Highlights
-
•
Hyperconnectivity of Sensorimotor and posterior Default Mode Networks during Moral Injury Retrieval in PTSD.
-
•
Enhanced and expanded functional connectivity of Sensorimotor Network during Moral Injury Retrieval in PTSD.
-
•
Higher subjective re-experiencing intensity reported in PTSD during Moral Injury retrieval.
-
•
Supramarginal gyrus may be a hub for re-experiencing/re-enactment processes in MI-related traumatic memory.
Keywords: PTSD, Trauma, Autobiographical memory, Sensorimotor, Moral injury
Abstract
Neural representations of sensory percepts and motor responses constitute key elements of autobiographical memory. However, these representations may remain as unintegrated sensory and motor fragments in traumatic memory, thus contributing toward re-experiencing and reliving symptoms in trauma-related conditions such as post-traumatic stress disorder (PTSD). Here, we investigated the sensorimotor network (SMN) and posterior default mode network (pDMN) using a group independent component analysis (ICA) by examining their functional connectivity during a script-driven memory retrieval paradigm of (potentially) morally injurious events in individuals with PTSD and healthy controls. Moral injury (MI), where an individual acts or fails to act in a morally aligned manner, is examined given its inherent ties to disrupted motor planning and thus sensorimotor mechanisms. Our findings revealed significant differences in functional network connectivity across the SMN and pDMN during MI retrieval in participants with PTSD (n = 65) as compared to healthy controls (n = 25). No such significant group-wise differences emerged during retrieval of a neutral memory. PTSD-related alterations included hyperconnectivity between the SMN and pDMN, enhanced within-network connectivity of the SMN with premotor areas, and increased recruitment of the supramarginal gyrus into both the SMN and the pDMN during MI retrieval. In parallel with these neuroimaging findings, a positive correlation was found between PTSD severity and subjective re-experiencing intensity ratings after MI retrieval. These results suggest a neural basis for traumatic re-experiencing, where reliving and/or re-enacting a past morally injurious event in the form of sensory and motor fragments occurs in place of retrieving a complete, past-contextualized narrative as put forth by Brewin and colleagues (1996) and Conway and Pleydell-Pearce (2000). These findings have implications for bottom-up treatments targeting directly the sensory and motoric elements of traumatic experiences.
1. Introduction
It is increasingly apparent that traumatic memories are not remembered, but instead relived as context-independent sensory and motor fragments in individuals with post-traumatic stress disorder (PTSD; Brewin et al., 1996, van der Kolk and Fisler, 1995). This reliving or re-experiencing of past traumatic events begs the question of how autobiographical memory processes are altered in this psychiatric condition, unique in its transparent linkage to life-threatening or terrifying experiences (American Psychiatric Association, 2013). While early models suggested a relatively basic, potentially modular, repository in the brain for autobiographical memories that was dissociable from perceptual and motor processes (Atkinson and Shiffrin, 1968, Fodor, 1983, Pylyshyn, 1984), more recent conceptualizations contend that memories must be grounded in sensory representations to explain how they once connected with the world (Barsalou, 1999, Barsalou, 2008, Damasio, 1989, Harnad, 1990, Ianì, 2019, Versace et al., 2014). This ‘bottom-up’ grounding of mental representations implies that memory-related processes rely, in part, on sensory affordances, with sensorimotor activations and their associated environmental stimuli becoming embodied in memory (Dijkstra and Zwaan, 2014, Foglia and Wilson, 2013, Tulving, 1995, Vogt, 2002). For example, a soldier who was once unable to help a wounded and dying comrade may re-experience the deep visceral and somatic experience of being “eaten up inside” at their inability to help while reliving vivid flashes of their comrade’s facial grimaces and anguished cries. The notion that sensorimotor representations contribute to autobiographical memory processes aligns with Brewin et al. (1996) Dual Representation Theory, which hypothesizes that traumatic events are encoded as two or more neural schemas that splinter higher-level self-referential information from lower-level sensory and affective information. Further, the self-memory system proposed by Conway and Pleydell-Pearce (2000) complements this notion, where event specific knowledge (ESK), or undifferentiated sensory-perceptual components that contribute to memory vividness, may lack organization into contextualizing autobiographical knowledge in PTSD. From these perspectives, traumatic memories may result from an imbalance or dissociation between the event’s sensory elements and its self- and time-bound context (Brewin, 2001, Herman, 1992, Janet, 1889, van der Kolk and Fisler, 1995, van der Kolk and Van der Hart, 1989), where decontextualized sensory fragments or ‘imprints’ are thought to form the basis of traumatic re-experiences or re-enactments (Brewin et al., 1996, Janet, 1889, Terr, 1991). Here, detachment from body-based perceptual experience or a ‘working-self’ (Conway and Pleydell-Pearce, 2000), as occurs during a dissociative response to an overwhelming event, may underly the inability to integrate sensorimotor information into an integrated, embodied autobiographical memory (van der Kolk and Fisler, 1995). In keeping with this hypothesis, experimentally induced out-of-body states are associated with deficits in episodic encoding of life events from a narrative first-person perspective in healthy adults (Bergouignan et al., 2014, Bergouignan et al., 2021).
One potential etiological impetus for the development of PTSD is moral injury (MI; Bryan et al., 2018, Farnsworth et al., 2014, Protopopescu et al., 2021, Williamson et al., 2018). MI is said to occur when an individual actively causes, witnesses, or fails to thwart an action that contradicts an internalized, embodied sense of morality or when a perceived betrayal occurs at the hand of an individual or organization with a duty of care (Litz et al., 2009, Nash, 2019, Te Brake and Nauta, 2022). Individuals with MI-related PTSD find themselves not only mired in deep feelings of guilt and shame (Brémault-Phillips et al., 2022, Nazarov et al., 2015, Vermetten and Jetly, 2018), but also in intrusive, sensorial, involuntary flashbacks of the morally injurious (traumatic) event (Brewin, 2015, Mensink et al., 2022). In instances of MI, threat to social group membership and/or mistrust in one’s social surroundings violates basic assumptions connected to survival (Copp, 2006). This can result in disordered relations between actions and outcomes and implications that the self and/or world is unpredictable and unsafe (Borges et al., 2022, Brewin et al., 1996, Foa et al., 1992). An inextricable link between sensorimotor processing and moral behavior can be gleaned from the defining components of morality: (1) the capacity to anticipate or predict the consequences of one’s own actions; (2) the ability to judge the value of certain events or actions; and (3) the capability of choice between reflexive responses and reflective alternative courses of action (Ayala, 2010). Morality, in some conceptualizations, is thus reserved for intentions or desires that are transformed into action, a complex process contingent upon the efficient operation of multiple convergent and redundant sensorimotor loops (Bizzi and Ajemian, 2020). As such, sensorimotor representations of morally injurious events are likely distributed across several neural networks underlying sensory integration, motor planning, and motor output (Versace et al., 2014, Wilson, 2002), a hypothesis in line with the multimodal reactivation of past events during memory retrieval (Brunel et al., 2009, Niedenthal et al., 2005). One such network may include the sensorimotor network (SMN), initially activated during the previously transpired somatosensory perception and action during the event (Bietti, 2012, Nyberg et al., 2001, Nyberg, 2002, Wheeler, 2000). Despite this, little is known about this network’s involvement in MIs and autobiographical memory processing more broadly.
Autobiographical memory processes engage the brain’s default mode network (DMN; Ino et al., 2011, Philippi et al., 2015, Spreng and Grady, 2010), a midline cortical network underlying self-referential processing and internal mentation (Qin and Northoff, 2011, Raichle, 2015). The more posterior aspects of this network constitute a reliably discernable DMN subcomponent (Damoiseaux et al., 2006, Lei et al., 2014) and contribute to the visual imagery and re-experiencing aspects of memory (Bréchet et al., 2018, Buckner et al., 1995, Fletcher et al., 1995, Knyazev et al., 2015) which may rely on functional inputs from the sensorimotor network (SMN; Beckmann et al., 2005, Heba et al., 2017). DMN functional connectivity has repeatedly been shown to be disrupted in PTSD (Akiki et al., 2018, Barredo et al., 2018, Bluhm et al., 2009, Koch et al., 2016; for reviews, see Lanius et al., 2010, Lanius et al., 2020, St Jacques et al., 2013, Wang et al., 2016) including in PTSD related to MI (Terpou et al., 2022). In particular, a decoupling of the DMN’s anterior and posterior nodes is prevalent (Akiki et al., 2017, Bluhm et al., 2009, DiGangi et al., 2016, Miller et al., 2017, Sripada et al., 2012), which may impact the anterior DMN’s ability to monitor and contextualize posterior (p)DMN-mediated online re-experiencing (Thome et al., 2019). However, MI-related PTSD symptomatology may correlate with disruptions not only in DMN functioning, but also in sensorimotor processes related to an incomplete moral action or to the completion of an action inconsistent with moral values. In essence, whereas the pDMN reflects upon and recreates personal narratives, supporting sensorimotor neural networks are necessary to put representations of intended (or unintended) action into motion and give them life.
A recent meta-analysis of seed-based resting-state functional connectivity (rsFC) studies in PTSD reported the SMN to be consistently hyperconnected to the DMN and affective brain networks, with these SMN-bound network alterations most commonly associated with PTSD-specific neurobiology and not merely trauma exposure (Bao et al., 2021). Although numerous rsFC studies of PTSD and trauma-related conditions have revealed altered connectivity with the pre- and/or post-central gyri (Chen et al., 2019, Harricharan et al., 2019, Leroy et al., 2022, Luo et al., 2022, Rabellino et al., 2018, Shaw et al., 2002), a paucity of studies have utilized these regions (or this network) as regions-of-interest (ROIs) for functional connectivity analyses (Bao et al., 2021). Functional connectivity differences have previously been found during script-driven imagery in PTSD as compared to trauma-exposed controls (Lanius et al., 2004), while the left postcentral gyrus appears to respond with increased activation to morally injurious memory recall in individuals with PTSD as compared to potential MI-exposed controls (Lloyd et al., 2021). Despite the growing body of evidence indicating SMN- and DMN-related alterations in participants with PTSD while at rest, relatively little is known about the functional connectivity of these networks during the recollection of a traumatic and/or morally injurious memory. This has the potential to have important treatment implications, particularly regarding sensorimotor approaches targeting the body’s somatic and visceral responses during MI (e.g., traumatic) memory recall (Levine, 2010, Ogden et al., 2006, Warner et al., 2014).
The current study therefore examined the functional connectivity of the sensorimotor network (SMN) and posterior default mode network (pDMN) during MI memory retrieval in PTSD and healthy controls, where both groups were exposed to (potentially) morally injurious events. Whereas main hubs of the pDMN are involved in online re-experiencing of emotional memory (Bréchet et al., 2018, Daselaar et al., 2008, Cavanna and Trimble, 2006, Gilboa et al., 2004, Knyazev et al., 2015, Spreng et al., 2009), the SMN is responsible for processing somatosensation and eliciting volitional action. We aimed further to use global measures of PTSD severity and dissociative symptoms, along with group dimensional analyses, in relation to symptomatology that exists on a continuum (Akiki et al., 2018). To our knowledge, this study is the first to examine SMN functional network connectivity on its own or in parallel with pDMN functional network connectivity during autobiographical memory retrieval of a morally injurious event. Based on the literature described, we hypothesized that: i) individuals with PTSD + MI would exhibit hyperconnectivity between the SMN and DMN in line with that observed in PTSD during resting state scanning (Bao et al., 2021); ii) individuals with PTSD + MI would show alterations to SMN functionality during script-driven MI memory retrieval, and iii) these alterations would increase in prominence as a function of increased symptom severity.
2. Methods
Participants were recruited via advertisements distributed in the London, Ontario community and local mental health treatment centers. Our sample included 90 participants in total; 65 participants who had a primary diagnosis of PTSD with concurrent exposure to a morally injurious event (PTSD + MI), and 25 control participants who had been exposed to a potentially morally injurious event but did not have any psychiatric diagnoses. Part of this sample has previously been analyzed by Lloyd et al. (2021) and Terpou et al. (2022). Exclusion criteria for both groups included past or current bipolar disorder, past or current psychotic disorder, substance use disorder within the 3 months prior to study participation, any neurological or developmental disorder, serious untreated medical illness, history of head injury involving loss of consciousness, and/or incompatibility with MRI safety standards. Additional exclusion criteria for control group-assigned participants included any current or past psychiatric disorder. Group demographic and clinical information is presented in Table 1. This study was approved by the Health Sciences Research Ethics Board at Western University, and participants were financially compensated for their time and participation.
Table 1.
Demographic and clinical information.
| Control | PTSD | |
|---|---|---|
| Participants, N |
25 | 65 |
| Female, N (%yes) |
16 (64.0%) | 30 (46.2%) |
| Age (years)* |
33 ± 11 | 48 ± 11† |
| Clinician-Administered PTSD Scale, total score* | 1 ± 3 | 40 ± 8† |
| Childhood Trauma Questionnaire, total score* | 32 ± 8 | 57 ± 24† |
| Beck Depression Inventory, total score* |
5 ± 8 | 29 ± 10† |
| Multiscale Dissociation Inventory, total score* | 37 ± 6 | 62 ± 18† |
| Moral Injury Events Scale, total score* |
23 ± 9 | 36 ± 7† |
| Major Depressive Disorder, recurrent, n | 0 | 40 |
| RSDI Reliving Difference Score (Reliving MI – Reliving Neutral)* | 0.83 ± 0.89 (n = 23) |
1.72 ± 1.05† (n = 61) |
PTSD = Post traumatic stress disorder; M = male; F = female.
*Values represent mean +/- standard deviation.
Significant differences in clinical symptom values relative to controls based on independent samples t-tests (p < 0.05).
2.1. Clinical questionnaires and interviews
For all participants, the Clinician-Administered PTSD Scale-5 (CAPS-5; Weathers et al., 2013) was administered to assess for clinical PTSD diagnosis and severity of PTSD symptoms. The Moral Injury Events Scale (MIES; Nash et al., 2013), an 11-item self-report scale, was used as a metric to determine presence and severity of moral injury; items 8–9 were omitted due to their specificity regarding military experience. The MIES uses a Likert scale ranging from 1 (strongly disagree) to 6 (strongly agree); all included participants selected a 6 in response to at least one item as a determination of exposure to a potentially morally injurious event. In addition, the Structured Clinical Interview for DSM-IV Axis-I Disorders (SCID; First et al., 2002) along with a battery of questionnaires were administered, including the Multiscale Dissociation Inventory (MDI; Briere et al., 2005), the Beck Depression Inventory (BDI; Beck et al., 1997), and the Childhood Trauma Questionnaire (CTQ; Bernstein and Fink, 1998).
During the initial clinical assessment, two individualized memory scripts consisting of eight short sentences were created for each participant in collaboration with the clinical assessor in line with prior autobiographical memory studies on psychological trauma (e.g., Hopper and van der Kolk, 2001, Palombo et al., 2015). The first script described a ‘neutral’ event devoid of positive or negative emotional valence (e.g., a trip to the grocery store) and the second script described a (potentially) morally injurious event. Definitions of MI were provided to the participant, and the clinical assessor supported participants in selecting an event which elicited strong moral emotions. The chosen MI memory script reflected a 5–8/10 SUDS level (subjective units of distress scale; 10 being most intense/severe) to elicit sufficiently strong moral emotions (while avoiding overwhelm) and to match subjective distress intensity between groups.
