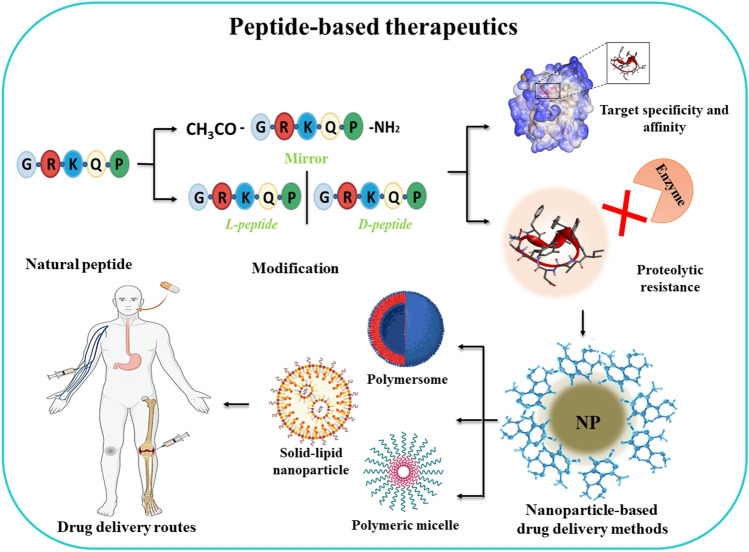
Abstract
In recent years, the occurrence of a wide variety of drug-resistant diseases has led to an increase in interest in alternate therapies. Peptide-based drugs as an alternate therapy hold researchers’ attention in various therapeutic fields such as neurology, dermatology, oncology, metabolic diseases, etc. Previously, they had been overlooked by pharmaceutical companies due to certain limitations such as proteolytic degradation, poor membrane permeability, low oral bioavailability, shorter half-life, and poor target specificity. Over the last two decades, these limitations have been countered by introducing various modification strategies such as backbone and side-chain modifications, amino acid substitution, etc. which improve their functionality. This has led to a substantial interest of researchers and pharmaceutical companies, moving the next generation of these therapeutics from fundamental research to the market. Various chemical and computational approaches are aiding the production of more stable and long-lasting peptides guiding the formulation of novel and advanced therapeutic agents. However, there is not a single article that talks about various peptide design approaches i.e., in-silico and in-vitro along with their applications and strategies to improve their efficacy. In this review, we try to bring different aspects of peptide-based therapeutics under one article with a clear focus to cover the missing links in the literature. This review draws emphasis on various in-silico approaches and modification-based peptide design strategies. It also highlights the recent progress made in peptide delivery methods important for their enhanced clinical efficacy. The article would provide a bird’s-eye view to researchers aiming to develop peptides with therapeutic applications.
Graphical Abstract
Keywords: Peptide therapeutics, Modifications, Computational strategies, Peptide-based drug delivery
Introduction
There are over 7000 natural peptides that have been recognized and are actively involved in a wide range of biological activities such as neurotransmission, hormonal functions, growth factors activity, antimicrobial activity, and immunomodulation (Buchwald et al. 2014; Fosgerau and Hoffmann 2015; Padhi et al. 2014; Souery and Bishop 2018). Peptide-based therapies exhibit significant efficacy in treating various diseases, including hormonal deficiencies, autoimmune disorders, infections, diabetes, and various types of cancers (Larché and Wraith 2005; Jones and Hattersley 2013; Souery and Bishop 2018). These therapeutics are selective and specific towards cell surface receptors, like G-protein-coupled receptors or ion channels, which in turn stimulate their intracellular effects (Fosgerau and Hoffmann 2015; Rastogi et al. 2019). The smaller size of peptides–based therapeutics allows them to penetrate deeper into tissues like skin, intestines, etc. as compared to other larger biomolecules such as antibodies which helps them to enter the bloodstream more quickly (Lee et al. 2019; Leonard 2019; Patel et al. 2019). They have low immunogenicity and high target specificity as compared to small drug molecules (Wagner et al. 2018). Small molecules target only 2–5% of the human genome but peptides are more selective for specific protein targets (Cirillo et al. 2011; Hopkins and Groom 2002; Lau and Dunn 2018; Rask-Andersen et al. 2014; Vargason et al. 2021). Additionally, peptides have a relatively lower production cost than recombinant proteins and antibodies (Sachdeva et al. 2019; Trier et al. 2019), and have a lower accumulation rate in the tissues (Groll et al. 2001). In recent times, several peptide-based drugs have entered the market showing efficiency against allergic diseases, infectious diseases, autoimmune diseases, fibrosis, asthma, etc. (Craik et al. 2013; Currier et al. 2008; Muheem et al. 2016; Semalty et al. 2007).
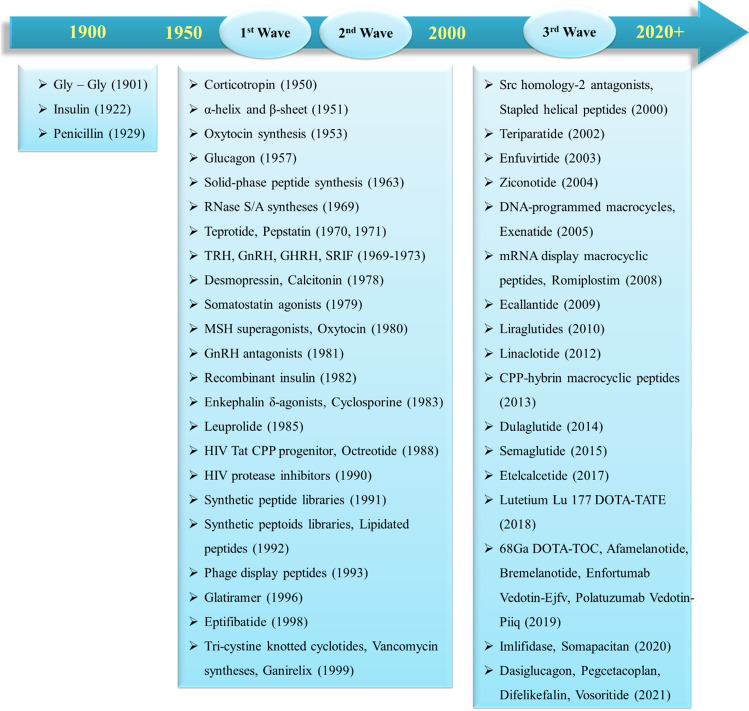
Peptide drug development had begun in the twentieth century with a strong emphasis on receptor targets such as G protein-coupled receptors. There are several key milestones (Fig. 1) that ought to be recognized as they represent some of the most notable achievements in the field of peptide drug discovery. However, over the past few years, the global market for peptide therapeutics has grown significantly in size and economic value. The US Food and Drug Administration (FDA) has approved a total number of 208 new drugs (150 chemical agents and 58 biologicals) in the last 6 years. Among the FDA-approved drugs in the past 6 years (2015–2020), 19 peptide-based drugs have been identified which are listed in Table 1 (Al Shaer et al. 2019, 2020; de la Torre and Albericio 2020a, b). D’Aloisio and his co-workers have designed PepTherDia (http://peptherdia.herokuapp.com/), a database containing the list for approved peptide-based drugs and diagnostics. As of 2023, PepTherDia lists 114 peptides approved for theranostic applications. The ultimate goal of this database is to aid the scientists in the early stage of the peptide-based drug discovery process to successfully design or pre-screen the peptide candidates (D’Aloisio et al. 2021). Apart from being used in therapeutics and diagnostics, these molecules are also playing a key role in drug delivery systems and as the foundation for new biomaterials especially in nano-range with a wide range of applications in medicine. Physical encapsulation or chemical conjugation procedures can be used to load drugs onto peptide nanomaterials, resulting in prolonged drug retention time and uptake rates (Yang et al. 2021). Peptide-drug conjugates as drug delivery systems fall under the prodrug strategy, which lowers the toxicity and increases the solubility of free drugs, thereby, improving the pharmacokinetic profile of the drug. It increases drug biocompatibility as well as encourages targeted delivery and controlled drug release (Goyal and Ramakrishnan 2019; Lian and Ji 2020; Tesauro et al. 2019).
Fig. 1.
Milestones in peptide and peptidomimetic drug discovery (FDA 2021; Muttenthaler et al. 2021; Zane et al. 2021)
Table 1.
Peptide-based drugs approved by FDA in the last 7 years (2015–2021) (de la Torre and Albericio 2020a; FDA 2021). [Copyright
© de la Torre and Albericio 2020a, b. Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0/)]
| Year | Active compound | Trade name | Disease treated |
|---|---|---|---|
| 2015 |
Insulin degludec Chain A (GIVEQCCTSICSLYQLENYCN) Chain B (FVNQLCGSHLVEALYLVCGERGFFYTP) |
Tresiba® | Diabetes |
| 2016 |
Adlyxin (HGEGTFTSDLSKQMEEEAVRLFIEWLKNGGPSSGAPPSKKKKK) |
Lixisenatide® | Diabetes |
| 2017 |
Abaloparatide (AVSEHQLLHDKGKSIQDLRRRELLEKLLXKLHTA) |
Tymlos® | Osteoporosis |
| 2017 |
Angiotensin II (DRVYIHPF) |
Giapreza® | Hypotension |
| 2017 |
Etelcalcetide [Ac-(CARRRAR)D-NH2] |
Parsabiv® | Hyperparathyroidism |
| 2017 |
Plecanatide (NDECELCVNVACTGCL) |
Trulance® | Chronic idiopathic constipation |
| 2017 |
Semaglutide (HXEGTFTSDVSSYLEGQAAKEFIAWLVRGRG) |
Ozempic® | Diabetes |
| 2018 |
177Lu DOTATATE (FDCYWDKTCT) |
Lutathera® | Neuroendocrine tumors, theranostic |
| 2019 |
68 Ga DOTATOC (FDCYDWKTCT) |
Edotreotide Gallium GA-68® | Neuroendocrine tumors, diagnostic |
| 2019 |
Afamelanotide (Ac-SYSXEHFDRWGKPV-NH2) |
Scenesse® | Skin damage and pain |
| 2019 |
Bremelanotide (DHFDRWK) |
Vyleesi® | Women hypoactive sexual desire |
| 2020 |
Bulevirtide acetate (GTNLSVPNPLGFFPDHQLDPAFGANSNNPDWDFNPNKDHWPEANKVG) |
Hepcludex® | Antiviral Hepatitis delta virus infection |
| 2020 |
Somapacitan (FPTIPLSRLFDNAMLRAHRLHQLAFDTYQEFEEAYIPKEQKYSFLQNPQTSLCFSESIPTPSNREETQQKSNLELLRISLLLIQSWLEPVQFLRSVFANSCVYGASDSNVYDLLKDLEEGIQTLMGRLEDGSPRTGQIFKQTYSKFDTNSHNDDALLKNYGLLYCFRKDMDKVETFLRIVQCRSVEGSCGF) |
Sogroya® | Growth hormone deficiency |
| 2020 |
Teriparatide acetate (SVSEIQLMHNLGKHLNSMERVEWLRKKLQDVHNF) |
Forteo/Forsteo® | Osteoporosis |
| 2021 |
Dasiglucagon (HSQGTFTSDYSKYLDXARAEEFVKWLEST) |
Zegalogue® | Hypoglycemia |
| 2021 |
Pegcetacoplan (ICVWQDWGAHRCTXK) |
Empaveli® | Nocturnal hemoglobinuria |
| 2021 |
Difelikefalin (FFLK)D-[ω(4-aminopiperidine-4-carboxylic acid)]-OH |
Korsuva® | Chronic kidney diseases |
| 2021 |
Vosoritide (PGQEHPNARKYKGANKKGLSKGCFGLKLDRIGSMSGLGC) |
Voxzogo® | Achondroplasia and open epiphyses |
Development of peptide therapeutics has established its potential in a new era in twenty-first century, which has significantly accelerated the breakthroughs in area of structural biology, recombinant biologics, and novel synthetic and analytical technologies. The development of peptide drugs now involves an intricate process encompassing novel peptide discovery, peptide design, peptide synthesis, modification of the structures, and its activity assessment. In this review article, we focus on various natural and synthetic peptides, that are currently being explored in the arena of peptide therapeutics. We attempt to combine various peptide-based therapeutic elements into a single piece which includes their limitations and highlights different strategies to improve their efficacy. It also draws attention to recent advancements in peptide delivery methods that are crucial for their improved therapeutic profile. This article can act as a one-stop point for the researchers to have a wide outlook in the field of peptide therapeutics.
Literature Search Method
This study was performed in accordance to the PRISMA guidelines (Fig. 2) (Moher et al. 2009). The databases PubMed, and Web of Science were used to conduct the electronic search strategy with combined keywords. We used the following search terms and other subject headings: ‘peptide therapeutics’, ‘classification of peptides’, ‘antimicrobial peptides’, ‘anticancer peptides’, ‘neurological peptide drugs’, ‘dermatological peptide drugs’, ‘cardiovascular peptide drugs’, ‘peptide vaccines’, ‘limitations of peptide therapeutic’, ‘peptide modification strategies’, ‘peptide drug delivery’, ‘peptide drug delivery routes’, ‘peptide-nanoparticle conjugates’, ‘peptide databases’, ‘peptide in-silico tools’. Articles published between 2010 and 2022 were included in the study. Review articles and articles written in English were only included. Some important articles were also sourced from the reference list of the included papers and some were recommended by experts in the field. A pool of 90,277 records was initially identified using the electronic search strategy, however, after removal of duplicates, 47,138 records remained. Among these, communications that were relevant to the topic, or in English language only, with full text were included (n = 211). In order to obtain detailed information on therapeutics under clinical trials or commercialized we also retrieved data from ClinicalTrials.gov., European medicines agency (www.ema.europa.eu) and U.S. Food & Drug Administration (www.fda.gov).
Fig. 2.
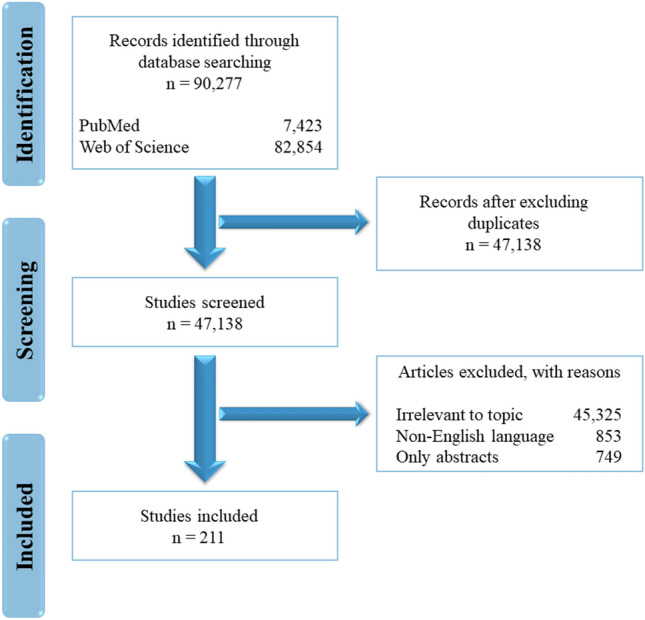
PRISMA flowchart representing the selection and exclusion of articles related to the topic
Peptides as Therapeutics
A peptide is a short chain of amino acids bound together by peptide bonds between the carboxyl group of one amino acid and the amino group of the other amino acid. A peptide is different from a protein as a peptide chain usually consists of 2 to 50 amino acids, whereas a protein is made up of 50 or more amino acids (Britannica 2016; "Peptide," 2014; Rogers). Peptides of diverse lengths have shown significant efficacy against various therapeutic conditions such as osteoporosis, cancer, microbial infections, hormonal deficiencies, diabetes, obesity, etc. (Thundimadathil 2012; Wetzler and Hamilton 2018). Due to the higher surface area of peptides, they are highly specific and sensitive to their native receptors, hence, exhibit minimal off-target effects causing less adverse effects in the patients. For nearly two decades it has been known that peptides are capable of triggering cellular apoptosis via caspase activation (Buckley et al. 1999; Philchenkov 2004). Studies and development in the area of apoptosis have gained the significant interest of researchers due to the increased prevalence of certain diseases like cancer, autoimmune disorders, neurodegenerative diseases, etc. at a rapid rate.
Peptides in Research and Market in the Recent Years
Research in peptide-based therapeutics has progressed extensively to have a broader range of structures from several natural sources or to use biomedical sciences beyond its traditional focus on endogenous human peptides. Almost a century ago, since the emergence of insulin, more than 80 peptide drugs have been approved for a variety of diseases such as diabetes, cancer, osteoporosis, multiple sclerosis, HIV infection, and chronic pain (Cabri et al. 2021). New peptide-based therapeutics are being developed at a steady pace, with more than 150 peptides in clinical trials and another 400–600 peptides in preclinical studies (Fosgerau and Hoffmann 2015; Lau and Dunn 2018; Muttenthaler et al. 2021). Peptide-based drugs occupy a distinct space in the pharmaceutical area accounting for 5% of the global pharmaceutical market exceeding US$ 50 billion of global sales in 2019. Over the last decades, peptide-based drugs have been steadily approved with an average growth rate of 7.7% for the global peptide therapeutic market (Global Peptide Therapeutics Sales Market Report 2020).
Peptides in Clinical Trials Phases
Lau and Dunn reported a series of peptides that entered human clinical trials including many peptides in active clinical development (Lau and Dunn 2018). Thus, the development of novel peptides and peptide-based therapies is becoming highly prevalent in combating multiple disorders. It is necessary to identify the lead compound during the drug discovery stage and establish a competent manufacturing method to assess the efficacy and safety of the new drug candidate during clinical trials. In the pre-clinical phase, various tests are carried out to determine safety before human testing is initiated. Clinical studies at all stages provide information to the organizations across the globe required to submit the regulatory approvals (Shojaei 1998).
A significant number of peptide-based drugs in the market are analogs that develop the intrinsic activity of natural hormones with enhanced therapeutic potential. Due to the existence of the endogenous peptide as a biological precedent, an analog drug development system is certainly safe concerning target validation. However, native peptide leads may have insufficient potency or selectivity (Lau and Dunn 2018). Thus, conjugation strategies have been developed as a significant approach for enhancing the properties of peptide therapeutics. The number of conjugated peptides has increased over time; since 2010, 30% of peptides that have undergone clinical development are conjugates (Lau and Dunn 2018).
These therapeutics have been categorized based on various diseases viz. anticancer peptides, antimicrobial peptides, immunogenic peptides, peptides against metabolic disorders, hematological disorders, neurodegenerative diseases, genetic disorders, etc. (Usmani et al. 2017). Among all these diseases, peptide therapeutics are most prevalently used in cancer therapies, microbial infections, metabolic disorders, neurodegenerative diseases, cardiovascular and dermatological diseases. Therefore, this article focuses on the peptide-based therapeutics that are being commonly used in these rampant areas.
Anti-cancer Peptides
ACPs are small amino acid sequences that are selective and harmful to cancer cells (Chiangjong et al. 2020). The conformation, net charge, and the secondary structure of peptides depend on the physicochemical properties, amino acid composition of the peptides, and chemical groups present in the chain. ACPs predominantly include the amino acid residues viz. glycine, lysine, and leucine that make up the hydrophobic component of the peptide (Chiangjong et al. 2020) (Shoombuatong et al. 2018). Charged amino acids, lysine, and arginine disrupt the integrity of the cell membrane and penetrate it, causing cytotoxicity in the cancer cells. Aspartic and glutamic acid also present anti-proliferative activity on cancer cells (Dai et al. 2017; Yamaguchi et al. 2016). l-asparaginase is a therapeutic enzyme used clinically for the treatment of pediatric acute lymphoblastic leukemia (Purwaha et al. 2014). This enzyme catalyzes the hydrolysis of l-asparagine into aspartic acid and ammonia (Purwaha et al. 2014; Shrivastava et al. 2016). Both normal and cancer cells require asparagine for growth and proliferation, but cancer cells cannot produce asparagine on their own and survive on the circulating asparagine (Jiang et al. 2021). Therefore, hydrolysis of asparagine in the body leads to cancer cell death ("l-asparaginase,"; Shrivastava et al. 2016). Similarly, glutamine, a derivative of glutamic acid is also an important substrate for cell growth (Cluntun et al. 2017; Dutta et al. 2013). l-glutamine synthetase converts l-glutamic acid into l-glutamine. Due to the lower reactivity of l-glutamine synthetase in tumor tissues, l-glutamine cannot be synthesized. Thus, an antagonist of this enzyme can interfere with the metabolic process of l-glutamine and act as an anticancer agent (Dutta et al. 2013; Luzzio et al. 2000). ACPs are either naturally occurring peptides or synthetic peptides which are modified by substituting amino acid residues or by the addition of chemical groups. Although various natural peptides are biocompatible and less cytotoxic, several natural peptides cannot account for active targeting, cellular uptake, and targeted delivery (Apostolopoulos et al. 2021; Lee et al. 2019; Serrill et al. 2016). Thus, natural peptides can be modified into novel synthetic peptides with improved specificity, higher therapeutic efficacy, cell permeability, and cancer cell cytotoxicity. A large number of ACPs kill the cancer cells by membrane lysis or pore formation via apoptosis and necrosis (Droin et al. 2009). These peptides are either molecularly targeted to specific cancer cells by penetrating or binding to the cells or can be bound to anticancer drugs to enhance their activity (Li et al. 2011; Peyressatre et al. 2015; Raucher and Ryu 2015).
The analogs of luteinizing hormone-releasing hormone (LHRH), a hypothalamic neuropeptide, and somatostatin, a tetradecapeptide hormone are the standard treatments for various cancers and provide a powerful forum for theranostics that helps in the advancement of treatments for cancer. The agonists of LHRH are reported to cause an early surge in luteinizing hormone (LH), follicle-stimulating hormone (FSH), and testosterone, and overstimulation of LHRH receptor suppresses LH leading to castrate level (50 ng/dL) of testosterone. It is essential for the therapeutic potential of LHRH agonists against prostate, endometrial, and breast cancers. Whereas, the antagonist is reported to block the signals of the LHRH receptor which causes persistent inhibition of LH, FSH, and testosterone. The most recently approved LHRH antagonist is degarelix which potentially induces competitive LHRH receptor blockade in the absence of an intrinsic agonist effect (Brunel et al. 2019; Klotz et al. 2008).
