Highlights
-
•
Bacterial inhibitory effect of cold plasma (CP) increased with increasing voltage.
-
•
Excessive CP voltage can lead to oxidation of fish proteins and lipids.
-
•
CP did not change the water-holding capacity, pH, and TVB-N of the samples.
-
•
CP voltage should be customized to balance the bactericidal and oxidation effects.
Keywords: Cold plasma, Golden pompano, Muscle quality, Protein structure, Microorganism
Abstract
Cold plasma (CP) is a non-thermal novel technology for the processing of heat-sensitive food products, but there is concern regarding its impact on food quality. Voltage is one of the most direct factors affecting the bacteriostatic effect of CP. Golden pompano (Trachinotus ovatus) was treated with CP at different voltages (10, 20, and 30 kV). The total viable count decreased as the CP voltage increased, reaching a maximum reduction of 1.54 lg CFU/g on golden pompano treated at 30 kV. No effects on water-holding capacity, pH, total volatile base nitrogen, and T2b relaxation time were observed, indicating that all CP treatments retained the freshness and bound water of the samples. However, as the CP voltage increased, peroxide value and thiobarbituric acid-reactive substances of golden pompano gradually increased, the protein tertiary structure unfolded, and α-helices converted to β-sheets, indicating inevitable lipid and protein oxidation caused by excessive CP voltage. Therefore, a suitable voltage of CP should be selected to inhibits the growth of microorganisms, which avoids deterioration of sea-foods quality.
1. Introduction
As a temperate fish with rapid growth, umami taste, and high nutritional value, golden pompano (Trachinotus ovatus) is an important marine commercial fish widely distributed in the southern sea area of China (Xun et al., 2022). However, the high moisture content, tissue fragility, and active endogenous enzymes in gold pompano render the fish susceptible to deterioration by the proliferation of the spoilage microorganisms (Lan et al., 2022, Liao et al., 2018). Therefore, certain measures need to be taken to inhibit the growth of microorganisms and extend the shelf life of golden pompano. Thermal treatment is currently the most effective means of sterilization, but for some thermosensitive foods, high-temperature sterilization is not suitable (Olatunde et al., 2021). For aquatic products, high-temperature sterilization will cause protein denaturation and impact the original flavor, color, texture, and other quality characteristics. Therefore, in the food industry, non-thermal sterilization technologies have gained widespread attention (Laroque et al., 2022).
Cold plasma (CP) is among the emerging non-thermal decontamination technologies for heat-sensitive foods due to its efficiency in activating pathogenic and spoilage microorganisms at ambient or sublethal temperatures and is being extensively investigated for application on raw and processed meat and seafood products (Lin et al., 2020, Nasiru et al., 2021, Olatunde et al., 2021). CP can be described as an ionized gas containing reactive species (such as reactive oxygen species [ROS] and reactive nitrogen species [RNS]), energized ions, charged particles, and ultraviolet (UV) radiation (Misra & Jo, 2017). CP has the advantages of low treatment temperature, no added chemicals, environmental friendliness, and low operating cost (Olatunde et al., 2021). CP shows antimicrobial, and fungicidal effects, prolonging food shelf life and replacing food additives (Laroque et al., 2022, Marcinkowska-Lesiak et al., 2022, Olatunde et al., 2019a). Although CP has good antibacterial activity, there is a possibility of deterioration of food quality, such as lipid and protein oxidation (Albertos et al., 2017, Laroque et al., 2022, Pérez-Andrés et al., 2020), and for this reason, it is necessary to understand and strike a balance between the beneficial effect of CP on microbial safety and quality losses.
In recent years, CP has been reported for the preservation of milk and milk products, fruits and vegetables, and poultry meat (Huang et al., 2019, Laroque et al., 2022, Yu et al., 2020), as well as aquatic products, such as salmon sashimi, herring, hairtail (Albertos et al., 2019, Koddy et al., 2021, Huang et al., 2021). Aquatic products, especially fish, are exceptional sources of high-quality fats, proteins, minerals, etc (Olatunde et al., 2021). However, excessive amounts of active substances produced by CP may cause lipid and protein oxidation, leading to deterioration of product quality, which limits the application of CP technology in the aquatic product industry. Protein alteration in CP is primarily triggered by reactions involving ROS and the synergistic effect of RNS (Barbhuiya et al., 2021). This causes oxidative reactions etching and cross-linking in protein. According to Nasiru et al (2022), CP treatment resulted in the modification of the tertiary structure of the lysozyme, which became more obvious with increased voltage. In addition, CP-based lipid oxidation can produce secondary oxidation products (Ucar et al., 2021). Moreover, primary oxidation products in seafood caused by CP can make lipids more vulnerable to oxidation during storage. Lipid oxidation may lead to loss of nutrients and sensory properties of food and alter organoleptic properties. Furthermore, Albertos et al (2019) observed that lipid oxidation is proportional to the applied CP energy. To confront this challenge, further studies are needed to fully elucidate the effects of CP on lipid and protein oxidation in aquatic products to select an appropriate CP treatment condition for application in aquatic product preservation.
Consequently, the present study aimed to comprehensively evaluate the effects of CP treatment at different voltages on the quality attributes of golden pompano, including antibacterial activity, physicochemical properties, lipid oxidation, and protein structure. A balance between microbial safety and quality attributes should be revealed by exploring the relationship between the change in each parameter and the CP treatment voltage. It is considered that the findings of this study would be beneficial for golden pompano preservation.
2. Materials and methods
2.1. Sample processing
The fresh golden pompano (average weight of 550 ± 50 g) was purchased from the Xiashan wholesale aquatic products market (Zhanjiang, China) and then transported to the laboratory in a box with oxygenated water within 30 min. The fish were killed by ice bath treatment. Then, they were cleaned with pure water and wiped dry with clean absorbent paper. The dorsa-lateral muscles of the fish were separated from the bone and skin and cut into 40 × 30 × 10 mm3 pieces.
2.2. Cold plasma treatment
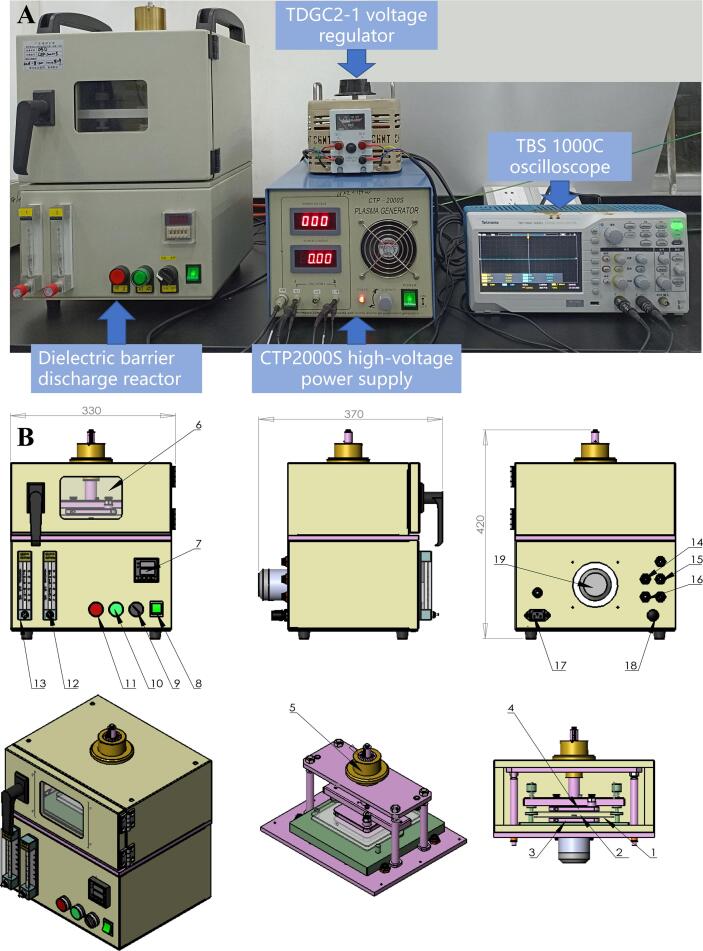
The CP system (Fig. 1) consisted of a DBD-100 reaction unit, a CTP2000S high-voltage power supply (output voltage: 0–60 kV; Suman, Nanjing, China), a TDGC2-1 voltage regulator (220 V, 50 Hz; Zhengtai, Yueqing, China), and a TBS 1000C oscilloscope (Tektronix, Beaverton, OR, USA). The CP discharge was carried out in a DBD-100 reaction unit consisting of quartz plates with a diameter of 100 mm and a spacing of 20 mm. The TBS 1000C oscilloscope was used to monitor the energy in the DBD-100 reaction unit.
