Summary
Background
Vaccine impact and cost-effectiveness models have mostly focused on acute burden. Shigella-attributable moderate-to-severe diarrhoea has been shown to be associated with childhood linear growth faltering. Evidence also links less severe diarrhoea to linear growth faltering. As Shigella vaccines are in late stages of clinical development, we aimed to estimate the potential impact and cost-effectiveness of vaccination against Shigella burden that includes stunting and the acute burden attributable to less severe diarrhoea and moderate-to-severe diarrhoea.
Methods
We used a simulation model to estimate Shigella burden and potential vaccination in children aged 5 years or younger from 102 low-income to middle-income countries from 2025 to 2044. Our model included stunting associated with Shigella-related moderate-to-severe diarrhoea and less severe diarrhoea and we explored vaccination impact on health and economic outcomes.
Findings
We estimate 109 million (95% uncertainty interval [UI] 39−204) Shigella-attributable stunting cases and 1·4 million (0·8–2·1) deaths in unvaccinated children over 20 years. We project that Shigella vaccination could avert 43 million (13−92) stunting cases and 590 000 (297 000–983 000) deaths over 20 years. The overall mean incremental cost-effectiveness ratio (ICER) was US$849 (95% uncertainty interval 423–1575; median $790 [IQR 635–1005]) per disability-adjusted life-year averted. Vaccination was most cost-effective in the WHO African region and in low-income countries. Including the burden of Shigella-related less severe diarrhoea improved mean ICERs by 47−48% for these groups and substantially improved ICERs for other regions.
Interpretation
Our model suggests that Shigella vaccination would be a cost-effective intervention, with a substantial impact in specific countries and regions. Other regions could potentially benefit upon the inclusion of the burden of Shigella-related stunting and less severe diarrhoea in the analysis.
Funding
Bill & Melinda Gates Foundation and Wellcome Trust.
Introduction
Traditional disease burden measures, vaccine impact analyses, and clinical trials generally focus on acute burden (primarily mortality). However, for enteric infections, downstream health effects of an acute episode can substantially affect an individual's health, quality of life, and survival. One of the potentially most detrimental long-term sequelae of enteric infections is linear growth faltering, when a child's height falls below the expected growth curve. Stunting is a specific case of linear growth faltering, when a child's height-for-age Z score (HAZ) is 2 SDs below WHO's child growth standards. Linear growth faltering and stunting are associated with important short-term and long-term outcomes in a child's life, such as increased susceptibility to other infections, exacerbation of undernutrition, physical and cognitive development delays, and reduced educational attainment and economic productivity.1, 2, 3 Researchers have subsequently expanded the burden definition for enteric diseases to include indirect and long-term effects; these expanded analyses report substantial burden and medical cost increases, indicating need for a broader approach.4, 5, 6, 7
Before the availability of more sensitive molecular diagnostics, empirical studies primarily focused on moderate-to-severe diarrhoeal disease when evaluating long-term impacts,8 with burden and cost-effectiveness models taking the same approach.6, 7 However, the increased use of molecular diagnostics in large-scale epidemiological studies has shown that less severe enteric illness is also associated with childhood linear growth faltering,9, 10 even showing that subclinical infections affect child growth.10 Shigella specifically has been found to have a robust relationship to childhood linear growth faltering.10, 11, 12, 13 Shigella is also associated with markers of environmental enteric dysfunction12, 14—the proposed link between enteric infection and growth deficits. Additionally, Shigella is the second leading cause of all-cause diarrhoeal mortality among all age groups (13% of all diarrhoeal deaths), accounting for almost 64 000 deaths among children aged 5 years or younger worldwide annually;15 Shigella has the highest incidence in young children after infancy (age 12–59 months),8, 9 and Shigella-attributable diarrhoea can have especially severe symptoms (eg, dysentery and protein-losing enteropathy).
Research in context.
Evidence before this study
We searched PubMed from inception to June 29, 2022, without language or location restrictions, using two search strings: one for the diarrhoeal disease aetiology in children younger than 5 years and another on diarrhoea and stunting. For diarrhoeal aetiology in children younger than 5 years, we found 74 publications with the search string (“shigella” OR “shigellosis”) AND (“children under five” OR “children under 5” OR “children <5”) AND (“morbidity” OR “illness” OR “stunting” OR “episodes” OR “events” OR “cases”) AND (“cause” OR “etiology” OR “aetiology”). For diarrhoea and stunting, we found 271 publications with the search string (“diarrhea” OR “diarrea” OR “diarrhoea”) AND (“stunting” OR “height for age” OR “height” OR “Z score” OR “linear growth faltering”) AND (“child*” OR “children under five” OR “children under 5” OR “children <5”) AND (“outcomes” OR “changes”). Two large-scale multisite studies—the Global Enteric Multicenter Study (GEMS) and the Malnutrition and Enteric Disease Study (MAL-ED)—have found that less severe Shigella-associated disease can also affect child growth. While several Shigella vaccines are in development, four studies have explored Shigella burden or cost-effectiveness of Shigella vaccination in children younger than 5 years. However, in our literature search, we did not find any studies that estimated the burden of less severe Shigella-attributable diarrhoea and stunting or associated vaccine impact and cost-effectiveness for children from low-income and middle-income countries (or any other population).
Added value of this study
We estimated Shigella-attributable morbidity, mortality, and stunting from episodes of less severe and moderate-to-severe diarrhoea for children younger than 5 years living in 102 low-income and middle-income countries. We adapted and refined our previously published approach with methods for including the diarrhoeal and stunting burden of less severe Shigella-attributable disease and estimated the health and economic impacts of reducing rates of less severe and moderate-to-severe Shigella disease through vaccination. Our analysis showed that Shigella vaccines are projected to be cost-effective in certain high-burden areas, even when using the most conservative model that only included moderate-to-severe episodes. Upon the inclusion of less severe episodes and the stunting-associated burden, vaccination was cost-effective in even more areas.
Implications of all the available evidence
Our study further illustrates the importance of including the broader effects of stunting for potential Shigella (and other enteric) vaccines when assessing their value. Having a broader approach allows countries or policy makers to have a more comprehensive picture of vaccination benefits—providing a best-case scenario—which can help guide policy decisions.