Somatostatin analogs are another major class of peptide-based therapeutics for cancer treatment. It is also known as growth hormone-inhibiting hormone (GHIH) or somatotropin release-inhibiting factor (SRIF). Along with growth hormone, it also suppresses cholecystokinin, insulin, thyroid-stimulating hormone (TSH), and glucagon (Mandarino et al. 1981). The efficiency of somatostatin ligands in cancer treatment has been indicated by discovering overexpression of somatostatin receptors in tumors which has led to the development and approval of somatostatin agonist, octreotide (Brown et al. 1977). Octreotide is a potent inhibitor of insulin and growth hormone and has significant efficiency against carcinoid syndrome, pancreatic, intestinal, and pituitary tumors (Modlin et al. 2010). In 2007, another somatostatin analog, lanreotide was approved which is structurally similar to octreotide and is used to treat various gastro-enteropancreatic-neuroendocrine tumors.
Several peptide-based cancer therapies have been developed using ACPs or ACPs in combination with various drugs and the efficacy of peptides to target the malignant cells have been tested in clinical trials. Table 2 summarises various synthetic peptide-based drugs and vaccines that are undergoing clinical trials.
Table 2.
Anticancer and antimicrobial peptide-based drugs and vaccines under clinical trials (Chiangjong et al. 2020; Håkansson et al. 2019; Niemeyer‐van der Kolk et al. 2020; Pan et al. 2019; Peek et al. 2020; "Peptides,"; Wang et al. 2018; Zhang and Yang 2022)
| Peptide | Peptide sequences | Target | Trial phase |
|---|---|---|---|
| SVN53-67/M57-KLH Peptide Vaccine | DLAQMFFCFKELEGW | Metastatic Pancreatic Neuroendocrine Tumor | Phase 1 |
| KRAS multipeptide vaccine |
Kras-G12D (KLVVVGADGVGKSALTI) Kras-61Wt (KLVVVGAGGVGKSALTI) Kras-63Wt (SALTIQLIQNHFVDE) Kras-68Wt (FLCVFAINNTKSFED) |
Pancreatic cancer | Phase 1 |
| Arginase-1 multipeptide vaccine |
ARG1-18 (AKDIVYIGLRDVDPGEHYIL), ARG1-19 (DVDPGEHYILKTLGIKYFSM),ARG1-20 (KTLGIKYFSMTEVDRLGIGK) |
Metastatic Solid Tumors | Phase 1 |
| MUC-1 peptide vaccine, MUC1 peptide-poly ICLC adjuvant vaccine | H2N-(GVTSAPDTRPAPGSTAPPAH)5-CONH2 | Breast cancer | Phase 1 |
| HER-2/neu peptide vaccine |
E75 (KIFGSLAFL) GP2 (IISAVVGIL) A37 (GVGSPYVSRLLGICL LRMK) |
Breast cancer | Phase 1 |
| HPV16 E7 peptide-based vaccine | GQAEPDRAHYNIVTF | Cervical cancer | Phase 1, Phase 2 |
| RNF43-721 | NSQPVWLCL | Colorectal cancer | Phase 1 |
| LY6K/VEGFR1/VEGFR2 multipeptide vaccine |
LY6K (RYCNLEGPPI) VEGFR1 (SYGVLLWEI) VEGFR2 (RFVPDGNRI) |
Esophageal cancer | Phase 1 |
| MAGE-3.A1 peptide-CpG 7909 adjuvant vaccine | EVDPIGHLY | Melanoma | Phase 1, Phase 2 |
| VEGFR1-1084, VEGFR2-169 |
VEGFR1 (SYGVLLWEI) VEGFR2 (RFVPDGNRI) |
Pancreatic cancer | Phase 1, Phase 2 |
| URLC10 peptides with adjuvant | RYCNLEGPPI | Lung cancer | Phase 1, Phase 2 |
| PD-L1 and Arginase 1 Dual peptide vaccine |
PD-L1Long1 (FMTYWHLLNAFTVTVPKDL) ArgLong2 (ISAKDIVYIGLRDVDPGEHYILKTLGIKYFSMTEVDRL) |
Myeloproliferative Neoplasms | Phase 1, Phase 2 |
| PD-L1/IDO peptide vaccine |
IO102 (DTLLKALLEIASCLEKALQVF) IO103 (FMTYWHLLNAFTVTVPKDL) |
Metastatic Melanoma | Phase 1, Phase 2 |
| IO102 peptide vaccine | DTLLKALLEIASCLEKALQVF | Squamous Cell Carcinoma | Phase 2 |
| WT 126-134 peptide vaccine | RMFPNAPYL | Leukemia | Phase 2 |
| PR1 peptide vaccine | VLQELNVTV | Leukemia | Phase 3 |
| Degarelix (LHRH antagonist) | Ac-XXXSXXLKPA-NH2 | Prostate cancer | Phase 4 |
| DPK-060 | GKHKNKGKKNGKHNGWKWWW | Staphylococcus aureus skin infection | Phase 2 |
| Omiganan | ILRWPWWPWRRK | Atopic dermatitis | Phase 2 |
| P60.4Ac | IGKEFKRIVERIKRFLRELVRPLR | Chronic suppurative otitis media | Phase 2 |
| Nal-P-113 | AKRXXGYKRKFX-NH2 | Periodontal Pathogenic infections | Phase 3 |
Anticancer peptides are classified into 3 major groups; (i) antimicrobial or pore-forming peptides, (ii) cell penetrating peptides, and (iii) tumour-targeting peptides.
Antimicrobial Peptides
Antimicrobial Peptides (AMPs) besides acting against microorganisms, they can also induce necrosis or apoptosis of cancer cell membranes. AMPs either cause cellular disruption of the negatively charged molecules present on the cancer cell membranes or breakdown of the mitochondrial membranes (Boohaker et al. 2012; Marqus et al. 2017). Margainin II, NRC-03, NRC-07, buforin II are a few pore-forming peptides that act against bladder cancer, breat cancer, cervical cancer, leukemia and lung cancer, respectively (Hilchie et al. 2013; Lehmann et al. 2006; Park et al. 2000).
Cell Penetrating Peptides
Cell Penetrating Peptides (CPPs) are hydrophobic peptides that can move through the plasma membrane and play the key role in transporting cargos such as DNA, siRNA, oligonucleotides, proteins, etc. Thus, CPPs are promising agents for drug delivery (Bidwell III and Raucher 2009; Regberg et al. 2012). BR2 and Tat are two CPPs derived from human immunodeficiency virus (HIV) and buforin II, respectively. BR2 actively targets colon cancer, cervical cancer, and melanoma, whereas, Tat in conjugation with doxorubicin targets breast and prostate cancer (Liang and Yang 2005; Lim et al. 2013).
Tumour-Targeting Peptides
Tumour-targeting Peptides (TPPs) target the receptors present on the tumour cell surfaces. The peptide RGD selectively binds to the integrin ανβ3 and ανβ5 that are expressed in melanoma, brain tumours, ovarian, lung and breast cancers (Wickham et al. 1993). Xiong and co-workers functionalised RGD onto a sterically stabilised liposome (SSL) and conjugated with doxorubicin (RGD-SSL-Dox) which resulted in enhanced efficacy against melanoma (Xiong et al. 2005).
Anticancer peptide can be further classified into 3 groups on the basis of their mode of actions: (i) targeting signal transduction pathways, (ii) cell cycle regulation, and (iii) cell death (Marqus et al. 2017).
Signal Transduction Pathways
TTPs that selectively bind to the receptors expressed on the cancer cell surface either result in stimulation or inhibition of the signalling pathways in cancer cells. Oncogenic signalling pathways are identified as the primary targets for peptides as predominantly control the cancer cell activity. Peptides binding to the receptors impairs the process of signal transduction leading to no cellular response which enhances the efficacy of the cancer treatment (Karami Fath et al. 2022). A 15 amino acid peptide, PNC-2 and a 13 amino acid peptide, PNC-7 were reported to actively target pancreatic cancer by inducing phenotypic reversion of Ras-transformed cells (Kanovsky et al. 2003; Lee et al. 1990).
Cell Cycle Regulation
Cell proliferation is essential for development and regeneration of eukaryotic organisms. Cell cycle involves four phases: G1-phase, S-phase, G2-phase, and M-phase. A number of cyclin-dependent kinases (Cdks) regulate the progression of cell through each phase of the cell cycle (Suryadinata et al. 2010). However, abnormal activation of Cdks in cancer results in abnormal cell proliferation. ACPs, by binding to specific Cdk inhibits the progression of the cells from one to phase to the next. A 22 amino acid synthetic peptide, p16 was reported as Cdk inhibitor that bound to Cdk4/6 in G1 phase which inhibited the complex formation of cyclin D-Cdk4 and prevented the breast and colon cancer cell progression to S-phase (Fåhraeus et al. 1998). Another Cdk inhibitor peptide, p21 was reported to induce cell cycle arrest at G1 phase and inhibited cell proliferation in colon cancer and lymphoma (Cayrol et al. 1998; Mutoh et al. 1999). When p21 was conjugated with biopolymer elastin-like polypeptide (ELP) and a CPP, Bac-7, the Bac-7-ELP-p21 polypeptide induced cell cycle arrest at S and G2 phase of cell cycle and inhibited cell proliferation in ovarian cancer (Massodi et al. 2010).
Cell Death
ACPs also function by causing cell death of the cancer cells by inducing apoptosis or necrosis by inducing membrane lysis or pore formation. A number of peptides, namely, Tat, CT20p, RRM-MV are reported to causes apoptosis against various cancers viz. lymphoma, melanoma, squamous carcinoma, pancreatic, breast and colon cancers.
Antimicrobial Peptides
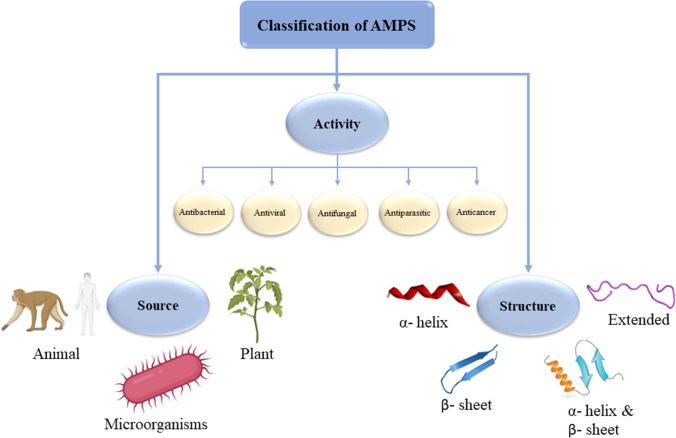
AMPs are short-chain proteins with a wide range of antimicrobial properties and immunomodulatory activities against bacterial pathogens, viruses, and fungi (Bardan et al. 2004). They have various advantages like broad‐spectrum activity, higher efficiency against various diseases, and lesser susceptibility to microbial resistance. They are naturally produced by ribosomal or non-ribosomal biogenic pathways and are structurally diverse. AMPs can be classified based on their (i) source, (ii) biological activity, (iii) structural charateristics (Fig. 3) (Elias and Choi 2005; Huan et al. 2020).
Fig. 3.
Classification of AMPs (Huan et al. 2020; Moretta et al. 2021)
AMPs Based on Sources
AMPs, based on its sources, can be found in different eukaryotes and prokaryotes. Primarily AMPs are derived from different animals, plants and microorganisms (Fig. 3).
AMPs Based on Biological Activity
AMPs are crucial part of innate immunity which possess a variety of biological activities, such as antibacterial, antiviral, antifungal, antiparasitic, or anticancer activities (Fig. 3).
AMPs Based on Their Structures
AMPs can be subcategorized into four groups on the basis of their secondary structures: (i) α-helical peptides, (ii) β-sheet peptides, (iii) both α-helix and β-sheet peptides, and (iv) linear extended peptides (Fig. 3). The secondary structure of the peptides is an essential element for their function as the bacterial membrane permeation by AMPs strongly rely on the secondary structure. It has been reported that folding of peptides into α-helix helps them penetrate the cell membrane (McKay et al. 2018). α-helical peptides exhibit amphipathic structure which separates the hydrophobic domain from the cationic one to the other side of the helix which leads to a favourable interaction between AMPs and cell membranes. Hydrophobic domain of the AMPs then disrupt the bacterial membrane followed by binding to the anionic endotoxin on the cell wall via electrostatic interactions (Liang et al. 2020). A number of AMPs such as LL-37, melittin, AH, C5A, kiadin-2, kiadin-6, etc. which exhibited α-helical structures resulted in potential antimicrobial activity. Considering the several studies reported (Johansson et al. 1998; Park et al. 2019; Rončević et al. 2018) it can be stated that the α-helical structures of the AMPs play an essential role in bacterial membrane disruption (Liang et al. 2020).
Among the most prevalent AMPs in nature, the cationic alpha-helical AMPs like cathelicidins LL-37, cecropin, magainin, and proline-rich AMPs can disrupt the bacterial cytoplasmic membranes resulting in apoptosis through an osmotic shock (Boparai and Sharma 2020). Various pathways hinder the development of microbial resistance. Cationic AMP residues electrostatically interact with the anionic bacterial cell wall which leads to bactericidal activity. AMPs also form pores on the membranes of bacterial cells which leads to apoptosis in bacteria (Kamaruzzaman et al. 2019; Namivandi-Zangeneh et al. 2019).
The peptides that are derived from ribosomes have lately shown significant therapeutic potential (Mahlapuu et al. 2016). Thus, in the field of AMPs, synthetic approaches to develop such peptides have risen significantly. Bacitracin, a peptide isolated from Brevibacillus brevis is used in combination with other antibiotics against gram-positive bacteria which interferes with the bacterial cell wall and peptidoglycan synthesis. In 2003, a lipidated cyclic depsipeptide, daptomycin was approved by FDA against Staphylococcus aureus caused complicated skin infections (Tótoli et al. 2015). Vancomycin, a glycopeptide also shows significant efficacy against gram-positive strains (Butler et al. 2014; Nicolaou et al. 1999). Echinocandins, lipidated cyclic hexapeptides exhibit substantial antifungal activity (Candida fungal infections) by potentially inhibiting the (1 → 3)‐β‐d‐glucan enzyme synthesis complex (Aguilar-Zapata et al. 2015; Nyfeler and Keller-Schierlein 1974). Peptides like defensins, insect-derived cecropins, and amphibian-originated antimicrobial peptides are also potent against various fungal infections and gram-positive and gram-negative strains. A cecropin analog, Hecate has recently shown inhibitory effects on several species of Acanthamoeba. SHIVA-11 is also a cecropin analog that is commonly used against different ocular infections (Warnke et al. 2013). In various local infections caused by multidrug-resistant bacterial strains, certain amphibian-derived peptides such as alyteserin, brevinin, ascaphin, pseudin, kassinatuerin, and temporin are used (Migoń et al. 2018). Another natural peptide, P113 derived from saliva has shown high in-vitro efficacy against Candida albicans and numerous other gram-positive and gram-negative bacteria (Shiffman and Low 2018). It is also used as a mouthwash to treat oral Candidiasis in human immunodeficiency virus (HIV) infected patients (Yu et al. 2017). Certain indolicidin-based peptides, MX-226 and MX-594AN have also used in the treatment of catheter-induced infections and Acne vulgaris respectively (Sachdeva 2017). Another peptide MBI-853NL is used to prevent the infections corresponding to Methicillin-Resistant Staphylococcus aureus (MRSA) (Levy 2004).
A wide range of advantages of AMPs such as high potency, efficacy, target specificity, low cytotoxicity, and low accumulation in tissues has led to the development of numerous peptide-based therapeutics and their appropriate preclinical and clinical trials (Table 2) (Bach 2018).
The investigation of AMPs, whether natural or synthetic, has been subjected to numerous studies over the last few decades, and the relevant information is available in several databases. Such ACP and AMP databases range from large general collections to specialized compilations which are summarized in Table 3.
Table 3.
Anticancer and antimicrobial peptide databases
| Database | Type | Number of peptides | URL | Year | References |
|---|---|---|---|---|---|
| DBAASP v3 | AMP | 16,180 | https://dbaasp.org/home | 2020 | Pirtskhalava et al. (2021) |
| LAMP2 | AMP | 23,253 | http://biotechlab.fudan.edu.cn/database/lamp/index.php | 2020 | Ye et al. (2020) |
| dbAMP | AMP | 12,389 | http://csb.cse.yzu.edu.tw/dbAMP/ | 2019 | Jhong et al. (2019) |
| DRAMP 2.0 | AMP | 19,899 | http://dramp.cpu-bioinfor.org/ | 2019 | Kang et al. (2019) |
| CancerPDF | ACP | 14,637 | http://crdd.osdd.net/raghava/cancerpdf/ | 2017 | Bhalla et al. (2017) |
| InverPep | AMP | 702 | https://ciencias.medellin.unal.edu.co/gruposdeinvestigacion/prospeccionydisenobiomoleculas/InverPep/public/home_en | 2017 | Gómez et al. (2017) |
| CAMPR3 | AMP | 11,118 | http://www.camp.bicnirrh.res.in/ | 2016 | Waghu et al. (2014) |
| APD3 |
AMP ACP |
AMP: 3,485 ACP: 185 |
http://aps.unmc.edu/AP/ | 2016 | Wang (2016) |
| CancerPPD | ACP |
ACP: 3491 Anticancer proteins: 121 |
http://crdd.osdd.net/raghava/cancerppd/ | 2015 | Tyagi (2015) |
| TumorHoPe | ACP | 744 | http://crdd.osdd.net/raghava/tumorhope/ | 2012 | Pallavi Kapoor (2012) |
| YADAMP | AMP | 2,525 | http://yadamp.unisa.it/about.aspx | 2012 | Piotto (2012) |
Animals
AMPs are primarily isolated from various vertebrates and invertebrates such as mammals, amphibians, fishes, insects, etc. They are identified at different sites of the body such as skin, mucosal barriers, eye, reproductive tract, saliva, milk, etc. Cathelicidins and defensins are the two major classes of mammalian AMPs found in humans, horses, rabbits, sheep, etc. (Lei et al. 2019). These peptides play major role in innate immune system and protect the host from foreign microbial infections. Human cathelicidin LL-37, beta-defensin 2, casein201, lactoferrin B, etc. are a few mammalian peptides isolated from skin, eyes, mouth, respiratory tract, intestines, and colostrum, respectively.
Amphibians are also a rich source of AMPs, especially frogs. Magainin is one of the most prevalent amphibian AMPs derived from frog skin which has potential activity against various bacteria, viruses, yeasts, and fungi. Other peptides viz. cancrin and esculentins are also derived from frogs which exhibit strong activity against several pathogens like C. albicans, P. aeruginosa, E. coli and S. aureus (Patocka et al. 2019). There several AMPs derived from fishes viz. piscidin, hepcidin, dicentracin, and NK lysine (Mabrouk 2022).
Several AMPs are also derived from blood cells and fat tissues of insects. Crecopin and jellein are the most famous AMPs isolated from insects such as silkworm, Drosophila, bees and show promising effects against several inflammatory diseases, cancers, and microorganisms.
Plants
Plants are well-known as one of the major sources of AMPs. Defensins, thionins, cyclotides etc. are commonly known plant-based AMPs and possess similar physiochemical properties like the animal-derived AMPs. PvD1, a plant defensin AMP and Snakin, a thionin peptide are the common examples of plant-based peptides derived from Phaseolus vulgaris and Ziziphus jujuba, respectively. These peptides exhibit potential activity against yeasts, fungi and bacteria. Although several AMPs derived from plants have been identified till date, none of them has been clinically approved yet (Saeed et al. 2022).
Microorganisms
Microorganisms like bacteria and fungi are known as reservoirs of AMPs. The most common bacterial peptides, also known as bacteriocins such as nisin, lacticin, gramicidin, mersacidin, etc. isolated from lactic acid bacteria, namely Lactococcus lactis, Bacillus subtilis, and Bacillus brevis (Lei et al. 2019). Nisin is 34 amino acid peptide sequence that has been commercially approved for the treatment of bovine mastitis (Li et al. 2021). Another 40 amino acid AMP, plectasin isolated from the fungus Pseudoplectania nigrella has exhibited strong bactericidal activity against multidrug resistant strains of S. aureus (Saeed et al. 2022).
Antibacterial Peptides
These peptides exert their biological activity by membrane or non-membrane mediated action. Bacterial cell walls are composed of anionic bacterial endotoxins such as lipopolysaccharides in gram-negative bacteria and lipotechoic acids in gram-positive bacteria. Cationic AMPs electrostatically or hydrophobically bind to these anionic components in the cell wall and results in membrane disruption leading to leakage of intracellular contents. AMPs like cathelicidin, defensin, nisin, cecropins, etc. are well-known for their potential inhibition activity towards various gram-positive and gram-negative bacteria (Huan et al. 2020; Q.-Y. Zhang et al. 2021a, b).
Antiviral Peptides
AMPs show broad-spectrum antiviral activity against viruses. These peptides exhibit their biological activity by (a) blocking the viral entry by inhibiting the attachment of virus to the host cell receptors, (b) destroying the viral envelope, or (c) inhibiting the virus replication (Jung et al. 2019). AMPs such as human cathelicidin LL-37, defensins, temporins, magainin, gramicidin, etc. exhibit potential antiviral activity against several viruses like HIV, influenza A virus, vaccinia virus, dengue virus, zika virus, etc.
Antifungal Peptides
Antifungal peptides primarily address the fungal infections caused by common pathogenic fungi viz. Aspergillus or Candida albicans. These peptides employ their biological activity on the targets by (a) inhibiting β-glucan synthesis, (b) inhibiting chitin biosynthesis in fungal cell wall, or (c) membrane permeation. Brevins, ranatuerin, cecropins, echinocandins, pneumocandins, etc. are some common examples of antifungal peptides (Fernández de Ullivarri et al. 2020).
Antiparasitic Peptides
Parasites essentially contribute to the statistics of human diseases worldwide, resulting in a significant global health burden. Malaria, leishmaniasis, trypanosomiasis, schistosomiasis, etc. are some common parasitic diseases that threaten the health of millions of populations. Increase in parasitic drug resistance has lead to substantial gain in interest towards AMP-based antiparasitic strategies. Halictine-2, attacin, cecropin, defensin 2, dragomide E, LZ1, phylloseptin-1, temporin, jellein, etc. are a few examples of AMPS that are currently being explored as antiparasitic therapeutic strategies (Huan et al. 2020; Q.-Y. Zhang et al. 2021a, b).
Anticancer peptides
Several cationic AMPs, alongside acting against microbes, also selectively target tumour cells by binding to the anionic phosphatidylserine moieties present on the cancer cell membranes (Wodlej et al. 2019). These peptides exert their anticancer activity by (a) blocking signalling pathways, (b) arresting cell cycles, or (c) causing cell death by apoptosis or necrosis. Tritrpticin, indolicidin, puroindoline are a few examples of AMPs that also act as ACPs.
