Abstract
Large-scale pigeon farming in China is gradually increasing. However, studies on the basic nutritional requirements of breeding pigeons during lactation, which greatly influence the productivity and economic benefits of pigeon breeding, remain scanty. The objective of this study was to determine the optimal dietary energy/protein ratio requirements for lactating pigeons in summer. A total of 576 pairs of Mimas breeding pigeons were randomly divided into 12 groups (n = 48 per treatment), and each pair bred 4 squabs. A two-way ANOVA design with different protein levels (15%, 16%, 17%, and 18%) for factor A and different energy levels (12.6 MJ/kg, 12.8 MJ/kg, and 13.0 MJ/kg) for factor B was used to design 12 groups of experimental diets for feeding. The experiment lasted for 28 d. We found that ME level had little effect on breeding pigeons, but the CP level and dietary energy/protein ratio significantly affected the reproductive and growth performance of the pigeons. The lowest total weight loss (P < 0.01), and the highest egg production (P < 0.01) were observed in group 11 (18% CP, 12.8 MJ/kg). It had no effect on egg quality. Both ME and CP levels significantly affected the growth performance, slaughter performance and meat quality of squabs, and there was a strong interaction between CP and ME. The fastest growth rate (P < 0.01) was observed in group 11 (18% CP, 12.8 MJ/kg). The best CP and ME combination for the eviscerated weight, pectoral muscle weight, organ weight, 45 min meat color (L⁎, a⁎, b⁎), pH, and muscle fiber characteristics were also group 11. Finally, the regression model revealed that the best dietary energy/protein ratio was 17.92 to 19.02 kcal/g for squabs and 16.72 kcal/g for the breeding pigeons. There was a strong interaction between energy and protein levels in breeding pigeons during the lactation period, and the best production performance was at 18% CP 12.8 MJ/kg. And this is recommended to be applied as the energy/protein ratio dietary requirement for breeding pigeons during lactation in the summer “2 + 4” pattern.
Key words: dietary energy/protein ratio, growth performance, reproductive performance, slaughter performance, meat quality
INTRODUCTION
In recent years feed material prices around the world have continued to rise. Many breeding companies pay more attention to the effectiveness of feed inputs and outputs. Therefore, in the actual production of livestock and poultry breeding, providing accurate nutrition to animals can achieve the best production performance and breeding efficiency (Geng et al., 2022). The pigeon farming industry in China has developed rapidly, with annual production and sales of nearly 700 million squabs, which is the highest producer in the world (Kokoszynski et al., 2020). However, unlike other poultry, squabs rely heavily on the “crop milk” of their parents to meet their nutritional requirements for growth and development (Dong et al., 2012; Gillespie et al., 2012). Feeding nutritionally irrational diets may lead to malnutrition or overnutrition in broiler pigeons, resulting in poor reproductive performance of the parents and even reducing the survival rate of squabs (Xie et al., 2019). Therefore, meeting the nutritional needs of breeding pigeons is key to achieving optimal production and economic benefits. Many farms have recently adopted the “2 + 4” high-yield breeding pattern, in which a pair of breeding pigeons feeds 4 squabs simultaneously. This high-production model not only increases the breeder's feeding burden but also requires a higher nutritional supply for the parents during the lactation period.
Metabolizable energy (ME) and crude protein (CP) levels of diets are important factors affecting the growth, development, and reproductive performance of livestock and poultry (Liu et al., 2015; Zeng et al., 2015; Xia et al., 2019; Heijmans et al., 2021). ME and CP are not independent of each other. There is a clear link between them. When the level of CP and ME in the diet is maintained in an appropriate proportion, it is beneficial for the health status and production performance of poultry, etc. (Chen et al., 2021). Therefore, specifying the optimal energy-to-protein ratio nutritional level in diets is an important basis for other poultry science studies. Bu et al. (2015) found that optimal body weight and egg production performance could be achieved at a dietary CP level of 14.0% and an ME level of 12.30 MJ/kg for laying pigeons. It was also found that for optimal production performance of breeding pigeons, the optimal level of diet was 17% CP at 12.50 MJ/kg in the “2 + 2” breeding pattern and 18% CP at 12.50 MJ/kg in the “2 + 3” breeding pattern (Li et al., 2022). In previous studies on single forms of CP and ME levels, the optimal CP level required for breeding pigeons was about 16.16 to 18%, while the optimal ME level was about 11.85 to 12.81 MJ/kg (Ye et al., 2015; Gao et al., 2016a; Wang et al., 2018; Zhu et al., 2021). The huge variation in the study findings may be due to the differences in the experimental breeding patterns and seasonal variations, which make it difficult to be applied in production practice. In particular, the effect of the energy/protein ratio on the production of breeding pigeons during the lactation period is still unclear. Therefore, this study aimed to determine the optimal energy and protein ratio requirement of breeding pigeon diet during lactation under the "2 + 4" feeding pattern in summer by adopting two-way ANOVA experiment with different levels of CP and ME, so as to provide a reference for formulating the diet of breeding pigeon during lactation and large-scale breeding of meat pigeon.
MATERIALS AND METHODS
All experimental procedures were carried out according to the Guidelines of Institutional Animal Care and approved by the Institutional Animal Ethics Committee of the Zhongkai University of Agricultural Engineering (Ethics Code: ZHKUMO-2021-075).
Study Design
In this study, 576 pairs of Mimas white pigeons of 12 to 14 months old with similar weight and reproductive performance were selected to carry out a two-way experiment. The factors are 4 CP levels (15%, 16%, 17%, and 18%) and 3 ME levels (12.6 MJ/kg, 12.8 MJ/kg, and 13.0 MJ/kg), respectively. A total of 12 treatments were tested (Table 1). It reveals that the energy/protein ratios of the 1 to 12 treatment groups were 20.12 kcal/g, 20.34 kcal/g, 20.72 kcal/g, 18.86 kcal/g, 19.09 kcal/g, 19.42 kcal/g, 17.63 kcal/g, 18.00 kcal/g, 18.22 kcal/g, 16.72 kcal/g, 17.00 kcal/g, 17.26 kcal/g, respectively. During the experiment, the pigeons were randomly divided into 48 pairs with 8 replicates per treatment group. The experiment lasted for 28 d, including 21 d of lactation and 7 d of the resting period. Notably, the “2 + 4” breeding pattern was used. The experimental pigeons and squabs were provided by Guangdong Meizhou Golden Green Modern Agricultural Development Company. The breed is European Mimas white pigeon.
Table 1.
Nutrient content of experimental diets for breeding pigeons during lactation.
| Items | I | II | III | IV | V | VI | VII | VIII | IX | X | XI | XII |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Corn | 24.00 | 24.00 | 24.00 | 20.00 | 20.00 | 20.00 | 17.00 | 17.00 | 17.00 | 15.00 | 15.00 | 15.00 |
| Sorghum | 15.00 | 15.00 | 15.00 | 15.00 | 15.00 | 15.00 | 15.00 | 15.00 | 15.00 | 13.00 | 13.00 | 13.00 |
| Wheat | 15.00 | 15.00 | 15.00 | 16.50 | 16.50 | 16.50 | 16.00 | 16.00 | 16.00 | 13.00 | 13.00 | 13.00 |
| Peas | 41.00 | 39.00 | 37.00 | 39.00 | 37.00 | 35.50 | 39.00 | 37.00 | 35.00 | 43.50 | 41.50 | 39.50 |
| Pal moil | 0.00 | 1.00 | 2.00 | 0.80 | 1.80 | 2.80 | 1.50 | 2.50 | 3.50 | 2.30 | 3.30 | 4.30 |
| Soybean meal | 5.00 | 6.00 | 7.00 | 8.70 | 9.70 | 10.20 | 11.50 | 12.50 | 13.50 | 13.20 | 14.20 | 15.20 |
| Total | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 |
| Nutrient levels1 | ||||||||||||
| CP (%) | 14.98 | 15.05 | 15.02 | 15.97 | 16.04 | 16.02 | 17.08 | 17.00 | 17.05 | 18.00 | 17.98 | 18.02 |
| EE (%) | 2.25 | 3.23 | 4.22 | 2.72 | 3.71 | 4.69 | 3.21 | 4.17 | 5.14 | 3.67 | 4.56 | 5.63 |
| Moisture (%) | 12.26 | 12.14 | 12.02 | 12.22 | 12.11 | 11.99 | 12.20 | 12.07 | 11.95 | 12.16 | 12.05 | 11.93 |
| Ash (%) | 1.95 | 1.96 | 1.96 | 2.09 | 2.10 | 2.10 | 2.25 | 2.24 | 2.25 | 2.38 | 2.38 | 2.39 |
| Ca (%) | 0.08 | 0.08 | 0.08 | 0.09 | 0.09 | 0.09 | 0.10 | 0.10 | 0.10 | 0.11 | 0.11 | 0.11 |
| TP (%) | 0.34 | 0.34 | 0.34 | 0.35 | 0.35 | 0.35 | 0.36 | 0.36 | 0.36 | 0.37 | 0.37 | 0.36 |
| ME (MJ/kg) | 12.61 | 12.81 | 13.02 | 12.60 | 12.81 | 13.02 | 12.60 | 12.80 | 13.00 | 12.59 | 12.79 | 13.01 |
| ME/CP (kcal/g) | 20.12 | 20.34 | 20.72 | 18.86 | 19.09 | 19.42 | 17.63 | 18.00 | 18.22 | 16.72 | 17.00 | 17.26 |
| SIDAA (%) | ||||||||||||
| Asp | 1.42 | 1.43 | 1.43 | 1.53 | 1.55 | 1.55 | 1.67 | 1.66 | 1.67 | 1.78 | 1.78 | 1.79 |
| Glu | 2.84 | 2.84 | 2.81 | 2.99 | 2.97 | 2.96 | 3.14 | 3.12 | 3.13 | 3.27 | 3.26 | 3.26 |
| Ser | 0.72 | 0.72 | 0.72 | 0.76 | 0.77 | 0.77 | 0.82 | 0.81 | 0.82 | 0.86 | 0.86 | 0.86 |
| His | 0.38 | 0.39 | 0.39 | 0.41 | 0.41 | 0.41 | 0.44 | 0.43 | 0.44 | 0.46 | 0.46 | 0.46 |
| Gly | 0.55 | 0.55 | 0.55 | 0.56 | 0.56 | 0.56 | 0.57 | 0.57 | 0.56 | 0.58 | 0.57 | 0.57 |
| Thr | 0.54 | 0.55 | 0.55 | 0.58 | 0.58 | 0.58 | 0.62 | 0.62 | 0.62 | 0.66 | 0.66 | 0.66 |
| Arg | 1.12 | 1.13 | 1.13 | 1.19 | 1.20 | 1.20 | 1.27 | 1.27 | 1.27 | 1.34 | 1.34 | 1.34 |
| Ala | 0.71 | 0.71 | 0.71 | 0.74 | 0.75 | 0.74 | 0.79 | 0.78 | 0.78 | 0.82 | 0.82 | 0.82 |
| Tyr | 0.31 | 0.31 | 0.30 | 0.31 | 0.31 | 0.31 | 0.32 | 0.32 | 0.31 | 0.32 | 0.32 | 0.32 |
| Val | 0.57 | 0.57 | 0.57 | 0.62 | 0.62 | 0.62 | 0.67 | 0.67 | 0.67 | 0.72 | 0.72 | 0.72 |
| Met | 0.13 | 0.14 | 0.14 | 0.17 | 0.17 | 0.18 | 0.21 | 0.21 | 0.21 | 0.24 | 0.25 | 0.25 |
| Phe | 0.71 | 0.71 | 0.71 | 0.75 | 0.76 | 0.76 | 0.81 | 0.80 | 0.80 | 0.85 | 0.85 | 0.85 |
| Ile | 0.52 | 0.53 | 0.53 | 0.57 | 0.57 | 0.57 | 0.62 | 0.62 | 0.62 | 0.67 | 0.67 | 0.67 |
| Leu | 1.30 | 1.30 | 1.30 | 1.35 | 1.36 | 1.35 | 1.42 | 1.41 | 1.41 | 1.48 | 1.47 | 1.47 |
| Lys | 0.79 | 0.79 | 0.80 | 0.85 | 0.86 | 0.86 | 0.92 | 0.92 | 0.93 | 0.99 | 0.98 | 0.99 |
All nutrient levels were calculated values.
