Abstract
Using a yeast two-hybrid human brain cDNA library screen, the cytoplasmic dynein light chain (LC8), a 10-kDa protein, was found to interact strongly with the phosphoprotein (P) of two lyssaviruses: rabies virus (genotype 1) and Mokola virus (genotype 3). The high degree of sequence divergence between these P proteins (only 46% amino acid identity) favors the hypothesis that this interaction is a common property shared by all lyssaviruses. The P protein-dynein LC8 interaction was confirmed by colocalization with laser confocal microscopy in infected cells and by coimmunoprecipitation. The dynein-interacting P protein domain was mapped to the 186 amino acid residues of the N-terminal half of the protein. Dynein LC8 is a component of both cytoplasmic dynein and myosin V, which are involved in a wide range of intracellular motile events, such as microtubule minus-end directed organelle transport in axon “retrograde transport” and actin-based vesicle transport, respectively. Our results provide support for a model of viral nucleocapsid axoplasmic transport. Furthermore, the role of LC8 in cellular mechanisms other than transport, e.g., inhibition of neuronal nitric oxide synthase, suggests that the P protein interactions could be involved in physiopathological mechanisms of rabies virus-induced pathogenesis.
Members of the Lyssavirus genus are nonsegmented negative-strand RNA viruses belonging to the Mononegavirales order, Rhabdoviridae family. On the basis of phylogenetic studies, seven genotypes have been distinguished among which genotype 1 (rabies virus, PV strain) and genotype 3 (Mokola virus) are the most divergent (5, 44). These enveloped viruses are responsible for rabies encephalomyelitis. Usually transmitted mechanically by bite, injury, or aerosol, lyssaviruses are highly neurotropic, migrating from inoculation point to the central nervous system (CNS) through peripheral nerves. Their viral cycle takes place in the cytoplasm, where the viral genetic information exclusively found in the form of a ribonucleoprotein (RNP) complex serves as a template for two distinct RNA synthesis functions: transcription of a leader RNA and 5′ capped and polyadenylated mRNAs encoding the different viral proteins (nucleoprotein [N], phosphoprotein [P], matrix protein [M], glycoprotein [G], and RNA polymerase [L]) and viral replication occurring in anti-genomic and new genomic RNA molecule synthesis. Transcription and replication are insured by the RNP complex composed of the L protein associated with the P protein and the genomic RNA tightly enwrapped by the N protein. The P protein via N:P complexes prevents nonspecific N protein aggregation while the L protein, considered the catalytic core, attaches to the N:RNA template through interactions with P. Thus, the P protein is considered to play a dual and pivotal role in this regulation. The P protein (297 amino acids [aa], PV strain [genotype 1]; 303 aa, Mokola virus [genotype 3]) is thought to be composed of two conserved domains: one is NH2 terminal (the first 60 aa residues) and the other is COOH terminal (encompassing residues 200 to 270) and a variable central hinge region. Both domains are implicated in various interactions with the N and L proteins (12, 13, 19) and with the P protein itself for homomultimerization (personal observation). Many discrete physiological changes in the neuron occurring during the course of viral infection have been described; these include changes in neurotransmitter release and binding (8, 26) and alterations of the actin-based cytoskeleton (10). Also, a dramatic increase of nitric oxide (NO) synthesis by activated macrophages or microglia via inducible NO synthase (iNOS) activity in the brains of rats infected with rabies virus and a parallel decrease of NO production in neurons regulated by neuronal NO synthase (nNOS) have been described (2, 21). Both types of deregulation seem to contribute to the neuropathogenesis of lyssavirus infection. Concerning axonal transport, several questions remain unsolved. The nature of the viral entity which is released into the cytoplasm and transported along the axon via a microtubule-dependent mechanism to the perikaryon is still unknown. It is not clear whether the RNP release takes place shortly after the synapse or only once it is in the perikaryon, implicating the retrograde transport of either the RNP or the endosomal vesicle, respectively. The following are unknown: which form of the newly synthesized viral constituents (RNP, M proteins, and G proteins) is transported, where they are assembled, and how they reach the next synapse, which seems to be the exclusive site from where the virus is transmitted. Some of these questions have been partially addressed; Gosztonyi suggested that within the axoplasm, the virus is probably carried in the form of an RNP (20). Outstanding questions regarding the viral cycle and the neurotropism of these viruses concern the participation of cellular factors beside neuronal receptors (29, 43, 46) in viral regulation and tropism. Such specific factors present in neuronal intracellular environment could be determinants for viral dissemination in the CNS and play a role at the replication level in neuronal perikarya, axonal transport, or transsynaptic spread.