Metabolic Disorders & Peptide Therapeutics
Peptide therapeutics have also played a crucial role in the management of metabolic diseases like type 1 diabetes mellitus (T1DM) and type 2 diabetes mellitus (T2DM). Two peptide hormones, insulin, and glucagon-like peptide-1 (GLP-1) produced by beta cells of pancreatic islets and intestinal l-cells respectively are involved in glucose homeostasis and are the basis of the most significant peptide therapeutics for metabolic diseases. Glucagon, a counter-regulatory hormone of insulin is used to reverse the insulin-induced hypoglycemic shock in type 1 diabetic patients (Müller et al. 2017; Pedersen-Bjergaard and Thorsteinsson 2017).
Several peptides have also been investigated for their potential in bone remodelling and healing. Calcitonin, a 32-amino acid peptide secreted by the parafollicular cells of the thyroid gland potentially sustains calcium homeostasis and bone turnover (Brunel et al. 2019; Kumar et al. 1963). It has shown greater pharmacological potency for post-menopausal osteoporosis, Paget’s disease, and hypercalcemia and enhances bone mineral density (Kumar et al. 1963). Peptides derived from bone morphogenic proteins (BMPs) like BMP‐7, BMP-9, belonging to the transforming growth factor (TGF‐β) family also play an integral role in the formation and development of bones (Bergeron et al. 2012, 2009; Hogan 1996; Kim et al. 2017; Urist 1997). There are currently several peptide-based drugs undergoing clinical development for gastrointestinal (GI) diseases such as larazotide for celiac diseases (Leffler et al. 2015), glucagon-like peptide-2 (GLP-2), for improved absorption of intestinal nutrient, intestinal growth, keratinocyte growth factor, and epidermal growth factor (Bahrami et al. 2010). Additionally, relamorelin, a ghrelin agonist, is also being investigated for chronic idiopathic constipation and gastroparesis (Camilleri and Acosta 2015).
Peptide-based drugs used to treat metabolic disorders are one of the therapeutic agents accounting for the largest revenue (Table 3) (Muttenthaler et al. 2021). Liraglutide, a GLP-1 analog is one of the top-selling peptide-based drugs for metabolic disorders having a sales revenue of 2 billion USD per annum (Lee et al. 2019). It is approved by the FDA and European Medicines Agency (EMA) for the management of obesity (Kumar 2019).
Neurodegenerative disorders
Neurodegenerative disorders are any anatomical or biochemical anomaly in the various parts of the nervous system caused by breakdown of the synapses which leads to mafunction of the entire nervous system. Brain-associated disorders, such as Alzheimer’s disease (AD), Parkinson’s diseases (PD), Huntington’s Disease (HD), Epilepsy, and Multiple Sclerosis (MS) have become a major cause of global deaths and disabilities in the recent years (Baig et al. 2018a, b), Many therapies had been developed for such disorders, however, they tend to fail in different stages of clinical trials due to toxicity and lack of immune response. One of the major challenges in treating neurodegenerative disorders is impermeability of the Blood Brain Barrier (BBB). However, in the last two decades, peptides are observed as promising agents to cross the BBB (Akhtar et al. 2021; Banks et al. 1992; Mendonça et al. 2021). Neuropeptides can be classified on the basis of ther origin as natural and synthetic peptides.
Numerous peptides have been explored for their potential against the neurodegenerative disorders. A 23 amino acids peptide, P42 (AASSGVSTPGSAGHDIITEQPRS) is reported to show potential therapeutic efficacy against Huntington’s disease. P42 is a part of Huntington (Htt) protein. It works by preventing the aggregation of polyQHtt protein which results in significant improvement in the symptoms of the disease (Marelli and Maschat 2016; Yadav et al. 2021). Another 11 amino acid peptide sequence, QBP1 (SNWKWWPGIFD) was reported against Huntington’s disease (Aharony et al. 2015). QBP1 has specifically binds to expanded polyQ stretch and prevents the proteins from misfolding by inhibiting the formation of β sheet structures which results in reduced aggregation in neurons (Yadav et al. 2021).
Soudy and his co-workers reported a peptide, R5 (SQELHRLQTYPR) derived from an amylin receptor antagonist, AC253 (Soudy et al. 2019). R5 is found to have neuroprotective properties against Aβ toxicity by reducing the Aβ plaque load and neuroinflammation in the brain. R5 attenuates the deleterious effects of Aβ on neurons and improves the cognitive capacity of patients affected with Alzheimer’s disease. Thus, it is noteworthy that R5 could serve as a potential therapy against neurodegenerative disorder (Yadav et al. 2021).
Cardiovascular Diseases
Cardiovascular diseases (CVD) have become one of the leading causes of morbidity and mortality across the globe. Various therapeutic strategies are being explored by scientists to improve the cardiovascular conditions whereas, only a few therapies are approved so far. Novel strategies with significant efficacy against CVD has become the need of the hour. Recently, peptides and peptidomimetics have gained increased attention as novel therapeutic approaches for modulation in CVD. Some of the most common CVDs include congestive heart failure, atherosclerosis, coronary artery disease, and pulmonary and systemic hypertension. Therapeutic peptides viz. Urotensin-II (Uro-II), a vasoconstrictor and Urocortins (UCNs) and Adrenomedullin (AM), vasodilators have recently gained significant attention in targeting the biomarkers of these CVDs.
Uro-II is a cyclic peptide derived from urophysis of teleost fishes (BERN et al. 1985). A number of peptides similar to Uro-II structure have also been derived from other amphibians and humans. Human Uro-II (ETPDCFWKYCV) is reported to be the most potent mammalian vasoconstrictor so far which targets the human G-protein coupled receptor (GPCR). GPCR is widely expressed in vascular muscles, myocardium, and endothelium and regulated cardiovascular homeostasis. Uro-II upon binding to GPCR mediates vasoconstriction by increasing the levels of phosphates released from sarcoplasmic reticulum and stimulating extracellular [Ca2+] influx (Grieco and Gomez-Monterrey 2019).
UCNs are peptide hormones that belong to the corticotropin-releasing factor (CRF) family. UCN was first isolated from rat brain, UCN1 (40 amino acids), followed by cloning of UCN2 (39 amino acids) and UCN3 (38 amino acids) from mouse and human cDNA libraries. UCNs are identified as potent and prolonged arterial vasodilators (Venkatasubramanian et al. 2013) which exert their effects in the target cell through p38 mitogen-activated protein kinase and protein kinase A pathways (Kageyama et al. 2012). UCNs mediate relaxation of pulmonary arteries by inhibiting a protein kinase C dependent contractile mechanism (Chan et al. 2004).
AM is vasodilatory peptide derived from human pheochromocytoma tissue (Kitamura et al. 1993) which potentially dilates the human coronary arteries and pulmonary arteries. Several studies have reported that AM increases the extracellular cAMP levels or activates potassium channels which mediates an endothelium-independent relaxation mechanism resulting in cell hyperpolarization in vascular smooth muscles (Grieco and Gomez-Monterrey 2019).
Dermatological Diseases
Atopic dermatitis, psoriasis or rosacea have become the most prevalent chronic inflammatory dermatological diseases nowadays. Atopic dermatitis is a common inflammatory skin disease impairing the patient’s quality of life. Various therapies, such as treatments with corticosteroids, calcineurin inhibitors, and antibody drugs, have been applied, but numerous side effects have been reported, including skin atrophy, burning, and infection. Functional peptides have lately been regarded as potential therapeutic agents to address such challenges due to their advantages of efficacy, safety, and low cost (Reinholz et al. 2012). Kim and co-workers reported the efficacy of a 5 amino acid wound healing peptide sequence, AES16-2M (REGRT) in attenuating the atopic dermatitis symptoms in the affected patients. The thickness of the epidermal layer was also improved by AES16-2M treatment. The results reported by Kim et al. suggests that AES16-2M can be a novel candidate for atopic dermatitis treatment (Kim et al. 2021).
Psoriasis is one of the most influential and fastest-growing inflammatory autoimmune diseases of the skin. It is a polygenic disease that activates the T-cells resulting in hyperproliferation of an array of cytokines, inflammatory cells, and keratinocytes (Das et al. 2009; Nestle and Conrad 2004). In the past two decades, muramyl peptides are widely used to treat the pathological conditions of psoriasis. Muramyl peptides are observed to normalize the immunocompetent T-cells and regulates the cytokines which play a crucial role during inflammation. These peptides have remarkable impact in treatment of psoriasis which suggests that muramyl peptides significantly influence the pathways of immune homeostasis (Guryanova et al. 2019).
It's widely believed that healthy and younger-looking skin symbolizes youth. Therefore, strategies to develop potential approaches for preventing the ageing process or skin diseases has gained significant interest in the research world. In the recent years, peptides in cosmetic formulations such as anti-aging skin creams, lotions, or skin brightening creams, etc. have gained notable attraction as anti-ageing strategies (Negahdaripour et al. 2019). Peptides play a crucial role in a variety of biological functions that are relevant to skin care, such as modulation of cell migration and proliferation, inflammation, melanogenesis, protein synthesis and regulation, etc. A large number of the peptides used are made of natural l-amino acids, which have non-immunogenic properties and easily breakdown over time to produce individual amino acids (Zhang and Falla 2009). In 1973, Pickart proposed the first cosmetic tripeptide, GHK that enhances collagen production (Pickart and Thaler 1973). Since then, a plethora of commercially available cosmetic peptides have been explored (Tables 4 and 5).
Table 4.
Top-selling peptide-based drugs for metabolic disorders in the market (Muttenthaler et al. 2021)
| Peptides | Brand names and their years of market introduction | Clinical indication | Sale in 2021/sales forecast to 2028 (in USD millions) | References |
|---|---|---|---|---|
| Insulin and analogues |
Humulin (1982) Insuman (1997) NovoRapid (1999) Lantus (2000) Novomix (2000) Toujeo (2000) Apidra (2004) Levemir (2004) Humalog (2005) Ryzodeg (2013) Tresiba (2013) Admelog (2017) |
Diabetes | 27,710 | ("Human Insulin Market Size 2021 | Is Anticipated to Reach USD 27.71 Billion and Exhibit a CAGR of 3.4% by 2026," 2021) |
|
Teduglutide (HGDGSFSDEMNTILDNLAARDFINWLIQTKITD) |
Gattex (2012) Revestive (2012) |
Short bowel syndrome | 4,600 | (Short Bowel Syndrome Market—Global Industry Analysis, Size, Share, Trends, Revenue, Forecast 2020 to 2027 2021) |
|
Dulaglutide (HAEGTETSDVSSYLEGQAAKEFIAWLVKGR) |
Trulicity (2014) | Diabetes | 4588.2 | ("Lilly Reports Robust Third-Quarter 2021 Financial Results as Pipeline Success Strengthens Future Growth Potential" 2021) |
|
Glatiramer (AKDY) |
Copaxone (1996) Glatopa (2015) |
Multiple sclerosis | 3,900 | ("Teva Reports Third Quarter 2021 Financial Results,") |
|
Semaglutide (HXEGTFTSDVSSYLEGQAAKEFIAWLVRGRG) |
Ozempic (2017) Rybelsus (2019) |
Diabetes, obesity | 3,494.72 and 458.33 | (Financial report for the period 1 January 2021 to 30 September 2021, 2021) |
|
Liraglutide (HAEGTFTSDVSSYLEGQAAKEFIAWLVRGRG) |
Victoza (2010) Saxenda (2015) |
Diabetes, obesity | 1,705 and 903.13 | (Financial report for the period 1 January 2021 to 30 September 2021 2021) |
|
Vasopressin (CYFQNCPRG) |
Vasostrict (2014) | Central diabetes insipidus | 785.6 | (Decker 2021) |
|
Teriparatide (SVSEIQLMHQLGKHLQSMERVEWLRKKLQDVHQF) |
Forteo (2002) | Osteoporosis | 650.1 | ("Lilly Reports Robust Third-Quarter 2021 Financial Results as Pipeline Success Strengthens Future Growth Potential" 2021) |
|
Lanreotide (NXCYDWKVCT) |
Somatuline (2007) | Acromegaly | 313.04 | ("Ipsen Delivers Encouraging Sales Growth in the First Quarter of 2021 Despite the Pandemic, and Confirms Its Full-Year Guidance" 2021) |
|
Etelcalcetide (Ac-CDADRDRDRDADRD-NH2) |
Parsabiv (2017) | Hyperparathyroidism | 71 | (Amgen Reports Second Quarter 2021 Financial Results 2021) |
Table 5.
Commercially available cosmetic peptides and their bioactivity (Errante et al. 2020) [Copyright
© 2020 Errante, Ledwoń, Latajka, Rovero and Papini. Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0/)]:
| Peptide name | Peptide sequence | Trade name | Bioactivity |
|---|---|---|---|
| Pentapeptide-3 | H-GPRPA-NH2 | Vialox | ACh receptor antagonist, disabling nerves’ function |
| Pentapeptide-18 | H-YAGFL-OH | Leuphasyl® | ACh decreased secretion in synaptic clefts |
| Acetyl octapeptide 1/-3 | Ac-EEMQRRAD-NH2 | SNAP-8™ | SNAP-8 competitive inhibitor, blocking SNARE complex formation |
| Palmitoyl hexapeptide-12 | Pal-VGVAPG-OH | Biopeptide EL™ | Matrix metalloproteases activity up-regulator, elastin down-regulator and collagen synthesis stimulator |
| Palmitoyl pentapeptide-4 | Pal-KTTKS-OH | Matrixyl® | Extracellular matrix proteins synthesis feedback modulator |
| Palmitoyl tripeptide-1 | Pal-GHK-OH | Biopeptide CL™ | Collagen and glycosaminoglycan synthesis stimulator |
| Palmitoyl tripeptide-5 | Pal-KVK-OH | Syn®-Coll | Transforming growth factor β stimulator inducing collagen synthesis |
| Tripeptide-10 citrulline | H-PVAPFP-OH | Decorinyl® | Collagen fibres diameter regulator, increasing endogenous collagen quality, without affecting its synthesis |
| SA1-III | Ac-MGKVVNPTQK-NH2 | KP1 | Collagen turnover modulator by protease inhibition |
Limitations of Peptide-Based Therapeutics
Peptides, despite being one of the most prevalent bio-drugs, have several limitations which hinder their therapeutic use. Their inadequate properties, such as poor permeability of the membrane, low oral bioavailability, shorter half-life, variable solubility, and poor metabolic stability usually complicate their systemic delivery (Haggag et al. 2018; Wetzler and Hamilton 2018). The list of bottlenecks of peptide-based therapeutics is rather long and is ascribed in the following points:
Drug delivery route: These drugs need to be delivered via injections. Although the oral drug administration route is the easiest and comfortable way, gastric acid and proteases in the digestive system and blood easily degrade the peptides. Intestinal absorption of these drugs is also restricted due to their poor membrane permeability (Sun 2013). Charge and polarity of peptides play a major role in exhibiting low permeability across gut membranes.
Shorter half-life: Proteolytic degradation of peptides in the digestive tract leads to their inactivation as well as rapid renal and hepatic clearance resulting in a shorter half-life (Haggag et al. 2018; MARKET; Wetzler and Hamilton 2018). Parenteral administration of frequent doses is usually required to maintain the drug at a clinically effective concentration.
Poor biodistribution: Peptides also have poor biodistribution because of their structural flexibility and folding which leads to poor selectivity towards receptors and also activates different target receptors resulting in certain side effects (Haggag et al. 2018).
Immunogenicity: Another key concern of peptide-based drugs is the possibility of peptide immunogenicity, i.e., undesirable immune responses (Haggag et al. 2018). Peptides tend to trigger an unwanted immune response against themselves. These immune responses lead to the activation of B cells which bind to the peptide molecules and reduce/eliminate their therapeutic effects. Thus, to have clinically safe peptide-based drugs, critical evaluation of their tendency to trigger immune response is a mandate (Sauna 2020).
Bacterial resistance: Peptide-based antimicrobials are promising agents; however, potential bacterial resistance is one of the major concerns. It has been reported that simple physicochemical features of AMPs dictate bacterial tendency to evolve resistance (Spohn et al. 2019). Interaction of AMPs with the extracellular bacterial enzymes leads to proteolytic degradation of AMPs which leads to resistance development in microorganisms. Resistance against the AMPs is also induced by modification of bacterial cell surfaces (Mukhopadhyay et al. 2020). Teichoic acid, an anionic linear polysaccharide is abundantly found on the cell walls of gram-positive bacteria which is responsible for negatively charged cell surfaces. D-alanylation on hydroxyl groups of teichoic acid adds a positive charge to the bacterial cell wall which lowers the attraction of cationic AMPs (Peschel et al. 1999). It also makes the cell wall denser which leads reduced surface permeability (Saar-Dover et al. 2012). Similarly, resistance to AMPs in gram-negative bacteria is also developed by alteration of cell surface charge and permeability. In gram-negative bacterial cell walls, anionic lipopolysaccharide (LPS) is the most abundantly found component. Lipid A, the innermost region of LPS, also known as endotoxin molecule is responsible for the toxicity of gram-negative bacteria (Valvano 2015). Dephosphorylation of lipid A by an amine-containing molecule such as aminoarabinose, glucosamine, galactosamine, alkaline phosphatase, etc. increases the positive charge on the cell surface which eventually prevents electrostatic binding of AMPs to the bacterial cell surface (Joo et al. 2016).
Thus, researchers have developed various strategies to counteract the drawbacks of peptide therapeutics to expand their uses for pharmaceutical purposes. These strategies lead to improved membrane permeability, protease resistance, increased drug retention time, and prolonged half-life of peptides making them least susceptible to resistance. Peptide-based therapeutics are hence becoming more easily manageable, thereby, leading to their rapid growth in the pharmaceutical industry (Craik and Kan 2021; Di 2015).
Strategies to Overcome the Limitations
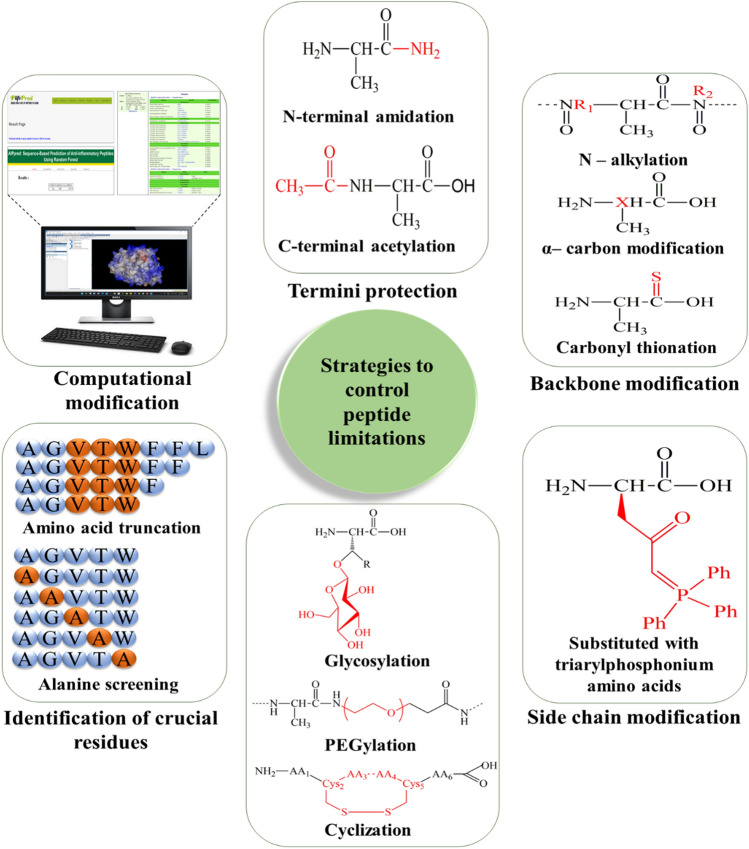
Substantial efforts have been made to establish strategies that can eradicate the limitations associated with peptide-based therapeutics and expand their uses in pharmaceutical fields. To overcome these limitations, several chemical modifications and computational approaches have been used that prevent their proteolytic degradation thereby, enhancing their half-life and ultimately improving their therapeutic efficacy (Fig. 4).
Fig. 4.
Various approaches explored to overcome the limitations of peptide-based drugs
Termini Protection
Peptidases like carboxypeptidases, serum aminopeptidases, and various other proteases, can lead to proteolysis at both N- and C-terminal of a peptide (Lee et al. 2019; Puente et al. 2005). It has been established that different amino acid residues at the N- or C- terminal result in different extents of degradation and proteolysis leading to poor bioavailability. Werle and Bernkop-Schnurch reported that peptides rich in Serine, Threonine, Glycine, Alanine, Valine, and Methionine residues at N-terminal are significantly resistant to degradation in plasma (Werle and Bernkop-Schnürch 2006). To improve the bioavailability, modification of the N or C terminal of the peptides can be done while maintaining their target affinity and specificity (Jambunathan and Galande 2014). Moreover, in an attempt to boost the in-vivo stability of peptides, N-terminal acetylation or C-terminal amidation can be also be done Georgieva et al. (2012). The same purpose may also be served by modifying the terminals with unnatural amino acid analogs (Goodwin et al. 2012; Varamini et al. 2012).
Backbone Modification
Backbone modifications can be carried out by substitution reactions such as by exchanging carbonyl oxygen for sulfur or replacement of H-atoms at the nitrogen or α-carbon at any position of the backbone. Thus, adequate procedures are required to perform regioselective manipulations. An additional stereogenic center occurs at α-carbon which also needs to be controlled (Deska and Kazmaier 2008). Peptide backbone modifications enhance the enzymatic stability of the peptide analogs and increase their biological membrane permeability (Ahn et al. 2002). Modifications of peptide backbones have been used in a wide range of fields, including HIV protease inhibitors and receptor mediators (Pu et al. 2019; von Recum and Pokorski 2013). The backbone can also trigger profound changes in molecular chirality, hybridization, conformation, and the self-assembly of peptide structures and nanoparticles (Shah et al. 2020).
N-Alkylation
Alkylation of nitrogen atoms in a peptide chain is an effective method from several perspectives. Peptides are commonly present in trans confirmation except when a peptide bond precedes a proline residue. N-alkylation leads to structural effects, resulting in an equilibrated cis- and trans— isomerization. Cis–trans isomerization can be used as a new molecular timer to help manage the amplitude and duration of a biological process, which might be a new therapeutic target. (Das et al. 2014; Lu et al. 2007). Moreover, elimination of the NH-group confines the number of feasible intra- and intermolecular H-bonds which can increase metabolic stability by conformational control or steric hindrance (Räder et al. 2018). Also, N-alkylated peptides are less prone to enzymatic cleavage. Consequently, N-alkylation leads to improved pharmaceutical properties like metabolic stability and selectivity of receptors (Urbańczyk et al. 2019).