Shigella is a WHO priority vaccine target because of its high burden and its high antibiotic resistance.16 Although no Shigella vaccines are approved for use, many are in the late stages of development.16 The potential for a Shigella vaccine to improve acute and long-term health in young children from high-risk settings and mitigate further antibiotic resistance highlights the importance of characterising the broader Shigella-related burden and vaccine impact.
Here, we explored how including episodes of less severe Shigella diarrhoea and their impact on childhood stunting and related mortality influence Shigella burden and vaccination-related effects. We adapted and expanded a previous model6, 7 to include the additional childhood burden from less severe Shigella infections, using updated methods and estimates. Then, we estimated the health and economic impacts over 20 years of potential Shigella vaccination, comparing the overall influence of moderate-to-severe and less severe diarrhoea on health outcomes, medical costs, and vaccine cost-effectiveness.
Methods
Overview
In this modelling study, we adapted and expanded our previous Shigella burden and impact models6, 7 to explore health and economic effects of future Shigella vaccination in children aged 5 years or younger from 102 low-income, lower-middle-income, and upper-middle-income countries (appendix p 5). First, we assessed Shigella-attributable morbidity (episodes of less severe diarrhoea and moderate-to-severe diarrhoea and Shigella-attributable childhood stunting) and mortality burden (total Shigella deaths, which included deaths from acute Shigella episodes and from other infections due to Shigella-attributable stunting).6 We estimated health (averted mortality, episodes, and stunting) and economic effects (medical costs and vaccine cost-effectiveness) of Shigella vaccines using a simulation model to project these outcomes up to 20 years after vaccination.
Study population and timeframe
We included children aged 5 years or younger from 102 low-income, lower-middle-income, and upper-middle-income countries according to World Bank classification (appendix p 5). Included countries must have had at least one estimated Shigella-attributable death annually according to the 2019 Global Burden of Disease Study (GBD)17 and available economic information. National estimates were aggregated by eligibility for Gavi, the Vaccine Alliance; World Bank income classification; and by WHO region to assess regional trends (African region, region of the Americas, Eastern Mediterranean region, South-East Asia region, and Western Pacific region). Countries’ Gavi eligibility status was based on 2020 criteria.18 Population estimates were based on UN Population Division country estimates and projections from 2025 to 2044.19
Shigella morbidity and mortality burden
We modelled Shigella morbidity and mortality burden in children aged 5 years or younger. Burden estimates depended upon Shigella-attributable proportions of diarrhoeal episodes and deaths from acute diarrhoea and deaths from other infections related to Shigella-attributable stunting (appendix p 1).6 Diarrhoeal morbidity was based on episodes per child from a systematic review.20 Mortality estimates for diarrhoeal disease were based on 2019 GBD estimates.17 We calculated Shigella-attributable deaths and diarrhoeal episodes (and cause-agnostic diarrhoeal episodes) and projected morbidity and mortality estimates from 2019 to 2044 (appendix p 2) using WHO region-specific aetiological fractions of Shigella-attributed morbidity and mortality.17 Further, we used fractions of episodes considered to be less severe diarrhoea and moderate-to-severe diarrhoea after visits to health-care facilities from the Global Enteric Multicenter Study (GEMS) 1A follow-on study9 in our estimates (appendix p 1).
Effects of Shigella-attributable stunting
We applied our previous methods6 to determine Shigella-attributable stunting, with some modifications (appendix p 12). First, we calculated the shift in child HAZ scores associated with episodes of Shigella-related less severe diarrhoea and moderate-to-severe diarrhoea using 2019 GEMS 1A follow-on study results9 (table; appendix p 1). In the absence of reliable community-level estimates for diarrhoea treatment, we assumed that, of diarrhoeal episodes when care was sought at a health facility, 25% were moderate-to-severe diarrhoea and 75% were less severe diarrhoea.9 Using data from the most recent Demographic and Health Surveys, we estimated the proportion of childhood diarrhoeal episodes for which care was sought by taking the mean proportion across all model countries reporting this variable and substituting the corresponding WHO regional average for those without a country-level estimate (table; appendix p 1). Using our previous approach,6 we estimated child mortality from infections for which stunting is a risk factor (eg, lower respiratory infections, malaria, measles, and other-cause diarrhoea; appendix p 2).1
Table.