In order to identify P protein-interactive cellular factors, the Saccharomyces cerevisiae two-hybrid approach (3, 17) was initially used with Mokola virus phosphoprotein (Pmok) as a bait. The diploid yeast strain CG1945:Y189 expressing the full-length Pmok gene fused to a GAL4 DNA binding domain (GAL4-BD) was transformed with a human brain cDNA library purchased from Clontech. This library was created in the pACTII vector by using whole brain mRNAs. The cDNAs obtained with both oligo(dT) and random priming are expressed in frame with the GAL4 activation domain (GAL4-AD). Five million clones were screened using the X-Gal (5- bromo-4-chloro-3-indolyl-β-d-galactopyranoside) overlay assay (18). Two main groups of positive clones were differentiated on the level of β-galactosidase activity (Table 1). Seventeen clones were found to be positive in ≤1 h (group A); 21 additional clones were positive in >1 h (group B). In group A, 47% (eight clones) contained sequences corresponding to cytoplasmic dynein light chain 1 (dynein LC8; GenBank accession no. Q15701), 23% (four clones) corresponded to a gene which is down-regulated in human immortalized cells and human tumor-derived cell lines (REIC/Dkk3 gene; GenBank accession no. AB034203), and 17% (three clones of Br5) corresponded to an open reading frame of unknown function found on human chromosome 22 (PAC clone DJ412A9; EST GenBank accession no. AA421950), and the remaining two clones corresponded to two single different sequences. The proportion of single different sequences dramatically increased to 81% (17 clones) in group B, while the proportion of the three other proteins decreased to 14% (3 clones) for LC8, 0% (no clone) for REIC/Dkk3 gene, and 5% (1 clone) for Br5. Sequencing of several clones classified in group C (very weak β-galactosidase activity) systematically identified sequences both nonredundant and different from those of the three candidates (not shown). Taken together, these results indicate that Pmok has specific and strong interactions with three different proteins encoded by cDNA of the library. A quantitative β-galactosidase liquid assay, using the LacZ chromogenic substrate ONPG (o-nitrophenyl-β-d-galactopyranoside) as previously described (35), established that the interaction of Pmok with each of the three proteins was of similar intensity (Table 2). Interestingly, the cytoplasmic LC8 sequences found in about 50% of the highly positive clones and in a total of 11 clones (8 from group A, 3 from group B) are characterized by GAL4-AD–LC8 fusion proteins in which the dynein-encoding sequence started in frame 78 to 102 nucleotides upstream from the classical start codon. In addition, an in-frame stop codon was systematically present between the GAL4-AD and the dynein open reading frames in such a way that the fusion protein could only be produced following a readthrough event. A similar feature was previously observed in two cDNA libraries screened either with the nNOS (22) or with IkB-α (15) as the bait. Both of these proteins demonstrated a strong interaction with cytoplasmic dynein LC8, and the selected sequence had a similar insertion of a 5′ untranslated sequence between the GAL4-AD and the dynein coding regions. This phenomenon probably represents a means of overcoming the observed toxicity for yeast cells when the LC8 coding region (89 aa) is fused in immediate contact to the GAL4-AD (construct denoted LC8 89aa). LC8 89aa in Table 2 resulted in very reduced growth of the yeast containing it, but the intensity of the interaction with Pmok detected by the quantitative β-galactosidase liquid assay was not affected (254 versus 202). In the following experiments, the genuine LC8 coding region was fused to either GAL4-AD or the GAL4-BD. Although LC8 is known to form dimers (4), this dimerization was not observed by the two-hybrid method due to the high toxicity resulting from coexpression of both LC8–GAL4-AD and LC8–GAL4-BD fusion proteins in yeast.
TABLE 1.
Screening of the cDNA library from human brain with the P protein of Mokola virusa
| Group | β-galactosidase activity in overlay assay | No. of clones | No. of clones per groupb
|
|||
|---|---|---|---|---|---|---|
| Dynein (LC8) | REIC/ Dkk3 | Clone Br5 | Other | |||
| A | ++ | 17 | 8 (47%) | 4 (23%) | 3 (18%) | 2 (12%) |
| B | + | 21 | 3 (14%) | 0 | 1 (5%) | 17 (81%) |
A total of 5 × 106 clones were tested.
LC8, dynein cytoplasmic light chain 1 (molecular mass of LC8, 10 kDa). REIC/Dkk3 is a gene whose expression is down-regulated in human immortalized cells and human tumor-derived cell lines. Clone Br5 matches the sequence of PAC clone DJ412A9 (EST GenBank accession no. AA421950, unknown function).
TABLE 2.
Quantitation of the Pmok interaction with the three major cDNA clones (preys) rescued from a human brain library screeninga
| Protein | β-galactosidase activity
|
|
|---|---|---|
| GAL4-BD | Pmok | |
| GAL4-AD | <1 | <1 |
| Dynein (LC8) | <1 | 202 |
| REIC/Dkk3 | <1 | 186 |
| Clone Br5 | <1 | 225 |
| LC8 89aa | <1 | 254 |
β-galactosidase activity is expressed in standard units multiplied by 1,000. GAL4-BD and GAL4-AD are controls with no insert in the vectors. LC8 89 aa corresponds to the direct fusion of the 89-aa coding fragment (deletion of the in-frame 5′ untranslated region) with the GAL4-AD domain.
In addition, the rabies virus P protein (PV strain) was also shown to exhibit a similarly strong interaction as Pmok with dynein LC8 (Table 3). This result reinforces the biological significance of this interaction since Mokola (genotype 3) and rabies (genotype 1) viruses have two of the most divergent genotypes of the Lyssavirus genus, sharing only 46% amino acid conservation in the P protein (5).
