Key Points
-
•
Comorbidities used to evaluate allogeneic transplantation candidates have differential impact on NRM in low-intensity conditioning settings.
-
•
Among prevalent comorbidities, cardiac disease (including arrhythmia and valvular disease) may confer significant additional NRM risk.
Visual Abstract
Abstract
Older age and a high burden of comorbidities often drive the selection of low-intensity conditioning regimens in allogeneic hematopoietic stem cell transplantation recipients. However, the impact of comorbidities in the low-intensity conditioning setting is unclear. We sought to determine the contribution of individual comorbidities and their cumulative burden on the risk of nonrelapse mortality (NRM) among patients receiving low-intensity regimens. In a retrospective analysis of adults (≥18 years) who underwent transplantation for acute myeloid leukemia in the first complete remission between 2008 and 2018, we studied recipients of low-intensity regimens as defined by the transplantation conditioning intensity (TCI) scale. Multivariable Cox models were constructed to study associations of comorbidities with NRM. Comorbidities identified as putative risk factors in the low-TCI setting were included in combined multivariable regression models assessed for overall survival, NRM, and relapse. A total of 1663 patients with a median age of 61 years received low-TCI regimens. Cardiac comorbidity (including arrhythmia/valvular disease) and psychiatric disease were associated with increased NRM risk (hazard ratio [HR], 1.54; 95% confidence interval [CI], 1.13-2.09 and HR, 1.69; 95% CI, 1.02-2.82, respectively). Moderate pulmonary dysfunction, though prevalent, was not associated with increased NRM. In a combined model, cardiac, psychiatric, renal, and inflammatory bowel diseases were independently associated with adverse transplantation outcomes. These findings may inform patient and regimen selection and reinforce the need for further investigation of cardioprotective transplantation approaches.
Introduction
Although nonrelapse mortality (NRM) has decreased over several years, it remains a considerable limitation to the use of allogeneic hematopoietic stem cell transplantation (HSCT).1,2 In an attempt to fit the treatment strategy to the patient and estimate transplantation-associated risk, patients undergo thorough evaluation of baseline comorbidities and physiologic status, as well as geriatric assessment when appropriate.3, 4, 5, 6 The cumulative burden of comorbidities, as codified by the hematopoietic cell transplantation–specific comorbidity index (HCT-CI), has proven informative in risk stratification and helps guide the selection of pretransplantation conditioning chemotherapy.7 However, the aggregation of comorbidities into a single value can lead to the loss of useful prognostic information.8, 9, 10 In previous retrospective analyses encompassing a broad range of transplantation indications, individual comorbidities included in the HCT-CI were associated with varying degrees of hazard. Moreover, some prevalent comorbidities appeared to have a limited independent contribution to the risk of NRM in the contemporary transplantation setting.8,11
Older or frailer patients undergoing HSCT are generally treated using conditioning regimens designed to reduce the risk of treatment-related morbidity and mortality. Historically, regimens have been categorized as either myeloablative or reduced-intensity/nonmyeloablative based on the degree of bone marrow suppression they induce.12,13 More recently, a new scale of conditioning regimen intensity, the transplantation conditioning intensity (TCI) score, has been introduced.14 This score is intended to overcome limitations in the traditional classification by capturing the extent of nonhematologic toxicity occurring with individual regimens. We hypothesized that in the low-intensity setting, as defined by the TCI, individual comorbidities may have a prognostically valuable, differential impact on NRM after transplantation. We therefore sought to characterize the influence of individual comorbidities on transplantation outcomes among low-TCI HSCT recipients. Although previous analyses have looked at the predictive value of cumulative comorbidity burden in patients receiving traditionally categorized reduced-intensity conditioning regimens,10,15 we believe this is the first large-scale attempt to identify particular hazards of individual concomitant diagnoses in the lowest intensity setting, especially as redefined by the TCI. Among patients already thought to be at elevated NRM risk by their transplant physician, these data may help to personalize decision-making based on the patient’s specific individual comorbidities.
To better isolate the relationship between comorbidity and outcome, we studied a population of patients restricted to those who underwent transplantation for acute myeloid leukemia (AML) in the first complete remission and who received HLA-matched grafts. Populations of patients with comorbidities are often excluded from clinical trials, and a randomized trial studying this question is not feasible. Therefore, transplantation registries are the most effective setting for exploring this clinically important question.
Methods
To study the impact of individual comorbidities in low-intensity conditioning regimens, we analyzed a retrospective cohort of adults undergoing allogeneic HSCT for AML included in the European Society for Blood and Marrow Transplantation (EBMT) registry. The EBMT maintains an audited registry of transplantations performed by more than 600 member institutions, primarily in Europe. Centers commit to obtaining informed consent in accordance with the local regulations applicable at the time of transplantation in order to report pseudonymized data to the EBMT. We included patients aged ≥18 years who underwent an allogeneic HSCT with low-intensity conditioning regimens (TCI ≤ 2) between 2008 and 2018 for AML in the first complete remission. The regimen TCI category was defined in accordance with the criteria specified by Spyridonidis et al (supplemental Table 1).14 To maximize generalizability, regimens including 6 common combinations of conditioning agents were included, as described in supplemental Table 2. All patients received grafts from matched sibling or 10/10 HLA-matched unrelated donors and had available data regarding the comorbidities included in the HCT-CI submitted to the registry. Patients with incomplete data for the covariates included in the regression model or for the outcome of NRM were excluded, as were patients who underwent ex vivo T-cell depletion. For a secondary comparison of comorbidities based on the TCI category, we included patients with similar inclusion criteria and conditioning with intermediate- and high-TCI regimens.
Comorbidities were defined per the standard definitions in the HCT-CI12 (supplemental Table 3). A composite cardiac comorbidity was constructed, including general cardiac disease, valvular disease, and arrhythmia, as has been described elsewhere.16 Disease-associated risk was adjusted using the disease risk stratification system,17 grouped into 3 tiers: low, intermediate-1, and combined intermediate-2/high risk. NRM was defined as death without the competing event of relapse after transplantation.
Population characteristics were tabulated for the study cohort. Overall outcomes of the cohort (overall survival [OS], NRM, and relapse incidence) were described using Kaplan-Meier and cumulative incidence estimators. Among patients treated with low-TCI regimens, individual comorbidities were studied for their association with NRM using cause-specific Cox proportional hazard models, including individual comorbidities, adjusted for age at transplantation, performance status, disease risk, donor type (matched sibling donor vs matched unrelated donor), donor/recipient cytomegalovirus serostatus, and donor/recipient sex mismatch. Finally, using stepwise selection, a multivariable model containing the most informative comorbidities was selected using the lower Akaike criteria. The same adjustment factors as described previously were included.