Feeding and Routine Management
This study was carried out from July 2021 to September 2021. The temperature in the loft during the summer trials ranged from 23°C to 35°C with an average of 27°C to 28°C and the humidity ranged from 46 to 73% with an average of 75 to 76%. Each pair was kept in separate cage and provided with enough food and water. The single-cage system ensured the best care was provided for each pair. The pigeons were fed manually at regular intervals from 7:00 am, and every evening at 19:00 they were fasted. During experimental period, we checked the feed through 5 times a day to ensure that there was still material when the material was finally withdrawn. In this way, the daily feed intake can be accurately measured by weighing the added and remaining feed weight of each cage. The pigeons were fed on complete-formula granulated feeds. It was also supplemented with health sand prepared only from ordinary shells and gravel (in a ratio of 1:2) to help the breeding pigeons digest. The specific nutritional composition of the experimental feeds is shown in Table 1.
Growth Performance of the Breeding Pigeons and Squabs During Lactation
The weights of the breeding pigeons before feeding and the 4 squabs nursed by each pair were weighed at 0 d, 7 d, 14 d, 21 d, and 28 d, respectively. The pigeons were counted separately by sex. The living condition of the squabs was observed and recorded daily promptly. The survival curve of the squabs during the whole period of the experiment was plotted based on the recorded data. Researchers recorded the daily additions and residuals to calculate the feed-to-gain ratio (F/G) of the squabs.
Reproductive Performance of the Breeding Pigeons During Lactation
The egg weight, egg-laying interval, egg-laying rate, fertilization rate, and hatching rate in the first breeding cycle during lactation were recorded. The birth weights of the squabs were also recorded.
Egg Quality During the Lactation Period
Thirty eggs in the second breeding cycle of lactation were randomly selected from each treatment group according to several similar studies (Zhang et al., 2021; Jin et al., 2022). Before measuring, all the eggs were stored at 4°C. The egg weight and yolk weight were determined using a universal electronic analytical balance (PWN124ZH/E). The egg shape index was calculated by measuring the longitudinal and transverse diameter with a vernier caliper (egg shape index = longitudinal diameter/transverse diameter). Eggshell strength was determined using a strength meter (EFR-01, ORKA Food Technology Ltd., Ramat Hasharon, Israel) with a blunt end for pigeon eggs. An automatic egg analyzer (EA-01, Robotmation Ltd., Tokyo, Japan) was used for determination at Haugh unit. The eggshells were washed with running water and dried naturally. Then the eggshells were weighed using a PWN124ZH/E 10,000-position electronic analytical balance. Finally, the shell membrane was removed to retain the calcified layer of eggshell. The thickness of the tip, middle and blunt end of eggshell was measured by a digital micrometer, and the average value of the 3 places was taken as the value of eggshell thickness.
Slaughter Performance of Squabs
The squabs were fasted for 12 h at 21 d of age, and then 24 squabs were randomly sampled from each treatment group for slaughter. The slaughter weight, semieviscerated weight, eviscerated weight, pectoral muscle weight, abdominal fat weight, liver weight, heart weight, kidney weight, glandular stomach weight, gizzard weight, pancreas weight, spleen weight, thymus weight, and bursa weight of the slaughter squabs were determined according to the metric statistics method specified in NY/T 823-2020 Poultry Production Performance Terms and Metric Statistical Methods (Ministry of Agriculture of the People's Republic of China, 2020).
Meat Physiochemical Quality of Slaughtered Squabs
The pectoral muscle samples were stored in sealed bags at 4°C after squab slaughtered for pH determination at 45 min and 24 h. The right pectoral muscle meat color (redness a⁎, yellowness b⁎, and lightness L⁎ values) and drip loss of the left pectoral muscle were determined according to the method of Petracci et al. (2013) at the stated intervals. Twelve squabs were randomly sampled from each treatment group of postslaughter pigeons. At the same time, about 1 cm3 muscle pieces were taken at the fixed position of the pectoral muscle and preserved in PFA fixative for backup. The fixed pectoral muscle samples were also sectioned (section thickness 10 μm) after gradient ethanol dehydration, xylene transparency, and paraffin embedding. These sections were rehydrated by gradient and stained using hematoxylin & eosin (HE). Then these stained sections were carefully observed and photographed under a 400× microscope. The muscle fiber area and muscle fiber diameter of individual muscle fibers in each photograph were measured using Image J software. The number of muscle fibers in 100*100 μm2 was marked according to the method adopted by Chen et al. (2020) using the counting tool in Image J software. And finally converted to the density of muscle fibers in a 1 mm2 photograph. The final data of muscle fiber traits were obtained for 12 groups for subsequent analysis.
Statistical Analysis
The data were analyzed using Excel and SPSS software, version 26.0. Differences between groups were compared using the Duncan's method, while the interaction between ME and CP was analyzed by 2-way ANOVA based on the general linear model (GLM). The significance test was performed using the LSD method, and P < 0.05 was considered statistically significant. The corresponding data were analyzed by linear and quadratic regression analysis of energy/protein ratio requirements.
RESULTS
Dietary Energy/Protein Ratio Affected the Growth Performance of Breeding Pigeons
High CP levels significantly reduced the weight loss of the breeding pigeons during lactation. Meanwhile, the weight loss of the breeding pigeons was lowest at 18% CP, which is significantly lower than in the other CP groups (P < 0.01). The ME level had little effect on the weight of the breeding pigeons. There was a strong interaction between CP and ME, which jointly affected the weight of the breeding pigeons during the lactation period. Group 12 (18% CP, 13.0 MJ/kg) had the lowest weight loss during lactation and the fastest weight recovery after the lactation period when the dietary energy/protein ratio was 17.26 kcal/g (Table 2).
Table 2.
Effect of dietary energy/protein ratios on the growth performance of breeding pigeons during lactation in summer.
| Treatments (n = 8) | CP/% | ME/MJ/kg | Male pigeon weight loss/g | Female pigeon weight loss/g | Total weight loss/g |
|---|---|---|---|---|---|
| I | 15 | 12.6 | 91.262CDE | 68.700CD | 159.962DEF |
| II | 15 | 12.8 | 102.725ABCD | 74.300CD | 177.025CDEF |
| III | 15 | 13.0 | 116.850AB | 87.025ABC | 203.875ABC |
| IV | 16 | 12.6 | 108.575ABC | 76.100CD | 184.675BCD |
| V | 16 | 12.8 | 107.812ABCD | 69.788CD | 177.538CDEF |
| VI | 16 | 13.0 | 123.525A | 102.375A | 225.900A |
| VII | 17 | 12.6 | 119.275AB | 97.900AB | 217.288AB |
| VIII | 17 | 12.8 | 113.562AB | 69.550CD | 183.288CDE |
| IX | 17 | 13.0 | 88.825CDE | 61.213D | 150.050EF |
| X | 18 | 12.6 | 76.550E | 79.075BCD | 156.775DEF |
| XI | 18 | 12.8 | 98.375BCDE | 61.763D | 155.613DEF |
| XII | 18 | 13.0 | 86.230DE | 64.210D | 149.710F |
| SEM | 2.673 | 2.364 | 3.994 | ||
| Main effect | |||||
| CP (n = 24) | 15% | 103.612A | 76.675 | 180.287A | |
| 16% | 113.304A | 82.754 | 196.037A | ||
| 17% | 107.221A | 76.221 | 183.542A | ||
| 18% | 87.495B | 67.663 | 153.852B | ||
| ME (n = 32) | 12.6 MJ/kg | 98.916 | 80.444 | 179.675 | |
| 12.8 MJ/kg | 105.619 | 68.850 | 173.366 | ||
| 13.0 MJ/kg | 104.190 | 78.191 | 182.249 | ||
| P values | CP | <0.001 | 0.126 | <0.001 | |
| ME | 0.457 | 0.059 | 0.547 | ||
| C*M | <0.001 | <0.001 | <0.001 |
Different superscript capital letters indicate extremely significant differences (P < 0.01), n = 8/group; C*M CP × ME; Total weight loss = (Male + Female) pigeon weight loss/g.
Dietary Energy/Protein Ratio Affected the Reproductive Performance of Breeding Pigeons
The interaction of dietary ME levels and CP levels had a significant effect on breeding pigeons' reproductive performance during the lactation period. The CP level positively correlated with the laying rate during the lactation period. The shortest average laying interval and the highest laying rate were observed at a CP rate of 18% (P < 0.01). The best reproductive performance was observed in groups 9 (17% CP, 13.0 MJ/kg) and 10 (18% CP, 12.6 MJ/kg) (P < 0.01), followed by group 11 (18% CP, 12.8 MJ/kg) (Table 3). In other words, the breeding efficiency can be improved when the ratio of energy/protein of 18.22 kcal/g and 16.72 kcal/g, followed by 17.00 kcal/g.
