Abstract
Three series of podophyllotoxin derivatives with various nitrogen-containing heterocycles were designed and synthesized. The antitumor activity of these podophyllotoxin derivatives was evaluated in vitro against a panel of human tumor cell lines. The results showed that podophyllotoxin-imidazolium salts and podophyllotoxin-1,2,4-triazolium salts a1–a20 exhibited excellent cytotoxic activity. Among them, a6 was the most potent cytotoxic compound with IC50 values of 0.04–0.29 μM. Podophyllotoxin-1,2,3-triazole derivatives b1–b5 displayed medium cytotoxic activity, and podophyllotoxin-amine compounds c1–c3 has good cytotoxic activity with IC50 value of 0.04–0.58 μM. Furthermore, cell cycle and apoptosis experiments of compound a6 were carried out and the results exhibited that a6 could induce G2/M cell cycle arrest and apoptosis in HCT-116 cells.
Keywords: podophyllotoxin, imidazolium salts, triazoles, antitumor activity, structure-activity relationships
1 Introduction
According to the data from the International Agency for Cancer Research (IARC), there would be around 19.3 million new cancer diagnoses and nearly 10 million cancer-related deaths in 2020 (Sung et al., 2021). Therefore, the development of innovative anticancer agents and therapeutic strategies is essential (Boshuizen and Peeper, 2020). Medicinal chemists have increasingly viewed natural products as valuable resources for developing anticancer drug (Choi et al., 2017). About 84% of antitumor small molecule drugs approved between 1981 and 2019 were derived from natural products or structural units containing natural products (Newman and Cragg, 2020). The design and rational synthesis of natural product-like libraries, from which lead compounds with high efficiency, high selectivity, and low toxicity can be screened and discovered for preclinical studies, is one of the significant approaches for developing new drugs (Liu et al., 2017).
Podophyllotoxin is a natural product with anticancer activity belonging to the lignans cyclolignolide family (Dagenais et al., 2020). Podophyllotoxin and its semi-synthetic glycoside derivatives Etoposide, Teniposide and Etoposide Phosphate have been proved to be highly active antitumor agents with excellent clinical effects and are essential drugs for the treatment of small cell lung cancer, leukemia, testicular cancer and other types of tumors (Zhang et al., 2018; Li et al., 2019; Guo and Jiang, 2021; Zhao et al., 2021). Numerous structural and pharmacological studies have demonstrated that C-4 derivatization could enhance the biological activity of this family of drugs (Xiao et al., 2020).
On the other side, nitrogen-containing heterocycles are widely used in drug design and discovery (Xu et al., 2014a; Vitaku et al., 2014). The unique structural features of imidazoles and triazoles possess desirable electron rich properties, which are more favorable for conjugation with other molecules, and the molecular activity could be improved after hybridization (Verma et al., 2013; Gaba and Mohan, 2015; Bozorov et al., 2019; Xu et al., 2019; Dixit et al., 2021; Sharma et al., 2021). Among them, imidazolium salts have attracted much attention for their important and extensive biological and pharmacological activities, especially antitumor activity (Cui et al., 2003; Yang et al., 2009). In this context, our group has devoted to the synthesis of novel imidazolium salt derivatives and found a series of promising compounds with antitumor activity (Chen et al., 2013; Wang et al., 2013; Xu et al., 2014b; Xu et al., 2015; Zhou et al., 2016a; Zhou et al., 2016b). Further mechanistic studies confirmed that these imidazolium salt derivatives can induce cell cycle arrest and apoptosis in tumor cells (Liu et al., 2013; Liu et al., 2015; Huang et al., 2019). The representative examples are an effective antitumor active diosgenin-imidazolium salt and a new mTOR pathway inhibitor B591 (Figure 1) (Deng et al., 2019; Zhou et al., 2019).
FIGURE 1.
Structures of podophyllotoxin and representative imidazolium salts with antitumor activity.
In the past three decades, molecular hybridization has played an important role in drug discovery (Zhang et al., 2017; Yang et al., 2021). In view of the potential anticancer activity of podophyllotoxin and nitrogen-containing heterocycles, we launched the synthesis of hybrid compounds of natural product podophyllotoxin and imidazolium/triazolium salts. Although some nitrogen-containing heterocycles-podophyllotoxin derivatives were prepared and found to possess anticancer and neuroactive activities (Chen et al., 2011; Shang et al., 2012; Vishnuvardhan et al., 2017; Hou et al., 2019), to the best of our knowledge, there are no reports on the synthesis and bioactivity of imidazolium/triazolium salt hybrids of podophyllotoxin. With this in mind, we turned our attention to the synthesis and antitumor activity of a series of novel podophyllotoxin nitrogen-containing heterocycles, especially imidazolium and triazolium salts.