Fig. 1.
(A) Dielectric barrier discharge (DBD) cold plasma device; (B) DBD reactor. 1. dielectric (current experimental dielectric is quartz); 2. DBD-100 reaction unit; 3. grounding electrode; 4. high voltage electrode; 5. reaction unit gap adjustment knob; 6. reaction unit viewing window; 7. timer; 8. switch; 9. timing knob; 10. discharge button; 11. stop button; 12. gas flow meters 1; 13. gas flow meters 2; 14. gas inlet 1; 15. gas inlet 2; 16. gas-vent; 17. power input port; 18. grounding electrode port.
The fish pieces were randomly divided into four groups: the negative control group (0 kV) without CP treatment and the three treatment groups (10, 20, and 30 kV) treated with CP at different voltages for 45 s. The reaction gas was clean air, and the treatment temperature was 25 ± 3 °C.
2.3. Microbiological analysis
As the microbial content of fresh fish is very low, according to the previous experimental results in our lab, the fish was placed at 20 °C for 24 h before CP treatment to achieve a certain microbial content. The total viable count (TVC) was determined according to the method of Huang et al. (2019) with minor modifications. The minced fish (10 g) was placed in a sterilized blender bag with sterile physiological saline (90 mL, 0.85% NaCl, w/v). Then, the samples were mixed with a stomacher (Truelab, BM-400HP, Shanghai, China) for 1 min. The samples (1 mL) were poured onto plate count agar after a series of dilutions in physiological saline. After the agar had solidified, it was incubated at 37 °C for 48 h, and the TVC was counted and reported as lg CFU/g.
2.4. Quality properties and biochemical analysis
2.4.1. Juice loss
Juice loss was determined according to the method of Du et al. (2022). The weight of each fish pieces was recorded before CP treatment (m0). After the CP treatment, the moisture on the surface of the sample was dried with filter paper, and then the sample was accurately weighed (m1), and the juice loss was calculated by the following equation:
2.4.2. Water-holding capacity
Water-holding capacity (WHC) was determined according to the method of Yang et al. (2022) with minor modifications. Approximately 5 g of fish sample (m2) was wrapped in blotting paper and centrifuged at 5,000 r/min, 4 °C for 10 min in a freezing centrifuge (Sigma, 3-30KS, Osterode am Harz, Germany). After removing the blotting paper, the weight of the fish pieces was recorded (m3), and the WHC was calculated by the following equation:
2.4.3. Color
Changes in the color parameters, including L* (lightness), a* (redness or greenness), and b* (yellowness or blueness), of the fish were evaluated as described by Yang et al. (2022) using a colorimeter (Konica Minolta, CR-20, Tokyo, Japan). The white reference tile (L*: 95.0, a*: −0.1, b*: 4.1) was used to calibrate the instrument.
2.4.4. Hardness
The hardness was measured according to the method of Yang et al. (2022) with minor modifications. The fish was cut into a 10 × 10 × 10 mm3 cube. The hardness of the sample was determined using a texture analyzer (Stable Micro Systems, TA.XTplusC, Surrey, UK) under the following test conditions: P/0.5 probe; trigger force, 5 g; pre-test, test, and post-test speed, 1 mm/s.
2.4.5. pH
The pH value was determined according to the method of Qian et al. (2021) with minor modifications. The minced fish (3 g) was added to deionized water (30 mL) and homogenized with a homogenizer (IKA, T25, Staufen, Germany) at 10,000 r/min for 1 min. After standing for 30 min, the pH value was measured with a PB-10 pH meter (Sartorius, Gottingen, Germany).
2.4.6. Total volatile base nitrogen
Total volatile base nitrogen (TVB-N) was determined according to the method of Lan et al. (2022a) with some modifications. About 5 g of minced fish was added to deionized water (37.5 mL) and mixed with a vortex mixer (Scilogex, SCI-VS, CT, USA) for 30 s. After standing for 30 min, the mixture was added to the distillation tube with MgO (0.5 g). A Vapodest Kjeldahl nitrogen analyzer (Gerhardt, Konigswinter, Germany) was used to determine the TVB-N under the following measurement settings: distillation time of 120 s, titration endpoint of pH 4.65. Hydrochloric acid (HCl) solution for volumetric analysis (0.1 M; Anpel Laboratory Technologies, Shanghai, China) was used to titrate. The TVB-N was calculated by the following equation:
where V1 is the volume of HCl solution for volumetric analysis in the mixture (mL); V2 is the volume of HCl solution for volumetric analysis in blank specimens (mL); c is the concentration of HCl solution for volumetric analysis (mol/L), and m is the mass of the fish (g).
2.5. Lipid oxidation
2.5.1. Peroxide value
Peroxide value (POV) was determined according to the method of Wang et al. (2018) with minor modifications. Twenty milliliters of cold chloroform:methanol (1:1, v/v) solution was added to about 5 g of minced fish. Then, the mixture was homogenized at 12,000 r/min for 1 min, followed by the addition of 6.16 mL of NaCl solution (0.5%, w/v), and then the mixture was centrifuged at 4,500 r/min, 4 °C for 8 min. Afterward, 3 mL of the bottom chloroform layer was aspirated by syringe and then mixed with 5 mL of cold chloroform:methanol solution (1:1, v/v), 100 μL of ammonium thiocyanate solution (3.94 M), and 100 μL of FeCl2 solution (18 mM), vortexed for 10 s. After standing for 20 min in the dark, the absorbance of the mixture was determined at 500 nm using a UV–visible spectrophotometer (Shimadzu, UV-2550, Kyoto, Japan). Finally, the POV value was calculated from the Fe3+ standard curve.
2.5.2. Thiobarbituric acid-reactive substances
TBARS was determined according to the method of Wang et al. (2018) with minor modifications. Twenty milliliters of trichloroacetic acid (20%, w/v) were added to 5 g of minced fish and homogenized at 12,000 r/min for 1 min. Then, the mixture was centrifuged for 15 min (8,000 r/min, 4 °C), and the supernatant was retained. Next, 4 mL of the supernatant was mixed with an equal amount of 2-thiobarbituric acid (20 mM) and heated in boiling water for 20 min. The mixed solution was cooled to room temperature with water. The absorbance of the solution was measured at 532 nm with a Varioskan Flash microplate reader (Thermo Fisher Scientific, Waltham, MA, USA). The TBARS value was calculated from the malondialdehyde (MDA) standard curve and expressed as milligrams of MDA per kilogram of meat.
2.6. Low-field nuclear magnetic resonance
Low-field nuclear magnetic resonance (LF-NMR) was performed according to the method of Yang et al. (2022) with some modifications. The fish was cut into a 30 × 30 × 10 mm3 pieces (about 10 g). The relaxation time (T2) and peak area of the samples were measured by the Carr–Purcell–Meiboom–Gill (CPMG) sequence with an NMR analyzer (Niumag Corp., NMI 20-060H-I, Suzhou, China) at 32 °C under the following measurement settings: stable frequency, 21 MHz; waiting time, 3,000 ms; echo count, 3,000; number of repeated scans, 8. The T2 relaxation time of the samples was obtained by inversion of the data using the Niumag NMR analysis software (Niumag Corp.) with the simultaneous iterative reconstruction technique.
