Abstract
The tumorigenic potential of the Burkitt lymphoma (BL) cell line Akata is dependent on the restricted latency program of Epstein-Barr virus (EBV) that is characteristically maintained in BL tumors. Within these cells, EBV-mediated inhibition of apoptosis correlates with an up-regulation of BCL-2 levels in concert with a down-regulation in c-MYC expression that occurs under growth-limiting conditions. Here we addressed whether EBV's effects on apoptosis and tumorigenicity are mediated by the EBV small RNAs EBER-1 and EBER-2. Stable expression of the EBERs in EBV-negative Akata BL cells, at levels comparable to those in EBV-positive cells, significantly enhanced the tumorigenic potential of EBV-negative BL cells in SCID mice, but did not fully restore tumorigenicity relative to EBV-positive Akata cells. Furthermore, wild-type or greater levels of EBER expression in EBV-negative Akata cells did not promote BL cell survival. These data therefore suggest that EBV can contribute to BL through at least two avenues: an EBER-dependent mechanism that enhances tumorigenic potential independent of a direct effect on apoptosis, and a second mechanism, mediated by an as-yet-unidentified EBV gene(s), that offsets the proapoptotic consequences of deregulated c-MYC in BL.
Burkitt lymphoma (BL) is a human B-cell tumor characterized by chromosomal translocations that juxtapose the c-MYC proto-oncogene and an immunoglobulin gene enhancer, resulting in the constitutive overexpression of c-MYC (reviewed in reference 35). Although deregulated expression of c-MYC is clearly a primary contributing factor in the development of BL, c-MYC overexpression itself is insufficient for the transformation of primary cells (30, 31), due to the activation of apoptotic pathways, particularly under growth-limiting conditions (2, 12). Recent analyses of B-cell tumors that arise in Eμ-myc transgenic mice have indicated that the proapoptotic properties of c-Myc are offset in 80% of these lymphomas by disruption of the ARF-Mdm2-p53 tumor suppressor pathway (10, 22, 46). Indeed, approximately one-third of actual BL tumors contain a mutated p53 gene (14), similar to the fraction of tumors in Eμ-myc mice that exhibit loss of p53 function (10). Based on these observations, inactivation of the ARF-Mdm2-p53 pathway is likely to be a key step in BL tumorigenesis, and one would predict that within BLs containing wild-type p53, other components of the pathway are targeted.
In addition to the genetic anomalies associated with BL, there is a high incidence of latent (noncytolytic) Epstein-Barr virus (EBV) infection of tumor cells in cases where BL is endemic, i.e., those that predominant in the equatorial belt of Africa (11, 35). EBV infection in endemic BL appears to occur prior to the clonal expansion of tumor cells, suggesting an important role for EBV in this tumor (38, 40). In support of this concept, loss of the EBV genome from cells of the Akata BL cell line is associated with loss of tumorigenic potential (6, 50), whereas reinfection of these cells with EBV restores tumorigenicity (27, 44). However, the EBV oncoprotein LMP-1 and other latency-associated EBV gene products essential for growth transformation (immortalization) of B lymphocytes in vitro by EBV are not expressed in BL tumors and many BL cell lines, including Akata (17, 43, 44, 50). The sole exception is EBNA-1, a protein required for maintenance of the episomal EBV genome during latent infection (32, 63). Although EBNA-1 is reported to have oncogenic potential (29, 62), enforced expression of EBNA-1 in EBV-negative Akata cells is not sufficient to confer tumorigenic potential to these BL cells (27, 44). The tumorigenic potential of Akata BL cells is therefore dependent on an EBV gene product or products other than EBNA-1 alone.
The mechanism or mechanisms by which EBV promotes the tumorigenic potential of BL cells are unclear. We and others have demonstrated that EBV-positive Akata BL cells are more resistant to the induction of apoptosis (e.g., by serum withdrawal) than their EBV-negative counterparts and that this resistance correlates in part with modestly elevated levels of the antiapoptotic BCL-2 protein in EBV-positive Akata cells (27, 44). However, the most striking difference in susceptibility to apoptosis between EBV-positive and -negative Akata BL cells occurs when cells are permitted to reach the stationary phase of their growth cycle. Under these conditions, c-MYC levels are down-regulated in EBV-positive cells (but not EBV-negative cells), which presumably contributes to BL cell survival in the absence of adequate growth factors (44). The apparent EBV-mediated up-regulation of BCL-2, in concert with the down-regulation of c-MYC under growth-limiting conditions, may therefore offset the proapoptotic consequences of a deregulated c-MYC gene to promote BL tumorigenicity.