This memory paradigm was utilized to provoke neurobiological alterations in PTSD, which can be strengthened by self-report measures assessing for symptoms as perceived by the participants. Thus, items adapted from the Responses to Script-Driven Imagery Scale (RSDI), a brief self-report measure of state PTSD and dissociative symptoms (Hopper et al., 2007), were administered after each condition using a 1–4 Likert scale. Scores of 1 and 4 indicated low and high frequency/intensity, respectively. For the re-experiencing item (did you feel as though past traumas were recurring, like you were reliving them?) difference scores (MI Remember – Neutral Remember) were calculated to determine reliving intensity during MI retrieval in relation to individuated baselines.
2.2. fMRI protocol/experimental procedures
A schematic of the fMRI paradigm is presented in Fig. 1. Pre- and post-experiment resting-state scans were conducted, each of eight-minute duration. For the Neutral Memory condition, participants were exposed to their neutral memory script, presented sentence by sentence to encourage participants to focus on details of the memory as vividly as possible (as opposed to a general impression of the memory as a whole). Sentences were presented visually and aurally in a neutral affective tone through MR-compatible headphones. After each sentence, participants were instructed to recall the memory in detail for 25 s. Following recall, a virtual avatar with either direct or averted gaze was presented (for the purposes of future analyses of the effect of direct eye gaze on social cognitive and threat processing), followed by a fixation cross. This experimental paradigm was then repeated for the moral injury memory script (creating the Moral Injury Remember condition). After each block (Neutral, Moral Injury) participants were asked to rate the presence/intensity of re-experiencing symptoms from the RSDI (Hopper et al., 2007) and of moral emotions (e.g. shame). In keeping with standard methods, stimuli were presented in a fixed order (neutral followed by morally injurious) for all subjects to prevent the effects of MI retrieval carrying over into the neutral memory condition (Bremner et al., 1999, Lanius et al., 2003). Stimuli were presented using E-Prime 3.0 software (Psychology Software Tools, Inc., 2016).
Fig. 1.
fMRI paradigm. Pre- and post-experiment resting-state scans were conducted, each of eight-minutes duration. For experimental conditions, participants were first exposed to their neutral memory script, followed by their moral injury memory script. Scripts consisted of eight sentences and were presented visually and aurally in a neutral affective tone, sentence by sentence. Following each sentence, participants were instructed to recall that part of the memory for 25 seconds. Following retrieval, a virtual avatar with either direct or averted gaze was displayed, followed by a fixation cross. This sequence was repeated for each sentence, for both Neutral Remember and Moral Injury Remember conditions.
2.3. fMRI image acquisition
Structural and functional brain images from each subject were acquired using a 3-T MRI Scanner (Biograph mMR; Siemens Medical Solutions) and a Siemens 32-channel head coil locally adapted to the scanner’s 4-plug interface. Participants’ heads were stabilized with foam padding to minimize motion artefacts. Collection of orthogonal scout images enabled prescription of a tri-dimensional T1-weighted anatomical image of the whole head with 1 mm isotropic resolution (Magnetization Prepared Rapid Gradient Echo- MP-RAGE). Transverse acquisition of functional whole-brain images with blood-oxygen-level-dependent (BOLD) contrast was conducted using the manufacturer’s gradient echo T2*-weighted blipped-echo-planar sequence (TR = 3000 ms, TE = 20 ms, FOV = 256 × 256 mm, flip angle = 90°, voxel size 2 mm isotropic, parallel imaging acceleration factor = 4). For experimental conditions, each volume consisted of 60 ascending interleaved slices and each experimental run consisted of 118 volumes. For the resting-state scans, each volume consisted of 60 ascending interleaved slices and each run consisted of 160 volumes.
2.4. fMRI data pre-processing & first-level specifications
Preprocessing of all (resting-state and experimental) functional images was conducted using the default preprocessing pipeline in the CONN toolbox (version 21.a; Whitfield-Gabrieli and Nieto-Castanon, 2012) operating within SPM12 (Wellcome Department of Cognitive Neurology) and MATLAB R2021b (Mathworks, Inc.). Although we did not conduct resting-state analyses, resting-state data were included for better approximation of underlying neural networks in ICA. The default pipeline includes functional realignment and unwarping, slice-time correction, structural segmentation, normalization to MNI (Montréal Neurological Institute) space, ART-based identification of outlier scans (Gabrieli Lab, McGovern Institute), and smoothing using a Gaussian kernel set to 6 mm FWHM.
After pre-processing, condition parameters, within-subjects (first-level) covariates, and between-subjects (second-level) covariates were specified. For the experimental conditions, onset times and durations were entered to allow for condition-specific individual subject-level maps, representing a measure of network expression and connectivity at each voxel, to be generated and later entered into second-level analyses. The following onset and duration times were entered: Neutral [Read; Remember; Avatar; Fixation (baseline)] and Moral [Read; Remember; Avatar; Fixation(baseline)]. This approach allows exploration of main effects of the Neutral and Moral Injury Remember conditions on BOLD signal time series.
CONN employs a de-noising step at the first level, which applies linear regression and band-pass filtering to remove unwanted motion, physiological effects, and other artefacts from the BOLD signal before connectivity measures are computed (Whitfield-Gabrieli and Nieto-Castanon, 2017). Here, noise components from cerebrospinal fluid (5 parameters) and white matter (5 parameters) were removed using anatomical component base noise reduction method (aCompCor) and scrubbing of ART-based identified outlier scans was performed. Offending scans were identified across all subjects and conditions (including resting-state and experimental conditions) and entered as regressors; an average of 8.77 (S.D. 13.57) volumes were scrubbed. 12 motion parameters (6 motion parameters and 6 first-order temporal derivatives) were included as additional covariates. As a quality control measure, each participant’s histogram of original and de-noised voxel-to-voxel connectivity (r-values) were visually inspected to ensure they were approximately normally distributed after de-noising. No outlier subjects were identified or removed.
3. Statistical analyses
3.1. Demographics and clinical information
In SPSS (v. 28.0.0.0), Fisher’s exact test was utilized to determine any difference in sex between groups (two-tailed, p < 0.05), independent samples t-tests were conducted to determine groupwise differences in age and clinical scores (two-tailed, p < 0.05), and a spearman’s rank order correlation was employed to assess the correlation between CAPS scores and RSDI reliving difference scores (two-tailed, p < 0.05).
3.2. Group independent component analysis
Independent component analysis (ICA) is a data-driven, computational approach used to identify whole-brain, voxel-wise functional network connectivity patterns (Calhoun et al., 2001, Calhoun et al., 2009). These functional network connectivity patterns are reflective of the temporal correlations among spatially distinct brain regions at the network level. Functional networks are defined through spatial correlations with masks composed of the key intrinsic connectivity networks reliably identified in multivariate decompositions of both resting-state and task-based fMRI data (Allen et al., 2011, Damoiseaux et al., 2006, Laird et al., 2009, Smith et al., 2009, Zuo et al., 2010). Here, we ran a group-ICA using Calhoun’s group-level approach (Calhoun et al., 2001) in CONN, where subject/condition BOLD signal data was concatenated along the temporal dimension across all participants (N = 90) to analyze inter-subject and inter-group differences regarding independent component spatial extent and amplitudes (Whitfield-Gabrieli and Nieto-Castanon, 2012). First, the number of components was estimated a-priori using the Group ICA of fMRI Toolbox (GIFT; Calhoun et al., 2001) across all subjects and conditions, which revealed 11 components using MDL (i.i.d. Sampling) criteria (mean = 11.2694, S.D. = 4.7917, median = 11). Therefore, we estimated 11 independent components within CONN, which utilizes the same computational algorithms as GIFT when conducting spatiotemporal group-ICA analysis (Calhoun et al., 2001) but is geared toward connectivity analyses and group comparisons. The group-level ICA approach in CONN implements the component-based noise correction method (CompCor) strategy for noise reduction, incorporates variance normalization pre-conditioning, and performs group-level dimensionality reduction (set to 64), fast-ICA, and GICA1 back-projection into single participant spatial maps and time courses (Calhoun et al., 2001, Whitfield-Gabrieli and Nieto-Castanon, 2012). Back-projected individual subject maps represent a measure of network expression and connectivity at each voxel along both spatial and temporal dimensions, which can be entered into second-level analyses for comparisons of functional network connectivity between groups and/or conditions (see Second-level Statistical Analyses).
3.3. Identification of functional connectivity networks using ICA
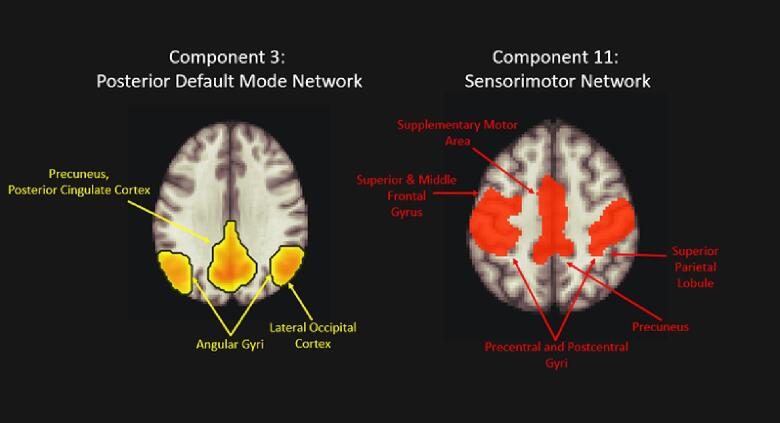
Group-ICA results were visually inspected for artefact or noise-related components (Component 2 revealed a diffuse network along the lateral edges of the cortex, which was deemed artifactual from motion and/or inhomogeneities in the magnetic field (Griffanti et al., 2017)). A spatial match to template revealed correlation (Pearson’s r) values for each of the 11 estimated independent components with spatial masks within the CONN toolbox (Default Mode, SensoriMotor, Visual, Salience, Dorsal Attention, Frontoparietal, Language, and Cerebellar). Of these networks, we aimed to focus on components correlating with the sensorimotor network (SMN), encompassing the pre- and post-central gyri extending from the superior bank of the Sylvian fissure to the medial aspect of the interhemispheric fissure, as well as the supplementary motor area (Beckmann et al., 2005, Heba et al., 2017) and default mode network (DMN). The highest correlation values for these networks were gleaned from component 3 with the default mode network (DMN; r = 0.489), and component 11 with the sensorimotor network (r = 0.422). Component 3 included key posterior DMN (pDMN) hubs (precuneus, posterior cingulate cortex, bilateral angular gyri) as well as lateral occipital cortices, and component 11 was comprised of the bilateral precentral and postcentral gyri, supplementary motor area, superior and middle frontal gyri, superior parietal lobule, and an anterior portion of the precuneus (Fig. 2).
Fig. 2.
Components 3 and 11, derived from ICA estimating 11 components, which were carried forward to statistical analyses. Component 3 correlated highest with the posterior nodes of the default mode network (r = 0.489), and Component 11 correlated highest with the sensorimotor network (r = 0.422). ICA. Independent Component Analysis.
3.4. Second-level statistical analyses
Groupwise comparisons of functional network connectivity were conducted by entering the back-projected subject-level spatial maps for each condition (Neutral Remember, MI Remember), representing estimated network expression and connectivity at each voxel, into second-level analyses. This approach utilizes unmasked data, organized into a matrix of voxel-to-voxel connectivity values, to characterize associations between the independent components (networks) and each voxel of the brain. Individual subject maps were assigned group membership using dummy-coded second-level covariates (Control and PTSD). As individual subject-level spatial maps were created for each condition, this allowed for condition-specific between-group comparisons. Thus, group differences could be interpreted as differences in independent component (network) expression and connectivity across the whole brain within the examined Neutral Remember and Moral Injury Remember conditions. We also ran correlations between functional network connectivity values of each network (SMN, DMN) and clinical scores for the CAPS, MDI total score, and MDI depersonalization/derealization subscale. This was conducted by entering a vector of scores as a co-variate at the second level for each clinical assessment.
Voxel-wise thresholding was set to p < 0.001 uncorrected (two-tailed). For between-group analyses of functional network connectivity, the significance threshold was set to p < 0.05 FDR-corrected for cluster-level significance. For the three correlational analyses with clinical scores (CAPS, MDI total, MDI depersonalization/derealization), we applied Bonferroni corrections (p < 0.05/3), resulting in a significance threshold of p < 0.017 cluster-level FDR-corrected. Brain regions were labeled using three methods: (1) CONN’s built-in brain atlas based on a combination of the Harvard-Oxford [https://fsl.fmrib.ox.ac.uk/fsl/fslwiki/Atlases] and Automatic Anatomical Labeling (AAL; Tzourio-Mazoyer et al., 2002) atlases, (2) the Neuromorphometrics atlas in SPM12 (Wellcome Department of Cognitive Neurology), and (3) visual inspection.
3.5. Post-hoc graph theoretical metrics
Post-hoc graph theoretical metrics were employed to further investigate the global properties of the between-group differences in functional network connectivity across the pDMN and SMN. Region of interest (ROI)-level graph metrics can provide a deeper and more nuanced understanding of the functional organization of brain regions or ‘nodes’, including the global efficiency of the nodes in communicating information and which nodes have ‘hub-like’ properties (Rubinov and Sporns, 2010, Shaw et al., 2021). Thus, ROIs included in the analysis were derived from the significant group comparison results found within the Moral Injury Remember condition for both the pDMN and SMN; this approach elaborates upon our results and provides insights into a nascent understanding of the global properties of PTSD-specific network differences (Akiki et al., 2018, Shaw et al., 2021). ROI masks were extracted from thresholded groupwise results (Table 2, Table 3) for both components and then included in an ROI-to-ROI analysis in CONN. For the ROI-to-ROI analysis, pairwise bivariate correlation coefficients were computed between each pair of ROIs using their preprocessed BOLD timeseries, which were Fisher transformed to permit groupwise comparisons (Rubinov and Sporns, 2010). A graph adjacency matrix was extracted from the ROI-to-ROI connectivity (RRC) matrix, and results were thresholded using an edge-defining threshold (cost-level) of 0.15 (one-sided) and FDR-correction threshold of pFDR < 0.05 (two-sided). From here, measures of network topology, including node-wise functional integration (global efficiency, path length), segregation (local efficiency, clustering coefficient) and centrality (betweenness centrality, cost, degree) were explored (for further discussion of graph theoretical measures, see Achard and Bullmore, 2007, Rubinov and Sporns, 2010). Notably, this post-hoc exploration of network properties was solely meant to elaborate on our existing results; network-restricted analyses using independently defined ROIs are required in future studies of how the pDMN and SMN behave in terms of network topology (Akiki et al., 2018).
Table 2.