2.7. Protein oxidation and structural changes
2.7.1. Extraction of myofibrillar protein
Myofibrillar proteins (MP) was extracted according to the method of Lan et al. (2022b) with minor modifications. Two grams of minced fish was mixed with 20 mL of Tris-buffer A (0.05 M KCl, 20 mM Tris-HCl, pH 7.00) and homogenized at 12,000 r/min for 1 min, The resultant homogenate was centrifuged at 10,000 r/min, 4 °C for 15 min, and the supernatant was discarded (This operation was repeated twice.). Then, 20 mL of Tris-buffer B (0.6 M KCl, 20 mM Tris-HCl, pH 7.00) was added to the precipitate and homogenized at 12,000 r/min for 1 min. After standing for 1 h, the mixture was centrifuged for 15 min (10,000 r/min, 4 °C). The supernatant was the MP solution. The MP content was determined by the Biuret Protein Quantitative Kit (PT0004; Leagene Biotechnology, Beijing, China).
2.7.2. Total sulfhydryl content
The total sulfhydryl content of the samples was determined by a colorimetric method with the Total Sulfhydryl Content Assay Kit (BC1375; Solarbio, Beijing, China), and the protein content was determined by the Biuret Protein Quantitative Kit (PT0004; Leagene Biotechnology). The absorbance value measured at 412 nm was substituted into the formula given in the instructions in the assay kit to calculate the total sulfhydryl content (mol/105 g protein).
2.7.3. Intrinsic fluorescence spectra
Intrinsic fluorescence was measured according to the method of Cheng et al. (2021b) with minor modifications. The protein concentration of MP was adjusted to 0.1 mg/mL. The fluorescence of the solution was measured using a fluorescence spectrophotometer (Shimadzu, RF-5301PC, Kyoto, Japan) under the following measurement settings: excitation wavelength, 280 nm; emission wavelength, 295–450 nm; scan speed, super; slit width, 5 nm.
2.7.4. Circular dichroism spectroscopy
Circular dichroism (CD) spectroscopy was performed according to the method of Sun et al. (2019a) with minor modifications. The protein concentration of MP was adjusted to 0.4 mg/mL. The CD spectroscopy of the solution was determined using a CD spectropolarimeter (Applied Photophysics, Chirascan V100, Surrey, UK) under the following measurement settings: wavelength range, 190–260 nm; scanning speed, 1 nm/s; quartz CD cuvette, 0.5 mm in thickness. The secondary structure contents were analyzed by the CD spectra deconvolution software (Institut für Biotechnologie, Saale, Germany).
2.8. Sensory evaluation
Sensory evaluation was determined according to the method of Olatunde et al. (2019a) with some modifications. Thirteen trained panelists (6 women and 7 men, aged 21 to 30 years) were asked to evaluate the appearance, texture, odor, and overall likeness of the fish. A 9-point hedonic scale was used for sensory evaluation (9: love extremely, 8: like very much, 7: like moderately, 6: like slightly, 5: neutral, 4: dislike slightly, 3: dislike moderately, 2: dislike very much, 1: dislike extremely). A mean score (5) was used as the acceptable threshold.
2.9. Statistical analysis
The data were analyzed by one-way analysis of variance (ANOVA) using JMP Pro 16 software (SAS Institute, Cary, NC, USA). Tukey’s honestly significant difference (HSD) multiple comparisons test was used to assess the significant differences between all samples, with P < 0.05 indicating a significant difference. All data were expressed as mean ± standard deviation. Three batches of fish samples were prepared for this study, and triplicate measurements were performed (Except that the hardness of the sample was measured five times).
3. Results and discussion
3.1. Microbiological analysis
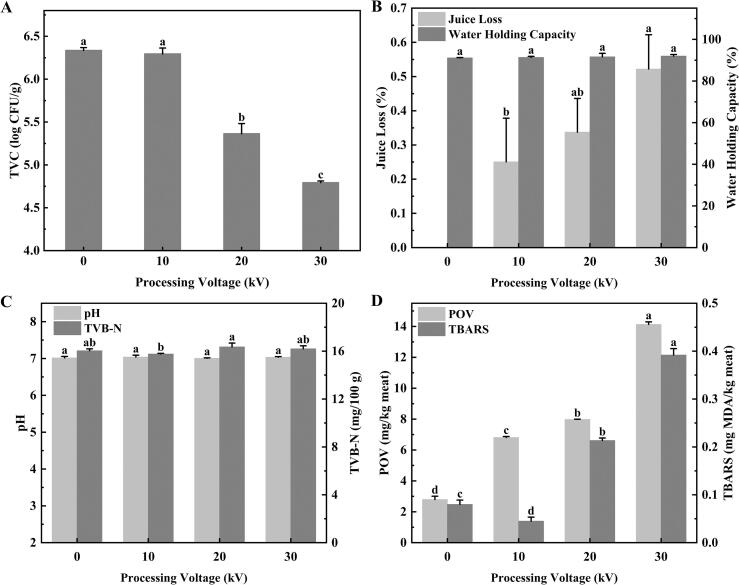
Microorganisms are one of the main causes of food spoilage during storage (Du et al., 2022). Changes in the TVC of the control and treated golden pompano samples are shown in Fig. 2A. The TVC of the control group was 6.33 lg CFU/g, indicating that after 24 h of standing at room temperature (20 °C), the microorganisms had proliferated substantially. However, Fig. 2A also shows that the TVC decreases with the increase in the treatment voltage of CP. There was no significant difference in the TVC between the group treated at the lowest voltage (10 kV) and the control group (P > 0.05), whereas the samples treated with CP at 20 kV and above showed a significant decrease in TVC (P < 0.05). Compared to the control group, the TVC of the 20 kV and 30 kV treatment groups decreased by 0.98 lg CFU/g and 1.54 lg CFU/g, respectively. Han et al. (2014) also found that increasing the voltage of CP increased the bactericidal effect, and the cell population of Escherichia coli ATCC 25922 treated with 70 kV CP was 1.6 lg CFU/mL less than that treated with 56 kV CP. The inactivation of microorganisms by CP may be related to the reactive species (such as ROS and RNS), energized ions, charged particles, and UV radiation (Misra and Jo, 2017, Nasiru et al., 2021). The reactive species produced by CP induce oxidative stress by interacting with cellular components, leading to cellular damage, and ultimately to cellular inactivation of microorganisms (Nasiru et al., 2021). In addition, the charged particles cause cell death through electrostatic disruption or electroporation. After low-voltage treatment, less reactive species and charged particles were produced, which were less effective for microbial inactivation. However, within a certain range, the production of reactive species and charged particles increased with the increase of treatment voltage, resulting in more microbial inactivation. Lin et al. (2020) also found that the antibacterial effect of CP increased with the voltage increase within a certain period of time.
Fig. 2.
(A) TVC; (B) Juice loss and water-holding capacity; (C) pH and TVB-N; and (D) POV and TBARS of golden pompano treated with cold plasma at different voltages. Different letters (a-d) indicate significant difference (P < 0.05) of means between the groups. TVC, total viable count; TVB-N, total volatile base nitrogen; POV, peroxide value, TBARS, thiobarbituric acid-reactive substances; MDA, malondialdehyde.
3.2. Juice loss and water holding capacity analysis
Juice loss reflects the water retention capacity of meat, which affects the extent of water exudation from the sample (Du et al., 2022). Fig. 1B shows the juice loss of golden pompano before and after the CP treatments. The juice loss of the sample increased with the CP voltage (P < 0.05), although the maximum juice loss was only 0.52%. Juice loss would lead to a decline in the nutritional value of fish, thereby deteriorating the quality and reducing the commercial value. It was speculated to be caused by a certain degree of oxidation of MP as the treatment voltage increases, resulting in a more pronounced loss of free water on the surface (Du et al., 2022). In the current study, the 45 s-CP treatment time was short enough to avoid substantial juice loss from fish.