TABLE 3.
Mapping of the Pmok interaction domain with LC8 89aa (bait) and interaction of P-rabies virus/PV (genotype 1) with LC8 89aa
| Protein | β-galactosidase activity
|
|
|---|---|---|
| GAL4-BD | LC8 89 aa | |
| GAL4-AD | <1 | <1 |
| Pmok WT | <1 | 192 |
| PmokΔ187–303 | <1 | 42 |
| PmokΔ1–175 | <1 | <1 |
| PmokΔ57–303 | <1 | <1 |
| P-rabies virus/PV WT | <1 | 265 |
In order to delineate more precisely the dynein LC8-binding domain, two constructs containing truncated Pmok in fusion with GAL4-AD were made by PCR using a specific set of primers: PmokΔ187–303 corresponding to the 186 NH2-terminal amino acids and PmokΔ1–175 corresponding to the 128 COOH-terminal amino acids. These fragments overlap by 11 residues. Each mutant protein was tested for its interaction with the dynein LC8 by using the quantitative β-galactosidase liquid assay (Table 3). Only the NH2-terminal segment exhibited a significant interaction (although it was only 22% of the value obtained with full-length Pmok), suggesting that the normal structure or folding of the P protein is required for an optimal interaction. This interacting NH2 segment, carrying both a P homomultimerization domain (data not shown), an N-P interaction site (12, 19), and an RNA polymerase L interacting domain (13), is composed of a highly conserved NH2 terminus (aa 1 to 56, 75% identity between rabies and Mokola P proteins) followed by a more variable region (aa 57 to 185, 24% identity between rabies and Mokola P proteins). However, PmokΔ58–303, containing the highly conserved 56 residues of the NH2 end, did not exhibit any interaction with dynein LC8.
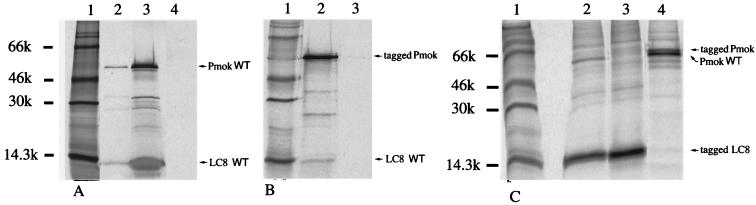
The interaction between dynein LC8 and Pmok has been verified by coimmunoprecipitation of the two proteins after in vitro coupled transcription-translation (TnT 7; Promega). Polyclonal anti-LC8 antibodies were able to coimmunoprecipitate LC8 and Pmok (Fig. 1A, lane 2). For the symmetrical coimmunoprecipitation, Pmok was Flag labeled with the IBI epitope (Kodak), because anti-Pmok specific antibodies were not available. Flag was added at the Pmok COOH terminus to avoid interference with the LC8 binding domain in the NH2 half of the P protein (see above). Anti-Flag antibodies coimmunoprecipitated the Flag-labeled Pmok and LC8 proteins (Fig. 1B, lane 2). These results confirm the strong interaction between Pmok and LC8 when the proteins are expressed in a mammalian system (rabbit reticulocyte lysate). This conclusion was verified ex vivo in neuroblastoma cells (Neuro 2A) transfected with plasmids expressing different Flag-labeled or non-Flag-labeled proteins and by subsequent immunoprecipitation with anti-Flag monoclonal antibody. Flag-labeled LC8 (IBI epitope at the NH2 side; apparent migration near the 14.3-kDa marker) was immunoprecipitated (Fig. 1C, lane 3) and was able to coimmunoprecipitate wild-type (WT) Pmok (Fig. 1C, lane 2) when the corresponding plasmids were cotransfected. Symmetrically, Flag-labeled Pmok was able to coimmunoprecipitate both WT Pmok from infected Neuro 2A cells and cellular LC8 (molecular mass, <14.3 kDa), although the latter is not very evident on the gel (Fig. 1C, lane 4). Taken together, these results indicate that Pmok is able both to form multimers and to establish strong interactions with LC8 in mammalian cells.
FIG. 1.
Coimmunoprecipitation of LC8 and P protein of Mokola virus. Pmok and LC8 genes were transcribed and translated in vitro using the TnT coupled reticulocyte lysate system (Promega) in the presence of [35S]methionine (>1,000 Ci/mmol; Amersham). The translated products (A, lane 3) were subjected to immunoprecipitation with protein A-Sepharose beads loaded with polyclonal anti-LC8 antibodies (A, lane 2) or with anti-Flag monoclonal antibody M2 (B, lane 2) or without any antibody (A, control lane 4, and B, control lane 3). Lanes 1, molecular size marker. Immunoprecipitates were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (20% polyacrylamide). (C) Ex vivo expression in Neuro 2A cells transfected with tagged LC8. The cells were labeled with [35S]methionine, and cell extracts were immunoprecipitated with anti-Flag monoclonal antibody M2 (lane 3). A similar experiment was performed after cotransfection with the Pmok WT (lane 2). Immunoprecipitation of cell extracts from Neuro 2A cells cotransfected with the LC8 WT and tagged Pmok plasmids, infected with Mokola virus (multiplicity of infection, 10) (lane 4).