α-Carbon Modification
α-carbon modification of peptide backbone is one of the most often used backbone modifications where a new amino acid can be introduced starting from a simple glycine subunit to a complex amino acid. α-carbon modification in a peptide chain can increase its biological activity or improve the pharmacokinetic properties by providing stability to enzymatic and chemical degradation and can be termed as protease inhibitors. It provides regioselectivity to the peptide that exhibits selective interaction with the targeted receptors (BEGUM et al. 2017). The major challenge in this modification is to manage the configuration of the new chiral center which is required to influence the transformations of the stereochemical outputs (Deska and Kazmaier 2008). Peptide chains with an altered chirality can break the secondary structure of peptides, thereby altering their assembly structures. Chirality of the peptide chains regulates the cell cytotoxicity of peptide assemblies (Zheng et al. 2021).
To introduce good selectivity, a fixed arrangement between the shielding side chain and reactive center is the basic requirement which can be introduced by having an adjacent side chain as a chiral auxiliary.
Whereas, induction through real chiral auxiliaries is another alternative that binds either N- or C- termini of a peptide to the reactive α-center or integrated into a cyclized moiety of imidazolinone.
It may be desirable to use external chiral materials, such as chiral catalysts or optically active substrates, as they only have to be added to the reaction process and no precedent attachment to the peptide is required (Deska and Kazmaier 2008; Urbańczyk et al. 2019).
Carbonyl Thionation
Researchers have drawn much attention to the isosteric substitution of amide bonds with thioamides, particularly in physiologically active peptides, as the secondary structure can be influenced by only a slight variation (C=O→C=S) (Choudhary and Raines 2011; Wildemann et al. 2007). The hydrogen bonding in peptides is affected by carbonyl thionation which is similar to N-alkylation. In comparison to amide oxygen, thioamide sulfur is a weak hydrogen acceptor and thus results in longer H-bonds (Chatterjee et al. 2021). The thioamide C–N bond, in comparison, displays a large rotational barrier, owing to the lower C=S double bond character (Deska and Kazmaier 2008).
Side-Chain Modification
Positional scanning usually provides substantial data to rationally modify or alter the main binding residues to increase the affinity and selectivity of the peptide. Natural amino acids have several close analogs which are extensively used and can be replaced at this point, and side chains that are non-natural often induce protease resistance. For example, lysine, ornithine, homoarginine, citrulline, and N-isopropylornithine are the substitutes of arginine (Henninot et al. 2018; Wisniewski et al. 2011). Aromatic residues have a very broad range of analogs, including unnatural heterocycles (Frey et al. 2008), and may also be benefited from the introduction of β-methyl groups which rigidify the conformation (Haskell-Luevano et al. 1997). Residues that are not strongly involved in binding interactions will rationally be substituted to change the physical properties of peptides, by increasing solubility or the addition of unnatural amino acids, resulting in proteolytic resistance (Sadowsky et al. 2007; Werner et al. 2016). But in some cases, certain non-critical residues have sites for conjugation or cyclization. The rational design of a triagonist by identifying and combining the active partial sequences for three individual peptide hormones viz. glucagon-like peptide-1 (GLP-1), glucose-dependent insulinotropic polypeptide (GIP), and glucagon is an impressive latest demonstration of sequence optimization (Finan et al. 2015). The subsequent single helical peptide, in this case, activates GLP-1, GIP, and glucagon receptors simultaneously which results in declined body weight and complications associated with diabetes in obese rodent models (Henninot et al. 2018).
Glycosylation, PEGylation, and Cyclization
Glycosylation
It is an efficient strategy to amplify the physicochemical characteristics of peptides and to enhance their absorption by biological membranes. Glycosylation is the process in which a carbohydrate is bound to the functional groups of other molecules like peptides, proteins, etc. which can improve its physiological properties. There are several advantages of peptide glycosylation such as enhancement in bio-distribution in tissues by targeting specific organs, improvement in membrane permeability, maintaining in-vivo stability and controlling clearance rate, maintaining and protecting amino acid side chains, specific receptor-binding, etc. (Costa et al. 2014; Moradi et al. 2016; Polt et al. 2005; Varamini et al. 2012) Peptides-sugar conjugates target glucose transporters on the cell membranes and stimulate the active transportation of modified compounds across cell membranes (Witczak 2006). To enhance the therapeutic potential, metabolic stability, and activity of the peptide conjugates, several essential factors include the arrangement, type, and several sugars (Bapst et al. 2009; Cudic 2013; Yamamoto et al. 2009). The position of the glycosyl unit attached to the peptide can influence the peptide–receptor interactions, biodistribution, and pharmacological activity of the glycosylated peptides (Bapst et al. 2009; Yamamoto et al. 2009). One of the major impacts of this process on the pharmaceutical properties of peptides is the enhancement in their oral bioavailability (Albert et al. 1993).
PEGylation
It is considered as the superior method to chemically modify the peptide therapeutics. It involves one or more chains of polyethylene glycol (PEG) attached to a peptide which changes the physical and chemical properties of peptides, such as its conformation, electrostatic binding, and hydrophobicity, and results in an improvement in its pharmacokinetic profile (Veronese and Mero 2008). It enhances its half-life, peptide immunogenicity (Freire Haddad et al. 2021), and in-vivo stability. When PEG is attached to a therapeutic peptide, it covers the peptide from the immune system of the host, resulting in reduced immunogenicity (Damodaran and Fee 2010). PEGylation of peptides decreases the rate of plasma clearance by preventing enzymatic degradation and prolongs their blood retention time (Harris and Chess 2003). This modification protects the peptides from proteolytic enzymes by increasing the molecular mass of peptides and improving their pharmacokinetic profiles (Harris and Chess 2003; Suk et al. 2016). It sustains peptide absorption and decreases the volume of distribution leading to decreased systemic clearance (Harris and Chess 2003). It also prevents reticuloendothelial system (RES) uptake, which eventually increases blood circulation time. Consequently, a longer blood circulation time reduces dosage frequency and encourages patient compliance (Uhrich and Abdelhamid 2016; Veronese and Mero 2008). The larger size of PEGylated protein for glomerular filtration sterically hinders the interaction of the peptides with the receptors which delays the metabolic activities and elimination resulting in prolonged circulation time (Harris and Chess 2003; Jambunathan and Galande 2014; Schiffter 2011) It also improves the potential of peptide drugs by playing a crucial role in drug delivery (Harris and Chess 2003).
Cyclization
It is a well-known method to improve the efficacy and half-life of peptides by restricting their conformational flexibility. It inhibits protease access to the amides of the backbone; these proteases normally bind their substrates in linear peptide confirmation (Henninot et al. 2018). Chemical linkers used in this process stabilize the peptides which eventually enhances their aqueous solubility by reducing their charges and the potential for H-bonding. Depending on the functional group, cyclization of a peptide can be done in four different ways: side chain-to-side chain, side chain-to-tail, head-to-side chain, or head-to-tail (White and Yudin 2011). The tripeptide Arg-Gly-Asp (RGD) is one of the most used examples of this strategy (Bogdanowich-Knipp et al. 1999; Kapp et al. 2017; Zhu et al. 2021). Linear RGD is highly susceptible to enzymatic degradation. Aspartic acid residue in the tripeptide is prone to chemical degradation and leads to the loss of biological activity (Zhu et al. 2021). Cyclization of RGD peptides via disulfide bond linkage can induce structural rigidity, thereby preventing aspartic acid residue mediated degradation (Bogdanowich-Knipp et al. 1999). Cyclization can also decrease the exposure of polar atoms to surroundings by folding peptides into bioactive conformations, leading to the increase of oral bioavailability (Zhu et al. 2021). Balkoves et al. reported an ionizable molecular entity by synthesizing a hydrophilic phosphate monoester derivative of a lipopeptide by phosphorylation of the phenolic hydroxyl group of a homotyrosine residue (Balkovec et al. 1992). Compared to the parent peptide, this prodrug exhibited remarkable hydrolytic stability and in vivo activity, which signifies that the prodrug has undergone enzymatic hydrolysis to generate the parent drug. It possesses enhanced hydrophilicity and sustained concentration in the body. Borchardt et al. reported cyclization of the linear peptide, [Leu]-enkephalin (H-Tyr-Gly-Gly-Phe-Leu-OH) and its metabolically stable analog DADLE (H-Tyr-D-Ala- Gly-Phe-D-Leu-OH) based on acyloxyalkoxy-, phenyl propionic acid- and coumarinic acid. It showed a substantial effect on their in-vivo stability to exo- and endo- peptidases and potential membrane permeability (e.g., intestinal walls, blood–brain barrier, etc.) (Borchardt 1999).
Identifying Crucial Residues
One of the most important strategies for the biological study of peptide-based drug design is the recognition of crucial residues. Firstly, the minimum amino acid residues necessary for peptide activity should be identified. It can be obtained by the repeated truncation of amino acids from either N- or C-terminal of the peptide sequence to determine the essential core peptide motif required for efficient bio-activities.
Secondly, a typical method of screening known as alanine scanning may be used to ascertain the contribution of each amino acid of the peptide to its activities (Gordee et al. 1988). Essential amino acids can be identified by screening the biological compatibility peptides in which particular amino acids have been replaced with alanine. Alanine is used due to its small size, moreover, it has uncharged side chains which do not interfere with the activities of adjacent side chains (Blaakmeer et al. 1991). More complicated scanning methods have been developed over the years which include the enantiomers of amino acids and properties such as hydrophobic or acidic or basic natures are also taken into account. However, these scanning methods need to be validated by molecular biology and in silico methods concerning stability, pharmacokinetics, and pharmacodynamics for effective bio-activities of the resulting peptides. These studies of the structure–activity relationship (SAR) can contribute to the identification of proteolytically-labile amino acids in peptides (Fournie-Zaluski et al. 1992).
Computational methods
Computational strategies cover a broad range, from the possible 3D structures of short oligopeptides in solution to the determination of peptide sequences that are ideally suitable to carry out certain biological activities to de novo estimates of the interaction of large proteins. The development of peptide therapies has focused on extracellular targets due to the poor permeability of peptides to the cell membrane. Thus, the strategies to enhance membrane permeability or active intracellular uptake of peptides are essential for the successful targeting of intracellular protein–protein interactions. Intracellular uptake of peptides can be improved by modulating their hydrophobicity and electrostatic charges. Conjugation of the active peptide drug to a cell-penetrating peptide (CPP) can also significantly improve its active transport. Hydrophilic peptides are witnessed to have enhanced bioavailability as the concentration of serum can be maintained easily at the desired level. Optimization of peptide hydrophilicity is majorly an empirical process to identify the unnecessary hydrophobic amino acids experimentally that can be substituted by charged or polar residues to upregulate the isoelectric point (pI) while maintaining the biological activities (Mahato et al. 2003).
To simplify this optimization, several bioinformatics tools have recently been developed (Xiao et al. 2008).
DeepSol is one of those tools which offers a single-stage protein solubility prediction system which outperforms all other sequence-based prediction tools. It uses Convolutional Neural Network which exploits k-mer structure of input protein sequence and constructs non-linear k-mer vector spaces. These spaces lead to more information regarding the k-mer structure of the input sequence required to predict the protein solubility. It is found to be highly sensitive for identifying soluble and insoluble protein as compared to other prediction servers such as PaRSnIP, PROSO II, etc. (Khurana et al. 2018). The best models and results of DeepSol are deposited and made accessible at https://zenodo.org.
CcSOL omics is one of the servers which offers large-scale solubility calculations for proteome-wide prediction and identifies the soluble motifs in any specific amino acid sequence (Zhang and Bulaj 2012). Validation on three independent sets indicates that CcSOL omics discriminates soluble and insoluble proteins with an accuracy of 74% on 31,760 proteins. It is useful in protein engineering studies because it enables the analysis of sequence variants in large datasets. Amin et al. reported a database, named, Protein Solubility Database (ProSol DB), which provides solubility confidence scores in E. coli for 2,40,016 characterized enzymes obtained from UniProtKB/Swiss-Prot (Amin et al. 2019). Solubility confidence scores for various proteins were computed using CcSOL omics and stored locally in the database. CcSOL omics showed an accuracy of 73.46% as compared to 46% accuracy for DeepSol S1. (Khurana et al. 2018). The high prediction accuracy of CcSOL omics justifies its use when computing solubility for various proteins. CcSOL omics can be freely accessed on the web at http://s.tartaglialab.com/page/ccsol_group (Agostini et al. 2014).
Protein-Sol is another tool for predicting protein solubility which freely accessible at https://protein-sol.manchester.ac.uk/. It reads the amino acid sequence and predicts the solubility and other properties such as pI, hydropathy, absolute charge, sequence entropy, etc. The predicted results are not valid for membrane proteins (Hebditch et al. 2017). Hasan et al 2019 conducted a study to design a non-allergic and immunogenic vaccines against avian influenza virus. The solubility of the vaccine was predicted using Protein-Sol server and calculated the distribution of charge, hydrophobicity, and stability at different pH (Hasan et al. 2019).
Servers to predict haemolytic profile of peptides:
Haemolytic Peptide Identification (HemoPI): It is a server that was designed for the estimation of haemolytic potency of peptides (http://crdd.osdd.net/raghava/hemopi/). Haemolytic peptides possess significant toxicity which impedes their further progress as therapeutic agents. Thus, it is important to recognize the haemolytic activities of peptides in the drug development process. Computational methods are an excellent way of evaluating the haemolytic behavior in large numbers and categorizing the peptides as haemolytic or non-haemolytic. Behzadipour et al. reported a study evaluating the SARS-CoV-2 Mpro inhibitory activity of bovine milk protein originated di- and tri-peptides. A set of 326 peptides were obtained from the virtual digestion of bovine milk proteins and screened via molecular docking. Among these, 5 peptides were selected based on their highest binding affinity for further characterization by ADME/Tox analyses. Hemolytic activity and isoelectric points of peptides were predicted using the HemoPI server and none of the peptides were predicted to be hemolytic (Behzadipour et al. 2021). Another novel peptide derived from the fragments of MARCKS as a DNA vaccine and drug delivery system was reported by Chen et al. Hemolytic potency of the novel peptide was predicted using an in-silico HemoPI server. Non-hemolytic property of the peptide predicted by the HemoPI server was further validated by wet-lab experiments (Chen et al. 2021). However, HemoPI did not provide an in-depth assessment of the mechanistic interpretation of the haemolysis behavior. To overcome these limitations, a new sequence-based prediction server named HemoPred is built for predicting the haemolytic behavior of peptides.
HemoPred is a new sequence-based user-friendly prediction webserver that predicts the haemolytic behavior of peptides. To overcome the limitations of HemoPI, HemoPred is developed and is freely accessible at http://codes.bio/hemopred/ (Win et al. 2017). Balmeh et al. studied a wide range of bio-peptides against the viral proteins playing vital roles in the processes of proliferation and infection of COVID-19. Based upon the best binding affinity scores as a result of molecular docking, they were further studied to predict their side-effects such as allergenicity, toxicity, anti-angiogenic, interleukin 4 inducing ability, anti-cancer ability, and haemolytic activity using different servers. To predict the hemolytic potential of the peptides, the HemoPred server was used and based on the obtained results, peptides were modified accordingly to overcome the predicted side-effects (Balmeh et al. 2021).
Hemolytic Activity Prediction for Peptides Employing Neural Networks (HAPPENN) is another tool for predicting haemolytic activity of peptides has been developed. To develop a deep neural network for a haemolytic or non-hemolytic prediction for peptides, the data from various databases, such as Database of Antimicrobial Activity and Structure of Peptides (DBAASP), Collection of Anti-Microbial Peptides (CAMP), and Hemolytik have been explored. This tool is available as a webserver at https://research.timmons.eu/happenn (Timmons and Hewage 2020). Lokhande et al. reported an in-silico study indicating the use of human antimicrobial peptide, LL-37 as a potential therapeutic agent against COVID-19. To predict any adverse effect of the peptide such as allergenicity, toxicity, and hemolytic activity when used as a therapeutic, further in-silico analyses had been done using Allergen FP v.1.0, AllerCatPro, ToxinPred, and HAPPENN. Based upon the results of HAPPENN server, the peptide was predicted to have low haemolytic activity with the scores of 0.073, 0.089, and 0.09 (Lokhande et al. 2020).
In recent years, peptide-based therapeutics are found to be promising agents against cancer, diabetes, and cardiovascular diseases (Mehta et al. 2014). Since peptides have a remarkable role in the treatment of cancers, it has become very essential to develop computational tools for anti-cancer peptide design and its prediction. One of the major mechanisms of an anti-cancer peptide is the induction of apoptosis of cancerous cells, hence, an SVM tool named Anti-cancer Peptide Predictor (ACPP) has been developed for the design and prediction of the anti-cancer peptide by assessing the presence of any apoptotic domain.
Anti-cancer Peptide Predictor (ACPP): The server was developed using Practical Extraction and Report Language Common Gateway Interface (PERL CGI) and is freely accessible at http://acpp.bicpu.edu.in/predict.php (Vijayakumar and Lakshmi 2015). E-kobon et al. reported an in-silico study predicting anticancer peptides from A. fulicamucus. Several peptides from two HPLC-separated mucous fractions (F2 and F5) were identified. These identified peptides were then screened for putative anticancer peptides by using anticancer prediction servers: ACPP and AntiCP based on amino acid composition, conserved features, and physicochemical properties. Among the identified peptides, 16 peptides were predicted to be putative anticancer peptides and were further studied for their toxicity and membrane permeability using ToxinPred and CellPPD servers (E-kobon et al. 2016).
PEPstrMOD: It was specifically designed to predict modified peptide structure that includes natural and non-natural / modified residues. The role of a peptide is well known to rely on its structure hence, it is important to anticipate its tertiary structure from the amino acid sequence. In this method for the prediction of peptide structures having non-natural amino acids and various forms of post-translation modifications, special force field libraries (Forcefield NCAA and Forcefield PTM) have been included. PEPstrMOD is freely available at http://osddlinux.osdd.net/raghava/pepstrmod/ (Singh et al. 2015). Gallego et al. reported the characterization study of an antioxidant peptide AEEEYPDL derived from Spanish dry-cured ham. Spanish dry-cured ham is reported as a good source of antioxidant peptides which can be used as an alternative to chemical food preservatives (Mora et al. 2014; Zhang et al. 2021a, b). In-silico studies were performed to predict the tertiary structure and the stability of the peptide during gastrointestinal digestion using PEPstrMOD and PeptideCutter of ExPASy, respectively. The predicted structure of the peptide in a hydrophilic environment showed electronic, steric, hydrophobic, and hydrogen bonding properties of amino acids at the C- and N-terminal regions which seem to be closely related to its antioxidant activity (Gallego et al. 2018).
There is increasing evidence that anti-microbial peptides having anti-infection and anti-inflammatory properties, and adjuvant and wound healing activities, have multiple immunomodulatory functions in mammals (Hilchie et al. 2013; Lai and Gallo 2009; Liu et al. 2017). It is essential to have methods to predict the antimicrobial behavior of any designed novel AMP to allow scientists to conduct rational experiments. It is therefore appealing to construct successful prediction models to classify possible peptides with desired activities. Table 6 summarizes a description of the current predictive methods (2015–2020) for AMP studies.
Table 6.
Antimicrobial peptides prediction server developed in past 5 years (2015–2020)
| Server | Function | Year | URL | References |
|---|---|---|---|---|
| ADAM | SVM | 2015 | http://bioinformatics.cs.ntou.edu.tw/adam/tool.html | Lee et al. (2015) |
| Antimicrobial Peptide Database (APD3) | Parameter space | 2016 | http://aps.unmc.edu/AP/ | Guangshun Wang (2016) |
| CAMP | RF, SVM, Dragonfly algorithm (DA) | 2016 | www.bicnirrh.res.in/antimicrobial | Waghu et al. (2014) |
| Antimicrobial Activity Prediction (AMAP) | SVM, Shapley Additive explanation (SHAP), t-distributed Stochastic Neighbor Embedding (t-SNE) | 2019 | http://faculty.pieas.edu.pk/fayyaz/software.html#AMAP | Gull et al. (2019) |
| Integrative Antimicrobial Peptides Evaluator (IAMPE) | Naïve Bayes (NB), KNN, SVM, RF, eXtreme Gradient Boosting (XGBoost) | 2020 | http://cbb1.ut.ac.ir/ | Kavousi et al. (2020) |
| Deep-AmPEP30 | Convolutional neural network (CNN), reduced AAC (RAAC) | 2020 | https://cbbio.cis.um.edu.mo/AxPEP | Yan et al. (2020) |
Peptide-Based Drug Delivery
Peptides are efficient drug candidates; yet, exposed to numerous setbacks, such as low oral bioavailability due to first-pass metabolism and inability to cross physiological barriers. Instead of systemic circulation, peptide drugs pass through portal circulation and get metabolized leading to hepatic and renal clearance. Peptides have always had a comparatively shorter half-life in systemic circulation as a large number of proteases lead to their degradation (Antosova et al. 2009). These attributes have restricted the routes of administration to the intravenous route which leads to poor patient compliance. Hence, substantial efforts have been made to develop a user-friendly and non-invasive approach by utilizing nanoparticle-based formulations. Some alternate routes such as oral, nasal, buccal, pulmonary, and transdermal delivery routes are considered to be favorable needle-free approaches beyond the unfavorable intravenous route of administration. These approaches are diagrammatically represented in Fig. 5 and discussed hereunder.
Fig. 5.
Peptide-nanoparticle conjugate and various routes of peptide-based drug delivery
Routes of Delivery
The classical routes of administration are intravenous (into veins), intramuscular (into muscles), and subcutaneous (under the skin), whereas, the alternate routes include the transdermal route, pulmonary route, nasal route, conventional oral route, and buccal route (Antosova et al. 2009; Vlieghe et al. 2010).