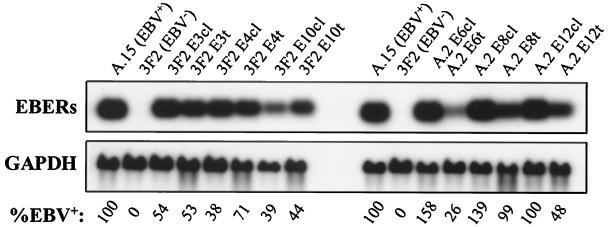
Here we have investigated the contribution of the EBV small noncoding nuclear RNAs EBER-1 and EBER-2 (166 and 172 nucleotides, respectively) to cell survival and tumorigenic potential in BL. The EBER genes, which are transcribed by cellular RNA polymerase III (16, 42), encode the most abundant EBV transcripts in BL tumors, BL-derived cell lines, and other latently infected cells (1, 42). To address the role of the EBERs in cell survival and tumorigenicity, we generated several lines of EBV-negative Akata BL cells that expressed different levels of the EBERs. Two approaches were taken to stably express the EBERs in these BL cells. First, a cassette containing the EBER-1 and EBER-2 genes and their transcriptional regulatory elements (19, 20) was cloned into a shuttle vector [pBluescript IIKS(+); Stratagene, La Jolla, Calif.] that was then used to generate EBV-negative EBER-positive (EBV−/EBER+) cell lines. These cell lines typically expressed EBERs at ∼10% of the levels expressed in EBV-positive BL cells (data not shown). Second, to generate cells that stably expressed higher levels of EBER-1 and EBER-2, the SacI-to-EcoRI fragment of the EBV EcoRI-J genomic restriction fragment that encodes both EBERs was cloned by blunt-end ligation into the SalI site of the amplicon BSAII adjacent to the EBV oriP element (61). Since the EBER genes reside immediately adjacent to oriP in the EBV genome, we reasoned that the high level of EBER gene expression in latently infected cells (∼5 × 106 copies per cell) (59) may be dependent on their position relative to an active oriP. Therefore, this construct was transfected by electroporation into EBV-negative 3F2 and A.2 Akata cells that stably express the EBV EBNA-1 protein (44), which binds to multiple elements within oriP to maintain the viral episome in proliferating cells (41, 63). As noted previously, EBNA-1 expression alone is not sufficient to confer tumorigenic potential to EBV-negative Akata cells or to protect them from apoptosis (27, 44). Following transfection, the cells were selected in medium containing 200 μg of G418 and 200 μg of hygromycin B (Gibco BRL, Rockville, Md.) per ml. Drug-resistant cells were then expanded and analyzed for EBER expression by RNA (Northern) blot analysis. As demonstrated in Fig. 1, the cell lines generated by this approach expressed 41 to 127% of the wild-type level of EBER expression. Furthermore, analysis of these lines over 18 months of continuous culture indicated that these levels of EBER expression were stably maintained and often increased by 10 to 25%. Additionally, when RNA blots were rehybridized to EBER-specific probes, the ratio of EBER-1 to EBER-2 expression in these EBV−/EBER+ cells was identical to that in the EBV-positive Akata cells (data not shown).
FIG. 1.
Restoration of EBER expression in EBV-negative Akata BL cells. EBER expression in EBV-positive Akata (A.15) cells and in EBV−/EBER+ cell lines derived from two clonal EBV-negative Akata lines (A.2 and 3F2) was assessed by Northern blot analysis with a probe spanning the EBER-1 and EBER-2 genes. Each lane contained 10 μg of total cellular RNA. The level of EBER expression is reported as a percentage of EBV-positive Akata cells (A.15) and was determined by phosphorimage analysis and normalization of values to GAPDH mRNA expression, which served as a loading control. EBER-1 and EBER-2 are not distinguishable on the blot due to their similar lengths; however, when the blot was sequentially stripped and rehybridized to EBER-specific probes, the ratios of EBER-1 to EBER-2 expression were identical in EBV-positive and EBV−/EBER+ Akata cells (data not shown).
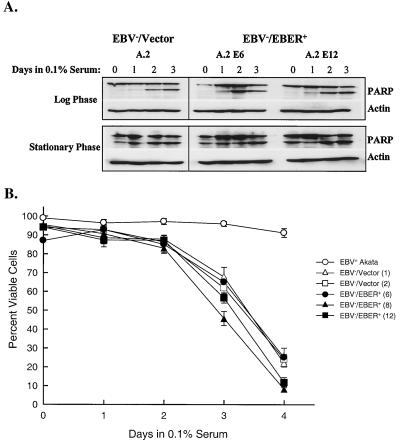
We next addressed whether the EBERs could provide a survival advantage in vitro, and thus account for the increased resistance of EBV-positive versus EBV-negative Akata BL cells to apoptosis (27, 44). We examined whether EBV− and EBV−/EBER+ Akata cells differed in their responses to serum withdrawal by measuring the cleavage of poly-(ADP-ribose)-polymerase (PARP), an early specific marker of apoptotic cell death (39), by immunoblotting with a polyclonal rabbit serum to PARP (Roche Biochemicals, Indianapolis, Ind.). As shown in Fig. 2A, the 89-kDa cleavage product of PARP continued to accumulate equally in EBV-negative (EBV−/Vector) and EBV−/EBER+ cells when shifted to 0.1% serum, regardless of whether they had been in the logarithmic or stationary phase (growth-restrictive conditions) of their growth cycle. In contrast, EBV-positive cells in the stationary phase exhibited little or no further increase in PARP cleavage (data not shown). Additionally, when cells from the stationary phase were shifted to 0.1% serum and their viability was monitored by trypan blue dye exclusion, we again observed no effect of EBER expression on cell survival in EBV-negative Akata cells (Fig. 2B). Note that whereas EBV-positive Akata cells maintained viability (>90%) over 4 days in low serum, as we previously reported (44), the EBV−/EBER+ cells rapidly died at a rate indistinguishable from that of the EBV-negative vector-control cells. Thus, the EBERs failed to promote BL cell survival under growth-restrictive conditions.
FIG. 2.