Group contrasts for independent component 3: posterior DMN.
| Condition | Contrast | Brain region | Cluster size (k) | MNI coordinates ×, y, z |
t statistic (peak) |
z-score (peak) |
pFDR (cluster) |
|---|---|---|---|---|---|---|---|
| Neutral Memory | Control > PTSD | n.s. | |||||
| Neutral Memory | PTSD > Control | n.s. | |||||
| MI Memory | Control > PTSD | n.s. | |||||
| MI Memory | PTSD > Control | Lateral occipital cortex (superior), Cuneus, Occipital pole (L) | 361 | −6, −88, 20 | 5.301 |
4.924 |
<0.001 |
| Occipital pole, Cuneus (R) | 123 | 10, −88, 24 | 5.116 |
4.773 |
0.001 | ||
| Lateral occipital cortex (superior), Occipital pole (R) | 69 | 24, −86, 36 | 4.186 |
3.986 |
0.017 | ||
| Lateral occipital cortex (inferior) (R) | 56 | 40, −88, 4 | 4.356 |
4.133 |
0.025 | ||
| Lateral occipital cortex (inferior) (L) | 48 | −44, −70, 2 | 4.330 |
4.111 |
0.040 | ||
| Superior parietal lobule (L) | 142 | −30, −56, 58 | 4.661 |
4.394 |
0.001 | ||
| Supramarginal gyrus, Postcentral gyrus (L) |
67 |
−64, −30, 28 |
4.289 |
4.076 |
0.017 |
||
| Supramarginal gyrus, Postcentral gyrus, Superior parietal lobule (L) | 63 | −36, −40, 46 | 4.446 |
4.211 |
0.065 | ||
| Precentral gyrus, Precentral gyrus (L) | 61 |
−8, −36, 58 |
4.745 | 4.465 |
0.019 |
||
| Supramarginal gyrus, Postcentral gyrus (R) | 101 | 30, −38, 38 | 4.395 |
4.167 |
0.003 |
A whole-brain, voxel-wise approach was utilized in correlating functional network connectivity across the pDMN-related independent component (3); two-tailed results were voxelwise thresholded at p < 0.001, and a significance threshold of pFDR < 0.05 (cluster-corrected) was applied.
pDMN = posterior default mode network; MI = Moral Injury; PTSD = posttraumatic stress disorder; MNI = Montreal Neurological Institute; FDR = false discovery rate.
Table 3.
Group contrasts for independent component 11: SMN.
| Condition | Contrast | Brain region | Cluster size (k) | MNI coordinates ×, y, z |
t statistic (peak) |
z-score (peak) |
pFDR (cluster) |
|---|---|---|---|---|---|---|---|
| Neutral Memory | Control > PTSD | n.s. | |||||
| Neutral Memory | PTSD > Control | n.s. | |||||
| MI Memory | Control > PTSD | Central operculum, Parietal operculum | 49 | −50, −20, 16 | 4.23 |
4.03 |
0.041 |
| MI Memory | PTSD > Control | Superior parietal lobule, Angular gyrus, Supramarginal gyrus, Lateral occipital cortex (superior) (R) | 1219 | 28, −66, 48 | 5.441 |
5.038 |
<0.001 |
| Angular gyrus, Lateral occipital gyrus (superior) (L) | 93 59 |
−42, −68, 52 −26, −72, 42 |
4.208 4.605 |
4.006 4.346 |
0.005 0.023 |
||
| Superior/middle frontal gyrus, Precentral gyrus (R) | 247 | 30, 6, 66 | 4.534 |
4.287 |
<0.001 | ||
| Precuneus (L) | 158 | −8, −68, 50 | 4.449 |
4.214 |
0.019 | ||
| Precuneus (R) |
89 | 10, −62, 52 | 4.238 | 4.031 | 0.005 | ||
| Supramarginal gyrus (L) | 66 | −50, −46, 58 | 4.186 | 3.986 | 0.017 |
A whole-brain, voxel-wise approach was utilized in correlating functional network connectivity across the SMN-related independent component (11). Two-tailed results were voxelwise thresholded at p < 0.001, and a significance threshold of pFDR < 0.05 (cluster-corrected) was applied.
SMN = sensorimotor network; MI = Moral Injury; PTSD = posttraumatic stress disorder MNI = Montreal Neurological Institute; FDR = false discovery rate.
4. Results
4.1. Demographic and clinical information
Demographic information and clinical scores are presented in Table 1. No statistically significant difference in the ratio of males to females between groups emerged (p = 0.09). Significant differences were found between the groups for age; therefore, we further examined whether an effect of age on BOLD signal differed for the PTSD group versus Control group across conditions. We conducted a 2 × 2 full-factorial ANOVA (group × condition) and entered ages of control participants and ages of PTSD participants as separate covariates in SPM to investigate whether the effect of age on BOLD signal differs for PTSD versus controls; no significant results emerged in component 3 nor 11.
As anticipated, the PTSD group had significantly higher scores on the CAPS, MDI, and MIES. High co-occurrence of depressive symptoms in PTSD, including overlapping symptomatology such as sleep disturbances, social withdrawal, and diminished interest in previously enjoyed activities, was reflected by significantly higher scores on the BDI in the PTSD group. Significantly higher scores on the CTQ were also observed among the PTSD group; this finding is reflective of the general population, where early childhood traumatic childhood experiences may predispose individuals toward the development of PTSD (Ackerman et al., 1998, Mikulincer et al., 2015, Ogle et al., 2015). Nevertheless, further exploration of the effect of CTQ scores on BOLD signal failed to demonstrate significant effects.
For available RSDI re-experiencing/reliving intensity scores (Control = 23, PTSD = 61), a significantly higher differential increase from the NR condition to MIR condition was shown for PTSD participants (n = 61, mean = 1.72, S.D. = 1.05) as compared to control participants (n = 23, mean = 0.83, SD = 0.89) (p < 0.001).
4.2. Between-group comparisons of functional network connectivity of the SMN and DMN
4.2.1. Posterior default mode network (pDMN)
An F-test revealed significant between-group differences across Neutral Remember (NR) and Moral Injury Remember (MIR) conditions. Follow-up post-hoc two-tailed t-tests explored between-group differences in functional network connectivity. No significant between-group differences were found for the NR condition. For the MIR condition, participants with PTSD showed increased pDMN functional network connectivity with the left pre/postcentral gyrus (pFDR = 0.019) and bilateral supramarginal gyri extending into the postcentral gyri (L: pFDR = 0.017 and 0.065; R: pFDR = 0.003). Increased connectivity was also found with the bilateral lateral occipital cortex/cuneus/occipital pole (L: pFDR < 0.001 and 0.040; R: pFDR = 0.001, 0.017 and 0.025) and left superior parietal lobule (pFDR < 0.001). Overall, increased pDMN network functional connectivity was found with nodes of the SMN (pre/postcentral gyri) extending into the SMG, as well as visual processing regions. Results are presented in Table 2 and Fig. 3.
Fig. 3.
Group contrast of PTSD greater than Control for independent component 3 (posterior DMN) in Moral Injury Memory and Neutral Memory conditions. The posterior DMN-correlated component shows increased functional network connectivity with the bilateral postcentral gyri, left precentral gyrus, left supramarginal gyrus, left superior parietal lobule, and bilateral occipital regions in the Moral Injury Remember condition only. Overlays are non-thresholded and thus are for visualization purposes only. Network labels in brackets depict networks those regions are typically associated with. DMN = default mode network; PTSD = posttraumatic stress disorder; SMN = sensorimotor network.
4.2.2. Sensorimotor network (SMN)
An F-test revealed significant group-wise differences across NR and MIR conditions. Post-hoc two-tailed t-tests explored between-group differences in functional connectivity. No between-group differences for the NR condition emerged. In the MIR condition, participants with PTSD showed increased functional network connectivity of the SMN with the bilateral angular gyri, where the right cluster spread into the superior parietal lobule, supramarginal gyrus and superior lateral occipital cortex (pFDR < 0.001) and two left clusters spread to the superior lateral occipital gyrus (pFDR = 0.005 and 0.023). Further, clusters were revealed within the right precentral gyrus extending into the superior and middle frontal gyri (pFDR < 0.001), bilateral precuneus (R: pFDR = 0.005; L: pFDR < 0.001) and left supramarginal gyrus (pFDR = 0.017). Here, the SMN showed right-lateralized enhancement and extension into premotor regions, as well as increased functional connectivity with nodes of the pDMN (precuneus, angular gyri), the SMG, and occipital regions. The MI-exposed control participants exhibited increased functional network connectivity with a cluster extending from the central to the parietal operculum as compared to PTSD (pFDR = 0.041). Results are presented in Table 3 and Fig. 4(A,B).
Fig. 4.
(A,B). Group contrasts for independent component 11 (SMN) in Moral Injury Memory and Neutral Memory conditions. Overlays are non-thresholded and thus are for visualization purposes only. Network labels in brackets depict networks these regions are typically associated with.
4A: PTSD greater than controls The SMN exhibited increased functional network connectivity with the right precentral gyrus, right superior and middle frontal gyri, bilateral precuneus, right supramarginal gyrus/angular gyrus/superior parietal lobule/lateral occipital cortex, left supramarginal gyrus, and left angular gyrus/lateral occipital cortex in the Moral Injury Remember condition only.
4B: Controls greater than PTSD The SMN exhibited increased functional connectivity with the left central/parietal opercula in the Moral Injury Remember condition only.
DMN = default mode network; SMN = sensorimotor network; PTSD = posttraumatic stress disorder.
4.2.3. Post-hoc graph theoretical analysis
No significant groupwise differences in global network properties among the above thresholded nodes emerged during the Neutral Remember condition. By contrast, in the Moral Injury Remember condition, graph metrics revealed significantly increased global efficiency (R: T(88) = 3.78, beta = 0.08, pFDR = 0.005; L: T(88) = 3.09, beta = 0.11, pFDR = 0.024), degree (R: T(88) = 2.99, beta = 1.39, pFDR = 0.032; L: T(88) = 2.84, beta = 1.09, pFDR = 0.033), and cost (L: T(88) = 2.99, beta = 0.08, pFDR = 0.032; R: T(88) = 2.84, beta = 0.06, pFDR = 0.033) for the node encompassing the right (R) angular gyrus/SMG (x,y,z = 37, −58, 51) and the node comprised of the left (L) SMG (x,y,z = -45, −45, 55) in PTSD compared to controls. Increased global efficiency indicates that those with PTSD transfer information more efficiently through these parietal regions while increased degree and cost suggest increased density of neighboring connections and thus some ‘hub-like’ properties of these nodes during recall of a MI-related memory (Rubinov and Sporns, 2010). Fig. 5 visualizes this phenomenon by presenting ROI-to-ROI analysis results for the PTSD > Control contrast (cluster-level threshold pFDR < 0.05, connection threshold p < 0.05 uncorrected), highlighting the hub-like properties of these nodes in the PTSD group.
Fig. 5.
ROI-to-ROI analysis and graph metrics results for PTSD > Control within the MI Remember condition, where ROIs were derived from groupwise comparison results (Table 2, Table 3) to depict global properties of PTSD-related functional network connectivity alterations. Red nodes depict significant (pFDR < 0.05, two-sided) graph theory metric changes in increased global efficiency, degree, and cost of the left supramarginal gyrus and right supramarginal/angular gyrus in coronal (left) and transverse (right) views. Connections (‘edges’) between ROIs were derived from ROI-to-ROI analysis for the PTSD > Control contrast (cluster-level pFDR < 0.05, two-sided) and are for visualization purposes. PTSD = post-traumatic stress disorder. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
4.3. Clinical correlations
The following available clinical scores from all participants were correlated with network-based functional connectivity of the SMN and DMN: CAPS severity scores (N = 85), MDI total (N = 84), and MDI depersonalization(dep)/derealization(der) subscale (N = 84). Significant correlations with functional network connectivity of the DMN and SMN emerged within the Moral Injury Remember condition only as depicted below and presented in Table 4 and Fig. 6. Further, RSDI reliving difference scores (Reliving MI Remember – Reliving Neutral Remember) were found to have a significant positive correlation with CAPS severity scores (N = 82, ρ = 0.404, p < 0.001).
Table 4.
Clinical correlations with functional network connectivity of the pDMN and SMN.
| Independent component | Condition | Clinical score (direction) | Brain region | Cluster size (k) | MNI coordinates ×, y, z | t statistic (peak) | z-score (peak) |
pFDR (cluster) |
|---|---|---|---|---|---|---|---|---|
| 3: pDMN |
Neutral Memory |
CAPS total (+) | n.s. | |||||
| CAPS total (-) | n.s. | |||||||
| MDI total (+) | n.s. | |||||||
| MDI total (-) | n.s. | |||||||
| MDI dep/der (+) | n.s. | |||||||
| MDI dep/der (-) | n.s. | |||||||
| Moral Injury Memory | CAPS total (+) | Lateral occipital cortex (superior), Cuneus (L) | 373 | −6, −88, 20 | 5.064 |
4.721 |
<0.001 | |
| Lateral occipital cortex (inferior) (L) | 85 | −44, −70, 2 | 5.055 |
4.713 |
0.009 | |||
| Cuneus, Occipital pole (R) | 113 | 12, −90, 22 | 4.958 |
4.634 |
0.003 | |||
| Postcentral gyrus, precentral gyrus Superior parietal lobule (R) | 82 | 30, −38, 38 | 4.365 |
4.134 |
0.009 | |||
| CAPS total (-) | n.s. | |||||||
| MDI total (+) | Precentral gyrus, Postcentral gyrus (R) | 86 | 32, –32, 58 | 5.001 |
4.658 |
0.016 | ||
| MDI total (-) | Medial prefrontal cortex | 104 | 0, 52, −12 | 4.395 |
4.150 |
0.012 | ||
| MDI dep/der (+) | Cuneus, occipital pole (R) | 125 | 10, −90, 24 | 4.6 |
4.33 |
0.002 | ||
| Precentral gyrus, Postcentral gyrus, Supramarginal gyrus (R) | 120 | 58, −20, 42 | 4.9 | 4.58 |
0.002 | |||
| Planum temporale (L) | 105 | −40, −28, 10 | 4.85 | 4.53 |
0.003 | |||
| MDI dep/der (-) | n.s. | |||||||
| 11: SMN | Neutral Memory | CAPS total (+) | n.s. | |||||
| CAPS total (-) | n.s. | |||||||
| MDI total (+) | n.s. | |||||||
| MDI total (-) | n.s. | |||||||
| MDI dep/der (+) | n.s. | |||||||
| MDI dep/der (-) | n.s. | |||||||
| Moral Injury Memory | CAPS total (+) | Superior parietal lobule, Supramarginal gyrus, Angular gyrus (R) | 752 | 28, −66, 50 | 5.202 |
4.833 |
<0.001 | |
| Precentral gyrus, superior/middle frontal gyrus (R) | 198 | 28, 8, 68 | 4.443 |
4.201 |
<0.001 | |||
| Precuneus, Superior parietal lobule (L) | 182 | −8, −68, 50 | 4.4778 |
4.231 |
<0.001 | |||
| CAPS total (-) | n.s. | |||||||
| MDI total (+) | n.s. | |||||||
| MDI total (-) | n.s. | |||||||
| MDI dep/der (+) | n.s. | |||||||
| MDI dep/der (-) | Lateral occipital cortex (inferior) (L) | 147 | −34, −80, −2 | 4.202 |
3.987 |
0.007 | ||
All correlational results were thresholded at p < 0.001 (two-tailed) and a significance threshold of pFDR < 0.017 (cluster-corrected) was utilized after Bonferroni corrections.
pDMN = posterior default mode network; SMN = Sensorimotor Network; MI = Moral Injury; PTSD = posttraumatic stress disorder; CAPS = Clinician Administered PTSD Scale (5th ed.); dep/der = depersonalization/derealization subscale; MNI = Montreal Neurological Institute; FDR = false discovery rate.