Table 3.
Effect of dietary energy/protein ratios on the reproductive performance of breeding pigeons during lactation in summer.
| Treatments (n = 8) | CP/% | ME/MJ/kg | Average laying interval/d | Average egg weight/g | Fertility rate/% | Hatchability/% | Average birth weight/g | 45-day laying rate/% | 50-day laying rate/% | 55-day laying rate/% |
|---|---|---|---|---|---|---|---|---|---|---|
| I | 15 | 12.6 | 50.150ABC | 22.993 | 91.706 | 88.060 | 14.680 | 12.500 | 37.499CD | 68.749 |
| II | 15 | 12.8 | 50.670ABC | 23.596 | 89.029 | 86.078 | 15.152 | 12.501 | 39.581CD | 77.082 |
| III | 15 | 13.0 | 50.596ABC | 24.759 | 86.285 | 78.959 | 14.642 | 12.500 | 41.668CD | 79.166 |
| IV | 16 | 12.6 | 51.883AB | 24.413 | 86.001 | 86.001 | 15.765 | 8.335 | 27.084D | 72.916 |
| V | 16 | 12.8 | 50.018ABC | 24.057 | 96.825 | 96.825 | 14.902 | 12.500 | 54.169BC | 77.081 |
| VI | 16 | 13.0 | 52.271AB | 22.722 | 94.463 | 84.444 | 14.851 | 10.416 | 33.334CD | 70.834 |
| VII | 17 | 12.6 | 52.775A | 22.662 | 96.875 | 94.236 | 14.703 | 6.250 | 22.918D | 64.582 |
| VIII | 17 | 12.8 | 50.621ABC | 23.268 | 97.500 | 88.750 | 14.867 | 17.085 | 40.832CD | 72.500 |
| IX | 17 | 13.0 | 48.020C | 23.054 | 96.086 | 89.960 | 14.877 | 20.834 | 81.249A | 95.833 |
| X | 18 | 12.6 | 47.696C | 22.677 | 92.103 | 84.504 | 14.742 | 15.416 | 77.915A | 88.749 |
| XI | 18 | 12.8 | 49.121BC | 23.383 | 92.311 | 90.923 | 14.879 | 23.334 | 73.749AB | 78.332 |
| XII | 18 | 13.0 | 49.175BC | 23.848 | 96.875 | 92.266 | 15.560 | 23.750 | 55.000BC | 87.500 |
| SEM | 0.309 | 0.210 | 1.029 | 1.247 | 0.076 | 1.610 | 2.823 | 2.096 | ||
| Main effect | ||||||||||
| CP (n = 24) | 15% | 50.472A | 23.782 | 89.007 | 84.365 | 14.825 | 12.500 | 39.583B | 74.999 | |
| 16% | 51.391A | 23.731 | 92.430 | 89.090 | 15.173 | 10.417 | 38.195B | 73.610 | ||
| 17% | 50.472A | 22.995 | 96.820 | 90.982 | 14.816 | 14.723 | 48.333B | 77.638 | ||
| 18% | 48.664B | 23.303 | 93.763 | 89.231 | 15.060 | 20.833 | 68.888A | 84.860 | ||
| ME (n = 32) | 12.6 MJ/kg | 50.626 | 23.186 | 91.671 | 88.200 | 14.972 | 10.625 | 41.354 | 73.749 | |
| 12.8 MJ/kg | 50.108 | 23.576 | 93.916 | 90.644 | 14.950 | 16.355 | 52.083 | 76.249 | ||
| 13.0 MJ/kg | 50.016 | 23.596 | 93.428 | 86.407 | 14.982 | 16.875 | 52.813 | 83.333 | ||
| P values | CP | 0.010 | 0.492 | 0.058 | 0.268 | 0.985 | 0.126 | <0.001 | 0.211 | |
| ME | 0.644 | 0.679 | 0.632 | 0.371 | 0.236 | 0.218 | 0.061 | 0.139 | ||
| C*M | 0.008 | 0.527 | 0.178 | 0.232 | 0.091 | 0.432 | <0.001 | 0.094 |
Different superscript capital letters indicate extremely significant differences (P < 0.01), n = 8/group; C*M CP × ME.
Dietary Energy/Protein Ratio Affected the Egg Quality of Breeding Pigeons
Quality test results of the second breeding cycle of eggs laid by breeding pigeons after the start of lactation are shown in Table 4, which showed that CP and ME levels had little effect on the egg quality of breeding pigeons in summer (P > 0.05), and there was no significant interaction between CP and ME on their effect on egg quality (P > 0.05).
Table 4.
Effect of dietary energy/protein ratios on egg production quality of breeding pigeons during lactation in summer.
| Treatments (n = 8) | CP/% | ME/MJ/kg | Egg weight/g | Relative yolk weight/g | Relative shell weight/g | Egg shape index | Shell strength/Pa | Shell thickness/mm | Haugh unit |
|---|---|---|---|---|---|---|---|---|---|
| I | 15 | 12.6 | 23.024 | 4.510 | 1.640 | 1.378 | 11.014 | 0.223 | 76.306 |
| II | 15 | 12.8 | 23.396 | 4.468 | 1.615 | 1.399 | 10.126 | 0.224 | 75.982 |
| III | 15 | 13.0 | 22.848 | 4.428 | 1.579 | 1.383 | 10.606 | 0.220 | 78.400 |
| IV | 16 | 12.6 | 24.432 | 4.482 | 1.679 | 1.468 | 10.786 | 0.222 | 77.433 |
| V | 16 | 12.8 | 23.404 | 4.457 | 1.596 | 1.392 | 10.196 | 0.220 | 76.194 |
| VI | 16 | 13.0 | 23.336 | 4.356 | 1.638 | 1.395 | 10.899 | 0.227 | 76.910 |
| VII | 17 | 12.6 | 22.520 | 4.348 | 1.585 | 1.486 | 11.210 | 0.225 | 75.418 |
| VIII | 17 | 12.8 | 23.652 | 4.486 | 1.631 | 1.375 | 10.168 | 0.219 | 77.852 |
| IX | 17 | 13.0 | 23.540 | 4.612 | 1.622 | 1.379 | 10.978 | 0.216 | 76.535 |
| X | 18 | 12.6 | 22.248 | 4.424 | 1.546 | 1.391 | 11.042 | 0.213 | 76.763 |
| XI | 18 | 12.8 | 23.404 | 4.558 | 1.619 | 1.388 | 11.419 | 0.219 | 77.040 |
| XII | 18 | 13.0 | 23.560 | 4.617 | 1.624 | 1.390 | 11.397 | 0.220 | 77.112 |
| SEM | 0.130 | 0.031 | 0.009 | 0.003 | 0.115 | 0.001 | 0.529 | ||
| Main effect | |||||||||
| CP (n = 24) | 15% | 23.089 | 4.469 | 1.611 | 1.387 | 10.582 | 0.222 | 76.896 | |
| 16% | 23.724 | 4.431 | 1.638 | 1.418 | 10.627 | 0.223 | 76.846 | ||
| 17% | 23.237 | 4.482 | 1.613 | 1.447 | 10.785 | 0.220 | 76.601 | ||
| 18% | 23.071 | 4.533 | 1.596 | 1.390 | 11.286 | 0.217 | 76.971 | ||
| ME (n = 32) | 12.6 MJ/kg | 23.056 | 4.441 | 1.613 | 1.431 | 11.013 | 0.221 | 76.480 | |
| 12.8 MJ/kg | 23.464 | 4.492 | 1.615 | 1.388 | 10.477 | 0.220 | 76.767 | ||
| 13.0 MJ/kg | 23.321 | 4.503 | 1.616 | 1.437 | 10.970 | 0.221 | 77.239 | ||
| P values | CP | 0.241 | 0.281 | 0.477 | 0.797 | 0.118 | 0.302 | 0.968 | |
| ME | 0.422 | 0.278 | 0.990 | 0.147 | 0.106 | 0.944 | 0.539 | ||
| C*M | 0.090 | 0.311 | 0.310 | 0.689 | 0.200 | 0.433 | 0.699 |
The absence of superscript letters in the same row of data indicates that the difference is not significant (P > 0.05), n = 8/group; C*M CP × ME.
Dietary Energy/Protein Ratio Affected the Growth Performance of Squabs
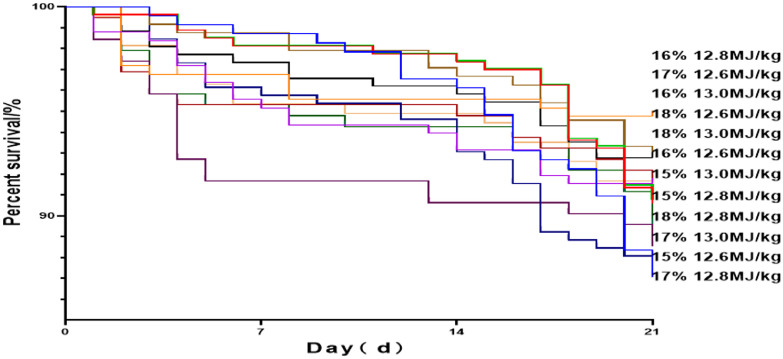
Figure 1 shows the survival rates of the squabs during the whole experimental period. The survival rate of squabs in all groups during the lactation period decreased with an increase in age, but CP and ME levels had little effect on this phenomenon (P > 0.05). There was no significant interaction between CP and ME on their effect on the survival rates of the squabs (P > 0.05).
Figure 1.
Full-term survival curve of pigeon squabs in different energy/protein ratios diet groups. The treatment groups on the right were ranked according to the squab survival rate at the end of the experiment.
In the early and middle growth stages (1–14 d), squab weight and average litter weight increased and then decreased with a further increase in CP levels. The fastest growth rate was observed at a CP content of 16% (P < 0.01). In the later growth stage (14–21 d), 18% CP was the optimal proportion for the nutritional needs of the squabs (P < 0.01). The ME level only affected the early growth stages of the squab (1–7 d), and the highest weight gain was observed at 12.8 MJ/kg (P < 0.05). The interaction between CP and ME had a significant effect on squab growth, and the highest squab weight gain (P < 0.01) during the lactation period was observed in groups 5 (17% CP, 13.0 MJ/kg) and 11 (18% CP, 12.8 MJ/kg). The average feed intake of breeding pigeons increased significantly (P < 0.01) with CP levels and a decrease in ME levels. Group 1 (15% CP, 12.6 MJ/kg) had the highest feed intake during the whole study period (P < 0.01). Combining with the litter weight gain of the squabs, we found that group 5 (16% CP, 12.8 MJ/kg) with energy/protein ratio of 19.09 kcal/g had the lowest full-term F/G and the highest production benefit (P < 0.01) (Table 5).