Human cytoplasmic LC8 is an 89-aa protein (10 kDa) which is highly conserved in mammals (100% with rat LC8), insects (94% with Drosophila melanogaster), nematodes (92% with Caenorhabditis elegans), bacteria (92% with C. reinhardtii), metazoa (63% with Schistosoma mansoni), plants (62% with Arabidopsis thaliana), and yeast (49% with S. cerevisiae). LC8 has been implicated in a variety of cellular functions, from cytoskeletal motors to neurotransmitter regulation. Moreover, null mutations in the Drosophila LC8 gene (39) are lethal mutations characterized by strong alterations of neuronal anatomy, suggesting that Drosophila LC8 plays a role in the regulation of axogenesis. LC8 is also a component of both microtubule- (23) and actin-based (16) motors. For microtubule-based transport, LC8 is a subunit of the dynein-dynactin complex required for retrograde axonal transport directed toward the minus end of the microtubule, i.e., from the synapse to the neuronal cell body (1, 47).
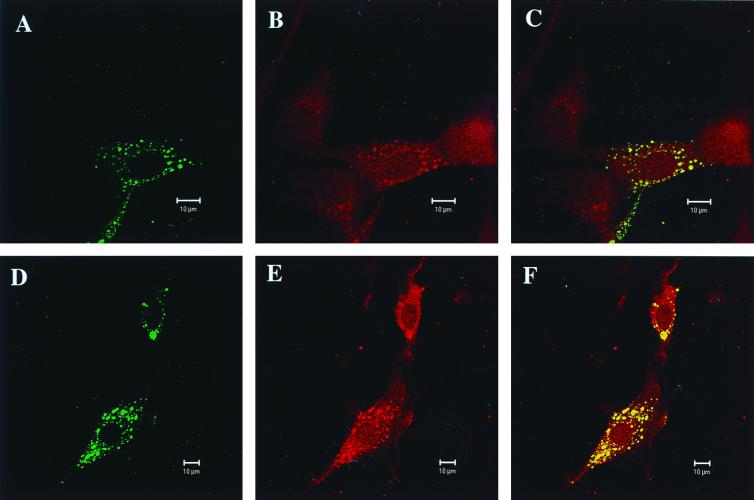
In order to appreciate cellular localization of LC8 and viral phosphoproteins during infection, confocal microscopy was performed. BHK-21 cells were infected for 48 h with either Mokola (Fig. 2A, B, and C) or rabies PV (Fig. 2D, E, and F) viruses. Infected cells were treated simultaneously with anti-LC8 rabbit polyclonal antibodies and with either anti-rabies RNP or anti-Mokola RNP mouse polyclonal antibodies. Immunostaining was carried out with either Texas Red or fluorescein-coupled secondary antibodies to detect rabbit (LC8) and mouse (RNP) primary antibodies, respectively. Fluorescein isothiocyanate staining exhibited a characteristic pattern consisting of a green punctuated perinuclear accumulation of RNP antigens (Fig. 2A and D). Texas Red staining (Fig. 2B and E) elicited a diffuse red cytoplasmic staining on the uninfected cells present in the microscopic field (Fig. 2B, uninfected cell on the right) and a punctuated accumulation of LC8 reminiscent of RNP inclusions in infected cells. Confocal microscopy and quantification analysis of the fluorogram (40) (not shown) clearly demonstrate the perfect colocalization of RNP inclusions and LC8 accumulation (Fig. 2C and F), arguing that the LC8-punctuated pattern resulted from trapping by RNP. Similar confocal microscopy results were obtained with an antibody directed against another component of the dynein complex: dynein LCA (data not shown). Since rabies virus has been shown to spread within the peripheral and central nervous systems through the axonal transport (9, 14, 25, 45), our data are in agreement with a model of viral nucleocapsid (RNP) transport along the microtubule network, as suggested by Gosztonyi (20), through the P protein-LC8 interaction. This dynein-driven transport could concern both the transport from the synapse to the perikaryon and the transport from the perikaryon of newly synthesized RNP within the dendrites to infect second-order neurons. Interestingly, the microtubule-mediated transport of another neurotropic virus (herpes simplex virus 1) (24), has also been shown to involve attachment of viral capsid to the nucleus (41).
FIG. 2.
Confocal microscopy analysis for LC8 and rabies virus or Mokola RNP. Analysis with a Zeiss Laser Scanning Microscope 510 of BHK-21 cells infected for 48 h with either Mokola (A, B, and C) or rabies (D, E, and F) viruses. Intracellular distribution in infected cells of RNP (A and D) immunostained with a fluorescein-coupled secondary antibody and LC8 (B and E) with a Texas Red secondary antibody. (C and F) Colocalization of both stainings characterized by yellow granulations. Also, note the diffuse punctuated staining characteristic of cellular LC8 visible in the uninfected cell (B), which is immediately on the right.