This study was approved by the Acute Leukemia Working Party and conducted in accordance with the Declaration of Helsinki guidelines. All analyses were performed using R version 4.2.1 with packages survival, cmprsk, ggplot2, and prodlim.
Results
Population characteristics of the cohort treated with low-TCI regimens
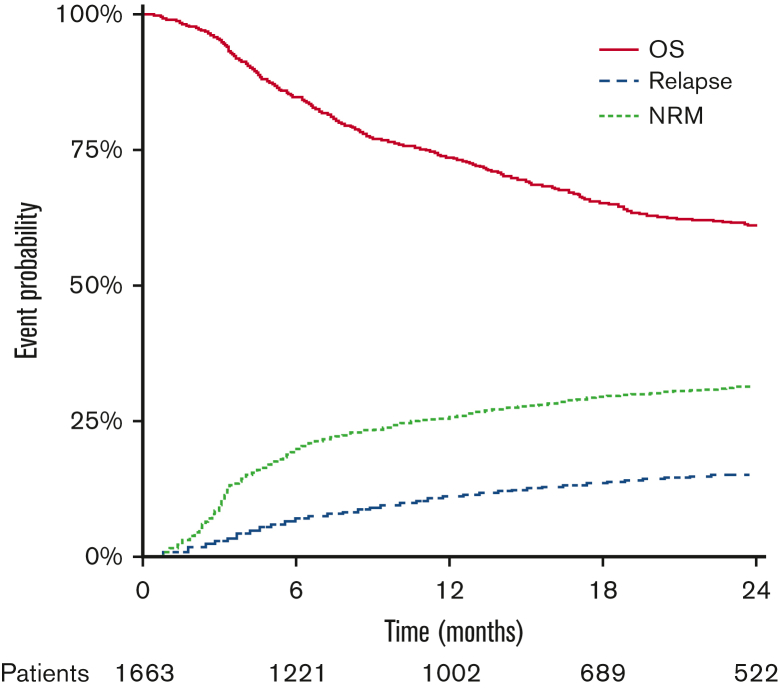
The cohort included 1663 patients with a median age of 61 years (interquartile range [IQR], 55-66) (Table 1). Patients were treated with a variety of low-TCI approaches, with the most common regimens being 150 mg/m2 fludarabine + 2 days busulfan (8 mg/kg by mouth or 6.4 mg/kg IV) and 150 mg/m2 fludarabine + 200 cGy total body irradiation (supplemental Table 2). Despite all patients being in the first complete remission, a large proportion of patients were in the intermediate-2/high disease risk stratification system category (40%, n = 666), with an additional 40% in the low-risk and 20% in intermediate-1 categories (n = 665 and n = 332, respectively). Nearly one-third of patients had a Karnofsky performance status <90 (31%, n = 513). A plurality of patients had none of the studied comorbidities (43%, n = 713), followed by 30% of patients with any 1 (n = 494), 18% with 2 (n = 298), and 9% with ≥3 individual studied comorbidities (n = 155). The most frequently observed comorbidities (Table 2) were moderate and severe pulmonary dysfunction (17%, n = 275% and 11%, n = 183), any cardiac disease (16%, n = 263), active infection (10%, n = 164), and prior malignancy (9%, n = 155). The median follow-up was 2.0 years (IQR, 1.1-3.5), as calculated using the reverse Kaplan-Meier method. Two-year OS and the incidence of NRM and relapse were 60.8% (58.1 and 63.5), 14.8% (12.9 and 16.7), and 31.2% (28.7 and 33.6), respectively (Figure 1). The most frequently identified cause of NRM was graft-versus-host disease, followed by infection and multiorgan failure (supplemental Figure 1).
Table 1.
Population characteristics
| Characteristic | TCI low (n = 1663)∗ |
|---|---|
| Age, median (interquartile range), y | 61 (55-66) |
| KPS, n (%) | |
| ≥90 | 1150 (69) |
| <90 | 513 (31) |
| Disease risk (disease risk stratification system), n (%) | |
| Low | 665 (40) |
| Intermediate-1 | 332 (20) |
| Intermediate-2/high | 666 (40) |
| Cytogenetic risk, n (%)† | |
| Favorable | 48 (4) |
| Intermediate | 1003 (73) |
| Poor | 314 (23) |
| Secondary AML, n (%) | 80 (5) |
| Donor, n (%) | |
| Matched sibling donor | 744 (45) |
| Matched unrelated donor | 919 (55) |
| Sex match, n (%) | |
| Other | 1367 (82) |
| Female-to-male | 296 (18) |
| Cytomegalovirus serostatus, n (%) | |
| D−/R− | 371 (22) |
| D−/R+ | 411 (25) |
| D+/R− | 162 (10) |
| D+/R+ | 719 (43) |
| HCT-CI, n (%) | |
| 0 | 713 (43) |
| 1-2 | 434 (26) |
| ≥3 | 516 (31) |
| Regimen, n (%) | |
| Flu + Bu | 1129 (68) |
| Bu + cyclophosphamide | 20 (1) |
| Flu + melphalan | 27 (2) |
| Flu + total body irradiation | 364 (22) |
| Flu + treosulfan | 65 (4) |
| Flu + Bu + thiotepa | 58 (3) |
| Graft-versus-host disease prophylaxis, n (%) | |
| Calcineurin inhibitor + methotrexate | 547 (33) |
| Calcineurin inhibitor + mycophenolate mofetil | 732 (44) |
| Cyclosporine A only | 272 (16) |
| Other/unknown | 111 (7) |
| T-cell depletion, n (%)‡ | |
| None | 610 (37) |
| Alemtuzumab | 113 (7) |
| Antithymocyte globulin | 879 (53) |
| PTCy | 58 (3) |
Bu, busulfan; D, donor; Flu, fludarabine; KPS, Karnofsky performance status; PTCy, posttransplantation cyclophosphamide; R, recipient.
Data presented as median (interquartile range) or n (%) as shown.
Per Medical Research Council schema. Missing for 298 patients.