EBERs fail to support BL cell survival following serum deprivation. (A) EBV−/Vector (A.2) and EBV−/EBER+ (A.2 E6 and A.2 E12) Akata BL cells were seeded in complete growth medium (containing 10% serum) at 2.5 × 105 cells per ml. Cells from the logarithmic (day 2 postseeding) and stationary (day 5 postseeding) phases of growth were washed three times and reseeded (5 × 105 per ml) in growth medium containing 0.1% serum. Cells (106) were then harvested immediately (day 0) and daily for 3 consecutive days for immunoblot analysis of the cleavage of PARP. Shown are the 113-kDa uncleaved and 89-kDa cleavage fragments of PARP. Detection of actin served as a loading control. In A.2 E6 and A.2 E12, the levels of EBER expression were 127 and 90%, respectively, of the level of expression observed in EBV-positive Akata cells (Fig. 1). (B) EBV-positive, EBV−/Vector and EBV−/EBER+ Akata cells were seeded at 2.5 × 105 per ml in growth medium containing 10% serum; when cells had reached the stationary phase of the growth cycle (day 5 postseeding), they were washed and reseeded in triplicate as described above in growth medium containing 0.1% serum. Percent viability was then determined daily by trypan blue dye exclusion. Numbers in parentheses indicate the clonal designation of individual vector control and EBER-expressing cell lines derived from the EBV-negative Akata cell line A.2. Note that the level of EBER expression in these EBV−/EBER+ cell lines (A.2 E6, E8, and E12) ranged from 90% to 127% of expression in EBV-positive Akata cells (Fig. 1).
EBV−/EBER+ Akata BL cell lines were next evaluated for their ability to induce tumors in SCID mice. These included one line (3F2 BSK E) that expressed EBERs at less than 10% of the levels expressed in EBV-positive BL cells and six lines (see Fig. 1) that expressed 41 to 127% of wild-type levels. Male C.B-17 SCID mice, obtained from the colony maintained by the St. Jude Children's Research Hospital Animal Resource Center, were injected subcutaneously in the hind flank with 2 × 107 cells in phosphate-buffered saline (200 μl). Animals were monitored for up to 20 weeks for tumor growth and were sacrificed, and their tumors were excised for analysis of EBER expression when tumors were approximately 1 cm in diameter. As summarized in Table 1, tumors were induced in 25 to 100% of mice injected with cells expressing EBERs at wild-type levels or greater. In contrast, only 1 of 24 mice injected with vector-control cells developed a tumor, and this occurred very late (19 weeks postinjection). All mice injected with EBV-positive Akata cells (Table 1 [A.15]) developed tumors at 4.5 weeks postinjection, similar to the rates and frequency previously established for EBV-positive and reinfected EBV-negative Akata cells (44).
TABLE 1.
EBERs enhance tumorigenic potential of EBV-negative Akata BL cells
| Cell line | Level of EBER expressiona | No. of mice that developed tumors/no. tested | Time to tumor development (wk) |
|---|---|---|---|
| 3F2 (EBV−) | − | 0/4 | NAb |
| A.15 (EBV+) | +++ | 4/4 | 4.5 |
| 3F2 E3 | ++ | 5/9c | 10–15 |
| 3F2 E4 | ++ | 9/12 | 12–18 |
| 3F2 E10 | ++ | 8/9d | 13–18 |
| 3F2 BSA vector 1 | − | 0/7 | NA |
| 3F2 BSA vector 2 | − | 0/4 | NA |
| 3F2 BSK E | + | 6/8 | 14–19 |
| 3F2 BSK vector | − | 1/5 | 19 |
| A.2 E6 | +++ | 1/4 | 12 |
| A.2 E8 | +++ | 1/4 | 10 |
| A.2 E12 | +++ | 3/3 | 7–10 |
| A.2 BSA vector 1 | − | 0/4 | NA |
| A.2 BSA vector 2 | − | 0/4 | NA |
+++, wild-type or greater level of EBER expression; ++, 40 to 55% wild-type expression; +, <10% wild-type expression.
NA, not applicable.
Two additional mice were injected, but died tumor free at 5 and 16 weeks postinjection of unknown causes.
Two additional mice were injected, but died tumor-free at 7 and 11 weeks postinjection of unknown causes.
The mean numbers of weeks required for EBV−/EBER+ BL cells to induce tumors were 10.1, 14.4, and 16.2 for the lines that expressed approximately 100, 50, and less than 10% of wild-type levels of EBERs, respectively. Thus, the EBERs were clearly capable of enhancing the tumorigenic potential of EBV-negative Akata BL cells, and there was a direct correlation between the level of EBER expression and the latency to tumor development. Interestingly, the observation that the EBV-negative lines that expressed wild-type or greater levels of EBERs did not induce tumors in all mice injected and took two to three times as long as EBV-positive Akata cells to induce tumors suggests that the EBERs may only partially contribute to EBV-dependent tumorigenicity. Certainly this is consistent with the failure of the EBERs to inhibit apoptosis in EBV-negative BL cells, whereas EBV efficiently blocks cell death (Fig. 2B) (27, 44). However, we cannot currently exclude the possibility that the apparent inability of the EBERs to restore tumorigenicity to the level of EBV-positive Akata cells is a reflection of inherent differences between cellular clones of EBV-negative and -positive Akata cells, and not the inability of the EBERs to enhance cell survival. Regardless of whether the EBERs are able to fully restore tumorigenicity or not, they were clearly able to potentiate tumorigenic potential independent of a measurable effect on cell survival.
