Abstract
Background
Common, operational definitions are crucial to assess interventions and outcomes related to pediatric mechanical ventilation. These definitions can reduce unnecessary variability among research and quality improvement efforts, to ensure findings are generalizable, and can be pooled to establish best practices.
Research Question
Can we establish operational definitions for key elements related to pediatric ventilator liberation using a combination of detailed literature review and consensus-based approaches?
Study Design and Methods
A panel of 26 international experts in pediatric ventilator liberation, two methodologists, and two librarians conducted systematic reviews on eight topic areas related to pediatric ventilator liberation. Through a series of virtual meetings, we established draft definitions that were voted upon using an anonymous web-based process. Definitions were revised by incorporating extracted data gathered during the systematic review and discussed in another consensus meeting. A second round of voting was conducted to confirm the final definitions.
Results
In eight topic areas identified by the experts, 16 preliminary definitions were established. Based on initial discussion and the first round of voting, modifications were suggested for 11 of the 16 definitions. There was significant variability in how these items were defined in the literature reviewed. The final round of voting achieved ≥ 80% agreement for all 16 definitions in the following areas: what constitutes respiratory support (invasive mechanical ventilation and noninvasive respiratory support), liberation and failed attempts to liberate from invasive mechanical ventilation, liberation from respiratory support, duration of noninvasive respiratory support, total duration of invasive mechanical ventilation, spontaneous breathing trials, extubation readiness testing, 28 ventilator-free days, and planned vs rescue use of post-extubation noninvasive respiratory support.
Interpretation
We propose that these consensus-based definitions for elements of pediatric ventilator liberation, informed by evidence, be used for future quality improvement initiatives and research studies to improve generalizability and facilitate comparison.
Key Words: airway extubation, extubation failure, high-flow nasal cannula, mechanical ventilation, noninvasive ventilation, pediatric ICU, ventilator weaning
Ventilator liberation is a daily practice in pediatric critical care, yet many aspects of pediatric ventilator liberation lack a clear evidence base.1, 2, 3, 4, 5, 6 There have been a multitude of studies published on aspects of pediatric ventilator liberation, but there is significant variability regarding definitions of interventions and outcomes. This variability makes it difficult to synthesize the evidence to establish best practices. Furthermore, as the field moves toward multi-national and platform-based clinical trials with ventilated children, it is increasingly important for there to be a shared framework for definitions of terms related to ventilated children and ventilator liberation.
As part of a larger project to establish clinical practice guidelines for pediatric ventilator liberation,7 we assembled a multi-professional panel of international experts in pediatric ventilator liberation. This work included systematic reviews of the literature to identify the most common definitions for interventions and outcomes related to pediatric ventilator liberation. The goal was to establish operational definitions that could be used for future research and quality improvement projects.
Study Design and Methods
A panel of 26 international experts was convened in April 2020 based on their published work in pediatric ventilator liberation in the last 10 years. In addition to the panelists, two methodologists and two librarians were recruited to support the project. Between April 2020 and October 2021, the expert panel had three virtual meetings to establish the definitions (Fig 1).
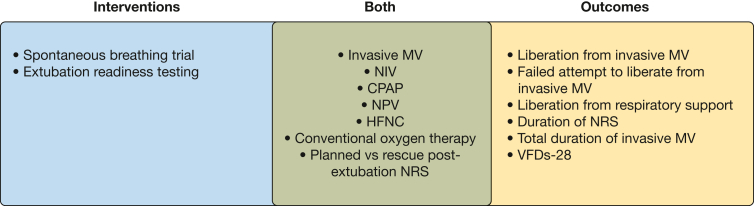
Figure 1.
Conceptual framework of pediatric ventilator liberation operational definitions. HFNC = high-flow nasal cannula; MV = mechanical ventilation; NIV = noninvasive ventilation; NPV = negative pressure ventilation; NRS = noninvasive respiratory support; VFDs-28 = 28 ventilator-free days.
Experts voted on the importance of establishing operational definitions for a list of topic areas related to pediatric ventilator liberation. Based on knowledge of the literature related to pediatric and adult ventilator liberation, the co-chairs of the pediatric ventilator liberation consensus conference (S. A. S. and R. G. K.) drafted initial definitions for discussion and voting. The proposed definitions were presented during a virtual meeting for initial discussion with real-time modification of definitions as necessary.
Subsequently, all experts participated in anonymous online voting (Qualtrics) with three options: (1) agree with definition as written; (2) agree with fundamental concept of definition but suggest following clarifications; or (3) disagree with fundamental concept of definition and would suggest the following instead. For options two and three, the experts could type comments for consideration.
The co-chairs modified definitions based on this feedback and presented the voting results and modified definitions to experts in a subsequent virtual meeting.
Systematic Reviews
In parallel, five systematic reviews were conducted as part of the parent project to answer eight PICO (Population, Intervention, Control, Outcomes, Study) questions related to pediatric ventilator liberation. For all PICO questions, the population of interest focused on children ventilated for at least 24 h. Key outcomes included the rates of liberation from invasive and noninvasive mechanical ventilation (MV), total duration of invasive MV, duration of noninvasive respiratory support (NRS), failure to liberate from invasive MV (including re-intubation rates), ventilator-free days (VFDs), PICU length of stay, hospital length of stay, effort/work of breathing, and mortality. The questions are summarized in Table 1 and focused on methods to conduct spontaneous breathing trials (SBTs), duration of SBTs, measures of respiratory muscle strength, post-extubation upper airway obstruction, NRS use after extubation, and sedation managment. Medline, Embase, and CINAHL databases were searched based on a combination of Medical Subject Headings terms and key words. There were no language or date limitations (e-Tables 1-5). Specific details about the inclusion and exclusion criteria and the methods for review have been published previously.7 For all articles that met inclusion and exclusion criteria for a given PICO question, experts extracted the definitions used in the individual studies related to proposed definition topic areas. Data extraction occurred in a REDCap database.
