Abstract
Oxidative stress and oxidative protein damage occur in various biological processes and diseases. The carbonyl group on amino acid side chains is the most widely used protein oxidation biomarker. Carbonyl groups are commonly detected indirectly through their reaction with 2,4-dinitrophenylhydrazine (DNPH) and subsequent labeling with an anti-DNP antibody. However, the DNPH immunoblotting method lacks protocol standardization, exhibits technical bias, and has low reliability. To overcome these shortcomings, we have developed a new blotting method in which the carbonyl group reacts with the biotin-aminooxy probe to form a chemically stable oxime bond. The reaction speed and the extent of the carbonyl group derivatization are increased by adding a p-phenylenediamine (pPDA) catalyst under neutral pH conditions. These improvements are crucial since they ensure that the carbonyl derivatization reaction reaches a plateau within hours and increases the sensitivity and robustness of protein carbonyl detection. Furthermore, derivatization under pH-neutral conditions facilitates a good SDS-PAGE protein migration pattern, avoids protein loss by acidic precipitation, and is directly compatible with protein immunoprecipitation. This work describes the new Oxime blot method and demonstrates its use in detecting protein carbonylation in complex matrices from diverse biological samples.
Keywords: Oxidative stress; Carbonylated proteins' western blot; 2,4 dinitrophenylhydrazine (DNPH); Age-related diseases; Alpha-synuclein; Biotin-aminooxy
Graphical abstract
Highlights
-
•
Optimized protein carbonyl derivatization protocol.
-
•
A simple, versatile, and reliable method, suitable for detecting protein oxidation in a range of biological matrices.
-
•
Detection of low-abundance protein carbonylation following immunoprecipitation.
1. Introduction
Life under aerobic conditions provides the benefit of efficient energy (ATP) production but adds the risk of harmful effects from constant exposure to oxygen radicals and their related oxidants. Reactive oxygen species (ROS) are short-lived, chemically reactive, oxygen-containing molecules that are typically by-products of cellular metabolism but that can also be generated under the influence of external factors such as ionizing and ultraviolet (UV) radiation, heat, cigarette smoking, alcohol, ischemia-reperfusion injury, nonsteroidal anti-inflammatory drugs, chronic infections, and inflammatory disorders [1,2]. Under physiological levels of ROS, cysteine, and methionine residues in proteins can be reversibly oxidized. These reversible oxidative post-translational modifications (PTMs) play an essential role in the redox signaling pathways that regulate various physiological processes such as signal transduction, cell cycling, gene expression, and cellular homeostasis [[3], [4], [5]]. Under normal conditions, published protein carbonyl content in E. coli was 3,0 nmol/mg [6], in recombinant bovine serum albumin (BSA) 1,5 nmol/mg [7], and in plasma from 0,7 to 2,4 nmol/mg [[8], [9], [10]]. The protein carbonyl content in various tissues varies from 0,2 to 0,4 nmol/mg in the brain, and about 10 to 30 nmol/mg in neutrophils. To ensure that ROS levels are maintained within the normal range, cells use several enzymatic antioxidant defense systems such as superoxide dismutase, glutathione peroxidase, peroxiredoxins, and catalase, as well as vitamins A, C, E, uric acid, ceruloplasmin, and glutathione as non-enzymatic antioxidants [11].
Pronounced oxidative stress, a state in which the production of ROS is much greater than the cellular ROS scavenging capacity, induces the irreversible oxidation of cellular and extracellular proteins, lipids, carbohydrates, metabolites, and nucleic acids, thus damaging their function. Therefore, an effective oxidative damage repair system is essential to fix the resulting biological damage and maintain cellular homeostasis. While the repair of mild oxidative damage by reductive processes is possible [12], extensively oxidized molecules are, in most cases, enzymatically degraded. Damaged proteins are degraded by the 20S and 26S proteasomes or lysosomal autophagy pathways. Therefore, the level of oxidative damage to the cell is determined by the opposing effects of free radicals production and the efficacy of antioxidant protection, repair, molecular clean-up, and turnover systems. It is widely accepted that the accumulation of oxidatively modified macromolecules contributes to cellular damage, aging, and initiation and progression of age-related diseases (ARDs) [[13], [14], [15], [16]].
The incidence of ARDs, such as cardiovascular diseases, type 2 diabetes, cancer, arthritis, cataracts, and neurodegenerative diseases, increases exponentially with age [17,18]. Proteins, directly or indirectly, perform all biological functions, and cumulative proteome damages might be the prime contributing factor in aging and ARDs [1]. It is known that the proteostatic capacity declines during aging, resulting in the accumulation of damaged proteins [19]. The increase of either proteasome activity [20,21] or the autophagic-lysosomal pathway [22] extends lifespan and protects model organisms from the development of ARDs [23]. Additionally, minor proteostatic disturbances can be amplified through a positive loop mechanism in which proteome damages accumulate with time since they are promoted by aging and ARDs that exhibit exponential growth [17]. Furthermore, the clinical significance of proteome damage was established by the detection of high levels of protein oxidation, especially protein carbonylation, in various ARDs such as Parkinson's disease [24], type 1 and type 2 diabetes [25,26], heart failure [27], cardiomyopathy [28], arterial hypertension [29], chronic renal failure [30,31], Alzheimer's disease [32,33], amyotrophic lateral sclerosis [34], rheumatoid arthritis [35], sepsis [36], myelodysplastic conditions [37], breast cancer risk [38,39], pancreatitis [40], and acute respiratory distress syndrome [41,42].
Carbonylation is one of proteins' most harmful, irreversible, non-enzymatic modifications. It consists of chemically diverse changes in which oxidative stress provokes the generation of reactive ketones and aldehydes on susceptible amino acids. It is induced directly by the oxidation of the amino acid side chains and protein backbones or indirectly by adding secondary oxidation products such as advanced lipoxidation or glycation end-products [43]. The exact mechanisms by which protein oxidation leads to ARDs are not fully understood. However, oxidative modifications to proteins can change their physicochemical properties, such as their solubility, structure, susceptibility to autophagy, ubiquitination, and proteasome-mediated degradation. These changes can lead to the loss or gain of enzyme activity, loss of protease inhibitor activity, protein aggregation, abnormal cellular uptake, change in protein-protein interactions, modification in gene transcription, and increase or induction of immunogenicity to endogenous proteins. All these changes could contribute to the development of ARDs [44].
Measuring and validating an individual protein's carbonylation is essential for understanding its role in a particular biological setting [45,46]. A bioconjugation reaction using DNPH is the most commonly used method to detect and measure protein carbonylation. It is an imine-based reaction that forms hydrazone bonds between the DNPH and the proteins' carbonyl (aldehyde or ketone) groups, followed by the detection of DNP moieties with an anti-DNP antibody [47]. This reaction is frequently detected by an enzyme-linked immunosorbent assay (ELISA) or a blot analysis [48]. However, detecting a given protein carbonylation by DNPH-based blotting is challenging due to the poor solubility of DNPH and its instability in solution. Strong acids are required to solubilize the DNPH, leading to acidic protein precipitation. The attempts to replace the trifluoroacetic acid (TFA) solubilization agent with 2 M hydrochloric acid (HCl) severely diminished the methods' sensitivity due to incomplete DNPH solubilization. A pH adjustment is necessary before the sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), which leads to an increased salt concentration that can distort protein migration patterns. Additionally, the solubilization of DNPH in TFA should be done immediately before the derivatization procedure due to its rapid degradation [49]. These issues can lead to limited derivatization of the carbonyl groups, inadequate representation of the low-abundance proteins, and distorted protein migration patterns demonstrating the questionable reliability of the DNPH-based detection method [50]. Thus, there is a need to develop a robust and reliable method for detecting protein carbonylation by immunoblotting.
While DNPH and aminooxy-containing reagents react with carbonyl groups and form stable complexes through a similar chemical reaction, the oxime bonds formed by the aminooxy groups are considerably more stable towards hydrolysis than the hydrazone bonds formed by DNPH (Fig. S1) [51]. Furthermore, aminooxy-containing reagents have higher reaction yields, but their reaction with carbonyls at neutral pH is relatively slow. However, this reaction can be accelerated by the addition of catalysts [52] such as anilines and substituted anilines (e.g., m-phenylenediamine, p-phenylenediamine (pPDA)) [[53], [54], [55]], to allow that the derivatization reaction reaches a plateau within hours and ensure consistent and reliable derivatization of the carbonylated proteins.
The above-described gaps in protein carbonyl detection prompted us to test the suitability of the aminooxy probe to detect protein carbonylation by immunoblotting in various experimental systems, such as bacteria, eukaryotic cell lines, and plasma, as well as its compatibility with different protein extraction buffers. We show that the aminooxy-based derivatization in the presence of the pPDA catalyst at a neutral pH enables reliable and sensitive detection of protein carbonylation with good protein separation patterns. Also, our method allows the immunoprecipitation of low-abundance proteins with subsequent detection of their expression and carbonylation level without additional cell lysate manipulation. We exemplify such a procedure by quantifying the carbonylation level of the α-synuclein (α-Syn) protein, which plays a vital role in the pathogenesis of Parkinson's disease [[56], [57], [58]]. Since we used a biotin-aminooxy probe, we named our approach the “Oxime blot method."