Finally, all tumors that arose in mice injected with the EBV−/EBER+ cells expressed EBERs (Fig. 3 and data not shown). In some instances, the level of EBERs detected in the tumor samples was lower (following normalization for RNA loading) than that seen within the parental cell line. This was most likely due to the amount of non-BL-derived cellular material within some tumor samples rather than loss of EBER expression in the EBV−/EBER+ cells. Note, however, that the tumor derived from A.2 E8 cells (A.2 E8t), which required 10 weeks to develop (Table 1), expressed EBERs at 99% of the level of EBV-positive Akata cells (Fig. 3). Only rarely did we observe an amplification of EBER expression in tumors, even from the cells that expressed less than 10% of wild-type levels of EBERs (data not shown). Therefore, the threshold of EBER expression needed to induce a tumor was less than 10%. Although there was a direct correlation between the level of EBER expression and tumorigenic potential, the minimal latency period of tumors obtained with EBV−/EBER+ cells that expressed 100% of the wild-type level was still twice as long as that obtained with EBV-positive cells, underscoring the concept that the EBERs are only partly responsible for the tumorigenic potential of these BL cells.
FIG. 3.
EBER expression is maintained within tumors induced by EBV−/EBER+ BL cells. EBER expression within each EBV-negative 3F2- and A.2-derived cell line (cl) that expressed EBERs at 41 to 127% of EBV-positive (A.15) Akata cells (Fig. 1) was compared to EBER expression within a respective tumor (t) by Northern blot analysis. Blots were also probed for GAPDH mRNA as a control for RNA loading and integrity. The level of EBER expression, normalized to GAPDH, is presented below each lane. When blots were sequentially stripped and rehybridized to EBER-specific probes, the ratio of EBER-1 to EBER-2 expression had not changed in the tumors relative to that in the parental cell lines (data not shown).
Given that neither tumorigenicity nor the ability to mediate increased resistance to apoptosis is dependent on the EBV EBNA-1 protein (27, 44), the current studies were initiated to identify which of the relatively few EBV genes expressed in BL are responsible for these phenomena. As demonstrated here and also recently by Komano et al. (26), expression of even modest levels of the EBERs markedly increases the tumorigenic potential of EBV-negative Akata BL cells. However, there are two notable differences between our results and those of Komano et al. First, the reduced tumorigenic potential of EBV−/EBER+ relative to EBV-positive Akata cells, also reported here, was previously attributed to the failure to restore EBER expression to wild-type levels and to the gradual loss of EBER expression during cell culture (26). Here we achieved wild-type or greater levels of EBER expression in EBV-negative Akata cells and did not observe loss of EBER expression. Therefore, the EBERs are sufficient to promote tumorigenicity of EBV-positive Akata BL cells, but the effects appear to be partial relative to EBV infection, which may require other viral gene products (see below). Second, Komano et al. also reported that an apparent induction of BCL-2 expression by the EBERs confers a modest survival advantage to EBV−/EBER+ relative to EBV− Akata cells under hypoxic conditions (26). In contrast, we have evaluated the levels of BCL-2 expressed in several lines each of vector-control and EBV−/EBER+ cells and find no evidence to support the notion that the EBERs induce expression of BCL-2 (data not shown). This is consistent with our observed lack of an effect by the EBERs on cell survival (Fig. 2). We have observed, however, that enforced expression of BCL-2 in EBV-negative Akata cells (at levels comparable to those in EBV-positive cells) markedly enhances cell survival and partially restores tumorigenic potential (I. K. Ruf and J. T. Sample, unpublished observations), suggesting that the EBV-induced expression of BCL-2 in Akata BL cells does contribute to tumorigenic potential. Also consistent with the failure of EBERs to protect BL cells from apoptosis, we found that restored EBER expression in EBV-negative Akata cells failed to down-regulate c-MYC (data not shown), as previously observed in EBV-positive and reinfected EBV-negative Akata cells.
The functions of the EBERs in tumorigenicity and EBV latency are unclear. Several cellular proteins that interact with one or both of the EBERs have been identified, including the autoantigen La (a component of cellular snRNP complexes) (16, 33), the ribosomal protein L22 (9, 58–60), and two proteins that mediate the antiviral effects of interferons: the double-stranded RNA-activated protein kinase PKR (7) and (2′—5′) oligoadenylate synthetase (48). However, the functional significance of these interactions with respect to EBV biology remains unresolved, primarily due to the absence of a phenotype associated with loss of EBER expression in vivo within EBV-immortalized B-lymphoblastoid cell lines (55, 56).
The formation of ribonucleoprotein complexes containing EBERs and La has been suggested to reduce pools of free La in the nucleus (16). This, in turn, might affect the stability and function of cellular polymerase III transcripts normally bound by La. The interaction of the EBERs with L22 and resulting relocalization of ∼50% of L22 to the nucleoplasm suggests that the EBERs may also target translation of a specific class of cellular or viral mRNAs (58). Perhaps the most intriguing (yet controversial) aspect of potential EBER function is their ability to bind and inhibit PKR (7, 8, 49), a pivotal mediator of the antiviral effects of interferons (54). In vitro, EBERs block PKR-mediated inhibition of translation, which involves PKR-dependent phosphorylation of the translation initiation factor eIF-2α (34, 37, 51). A dominant-negative form of PKR is capable of transforming immortal murine fibroblasts (NIH 3T3 cells), suggesting that PKR possesses a tumor suppressor function (28, 36). Thus, EBERs could possibly promote tumorigenesis through inhibition of PKR. However, the majority of EBERs are present within the cell nucleus (21), whereas PKR is primarily cytoplasmic (23, 24, 47). Moreover, deletion of the EBER genes from the EBV genome does not impair the antiviral effects of interferon in EBV-immortalized B-lymphoblastoid cell lines (55). Thus, at this juncture, it is unclear if the EBERs promote tumorigenesis vis-a-vis effects on PKR function.
