Abstract
The abscopal effect is a rare phenomenon, in which tumor shrinkage in the nonirradiated metastatic region is observed after radiotherapy. Certainly, this response is sometimes reported with the combined use of immune-checkpoint inhibitors, but a pure abscopal effect is extremely rare, especially in endometrial cancer. We present the case of a 79-year-old woman with an advanced endometrial carcinosarcoma. She was treated with surgical reduction of the primary lesion, followed by radiotherapy of the metastatic regional lymph nodes. Distant metastases were detected in radiological imaging test 2 months after the completion of radiotherapy, and we carefully followed up without any treatment considering the patient's tolerability for further procedures. Six months after recurrence, she experienced cytoreduction in the metastatic lesions confirmed through imaging findings, which was believed to be an abscopal effect, and maintained this shrinking state for 15 months. Herein, we describe this pure abscopal effect from the perspective of imaging, pathological and molecular findings, and therapeutic strategies.
Keywords: Abscopal effect, Endometrial cancer, Carcinosarcoma
Introduction
The abscopal effect was first described by RH Mole in 1953, in which shrinkage of nonirradiated metastatic tumors was observed following radiotherapy (RT) [1]. RT has long been used for the treatment of gynecological malignancies, especially uterine cancer. In terms of uterine neoplasms, external beam RT (EBRT) is commonly used and directed to sites of known or suspected tumor involvement, generally to the pelvis, with or without the paraaortic region [2]. Historically, RT-induced tumor cell death has been considered to be a local effect of direct or indirect DNA damage in the form of single-strand breaks or double-strand breaks [3]. However, RT-induced immunostimulatory activity has been recognized, and the detailed mechanism has been gradually elucidated in recent years. Given the compatibility between RT and the immune system, RT and immune checkpoint inhibitor (ICI) combination therapy has been increasingly highlighted [4]. In endometrial cancer (EC), which is the most common gynecological malignancy worldwide, some reports have described the abscopal effect in the ICI combination setting, but reports of pure abscopal effect are extremely rare [5], [6], [7]. Herein, we report a case of uterine carcinosarcoma with pure abscopal effect.
Case report
A 79-year-old parous woman with a history of atypical vaginal bleeding was referred to our hospital for endometrial cancer. The patient had a medical history of hypertension, hyperlipidemia, and hyperuricemia. Pathological examination of endometrial tissue revealed an undifferentiated carcinoma. Blood analysis showed elevated levels of tumor markers, including CA19-9 107.0 U/mL (<40 U/mL) and CA125 473.0 U/mL (<55 U/mL). Contrast-enhanced magnetic resonance imaging showed an enlarged uterine intrauterine cavity containing a large tumor mass hyposignal on T2-weighted images (Fig.1). The junctional zone at the uterine fundus was disrupted, and myometrial invasion >50% was strongly suspected (Fig. 1). Pelvic lymph node enlargement was also observed. 18F-fluorodeoxyglucose positron emission tomography/computed tomography (FDG-PET/CT) was performed to identify any metastatic lesions, which revealed intense FDG uptake in the uterine mass (SUVmax 26.4), as well as in multiple lymph nodes, including paraaortic, bilateral iliac, left internal iliac, and left obturator nodes (Fig. 2). The patient's performance status was 2. Considering the patient's tolerability for treatment, we determined that total hysterectomy and bilateral salpingo-oophorectomy followed by radiation for the metastatic lesions were the most suitable. The postoperative histopathological result indicated carcinosarcoma of the uterine corpus (Figs. 3A and B). The patient subsequently underwent RT; conventional treatment fields were used to deliver a 50.4 Gy/ 28 fraction (fr) to the whole pelvis and 39.6 Gy/ 22 fr to the paraaortic area. The additional boost dose to the positive lymph nodes was 10 Gy/5 fr to the pelvic nodes and 20 Gy/10 fr to the paraaortic nodes. Two months after the completion of RT, recurrence was confirmed using follow-up FDG-PET/CT in distant nodes, including the mediastinal and left supraclavicular nodes, and a favorable response to the irradiated region was shown (Figs. 4A and B). RT could not be applied to newly metastatic lesions due to their extensive spread. The patient had no symptoms; therefore, we decided to closely follow-up until she developed any associated symptoms. Three months after the diagnosis of recurrence, follow-up FDG-PET/CT showed further increased FDG uptake in the metastatic lesions (SUV max 12.56 in the left supraclavicular nodes). The patient remained asymptomatic; therefore, no further action was taken. Three months later, follow-up computed tomography demonstrated tumor shrinkage in all metastatic nodes, and the serum levels of the tumor markers returned to normal (Fig. 4C). We recognized that the patient experienced an abscopal effect. Disease progression was confirmed using diagnostic imaging 15 months after the appearance of the abscopal effect; however, the patient remained free of disease-related symptoms.