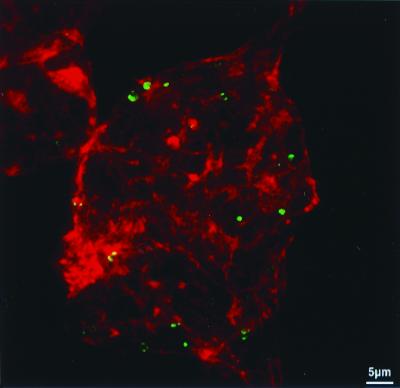
Considering LC8 participation in the myosin V complex implicated in actin-based motor transport of endoplasmic reticulum vesicles in brain neurons, a double labeling experiment of rabies virus RNP and F-actin was realized. Neuroblastoma cell lines (NIE-115) infected for 24 h with rabies virus (strain CVS) were labeled simultaneously for F-actin (Bodipy phalloidin) and for rabies virus RNP (anti-RNP fluorescent conjugate). Confocal microscopy revealed that the viral RNP inclusions are in close contact to F-actin fibers that cross the cytoplasm or lay beneath the cell membrane (Fig. 3). This observation is in agreement with the presence of actin in rabies virus particles (37) and previous studies showing the requirement of the actin network in the early steps of infection and its further alteration in late stages (10, 31).
FIG. 3.
Confocal microscopy analysis for F-actin and rabies virus RNP. Confocal microscopy analysis of F-actin and rabies virus RNP in N1E-115 cell lines infected for 24 h with CVS rabies virus (multiplicity of infection, 10). Cells were processed for detection of F-actin (with Bodipy phalloidin, red staining) and rabies virus RNP (with anti-RNP fluorescent conjugate, green staining) as described in the text. Observation was performed with a confocal laser scanning microscope (Wild Leitz Instruments, Heidelberg, Germany) which uses an argon-krypton laser operating in multi-line mode. Fluorescein conjugate and Bodipy 558-568-phalloidin were sequentially analyzed at 488- and 567-nm excitation wavelengths and detected, respectively, through a narrow-band filter centered at 535 nm and through a long-wave pass filter OG590. The 0.5-mm-wide optical section shows rabies virus RNP inclusions in close contact with the F-actin fibers.
The involvement of both actin- and microtubule-dependent motors is likely to be a general principle in trafficking and transport (36). Thus, a dual filament model for organelle transport in neurons has been proposed, in which microtubules provide the tracks for movement over long distances while actin filaments provide the tracks for movement locally (27, 28). According to this model, dynein LC8 could play a pivotal role in actin- and microtubule-based transport of rabies virus RNP and in the switch between the two phenomena.
In addition, LC8 has been identified in a two-hybrid screening as an inhibitor of nNOS (22). Three independent NOSs regulate the NO level in vivo (6, 7, 32, 34). One is iNOS and is expressed in a variety of cells (38, 42). The others are constitutively expressed NOS (cNOS) in specific tissues and are active in a Ca2+-dependent calmodulin response. The endothelial cNOS (ecNOS) is implicated in vasodilatation and blood flow regulation (33), and the neuronal cNOS (ncNOS) is required for N-methyl-d-aspartate (NMDA) receptor-mediated neurotoxicity and 3′,5′-cyclic GMP (cGMP) elevation and is also involved in neurotransmission, neuronal development, and apoptosis (11). Rabies virus infection in rat brain provokes in parallel an increase of the iNOS activity in macrophages or microglia that is correlated with clinical severity and a decrease of ncNOS activity (2). Thus, one might suggest that some of the NO synthesis changes that we observed during rabies virus infection are indeed due to interaction between P protein and LC8.
Recently, X-ray diffraction studies have resolved the structure of LC8 in the presence of an ncNOS-derived peptide (residues 225 to 237) and showed that two LC8 monomers form a homodimer (4, 30). These structural studies indicated that a D-T-x-I-Q-V-D-x sequence from ncNOS (x, any amino acid) could bind to the dimer and that T and V could be replaced, respectively, by S and by I or L. This work also suggested that the LC8 dimer could interact with human myosin V at the D-T-Q-I-Q-L-D sequence (residues 1652 to 1658), and it was noted that human dynamin has a D-S-W-L-Q-V-Q sequence (residues 760 to 766). Interestingly, a similar D-T-K-S-I-Q-I-Q sequence is found in position 140 to 147 in Pmok, and P-PV, in spite of a positional shift of 3 aa residues, presents a nearly analogous sequence (Table 4). This potential interaction site is within the 186-aa fragment required for interaction with LC8. This observation lends further weight to the hypothesis that P protein interaction with LC8 might directly affect NOS activity. In this respect, it will be important to determine whether a peptide corresponding to aa 140 to 147 of Pmok can interact with LC8 and displace NOS.
TABLE 4.
Sequence alignment of putative LC8-interacting domainsa
| Protein | Domain sequence |
|---|---|
| ncNOS peptide | D T(S) x I Q V(I/L) D x |
| Myosin V | D T Q I Q L D |
| Dynamin | D S W L Q V Q |
| PMok (140–147) | D T KS I Q I Q |
| P-PV (143–149) | D —b KS T Q T T |
x, any amino acid. Amino acids in parentheses are possible replacements.