One of the most important factors affecting the economic value and quality of meat is the WHC, which is closely related to the color and texture of raw meat (Barbera, 2019, Yang et al., 2022). As shown in Fig. 2B, there was no significant difference in the WHC among all the groups (P > 0.05). The WHC depends largely on the water content of the aquatic product itself (Szmanko et al., 2021). The 45 s-CP treatment caused significant juice loss (≤0.6%, Fig. 2B), but it had little effect on the WHC of the samples containing a large amount of water. Koddy et al. (2021) asserted that the reactive species generated by CP might have the ability to induce aggregation reactions. They found that the WHC of hairtail treated at 50 kV for 60 s increased compared to the untreated group. The impact of the treatment depends on a range of factors, such as the CP equipment, treatment voltage, and raw materials.
3.3. Color analysis
Color is the first quality attribute those consumers use to assess meat quality, which is more intuitive than any other sensory attribute (Huang et al., 2019). The color changes in golden pompano induced by CP at different voltages are shown in Table 1. There was no significant difference in the L*-value between the control group and the 10 kV CP group (P > 0.05), perhaps due to the small processing voltage. However, the L*-value increased significantly when the treatment voltage exceeded 20 kV (P < 0.05). An increase in the L*-value suggests that CP treatment caused the migration of water from inside the fish to the surface (Yang et al., 2022), perhaps due to a certain degree of oxidation of MP as the treatment voltage is increased (Du et al., 2022).
Table 1.
Color, hardness, and sensory evaluation of golden pompano treated by cold plasma at different voltages.
| Processing voltage (kV) | L* | a* | b* | Hardness (g) | Appearance | Texture | Odor | Overall likeness |
|---|---|---|---|---|---|---|---|---|
| 0 | 45.96 ± 1.07c | −1.22 ± 0.15a | 0.86 ± 0.18b | 1517.1 ± 79.33b | 8.42 ± 0.53a | 8.35 ± 0.47a | 8.58 ± 0.49a | 8.38 ± 0.46a |
| 10 | 46.76 ± 0.96bc | −1.66 ± 0.23b | 1.08 ± 0.22b | 1493.56 ± 39.49b | 7.94 ± 0.54ab | 7.77 ± 1.25a | 8.10 ± 0.66a | 7.79 ± 0.88a |
| 20 | 48.26 ± 1.02b | −2.10 ± 0.16c | 1.94 ± 0.38a | 1290.73 ± 95.60c | 7.70 ± 0.82b | 7.92 ± 0.7a | 6.95 ± 1.09b | 7.81 ± 0.7a |
| 30 | 50.96 ± 0.99a | −2.64 ± 0.11d | 2.28 ± 0.72a | 1704.81 ± 69.32a | 6.27 ± 0.73c | 6.38 ± 0.89b | 5.98 ± 1.34c | 6.42 ± 0.88b |
Different letters (a-d) indicate significant difference (P < 0.05) of means between the groups.
In addition to the change in the L*-value, there was a dramatic decrease in the a*-value with the increase of the voltage (P < 0.05). This change may result from the oxidation of pigments and lipids caused by the reactive species produced by CP, especially ozone (O3), and metmyoglobin formation could be promoted when myoglobin reacts with reactive species (Olatunde et al., 2019b). Cho et al. (2014) showed that O3 treatment decreased the a*-value of beef. In addition, the decrease in the a*-value of fish might be associated with the degradation of the heme porphyrin rings induced by plasma treatment (Cheng et al., 2021a).
There was no significant difference in the b*-value between the control group and the 10 kV CP group (P > 0.05) but the b*-value increased significantly when the treatment voltage exceeded 20 kV (P < 0.05). Huang et al. (2021) also found that the b*-value of salmon sashimi increased after CP jet treatment. It may be because of lipid and pigment oxidation induced by CP, or the lipid oxidation products might be involved in browning processes by reacting with proteins and related compounds (Huang et al., 2021). In summary, the changes in the color parameters were mainly due to the oxidation of proteins, pigments, and lipids caused by the reactive species produced by CP (Nasiru et al., 2021).
3.4. Hardness analysis
The hardness is a sensory characteristic of food (Yang et al., 2022). It can be seen from Table 1 that with the increase of processing voltage, the hardness of golden pompano first decreased and then increased. There was no significant difference in hardness between samples treated with 10 kV CP and fresh samples (P > 0.05). After low-voltage treatment (10 kV), the lipid and protein products of oxidation caused by the reactive species produced by CP were not enough to change the hardness of the fish. However, the hardness decreased significantly when the voltage was 20 kV (P < 0.05). This decrease in the fish hardness might be related to the bombardment of fish by charged particles generated by CP, which damaged muscle fibers and destroyed the protein spatial structure (Bao & Ertbjerg, 2019). At the same time, the decrease in fish hardness also reflected an improvement in the tenderness. Nevertheless, the hardness of fish treated with 30 kV CP was significantly higher than that of the fresh samples (P < 0.05), which can be explained by the severe dehydration of the surface of the fish muscle, protein oxidation leading to crosslinking, and a dense protein network (Koddy et al., 2021).
3.5. pH and total volatile base nitrogen analysis
The pH value is an important indicator of meat quality. Changes in the pH value of golden pompano treated with CP at different voltages are shown in Fig. 2C. The pH values of all the samples ranged from 7.01 to 7.04 (P > 0.05). Generally, the pH value of fresh fish varies from 5.80 to 7.20, which is related to the species, growth environment, and fishing methods (Huang et al., 2021). Based on their observations that the pH value of sashimi treated with CP for 3 min did not change significantly, Huang et al. (2021) suggested that the CP jet treatment could retain the pH value of sashimi at normal levels. Moreover, Olatunde et al. (2019a) found that the pH value of Asian sea bass slices did not change with the extension of plasma treatment time. Changes in the pH value may be related to the accumulation of basic compounds from the decomposition or degradation of nucleotides and proteins mediated by autolysis and microbial proliferation (Olatunde et al., 2019a). From the results of the present study, short-term CP treatment does not affect the pH value of golden pompano.
TVB-N is an important indicator for assessing fish freshness. It is a general term for the basic volatile compounds produced due to the degradation of protein and non-protein nitrogen compounds (Sun et al., 2019b). As shown in Fig. 2C, the TVB-N values of golden pompano before and after CP treatments ranged from 15.76 to 16.36 mg/100 g (P > 0.05). An increase in the TVB-N value is mainly due to the role of microorganisms. Olatunde et al. (2019a) also found that there was no significant difference in the TVB-N value of fish after CP treatment without storage (P > 0.05).
3.6. Peroxide value and thiobarbituric acid-reactive substances analysis
Lipid oxidation can result in the generation of hydroperoxides in the initial stage, and POV values were measured to reflect the amount of hydroperoxides formed during lipid oxidation (Fang et al., 2022). As shown in Fig. 2D, the POV value of golden pompano treated with CP gradually increased with the processing voltage (P < 0.05). These results mirrored the observations of Albertos et al. (2017) that the POV value of mackerel increased after CP treatment. In general, the increase in the POV value indicated that CP treatment caused lipid oxidation reactions. The mechanism of CP action has been confirmed, and the ROS and RNS produced by CP are the most important components (Misra & Jo, 2017). Although the bactericidal efficiency of ROS and RNS cannot be underrated, these reactive chemical species also have adverse effects on lipids.