2. Material and methods
2.1. Chemicals
Ammonium persulfate (APS), bromophenol blue, CelLytic™ M, dimethyl sulfoxide (DMSO), 2,4-dinitrophenylhydrazine (DNPH), rabbit polyclonal anti-DNP antibody, glutaraldehyde, hydroxylamine hydrochloride (NH2OH), p-phenylenediamine (pPDA), paraformaldehyde, Ponceau S, sodium borohydride, sodium deoxycholate, trifluoroacetic acid (TFA), tris(hydroxymethyl)aminomethane (Tris–base), and Dulbecco's Modified Eagle's medium (DMEM) containing sodium pyruvate were purchased from Sigma Aldrich GmbH (Taufkirchen, Germany). Dithiothreitol (DTT), glycerol, disodium hydrogen phosphate, sodium dihydrogen phosphate, sodium dodecyl sulfate (SDS), and urea were obtained from SERVA Electrophoresis GmbH (Heidelberg, Germany). Biotin hydrazide and biotin aminooxy (EZ-Link™ alkoxyamine-PEG4-biotin), ethylenediaminetetraacetic acid (EDTA), Halt™ protease inhibitor cocktail, goat anti-rabbit IgG Alexa Fluor®700 antibody, goat anti-rabbit IgG horseradish peroxidase (HRP)-conjugated antibody, nonyl phenoxypolyethoxylethanol (NP-40), streptavidin-AlexaFluor®700, streptavidin-HRP, and Tween 20 were purchased from Thermo Fisher Scientific, Inc. (Karlsruhe, Germany). Ethanol, hydrochloric acid, magnesium sulfate, sodium acetate, sodium chloride (NaCl), and sodium hydroxide were obtained from Kemika (Zagreb, Croatia). Western blotting luminol reagent was purchased from Santa Cruz Biotechnology (Dallas, TX, USA). Acrylamide/bis-acrylamide 40% and protein A magnetic beads (SureBeads™) were obtained from Bio-Rad Laboratories (Hercules, CA, USA). N,N,N′,N′-tetramethylethylenediamine (TEMED), powdered milk, and bovine serum albumin fraction V were purchased from Carl Roth GmbH & Co (Karlsruhe, Germany). Fetal bovine serum (FBS), trypsin, and penicillin/streptomycin antibiotic mixture were purchased from Capricorn Scientific GmbH (Ebsdorfergrund, Germany). Anti-α-Syn monoclonal antibody (clone EPR20535) was purchased from Abcam (Cambridge, UK). Ficoll-Paque® Plus was purchased from Cytiva Europe GmbH (Freiburg, Germany).
2.2. Cell cultures and isolation of red blood cells
E. coli MG1655 cells were grown exponentially in 200 mL of lysogeny broth (LB – Miller's formulation) media at 37 °C with shaking (250 rpm) until reaching an OD600nm of 0.4, resulting in 4–8 x1010 bacteria per batch. Cells were collected, pelleted, and washed three times with phosphate-buffered saline (PBS) before lysis.
Expi293F™ cells (Thermo Fisher Scientific), a Human Embryonic Kidney 293 (HEK 293) cell line optimized for high-yield protein expression, were grown in 200 mL of Expi293 Expression Medium (Thermo Fisher Scientific) in 1 L Erlenmeyer flasks at 37 °C with agitation at 100 rpm in an 8% CO2 atmosphere. Cells were collected, pelleted, and washed twice with PBS before lysis.
Michigan Cancer Foundation-7 (MCF-7), an estrogen and progesterone receptor-positive human breast cancer cell line, was grown in DMEM, which contains sodium pyruvate and is supplemented with 10% heat-inactivated FBS and penicillin/streptomycin (100 μg/mL) in a humidified atmosphere with 5% CO2 at 37 °C. Cells were collected by trypsinization and washed with PBS twice.
The blood from a healthy volunteer was collected by venipuncture into BD tubes containing citrate anticoagulant. Red blood cells (RBC) were isolated after centrifugation on Ficoll®-Paque Plus according to the manufacturer's instructions, washed twice with PBS, and pelleted.
2.3. Protein extraction
Bacterial proteins were extracted by resuspending the bacterial pellet in 3 mL of the phosphate radioimmunoprecipitation (RIPA) lysis buffer (10 mM sodium phosphate - pH 7.5, 150 mM NaCl, 1% NP-40, and 0.1% sodium deoxycholate), with phosphatase and protease inhibitors (10 mM sodium fluoride and Halt™ protease inhibitor cocktail) added.
HEK 293 cells (107) were lysed in 250 μL of CelLytic™ M lysis buffer containing Halt™ protease inhibitor cocktail.
MCF-7 cells were lysed in two cell pellet volumes of 8 M urea in 10 mM phosphate buffer (pH 7.5).
RBC pellets were lysed in 3 pellet volumes of phosphate RIPA supplemented with a Halt™ protease inhibitor cocktail.
Cell lysates were incubated on a thermomixer at 4 °C at 500 rpm for 30 min, with additional periodic mixing by pipetting. Cell lysates were cleared of insoluble proteins and debris by centrifugation at 15,000×g for 15 min at 4 °C. Protein concentration was quantified using a BCA assay (Thermo Fisher Scientific) according to the manufacturer's instructions.
2.4. Plasma protein preparation
Peripheral blood was collected from healthy volunteers in BD Vacutainer® EDTA plasma collection tubes. The tubes were centrifuged at 1100×g for 10 min at room temperature (RT). The plasma was diluted 10 x with a 10 mM phosphate buffer (pH 7) supplemented with a Halt™ protease inhibitor cocktail and incubated at RT with shaking at 500 rpm for 10 min.
2.5. Oxidation by UV irradiation
Cell lysates with a 5 mg/mL protein concentration were oxidized with UV irradiation doses ranging from 0.25 J/cm2 to 3 J/cm2 in an irradiation chamber BS-02 (Opsytec Dr. Gröbel GmbH, Ettlingen, Germany) with a 1:2 ratio of UVC (254 nm) and UVB (280–315 nm).
2.6. Immunoprecipitation of carbonylated α-Syn
Cleared RBC lysates corresponding to 1 mL of blood were placed in plastic 3 mL Petri dish plates and irradiated with total UV doses of 0.75 J/cm2 and 1.5 J/cm2, respectively. The samples were then transferred to a 2 mL microcentrifuge tube and incubated overnight at 4 °C with rotation with 3 μg of anti-α-Syn monoclonal antibody (Abcam, AB212184). The antibody-protein complex was captured by adding 50 μL of protein A to magnetic beads (SureBeads™, BioRad) for 3 h with rotation at 4 °C. The beads were washed three times with phosphate-buffered saline containing 0.05% Tween (PBS-T), and the carbonylated α-Syn was derivatized and blotted as described below.
2.7. Carbonyl labeling
Carbonyl labeling of purified protein or cell lysates was performed in phosphate buffer pH 7 at 5 mg/mL protein concentration. HEPES, PIPES, or MOPS buffers could also be used, but their suitability was not formally tested. For the carbonyl labeling with biotin-aminooxy, biotin-hydrazide, or DNPH, it is crucial to avoid the Tris buffer or other buffers containing primary amines, as these buffers will quench the reaction and prevent carbonyl labeling. Furthermore, the reaction buffers or reagents should not have carbonyl groups (aldehydes or ketones) since they would act as competitive inhibitors in the derivatization reaction.
Samples were derivatized with the DNPH probe as described previously [49]. Briefly, the initial volume of the lysate was diluted with an equal volume of 12% SDS solution (1:1 ratio), as it is crucial to have at least 6% of SDS before adding the TFA. The 200 mM DNPH stock solution in TFA was diluted 10 times with distilled water (1:9 ratio). The derivatization was initiated by adding an equal volume of diluted DNPH to the sample (1:1 ratio), resulting in a 10 mM final concentration of DNPH, and incubated in a thermomixer at 25 °C and 500 rpm for 30 min. The mixture was then neutralized by adding approximately 1.5 derivatization reaction volumes of 2 M Tris/30% glycerol (1:1,5 ratio). When the solution was neutralized, a noticeable change in color from light yellow to orange occurred.
Derivatization with biotin-aminooxy or biotin-hydrazide probes was done by incubating samples in a neutral pH buffer with 10 mM of the probe (from a freshly thawed aliquot of 100 mM stock solution in DMSO; aliquots of biotin-aminooxy are stable for at least 6 months when kept at −20 °C) in the presence of 50 mM of pPDA catalyst. The pPDA has the highest catalytic efficacy at a neutral pH. Its stock solution (0.5 M pPDA in DMSO with 5 mM DTT) was freshly prepared since the pPDA is susceptible to oxidation and inactivation [55]. Samples were derivatized by incubating in a thermomixer at 25 °C and 500 rpm for 3 h. The reaction was terminated by adding an excess of NH2OH (100 mM final) from a freshly prepared 1 M stock solution in DMSO, and the samples were snap-frozen before further analysis.
For derivatization of α-Syn, the SureBeads were resuspended in 8 μL of 10 mM phosphate buffer pH 7, with 1 μL of biotin-aminooxy (100 mM stock in DMSO) and 1 μL of pPDA (0.5 M stock dissolved in DMSO) and incubated in a thermomixer at 25 °C and 500 rpm for 3 h. The reaction was stopped by adding 10 μL of 2x Laemmli sample buffer, and samples were frozen before further analysis.
2.8. Western blot analysis
The protein samples were mixed with 4x Laemmli sample buffer (250 mM Tris-HCl pH 6.8, 40% glycerol (v/v), 8% SDS (w/v), and 0.01% bromophenol blue) and heated at 95 °C for 5 min. Denatured proteins (20 μg/lane) were resolved by 12.5% SDS-polyacrylamide gels, unless otherwise indicated, using the Mini-Protean® Tetra Cell system (Bio-Rad). Proteins were then transferred to 0.22 μm polyvinylidene difluoride (PVDF) membranes (Merck-Millipore) using the Trans-Blot® Turbo™ Transfer system (Bio-Rad) with home-made buffers as described by Jiménez-Soto [59]. For alpha-Syn detection, PVDF membranes were cross-linked with 4% of paraformaldehyde and 0.1% glutaraldehyde in PBS at RT for 30 min. This step is essential for preventing α-Syn detachment from the membrane during subsequent incubations [60]. The proteins on the membranes were visualized after a short incubation in Ponceau S staining buffer. The stained membranes were blocked with 5% powdered milk (w/v) at RT for 30 min with agitation. Blocking and all other incubation steps were done in PBS-T. At each stage after blocking, the membranes were washed four times with PBS-T at RT for 5 min.