Fig. 6.
Clinical correlations of CAPS total severity scores, MDI total scores, and MDI depersonalization/derealization subscales with functional network connectivity of the posterior DMN and SMN during the Moral Injury Remember condition for all participants. No significant correlations emerged for any clinical scores in either network during the Neutral Remember condition. Overlays are non-thresholded and thus are for visualization purposes only. Network labels in brackets depict networks these regions are typically associated with.
Panels A-C represent functional network connectivity of the pDMN as correlated with (A) CAPS total scores, (B) MDI total scores, and (C) MDI depersonalization/derealization subscales.
(A): CAPS total scores correlated positively with pDMN recruitment of right precentral/postcentral gyrus (extending into the superior parietal lobule; pFDR = 0.009) and bilateral lateral occipital cortex/cuneus (L: pFDR < 0.001 and 0.009/ R: pFDR = 0.003).
(B) Increased dissociative symptoms (MDI total scores) correlated positively with pDMN recruitment of precentral/postcentral gyri (pFDR = 0.016), regions typically associated with the SMN, and negatively with recruitment of the medial prefrontal cortex (pFDR = 0.012), a key anterior hub of the DMN.
(C) MDI dep/der subscales correlated positively with pDMN recruitment of right cuneus/occipital pole (pFDR = 0.002), right postcentral gyrus extending into supramarginal gyrus (pFDR = 0.002); and left planum temporale/Heschl’s gyrus (pFDR = 0.003).
Panels D-F represent functional network connectivity of the SMN as correlated with (D) CAPS total scores, (E) MDI total scores, and (F) MDI depersonalization/derealization subscales.
(D): CAPS total scores correlated positively with recruitment of the right precentral gyrus (a region associated with the SMN) extending into the middle/frontal gyri (pFDR < 0.001), right supramarginal gyrus and angular gyrus (the latter typically associated with the pDMN; (pFDR < 0.001), and left precuneus (a region of the posterior DMN; pFDR < 0.001) into the SMN.
(E): No significant correlations emerged for the MDI total scores with functional connectivity of the SMN.
(F): MDI depersonalization/derealization scores correlated negatively with the left lateral occipital cortex (pFDR = 0.007).
CAPS = Clinician Administered PTSD Scale; dep/der = depersonalization/derealization; DMN = default mode network; MDI = Multiscale Dissociation Inventory; SMN = sensorimotor network.
5. Discussion
In the present study, we explored the functional network connectivity of the SMN and the posterior DMN during a script-driven memory retrieval paradigm in participants with moral injury-related PTSD (PTSD + MI) and controls who had experienced a potentially morally injurious event. Our stimuli consisted of an emotionally provocative, individualized memory script as well as a neutral memory script to highlight differences in memory processes specific to MI. Group-ICA was employed to define these networks and compare their functional network connectivity differences across the two groups. ICA defined a component reflecting the SMN (pre- and postcentral gyri, supplementary motor area) as well as a component corresponding to the posterior DMN (pDMN; posterior cingulate cortex, precuneus, and angular gyri). The pDMN-correlated component likely reflects a DMN subsystem governing autobiographical memory elaboration, first-person perspective, and visual imagery processes (Cabeza and St Jacques, 2007, Qin and Northoff, 2011, Raichle et al., 2001, Ritchey and Cooper, 2020). Interestingly, posterior regions of the DMN have been shown to be more modular and/or disrupted in PTSD as evidenced by reduced resting-state functional connectivity across the DMN (Akiki et al., 2017, Bluhm et al., 2009, DiGangi et al., 2016, Miller et al., 2017, Sripada et al., 2012).
Significant between-group alterations in functional network connectivity emerged across the pDMN and SMN-related components during the MI Remember condition; no such significant differences were observed in the Neutral Remember condition. These alterations, expanded upon below, include hyperconnectivity between nodes of the pDMN and SMN, increased functional network connectivity and expansion of the SMN, and increased recruitment of the SMG and occipital regions into both networks in individuals with PTSD + MI during moral injury recall. Post-hoc graph metrics exploring network-restricted properties revealed increased ‘hub-like’ properties of the bilateral SMG extending into the right angular gyrus, a region previously associated with action sequencing-related information processing, relative to other PTSD-specific pDMN and SMN functional connectivity alterations. Correlational analysis of PTSD severity scores revealed a similar pattern of findings to groupwise comparisons during MI recall, which occurred in tandem with a significant positive correlation between differential increases in in situ re-experiencing/reliving intensity ratings and PTSD severity, suggesting that such neurobiological alterations may occur on a dimensional basis and relate to intensified re-experiencing/reliving symptomatology. Additionally, increased MDI scores (corresponding to greater dissociative symptoms) correlated with diminished coherence across posterior and anterior DMN hubs and increased recruitment of primary auditory, visual, and somatosensory regions into the pDMN. Overall, these results provide neurobiological evidence for the possibility that fragmented sensorimotor representations (Brewin et al., 1996) or event-specific knowledge (Conway and Pleydell-Pearce, 2000) of morally injurious memories lack appropriate integration into an autobiographical knowledge base during recall of MI among individuals with PTSD. Critically, decoupling of the anterior and posterior DMN may correspond with a lack of monitoring and contextualization during morally injurious memory retrieval in PTSD with dissociative symptoms (Thome et al., 2019), resulting in sensorimotor influences infiltrating or accentuating posterior DMN re-experiencing processes (Conway and Pleydell-Pearce, 2000). Increased functional network connectivity with primary sensory processing regions may contribute toward disruptions to DMN coherence in PTSD (Akiki et al., 2018, Barredo et al., 2018, Sripada et al., 2012; for reviews, see Koch et al., 2016, Lanius et al., 2010, Lanius et al., 2020, St Jacques et al., 2013, Wang et al., 2016). Of clinical importance, these results highlight how sensorimotor approaches to psychotherapy may be relevant for memory re-contextualization and healing in those with MI-related PTSD.
5.1. Hyperconnectivity between the SMN and pDMN during MI recall in PTSD
In PTSD + MI as compared to controls, regions of the SMN-correlated component were more functionally connected with the pDMN-correlated component and vice versa, suggesting spatiotemporal overlap and altered functionality of these networks during MI memory retrieval. The pDMN recruited the left primary somatosensory cortex, while the SMN coupled with the precuneus and angular gyri. Using clinical correlations as an adjunctive analysis, SMN-DMN hyperconnectivity was found to correlate positively with CAPS scores indexing PTSD symptomatology along a continuum. Notably, these findings corroborate the meta-analysis by Bao and colleagues (2021), where resting-state coupling of the SMN and DMN was found to be most reflective of a clinical PTSD diagnosis (Bao et al., 2021).
Though hypothetical, DMN-SMN hyperconnectivity may reflect a failure of individuals with PTSD + MI to have organized the sensorimotor features of the morally injurious event into a historical narrative, leading to the intrusion of unintegrated, fragmented sensorimotor imprints (or event-specific knowledge as depicted by Conway and Pleydell-Pearce, 2000) of the traumatic event during recall (Brewin et al., 1996, Janet, 1909). Generally, the DMN’s role in abstract internal thought processes contrasts with the SMN’s concern with the physical ‘here-and-now’ (Margulies et al., 2016, Smallwood et al., 2021). Hyperconnectivity of these networks may therefore support accounts of timeless re-experiencing/reliving of traumatic experiences (Brewin et al., 1996, van der Kolk and Van der Hart, 1989). A putatively unsuccessful integration of the event’s sensorimotor elements into self-referential processing or narratives may leave the residual sensory and motoric fragments without context, meaning, or anchored accommodation (Conway and Pleydell-Pearce, 2000, Janet, 1909, van der Kolk, 2006).
5.2. Enhancement and extension of the SMN during MI recall in PTSD
A PTSD-related pattern of enhanced functional network connectivity of the SMN with the right precentral gyrus, which expanded into the posterior portions of the superior and middle frontal gyri (SFG/MFG), was evident from both groupwise comparisons and CAPS severity correlations during MI retrieval. The posterior regions of the SFG and MFG, or Brodmann’s area 8 (Petrides and Pandya, 2012), are involved in motor processes given their dense composition of pyramidal cells as well as their proximity to and functional connectivity with the precentral gyrus (El-Baba and Schury, 2022, Li et al., 2013). In particular, the right posterior SFG is involved in modulating intentional motor inhibition and motor urgency (Hu et al., 2016, Schel et al., 2014). Potentially, this enhanced and expanded SMN functional network connectivity during MI-related memory recall may reflect an exaggeration of the purported sensorimotor imprints of the trauma and a preparedness for action or inaction triggered by recollection (implicit or explicit) of the past event (Janet, 1909, van der Kolk and Van der Hart, 1989).
5.3. Supramarginal gyrus as a hub in concert with the DMN and SMN: Toward re-enactment
The supramarginal gyrus (SMG), an inferior parietal region considered part of the temporo-parietal junction (TPJ), revealed increased functional connectivity with both the pDMN and SMN as well as hub-like characteristics in consideration of network functionality in PTSD + MI participants compared to controls during MI recall. The SMG has been implicated in the mental sequencing and simulation of action-related memory, suggesting a vital role in the cognitive reconstruction of action sequences (Grèzes and Decety, 2000, Guidali et al., 2019, Ohgami et al., 2004, Rumiati, 2003, Sirigu et al., 1996, Stephan et al., 1995, Vingerhoets et al., 2011). The SMG is also central to the ‘enactment effect’, a phenomenon where action-related words are better remembered when physically acted out versus read or heard (Nilsson, 2000, Russ et al., 2003), indicating a supporting role in memory processes. In addition, transcranial magnetic stimulation (TMS) over the posterior aspect of the SMG disrupts one’s sense of bodily orientation (Kheradmand et al., 2015), suggesting a role in multimodal integration and bodily self-consciousness (Bekrater-Bodmann et al., 2014, Blanke et al., 2015, Serino et al., 2013). Thus, alterations to SMG functionality may impact one’s kinesthetic awareness of their body in extrapersonal space, ultimately impacting motor planning (Ben-Shabat et al., 2015, Mountcastle et al., 1975). Speculatively, a lack of differentiation of the pDMN and SMN from the SMG suggests these networks may adapt to enhance SMG-mediated processes such as re-enactment and the underlying sequencing of action-related memory. Here, a repeated sequencing of an act misaligned with moral intention (“I should have acted differently”) may govern autobiographical narratives. Critically, traumatic re-enactments, or automatic experiences of reliving the past, are frequently reported amongst those with PTSD (Birrer et al., 2007, Brewin, 2015, Lanius et al., 2020, Reynolds and Brewin, 1998, van der Kolk, 1989, van der Kolk and Van der Hart, 1989) and are described as a complete disconnect from the ‘here and now’ (Brewin et al., 1996, Ehlers et al., 2004) as partially governed by the SMG’s mediation of body in space.
5.4. The central/parietal operculum in concert with the SMN: Toward cortical multisensory integration for embodied emotional memory processing
The left central/parietal operculum emerged as less functionally connected with the SMN in the PTSD + MI participants as compared to controls during MI recall. This anterior temporal region is heavily involved in multisensory integration at the cortical level (Blakemore et al., 2005, Bowsher et al., 2004, Dieterich and Brandt, 2018, Frank and Greenlee, 2018, Lopez et al., 2012, Macaluso and Driver, 2001, Yu et al., 2018). In particular, the parietal operculum is considered the secondary sensory area (SII; Bowsher et al., 2004) and cortical hub for vestibular processing (Huber et al., 2021, Ibitoye et al., 2022, zu Eulenburg, 2012), integrating feedback from the body’s posture and location in space to give rise to an embodied first-person perspective (Deroualle et al., 2015, Dieterich and Brandt, 2015, Ferrè et al., 2014, Ibitoye et al., 2022, Pfeiffer et al., 2014). Intriguingly, the parietal operculum is activated in rodents during the formation and storage of pain-related memories (Debowska et al., 2011), particularly those with emotional salience (Sacco and Sacchetti, 2010), and may thus be where integrated sensory content is incorporated into emotional memory. Here, decreased functional network connectivity of the SMN with the central and parietal opercula during MI recall in PTSD may reflect deficient integration of sensorimotor representations into an embodied first-person representation of the morally injurious event (Ibanez et al., 2010, Yu et al., 2018). While many individuals with PTSD are thought to lack the capacity to harness emotional awareness to guide effective action (Frewen et al., 2012, Lanius et al., 2011), particularly during traumatic memory recall (Frewen et al., 2008, van der Kolk, 2006), it is conversely possible that an inability to carry out effective action alters the ability to process and embody emotionally laden memories.
5.5. Dissociative symptom Correlations: MDI total and depersonalization/derealization subscale
Although we did not include the dissociative subtype of PTSD (PTSD + DS) as a separate group in our analysis due to an underpowered sample size for PTSD + DS, this subtype is substantially prevalent in the general population, occurring in up to 44% of individuals with PTSD (Blevins et al., 2014, Lanius et al., 2010, Wolf et al., 2012, Wolf et al., 2017; for reviews, see Hansen et al., 2017, White et al., 2022). Accordingly, we co-varied MDI scores with the functional network connectivity of the SMN and pDMN-related components to explore effects of dissociative symptom severity during MI retrieval.
Higher levels of dissociative symptoms, previously associated with increased traumatization frequency and severity (Deen et al., 2022, Stein et al., 2013, Schiavone et al., 2018), correlated with reduced functional network connectivity between the pDMN and the medial prefrontal cortex (mPFC). The mPFC is a main anterior hub of the DMN governing semantic and contextual contributions to autobiographical memory (Buckner and Carroll, 2007, Cabeza and St Jacques, 2007, Svoboda et al., 2006). This diminished coherence is in line with previous findings of greater segregation among DMN regions in PTSD (Akiki et al., 2018, Bluhm et al., 2009, Clancy et al., 2020, DiGangi et al., 2016, Miller et al., 2017, Sripada et al., 2012). Disconnect of the posterior DMN regions involved in re-experiencing and emotional processes from the anterior DMN may occur at the expense of online monitoring and contextualization in those with higher dissociative symptoms (Barredo et al., 2018, Buckner et al., 2008, Daniels et al., 2011, Frewen et al., 2020, Kim, 2010, Philippi et al., 2015, van der Kolk et al., 2005).
