Abstract
Background
Delirium is among the most common complications following major surgery. Delirium following medical illness is associated with the development of chronic cognitive decline. The objective of this study was to determine the association of postoperative delirium with dementia in the year following surgery.
Materials and Methods:
This was a retrospective cohort study in a large health network (1/2013–12/2019). All patients over age 50 undergoing surgery requiring an inpatient stay were included. Our main exposure was an episode of delirium. The primary outcome was a new dementia diagnosis in the one year following discharge. Secondary outcomes included hospital length of stay, non-home discharge destination, mortality and rehospitalizations in one year.
Results:
There were 39,665 patients included, with a median age of 66. There were 4,156 / 39,665 emergencies (10.5%). Specialties were general surgery (12,285 / 39,665, 31%) and orthopedics (11,503/39,665, 29%). There were 3,327 (8.4%) patients with delirium. Delirious patients were older and were more likely to have comorbid conditions and undergone complex procedures. There were 1,353 / 39,665 (3.5%) patients who developed dementia in the year following their surgery; 4,930/39,665 (12.4%) who died; and 8,200/39,665 (20.7%) who were readmitted. Delirium was associated with a new dementia diagnosis after adjusting for baseline characteristics (Odds ratio [OR] 13.9; 95% CI, 12.2–15.7). Similarly, delirium was also associated with one-year mortality (OR 3.1; 95% CI 2.9–3.4) and readmission (OR 1.9, 95% CI 1.7–2.0).
Conclusion:
Postoperative delirium is the strongest factor associated with development of dementia in the year following a major operation. Strategies to prevent, identify, and treat delirium in the postoperative setting may improve long-term cognitive recovery.
Keywords: postoperative delirium, dementia, geriatrics, surgery
Introduction
Postoperative delirium is one of the most common complications following elective and emergent operations, occurring in 15–80% of patients, depending on the particular population.1 It is a syndrome of altered attention, cognition, and awareness, acute in onset and waxing and waning over time, that is also known as acute brain failure. Delirium occurs as a response to physiologic stress such as an acute illness, trauma, medication use, or a major operation. Not only is this disease entity harmful itself, but it is also associated with many other adverse and downstream effects, including other surgical complications, prolonged length of stay, increased cost, increased rates of post-discharge institutionalization, and poor functional outcomes.2
Delirium has a complex relationship with other disorders of cognition, including dementia, which is a condition of chronic cognitive decline. Though delirium and pre-existing cognitive problems are related and associated with the subsequent development of dementia, they have important distinctions.3–6 Many cases of delirium, especially postoperative delirium, are preventable. Delirium in the elective postoperative setting is associated with a number of adverse outcomes and increased cost, and we are beginning to understand its impact on long term outcomes such as dementia. Though clinically rigorous, these studies are limited by either surgical population or small sample sizes.7–11
We sought to characterize the burden of adverse postoperative cognitive outcomes in a large Midwestern health system and determine the relationship between an episode of postoperative delirium on subsequent development of dementia in the year following discharge. Our hypothesis was that an episode of postoperative delirium would be associated with an increased risk of dementia at one year when compared to a patient without delirium.
Methods
Data source and patients
Hospital encounter data were obtained from the Indiana University (IU) Health electronic data warehouse. IU Health is a non-profit health system comprising 18 hospitals and numerous health centers statewide, linked by a common medical record system. Inclusion criteria were patients 50 or older undergoing a major procedure, as defined by an established list of Current Procedure Terminology (CPT) codes12,13 (January 2013 – December 2019), which required an inpatient stay of at least one day. We excluded patients missing key demographic information as well as those patients that exhibited delirium at the time of admission (Figure 1).
Figure 1.