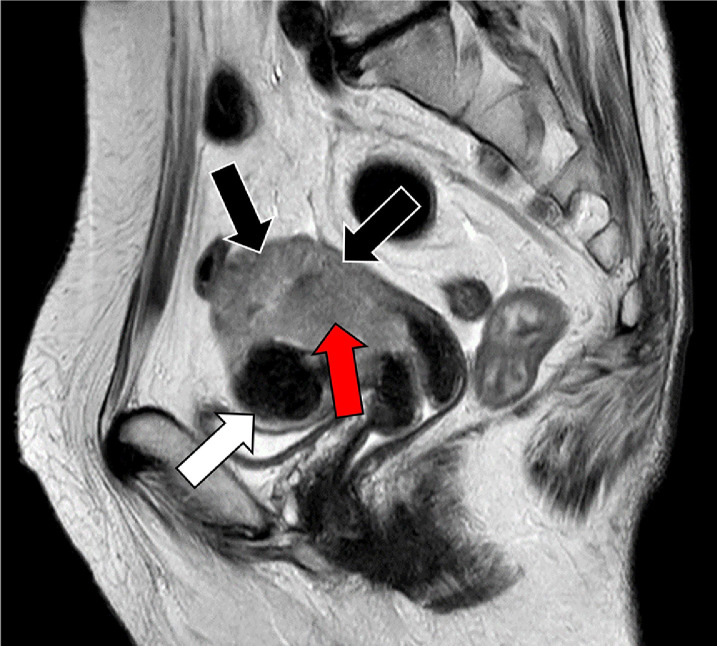
Fig. 1.
Pelvic magnetic resonance imaging, sagittal T2-weighted image. Intrauterine cavity contains large amount of hyposignal tumor mass (red arrow), and deep myometrial invasion is strongly suspected (black arrows). Benign uterine fibroid is seen in the anterior uterine wall (white arrow).
Fig. 2.
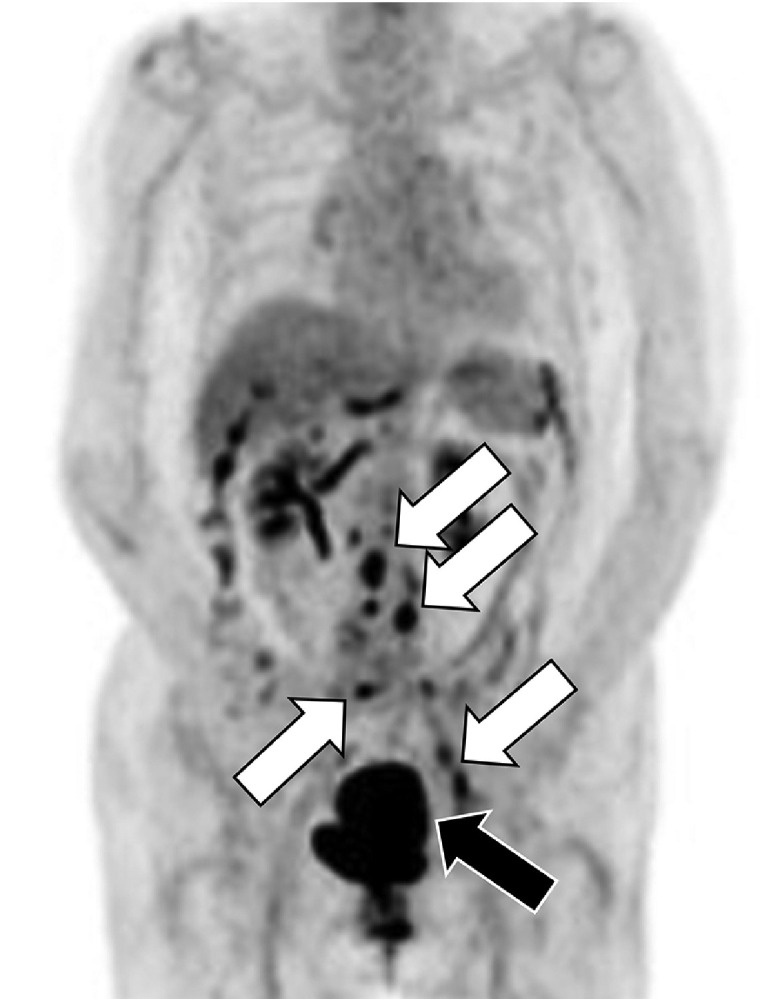
18F-fluorodeoxyglucose positron emission tomography/computed tomography (FDG-PET/CT). Along with primary lesion, increased FDG uptake is seen in multiple regional lymph nodes both in pelvic and paraaortic lesions (arrows). Distant metastasis is not seen.