2 Results and discussion
2.1 Chemistry
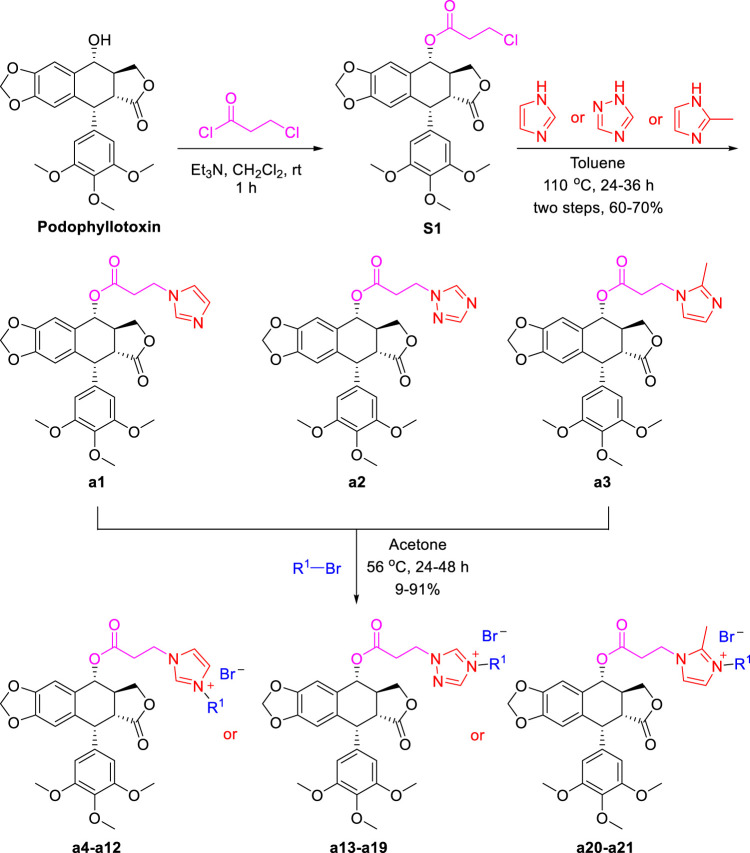
As shown in Scheme 1, firstly, to synthesize podophyllotoxin nitrogen-containing heterocycles, imidazole, 1,2,4-triazole, 2-methylimidazole, 1,2,3-triazole and amines were used for reaction. Using the commercial podophyllotoxin as starting material, the esterification reaction with 2-chloropropionyl chloride was carried out to obtain the ester S1. Next, S1 reacted with imidazole, 1,2,4-triazole and 2-methylimidazole to obtain the nitrogen-containing heterocycles a1–a3 (60%–70% yields, two steps). Then, treatment of a1–a3 with various bromides generated the podophyllotoxin imidazolium/triazolium salts a4–a21 (9%–91% yields). Secondly, as shown in Scheme 2, 4-chlorinated podophyllotoxin S2 was obtained by commercial podophyllotoxin reacting with thionyl chloride. Next, a nucleophilic substitution reaction with sodium azide was conducted to obtain compound S3 (46% yield, two steps). Then, azide S3 reacted with various terminal alkynes under Click reaction condition to get the podophyllotoxin-1,2,3-triazole derivatives b1–b5 (31%–47% yields). Finally, as shown in Scheme 3, using podophyllotoxin as the starting material, esterification reaction with 2-chloropropionyl chloride was performed to obtain the ester S1, which then underwent a nucleophilic substitution reaction with commercial cyclic amines (pyrrole, piperidine and morpholine) to furnish the podophyllotoxin-amines c1-c3 (48%–61% yields, two steps). To summarize, the structures and yields of all new podophyllotoxin nitrogen-containing heterocycle derivatives were shown in Table 1.
SCHEME 1.
Synthesis of podophyllotoxin nitrogenous derivatives a1–a21.
SCHEME 2.
Synthesis of podophyllotoxin nitrogenous derivatives b1–b5.
SCHEME 3.
Synthesis of podophyllotoxin nitrogenous derivatives c1–c3.
TABLE 1.
