia W, Orringer JS, et al. Enhancing structural support of the dermal microenvironment activates fibroblasts, endothelial cells, and keratinocytes in aged human skin in vivo. J Invest Dermatol. 2013;133:658–67. doi: 10.1038/jid.2012.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Robert L, Robert AM, Jacotot B. Elastin-elastase-atherosclerosis revisited. Atherosclerosis. 1998;140:281–95. doi: 10.1016/S0021-9150(98)00171-3. [DOI] [PubMed] [Google Scholar]
- 11.Sellami M, Meghraoui-Kheddar A, Terryn C, Fichel C, Bouland N, Diebold MD, et al. Induction and regulation of murine emphysema by elastin peptides. Am J Physiol Lung Cell Mol Physiol. 2016;310:L8–23. doi: 10.1152/ajplung.00068.2015. [DOI] [PubMed] [Google Scholar]
- 12.Baló J, Banga I. Elastase and elastase-inhibitor. Nature. 1949;164:491. doi: 10.1038/164491a0. [DOI] [PubMed] [Google Scholar]
- 13.Maquart FX, Pasco S, Ramont L, Hornebeck W, Monboisse JC. An introduction to matrikines: Extracellular matrix-derived peptides which regulate cell activity—implication in tumor invasion. Crit Rev Oncol/Hematol. 2004;49:199–202. doi: 10.1016/j.critrevonc.2003.06.007. [DOI] [PubMed] [Google Scholar]
- 14.Donet M, Brassart-Pasco S, Salesse S, Maquart FX, Brassart B. Elastin peptides regulate HT-1080 fibrosarcoma cell migration and invasion through an Hsp90-dependent mechanism. Br J Cancer. 2014;111:139–48. doi: 10.1038/bjc.2014.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Toupance S, Brassart B, Rabenoelina F, Ghoneim C, Vallar L, Polette M, et al. Elastin-derived peptides increase invasive capacities of lung cancer cells by post-transcriptional regulation of MMP-2 and uPA. Clin Exp Metastasis. 2012;29:511–22. doi: 10.1007/s10585-012-9467-3. [DOI] [PubMed] [Google Scholar]
- 16.Da Silva J, Lameiras P, Beljebbar A, Berquand A, Villemin M, Ramont L, et al. Structural characterization and in vivo pro-tumor properties of a highly conserved matrikine. Oncotarget. 2018;9:17839–57. doi: 10.18632/oncotarget.24894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Castronovo V, Claysmith AP, Barker KT, Cioce V, Krutzsch HC, Sobel ME. Biosynthesis of the 67 kDa high affinity laminin receptor. Biochem Biophys Res Commun. 1991;177:177–83. doi: 10.1016/0006-291X(91)91965-F. [DOI] [PubMed] [Google Scholar]
- 18.Digiacomo V, Meruelo D. Looking into laminin receptor: critical discussion regarding the non-integrin 37/67-kDa laminin receptor/RPSA protein. Biol Rev. 2016;91:288–310. doi: 10.1111/brv.12170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mecham RP, Hinek A, Griffin GL, Senior RM, Liotta LA. The elastin receptor shows structural and functional similarities to the 67-kDa tumor cell laminin receptor. J Biol Chem. 1989;264:16652–7. doi: 10.1016/S0021-9258(19)84755-5. [DOI] [PubMed] [Google Scholar]
- 20.Mercurio AM. Laminin receptors: achieving specificity through cooperation. Trends Cell Biol. 1995;5:419–23. doi: 10.1016/S0962-8924(00)89100-X. [DOI] [PubMed] [Google Scholar]
- 21.Lefebvre T, Rybarczyk P, Bretaudeau C, Vanlaeys A, Cousin R, Brassart-Pasco S, et al. TRPM7/RPSA complex regulates pancreatic cancer cell migration. Front Cell Dev Biol. 2020;8:549. doi: 10.3389/fcell.2020.00549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Treadwell JR, Zafar HM, Mitchell MD, Tipton K, Teitelbaum U, Jue J. Imaging tests for the diagnosis and staging of pancreatic adenocarcinoma: a meta-analysis. Pancreas. 2016;45:789–95. doi: 10.1097/MPA.0000000000000524. [DOI] [PubMed] [Google Scholar]