Table 1.
List of Pediatric Ventilator Liberation Guideline PICO Questions
| PICO | Question |
|---|---|
| 1. |
SBT method In acutely hospitalized children receiving conventional mechanical ventilation for > 24 h who are undergoing an SBT as part of extubation readiness assessments, should inspiratory pressure augmentation (ie, pressure support or automatic tube compensation) be used? |
| 2. |
SBT duration In acutely hospitalized children receiving conventional mechanical ventilation for > 24 h who are undergoing an SBT to assess for extubation readiness, should the SBT be conducted for 30 min or 60-120 min? |
| 3. |
Utility of measuring respiratory muscle strength/function In acutely hospitalized children receiving conventional mechanical ventilation for > 24 h should a measure of respiratory muscle strength during airway occlusion (ie, the negative inspiratory force or maximal inspiratory pressure during airway occlusion) be included in determining extubation readiness? |
| 4. |
Utility of using the air leak test to predict upper airway obstruction In acutely hospitalized children receiving conventional mechanical ventilation for > 24 h, should an endotracheal tube air leak test be measured prior to extubation to predict post-extubation upper airway obstruction? |
| 5. |
Utility of using corticosteroids to prevent upper airway obstruction In acutely hospitalized children receiving conventional mechanical ventilation for > 24 h, should systemic corticosteroids be administered prior to extubation to prevent post-extubation upper airway obstruction? |
| 6. |
Post-extubation noninvasive respiratory support vs conventional oxygen therapy In acutely hospitalized children receiving conventional mechanical ventilation for > 24 h, should planned noninvasive respiratory support (HFNC, CPAP, or NIV) be used post-extubation? |
| 7. |
Post-extubation NIV/CPAP vs HFNC In acutely hospitalized children being extubated to planned noninvasive respiratory support (NIV, CPAP or HFNC), would NIV/CPAP be superior to HFNC? |
| 8. |
Sedation management In acutely hospitalized children receiving conventional mechanical ventilation for > 24 h, should a goal-directed sedation protocol be used compared with non-protocolized sedation management to guide sedation management during mechanical ventilation and endotracheal extubation? |
HFNC = high-flow nasal cannula; NIV = noninvasive ventilation; PICO = Population, Intervention, Control, Outcomes, Study; SBT = spontaneous breathing trial.
For each of the proposed definitions, the co-chairs (S. A. S. and R. G. K.) synthesized the extracted data from the published studies related to each definition and presented these summary findings to the expert panel for consideration during a virtual meeting. The data presented included the number of studies that explicitly defined the term of interest and specifics about the definitions. Synthesis focused on common elements for each definition, as well as areas which differed (eg, whether the study used a time frame for re-intubation such as 24, 48, or > 48 h of planned extubation). Subsequently, final modifications were made to the definitions. A second round of anonymous online voting (Qualtrics) was conducted, where experts were given only two options (agree/disagree), with the disagree option allowing inclusion of comments in a text box. An 80% agreement threshold was required to constitute agreement for a definition. Comments related to disagreement are synthesized into the rationale provided below for each definition.
Recommendations and Rationale
There were eight topic areas identified by experts with 16 preliminary definitions established. Based on initial discussion and the first voting, modifications were suggested for 11 of the 16 definitions that did not reach the 80% agreement threshold: noninvasive ventilation (NIV), CPAP, high-flow nasal cannula (HFNC), conventional oxygen therapy, liberation from invasive MV, failed attempt to liberate from invasive MV, duration of NRS, total duration of invasive MV, SBT, extubation readiness testing (ERT), and 28 VFDs (VFDs-28) (e-Table 6).
The systematic review yielded 49 articles for which definitions were extracted, although not all topics for definitions were addressed explicitly in the articles. In these circumstances, the panelists were informed that no studies were identified. The articles that were used to inform the definitions are cited in Table 2.8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53 During the final voting round, the expert panel agreed on all modified definitions (e-Table 7). Final definitions are shown in Table 2 and reported below.
Table 2.
Pediatric Ventilator Liberation Operational Definitions
| Topic | Definition |
|---|---|
| A. |
Respiratory Support: respiratory support includes invasive mechanical ventilation and noninvasive respiratory support
|
| B. |
|
| C. | |
| D. |
|
| E. |
|
| F. |
|
| G. |
|
| H. |
Planned vs rescue post-extubation NRS use9,14,39,41,49:
|
Respiratory Support
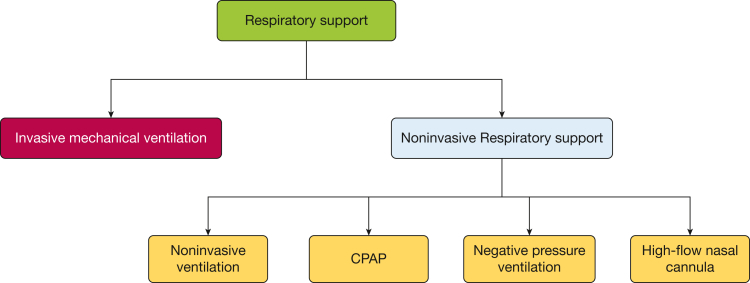
Respiratory support includes invasive MV and NRS. NRS includes NIV, CPAP, negative pressure ventilation (NPV), and HFNC (Fig 2).
Figure 2.
Respiratory support types.
Definition 1. Invasive Mechanical Ventilation (MV) (100% Agreement)
Positive pressure ventilation delivered via an artificial airway (ie, endotracheal tube [ETT] or tracheostomy tube) into the trachea.