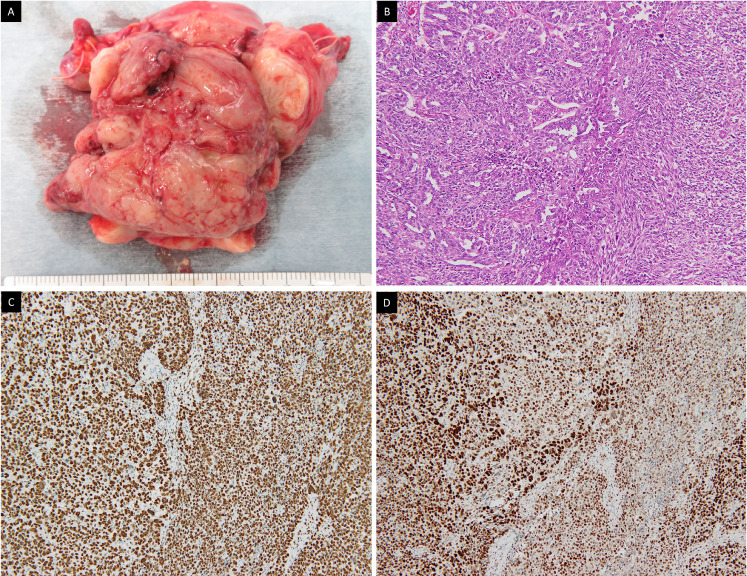
Fig. 3.
Histopathological findings. (A) Excised specimen. (B) Hematoxylin-eosin staining demonstrating carcinomatous (left side) and sarcomatous (right side) component regarded as carcinosarcoma. Immunostaining of MSH6 (C) and PMS2 (D) showing the expression of those MMR proteins regarded as MMR proficient.
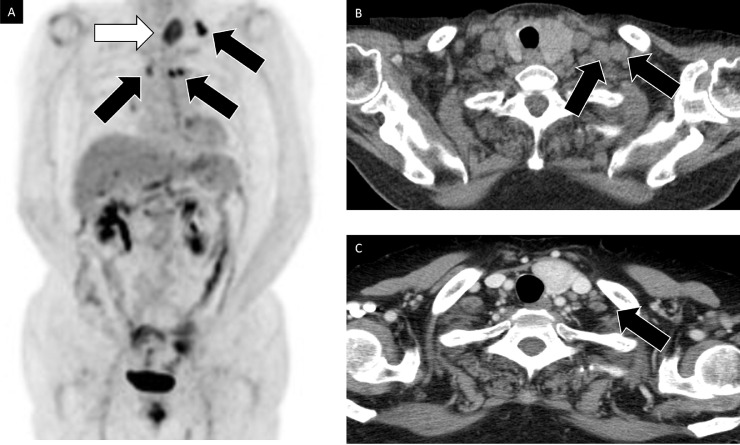
Fig. 4.
Imaging findings of 18F-fluorodeoxyglucose positron emission tomography/computed tomography (FDG-PET/CT) and enhanced computed tomography. (A) Increased FDG uptake in mediastinal node and left supraclavicular node (arrows). No FDG uptake in regional retroperitoneum lymph nodes. Incidentally found benign adenomatoid goiter (white arrow). (B) Enlarged supraclavicular nodes (arrows). (C) Tumor shrinkage in supraclavicular nodes (arrow).
Discussion
Recently, the abscopal effect has gained recognition owing to the accumulation of clinical experience. As its anticancer effect is driven by RT-evoking systemic immune responses, combination therapy with ICIs, which enhances immunity, has been increasingly highlighted [4]. Certainly, there are multiple case reports of the abscopal effect in some types of cancers, such as melanoma or renal cell carcinoma, where ICIs are regarded as the first-line therapy, but there are only a few reports on the subject of EC [3,[6], [7], [8].