In transdermal administration, active ingredients are delivered for systemic distribution through the skin either in the form of a patch or ointment. Insulin, vasopressin, LHRH, ACTH (adrenocorticotropic hormone), etc. are a few examples of drugs including peptide hormones and vasoactive peptides (Sachdeva 2017). In the pulmonary route of administration, drugs are administered by inhalation through the mouth which is atomized into fine droplets and are further deposited in the lower airways so that the drugs can move through the trachea and into the lungs (Scheuch and Siekmeier 2007). While administering drugs via nasal route, also known as snorting, drugs are insufflated through the nose. Drugs that are administered through the nasal cavity enter the olfactory mucosa which carries the drugs directly to the cerebrospinal fluid and brain through the olfactory receptor neurons (Shah et al. 2020). Numerous drugs are marketed such as LHRH, 8-arginine vasopressin (ADH), etc. which are administered intranasally (Sachdeva 2017). These routes further provide uniform drug distribution and higher bioavailability which decreases the frequency of dosing (Andrade et al. 2011; Kammona and Kiparissides 2012; Smola et al. 2008; Tomoda et al. 2008). Besides these, oral route of drug delivery is the most user-friendly route, yet have poor bioavailability as they are less susceptible to GI tract permeation and stability (Reinholz et al. 2012). However, chemical modification of peptides including amino acid substitution, carboxyl reduction, or olefin reduction remarkably contributes to drug stability and increased half-life (Sachdeva 2017). Alongside conventional oral route, buccal route is special type to oral delivery route where a drug is administered through the mucosal lining of the cheeks. Drug administered through buccal route (Johnston 2017) are not degraded in the GI tract leading to no first-pass metabolism which enhances the drug bioavailability (Ahmady 2014; Caon et al. 2015; Merkle and Wolany 1992; Pauletti et al. 1996).
Methods of Delivery
Chemistry and Nanophysics-Based Formulations
Various chemistry and nanophysics-based therapeutic formulations were designed to aid advanced peptide-based drugs that are responsible for the advancement of the pharmaceutical industry to fix the physicochemical constraints. The chemical incorporation of sugars such as glucose, maltose, sucrose, and trehalose, as discussed above in "Microorganisms" section, has shown improved stability and solubility of in-vivo peptides. It has been reported that the distribution of peptides across tissue membranes is enhanced by ionic surfactants such as cetrimide and sodium dodecyl sulfate (SDS). Various protease inhibitors such as bacitracin, sodium glycolate, or camostat mesilate are administered to inhibit the proteolysis of proteins. The bioavailability of the peptide-based drugs is enhanced by encapsulating them into nanoparticles that act as drug carriers or coupled with certain polymers such as polyvinylpyrrolidone (PVP) and PEG (Antosova et al. 2009).
Nanocarrier Technology
An ideal nano-sized drug carrier must be inert and biodegradable and must be able to encapsulate and protect the drug against degeneration. It also has to be adequately competent for targeted delivery of the drug (Win et al. 2017). In pharmacokinetic studies, nanocarriers such as micelles or liposomes are promising advancements towards the efficient distribution of drugs in the body. The active compounds of drugs are often destabilized by certain external threats like peptidases responsible for the breakdown of peptides into amino acids. Drugs that are encapsulated into these nano-sized drug carriers are protected against the peptidases which prevent their breakdown into amino acids. This technology also has the benefit of sustained drug release at the target sites to enhance efficiency (Andrade et al. 2011). Surfactant-assisted polymers like polylactic acid (PLA) and polylactic-co-glycolic acid (PLGA) are the other viable substitutes used as drug carriers. These polymers provide a consistent release of drugs as well as optimal safety is ensured due to their significant biodegradability (Andrade et al. 2011; Csaba et al. 2009; Kammona and Kiparissides 2012).
The development of nanostructured materials which can selectively interact with various nano and micro-sized biomaterials is desirable due to the ultra-small size and high surface area to volume ratio of nanomaterials (Lau and Dunn 2018; Shojaei 1998). Peptides in conjugation with nanoparticles provide improved control over structural properties of nanostructures and enable facile modification of overall structure, dimensions, and size of conjugates by designing nanoparticle scaffolds optimized for specific purposes (Shojaei 1998). Besides this, peptide-nanoparticle conjugates play a key role in overcoming the limitations of the current peptide-based drug delivery system by increasing the plasma circulation time and selectively delivering the drugs to the targeted tissue. Table 7 summarises the various targeting peptides on different types of nanoparticles which researchers have conjugated to provide more efficient drug delivery systems.
Table 7.
Peptide-nanoparticle conjugates enhance the efficiency of peptide-based theranostics
| Peptide | Nanoparticles | Target | Application | References |
|---|---|---|---|---|
|
TAT (GRKKRRQRRRPQ) |
Mesoporous Silica | Targets importin α and importin β | Nuclear target drug delivery | Zou et al. (2018) |
| Gold nanoparticle | Assists with membrane disruption and cellular uptake | Transdermal drug delivery | Niu et al. (2017) | |
| Nano lipid crystal nanoparticles | Binds to stratum corneum and assists movement of nanoparticles into epidermal layers | Transdermal drug delivery | Patlolla et al. (2010) | |
|
Adenoviral NLS (CGGFSTSLRARKA) |
Gold nanoparticles | Nuclear pore complex for nuclear uptake | Nuclear target drug delivery | Li et al., (2017) |
| BSA (Bovine Serum Albumin)-coated gold nanoparticles | Nuclear pore complex for nanoparticle entrance into the nucleus | Nuclear target drug delivery | Tkachenko et al. (2003) | |
|
TD (ACSSSPSKHCG) |
Liposome | Targets the Na+/K+-ATPase beta-subunit of the stratum corneum for enhanced skin permeability | Transdermal drug delivery | Zou et al. (2018) |
|
G23 (HLNILSTLWKYRC) |
Polymersome | Targets gangliosides GM1 and GT1b | Blood–brain barrier drug delivery | Georgieva et al. (2012) |
|
LNP (KKRTLRKNDRKKRC) |
DGL-PEG | Cell-penetrating peptide for cellular uptake | Blood–brain barrier drug delivery | Yao et al., (2015) |
|
RGD (ADDADW) |
Fluorescent cyclic peptide nanoparticle | αvβ3 Integrin | Molecular imaging | Fan et al., (2018), Fan et al. (2016) |
|
RGD (RGDFDC) |
Au-tripods | αvβ3 Integrin | Molecular imaging | Cheng et al. (2014) |
|
RGD (CRGDC) |
Poly(ethylene oxide) dendrimer | αvβ3 Integrin | Molecular imaging | Almutairi et al. (2009) |
|
RGD (cRGD) |
Iron oxide nanoparticles | αvβ3 Integrin | Molecular imaging | Xie et al., (2008) |
|
Angiopep-2 (TFFYGGSRGKRNN FKTEEY) |
DTX-loaded PLGA@Au-nanoparticles | Targets glioma | Molecular imaging | Hao et al., (2015) |
Conclusion and Future Perspective
Peptides have received extensive interest in recent decades and the number of approved peptide-based biotherapeutics has been increasing with every passing year. More than 80 peptide medications have entered the market, and several hundred novel therapeutic peptides are under preclinical and clinical trials, and this development will significantly streamline in the coming years. The challenges to the delivery of peptide-based drugs are successfully being resolved with the development of the various strategies discussed in this review. Advances in computational structural prediction and various chemical modifications have been attractive approaches due to their ability to enhance stability, affinity, and specificity. Substantial efforts are being put towards the development of peptides with promising compositions, and modes of action which resulted in enhanced functionality of peptides making them suitable therapeutic agents. Taking a retrospective look at the odyssey of peptide therapeutics, it can be stated that the peptide therapeutics have remarkably flourished and their foreseeable future in treating unmet clinical challenges is impregnable. However, the cost of synthesizing such compounds as compared to small molecules has handicapped the overall implementation of peptide-based therapies. Protected amino acids and extensive solvents used for synthesis remain to be cost-intensive factors in the production process, overcoming which will yield potent as well as cost-effective therapeutics that can act on a wide range of diseases. An extensive array of process optimization like resins loading and swelling, reaction time for amino acid coupling, etc., and advancement in instruments might reduce the production time, cost, and solvent usage. Moreover, most of the solvents used in this process viz. methylpyrrolidone, N,N-dimethylformamide, N,N-dimethylacetamide, etc. are reported to have adverse effects on the environment. Thus, replacement of these solvents is likely to make peptide synthesis greener and also may reduce the required solvent volumes and facilitate solvent recycling which would ultimately result in cost-efficient peptide synthesis.
Author Contributions
PB: Data curation, Formal analysis, Investigation, Writing—original draft, Writing—review & editing, SJ: Literature search, Writing—review & editing, SS: Writing—review & editing, SP: Conceptualization, Scheme administration, Writing—review & editing, Supervision.SS Conceptualization, Scheme administration, Writing—review & editing, Supervision, AS Conceptualization, Scheme administration, Writing—review & editing, Supervision.
Declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Agostini F, Cirillo D, Livi CM, Delli Ponti R, Tartaglia GG. cc SOL omics: a webserver for solubility prediction of endogenous and heterologous expression in Escherichia coli. Bioinformatics. 2014;30(20):2975–2977. doi: 10.1093/bioinformatics/btu420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguilar-Zapata D, Petraitiene R, Petraitis V. Echinocandins: the expanding antifungal armamentarium. Clin Infect Dis. 2015;61(6):S604–S611. doi: 10.1093/cid/civ814. [DOI] [PubMed] [Google Scholar]
- Aharony I, Ehrnhoefer DE, Shruster A, Qiu X, Franciosi S, Hayden MR, Offen D. A Huntingtin-based peptide inhibitor of caspase-6 provides protection from mutant Huntingtin-induced motor and behavioral deficits. Hum Mol Genet. 2015;24(9):2604–2614. doi: 10.1093/hmg/ddv023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmady A (2014) Buccal delivery of peptides and proteins.
- Ahn J-M, Boyle NA, Macdonald MT, Janda KD. Peptidomimetics and peptide backbone modifications. Mini Rev Med Chem. 2002;2(5):463–473. doi: 10.2174/1389557023405828. [DOI] [PubMed] [Google Scholar]
- Akhtar A, Andleeb A, Waris TS, Bazzar M, Moradi A-R, Awan NR, Yar M. Neurodegenerative diseases and effective drug delivery: a review of challenges and novel therapeutics. J Control Release. 2021;330:1152–1167. doi: 10.1016/j.jconrel.2020.11.021. [DOI] [PubMed] [Google Scholar]
- Al Shaer D, Al Musaimi O, Albericio F, De La Torre BG. 2018 FDA tides harvest. Pharmaceuticals. 2019;12(2):52. doi: 10.3390/ph12020052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al Shaer D, Al Musaimi O, Albericio F, De La Torre BG. 2019 FDA TIDES (peptides and oligonucleotides) harvest. Pharmaceuticals. 2020;13(3):40. doi: 10.3390/ph13030040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert R, Marbach P, Bauer W, Briner U, Fricker G, Brums C, Pless J. SDZ CO 611: a highly potent glycated analog of somatostatin with improved oral activity. Life Sci. 1993;53(6):517–525. doi: 10.1016/0024-3205(93)90703-6. [DOI] [PubMed] [Google Scholar]
- Almutairi A, Rossin R, Shokeen M, Hagooly A, Ananth A, Capoccia B, Guillaudeu S, Abendschein D, Anderson CJ, Welch MJ. Biodegradable dendritic positron-emitting nanoprobes for the noninvasive imaging of angiogenesis. Proc Natl Acad Sci. 2009;106(3):685–690. doi: 10.1073/pnas.0811757106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amgen Reports Second Quarter 2021 Financial Results (2021) https://www.amgen.com/newsroom/press-releases/2021/08/amgen-reports-second-quarter-2021-financial-results
- Amin SA, Endalur Gopinarayanan V, Nair NU, Hassoun S. Establishing synthesis pathway-host compatibility via enzyme solubility. Biotechnol Bioeng. 2019;116(6):1405–1416. doi: 10.1002/bit.26959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade F, Videira M, Ferreira D, Sarmento B. Nanocarriers for pulmonary administration of peptides and therapeutic proteins. Nanomedicine. 2011;6(1):123–141. doi: 10.2217/nnm.10.143. [DOI] [PubMed] [Google Scholar]
- Antosova Z, Mackova M, Kral V, Macek T. Therapeutic application of peptides and proteins: parenteral forever? Trends Biotechnol. 2009;27(11):628–635. doi: 10.1016/j.tibtech.2009.07.009. [DOI] [PubMed] [Google Scholar]
- Apostolopoulos V, Bojarska J, Chai T-T, Elnagdy S, Kaczmarek K, Matsoukas J, New R, Parang K, Lopez OP, Parhiz H. A global review on short peptides: frontiers and perspectives. Molecules. 2021;26(2):430. doi: 10.3390/molecules26020430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atul Tyagi AT, Anand P, Gupta S, Sharma M, Mathur D, Joshi A, Singh S, Gautam A, Raghava GPS. CancerPPD: a database of anticancer peptides and proteins. Nucleic Acids Res. 2015;43(D1):D837–D843. doi: 10.1093/nar/gku892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach H. A new era without antibiotics. Technol Sci Cult. 2018;1:25. [Google Scholar]
- Bahrami J, Longuet C, Baggio LL, Li K, Drucker DJ. Glucagon-like peptide-2 receptor modulates islet adaptation to metabolic stress in the ob/ob mouse. Gastroenterology. 2010;139(3):857–868. doi: 10.1053/j.gastro.2010.05.006. [DOI] [PubMed] [Google Scholar]
- Baig MH, Ahmad K, Rabbani G, Danishuddin M, Choi I. Computer aided drug design and its application to the development of potential drugs for neurodegenerative disorders. Curr Neuropharmacol. 2018;16(6):740–748. doi: 10.2174/1570159X15666171016163510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baig MH, Ahmad K, Saeed M, Alharbi AM, Barreto GE, Ashraf GM, Choi I. Peptide based therapeutics and their use for the treatment of neurodegenerative and other diseases. Biomed Pharmacother. 2018;103:574–581. doi: 10.1016/j.biopha.2018.04.025. [DOI] [PubMed] [Google Scholar]
- Balkovec JM, Black RM, Hammond ML, Heck JV, Zambias RA, Abruzzo G, Bartizal K, Kropp H, Trainor C. Synthesis, stability, and biological evaluation of water-soluble prodrugs of a new echinocandin lipopeptide: discovery of a potential clinical agent for the treatment of systemic candidiasis and Pneumocystis carinii pneumonia (PCP) J Med Chem. 1992;35(1):194–198. doi: 10.1021/jm00079a027. [DOI] [PubMed] [Google Scholar]
- Balmeh N, Mahmoudi S, Fard NA. Manipulated bio antimicrobial peptides from probiotic bacteria as proposed drugs for COVID-19 disease. Inform Med Unlocked. 2021;23:100515. doi: 10.1016/j.imu.2021.100515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks WA, Audus KL, Davis TP. Permeability of the blood-brain barrier to peptides: an approach to the development of therapeutically useful analogs. Peptides. 1992;13(6):1289–1294. doi: 10.1016/0196-9781(92)90037-4. [DOI] [PubMed] [Google Scholar]
- Bapst J-P, Calame M, Tanner H, Eberle AN. Glycosylated DOTA−α-melanocyte-stimulating hormone analogues for melanoma targeting: influence of the site of glycosylation on in vivo biodistribution. Bioconjug Chem. 2009;20(5):984–993. doi: 10.1021/bc900007u. [DOI] [PubMed] [Google Scholar]
- Bardan A, Nizet V, Gallo RL. Antimicrobial peptides and the skin. Expert Opin Biol Ther. 2004;4(4):543–549. doi: 10.1517/14712598.4.4.543. [DOI] [PubMed] [Google Scholar]
- Begum A, Sujatha D, Prasad K, Bharathi K. A review on Azapeptides: the promising Peptidomimetics. Asian J Chem. 2017;29(9):1879–1887. doi: 10.14233/ajchem.2017.20802. [DOI] [Google Scholar]
- Behzadipour Y, Gholampour M, Pirhadi S, Seradj H, Khoshneviszadeh M, Hemmati S. Viral 3CL(pro) as a target for antiviral intervention using milk-derived bioactive peptides. Int J Pept Res Ther. 2021;58:1–14. doi: 10.1007/s10989-021-10284-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergeron E, Leblanc E, Drevelle O, Giguere R, Beauvais S, Grenier G, Faucheux N. The evaluation of ectopic bone formation induced by delivery systems for bone morphogenetic protein-9 or its derived peptide. Tissue Eng Part A. 2012;18(3–4):342–352. doi: 10.1089/ten.tea.2011.0008. [DOI] [PubMed] [Google Scholar]
- Bergeron E, Senta H, Mailloux A, Park H, Lord E, Faucheux N. Murine preosteoblast differentiation induced by a peptide derived from bone morphogenetic proteins-9. Tissue Eng Part A. 2009;15(11):3341–3349. doi: 10.1089/ten.tea.2009.0189. [DOI] [PubMed] [Google Scholar]
- Bern HA, Pearson D, Larson BA, Nishioka RS (1985) Neurohormones from fish tails: the caudal neurosecretory system: I.“Urophysiology” and the caudal neurosecretory system of fishes. Proceedings of the 1984 Laurentian Hormone Conference [DOI] [PubMed]
- Bhalla S, Verma R, Kaur H, Kumar R, Usmani SS, Sharma S, Raghava GP. CancerPDF: a repository of cancer-associated peptidome found in human biofluids. Sci Rep. 2017;7(1):1–8. doi: 10.1038/s41598-017-01633-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidwell GL, III, Raucher D. Therapeutic peptides for cancer therapy: part I–peptide inhibitors of signal transduction cascades. Expert Opin Drug Deliv. 2009;6(10):1033–1047. doi: 10.1517/17425240903143745. [DOI] [PubMed] [Google Scholar]
- Blaakmeer J, Tijsse-Klasen T, Tesser G. Enhancement of solubility by temporary dimethoxybenzyl-substitution of peptide bonds: towards the synthesis of defined oligomers of alanine and of lysylglutamyl-glycine. Int J Pept Protein Res. 1991;37(6):556–564. doi: 10.1111/j.1399-3011.1991.tb00775.x. [DOI] [PubMed] [Google Scholar]
- Bogdanowich-Knipp SJ, Chakrabarti S, Williams TD, Dillman RK, Siahaan TJ. Solution stability of linear vs cyclic RGD peptides. J Pept Res. 1999;53(5):530–541. doi: 10.1034/j.1399-3011.1999.00052.x. [DOI] [PubMed] [Google Scholar]
- Boparai JK, Sharma PK. Mini review on antimicrobial peptides, sources, mechanism and recent applications. Protein Pept Lett. 2020;27(1):4–16. doi: 10.2174/18755305MTAwENDE80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borchardt RT. Optimizing oral absorption of peptides using prodrug strategies. J Control Release. 1999;62(1–2):231–238. doi: 10.1016/S0168-3659(99)00042-5. [DOI] [PubMed] [Google Scholar]
- Britannica, TEOE (2016) Peptide. Encyclopedia Britannica. Retrieved 27th September from https://www.britannica.com/science/peptide
- Brown M, Rivier J, Vale W. Somatostatin: analogs with selected biological activities. Science. 1977;196(4297):1467–1469. doi: 10.1126/science.867045. [DOI] [PubMed] [Google Scholar]
- Brunel FM, Liu F, Mayer JP. Trends in peptide therapeutics. Successful Drug Discov. 2019;4:1–33. [Google Scholar]
- Buchwald H, Dorman RB, Rasmus NF, Michalek VN, Landvik NM, Ikramuddin S. Effects on GLP-1, PYY, and leptin by direct stimulation of terminal ileum and cecum in humans: implications for ileal transposition. Surg Obes Relat Dis. 2014;10(5):780–786. doi: 10.1016/j.soard.2014.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley CD, Pilling D, Henriquez NV, Parsonage G, Threlfall K, Scheel-Toellner D, Simmons DL, Akbar AN, Lord JM, Salmon M. RGD peptides induce apoptosis by direct caspase-3 activation. Nature. 1999;397(6719):534–539. doi: 10.1038/17409. [DOI] [PubMed] [Google Scholar]
- Butler MS, Hansford KA, Blaskovich MA, Halai R, Cooper MA. Glycopeptide antibiotics: back to the future. J Antibiot. 2014;67(9):631–644. doi: 10.1038/ja.2014.111. [DOI] [PubMed] [Google Scholar]
- Cabri W, Cantelmi P, Corbisiero D, Fantoni T, Ferrazzano L, Martelli G, Mattellone A, Tolomelli A. Therapeutic peptides targeting PPI in clinical development: overview, mechanism of action and perspectives. Front Mol Biosci. 2021;8:697586–697586. doi: 10.3389/fmolb.2021.697586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camilleri M, Acosta A. Emerging treatments in Neurogastroenterology: relamorelin: a novel gastrocolokinetic synthetic ghrelin agonist. Neurogastroenterol Motil. 2015;27(3):324–332. doi: 10.1111/nmo.12490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caon T, Jin L, Simões CMO, Norton RS, Nicolazzo JA. Enhancing the buccal mucosal delivery of peptide and protein therapeutics. Pharm Res. 2015;32(1):1–21. doi: 10.1007/s11095-014-1485-1. [DOI] [PubMed] [Google Scholar]
- Cayrol C, Knibiehler M, Ducommun B. p21 binding to PCNA causes G1 and G2 cell cycle arrest in p53-deficient cells. Oncogene. 1998;16(3):311–320. doi: 10.1038/sj.onc.1201543. [DOI] [PubMed] [Google Scholar]
- Chan Y, Yao X, Lau C, Chan F, He G, Bourreau J-P, Huang Y. The relaxant effect of urocortin in rat pulmonary arteries. Regul Pept. 2004;121(1–3):11–18. doi: 10.1016/j.regpep.2004.03.019. [DOI] [PubMed] [Google Scholar]
- Chatterjee J, Khatri B, Raghunathan S, Chakraborti S, Kumaran S, Tadala R, Wagh P, Priyakumar UD, Rahisuddin R (2021) Desolvation of peptide bond by O to S substitution impacts protein stability. Angewandte Chemie International Edition. [DOI] [PubMed]
- Chen L, Guo X, Wang L, Geng J, Wu J, Hu B, Wang T, Li J, Liu C, Wang H. In silico identification and experimental validation of cellular uptake by a new cell penetrating peptide P1 derived from MARCKS. Drug Deliv. 2021;28(1):1637–1648. doi: 10.1080/10717544.2021.1960922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng K, Kothapalli S-R, Liu H, Koh AL, Jokerst JV, Jiang H, Yang M, Li J, Levi J, Wu JC. Construction and validation of nano gold tripods for molecular imaging of living subjects. J Am Chem Soc. 2014;136(9):3560–3571. doi: 10.1021/ja412001e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiangjong W, Chutipongtanate S, Hongeng S. Anticancer peptide: physicochemical property, functional aspect and trend in clinical application. Int J Oncol. 2020;57(3):678–696. doi: 10.3892/ijo.2020.5099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhary A, Raines RT. An evaluation of peptide-bond isosteres. ChemBioChem. 2011;12(12):1801–1807. doi: 10.1002/cbic.201100272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirillo D, Pentimalli F, Giordano A. Peptides or small molecules? Different approaches to develop more effective CDK inhibitors. Curr Med Chem. 2011;18(19):2854–2866. doi: 10.2174/092986711796150496. [DOI] [PubMed] [Google Scholar]
- Cluntun AA, Lukey MJ, Cerione RA, Locasale JW. Glutamine metabolism in cancer: understanding the heterogeneity. Trends Cancer. 2017;3(3):169–180. doi: 10.1016/j.trecan.2017.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa AR, Rodrigues ME, Henriques M, Oliveira R, Azeredo J. Glycosylation: impact, control and improvement during therapeutic protein production. Crit Rev Biotechnol. 2014;34(4):281–299. doi: 10.3109/07388551.2013.793649. [DOI] [PubMed] [Google Scholar]
- Craik DJ, Fairlie DP, Liras S, Price D. The future of peptide-based drugs. Chem Biol Drug Des. 2013;81(1):136–147. doi: 10.1111/cbdd.12055. [DOI] [PubMed] [Google Scholar]
- Craik DJ, Kan M-W. How can we improve peptide drug discovery? Learning from the past. Expert Opin Drug Discov. 2021;22:1–4. doi: 10.1080/17460441.2021.1961740. [DOI] [PubMed] [Google Scholar]
- Csaba N, Garcia-Fuentes M, Alonso MJ. Nanoparticles for nasal vaccination. Adv Drug Deliv Rev. 2009;61(2):140–157. doi: 10.1016/j.addr.2008.09.005. [DOI] [PubMed] [Google Scholar]
- Cudic P. Peptide modifications to increase metabolic stability and activity. New York: Springer; 2013. [Google Scholar]
- Currier JR, Galley LM, Wenschuh H, Morafo V, Ratto-Kim S, Gray CM, Maboko L, Hoelscher M, Marovich MA, Cox JH. Peptide impurities in commercial synthetic peptides and their implications for vaccine trial assessment. Clin Vaccine Immunol. 2008;15(2):267–276. doi: 10.1128/CVI.00284-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’aloisio V, Dognini P, Hutcheon GA, Coxon CR. PepTherDia: database and structural composition analysis of approved peptide therapeutics and diagnostics. Drug Discovery Today. 2021;26(6):1409–1419. doi: 10.1016/j.drudis.2021.02.019. [DOI] [PubMed] [Google Scholar]
- Dai Y, Cai X, Shi W, Bi X, Su X, Pan M, Li H, Lin H, Huang W, Qian H. Pro-apoptotic cationic host defense peptides rich in lysine or arginine to reverse drug resistance by disrupting tumor cell membrane. Amino Acids. 2017;49(9):1601–1610. doi: 10.1007/s00726-017-2453-y. [DOI] [PubMed] [Google Scholar]
- Damodaran VB, Fee C. Protein PEGylation: an overview of chemistry and process considerations. Eur Pharm Rev. 2010;15(1):18–26. [Google Scholar]
- Das RP, Jain AK, Ramesh V. Current concepts in the pathogenesis of psoriasis. Indian J Dermatol. 2009;54(1):7–12. doi: 10.4103/0019-5154.48977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das S, Ramakumar S, Pal D. Identifying functionally important cis-peptide containing segments in proteins and their utility in molecular function annotation. FEBS J. 2014;281(24):5602–5621. doi: 10.1111/febs.13100. [DOI] [PubMed] [Google Scholar]
- De La Torre BG, Albericio F (2020a) Peptide Therapeutics 2.0. In: Multidisciplinary Digital Publishing Institute.