- 23.Tamm EP, Balachandran A, Bhosale PR, Katz MH, Fleming JB, Lee JH, et al. Imaging of pancreatic adenocarcinoma: update on staging/resectability. Radiol Clin North Am. 2012;50:407–28. doi: 10.1016/j.rcl.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 24.Hee SP, Jeong ML, Hei KC, Sung HH, Joon KH, Byung IC. Preoperative evaluation of pancreatic cancer: comparison of gadolinium-enhanced dynamic MRI with MR cholangiopancreatography versus MDCT. J Magn Reson Imaging. 2009;30:586–95. doi: 10.1002/jmri.21889. [DOI] [PubMed] [Google Scholar]
- 25.Wang Y, Miller FH, Chen ZE, Merrick L, Mortele KJ, Hoff FL, et al. Diffusion-weighted MR imaging of solid and cystic lesions of the pancreas. Radiographics. 2011;31:E47–64. doi: 10.1148/rg.313105174. [DOI] [PubMed] [Google Scholar]
- 26.Mitchell RA, Stanger D, Shuster C, Telford J, Lam E, Enns R. Repeat endoscopic ultrasound-guided fine-needle aspiration in patients with suspected pancreatic cancer: diagnostic yield and associated change in access to appropriate care. Can J Gastroenterol Hepatol. 2016;2016:7678403. doi: 10.1155/2016/7678403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Contag CH, Spilman SD, Contag PR, Oshiro M, Eames B, Dennery P, et al. Visualizing gene expression in living mammals using a bioluminescent reporter. Photochem Photobiol. 1997;66:523–31. doi: 10.1111/j.1751-1097.1997.tb03184.x. [DOI] [PubMed] [Google Scholar]
- 28.Hilderbrand SA, Weissleder R. Near-infrared fluorescence: application to in vivo molecular imaging. Curr Opin Chem Biol. 2010;14:71–9. doi: 10.1016/j.cbpa.2009.09.029. [DOI] [PubMed] [Google Scholar]
- 29.Koh DM, Collins DJ. Diffusion-weighted MRI in the body: applications and challenges in oncology. Am J Roentgenol. 2007;188:1622–35. doi: 10.2214/AJR.06.1403. [DOI] [PubMed] [Google Scholar]
- 30.Koh DM, Padhani AR. Diffussion-weighted MRI: a new functional clinical technique for tumour imaging. Br J Radiol. 2006;79:633–5. [DOI] [PubMed]
- 31.Leten C, Roobrouck VD, Struys T, Burns TC, Dresselaers T, Vande Velde G, et al. Controlling and monitoring stem cell safety in vivo in an experimental rodent model. Stem Cells. 2014;32:2833–44. doi: 10.1002/stem.1819. [DOI] [PubMed] [Google Scholar]
- 32.Wu Y, Tan X, Liu P, Yang Y, Huang Y, Liu X, et al. ITGA6 and RPSA synergistically promote pancreatic cancer invasion and metastasis via PI3K and MAPK signaling pathways. Exp Cell Res. 2019;379:30–47. doi: 10.1016/j.yexcr.2019.03.022. [DOI] [PubMed] [Google Scholar]
- 33.Long MM, King VJ, Prasad KU, Urry DW. Chemotaxis of fibroblasts toward nonapeptide of elastin. Biochim Biophys Acta. 1988;968:300–11. doi: 10.1016/0167-4889(88)90021-3. [DOI] [PubMed] [Google Scholar]
- 34.Senior RM, Griffin GL, Mecham RP, Wrenn DS, Prasad KU, Urry DW. Val-Gly-Val-Ala-Pro-Gly, a repeating peptide in elastin, is chemotactic for fibroblasts and monocytes. J Cell Biol. 1984;99:870–4. doi: 10.1083/jcb.99.3.870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mochizuki S, Brassart B, Hinek A. Signaling pathways transduced through the elastin receptor facilitate proliferation of arterial smooth muscle cells. J Biol Chem. 2002;277:44854–63. doi: 10.1074/jbc.M205630200. [DOI] [PubMed] [Google Scholar]