Background: Respiratory support modalities carry different risk/benefit profiles for patients and different values for critical care providers, caregivers, and policy makers. Invasive MV is often thought to have the highest risk profile due to known complications such as ventilator-induced lung injury, ventilator-associated events, airway trauma, exposure to opioids and sedatives, critical illness myopathy/neuropathy, cost, and long-term pediatric post-intensive care syndrome.54, 55, 56, 57, 58 Hence clear delineation of the course of invasive MV was felt to be crucial.
Summary of deliberations, studies, and implementation: The definition of invasive MV was relatively straightforward and consistent across reviewed studies.8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53 In most circumstances, ventilators provide invasive MV through the endotracheal or tracheostomy tube, but in rare instances, hand-bag ventilation can be used, particularly in low-resource settings. For this reason, the definition focuses on any positive pressure being delivered through a tube which passes into the trachea.
Definition 2. Noninvasive Ventilation (NIV) (87% Agreement)
Positive pressure with variable levels of pressure delivered without an artificial airway via any interface which aims to provide an occlusive fit (eg, nasal mask, nasal pillows/prongs, full face mask or helmet). Examples include bi-level positive airway pressure or nasal high-frequency oscillation ventilation.
Definition 3. CPAP (91% Agreement)
Positive pressure with a single continuous distending pressure delivered without an artificial airway via any interface which aims to provide an occlusive fit (eg, nasal mask, nasal pillows/prongs, full face mask or helmet).
Background: There are increasing varieties of interfaces and noninvasive modes which are used to deliver positive pressure. Interface fit, as well as the modality of support, are crucial components to the benefits and risks of noninvasive modes. It is often difficult to generalize findings from individual studies related to NIV or CPAP without a clear description of the interface and systems used.59 In addition, the therapeutic target of NIV may differ from CPAP, although these terms are often combined or used interchangeably in the literature.
Summary of deliberations, studies, and implementation: Ten (20.4%) of the 49 articles examined during the systematic review reported on the use of NIV or CPAP post-extubation.14,15,17,19,26,32,34,41,42,49 Post-extubation NIV and CPAP use was not clearly specified in four articles,15,17,19,32 while three articles combined NIV with CPAP,14,26,49 two reported NIV alone,34,42 and one study reported CPAP alone.41 The CPAP/NIV interface varied; four studies used full face or oro-nasal mask,14,34,42,49 two used nasal pillows/occlusive prongs,14,49 one used nonocclusive oral mask,41 one used helmet,49 two used oral mask,41,49 and five did not report the interface used.15,17,19,26,32
In discussion with the panelists, the largest area for disagreement in defining CPAP or NIV was related to the occlusiveness of the interface. This affects the amount of pressure and oxygen delivered to the lungs. As an example, many studies of CPAP/NIV report using nasal cannula-type interfaces, which most panel experts considered to deliver a different level of support than occlusive nasal interfaces (eg, prongs or pillows) or oro-nasal interfaces.60 These interfaces also have different risk profiles for pressure injury and patient comfort.61 Therefore, almost all panelists felt that occlusive fit was necessary to label a therapy CPAP or NIV. In addition, the panel felt it important to differentiate CPAP from NIV, because the addition of inspiratory pressure augmentation with NIV likely represents a different therapeutic target than positive end-expiratory pressure alone with CPAP. These were also considered to have different risk/benefit profiles and potentially different levels of tolerance among patients. Future studies in pediatric ventilation liberation should report the specific interface used for CPAP/NIV and treat NIV and CPAP as different interventions.62
Definition 4. Negative Pressure Ventilation (NPV) (96% Agreement)
A type of respiratory support in which the surface of the thorax and/or abdomen is exposed to sub-atmospheric pressure (ie, negative pressure).
Background: NPV is typically delivered through a cuirass-type device that can synchronize with patient effort to augment a reduction in pleural pressure to stimulate airflow delivery. While there are limited studies of NPV related to ventilator liberation in the PICU, devices are commercially available and have been used in some PICUs to provide respiratory support in addition to or in place of positive pressure ventilation.63
Summary of deliberations, studies, and implementation: The definition of NPV was relatively straightforward, with minimal debate among the panelists. The panelists did feel that NPV constituted a form of respiratory support, and that NPV should be explicitly differentiated from other forms of respiratory support, in addition to reporting its concomitant use with other modes of respiratory support.
Definition 5. High-Flow Nasal Cannula (HFNC) (87% Agreement)
Flow that is delivered through a heated humidified nasal cannula circuit and interface at a flow rate, which is:
-
a.
≥ 1 L/kg/min for patients up to 10 kg.
-
b.
≥ 10 L/min for patients above 10 kg.
When the HFNC flow falls below the above rates, the patient is considered to be receiving conventional oxygen therapy (see Definition 6).
Definition 6. Conventional Oxygen Therapy (96% Agreement)
In the context of defining liberation from respiratory support, conventional oxygen therapy is not considered a respiratory support.
Conventional oxygen therapy is defined as the provision of > 0.21 oxygen by any of the following devices applied to a spontaneously breathing patient regardless of presence of humidification:
-
a.
Face mask oxygen delivered via any type of nonocclusive mask
-
b.
Nasal cannula at flow rates less than HFNC rates (see Definition 5)
-
c.
Tracheostomy collar without positive pressure
Background: HFNC is increasingly used in PICU for various indications64,65 but with significant controversy. Controversy even exists about the most appropriate terminology: HFNC; heated, humidified high-flow nasal cannula (HHHFNC); or high-flow nasal oxygen (HFNO). Fundamentally, there is a need to differentiate HFNC from conventional oxygen therapy, CPAP, and NIV, given different benefits, risks, and cost. There is inconsistency in the definition of HFNC, and whether this should be based on a minimum flow rate, the device or interface used, and whether there is a requirement for the gas to be heated and humidified. There is also inconsistency as to whether supplemental oxygen is required for HFNC, given that HFNC is often used without supplemental oxygen for children who have high work of breathing.