Model inputs and parameters for best-case scenario and ranges used in uncertainty and sensitivity analyses
| Values | Sensitivity range | Distribution | Source | |
|---|---|---|---|---|
| Burden | ||||
| Population estimates | Varies by country | .. | NA | UNDP 2025-4519 |
| Diarrhoeal mortality for children aged ≤5 years | Varies by country | 10% | Log normal | GBD 201917 |
| Diarrhoeal mortality rate of change (2025–44) | Varies by region and income classification | 25% | Normal | .. |
| Other infectious disease mortality for children aged ≤5 years | Varies by country | .. | Log normal | GBD 201917 |
| Other infectious disease mortality rate of change (2025–44) | Varies by region and income classification | .. | Normal | .. |
| Annual rate of diarrhoeal episodes in children aged ≤5 years (95% UI) | African region 3·3 (2·1–5·0); region of the Americas 3·2 (2·6–3·8); Eastern Mediterranean region 2·9 (1·6–4·2); European region 2·8 (2·3–3·3); South-East Asia region 2·4 (1·5–3·3); and Western Pacific region: 2·2 (1·3–2·5) | 25% | Log normal | Anderson et al6 and Walker et al21 |
| Diarrhoeal episodes annual rate of decline (2025–44) | 0·37% (SD 0·09%) | 25% | Normal | GBD 201917 |
| Aetiological fraction of Shigella-attributable mortality by WHO region (95% UI) | African region 22·4% (9·6–40·3); region of the Americas 13·4% (5·4–26·8%); Eastern Mediterranean region 11·7% (4·2–24·6%); European region 10·2% (3·8–21·7%); South-East Asia region 9·0% (3·4–19·5%); and Western Pacific region 11·2% (4·4–22·5%) | 50% | Beta | GBD 201917 |
| Aetiological fraction of Shigella-attributable morbidity by WHO region (95% UI) | African region 16·6% (9·1–27·7%); region of the Americas; 12·6% (6·9–20·4%); Eastern Mediterranean region 10·8% (5·8–18·5%); European region 6·8% (3·7–11·3%); South-East Asia region 8·2% (4·5–13·6%); and Western Pacific region 7·5% (4·1–12·2%) | 50% | Beta | GBD 201917 |
| Moderate and severe stunting estimates | Varies by country | .. | .. | DHS and JME22 |
| Annual stunting rate of decline | African region 1·7%; region of the Americas 2·6%; Eastern Mediterranean region 1·5%; European region 3·7%; South-East Asia region 2·3%; and Western Pacific region 1·6% | .. | .. | JME22 |
| Shigella-attributable stunting cases (by episodes of moderate-to-severe diarrhoea) | 0·072 (SD 0·017) shift in HAZ | 25% | Normal | GEMS 1A9 |
| Shigella-attributable stunting cases (by episodes of less severe diarrhoea) | 0·052 (SD 0·018) shift in HAZ | 25% | Normal | GEMS 1A9 |
| Percentage of Shigella-attributable episodes of moderate-to-severe diarrhoea | 25% of children who sought care at a health-care facility | .. | NA | GEMS 1A9 |
| Percentage of Shigella-attributable episodes of less severe diarrhoea | 75% of children who sought care at a health-care facility | .. | NA | GEMS 1A9 |
| Percentage of caretakers who sought care at a health facility after a child's diarrhoeal episode (95% UI) | 57% (55–59) | .. | Beta | DHS data |
| Vaccination inputs and medical costs | ||||
| Shigella vaccine effectiveness against mortality and episodes of moderate-to-severe diarrhoea (95% UI) | 60% (40–80) | 33% | Beta | iTPP23 assumption |
| Shigella vaccine effectiveness against episodes of less severe diarrhoea (95% UI) | 40% (20–60) | 33% | Beta | iTPP23 assumption |
| Gavi-eligible dose price (95% UI) | $2·00 (1·00–3·00) | 50% | Gamma | iTPP23 assumption |
| Gavi-ineligible dose price in lower-middle-income countries (95% UI) | $7·10 (3·55–10·65) | 50% | Gamma | MI4A24 analysis |
| Gavi-ineligible dose price in upper-middle-income countries (95% UI) | $9·73 (4·87–14·60) | 50% | Gamma | MI4A24 analysis |
| Gavi-ineligible dose price in region of the Americas (95% UI) | $6·75 (3·73–10·12) | 50% | Gamma | MI4A24 analysis |
| Doses for full course | 2 | .. | NA | iTPP23 assumption |
| Administration cost | $1·37 in low-income countries, $2·05 in lower-middle-income countries, and $2·05 in upper-middle-income countries | 40% | Gamma | Debellut et al25 |
| Cost of Shigella illness | Varies by country; outpatient median $7·08 per episode and inpatient mean $71·48 per episode | 40% | Gamma | Baral et al26 |
| Inpatient visit rate (95% UI) | African region 2·2% (1·7–2·5); region of the Americas 1·9% (1·8–2·0); Eastern Mediterranean region 2·1% (1·7–2·8); European region 1·6% (1·5–1·7); South-East Asia region 2·2% (1·9–2·6); and Western Pacific region 2·3% (2·2–2·6) | .. | Beta | Debellut et al25 and Walker et al21 |
| Outpatient visit rate (cases taken to health-care facility; 95% UI) | 57% (55–59) of Shigella cases sought treatment annually | .. | Beta | DHS data |
All costs are presented in 2019 US$. DHS=Demographic and Health Surveys. GBD=Global Burden of Disease Study. GEMS=Global Enteric Multicenter Study. HAZ=height-for-age Z score. iTPP=Intervention Target Product Profile. JME=Joint Child Malnutrition Estimates. MI4A=Market Information for Access to Vaccines. NA=not applicable. UI=uncertainty interval.
Burden outcome measures
Burden outcome measures included episodes of Shigella-attributable moderate-to-severe diarrhoea and less severe diarrhoea, Shigella-attributable direct mortality, stunting attributable to Shigella-related moderate-to-severe diarrhoea and less severe diarrhoea, stunting-related deaths from other infections, and disability-adjusted life-years (DALYs). Although childhood stunting is a risk factor associated with other infection-related deaths, it is not directly included in DALY calculations. All mortality outcomes, including stunting-related deaths from other infections, were translated to DALYs27 that were not age-weighted but were discounted 3% annually from 2025 to 2044 (appendix p 1). We calculated all outcomes annually and cumulatively from vaccine introduction.
Vaccine-related assumptions and inputs
We assessed the effect of the national introduction of Shigella standalone vaccine candidates administered to children after age 6 months, assuming a 2025 introduction by all countries. We examined 20 annual birth cohorts of children over the first 5 years of life (following each cohort until 2044). The vaccine was assumed to be 60% effective23, 28 in preventing deaths and episodes attributable to Shigella-related moderate-to-severe diarrhoea and 40% effective in preventing deaths and episodes attributable to Shigella-related less severe diarrhoea (table). Additionally, we assumed that protection is conferred after the second dose, with no protection for partially vaccinated children; that effectiveness does not wane during the first 5 years after immunisation; and that herd protection is not conferred by vaccination. We did not include vaccine effectiveness after age 5 years. We calculated vaccination coverage using country-specific 2020 coverage estimates for the third dose of diphtheria-pertussis-tetanus vaccine (DPT3; DPT2 coverage not reported29). In countries with estimates of below 90%, we projected vaccination coverage as improving 1% per year up to 90%.
As vaccine price is uncertain, we used a 2021 Shigella vaccine product profile23 to inform our base price (US$2·00/dose) for Gavi-eligible countries, exploring prices ranging from $1·00 to $3·00 per dose. For lower-middle-income and upper-middle-income countries that were ineligible for Gavi in 2020, we used vaccine market data from the WHO Market Information for Access to Vaccines24 to estimate vaccine prices. However, we calculated separate prices for Gavi-ineligible countries in the region of the Americas regardless of income class because vaccines in this region are procured via the Pan American Health Organization Revolving Fund (table; appendix p 3).