—, space in sequence introduced for alignment.
Taken together, our data provide strong evidence for interactions between RNP and actin and microtubule networks, both of which are involved in early steps of viral entry and further axonal transport. Thus, LC8 might play a switching role between actin filaments and microtubules. Moreover, major changes in LC8 cellular distribution during infection could alter NO regulation or affect neurotransmitter via perturbed synaptic vesicle transport. We are currently investigating these potential physiopathological mechanisms of rabies virus-induced neuronal dysfunction.
Acknowledgments
We thank Pierre Legrain, Micheline Fromont-Racine, Jean-Christophe Rain, and Fredj Tekaia for helpful discussions and the generous gift of plasmids and yeast strains; Samie R. Jaffrey and Solomon H. Snyder for anti-LC8 antibodies; Janis Burkhardt for JH92 antibodies against dynein LCA; Pierre Perrin for Mokola and rabies virus stocks and anti-RNP antibodies; Raymond Hellio for technical expertise in confocal microscopy; and Arielle Blocker and Charlie Roth for helpful comments and suggestions. We are greatly indebted to Yvette Forteville for technical assistance.
The confocal microscope was purchased with a donation from Marcel and Liliane Pollack.
ADDENDUM
Results similar to those found in this article were obtained independently with a rat pheochromocytoma cDNA library using the P protein of CVS rabies virus strain by Raux et al. (39a).
REFERENCES
- 1.Ahmad F J, Echteverri C J, Vallee R B, Baas P W. Cytoplasmic dynein and dynactin are required for the transport of microtubules into the axon. J Cell Biol. 1998;140:391–401. doi: 10.1083/jcb.140.2.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akaike T, Weihe E, Schaefer M, Fu Z F, Zheng Y M, Vogel W, Schmidt H, Koprowski H, Dietzschold B. Effect of neurotropic virus infection on neuronal and inducible nitric oxide synthase activity in rat brain. J Neurovirol. 1995;1:118–125. doi: 10.3109/13550289509111016. [DOI] [PubMed] [Google Scholar]
- 3.Bartel P L, Fields S. Analyzing protein-protein interactions using two-hybrid system. Methods Enzymol. 1995;254:241–263. doi: 10.1016/0076-6879(95)54018-0. [DOI] [PubMed] [Google Scholar]
- 4.Benashski S E, Harrison A, Patel-King R S, King S M. Dimerization of the highly conserved light chain shared by dynein and myosin V. J Biol Chem. 1997;272:20929–20935. doi: 10.1074/jbc.272.33.20929. [DOI] [PubMed] [Google Scholar]
- 5.Bourhy H, Kissi B, Tordo N. Molecular diversity of the Lyssavirus genus. Virology. 1993;194:70–81. doi: 10.1006/viro.1993.1236. [DOI] [PubMed] [Google Scholar]
- 6.Bredt D S, Snyder S H. Nitric oxide: a physiologic messenger molecule. Annu Rev Biochem. 1994;63:175–195. doi: 10.1146/annurev.bi.63.070194.001135. [DOI] [PubMed] [Google Scholar]
- 7.Brenman J E, Bredt D S. Nitric oxide signaling in the nervous system. In: Doolittle R F, editor. Methods in enzymology. Vol. 269. New York, N.Y: Academic Press; 1996. pp. 119–129. [DOI] [PubMed] [Google Scholar]
- 8.Ceccaldi P E, Fillion M P, Ermine A, Tsiang H, Fillion G. Rabies virus selectively alters 5-HT1 receptor subtypes in rat brain. Eur J Pharmacol. 1993;245:129–138. doi: 10.1016/0922-4106(93)90120-x. [DOI] [PubMed] [Google Scholar]
- 9.Ceccaldi P E, Gillet J P, Tsiang H. Inhibition of the transport of rabies virus in the central nervous system. J Neuropathol Exp Neurol. 1989;48:620–630. doi: 10.1097/00005072-198911000-00004. [DOI] [PubMed] [Google Scholar]
- 10.Ceccaldi P E, Valtorta F, Braud S, Hellio R, Tsiang H. Alteration of the actin-based cytoskeleton by rabies virus. J Gen Virol. 1997;78:2831–2835. doi: 10.1099/0022-1317-78-11-2831. [DOI] [PubMed] [Google Scholar]
- 11.Chalimoniuk M, Strosznajder J. NMDA receptor-dependent nitric oxide and cGMP synthesis in brain hemispheres and cerebellum during reperfusion after transient forebrain ischemia in gerbils: effect of 7-nitroindazole. J Neurosci Res. 1998;54:681–690. doi: 10.1002/(SICI)1097-4547(19981201)54:5<681::AID-JNR13>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 12.Chenik M, Chebli K, Gaudin Y, Blondel D. In vivo interaction of rabies virus phosphoprotein (P) and nucleoprotein (N): existence of two N-binding sites on P protein. J Gen Virol. 1994;75:2889–2896. doi: 10.1099/0022-1317-75-11-2889. [DOI] [PubMed] [Google Scholar]