Summary of deliberations, studies, and implementation: Six studies reported post-extubation HFNC.19,32,39,40,49,50 Three studies defined HFNC based on a flow of 1 to 1.99 L/kg/min,39,40,50 two did not specify a flow rate,19,32 while one study defined HFNC as 2 L/kg/min for children below 10 kg and specified minimum flow rates for different weight brackets.49 Description of HFNC humidification and heating were only reported in two-thirds of the included studies.39,40,49,50 The definition used to delineate the end of HFNC was not reported in the majority of studies,32,39,40,50 and one study defined it as removal of HFNC interface regardless of flow rate.49
There was extensive discussion among the expert panel with a general belief that the definition of HFNC should not be based on the interface or device type being used. Areas of disagreement focused mainly on the minimum flow rate for inclusion, particularly when considering how to define discontinuation of HFNC. While the minimal effective dose of HFNC remains somewhat controversial, existing physiological studies were used to support inclusion in the definition of a minimal flow rate of 1 L/kg/min for children less than 10 kg, based primarily on its effect on work of breathing. For children over 10 kg,66,67 a minimum flow rate of 10 L/min was considered pragmatic, to differentiate HFNC from conventional oxygen therapy. Moreover, since the intent of HFNC is often to reduce work of breathing, and not simply to deliver oxygen, experts did not feel oxygen supplementation was a necessary element in the definition. The use of heating and humidification was considered a crucial element of the potential therapeutic benefit and patient tolerance of the therapy, hence the panel believed these should be contained in the definition.
There may be challenges to implementing this HFNC definition, as it will necessitate consideration of patient-related factors (weight) to define the commencement and discontinuation of the therapy, rather than simply the interface being used. Weight is crucial for many elements of pediatric medicine, so it is likely widely available. The additional burden may relate to explicitly reporting the flow rate of HFNC. On balance, this additional burden was outweighed by the benefits of more clearly defining the time frame in which the patient is truly receiving what is believed to be HFNC therapy.
Definition 7. Liberation From Invasive MV (96% Agreement)
A patient is considered to be liberated from invasive MV when:
-
a.
ETT: An ETT is removed and is not reinserted within 48 h.∗
-
b.
Tracheostomy tube: Positive pressure ventilation is no longer being delivered through a tracheostomy tube and is not re-initiated within 48 h.∗ This includes application of controlled, assisted, supported, or CPAP modes of positive pressure via a tracheostomy tube for any period during the day/night.
Definition 8. Failed Attempt to Liberate From Invasive MV (ie, Extubation Failure) (96% Agreement)
-
a.
ETT: Re-intubation within 48 h following extubation or a placement of a new tracheostomy with delivery of positive pressure ventilation for any period of the day.∗
-
b.
Tracheostomy tube: Re-institution of positive pressure ventilation within 48 h after attempt of liberation from invasive mechanical ventilation.∗ This includes application of controlled, assisted, supported, or CPAP modes of positive pressure via a tracheostomy tube for any period during the day/night.
Background: Successful liberation from invasive MV is an important outcome reported in nearly all studies of ventilated children, yet there is significant inconsistency in the literature in terms of how it is defined. This inconsistency is complicated by increasing use of NIV after extubation,68 which may prevent re-intubation for some or simply prolong the time to re-intubation for others. It is unclear whether a patient who is re-intubated several days following extubation should be considered to have failed extubation, or whether the reason for re-intubation should be factored into the definition, as the need for re-intubation may relate to a new event such as development of a hospital-acquired pneumonia.
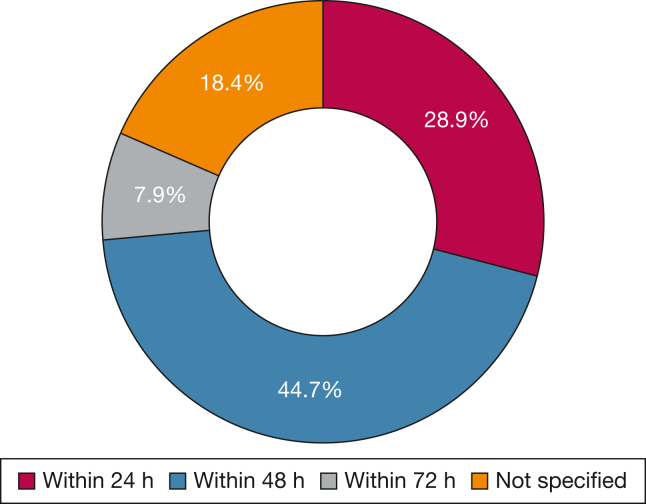
Summary of deliberations, studies, and implementation: Most studies (36 of 49 [73.5%]) used re-intubation as the definition for extubation failure8, 9, 10, 11, 12, 13, 14,16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38,40, 41, 42, 43, 44,53; only two studies considered re-intubation and/or use of NIV post-extubation as extubation failure.15,39 The most common reported time frame for extubation failure was 48 h (44.7%), followed by 24 h (28.9%) and 72 h (7.9%) (Fig. 3). None of the analyzed studies included patients with tracheostomy with home MV, while one study included patients with tracheostomy without home MV who were receiving MV in the PICU.43
Figure 3.
Reported extubation failure time frame (n = 38).