The apparent inability of the EBERs to fully restore tumorigenic potential or to effect cell survival implicates the involvement of an additional EBV gene(s). The reported survival function of the EBV LMP-2A gene (4), expression of which is detectable in some BL cell lines and tumor biopsies by reverse transcription-PCR (27, 57), suggests LMP-2A as a likely candidate. However, we have not detected LMP-2A protein within tumors derived from EBV-positive Akata cells, and enforced expression of LMP-2A does not confer tumorigenic potential to EBV-negative Akata cells (I. K. Ruf, R. Longnecker, and J. T. Sample, unpublished observations). The only remaining known latency-associated EBV gene expressed in BL is that which encodes the BamHI-A rightward transcripts (BARTs), a family of alternatively spliced polyadenylated RNAs that contain several short open reading frames (3, 5, 15, 18, 25, 45, 52, 53). The true coding capacity of these transcripts and the functions of the putative proteins encoded by this gene are currently undefined.
In summary, we have demonstrated that the EBV EBER RNAs can contribute to the tumorigenic potential of BL cells independent of an effect upon apoptosis. Thus, EBV likely contributes to c-MYC-induced lymphomagenesis through at least two avenues: an EBER-dependent mechanism that may enhance proliferative potential, and a second mechanism, mediated by an as-yet-unidentified EBV gene(s), that counters the proapoptotic consequences of the inappropriate expression of c-MYC in BL cells. The latter appears to occur through the induction of BCL-2 expression and the down-regulation of c-MYC under growth-limiting conditions (27, 44). Intriguingly, these data also indicate that even in BL cells such as Akata, in which the ARF-Mdm2-p53 tumor suppressor pathway has been inactivated (13), additional inhibition of c-MYC-induced apoptosis is an important facet of BL tumorigenesis.
Acknowledgments
We thank Fred Wang for the BSAII amplicon, Daniel Henson and Elsie White for excellent technical assistance, and Gerard Zambetti for helpful comments and critical reading of the manuscript.
This work was supported by Public Health Service (PHS) grants CA76379 and DK44158 to J.L.C. and CA73544 and CA56639 to J.T.S., Cancer Center support grant CA21765, and the American Lebanese Syrian Associated Charities (ALSAC). I.K.R. was supported by PHS grant T32-AI07372.
REFERENCES
- 1.Arrand J R, Rymo L. Characterization of the major Epstein-Barr virus-specific RNA in Burkitt lymphoma-derived cells. J Virol. 1982;41:376–389. doi: 10.1128/jvi.41.2.376-389.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Askew D S, Ashmun R A, Simmons B C, Cleveland J L. Constitutive c-myc expression in an IL-3-dependent myeloid cell line suppresses cell cycle arrest and accelerates apoptosis. Oncogene. 1991;6:1915–1922. [PubMed] [Google Scholar]
- 3.Brooks L A, Lear A L, Young L S, Rickinson A B. Transcripts from the Epstein-Barr virus BamHI A fragment are detectable in all three forms of virus latency. J Virol. 1993;67:3182–3190. doi: 10.1128/jvi.67.6.3182-3190.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Caldwell R G, Wilson J B, Anderson S J, Longnecker R. Epstein-Barr virus LMP2A drives B cell development and survival in the absence of normal B cell receptor signals. Immunity. 1998;9:405–411. doi: 10.1016/s1074-7613(00)80623-8. [DOI] [PubMed] [Google Scholar]
- 5.Chen H L, Lung M M, Sham J S, Choy D T, Griffin B E, Ng M H. Transcription of BamHI-A region of the EBV genome in NPC tissues and B cells. Virology. 1992;191:193–201. doi: 10.1016/0042-6822(92)90181-n. [DOI] [PubMed] [Google Scholar]
- 6.Chodosh J, Holder V P, Gan Y J, Belgaumi A, Sample J, Sixbey J W. Eradication of latent Epstein-Barr virus by hydroxyurea alters the growth-transformed cell phenotype. J Infect Dis. 1998;177:1194–1201. doi: 10.1086/515290. [DOI] [PubMed] [Google Scholar]
- 7.Clarke P A, Schwemmle M, Schickinger J, Hilse K, Clemens M J. Binding of Epstein-Barr virus small RNA EBER-1 to the double-stranded RNA-activated protein kinase DAI. Nucleic Acids Res. 1991;19:243–248. doi: 10.1093/nar/19.2.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clarke P A, Sharp N A, Clemens M J. Translational control by the Epstein-Barr virus small RNA EBER-1: reversal of the double-stranded RNA-induced inhibition of protein synthesis in reticulocyte lysates. Eur J Biochem. 1990;193:635–641. doi: 10.1111/j.1432-1033.1990.tb19381.x. [DOI] [PubMed] [Google Scholar]