To detect carbonylated proteins by the DNPH-based method, membranes were incubated for 1 h with a 1:2000 dilution of rabbit anti-DNP antibody, followed by a 1-h incubation with a 1:2000 dilution of secondary anti-rabbit antibodies conjugated with Alexa Fluor®700 (Alexa700) or HRP (Fig. S2) at RT. For detection by the Oxime blot method, membranes were incubated with a 1:2000 dilution of Alexa700-or HRP-streptavidin (Fig. S2) conjugates. Fluorescent signals were acquired by the Typhoon™ FLA 9500 biomolecular imager (GE Healthcare Life Science). Luminescent signals were measured using photographic films. The membranes were incubated for 1 min in 1:5 diluted Western Blotting Luminol Reagent (Santa Cruz Biotechnology) at RT. After a 30-s exposure, photographic films were developed using a film developer (Fuji medical film processor FPM-100A) and digitized with the BIO-5000 Plus VIS Gel Scanner (SERVA Electrophoresis GmbH).
To detect α-Syn, the membrane was first incubated with a monoclonal anti-α-Syn antibody (1:1000 dilution in PBS-T) at RT for 1 h and then washed three times in PBS-T for 5 min. The membrane was then incubated with anti-rabbit-HRP (1:2000 dilution in PBS-T) secondary antibody at RT for 1 h and then washed three times in PBS-T for 5 min. Membranes’ luminescence was then detected, as described in the previous paragraph.
2.9. Quantification and statistical analysis
Quantifications were performed by densitometric analysis using Image Lab software (Bio-Rad), except for quantification in Fig. 1, which was performed using TotalLab Quant software (TotalLab, Newcastle-Upon-Tyne, UK). The differences in the sample loading were corrected using Coomassie-stained gel or the Ponceau S-stained membrane, as indicated. All data are representative of three independent experiments and shown as the standard error of the mean (±SEM). One-way analysis of variance (ANOVA) was performed for all experiments that require a comparison between three or more groups within one categorical variable followed by a Dunnett's or Tuckey's post hoc test as indicated. A paired t-test was used to compare signal and blank for every quantity in Fig. 2 and S3. In all analyses, significance was accepted with *p < 0.05, **p < 0.01, ***p < 0.01, and ****p < 0.0001. All statistical calculations and graphical illustrations were performed using GraphPad Prism 8.4.3 software (GraphPad Software Inc, CA).
Fig. 1.
Characterization of Oxime blot derivatization reaction and detection of carbonylated proteins in E. coli by different blotting methods.
(A) Quantification of carbonyl derivatization kinetics. Data from panels B to F and one additional experiment were analyzed. Data are presented as mean ± SEM. (B–F) Oxime blot analysis of the kinetics of E. coli protein carbonyl derivatization using 10 mM of the biotin-aminooxy probe in the presence of the indicated pPDA concentration. Blank (Blk) corresponds to the sample incubated in the same buffer as the other samples without the biotin aminooxy probe. (G) Ponceau S staining of the transferred proteins. (H) Detection of carbonylated proteins derivatized by DNPH, biotin-hydrazide, or biotin-aminooxy probes in the presence or absence of the catalyst (pPDA) as indicated. (I) Quantification of the relative carbonyl levels was obtained by dividing the total signal intensity of the carbonyl content in panel (H) by the level of total signal intensity of the proteins in panel (G). The graph represents the quantification of one representative experiment. The relative m. w. (kDa) is indicated on the left side of each blot (B–H).
Fig. 2.
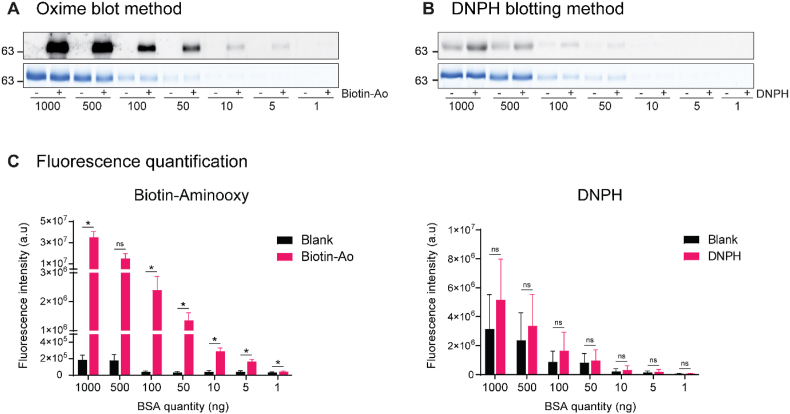
Evaluation of the specificity of the Oxime and DNPH blotting methods using fluorescence detection and BSA as a model protein.
Fluorescence imaging was used to detect BSA carbonylation using the Oxime (A) and DNPH (B) blotting methods. Decreasing amounts of BSA were resolved in parallel gels by SDS-PAGE. For each method, one gel was stained with Coomassie blue for total protein content (A and B, the lower panels) while the proteins from the other gel were transferred onto a PVDF membrane and analyzed for carbonylation (A and B, the upper panels, respectively). Minus (−) is blank in panels A and B and corresponds to the BSA detected without the carbonyl derivatization probe. The relative m. w. (kDa) is indicated on the left side. (C) Quantification of the fluorescent signal intensity of the bands for carbonylation detected by the Oxime or DNPH blotting method is displayed below each panel. Data are presented as mean ± SEM (n = 3 independent experiments). The statistical significance between the blank and the labeled samples was calculated using a paired t-test. *p < 0,05, and ns, not significant. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
3. Results
3.1. Detection of protein carbonylation by oxime blot
To overcome the limitations of the DNPH-based blotting method for the detection of protein carbonylation, we developed a new approach where the protein carbonyls were derivatized by a biotin-aminooxy probe in the presence of the pPDA catalyst at a neutral pH. We took advantage of the presence of the carbonylated proteins in E. coli lysate under normal growing conditions [6]. A range of pPDA concentrations and incubation times were tested, and the optimal conditions were determined by a blotting approach (Fig. 1A–F). Upon derivatization, the proteins were resolved by SDS-PAGE, transferred to a PVDF membrane, and stained with Ponceau S (Fig. S2). The derivatized proteins were detected with a fluorescent streptavidin conjugate (Fig. 1B–F). The pPDA catalyst increased protein carbonyl derivatization with the biotin-aminooxy probe in a dose- and time-dependent manner in the 10 mM–50 mM concentration range (Fig. 1B–E). However, when the catalyst concentration was further increased to 100 mM, the carbonyl derivatization was significantly reduced (Fig. 1A, F). The derivatization reaction reached a near-maximal intensity after 3 h of incubation with 50 mM pPDA, with minimal gains thereafter. These conditions were used in all subsequent derivatization experiments with biotin-hydrazide and -aminooxy probes.
Next, we compared the performances of DNPH, biotin-hydrazide, and biotin-aminooxy in detecting protein carbonyls in E. coli lysates (Fig. 1G–I). Ponceau S staining revealed significant differences in protein band intensities and migration patterns (Fig. 1G). The most striking finding was that the DNPH derivatization led to a loss of about 70% of the total proteins and distortion and streaking in the protein migration pattern. In contrast, the quantity and migration patterns of proteins derivatized with hydrazide- or biotin-aminooxy probes were excellent, with no discernible differences between samples. The membrane was then cut to separately process the DNPH and biotin-hydrazide/aminooxy derivatized samples with their specific detection methods using streptavidin conjugate (for biotin derivatization) or secondary antibody (for DNPH The most abundant carbonyl signal was observed in samples derivatized with the biotin-aminooxy probe catalyzed with the pPDA. The detection of the carbonylated proteins by the biotin-hydrazide probe was lower, with little to no benefit from the catalyst addition. The weakest protein carbonyl signal was observed following derivatization with DNPH (Fig. 1H). Two bands with an apparent molecular weight (m.w.) of around 18 kDa were observed in the non-derivatized samples when the streptavidin-Alexa700 conjugate was used with the biotin-hydrazide or aminooxy probe systems. In contrast, several faint non-specific bands were detected in the DNPH-derivatized samples labeled with the secondary antibody-Alexa700 conjugate. The proteins observed in the non-derivatized samples could be related to the biotin carboxyl carrier protein (BCCP), a subunit of the acetyl-CoA carboxyl, the only known endogenous E. coli protein that binds streptavidin conjugates [61]. These false positive proteins are not seen with other samples analyzed in this study.
The carbonylated proteins detected by these methods were quantified and corrected for protein loading (Fig. 1I). Biotin-hydrazide- and biotin-aminooxy-based derivatization yielded 9- and 33-times higher relative carbonylation signals than DNPH. Using the catalyst with the biotin-aminooxy-based derivatization increased the signal by an additional 60%. These results demonstrate that derivatization of the carbonyls with a biotin-aminooxy probe catalyzed with pPDA is superior to the DNPH method for assessing protein carbonylation by western blotting.
3.2. Specificity of aminooxy-pPDA and DNPH-based carbonyl detection methods
Specificity of the Oxime- and the DNPH-blotting methods were compared using purified BSA as a model protein (Fig. 2). Decreasing amounts of BSA derivatized by biotin-aminooxy (Fig. 2A), or DNPH probes (Fig. 2B) were loaded and resolved by 1D SDS-PAGE. The protein was either stained with Coomassie blue G250 (Fig. 2A and B, lower panel) or transferred to a PVDF membrane and analyzed for carbonylation by blotting using either fluorescence (Fig. 2A and B, upper panel) or luminescence (Fig. S3) detection. The whole gel images are shown in Fig. S4. The Coomassie blue staining revealed similar protein quantities in both derivatized and non-derivatized samples, confirming that the BSA carbonyl derivatization did not impact the protein yield.
The BSA carbonylation level was detected by either fluorescence (Fig. 2) or luminescence (Fig. S3). The non-derivatized proteins labeled “blank” were used to test for any non-specific signal. While the reaction of BSA with the antibody detection system used in the DNPH method was easily noticeable (Fig. 2B and S3B), there was practically no reactivity with the blank samples in the Oxime blot method (Fig. 2A andS3A). Furthermore, the fluorescence carbonylation signal was not visible below 50 ng of BSA with the DNPH method (Fig. 2B) but was stronger and visible even at 5 ng of BSA with the Oxime blot method (Fig. 2A). The carbonyl signals for the derivatized and blank samples were quantified for the fluorescence and luminescence detection systems and are presented on the bottom panels of Fig. 2C and S3C, respectively. The non-specific signal observed with the fluorescence detection method, determined as the ratio between non-derivatized and derivatized protein samples, was 61% for the DNPH method and only 0.5% for the Oxime blot method for the condition using 1 μg of BSA. Similar values of these non-specific signals were observed with lower protein quantities. The best signal-to-noise ratios of the Oxime blot method were not dependent on the nature of the detection method since similar results were obtained with luminescence detection (Fig. S3), where the non-specific signal with the DNPH method was 72% and only 2.9% for the Oxime blot method when using 1 μg of BSA. These results demonstrate that the Oxime blot method has greater specificity and a lower detection limit than the DNPH method.