Structures and yields of podophyllotoxin nitrogen-containing heterocycles a1–a21/b1–b5/c1–c3.
| Entry | Compound | R1 | R2 | R3 | Yields (%) |
|---|---|---|---|---|---|
| 1 | a1 | — | — | — | 68 |
| 2 | a2 | — | — | — | 70 |
| 3 | a3 | — | — | — | 60 |
| 4 | a4 | 2-naphthylacyl | — | — | 38 |
| 5 | a5 | 4-bromophenacyl | — | — | 91 |
| 6 | a6 | 2-naphthylmethyl | — | — | 58 |
| 7 | a7 | phenacyl | — | — | 44 |
| 8 | a8 | 4-methoxyphenacyl | — | — | 53 |
| 9 | a9 | 4-bromobenzy | — | — | 82 |
| 10 | a10 | 4-methylbenzyl | — | — | 46 |
| 11 | a11 | 2-bromobenzyl | — | — | 81 |
| 12 | a12 | 5-bromomethyl | — | — | 78 |
| 13 | a13 | 2-naphthylacyl | — | — | 30 |
| 14 | a14 | 4-bromophenacyl | — | — | 34 |
| 15 | a15 | 2-naphthylmethyl | — | — | 63 |
| 16 | a16 | phenacyl | — | — | 37 |
| 17 | a17 | 4-methoxyphenacyl | — | — | 80 |
| 18 | a18 | 4-bromobenzy | — | — | 71 |
| 19 | a19 | 4-methylbenzyl | — | — | 9 |
| 20 | a20 | 4-bromophenacyl | — | — | 58 |
| 21 | a21 | phenacyl | — | — | 50 |
| 22 | b1 | — | F | — | 31 |
| 23 | b2 | — | Br | — | 47 |
| 24 | b3 | — | OMe | — | 33 |
| 25 | b4 | — | Pyridine | — | 38 |
| 26 | b5 | — | Naphthalene | — | 43 |
| 27 | c1 | — | — | Pyrrolidine | 58 |
| 28 | c2 | — | — | Piperidine | 61 |
| 29 | c3 | — | — | Morpholine | 48 |
2.2 Biological evaluation and structure-activity relationship analysis
2.2.1 Biological assay procedures and results
The synthesized twenty-nine podophyllotoxin nitrogen-containing derivatives were evaluated in vitro antitumor cytotoxic activity screening by MTS method (Perchellet et al., 2005). Four human cancer cell lines including hepatocellular carcinoma cells (HepG-2), non-small cell lung cancer cells (A-549), breast cancer cells (MDA-MB-231) and colon cancer cells (HCT-116) were selected to determine in vitro cytotoxic activity. DDP (Cisplatin), Etoposide, and Paclitaxel were chosen as positive controls. The results were listed in Table 2.
TABLE 2.
Cytotoxic activities of podophyllotoxin nitrogen-containing heterocycles a1–a21/b1–b5/c1–c3 in vitro a (IC50, μM b ).
| Entry | Compound No. | HepG-2 | A-549 | MDA-MB-231 | HCT-116 |
|---|---|---|---|---|---|
| 1 | a1 | 0.31 ± 0.02 | 0.76 ± 0.12 | 0.47 ± 0.01 | 0.04 ± 0.00 |
| 2 | a2 | 0.23 ± 0.01 | 0.30 ± 0.02 | 0.45 ± 0.03 | 0.15 ± 0.01 |
| 3 | a3 | 0.32 ± 0.06 | 0.65 ± 0.03 | 0.38 ± 0.04 | 0.31 ± 0.04 |
| 4 | a4 | 0.33 ± 0.01 | 1.11 ± 0.04 | 0.53 ± 0.01 | 0.04 ± 0.00 |
| 5 | a5 | 0.29 ± 0.00 | 0.76 ± 1.54 | 0.51 ± 0.05 | 0.04 ± 0.00 |
| 6 | a6 | 0.07 ± 0.00 | 0.29 ± 0.04 | 0.11 ± 0.01 | 0.04 ± 0.00 |
| 7 | a7 | 0.18 ± 0.01 | 1.08 ± 0.20 | 0.48 ± 0.02 | 0.04 ± 0.00 |
| 8 | a8 | 0.26 ± 0.01 | 0.65 ± 0.39 | 0.55 ± 0.02 | 0.04 ± 0.00 |
| 9 | a9 | 0.25 ± 0.01 | 0.44 ± 0.10 | 0.49 ± 0.02 | 0.29 ± 0.00 |
| 10 | a10 | 0.25 ± 0.01 | 0.25 ± 0.00 | 0.45 ± 0.09 | 0.30 ± 0.05 |