Table 5.
Effect of dietary energy/protein ratios on the growth performance of squabs in summer.
| Treatments (n = 8) | CP/% | ME/MJ/kg | 0-day SW/g | 7-day SW/g | 14-day SW/g | 21-day SW/g | 0-day LW/g | 7-day LW/g | 14-day LW/g | 21-day LW/g | Total feed intake/g | First week F/G | Second week F/G | Third week F/G | Full-term F/G |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| I | 15 | 12.6 | 16.037 | 105.526BC | 243.316ABC | 351.234AB | 64.149 | 422.666CD | 964.282AB | 1400.532A | 3727.765A | 1.487DE | 2.503CD | 3.957DEF | 2.740BCD |
| II | 15 | 12.8 | 15.790 | 108.341B | 231.666CDE | 277.831F | 63.159 | 435.918BC | 937.015BCD | 1135.606E | 3370.025CD | 1.633BCD | 2.837ABC | 6.620A | 3.147A |
| III | 15 | 13.0 | 16.135 | 98.989C | 222.769E | 287.684EF | 64.534 | 396.794D | 889.843D | 1175.340DE | 3082.219E | 1.523CDE | 2.565CD | 5.144BC | 2.784BC |
| IV | 16 | 12.6 | 15.933 | 103.310BC | 248.335AB | 326.999CD | 63.726 | 408.135CD | 996.613AB | 1308.000BC | 3481.571ABC | 1.810A | 2.328D | 5.308BC | 2.814BC |
| V | 16 | 12.8 | 16.124 | 116.981A | 251.740A | 352.593A | 64.498 | 474.913A | 1007.270A | 1413.779A | 3115.514E | 1.369E | 2.432D | 3.445EF | 2.311E |
| VI | 16 | 13.0 | 16.237 | 114.629AB | 249.633A | 322.917D | 64.948 | 462.066AB | 996.590AB | 1297.692C | 3162.103DE | 1.569CD | 2.342D | 4.596CD | 2.565D |
| VII | 17 | 12.6 | 16.064 | 100.134C | 232.767BCDE | 289.662EF | 64.259 | 405.000CD | 938.550BCD | 1170.774DE | 3204.474DE | 1.736AB | 2.443D | 5.881AB | 2.901B |
| VIII | 17 | 12.8 | 16.126 | 103.616BC | 224.261DE | 302.463E | 64.500 | 418.435CD | 894.975CD | 1217.481D | 3089.408E | 1.668ABC | 2.691BCD | 4.270CDE | 2.688BCD |
| IX | 17 | 13.0 | 16.173 | 99.319C | 238.958ABCD | 331.998BCD | 64.693 | 395.244D | 958.450ABC | 1323.750BC | 3502.365ABC | 1.483DE | 2.484CD | 4.528CD | 2.783BC |
| X | 18 | 12.6 | 16.036 | 100.503BC | 221.541E | 323.387D | 64.136 | 403.925CD | 895.406CD | 1316.762BC | 3628.470AB | 1.597BCD | 3.064A | 3.818DE | 2.900B |
| XI | 18 | 12.8 | 16.059 | 104.411BC | 224.679DE | 352.049A | 64.231 | 417.543CD | 894.933CD | 1422.587A | 3584.260ABC | 1.664ABC | 3.047AB | 3.173F | 2.640CD |
| XII | 18 | 13.0 | 16.228 | 103.389BC | 218.069E | 344.090ABC | 64.900 | 413.554CD | 872.271D | 1372.329AB | 3399.979BCD | 1.552CD | 3.044AB | 3.039F | 2.605CD |
| SEM | 0.171 | 3.967 | 7.968 | 12.397 | 0.043 | 0.959 | 1.952 | 3.255 | 32.923 | 0.019 | 0.045 | 0.150 | 0.029 | ||
| Main effect | |||||||||||||||
| CP (n = 24) | 15% | 15.988 | 104.285B | 232.584B | 305.584B | 63.947 | 418.459B | 930.379B | 1237.159B | 3393.336AB | 1.548 | 2.635B | 5.240A | 2.890A | |
| 16% | 16.098 | 111.640A | 249.903A | 334.170A | 64.390 | 448.4371A | 1000.157A | 1339.824A | 3253.063B | 1.583 | 2.367C | 4.450B | 2.563C | ||
| 17% | 16.121 | 101.023B | 231.995B | 308.041B | 64.484 | 406.226B | 930.658B | 1237.335B | 3265.415B | 1.629 | 2.539BC | 4.893AB | 2.791AB | ||
| 18% | 16.108 | 102.767B | 221.430C | 339.842A | 64.423 | 411.674B | 887.537C | 1370.560A | 3537.570A | 1.604 | 3.052A | 3.343B | 2.715B | ||
| ME (n = 32) | 12.6 MJ/kg | 16.018 | 102.368b | 236.490 | 322.821 | 64.068 | 409.931B | 948.713 | 1299.0167 | 3510.570A | 1.658A | 2.584 | 4.741 | 2.839A | |
| 12.8 MJ/kg | 16.025 | 108.337a | 233.087 | 321.234 | 64.097 | 436.702A | 933.548 | 1297.363 | 3289.802B | 1.583AB | 2.752 | 5.377 | 2.696B | ||
| 13.0 MJ/kg | 16.193 | 104.081b | 232.357 | 321.672 | 64.768 | 416.914B | 929.288 | 1292.278 | 3286.666B | 1.532B | 2.609 | 4.327 | 2.684B | ||
| P values | CP | 0.689 | <0.001 | <0.001 | <0.001 | 0.684 | <0.001 | <0.001 | <0.001 | <0.001 | 0.346 | <0.001 | <0.001 | <0.001 | |
| ME | 0.183 | 0.012 | 0.553 | 0.948 | 0.183 | 0.004 | 0.478 | 0.932 | 0.001 | 0.009 | 0.148 | 0.258 | 0.009 | ||
| C*M | 0.747 | <0.001 | <0.001 | <0.001 | 0.745 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.001 | <0.001 |
Different superscript lowercase letters in the data of the same row indicate significant differences (P < 0.05), and A-Fdifferent superscript capital letters indicate extremely significant differences (P < 0.01), n = 8/group; C*M CP × ME; SW, squab weight; LW, average litter weight; F/G, feed-to-gain ratio.
Dietary Energy/Protein Ratio Affected the Slaughter Performance of Squabs
Both ME and CP levels significantly affected the slaughter performance of squabs, and the semieviscerated weight, eviscerated weight, and heart weight of the squabs decreased with an increase in ME level (P < 0.05). Compared with other CP levels, the slaughter weight, semieviscerated weight, eviscerated weight, abdominal fat, and gizzard weights were significantly high at 18% CP level (P < 0.05). The interaction between ME and CP significantly affected the slaughter weight, semieviscerated weight, eviscerated weight, pectoral muscle weight, abdominal fat, gizzard, and kidney weights. Group 11 (18% CP, 12.8 MJ/kg) with an energy/protein ratio of 17.00 kcal/g had the best body fat and organ development (P < 0.05) (Table 6).
Table 6.
Effect of dietary energy/protein ratios on the slaughter performance of squabs in summer.
| Treatments (n = 24) | CP/% | ME/MJ/kg | Live body weight/g | Slaughter weight/g | Semieviscerated weight/g | Eviscerated weight/g | Pectoral muscle weight/g | Abdominal fat/g | Liver/g | Spleen/g | Thymus/g | Pancreas/g | Glandular stomach/g | Gizzard/g | Kidney/g | Bursa/g | Heart/g |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| I | 15 | 12.6 | 369.688AB | 289.858ab | 168.863AB | 103.046AB | 26.507ab | 1.538b | 12.150 | 0.383 | 0.932 | 2.276 | 1.253 | 6.504ABC | 2.566c | 0.493AB | 3.315A |
| II | 15 | 12.8 | 301.225E | 246.220c | 140.515D | 82.906D | 19.857c | 1.688ab | 10.498 | 0.297 | 0.827 | 2.029 | 1.228 | 6.108CD | 2.871abc | 0.285C | 2.718BC |
| III | 15 | 13.0 | 301.068E | 242.827c | 144.043CD | 84.931CD | 23.211abc | 1.680ab | 10.693 | 0.311 | 1.178 | 2.104 | 1.196 | 5.694CD | 2.852abc | 0.373BC | 2.619C |
| IV | 16 | 12.6 | 309.458DE | 253.233bc | 152.304ABCD | 93.241ABCD | 24.062abc | 2.573a | 11.289 | 0.293 | 1.167 | 2.185 | 1.103 | 5.683CD | 2.742abc | 0.323C | 2.767BC |
| V | 16 | 12.8 | 334.000BCD | 272.171ab | 155.913ABCD | 98.410ABC | 21.904abc | 1.718ab | 11.683 | 0.404 | 0.965 | 2.186 | 1.221 | 5.843CD | 2.549c | 0.533A | 2.982AB |
| VI | 16 | 13.0 | 309.300DE | 261.105bc | 143.213CD | 84.439CD | 19.077c | 1.907ab | 10.001 | 0.369 | 0.747 | 2.094 | 1.111 | 5.424D | 2.571bc | 0.324C | 2.702BC |