3.3. Detection of carbonylated proteins in various biological systems
A reliable method for protein carbonylation detection is a vital tool in many fields of biology and medicine. Since protein extracts can be produced in various ways and from multiple biological sources, we tested the compatibility of different frequently used extraction buffers with the Oxime blot method in bacterial and eukaryotic systems and models [62] and the ability of the Oxime blot method to detect UV-induced protein carbonylation.
Firstly, the detection of the UV-induced protein carbonylation inE. coli cells lysed in a modified RIPA buffer made with phosphate instead of Tris buffer was tested (Fig. 3A). Membrane staining with Ponceau S revealed well-separated and uniform protein bands in all lanes (Fig. 3A, right panel). Aside from the two faint and non-specific bands in the blank sample that were not derivatized with the biotin-aminooxy probe (Fig. 3A, left panel), a marked UV dose-dependent increase in the total protein carbonyl signal intensity and excellent protein resolution were observed. The quantification of the relative carbonyl levels from 5 independent experiments revealed a 166% and a 362% increase in the total protein carbonylation with 0.25 and 1.25 J/cm2 of UV irradiation, respectively (Fig. 3B).
Fig. 3.
Detection of endogenous and UV-induced protein carbonylation by the Oxime blot method.
Carbonylation (left) and Ponceau S (right) staining of (A)E. coli or (C) HEK 293 lysates exposed to 0.25 and 1.25 J/cm2 or 0.5, 1, and 3 J/cm2 of UV, respectively, as indicated. Blank (Blk) corresponds to the non-irradiated sample incubated in the same buffer as the other samples without the biotin-aminooxy probe. The control samples (0) correspond to the derivatized, non-irradiated samples. The relative m. w. (kDa) is indicated on the left side. Bar graphs for E. coli(B) and HEK293 (D) represent the total lane fluorescence carbonylation signal normalized with the corresponding Ponceau S signal. Data are expressed as a percentage of the control for each replicate. Data are presented as mean ± SEM (n = 5 independent experiments (B) and n = 4 independent experiments (D)). One-way ANOVA was performed for both experiments, followed by Dunnett's post hoc test. *p < 0.05, **p < 0.01 and ****p < 0.0001.
Additionally, we chose a HEK 293 subline, the Expi293F™, lysed in commercially available CelLytic M buffer (Fig. 3C), as a second model due to its wide use for recombinant protein expression and production. The Expi293F™ cell lysates were subjected to oxidative stress via UV irradiation at various doses, and the Oxime blot protocol was performed. The Ponceau S staining revealed well-resolved proteins in both derivatized and non-derivatized samples with similar intensities, indicating no derivatization-induced protein loss. No carbonylated protein bands were visible in the blank (non-derivatized sample). A strong protein carbonyl signal was observed, and the carbonylated proteins were well separated. The protein carbonylation increased dose-dependently, with 17%, 26%, and 45% increases at 0.5, 1.0, and 3.0 J/cm2 of UV irradiation, respectively (Fig. 3D). The marked, dose-dependent increase of well-resolved carbonylated proteins indicates the suitability of the Oxime blot method for detecting carbonylated proteins in the eukaryotic cell system as well.
Lastly, we extended this study by including the MCF-7 cell line and plasma, as it is one of the most frequently used specimens in the study of the link between protein oxidation and age-related diseases (Fig. S5). As before, only a negligible carbonylation signal was detected in blank samples. Well-resolved spontaneous carbonylation signals were detected in both MCF-7 and plasma specimens.
3.4. Immunoprecipitation of carbonylated α-Syn
Measuring carbonylation in low-abundance proteins isolated by immunoprecipitation from a complex specimen is challenging. To evaluate the suitability of the Oxime blot method for such purposes, we tested its ability to detect basal levels and UV-induced carbonylation of α-Syn after immunoprecipitation from RBC lysates (Fig. 4A). Following RBC protein extraction, cell lysates were exposed to increasing doses of UV irradiation, and α-Syn was immunoprecipitated. The protein samples were derivatized, divided, and analyzed in two separate gels that were run in parallel. The first gel was stained with Coomassie, and the bands corresponding to the α-Syn protein had a similar intensity, just below 17 kDa. The other gel was blotted to measure the carbonylation level and α-Syn protein expression. While UV irradiation could induce photodegradation of proteins [[63], [64], [65]], oxidation [[66], [67], [68]] or fragmentation [69] of α-Syn causes its cross-linking and aggregation [[66], [67], [68], [69]]. The whole gel images are shown in Fig. 4A, changes in the monomeric form of α-Syn are shown in Fig. 4B and their quantification in Fig. 4C. Putative oligomers and truncated carbonylated α-Syn are readily seen. The monomeric α-Syn protein carbonylation signal analysis revealed a marked UV-induced increase in α-Syn carbonylation. Quantifying the α-Syn carbonylation band relative to its expression showed an increase of 37% and 56% in samples irradiated with 0.75 and 1.5 J/cm2 doses of UV compared to the physiological α-Syn carbonylation level in the non-irradiated sample (Fig. 4C). Therefore, the Oxime blot method detects carbonylation of endogenous α-Syn protein immunoprecipitated from RBC and could be used to study the oxidative damage of low-abundance proteins.
Fig. 4.
Immunoprecipitation of UV carbonylated α-Syn from RBC.
(A) Human RBC lysates were exposed to the indicated UV doses, and α-Syn was immunoprecipitated. The samples were resolved on parallel SDS-PAGE gels. One gel was stained with Coomassie blue (left panel), and the other was transferred to a PVDF membrane and probed for the detection of the carbonylation signal by Oxime blot (middle panel) and the α-Syn expression (right panel). The positions of α-Syn monomer and oligomers species are indicated on the right side. The relative m. w. (kDa) is indicated on the left side. (B) Bands corresponding to the α-Syn monomer from (A). (C) Quantification of the monomeric α-Syn carbonylation. The α-Syn band carbonyl signal intensity from the middle panel was normalized using the α-Syn expression band signal intensity from the bottom panel. Data are presented as a mean ± SEM (n = 3 independent experiments). One-way ANOVA was performed, followed by Dunnett's post hoc test. **p < 0.01 and ***p < 0.001. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
4. Discussion
Protein carbonylation is the most widely used marker of oxidative stress. While DNPH-based spectrophotometric and ELISA methods are widely used for quantifying oxidative damage, they cannot effectively determine the carbonylation of an individual protein, which is necessary to understand the role of oxidative damage in a particular biological setting [45,46]. Western blot is ideally suited for such purposes due to its widespread accessibility, versatility, and relatively low cost. Unfortunately, DNPH-based immunoblotting is rarely used to determine the carbonylation of an individual protein due to the numerous issues associated with its use. The low pH of the DNPH derivatization mix leads to massive protein loss. Furthermore, the increased salt concentration required for reaction mix neutralization alters the protein migration pattern. The presence of reducing agents such as DTT in some protocols and the low pH and high salt concentration from the DNPH derivatization interfere with carbonylated proteins' immunoprecipitation and prevent its efficient use for the characterization of individual protein carbonylation. These issues lead to the suboptimal reliability and reproducibility of the DNPH-based detection of oxidized proteins by immunoblotting [50]. Therefore, there is a need for a new method that would enable sensitive, specific, and reliable detection of protein carbonylation in diverse biological systems.
By combining the favorable physicochemical properties of the biotin-aminooxy with the pPDA-catalyzed derivatization of reactive carbonyl groups, we resolved the most significant issues associated with the DNPH-based immunoblotting of carbonylated proteins, especially the ones related to protein loss, the quality of the electrophoretic protein separation pattern, the quantity of detected carbonyl, the sensitivity, and the specificity as demonstrated in Fig. 1 G-I. The biotin-aminooxy probe is easily solubilized in DMSO, and the derivatization step is performed at neutral pH in aqueous solutions. Additionally, the probe forms oxime bonds that are considerably more stable against hydrolysis than the hydrazone bonds of DNPH, allowing higher reaction yields that could be further enhanced using the appropriate catalyst [51]. Since an excess of catalyst can block the derivatization reaction [54], it is essential to optimize the probe and the catalyst concentration ratio. When optimizing the aminooxy/pPDA ratio, we observed an increase in the protein carbonyl signal with up to 50 mM of pPDA. The derivatization plateau was reached after 3–6 h of incubation. An increase in catalyst concentration to 100 mM negatively affected the carbonyl derivatization (Fig. 1A–F). For two reasons, we have decided to perform all subsequent experiments using the 3-h derivatization step with 50 mM of pPDA. Doubling the incubation time resulted in a slight increase in protein carbonyl detection. Additionally, a shorter incubation time allows users to complete the Oxime blot protocol, like the DNPH labeling, in about 7 h. These optimized derivatization conditions reduce the effects of slight variations in reagents concentration, pH, temperature, or incubation time on protein oxidation detection. As a result, the Oxime blot method provides very effective, sensitive, and robust detection of carbonylated proteins in biological samples with a 50 times higher carbonyl signal detection than the DNPH method (Fig. 1G–I).
The higher detection, signal intensity, and specificity reflect the additional benefits of the efficient carbonyl derivatization and low intrinsic interaction of the biotin-aminooxy probe with proteins. These characteristics were tested by comparing the detection of carbonylated BSA (Fig. 2 and S3) with the Oxime blot method and the reference DNPH method. Considerable non-specific interaction of antibodies used in the DNPH-based methods was detected in the blank samples, whereas fluorescently or HRP-labeled streptavidin conjugates hardly interacted with the BSA. Combining specific, efficient, and optimized aminooxy-based carbonyl derivatization with a low non-specific protein interaction of conjugated streptavidin resulted in a 12-fold (Fig. S3) to 18-fold (Fig. 2) higher signal-to-blank ratio of the Oxime blot than the DNPH-based immunoblotting method. Reduced non-specific interactions were observed in HEK and MCF-7 eukaryotic cell lines and plasma samples as well (Fig. 3 and S5). However, in E. coli non-derivatized blank samples, two 18–19 kDa bands were observed. Their identity is not known but could correspond to the BCCP isoforms, the only known endogenously biotinylated protein in E. coli [61]. These biotin-binding proteins could easily be depleted using streptavidin beads if required.