In the absence of published medical costs of Shigella diarrhoea for study countries, we used country-specific estimates of direct inpatient and outpatient medical costs associated with moderate-to-severe diarrhoea.26 We assumed that patients with severe diarrhoea were referred for inpatient care using published estimates (table).21 We assumed that vaccine administration costs were similar to those of rotavirus vaccines.25 All costs were adjusted to30 and reported in 2019 US$ and were discounted at 3% per year.
Cost-effectiveness analysis
We used a health-system perspective to calculate cost-effectiveness estimates. Vaccination costs include vaccine administration cost, vaccine price, vaccine demand, and 10% wastage from 2025 to 2044. We calculated net costs for each country and aggregated results by WHO region, World Bank income classification, and Gavi eligibility (appendix p 3). We calculated vaccine benefits for each aggregate over 20 years, using projected benefits for the first 5 years of life for children vaccinated in each annual birth cohort (appendix p 3). We calculated primary cost-effectiveness measures—mean and median incremental cost-effectiveness ratios (ICERs)—by dividing incremental costs associated with vaccine introduction by DALYs averted for each aggregate (appendix p 3). Our comparator scenario was no vaccination.
Scenario analyses
We calculated three ICERs representing different scenarios: assuming vaccination is effective against moderate-to-severe diarrhoea episodes, mortality, and medical costs attributable to Shigella (scenario 1); scenario 1 plus less severe diarrhoea episodes, mortality, and medical costs attributable to Shigella (scenario 2); and scenario 2 plus stunting attributable to moderate-to-severe diarrhoea and less severe diarrhoea attributable to Shigella and associated mortality from other infections (scenario 3, total Shigella burden). We also explored a scenario in which vaccination is only effective against the burden associated with Shigella-attributable moderate-to-severe diarrhoea (ie, episodes, mortality, and stunting), which we termed MSD+S only.
Simulations, uncertainty, and sensitivity analyses
We used Monte Carlo simulation methods to generate model point estimates and uncertainty and sensitivity analyses in R (version 4.1.2). We characterised key input variables as distributions (table) and simulated 1000 replicates. Uncertainty is presented as 95% uncertainty intervals (UIs) representing 2·5% and 97·5% quantile estimates. For ICERs we also present IQRs. We used one-way sensitivity analyses to estimate the effect of changing select input values on ICERS, presented as ranges and percentages of the total variance explained by each input.
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
From 2025 to 2044, we estimated that Shigella would be associated with 1·6 billion (95% UI 1·0−2·4) episodes of less severe diarrhoea and 543 million (341−804) episodes of moderate-to-severe diarrhoea in children younger than 5 years across all countries. An estimated 109 million (39−204) children would have Shigella-attributable stunted growth (appendix p 6). Total deaths from Shigella-related diarrhoea in children younger than 5 years without vaccination would be 1·4 million (0·8−2·1) and would cost country health-care systems $11·1 billion (7·5−15·4; appendix p 6).
Our results indicate that, over 20 years, the WHO African region would have the highest estimated Shigella-attributable health burden, including the highest burden of episodes of moderate-to-severe diarrhoea and less severe diarrhoea, stunting cases, total mortality, and DALY rates across regions (figure 1; appendix pp 6–10). The African region's burden would be substantially higher than that of other regions, with morbidity rates (Shigella-attributable diarrhoeal episodes and stunting cases) twice as high and total Shigella-attributable mortality and DALY rates four times as high as those of the Eastern Mediterranean region, the region with the second highest health burden (figure 1; appendix pp 6–10). The region of the Americas would have the highest Shigella-attributable medical cost rate, the second highest episode rate of Shigella-attributable diarrhoeal episodes (figure 1A, C), but low mortality and DALY rates (figure 1C, D). When assessed by income classification, low-income countries would have the highest Shigella-attributable health burden, with the highest rates of episodes of moderate-to-severe diarrhoea and less severe diarrhoea, stunting cases, total mortality, and DALYs (figure 1; appendix p 6), whereas upper-middle-income countries would have the highest medical cost rates (figure 1C). Also, Gavi-eligible countries would have 1·5 times higher diarrhoeal episodic rates, nearly 3 times higher stunting rates, and 10 times higher mortality rates than those of Gavi-ineligible countries (figure 1A, B, D; appendix p 9).
Figure 1.
Health and economic burden rates of Shigella-attributable diarrhoea and stunting by WHO Region, World Bank income classification, and Gavi eligibility
DALY=disability-adjusted life-year. YLD=year lived with disability. YLL=year of life lost.
When considering the incremental effect of including the burden of less severe diarrhoea, projected diarrhoeal episodes increased by 75% and stunting cases by 68% (appendix p 6). Including stunting cases attributable to Shigella-related less severe diarrhoea increased mortality estimates by 17% (region of the Americas) to 25% (South-East Asia region) in WHO regions, 15% (upper-middle income) to 18% (low income and lower-middle income) in income classes, and 15% (ineligible) to 17% (eligible) by Gavi eligibility status (figure 1D; appendix p 8). Inclusion of years lived with disability from less severe diarrhoea and years of life lost from acute Shigella episodes and from other infections due to Shigella-attributable stunting increased DALYs by 18% (African region) to 27% (South-East Asia region) in WHO regions, 18% (low income) to 24% (upper-middle income) in income classes, and 19% (eligible) to 20% (ineligible) by Gavi status (figure 1E; appendix p 9). Including medical costs of less severe diarrhoea resulted in increasing costs across WHO regions (2·2 times higher in the region of the Americas and Western Pacific region and 2·5 times higher in the African, Eastern Mediterranean, and South-East Asia regions), income classes (2·2 times higher in upper-middle-income countries and 2·7 times in low-income countries), and Gavi eligibility status (2·2 times higher in eligible countries and 2·6 times higher in ineligible countries; figure 1C; appendix p 9).