In addition, MDI total scores correlated positively with pDMN functional connectivity with a cluster within the right pre- and postcentral gyri. To explore the extent to which the characteristic depersonalization and derealization (dep/der) symptoms of PTSD + DS were driving this correlation, we also co-varied the MDI dep/der subscale which revealed a significant positive correlation of pDMN with the right postcentral gyrus/SMG, right occipital pole, and left planum temporale/Heschl’s gyrus. Increasing pDMN hyperconnectivity with primary sensorimotor, visual, and auditory processing regions suggests widespread neural disinhibition of cortically mediated sensory influences on MI-related memory processes in those with more severe dissociative symptoms. Contrarily, MDI dep/der symptoms correlated negatively with SMN functional recruitment of the left lateral occipital cortex. We speculate that suppression of visual processes in SMN functionality may bias the individual with high dissociative symptoms toward sensorimotor re-experiencing while altering exteroceptive visual perception as experienced in derealization. Nevertheless, future research differentiating between PTSD and PTSD + DS is warranted to explore potential differences in SMN functional connectivity between these groups.
6. Limitations and Future Directions
A limitation of this study was that we did not identify those participants with PTSD who met criteria for the dissociative subtype (PTSD + DS) as a separate group. Thus, traumatogenic dissociative symptomatology may have contributed to heterogeneity within our PTSD group. Nevertheless, correlating MDI scores allowed us to explore the effects of dissociative symptoms as a transdiagnostic phenomenon, occurring on a spectrum of intensity and frequency. Moreover, our study was limited to MI-related PTSD; further research is required with other forms of traumatic events to generalize these results to PTSD with different etiologies. The spatial resolution of the current dataset was not conducive to exploring brainstem-level functional connectivity. Phylogenetically ancient motor circuitry originating from the brainstem and limbic system has been hypothesized to provide foundational stability for higher-order self-referential processing (Panksepp, 1998), and thus higher spatially resolute imaging analyses of brainstem and midbrain roots of sensorimotor processing from which cortical sensorimotor circuits may reverberate (Doubell et al., 2003, Hall and Moschovakis, 2003, Isa et al., 2021, McHaffie et al., 2005) is an important avenue for future investigation. In future endeavors, inclusion of networks that process sensorimotor information such as the SMN are crucial to consider alongside the more widely examined networks comprising the triple network model of psychopathology (Menon, 2011, Menon, 2019, Weng et al., 2019). Continued exploration of how sensorimotor representations associated with memory traces may serve as a critical contributor to re-experiencing symptoms is warranted.
7. Conclusion
Returning to the seminal work of Pierre Janet (1889), successful action upon the environment is necessary for integration of sensorimotor traces from events into self-referential memory. Actions, or prevented actions, perceived to be misaligned with one’s moral code may not only pose a threat to personal goal attainment (Conway and Pleydell-Pearce, 2000) but may also be considered unsuccessful sensorimotor adaptions and stored as fragmented sensorimotor representations. Our findings of diminished differentiation of the SMN and pDMN and of these networks' enhanced recruitment of the SMG in PTSD during MI retrieval offer a neural basis for putative sensorimotor imprints which lack appropriate integration into autobiographical narratives, thus contributing toward vivid and distressing symptoms of re-experiencing/reliving or re-enactment (Brewin et al., 1996, Conway and Pleydell-Pearce, 2000). Crucially, these findings may also have important implications for neuroscientifically-guided treatments for PTSD. Sensorimotor-based treatment approaches which utilize different, or differently contextualized, sensorimotor feedback coupled with intentional action may encourage integration of the traumatic or morally injurious memory (Borges et al., 2022, Ianì, 2019, Levine, 2010, Ogden et al., 2006, Payne et al., 2015, Warner et al., 2014). Bodily movement and postural manipulations may allow for therapeutic interventions to alter how these sensory and motor traces are re-experienced as a successful, as opposed to an unsuccessful, action sequence in a safe physical and social environment (Brewin et al., 1996, Dijkstra and Zwaan, 2014, Ianì, 2019, van der Kolk, 2006). These alterations may also foster the contextualization and integration of sensorimotor fragments into dynamic, verbally accessible autobiographical narratives (Brewin et al., 1996, Conway and Pleydell-Pearce, 2000), thereby optimizing verbal-cognitive approaches to treatment. Importantly, sensorimotor mechanistic neural networks should be further considered in ongoing research targeting our understanding and treatment of psychological trauma, thus providing a window into “how the body keeps the score” (van der Kolk, 2015).
Funding
This work was supported by infrastructure funds from the Canada Foundation for Innovation Grant (Théberge; grant number: 31724) and Lawson Health Research Institute, as well as operating funds from Innovation for Defense Excellence and Security (IDEaS) (Lanius; grant number: CovCA-0642), the Canadian Institute of Military and Veteran Health Research, Green Shield Canada, the Centre of Excellence on PTSD, Canada, and the Canadian Institutes of Health Research (McKinnon and Lanius; grant number: 148784). R. Lanius is supported by the Harris-Woodman Chair in Psyche and Soma at Western University, and M. McKinnon is supported by the Homewood Chair in Mental Health and Trauma at McMaster University.
CRediT authorship contribution statement
Breanne E. Kearney: Writing – original draft, Conceptualization, Formal analysis, Visualization. Braeden A. Terpou: Writing – review & editing, Conceptualization, Formal analysis. Maria Densmore: Writing – review & editing, Conceptualization, Formal analysis, Visualization. Saurabh B. Shaw: Writing – review & editing, Conceptualization, Formal analysis. Jean Théberge: Writing – review & editing, Resources, Investigation, Funding acquisition. Rakesh Jetly: Writing – review & editing, Conceptualization. Margaret C. McKinnon: Writing – review & editing, Conceptualization, Funding acquisition. Ruth A. Lanius: Writing – original draft, Conceptualization, Formal analysis, Visualization, Supervision, Funding acquisition.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors would like to thank all the individuals who participated, as well as Homewood Health in Guelph, Ontario, Canada, who facilitated referrals. We are also grateful to our dedicated research and clinical team, without whom we could not have done this work.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2023.103426.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- Achard S., Bullmore E. Efficiency and cost of economical brain functional networks. PLoS Comput. Biol. 2007;3:e17. doi: 10.1371/journal.pcbi.0030017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ackerman P.T., Newton J.E.O., McPherson W.B., Jones J.G., Dykman R.A. Prevalence of post traumatic stress disorder and other psychiatric diagnoses in three groups of abused children (sexual, physical, and both) Child Abuse Negl. 1998;22(8):759–774. doi: 10.1016/S0145-2134(98)00062-3. [DOI] [PubMed] [Google Scholar]
- Akiki T.J., Averill C.L., Abdallah C.G. A network-based neurobiological model of PTSD: evidence from structural and functional neuroimaging studies. Curr. Psychiatry Rep. 2017;19(11):1–10. doi: 10.1007/s11920-017-0840-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiki T.J., Averill C.L., Wrocklage K.M., Scott J.C., Averill L.A., Schweinsburg B., Abdallah C.G. Default mode network abnormalities in posttraumatic stress disorder: a novel network-restricted topology approach. Neuroimage. 2018;176:489–498. doi: 10.1016/j.neuroimage.2018.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen E.A., Erhardt E.B., Damaraju E., Gruner W., Segall J.M., Silva R.F., Havlicek M., Rachakonda S., Fries J., Kalyanam R., Michael A.M., Caprihan A., Turner J., Eichele T., Adelsheim S., Bryan A.D., Bustillo J., Clark V.P., Feldstein Ewing S.W., Filbey F., Ford C.C., Hutchison K., Jung R.E., Kiehl K.A., Kodituwakku P., Komesu Y.M., Mayer A.R., Pearlson G.D., Philips J.P., Sadek J.R., Stevens M., Teuscher U., Thoma R.J., Calhoun V.D. A baseline for the multivariate comparison of resting state networks. Front. Neurosci. 2011;5(2):1–23. doi: 10.3389/fnsys.2011.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (5th ed.).
- Atkinson R.C., Shiffrin R.M. In: Spence K.W., Spence J.T., editors. Vol. 2. Academic Press; New York: 1968. Human memory: a proposed system and its control processes; pp. 89–195. (Psychology of learning and motivation). [Google Scholar]
- Ayala F.H. The difference of being human. Biol. Sci. 2010;107(Suppl 2):9015–9022. doi: 10.1073/pnas.0914616107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao W., Gao Y., Cao L., Li H., Liu J., Liang K., Hu X., Zhang L., Hu X., Gong Q., Huang X. Alterations in large-scale functional networks in adult posttraumatic stress disorder: a systematic review and meta-analysis of resting-state functional connectivity studies. Neurosci. Biobehav. Rev. 2021;131:1027–1036. doi: 10.1016/j.neubiorev.2021.10.017. [DOI] [PubMed] [Google Scholar]
- Barredo, J., Aiken, E., Van ’T Wout-Frank, M., Greenberg, B. D., Carpenter, L. L., and Philip, N. S. (2018). Network functional architecture and aberrant functional connectivity in post-traumatic stress disorder: A clinical application of network convergence. Brain Connectivity, 8(9): 549–557. DOI: 10.1089/brain.2018.0634. [DOI] [PubMed]
- Barsalou L.W. Perceptual symbol systems. Behav. Brain Sci. 1999;22(4):577–660. doi: 10.1017/S0140525X99002149. [DOI] [PubMed] [Google Scholar]
- Barsalou L.W. Grounded cognition. Annu. Rev. Psychol. 2008;59(1):617–645. doi: 10.1146/annurev.psych.59.103006.093639. [DOI] [PubMed] [Google Scholar]
- Beck A.T., Guth D., Steer R.A., Ball R. Screening for major depression disorders in medical inpatients with the Beck Depression Inventory for Primary Care. Behav. Res. Ther. 1997;35(8):785–791. doi: 10.1016/S0005-7967(97)00025-9. [DOI] [PubMed] [Google Scholar]
- Beckmann C., DeLuca M., Devlin J., Smith S. Investigations into resting-state connectivity using independent component analysis. Philos. Trans. R. Soc., B. 2005;360(1457):1001–1013. doi: 10.1098/rstb.2005.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekrater-Bodmann R., Foell J., Diers M., Kamping S., Rance M., Kirsch P., Trojan J., Fuchs X., Bach F., Çakmak H., Maaß H., Flor H. The importance of synchrony and temporal order of visual and tactile input for illusory limb ownership experiences – An fMRI study applying virtual reality. PLoS One. 2014;9(1):e87013. doi: 10.1371/journal.pone.0087013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Shabat E., Matyas T.A., Pell G.S., Brodtmann A., Carey L.M. The right supramarginal gyrus is important for proprioception in healthy and stroke-affected participants: a functional MRI study. Front. Neurol. 2015;6(248):1–14. doi: 10.3389/fneur.2015.00248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergouignan, L., Nyberg, L., and Ehrsson, H. H. (2014). Out-of-body-induced hippocampal amnesia. Proceedings of the National Academy of Sciences in the United States of America, 111(12): 4421-4426. 10.1073/pnas.1318801111. [DOI] [PMC free article] [PubMed]
- Bergouignan L., Nyberg L., Ehrsson H.H. Out-of-body memory encoding causes third-person perspective at recall. J. Cogn. Psychol. 2021;34(1):160–178. doi: 10.1080/20445911.2021.1958823. [DOI] [Google Scholar]
- Bernstein D., Fink L. Psychological Corporation; San Antonio, TX: 1998. Childhood trauma questionnaire: A retrospective self-report: Manual. [Google Scholar]
- Bietti L.M. Towards a cognitive pragmatics of collective remembering. Pragmat. Cogn. 2012;20(1):32–61. doi: 10.1075/pc.20.1.02bie. [DOI] [Google Scholar]
- Birrer E., Michael T., Munsch S. Intrusive images in PTSD and in traumatised and non-traumatised depressed patients: a cross-sectional clinical study. Behav. Res. Ther. 2007;45:2053–2065. doi: 10.1016/j.brat.2007.03.005. [DOI] [PubMed] [Google Scholar]
- Bizzi E., Ajemian R. From motor planning to execution: a sensorimotor loop perspective. J. Neurophysiol. 2020;124:1815–1823. doi: 10.1152/jn.00715.2019. [DOI] [PubMed] [Google Scholar]
- Blakemore S.J., Bristow D., Bird G., Frith C., Ward J. Somatosensory activations during the observation of touch and a case of vision-touch synesthesia. Brain. 2005;128(pt 7):1571–1583. doi: 10.1093/brain/awh500. [DOI] [PubMed] [Google Scholar]
- Blanke O., Slater M., Serino A. Behavioral, neural, and computational principles of bodily self-consciousness. Neuron. 2015;88(1):145–166. doi: 10.1016/j.neuron.2015.09.029. [DOI] [PubMed] [Google Scholar]
- Blevins C.A., Weathers F.W., Witte T.K. Dissociation and posttraumatic stress disorder: a latent profile analysis. J. Trauma. Stress. 2014;27(4):388–396. doi: 10.1002/jts.21933. [DOI] [PubMed] [Google Scholar]