- De La Torre BG, Albericio F. The pharmaceutical industry in 2019. An analysis of FDA drug approvals from the perspective of molecules. Molecules. 2020;25(3):745. doi: 10.3390/molecules25030745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decker CYaS (2021) Endo’s patent loss over blood pressure drug adds to its troubles. Bloomberg. Retrieved 6th December from https://www.bloomberg.com/news/articles/2021-08-31/endo-s-par-loses-ruling-over-eagle-s-proposed-vasostrict-copy
- Deska J, Kazmaier U. Peptide backbone modifications. Curr Org Chem. 2008;12(5):355. doi: 10.2174/138527208783743697. [DOI] [Google Scholar]
- Di L. Strategic approaches to optimizing peptide ADME properties. AAPS J. 2015;17(1):134–143. doi: 10.1208/s12248-014-9687-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Droin N, Hendra J-B, Ducoroy P, Solary E. Human defensins as cancer biomarkers and antitumour molecules. J Proteomics. 2009;72(6):918–927. doi: 10.1016/j.jprot.2009.01.002. [DOI] [PubMed] [Google Scholar]
- Dutta S, Ray S, Nagarajan K. Glutamic acid as anticancer agent: an overview. Saudi Pharm J. 2013;21(4):337–343. doi: 10.1016/j.jsps.2012.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E-Kobon T, Thongararm P, Roytrakul S, Meesuk L, Chumnanpuen P. Prediction of anticancer peptides against MCF-7 breast cancer cells from the peptidomes of Achatina fulica mucus fractions. Comput Struct Biotechnol J. 2016;14:49–57. doi: 10.1016/j.csbj.2015.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias PM, Choi EH. Interactions among stratum corneum defensive functions. Exp Dermatol. 2005;14(10):719–726. doi: 10.1111/j.1600-0625.2005.00363.x. [DOI] [PubMed] [Google Scholar]
- Errante F, Ledwoń P, Latajka R, Rovero P, Papini AM (2020) Cosmeceutical peptides in the framework of sustainable wellness economy [Mini Review]. Front Chem, 8. [DOI] [PMC free article] [PubMed]
- Fåhraeus R, Laín S, Ball KL, Lane DP. Characterization of the cyclin-dependent kinase inhibitory domain of the INK4 family as a model for a synthetic tumour suppressor molecule. Oncogene. 1998;16(5):587–596. doi: 10.1038/sj.onc.1201580. [DOI] [PubMed] [Google Scholar]
- Fan Z, Chang Y, Cui C, Sun L, Wang DH, Pan Z, Zhang M. Near infrared fluorescent peptide nanoparticles for enhancing esophageal cancer therapeutic efficacy. Nat Commun. 2018;9(1):1–11. doi: 10.1038/s41467-018-04763-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Z, Sun L, Huang Y, Wang Y, Zhang M. Bioinspired fluorescent dipeptide nanoparticles for targeted cancer cell imaging and real-time monitoring of drug release. Nat Nanotechnol. 2016;11(4):388–394. doi: 10.1038/nnano.2015.312. [DOI] [PubMed] [Google Scholar]
- Fda (2021) Novel drug approvals for 2021. U.S. food and drug administration. Retrieved October 12 from https://www.fda.gov/drugs/new-drugs-fda-cders-new-molecular-entities-and-new-therapeutic-biological-products/novel-drug-approvals-2021
- Fernández De Ullivarri M, Arbulu S, Garcia-Gutierrez E, Cotter PD. Antifungal peptides as therapeutic agents [Review] Front Cell Infection Microbiol. 2020;10:12. doi: 10.3389/fcimb.2020.00105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finan B, Yang B, Ottaway N, Smiley DL, Ma T, Clemmensen C, Chabenne J, Zhang L, Habegger KM, Fischer K. A rationally designed monomeric peptide triagonist corrects obesity and diabetes in rodents. Nat Med. 2015;21(1):27–36. doi: 10.1038/nm.3761. [DOI] [PubMed] [Google Scholar]
- Financial report for the period 1 January 2021 to 30 September 2021 (2021) N Nordisk. https://ml-eu.globenewswire.com/Resource/Download/55ede868-1686-4d59-9a68-ee0b4470d4e7
- Fosgerau K, Hoffmann T. Peptide therapeutics: current status and future directions. Drug Discovery Today. 2015;20(1):122–128. doi: 10.1016/j.drudis.2014.10.003. [DOI] [PubMed] [Google Scholar]
- Fournie-Zaluski MC, Coric P, Turcaud S, Lucas E, Noble F, Maldonado R, Roques BP. Mixed-inhibitor-prodrug as a new approach toward systemically active inhibitors of enkephalin-degrading enzymes. J Med Chem. 1992;35(13):2473–2481. doi: 10.1021/jm00091a016. [DOI] [PubMed] [Google Scholar]
- Freire Haddad H, Burke JA, Scott EA, Ameer GA (2021) Clinical relevance of pre-existing and treatment-induced anti-poly(ethylene glycol) antibodies. Regenerative Engineering and Translational Medicine. [DOI] [PMC free article] [PubMed]
- Frey V, Viaud J, Subra G, Cauquil N, Guichou J-F, Casara P, Grassy G, Chavanieu A. Structure–activity relationships of Bak derived peptides: affinity and specificity modulations by amino acid replacement. Eur J Med Chem. 2008;43(5):966–972. doi: 10.1016/j.ejmech.2007.06.008. [DOI] [PubMed] [Google Scholar]
- Gallego M, Mora L, Toldrá F. Characterisation of the antioxidant peptide AEEEYPDL and its quantification in Spanish dry-cured ham. Food Chem. 2018;258:8–15. doi: 10.1016/j.foodchem.2018.03.035. [DOI] [PubMed] [Google Scholar]
- Georgieva JV, Brinkhuis RP, Stojanov K, Weijers CA, Zuilhof H, Rutjes FP, Hoekstra D, Van Hest JC, Zuhorn IS. Peptide-mediated blood–brain barrier transport of polymersomes. Angew Chem Int Ed. 2012;51(33):8339–8342. doi: 10.1002/anie.201202001. [DOI] [PubMed] [Google Scholar]
- Global Peptide Therapeutics Sales Market Report 2020 (2020) (2200518). Q Research. https://www.qyresearch.com/index/detail/2200518/global-peptide-therapeutics-sales%20market
- Gómez EA, Giraldo P, Orduz S. InverPep: A database of invertebrate antimicrobial peptides. J Global Antimicrob Resistance. 2017;8:13–17. doi: 10.1016/j.jgar.2016.10.003. [DOI] [PubMed] [Google Scholar]
- Goodwin D, Simerska P, Toth I. Peptides as therapeutics with enhanced bioactivity. Curr Med Chem. 2012;19(26):4451–4461. doi: 10.2174/092986712803251548. [DOI] [PubMed] [Google Scholar]
- Gordee RS, Zeckner DJ, Howard LC, Alborn WE, Jr, Debono M. Anti-candida activity and toxicology of LY121019, a Novel semisynthetic polypeptide antifungal antibiotic a. Ann N Y Acad Sci. 1988;544(1):294–309. doi: 10.1111/j.1749-6632.1988.tb40415.x. [DOI] [PubMed] [Google Scholar]
- Goyal R, Ramakrishnan V. Chapter 2 - peptide-based drug delivery systems. In: Mohapatra SS, Ranjan S, Dasgupta N, Mishra RK, Thomas S, editors. Characterization and biology of nanomaterials for drug delivery. Amsterdam: Elsevier; 2019. pp. 25–45. [Google Scholar]
- Grieco P, Gomez-Monterrey I. Natural and synthetic peptides in the cardiovascular diseases: An update on diagnostic and therapeutic potentials. Arch Biochem Biophys. 2019;662:15–32. doi: 10.1016/j.abb.2018.11.021. [DOI] [PubMed] [Google Scholar]
- Groll AH, Mickiene D, Petraitis V, Petraitiene R, Ibrahim KH, Piscitelli SC, Bekersky I, Walsh TJ. Compartmental Pharmacokinetics and Tissue Distribution of the Antifungal Echinocandin Lipopeptide Micafungin (FK463) in Rabbits. Antimicrob Agents Chemother. 2001;45(12):3322–3327. doi: 10.1128/AAC.45.12.3322-3327.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Li X, Wang Z. APD3: the antimicrobial peptide database as a tool for research and education. Nucleic Acids Res. 2016;44(D1):D1087–D1093. doi: 10.1093/nar/gkv1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gull S, Shamim N, Minhas F. AMAP: Hierarchical multi-label prediction of biologically active and antimicrobial peptides. Comput Biol Med. 2019;107:172–181. doi: 10.1016/j.compbiomed.2019.02.018. [DOI] [PubMed] [Google Scholar]
- Guryanova S, Udzhukhu V, Kubylinsky A. Pathogenetic therapy of psoriasis by muramyl peptide. Front Immunol. 2019;10:1275. doi: 10.3389/fimmu.2019.01275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haggag YA, Donia AA, Osman MA, El-Gizawy SA. Peptides as drug candidates: limitations and recent development perspectives. Biomed J. 2018;1:3. [Google Scholar]
- Håkansson J, Ringstad L, Umerska A, Johansson J, Andersson T, Boge L, Rozenbaum RT, Sharma PK, Tollbäck P, Björn C. Characterization of the in vitro, ex vivo, and in vivo efficacy of the antimicrobial peptide DPK-060 used for topical treatment. Front Cell Infect Microbiol. 2019;9:174. doi: 10.3389/fcimb.2019.00174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao Y, Zhang B, Zheng C, Ji R, Ren X, Guo F, Sun S, Shi J, Zhang H, Zhang Z. The tumor-targeting core–shell structured DTX-loaded PLGA@ Au nanoparticles for chemo-photothermal therapy and X-ray imaging. J Control Release. 2015;220:545–555. doi: 10.1016/j.jconrel.2015.11.016. [DOI] [PubMed] [Google Scholar]
- Harris JM, Chess RB. Effect of pegylation on pharmaceuticals. Nat Rev Drug Discovery. 2003;2(3):214–221. doi: 10.1038/nrd1033. [DOI] [PubMed] [Google Scholar]
- Hasan M, Ghosh PP, Azim KF, Mukta S, Abir RA, Nahar J, Khan MMH. Reverse vaccinology approach to design a novel multi-epitope subunit vaccine against avian influenza A (H7N9) virus. Microb Pathog. 2019;130:19–37. doi: 10.1016/j.micpath.2019.02.023. [DOI] [PubMed] [Google Scholar]
- Haskell-Luevano C, Toth K, Boteju L, Job C, Castrucci AMDL, Hadley ME, Hruby VJ. β-Methylation of the Phe7 and Trp9 melanotropin side chain pharmacophores affects ligand− receptor interactions and prolonged biological activity. J Med Chem. 1997;40(17):2740–2749. doi: 10.1021/jm970018t. [DOI] [PubMed] [Google Scholar]
- Hebditch M, Carballo-Amador MA, Charonis S, Curtis R, Warwicker J. Protein–Sol: a web tool for predicting protein solubility from sequence. Bioinformatics. 2017;33(19):3098–3100. doi: 10.1093/bioinformatics/btx345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henninot A, Collins JC, Nuss JM. The current state of peptide drug discovery: back to the future? J Med Chem. 2018;61(4):1382–1414. doi: 10.1021/acs.jmedchem.7b00318. [DOI] [PubMed] [Google Scholar]
- Hilchie AL, Wuerth K, Hancock RE. Immune modulation by multifaceted cationic host defense (antimicrobial) peptides. Nat Chem Biol. 2013;9(12):761–768. doi: 10.1038/nchembio.1393. [DOI] [PubMed] [Google Scholar]
- Hogan B. Bone morphogenetic proteins: multifunctional regulators of vertebrate development. Genes Dev. 1996;10(13):1580–1594. doi: 10.1101/gad.10.13.1580. [DOI] [PubMed] [Google Scholar]
- Hopkins AL, Groom CR. The druggable genome. Nat Rev Drug Discovery. 2002;1(9):727–730. doi: 10.1038/nrd892. [DOI] [PubMed] [Google Scholar]
- Huan Y, Kong Q, Mou H, Yi H. Antimicrobial peptides: classification, design, application and research progress in multiple fields [Review] Front Microbiol. 2020;11:158. doi: 10.3389/fmicb.2020.582779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Human Insulin Market Size 2021|Is Anticipated to Reach USD 27.71 Billion and Exhibit a CAGR of 3.4% by 2026 (2021) GlobeNewswire. Retrieved 6th December from https://www.globenewswire.com/news-release/2021/04/26/2216727/0/en/Human-Insulin-Market-Size-2021-Is-Anticipated-to-Reach-USD-27-71-Billion-and-Exhibit-a-CAGR-of-3-4-by-2026.html
- Ipsen Delivers Encouraging Sales Growth in the First Quarter of 2021 Despite the Pandemic, and Confirms Its Full-Year Guidance (2021) Businesswire. Retrieved 6th december from https://www.businesswire.com/news/home/20210421006087/en/Ipsen-Delivers-Encouraging-Sales-Growth-in-the-First-Quarter-of-2021-Despite-the-Pandemic-and-Confirms-Its-Full-Year-Guidance
- Boohaker JR, Lee MW, Vishnubhotla P, Perez JLM, Khaled AR. The use of therapeutic peptides to target and to kill cancer cells. Curr Med Chem. 2012;19(22):3794–3804. doi: 10.2174/092986712801661004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jambunathan K, Galande KA. Design of a serum stability tag for bioactive peptides. Protein Pept Lett. 2014;21(1):32–38. doi: 10.2174/09298665113209990069. [DOI] [PubMed] [Google Scholar]
- Jhong J-H, Chi Y-H, Li W-C, Lin T-H, Huang K-Y, Lee T-Y. dbAMP: an integrated resource for exploring antimicrobial peptides with functional activities and physicochemical properties on transcriptome and proteome data. Nucleic Acids Res. 2019;47(D1):D285–D297. doi: 10.1093/nar/gky1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang J, Batra S, Zhang J. Asparagine: a metabolite to be targeted in cancers. Metabolites. 2021;11(6):402. doi: 10.3390/metabo11060402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson J, Gudmundsson GH, Rottenberg MNE, Berndt KD, Agerberth B. Conformation-dependent antibacterial activity of the naturally occurring human peptide LL-37. J Biol Chem. 1998;273(6):3718–3724. doi: 10.1074/jbc.273.6.3718. [DOI] [PubMed] [Google Scholar]
- Johnston TA (2017) The formulation and delivery of a novel peptide drug via the buccal route by an orally disintegrating tablet.