The time frame of liberation for invasive MV and extubation failure was a major discussion point among the panelists. Ultimately, the panel elected for a 48 h time frame to define extubation failure for several reasons. First, 48 h is most commonly reported in the literature and is also consistent with adult ventilator liberation definitions.69 Second, 24 h was perceived as too short, given the increasing use of NRS following extubation, which may prolong the time to re-intubation. Third, extubation failures beyond 48 h were thought to be less attributable to the primary ventilation course. Additional time frames (ie, 72 h or 7 days) were considered to be beneficial as secondary outcomes for certain patient populations such as those with cardiac disease, chronic critical illness, neuromuscular disorders, and traumatic brain injury.
We added “new tracheostomy with delivery of positive pressure ventilation” to the extubation failure definition for patients with an ETT, to explicitly characterize this as extubation failure. The panel felt that because invasive MV, NIV, and HFNC carry different benefit/risk profiles, failure to liberate from invasive MV and the time on invasive MV should be specifically differentiated from time on NIV and HFNC.
For patients with existing tracheostomy without home MV but who are receiving invasive MV in the PICU, the panel felt it important to clarify that all modes of positive pressure delivered through the tracheostomy constituted invasive MV. This was a point of discussion because the use of NIV was unlikely in these patients given that they have an existing invasive airway. Patients with a tracheostomy and home ventilation are not commonly included in pediatric ventilator liberation research, but they are a growing population in the PICU. Future studies should specifically establish definitions related to pediatric ventilator liberation for this population.
Definition 9. Liberation From Respiratory Support (100% Agreement)
A patient is considered liberated from respiratory support when the patient is no longer receiving invasive MV or NRS and it is not re-initiated within 48 h.
Definition 10. Duration of NRS (100% Agreement)
A measure of the total duration in which any of the NRS modes (Definitions 2-5) are applied.
-
•
If NRS is resumed > 48 h after an initial attempt to liberate from NRS, it is considered a new NRS course.
-
•
If one of above NRS is re-initiated ≤ 48 h from an attempt to liberate from NRS, it is considered a failed liberation attempt, and the duration of NRS should include the time (≤ 48 h) that the patient was not receiving one of these therapies.
Background: The last decade has seen increased use of NRS in the PICU. At times, reductions in length of invasive MV may be traded for increased use or duration of NRS.70, 71, 72 These treatment modalities may have differential impact and importance for families, patients, health-care professionals, and policy makers.
Summary of deliberations, studies, and implementation: Definitions of NRS discontinuation were only explicitly reported in two studies and related to physical removal of the machine delivering NRS.19,41 Experts felt the concepts of NRS liberation should mimic the definition and time frame (ie, 48 h) of liberation from invasive MV. In addition, most patients are liberated from NRS within 48 h of extubation.73 The panel discussed the potential importance of identifying the subset of patients who receive prolonged NRS or those who go on to receive chronic NRS after PICU discharge. Furthermore, because tolerance and risk/benefit profiles differ based on NRS modalities, it was felt that studies specifically focused on NRS following extubation should report the duration of different NRS modalities. Panel members did acknowledge that the additional resources required to gather these data may not always be available. Important areas for additional research were identified, including patient, family member, policy maker, and clinician perspectives regarding trade-offs between the use of invasive MV vs NRS and prolonged NRS. Additional areas of research included methods to incorporate preexisting use of NRS and nocturnal NRS in the definitions of NRS use and NRS duration, as well as appropriate benchmarks for optimal rate and duration of NRS use following extubation vs duration of invasive MV and extubation failure.
Definition 11. Total Duration of Invasive MV (91% Agreement)
Time from initiation of invasive MV until successful liberation from invasive MV or death.
-
•
If invasive MV is resumed > 48 h after an initial attempt to liberate from invasive MV, it is considered a new ventilation course.
-
•
If invasive MV is resumed ≤ 48 h of an initial attempt to liberate from invasive MV, it is considered a failed liberation attempt, and the duration of invasive MV should include the time (≤ 48 h) that the patient was not receiving invasive MV.
Background: Duration of invasive MV is one of the most important outcomes for pediatric ventilator liberation, and it is used as a balancing measure to extubation failure. It is also an important metric for policy makers for considering resource allocation and utilization tracking. There is general consensus on how to define duration of invasive MV, although there remains some inconsistency in its measurement and reporting in randomized controlled trials.
Summary of deliberations, studies, and implementation: Almost all studies reported invasive MV duration. Only six of the analyzed studies reported the combination of invasive MV and NIV duration,10,12,13,15,30,33 while one study separately reported the duration of NRS from duration of invasive MV.14 Most studies (36 of 49 [73.5%]) used initiation of invasive MV as the commencement anchoring point to calculate the duration of invasive MV, although two studies used randomization in the study as an anchoring point.33,40
Panel experts selected the initiation of invasive MV as opposed to time of study randomization as the anchoring point to identify commencement of invasive MV for the calculation of total invasive MV duration because it captures the whole course of invasive MV and its associated risks. Moreover, with effective randomization, duration of invasive MV prerandomization should be similar. This definition can also be applied across study types (cohort, case control, randomized trials). There was also discussion about how to consider patients who die on invasive MV. The panel felt that length of invasive MV should be reported only in survivors, particularly when mortality rates are different between treatment groups. Use of composite outcomes such as VFDs (see Definition 14) may be more appropriate for studies with a significant number of patients who die while on invasive MV. Important areas for research included establishing benchmarks for invasive MV duration in subpopulations of children (based on presenting illnesses, comorbidities, and severity of illness) to be used by PICU providers, researchers, and policy makers.
Definition 12. Spontaneous Breathing Trial (SBT) (91% Agreement)
SBT is defined as a systematic method of reduction of invasive MV support to predetermined settings to assess the likelihood that a patient will be able to independently maintain adequate minute ventilation and gas exchange without excessive respiratory effort if liberated from invasive MV.