- 13.Chenik M, Schnell M, Conzelmann K K, Blondel D. Mapping the interacting domains between the rabies virus polymerase and phosphoprotein. J Virol. 1998;72:1925–1930. doi: 10.1128/jvi.72.3.1925-1930.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coulon P, Derbin C, Kucera P, Lafay F, Prehaud C, Flamand A. Invasion of the peripheral nervous systems of adult mice by the CVS strain of rabies virus and its avirulent derivate AvO1. J Virol. 1989;63:3550–3554. doi: 10.1128/jvi.63.8.3550-3554.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crepieux P, Kwon H, Leclerc N, Spencer W, Richard S, Lin R, Hiscott J. I kappaB alpha physically interacts with a cytoskeleton-associated protein through its signal response domain. Mol Cell Biol. 1997;17:7375–7385. doi: 10.1128/mcb.17.12.7375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Espindola F S, Cheney R E, King S M, Suter D M, Mooseker M S. Myosin-V and dynein share a similar light chain. Mol Biol Cell. 1996;7:372a. [Google Scholar]
- 17.Fields S, Sternglanz R. The two-hybrid system: an assay for protein-protein interactions. Trends Genet. 1994;10:286–292. doi: 10.1016/0168-9525(90)90012-u. [DOI] [PubMed] [Google Scholar]
- 18.Fromont-Racine M, Rain J C, Legrain P. Toward a functional analysis of the yeast genome through exhaustive two-hybrid screens. Nat Genet. 1997;16:277–282. doi: 10.1038/ng0797-277. [DOI] [PubMed] [Google Scholar]
- 19.Fu Z F, Zengh Y, Wunner W H, Koprowski H, Dietzschold B. Both the N- and the C-terminal domains of the nominal phosphoprotein of rabies virus are involved in the binding to the N protein. Virology. 1994;200:590–597. doi: 10.1006/viro.1994.1222. [DOI] [PubMed] [Google Scholar]
- 20.Gosztonyi G. Reproduction of lyssaviruses: ultrastructural composition of Lyssavirus and functional aspects of pathogenesis. In: Rupprecht C E, Dietzschold B, Koprowski H, editors. Lyssaviruses. Berlin, Germany: Springer-Verlag; 1994. pp. 43–68. [DOI] [PubMed] [Google Scholar]
- 21.Hooper D C, Ohnishi S T, Kean R, Numagami Y, Dietzschold B, Koprowski H. Local nitric oxide production in viral and autoimmune diseases of the central nervous system. Proc Natl Acad Sci USA. 1995;92:5312–5316. doi: 10.1073/pnas.92.12.5312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jaffrey S R, Snyder S H. PIN: an associated protein inhibitor of neuronal nitric oxide synthase. Science. 1996;274:774–777. doi: 10.1126/science.274.5288.774. [DOI] [PubMed] [Google Scholar]
- 23.King S M, Barbarese E, Dillman III J F, Patel-King R S, Carson J H, Pfister K K. Brain cytoplasmic and flagellar outer arm dyneins share a highly conserved Mr 8,000 light chain. J Biol Chem. 1996;271:19358–19366. doi: 10.1074/jbc.271.32.19358. [DOI] [PubMed] [Google Scholar]
- 24.Kristensson K, Lycke E, Roytta M, Svennerholm B, Vahlne A. Neuritic transport of herpes simplex virus in rat sensory neurons in vitro. Effects of substances interacting with microtubular function and axonal flow. J Gen Virol. 1986;67:2023–2028. doi: 10.1099/0022-1317-67-9-2023. [DOI] [PubMed] [Google Scholar]
- 25.Kucera P, Dolivo P, Coulon P, Flamand A. Pathways of the early propagation of virulent and avirulent rabies strains from the eye to the brain. J Virol. 1985;55:158–162. doi: 10.1128/jvi.55.1.158-162.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ladogana A, Bouzamondo E, Pocchiari M, Tsiang H. Modification of tritiated gamma-amino-n-butyric acid transport in rabies virus-infected primary cortical cultures. J Gen Virol. 1994;75:623–627. doi: 10.1099/0022-1317-75-3-623. [DOI] [PubMed] [Google Scholar]
- 27.Langford G M, Kuznetsov S A, Johnson D, Cohen D L, Weiss D G. Movement of axoplasmic organelles on actin filaments assembled on acrosomal processes: evidence for a barbed-end-directed organelle motor. J Cell Sci. 1994;107:2291–2298. doi: 10.1242/jcs.107.8.2291. [DOI] [PubMed] [Google Scholar]
- 28.Langford G M, Molyneaux B J. Myosin V in the brain: mutations lead to neurological defects. Brain Res Rev. 1998;28:1–8. doi: 10.1016/s0165-0173(98)00020-4. [DOI] [PubMed] [Google Scholar]
- 29.Lentz T L, Burrage T G, Smith A L, Crick J, Tignor G H. Is the acetylcholine receptor a rabies virus receptor? Science. 1982;215:182–184. doi: 10.1126/science.7053569. [DOI] [PubMed] [Google Scholar]