- 9.Dobbelstein M, Shenk T. In vitro selection of RNA ligands for the ribosomal L22 protein associated with Epstein-Barr virus-expressed RNA by using randomized and cDNA-derived RNA libraries. J Virol. 1995;69:8027–8034. doi: 10.1128/jvi.69.12.8027-8034.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eischen C M, Weber J D, Roussel M F, Sherr C J, Cleveland J L. Disruption of the ARF-Mdm2-p53 tumor suppressor pathway in Myc-induced lymphomagenesis. Genes Dev. 1999;13:2658–2669. doi: 10.1101/gad.13.20.2658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Epstein M A, Achong B G, Barr Y M. Virus particles in cultured lymphoblasts from Burkitt's lymphoma. Lancet. 1964;i:702–703. doi: 10.1016/s0140-6736(64)91524-7. [DOI] [PubMed] [Google Scholar]
- 12.Evan G I, Wyllie A H, Gilbert C S, Littlewood T D, Land H, Brooks M, Waters C M, Penn L Z, Hancock D C. Induction of apoptosis in fibroblasts by c-myc protein. Cell. 1992;69:119–128. doi: 10.1016/0092-8674(92)90123-t. [DOI] [PubMed] [Google Scholar]
- 13.Farrell P J, Allan G J, Shanahan F, Vousden K H, Crook T. p53 is frequently mutated in Burkitt's lymphoma cell lines. EMBO J. 1991;10:2879–2887. doi: 10.1002/j.1460-2075.1991.tb07837.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gaidano G, Ballerini P, Gong J Z, Inghirami G, Neri A, Newcomb E W, Magrath I T, Knowles D M, Dalla-Favera R. p53 mutations in human lymphoid malignancies: association with Burkitt lymphoma and chronic lymphocytic leukemia. Proc Natl Acad Sci USA. 1991;88:5413–5417. doi: 10.1073/pnas.88.12.5413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gilligan K, Sato H, Rajadurai P, Busson P, Young L, Rickinson A, Tursz T, Raab-Traub N. Novel transcription from the Epstein-Barr virus terminal EcoRI fragment, DIJhet, in a nasopharyngeal carcinoma. J Virol. 1990;64:4948–4956. doi: 10.1128/jvi.64.10.4948-4956.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Glickman J N, Howe J G, Steitz J A. Structural analyses of EBER1 and EBER2 ribonucleoprotein particles present in Epstein-Barr virus-infected cells. J Virol. 1988;62:902–911. doi: 10.1128/jvi.62.3.902-911.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gregory C D, Rowe M, Rickinson A B. Different Epstein-Barr virus-B cell interactions in phenotypically distinct clones of a Burkitt's lymphoma cell line. J Gen Virol. 1990;71:1481–1495. doi: 10.1099/0022-1317-71-7-1481. [DOI] [PubMed] [Google Scholar]
- 18.Hitt M M, Allday M J, Hara T, Karran L, Jones M D, Busson P, Tursz T, Ernberg I, Griffin B E. EBV gene expression in an NPC-related tumour. EMBO J. 1989;8:2639–2651. doi: 10.1002/j.1460-2075.1989.tb08404.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Howe J G, Shu M D. Epstein-Barr virus small RNA (EBER) genes: unique transcription units that combine RNA polymerase II and III promoter elements. Cell. 1989;57:825–834. doi: 10.1016/0092-8674(89)90797-6. [DOI] [PubMed] [Google Scholar]
- 20.Howe J G, Shu M-D. Upstream basal promoter element important for exclusive RNA polymerase III transcription of the EBER 2 gene. Mol Cell Biol. 1993;13:2655–2665. doi: 10.1128/mcb.13.5.2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Howe J G, Steitz J A. Localization of Epstein-Barr virus-encoded small RNAs by in situ hybridization. Proc Natl Acad Sci USA. 1986;83:9006–9010. doi: 10.1073/pnas.83.23.9006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jacobs J J, Scheijen B, Voncken J W, Kieboom K, Berns A, van Lohuizen M. Bmi-1 collaborates with c-Myc in tumorigenesis by inhibiting c-Myc-induced apoptosis via INK4a/ARF. Genes Dev. 1999;13:2678–2690. doi: 10.1101/gad.13.20.2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jeffrey I W, Kadereit S, Meurs E F, Metzger T, Bachmann M, Schwemmle M, Hovanessian A G, Clemens M J. Nuclear localization of the interferon-inducible protein kinase PKR in human cells and transfected mouse cells. Exp Cell Res. 1995;218:17–27. doi: 10.1006/excr.1995.1126. [DOI] [PubMed] [Google Scholar]
- 24.Jimenez-Garcia L F, Green S R, Matthews M B, Spector D L. Organization of the double-stranded RNA-activated protein kinase DAI and virus-associated VA RNAI in adenovirus-2-infected HeLa cells. J Cell Sci. 1993;106:11–22. doi: 10.1242/jcs.106.1.11. [DOI] [PubMed] [Google Scholar]
- 25.Karran L, Gao Y, Smith P R, Griffin B E. Expression of a family of complementary-strand transcripts in Epstein-Barr virus-infected cells. Proc Natl Acad Sci USA. 1992;89:8058–8062. doi: 10.1073/pnas.89.17.8058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Komano J, Maruo S, Kurozumi K, Oda T, Takada K. Oncogenic role of Epstein-Barr virus-encoded RNAs in Burkitt's lymphoma cell line Akata. J Virol. 1999;73:9827–9831. doi: 10.1128/jvi.73.12.9827-9831.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Komano J, Sugiura M, Takada K. Epstein-Barr virus contributes to the malignant phenotype and to apoptosis resistance in Burkitt's lymphoma cell line Akata. J Virol. 1998;72:9150–9156. doi: 10.1128/jvi.72.11.9150-9156.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koromilas A E, Roy S, Barber G N, Katze M G, Sonenberg N. Malignant transformation by a mutant of the IFN-inducible dsRNA-dependent protein kinase. Science. 1992;257:1685–1689. doi: 10.1126/science.1382315. [DOI] [PubMed] [Google Scholar]