| VII | 17 | 12.6 | 343.591ABCD | 278.518ab | 160.291ABC | 100.977AB | 25.525ab | 1.936ab | 11.144 | 0.321 | 1.435 | 2.522 | 1.270 | 6.186BC | 3.151a | 0.376BC | 2.859BC |
| VIII | 17 | 12.8 | 329.100BCDE | 261.725bc | 147.820BCD | 89.794BCD | 23.276abc | 2.102ab | 10.006 | 0.289 | 1.247 | 2.143 | 1.288 | 6.089CD | 3.033abc | 0.380BC | 2.623C |
| IX | 17 | 13.0 | 325.350CDE | 274.135abc | 151.530ABCD | 91.752ABCD | 21.735bc | 1.862ab | 10.717 | 0.306 | 0.488 | 2.116 | 1.238 | 6.005CD | 2.594bc | 0.331C | 2.778BC |
| X | 18 | 12.6 | 336.238BCD | 279.357ab | 165.656A | 104.814A | 23.568abc | 2.341ab | 11.473 | 0.332 | 0.824 | 2.266 | 1.178 | 6.207BC | 2.609abc | 0.345C | 2.975BC |
| XI | 18 | 12.8 | 360.700ABC | 287.400ab | 169.811AB | 103.807A | 27.849a | 2.556a | 11.416 | 0.331 | 1.258 | 2.459 | 1.306 | 7.078A | 3.106ab | 0.54BC | 2.796BC |
| XII | 18 | 13.0 | 383.700A | 295.775a | 161.720ABC | 99.531AB | 25.871ab | 2.263ab | 10.789 | 0.297 | 1.356 | 2.278 | 1.361 | 6.848AB | 2.994abc | 0.418BC | 2.912BC |
| SEM | 3.823 | 3.020 | 1.870 | 1.469 | 0.535 | 0.087 | 0.149 | 0.009 | 0.045 | 0.037 | 0.021 | 0.072 | 0.050 | 0.015 | 0.035 | ||
| Main effect | |||||||||||||||||
| CP (n = 72) | 15% | 323.008B | 259.020B | 150.397b | 90.294b | 23.043 | 1.651b | 10.993 | 0.328 | 0.984 | 2.133 | 1.248 | 6.150B | 2.739 | 0.390 | 2.904 | |
| 16% | 323.933B | 267.085B | 150.154b | 92.029b | 22.331 | 2.239a | 11.159 | 0.363 | 1.026 | 2.164 | 1.146 | 5.756C | 2.650 | 0.410 | 2.836 | ||
| 17% | 334.050B | 272.552B | 153.797b | 94.174b | 23.653 | 1.969ab | 10.669 | 0.307 | 1.072 | 2.260 | 1.274 | 6.118BC | 2.927 | 0.365 | 2.763 | ||
| 18% | 361.733A | 288.753A | 165.397a | 102.717a | 25.976 | 2.386a | 11.263 | 0.322 | 1.155 | 2.338 | 1.287 | 6.724A | 2.903 | 0.372 | 2.900 | ||
| ME (n = 96) | 12.6 MJ/kg | 343.325 | 278.309 | 163.459A | 100.519A | 25.296 | 2.184 | 11.638A | 0.339 | 1.137 | 2.306 | 1.229 | 6.224 | 2.786 | 0.393 | 2.990a | |
| 12.8 MJ/kg | 333.919 | 268.786 | 153.630B | 93.729ab | 23.520 | 2.058 | 10.881B | 0.331 | 1.098 | 2.204 | 1.252 | 6.300 | 2.877 | 0.398 | 2.801b | ||
| 13.0 MJ/kg | 329.800 | 268.463 | 149.960B | 90.163b | 22.436 | 1.942 | 10.544B | 0.319 | 0.942 | 2.161 | 1.236 | 6.037 | 2.751 | 0.362 | 2.760b | ||
| P values | CP | <0.001 | 0.003 | 0.019 | 0.011 | 0.079 | 0.014 | 0.504 | 0.168 | 0.517 | 0.179 | 0.065 | <0.001 | 0.141 | 0.695 | 0.407 | |
| ME | 0.280 | 0.290 | 0.008 | 0.011 | 0.080 | 0.509 | 0.009 | 0.674 | 0.134 | 0.248 | 0.895 | 0.266 | 0.558 | 0.542 | 0.013 | ||
| C*M | <0.001 | 0.020 | 0.004 | 0.003 | 0.021 | 0.050 | 0.073 | 0.163 | 0.661 | 0.217 | 0.406 | <0.001 | 0.027 | 0.003 | 0.001 |
Different superscript lowercase letters in the data of the same row indicate significant differences (P < 0.05), and A-Edifferent superscript capital letters indicate extremely significant differences (P < 0.01), n = 24/group; C*M CP × ME.
Dietary Energy/Protein Ratio Affected the Meat Physiochemical Quality of Slaughtered Squabs
Both ME and CP levels also had a significant effect on the physicochemical traits of the pectoral muscle of the squabs. Furthermore, there was a strong interaction between ME and CP levels regarding the meat quality of the pectoral muscle of the squabs. Overall, 18% CP affected the meat color the most (lightness L⁎, redness a⁎, yellowness b⁎) at 45 min and pH after slaughter (P < 0.01). The lowest pH after 24 h was observed in the 12.8 MJ/kg group (P < 0.05) (Table 7).
Table 7.
Effect of dietary energy/protein ratios on the meat quality of squabs in summer.
| Treatments (n = 24) | CP/% | ME/MJ/kg | 45-min L⁎ | 45-min a⁎ | 45-min b⁎ | pH 45 min | pH 24 h | Drip loss/% |
|---|---|---|---|---|---|---|---|---|
| I | 15 | 12.6 | 41.614CD | 9.995ABC | 8.881ABCD | 6.223A | 5.822ABCD | 1.989ab |
| II | 15 | 12.8 | 42.674CD | 10.068AB | 8.961ABC | 6.156A | 5.787CD | 1.660ab |
| III | 15 | 13.0 | 47.775A | 9.157BCD | 9.256AB | 6.189A | 5.932AB | 2.214a |
| IV | 16 | 12.6 | 42.919CD | 9.170BCD | 8.417ABC | 6.118AB | 5.946A | 1.736ab |
| V | 16 | 12.8 | 41.535CD | 11.276A | 9.814A | 6.287A | 5.878ABCD | 2.014a |
| VI | 16 | 13.0 | 42.557CD | 9.553ABCD | 8.080ABCD | 6.172A | 5.862ABC | 1.255b |
| VII | 17 | 12.6 | 44.351BC | 8.446BCD | 7.014D | 5.980BC | 5.800BCD | 2.077a |
| VIII | 17 | 12.8 | 46.386AB | 8.148CD | 8.698ABC | 6.096ABC | 5.938A | 1.603ab |
| IX | 17 | 13.0 | 42.933CD | 8.663BCD | 7.274CD | 6.237A | 5.821A | 1.250b |
| X | 18 | 12.6 | 44.100BCD | 8.110D | 7.518CD | 6.118AB | 5.848ABCD | 1.525ab |
| XI | 18 | 12.8 | 42.458D | 8.617BCD | 7.010BCD | 5.955C | 5.721D | 1.548ab |
| XII | 18 | 13.0 | 43.874BCD | 8.429BCD | 6.983CD | 6.114AB | 5.850ABCD | 1.586ab |
| SEM | 0.284 | 0.131 | 0.139 | 0.015 | 0.013 | 0.061 | ||
| Main effect | ||||||||
| CP (n = 72) | 15% | 44.129a | 9.728A | 9.002A | 6.201A | 5.851ab | 1.954 | |
| 16% | 42.462b | 9.806A | 8.677A | 6.183A | 5.883a | 1.669 | ||
| 17% | 44.620a | 8.396B | 7.575B | 6.108B | 5.894a | 1.643 | ||
| 18% | 43.419ab | 8.351B | 7.070B | 6.064B | 5.801b | 1.666 | ||
| ME (n = 96) | 12.6 MJ/kg | 43.398 | 8.864 | 7.809b | 6.122 | 5.854ab | 1.832 | |
| 12.8 MJ/kg | 43.257 | 9.410 | 8.528a | 6.114 | 5.820b | 1.706 | ||
| 13.0 MJ/kg | 44.318 | 8.936 | 7.905b | 6.181 | 5.898a | 1.661 | ||
| P values | CP | 0.026 | <0.001 | <0.001 | 0.003 | 0.031 | 0.155 | |
| ME | 0.209 | 0.145 | 0.048 | 0.121 | 0.034 | 0.280 | ||
| C*M | <0.001 | <0.001 | <0.001 | <0.001 | 0.001 | 0.039 |
Different superscript lowercase letters in the data of the same row indicate significant differences (P < 0.05), and A-Ddifferent superscript capital letters indicate extremely significant differences (P < 0.01), n = 24/group; C*M CP × ME; L⁎ stands for brightness; a⁎ stands for redness; b⁎ stands for yellowness.
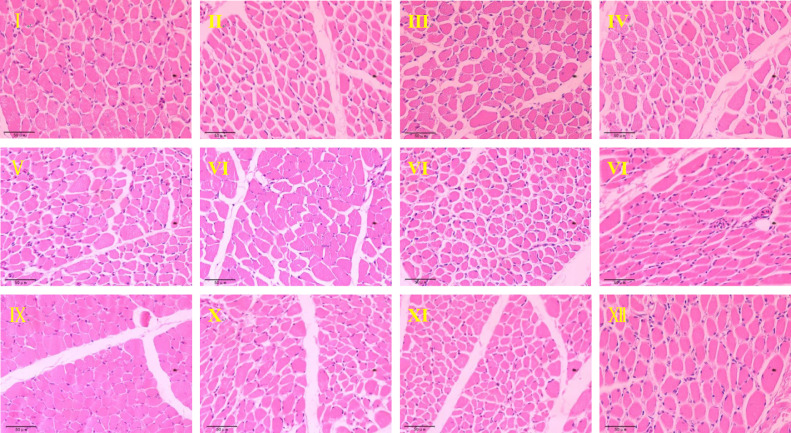
Further investigation revealed that the ME level had a significant effect on the tissue biological properties of muscle fiber in pigeons, especially in the 12.8 MJ/kg group, which had the smallest cross-sectional area and diameter of muscle fiber (P < 0.01) and the highest muscle fiber density (P < 0.01) (Table 8). As shown in Figure 2, there was also a significant interaction between ME and CP levels. Compared to the other test groups, test group 11 (18% CP, 12.8 MJ/kg) significantly increased the muscle fiber density and decreased the cross-sectional area of pectoral muscle fibers (P < 0.01). Therefore, 18% CP and 12.8 MJ/kg was the optimal combination to improve the quality of pigeon breast with a corresponding energy/protein ratio of 17.00 kcal/g.
Table 8.