Definition 13. Extubation Readiness Testing (ERT) (96% Agreement)
ERT is defined as a bundle of elements used to assess the patient’s eligibility to be liberated from invasive MV. In addition to the SBT, this may include factors such as assessment of sedation level, adequacy of neurologic control of the airway (ie, cough and gag), likelihood of post-extubation upper airway obstruction, assessment of respiratory muscle strength, magnitude of airway secretions, hemodynamic status, and a plan for post-extubation respiratory support.
Background: SBT and ERT are often used interchangeably in the literature, although they represent different concepts, with an SBT often being a component of an ERT.
Summary of deliberations, studies, and implementation: Panelists built on the conceptual framework that the SBT is an element of the ERT. The SBT gauges whether the patient will be able to initiate spontaneous breaths and breathe independently without excessive respiratory effort after extubation. The SBT is an important element of the ERT bundle. However, there are other elements that need to be assessed to achieve successful extubation. The ERT bundle may additionally include elements such as sedation level, adequacy of neurologic control of the airway (ie, cough and gag), likelihood of post-extubation upper airway obstruction, assessment of respiratory muscle strength, magnitude of airway secretions, hemodynamic status, and a plan for post-extubation respiratory support.
There was general agreement on the SBT and ERT definitions. The SBT definition was clarified by adding “reduction of ventilator support to predetermined settings” to distinguish it from gradual reduction of ventilatory support. ERT elements were discussed to ensure inclusiveness of all important elements reported in the literature, although panelists felt it was necessary to allow for inclusion of other elements which may be important based on local practice or patient-specific risk factors. Panelists also acknowledged that the individual elements proposed for ERTs were not all mandatory to constitute an ERT.
Definition 14. 28 Ventilator-Free Days (VFDs-28) (91% Agreement)
-
a.
For survivors: equals 28 minus the sum of invasive MV days during the first 28 days following initiation of invasive MV.
-
b.
For non-survivors: VFDs-28 would be ZERO if death occurred within 28 days of initiation of invasive MV. If death occurs after 28 days, VFDs-28 are calculated in the same way as for survivors.
Background: VFDs-28 are commonly reported in trials of mechanically ventilated patients as they capture a composite outcome of mortality and length of ventilation.74 Because length of ventilation is influenced by the above definitions related to ventilator liberation, the panel felt it was important to specifically address VFDs in these definitions.
Summary of deliberations, studies, and implementation: The definition of VFDs-28 was not clearly reported in any of the studies included in our systematic reviews. The panel felt it important to stay consistent with existing definitions for VFDs-28, incorporating the definitions for duration of invasive MV reported above. The panel felt it may be relevant to use similar definitions for 28 NRS-free days (28 NIV-free days, 28 CPAP-free days, and 28 HFNC-free days), although the relevance of these outcomes was uncertain. Examples of VFDs-28 calculation are shown in e-Table 8.
Definition 15. Planned NRS Post-extubation Use (100% Agreement)
The application of NRS (NIV, CPAP, NPV, or HFNC) which was planned to be initiated immediately after an attempt of liberation from invasive MV.
Definition 16. Rescue NRS Post-extubation Use (100% Agreement)
The application of NRS (NIV, CPAP, NPV, or HFNC) within 48 h after an attempt of liberation from invasive MV which was NOT planned prior to the invasive MV liberation attempt.
Background: NRS is sometimes applied in a planned fashion (ie, the practitioner intends to use it regardless of clinical status after extubation), while other times it is used when the patient is failing conventional therapies (rescue). The efficacy of using NRS post-extubation to prevent extubation failure in the pediatric population is still under investigation.68,75 It is still unclear if planned NRS use provides any advantage over rescue or delayed NRS use.
Summary of deliberations, studies, and implementation: Definitions of planned vs rescue NRS use post-extubation varied between studies.9,14,39,41,49 Intention to use NRS post-extubation defined planned NRS in three studies,39,41,49 while another study defined it as the initiation of NRS within 60 min of extubation.9 A focus of discussion among the panel was whether a specific time frame after extubation for initiation of NRS could be used to define planned vs rescue, given that it may be impossible to ascertain whether the therapy was planned simply by reviewing the medical record. For example, if NRS is started 30 min following extubation, this could be in response to the patient failing conventional therapies, or as part of a predetermined treatment plan. As such, the panel felt the definition should not be based on time to initiate NRS but rather clinician intent. Ascertainment of this may require some discussion with the care team. Using a specific data collection form to differentiate planned from rescue therapy or implementing documentation within the electronic health record would assist in making this data collection more feasible and accurate.
Potential Gaps With These Proposed Definitions
These proposed definitions are intended to represent the spectrum of respiratory support for pediatric ventilator liberation. However, there are some gaps. First, given the changing landscape of respiratory support devices, the panel was unclear how to best characterize CPAP/NIV delivered with nonocclusive interfaces. The panel felt strongly that these types of interfaces (ie, nasal cannula) did not provide the same level of support as CPAP/NIV delivered with occlusive interfaces and should be treated separately. At the same time, they are likely distinct from HFNC and conventional oxygen therapy. At this point, the panel did not suggest a clear definition or label for this group of patients, and encourages future studies capture data related to the occlusive fit of CPAP/NIV interfaces to inform future definitions.
Second, there was not clear consensus about how to characterize respiratory support for children who are receiving HFNC or “conventional oxygen” with 0.21 Fio2. The panel felt that when heating and humification were used with flow rates exceeding 1 L/kg or 10 L, that these patients met the definitions for HFNC. It remains unclear how to categorize these patients when flow rates fall below HFNC flow rates but 0.21 Fio2 is used. Technically, these patients do not meet our proposed definitions for conventional oxygen therapy, and likely represent a different group.