- 36.Huet E, Brassart B, Cauchard JH, Debelle L, Birembaut P, Wallach J, et al. Cumulative influence of elastin peptides and plasminogen on matrix metalloproteinase activation and type I collagen invasion by HT-1080 fibrosarcoma cells. Clin Exp Metastasis. 2002;19:107–17. doi: 10.1023/A:1014547324918. [DOI] [PubMed] [Google Scholar]
- 37.Brassart B, Randoux A, Hornebeck W, Emonard H. Regulation of matrix metalloproteinase-2 (gelatinase A, MMP-2), membrane-type matrix metalloproteinase-1 (MT1-MMP) and tissue inhibitor of metalloproteinases-2 (TIMP-2) expression by elastin-derived peptides in human HT-1080 fibrosarcoma cell line. Clin Exp Metastasis. 1998;16:489–500. doi: 10.1023/A:1006550503612. [DOI] [PubMed] [Google Scholar]
- 38.Brassart B, Da Silva J, Donet M, Seurat E, Hague F, Terryn C, et al. Tumour cell blebbing and extracellular vesicle shedding: key role of matrikines and ribosomal protein SA. Br J Cancer. 2019;120:453–65. doi: 10.1038/s41416-019-0382-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ntayi C, Labrousse AL, Debret R, Birembaut P, Bellon G, Antonicelli F, et al. Elastin-derived peptides upregulate matrix metalloproteinase-2-ediated melanoma cell invasion through elastin-binding protein. J Invest Dermatol. 2004;122:256–65. doi: 10.1046/j.0022-202X.2004.22228.x. [DOI] [PubMed] [Google Scholar]
- 40.Robinet A, Fahem A, Cauchard JH, Huet E, Vincent L, Lorimier S, et al. Elastin-derived peptides enhance angiogenesis by promoting endothelial cell migration and tubulogenesis through upregulation of MT1-MMP. J Cell Sci. 2005;118:343–56. [DOI] [PubMed]
- 41.Dale MA, Xiong W, Carson JS, Suh MK, Karpisek AD, Meisinger TM, et al. Elastin-derived peptides promote abdominal aortic aneurysm formation by modulating M1/M2 macrophage polarization. J Immunol. 2016;196:4536–43. doi: 10.4049/jimmunol.1502454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hance KA, Tataria M, Ziporin SJ, Lee JK, Thompson RW. Monocyte chemotactic activity in human abdominal aortic aneurysms: role of elastin degradation peptides and the 67-kD cell surface elastin receptor. J Vasc Surg Publ Soc Vasc Surg Int Soc Cardiovasc Surg North Am Chapter. 2002;35:254–61. doi: 10.1067/mva.2002.120382. [DOI] [PubMed] [Google Scholar]
- 43.Huet E, Brassart B, Wallach J, Debelle L, Haye B, Emonard H, et al. Effect of elastin peptides on the production of matrix metalloproteinase 2 by human skin fibroblasts in culture. J Soc Biol. 2001;195:165–72. doi: 10.1051/jbio/2001195020165. [DOI] [PubMed] [Google Scholar]
- 44.Brassart B, Fuchs P, Huet E, Alix AJP, Wallach J, Tamburro AM, et al. Conformational dependence of collagenase (matrix metalloproteinase-1) up-regulation by elastin peptides in cultured fibroblasts. J Biol Chem. 2001;276:5222–7. doi: 10.1074/jbc.M003642200. [DOI] [PubMed] [Google Scholar]
- 45.Hinek A, Boyle J, Rabinovitch M. Vascular smooth muscle cell detachment from elastin and migration through elastic laminae is promoted by chondroitin sulfate-induced “shedding” of the 67-kDa cell surface elastin binding protein. Exp Cell Res. 1992;203:344–53. doi: 10.1016/0014-4827(92)90008-V. [DOI] [PubMed] [Google Scholar]
- 46.Coquerel B, Poyer F, Torossian F, Dulong V, Bellon G, Dubus I, et al. Elastin-derived peptides: matrikines critical for glioblastoma cell aggressiveness in a 3-D system. Glia. 2009;57:1716–26. doi: 10.1002/glia.20884. [DOI] [PubMed] [Google Scholar]