Effect of dietary energy/protein ratios on the muscle fiber characteristics of squabs in summer.
| Treatments (n = 12) | CP/% | ME/MJ/kg | Muscle fiber area/μm2 | Muscle fiber diameter/μm | Muscle fiber density/n*mm−2 |
|---|---|---|---|---|---|
| I | 15 | 12.6 | 336.134A | 20.455A | 2108.333D |
| II | 15 | 12.8 | 242.099DE | 17.167DE | 3358.333A |
| III | 15 | 13.0 | 257.148CDE | 17.644CDE | 2983.333AB |
| IV | 16 | 12.6 | 277.801BCD | 18.391BCD | 2358.333BCD |
| V | 16 | 12.8 | 238.056DE | 17.135DE | 2808.333ABC |
| VI | 16 | 13.0 | 273.971BCD | 18.390BCD | 2891.667ABC |
| VII | 17 | 12.6 | 247.163CDE | 17.549CDE | 3100.000A |
| VIII | 17 | 12.8 | 258.318CDE | 17.775CDE | 3225.000A |
| IX | 17 | 13.0 | 259.244CDE | 18.023BCD | 2850.000ABC |
| X | 18 | 12.6 | 281.403ABCD | 18.550ABCD | 2300.000CD |
| XI | 18 | 12.8 | 208.752E | 15.920E | 3433.333A |
| XII | 18 | 13.0 | 302.236ABC | 19.294ABC | 2000.000D |
| SEM | 6.179 | 0.214 | 76.632 | ||
| Main effect | |||||
| CP (n = 36) | 15% | 278.461 | 18.422 | 2816.667 | |
| 16% | 263.276 | 17.972 | 2686.111 | ||
| 17% | 254.908 | 17.782 | 3058.333 | ||
| 18% | 264.130 | 17.921 | 2577.778 | ||
| ME (n = 48) | 12.6 MJ/kg | 285.625A | 18.736A | 2466.667B | |
| 12.8 MJ/kg | 236.806B | 16.999B | 3206.250A | ||
| 13.0 MJ/kg | 273.149A | 18.338A | 2681.250B | ||
| P values | CP | 0.551 | 0.701 | 0.084 | |
| ME | 0.002 | 0.002 | <0.001 | ||
| C*M | 0.004 | 0.004 | <0.001 |
Different superscript capital letters indicate extremely significant differences (P < 0.01), n = 12/group;C*M CP × ME.
Figure 2.
Effect of dietary energy/protein ratio on the muscle fiber development of slaughter squabs (400×). The Roman numerals I to XII at the top left of the image represent different processing groups.
Regression Models to Estimate the Dietary Energy/Protein Ratio Requirements of Breeding Pigeons
The 1 to 21-day ADG, slaughter rate, drip loss, the 50-day laying rate, total weight loss of the breeding pigeons, average laying interval, and full-term F/G all showed significant quadratic curve changes with the change of energy/protein ratio level in the dietary. As shown in Table 9, the optimal energy/protein ratio for lactating breeding pigeons in summer varies according to different indices. For the slaughter rate and F/G of squabs, the optimal dietary energy/protein ratio was 17.919 to 19.020 kcal/g; for the laying period and weight recovery after lactation, the optimal dietary energy/protein ratio for breeding pigeons was 16.720 kcal/g.
Table 9.
Regression analysis of dietary energy/protein ratio requirements for lactating pigeons.
| Items | Regression model | Coefficient (R2) | P value | Dietary energy/protein ratio additive amount/(kcal/g) |
|---|---|---|---|---|
| Pigeon squabs 1–21-day ADG /g | y = 21.235 − 0.319x | 0.068 | 0.010 | 17.919 (max) |
| y = −0.203x2 + 7.275x − 49.465 | 0.081 | 0.007 | ||
| Slaughter rate/% | y = 75.591 + 0.294x | 0.004 | 0.308 | 19.020 (max) |
| y = −0.510x2 + 19.400x − 102.379 | 0.012 | 0.094 | ||
| Drip loss/% | y = 0.066 + 0.001x | 0.008 | 0.749 | 19.188 (min) |
| y = 0.008x2 − 0.307x + 2.945 | 0.009 | 0.129 | ||
| The 50-day laying rate/% | y = 186.834 − 7.418x | 0.122 | <0.001 | 19.665 (min) |
| y = 3.877x2 − 152.482x + 1537.245 | 0.161 | <0.001 | ||
| Total heavy loss of breeding pigeons/g | y = 40.410 + 7.417x | 0.057 | 0.019 | 19.731 (max) |
| y = −3.622x2 + 142.930x − 1221.90 | 0.054 | 0.029 | ||
| Average laying interval/d | y = 41.461 + 0.473x | 0.040 | 0.049 | 19.289 (max) |
| y = −0.407x2 + 15.701x − 100.290 | 0.056 | 0.025 | ||
| Full-term F/G | y = 2.403 + 0.018x | 0.007 | 0.424 | 18.404 (min) |
| y = 0.057x2 − 2.098x + 22.102 | 0.066 | 0.016 |
DISCUSSION
Appropriate levels of ME and CP ensure the nutritional quality of the diet and have important implications for promoting poultry growth and development, immune function, and reproduction of offspring (Ye et al., 2015). Lesuisse et al. (2017, 2018) obtained a higher energy/protein ratio by reducing the CP level in the diet, which resulted in a higher body fat ratio during the actual production of breeding pigeons. And such a higher body fat amount obtained by consuming less CP may be detrimental to the breeder's productive performance (Heijmans et al., 2021). If the energy/protein ratio of the dietary is not balanced, it will directly affect the growth and reproductive performance of breeding pigeons. Herein, we first evaluated the effects of different energy/protein levels on the growth and reproductive performance, “crop milk” quality, and egg quality of breeding pigeons. We found that as the dietary CP level increased to 18%, the weight loss amount of breeding pigeons in the lactation period decreased significantly, which is consistent with previous results by Gao et al. (2016a) and Chen et al. (2016). ME level had no significant effect on the weight of the breeding pigeon, but there was a significant interaction between ME and CP on their effect on breeding pigeon weight and reproductive production. Notably, high ME in the diet but low CP levels (15–16%) caused weight loss in breeding pigeons, while high CP levels (17–18%) caused the opposite effect. This means that a very high or too low energy/protein ratio reduces the production performance of breeding pigeons. Also, high CP levels significantly shortened the average egg-laying interval during the lactation period and positively correlated with the laying rate of breeding pigeons, which is consistent with Gao et al. (2016a) and Ding et al. (2016) findings. However, too high CP levels in actual production tend to deposit too much body fat in breeders, which prolong the egg-laying interval of female breeders and even cause difficulties in the emergence of offspring, etc. (Costantini, 2010; Ding et al., 2016; Kriseldi et al., 2018). Test group 12 (18% CP, 13.0 MJ/kg), which had the lowest weight loss during lactation, did not perform as well as group 9 (17% CP, 13.0 MJ/kg) and group 10 (18% CP, 12.6 MJ/kg) in reproductive production. This study also revealed that test group 12 (18% CP, 13.0 MJ/kg), which had the lowest weight loss during lactation, did not perform as well as group 9 (17% CP, 13.0 MJ/kg) and group 10 (18% CP, 12.6 MJ/kg) in reproductive production. It has been found that changes in the composition of breeder diet formulations may affect nutrient deposition in eggs (Junqueira et al., 2006). In the present study, we found no significant effect of the interaction of CP and ME levels in breeding pigeon diets on egg quality, including mean egg weight, egg shape index, and yolk weight, among others. Similar studies have also observed in laying hens that dietary energy/protein ratios do not affect yolk weight, Haugh unit, or shell thickness (Ding et al., 2016; Heijmans et al., 2022). Therefore, an appropriate level of energy/protein ratio in the diet will be more favorable to the best weight recovery of pigeons after the lactation period, thus optimizing reproductive efficiency could achieve sustainable pigeon farming.
Given that squabs are the main source of meat in the pigeon market, the fundamental objective of the research on the energy/protein ratio requirements of lactating breeding pigeons is also to improve the survival rate and slaughter performance of squabs. Furthermore, a reasonable energy/protein ratio can also optimize the F/G to reduce production costs and maximize the output (Hu, 2016; Wang, 2018). A poor combination of protein and energy levels also affects the quality of “crop milk” from the breeding pigeon, which in turn affects the growth and development of the squabs (Gillespie et al., 2013; Fouad and El-Senousey, 2014; Gao et al., 2016b). The market weight and F/G of squabs are also the most intuitive indicators to examine the feasibility of the ration formula and the feeding effect. In this study, we found that CP and ME levels had little effect on the mortality of squabs in summer, but there was a strong interaction between ME and CP levels on their effect on the body weight and F/G of squabs. Among them, dietary ME level only influenced the feeding and body weight gain in the early growth stage of the squabs, and increasing ME level reduced feed intake. This could be due to the actual nutrient intake and feed utilization efficiency increased with an increase in the dietary nutrient concentration, which is consistent with a previous study (Sahin et al., 2003). However, we also found that the growth rate of squabs in the early growth stage showed a tendency to increase and then decrease as the ME level increased, which means that too high a level of ME in the diet can have a negative effect. Studies have shown that increasing the protein level in the diet can significantly improve feed utilization efficiency (Liu et al., 2005; Wu et al., 2005). In contrast, in the present study, we found that the optimal CP level for squabs was not the same in different growth stages. In the first and middle growth stages (1–14 d), the fastest growth rate of the squabs occurred at 16% CP level, while F/G was the lowest. In the later growth stage (14–21 d), 18% CP was optimal for weight gain and feed efficiency rate of the squabs. Based on this, we recommend that when we go on to further study the nutritional requirements of squabs, perhaps we can break down the lactation period to explore it in stages.
Slaughter performance is an important indicator of the growth performance of meat poultry, which reflects the differences in the number of nutrients deposited in different tissue parts of the diet (Chen et al., 2017). It is also an important reference indicator to evaluate the feeding management status and nutritional status of meat pigeons. These indicators clearly reflect the growth and development of squabs, while helping to better improve production efficiency. In terms of poultry slaughter performance, dietary ME levels can have a significant effect on abdominal fat deposition, while diets with higher levels of CP are more favorable for the growth of breast muscle (Cao et al., 2014). In mammals, differences in CP levels in parental diets can affect the organ or tissue quality of offspring by influencing protein or amino acid levels in milk (Wang, 2011), and a similar pattern may exist in domestic pigeons. Similar to this result, our results showed that with the change in ME and CP levels, the differences in pectoral muscle weight, abdominal fat, and organ weight between the groups of squabs were significant. High CP levels significantly improved carcass quality and the development of digestive-related organs such as the gizzard weight in squabs. High ME levels exerted the opposite effect. It is manifested in the reduction of eviscerated weight and the development of vital organs such as the heart and liver. The abdominal fat ratio is also an important indicator of lipid deposition (Zhang et al., 2022). In general, when animals consume diets with high CP levels, the bodies need to consume more energy to drive the excretion of nitrogen, which disrupts the deposition of excess fats (Wang et al., 2021). Rozenboim et al. (2016) also showed that the abdominal fat rate of broiler chickens increased with increasing dietary energy level and decreased with increasing dietary CP levels within a certain range. The present study revealed conflicting findings, in which the abdominal fat content of squabs increased with increasing CP levels. It may be necessary to explain this phenomenon further based on serum biochemical indices in squabs.