According to our estimates, Shigella vaccination would prevent 548 million (95% UI 244−1010) episodes of less severe diarrhoea and 280 million (150−461) episodes of moderate-to-severe diarrhoea across all countries over 20 years (appendix p 6). Vaccination would prevent 43 million (13−92) children from having Shigella-attributable stunted growth, 590 000 (297 000–983 000) deaths, and 20 million (11–33) DALYs. Vaccination would avert $4·4 billion (2·2–7·4) in medical costs and result in an ICER (scenario 3) of $849 (423–1575; median $790 [IQR 635–1005]) per DALY averted.
Shigella vaccination would avert the greatest amount of DALY burden in the African region—3·4 times that of the Eastern Mediterranean region, the region with the second highest rates of averted DALYs (figure 2A; appendix p 9). Although the African region would have the highest reduction in stunting episode rates, the region of the Americas, the Eastern Mediterranean region, and the South-East Asia region would also have substantial reductions (figure 2B; appendix p 9). The region of the Americas would have the greatest reduction in estimated medical cost rate (figure 2C; appendix p 9). Low-income and Gavi-eligible countries would have the greatest reduction in estimated DALYs and stunting rates (figure 2A, B; appendix p 9), and upper-middle-income and Gavi-ineligible countries would have the greatest reduction in medical costs (figure 2C; appendix p 9).
Figure 2.
Potential vaccination-averted Shigella-attributable health and economic burden rates by WHO region, World Bank income classification, and Gavi eligibility
All costs are presented in 2019 US$. DALY=disability-adjusted life-year.
When including the full burden of episodes of Shigella-attributable less severe diarrhoea and moderate-to-severe diarrhoea and stunting (scenario 3), vaccination would be most cost-effective (lowest ICERs) in the African region ($161 [95% UI 18–383]; median $145 [IQR 99–206]) and Eastern Mediterranean region ($1210 [434–2575; median $1106 [797–1505]) per DALY averted, low-income ($143 [33–332]; median $129 [92–177]) and lower-middle-income countries ($644 [304–1169]; median $604 [484–752]) per DALY averted; and Gavi-eligible countries ($308 [124–589]; median $285 [219–369]) per DALY averted (figure 3; appendix pp 9–11). The MSD+S only scenario was more cost-effective than scenario 1, but less cost-effective than scenarios 2 and 3 (appendix pp 9–11).
Figure 3.
Change in ICERs when including additional burden
Scenario 1 ICERs were calculated by including the DALYs associated with episodes of moderate-to-severe diarrhoea (years of life lost and years lived with disability). Scenario 2 ICERs were calculated with DALYs associated with episodes of moderate-to-severe diarrhoea and less severe diarrhoea (years of life lost and years lived with disability). Scenario 3 ICERs were calculated with DALYs associated with episodes of moderate-to-severe diarrhoea and less severe diarrhoea plus stunting attributable to moderate-to-severe diarrhoea and less severe diarrhoea. Lines around points represent 95% uncertainty intervals around ICERs. For ease of visual comprehension, ICERs of 4000 or greater are classified as high and ICERS below 4000 are classified as low. All ICERs are presented in 2019 US$. DALY=disability-adjusted life-year. ICER=incremental cost-effectiveness ratio.
The addition of less severe diarrhoea episodes attributable to Shigella and medical costs (scenario 2) resulted in improved ICERs for the African region (34% reduction), region of the Americas (35% reduction), low-income countries (32% reduction), and Gavi-eligible countries (23% reduction; figure 4). The further inclusion of stunting attributable to Shigella-related less severe diarrhoea and moderate-to-severe diarrhoea (percentage difference between scenarios 2 and 3) showed the greatest effect on the mean ICERs of the Eastern Mediterranean region (21% reduction), the South-East Asia region (19% reduction), lower-middle-income countries (18% reduction), and Gavi-eligible countries (18% reduction; figure 4).
Figure 4.
Percentage decline in ICERs when adding episodes of less severe diarrhoea and stunting attributable to moderate-to-severe diarrhoea and less severe diarrhoea to Shigella burden
Scenario 1 ICERs were calculated by including the DALYs associated with episodes of moderate-to-severe diarrhoea (years of life lost and years lived with disability). Scenario 2 ICERs were calculated with DALYs associated with episodes of moderate-to-severe diarrhoea and less severe diarrhoea (years of life lost and years lived with disability). Scenario 3 ICERs were calculated with DALYs associated with episodes of moderate-to-severe diarrhoea and less severe diarrhoea plus stunting attributable to moderate-to-severe diarrhoea and less severe diarrhoea. DALY=disability-adjusted life-year. ICER=incremental cost-effectiveness ratio.
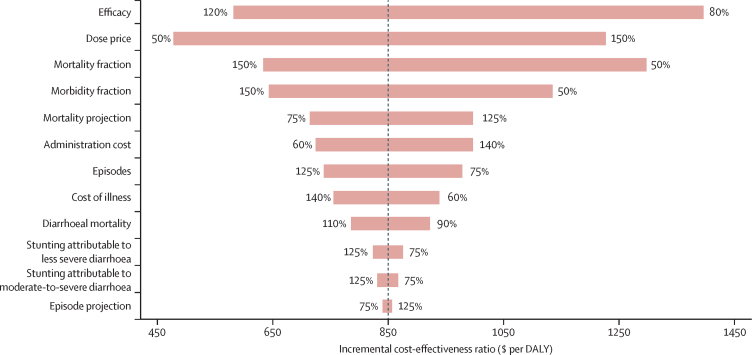
According to the ICER one-way sensitivity analysis for all countries, four variables accounted for 88% of the variance (figure 5). The scenario 3 ICER ($849 [95% UI 423–1575; median $790 [IQR635–1005] per DALY averted) was most sensitive to changes in vaccine efficacy (31% of variance), vaccine price (26%), Shigella-attributable mortality (20%), and Shigella-attributable morbidity (11%). Mortality projections accounted for 3% of variance, administrative costs accounted for 4%, and cost of illness accounted for 2%. Remaining variables accounted for 1% or less.