The Western blot should reliably detect changes in protein carbonylation in complex biological samples to be suitable for extensive use in biomedical research. The Oxime blot method fulfills these requirements perfectly. It efficiently detects spontaneous and UV-induced protein carbonylation in bacteria and eukaryotic cell lines, as well as carbonylated proteins from plasma (Fig. 3 and S5). Since Oxime blot derivatization time is relatively long, there is a legitimate concern that artificial, metal-catalyzed protein oxidation contributes to the observed results. We add 250 μM of DTT in the sample mix (along with the pPDA reagent) to avoid artifactual oxidation during the derivatization step. If metal-catalyzed oxidation during derivatization time contributes to protein carbonylation, one would expect to see decreased carbonylation with the chelation of metal cations. However, adding EDTA did not affect BSA or E. coli protein's carbonylation level, confirming that the carbonylation observed throughout is not an experimentally induced artifact (Fig. S6). When there is a known or suspected presence of metals, it is prudent to do the Oxime blot derivatization in the presence of metal chelators such as EDTA. Therefore, the Oxime blot method is suitable for investigating oxidative damage in various experimental systems.
Protein carbonylation plays a fundamental role in aging and age-related diseases. The lack of consensus and the stagnation of methodological developments on DNPH-based methods have slowed down the study of protein carbonylation as one of the main factors in the onset of age-related diseases and aging [70]. The development of a method that reliably measures the carbonylation of a particular protein in a relevant biological or pathological context can deepen our understanding of its role in the pathophysiology of aging [45,46,71] and significantly increase the method's applicability. Unfortunately, the carbonylation of a specific protein using DNPH-based Western blot was shown only for a handful of proteins such as albumin, immunoglobulin, α2-macroglobulin, transferrin, and fibrinogen in plasma samples [72,73], heat shock protein 90β (HSP90β), filamin A (FLNA), bifunctional glutamate/proline-tRNA ligase (EPRS) [74], and superoxide dismutase 1 (SOD1) in cell lysates [75]. However, most of these proteins are either abundant or highly susceptible to protein carbonylation, which makes the detection of their carbonylation easy. The protein concentration in plasma is around 60–80 mg/mL [76], of which about 60% are albumins, 25% are IgG and IgA globulins, 5% are transferrin, 4% are fibrinogen and α2-macroglobulin. Interestingly, fibrinogen is the most sensitive protein to oxidative stress [72]. The previously mentioned cellular proteins also belong to abundant cellular proteins. Heat shock proteins (HSPs) are one of the most highly expressed classes of cellular proteins. The two equally represented HSP90 isoforms, HSP90α and HSP90β, are critically important for the viability of eukaryotic cells and constitutively compose 1–2% of cytosolic proteins. When cells are heated or stressed, their fraction increases to 4–6% of cellular proteins [77]. Additionally, the FLNA is a 280 kDa cytoskeletal actin-binding protein that plays a role in cytoskeleton organization, membrane stabilization, and the anchoring of many membrane receptors and intracellular signaling molecules. It plays a vital role in regulating cell adhesion, locomotion, and signaling [78,79]. EPRS is found in the multi-aminoacyl-tRNA synthetase complex and catalyzes the ligation of glutamic acid and proline to their respective tRNAs [80]. We could not find good quantification data for EPRS. According to the human proteome atlas, it is highly abundant in endothelial cells [81]. Last, SOD1 is a ubiquitously and highly expressed antioxidant enzyme involved in amyotrophic lateral sclerosis (ALS) that constitutes 1–2% of the total soluble protein in the central nervous system [82].
We chose to investigate the carbonylation of α-Syn to test the suitability of the Oxime blot method for detecting the carbonylation of immunoprecipitated proteins due to its biological relevance, ease of sample access, and expression level. It is well-known that α-Syn plays a crucial role in the onset and development of Parkinson's disease [83,84]. Increased oxidation of the protein leads to its misfolding, aggregation, and oligomer formation [[66], [67], [68]], affecting the onset and severity of the disease [[56], [57], [58]]. The α-Syn protein is present in the blood at a low concentration of 26 ng/mL, primarily in RBC [85]. Its concentrations are approximately 1350, 550, 110, 90, 385, and 18-fold lower than that of albumin, immunoglobulins, transferrin, fibrinogen/α2-macroglobulin, HSP90β, or SOD1. The Oxime blot method successfully detected an increase in α-Syn protein carbonylation and oxidation-induced formation of higher molecular weight aggregates, indicating its suitability for measuring carbonylation of low-abundance proteins (Fig. 4). However, due to the differences in antibody recognition of monomeric and oligomeric forms of α-Syn [69], potential UV-induced degradation [[63], [64], [65]], non-specific protein recognition by α-Syn antibodies and interplay of these factors in α-Syn cross-linking and aggregation [[66], [67], [68], [69]], the precise identification and analysis of α-Syn oligomers and truncated products are difficult and beyond the scope of the manuscript. Since the signals of α-Syn expression and carbonylation in western blotting are similar, it appears that the Oxime blot method can detect carbonylation of a given protein if its expression is detectable by western blotting. These findings suggest that the Oxime blot may be the most effective method for studying an individual protein oxidative damage.
The Oxime blot method could be further refined for the simultaneous detection of proteins with disparate carbonylation and expression patterns by designing multiple-color panels in which a different dye brightness is adjusted to a particular need. For example, the Enhanced ChemiLuminescence (ECL) detection lowered the detection of carbonylated BSA by about 5-fold compared to the Alexa700 (Fig. 2 and S3). Since the Alexa700 is a relatively faint dye, the detection sensitivity could easily be increased by substituting it with a brighter fluorochrome such as Qdot 800, APC, PE, Texas Red, or similar. Additionally, the use of bright, near-infrared dyes such as Qdot 800 or Qdot 705 would be advantageous since they enable detection sensitivity comparable to or better than that of the ECL, with a much higher linear dynamic range. Thus, the careful selection of various dyes allows sensitive detection and quantification of protein carbonylation and the expression of changes in biological systems [86].
The Oxime blot method overcomes significant issues associated with the DNPH-based immunoblotting of carbonylated proteins. Since the carbonyl derivatization step reaches a plateau, the Oxime blotting enables sensitive, simple, and reproducible detection of protein carbonylation in complex biological samples. Therefore, the Oxime blot method could facilitate the studies investigating the effects of protein carbonylation in various signaling pathways [87], the validation of the carbonylation status of candidate proteins identified with screening methods such as 2D-OxiDIGE [88], and mass spectrometry (MS) [89], the measurement of therapeutic proteins and peptides oxidation to monitor their degradation [90], and the characterization of target proteins in diverse biological settings. The Oxime blot will be particularly beneficial when paired with other methods for detecting protein oxidation, such as ELISA and mass spectrometry, using the same underlying chemistry. Our preliminary data indicate that aminooxy-based ELISA could be used to quantify total protein carbonyls in complex samples, including those with aggregated proteins. This result is consistent with previously published data that the aminooxy-mass tagged derivative combined with the anti-tag antibody could detect oxidized proteins by ELISA [91]. Furthermore, aminooxy-containing reagents [91,92] and biotin-aminooxy [89,93] were used to identify protein carbonyl sites by MS or by Western blot using the anti-tag antibody [[91], [92], [93]]. We have generated an aminooxy derivative for a vastly improved MS identification of carbonylated protein sites (R. Ladouce, M. Girod, and M. Merćep, manuscript in preparation). When combined, these methods will significantly increase our ability to measure, identify and characterize oxidative protein damage and facilitate understanding of the role of protein oxidation in biological processes and ARDs.
Author contributions
R.L. and G.F.C. contributed equally to the conception and design of the study, the experimental work, the acquisition of data, the analysis, the interpretation of data, and the writing and the critical revision of the manuscript. I.D.H. contributed to the experimental work during the revision of the manuscript, the interpretation of the data, and the critical revision of the manuscript. K.T. was involved in the analysis and interpretation of the data and contributed to the critical revision of the manuscript and the experimental work during the revision. M.M. contributed to the concept and design of the study, the analysis, and interpretation of the data, the critical revision of the manuscript, and the study supervision. All authors approved the submission of this manuscript version for publication.
Ethics statement
The Ethics Committee of the Mediterranean Institute of Life Sciences approved the use of material from human subjects in this study.
Funding
This research was funded from the MedILS institute budget acquired by Prof. Radman through sponsorship from NAOS Group and its founder, Mr. Jean-Noel Thorel, and former collaborative contracts with Methuselah Health and Divide & Conquer companies.
G.F.C. and K.T. are supported by the project STIM-REI, Contract Number: KK.01.1.1.01.0003, funded by the European Union through the European Regional Development Fund—the Operational Programme Competitiveness and Cohesion 2014–2020 (KK.01.1.1.01). K.T. is also supported by the European Union's Horizon 2020 research and innovation program under the Marie Sklodowska-Curie grant agreement No 898685. I.D.H. was supported by the project, funded by the Croatian Ministry of Science and Education (number 141-0000000-0080) and Program funds for science, University of Split School of Medicine.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We thank Prof. Miroslav Radman for securing funding for our research and the critical reading of this manuscript. We are grateful to Drs. Siniša Volarević and Jamie Snider for a critical review of the manuscript. We thank Nevena Nikolac, Antonija Rubić, and Josipa Dunatov for their expert technical assistance.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.redox.2023.102743.
Contributor Information
Guillaume Fabien Combes, Email: guillaume.combes@medils.hr.
Mladen Merćep, Email: mladen.mercep@uniri.hr.