- 30.Liang J, Jaffrey S R, Guo W, Snyder S H, Clardy J. Structure of the PIN/LC8 dimer with a bound peptide. Nat Struct Biol. 1999;6:735–740. doi: 10.1038/11501. [DOI] [PubMed] [Google Scholar]
- 31.Lockhart B P, Tsiang H. Actin-independent maturation of rabies virus in neuronal cultures. J Gen Virol. 1991;72:2257–2261. doi: 10.1099/0022-1317-72-9-2257. [DOI] [PubMed] [Google Scholar]
- 32.Marletta M A. Nitric oxide synthase: aspects concerning structure and catalysis. Cell. 1994;78:927–930. doi: 10.1016/0092-8674(94)90268-2. [DOI] [PubMed] [Google Scholar]
- 33.Marsden P A, Schappert K T, Chen H S, Flowers M, Sundell C L, Wilcox J N, Lamas S, Michel T. Molecular cloning and characterization of human endothelial nitric oxide synthase. FEBS Lett. 1992;307:287–293. doi: 10.1016/0014-5793(92)80697-f. [DOI] [PubMed] [Google Scholar]
- 34.Masters B S, McMillan K, Sheta E A, Nishimura J S, Roman L J, Martasek P. Neuronal nitric oxide synthase, a modular enzyme formed by convergent evolution: structure studies of a cysteine thiolate-liganded heme protein that hydroxylates L-arginine to produce NO as a cellular signal. FASEB J. 1996;10:552–558. doi: 10.1096/fasebj.10.5.8621055. [DOI] [PubMed] [Google Scholar]
- 35.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1972. [Google Scholar]
- 36.Moreau V, Way M. In vitro approaches to study actin and microtubule dependent cell processes. Curr Opin Cell Biol. 1999;11:152–158. doi: 10.1016/s0955-0674(99)80019-2. [DOI] [PubMed] [Google Scholar]
- 37.Naito S, Matsumoto S. Identification of cellular actin within the rabies virus. Virology. 1978;91:151–163. doi: 10.1016/0042-6822(78)90363-x. [DOI] [PubMed] [Google Scholar]
- 38.Nomura Y, Kitamura Y. Inducible nitric oxide synthase in glial cells. Neurosci Res. 1993;18:103–107. doi: 10.1016/0168-0102(93)90013-g. [DOI] [PubMed] [Google Scholar]
- 39.Phillis R, Statton D, Caruccio P, Murphey R K. Mutations in the 8kDa dynein light chain gene disrupt sensory axon projections in the Drosophila imaginal CNS. Development. 1996;122:2955–2963. doi: 10.1242/dev.122.10.2955. [DOI] [PubMed] [Google Scholar]
- 39a.Raux H, Flamand A, Blondel D. Interaction of the rabies virus P protein with the LC8 dynein light chain. J Virol. 2000;74:10212–10216. doi: 10.1128/jvi.74.21.10212-10216.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Salamero J, Humbert M, Cosson P, Davoust J. Mouse B lymphocytes specific endocytosis and recycling of MHC class II molecules. EMBO J. 1990;11:3489–3496. doi: 10.1002/j.1460-2075.1990.tb07557.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sodeik B, Ebershold M W, Helenius A. Microtubule-mediated transport of incoming Herpes Simplex virus 1 capsids to the nucleus. J Cell Biol. 1997;136:1007–1021. doi: 10.1083/jcb.136.5.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sparrow J R. Inducible nitric oxide synthase in the central nervous system. J Mol Neurosci. 1994;5:219–229. doi: 10.1007/BF02736723. [DOI] [PubMed] [Google Scholar]
- 43.Thoulouze M I, Lafage M, Schachner M, Hartmann U, Cremer H, Lafon M. The neural cell adhesion molecule is a receptor for rabies virus. J Virol. 1998;72:7181–7190. doi: 10.1128/jvi.72.9.7181-7190.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tordo N, Badrane H, Bourhy H, Sacramento D. Molecular epidemiology of Lyssaviruses: focus on the glycoprotein and pseudogenes. Onderstepoort J Vet Res. 1993;60:315–323. [PubMed] [Google Scholar]
- 45.Tsiang H. Evidence for an intraaxonal transport of fixed and street rabies virus. J Neuropathol Exp Neurol. 1979;38:286–296. doi: 10.1097/00005072-197905000-00008. [DOI] [PubMed] [Google Scholar]
- 46.Tuffereau C, Benejean J, Roque Alfonso A-M, Flamand A, Fishman M C. Neuronal cell surface molecules mediate specific binding to rabies virus glycoprotein expressed by a recombinant baculovirus on the surfaces of lepidoptera cells. J Virol. 1998;72:1085–1091. doi: 10.1128/jvi.72.2.1085-1091.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Waterman-Storer C M, Karki S B, Kuznetsov S A, Tabb J S, Weiss D G, Langford G M, Holzbaur E L F. The interaction between cytoplasmic dynein and dynactin is required for axonal transport. Proc Natl Acad Sci USA. 1997;94:12180–12185. doi: 10.1073/pnas.94.22.12180. [DOI] [PMC free article] [PubMed] [Google Scholar]