Figure 5.
Tornado diagram of one-way probabilistic sensitivity analysis of the key input variables on global Shigella vaccination cost-effectiveness in 102 countries from 2025 to 2044
Ranges of variables (listed in the table) are displayed at the ends of the corresponding bars. Mortality fraction and morbidity fraction represent the variation in the fraction of overall Shigella-attributable diarrhoeal mortality or morbidity. Mortality projection and episode projection are the variations in the rates of diarrhoeal mortality and episodes projected from 2025 to 2044. Stunting attributable to less severe diarrhoea or moderate-to-severe diarrhoea refers to the number of other infectious disease deaths caused by stunting attributable to Shigella-related less severe diarrhoea and moderate-to-severe diarrhoea. All ICERs are presented in 2019 US$. All burden estimates are without vaccination. DALY=disability-adjusted life-year. ICER=incremental cost-effectiveness ratio.
Discussion
Based on the vaccine efficacy assumptions outlined in the target product profiles, our model indicates that Shigella vaccination could potentially prevent a third of episodes of less severe diarrhoea and stunting cases attributable to Shigella, half of moderate-to-severe diarrhoea episodes attributable to Shigella, and almost half of Shigella-attributable total mortality and DALY burden across all countries over 20 years. These results show that a potential Shigella vaccine could have a considerable public health benefit, even at a fairly modest range of effectiveness explored in our model. Our estimates indicate that Shigella vaccination would avert the most mortality and DALY burden in groups with the highest Shigella-attributable health burden—the African region, Eastern Mediterranean region, low-income countries, and Gavi-eligible countries. If Shigella vaccination could reduce childhood stunting as simulated in our model, the African region, Eastern Mediterranean region, South-East Asia region, and low-income countries would benefit the most. The region of the Americas, upper-middle-income countries, and Gavi-ineligible countries had the largest economic burden and, accordingly, medical costs averted by vaccination. When compared with Shigella-attributable moderate-to-severe diarrhoea only (scenario 1), ICER estimates decreased by 17% upon inclusion of episodes of less severe diarrhoea (scenario 2) and 36% upon inclusion of stunting attributable to moderate-to-severe diarrhoea and less severe diarrhoea (scenario 3) across all countries. Including medical costs associated with less severe diarrhoea doubled economic burden, resulting in substantial improvement in ICERs, especially for high-burden regions and low-income and Gavi-eligible countries. Vaccination was most cost-effective in the African region, low-income countries, and Gavi-eligible countries across all scenarios.
When including Shigella-attributable episodes of less severe diarrhoea (scenario 2 vs scenario 1) in ICERs, cost-effectiveness improved most for the region of the Americas because of high rates of less severe diarrhoea medical costs, all diarrhoea episodes, and Shigella-attributable aetiological fractions, although the mean ICER remained high (>$4700). The inclusion of stunting associated with Shigella-attributable moderate-to-severe and less severe diarrhoea (scenario 3 vs scenario 2) in ICERs resulted in the Eastern Mediterranean region, South-East Asia region, low-income countries, and lower-middle income-countries had the greatest improvement, stemming from high mortality from stunting associated with Shigella-attributable less severe diarrhoea. These results indicate that, although vaccination might not have a substantial short-term benefit for certain countries or regions, if the vaccine can reduce childhood stunting and its downstream effects, areas with considerable childhood stunting might experience long-term benefits.
When including the burden attributable to less severe diarrhoea, Shigella vaccination is still less cost-effective than malaria vaccination (ICERs of $80−87 in African settings),31 but is approaching cost-effectiveness of pneumococcal32 and live oral rotavirus vaccination for certain regions.25 For example, an analysis of rotavirus vaccination reported ICERs of $108 for the African region and $264 for Gavi-eligible countries,25 and we report means of $161 for the African region and $308 for Gavi-eligible countries. However, ICERs for other regions were considerably higher than those for rotavirus. Despite this finding, Shigella vaccination should still be considered for introduction in high-burden areas.
The sensitivity analysis showed that the two most influential inputs were vaccine efficacy and vaccine price, both of which are still unknown. Ongoing clinical trials (NCT05073003, NCT04602975, NCT04634513, and NCT04242264) will provide key information on vaccine efficacy, dose schedule, timing, and combination potential, influencing future vaccine impact estimates. Shigella vaccine efficacy, which is currently unknown, probably differs against moderate-to-severe diarrhoea and less severe diarrhoea and affects the estimates of averted medical costs and stunting. The next two most influential inputs were the aetiological fractions of Shigella-attributable diarrhoeal mortality and morbidity.
Comparing Shigella aetiological fraction estimates used here to those used in our previous burden model provides additional insight into their importance. Previously, we used culture-based aetiological fractions adjusted for molecular sensitivity,6 resulting in the Eastern Mediterranean region having the highest Shigella mortality rate and the lowest ICERs.7 By contrast, 2019 GBD Shigella aetiological fraction estimates were highest for the African region and considerably higher for the European region, South-East Asia region, and Western Pacific region than in our previous model iteration.6 As a result, Shigella vaccination was most cost-effective in the African region and more cost-effective in the European region, South-East Asia region, and Western Pacific region compared with in previous estimates.
Although our results are based on the best available data and empirical evidence, several limitations remain. An underlying assumption is that Shigella infection and disease results in childhood stunting. Although large multisite and other studies have linked Shigella-attributable disease to growth impacts10, 11, 13 and inflammatory markers suspected to mediate this relationship,12, 14 the underlying mechanism between the two remains an area of ongoing research. Many aspects of this relationship remain largely uncharacterised—the most notable being whether a vaccine can ameliorate enteric infection-related growth impacts. Multiple Shigella vaccines are in preclinical studies and phase 1 or phase 2 clinical trials; however, trials in young children are pending. Although our results show the potential ability of Shigella vaccination to avert stunting, once such a vaccine is developed the crucial next step will be vaccine probe studies that include secondary endpoints on prevention of linear growth faltering and childhood stunting.33 Also, although we include the burden of less severe diarrhoea that is based on the findings of one study,9 other studies10, 34 have shown an association between subclinical Shigella infections and growth faltering, indicating that the inclusion of less severe diarrhoea is conservative. As the contribution of subclinical infections to linear growth faltering is better characterised, future burden models should integrate the effects of subclinical Shigella infections on childhood linear growth faltering and stunting. Details of the delivery schedule will be important for future cost and impact estimates but are currently not known.