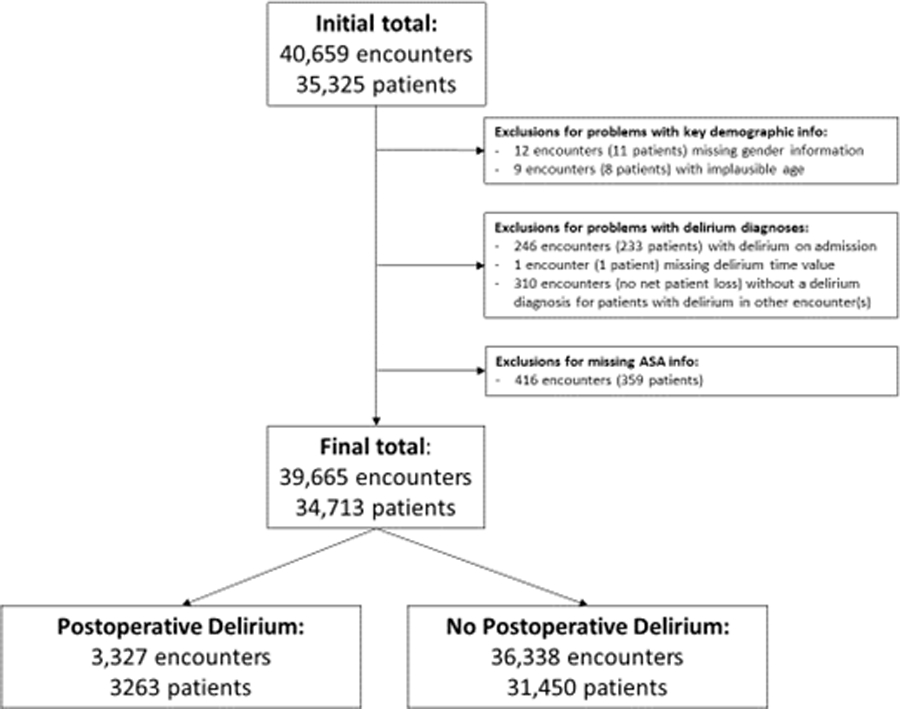
Flow diagram of study cohort undergoing inpatient surgery in Indiana, 2013–2019
Baseline demographic data including age, sex, and race were collected, as were data on zip code and insurance type. Clinical data from each encounter included American Society of Anesthesiologists (ASA) class, comorbidity data which was used to calculate a Charlson score, and a diagnosis of mild cognitive impairment (ICD-9: 331.83; ICD-10: G31.84). Patients were classified as undergoing an emergency operation based on ASA class. We characterized operation types in several ways. First, individual CPT codes were grouped into operating specialties based on National Surgical Quality Improvement Program inclusion and exclusion criteria. After this initial grouping, CPT codes that remained uncategorized and had a frequency greater than 1% were examined individually and grouped based on their description. Second, to capture indicators of case complexity, we collected all major procedure codes for a given encounter. For scenarios in which there were four or more CPT codes falling into two or more different specialties, patients were placed into a multispecialty category. Distinct from this categorization, patients that had a “22” modifier on the primary procedure CPT code, indicating services rendered that are significantly more complex than otherwise indicated; underwent a reoperative procedure (CPT 49002); or underwent four or more procedures were given a complex case designation. Our primary exposure variable was an inpatient diagnosis of delirium using a set of ICD-9 and ICD-10 codes published previously.14
Outcomes
The primary aim of this study was to examine the impact of postoperative delirium on subsequent diagnosis of dementia or mild cognitive impairment in the following year. All outpatient encounters in the year following the index procedure and hospital stay were examined for documented diagnoses of dementia or mild cognitive impairment.15 Secondary outcomes included hospital length of stay, discharge disposition, all-cause mortality at one year, and rehospitalizations in one year. For hospital length of stay, we determined the interquartile range of length of stay for all procedures in each surgical specialty and defined prolonged length of stay as > 75th percentile of length of stay for that specialty.
Statistical analysis
Descriptive statistics were calculated for baseline demographic and clinical data and compared between groups with and without delirium. Means and standard deviations were used for normally distributed continuous variables and medians with interquartile ranges for non-normally distributed continuous variables. We reported categorical variables using frequencies and percentages. Characteristics were compared between the two groups using Chi-squared tests for categorical variables and the Wilcoxon rank sum test for non-normally distributed continuous variables. Multivariable logistic regression models were then developed to identify factors associated with a new diagnosis of dementia in the year following and each of our secondary outcomes. We included variables in models if they were significant on initial analysis with a p-value < 0.20. Final models included age, sex, Charlson score, American Society of Anesthesiologists (ASA) category, case complexity, and operating surgical specialty. Given the known risk factors for delirium of vascular and primary brain pathology, we also developed a separate set of models excluding these specialties – our primary results were not significantly different, and so we present all specialties in this study. This research study was deemed exempt by the Indiana University Institutional Review Board. Statistical significance was set at an alpha level of 0.05 or lower. All statistical analyses were performed using SAS, version 9.4 (Cary, NC).