11 patients received PTCy + antithymocyte globulin and 1 received PTCy + alemtuzumab; these are listed as PTCy-based. T-cell depletion status is unknown for 3 patients.
Table 2.
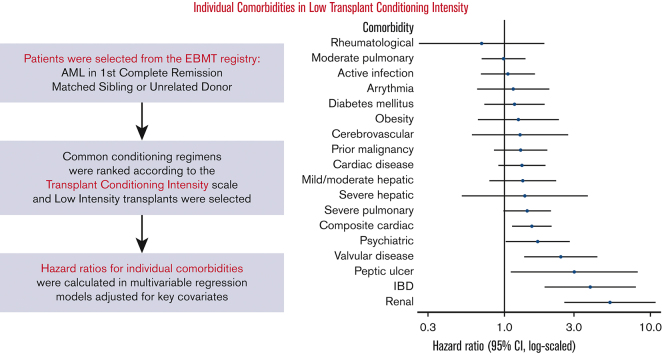
HRs of NRM associated with individual comorbidities in patients receiving low-TCI regimens
| Comorbidity | N (%) | Adjusted∗ HR (95% CI) | P |
|---|---|---|---|
| Rheumatologic | 40 (2) | 0.70 (0.26-1.90) | .489 |
| Moderate pulmonary | 275 (17) | 0.99 (0.70-1.41) | .954 |
| Active infection | 164 (10) | 1.06 (0.69-1.63) | .785 |
| Arrythmia | 73 (4) | 1.15 (0.65-2.02) | .636 |
| Diabetes mellitus | 112 (7) | 1.17 (0.73-1.88) | .509 |
| Obesity | 58 (3) | 1.25 (0.66-2.36) | .494 |
| Cerebrovascular | 38 (2) | 1.28 (0.60-2.74) | .529 |
| Prior malignancy | 155 (9) | 1.29 (0.85-1.94) | .230 |
| Cardiac disease† | 182 (11) | 1.32 (0.91-1.90) | .143 |
| Mild/moderate hepatic | 90 (5) | 1.34 (0.79-2.28) | .273 |
| Severe hepatic | 26 (2) | 1.38 (0.51-3.74) | .530 |
| Severe pulmonary | 183 (11) | 1.43 (0.99-2.07) | .058 |
| Composite cardiac‡ | 263 (16) | 1.54 (1.13-2.09) | .006 |
| Psychiatric | 75 (5) | 1.69 (1.02-2.82) | .043 |
| Valvular disease | 38 (2) | 2.44 (1.37-4.35) | .002 |
| Peptic ulcer | 14 (1) | 3.01 (1.11-8.15) | .031 |
| IBD | 26 (2) | 3.88 (1.90-7.96) | <.001 |
| Renal | 20 (1) | 5.29 (2.58-10.85) | <.001 |
| HCT-CI§ | |||
| 1-2 points | 434 (26) | 1.17 (0.83-1.64) | .363 |
| ≥3 points | 516 (31) | 1.70 (1.25-2.30) | .001 |
| Additional covariates‖ | |||
| KPS <90 | 523 (29) | 1.13 (0.86-1.49) | .365 |
| Age (by decade) | 1.71 (1.42-2.07) | <.001 |
Adjusted for age, KPS, disease risk (disease risk stratification system), donor type, sex mismatch, and donor/recipient cytomegalovirus serostatus.
HCT-CI definition.
HCT-CI definition plus valvular disease and arrhythmia.
The HCT-CI was not included in the model; HRs adjusted using the same covariates as in ∗ are shown here for comparison.
Studied as the primary effect in models with the same adjustment covariates.
Figure 1.
Overall outcomes for low-TCI recipients. OS, NRM, and relapse incidence are shown. At-risk numbers are shown for the OS outcome.
Comparison of comorbidity prevalence across TCI levels
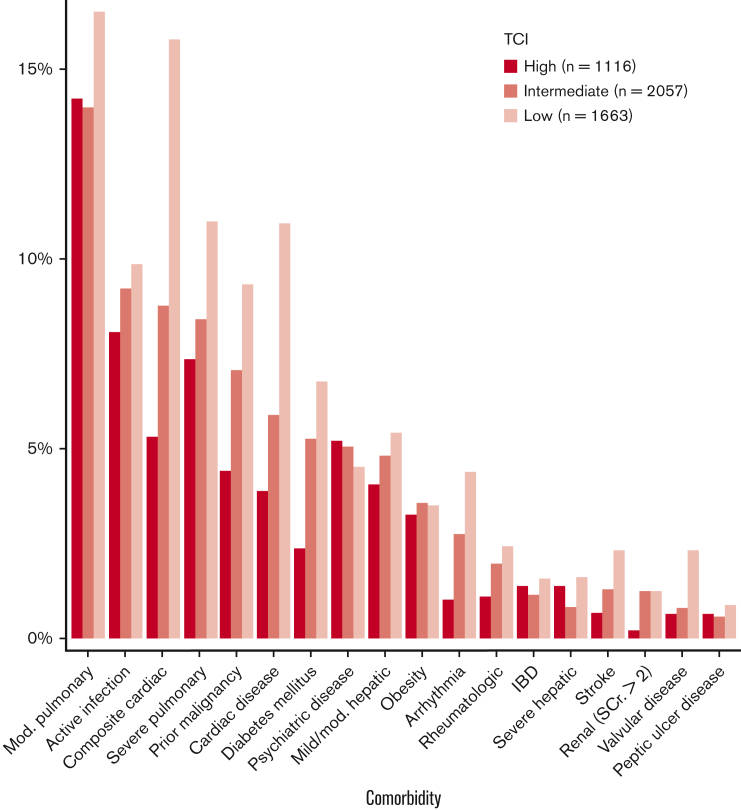
To better understand the role of comorbidities in selecting low-TCI recipients, we included an expanded cohort with similar inclusion/exclusion criteria, adding patients who received intermediate- and high-TCI regimens. This analysis included a total of 4836 patients (intermediate-TCI [2.5-3.5] 43%, n = 2057; high-TCI [≥4] 23%, n = 1116). Reporting of comorbidities is not mandated by the EBMT, and thus, complete comorbidity data were available for only approximately half of the patients meeting the remaining inclusion criteria (n = 4836/9386, 52%). The overall median age was 54 years (IQR, 44-62), with median ages of 54 years (IQR, 45-61) and 41 years (IQR, 31-48) for intermediate- and high-TCI groups, respectively. Additional characteristics are described in supplemental Table 4. As in the low-TCI group, moderate pulmonary disease was the most prevalent comorbidity (15%, n = 722) (Figure 2), with close prevalence across all 3 TCI levels. In contrast, although among the next most common comorbidities overall was any cardiac disease (10%, n = 502; per the composite definition), prevalence was markedly different between the groups: 5% (n = 59 of 1116) in the high-TCI recipients and 9% (n = 180 of 2057) in the intermediate-TCI group as compared with 16% in the low-TCI group as described above. Additional comorbidities are depicted in Figure 2. Most notably among these, prior malignancy (7% overall, n = 349) was present in 4% of the high-TCI recipients (n = 49 of 1116), 7% of the intermediate-TCI recipients (n = 145 of 2057) vs 9% of the low-TCI recipients as described above.