The TBARS value represents the content of secondary lipid oxidation products mainly derived from the degradation of polyunsaturated fatty acids (Sun et al., 2019b). As shown in Fig. 2D, the TBARS value of golden pompano increased significantly after CP treatment (except for the 10 kV) (P < 0.05), which indicated that the lipid oxidation increased with the increase of the treatment voltage. The change trend of the TBARS value (except for the 10 kV) almost mirrored that of the POV. Olatunde et al. (2019b) proposed that the increase in the TBARS value of Asian sea bass with the extension of CP treatment time was due to the increasing production of O3. It is well known that an increase in the treatment voltage will also lead to an increase in O3 formation (Albertos et al., 2019). Intriguingly, the TBARS value of golden pompano treated with CP at 10 kV was lower than that of the fresh sample. It seems plausible that the protein binding efficiency of MDA and the active substances and charged particles produced by CP promote this process, resulting in a decrease in the TBARS value (Wang et al., 2021). Nevertheless, with further increments in the treatment voltage, the yield of MDA caused by lipid oxidation exceeded the amount needed to bind to the protein, and the TBARS value increased. In general, the threshold level for acidification of the TBARS is 2.0–2.5 mg/kg (Wang et al., 2018). However, in the present study, the TBARS values of all the golden pompano samples were well below this threshold level.
From these results, the amount of hydroperoxides and MDA increased with the CP treatment voltage, which proved that the degree of fat oxidation increased with the CP voltage. Although the CP treatment did not affect the acceptability of golden pompano, the lipid oxidation caused by the CP treatment of golden pompano would adversely affect the shelf life. Further experiments are needed to determine if the prior treatment of seafood with some antioxidant technology will circumvent this limitation.
3.7. Low-field nuclear magnetic resonance F-NMR analysis
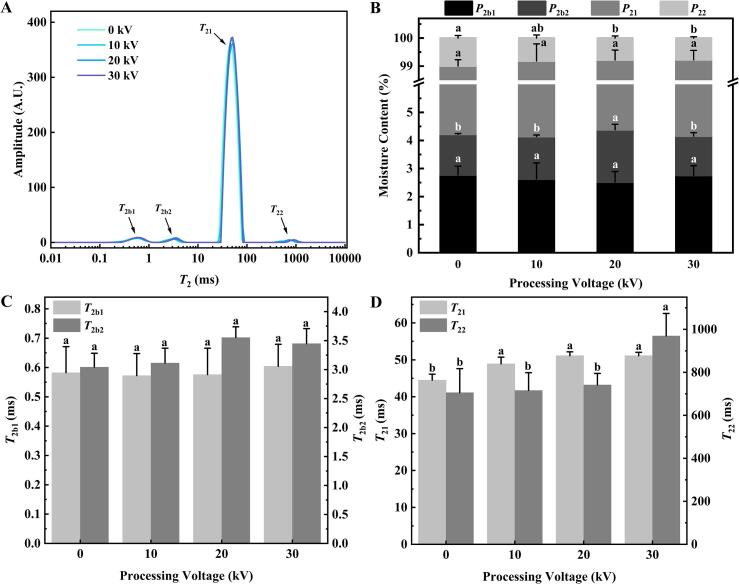
The water mobility and distribution in golden pompano were evaluated by measuring the proton spin–spin relaxation time (T2) using an LF-NMR spectrometer (Yang et al., 2022). The water distribution of all the samples is shown in Fig. 3A–D. Four water peaks were observed on the LF-NMR curves (Fig. 3A): T2b1 (0–1 ms) and T2b2 (1–10 ms) both represent bound water, T21 (10–100 ms) represents immobilized water, and T22 (100–10,000 ms) represents free water. The changes in the T2b1, T2b2, T21, and T22 relaxation times are shown in Fig. 3C and 3D. The peak area reflects the amount of water in the samples. The contribution of the peak area of each component to the total peak area is shown in Fig. 3B.
Fig. 3.
Water properties of golden pompano treated with cold plasma at different voltages. (A) T2 relaxation times, (B) different types of water content, (C) T2b1 and T2b2 relaxation times, (D) T21 and T22 relaxation times. Different letters (a-b) indicate significant difference (P < 0.05) of means between the groups.
As shown in Fig. 3C, there were no significant changes in T2b1 and T2b2 among the different treatment groups, and correspondingly, there were also no significant differences in P2b1 and P2b2 (Fig. 3B) between each treatment group (P > 0.05). The bound water is tightly bound to proteins or other biological macromolecules and is not easy to migrate to other regions. Albertos et al. (2017) also noticed that strongly bound water (T2b) was not influenced by CP treatment. A significant increase in the T21 relaxation time (Fig. 3D) was observed for the CP-treated golden pompano samples compared with the fresh sample (P < 0.05), causing a decline in the binding capacity of the muscle tissue with water, and water mobility increased. Simultaneously, the T22 relaxation time increased as the treatment voltage increased (Fig. 3D), which was consistent with Albertos et al. (2017) that T22 after CP treatment at 80 kV was significantly higher than that obtained after CP treatment at 70 kV (P < 0.05). The increase in the T21 and T22 relaxation times may be related to the oxidation of proteins caused by CP, resulting in a certain degree of conversion of immobilized water to free water (Liu et al., 2022) and subsequent loss of water. This corresponded with the result of juice loss. As shown in Fig. 3A and 3B, P21 was not affected (P > 0.05) by the intensity of the treatment voltage, indicating that the proportion of immobilized water to total water remained constant, which was verified by the change in the WHC (Fig. 2B). In addition, P22 decreased significantly (P < 0.05) with further increments of the treatment voltage (≥20 kV) compared with the fresh fish, signifying that the free water decreased. This may be due to the evaporation of free water at the increased treatment voltage, resulting in juice loss.
3.8. Total sulfhydryl content analysis
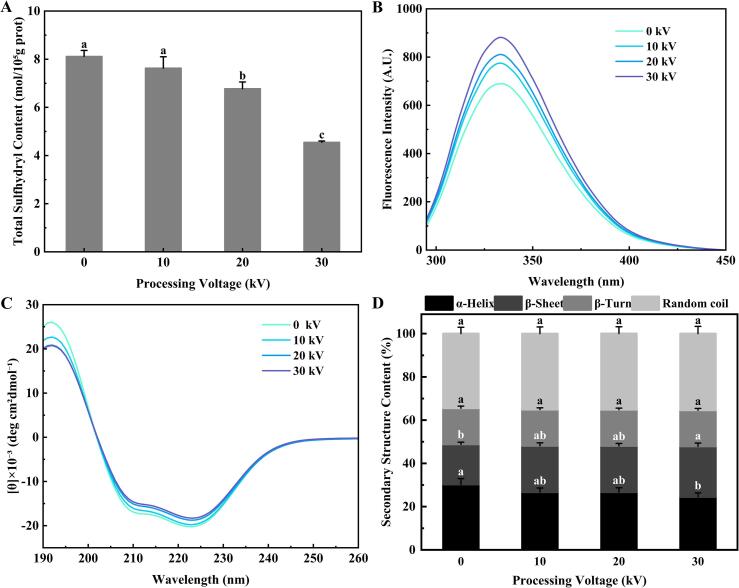
Protein oxidation leads to a variety of changes, including carbonylation, reduction of sulfhydryl groups, and crosslinking formation (Nawaz et al., 2022). The sulfhydryl content of all samples was evaluated to identify the extent of oxidation of the fish with CP treatment. As revealed in Fig. 4A, the sulfhydryl content of golden pompano tended to decrease with the increase in the processing voltage, but the magnitude of the decrease was significant only from 20 to 30 kV (P < 0.05). The sulfhydryl content decreased from 8.13 mol/105g protein (control sample) to 5.57 mol/105g protein after being treated with 30 kV. This may be because of the particular susceptibility of the sulfhydryl groups in fish protein to oxidation induced by ROS (Huang et al., 2019). The reactive species produced by CP lead to either strong oxidation of side chains of sulfur-containing proteins or aggregation of disulfide bonds through sulfhydryl crosslinking in sulfur amino acids (Koddy et al., 2021). The decrease in sulfhydryl content indicates the loss of most sulfhydryl functional groups and usually indicates protein oxidation. Consistent with the present results, Koddy et al. (2021) reported that the sulfhydryl content of hairtail was notably affected by CP treatment.