Results
Between 2013 and 2019, our final cohort included 39,665 inpatient encounters and 34,713 unique patients (Figure 1). Median age was 66 (IQR 59–73) with a female predominance (n=20,698/39,665; 52%). The majority of patients were White or Caucasian (n=35,098/39,665; 89%) with public insurance (n=27,176/39,665; 69%). More than half to three-quarters of patients had Charlson scores (n=21,315/39,665; 54%) and ASA class (n=21,124/39,665; 73%). Less than 1% of patients had mild cognitive impairment. About 10% of patients underwent procedures that were deemed emergencies. More than half of the procedures performed on this cohort were either general surgical or orthopedic procedures, with significant numbers of cardiothoracic, neurosurgery, urologic and gynecologic, and vascular procedures as well (Table 1).
Table 1:
Baseline population characteristics for surgery patients in the Indiana University Health system from 2014–2019
| Entire Sample N=39,665 |
Postoperative Delirium N=3,327 |
No Postoperative Delirium N=36,338 |
P-value | |
|---|---|---|---|---|
| Age, median (IQR) | 66 (59,73) | 71 (62,79) | 66 (59,73) | <0.0001 |
| Sex, N (%) | <0.0001 | |||
| Male | 18,967 (47.8) | 1,737 (52.2) | 17,230 (47.4) | |
| Female | 20,698 (52.2) | 1,590 (47.8) | 19,108 (52.6) | |
| Race, N (%) | <0.0001 | |||
| American Indian, Alaska Native, Native Hawaiian, or other Pacific Islander | 92 (0.23) | 5 (0.15) | 87 (0.24) | |
| Asian | 273 (0.69) | 22 (0.66) | 251 (0.69) | |
| Black or African American | 3,831 (9.66) | 392 (11.8) | 3,439 (9.46) | |
| White or Caucasian | 35,098 (88.5) | 2,857 (85.8) | 32,241 (88.73) | |
| Unknown | 371 (0.93) | 51 (1.53) | 320 (0.88) | |
| Ethnicity, N (%) | <0.0001 | |||
| Hispanic or Latino | 472 (1.19) | 31 (0.93) | 441 (1.21) | |
| Not Hispanic or Latino | 38,791 (97.8) | 3,223 (96.9) | 35,568 (97.9) | |
| Unknown | 402 (1.01) | 73 (2.19) | 329 (0.91) | |
| Mild cognitive impairment | 100 (0.25) | 29 (0.87) | 71 (0.20) | <0.0001 |
| Charlson Index, N (%) | <0.0001 | |||
| No comorbid disease | 18,350 (46.3) | 1,316 (39.5) | 17,034 (46.9) | |
| Mild comorbid disease | 11,694 (29.5) | 846 (25.4) | 10,848 (29.8) | |
| Moderate comorbid disease | 4,717 (11.9) | 481 (14.5) | 4,236 (11.7) | |
| Severe comorbid disease | 4,904 (12.3) | 684 (20.6) | 4,220 (11.6) | |
| ASA, N (%) | <0.0001 | |||
| 1–2 | 6,385 (16.1) | 102 (3.07) | 6,283 (17.3) | |
| 3–4 | 29,124 (73.4) | 2,324 (69.9) | 26,800 (73.8) | |
| Emergent (5 or ‘E’) | 4,156 (10.5) | 901 (27.1) | 3,255 (8.96) | |
| Encounter Specialty | <0.0001 | |||
| CT | 3,191 (8.04) | 387 (11.6) | 2,804 (7.72) | |
| ENT | 741 (1.87) | 55 (1.65) | 686 (1.89) | |
| General | 12,285 (31.0) | 1,040 (31.3) | 11,245 (30.9) | |
| Multiple* | 1,950 (4.92) | 206 (6.19) | 1,744 (4.80) | |
| Neurosurgery | 3,784 (9.54) | 314 (9.44) | 3,470 (9.55) | |
| Orthopedic | 11,503 (29.0) | 826 (24.8) | 10,677 (29.4) | |
| Plastic | 253 (0.66) | 23 (0.69) | 240 (0.66) | |
| Urology/Gynecology | 2,926 (7.38) | 97 (2.92) | 2,829 (7.79) | |
| Vascular | 3,022 (7.62) | 379 (11.4) | 2,643 (7.27) | |
| Complex Case | 1,324 (3.34) | 337 (10.1) | 987 (2.72) | <0.0001 |
| Insurance Type | <0.0001 | |||
| Private | 12,093 (30.4) | 526 (15.8) | 11,513 (31.7) | |
| Public | 27,176 (68.5) | 2,756 (82.8) | 24,420 (67.2) | |
| Uninsured | 450 (1.13) | 45 (1.35) | 405 (1.11) |
Multiple refers to cases wherein procedure codes were attributed to > 2 surgical specialties
Abbreviations: IQR, interquartile range; ASA, American Society of Anesthesiologists physical classification score; CT, cardiothoracic; ENT, otolaryngology.