Figure 2.
Prevalence of individual comorbid diseases in each TCI stratum. Mod., moderate; SCr., serum creatinine; IBD, inflammatory bowel disease.
Role of comorbidities in low-TCI recipients
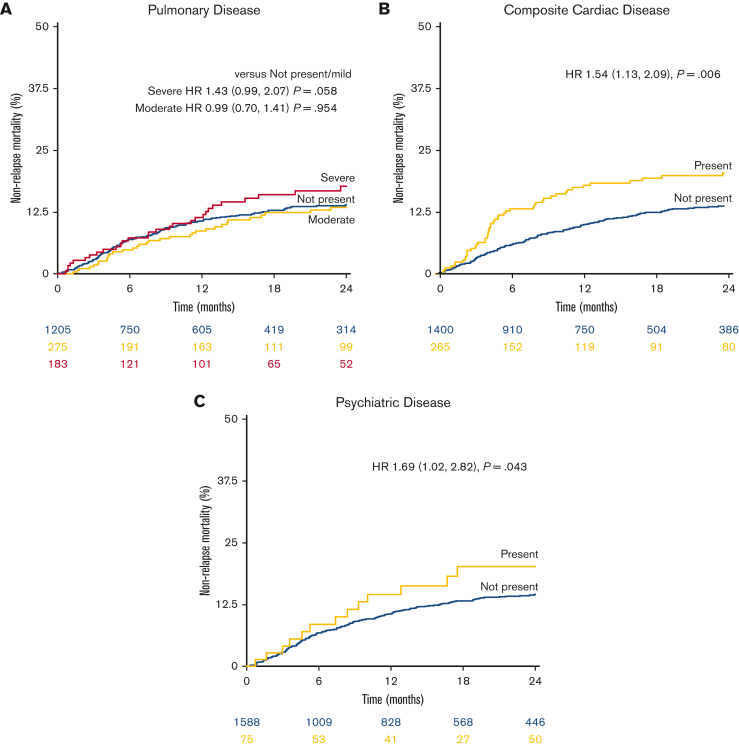
Most studied comorbidities were not significantly associated with NRM in this large cohort of low-TCI recipients (Table 2). These included the most prevalent comorbidities, such as moderate pulmonary dysfunction (hazard ratio [HR], 0.99; 95% confidence interval [CI], 0.70-1.41) (Figure 3A), mild/moderate hepatic dysfunction (n = 90 [5%]; HR, 1.34; 95% CI, 0.79-2.28), and prior malignancy (HR, 1.29; 95% CI, 0.85-1.94). A notable exception was composite cardiac disease (HR, 1.54; 95% CI, 1.13-2.09) (Figure 2B), whereas the HCT-CI definition of cardiac disease, excluding valvular disease and arrhythmia, was not significantly associated with higher risk (HR, 1.32; 95% CI, 0.91-1.90). Psychiatric diagnoses were associated with an elevated risk of NRM (n = 75; HR, 1.69; 95% CI, 1.02-2.82) (Figure 2C). The strongest comorbidity predictors of NRM were rare comorbidities, including valvular disease (HR, 2.44; 95% CI, 1.37-4.35), peptic ulcer disease (HR, 3.01; 95% CI, 1.11-8.15), inflammatory bowel disease (IBD; HR, 3.88; 95% CI, 1.90-7.96), and renal disease (HR, 5.29; 95% CI, 2.58-10.85) (per the HCT-CI definition, including those with serum creatinine >2). Independent of individual comorbidities, increasing age was strongly associated with NRM (by decade HR, 1.71; 95% CI, 1.42-2.07); in contrast, performance status (Karnofsky performance status <90) was not significantly associated with NRM in the multivariable setting even when comorbidities were excluded from the model (HR, 1.13; 95% CI, 0.86-1.49) (Table 3).
Figure 3.
NRM among individual comorbid conditions. (A) Patients with no/mild, moderate, and severe pulmonary comorbidity. (B) Patients with or without any cardiac comorbidity. (C) Patients with vs without baseline psychiatric disease. Adjusted HRs are shown.
Table 3.