| 11 | a11 | 0.27 ± 0.00 | 0.42 ± 0.09 | 0.33 ± 0.06 | 0.30 ± 0.08 |
| 12 | a12 | 0.26 ± 0.05 | 0.53 ± 0.10 | 0.51 ± 0.02 | 0.21 ± 0.01 |
| 13 | a13 | 0.34 ± 0.02 | 1.10 ± 0.14 | 0.41 ± 0.06 | 0.10 ± 0.02 |
| 14 | a14 | 0.42 ± 0.03 | 1.53 ± 0.23 | 0.33 ± 0.04 | 0.05 ± 0.02 |
| 15 | a15 | 0.29 ± 0.01 | 0.75 ± 0.07 | 0.30 ± 0.00 | 0.27 ± 0.15 |
| 16 | a16 | 0.28 ± 0.02 | 0.75 ± 0.07 | 0.25 ± 0.02 | 0.20 ± 0.06 |
| 17 | a17 | 0.25 ± 0.02 | 0.74 ± 0.19 | 0.28 ± 0.05 | 0.04 ± 0.09 |
| 18 | a18 | 0.28 ± 0.03 | 0.58 ± 0.01 | 0.23 ± 0.02 | 0.04 ± 0.00 |
| 19 | a19 | 0.25 ± 0.00 | 0.65 ± 0.01 | 0.26 ± 0.00 | 0.04 ± 0.00 |
| 20 | a20 | 7.98 ± 0.51 | 15.84 ± 0.04 | >20 | 6.80 ± 0.11 |
| 21 | a21 | 0.40 ± 0.03 | 0.28 ± 0.05 | 0.11 ± 0.03 | 0.07 ± 0.01 |
| 22 | b1 | 1.86 ± 0.15 | 3.60 ± 0.56 | 2.03 ± 0.14 | 0.04 ± 0.33 |
| 23 | b2 | 2.14 ± 0.04 | 7.31 ± 0.12 | 1.74 ± 0.47 | 6.58 ± 1.87 |
| 24 | b3 | 1.60 ± 0.00 | 3.48 ± 0.03 | 0.49 ± 0.01 | 0.90 ± 0.42 |
| 25 | b4 | 4.59 ± 0.37 | 9.64 ± 0.62 | 7.57 ± 0.62 | 1.49 ± 1.76 |
| 26 | b5 | >20 | >20 | >20 | >20 |
| 27 | c1 | 0.28 ± 0.01 | 0.58 ± 0.02 | 0.04 ± 0.03 | 0.05 ± 0.01 |
| 28 | c2 | 0.10 ± 0.01 | 0.39 ± 0.03 | 0.10 ± 0.00 | 0.10 ± 0.02 |
| 29 | c3 | 0.21 ± 0.03 | 0.39 ± 0.01 | 0.36 ± 0.11 | 0.06 ± 0.11 |
| 30 | DDP | 1.85 ± 0.34 | 5.52 ± 0.21 | 12.77 ± 2.71 | 10.92 ± 0.26 |
| 31 | Etoposide | 16.95 ± 2.00 | 14.77 ± 0.26 | 1.92 ± 0.96 | 14.19 ± 0.13 |
| 32 | Paclitaxel | <0.008 | <0.008 | <0.008 | <0.008 |
Data represent the mean values of three independent determinations.
Cytotoxicity as IC50 for each cell line, is the concentration of compound which reduced by 50% the optical density of treated cells with respect to untreated cells using the MTS assay.
As presented in Table 2, the majority of podophyllotoxin nitrogen-containing heterocycles showed potent inhibitory activity than positive controls Etoposide and DDP. Notably, these derivatives have obvious selective inhibitory against HCT-116 cell lines. The results showed that the structures of podophyllotoxin nitrogen-containing heterocycles plays a crucial role in regulating cytotoxic activity.
For pharmacophores of nitrogen-containing heterocycles, podophyllotoxin-imidazole and its salts (a1/a3/a4-a12/a20/a21) and podophyllotoxin-1,2,4-triazole and its salts (a2/a13–a19) exhibited excellent cytotoxic activity with IC50 values of 0.04–1.53 μM except a20. Among them, a6 was the most potent cytotoxic compound and its IC50 values for HepG2, A-549, MDA-MB-231 and HCT-116 were 0.07, 0.29, 0.11 and 0.04 μM, respectively. Secondly, the introduction of 1,2,3-triazole derivatives b1–b5 by Click reaction showed medium cytotoxic activity with IC50 values of 0.04–9.64 μM except b5. Finally, while compounds c1–c3 introduced with cyclic amines also showed excellent cytotoxic activity with IC50 values of 0.04–0.58 μM.