Meat physiochemical quality of squabs is also closely related to the nutritional level of the diet, and appropriate dietary energy and protein levels significantly improve the meat quality of domestic poultry (Shi et al., 2009; Lin et al., 2020). The quality of muscle is usually reflected in the meat color, pH, and drip loss. Among them, meat color is the most visual indicator of physiological and biochemical changes in muscle. Muscle brightness values are influenced by the myoglobin and fat deposition content of the muscle, with red values reflecting the myoglobin content and yellow values reflecting the influence of ration pigments (Mir et al., 2017). Many previous studies on the assessment of pectoral muscle meat color have shown that the smaller the L⁎ value, the larger the a⁎ value, and the smaller the b⁎ value, the better the muscle quality and vice versa (Sun, 2004; Wu et al., 2018; Wen et al., 2020). pH is an important index of muscle quality and can affect muscle color and tethering power. The lower the drip loss, the higher the turgidity and tenderness of the muscle (Zhang et al., 2015; Bernad et al., 2018). Tang et al. (2007) showed that increasing the level of ME in the diet significantly increased the b⁎ value and the 24-h pH of broiler pectoral muscle and decreased the drip loss rate of the muscle. In this experiment, the b⁎ value and the 24h pH of the pigeon pectoral muscle increased and then decreased with an increase in the ME level. This indicated that the dietary ME level should not be too high or too low, and 12.8 MJ/kg ME was the optimal ME level for the best quality pigeon meat. An increase in CP level significantly affected the 45 min meat color (L⁎, a⁎, b⁎) and pH (45 min, 24 h) after slaughter. Generally, a high CP level had more beneficial than harmful to the overall pectoral muscle meat color. The histological properties of muscle fiber are the histological basis of meat quality and are a major indicator for assessing meat quality. Some studies have shown that as the diameter of muscle fibers increases, muscle tenderness decreases accordingly (Zhu et al., 2020). Therefore, the finer and denser the muscle fibers are, the more tender and juicy the meat will be, and Jackson et al. (1982) further showed that diets with high ME and high CP could improve muscle fiber density. This experiment also showed that the ME and CP levels of 12.8 MJ/kg and 18%, respectively, significantly increased the muscle fiber density and reduced the cross-sectional area of pectoral muscle fibers in squabs. This demonstrates that appropriate energy/protein ratio levels greatly improve the meat quality of squabs, which ensures better slaughter performance.
Finally, a linear regression analysis was performed to estimate the energy/protein ratio requirements in the lactation diets of breeding pigeons in summer “2 + 4” breeding pattern. The correlation coefficient (R2) between each index and the energy/protein ratio level in the diets was low. This could be the effect of different energy/protein ratios was a reciprocal effect between the combined two factors, resulting in a poorer mean of the data for each treatment group. The optimal requirements for the laying rate, average laying interval, and total weight loss of breeding pigeons were all negatively correlated with the optimal requirements for actual production. For example, the model equations for the laying rate only yielded minimum values. Therefore we can only infer the optimal requirement from the range of energy/protein ratios provided in this experiment. If the F/G is the primary objective, the recommended level of energy/protein ratio in the diet of breeding pigeons is 17.92 to 19.02 kcal/g; if the reproductive efficiency is the primary objective, then the recommended energy/protein ratio in the breeder's diet is 16.72 kcal/g.
CONCLUSIONS
The energy level in summer diet has little effect on the production of breeding pigeons during the lactation period, but the protein level significantly affects the reproductive performance of these birds. There is a strong interaction between energy and protein levels in pigeons. The best reproductive performance, the fastest growth of squabs, and better meat quality of pigeons in summer are achieved at 18% CP and 12.8 MJ/kg. Finally, the regression model revealed that the best dietary energy/protein ratio was 17.92 to 19.02 kcal/g for squabs and 16.72 kcal/g for the breeding pigeons. Therefore, 18% CP and 12.8 MJ/kg can be considered the best energy/protein ratio requirement for breeding pigeons in the lactation period under the summer “2 + 4” breeding pattern.
ACKNOWLEDGMENTS
We acknowledge Guangdong Meizhou Jinlv Modern Agriculture Development Co. Ltd. for providing us with the experimental site. This research was supported by Key Realm R&D Program of Guangdong Province (2020B0202080002), Guangdong Basic and Applied Basic Research Foundation (2021A1515012417), Innovative Team Projects of Ordinary Colleges and Universities in Guangdong Province (2020KCXTD019), Provincial-level agricultural technology innovation promotion and agricultural resources and ecological environmental protection construction projects (2021KJ115), Technical Service of Xingning Pigeon Industrial Park “Top ranking” project (D122222G902).
DISCLOSURES
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work, and there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the content of this paper.
REFERENCES
- Bernad L., Casado P.D., Murillo N.L., Picallo A.B., Garriz C.A., Maceira N.O. Meat quality traits in the Greater rhea (Rhea americana) as influenced by muscle, sex and age. Poult. Sci. 2018;97:1579–1587. doi: 10.3382/ps/pey005. [DOI] [PubMed] [Google Scholar]
- Bu Z., Xie P., Fu S.Y., Tong H.B., Dai X. Effect of energy and protein levels on performance, egg quality, and nutrient digestibility of laying pigeons. J. Appl. Poult. Res. 2015;24:371–379. [Google Scholar]
- Cao Z., Gao Z.H., Chen G.X., Li Q.P., Chen C.W., Zhang S.C., Huang Z.P., Ding Z.P. Effects of metabolizable energy and crude protein levels on performance, slaughter performance and serum biochemical indexes in Cobb Broilers. Chin. J. Anim. Nutr. 2014;26:2553–2564. (in Chinese) [Google Scholar]
- Chen J.Y., Long J.F., Chen F.M., Zhao Y.R. Effects of diets with different energy and protein levels on growth performance, carcass traits and meat quality of Ningxiang pigs. Chin. J. Anim. Nutr. 2021;33:208–216. (in Chinese) [Google Scholar]
- Chen X.S., Yang H.M., Meng J., Wang Z.Y., Zhou H., Yang Z.F. Effects of compound probiotics on growth performance, slaughter performance, immune organ index and serum biochemical parameters of squabs. Chin. J. Anim. Nutr. 2017;29:2384–2390. (in Chinese) [Google Scholar]
- Chen Y., Ye C., Cao S.M., Li D.H., Li A. Effects of dietary different protein levels of breeding pigeon on growth performance and intestinal morphology for Carrier pigeon at 1 to 28 days of age. China Poultry. 2016;38:33–36. (in Chinese) [Google Scholar]
- Chen Y.F., Zhen H., Lin J., Zhen X.T., Wu W.B., Yang Y.X., Xu S.F., Liang H.Z. Effect of scalding on the process of rigor mortis and meat quality of broilers as studied by low-field nuclear magnetic resonance. Food Sci. Technol. 2020;45:159–165. (in Chinese) [Google Scholar]
- Costantini D. Complex trade-offs in the pigeon (Columba livia): egg antioxidant capacity and female serum oxidative status in relation to diet quality. J. Comp. Physiol. B. 2010;180:731–739. doi: 10.1007/s00360-010-0456-z. [DOI] [PubMed] [Google Scholar]
- Ding Y., Bu X., Zhang N., Li L., Zou X. Effects of metabolizable energy and crude protein levels on laying performance, egg quality and serum biochemical indices of Fengda-1 layers. Anim. Nutr. 2016;2:93–98. doi: 10.1016/j.aninu.2016.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong X.Y., Wang Y.M., Dai L., Azzam M.M., Wang C., Zou X.T. Posthatch development of intestinal morphology and digestive enzyme activities in domestic pigeons (Columba livia) Poult. Sci. 2012;91:1886–1892. doi: 10.3382/ps.2011-02091. [DOI] [PubMed] [Google Scholar]
- Fouad A.M., El-Senousey H.K. Nutritional factors affecting abdominal fat deposition in poultry: a review. Asian-Australas. J. Anim. Sci. 2014;27:1057–1068. doi: 10.5713/ajas.2013.13702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao C.Q., Wang X.H., Hu X.C., Yan H.C., Wang X.Q. Effect of dietary crude protein levels on growth performance, carcass characteristics, meat quality of squabs and laying performance of breeding pigeons. J. South China Agric. Univ. 2016;37:1–6. (in Chinese) [Google Scholar]
- Gao C.Q., Yang J.X., Chen M.X., Yan H.C., Wang X.Q. Growth curves and age-related changes in carcass characteristics, organs, serum parameters, and intestinal transporter gene expression in domestic pigeon (Columba livia) Poult. Sci. 2016;95:867–877. doi: 10.3382/ps/pev443. [DOI] [PubMed] [Google Scholar]
- Geng A.L., Zhang Q.Q., Chang C., Wang H.H., Chu Q., Zhang J., Yan Z.X., Liu H.G. Dietary metabolizable energy and crude protein levels affect the performance, egg quality and biochemical parameters of a dual-purpose chicken. Anim. Biotechnol. 2022;26:1–10. doi: 10.1080/10495398.2022.2114001. [DOI] [PubMed] [Google Scholar]
- Gillespie M.J., Crowley T.M., Haring V.R., Wilson S.L., Harper J.A., Payne J.S., Green D., Monaghan P., Stanley D., Donald J.A., Nicholas K.R., Moore R.J. Transcriptome analysis of pigeon milk production – role of cornification and triglyceride synthesis genes. BMC Genom. 2013;14:169. doi: 10.1186/1471-2164-14-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie M.J., Stanley D., Chen H., Donald J.A., Nicholas K.R., Moore R.J., Crowley T.M. Functional similarities between pigeon 'milk' and mammalian milk: induction of immune gene expression and modification of the microbiota. PLoS One. 2012;7:e48363. doi: 10.1371/journal.pone.0048363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heijmans J., Duijster M., Gerrits W.J.J., Kemp B., Kwakkel R.P., van den Brand H. Impact of growth curve and dietary energy-to-protein ratio on productive performance of broiler breeders. Poult Sci. 2021;100 doi: 10.1016/j.psj.2021.101131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heijmans J., Duijster M., Gerrits W.J.J., Kemp B., Kwakkel R.P., van den Brand H. Impact of growth curve and dietary energy-to-protein ratio of broiler breeders on egg quality and egg composition. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2022.101946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X.C. Crop Physiological Structure at Different Reproductive Cycles and the Mechanism of Milk Protein Synthesis of Domestic Pigeon (Columba Livia), 2016, South China Agricultural University; Guangzhou, PhD Diss.(in Chinese).