Cox regression models including selected comorbidities for NRM, relapse, and OS
| Variable | NRM |
Relapse |
OS |
|||
|---|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | |
| Preexisting malignancy | 1.34 (0.88-2.02) | .16 | 1.39 (1.05-1.82) | .020 | 1.47 (1.16-1.87) | .002 |
| Composite cardiac | 1.52 (1.12-2.07) | .008 | 1.13 (0.89-1.43) | .307 | 1.39 (1.14-1.69) | .001 |
| Psychiatric | 1.70 (1.01-2.83) | .044 | 0.83 (0.53-1.30) | .412 | 1.09 (0.75-1.58) | .669 |
| Renal | 5.59 (2.72-11.50) | <.001 | 1.27 (0.53-3.08) | .594 | 2.75 (1.54-4.90) | <.001 |
| IBD | 3.66 (1.78-7.55) | <.001 | 1.86 (1.02-3.41) | .043 | 1.99 (1.19-3.35) | .009 |
| Age (by decade), y | 1.76 (1.46-2.13) | <.001 | 0.98 (0.89-1.08) | .634 | 1.22 (1.11-1.35) | <.001 |
| KPS | ||||||
| ≥90 | reference | |||||
| <90 | 0.87 (0.66-1.14) | .317 | 1.00 (0.83-1.22) | .966 | 0.90 (0.76-1.07) | .234 |
| Disease risk (disease risk stratification system) | ||||||
| Low | reference | |||||
| Intermediate-1 | 1.04 (0.73-1.47) | .846 | 1.34 (1.03-1.74) | .031 | 1.24 (0.99-1.56) | .067 |
| Intermediate-2/high | 1.10 (0.82-1.47) | .521 | 1.89 (1.54-2.32) | <.001 | 1.60 (1.33-1.92) | <.001 |
| Donor | ||||||
| Matched sibling donor | Reference | |||||
| Matched unrelated donor | 1.24 (0.93-1.65) | .135 | 0.83 (0.69-1.01) | .058 | 1.02 (0.86-1.21) | .850 |
| Female-to-male | 1.96 (1.44-2.67) | <.001 | 0.92 (0.72-1.18) | 0524 | 1.24 (1.01-1.53) | .041 |
| Cytomegalovirus | ||||||
| D−/R− | reference | |||||
| D−/R+ | 1.43 (0.98-2.08) | .065 | 0.99 (0.77-1.28) | .954 | 1.13 (0.90-1.42) | .293 |
| D+/R− | 1.03 (0.61-1.75) | .914 | 0.92 (0.65-1.31) | .657 | 0.94 (0.69-1.30) | .718 |
| D+/R+ | 1.12 (0.78-1.60) | .528 | 0.90 (0.71-1.13) | .370 | 0.95 (0.77-1.18) | .666 |
Using stepwise selection in accordance with the Akaike information criterion, a composite model of comorbidities linked to the outcome of NRM in the low-TCI setting was selected. Psychiatric, cardiac (composite), renal, and IBD as well as prior malignancy were included together with the adjustment covariates previously described. Cardiac comorbidity, arrhythmia, and valve disease were combined into a composite cardiac category. Most patients (71%, n = 1183) had none of the included comorbidities, and only 3% (n = 54) had ≥2. In a composite model for the event of NRM, cardiac (HR, 1.52; 95% CI, 1.12-2.07) and psychiatric disease (HR, 1.70; 95% CI, 1.01-2.83) were associated with similar degrees of risk (Table 3). IBD and renal disease remained significant, albeit rare, in the population, whereas prior malignancy remained as statistically not significant in this model. In a multivariable composite model for OS, cardiac disease (HR, 1.39; 95% CI, 1.14-1.69), IBD (HR, 1.99; 95% CI, 1.19-3.35), and renal disease (HR, 2.75; 95% CI, 1.54-4.90) were each identified as significant risk factors; prior malignancy was also strongly associated with early mortality (HR, 1.47; 95% CI, 1.16-1.87) (Table 3).
Finally, using the comorbidities determined individually to contribute to NRM, a simplified model was constructed to compare patients with any of these comorbidities (cardiac [composite], psychiatric, renal, IBD, or prior malignancy) with those with none. The same adjustment covariates were used. Low-TCI recipients with any of the proposed risk factors (29%, n = 480) had an increased HR for NRM of 1.74 (95% CI, 1.34-2.26) and OS of 1.47 (95% CI, 1.24-1.73) (supplemental Table 5). Increased relapse was also observed (HR, 1.22; 95% CI, 1.01-1.47).
Discussion
In this registry-based analysis of patients undergoing allogeneic HSCT, we found that individual comorbidities vary widely in their clinical significance for NRM in the low-TCI setting. Intensity selection is strongly linked to comorbidity burden.7 However, the prognostic importance of individual comorbidities among patients selected as requiring the lowest intensity conditioning remains poorly understood. In accordance with our initial hypothesis, we identify a subset of comorbidities independently associated with NRM among patients receiving low-TCI conditioning. At the same time, we were unable to draw a conclusive connection for other major comorbidities, such as pulmonary and liver disorders, despite the large size of the cohort.
Spyridonidis et al proposed the TCI to overcome a major limitation in the traditional approach for ranking conditioning regimen toxicity.14 Classifying regimens as myeloablative or reduced intensity, as introduced by Bacigalupo et al,12 captures the degree of bone marrow suppression associated with a regimen well. However, damage to other organ systems is not strictly correlated with myeloablation. The TCI attempts to remedy this by ranking regimens based on their associated risk of transplantation-related mortality historically observed among patients with AML aged 45 to 65 years. Using such a model, we believe we isolated a broad swath of patients who were selected for and underwent transplantation with chemotherapy designed to absolutely minimize transplantation-related toxicity.
Importantly, patients in this cohort were selected for these low-intensity regimens, either because of factors captured in the data sets, such as age or comorbidity burden by HCT-CI or because of other unmeasured but likely similar attributes. Certain comorbidities were clearly associated with physician preference for low-intensity regimens, most strongly with cardiac disease and prior malignancy; others bore only weak association with a low-intensity regimen. When considering NRM in low-intensity regimens among patients with and without a given comorbidity, we can infer that a degree of the treating physician-anticipated adverse risk has already been factored in. A finding of an increased HR in this cohort, then, indicates a risk factor that may reflect still further adverse outcomes. We observed that rare comorbidities, such as IBD and peptic ulcer disease, were associated with the greatest NRM disadvantage. Renal disease, here defined as a serum creatinine >2.0 mg/dL, was uncommon at baseline before transplantation but associated with the greatest risk of NRM. Previous studies using a more liberal definition of estimated creatinine clearance <60 mg/dL per 1.73 m2 have also found chronic kidney disease to be powerfully prognostic of NRM.6,8,18,19 Given the growing evidence in the literature of its prognostic importance, collecting detailed pretransplant renal function data should be a key focus of transplantation registries moving forward.