Fig. 4.
(A) Total sulfhydryl content; (B) Intrinsic fluorescence; (C) Circular dichroism spectra; and (D) Secondary structure content of golden pompano treated with cold plasma at different voltages. Different letters (a-c) indicate significant difference (P < 0.05) of means between the groups.
3.9. Intrinsic fluorescence analysis
The fluorescence spectroscopy of tryptophan (Trp), which shows high sensitivity to its microenvironment, is conventionally used to observe the changes in protein tertiary structures (Lan et al., 2022b). As shown in Fig. 4B, there was a difference in the fluorescence intensity between the control sample of golden pompano and the CP-treated samples, and the fluorescence intensity increased with the increase of the treatment voltage. This could be attributed to the etching and oxidation induced by CP that exposed the Trp residues to the outer surface, increasing the degree of MP unfolding (Yu et al., 2020), which becomes more pronounced with increasing treatment voltage. Another study showed that the destruction of the protein hydrophobic regions would lead to the exposure of aromatic groups, thus increasing the fluorescence intensity (Lan et al., 2022b). Similarly, Yu et al. (2020) found that CP treatment could increase the fluorescence intensity of peanut isolate protein, and Nasiru et al. (2022) observed a similar phenomenon in lysozyme. The changes in the sulfhydryl content and fluorescence intensity reflected a certain degree of destruction of the structure of MP in the fish. The destruction of the protein structure is not conducive to subsequent storage, so certain measures need to be taken to inhibit protein oxidation caused by CP.
3.10. Circular dichroism spectroscopy analysis
CD spectroscopy analysis examines the conformational changes connected to the polypeptide backbone structure of proteins, including α-helix, β-turn, β-sheet, and random coil (Nasiru et al., 2022, Sun et al., 2019). The CD spectra of golden pompano samples before and after CP treatment at different voltages are shown in Fig. 4C. The peak position of each treatment group was the same. However, compared with the fresh sample, the negative peaks of the other treatment groups shifted upward, and the positive peaks decreased as the voltage increased, indicating that the secondary structure of the protein was transformed by the CP treatment (Nasiru et al., 2022). However, it should be noted that these changes were very small (Fig. 4D). With the increase of the CP treatment voltage, the α-helix content of the samples gradually decreased, and the β-sheet content gradually increased, but there was no significant change in the content of random coil and β-turns, which indicated that the conformation of protein was partially transformed from stable to relatively disordered alongside the denaturation and unfolding of the protein (Han et al., 2019). This may be because of the high reactivity of methionine, phenylalanine, and tyrosine in the fish protein, which can be rapidly oxidized when exposed to reactive species (ROS and RNS) produced by CP (Qian et al., 2021). However, the secondary structure content changes were slight, with significant differences only between the 30 kV group and the control group (P < 0.05). The CD results suggested that CP treatment can change the MP secondary structure and that an appropriate treatment voltage (≤20 kV) has no significant effect on the protein secondary structure.
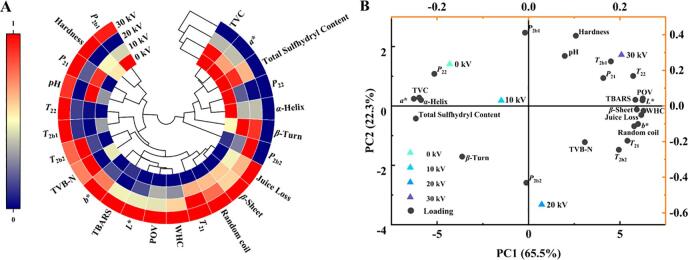
3.12. Correlation analysis and principal component analysis
The quality of golden pompano after CP treatment was determined by its microbial content and physical and chemical properties. Thus, to predict the effect of CP on the quality of golden pompano, the complex relationships between various variables must be resolved. In the present study, correlation analysis (Fig. 5A) and principal component analysis (PCA) (Fig. 5B) were conducted to clarify the relationships among the TVC, juice loss, WHC, hardness, color, pH, TVB-N, water properties (T2b1, T2b2, T21, T22, P2b1, P2b2, P21, and P22), lipid oxidation (POV, TBARS) and protein structure (total sulfhydryl content, α-helix, β-turn, β-sheet, and random coil) of the CP treatment at different voltages. It can be approximately considered that the TVC, a*-value, total sulfhydryl content, P22, α-helix, β-turn, and P2b2 were negatively correlated with T2b1, T2b2, T21, T22, P2b1, P21, pH, TVB-N, juice loss, WHC, hardness, POV, TBARS, L*-value, b*-value, β-sheet, and random coil. In addition, the TVC had a significantly negative correlation with TBARS, L*-value, and b*-value (P < 0.05) and a significantly positive correlation with the a*-value (P < 0.05). Therefore, as a more convenient experiment, only the TVC, TBARS, and color need to be studied to allow a rapid investigation of the optimal conditions of the CP treatment.
Fig. 5.
(A) Correlation analysis; and (B) Principal component analysis.
At the same time, cluster analysis of the different treatment groups divided the groups into three categories. The first group included the control group and the 10 kV group; the second group was 20 kV, which significantly reduced the TVC. The third group was 30 kV, which further reduced the TVC. With the increase of the treatment voltage, the components of reactive species and charged particles also increased, which reduced the microbial content, but aggravated the deterioration of the lipid.
The PCA of each indicator of the sample is shown in Fig. 5B, and the contribution rates of the first two principal components (PC1 and PC2) were 65.5% and 22.3%, respectively, with a cumulative contribution rate of 87.8% (>85%), which can well reflect the information of the original data. The PCA also showed similar results to the cluster analysis, with the control (0 kV) and 10 kV groups in the second quadrant, 20 kV in the fourth quadrant, and 30 kV in the first quadrant. Moreover, TVC was also located in the second quadrant, indicating that the CP antibacterial effect was not significant under low-voltage conditions. Meanwhile, POV and TBARS were in the first quadrant, indicating that high-voltage CP treatment improved the antimicrobial effects but promoted lipid oxidation. However, the 20 kV group was far from TVC, b*-value, a*-value, POV, and TBARS on the coordinate axis, indicating that this group had a good inhibition effect and maintained good quality. Therefore, CP treatment at 20 kV may be used for the sterilization and preservation of golden pompano.
3.12. Sensory evaluation analysis
Sensory analysis is important in any food-quality evaluation procedure because the ultimate criterion for judgment is human response. As shown in Table.1, there was no significant difference in the appearance, texture, odor, and overall likeness between the group treated at the 10 kV CP group and the control group (P > 0.05), which may be due to the low treatment voltage. Whereas the samples treated with CP at 20 kV showed a significant decrease in appearance and odor (P < 0.05). This may be due to the fact that as the treatment voltage increases further, more reactive species were produced, especially O3 (Olatunde et al., 2019b), which caused a change in the surface color of the fish and produces an off-flavor. Although the appearance and odor scores decreased in the 20 kV group, the texture and overall likeness were not significantly different from the control group (P > 0.05). As the voltage reached 30 kV, the appearance, texture, and odor scores of the fish decreased significantly, as did the overall likeness. This may be due to the higher voltage, which caused strong off-flavor of fish. In addition, it also resulted in severe water loss from the surface of the fish and the fish became tougher, which was consistent with the hardness results (Table 1). Overall, the acceptability of the golden pompano was not affected at the appropriate CP treatment voltage.