Abbreviations
- Alexa700
Alexa Fluor®700
- α-Syn
alpha-synuclein
- ARDs
age-related diseases
- BCCP
biotin carboxyl carrier protein
- BSA
Bovine serum albumin
- DMEM
Dulbecco's Modified Eagle's medium
- DMSO
dimethyl sulfoxide
- DNP
2,4-dinitrophenyl
- DNPH
2,4-dinitrophenylhydrazine
- DTT
dithiothreitol
- ECL
enhanced chemiluminescence
- EDTA
ethylenediaminetetraacetic acid
- ELISA
enzyme-linked immunosorbent assay
- EPRS
bifunctional glutamate/proline-tRNA ligase
- FBS
fetal bovine serum
- FLNA
filamin A
- HCl
hydrochloric acid
- HEK 293
Human Embryonic Kidney 293
- HRP
streptavidin-horseradish peroxidase
- HSPs
heat shock proteins
- HSP90
heat shock protein 90
- LB
Lysogeny broth media
- MCF-7
Michigan Cancer Foundation-7
- MS
mass spectrometry
- m.w.
molecular weight
- NaCl
sodium chloride
- NH2OH
hydroxylamine hydrochloride
- NP-40
nonyl phenoxypolyethoxylethanol
- PBS
phosphate-buffered saline
- PBS-T
phosphate-buffered saline containing 0.05% Tween
- pPDA
p-phenylenediamine
- PTMs
posttranslational modifications
- PVDF
polyvinylidene difluoride
- RBC
red blood cells
- RIPA
radioimmunoprecipitation lysis buffer
- ROS
reactive oxygen species
- RT
room temperature
- SDS
sodium dodecyl sulfate
- SDS-PAGE
sodium dodecyl sulfate-polyacrylamide gel electrophoresis
- SOD1
superoxide dismutase 1
- TEMED
N,N,N′,N′-tetramethylethylenediamine
- TFA
trifluoroacetic acid
- UV
ultraviolet
Appendix A. Supplementary data
The following is the Supplementary data to this article.
Data availability
Data will be made available on request.
References
- 1.Stadtman E.R., Berlett B.S. Reactive oxygen-mediated protein oxidation in aging and disease. Drug Metab. Rev. 1998;30:225–243. doi: 10.3109/03602539808996310. [DOI] [PubMed] [Google Scholar]
- 2.Halliwell B., Gutteridge J.M.C. Oxygen toxicity, oxygen radicals, transition metals and disease. Biochem. J. 1984;219:1–14. doi: 10.1042/bj2190001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Drazic A., Winter J. The physiological role of reversible methionine oxidation. Biochim. Biophys. Acta, Proteins Proteomics. 2014;1844:1367–1382. doi: 10.1016/j.bbapap.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 4.Meng T.C., Fukada T., Tonks N.K. Reversible oxidation and inactivation of protein tyrosine phosphatases in vivo. Mol. Cell. 2002;9:387–399. doi: 10.1016/S1097-2765(02)00445-8. [DOI] [PubMed] [Google Scholar]
- 5.Krisko A., Radman M. Protein damage, ageing and age-related diseases. Open Biol. 2019;9 doi: 10.1098/rsob.180249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tamarit J., Cabiscol E., Ros J. Identification of the major oxidatively damaged proteins in Escherichia coli cells exposed to oxidative stress. J. Biol. Chem. 1998;273:3027–3032. doi: 10.1074/jbc.273.5.3027. [DOI] [PubMed] [Google Scholar]
- 7.Robinson C.E., Keshavarzian A., Pasco D.S., Frommel T.O., Winship D.H., Holmes E.W. Determination of protein carbonyl groups by immunoblotting. Anal. Biochem. 1999;266:48–57. doi: 10.1006/abio.1998.2932. [DOI] [PubMed] [Google Scholar]
- 8.Chevion S., Moran D.S., Heled Y., Shani Y., Regev G., Abbou B., Berenshtein E., Stadtman E.R., Epstein Y. Plasma antioxidant status and cell injury after severe physical exercise. Proc. Natl. Acad. Sci. U.S.A. 2003;100:5119–5123. doi: 10.1073/pnas.0831097100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ahmad A., Shameem M., Husain Q. Relation of oxidant-antioxidant imbalance with disease progression in patients with asthma. Ann. Thorac. Med. 2012;7:226–232. doi: 10.4103/1817-1737.102182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Al-Shobaili H.A., Al Robaee A.A., Alzolibani A., Khan M.I., Rasheed Z. Hydroxyl radical modification of immunoglobulin g generated cross-reactive antibodies: its potential role in systemic lupus erythematosus. Clin. Med. Insights Arthritis Musculoskelet. Disord. 2011;4:11–19. doi: 10.4137/CMAMD.S6793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cecarini V., Gee J., Fioretti E., Amici M., Angeletti M., Eleuteri A.M., Keller J.N. Protein oxidation and cellular homeostasis: emphasis on metabolism. Biochim. Biophys. Acta Mol. Cell Res. 2007;1773:93–104. doi: 10.1016/j.bbamcr.2006.08.039. [DOI] [PubMed] [Google Scholar]
- 12.Moskovitz J., Bar-Noy S., Williams W.M., Requena J., Berlett B.S., Stadtman E.R. Methionine sulfoxide reductase (MsrA) is a regulator of antioxidant defense and lifespan in mammals. Proc. Natl. Acad. Sci. U.S.A. 2001;98:12920–12925. doi: 10.1073/pnas.231472998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pacifici R.E., Davies K.J.A. Protein, lipid and DNA repair systems in oxidative stress: the free-radical theory of aging revisited. Gerontology. 1991;37:166–180. doi: 10.1159/000213257. [DOI] [PubMed] [Google Scholar]
- 14.Nyström T. Role of oxidative carbonylation in protein quality control and senescence. EMBO J. 2005;24:1311–1317. doi: 10.1038/sj.emboj.7600599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liguori I., Russo G., Curcio F., Bulli G., Aran L., Della-Morte D., Gargiulo G., Testa G., Cacciatore F., Bonaduce D., Abete P. Oxidative stress, aging, and diseases. Clin. Interv. Aging. 2018;13:757–772. doi: 10.2147/CIA.S158513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Madreiter-Sokolowski C.T., Thomas C., Ristow M. Interrelation between ROS and Ca2+ in aging and age-related diseases. Redox Biol. 2020;36 doi: 10.1016/j.redox.2020.101678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Belikov A.V. Age-related diseases as vicious cycles. Ageing Res. Rev. 2019;49:11–26. doi: 10.1016/j.arr.2018.11.002. [DOI] [PubMed] [Google Scholar]
- 18.Katzir I., Adler M., Karin O., Mendelsohn-Cohen N., Mayo A., Alon U. Senescent cells and the incidence of age-related diseases. Aging Cell. 2021;20 doi: 10.1111/acel.13314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.López-Otín C., Blasco M.A., Partridge L., Serrano M., Kroemer G. The hallmarks of aging. Cell. 2013;153:1194. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saez I., Vilchez D. The mechanistic links between proteasome activity, aging and agerelated diseases. Curr. Genom. 2014;15:38–51. doi: 10.2174/138920291501140306113344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nguyen N.N., Rana A., Goldman C., Moore R., Tai J., Hong Y., Shen J., Walker D.W., Hur J.H. Proteasome β5 subunit overexpression improves proteostasis during aging and extends lifespan in Drosophila melanogaster. Sci. Rep. 2019;9:3170. doi: 10.1038/s41598-019-39508-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hansen M., Rubinsztein D.C., Walker D.W. Autophagy as a promoter of longevity: insights from model organisms. Nat. Rev. Mol. Cell Biol. 2018;19:579–593. doi: 10.1038/s41580-018-0033-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vilchez D., Saez I., Dillin A. The role of protein clearance mechanisms in organismal ageing and age-related diseases. Nat. Commun. 2014;5:5659. doi: 10.1038/ncomms6659. [DOI] [PubMed] [Google Scholar]
- 24.Alam Z.I., Daniel S.E., Lees A.J., Marsden D.C., Jenner P., Halliwell B. A generalised increase in protein carbonyls in the brain in Parkinson's but not incidental Lewy body disease. J. Neurochem. 1997;69:1326–1329. doi: 10.1046/j.1471-4159.1997.69031326.x. [DOI] [PubMed] [Google Scholar]
- 25.Dominguez C., Ruiz E., Gussinye M., Carrascosa A. Oxidative stress at onset and in early stages of type 1 diabetes in children and adolescents. Diabetes Care. 1998;21:1736–1742. doi: 10.2337/diacare.21.10.1736. [DOI] [PubMed] [Google Scholar]
- 26.Telci A., Çakatay U., Kayali R., Erdogan C., Orhan Y., Sivas A., Akçay T. Oxidative protein damage in plasma of Type 2 diabetic patients. Horm. Metab. Res. 2000;32:40–43. doi: 10.1055/s-2007-978584. [DOI] [PubMed] [Google Scholar]
- 27.Banfi C., Brioschi M., Barcella S., Veglia F., Biglioli P., Tremoli E., Agostoni P.G. Oxidized proteins in plasma of patients with heart failure: role in endothelial damage. Eur. J. Heart Fail. 2008;10:244–251. doi: 10.1016/j.ejheart.2008.01.016. [DOI] [PubMed] [Google Scholar]
- 28.Ichihara S., Suzuki Y., Chang J., Kuzuya K., Inoue C., Kitamura Y., Oikawa S. Involvement of oxidative modification of proteins related to ATP synthesis in the left ventricles of hamsters with cardiomyopathy. Sci. Rep. 2017;7:9243. doi: 10.1038/s41598-017-08546-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rodrigo R., Libuy M., Feliú F., Hasson D. Oxidative stress-related biomarkers in essential hypertension and ischemia-reperfusion myocardial damage. Dis. Markers. 2013;35:773–790. doi: 10.1155/2013/974358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Himmelfarb J., McMonagle E., McMenamin E. Plasma protein thiol oxidation and carbonyl formation in chronic renal failure. Kidney Int. 2000;58:2571–2578. doi: 10.1046/j.1523-1755.2000.00443.x. [DOI] [PubMed] [Google Scholar]