For the groups at position-3 of imidazolium and triazolium salts (a4–a21), the cytotoxic activities of most substituted benzyl groups were superior to those of substituted phenacyl groups. Among them, 2-naphthylmethyl substituent at position-3 of the imidazole ring (a6 and a15) showed excellent cytotoxic activity with IC50 values of 0.04–0.75 μM and a6 was the most powerful compound. Similarly, 4-bromobenzyl, 4-methylbenzyl, 4-methoxybenzoyl and 2-bromobenzyl groups at position-3 of the imidazole ring exhibited good cytotoxic activity with IC50 values of 0.04–0.65 μM.
For the groups at position-4 of 1,2,3-triazole ring (b1–b5), when the substituent was replaced with electron donating groups (R2 = OMe), b3 exhibits higher inhibitory activity with IC50 values of 0.49–3.48 μM. In contrast, when the substituent was charged with electron-withdrawing groups (R2 = F, Br), b1 and b2 were decreased slightly with IC50 values of 0.04–7.31 μM. When the substituent group was pyridine, b4 exhibited poor inhibitory activity with IC50 values of 1.49–9.64 μM, due to the electron-withdrawing effect of pyridine. When the substituent was a naphthalene ring, b5 did not exhibit any inhibitory activity.
For the cyclic amines (c1–c3), piperidine derivative of podophyllotoxin (c2) displayed excellent cytotoxic activity with IC50 values of 0.10–0.39 μM, which was superior to pyrrole derivative (0.04–0.58 μM) and morpholine derivative (0.06–0.39 μM). Notably, compound c1 has selective inhibitory against MDA-MB-231 cell lines with an IC50 value of 0.04 μM.
The results demonstrated that the introduction of imidazole ring into podophyllotoxin and a 2-naphthyl methyl substituent at the imidazolium salt’s 3-position play a critical role in enhancing cytotoxic activity. The preliminary structure activity relationships (SARs) of the derivatives were summarized in Scheme 4.
SCHEME 4.
Structure-activity relationship of podophyllotoxin derivatives.
2.2.2 Compound a6 induced G2/M cell cycle arrest and apoptosis
To determine the possible mechanism of compound a6 induced proliferation inhibition, cell cycle and apoptosis analysis were performed with flow cytometry. Firstly, HCT-116 cells were treated with indicated concentrations of compound a6 for 24 h and the cell cycle phase distribution of a6-treated cells was determined with propidium iodide (PI) staining. As shown in Figure 2, a6 exposure caused G2/M phase arrest in HCT-116 cells when compared with the control group, indicating that compound a6 inhibited cell proliferation through inducing G2/M cell cycle arrest.
FIGURE 2.
Compound a6 induced G2/M phase arrest in HCT-116 cells. (A) Cells were treated with different concentrations of compound a6 (25, 50 and 200 nM) for 24 h, and cell cycle was determined by cell cytometry with PI staining. (B) The percentages of cells in different phases were quantified.
The compound a6 induced cell apoptosis was also determined with Annexin V-FITC/PI staining. As shown in Figure 3, after treated with compound a6 at 25, 50 and 200 nM for 48 h, the apoptotic rate of HCT-116 cells remarkably elevated to 5.37 ± 0.37%, 10.45 ± 0.20% and 64.98 ± 2.40%, respectively. The results suggested that compound a6 inhibited cell proliferation through induction of G2/M cell cycle arrest and apoptosis of HCT-116 cells.
FIGURE 3.
Compound a6 induced apoptosis of HCT-116 cells. (A) Cells were treated with 25, 50 and 200 nM compound a6 for 48 h. Cell apoptosis was determined by Annexin V-FITC/PI staining analysis. (B) The quantification of apoptotic cells.
3 Conclusion
In conclusion, a series of novel podophyllotoxin nitrogen-containing heterocycle derivatives with potential antitumor activity were prepared using a straightforward synthetic approach. The results showed that the imidazole-substituted derivatives demonstrated more effective inhibitory activity than 1,2,4-triazole-substituted and 1,2,3-triazole-substituted equivalents. The biological activity was significantly improved when the imidazole or imidazolium salt group was introduced into the structure of podophyllotoxin. Among them, imidazolium salt a6 was the most potent cytotoxic activity with IC50 values of 0.04–0.29 μM. It has an obvious selective inhibitory against HCT-116 cell lines with an IC50 value of 0.04 μM and could induce G2/M cell cycle arrest and apoptosis in HCT-116 cells. Podophyllotoxin-imidazolium salt a6 could be employed as a promising lead compound for further structural modification and in-depth activity research to identify new starting points for more effective anticancer agents.