Among ECs, carcinosarcomas classified as high-grade carcinomas are rare but aggressive tumors, leading to poor prognosis [9]. To our knowledge, this is the first report to describe the pure abscopal effect in a patient with uterine carcinosarcoma.
Although established predictive biomarkers of the abscopal effect have not been identified, some potential candidates have been proposed.
First, RT dose and fractionation schemes might influence the emergence of the abscopal effect [4]. One clinical review suggested that a high-dose per fractionation RT regimen could induce an enhanced immune response without depleting the recruited immune cells, while the conventional RT scheme was negative for immunostimulant activities [10]. Schaue et al. [11] reported that medium-size RT doses of 7.5 Gy/fr provided the best antitumor immunity. However, the above studies that examined the optimal RT dosing or fractionation regimen to induce the abscopal effect involved treatments other than EC. Moreover, according to the NCCN or ESGO/ESTRO/ESP guidelines, doses of 45-50.4 Gy/5-6 weeks are typically used in the setting of EBRT for EC, and high-dose-rate regimens are applied only for brachytherapy [2,12]. Under these circumstances, high-dose fractionated RT therapy cannot be easily applied in the treatment of EC. In the present case, the conventional fraction scheme was indicated without combination with ICI, and metastatic tumor shrinkage was recognized as an abscopal effect. High-dose fractionation RT and concomitant treatment with ICI might not be indispensable for the development of the abscopal effect in the treatment course of EC.
Second, the molecular biological status of tumors could play another role in producing the abscopal effect. Irradiated tumor cells release tumor antigens along with cell death, and immune cells recognize these neoantigens [4]. Immune cells activated by perceiving neoantigens migrate to distant tumor sites and elicit an abscopal effect [4]. Given this feature, highly immunogenic tumors are more likely to have an abscopal effect. In EC, mismatch repair (MMR) status is one of the most notable characteristics that could boost the immune system, as MMR-deficient (dMMR) tumors are associated with increased genetic mutations and consequently produce more tumor-specific antigens [13,14]. In general, 20%-40% of the ECs demonstrate dMMR, while carcinosarcoma has a low frequency of dMMR reported as 10.5% [14,15].
In the current case, MMR status was confirmed as MMR-proficient using immunostaining for MMR proteins, including PMS2 and MSH6 (Figs. 3C and D). The dMMR status may not necessarily be required to provoke an abscopal effect. However, from the aspect of exposure to neoantigens, regardless of their immunogenicity, surgery itself could introduce systemic exposure of neoantigens, allowing immune cells to recognize them [3]. In this case, although a high immunogenic status was not detected, surgery might have had a positive impact on the abscopal effect.
Third, the local response of RT to the irradiated field might be a potential predictive factor of the abscopal effect. Some studies have demonstrated a significant correlation between responses detected in radiated and non-radiated fields [16,17]. In this case, at the time of distant metastases observed on follow-up FDG-PET/CT, a favorable response was observed at the radiation sites.
The median time to the development of the abscopal effect and its duration were reported 2 (0-24 months) and 6 months (0.7-14 months), respectively [8]. In this case, we judged the patient's background in a comprehensive manner after recurrence was observed and decided to follow closely without therapy. As the patient experienced an abscopal effect and maintained its phenomenon for a relatively long period, our decision turned out to be the best option. However, we should not follow patients with relapse without any treatment, expecting this unpredictable effect in normal clinical practice.
In conclusion, we report the first case of pure abscopal effect in a patient with advanced uterine carcinosarcoma. We herein provide useful information that high-dose per-fractionation RT, combined use of ICI treatment, and high immunogenic (dMMR) status are not always necessary for the emergence of the abscopal effect. This phenomenon can occur and produce its effect even in carcinosarcoma.
Patient consent
I confirm that a written informed consent for publication of this case was obtained from the involved patient.