- Bluhm, R. L., Williamson, P. C., Osuch, E. A., Frewen, P. A., Stevens, T. K., Boksman, K., andLanius, R. A. (2009). Alterations in default network connectivity in posttraumatic stress disorder related to early-life trauma. Journal of Psychiatry and Neuroscience, 34(3), 187–194. [PMC free article] [PubMed]
- Borges L.M., Barnes S.M., Farnsworth J.K., Drescher K.D., Walser R.D. Case conceptualizing in acceptance and commitment therapy for moral injury: an active and ongoing approach to understanding and intervening on moral injury. Front. Psych. 2022;13 doi: 10.3389/fpsyt.2022.910414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowsher D., Brooks J., Enevoldson P. Central representations of somatic sensations in the parietal operculum (SII) and insula. Eur. Neurol. 2004;52(4):211–225. doi: 10.1159/000082038. [DOI] [PubMed] [Google Scholar]
- Bréchet L., Grivaz P., Gauthier B., Blanke O. Common recruitment of angular gyrus in episodic autobiographical memory and bodily self-consciousness. Front. Behav. Neurosci. 2018;12(270):1–10. doi: 10.3389/fnbeh.2018.00270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brémault-Phillips S., Cherwick T., Smith-MacDonald L.A., Huh J., Vermetten E. Forgiveness: a key component of healing from moral injury? Front. Psychol. 2022;13 doi: 10.3389/fpsyt.2022.906945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner J.D., Narayan M., Staib L.H., Southwick S.M., McGlashan T., Charney D.S. Neural correlates of memories of childhood sexual abuse in women with and without posttraumatic stress disorder. Am. J. Psychiatry. 1999;156(11):1787–1795. doi: 10.1176/ajp.156.11.1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewin C.R. Memory processes in post-traumatic stress disorder. Int. Rev. Psychiatry. 2001;13(3):159–163. doi: 10.1080/09540260120074019. [DOI] [Google Scholar]
- Brewin C.R. Re-experiencing traumatic events in PTSD: New avenues in research on intrusive memories and flashbacks. Eur. J. Psychotraumatol. 2015;6(s4):1–5. doi: 10.3402/ejpt.v6.27180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewin C.R., Dalgleish T., Joseph S. A dual representation theory of posttraumatic stress disorder. Psychol. Rev. 1996;103(4):670–686. doi: 10.1037/0033-295X.103.4.670. [DOI] [PubMed] [Google Scholar]
- Briere J., Weathers F.W., Runtz M. Is dissociation a multidimensional construct? Data from the multiscale dissociation inventory. J. Trauma. Stress. 2005;18(3):221–231. doi: 10.1002/jts.20024. [DOI] [PubMed] [Google Scholar]
- Brunel L., Labeye É., Lesourd M., Versace R. The sensory nature of episodic memory: sensory priming effects due to memory trace activation. J. Exp. Psychol. Learn. Mem. Cogn. 2009;35(4):1081–1088. doi: 10.1037/a0015537. [DOI] [PubMed] [Google Scholar]
- Bryan C.J., Bryan A.O., Roberge E., Leifker F.R., Rozek D.C. Moral injury, posttraumatic stress disorder, and suicidal behavior among National Guard personnel. Psychol. Trauma. 2018;10(1):36–45. doi: 10.1037/tra0000290. [DOI] [PubMed] [Google Scholar]
- Buckner R.L., Carroll D.C. Self-projection and the brain. Trends Cogn. Sci. 2007;11(2):49–57. doi: 10.1016/j.tics.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Buckner R.L., Peterson S.E., Ojemann J.G., Miezin F.M., Squire L.R., Raichle M.E. Functional anatomical studies of explicit and implicit memory retrieval tasks. J. Neurosci. 1995;15(1):12–29. doi: 10.1523/JNEUROSCI.15-01-00012.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner R.L., Andrews-Hanna J.R., Schacter D.L. The brain’s default network: anatomy, function, and relevance to disease. Ann. N. Y. Acad. Sci. 2008;1124(1):1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Cabeza R., St Jacques P. Functional neuroimaging of autobiographical memory. Trends Cogn. Sci. 2007;11(5):219–227. doi: 10.1016/j.tics.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Calhoun V.D., Adali T., Pearlson G.D., Pekar J.J. A method for making group inferences from functional MRI data using independent component analysis. Hum. Brain Mapp. 2001;14(3):140–151. doi: 10.1002/hbm.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun V.D., Liu J., Adali T. A review of group ICA for fMRI data and ICA for joint inference of imaging, genetic, and ERP data. Neuroimage. 2009;45(1 Suppl):163–172. doi: 10.1016/j.neuroimage.2008.10.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanna A.E., Trimble M.R. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006;129:564–583. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- Chen, H. Zhang, L., Ke, J. Qi, R., Xu, Q., Zhong, Y., Pan, M., Li, J., Lu, G., and Chen, F. (2019). Altered resting-state dorsal anterior cingulate cortex functional connectivity in patients with post-traumatic stress disorder. Australian and New Zealand Journal of Psychiatry, 53(1): 10.1177/0004867418812674. [DOI] [PubMed]
- Clancy K.J., Andrzejewski J.A., Simon J., Ding M., Schmidt N.B., Li W. Posttraumatic stress disorder is associated with a dysrhythmia across the visual cortex and the default mode network. eNeuro. 2020;7(4):1–12. doi: 10.1523/ENEURO.0053-20.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway M.A., Pleydell-Pearce C.W. The construction of autobiographical memories in the self-memory system. Psychol. Rev. 2000;107(2):261–288. doi: 10.1037/0033-295X.107.2.261. [DOI] [PubMed] [Google Scholar]
- Copp D., editor. The Oxford Handbook of Ethical Theory. Oxford University Press; 2006. [Google Scholar]
- Damasio A.R. Time-locked multiregional retroactivation: a systems-level proposal for the neural substrates of recall and recognition. Cognition. 1989;33:25–62. doi: 10.1016/0010-0277(89)90005-X. [DOI] [PubMed] [Google Scholar]
- Damoiseaux, J.S., Rombouts, S.A., Barkhof, F., Scheltens, P., Stam C.J., Smith, S.M., and Beckmann, C. F. (2006). Consistent resting-state networks across healthy subjects. Proceedings of the National Academy of Sciences, U.S.A., 103(37): 13848–13853. 10.1073/pnas.0601417103. [DOI] [PMC free article] [PubMed]
- Daniels J.K., Frewen P., McKinnon M.C., Lanius R.A. Default mode alterations in posttraumatic stress disorder related to early-life trauma: a developmental perspective. J. Psychiatry Neurosci. 2011;36(1):56–59. doi: 10.1503/jpn.100050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daselaar S.M., Rice H.J., Greenberg D.L., Cabeza R., LaBar K.S., Rubin D.C. The spatiotemporal dynamics of autobiographical memory: Neural correlates of recall, emotional intensity, and reliving. Cereb. Cortex. 2008;18(1):217–229. doi: 10.1093/cercor/bhm048. [DOI] [PubMed] [Google Scholar]
- Debowska W., Liguz-Lecznar M., Kossut M. Bilateral plasticity of vibrissae SII representation induced by classical conditioning in mice. J. Neurosci. 2011;31(14):5447–5453. doi: 10.1523/JNEUROSCI.5989-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deen A., Biedermann S.V., Lotzin A., Krüger-Gottschalk A., Dyer A., Knaevelsrud C., Rau H., Schellong J., Ehring T., Schäfer I. The dissociative subtype of PTSD in trauma-exposed individuals: a latent class analysis and examination of clinical covariates. Eur. J. Psychotraumatol. 2022;13(1):2031591. doi: 10.1080/20008198.2022.2031591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deroualle D., Borel L., Devèze A., Lopez C. Changing perspective: the role of vestibular signals. Neuropsycholgia. 2015;79(B):175–185. doi: 10.1016/j.neuropsychologia.2015.08.022. [DOI] [PubMed] [Google Scholar]
- Dieterich, M., and Brandt, T. (2018). Chapter 6- The parietal lobe and the vestibular system. Handbook of Clinical Neurology (Vo. 151), 119-140. Academic Publishing- Elsevier. 10.1016/B978-0-444-63622-5.00006-1. [DOI] [PubMed]
- Dieterich M., Brandt T. The bilateral central vestibular system: Its pathways, functions, and disorders. Ann. N. Y. Acad. Sci. 2015;1343(1):10–26. doi: 10.1111/nyas.12585. [DOI] [PubMed] [Google Scholar]
- DiGangi J.A., Tadayyon A., Fitzgerald D.A., Rabinak C.A., Kennedy A., Klumpp H., Phan K.L. Reduced default mode network connectivity following combat trauma. Neurosci. Lett. 2016;615:37–43. doi: 10.1016/j.neulet.2016.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijkstra K., Zwaan R.A. In: The Routledge handbook of embodied cognition. Shapiro L.A., editor. Taylor & FrancisBooks; Abingdon, England: 2014. Memory and action; pp. 296–305. [Google Scholar]
- Doubell T.P., Skaliora I., Baron J., King A. Functional connectivity between the superficial and deep layers of the superior colliculus: an anatomical substrate for sensorimotor integration. J. Neurosci. 2003;23(16):6595–6607. doi: 10.1523/JNEUROSCI.23-16-06596.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers A., Hackmann A., Michael T. Intrusive re-experiencing in post-traumatic stress disorder: phenomenology, theory, and therapy. Memory. 2004;12:403–415. doi: 10.1080/09658210444000025. [DOI] [PubMed] [Google Scholar]
- El-Baba, R. M., and Schury, M. P. (2022). Neuroanatomy, frontal cortex. [Updated 2022 Jun 5]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing. Available from: https://www.ncbi.nlm.nih.gov/books/NBK554483. [PubMed]
- Farnsworth J.K., Drescher K.D., Nieuwsma J.A., Walser R.B., Currier J.M. The role of moral emotions in military trauma: Implications for the study and treatment of moral injury. Rev. Gen. Psychol. 2014;18(4):249–262. doi: 10.1037/gpr0000018. [DOI] [Google Scholar]
- Ferrè E.R., Lopez C., Haggard P. Anchoring the self to the body: Vestibular contribution to the sense of self. Psychol. Sci. 2014;25(11):2106–2108. doi: 10.1177/0956797614547917. [DOI] [PubMed] [Google Scholar]
- First, M., Spitzer, R., Gibbon, M., and Williams, J. (2002). Structured Clinical Interview for DSM‐IV‐TR Axis I disorders (Research version, patient edition). New York, NY: Biometrics Research, New York State Psychiatric Institute.
- Fletcher P.C., Frith C.D., Baker S.C., Shallice T., Frackowiak R.S., Dolan R.J. The mind's eye—precuneus activation in memory-related imagery. Neuroimage. 1995;2:195–200. doi: 10.1006/nimg.1995.1025. [DOI] [PubMed] [Google Scholar]
- Foa E.B., Zinbarg R., Rothbaum B.O. Uncontrollability and unpredictability in posttraumatic stress disorder: animal model. Psychol. Bull. 1992;112(2):218–238. doi: 10.1037/0033-2909.112.2.218. [DOI] [PubMed] [Google Scholar]
- Fodor J.A. MIT Press; Cambridge, MA: 1983. The Modularity of Mind. [Google Scholar]
- Foglia L., Wilson R.A. Embodied cognition. WIREs Cognit. Sci. 2013;4(3):319–325. doi: 10.1002/wcs.1226. [DOI] [PubMed] [Google Scholar]
- Frank S.M., Greenlee M.W. The parieto-insular vestibular cortex in humans: more than a single area? J. Neurophysiol. 2018;120:1438–1450. doi: 10.1152/jn.00907.2017. [DOI] [PubMed] [Google Scholar]
- Frewen P.A., Lane R.D., Neufeld R.W.J., Densmore M., Stevens T., Lanius R.A. Neural correlates of levels of emotional awareness during trauma script-imagery in posttraumatic stress disorder. Psychosom. Med. 2008;70(1):27–31. doi: 10.1097/pSY0b013e31815f66d4. [DOI] [PubMed] [Google Scholar]
- Frewen P.A., Dozois D.J.A., Neufeld R.W.J., Lanius R.A. Disturbances of emotional awareness and expression in posttraumatic stress disorder: Meta-mood, emotion regulation, mindfulness, and interference of emotional expressiveness. Psychol. Trauma Theory Res. Pract. Policy. 2012;4(2):152–161. doi: 10.1037/a0023114. [DOI] [Google Scholar]
- Frewen P.A., Schroeter M.L., Riva G., Cipresso P., Fairfield B., Padulo C., Kemp A.H., Palaniyappan L., Owolabi M., Kusi-Mensah K., Polyakova M., Fehertoi N., D’Andrea W., Lowe L., Northoff G. Neuroimaging the consciousness of self: Review and conceptual-methodological framework. Neurosci. Biobehav. Rev. 2020;112:164–212. doi: 10.1016/j.neubiorev.2020.01.023. [DOI] [PubMed] [Google Scholar]
- Gilboa A., Winocur G., Grady C.L., Hevenor S.J., Moscovitch M. Remembering our past: functional neuroanatomy of recollection of recent and very remote personal events. Cereb. Cortex. 2004;14(11):1214–1225. doi: 10.1093/cercor/bhh082. [DOI] [PubMed] [Google Scholar]
- Grèzes J., Decety J. Functional anatomy of execution, mental simulation, observation, and verb generation of actions: a meta-analysis. Hum. Brain Mapp. 2000;12(1):1–19. doi: 10.1002/1097-0193(200101)12:1<1::AID-HBM10>3.0.CO;2-V. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffanti L., Douaud G., Bijsterbosch J., Evangelisti S., Alfaro-Almagro F., Glasser M.F., Duff E.P., Sitzgibbon S., Westphal R., Carone D., Beckmann C.F., Smith S.M. Hand classification of fMRI ICA noise components. Neuroimage. 2017;154:188–205. doi: 10.1016/j.neuroimage.2016.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidali G., Pisoni A., Bolognini N., Papagno C. Keeping order in the brain: the supramarginal gyrus and serial order in short-term memory. Cortex. 2019;119:89–99. doi: 10.1016/j.cortex.2019.04.009. [DOI] [PubMed] [Google Scholar]
- Hall W.C., Moschovakis A.K., editors. The superior colliculus: New approaches for studying sensorimotor integration. (1st ed.). CRC Press; 2003. [DOI] [Google Scholar]
- Hansen M., Ross J., Armour C. Evidence of the dissociative PTSD subtype: a systematic literature review of latent class and profile analytic studies of PTSD. J. Affect. Disord. 2017;213:59–69. doi: 10.1016/j.jad.2017.02.004. [DOI] [PubMed] [Google Scholar]
- Harnad S. The symbol grounding problem. Physica D. 1990;42(1–3):335–346. doi: 10.1016/0167-2789(90)90087-6. [DOI] [Google Scholar]
- Harricharan S., Nicholson A.A., Thome J., Densmore M., McKinnon M.C., Théberge J., Frewen P.A., Neufeld R.W., Lanius R.A. PTSD and its dissociative subtype through the lens of the insula: anterior and posterior insula resting-state functional connectivity and its predictive validity using machine learning. Psychophysiology. 2019;57(1):e13472. doi: 10.1111/psyp.13472. [DOI] [PubMed] [Google Scholar]