Our estimates have wide UIs, reflecting uncertainty of inputs and assumptions. Aetiological fractions were based on GBD 2019 data and limited by data availability issues in neonatal age groups and some geographical regions.15 To improve the accuracy of estimates of the Shigella health and economic burden, future models should use region-representative episode prevalence and associated medical costs from community surveillance data. We used a comparator of no vaccination—other comparators could be examined as described in our companion analysis.33 Including other secondary benefits of vaccination, such as herd immunity and effects on antibiotic resistance, will also improve future estimates.
Our companion analysis shows the importance of including the long-term economic benefits of Shigella vaccination and macroeconomic implications of this relationship.33 Including future gains in adult productivity among vaccination benefits resulted in the vaccine being cost-effective in almost all regions or country groupings studied. Future economic analyses from the societal perspective would provide a necessary, more comprehensive understanding of Shigella burden.
This study builds on our previous analysis, which focused on the effects of reducing the burden of moderate-to-severe diarrhoea. Our model improves our cost-effectiveness projections by accounting for the burden of less severe diarrhoea attributable to Shigella. Our results show the importance of assessing the full burden of disease and how doing so might uncover patterns or factors to consider when developing vaccines and planning implementation. Our findings also enhance the estimated value of Shigella vaccination, which is important as policy makers consider a multiplicity of other public health interventions.35 These findings require assessment by large-scale vaccine trials in populations at high risk, ideally showing that non-acute health effects, such as linear growth faltering and stunting, even from less severe disease, can be ameliorated by vaccination.
Data sharing
Data for this study were taken from publicly available sources that are documented in this Article. Individual participant data were not used in this research. The model has been described in sufficient detail in the Article and appendix. There are no plans to make the model publicly available.
For the Demographic and Health Surveys see https://www.statcompiler.com/en
Declaration of interests
We declare no competing interests.
Acknowledgments
Acknowledgments
This study was funded by the Bill & Melinda Gates Foundation (INV-018460) and the Wellcome Trust (221988-Z-20-Z). We would like to thank Emily Hsu from PATH for her logistical and general support and Ben Toh for his suggestions regarding analysis in R software and GitHub.
Contributors
JDA co-led study conceptualisation, collected and analysed the data, generated the figures, interpreted the results of the analysis, and co-wrote the manuscript. KHB co-led study conceptualisation, guided the analysis, co-interpreted the results, and wrote the manuscript. CJP assisted in vaccine pricing estimation and provided critical guidance on the economic analysis and feedback on the manuscript. FM, CAP, and WPH provided critical review and input on the analysis and manuscript. SS provided overall guidance on the study, critical feedback on the manuscript, and assistance in developing the manuscript.
Supplementary Material
References
- 1.Black RE, Allen LH, Bhutta ZA, et al. Maternal and child undernutrition: global and regional exposures and health consequences. Lancet. 2008;371:243–260. doi: 10.1016/S0140-6736(07)61690-0. [DOI] [PubMed] [Google Scholar]
- 2.Victora CG, Adair L, Fall C, et al. Maternal and child undernutrition: consequences for adult health and human capital. Lancet. 2008;371:340–357. doi: 10.1016/S0140-6736(07)61692-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guerrant RL, DeBoer MD, Moore SR, Scharf RJ, Lima AAM. The impoverished gut—a triple burden of diarrhoea, stunting and chronic disease. Nat Rev Gastroenterol Hepatol. 2013;10:220–229. doi: 10.1038/nrgastro.2012.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khalil IA, Troeger C, Rao PC, et al. Morbidity, mortality, and long-term consequences associated with diarrhoea from Cryptosporidium infection in children younger than 5 years: a meta-analyses study. Lancet Glob Health. 2018;6:e758–e768. doi: 10.1016/S2214-109X(18)30283-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Troeger C, Colombara DV, Rao PC, et al. Global disability-adjusted life-year estimates of long-term health burden and undernutrition attributable to diarrhoeal diseases in children younger than 5 years. Lancet Glob Health. 2018;6:e255–e269. doi: 10.1016/S2214-109X(18)30045-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anderson JD, 4th, Bagamian KH, Muhib F, et al. Burden of enterotoxigenic Escherichia coli and shigella non-fatal diarrhoeal infections in 79 low-income and lower middle-income countries: a modelling analysis. Lancet Glob Health. 2019;7:e321–e330. doi: 10.1016/S2214-109X(18)30483-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anderson JD, 4th, Bagamian KH, Muhib F, et al. Potential impact and cost-effectiveness of future ETEC and Shigella vaccines in 79 low- and lower middle-income countries. Vaccine X. 2019;2 doi: 10.1016/j.jvacx.2019.100024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kotloff KL, Nataro JP, Blackwelder WC, et al. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet. 2013;382:209–222. doi: 10.1016/S0140-6736(13)60844-2. [DOI] [PubMed] [Google Scholar]