Footnotes
Competing Interests: The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Mole RH. Whole body irradiation; radiobiology or medicine? Br J Radiol. 1953;26:234–241. doi: 10.1259/0007-1285-26-305-234. [DOI] [PubMed] [Google Scholar]
- 2.Abu-Rustum NR, Yashar CM, Bradley K, Campos SM, Chino J, Chon HS, et al.. NCCN Guidelines® insights: uterine neoplasms, Version 3.2021. J Natl Compr Cancer Netw. 2021;19:888–95. https://doi.org/10.6004/jnccn.2021.0038. [DOI] [PubMed]
- 3.Craig DJ, Nanavaty NS, Devanaboyina M, Stanbery L, Hamouda D, Edelman G, et al. The abscopal effect of radiation therapy. Future Oncol. 2021;17:1683–1694. doi: 10.2217/fon-2020-0994. [DOI] [PubMed] [Google Scholar]
- 4.Liu Y, Dong Y, Kong L, Shi F, Zhu H, Yu J. Abscopal effect of radiotherapy combined with immune checkpoint inhibitors. J Hematol Oncol. 2018;11:104. doi: 10.1186/s13045-018-0647-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023;73:17–48. doi: 10.3322/caac.21763. [DOI] [PubMed] [Google Scholar]
- 6.Oh MS, Chae YK. Repeated abscopal effect with radiotherapy and programmed death 1 blockade in mismatch repair-deficient endometrial cancer. JCO Precis Oncol. 2018;2:1–8. doi: 10.1200/PO.18.00085. [DOI] [PubMed] [Google Scholar]
- 7.Tomita N, Ogawa S, Aikawa G. Abscopal effect of pelvic intensity modulated radiation therapy on lung metastases in a patient with recurrent endometrial cancer. Adv Radiat Oncol. 2020;22 doi: 10.1016/j.adro.2020.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abuodeh Y, Venkat P, Kim S. Systematic review of case reports on the abscopal effect. Curr Probl Cancer. 2016;40:25–37. doi: 10.1016/j.currproblcancer.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 9.Matsuzaki S, Klar M, Matsuzaki S, Roman LD, Sood AK, Matsuo K. Uterine carcinosarcoma: contemporary clinical summary, molecular updates, and future research opportunity. Gynecol Oncol. 2021;160:586–601. doi: 10.1016/j.ygyno.2020.10.043. [DOI] [PubMed] [Google Scholar]
- 10.Siva S, MacManus MP, Martin RF, Martin OA. Abscopal effects of radiation therapy: a clinical review for the radiobiologist. Cancer Lett. 2015;356:82–90. doi: 10.1016/j.canlet.2013.09.018. [DOI] [PubMed] [Google Scholar]
- 11.Schaue D, Ratikan JA, Iwamoto KS, McBride WH. Maximizing tumor immunity with fractionated radiation. Int J Radiat Oncol Biol Phys. 2012;83:1306–1310. doi: 10.1016/j.ijrobp.2011.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Concin N, Matias-Guiu X, Vergote I, Cibula D, Mirza MR, Marnitz S, et al. ESGO/ESTRO/ESP guidelines for the management of patients with endometrial carcinoma. Int J Gynecol Cancer. 2021;31:12–39. doi: 10.1136/ijgc-2020-002230. [DOI] [PubMed] [Google Scholar]
- 13.Cancer Genome Atlas Research Network. Kandoth C, Schultz N, Cherniack AD, Akbani R, Liu Y, et al. Integrated genomic characterization of endometrial carcinoma. Nature. 2013;497:67–73. doi: 10.1038/nature12113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Okamoto K, Nakamura K, Haraga J, Masuyama H. Molecular characteristics of metastatic lesions have superior prognostic impact on endometrial cancer. Anticancer Res. 2022;42:4535–4543. doi: 10.21873/anticanres.15956. [DOI] [PubMed] [Google Scholar]
- 15.Saijo M, Nakamura K, Ida N, Nasu A, Yoshino T, Masuyama H, et al. Histologic appearance and immunohistochemistry of DNA mismatch repair protein and p53 in endometrial carcinosarcoma: impact on prognosis and insights into tumorigenesis. Am J Surg Pathol. 2019;43:1493–1500. doi: 10.1097/PAS.0000000000001353. [DOI] [PubMed] [Google Scholar]
- 16.Grimaldi AM, Simeone E, Giannarelli D, Muto P, Falivene S, Borzillo V, et al. Abscopal effects of radiotherapy on advanced melanoma patients who progressed after ipilimumab immunotherapy. Oncoimmunology. 2014;3:e28780. doi: 10.4161/onci.28780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roger A, Finet A, Boru B, Beauchet A, Mazeron JJ, Otzmeguine Y, et al. Efficacy of combined hypo-fractionated radiotherapy and anti-PD-1 monotherapy in difficult-to-treat advanced melanoma patients. Oncoimmunology. 2018;7 doi: 10.1080/2162402X.2018.1442166. [DOI] [PMC free article] [PubMed] [Google Scholar]