- 29.Kube D, Vockerodt M, Weber O, Hell K, Wolf J, Haier B, Grässer F A, Müller-Lantzsch N, Kieff E, Diehl V, Tesch H. Expression of Epstein-Barr virus nuclear antigen 1 is associated with enhanced expression of CD25 in the Hodgkin cell line L428. J Virol. 1999;73:1630–1636. doi: 10.1128/jvi.73.2.1630-1636.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Land H, Chen A C, Morgenstern J P, Parada L F, Weinberg R A. Behavior of myc and ras oncogenes in transformation of rat embryo fibroblasts. Mol Cell Biol. 1986;6:1917–1925. doi: 10.1128/mcb.6.6.1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Land H, Parada L F, Weinberg R A. Tumorigenic conversion of primary embryo fibroblasts requires at least two cooperating oncogenes. Nature. 1983;304:596–602. doi: 10.1038/304596a0. [DOI] [PubMed] [Google Scholar]
- 32.Lee M-A, Diamond M E, Yates J L. Genetic evidence that EBNA-1 is needed for efficient, stable latent infection by Epstein-Barr virus. J Virol. 1999;73:2974–2982. doi: 10.1128/jvi.73.4.2974-2982.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lerner M R, Andrews N C, Miller G, Steitz J A. Two small RNAs encoded by Epstein-Barr virus and complexed with protein are precipitated by antibodies from patients with systemic lupus erythematosus. Proc Natl Acad Sci USA. 1981;78:805–809. doi: 10.1073/pnas.78.2.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Levin D H, Petryshyn R, London I M. Characterization of double-stranded-RNA-activated kinase that phosphorylates alpha subunit of eukaryotic initiation factor 2 (eIF-2 alpha) in reticulocyte lysates. Proc Natl Acad Sci USA. 1980;77:832–836. doi: 10.1073/pnas.77.2.832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Magrath I. The pathogenesis of Burkitt's lymphoma. Adv Cancer Res. 1990;55:133–270. doi: 10.1016/s0065-230x(08)60470-4. [DOI] [PubMed] [Google Scholar]
- 36.Meurs E F, Galabru J, Barber G N, Katze M G, Hovanessian A G. Tumor suppressor function of the interferon-induced double-stranded RNA-activated protein kinase. Proc Natl Acad Sci USA. 1993;90:232–236. doi: 10.1073/pnas.90.1.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meurs E F, Watanabe Y, Kadereit S, Barber G N, Katze M G, Chong K, Williams B R, Hovanessian A G. Constitutive expression of human double-stranded RNA-activated p68 kinase in murine cells mediates phosphorylation of eukaryotic initiation factor 2 and partial resistance to encephalomyocarditis virus growth. J Virol. 1992;66:5804–5814. doi: 10.1128/jvi.66.10.5805-5814.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Neri A, Barriga F, Inghirami G, Knowles D M, Neequaye J, Magrath I T, Dalla-Favera R. Epstein-Barr virus infection precedes clonal expansion in Burkitt's and acquired immunodeficiency syndrome-associated lymphoma. Blood. 1991;77:1092–1095. [PubMed] [Google Scholar]
- 39.Nicholson D W, Ali A, Thornberry N A, Vaillancourt J P, Ding C K, Gallant M, Gareau Y, Griffin P R, Labelle M, Lazebnik Y A. Identification and inhibition of the ICE/CED-3 protease necessary for mammalian apoptosis. Nature. 1995;376:37–43. doi: 10.1038/376037a0. [DOI] [PubMed] [Google Scholar]
- 40.Raab-Traub N, Flynn K. The structure of the termini of the Epstein-Barr virus as a marker of clonal cellular proliferation. Cell. 1986;47:883–889. doi: 10.1016/0092-8674(86)90803-2. [DOI] [PubMed] [Google Scholar]
- 41.Rawlins D R, Milman G, Hayward S D, Hayward G S. Sequence-specific DNA binding of the Epstein-Barr virus nuclear antigen (EBNA-1) to clustered sites in the plasmid maintenance region. Cell. 1985;42:859–868. doi: 10.1016/0092-8674(85)90282-x. [DOI] [PubMed] [Google Scholar]
- 42.Rosa M D, Gottlieb E, Lerner M R, Steitz J A. Striking similarities are exhibited by two small Epstein-Barr virus-encoded ribonucleic acids and the adenovirus-associated ribonucleic acids VAI and VAII. Mol Cell Biol. 1981;1:785–796. doi: 10.1128/mcb.1.9.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rowe D T, Rowe M, Evan G I, Wallace L E, Farrell P J, Rickinson A B. Restricted expression of EBV latent genes and T-lymphocyte-detected membrane antigen in Burkitt's lymphoma cells. EMBO J. 1986;5:2599–2607. doi: 10.1002/j.1460-2075.1986.tb04540.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ruf I K, Rhyne P W, Yang H, Borza C M, Hutt-Fletcher L M, Cleveland J L, Sample J T. Epstein-Barr virus regulates c-MYC, apoptosis, and tumorigenicity in Burkitt lymphoma. Mol Cell Biol. 1999;19:1651–1660. doi: 10.1128/mcb.19.3.1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sadler R H, Raab-Traub N. Structural analyses of the Epstein-Barr virus BamHI A transcripts. J Virol. 1995;69:1132–1141. doi: 10.1128/jvi.69.2.1132-1141.