Third, we did not address use of extracorporeal therapies (ie, extracorporeal membrane oxygenation and extracorporeal CO2 removal) which may provide respiratory support. It is certainly possible that some patients could meet our definitions for liberation from respiratory support but are still receiving extracorporeal therapies. This is likely to constitute a small proportion of patients in most studies of pediatric ventilator liberation, but investigators should specifically address these scenarios in studies where there are likely to be a significant number of patients on extracorporeal support.
Limitations
In addition to the potential gaps identified above, there are important limitations of this work. First, the expert panel was chosen based on the criterion of having published on pediatric ventilator liberation in the last 10 years. While this has advantages of experts with experience in this domain, it may lead to underrepresentation of experts from resource-limited settings, or more junior investigators. To overcome this limitation, we attempted to focus on including more junior investigators, as well as multi-professional international representatives. Second, there is a risk in consensus-based approaches that people feel obligated to agree with definitions. We attempted to reduce the impact of this by using anonymous online voting. Third, while we conducted systematic reviews to identify relevant evidence, we analyzed only articles included in the systematic reviews related to the parent project focused on developing pediatric ventilator liberation guidelines.7 We did not conduct a separate search to specifically identify all the pediatric respiratory evidence related to these modalities. Finally, we chose topic areas which we felt were most relevant to standardize in studies of pediatric ventilator liberation but acknowledge that there are likely many more topic areas which would benefit from this type of approach.
Conclusions
Although we have made substantial progress in research related to pediatric ventilator liberation, there continue to be many unanswered questions. It is imperative that definitions for important elements in pediatric ventilator liberation are standardized to facilitate pooling of data across studies and help generalize findings from research into clinical practice. We propose that these pediatric ventilator liberation operational definitions be used in future quality improvement and research studies. Future work is needed to study the feasibility of implementing these definitions in different ICU settings and populations and identify areas in need of refinement.
Funding/Support
The project was funded by Eunice Kennedy Shriver National Institute of Child Health (NICHD) and Human Development National Heart, Lung, and Blood Institute (NHLBI) of the National Institutes of Health (NIH) (R13HD102137), in addition to funds from the Department of Pediatrics at Indiana University School of Medicine, Indianapolis, IN.
Financial/Nonfinancial Disclosures
None declared.
Acknowledgments
Author contributions: All authors contributed to the conception or design of the work, or the acquisition, analysis, or interpretation of data for the work. All authors participated in drafting the work or revising it critically for important intellectual content; they approved and are responsible for the final version submitted for publication.
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
DISCLAIMER: American College of Chest Physician guidelines are intended for general information only, are not medical advice, and do not replace professional medical care and physician advice, which always should be sought for any medical condition. The complete disclaimer for this guideline can be accessed at https://www.chestnet.org/Guidelines-and-Resources.
Excluding use for temporary procedures.
Excluding use for temporary procedures.
Supplementary Data
References
- 1.Newth C.J., Venkataraman S., Willson D.F., et al. Weaning and extubation readiness in pediatric patients. Pediatr Crit Care Med. 2009;10(1):1–11. doi: 10.1097/PCC.0b013e318193724d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fan E., Zakhary B., Amaral A., et al. Liberation from mechanical ventilation in critically ill adults. An Official ATS/ACCP Clinical Practice Guideline. Ann Am Thorac Soc. 2017;14(3):441–443. doi: 10.1513/AnnalsATS.201612-993CME. [DOI] [PubMed] [Google Scholar]
- 3.Abu-Sultaneh S., Mastropietro C.W. In: Pediatric Critical Care. Mastropietro C., Valentine K., editors. Springer; 2019. Weaning and extubation readiness assessment in pediatric patients; pp. 43–62. [Google Scholar]
- 4.Newth C.J., Hotz J.C., Khemani R.G. Ventilator liberation in the pediatric ICU. Respir Care. 2020;65(10):1601–1610. doi: 10.4187/respcare.07810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Loberger J.M., Campbell C.M., Colleti J., Jr., Borasino S., Abu-Sultaneh S., Khemani R.G. Ventilation liberation practices among 380 international PICUs. Crit Care Explor. 2022;4(6) doi: 10.1097/CCE.0000000000000710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Dijk J., Blokpoel R.G.T., Abu-Sultaneh S., Newth C.J.L., Khemani R.G., Kneyber M.C.J. Clinical challenges in pediatric ventilation liberation: a meta-narrative review. Pediatr Crit Care Med. 2022;23(12):999–1008. doi: 10.1097/PCC.0000000000003025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abu-Sultaneh S., Iyer N.P., Fernandez A., et al. Executive Summary: International Clinical Practice Guidelines for Pediatric Ventilator Liberation, A Pediatric Acute Lung Injury and Sepsis Investigators (PALISI) Network Document. Am J Respir Crit Care Med. 2023;207(1):17–28. doi: 10.1164/rccm.202204-0795SO. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khemani R.G., Hotz J., Morzov R., et al. Evaluating risk factors for pediatric post-extubation upper airway obstruction using a physiology-based tool. Am J Respir Crit Care Med. 2016;193(2):198–209. doi: 10.1164/rccm.201506-1064OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khemani R.G., Hotz J., Morzov R., et al. Pediatric extubation readiness tests should not use pressure support. Intensive Care Med. 2016;42(8):1214–1222. doi: 10.1007/s00134-016-4387-3. [DOI] [PubMed] [Google Scholar]