There were 3,327 (8.4%) patients with delirium following major surgery. This varied across specialties, with the highest delirium prevalence in cardiothoracic and vascular surgical procedures (Figure 2). When compared to patients without delirium, patients with delirium were older (median age 71 v. 66) and were more likely to be male (n=1,737/3,327; 52% v. 17,230/36,338; 47%) and black. Delirious patients also had higher Charlson scores and were more likely to have undergone vascular, cardiothoracic, multispecialty, and complex procedures. Complete bivariable comparisons are shown in Table 1.
Figure 2.
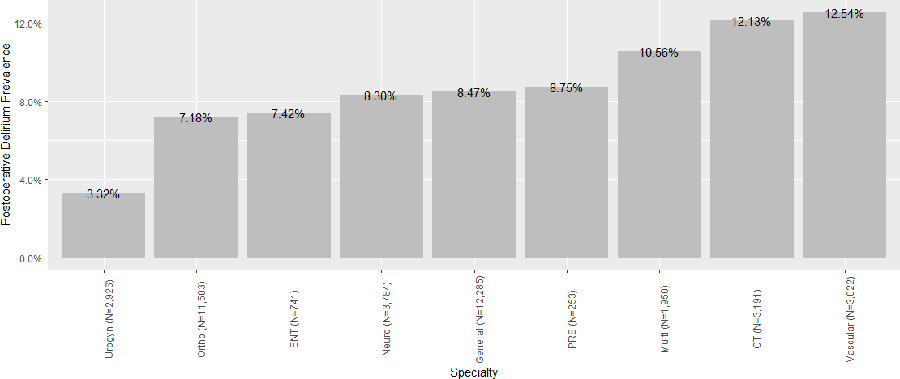
Postoperative delirium prevalence across surgical specialties in Indiana, 2013–2019
Abbreviations: ENT, otolaryngology; PRS, plastic and reconstructive surgery; CT, cardiothoracic
Outcomes
In the overall sample, 3.5% (1,353/39,665) developed a new diagnosis of dementia in the year following their discharge. Patients with delirium had higher rates of dementia (23.6%, 785/3,327) than those without delirium (1.5%; 568/36,338). For our secondary outcomes, 12.4% (4,930/39,665) died, 20.7% (8,200/39,665) were readmitted, and 25.4% (10,056 /39,665) were discharged to non-home settings overall. These rates also differed significantly by delirium status. Delirium was associated with a new dementia diagnosis within one year of discharge (Adjusted odds ratio [OR] 13.9; 95% CI, 12.3–15.7) (Table 2). Similarly, delirium was also associated with both mortality and readmission within one year (Adjusted OR 1.92; 95% CI 1.76–2.10 and OR 1.47, 95% CI 1.35–1.59, respectively). Finally, delirium was also associated with discharge to non-home settings and prolonged length of stay, regardless of surgery type. These associations remained significant after adjustment for age, sex, Charlson score, ASA class, mild cognitive impairment, case complexity, and encounter specialty (Table 3).
Table 2.