4. Conclusion
CP inhibited the growth of microorganisms, thus reducing the TVC, and the inhibits effect increased with the increase of the CP voltage. However, excessive voltage (30 kV) inevitably leads to problems, such as fat oxidation, protein oxidation, and juice loss, while at an appropriate voltage, CP treatment had inapparent or small effects on the fundamental properties of golden pompano, such as texture, color, water distribution, lipid oxidation, and protein structure. This indicates that the quality of golden pompano can be maintained after CP treatment at an appropriate voltage. In this context, CP could be considered as a potential alternative method for the preservation of seafood products in the future. Moreover, if the oxidation problem caused by high-voltage CP can be solved, it will broaden the application prospect of CP.
Funding
This work was supported by the Scientific and Technological Innovation Strategy of Guangdong Province [grant number 2022A05036]; Guangdong Basic and Applied Basic Research Foundation [grant number 2023A1515011513]; Zhanjiang Ocean Youth Talents Innovation Project [grant number 2021E05015]; General transfer payment fund project of fishery development support policy in Guangdong Province in 2022 [grant number 2022-440000-45060100-9680]; and Guangdong Innovation Team of Seafood Green Processing Technology [grant number 2019KCXTD011].
CRediT authorship contribution statement
Jie Xu: Data curation, Writing – original draft, Visualization. Qinxiu Sun: Conceptualization, Methodology, Writing – review & editing. Xiuping Dong: Supervision. Jialong Gao: Investigation, Validation. Zefu Wang: Investigation, Validation. Shucheng Liu: Conceptualization, Methodology, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors wish to express their gratitude to Wenqing Xu (Undergraduate student; Guangdong Ocean University, Zhanjiang, China) for her great efforts in the whole research project. In addition, the author also thanks Huiyuan Situ, Jinzhen Li, Zhendong Tang, and Yu Yang (Postgraduate student; Guangdong Ocean University, Zhanjiang, China) for assistance in the raw material processing, instrument operation, and charting of this research.
Contributor Information
Qinxiu Sun, Email: sunqinxiugo@163.com.
Shucheng Liu, Email: Lsc771017@163.com.
Data availability
Data will be made available on request.
References
- Albertos I., Martín-Diana A.B., Cullen P.J., Tiwari B.K., Ojha S.K., Bourke P.…Rico D. Effects of dielectric barrier discharge (DBD) generated plasma on microbial reduction and quality parameters of fresh mackerel (Scomber scombrus) fillets. Innovative Food Science and Emerging Technologies. 2017;44:117–122. doi: 10.1016/j.ifset.2017.07.006. [DOI] [Google Scholar]
- Albertos I., Martin-Diana A.B., Cullen P.J., Tiwari B.K., Ojha K.S., Bourke P., Rico D. Shelf-life extension of herring (Clupea harengus) using in-package atmospheric plasma technology. Innovative Food Science and Emerging Technologies. 2019;53:85–91. doi: 10.1016/j.ifset.2017.09.010. [DOI] [Google Scholar]
- Bao Y.L., Ertbjerg P. Effects of protein oxidation on the texture and water-holding of meat: A review. Critical Reviews in Food Science and Nutrition. 2019;59(22):3564–3578. doi: 10.1080/10408398.2018.1498444. [DOI] [PubMed] [Google Scholar]
- Barbera S. WHCtrend, an up-to-date method to measure water holding capacity in meat. Meat Science. 2019;152:134–140. doi: 10.1016/j.meatsci.2019.02.022. [DOI] [PubMed] [Google Scholar]
- Barbhuiya R.I., Singha P., Singh S.K. A comprehensive review on impact of non-thermal processing on the structural changes of food components. Food Research International. 2021;149 doi: 10.1016/j.foodres.2021.110647. [DOI] [PubMed] [Google Scholar]
- Cheng J.H., Chen Y.Q., Sun D.W. Effects of plasma activated solution on the colour and structure of metmyoglobin and oxymyoglobin. Food Chemistry. 2021;353 doi: 10.1016/j.foodchem.2021.129433. [DOI] [PubMed] [Google Scholar]
- Cheng L.N., Zhu Z.W., Sun D.W. Impacts of high pressure assisted freezing on the denaturation of polyphenol oxidase. Food Chemistry. 2021;335 doi: 10.1016/j.foodchem.2020.127485. [DOI] [PubMed] [Google Scholar]
- Cho, Y., Muhlisin, Choi, J. H., Hahn, T. W., & Lee, S. K. (2014). Effect of gaseous ozone exposure on the bacteria counts and oxidative properties of ground hanwoo beef at refrigeration temperature. Korean Journal for Food Science of Animal Resources, 34(4), 525-532. doi: 10.5851/kosfa.2014.34.4.525. [DOI] [PMC free article] [PubMed]
- Du M.T., Huang L., Gao M.L., Li K., Xiang Q.S., Bai Y.H. Effect of dielectric barrier discharge low temperature plasma treatment on mutton quality. Food Science. 2022;43(21):87–92. doi: 10.7506/spkx1002-6630-20210628-316. [DOI] [Google Scholar]
- Fang C.D., Chen H.S., Yan H.B., Shui S.S., Benjakul S., Zhang B. Investigation of the changes in the lipid profiles in hairtail (Trichiurus haumela) muscle during frozen storage using chemical and LC/MS-based lipidomics analysis. Food Chemistry. 2022;390 doi: 10.1016/j.foodchem.2022.133140. [DOI] [PubMed] [Google Scholar]
- Han L., Patil S., Keener K.M., Cullen P.J., Bourke P. Bacterial inactivation by high-voltage atmospheric cold plasma: Influence of process parameters and effects on cell leakage and DNA. Journal of Applied Microbiology. 2014;116(4):784–794. doi: 10.1111/jam.12426. [DOI] [PubMed] [Google Scholar]
- Han Y.X., Cheng J.H., Sun D.W. Changes in activity, structure and morphology of horseradish peroxidase induced by cold plasma. Food Chemistry. 2019;301 doi: 10.1016/j.foodchem.2019.125240. [DOI] [PubMed] [Google Scholar]
- Huang M.M., Wang J.M., Zhuang H., Yan W.J., Zhao J.Y., Zhang J.H. Effect of in-package high voltage dielectric barrier discharge on microbiological, color and oxidation properties of pork in modified atmosphere packaging during storage. Meat Science. 2019;149:107–113. doi: 10.1016/j.meatsci.2018.11.016. [DOI] [PubMed] [Google Scholar]
- Huang Y.M., Chang W.C., Hsu C.L. Inactivation of norovirus by atmospheric pressure plasma jet on salmon sashimi. Food Research International. 2021;141 doi: 10.1016/j.foodres.2021.110108. [DOI] [PubMed] [Google Scholar]
- Koddy J.K., Miao W.H., Hatab S., Tang L.L., Xu H.Q., Nyaisaba B.M.…Deng S.G. Understanding the role of atmospheric cold plasma (ACP) in maintaining the quality of hairtail (Trichiurus Lepturus) Food Chemistry. 2021;343 doi: 10.1016/j.foodchem.2020.128418. [DOI] [PubMed] [Google Scholar]
- Lan W.Q., Sun Y.Q., Liu S.C., Guan Y., Zhu S.Y., Xie J. Effects of ultrasound-assisted chitosan grafted caffeic acid coating on the quality and microbial composition of pompano during ice storage. Ultrasonics Sonochemistry. 2022;86 doi: 10.1016/j.ultsonch.2022.106032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan W.Q., Zhang B.J., Zhang X., Xie J. Effects of slightly acidic electrolyzed water combined with compound preservative on the protein characteristics of Pacific white shrimp (Litopenaeus vannamei) during refrigerated storage. Journal of Guangdong Ocean University. 2022;42(1):98–105. https://kns.cnki.net/kcms/detail/44.1635.N.20211224.1820.002.html [Google Scholar]