- 47.Fahem A, Robinet A, Cauchard JH, Duca L, Soula-Rothhut M, Rothhut B, et al. Elastokine-mediated up-regulation of MT1-MMP is triggered by nitric oxide in endothelial cells. Int J Biochem Cell Biol. 2008;40:1581–96. doi: 10.1016/j.biocel.2007.11.022. [DOI] [PubMed] [Google Scholar]
- 48.Nackman GB, Karkowski FJ, Halpern VJ, Gaetz HP, Tilson MD. Elastin degradation products induce adventitial angiogenesis in the Anidjar/Dobrin rat aneurysm model. Surgery. 1997;122:39–44. doi: 10.1016/S0039-6060(97)90262-2. [DOI] [PubMed] [Google Scholar]
- 49.Debret R, Le Naour RR, Sallenave JM, Deshorgue A, Hornebeck WG, Guenounou M, et al. Elastin fragments induce IL-1β upregulation via NF-κB pathway in melanoma cells. J Invest Dermatol. 2006;126:1860–8. doi: 10.1038/sj.jid.5700337. [DOI] [PubMed] [Google Scholar]
- 50.Szychowski KA, Gmiński J. Impact of elastin-derived VGVAPG peptide on bidirectional interaction between peroxisome proliferator-activated receptor gamma (Pparγ) and beta-galactosidase (β-Gal) expression in mouse cortical astrocytes in vitro. Naunyn Schmiedebergs Arch Pharm. 2019;392:405–13. doi: 10.1007/s00210-018-1591-4. [DOI] [PubMed] [Google Scholar]
- 51.Pocza P, Süli-Vargha H, Darvas Z, Falus A. Locally generated VGVAPG and VAPG elastin-derived peptides amplify melanoma invasion via the galectin-3 receptor. Int J Cancer. 2008;122:1972–80. doi: 10.1002/ijc.23296. [DOI] [PubMed] [Google Scholar]
- 52.Timár J, Diczházi C, Ladányi A, Rásó E, Hornebeck W, Robert L, et al. Interaction of tumour cells with elastin and the metastatic phenotype. Ciba Found Symp. 1995;192:321–35. [PubMed] [Google Scholar]
- 53.Yusa T, Blood CH, Zetter BR. Tumor cell interactions with elastin: implications for pulmonary metastasis. Am Rev Respir Dis. 1989;140:1458–62. doi: 10.1164/ajrccm/140.5.1458. [DOI] [PubMed] [Google Scholar]
- 54.Liot S, Balas J, Aubert A, Prigent L, Mercier-Gouy P, Verrier B, et al. Stroma involvement in pancreatic ductal adenocarcinoma: an overview focusing on extracellular matrix proteins. Front Immunol. 2021;12:612271. doi: 10.3389/fimmu.2021.612271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li KY, Yuan JL, Trafton D, Wang JX, Niu N, Yuan CH, et al. Pancreatic ductal adenocarcinoma immune microenvironment and immunotherapy prospects. Chronic Dis Transl Med. 2020;6:6–17. doi: 10.1016/j.cdtm.2020.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vasseur R, Skrypek N, Duchêne B, Renaud F, Martínez-Maqueda D, Vincent A, et al. The mucin MUC4 is a transcriptional and post-transcriptional target of K-ras oncogene in pancreatic cancer. Implication of MAPK/AP-1, NF-κB and RalB signaling pathways. Biochim Biophys Acta. 2015;1849:1375–84. doi: 10.1016/j.bbagrm.2015.10.014. [DOI] [PubMed] [Google Scholar]
- 57.Timar J, Kashofer K. Molecular epidemiology and diagnostics of KRAS mutations in human cancer. Cancer Metastasis Rev. 2020;39:1029–38. doi: 10.1007/s10555-020-09915-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Buscail L, Bournet B, Cordelier P. Role of oncogenic KRAS in the diagnosis, prognosis and treatment of pancreatic cancer. Nat Rev Gastroenterol Hepatol. 2020;17:153–68. doi: 10.1038/s41575-019-0245-4. [DOI] [PubMed] [Google Scholar]