Funding Statement
This work was supported by grants from the National Key R&D Program of China (2019YFE0109200), the Central Government Guides Local Science and Technology Development Fund (202207AA110007, 202207AB110002), Yunnan Science and Technology Department and Yunnan University Joint Fund Project (2019FY003010), Program for Xingdian Talents (Yun-Ling Scholars) and IRTSTYN, and the Project of Yunnan Characteristic Plant Screening and R&D Service CXO Platform (2022YKZY001).
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Author contributions
XY, JZ, and YL conceived and designed the experiments. MY, YF, XS, MX, CZ, and YaM performed the experiments. MY, YF, ZZ, LK, and YiM analyzed the data. XY, YF, and LK wrote the article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fchem.2023.1191498/full#supplementary-material
References
- Boshuizen J., Peeper D. S. (2020). Rational cancer treatment combinations: An urgent clinical need. Mol. Cell. 78 (6), 1002–1018. 10.1016/j.molcel.2020.05.031 [DOI] [PubMed] [Google Scholar]
- Bozorov K., Zhao J., Aisa H. A. (2019). 1,2,3-Triazole-containing hybrids as leads in medicinal chemistry: A recent overview. Bioorg. Med. Chem. 27 (16), 3511–3531. 10.1016/j.bmc.2019.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H., Zuo S., Wang X., Tang X., Zhao M., Lu Y., et al. (2011). Synthesis of 4β-triazole-podophyllotoxin derivatives by azide–alkyne cycloaddition and biological evaluation as potential antitumor agents. Eur. J. Med. Chem. 46 (9), 4709–4714. 10.1016/j.ejmech.2011.07.024 [DOI] [PubMed] [Google Scholar]
- Chen W., Deng X. Y., Li Y., Yang L. J., Wan W. C., Wang X. Q., et al. (2013). Synthesis and cytotoxic activities of novel hybrid 2-phenyl-3-alkylbenzofuran and imidazole/triazole compounds. Bioorg. Med. Chem. Lett. 23 (15), 4297–4302. 10.1016/j.bmcl.2013.06.001 [DOI] [PubMed] [Google Scholar]
- Choi H., Cho S. Y., Pak H. J., Kim Y., Choi J.-y., Lee Y. J., et al. (2017). Npcare: Database of natural products and fractional extracts for cancer regulation. J. Cheminformatics 9 (1), 2. 10.1186/s13321-016-0188-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui B. L., Zheng B. L., He K., Zheng Q. Y. (2003). Imidazole alkaloids from lepidium meyenii. J. Nat. Prod. 66, 1101–1103. 10.1021/np030031i [DOI] [PubMed] [Google Scholar]
- Dagenais G. R., Leong D. P., Rangarajan S., Lanas F., Lopez-Jaramillo P., Gupta R., et al. (2020). Variations in common diseases, hospital admissions, and deaths in middle-aged adults in 21 countries from five continents (PURE): A prospective cohort study. Lancet 395 (10226), 785–794. 10.1016/s0140-6736(19)32007-0 [DOI] [PubMed] [Google Scholar]
- Deng G., Zhou B., Wang J., Chen Z., Gong L., Gong Y., et al. (2019). Synthesis and antitumor activity of novel steroidal imidazolium salt derivatives. Eur. J. Med. Chem. 168, 232–252. 10.1016/j.ejmech.2019.02.025 [DOI] [PubMed] [Google Scholar]
- Dixit D., Verma P. K., Marwaha R. K. (2021). A review on ‘triazoles’: Their chemistry, synthesis and pharmacological potentials. J. Iran. Chem. Soc. 18 (10), 2535–2565. 10.1007/s13738-021-02231-x [DOI] [Google Scholar]
- Gaba M., Mohan C. (2015). Development of drugs based on imidazole and benzimidazole bioactive heterocycles: Recent advances and future directions. Med. Chem. Res. 25 (2), 173–210. 10.1007/s00044-015-1495-5 [DOI] [Google Scholar]
- Guo Q., Jiang E. (2021). Recent advances in the application of podophyllotoxin derivatives to fight against multidrug-resistant cancer cells. Curr. Top. Med. Chem. 21 (19), 1712–1724. 10.2174/1568026621666210113163327 [DOI] [PubMed] [Google Scholar]