- Joo HS, Fu CI, Otto M. Bacterial strategies of resistance to antimicrobial peptides. Philos Trans R Soc Lond B. 2016;371(1695):15. doi: 10.1098/rstb.2015.0292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones AG, Hattersley AT. The clinical utility of C-peptide measurement in the care of patients with diabetes. Diabet Med. 2013;30(7):803–817. doi: 10.1111/dme.12159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung Y, Kong B, Moon S, Yu S-H, Chung J, Ban C, Chung W-J, Kim S-G, Kweon D-H. Envelope-deforming antiviral peptide derived from influenza virus M2 protein. Biochem Biophys Res Commun. 2019;517(3):507–512. doi: 10.1016/j.bbrc.2019.07.088. [DOI] [PubMed] [Google Scholar]
- Kageyama K, Teui K, Tamasawa N, Suda T. Regulation and roles of urocortins in the vascular system. Int J Endocrinol. 2012;2012:873723. doi: 10.1155/2012/873723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamaruzzaman NF, Tan LP, Hamdan RH, Choong SS, Wong WK, Gibson AJ, Chivu A, Pina MDF. Antimicrobial polymers: the potential replacement of existing antibiotics? Int J Mol Sci. 2019;20(11):2747. doi: 10.3390/ijms20112747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kammona O, Kiparissides C. Recent advances in nanocarrier-based mucosal delivery of biomolecules. J Control Release. 2012;161(3):781–794. doi: 10.1016/j.jconrel.2012.05.040. [DOI] [PubMed] [Google Scholar]
- Kang X, Dong F, Shi C, Liu S, Sun J, Chen J, Li H, Xu H, Lao X, Zheng H. DRAMP 2.0, an updated data repository of antimicrobial peptides. Sci Data. 2019;6(1):1–10. doi: 10.1038/s41597-019-0154-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanovsky M, Michl J, Botzolaki G, Morin J, Kovac C, Chung DL, Chie L, Friedman FK, Pincus MR. Peptides designed from molecular modeling studies of the ras-p21 protein induce phenotypic reversion of a pancreatic carcinoma cell line but have no effect on normal pancreatic acinar cell growth. Cancer Chemother Pharmacol. 2003;52:202–208. doi: 10.1007/s00280-003-0639-3. [DOI] [PubMed] [Google Scholar]
- Kapp TG, Rechenmacher F, Neubauer S, Maltsev OV, Cavalcanti-Adam EA, Zarka R, Reuning U, Notni J, Wester H-J, Mas-Moruno C, Spatz J, Geiger B, Kessler H. A comprehensive evaluation of the activity and selectivity profile of ligands for RGD-binding integrins. Sci Rep. 2017;7(1):39805. doi: 10.1038/srep39805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karami Fath M, Babakhaniyan K, Zokaei M, Yaghoubian A, Akbari S, Khorsandi M, Soofi A, Nabi-Afjadi M, Zalpoor H, Jalalifar F, Azargoonjahromi A, Payandeh Z, Alagheband Bahrami A. Anti-cancer peptide-based therapeutic strategies in solid tumors. Cell Mol Biol Lett. 2022;27(1):33. doi: 10.1186/s11658-022-00332-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavousi K, Bagheri M, Behrouzi S, Vafadar S, Atanaki FF, Lotfabadi BT, Ariaeenejad S, Shockravi A, Moosavi-Movahedi AA. IAMPE: NMR-assisted computational prediction of antimicrobial peptides. J Chem Inf Model. 2020;60(10):4691–4701. doi: 10.1021/acs.jcim.0c00841. [DOI] [PubMed] [Google Scholar]
- Khurana S, Rawi R, Kunji K, Chuang GY, Bensmail H, Mall R. DeepSol: a deep learning framework for sequence-based protein solubility prediction. Bioinformatics. 2018;34(15):2605–2613. doi: 10.1093/bioinformatics/bty166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HK, Lee JS, Kim JH, Seon JK, Park KS, Jeong MH, Yoon TR. Bone-forming peptide-2 derived from BMP-7 enhances osteoblast differentiation from multipotent bone marrow stromal cells and bone formation. Exp Mol Med. 2017;49(5):e328–e328. doi: 10.1038/emm.2017.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MS, Song J, Park S, Kim TS, Park HJ, Cho D. The wound healing peptide, AES16-2M, ameliorates atopic dermatitis in vivo. Molecules. 2021;26(4):1168. doi: 10.3390/molecules26041168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura K, Kangawa K, Kawamoto M, Ichiki Y, Nakamura S, Matsuo H, Eto T. Adrenomedullin: a novel hypotensive peptide isolated from human pheochromocytoma. Biochem Biophys Res Commun. 1993;192(2):553–560. doi: 10.1006/bbrc.1993.1451. [DOI] [PubMed] [Google Scholar]
- Klotz L, Boccon-Gibod L, Shore ND, Andreou C, Persson B-E, Cantor P, Jensen J-K, Olesen TK, Schröder FH. The efficacy and safety of degarelix: a 12-month, comparative, randomized, open-label, parallel-group. BJU Int. 2008;2:102. doi: 10.1111/j.1464-410X.2008.08183.x. [DOI] [PubMed] [Google Scholar]
- Kumar MA, Foster G, Macintyre I. Further evidence for calcitonin a rapid-acting hormone which lowers plasma-calcium. The Lancet. 1963;282(7306):480–482. doi: 10.1016/S0140-6736(63)90224-1. [DOI] [PubMed] [Google Scholar]
- Kumar MS. Peptides and Peptidomimetics as potential antiobesity agents: overview of current status. Front Nutr. 2019;6(11):125. doi: 10.3389/fnut.2019.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- L-asparaginase. Chemocare. https://chemocare.com/chemotherapy/drug-info/Lasparaginase.aspx
- Lai Y, Gallo RL. AMPed up immunity: how antimicrobial peptides have multiple roles in immune defense. Trends Immunol. 2009;30(3):131–141. doi: 10.1016/j.it.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau JL, Dunn MK. Therapeutic peptides: Historical perspectives, current development trends, and future directions. Bioorg Med Chem. 2018;26(10):2700–2707. doi: 10.1016/j.bmc.2017.06.052. [DOI] [PubMed] [Google Scholar]
- Lee AC-L, Harris JL, Khanna KK, Hong J-H. A comprehensive review on current advances in peptide drug development and design. Int J Mol Sci. 2019;20(10):2383. doi: 10.3390/ijms20102383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee G, Ronai Z, Pincus M, Murphy R, Delohery T, Nishimura S, Yamaizumi Z, Weinstein I, Brandt-Rauf P. Inhibition of ras oncogeneencoded P21 protein-induced pinocytotic activity by a synthetic peptide corresponding to an effector domain of the protein. Med Sci Res. 1990;18(19):771–772. [Google Scholar]
- Lee H-T, Lee C-C, Yang J-R, Lai JZ, Chang KY (2015) A large-scale structural classification of antimicrobial peptides. BioMed Res Int, 2015. [DOI] [PMC free article] [PubMed]
- Leffler DA, Kelly CP, Green PH, Fedorak RN, Dimarino A, Perrow W, Rasmussen H, Wang C, Bercik P, Bachir NM. Larazotide acetate for persistent symptoms of celiac disease despite a gluten-free diet: a randomized controlled trial. Gastroenterology. 2015;148(7):1311–1319. doi: 10.1053/j.gastro.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann J, Retz M, Sidhu SS, Suttmann H, Sell M, Paulsen F, Harder J, Unteregger G, Stöckle M. Antitumor activity of the antimicrobial peptide magainin II against bladder cancer cell lines. Eur Urol. 2006;50(1):141–147. doi: 10.1016/j.eururo.2005.12.043. [DOI] [PubMed] [Google Scholar]
- Lei J, Sun L, Huang S, Zhu C, Li P, He J, Mackey V, Coy DH, He Q. The antimicrobial peptides and their potential clinical applications. Am J Transl Res. 2019;11(7):3919. [PMC free article] [PubMed] [Google Scholar]
- Leonard J (2019) What to know about peptides for health. Medical News Today. https://www.medicalnewstoday.com/articles/326701
- Levy O. Antimicrobial proteins and peptides: anti-infective molecules of mammalian leukocytes. J Leukoc Biol. 2004;76(5):909–925. doi: 10.1189/jlb.0604320. [DOI] [PubMed] [Google Scholar]
- Li J, Hu S, Jian W, Xie C, Yang X. Plant antimicrobial peptides: structures, functions, and applications. Bot Stud. 2021;62(1):1–15. doi: 10.1186/s40529-021-00312-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Tan S, Chen X, Zhang C-Y, Zhang Y. Peptide aptamers with biological and therapeutic applications. Curr Med Chem. 2011;18(27):4215–4222. doi: 10.2174/092986711797189583. [DOI] [PubMed] [Google Scholar]
- Li N, Yang H, Yu Z, Li Y, Pan W, Wang H, Tang B. Nuclear-targeted siRNA delivery for long-term gene silencing. Chem Sci. 2017;8(4):2816–2822. doi: 10.1039/C6SC04293G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lian Z, Ji T. Functional peptide-based drug delivery systems. J Mater Chem B. 2020;8(31):6517–6529. doi: 10.1039/D0TB00713G. [DOI] [PubMed] [Google Scholar]
- Liang JF, Yang VC. Synthesis of doxorubicin–peptide conjugate with multidrug resistant tumor cell killing activity. Bioorg Med Chem Lett. 2005;15(22):5071–5075. doi: 10.1016/j.bmcl.2005.07.087. [DOI] [PubMed] [Google Scholar]
- Liang Y, Zhang X, Yuan Y, Bao Y, Xiong M. Role and modulation of the secondary structure of antimicrobial peptides to improve selectivity. Biomater Sci. 2020;8(24):6858–6866. doi: 10.1039/D0BM00801J. [DOI] [PubMed] [Google Scholar]
- Lilly Reports Robust Third-Quarter 2021 Financial Results as Pipeline Success Strengthens Future Growth Potential (2021) Lilly Investors. Retrieved 6th December from https://investor.lilly.com/news-releases/news-release-details/lilly-reports-robust-third-quarter-2021-financial-results
- Lim KJ, Sung BH, Shin JR, Lee YW, Kim DJ, Yang KS, Kim SC. A cancer specific cell-penetrating peptide, BR2, for the efficient delivery of an scFv into cancer cells. PLoS ONE. 2013;8(6):e66084. doi: 10.1371/journal.pone.0066084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Fan L, Sun J, Lao X, Zheng H. Computational resources and tools for antimicrobial peptides. J Pept Sci. 2017;23(1):4–12. doi: 10.1002/psc.2947. [DOI] [PubMed] [Google Scholar]
- Lokhande KB, Banerjee T, Swamy KV, Deshpande M. (2020). An In silico scientific basis for LL-37 as a therapeutic and Vitamin D as preventive for Covid-19. [DOI] [PMC free article] [PubMed]
- Lu KP, Finn G, Lee TH, Nicholson LK. Prolyl cis-trans isomerization as a molecular timer. Nat Chem Biol. 2007;3(10):619–629. doi: 10.1038/nchembio.2007.35. [DOI] [PubMed] [Google Scholar]
- Luzzio FA, Mayorov AV, Figg WD. Thalidomide metabolites: Part 1: Derivatives of (+)-2-(N-phthalimido)-γ-hydroxyglutamic acid. Tetrahedron Lett. 2000;41(14):2275–2278. doi: 10.1016/S0040-4039(00)00160-X. [DOI] [Google Scholar]
- Mabrouk DM. Antimicrobial peptides: features, applications and the potential use against covid-19. Mol Biol Rep. 2022;49(10):10039–10050. doi: 10.1007/s11033-022-07572-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahato RI, Narang AS, Thoma L, Miller DD. Emerging trends in oral delivery of peptide and protein drugs. Crit Rev Ther Drug Carrier Syst. 2003;20:2–3. doi: 10.1615/CritRevTherDrugCarrierSyst.v20.i23.30. [DOI] [PubMed] [Google Scholar]
- Mahlapuu M, Håkansson J, Ringstad L, Björn C. Antimicrobial peptides: an emerging category of therapeutic agents. Front Cell Infect Microbiol. 2016;6:194. doi: 10.3389/fcimb.2016.00194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandarino L, Stenner D, Blanchard W, Nissen S, Gerich J, Ling N, Brazeau P, Bohlen P, Esch F, Guillemin R. Selective effects of somatostatin-14,-25 and-28 on in vitro insulin and glucagon secretion. Nature. 1981;291(5810):76–77. doi: 10.1038/291076a0. [DOI] [PubMed] [Google Scholar]
- Marelli C, Maschat F. The P42 peptide and Peptide-based therapies for Huntington's disease. Orphanet J Rare Dis. 2016;11:24. doi: 10.1186/s13023-016-0405-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larché DM, Wraith C. Peptide-based therapeutic vaccines for allergic and autoimmune diseases. Nat Med. 2005;11(S4):S69–S76. doi: 10.1038/nm1226. [DOI] [PubMed] [Google Scholar]
- Market AE. Therapeutic Peptides under the Spotlight.
- Marqus S, Pirogova E, Piva TJ. Evaluation of the use of therapeutic peptides for cancer treatment. J Biomed Sci. 2017;24(1):21. doi: 10.1186/s12929-017-0328-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massodi I, Moktan S, Rawat A, Bidwell Iii GL, Raucher D. Inhibition of ovarian cancer cell proliferation by a cell cycle inhibitory peptide fused to a thermally responsive polypeptide carrier. Int J Cancer. 2010;126(2):533–544. doi: 10.1002/ijc.24725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mckay M, Afrose F, Koeppe R. 2nd and DV Greathouse. Biochim Biophys Acta Biomembr. 2018;1860:2108–2117. doi: 10.1016/j.bbamem.2018.02.010. [DOI] [PubMed] [Google Scholar]
- Mehta D, Anand P, Kumar V, Joshi A, Mathur D, Singh S, Tuknait A, Chaudhary K, Gautam SK, Gautam A (2014) ParaPep: a web resource for experimentally validated antiparasitic peptide sequences and their structures. Database, 2014. [DOI] [PMC free article] [PubMed]
- Mendonça DA, Bakker M, Cruz-Oliveira C, Neves V, Jiménez MA, Defaus S, Cavaco M, Veiga AS, Cadima-Couto I, Castanho MaRB, Andreu D, Todorovski T. Penetrating the blood-brain barrier with new peptide-porphyrin conjugates having anti-HIV activity. Bioconjug Chem. 2021;32(6):1067–1077. doi: 10.1021/acs.bioconjchem.1c00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merkle HP, Wolany G. Buccal delivery for peptide drugs. J Control Release. 1992;21(1):155–164. doi: 10.1016/0168-3659(92)90017-L. [DOI] [Google Scholar]
- Migoń D, Neubauer D, Kamysz W. Hydrocarbon stapled antimicrobial peptides. Protein J. 2018;37(1):2–12. doi: 10.1007/s10930-018-9755-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modlin I, Pavel M, Kidd M, Gustafsson B. Somatostatin analogues in the treatment of gastroenteropancreatic neuroendocrine (carcinoid) tumours. Aliment Pharmacol Ther. 2010;31(2):169–188. doi: 10.1111/j.1365-2036.2009.04174.x. [DOI] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG, risma Group*, T Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- Mora L, Escudero E, Fraser PD, Aristoy MC, Toldrá F. Proteomic identification of antioxidant peptides from 400 to 2500Da generated in Spanish dry-cured ham contained in a size-exclusion chromatography fraction. Food Res Int. 2014;56:68–76. doi: 10.1016/j.foodres.2013.12.001. [DOI] [Google Scholar]
- Moradi SV, Hussein WM, Varamini P, Simerska P, Toth I. Glycosylation, an effective synthetic strategy to improve the bioavailability of therapeutic peptides. Chem Sci. 2016;7(4):2492–2500. doi: 10.1039/C5SC04392A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moretta A, Scieuzo C, Petrone AM, Salvia R, Manniello MD, Franco A, Lucchetti D, Vassallo A, Vogel H, Sgambato A, Falabella P. Antimicrobial Peptides: A New Hope in Biomedical and Pharmaceutical Fields. Front Cell Infect Microbiol. 2021;11:15. doi: 10.3389/fcimb.2021.668632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muheem A, Shakeel F, Jahangir MA, Anwar M, Mallick N, Jain GK, Warsi MH, Ahmad FJ. A review on the strategies for oral delivery of proteins and peptides and their clinical perspectives. Saudi Pharm J. 2016;24(4):413–428. doi: 10.1016/j.jsps.2014.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhopadhyay S, Prasad AB, Mehta CH, Nayak UY. Antimicrobial peptide polymers: no escape to ESKAPE pathogens: a review. World J Microbiol Biotechnol. 2020;36(9):1–14. doi: 10.1007/s11274-020-02907-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller T, Finan B, Clemmensen C, Dimarchi R, Tschöp M. The new biology and pharmacology of glucagon. Physiol Rev. 2017;97(2):721–766. doi: 10.1152/physrev.00025.2016. [DOI] [PubMed] [Google Scholar]
- Mutoh M, Lung F-DT, Long Y-Q, Roller PP, Sikorski RS, O’connor PM. A p21Waf1/Cip1 carboxyl-terminal peptide exhibited cyclin-dependent kinase-inhibitory activity and cytotoxicity when introduced into human cells. Cancer Res. 1999;59(14):3480–3488. [PubMed] [Google Scholar]
- Muttenthaler M, King GF, Adams DJ, Alewood PF. Trends in peptide drug discovery. Nat Rev Drug Discovery. 2021;20(4):309–325. doi: 10.1038/s41573-020-00135-8. [DOI] [PubMed] [Google Scholar]
- Namivandi-Zangeneh R, Sadrearhami Z, Dutta D, Willcox M, Wong EH, Boyer C. Synergy between synthetic antimicrobial polymer and antibiotics: a promising platform to combat multidrug-resistant bacteria. ACS Infectious Dis. 2019;5(8):1357–1365. doi: 10.1021/acsinfecdis.9b00049. [DOI] [PubMed] [Google Scholar]
- Negahdaripour M, Owji H, Eslami M, Zamani M, Vakili B, Sabetian S, Nezafat N, Ghasemi Y. Selected application of peptide molecules as pharmaceutical agents and in cosmeceuticals. Expert Opin Biol Ther. 2019;19(12):1275–1287. doi: 10.1080/14712598.2019.1652592. [DOI] [PubMed] [Google Scholar]
- Nestle FO, Conrad C. Mechanisms of psoriasis. Drug Discov Today Dis Mech. 2004;1(3):315–319. doi: 10.1016/j.ddmec.2004.11.005. [DOI] [Google Scholar]
- Nicolaou K, Boddy CN, Bräse S, Winssinger N. Chemistry, biology, and medicine of the glycopeptide antibiotics. Angew Chem Int Ed. 1999;38(15):2096–2152. doi: 10.1002/(SICI)1521-3773(19990802)38:15<2096::AID-ANIE2096>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Niemeyer-Van Der Kolk T, Van Der Wall H, Hogendoorn GK, Rijneveld R, Luijten S, Van Alewijk DC, Van Den Munckhof EH, De Kam ML, Feiss GL, Prens EP. Pharmacodynamic effects of topical omiganan in patients with mild to moderate atopic dermatitis in a randomized, placebo-controlled, phase II trial. Clin Transl Sci. 2020;13(5):994–1003. doi: 10.1111/cts.12792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu J, Chu Y, Huang Y-F, Chong Y-S, Jiang Z-H, Mao Z-W, Peng L-H, Gao J-Q. Transdermal gene delivery by functional peptide-conjugated cationic gold nanoparticle reverses the progression and metastasis of cutaneous melanoma. ACS Appl Mater Interfaces. 2017;9(11):9388–9401. doi: 10.1021/acsami.6b16378. [DOI] [PubMed] [Google Scholar]
- Nyfeler R, Keller-Schierlein W. Metabolites of microorganisms. 143. Echinocandin B, a novel polypeptide-antibiotic from Aspergillus nidulans var: echinulatus: isolation and structural components. Helv Chim Acta. 1974;57(8):2459–2477. doi: 10.1002/hlca.19740570818. [DOI] [PubMed] [Google Scholar]
- Padhi A, Sengupta M, Sengupta S, Roehm KH, Sonawane A. Antimicrobial peptides and proteins in mycobacterial therapy: current status and future prospects. Tuberculosis. 2014;94(4):363–373. doi: 10.1016/j.tube.2014.03.011. [DOI] [PubMed] [Google Scholar]
- Pallavi Kapoor HS, Gautam A, Chaudhary K, Kumar R, Raghava GPS. TumorHoPe: a database of tumor homing peptides. PLoS ONE. 2012;7(4):e35187. doi: 10.1371/journal.pone.0035187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan J, Zhang Q, Palen K, Wang L, Qiao L, Johnson B, Sei S, Shoemaker RH, Lubet RA, Wang Y, You M. Potentiation of Kras peptide cancer vaccine by avasimibe, a cholesterol modulator. Biomedicine. 2019;49:72–81. doi: 10.1016/j.ebiom.2019.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park CB, Yi K-S, Matsuzaki K, Kim MS, Kim SC. Structure–activity analysis of buforin II, a histone H2A-derived antimicrobial peptide: the proline hinge is responsible for the cell-penetrating ability of buforin II. Proc Natl Acad Sci. 2000;97(15):8245–8250. doi: 10.1073/pnas.150518097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S, Jackman JA, Cho N-J. Comparing the membrane-interaction profiles of two antiviral peptides: insights into structure–function relationship. Langmuir. 2019;35(30):9934–9943. doi: 10.1021/acs.langmuir.9b01052. [DOI] [PubMed] [Google Scholar]
- Patel SG, Sayers EJ, He L, Narayan R, Williams TL, Mills EM, Allemann RK, Luk LYP, Jones AT, Tsai Y-H. Cell-penetrating peptide sequence and modification dependent uptake and subcellular distribution of green florescent protein in different cell lines. Sci Rep. 2019;9(1):6298. doi: 10.1038/s41598-019-42456-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patlolla RR, Desai PR, Belay K, Singh MS. Translocation of cell penetrating peptide engrafted nanoparticles across skin layers. Biomaterials. 2010;31(21):5598–5607. doi: 10.1016/j.biomaterials.2010.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patocka J, Nepovimova E, Klimova B, Wu Q, Kuca K. Antimicrobial peptides: amphibian host defense peptides. Curr Med Chem. 2019;26(32):5924–5946. doi: 10.2174/0929867325666180713125314. [DOI] [PubMed] [Google Scholar]
- Pauletti GM, Gangwar S, Knipp GT, Nerurkar MM, Okumu FW, Tamura K, Siahaan TJ, Borchardt RT. Structural requirements for intestinal absorption of peptide drugs. J Control Release. 1996;41(1–2):3–17. doi: 10.1016/0168-3659(96)01352-1. [DOI] [Google Scholar]
- Pedersen-Bjergaard U, Thorsteinsson B. Reporting severe hypoglycemia in type 1 diabetes: facts and pitfalls. Curr Diab Rep. 2017;17(12):1–11. doi: 10.1007/s11892-017-0965-1. [DOI] [PubMed] [Google Scholar]
- Peek NF, Nell MJ, Brand R, Jansen-Werkhoven T, Van Hoogdalem EJ, Verrijk R, Vonk MJ, Wafelman AR, Valentijn ARP, Frijns JH. Ototopical drops containing a novel antibacterial synthetic peptide: Safety and efficacy in adults with chronic suppurative otitis media. PLoS ONE. 2020;15(4):e0231573. doi: 10.1371/journal.pone.0231573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peptide (2014) Nature education. Retrieved 27th September from https://www.nature.com/scitable/definition/peptide-317/
- Peptides. Clinicaltrials.gov. Retrieved 5th December from https://clinicaltrials.gov/ct2/home
- Peschel A, Otto M, Jack RW, Kalbacher H, Jung G, GöTz F. Inactivation of the dlt operon in Staphylococcus aureus confers sensitivity to defensins, protegrins, and other antimicrobial peptides. J Biol Chem. 1999;274(13):8405–8410. doi: 10.1074/jbc.274.13.8405. [DOI] [PubMed] [Google Scholar]
- Peyressatre M, Prével C, Pellerano M, Morris MC. Targeting cyclin-dependent kinases in human cancers: from small molecules to peptide inhibitors. Cancers (basel) 2015;7(1):179–237. doi: 10.3390/cancers7010179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philchenkov A. Caspases: potential targets for regulating cell death. J Cell Mol Med. 2004;8(4):432–444. doi: 10.1111/j.1582-4934.2004.tb00468.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickart L, Thaler M. Tripeptide in human serum which prolongs survival of normal liver cells and stimulates growth in neoplastic liver. Nat New Biol. 1973;243(124):85–87. [PubMed] [Google Scholar]
- Pirtskhalava M, Amstrong AA, Grigolava M, Chubinidze M, Alimbarashvili E, Vishnepolsky B, Gabrielian A, Rosenthal A, Hurt DE, Tartakovsky M. DBAASP v3: database of antimicrobial/cytotoxic activity and structure of peptides as a resource for development of new therapeutics. Nucleic Acids Res. 2021;49(D1):D288–D297. doi: 10.1093/nar/gkaa991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polt R, Dhanasekaran M, Keyari CM. Glycosylated neuropeptides: a new vista for neuropsychopharmacology? Med Res Rev. 2005;25(5):557–585. doi: 10.1002/med.20039. [DOI] [PubMed] [Google Scholar]
- Pu J, Wang Q, Xu W, Lu L, Jiang S. Development of protein- and peptide-based HIV entry inhibitors targeting gp120 or gp41. Viruses. 2019;11(8):705. doi: 10.3390/v11080705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puente XS, Gutiérrez-Fernández A, Ordóñez GR, Hillier LW, López-Otín C. Comparative genomic analysis of human and chimpanzee proteases. Genomics. 2005;86(6):638–647. doi: 10.1016/j.ygeno.2005.07.009. [DOI] [PubMed] [Google Scholar]
- Purwaha P, Lorenzi PL, Silva LP, Hawke DH, Weinstein JN. Targeted metabolomic analysis of amino acid response to L-asparaginase in adherent cells. Metabolomics. 2014;10(5):909–919. doi: 10.1007/s11306-014-0634-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Räder AFB, Reichart F, Weinmüller M, Kessler H. Improving oral bioavailability of cyclic peptides by N-methylation. Bioorg Med Chem. 2018;26(10):2766–2773. doi: 10.1016/j.bmc.2017.08.031. [DOI] [PubMed] [Google Scholar]
- Rask-Andersen M, Masuram S, Schiöth HB. The druggable genome: evaluation of drug targets in clinical trials suggests major shifts in molecular class and indication. Annu Rev Pharmacol Toxicol. 2014;54:9–26. doi: 10.1146/annurev-pharmtox-011613-135943. [DOI] [PubMed] [Google Scholar]
- Rastogi S, Shukla S, Kalaivani M, Singh GN. Peptide-based therapeutics: quality specifications, regulatory considerations, and prospects. Drug Discovery Today. 2019;24(1):148–162. doi: 10.1016/j.drudis.2018.10.002. [DOI] [PubMed] [Google Scholar]
- Raucher D, Ryu JS. Cell-penetrating peptides: strategies for anticancer treatment. Trends Mol Med. 2015;21(9):560–570. doi: 10.1016/j.molmed.2015.06.005. [DOI] [PubMed] [Google Scholar]
- Regberg J, Srimanee A, Langel Ü. Applications of cell-penetrating peptides for tumor targeting and future cancer therapies. Pharmaceuticals. 2012;5(9):991–1007. doi: 10.3390/ph5090991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinholz M, Ruzicka T, Schauber J. Cathelicidin LL-37: an antimicrobial peptide with a role in inflammatory skin disease. Ann Dermatol. 2012;24(2):126–135. doi: 10.5021/ad.2012.24.2.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers K. What is the difference between a peptide and a protein? Encyclopedia Britannica. Retrieved 27th Spetember from https://www.britannica.com/story/what-is-the-difference-between-a-peptide-and-a-protein.