- Jackson S., Summers J.D., Leeson S. Effect of dietary protein and energy on broiler carcass composition and efficiency of nutrient utilization. Poult. Sci. 1982;61:2224–2231. doi: 10.3382/ps.0612224. [DOI] [PubMed] [Google Scholar]
- Jin Y.Y., Zheng C.T., Chen W., Wang S., Xia W.G., Liu W.C., Cheng J.R., Zhang Y.N. Effects of sodium selenite and selenium yeast on laying performance, egg quality, egg selenium content and egg selenium conversion rate of laying ducks. J. Anim. Nutr. 2022;20:1–16. (in Chinese) [Google Scholar]
- Junqueira O.M., Laurentiz A.C.d., Filardi R.d.S., Rodrigues E.A., Casartelli E.M.C. Effects of energy and protein levels on egg quality and performance of laying hens at early second production cycle. J. Appl. Poult. Res. 2006;15:110–115. [Google Scholar]
- Kokoszynski D., Steczny K., Zochowska-Kujawska J., Sobczak M., Kotowicz M., Saleh M., Fik M., Arpasova H., Hrncar C., Wlodarczyk K. Carcass characteristics, physicochemical properties, and texture and microstructure of the meat and internal organs of carrier and king pigeons. Animals (Basel) 2020;10:1315. doi: 10.3390/ani10081315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriseldi R., Tillman P.B., Jiang Z., Dozier, 3rd W.A. Effects of feeding reduced crude protein diets on growth performance, nitrogen excretion, and plasma uric acid concentration of broiler chicks during the starter period. Poult. Sci. 2018;97:1614–1626. doi: 10.3382/ps/pex395. [DOI] [PubMed] [Google Scholar]
- Lesuisse J., Li C., Schallier S., Climaco W.L.S., Bautil A., Everaert N., Buyse J. Multigenerational effects of a reduced balanced protein diet during the rearing and laying period of broiler breeders. 1. Performance of the F1 breeder generation. Poult. Sci. 2018;97:1651–1665. doi: 10.3382/ps/pey013. [DOI] [PubMed] [Google Scholar]
- Lesuisse J., Li C., Schallier S., Leblois J., Everaert N., Buyse J. Feeding broiler breeders a reduced balanced protein diet during the rearing and laying period impairs reproductive performance but enhances broiler offspring performance. Poult. Sci. 2017;96:3949–3959. doi: 10.3382/ps/pex211. [DOI] [PubMed] [Google Scholar]
- Li Y., Li F.H., She S.J., Zhang S., Wang Y.C., Guo k.J. Effect of different feeding patterns and protein levels on growth performance, slaughter performance and nitrogen balance of squabs. Chin. J. Anim. Sci. 2022;58:161–166. (in Chinese) [Google Scholar]
- Lin X., Gou Z., Wang Y., Li L., Fan Q., Ding F., Zheng C., Jiang S. Effects of dietary iron level on growth performance, immune organ indices and meat quality in Chinese yellow broilers. Animals (Basel) 2020;10:670. doi: 10.3390/ani10040670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Kong X., Jiang G., Tan B., Deng J., Yang X., Li F., Xiong X., Yin Y. Effects of dietary protein/energy ratio on growth performance, carcass trait, meat quality, and plasma metabolites in pigs of different genotypes. J. Anim. Sci. Biotechnol. 2015;6:36. doi: 10.1186/s40104-015-0036-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z., Wu G., Bryant M.M., Roland D.A. Influence of added synthetic lysine in low-protein diets with the methionine plus cysteine to lysine ratio maintained at 0.75. J. Appl. Poult. Res. 2005;14:174–182. [Google Scholar]
- Ministry of Agriculture of the People's Republic of China . Standards Press of China; Beijing, China: 2020. NY/T 823-2020 Poultry Production Performance Terms and Metric Statistical Methods. (in Chinese) [Google Scholar]
- Mir N.A., Rafiq A., Kumar F., Singh V., Shukla V. Determinants of broiler chicken meat quality and factors affecting them: a review. J. Food Sci. Technol. 2017;54:2997–3009. doi: 10.1007/s13197-017-2789-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petracci M., Sirri F., Mazzoni M., Meluzzi A. Comparison of breast muscle traits and meat quality characteristics in 2 commercial chicken hybrids. Poult. Sci. 2013;92:2438–2447. doi: 10.3382/ps.2013-03087. [DOI] [PubMed] [Google Scholar]
- Rozenboim I., Mahato J., Cohen N.A., Tirosh O. Low protein and high-energy diet: a possible natural cause of fatty liver hemorrhagic syndrome in caged White Leghorn laying hens. Poult. Sci. 2016;95:612–621. doi: 10.3382/ps/pev367. [DOI] [PubMed] [Google Scholar]
- Sahin K., Onderci M., Sahin N., Gursu M.F., Kucuk O. Dietary vitamin C and folic acid supplementation ameliorates the detrimental effects of heat stress in Japanese quail. J. Nutr. 2003;133:1882–1886. doi: 10.1093/jn/133.6.1882. [DOI] [PubMed] [Google Scholar]
- Shi J.S., Liu F.Z., Niu Z.Y. Study on the requirements of energy and protein for broilers during finishing phase. Acta Agric. Boreali-occidentalis Sin. 2009;18:67–73. (in Chinese) [Google Scholar]
- Sun J.X. Nanjing Agricultural University; Nanjing: 2004. Analysis and assessment of chilled pork color and its color stability. PhD Diss. (in Chinese) [Google Scholar]
- Tang M.Y., Ma Q.G., Chen X.D., Ji C. Effects of dietary metabolizable energy and lysine on carcass characteristics and meat quality in Arbor Acres broilers. Asian Austral. J. Anim. 2007;20:1865–1873. [Google Scholar]
- Wang J.Q. Effect of Maternal Dietary Protein Level on Characteristics of Skeletal Muscle in Weaning and Finishing Meishan Pigs and Its Epigenetic Mechanisms Involved, 2011, Nanjing Agricultural University; Nanjing, PhD Diss. (in Chinese).
- Wang X.P. Research on the Regulation Mechanism of Lipid Formation in Pigeon Milk, 2018, Zhejiang University; Zhejiang, PhD Diss. (in Chinese).
- Wang Z., Hu Z.H., Li S.T., Gui X.E., Feng S.B., Li Y., Wang X.C., Li J.C., Wu J.J. Study on serum metabonomics of goslings with hyperuricemia induced by high protein diet. J. Nanjing Agric. Univ. 2021;44:1169–1176. (in Chinese) [Google Scholar]
- Wang F., Li F.H., Zhang S.J., Chen Y., Zhu X.J., Guo K.J. Study on the energy and protein requirements of meat pigeon under 2+3 feeding pattern. J. Beijing Univ. Agric. 2018;33:58–63. (in Chinese) [Google Scholar]
- Wen Y.Y., Liu H.H., Liu K., Cao H.Y., Mao H.G., Dong X.Y., Yin Z.Z. Analysis of the physical meat quality in partridge (Alectoris chukar) and its relationship with intramuscular fat. Poult. Sci. 2020;99:1225–1231. doi: 10.1016/j.psj.2019.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G., Bryant M.M., Voitle R.A., Roland D.A. Effect of dietary energy on performance and egg composition of Bovans White and Dekalb White hens during phase I. Poult. Sci. 2005;84:1610–1615. doi: 10.1093/ps/84.10.1610. [DOI] [PubMed] [Google Scholar]
- Wu S., Jiang B.Y., Song Z.H., Hou D.X., Shi S.R., He X. Effects of botanical polyphenol on antioxidant capacity, intestinal morphology and meat quality of yellow broilers. Chin. J. Anim. Nutr. 2018;30:5118–5126. (in Chinese) [Google Scholar]
- Xia W.G., Abouelezz K.F.M., Fouad A.M., Chen W., Ruan D., Wang S., Azzam M.M.M., Luo X., Fan Q.L., Zhang Y.N., Zheng C.T. Productivity, reproductive performance, and fat deposition of laying duck breeders in response to concentrations of dietary energy and protein. Poult. Sci. 2019;98:3729–3738. doi: 10.3382/ps/pez061. [DOI] [PubMed] [Google Scholar]
- Xie W.Y., Fu Z., Pan N.X., Yan H.C., Wang X.Q., Gao C.Q. Leucine promotes the growth of squabs by increasing crop milk protein synthesis through the TOR signaling pathway in the domestic pigeon (Columba livia) Poult. Sci. 2019;98:5514–5524. doi: 10.3382/ps/pez296. [DOI] [PubMed] [Google Scholar]
- Ye C., Lǚ X.C., Ji S.B., Luo G., Li A. Effect of the meal with different ME and CP on growth performance and blood biochemistry indexes in 28 to 56-day-old pigeons. Chin. Poult. 2015;37:26–30. (in Chinese) [Google Scholar]
- Zeng Q.F., Cherry P., Doster A., Murdoch R., Adeola O., Applegate T.J. Effect of dietary energy and protein content on growth and carcass traits of Pekin ducks. Poult. Sci. 2015;94:384–394. doi: 10.3382/ps/peu069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C., Luo J.Q., Yu B., Zheng P., Huang Z.Q., Mao X.B., He J., Yu J., Chen J.L., Chen D.W. Dietary resveratrol supplementation improves meat quality of finishing pigs through changing muscle fiber characteristics and antioxidative status. Meat Sci. 2015;102:15–21. doi: 10.1016/j.meatsci.2014.11.014. [DOI] [PubMed] [Google Scholar]
- Zhang R., Ma H., Han P., Li Y., Sun Y., Yuan J., Wang Y., Ni A., Zong Y., Bian S., Zhao J., Chen J. Effects of feed systems on growth performance, carcass characteristics, organ index, and serum biochemical parameters of pigeon. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2022.102224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y.N., Wang S., Deng Y.Z., Huang X.B., Li K.C., Chen W., Ruan D., Xia W.G., Wang S.L., Zheng C.T. The application of reduced dietary crude protein levels supplemented with additional amino acids in laying ducks. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Z., Gu C., Hu S., Li B., Zeng X., Yin J. Dietary guanidinoacetic acid supplementation improved carcass characteristics, meat quality and muscle fibre traits in growing-finishing gilts. J. Anim. Physiol. Anim. Nutr. (Berl.) 2020;104:1454–1461. doi: 10.1111/jpn.13410. [DOI] [PubMed] [Google Scholar]
- Zhu L.H., Xiao C.F., Hou H.B., Lǚ W.W., Zhao W.M., Li F.H., Yang C.S. Effects of different protein levels on growth performance, slaughter performance and meat quality of Tianshan snow squabs. Chin. J. Anim. Nutr. 2021;57:173–178. (in Chinese) [Google Scholar]