- Laroque D.A., Seó S.T., Valencia G.A., Laurindo J.B., Carciofi B.A.M. Cold plasma in food processing: Design, mechanisms, and application. Journal of Food Engineering. 2022;312 doi: 10.1016/j.jfoodeng.2021.110748. [DOI] [Google Scholar]
- Liao X.Y., Su Y., Liu D.L., Chen S.G., Hu Y.Q., Ye X.Q.…Ding T. Application of atmospheric cold plasma-activated water (PAW) ice for preservation of shrimps (Metapenaeus ensis) Food Control. 2018;94:307–314. doi: 10.1016/j.foodcont.2018.07.026. [DOI] [Google Scholar]
- Lin L., Liao X., Li C.Z., Abdel-Samie M.A., Cui H.Y. Inhibitory effect of cold nitrogen plasma on Salmonella Typhimurium biofilm and its application on poultry egg preservation. LWT-Food Science and Technology. 2020;126 doi: 10.1016/j.lwt.2020.109340. [DOI] [Google Scholar]
- Liu J., Liu D.H., Zheng A.R., Ma Q. Haem-mediated protein oxidation affects water-holding capacity of beef during refrigerated storage. Food Chemistry: X. 2022;14 doi: 10.1016/j.fochx.2022.100304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcinkowska-Lesiak M., Wojtasik-Kalinowska I., Onopiuk A., Stelmasiak A., Wierzbicka A., Poltorak A. Application of atmospheric pressure cold plasma activated plant protein preparations solutions as an alternative curing method for pork sausages. Meat Science. 2022;187 doi: 10.1016/j.meatsci.2022.108751. [DOI] [PubMed] [Google Scholar]
- Misra N.N., Jo C. Applications of cold plasma technology for microbiological safety in meat industry. Trends in Food Science and Technology. 2017;64:74–86. doi: 10.1016/j.tifs.2017.04.005. [DOI] [Google Scholar]
- Nasiru M.M., Boateng E.F., Wang Z.B., Yan W.J., Zhuang H., Zhang J.H. Ultrasound-assisted high-voltage cold atmospheric plasma treatment on the inactivation and structure of lysozyme: Effect of treatment voltage. Food and Bioprocess Technology. 2022;15:1866–1880. doi: 10.1007/s11947-022-02842-z. [DOI] [Google Scholar]
- Nasiru M.M., Frimpong E.B., Muhammad U., Qian J., Mustapha A.T., Yan W.J.…Zhang J.H. Dielectric barrier discharge cold atmospheric plasma: Influence of processing parameters on microbial inactivation in meat and meat products. Comprehensive Reviews in Food Science and Food Safety. 2021;20(3):2626–2659. doi: 10.1111/1541-4337.12740. [DOI] [PubMed] [Google Scholar]
- Nawaz A., Irshad S., Ali Khan I., Khalifa I., Walayat N., Muhammad Aadil R.…Lorenzo J.M. Protein oxidation in muscle-based products: Effects on physicochemical properties, quality concerns, and challenges to food industry. Food Research International. 2022;157 doi: 10.1016/j.foodres.2022.111322. [DOI] [PubMed] [Google Scholar]
- Olatunde O.O., Benjakul S., Vongkamjan K. Dielectric barrier discharge high voltage cold atmospheric plasma: An innovative nonthermal technology for extending the shelf-life of Asian sea bass slices. Journal of Food Science. 2019;84(7):1871–1880. doi: 10.1111/1750-3841.14669. [DOI] [PubMed] [Google Scholar]
- Olatunde O.O., Benjakul S., Vongkamjan K. High voltage cold atmospheric plasma: Antibacterial properties and its effect on quality of Asian sea bass slices. Innovative Food Science and Emerging Technologies. 2019;52:305–312. doi: 10.1016/j.ifset.2019.01.011. [DOI] [Google Scholar]
- Olatunde O.O., Shiekh K.A., Benjakul S. Pros and cons of cold plasma technology as an alternative non-thermal processing technology in seafood industry. Trends in Food Science and Technology. 2021;111:617–627. doi: 10.1016/j.tifs.2021.03.026. [DOI] [Google Scholar]
- Pérez-Andrés J.M., de Alba M., Harrison S.M., Brunton N.P., Cullen P.J., Tiwari B.K. Effects of cold atmospheric plasma on mackerel lipid and protein oxidation during storage. LWT-Food Science and Technology. 2020;118 doi: 10.1016/j.lwt.2019.108697. [DOI] [Google Scholar]
- Qian J., Wang C., Zhuang H., Nasiru M.M., Zhang J.H., Yan W.J. Evaluation of meat-quality and myofibrillar protein of chicken drumsticks treated with plasma-activated lactic acid as a novel sanitizer. LWT-Food Science and Technology. 2021;138 doi: 10.1016/j.lwt.2020.110642. [DOI] [Google Scholar]
- Sun Q.X., Chen Q., Xia X.F., Kong B.H., Diao X.P. Effects of ultrasound-assisted freezing at different power levels on the structure and thermal stability of common carp (Cyprinus carpio) proteins. Ultrasonics Sonochemistry. 2019;54:311–320. doi: 10.1016/j.ultsonch.2019.01.026. [DOI] [PubMed] [Google Scholar]
- Sun Q.X., Sun F.D., Xia X.F., Xu H.H., Kong B.H. The comparison of ultrasound-assisted immersion freezing, air freezing and immersion freezing on the muscle quality and physicochemical properties of common carp (Cyprinus carpio) during freezing storage. Ultrasonics Sonochemistry. 2019;51:281–291. doi: 10.1016/j.ultsonch.2018.10.006. [DOI] [PubMed] [Google Scholar]
- Szmanko T., Lesiow T., Gorecka J. The water-holding capacity of meat: A reference analytical method. Food Chemistry. 2021;357 doi: 10.1016/j.foodchem.2021.129727. [DOI] [PubMed] [Google Scholar]
- Ucar Y., Ceylan Z., Durmus M., Tomar O., Cetinkaya T. Application of cold plasma technology in the food industry and its combination with other emerging technologies. Trends in Food Science and Technology. 2021;114:355–371. doi: 10.1016/j.tifs.2021.06.004. [DOI] [Google Scholar]
- Wang Z.M., He Z.F., Gan X., Li H.J. Interrelationship among ferrous myoglobin, lipid and protein oxidations in rabbit meat during refrigerated and superchilled storage. Meat Science. 2018;146:131–139. doi: 10.1016/j.meatsci.2018.08.006. [DOI] [PubMed] [Google Scholar]
- Wang Z.F., He Z.F., Zhang D., Chen X.S., Li H.J. The effect of linalool, limonene and sabinene on the thermal stability and structure of rabbit meat myofibrillar protein under malondialdehyde-induced oxidative stress. LWT-Food Science and Technology. 2021;148 doi: 10.1016/j.lwt.2021.111707. [DOI] [Google Scholar]
- Xun, P. W., Zhou, C. P., Huang, X. L., Huang, Z., Yu, W., Yang, Y. K., Li, T. Huang, J. B. Wu, Y., & Lin, H. T. (2022). Effects of dietary potassium diformate on growth performance, fillet quality, plasma indices, intestinal morphology and liver health of juvenile golden pompano (Trachinotus ovatus). Aquaculture Reports, 24, 101110. doi: 10.1016/j.aqrep.2022.101110.
- Yang Z.M., Liu S.C., Sun Q.X., Zheng O.Y., Wei S., Xia Q.Y.…Xu J. Insight into muscle quality of golden pompano (Trachinotus ovatus) frozen with liquid nitrogen at different temperatures. Food Chemistry. 2022;374 doi: 10.1016/j.foodchem.2021.131737. [DOI] [PubMed] [Google Scholar]
- Yu J.J., Ji H., Chen Y., Zhang Y.F., Zheng X.C., Li S.H., Chen Y. Analysis of the glycosylation products of peanut protein and lactose by cold plasma treatment: Solubility and structural characteristics. International Journal of Biological Macromolecules. 2020;158:1194–1203. doi: 10.1016/j.ijbiomac.2020.04.255. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.