- Heba S., Lenz M., Kalisch T., Höffken O., Schweizer L., Glaubitz B., Puts N., Tegenthoff M., Dinse H., Schmidt-Wilcke T. Regionally specific regulation of sensorimotor network connectivity following tactile improvement. Neural Plast. 2017;2017:5270532. doi: 10.1155/2017/5270532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman J.L. Basic Books; New York: 1992. Trauma and Recovery. [Google Scholar]
- Hopper J.W., Frewen P.A., Sack M., Lanius R.A., van der Kolk B.A. The responses to script-driven imagery scale (RSDI): assessment of state posttraumatic symptoms for psychobiological and treatment research. J. Psychopathol. Behav. Assess. 2007;29(4):249–268. doi: 10.1007/s10862-007-9046-0. [DOI] [Google Scholar]
- Hopper J.W., van der Kolk B.A. Retrieving, assessing, and classifying traumatic memories: a preliminary report on three case studies of a new standardized method. J. Aggress. Maltreat. Trauma. 2001;4(2):33–71. doi: 10.1300/J146v04n02_03. [DOI] [Google Scholar]
- Hu S., Ide J.S., Zhang S., Li C.-S.-R. The right superior frontal gyrus and individual variation in proactive control of impulsive response. J. Neurosci. 2016;36(50):12688–12696. doi: 10.1523/JNEUROSCI.1175-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber, J., Ruehl, M., Flanagin, V., and zu Eulenburg, P. (2021). Delineating neural responses and functional connectivity changes during vestibular and nociceptive stimulation reveal the uniqueness of cortical vestibular processing. Brain Structure and Function, 227: 779-791. 10.1007/s00429-021-02394-6. [DOI] [PMC free article] [PubMed]
- Ianì F. Embodied memories: reviewing the role of the body in memory processes. Psychon. Bull. Rev. 2019;26:1747–1766. doi: 10.3758/s13423-019-01674-x. [DOI] [PubMed] [Google Scholar]
- Ibanez A., Gleichgerrcht E., Manes F. Clinical effects of insular damage in humans. Brain Struct. Funct. 2010;214:397–410. doi: 10.1007/s00429-010-0256-y. [DOI] [PubMed] [Google Scholar]
- Ibitoye R.T., Mallas E.-J., Bourke N.J., Kaski D., Bronstein A.M., Sharp D.J. The human vestibular cortex: functional anatomy of OP2, its connectivity and the effect of vestibular disease. Cereb. Cortex. 2022;00:1–16. doi: 10.1093/cercor/bhac085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ino T., Nakai R., Azuma T., Kimura T., Fukuyama H. Brain activation during autobiographical memory retrieval with special reference to default mode network. Open Neuroimag. J. 2011;5:14–23. doi: 10.2174/1874440001105010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isa T., Marquez-Legorreta E., Grillner S., Scott E.K. The tectum/superior colliculus as the vertebrate solution for spatial sensory integration and action. Curr. Biol. 2021;31(11):R741–R762. doi: 10.1016/j.cub.2021.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janet P. Alcan; Paris: 1889. L’automatisme psychologique. [Google Scholar]
- Janet P. Problèmes psychologiques de l’émotion. Rev. Neurol. 1909;17(2):1551–1672. [Google Scholar]
- Kheradmand A., Lasker A., Zee D.S. Transcranial magnetic stimulation (TMS) of the supramarginal gyrus: a window to perception of upright. Cereb. Cortex. 2015;25(3):765–771. doi: 10.1093/cercor/bht267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H. Dissociating the roles of the default-mode, dorsal, and ventral networks in episodic memory retrieval. Neuroimage. 2010;50(4):1648–1657. doi: 10.1016/j.neuroimage.2010.01.051. [DOI] [PubMed] [Google Scholar]
- Knyazev G.G., Savostyanov A.N., Bocharov A.V., Dorosheva E.A., Tamozhnikov S.S., Saprigyn A.E. Oscillatory correlates of autobiographical memory. Int. J. Psychophysiol. 2015;95(3):322–332. doi: 10.1016/j.ijpsycho.2014.12.006. [DOI] [PubMed] [Google Scholar]
- Koch S.B.J., van Zuiden M., Nawijn L., Frijling J.L., Veltman D.J., Olff M. Aberrant resting-state brain activity in posttraumatic stress disorder: a meta-analysis and systematic review. Depress. Anxiety. 2016;33(7):592–605. doi: 10.1002/da.22478. [DOI] [PubMed] [Google Scholar]
- Laird A.G., Eickhoff S.B., Li K., Robin D.A., Glahn D.C., Fox P.T. Investigating the functional heterogeneity of the default mode network using coordinate-based meta-analytic modeling. J. Neurosci. 2009;29(46):14496–14505. doi: 10.1523/JNEUROSCI.4004-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanius R.A., Williamson P.C., Hopper J., Densmore M., Boksman K., Gupta M.A., Neufeld R.W.J., Gati J.S., Menon R.S. Recall of emotional states in posttraumatic stress disorder: an fMRI investigation. Biol. Psychiatry. 2003;53(3):204–210. doi: 10.1016/S0006-3223(02)01466-X. [DOI] [PubMed] [Google Scholar]
- Lanius R.A., Williamson P.C., Densmore M., Boksman K., Neufeld R.W., Gati J.S., Menon R.S. The nature of traumatic memories: a 4-T fMRI functional connectivity analysis. Am. J. Psychiatry. 2004;161(1):36–44. doi: 10.1176/appi.ajp.161.1.36. [DOI] [PubMed] [Google Scholar]
- Lanius R.A., Bluhm R.L., Coupland N.J., Hegadoren K.M., Rowe B., Théberge J., Neufeld R.W.J., Williamson P.C., Brimson M. Default mode network connectivity as a predictor of post-traumatic stress disorder symptom severity in acutelytraumatized subjects. Acta Psychiatr. Scand. 2010;121(1):33–40. doi: 10.1111/j.1600-0447.2009.01391.x. [DOI] [PubMed] [Google Scholar]
- Lanius R.A., Bluhm R.L., Frewen P.A. How understanding the neurobiology of complex post-traumatic stress disorder can inform clinical practice: a social cognitive and affective neuroscience approach. Acta Psychiatr. Scand. 2011;124(5):331–348. doi: 10.1111/j.1600-0447.2011.01755.x. [DOI] [PubMed] [Google Scholar]
- Lanius R.A., Terpou B.A., McKinnon M.C. The sense of self in the aftermath of trauma: lessons from the default mode network in posttraumatic stress disorder. Eur. J. Psychotraumatol. 2020;11(1):1807703. doi: 10.1080/20008198.2020.1807703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei X., Wang Y., Yuan H., Mantini D. Neuronal oscillations and functional interactions between resting state networks. Hum. Brain Mapp. 2014;35(7):3517–3528. doi: 10.1002/hbm.22418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leroy A., Very E., Birmes P., Yger P., Szaffarczyk S., Lopes R., Outteryck O., Faure C., Duhem S., Grandgenevre P., Warembourg F., Vaiva G., Jardri R. Intrusive experiences in posttraumatic stress disorder: treatment response induces changes in the directed functional connectivity of the anterior insula. NeuroImage Clin. 2022;34 doi: 10.1016/j.nicl.2022.102964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine P.A. North Atlantic Books; Berkeley, CA: 2010. In an Unspoken Voice: How The Body Releases Trauma and Restores Goodness. [Google Scholar]
- Li W., Qin W., Liu H., Fan L., Wang J., Jiang T., Yu C. Subregions of the human superior frontal gyrus and their connections. Neuroimage. 2013;78:46–58. doi: 10.1016/j.neuroimage.2013.04.011. [DOI] [PubMed] [Google Scholar]
- Litz B.T., Stein N., Delaney E., Lebowitz L., Nash W.P., Silva C., Maguen S. Moral injury and moral repair in war veterans: a preliminary model and intervention strategy. Clin. Psychol. Rev. 2009;29(8):695–706. doi: 10.1016/j.cpr.2009.07.003. [DOI] [PubMed] [Google Scholar]
- Lloyd C.S., Nicholson A.A., Densmore M., Théberge J., Neufeld R.W.J., Jetly R., McKinnon M.C., Lanius R.A. Shame on the brain: neural correlates of moral injury event recall in posttraumatic stress disorder. Depress. Anxiety. 2021;38(6):596–605. doi: 10.1002/da.23128. [DOI] [PubMed] [Google Scholar]
- Lopez C., Blanke O., Mast F.W. The human vestibular cortex revealed by coordinate-based activation likelihood estimation meta-analysis. Neuroscience. 2012;212:159–179. doi: 10.1016/j.neuroscience.2012.03.028. [DOI] [PubMed] [Google Scholar]
- Luo Q., Chen J., Li Y., Wu Z., Lin X., Yao J., Yu H., Peng H., Wu H. Altered regional brain activity and functional connectivity patterns in major depressive disorder: A function of childhood trauma or diagnosis? J. Psychiatry Res. 2022;147:237–247. doi: 10.1016/j.jpsychires.2022.01.038. [DOI] [PubMed] [Google Scholar]
- Macaluso E., Driver J. Spatial attention and crossmodal interactions between vision and touch. Neuropsychologia. 2001;39:1304–1316. doi: 10.1016/S0028-3932(01)00119-1. [DOI] [PubMed] [Google Scholar]
- Margulies, D. S., Ghosh, S. S., Goulas, A., Falkiewicz, M., Huntenburg, J. M., Langs, G., Bezgin, G., Eickhoff, S. B., Castellanos, F. X., Petrides, M., Jefferies, E., and Smallwood, J. (2016). Situating the default-mode network along a principal gradient of macroscale cortical organization. Proceedings of the National Academy of Sciences, U.S.A., 113(44): 12574-12579. 10.1073/pnas.1608282113. [DOI] [PMC free article] [PubMed]
- McHaffie, J. G., Stanford, T. R., Stein, B. E., ZCoizet, V., and Redgrave, P. (2005). Subcortical loops through the basal ganglia. Trends in Neurosciences, 28(8): 401-407. 10.1016/j.tins.2005.06.006. [DOI] [PubMed]
- Menon V. Large-scale brain networks and psychopathology: Unifying triple network model. Trends Cogn. Sci. 2011;15(10):483–506. doi: 10.1016/j.tics.2011.08.003. [DOI] [PubMed] [Google Scholar]
- Menon B. Towards a new model of understanding- The triple network, psychopathology and the structure of the mind. Med. Hypotheses. 2019;133(109385) doi: 10.1016/j.mehy.2019.109385. [DOI] [PubMed] [Google Scholar]
- Mensink B., van Schagen A., van der Aa N., June J., ter Heide F. Moral injury in trauma-exposed, treatment-seeking police officers and military veterans: Latent class analysis. Front. Psych. 2022;13 doi: 10.3389/fpsyt.2022.904659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikulincer, M., Shaver, P. R., and Solomon, Z. (2015). “An attachment perspective on traumatic and posttraumatic reactions,” in Future directions in post-traumatic stress disorder: Prevention, diagnosis, and treatment, eds M. Safir, H. Wallach, and A. Rizzo (New York: Springer), 79–96. 10.1007/978-1-4899-7522-5_4.
- Miller D.R., Hayes S.M., Hayes J.P., Spielberg J.M., Lafleche G., Verfaellie M. Default mode network subsystems are differentially disrupted in posttraumatic stress disorder. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging. 2017;2(4):363–371. doi: 10.1016/j.bpsc.2016/12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mountcastle V.B., Lynch J.C., Georgopoulos A., Sakata H., Acuna C. Posterior parietal association cortex of the monkey: Command functions for operation within extrapersonal space. J. Neurophysiol. 1975;38:871–908. doi: 10.1152/jn.1975.38.4.871. [DOI] [PubMed] [Google Scholar]
- Nash J.A. In: Environmental risk decision making: Values, perceptions, & ethics. (1st ed., Cothern I.C.R., editor. CRC Press; 2019. Moral values in risk decisions; pp. 195–212. [Google Scholar]
- Nash W.P., Marino Carper T.L., Mills M.A., Au T., Goldsmith A., Litz B.T. Psychometric evaluation of the moral injury events scale. Mil. Med. 2013;178(6):646–652. doi: 10.7205/MILMED-D-13-00017. [DOI] [PubMed] [Google Scholar]
- Nazarov A., Jetly R., McNeely H., Kiang M., Lanius R.A., McKinnon M.C. Role of morality in the experience of guilt and shame within armed forces. Acta Psychiatr. Scand. 2015;132(1):4–19. doi: 10.1111/acps.12406. [DOI] [PubMed] [Google Scholar]
- Niedenthal P.M., Barsalou L.W., Ric F., Krauth-Gruber S. In: Emotion: Conscious and unconscious. Feldman-Barrett L., Niedenthal P.M., Winkielman P., editors. Guilford Press; New York: 2005. Embodiment in the acquisition and use of emotion knowledge; pp. 21–50. [Google Scholar]
- Nilsson, L.G. (2000). Remembering actions and words. In: E. Tulving E and F. I. M. Craik (eds), The Oxford handbook of memory. Oxford: Oxford University Press, 137–148.
- Nyberg L. Levels of processing: a view from functional brain imaging. Memory. 2002;10(5–6):345–348. doi: 10.1080/09658210244000171. [DOI] [PubMed] [Google Scholar]
- Nyberg L., Petersson K.M., Nilsson L.-G., Sandblom J., Åberg C., Ingvar M. Reactivation of motor brain areas during explicit memory for actions. Neuroimage. 2001;14(2):521–528. doi: 10.1006/nimg.2001.0801. [DOI] [PubMed] [Google Scholar]
- Ogden P., Pain C., Fisher J. A sensorimotor approach to the treatment of trauma and dissociation. Psychiatr. Clin. N. Am. 2006;29(1):263–279. doi: 10.1016/j.psc.2005.10.012. [DOI] [PubMed] [Google Scholar]
- Ogle C.M., Rubin D.C., Siegler I.C. The relation between insecure attachment and posttraumatic stress: Early life versus adulthood traumas. Psychol. Trauma. 2015;7:324–332. doi: 10.1037/tra0000015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohgami Y., Matsuo K., Uchida N., Nakai T. An fMRI study of tool-use gestures: body part as object and pantomime. Neuroreport. 2004;15(12):1903–1906. doi: 10.1097/00001756-200408260-00014. [DOI] [PubMed] [Google Scholar]
- Palombo D.J., McKinnon M.C., McIntosh A.R., Anderson A.K., Todd R.M., Levine B. The neural correlates of memory for a life-threatening event; an fMRI study of passengers from flight AT236. Clin. Psychol. Sci. 2015;4(2):312–319. doi: 10.1177/2167702615589308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panksepp J. Affective neuroscience: The foundations of human and animal emotions. Oxford University Press; Oxford: 1998. [Google Scholar]
- Payne P., Levine P.A., Crane-Godreau M.A. Somatic experiencing: using interoception and proprioception as core elements of trauma therapy. Front. Psychol. 2015;6(93):1–18. doi: 10.3389/fpsyg.2015.00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrides, M., and Pandya, D. N. (2012). The Frontal Cortex. In The human nervous system (3rd ed), ed. J. K. Mai and G. Paxinos (Elsevier: Academic Press), 988-1011. 10.1016/B978-0-12-374236-0.10026-4.