- RončEvic T, VukičEvic D, Ilic N, Krce L, Gajski G, Tonkic M, Goić-BarišIc I, Zoranic L, Sonavane Y, Benincasa M. Antibacterial activity affected by the conformational flexibility in glycine–lysine based α-helical antimicrobial peptides. J Med Chem. 2018;61(7):2924–2936. doi: 10.1021/acs.jmedchem.7b01831. [DOI] [PubMed] [Google Scholar]
- Saar-Dover R, Bitler A, Nezer R, Shmuel-Galia L, Firon A, Shimoni E, Trieu-Cuot P, Shai Y. D-alanylation of lipoteichoic acids confers resistance to cationic peptides in group B streptococcus by increasing the cell wall density. Expert Rev Mol Med. 2012;10:27. doi: 10.1371/journal.ppat.1002891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachdeva S. Peptides as ‘drugs’: the journey so far. Int J Pept Res Ther. 2017;23(1):49–60. doi: 10.1007/s10989-016-9534-8. [DOI] [Google Scholar]
- Sachdeva S, Joo H, Tsai J, Jasti B, Li X. A rational approach for creating peptides mimicking antibody binding. Sci Rep. 2019;9(1):997. doi: 10.1038/s41598-018-37201-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadowsky JD, Murray JK, Tomita Y, Gellman SH. Exploration of backbone space in foldamers containing α-and β-amino acid residues: developing protease-resistant oligomers that bind tightly to the BH3-recognition cleft of Bcl-xL. ChemBioChem. 2007;8(8):903–916. doi: 10.1002/cbic.200600546. [DOI] [PubMed] [Google Scholar]
- Saeed SI, Mergani A, Aklilu E, Kamaruzzaman NF. Antimicrobial peptides: bringing solution to the rising threats of antimicrobial resistance in livestock. Front Vet Sci. 2022;9:15. doi: 10.3389/fvets.2022.851052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauna ZE (2020) Immunogenicity of protein-based therapeutics. U.S. Food and Drug Administration. Retrieved 6 November from https://www.fda.gov/vaccines-blood-biologics/biologics-research-projects/immunogenicity-protein-based-therapeutics
- Scheuch G, Siekmeier R. Novel approaches to enhance pulmonary. J Physiol Pharmacol. 2007;58(5):615–625. [PubMed] [Google Scholar]
- Schiffter H. The delivery of drugs–peptides and proteins. Curr Pharm Des. 2011;13(1):99–117. doi: 10.2174/138161207779313795. [DOI] [PubMed] [Google Scholar]
- Semalty A, Semalty M, Singh R, Saraf S, Saraf S. Properties and formulation of oral drug delivery systems of protein and peptides. Indian J Pharm Sci. 2007;69(6):741. doi: 10.4103/0250-474X.39426. [DOI] [Google Scholar]
- Serrill JD, Wan X, Hau AM, Jang HS, Coleman DJ, Indra AK, Alani AW, Mcphail KL, Ishmael JE. Coibamide A, a natural lariat depsipeptide, inhibits VEGFA/VEGFR2 expression and suppresses tumor growth in glioblastoma xenografts. Invest New Drugs. 2016;34(1):24–40. doi: 10.1007/s10637-015-0303-x. [DOI] [PubMed] [Google Scholar]
- Shah SS, Casanova N, Antuono G, Sabatino D. Polyamide backbone modified cell targeting and penetrating peptides in cancer detection and treatment. Front Chem. 2020;8:218–218. doi: 10.3389/fchem.2020.00218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman MA, Low M. Recent clinical techniques, results, and research in wounds. Berlin: Springer International Publishing; 2018. [Google Scholar]
- Shojaei AH. Buccal mucosa as a route for systemic drug delivery: a review. J Pharm Pharm Sci. 1998;1(1):15–30. [PubMed] [Google Scholar]
- Shoombuatong W, Schaduangrat N, Nantasenamat C. Unraveling the bioactivity of anticancer peptides as deduced from machine learning. Excli J. 2018;17:734. doi: 10.17179/excli2018-1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short Bowel Syndrome Market—Global Industry Analysis, Size, Share, Trends, Revenue, Forecast 2020 to 2027 (2021) https://www.mynewsdesk.com/us/medical-technology-news/pressreleases/short-bowel-syndrome-market-global-industry-analysis-size-share-trends-revenue-forecast-2020-to-2027-3069433
- Shrivastava A, Khan AA, Khurshid M, Kalam MA, Jain SK, Singhal PK. Recent developments in l-asparaginase discovery and its potential as anticancer agent. Crit Rev Oncol Hematol. 2016;100:1–10. doi: 10.1016/j.critrevonc.2015.01.002. [DOI] [PubMed] [Google Scholar]
- Singh S, Singh H, Tuknait A, Chaudhary K, Singh B, Kumaran S, Raghava GP. PEPstrMOD: structure prediction of peptides containing natural, non-natural and modified residues. Biol Direct. 2015;10(1):1–19. doi: 10.1186/s13062-015-0103-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smola M, Vandamme T, Sokolowski A. Nanocarriers as pulmonary drug delivery systems to treat and to diagnose respiratory and non respiratory diseases. Int J Nanomed. 2008;3(1):1. [PMC free article] [PubMed] [Google Scholar]
- Soudy R, Kimura R, Patel A, Fu W, Kaur K, Westaway D, Yang J, Jhamandas J. Short amylin receptor antagonist peptides improve memory deficits in Alzheimer’s disease mouse model. Sci Rep. 2019;9(1):10942. doi: 10.1038/s41598-019-47255-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souery WN, Bishop CJ. Clinically advancing and promising polymer-based therapeutics. Acta Biomater. 2018;67:1–20. doi: 10.1016/j.actbio.2017.11.044. [DOI] [PubMed] [Google Scholar]
- Spohn R, Daruka L, Lázár V, Martins A, Vidovics F, Grézal G, Méhi O, Kintses B, Számel M, Jangir PK, Csörgő B, Györkei Á, Bódi Z, Faragó A, Bodai L, Földesi I, Kata D, Maróti G, Pap B, Wirth R, Papp B, Pál C. Integrated evolutionary analysis reveals antimicrobial peptides with limited resistance. Nat Commun. 2019;10(1):4538. doi: 10.1038/s41467-019-12364-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piotto SP, Sessa L, Concilio S, Iannelli P. YADAMP: yet another database of antimicrobial peptides. Int J Antimicrob Agents. 2012;39(4):346–351. doi: 10.1016/j.ijantimicag.2011.12.003. [DOI] [PubMed] [Google Scholar]
- Suk JS, Xu Q, Kim N, Hanes J, Ensign LM. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv Drug Deliv Rev. 2016;99(Pt A):28–51. doi: 10.1016/j.addr.2015.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L. Peptide-based drug development. Mod Chem Appl. 2013;1(1):1–2. doi: 10.4172/2329-6798.1000e103. [DOI] [Google Scholar]
- Suryadinata R, Sadowski M, Sarcevic B. Control of cell cycle progression by phosphorylation of cyclin-dependent kinase (CDK) substrates. Biosci Rep. 2010;30(4):243–255. doi: 10.1042/BSR20090171. [DOI] [PubMed] [Google Scholar]
- Tesauro D, Accardo A, Diaferia C, Milano V, Guillon J, Ronga L, Rossi F. Peptide-based drug-delivery systems in biotechnological applications: recent advances and perspectives. Molecules. 2019;24(2):1–8. doi: 10.3390/molecules24020351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teva Reports Third Quarter 2021 Financial Results. Businesswire. Retrieved 6th December from https://www.businesswire.com/news/home/20211027005516/en/Teva-Reports-Third-Quarter-2021-Financial-Results
- Thundimadathil J (2012) Cancer treatment using peptides: current therapies and future prospects. J Amino Acids, 2012. [DOI] [PMC free article] [PubMed]
- Timmons PB, Hewage CM. HAPPENN is a novel tool for hemolytic activity prediction for therapeutic peptides which employs neural networks. Sci Rep. 2020;10(1):1–18. doi: 10.1038/s41598-020-67701-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tkachenko AG, Xie H, Coleman D, Glomm W, Ryan J, Anderson MF, Franzen S, Feldheim DL. Multifunctional gold nanoparticle− peptide complexes for nuclear targeting. J Am Chem Soc. 2003;125(16):4700–4701. doi: 10.1021/ja0296935. [DOI] [PubMed] [Google Scholar]
- Tomoda K, Ohkoshi T, Nakajima T, Makino K. Preparation and properties of inhalable nanocomposite particles: effects of the size, weight ratio of the primary nanoparticles in nanocomposite particles and temperature at a spray-dryer inlet upon properties of nanocomposite particles. Colloids Surf B. 2008;64(1):70–76. doi: 10.1016/j.colsurfb.2008.01.016. [DOI] [PubMed] [Google Scholar]
- Tótoli EG, Garg S, Salgado HRN. Daptomycin: physicochemical, analytical, and pharmacological properties. Ther Drug Monit. 2015;37(6):699–710. doi: 10.1097/FTD.0000000000000222. [DOI] [PubMed] [Google Scholar]
- Trier N, Hansen P, Houen G. Peptides, antibodies, peptide antibodies and more. Int J Mol Sci. 2019;20(24):6289. doi: 10.3390/ijms20246289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhrich KE, Abdelhamid D. 3 - Biodegradable and bioerodible polymers for medical applications. In: Poole-Warren L, Martens P, Green R, editors. Biosynthetic polymers for medical applications. Sawston: Woodhead Publishing; 2016. pp. 63–83. [Google Scholar]
- Urbańczyk M, Jewgiński M, Krzciuk-Gula J, Góra J, Latajka R, Sewald N. Synthesis and conformational preferences of short analogues of antifreeze glycopeptides (AFGP) Beilstein J Org Chem. 2019;15(1):1581–1591. doi: 10.3762/bjoc.15.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urist MR. Bone morphogenetic protein: the molecularization of skeletal system development. J Bone Miner Res. 1997;12(3):343–346. doi: 10.1359/jbmr.1997.12.3.343. [DOI] [PubMed] [Google Scholar]
- Usmani SS, Bedi G, Samuel JS, Singh S, Kalra S, Kumar P, Ahuja AA, Sharma M, Gautam A, Raghava GP. THPdb: Database of FDA-approved peptide and protein therapeutics. PLoS ONE. 2017;12(7):e0181748. doi: 10.1371/journal.pone.0181748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valvano MA. Chapter 4 - genetics and biosynthesis of lipopolysaccharide. In: Tang Y-W, Sussman M, Liu D, Poxton I, Schwartzman J, editors. Molecular Medical Microbiology. 2. Boston: Academic Press; 2015. pp. 55–89. [Google Scholar]
- Varamini P, Mansfeld FM, Blanchfield JT, Wyse BD, Smith MT, Toth I. Synthesis and biological evaluation of an orally active glycosylated endomorphin-1. J Med Chem. 2012;55(12):5859–5867. doi: 10.1021/jm300418d. [DOI] [PubMed] [Google Scholar]
- Vargason AM, Anselmo AC, Mitragotri S. The evolution of commercial drug delivery technologies. Nature Biomedical Engineering. 2021;5(9):951–967. doi: 10.1038/s41551-021-00698-w. [DOI] [PubMed] [Google Scholar]
- Venkatasubramanian S, Griffiths ME, Mclean SG, Miller MR, Luo R, Lang NN, Newby DE. Vascular effects of urocortins 2 and 3 in healthy volunteers. J Am Heart Assoc. 2013;2(1):e004267. doi: 10.1161/JAHA.112.004267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veronese FM, Mero A. The impact of PEGylation on biological therapies. BioDrugs. 2008;22(5):315–329. doi: 10.2165/00063030-200822050-00004. [DOI] [PubMed] [Google Scholar]
- Vijayakumar S, Lakshmi P. ACPP: A web server for prediction and design of anti-cancer peptides. Int J Pept Res Ther. 2015;21(1):99–106. doi: 10.1007/s10989-014-9435-7. [DOI] [Google Scholar]
- Vlieghe P, Lisowski V, Martinez J, Khrestchatisky M. Synthetic therapeutic peptides: science and market. Drug Discovery Today. 2010;15(1–2):40–56. doi: 10.1016/j.drudis.2009.10.009. [DOI] [PubMed] [Google Scholar]
- Von Recum HA, Pokorski JK. Peptide and protein-based inhibitors of HIV-1 co-receptors. Exp Biol Med (maywood) 2013;238(5):442–449. doi: 10.1177/1535370213480696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waghu FH, Gopi L, Barai RS, Ramteke P, Nizami B, Idicula-Thomas S. CAMP: collection of sequences and structures of antimicrobial peptides. Nucleic Acids Res. 2014;42(D1):D1154–D1158. doi: 10.1093/nar/gkt1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner AM, Gran MP, Peppas NA. Designing the new generation of intelligent biocompatible carriers for protein and peptide delivery. Acta Pharm Sin B. 2018;8(2):147–164. doi: 10.1016/j.apsb.2018.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Ai L, Zhang Y, Cheng J, Yu H, Li C, Zhang D, Pan Y, Lin L (2018) The effects of antimicrobial peptide Nal-P-113 on inhibiting periodontal pathogens and improving periodontal status. BioMed Res Int 2018. [DOI] [PMC free article] [PubMed]
- Warnke PH, Voss E, Russo PA, Stephens S, Kleine M, Terheyden H, Liu Q. Antimicrobial peptide coating of dental implants: biocompatibility assessment of recombinant human beta defensin-2 for human cells. Int J Oral Maxillofac Implants. 2013;28(4):78. doi: 10.11607/jomi.2594. [DOI] [PubMed] [Google Scholar]
- Werle M, Bernkop-Schnürch A. Strategies to improve plasma half life time of peptide and protein drugs. Amino Acids. 2006;30(4):351–367. doi: 10.1007/s00726-005-0289-3. [DOI] [PubMed] [Google Scholar]
- Werner HM, Cabalteja CC, Horne WS. Peptide backbone composition and protease susceptibility: impact of modification type, position, and tandem substitution. ChemBioChem. 2016;17(8):712. doi: 10.1002/cbic.201500312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetzler, M, Hamilton, P. (2018). Peptides as therapeutics. In: Peptide applications in biomedicine, biotechnology and bioengineering. Elsevier, pp 215–230
- White CJ, Yudin AK. Contemporary strategies for peptide macrocyclization. Nat Chem. 2011;3(7):509–524. doi: 10.1038/nchem.1062. [DOI] [PubMed] [Google Scholar]
- Wickham TJ, Mathias P, Cheresh DA, Nemerow GR. Integrins αvβ3 and αvβ5 promote adenovirus internalization but not virus attachment. Cell. 1993;73(2):309–319. doi: 10.1016/0092-8674(93)90231-E. [DOI] [PubMed] [Google Scholar]
- Wildemann D, Schiene-Fischer C, Aumüller T, Bachmann A, Kiefhaber T, Lücke C, Fischer G. A nearly isosteric photosensitive amide-backbone substitution allows enzyme activity switching in Ribonuclease S. J Am Chem Soc. 2007;129(16):4910–4918. doi: 10.1021/ja069048o. [DOI] [PubMed] [Google Scholar]
- Win TS, Malik AA, Prachayasittikul V, Wikberg S, Nantasenamat JEC, Shoombuatong W. HemoPred: a web server for predicting the hemolytic activity of peptides. Future Med Chem. 2017;9(3):275–291. doi: 10.4155/fmc-2016-0188. [DOI] [PubMed] [Google Scholar]
- Wisniewski K, Galyean R, Tariga H, Alagarsamy S, Croston G, Heitzmann J, Kohan A, Wisniewska H, Laporte R, Riviere PJ. New, potent, selective, and short-acting peptidic V1a receptor agonists. J Med Chem. 2011;54(13):4388–4398. doi: 10.1021/jm200278m. [DOI] [PubMed] [Google Scholar]
- Witczak ZJ (2006) Carbohydrate therapeutics: new developments and strategies.
- Wodlej C, Riedl S, Rinner B, Leber R, Drechsler C, Voelker DR, Choi J-Y, Lohner K, Zweytick D. Interaction of two antitumor peptides with membrane lipids–Influence of phosphatidylserine and cholesterol on specificity for melanoma cells. PLoS ONE. 2019;14(1):e0211187. doi: 10.1371/journal.pone.0211187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao J, Burn A, Tolbert TJ. Increasing solubility of proteins and peptides by site-specific modification with betaine. Bioconjug Chem. 2008;19(6):1113–1118. doi: 10.1021/bc800063k. [DOI] [PubMed] [Google Scholar]
- Xie J, Chen K, Lee H-Y, Xu C, Hsu AR, Peng S, Chen X, Sun S. Ultrasmall c (RGDyK)-coated Fe3O4 nanoparticles and their specific targeting to integrin αvβ3-rich tumor cells. J Am Chem Soc. 2008;130(24):7542–7543. doi: 10.1021/ja802003h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong X-B, Huang Y, Wan-Liang L, Zhang X, Zhang H, Nagai T, Zhang Q. Intracellular delivery of doxorubicin with RGD-modified sterically stabilized liposomes for an improved antitumor efficacy: in vitro and in vivo. J Pharm Sci. 2005;94(8):1782–1793. doi: 10.1002/jps.20397. [DOI] [PubMed] [Google Scholar]
- Yadav A, Pandey D, Ashraf GM. Peptide based therapy for neurological disorders. Curr Protein Pept Sci. 2021;22(9):656–665. doi: 10.2174/1389203722666210920151810. [DOI] [PubMed] [Google Scholar]
- Yamaguchi Y, Yamamoto K, Sato Y, Inoue S, Morinaga T, Hirano E. Combination of aspartic acid and glutamic acid inhibits tumor cell proliferation. Biomed Res. 2016;37(2):153–159. doi: 10.2220/biomedres.37.153. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Nair P, Jacobsen NE, Vagner J, Kulkarni V, Davis P, Ma S-W, Navratilova E, Yamamura HI, Vanderah TW. Improving metabolic stability by glycosylation: bifunctional peptide derivatives that are opioid receptor agonists and neurokinin 1 receptor antagonists. J Med Chem. 2009;52(16):5164–5175. doi: 10.1021/jm900473p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan J, Bhadra P, Li A, Sethiya P, Qin L, Tai HK, Wong KH, Siu SW. Deep-AmPEP30: improve short antimicrobial peptides prediction with deep learning. Mol Therapy-Nucleic Acids. 2020;20:882–894. doi: 10.1016/j.omtn.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, An H-W, Wang H. Self-assembled peptide drug delivery systems. ACS Appl Bio Mater. 2021;4(1):24–46. doi: 10.1021/acsabm.0c00707. [DOI] [PubMed] [Google Scholar]
- Yao H, Wang K, Wang Y, Wang S, Li J, Lou J, Ye L, Yan X, Lu W, Huang R. Enhanced blood–brain barrier penetration and glioma therapy mediated by a new peptide modified gene delivery system. Biomaterials. 2015;37:345–352. doi: 10.1016/j.biomaterials.2014.10.034. [DOI] [PubMed] [Google Scholar]
- Ye G, Wu H, Huang J, Wang W, Ge K, Li G, Zhong J, Huang Q (2020) LAMP2: a major update of the database linking antimicrobial peptides. Database, 2020. [DOI] [PMC free article] [PubMed]
- Yu K, Lo JC, Yan M, Yang X, Brooks DE, Hancock RE, Lange D, Kizhakkedathu JN. Anti-adhesive antimicrobial peptide coating prevents catheter associated infection in a mouse urinary infection model. Biomaterials. 2017;116:69–81. doi: 10.1016/j.biomaterials.2016.11.047. [DOI] [PubMed] [Google Scholar]
- Zane D, Feldman PL, Sawyer T, Sobol Z, Hawes J. Development and regulatory challenges for peptide therapeutics. Int J Toxicol. 2021;40(2):108–124. doi: 10.1177/1091581820977846. [DOI] [PubMed] [Google Scholar]
- Zhang C, Yang M. Antimicrobial peptides: from design to clinical application. Antibiotics (basel) 2022;11(3):15. doi: 10.3390/antibiotics11030349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Bulaj G. Converting peptides into drug leads by lipidation. Curr Med Chem. 2012;19(11):1602–1618. doi: 10.2174/092986712799945003. [DOI] [PubMed] [Google Scholar]
- Zhang L, Falla TJ. Cosmeceuticals and peptides. Clin Dermatol. 2009;27(5):485–494. doi: 10.1016/j.clindermatol.2009.05.013. [DOI] [PubMed] [Google Scholar]
- Zhang Q-Y, Yan Z-B, Meng Y-M, Hong X-Y, Shao G, Ma J-J, Cheng X-R, Liu J, Kang J, Fu C-Y. Antimicrobial peptides: mechanism of action, activity and clinical potential. Mil Med Res. 2021;8(1):48. doi: 10.1186/s40779-021-00343-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Luo L, Sun X, Ma A. Bioactive peptides: a promising alternative to chemical preservatives for food preservation. J Agric Food Chem. 2021;69(42):12369–12384. doi: 10.1021/acs.jafc.1c04020. [DOI] [PubMed] [Google Scholar]
- Zheng Y, Mao K, Chen S, Zhu H. Chirality effects in peptide assembly structures. Front Bioeng Biotechnol. 2021;9:517. doi: 10.3389/fbioe.2021.703004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Q, Chen Z, Paul PK, Lu Y, Wu W, Qi J. Oral delivery of proteins and peptides: challenges, status quo and future perspectives. Acta Pharm Sin B. 2021;11(8):2416–2448. doi: 10.1016/j.apsb.2021.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou L, Ding W, Zhang Y, Cheng S, Li F, Ruan R, Wei P, Qiu B. Peptide-modified vemurafenib-loaded liposomes for targeted inhibition of melanoma via the skin. Biomaterials. 2018;182:1–12. doi: 10.1016/j.biomaterials.2018.08.013. [DOI] [PubMed] [Google Scholar]