- 59.Sato N, Cheng XB, Kohi S, Koga A, Hirata K. Targeting hyaluronan for the treatment of pancreatic ductal adenocarcinoma. Acta Pharm Sin B. 2016;6:101–5. doi: 10.1016/j.apsb.2016.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Delaney LJ, Eisenbrey JR, Brown D, Brody JR, Jimbo M, Oeffinger BE, et al. Gemcitabine-loaded microbubble system for ultrasound imaging and therapy. Acta Biomater. 2021;130:385–94. doi: 10.1016/j.actbio.2021.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kobes JE, Daryaei I, Howison CM, Bontrager JG, Sirianni RW, Meuillet EJ, et al. Improved treatment of pancreatic cancer with drug delivery nanoparticles loaded with a novel AKT/PDK1 inhibitor. Pancreas. 2016;45:1158–66. doi: 10.1097/MPA.0000000000000607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang X, Wojtkowiak JW, Martinez GV, Cornnell HH, Hart CP, Baker AF, et al. MR imaging biomarkers to monitor early response to hypoxia-activated prodrug TH-302 in pancreatic cancer xenografts. PLoS ONE. 2016;11:e0155289. doi: 10.1371/journal.pone.0155289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kishimoto S, Brender JR, Crooks DR, Matsumoto S, Seki T, Oshima N, et al. Imaging of glucose metabolism by 13C-MRI distinguishes pancreatic cancer subtypes in mice. Elife. 2019;8:e46312. doi: 10.7554/eLife.46312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kim H, Rigell CJ, Zhai G, Lee SK, Samuel SL, Martin A, et al. Antagonistic effects of anti-EMMPRIN antibody when combined with chemotherapy against hypovascular pancreatic cancers. Mol Imaging Biol. 2014;16:85–94. doi: 10.1007/s11307-013-0665-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wachsmuth L, Mensen A, Barca C, Wiart M, Tristão-Pereira C, Busato A, et al. Contribution of preclinical MRI to responsible animal research: living up to the 3R principle. Magn Reson Mat Phys Biol Med. 2021;34:469–74. doi: 10.1007/s10334-021-00929-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kubo A, Kajimura M, Suematsu M. Matrix-assisted laser desorption/ionization (MALDI) imaging mass spectrometry (IMS): a challenge for reliable quantitative analyses. Mass Spectrom (Tokyo, Jpn) 2012;1:A0004–A0004. doi: 10.5702/massspectrometry.A0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ryan DJ, Spraggins JM, Caprioli RM. Protein identification strategies in MALDI imaging mass spectrometry: a brief review. Curr Opin Chem Biol. 2019;48:64–72. doi: 10.1016/j.cbpa.2018.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bhardwaj A, Srivastava SK, Singh S, Tyagi N, Arora S, Carter JE, et al. MYB promotes desmoplasia in pancreatic cancer through direct transcriptional up-regulation and cooperative action of sonic hedgehog and adrenomedullin. J Biol Chem. 2016;291:16263–70. doi: 10.1074/jbc.M116.732651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wegner CS, Gaustad JV, Andersen LMK, Simonsen TG, Rofstad EK. Diffusion-weighted and dynamic contrast-enhanced MRI of pancreatic adenocarcinoma xenografts: associations with tumor differentiation and collagen content. J Transl Med. 2016;14:161. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
In vivo Magnetic Resonance Imaging (MRI) assessment of the elastin-derived peptide on tumour growth
Overview of MRI sequences and scan parameters
Summary of MRI data for tumour volume (3D T2-weighted MRI) and tumour vascularisation (3D MRI angiography)
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.