- Hou W., Zhang G., Luo Z., Su L., Xu H. (2019). Click chemistry‐based synthesis and cytotoxic activity evaluation of 4α‐triazole acetate podophyllotoxin derivatives. Chem. Biol. Drug Des. 93 (4), 473–483. 10.1111/cbdd.13436 [DOI] [PubMed] [Google Scholar]
- Huang M., Duan S., Ma X., Cai B., Wu D., Li Y., et al. (2019). Synthesis and antitumor activity of aza-brazilan derivatives containing imidazolium salt pharmacophores. Med. Chem. Commun. 10 (6), 1027–1036. 10.1039/c9md00112c [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Chen M., Yao B., Lu X., Zhang X., He P., et al. (2019). Transferrin receptor-targeted redox/pH-sensitive podophyllotoxin prodrug micelles for multidrug-resistant breast cancer therapy. J. Mat. Chem. B 7 (38), 5814–5824. 10.1039/c9tb00651f [DOI] [PubMed] [Google Scholar]
- Liu L.-X., Wang X.-Q., Yan J.-M., Li Y., Sun C.-J., Chen W., et al. (2013). Synthesis and antitumor activities of novel dibenzo[b,d]furan–imidazole hybrid compounds. Eur. J. Med. Chem. 66, 423–437. 10.1016/j.ejmech.2013.06.011 [DOI] [PubMed] [Google Scholar]
- Liu L. X., Wang X. Q., Zhou B., Yang L. J., Li Y., Zhang H. B., et al. (2015). Synthesis and antitumor activity of novel N-substituted carbazole imidazolium salt derivatives. Sci. Rep. 5, 13101. 10.1038/srep13101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z., Zhang C., Duan S., Liu Y., Chen W., Li Y., et al. (2017). Synthesis and cytotoxic activity of novel hybrid compounds between indolo[b]tetrahydrofuran and imidazolium salts. Chin. J. Org. Chem. 37 (6), 1506–1515. 10.6023/cjoc201610043 [DOI] [Google Scholar]
- Newman D. J., Cragg G. M. (2020). Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J. Nat. Prod. 83 (3), 770–803. 10.1021/acs.jnatprod.9b01285 [DOI] [PubMed] [Google Scholar]
- Perchellet E. M., Perchellet J.-P., Baures P. W. (2005). Imidazole-4,5-dicarboxamide derivatives with antiproliferative activity against HL-60 cells. J. Med. Chem. 48 (19), 5955–5965. 10.1021/jm050160r [DOI] [PubMed] [Google Scholar]
- Shang H., Chen H., Zhao D., Tang X., Liu Y., Pan L., et al. (2012). Synthesis and biological evaluation of 4α/4β-imidazolyl podophyllotoxin analogues as antitumor agents. Arch. Pharm. Chem. Life Sci. 345 (1), 43–48. 10.1002/ardp.201100094 [DOI] [PubMed] [Google Scholar]
- Sharma P., LaRosa C., Antwi J., Govindarajan R., Werbovetz K. A. (2021). Imidazoles as potential anticancer agents: An update on recent studies. Molecules 26 (14), 4213. 10.3390/molecules26144213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung H., Ferlay J., Siegel R. L., Laversanne M., Soerjomataram I., Jemal A., et al. (2021). Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71 (3), 209–249. 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- Verma A., Joshi S., Singh D. (2013). Imidazole: Having versatile biological activities. J. Chem. 2013, 1–12. 10.1155/2013/329412 [DOI] [Google Scholar]
- Vishnuvardhan M., V S. R., Chandrasekhar K., Lakshma Nayak V., Sayeed I. B., Alarifi A., et al. (2017). Click chemistry-assisted synthesis of triazolo linked podophyllotoxin conjugates as tubulin polymerization inhibitors. Med. Chem. Commun. 8 (9), 1817–1823. 10.1039/c7md00273d [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitaku E., Smith D. T., Njardarson J. T. (2014). Analysis of the structural diversity, substitution patterns, and frequency of nitrogen heterocycles among U.S. FDA approved pharmaceuticals. J. Med. Chem. 57 (24), 10257–10274. 10.1021/jm501100b [DOI] [PubMed] [Google Scholar]
- Wang X. Q., Liu L. X., Li Y., Sun C. J., Chen W., Li L., et al. (2013). Design, synthesis and biological evaluation of novel hybrid compounds of imidazole scaffold-based 2-benzylbenzofuran as potent anticancer agents. Eur. J. Med. Chem. 62, 111–121. 10.1016/j.ejmech.2012.12.040 [DOI] [PubMed] [Google Scholar]