Among the most prevalent comorbidities, the spectrum of cardiac disease is noteworthy for its strong association with NRM. Cardiac disease has previously been shown to be associated with a poorer prognosis after transplantation.8 Notably, patients treated with HSCT have generally been exposed to multiple cardiotoxic agents in earlier lines of therapy, perhaps contributing to the high prevalence (16% composite, ie, heart failure, coronary artery disease, valvular disease, and arrhythmia vs 10% by HCT-CI definition) of cardiac comorbidity in the cohort. Based on the significantly increased hazard, it appears that decreased conditioning intensity cannot overcome the risk associated with cardiac disease. Stillwell et al have previously shown in an analysis of patients treated with either autologous or allogeneic HSCT between 1999 and 2009 that there was no difference in outcomes (including both 1-year survival and posttransplantation cardiac events) between patients with and without a history of coronary artery disease.20 Similarly, in a study of patients with reduced left ventricular ejection fraction (<45%), outcomes paralleled those of matched controls with normal systolic function.21 However, these single-center studies were performed on patients who underwent transplantation more than a decade ago and across indications and transplantation approaches. Among allogeneic HSCT recipients, patients treated for AML are likely to be at particularly high cardiac risk given the role of intensive induction with anthracyclines and cytarabine.22 Notably, only 3% of patients received posttransplantation cyclophosphamide for T-cell depletion in this study. Although cyclophosphamide has been linked broadly to cardiotoxicity, recent studies have not shown increased cardiac morbidity in patients receiving posttransplantation cyclophosphamide.23,24 Increasing use of this strategy in matched donor transplantation may reveal a more subtle association. Future studies benefiting from more detailed cardiac screening data may better elucidate the relative NRM risk associated with individual parameters of heart disease. Advances in the management of heart failure, including the broader adoption of guideline-directed medical therapy for reduced ejection fraction,25,26 may change overall outcomes for patients with heart failure who underwent transplantation. Collaborative studies with the nascent field of cardio-oncology are critical, and normalizing pretransplantation referral to a cardio-oncologist could provide meaningful benefit.
Pulmonary disease, broadly defined in this analysis (encompassing both obstructive and restrictive pulmonary function patterns), was the most prevalent comorbidity studied. Among low-TCI recipients, nearly one-quarter of all patients had any degree of pulmonary dysfunction, which may be the sequelae of prior treatments, lung infections in the setting of treatment-induced neutropenia, or preexisting disease.27 Remarkably, however, moderate pulmonary disease was not linked to an increased risk of NRM, with an HR of nearly 1.0. Pulmonary function test (PFT) abnormalities that define this comorbidity were established as adverse risk markers in a large retrospective analysis of patients treated between 1991 and 2001 at a single center.28 However, these results were obtained in a cohort treated almost exclusively with high-dose total body irradiation–containing regimens. Studies of pulmonary disease in reduced-intensity regimens have been more limited in scale. A single-center study of PFTs in the reduced-intensity conditioning setting, in which patients with AML were conditioned with low-dose fludarabine/busulfan, showed that worse outcomes, including increased NRM, were associated with PFT abnormalities.29 Notably, different cutoffs were used for forced expiratory volume in 1 second and diffusion lung capacity for carbon monoxide (DLCo) compared with the cutoffs in the HCT-CI definitions, making comparisons between the studies challenging. Though nonsignificant in this cohort, our results may be consistent with their finding that severe pulmonary dysfunction is adverse even in the low-intensity conditioning setting. The accepted definition of pulmonary disease, combining both diminished forced expiratory volume in 1 second and DLCo, obscures a greater adverse impact of one criterion over the other. Notably, neither PFT abnormality reached statistical significance by multivariate analysis in the described analysis.29 A more recent study by Le Bourgeois et al suggests that incorporating the diffusion lung capacity for nitric oxide, a more sensitive test of alveolar membrane conductance than DLCo, may improve the detection of patients at high risk for pulmonary complications and subsequent NRM after transplantation.30 Given the prevalence of the disease, further studies of protective strategies among patients with advanced pulmonary disease are undoubtedly warranted.
The strong association between psychiatric disease at baseline and NRM in this cohort is a striking finding that has been underexplored in the literature to date. Depression and depressive symptoms have long been established as adverse markers of transplantation outcome.31,32 Studies of psychiatric interventions in allogeneic transplantation often address the psychologic distress resulting from transplantation itself. A 2015 systematic review of 9 reported interventions for distress after transplantation demonstrated significant improvements in a variety of patient-reported quality-of-life measurements among patients treated with nonpharmacologic therapies.33 Cognitive behavioral therapies in particular were found to have some efficacy, though survival was not a studied end point. An investigation of incorporating in-patient palliative care into HSCT treatment showed improved patient-reported quality of life, though survival outcomes were similarly not studied.34 A single-institution randomized trial of sertraline in transplantation recipients found a significant reduction in 6-month overall mortality (1 out of 28 in the treatment arm vs 6 out of 28 in the control arm) without noteworthy toxicity;35 importantly, patients with baseline psychiatric conditions as well as several other common comorbidities were not included in this study. Psychiatric assessment and intervention early in the disease course, before transplantation, could yield improvements in both quality of life and survival for this population.
This study bears several limitations, many inherent to registry analyses. As in all retrospective analyses of registry data sets, these results are observational, and we cannot fully account for selection bias. To some extent, our analysis attempts to capitalize on selection bias by interrogating the role of these concomitant conditions among patients already deemed to be at the highest risk. That said, subtler biases included among the individual regimens likely still confound a full understanding of the comorbidity impact. Comorbidity data were only captured in approximately half of the EBMT cases, which again may reflect institutional biases. Despite including only a subset of all patients, overall outcomes for the cohort are consistent with reported outcomes for other large cohorts during this time period.36,37 Although this is a fundamental challenge of registry studies, the future incorporation of data capture directly from the electronic medical record may help address this limitation. We wholeheartedly recommend that collecting detailed comorbidity data, including those from baseline laboratory studies, such as serum creatinine/creatinine clearance as well as cardiac and pulmonary evaluations, be strongly encouraged by transplantation registries. Analyses of these data have an ongoing and important impact on patient-level risk assessment and determining the appropriateness of patients for allogeneic HSCT. A decade-long interval of patient data were included in this study, during which time supportive care and patient outcomes might have evolved; however, we note that a large proportion of included patients come from more recent years, weighting the analysis toward contemporary practice and results. As always, “the absence of evidence is not evidence of absence”; comorbidities without statistically significant association with NRM in this study may meet the P < .05 threshold in a still-larger cohort. However, to our knowledge, this study represents the largest real-world analysis of HSCT comorbidities in a single-indication setting. Therefore, we believe the likelihood of clinically significant effects as the HR of a comorbidity approaches 1.0 can be expected to be modest. Patient-reported outcomes, newly a major focus of transplantation data collection, will surely deepen our understanding of the impacts of transplantation morbidity and clarify comorbidity’s role in transplantation survivorship, a critical and burgeoning field. The ongoing Bone and Marrow Transplant Clinical Trials Network 1704 trial is designed specifically to further our understanding of transplant risk in older and frailer patients and may help to refine patient selection in this population. Finally, individual comorbidity definitions in our analysis were based on those codified in the HCT-CI.7 As repeatedly highlighted above, objective, biomarker-driven investigation of comorbid disease could yield more specific insight into the role conditions play in transplantation-related morbidity and mortality.