Adjusted odds of dementia at one year
| OR(95% CI) | |
|---|---|
| Postoperative delirium | 13.9 (12.3–15.7) |
| Male sex (v. female) | 1.15 (1.01–1.30) |
| Age | |
| 50–59 | [referent] |
| 60–66 | 1.36 (1.06–1.74) |
| 67–74 | 2.09 (1.66–2.63) |
| >74 | 5.63 (4.59–6.97) |
| Mild cognitive impairment | 2.70 (1.51–4.66) |
| Charlson score | |
| No comorbid disease | [referent] |
| Mild comorbid disease | 0.73 (0.63–0.85) |
| Moderate comorbid disease | 0.79 (0.65–0.95) |
| Severe comorbid disease | 0.78 (0.66–0.94) |
| ASA classification | |
| 1–2 | [referent] |
| 3–4 | 2.46 (1.85–3.36) |
| 5 or ‘E’ | 3.07 (2.23–4.31) |
| Complex case status | 1.21 (0.90–1.61) |
Abbreviations: OR, odds ratio; CI, confidence interval; ASA, American Society of Anesthesiologists;
In addition to those factors listed, model also adjusted for operating surgical specialty
Table 3.
Odds of mortality, readmission, prolonged length of stay, and non-home discharge
| OR (95% CI) | |||||
|---|---|---|---|---|---|
| Mortality | Readmission | Prolonged LOS* | Non-home discharge | ||
| Postoperative delirium | 1.92 (1.76–2.10) | 1.47 (1.35–1.59) | 7.31 (6.65–8.03) | 6.66 (6.07–7.32) | |
| Male sex (v. female) | 1.14 (1.07–1.22) | 1.00 (0.95–1.05) | 1.11 (1.05–1.17) | 0.71 (0.67–0.75) | |
| Age quartile | |||||
| 50–59 | [referent] | [referent] | [referent] | [referent] | |
| 60–66 | 1.17 (1.06–1.30) | 0.89 (0.83–0.96) | 0.97 (0.90–1.04) | 1.21 (1.12–1.33) | |
| 67–74 | 1.46 (1.32–1.61) | 0.91 (0.85–0.98) | 0.94 (0.87–1.01) | 1.68 (1.7–1.97) | |
| >74 | 2.44 (2.22–2.68) | 0.99 (0.93–1.1) | 1.21 (1.12–1.30) | 4.39 (4.07–4.74) | |
| Mild cognitive impairment | 1.44 (0.88–2.31) | 0.91 (0.55–1.46) | 1.71 (1.04–2.78) | 3.14 (1.87–5.42) | |
| Charlson score | |||||
| No comorbid disease | [referent] | [referent] | [referent] | [referent] | |
| Mild comorbid disease | 1.28 (1.18–1.39) | 1.13 (1.06–1.21) | 0.80 (0.75–0.86) | 0.88 (0.82–0.93) | |
| Moderate comorbid disease | 2.0 (1.8–2.2) | 1.70 (1.57–1.84) | 1.03 (0.95–1.13) | 1.19 (1.10–1.30) | |
| Severe comorbid disease | 3.69 (3.38–4.02) | 2.88 (2.67–3.10) | 1.30 (1.20–1.42) | 1.48 (1.37–1.61) | |
| ASA classification | |||||
| 1–2 | [referent] | [referent] | [referent] | [referent] | |
| 3–4 | 3.56 (3.02–4.24) | 1.90 (1.74–2.08) | 4.10 (3.69–4.56) | 3.56 (3.23–3.92) | |
| 5 or ‘E’ | 6.24 (5.20–7.54) | 2.54 (2.27–2.85) | 13.2 (11.6–15.0) | 11.2 (9.91–12.7) | |
| Complex case status | 1.51 (1.29–1.77) | 1.18 (1.02–1.37) | 3.30 (2.78–3.92) | 2.39 (2.03–2.82) | |
Abbreviations: OR, odds ratio; CI, confidence interval; LOS, length of stay; ASA, American Society of Anesthesiologists; CT, cardiothoracic; ENT, otolaryngology
LOS considered to be prolonged if >75th percentile for surgical specialty
In addition to those variables listed, models also adjusted for operating surgical specialty.
Discussion
This study describes an episode of postoperative delirium as the most significant factor associated with a subsequent dementia diagnosis in the year following surgery, irrespective of surgery type. We also substantiate the relationship between delirium and other short- and long-term outcomes, including mortality, prolonged length of stay, and readmission.