- Xiao J., Gao M., Sun Z., Diao Q., Wang P., Gao F. (2020). Recent advances of podophyllotoxin/epipodophyllotoxin hybrids in anticancer activity, mode of action, and structure-activity relationship: An update (2010-2020). Eur. J. Med. Chem. 208, 112830. 10.1016/j.ejmech.2020.112830 [DOI] [PubMed] [Google Scholar]
- Xu H., Tang H., Feng H., Li Y. (2014a). Design, synthesis and anticancer activity evaluation of novel C14 heterocycle substituted epi-triptolide. Eur. J. Med. Chem. 73, 46–55. 10.1016/j.ejmech.2013.11.044 [DOI] [PubMed] [Google Scholar]
- Xu X. L., Wang J., Yu C. L., Chen W., Li Y. C., Li Y., et al. (2014b). Synthesis and cytotoxic activity of novel 1-((indol-3-yl)methyl)-1H-imidazolium salts. Bioorg. Med. Chem. Lett. 24 (21), 4926–4930. 10.1016/j.bmcl.2014.09.045 [DOI] [PubMed] [Google Scholar]
- Xu X. L., Yu C. L., Chen W., Li Y. C., Yang L. J., Li Y., et al. (2015). Synthesis and antitumor activity of novel 2-substituted indoline imidazolium salt derivatives. Org. Biomol. Chem. 13 (5), 1550–1557. 10.1039/c4ob02385d [DOI] [PubMed] [Google Scholar]
- Xu Z., Zhao S. J., Liu Y. (2019). 1,2,3-Triazole-containing hybrids as potential anticancer agents: Current developments, action mechanisms and structure-activity relationships. Eur. J. Med. Chem. 183, 111700. 10.1016/j.ejmech.2019.111700 [DOI] [PubMed] [Google Scholar]
- Yang X. D., Zeng X. H., Zhang Y. L., Qing C., Song W. J., Li L., et al. (2009). Synthesis and cytotoxic activities of novel phenacylimidazolium bromides. Bioorg. Med. Chem. Lett. 19 (7), 1892–1895. 10.1016/j.bmcl.2009.02.065 [DOI] [PubMed] [Google Scholar]
- Yang Z., Zhou Z., Luo X., Luo X., Luo H., Luo L., et al. (2021). Design and synthesis of novel podophyllotoxins hybrids and the effects of different functional groups on cytotoxicity. Molecules 27 (1), 220. 10.3390/molecules27010220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Tian Y., Kang D., Huo Z., Zhou Z., Liu H., et al. (2017). Discovery of uracil-bearing DAPYs derivatives as novel HIV-1 NNRTIs via crystallographic overlay-based molecular hybridization. Eur. J. Med. Chem. 130, 209–222. 10.1016/j.ejmech.2017.02.047 [DOI] [PubMed] [Google Scholar]
- Zhang X., Rakesh K. P., Shantharam C. S., Manukumar H. M., Asiri A. M., Marwani H. M., et al. (2018). Podophyllotoxin derivatives as an excellent anticancer aspirant for future chemotherapy: A key current imminent needs. Bioorg. Med. Chem. 26 (2), 340–355. 10.1016/j.bmc.2017.11.026 [DOI] [PubMed] [Google Scholar]
- Zhao W., Cong Y., Li H. M., Li S., Shen Y., Qi Q., et al. (2021). Challenges and potential for improving the druggability of podophyllotoxin-derived drugs in cancer chemotherapy. Nat. Prod. Rep. 38 (3), 470–488. 10.1039/d0np00041h [DOI] [PubMed] [Google Scholar]
- Zhou B., Liu Z. F., Deng G. G., Chen W., Li M. Y., Yang L. J., et al. (2016a). Synthesis and antitumor activity of novel N-substituted tetrahydro-beta-carboline-imidazolium salt derivatives. Org. Biomol. Chem. 14 (39), 9423–9430. 10.1039/c6ob01495j [DOI] [PubMed] [Google Scholar]
- Zhou H., Yu C., Kong L., Xu X., Yan J., Li Y., et al. (2019). B591, a novel specific pan-PI3K inhibitor, preferentially targets cancer stem cells. Oncogene 38 (18), 3371–3386. 10.1038/s41388-018-0674-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., Duan K., Zhu L., Liu Z., Zhang C., Yang L., et al. (2016b). Synthesis and cytotoxic activity of novel hexahydropyrrolo[2,3-b]indole imidazolium salts. Bioorg. Med. Chem. Lett. 26 (2), 460–465. 10.1016/j.bmcl.2015.11.092 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.