- 9.Kotloff KL, Nasrin D, Blackwelder WC, et al. The incidence, aetiology, and adverse clinical consequences of less severe diarrhoeal episodes among infants and children residing in low-income and middle-income countries: a 12-month case-control study as a follow-on to the Global Enteric Multicenter Study (GEMS) Lancet Glob Health. 2019;7:e568–e584. doi: 10.1016/S2214-109X(19)30076-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rogawski ET, Liu J, Platts-Mills JA, et al. Use of quantitative molecular diagnostic methods to investigate the effect of enteropathogen infections on linear growth in children in low-resource settings: longitudinal analysis of results from the MAL-ED cohort study. Lancet Glob Health. 2018;6:e1319–e1328. doi: 10.1016/S2214-109X(18)30351-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nasrin D, Blackwelder WC, Sommerfelt H, et al. Pathogens associated with linear growth faltering in children with diarrhea and impact of antibiotic treatment: The Global Enteric Multicenter Study. J Infect Dis. 2021 doi: 10.1093/infdis/jiab434. published online Sept 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schnee AE, Haque R, Taniuchi M, et al. Identification of etiology-specific diarrhea associated with linear growth faltering in Bangladeshi infants. Am J Epidemiol. 2018;187:2210–2218. doi: 10.1093/aje/kwy106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Black RE, Brown KH, Becker S. Effects of diarrhea associated with specific enteropathogens on the growth of children in rural Bangladesh. Pediatrics. 1984;73:799–805. [PubMed] [Google Scholar]
- 14.Rogawski McQuade ET, Shaheen F, Kabir F, et al. Epidemiology of Shigella infections and diarrhea in the first two years of life using culture-independent diagnostics in 8 low-resource settings. PLoS Negl Trop Dis. 2020;14 doi: 10.1371/journal.pntd.0008536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khalil IA, Troeger C, Blacker BF, et al. Morbidity and mortality due to shigella and enterotoxigenic Escherichia coli diarrhoea: the Global Burden of Disease Study 1990–2016. Lancet Infect Dis. 2018;18:1229–1240. doi: 10.1016/S1473-3099(18)30475-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hausdorff WP, Anderson JD, Bagamian KH, et al. Vaccine value profile for Shigella. Vaccine (in press). [DOI] [PubMed]
- 17.Global Burden of Disease Collaborative Network . Institute for Health Metrics and Evaluation (IHME); Seattle, USA: 2019. Global Burden of Disease Study 2019 (GBD 2019) results.http://ghdx.healthdata.org/gbd-results-tool [Google Scholar]
- 18.Gavi The Vaccine Alliance Eligibility. https://www.gavi.org/types-support/sustainability/eligibility
- 19.UN. Department of Economic and Social Affairs. Population Division World Population Prospects 2019. Online Edition. 2019. https://population.un.org/wpp/
- 20.Lanata CF, Fischer-Walker CL, Olascoaga AC, Torres CX, Aryee MJ, Black RE. Global causes of diarrheal disease mortality in children <5 years of age: a systematic review. PLoS One. 2013;8 doi: 10.1371/journal.pone.0072788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Walker CLF, Rudan I, Liu L, et al. Global burden of childhood pneumonia and diarrhoea. Lancet. 2013;381:1405–1416. doi: 10.1016/S0140-6736(13)60222-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.WHO The UNICEF/WHO/WB Joint Child Malnutrition Estimates (JME) group released new data for 2021. 2021. https://www.who.int/news/item/06-05-2021-the-unicef-who-wb-joint-child-malnutrition-estimates-group-released-new-data-for-2021
- 23.Hausdorff WP, Anderson IV JD, Bagamian KH, et al. Vaccine value profile for Shigella vaccine. Vaccine (in press). [DOI] [PubMed]
- 24.WHO Market information for access to vaccines (MI4A) 2020. https://www.who.int/teams/immunization-vaccines-and-biologicals/vaccine-access/mi4a/mi4a-vaccine-purchase-data
- 25.Debellut F, Clark A, Pecenka C, et al. Re-evaluating the potential impact and cost-effectiveness of rotavirus vaccination in 73 Gavi countries: a modelling study. Lancet Glob Health. 2019;7:e1664–e1674. doi: 10.1016/S2214-109X(19)30439-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baral R, Nonvignon J, Debellut F, Agyemang SA, Clark A, Pecenka C. Cost of illness for childhood diarrhea in low- and middle-income countries: a systematic review of evidence and modelled estimates. BMC Public Health. 2020;20:619. doi: 10.1186/s12889-020-08595-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murray CJL, Lopez AD, WHO. World Bank & Harvard School of Public Health The global burden of disease: a comprehensive assessment of mortality and disability from diseases, injuries, and risk factors in 1990 and projected to 2020: summary. 1996. http://www.who.int/iris/handle/10665/41864
- 28.WHO . World Health Organization; Geneva: 2021. WHO preferred product characteristics for vaccines against Shigella. [Google Scholar]
- 29.WHO. UNICEF WHO/UNICEF estimates of national immunization coverage. 2020. https://www.who.int/teams/immunization-vaccines-and-biologicals/immunization-analysis-and-insights/global-monitoring/immunization-coverage/who-unicef-estimates-of-national-immunization-coverage
- 30.US Bureau of Labor Statistics Consumer Price Index Inflation Calculator. 2021. https://www.bls.gov/data/inflation_calculator.htm
- 31.Penny MA, Verity R, Bever CA, et al. Public health impact and cost-effectiveness of the RTS,S/AS01 malaria vaccine: a systematic comparison of predictions from four mathematical models. Lancet. 2016;387:367–375. doi: 10.1016/S0140-6736(15)00725-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen C, Cervero Liceras F, Flasche S, et al. Effect and cost-effectiveness of pneumococcal conjugate vaccination: a global modelling analysis. Lancet Glob Health. 2019;7:e58–e67. doi: 10.1016/S2214-109X(18)30422-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Puett C, Anderson JD, Bagamian KH, et al. Projecting the long-term economic benefits of reducing Shigella-attributable linear growth faltering with a potential vaccine: a modelling study. Lancet Glob Health. 2023;11:e892–e902. doi: 10.1016/S2214-109X(23)00050-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.George CM, Burrowes V, Perin J, et al. Enteric infections in young children are associated with environmental enteropathy and impaired growth. Trop Med Int Health. 2018;23:26–33. doi: 10.1111/tmi.13002. [DOI] [PubMed] [Google Scholar]
- 35.Hausdorff WP, Scheele S, Giersing BK. What drives the value of a Shigella vaccine? Vaccines (Basel) 2022;10:282. doi: 10.3390/vaccines10020282. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data for this study were taken from publicly available sources that are documented in this Article. Individual participant data were not used in this research. The model has been described in sufficient detail in the Article and appendix. There are no plans to make the model publicly available.
For the Demographic and Health Surveys see https://www.statcompiler.com/en