The present study is distinguished primarily because of its population-based approach, inclusion of several hospital systems, and much larger cohort size, including a broad cross section of elective, inpatient surgical populations, encompassing some specialties that have not been well studied. Our delirium rate is lower than in some studies – particularly those with rigorous clinical assessments. The Successful Aging after Elective Surgery (SAGES) study is a long term prospective cohort study of 566 patients aged 70 or older at three academic medical centers in the Northeast. This largely elective orthopedic surgical population had a delirium rate of 24%.16 Other studies using clinical assessments in postoperative patients requiring ICU admissions and emergency general surgery patients demonstrated rates of 39% and 26%, respectively.17,18 In a larger study using a clinical registry with trained abstractors and a chart-based method for delirium assessment, the rate of postoperative delirium were estimated at 12%.19
There is controversy over whether postoperative delirium represents a catalyst for subsequent cognitive decline or unmasks pre-existing cognitive vulnerability. Several other studies, generally in medical and critical care settings, have demonstrated that delirium is associated with long term cognitive impairment. For example, in one study using data from a prospective cohort study in Massachusetts and chart-based delirium assessments, patients with Alzheimer’s disease who developed delirium while hospitalized (n=195) had an accelerated cognitive decline when compared to those that did not develop delirium during the study period.5,6,20 Two reviews in 2004 and 2009 summarized the literature on the relationship between delirium and long-term cognitive decline and dementia. Together, these reviews included eighteen studies (3,995 patients), including several studies in orthopedic and cardiac surgery populations and one study in an elective abdominal surgery population. In sum, though there were persistent issues with small sample sizes as well as substantial variability in delirium and cognitive assessments and follow up time (ranging from 3 months to 5 years), the evidence indicates a link between delirium and dementia.3,4 More recently, several studies with strong methodological bases focused on surgical patients. In the first, 184 patients undergoing elective cardiac surgery underwent preoperative neuropsychological testing. They documented cognitive function in the year following surgery, and noted that postoperative delirium was independently associated with cognitive decline at one month, but that the patients recovered over time.21 In the second, using the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU) for delirium assessment and several tests evaluating cognition before and after surgery, 2,018 patients undergoing surgery at the Mayo Clinic were followed prospectively. This study found that patients who were cognitively normal at baseline who experienced postoperative delirium were more likely to develop mild cognitive impairment or dementia.8 Notably, only 36% of the patients in this study were admitted to the hospital following surgery. Both studies had strong evaluation of delirium and cognitive function throughout the study period, with follow up time out to nine months. The SAGES study22 and a second study in cardiac surgery patients23 also demonstrated associations between delirium and subsequent cognitive decline. Both studies highlighted the importance of preoperative cognitive function and its contribution as a risk factor for poor long-term outcomes.
Postoperative delirium is more common than many other commonly reported adverse postoperative outcomes such as surgical site infections. Its association with other complications is well-known, particularly in the intensive care unit setting. More recently, delirium has been found to be related to changes in functional recovery trajectories of elective surgery patients.24 The current study adds to the existing literature about the potential long-term effects of an episode of postoperative delirium on patients’ subsequent cognitive function. This information is critical to discussions around informed consent in high-risk major surgery. Moreover, as a significant proportion of postoperative delirium episodes are preventable, this raises the question about whether cognitive trajectories would also be altered, avoiding further cognitive decline following surgery, if we deploy system-based delirium prevention strategies.