In conclusion, we find that a subset of key comorbid conditions is most strongly associated with NRM after allogeneic HSCT with TCI–low-intensity regimens, particularly cardiac and psychiatric disease. As the median age of transplantation recipients continues to increase, further investigation toward optimizing therapy for patients with these comorbidities should be a priority for the transplantation community.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Acknowledgments
The authors thank the data managers for their excellent work and the patients who provided data. They also thank all of the European Society for Blood and Marrow Transplantation centers and national registries for contributing patients to this study (supplemental Appendix).
This work is supported by funding and a grant from the American Society for Hematology HONORS award (J.A.F.) and the Memorial Sloan Kettering Cancer Center Core grant, National Cancer Institute, National Institutes of Health (grant P30 CA008748) (R.S.).
The scientific boards of the Acute Leukemia Working Party (ALWP) of the EBMT approved this study.
Authorship
Contribution: J.A.F. and R.S. designed the study and performed the analysis; J.-E.G. and M.L. provided statistical oversight and guidance; R.S., M.M., and A.N. supervised the study; J.A.F. wrote the initial draft; and all authors provided critical feedback, helped to shape the research, discussed the results, and commented on and approved the final manuscript.
Footnotes
∗J.A.F. and R.S. contributed equally to this study.
Qualified researchers may request access to data by applying to the European Society for Blood and Marrow Transplantation and Acute Leukemia Working Party.
Data are available on request from the corresponding author, Arnon Nagler (arnon.nagler@sheba.health.gov.il).
The full-text version of this article contains a data supplement.
Supplementary Material
References
- 1.McDonald GB, Sandmaier BM, Mielcarek M, et al. Survival, nonrelapse mortality, and relapse-related mortality after allogeneic hematopoietic cell transplantation: comparing 2003-2007 versus 2013-2017 cohorts. Ann Intern Med. 2020;172(4):229–239. doi: 10.7326/M19-2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gooley TA, Chien JW, Pergam SA, et al. Reduced mortality after allogeneic hematopoietic-cell transplantation. N Engl J Med. 2010;363(22):2091–2101. doi: 10.1056/NEJMoa1004383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hamadani M, Craig M, Awan FT, Devine SM. How we approach patient evaluation for hematopoietic stem cell transplantation. Bone Marrow Transplant. 2010;45(8):1259–1268. doi: 10.1038/bmt.2010.94. [DOI] [PubMed] [Google Scholar]
- 4.Muffly LS, Kocherginsky M, Stock W, et al. Geriatric assessment to predict survival in older allogeneic hematopoietic cell transplantation recipients. Haematologica. 2014;99(8):1373–1379. doi: 10.3324/haematol.2014.103655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lin RJ, Elko TA, Devlin SM, et al. Impact of geriatric vulnerabilities on allogeneic hematopoietic cell transplantation outcomes in older patients with hematologic malignancies. Bone Marrow Transplant. 2020;55(1):157–164. doi: 10.1038/s41409-019-0654-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Olin RL, Fretham C, Pasquini MC, et al. Geriatric assessment in older alloHCT recipients: association of functional and cognitive impairment with outcomes. Blood Adv. 2020;4(12):2810–2820. doi: 10.1182/bloodadvances.2020001719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sorror ML, Maris MB, Storb R, et al. Hematopoietic Cell Transplantation (HCT)-specific Comorbidity Index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106(8):2912–2919. doi: 10.1182/blood-2005-05-2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fein JA, Shimoni A, Labopin M, et al. The impact of individual comorbidities on non-relapse mortality following allogeneic hematopoietic stem cell transplantation. Leukemia. 2018;32(8):1787–1794. doi: 10.1038/s41375-018-0185-y. [DOI] [PubMed] [Google Scholar]
- 9.Shouval R, Fein JA, Shouval A, et al. External validation and comparison of multiple prognostic scores in allogeneic hematopoietic stem cell transplantation. Blood Adv. 2019;3(12):1881–1890. doi: 10.1182/bloodadvances.2019032268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Versluis J, Labopin M, Niederwieser D, et al. Prediction of non-relapse mortality in recipients of reduced intensity conditioning allogeneic stem cell transplantation with AML in first complete remission. Leukemia. 2015;29(1):51–57. doi: 10.1038/leu.2014.164. [DOI] [PubMed] [Google Scholar]
- 11.Penack O, Peczynski C, Mohty M, et al. Association of pre-existing comorbidities with outcome of allogeneic hematopoietic cell transplantation. A retrospective analysis from the EBMT. Bone Marrow Transplant. 2022;57(2):183–190. doi: 10.1038/s41409-021-01502-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bacigalupo A, Ballen K, Rizzo D, et al. Defining the intensity of conditioning regimens: working definitions. Biol Blood Marrow Transplant. 2009;15(12):1628–1633. doi: 10.1016/j.bbmt.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Giralt S, Ballen K, Rizzo D, et al. Reduced-intensity conditioning regimen workshop: defining the dose spectrum. report of a workshop convened by the Center for International Blood and Marrow Transplant Research. Biol Blood Marrow Transplant. 2009;15(3):367–369. doi: 10.1016/j.bbmt.2008.12.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spyridonidis A, Labopin M, Savani BN, et al. Redefining and measuring transplant conditioning intensity in current era: a study in acute myeloid leukemia patients. Bone Marrow Transplant. 2020;55(6):1114–1125. doi: 10.1038/s41409-020-0803-y. [DOI] [PubMed] [Google Scholar]