- Pfeiffer C., Serino A., Blanke O. The vestibular system: a spatial reference for bodily self-consciousness. Front. Integr. Neurosci. 2014;8(31):1–13. doi: 10.3389/fnint.2014.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippi C.L., Tranel D., Duff M., Rudrauf D. Damage to the default mode network disrupts autobiographical memory retrieval. Soc. Cogn. Affect. Neurosci. 2015;10(3):318–326. doi: 10.1093/scan/nsu070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Protopopescu A., Boyd J.E., O’Connor C., Rhind S.G., Jetly R., Lanius R.A., McKinnon M.C. Examining the associations among moral injury, difficulties with emotion regulation, and symptoms of PTSD, depression, anxiety, and stress among Canadian military members and veterans: A preliminary study. J. Military Vet. Fam. Health. 2021;7(2):71–80. doi: 10.3138/jmvfh-2020-0036. [DOI] [Google Scholar]
- Pylyshyn Z.W. MIT Press; Cambridge, MA: 1984. Computation and Cognition. [Google Scholar]
- Qin P., Northoff G. How is our self related to midline regions and the default-mode network? Neuroimage. 2011;37(3):1221–1233. doi: 10.1016/j.neuroimage.2011.05.028. [DOI] [PubMed] [Google Scholar]
- Rabellino D., Densmore M., Théberge J., McKinnon M.C., Lanius R.A. The cerebellum after trauma: resting-state functional connectivity of the cerebellum in posttraumatic stress disorder and its dissociative subtype. Hum. Brain Mapp. 2018;39(8):3354–3374. doi: 10.1002/hbm.24081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle M.E. The brain’s default mode network. Annu. Rev. Neurosci. 2015;38:433–447. doi: 10.1146/annurev-neuro-071013-014030. [DOI] [PubMed] [Google Scholar]
- Raichle M.E., MacLeod A.M., Snyder A.Z., Shulman G.L. A default mode of brain function. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds M., Brewin C.R. Intrusive cognitions, coping strategies and emotional responses in depression, post-traumatic stress disorder and a non-clinical population. Behav. Res. Ther. 1998;36:135–147. doi: 10.1016/s0005-7967(98)00013-8. [DOI] [PubMed] [Google Scholar]
- Ritchey M., Cooper R.A. Deconstructing the posterior medial episodic network. Trends Cogn. Sci. 2020;24:451–465. doi: 10.1016/j.tics.2020.03.006. [DOI] [PubMed] [Google Scholar]
- Rubinov M., Sporns O. Complex network measures of brain connectivity: uses and interpretations. Neuroimage. 2010;52(3):1059–1069. doi: 10.1016/j.neuroimage.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Rumiati, R., I., Weiss, P. H., Shallice, T., Ottoboni, G., Noth, J., Zilles, K., and Fink, G. R. (2003). Neural basis of pantomiming the use of visually presented objects. NeuroImage, 21(4): 1224-1231. 10.1016/j.neuroimage.2003.11.01. [DOI] [PubMed]
- Russ M.O., Mack W., Grama C.-R., Lanfermann H., Knopf M. Enactment effect in memory: evidence concerning the function of the supramarginal gyrus. Exp. Brain Res. 2003;149:497–504. doi: 10.1007/s00221-003-1398-4. [DOI] [PubMed] [Google Scholar]
- Sacco T., Sacchetti B. Role of secondary sensory cortices in emotional memory storage and retrieval in rats. Science. 2010;329(5992):649–656. doi: 10.1126/science.1183165. [DOI] [PubMed] [Google Scholar]
- Schel M.A., Kühn S., Brass M., Haggard P., Ridderinkhof K.R., Crone E.A. Neural correlates of intentional and stimulus-driven inhibition: a comparison. Front. Hum. Neurosci. 2014;8(27):1–10. doi: 10.3389/fnhum.2014.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiavone F.L., Frewen P., McKinnon M., Lanius R.A. The dissociative subtype of PTSD: an update on the literature. PTSD Res. Quart. 2018;29(3):1–13. [Google Scholar]
- Serino A., Alsmith A., Costantini M., Mandrigin A., Tajadura-Jimenez A., Lopez C. Bodily ownership and self-location: components of bodily self-consciousness. Conscious. Cogn. 2013;22(4):1239–1252. doi: 10.1016/j.concog.2013.08.013. [DOI] [PubMed] [Google Scholar]
- Shaw S.B., McKinnon M.C., Heisz J., Becker S. Dynamic task-linked switching between brain networks – A tri-network perspective. Brain Cogn. 2021;151(105725):1–18. doi: 10.1016/j.bandc.2021.105725. [DOI] [PubMed] [Google Scholar]
- Shaw M., Strother S., McFarlane A., Morris P., Anderson J., Clark C., Egan G. Abnormal functional connectivity in posttraumatic stress disorder. Neuroimage. 2002;15(3):661–674. doi: 10.1006/nimg.2001.1024. [DOI] [PubMed] [Google Scholar]
- Sirigu A., Duhamel J.-R., Cohen L., Pillon B., DuBois B., Agid Y. The mental representation of hand movements after parietal cortex damage. Science. 1996;273(5281):1564–1568. doi: 10.1126/science.273.5281.1564. [DOI] [PubMed] [Google Scholar]
- Smallwood J., Bernhardt B.B., Leech R., Bzdok D., Jefferies E., Margulies D.S. The default mode network in cognition: A topographical perspective. Nat. Rev. Neurosci. 2021;22:503–513. doi: 10.1038/s41583-021-00474-4. [DOI] [PubMed] [Google Scholar]
- Smith, S.M., Fox, P.T., Miller, K.L., Glahn, D.C., Fox, P.M., Mackay, C.E., Filippini, N., Watkins, K. E., Toro, R., Laird, A. R., and Beckmann, C. F. (2009). Correspondence of the brain’s functional architecture during activation and rest. Proceedings of the National Academy of Sciences, U.S.A., 106(31): 13040–13045. 10.1073/pnas.0905267106. [DOI] [PMC free article] [PubMed]
- Spreng R.N., Grady C.L. Patterns of brain activity supporting autobiographical memory, prospection, and theory of mind, and their relationship to the default mode network. J. Cogn. Neurosci. 2010;22(6):1112–1123. doi: 10.1162/jocn.2009.21282. [DOI] [PubMed] [Google Scholar]
- Spreng R.N., Mar R.A., Kim A.S.N. The common neural basis of autobiographical memory, prospection, navigation, theory of mind, and the default mode: a quantitative meta-analysis. J. Cogn. Neurosci. 2009;21(3):489–510. doi: 10.1162/jocn.2008.21029. [DOI] [PubMed] [Google Scholar]
- Sripada R.K., King A.P., Welsh R.C., Garfinkel S.N., Wang X., Sripada C.A., Liberzon I. Neural dysregulation in posttraumatic stress disorder: Evidence for disrupted equilibrium between salience and default mode brain networks. Psychosom. Med. 2012;74(9):904–911. doi: 10.1097/PSY.0b013e318273bf33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Jacques P.L., Kragel P.A., Rubin D.C. Neural networks supporting autobiographical memory retrieval in posttraumatic stress disorder. Cogn. Affect. Behav. Neurosci. 2013;13(3):554–566. doi: 10.3758/s13415-013-0157-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein D.J., Koenen K.C., Friedman M.J., Hill E., McLaughlin K.A., Petukhova M., Ruscio A.M., Shahly V., Spiegel D., Borges G., Bunting B., Caldas-De-Almeida J.M., de Girolamo G., Demyttenaere K., Florescu S., Haro J.M., Karam E.G., Kovess-Masfety V., Lee S., Kessler R.C. Dissociation in posttraumatic stress disorder: Evidence from the world mental health surveys. Biol. Psychiatry. 2013;73(4):302–312. doi: 10.1016/j.biopsych.2012.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan K.M., Fink G.R., Passingham R.E., Silbersweig D., Ceballos-Baumann A.O., Frith C.D., Frackowiak R.S.J. Functional anatomy of the mental representation of upper extremity movements in healthy subjects. J. Neurophysiol. 1995;73(1):373–386. doi: 10.1152/jn.1995.73.1.373. [DOI] [PubMed] [Google Scholar]
- Svoboda E., McKinnon M.C., Levine B. The functional neuroanatomy of autobiographical memory: a meta-analysis. Neuropsychologia. 2006;44(12):2189–2208. doi: 10.1016/j.neuropsychologia.2006.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Te Brake H., Nauta B. Caught between is and ought: The moral dissonance model. Front. Psychol. 2022;13 doi: 10.3389/fpsyt.2022.906231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terpou B.A., Lloyd C.S., Densmore M., McKinnon M.C., Théberge J., Neufeld R.W.J., Jetly R., Lanius R.A. Moral wounds run deep: Exaggerated midbrain functional network connectivity across the default mode network in posttraumatic stress disorder. J. Psychiatry Neurosci. 2022;47(1):E56–E66. doi: 10.1503/jpn.210117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terr L.C. Childhood traumas: an outline and overview. Am. J. Psychiatry. 1991;148(1):10–20. doi: 10.1176/ajp.148.1.10. [DOI] [PubMed] [Google Scholar]
- Thome J., Terpou B.A., McKinnon M.C., Lanius R.A. The neural correlates of trauma-related autobiographical memory in posttraumatic stress disorder: a meta-analysis. Depress. Anxiety. 2019;37(4):321–345. doi: 10.1002/da.22977. [DOI] [PubMed] [Google Scholar]
- Tulving E. In: The cognitive neurosciences. Gazzaniga M.S., editor. MIT Press; Cambridge, MA: 1995. Organization of memory: quo vadis? pp. 839–853. [Google Scholar]
- Tzourio-Mazoyer N., Landeau B., Papathanassiou D., Crivello F., Etard O., Delcroix N., Mazoyer B., Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15(1):273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- van der Kolk B.A. The compulsion to repeat the trauma. Re-enactment, revictimization, and masochism. Psychiatr. Clin. N. Am. 1989;12(2):389–411. doi: 10.1016/s0193-953x(18)30439-8. [DOI] [PubMed] [Google Scholar]
- van der Kolk B.A. Clinical Implications of Neuroscience Research in PTSD. Ann. N.Y. Acad. Sci. 2006;1071:277–293. doi: 10.1196/annals.1364.022277. [DOI] [PubMed] [Google Scholar]
- van der Kolk B.A. Viking; New York: 2015. The Body Keeps the Score: Brain, Mind, and Body in the Healing Of Trauma. [Google Scholar]
- van der Kolk B.A., Fisler R. Dissociation and the fragmentary nature of traumatic memories: overview and exploratory study. J. Trauma. Stress. 1995;8(4):501–726. doi: 10.1002/jts.2490080402. [DOI] [PubMed] [Google Scholar]
- van der Kolk B.A., Van der Hart O. Pierre Janet and the breakdown of adaptation in psychological trauma. Am. J. Psychiatry. 1989;146:1530–1540. doi: 10.1176/ajp.146.12.1530. [DOI] [PubMed] [Google Scholar]
- van der Kolk B.A., Roth S., Pelcovitz D., Sunday S., Spinazzola J. Disorders of extreme stress: the empirical foundation of a complex adaptation to trauma. J. Trauma. Stress. 2005;18(5):389–399. doi: 10.1002/jts.20047. [DOI] [PubMed] [Google Scholar]
- Vermetten E., Jetly R. A critical outlook on combat-related PTSD: review and case reports of guilt and shame as drivers for moral injury. Mil. Behav. Health. 2018;6(2):156–164. doi: 10.1080/21635781.2018.1459973. [DOI] [Google Scholar]
- Versace R., Vallet G.T., Riou B., Lesourd M., Labeye É., Brunel L. Act-In: an integrated view of memory mechanisms. J. Cogn. Psychol. 2014;26(3):280–306. doi: 10.1080/20445911.2014.892113. [DOI] [Google Scholar]
- Vingerhoets G., Vandekerckhove E., Honore P., Vandemaele P., Achten E. Neural correlates of pantomiming familiar and unfamiliar tools: action semantics versus mechanical problem solving? Hum. Brain Mapp. 2011;32(6):905–918. doi: 10.1002/hbm.21078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt P. The physical symbol grounding problem. Cogn. Syst. Res. 2002;3(3):429–457. doi: 10.1016/S1389-0417(02)00051-7. [DOI] [Google Scholar]
- Wang T., Liu J., Zhang J., Zhan W., Li L., Wu M., Gong Q. Altered resting-state functional activity in posttraumatic stress disorder: a quantitative meta-analysis. Sci. Rep. 2016;6(1):1–14. doi: 10.1038/srep27131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner E., Spinazzola J., Westcott A., Gunn C., Hodgdon H. The body can change the score: empirical support for somatic regulation in the treatment of traumatized adolescents. J. Child Adolesc. Trauma. 2014;7(4):237–246. doi: 10.1007/s40653-014-0030-z. [DOI] [Google Scholar]
- Weathers, F. W., Blake, D. D., Schnurr, P. P., Kaloupek, D. G., Marx, B., P., and Keane, T. M. (2013). The clinician-administered PTSD scale for DSM-5 (CAPS-5). Interview available from the National Center for PTSD at www.ptsd.va.gov.
- Weng Y., Qi R., Zhang L., Luo Y., Ke J., Xu Q., Yuan Z., Li J., Chen F., Cao Z., Lu G. Disturbed effective connectivity patterns in an intrinsic triple network model are associated with posttraumatic stress disorder. Neurol. Sci. 2019;40:339–349. doi: 10.1007/s10072-018-3638-1. [DOI] [PubMed] [Google Scholar]
- Wheeler M.A. In: The Oxford handbook of memory. Tulving E., Craik F.I.M., editors. Oxford University Press; Oxford: 2000. Episodic memory and autonoetic awareness; pp. 597–608. [Google Scholar]
- White W.F., Burgess A., Dalgleish T., Halligan S., Hiller R., Oxley A., Smith P., Meiser-Stedman R. Prevalence of the dissociative subtype of post-traumatic stress disorder: a systematic review and meta-analysis. Psychol. Med. 2022;52(9):1629–1644. doi: 10.1017/S0033291722001647. [DOI] [PubMed] [Google Scholar]
- Whitfield-Gabrieli, S. and Nieto-Castanon, A. (2017). CONN functional connectivity toolbox (RRID:SCR_009550) version 17. Hilbert Press. 10.56441/hilbertpress.1744.6736.
- Whitfield-Gabrieli S., Nieto-Castanon A. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. 2012;2(3):125–141. doi: 10.1089/brain.2012.0073. [DOI] [PubMed] [Google Scholar]
- Williamson V., Stevelink S.A.M., Greenberg N. Occupational moral injury and mental health: Systematic review and meta-analysis. Br. J. Psychiatry. 2018;212(6):339–346. doi: 10.1192/bjp.2018.55. [DOI] [PubMed] [Google Scholar]
- Wilson M. Six views of embodied cognition. Psychon. Bull. Rev. 2002;9:625–636. doi: 10.3758/BF03196322. [DOI] [PubMed] [Google Scholar]
- Wolf E.J., Miller M.W., Reardon A.F., Ryabchenko K.A., Castillo D., Freund R. A latent class analysis of dissociation and posttraumatic stress disorder: evidence for a dissociative subtype. Arch. Gen. Psychiatry. 2012;69(7):698–705. doi: 10.1001/archgenpsychiatry.2011.1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf E.J., Mitchell K.S., Sadeh N., Hein C., Fuhrman I., Pietrzak R.H., Miller M.W. The dissociative subtype of PTSD scale: Initial evaluation in a national sample of trauma-exposed veterans. Assessment. 2017;24(4):503–516. doi: 10.1177/1073191115615212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu K., Yu T., Qiao L., Liu C., Wang X., Zhou X., Ni D., Zhang G., Li Y. Electrical stimulation of the insulo-opercular region: Visual phenomena and altered body-ownership symptoms. Epilepsy Res. 2018;148:96–106. doi: 10.1016/j.eplepsyres.2018.09.014. [DOI] [PubMed] [Google Scholar]
- zu Eulenburg, P., Caspers, S., Roski, C., and Eickhoff, S. B. (2012). Meta-analytical definition and functional connectivity of the human vestibular cortex. NeuroImage, 60(1): 162-169). 10.1016/j.neuroimage.2011.12.032. [DOI] [PubMed]
- Zuo X.N., Kelly C., Adelstein J.S., Klein D.F., Castellanos F.X., Milham M.P. Reliable intrinsic connectivity networks: Test-retest evaluation using ICA and dual regression approach. Neuroimage. 2010;49(3):2163–2177. doi: 10.1016/j.neuroimage.2009.10.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.