Strengths and Limitations
This is a population-based analysis in a large, tertiary Midwestern health system with a robust healthcare data infrastructure. Other studies of postoperative delirium have been smaller in size and scope and have also been in studies performed in other geographies. Ascertainment of both delirium and dementia using diagnosis codes is imperfect. However, the IU Health system, which benefits from a partnership with the IU School of Medicine, has a long history of demonstrated attention to the cognitive health of older adults, which may be one reason our rates of postoperative delirium based on diagnosis codes alone were higher than other population-based studies in the past. Notably, however, our delirium and dementia rates were lower than some studies that have reported using clinically-based assessments of delirium or dementia. Under-recognition and inaccurate assessment of postoperative delirium and dementia in the community and in inpatient settings remains a persistent problem due to lack of active screening and inaccurate or incomplete documentation. Consequently, our numbers likely represent underestimates. The lack of clinical testing is also an important limitation; however this type of assessment can be difficult to do at the system scale. We did not have follow up time or date data for our readmission and mortality data, which would have allowed for a time-to-event analysis rather than analysis of a dichotomous outcome. These data also did not contain structured information on patients’ education level, family history, or granular data specifying stroke or substance use history – all factors known to impact delirium risk. Unique to this study was the level of detail regarding the particular procedures and their specialties; unfortunately, similar to ACS NSQIP, we did rely on CPT codes, which are designed for billing rather than research purposes. Finally, as this study was limited to those patients cared for at IU Health facilities, we do not have data (e.g., mortality) on patients who may have been cared for at other facilities during the time period of the study.
This preliminary works highlights several gaps in knowledge, including the degree to which these episodes of postoperative delirium represents a risk factor that alters the trajectory of cognitive recovery following surgery versus a kind of “stress test” that suggests either undiagnosed dementia or vulnerability to chronic cognitive decline. Ultimately, patients who develop delirium may benefits from long term follow up and perhaps referral for formal, clinical dementia screening, evaluation, and treatment. Finally, our group is working on developing automated both dementia and delirium screening strategies using historical electronic diagnosis data – so-called passive digital markers – to allow for large scale prediction of postoperative delirium and exploration of long-term effects of this complication in surgical patients in Indiana.22
Conclusion
Despite the above-mentioned limitations, the findings of this study are sobering. After adjustment for comorbidities, surgical specialty and complexity, and emergency case status, postoperative delirium is the factor most strongly associated with a new dementia diagnosis in the year following a major operation. With over 10 million inpatient procedures occurring annually,23 these data would suggest more than 800,000 episodes of delirium, many of them potentially preventable.1 Future studies examining cognitive function with both electronic medical record- and clinical assessments will provide further insight on this relationship.
Footnotes
Disclosures
The authors report no proprietary or commercial interest in any product mentioned or concept discussed in this article.
References
- 1.Inouye SK, Westendorp RGJ, Saczynski JS. Delirium in elderly people. Lancet 2014;383(9920):911–922. doi: 10.1016/S0140-6736(13)60688-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.AGS Expert Panel on Postoperative Delirium in Older Adults. American Geriatrics Society Clinical Practice Guideline for Postoperative Delirium in Older Adults. J Am Geriatr Soc 2015;63(1):142–50. doi: 10.1111/jgs.13281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jackson JC, Gordon SM, Hart RP, et al. The Association Between Delirium and Cognitive Decline: A Review of the Empirical Literature. Neuropsychol Rev 2004;14(2):87–98. doi: 10.1023/B:NERV.0000028080.39602.17 [DOI] [PubMed] [Google Scholar]
- 4.MacLullich AMJ, Beaglehole A, Hall RJ, Meagher DJ. Delirium and long-term cognitive impairment. Int Rev Psychiatry 2009;21(1):30–42. doi: 10.1080/09540260802675031 [DOI] [PubMed] [Google Scholar]