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schmitt C A, McCurrach M E, de Stanchina E, Wallace-Brodeur R R, Lowe S W. INK4a/ARF mutations accelerate lymphomagenesis and promote chemoresistance by disabling p53. Genes Dev. 1999;13:2670–2677. doi: 10.1101/gad.13.20.2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schwemmle M, Clemens M J, Hilse K, Pfeifer K, Troster H, Muller W E, Bachmann M. Localization of Epstein-Barr virus-encoded RNAs EBER-1 and EBER-2 in interphase and mitotic Burkitt lymphoma cells. Proc Natl Acad Sci USA. 1992;89:10292–10296. doi: 10.1073/pnas.89.21.10292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sharp T V, Raine D A, Gewert D R, Joshi B, Jagus R, Clemens M J. Activation of the interferon-inducible (2′–5′) oligoadenylate synthetase by the Epstein-Barr virus RNA, EBER-1. Virology. 1999;257:303–313. doi: 10.1006/viro.1999.9689. [DOI] [PubMed] [Google Scholar]
- 49.Sharp T V, Schwemmle M, Jeffrey I, Laing K, Mellor H, Proud C G, Hilse K, Clemens M J. Comparative analysis of the regulation of the interferon-inducible protein kinase PKR by Epstein-Barr virus RNAs EBER-1 and EBER-2 and adenovirus VAI RNA. Nucleic Acids Res. 1993;21:4483–4490. doi: 10.1093/nar/21.19.4483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shimizu N, Tanabe-Tochikura A, Kuroiwa Y, Takada K. Isolation of Epstein-Barr virus (EBV)-negative cell clones from the EBV-positive Burkitt's lymphoma (BL) line Akata: malignant phenotypes of BL cells are dependent on EBV. J Virol. 1994;68:6069–6073. doi: 10.1128/jvi.68.9.6069-6073.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Siekierka J, Mariano T M, Reichel P A, Mathews M B. Translational control by adenovirus: lack of virus-associated RNAI during adenovirus infection results in phosphorylation of initiation factor eIF-2 and inhibition of protein synthesis. Proc Natl Acad Sci USA. 1985;82:1959–1963. doi: 10.1073/pnas.82.7.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Smith P R, de Jesus O, Turner D, Hollyoake M, Karstegl C E, Griffin B E, Karran L, Wang Y, Hayward S D, Farrell P J. Structure and coding content of CST (BART) family RNAs of Epstein-Barr virus. J Virol. 2000;74:3082–3092. doi: 10.1128/jvi.74.7.3082-3092.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Smith P R, Gao Y, Karran L, Jones M D, Snudden D, Griffin B E. Complex nature of the major viral polyadenylated transcripts in Epstein-Barr virus-associated tumors. J Virol. 1993;67:3217–3225. doi: 10.1128/jvi.67.6.3217-3225.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stark G R, Kerr I M, Williams B R, Silverman R H, Schreiber R D. How cells respond to interferons. Annu Rev Biochem. 1998;67:227–264. doi: 10.1146/annurev.biochem.67.1.227. [DOI] [PubMed] [Google Scholar]
- 55.Swaminathan S, Huneycutt B S, Reiss C S, Kieff E. Epstein-Barr virus-encoded small RNAs (EBERs) do not modulate interferon effects in infected lymphocytes. J Virol. 1992;66:5133–5136. doi: 10.1128/jvi.66.8.5133-5136.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Swaminathan S, Tomkinson B, Kieff E. Recombinant Epstein-Barr virus with small RNA (EBER) genes deleted transforms lymphocytes and replicates in vitro. Proc Natl Acad Sci USA. 1991;88:1546–1550. doi: 10.1073/pnas.88.4.1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tao Q, Robertson K D, Manns A, Hildesheim A, Ambinder R F. Epstein-Barr virus (EBV) in endemic Burkitt's lymphoma: molecular analysis of primary tumor tissue. Blood. 1998;91:1373–1381. [PubMed] [Google Scholar]
- 58.Toczyski D P, Matera A G, Ward D C, Steitz J A. The Epstein-Barr virus (EBV) small RNA EBER1 binds and relocalizes ribosomal protein L22 in EBV-infected human B lymphocytes. Proc Natl Acad Sci USA. 1994;91:3463–3467. doi: 10.1073/pnas.91.8.3463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Toczyski D P, Steitz J A. EAP, a highly conserved cellular protein associated with Epstein-Barr virus small RNAs (EBERs) EMBO J. 1991;10:459–466. doi: 10.1002/j.1460-2075.1991.tb07968.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Toczyski D P, Steitz J A. The cellular RNA-binding protein EAP recognizes a conserved stem-loop in the Epstein-Barr virus small RNA EBER 1. Mol Cell Biol. 1993;13:703–710. doi: 10.1128/mcb.13.1.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang F, Li X, Annis B, Faustman D. Tap-1 and Tap-2 gene therapy selectively restores conformationally dependent HLA class I expression in type I diabetic cells. Hum Gene Ther. 1995;6:1005–1017. doi: 10.1089/hum.1995.6.8-1005. [DOI] [PubMed] [Google Scholar]
- 62.Wilson J B, Bell J L, Levine A J. Expression of Epstein-Barr virus nuclear-antigen-1 induces B cell neoplasia in transgenic mice. EMBO J. 1996;15:3117–3126. [PMC free article] [PubMed] [Google Scholar]
- 63.Yates J L, Warren N, Sugden B. Stable replication of plasmids derived from Epstein-Barr virus in various mammalian cells. Nature. 1985;313:812–815. doi: 10.1038/313812a0. [DOI] [PubMed] [Google Scholar]