- 15.Barba P, Piñana JL, Martino R, et al. Comparison of two pretransplant predictive models and a flexible HCT-CI using different cut off points to determine low-, intermediate-, and high-risk groups: the flexible HCT-CI is the best predictor of NRM and OS in a population of patients undergoing allo-RIC. Biol Blood Marrow Transplant. 2010;16(3):413–420. doi: 10.1016/j.bbmt.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 16.Shouval R, Fein JA, Cho C, et al. The Simplified Comorbidity Index: a new tool for prediction of nonrelapse mortality in allo-HCT. Blood Adv. 2022;6(5):1525–1535. doi: 10.1182/bloodadvances.2021004319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shouval R, Fein JA, Labopin M, et al. Development and validation of a disease risk stratification system for patients with haematological malignancies: a retrospective cohort study of the European Society for Blood and Marrow Transplantation registry. Lancet Haematol. 2021;8(3):e205–e215. doi: 10.1016/S2352-3026(20)30394-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Farhadfar N, Dias A, Wang T, et al. Impact of pretransplantation renal dysfunction on outcomes after allogeneic hematopoietic cell transplantation. Transplant Cell Ther. 2021;27(5):410–422. doi: 10.1016/j.jtct.2021.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shouval R, de Jong CN, Fein J, et al. Baseline renal function and albumin are powerful predictors for allogeneic transplantation-related mortality. Biol Blood Marrow Transplant. 2018;24(8):1685–1691. doi: 10.1016/j.bbmt.2018.05.005. [DOI] [PubMed] [Google Scholar]
- 20.Stillwell EE, Wessler JD, Rebolledo BJ, et al. Retrospective outcome data for hematopoietic stem cell transplantation in patients with concurrent coronary artery disease. Biol Blood Marrow Transplant. 2011;17(8):1182–1186. doi: 10.1016/j.bbmt.2010.12.698. [DOI] [PubMed] [Google Scholar]
- 21.Qazilbash MH, Amjad AI, Qureshi S, et al. Outcome of allogeneic hematopoietic stem cell transplantation in patients with low left ventricular ejection fraction. Biol Blood Marrow Transplant. 2009;15(10):1265–1270. doi: 10.1016/j.bbmt.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Neuendorff NR, Loh KP, Mims AS, et al. Anthracycline-related cardiotoxicity in older patients with acute myeloid leukemia: a Young SIOG review paper. Blood Adv. 2020;4(4):762–775. doi: 10.1182/bloodadvances.2019000955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yeh J, Whited L, Saliba RM, et al. Cardiac toxicity after matched allogeneic hematopoietic cell transplant in the posttransplant cyclophosphamide era. Blood Adv. 2021;5(24):5599–5607. doi: 10.1182/bloodadvances.2021004846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin C-J, Vader JM, Slade M, et al. Cardiomyopathy in patients after posttransplant cyclophosphamide–based hematopoietic cell transplantation. Cancer. 2017;123(10):1800–1809. doi: 10.1002/cncr.30534. [DOI] [PubMed] [Google Scholar]
- 25.Al-Kindi SG, Oliveira GH. Prevalence of Preexisting cardiovascular disease in patients with different types of cancer: the unmet need for onco-cardiology. Mayo Clin Proc. 2016;91(1):81–83. doi: 10.1016/j.mayocp.2015.09.009. [DOI] [PubMed] [Google Scholar]
- 26.Cardinale D, Colombo A, Bacchiani G, et al. Early detection of anthracycline cardiotoxicity and improvement with heart failure therapy. Circulation. 2015;131(22):1981–1988. doi: 10.1161/CIRCULATIONAHA.114.013777. [DOI] [PubMed] [Google Scholar]
- 27.Cheng G-S. Pulmonary function and pretransplant evaluation of the hematopoietic cell transplant candidate. Clin Chest Med. 2017;38(2):307–316. doi: 10.1016/j.ccm.2016.12.014. [DOI] [PubMed] [Google Scholar]
- 28.Parimon T, Madtes DK, Au DH, Clark JG, Chien JW. Pretransplant lung function, respiratory failure, and mortality after stem cell transplantation. Am J Respir Crit Care Med. 2005;172(3):384–390. doi: 10.1164/rccm.200502-212OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Piñana JL, Martino R, Barba P, et al. Pulmonary function testing prior to reduced intensity conditioning allogeneic stem cell transplantation in an unselected patient cohort predicts posttransplantation pulmonary complications and outcome. Am J Hematol. 2012;87(1):9–14. doi: 10.1002/ajh.22183. [DOI] [PubMed] [Google Scholar]
- 30.Le Bourgeois A, Malard F, Chevallier P, et al. Impact of pre-transplant diffusion lung capacity for nitric oxide (DLNO) and of DLNO/pre-transplant diffusion lung capacity for carbon monoxide (DLNO/DLCO) ratio on pulmonary outcomes in adults receiving allogeneic stem cell transplantation for hematological diseases. Bone Marrow Transplant. 2016;51(4):589–592. doi: 10.1038/bmt.2015.284. [DOI] [PubMed] [Google Scholar]
- 31.Loberiza FR, Rizzo JD, Bredeson CN, et al. Association of depressive syndrome and early deaths among patients after stem-cell transplantation for malignant diseases. J Clin Oncol. 2002;20(8):2118–2126. doi: 10.1200/JCO.2002.08.757. [DOI] [PubMed] [Google Scholar]
- 32.Prieto JM, Atala J, Blanch J, et al. Role of depression as a predictor of mortality among cancer patients after stem-cell transplantation. J Clin Oncol. 2005;23(25):6063–6071. doi: 10.1200/JCO.2005.05.751. [DOI] [PubMed] [Google Scholar]
- 33.Baliousis M, Rennoldson M, Snowden JA. Psychological interventions for distress in adults undergoing haematopoietic stem cell transplantation: a systematic review with meta-analysis. Psychooncology. 2016;25(4):400–411. doi: 10.1002/pon.3925. [DOI] [PubMed] [Google Scholar]
- 34.El-Jawahri A, Traeger L, Greer JA, et al. Effect of inpatient palliative care during hematopoietic stem-cell transplant on psychological distress 6 months after transplant: results of a randomized clinical trial. J Clin Oncol. 2017;35(32):3714–3721. doi: 10.1200/JCO.2017.73.2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tavakoli-Ardakani M, Kheshti R, Maryam M. Effect of sertraline on complications and survival after hematopoietic stem-cell transplantation, a double-blind, placebo-controlled clinical study. Int J Hematol. 2017;106(6):832–841. doi: 10.1007/s12185-017-2309-y. [DOI] [PubMed] [Google Scholar]
- 36.D’Souza A, Fretham C, Lee SJ, et al. Current use of and trends in hematopoietic cell transplantation in the United States. Biol Blood Marrow Transplant. 2020;26(8):e177–e182. doi: 10.1016/j.bbmt.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maakaron JE, Zhang M-J, Chen K, et al. Age is no barrier for adults undergoing HCT for AML in CR1: contemporary CIBMTR analysis. Bone Marrow Transplant. 2022;57(6):911–917. doi: 10.1038/s41409-022-01650-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.