- 31.Colombo G., Reggiani F., Angelini C., Finazzi S., Astori E., Garavaglia M.L., Landoni L., Portinaro N.M., Giustarini D., Rossi R., Santucci A., Milzani A., Badalamenti S., Dalle-Donne I. Plasma protein carbonyls as biomarkers of oxidative stress in chronic kidney disease, dialysis, and transplantation. Oxid. Med. Cell. Longev. 2020;2020 doi: 10.1155/2020/2975256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smith M.A., Perry G., Richey P.L., Sayre L.M., Anderson V.E., Beal M.F., Kowall N. Oxidative damage in Alzheimer's [6] Nature. 1996;382:120–121. doi: 10.1038/382120b0. [DOI] [PubMed] [Google Scholar]
- 33.Bizzozero O.A. In: Handb. Neurochem. Mol. Neurobiol. Lajtha A., Banik N., Ray S.K., editors. Springer US; Boston, MA: 2009. Protein carbonylation in neurodegenerative and demyelinating CNS diseases; pp. 543–562. [DOI] [Google Scholar]
- 34.Pedersen W.A., Fu W., Keller J.N., Markesbery W.R., Appel S., Smith R.G., Kasarskis E., Mattson M.P. Protein modification by the lipid peroxidation product 4-hydroxynonenal in the spinal cords of amyotrophic lateral sclerosis patients. Ann. Neurol. 1998;44:819–824. doi: 10.1002/ana.410440518. [DOI] [PubMed] [Google Scholar]
- 35.Renke J., Popadiuk S., Korzon M., Bugajczyk B., Woźniak M.X. Protein carbonyl groups' content as a useful clinical marker of antioxidant barrier impairment in plasma of children with juvenile chronic arthritis. Free Radic. Biol. Med. 2000;29:101–104. doi: 10.1016/S0891-5849(00)00288-4. [DOI] [PubMed] [Google Scholar]
- 36.Winterbourn C.C., Buss I.H., Chan T.P., Plank L.D., Clark M.A., Windsor J.A. Protein carbonyl measurements show evidence of early oxidative stress in critically ill patients. Crit. Care Med. 2000;28:143–149. doi: 10.1097/00003246-200001000-00024. [DOI] [PubMed] [Google Scholar]
- 37.Hlaváčková A., Štikarová J., Pimková K., Chrastinová L., Májek P., Kotlín R., Čermák J., Suttnar J., Dyr J.E. Enhanced plasma protein carbonylation in patients with myelodysplastic syndromes. Free Radic. Biol. Med. 2017;108:1–7. doi: 10.1016/j.freeradbiomed.2017.03.007. [DOI] [PubMed] [Google Scholar]
- 38.Rossner P., Terry M.B., Gammon M.D., Agrawal M., Zhang F.F., Ferris J.S., Teitelbaum S.L., Eng S.M., Gaudet M.M., Neugut A.I., Santella R.M. Plasma protein carbonyl levels and breast cancer risk. J. Cell Mol. Med. 2007;11:1138–1148. doi: 10.1111/j.1582-4934.2007.00097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zipprich J., Beth Terry M., Liao Y., Agrawal M., Gurvich I., Senie R., Santella R.M. Plasma protein carbonyls and breast cancer risk in sisters discordant for breast cancer from the New York Site of the breast cancer family registry. Cancer Res. 2009;69:2966–2972. doi: 10.1158/0008-5472.CAN-08-3418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Winterbourn C.C., Bonham M.J.D., Buss H., Abu-Zidan F.M., Windsor J.A. Elevated protein carbonyls as plasma markers of oxidative stress in acute pancreatitis. Pancreatology. 2003;3:375–382. doi: 10.1159/000073652. [DOI] [PubMed] [Google Scholar]
- 41.Gole M.D., Souza J.M., Choi I., Hertkorn C., Malcolm S., Foust R.F., Finkel B., Lanken P.N., Ischiropoulos H. Plasma proteins modified by tyrosine nitration in acute respiratory distress syndrome. Am. J. Physiol. Lung Cell Mol. Physiol. 2000;278:L961. doi: 10.1152/ajplung.2000.278.5.l961. –L967. [DOI] [PubMed] [Google Scholar]
- 42.Dalle-Donne I., Giustarini D., Colombo R., Rossi R., Milzani A. Protein carbonylation in human diseases. Trends Mol. Med. 2003;9:169–176. doi: 10.1016/S1471-4914(03)00031-5. [DOI] [PubMed] [Google Scholar]
- 43.Fedorova M. Protein Carbonylation Princ. Anal. Biol. Implic.; 2017. Diversity of protein carbonylation pathways: direct oxidation, glycoxidation, and modifications by lipid peroxidation products; pp. 48–82. [DOI] [Google Scholar]
- 44.Shacter E. Quantification and significance of protein oxidation in biological samples. Drug Metab. Rev. 2000;32:307–326. doi: 10.1081/DMR-100102336. [DOI] [PubMed] [Google Scholar]
- 45.Aryal B., Jeong J., Rao V.A. Doxorubicin-induced carbonylation and degradation of cardiac myosin binding protein C promote cardiotoxicity. Proc. Natl. Acad. Sci. U.S.A. 2014;111:2011–2016. doi: 10.1073/pnas.1321783111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Magherini F., Abruzzo P.M., Puglia M., Bini L., Gamberi T., Esposito F., Veicsteinas A., Marini M., Fiorillo C., Gulisano M., Modesti A. Proteomic analysis and protein carbonylation profile in trained and untrained rat muscles. J. Proteonomics. 2012;75:978–992. doi: 10.1016/j.jprot.2011.10.017. [DOI] [PubMed] [Google Scholar]
- 47.Levine R.L., Garland D., Oliver C.N., Amici A., Climent I., Lenz A.G., Ahn B.W., Shaltiel S., Stadtman E.R. Methods Enzymol. Academic Press; 1990. Determination of carbonyl content in oxidatively modified proteins; pp. 464–478. [DOI] [PubMed] [Google Scholar]
- 48.Dalle-Donne I., Rossi R., Giustarini D., Milzani A., Colombo R. Protein carbonyl groups as biomarkers of oxidative stress. Clin. Chim. Acta. 2003;329:23–38. doi: 10.1016/S0009-8981(03)00003-2. [DOI] [PubMed] [Google Scholar]
- 49.Wang P., Powell S.R. Decreased sensitivity associated with an altered formulation of a commercially available kit for detection of protein carbonyls. Free Radic. Biol. Med. 2010;49:119–121. doi: 10.1016/j.freeradbiomed.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Augustyniak E., Adam A., Wojdyla K., Rogowska-Wrzesinska A., Willetts R., Korkmaz A., Atalay M., Weber D., Grune T., Borsa C., Gradinaru D., Chand Bollineni R., Fedorova M., Griffiths H.R. Validation of protein carbonyl measurement: a multi-centre study. Redox Biol. 2015;4:149–157. doi: 10.1016/j.redox.2014.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kalia J., Raines R.T. Hydrolytic stability of hydrazones and oximes. Angew. Chem., Int. Ed. 2008;47:7523–7526. doi: 10.1002/anie.200802651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kölmel D.K., Kool E.T. Oximes and hydrazones in bioconjugation: mechanism and catalysis. Chem. Rev. 2017;117:10358–10376. doi: 10.1021/acs.chemrev.7b00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dirksen A., Hackeng T.M., Dawson P.E. Nucleophilic catalysis of oxime ligation. Angew. Chem., Int. Ed. 2006;45:7581–7584. doi: 10.1002/anie.200602877. [DOI] [PubMed] [Google Scholar]
- 54.Mahmoodi M.M., Rashidian M., Zhang Y., Distefano M.D. Application of meta- and para-phenylenediamine as enhanced oxime ligation catalysts for protein labeling, PEGylation, immobilization, and release. Curr. Protoc. Protein Sci. 2015;2015:15. doi: 10.1002/0471140864.ps1504s79. 4.1-15.4.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wendeler M., Grinberg L., Wang X., Dawson P.E., Baca M. Enhanced catalysis of oxime-based bioconjugations by substituted anilines. Bioconjugate Chem. 2014;25:93–101. doi: 10.1021/bc400380f. [DOI] [PubMed] [Google Scholar]
- 56.Zhou W., Zhu M., Wilson M.A., Petsko G.A., Fink A.L. The oxidation state of DJ-1 regulates its chaperone activity toward α-synuclein. J. Mol. Biol. 2006;356:1036–1048. doi: 10.1016/j.jmb.2005.12.030. [DOI] [PubMed] [Google Scholar]
- 57.Schildknecht S., Gerding H.R., Karreman C., Drescher M., Lashuel H.A., Outeiro T.F., Di Monte D.A., Leist M. Oxidative and nitrative alpha-synuclein modifications and proteostatic stress: implications for disease mechanisms and interventions in synucleinopathies. J. Neurochem. 2013;125:491–511. doi: 10.1111/jnc.12226. [DOI] [PubMed] [Google Scholar]
- 58.Bridi J.C., Hirth F. Mechanisms of α-Synuclein induced synaptopathy in Parkinson's disease. Front. Neurosci. 2018;12:80. doi: 10.3389/fnins.2018.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jiménez-Soto L.F. Trans-Blot® TurboTM transfer with home-made buffers. Protocol.Io. 2016 doi: 10.17504/protocols.io.ghhbt36. [DOI] [Google Scholar]
- 60.Sasaki A., Arawaka S., Sato H., Kato T. Sensitive western blotting for detection of endogenous Ser129-phosphorylated α-synuclein in intracellular and extracellular spaces. Sci. Rep. 2015;5 doi: 10.1038/srep14211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chapman-Smith A., Cronan J.E. The enzymatic biotinylation of proteins: a post-translational modification of exceptional specificity. Trends Biochem. Sci. 1999;24:359–363. doi: 10.1016/S0968-0004(99)01438-3. [DOI] [PubMed] [Google Scholar]
- 62.Weber D., Davies M.J., Grune T. Determination of protein carbonyls in plasma, cell extracts, tissue homogenates, isolated proteins: focus on sample preparation and derivatization conditions. Redox Biol. 2015;5:367–380. doi: 10.1016/j.redox.2015.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.V Zabuga A., Kamrath M.Z., V Boyarkin O., Rizzo T.R. Fragmentation mechanism of UV-excited peptides in the gas phase. J. Chem. Phys. 2014;141 doi: 10.1063/1.4897158. [DOI] [PubMed] [Google Scholar]