- 5.Pandharipande PP, Girard TD, Jackson JC, et al. Long-term cognitive impairment after critical illness. N Engl J Med 2013;369(14):1306–1316. doi: 10.1056/NEJMoa1301372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Girard TD, Jackson JC, Pandharipande PP, et al. Delirium as a predictor of long-term cognitive impairment in survivors of critical illness. Crit Care Med 2010;38(7):1513–1520. doi: 10.1097/CCM.0b013e3181e47be1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Inouye SK, Marcantonio ER, Kosar CM, et al. The short-term and long-term relationship between delirium and cognitive trajectory in older surgical patients. Alzheimer’s & Dementia 2016;12(7):766–775. doi: 10.1016/j.jalz.2016.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sprung J, Roberts RO, Weingarten TN, et al. Postoperative delirium in elderly patients is associated with subsequent cognitive impairment. British Journal of Anaesthesia 2017;119(2):316–323. doi: 10.1093/bja/aex130 [DOI] [PubMed] [Google Scholar]
- 9.Morandi A, Davis D, Fick DM, et al. Delirium superimposed on dementia strongly predicts worse outcomes in older rehabilitation inpatients. J Am Med Dir Assoc 2014;15(5):349–354. doi: 10.1016/j.jamda.2013.12.084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vasunilashorn SM, Fong TG, Albuquerque A, et al. Delirium Severity Post-Surgery and its Relationship with Long-Term Cognitive Decline in a Cohort of Patients without Dementia. J Alzheimers Dis 2018;61(1):347–358. doi: 10.3233/JAD-170288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gou RY, Hshieh TT, Marcantonio ER, et al. One-Year Medicare Costs Associated With Delirium in Older Patients Undergoing Major Elective Surgery. JAMA Surgery Published online February 24, 2021. doi: 10.1001/jamasurg.2020.7260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khuri SF, Daley J, Henderson W, et al. The Department of Veterans Affairs’ NSQIP: the first national, validated, outcome-based, risk-adjusted, and peer-controlled program for the measurement and enhancement of the quality of surgical care. National VA Surgical Quality Improvement Program. Ann Surg 1998;228(4):491–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khuri SF, Henderson WG, Daley J, et al. Successful implementation of the Department of Veterans Affairs’ National Surgical Quality Improvement Program in the private sector: the Patient Safety in Surgery study. Ann Surg 2008;248(2):329–336. doi: 10.1097/SLA.0b013e3181823485 [DOI] [PubMed] [Google Scholar]
- 14.Boustani M, Baker MS, Campbell N, et al. Impact and Recognition of Cognitive Impairment among Hospitalized Elders. J Hosp Med 2010;5(2):69–75. doi: 10.1002/jhm.589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.St. Germaine-Smith C, Metcalfe A, Pringsheim T, et al. Recommendations for optimal ICD codes to study neurologic conditions. Neurology 2012;79(10):1049–1055. doi: 10.1212/WNL.0b013e3182684707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schmitt EM, Saczynski JS, et al. The Successful Aging after Elective Surgery (SAGES) Study: Cohort Description and Data Quality Procedures. J Am Geriatr Soc 2015:63(12):2463–2471. doi: 10.1111/jgs.13793. Epub 2015 Dec 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Robinson TN, Dun CL, et al. Tryptophan Supplementation and Postoperative Delirium – A Randomized Controlled Trial. J Am Geriatr Soc 2014:62(9):1764–1771. doi: 10.1111/jgs.12972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saljuqi AT, Hanna K, et al. Prospective Evaluation of Delirium in Geriatric Patients Undergoing Emergency General Surgery. J Am Coll Surg 2020:230(5):758–765. doi: 10.1016/j.jamcollsurg.2020.01.029. Epub 2020 Feb 21. [DOI] [PubMed] [Google Scholar]
- 19.Berian JR, Zhou L, et al. Postoperative Delirium as a Target for Surgical Quality Improvement. Ann Surg. 2018:268(1):93–99. doi: 10.1097/SLA.0000000000002436. [DOI] [PubMed] [Google Scholar]
- 20.Fong TG, Jones RN, Shi P, et al. Delirium accelerates cognitive decline in Alzheimer disease. Neurology 2009;72(18):1570–1575. doi: 10.1212/WNL.0b013e3181a4129a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sauër AC, Veldhuijzen DS, Ottens TH, et al. Association between delirium and cognitive change after cardiac surgery. British Journal of Anaesthesia 2017;119(2):308–315. doi: 10.1093/bja/aex053 [DOI] [PubMed] [Google Scholar]
- 22.Devore EE, Fong TG, Marcantonio ER, et al. Prediction of Long-term Cognitive Decline Following Postoperative Delirium in Older Adults. The Journals of Gerontology: Series A 2017;72(12):1697–1702. doi: 10.1093/gerona/glx030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saczynski JS, Marcantonio ER, Quach L, et al. Cognitive Trajectories after Postoperative Delirium. N Engl J Med 2012;367(1):30–39. doi: 10.1056/NEJMoa1112923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hshieh TT, Saczynski J, Gou RY, et al. Trajectory of Functional Recovery After Postoperative Delirium in Elective Surgery. Ann Surg 2017;265(4):647–653. doi: 10.1097/SLA.0000000000001952 [DOI] [PMC free article] [PubMed] [Google Scholar]