- 64.Thomson B., Hepburn C.D., Lamare M., Baltar F. Temperature and UV light affect the activity of marine cell-free enzymes. Biogeosciences. 2017;14:3971–3977. doi: 10.5194/bg-14-3971-2017. [DOI] [Google Scholar]
- 65.Schöneich C. Photo-degradation of therapeutic proteins: mechanistic aspects. Pharm. Res. (N. Y.) 2020;37:45. doi: 10.1007/s11095-020-2763-8. [DOI] [PubMed] [Google Scholar]
- 66.Fauvet B., Mbefo M.K., Fares M.-B., Desobry C., Michael S., Ardah M.T., Tsika E., Coune P., Prudent M., Lion N., Eliezer D., Moore D.J., Schneider B., Aebischer P., El-Agnaf O.M., Masliah E., Lashuel H.A. α-Synuclein in central nervous system and from erythrocytes, mammalian cells, and Escherichia coli exists predominantly as disordered monomer. J. Biol. Chem. 2012;287:15345–15364. doi: 10.1074/jbc.M111.318949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dias V., Junn E., Mouradian M.M. The role of oxidative stress in Parkinson's disease. J. Parkinsons Dis. 2013;3:461–491. doi: 10.3233/JPD-130230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dettmer U., Newman A.J., Luth E.S., Bartels T., Selkoe D. In vivo cross-linking reveals principally oligomeric forms of α-synuclein and β-synuclein in neurons and non-neural cells. J. Biol. Chem. 2013;288:6371–6385. doi: 10.1074/jbc.M112.403311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chakroun T., Evsyukov V., Nykänen N.-P., Höllerhage M., Schmidt A., Kamp F., Ruf V.C., Wurst W., Rösler T.W., Höglinger G.U. Alpha-synuclein fragments trigger distinct aggregation pathways. Cell Death Dis. 2020;11:84. doi: 10.1038/s41419-020-2285-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nedić O., Rogowska-Wrzesinska A., Rattan S.I.S. Standardization and quality control in quantifying non-enzymatic oxidative protein modifications in relation to ageing and disease: why is it important and why is it hard? Redox Biol. 2015;5:91–100. doi: 10.1016/j.redox.2015.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Guareschi S., Cova E., Cereda C., Ceroni M., Donetti E., Bosco D.A., Trotti D., Pasinelli P. An over-oxidized form of superoxide dismutase found in sporadic amyotrophic lateral sclerosis with bulbar onset shares a toxic mechanism with mutant SOD1. Proc. Natl. Acad. Sci. USA. 2012;109:5074–5079. doi: 10.1073/PNAS.1115402109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shacter E., Williams J.A., Lim M., Levine R.L. Differential susceptibility of plasma proteins to oxidative modification: examination by western blot immunoassay. Free Radic. Biol. Med. 1994;17:429–437. doi: 10.1016/0891-5849(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 73.Han C.Y., Pichon T.J., Wang X., Ringgold K.M., St John A.E., Stern S.A., White N.J. Leukocyte activation primes fibrinogen for proteolysis by mitochondrial oxidative stress. Redox Biol. 2022;51 doi: 10.1016/j.redox.2022.102263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Aryal B., Rao V.A. Specific protein carbonylation in human breast cancer tissue compared to adjacent healthy epithelial tissue. PLoS One. 2018;13 doi: 10.1371/journal.pone.0194164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Andrus P.K., Fleck T.J., Gurney M.E., Hall E.D. Protein oxidative damage in a transgenic mouse model of familial amyotrophic lateral sclerosis. J. Neurochem. 1998;71:2041–2048. doi: 10.1046/j.1471-4159.1998.71052041.x. [DOI] [PubMed] [Google Scholar]
- 76.Leeman M., Choi J., Hansson S., Storm M.U., Nilsson L. Proteins and antibodies in serum, plasma, and whole blood—size characterization using asymmetrical flow field-flow fractionation (AF4) Anal. Bioanal. Chem. 2018;410:4867–4873. doi: 10.1007/s00216-018-1127-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Garrido C., Gurbuxani S., Ravagnan L., Kroemer G. Heat shock proteins: endogenous modulators of apoptotic cell death. Biochem. Biophys. Res. Commun. 2001;286:433–442. doi: 10.1006/bbrc.2001.5427. [DOI] [PubMed] [Google Scholar]
- 78.Calderwood D.A., Shattil S.J., Ginsberg M.H. Integrins and actin filaments: reciprocal regulation of cell adhesion and signaling. J. Biol. Chem. 2000;275:22607–22610. doi: 10.1074/jbc.R900037199. [DOI] [PubMed] [Google Scholar]
- 79.Stossel T.P., Condeelis J., Cooley L., Hartwig J.H., Noegel A., Schleicher M., Shapiro S.S. Filamins as integrators of cell mechanics and signalling. Nat. Rev. Mol. Cell Biol. 2001;2:138–145. doi: 10.1038/35052082. [DOI] [PubMed] [Google Scholar]
- 80.Eswarappa S.M., Potdar A.A., Sahoo S., Sankar S., Fox P.L. Metabolic origin of the fused aminoacyl-tRNA synthetase, glutamyl-prolyl-tRNA synthetase. J. Biol. Chem. 2018;293:19148–19156. doi: 10.1074/jbc.RA118.004276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Karlsson M., Zhang C., Méar L., Zhong W., Digre A., Katona B., Sjöstedt E., Butler L., Odeberg J., Dusart P., Edfors F., Oksvold P., von Feilitzen K., Zwahlen M., Arif M., Altay O., Li X., Ozcan M., Mardonoglu A., Fagerberg L., Mulder J., Luo Y., Ponten F., Uhlén M., Lindskog C. A single–cell type transcriptomics map of human tissues. Sci. Adv. 2021;7:eabh2169. doi: 10.1126/sciadv.abh2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pardo C.A., Xu Z., Borchelt D.R., Price D.L., Sisodia S.S., Cleveland D.W. Superoxide dismutase is an abundant component in cell bodies, dendrites, and axons of motor neurons and in a subset of other neurons. Proc. Natl. Acad. Sci. U.S.A. 1995;92:954–958. doi: 10.1073/pnas.92.4.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Maries E., Dass B., Collier T.J., Kordower J.H., Steece-Collier K. The role of α-synuclein in Parkinson's disease: insights from animal models. Nat. Rev. Neurosci. 2003;4:727–738. doi: 10.1038/nrn1199. [DOI] [PubMed] [Google Scholar]
- 84.Ganguly U., Singh S., Pal S., Prasad S., Agrawal B.K., Saini R.V., Chakrabarti S. Alpha-synuclein as a biomarker of Parkinson's disease: good, but not good enough. Front. Aging Neurosci. 2021;13 doi: 10.3389/fnagi.2021.702639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Barbour R., Kling K., Anderson J.P., Banducci K., Cole T., Diep L., Fox M., Goldstein J.M., Soriano F., Seubert P., Chilcote T.J. Red blood cells are the major source of alpha-synuclein in blood. Neurodegener. Dis. 2008;5:55–59. doi: 10.1159/000112832. [DOI] [PubMed] [Google Scholar]
- 86.Schutz-geschwender A., Zhang Y., Holt T., Mcdermitt D., Olive D.M., Biosciences L. 2004. Quantitative , Two-Color Western Blot Detection with Infrared Fluorescence.http://cdn.licor.com/bio/PDF/IRquant.pdf [Google Scholar]
- 87.Wong C.M., Marcocci L., Liu L., Suzuki Y.J. Cell signaling by protein carbonylation and decarbonylation. Antioxidants Redox Signal. 2010;12:393–404. doi: 10.1089/ars.2009.2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Baraibar M.A., Ladouce R., Friguet B. Proteomic quantification and identification of carbonylated proteins upon oxidative stress and during cellular aging. J. Proteonomics. 2013;92:63–70. doi: 10.1016/j.jprot.2013.05.008. [DOI] [PubMed] [Google Scholar]
- 89.Bollineni R.C., Hoffmann R., Fedorova M. Proteome-wide profiling of carbonylated proteins and carbonylation sites in HeLa cells under mild oxidative stress conditions. Free Radic. Biol. Med. 2014;68:186–195. doi: 10.1016/j.freeradbiomed.2013.11.030. [DOI] [PubMed] [Google Scholar]
- 90.Torosantucci R., Schöneich C., Jiskoot W. Oxidation of therapeutic proteins and peptides: structural and biological consequences. Pharm. Res. (N. Y.) 2014;31:541–553. doi: 10.1007/s11095-013-1199-9. [DOI] [PubMed] [Google Scholar]
- 91.Lee S., Young N.L., Whetstone P.A., Cheal S.M., Benner W.H., Lebrilla C.B., Meares C.F. Method to site-specifically identify and quantitate carbonyl end products of protein oxidation using oxidation-dependent element coded affinity tags (O-ECAT) and nanoliquid chromatography Fourier transform mass spectrometry. J. Proteome Res. 2006;5:539–547. doi: 10.1021/pr050299q. [DOI] [PubMed] [Google Scholar]
- 92.Afiuni-Zadeh S., Rogers J.C., Snovida S.I., Bomgarden R.D., Griffin T.J. AminoxyTMT: a novel multi-functional reagent for characterization of protein carbonylation. Biotechniques. 2016;60:186–188. doi: 10.2144/000114402. 190,192-196. [DOI] [PubMed] [Google Scholar]
- 93.Combes G.F., Fakhouri H., Moulin C., Girod M., Bertorelle F., Basu S., Ladouce R., Bakulić M.P., Maršić Ž.S., Russier-Antoine I., Brevet P.-F., Dugourd P., Krisko A., Trajković K., Radman M., Bonačić-Koutecký V., Antoine R. Functionalized Au15 nanoclusters as luminescent probes for protein carbonylation detection. Commun. Chem. 2021;4:69. doi: 10.1038/s42004-021-00497-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.