- 10.van Dijk J., Blokpoel R.G.T., Koopman A.A., Dijkstra S., Burgerhof J.G.M., Kneyber M.C.J. The effect of pressure support on imposed work of breathing during paediatric extubation readiness testing. Ann Intensive Care. 2019;9(1):78. doi: 10.1186/s13613-019-0549-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abdel Rahman D.A., Saber S., El-Maghraby A. Diaphragm and lung ultrasound indices in prediction of outcome of weaning from mechanical ventilation in pediatric intensive care unit. Indian J Pediatr. 2020;87(6):413–420. doi: 10.1007/s12098-019-03177-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harikumar G., Egberongbe Y., Nadel S., et al. Tension-time index as a predictor of extubation outcome in ventilated children. Am J Respir Crit Care Med. 2009;180(10):982–988. doi: 10.1164/rccm.200811-1725OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnston C., de Carvalho W.B., Piva J., Garcia P.C., Fonseca M.C. Risk factors for extubation failure in infants with severe acute bronchiolitis. Respir Care. 2010;55(3):328–333. [PubMed] [Google Scholar]
- 14.Khemani R.G., Sekayan T., Hotz J., et al. Risk factors for pediatric extubation failure: the importance of respiratory muscle strength. Crit Care Med. 2017;45(8):e798–e805. doi: 10.1097/CCM.0000000000002433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Noizet O., Leclerc F., Sadik A., et al. Does taking endurance into account improve the prediction of weaning outcome in mechanically ventilated children? Crit Care. 2005;9(6):R798–R807. doi: 10.1186/cc3898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thiagarajan R.R., Bratton S.L., Martin L.D., Brogan T.V., Taylor D. Predictors of successful extubation in children. Am J Respir Crit Care Med. 1999;160(5 pt 1):1562–1566. doi: 10.1164/ajrccm.160.5.9810036. [DOI] [PubMed] [Google Scholar]
- 17.Wolf G.K., Walsh B.K., Green M.L., Arnold J.H. Electrical activity of the diaphragm during extubation readiness testing in critically ill children. Pediatr Crit Care Med. 2011;12(6):e220–e224. doi: 10.1097/PCC.0b013e3181fe28fc. [DOI] [PubMed] [Google Scholar]
- 18.Xue Y., Zhang Z., Sheng C.Q., Li Y.M., Jia F.Y. The predictive value of diaphragm ultrasound for weaning outcomes in critically ill children. BMC Pulm Med. 2019;19(1):270. doi: 10.1186/s12890-019-1034-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.IJland M.M., Lemson J., van der Hoeven J.G., Heunks L.M.A. The impact of critical illness on the expiratory muscles and the diaphragm assessed by ultrasound in mechanical ventilated children. Ann Intensive Care. 2020;10(1):115. doi: 10.1186/s13613-020-00731-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shimada Y., Yoshiya I., Tanaka K., Yamazaki T., Kumon K. Crying vital capacity and maximal inspiratory pressure as clinical indicators of readiness for weaning of infants less than a year of age. Anesthesiology. 1979;51(5):456–459. doi: 10.1097/00000542-197911000-00017. [DOI] [PubMed] [Google Scholar]
- 21.Farias J.A., Alia I., Retta A., et al. An evaluation of extubation failure predictors in mechanically ventilated infants and children. Intensive Care Med. 2002;28(6):752–757. doi: 10.1007/s00134-002-1306-6. [DOI] [PubMed] [Google Scholar]
- 22.el-Khatib M.F., Baumeister B., Smith P.G., Chatburn R.L., Blumer J.L. Inspiratory pressure/maximal inspiratory pressure: does it predict successful extubation in critically ill infants and children? Intensive Care Med. 1996;22(3):264–268. doi: 10.1007/BF01712248. [DOI] [PubMed] [Google Scholar]
- 23.Toida C., Muguruma T., Miyamoto M. Detection and validation of predictors of successful extubation in critically ill children. PLoS One. 2017;12(12) doi: 10.1371/journal.pone.0189787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Venkataraman S.T., Khan N., Brown A. Validation of predictors of extubation success and failure in mechanically ventilated infants and children. Crit Care Med. 2000;28(8):2991–2996. doi: 10.1097/00003246-200008000-00051. [DOI] [PubMed] [Google Scholar]
- 25.Khan N., Brown A., Venkataraman S.T. Predictors of extubation success and failure in mechanically ventilated infants and children. Crit Care Med. 1996;24(9):1568–1579. doi: 10.1097/00003246-199609000-00023. [DOI] [PubMed] [Google Scholar]
- 26.Wratney A.T., Benjamin D.K., Jr., Slonim A.D., He J., Hamel D.S., Cheifetz I.M. The endotracheal tube air leak test does not predict extubation outcome in critically ill pediatric patients. Pediatr Crit Care Med. 2008;9(5):490–496. doi: 10.1097/PCC.0b013e3181849901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mhanna M.J., Zamel Y.B., Tichy C.M., Super D.M. The “air leak” test around the endotracheal tube, as a predictor of postextubation stridor, is age dependent in children. Crit Care Med. 2002;30(12):2639–2643. doi: 10.1097/00003246-200212000-00005. [DOI] [PubMed] [Google Scholar]
- 28.Schneider J., Mulale U., Yamout S., Pollard S., Silver P. Impact of monitoring endotracheal tube cuff leak pressure on postextubation stridor in children. J Crit Care. 2016;36:173–177. doi: 10.1016/j.jcrc.2016.06.033. [DOI] [PubMed] [Google Scholar]
- 29.Suominen P.K., Tuominen N.A., Salminen J.T., et al. The air-leak test is not a good predictor of postextubation adverse events in children undergoing cardiac surgery. J Cardiothorac Vasc Anesth. 2007;21(2):197–202. doi: 10.1053/j.jvca.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 30.Anene O., Meert K.L., Uy H., Simpson P., Sarnaik A.P. Dexamethasone for the prevention of postextubation airway obstruction: a prospective, randomized, double-blind, placebo-controlled trial. Crit Care Med. 1996;24(10):1666–1669. doi: 10.1097/00003246-199610000-00011. [DOI] [PubMed] [Google Scholar]
- 31.Cesar R.G., de Carvalho W.B. L-epinephrine and dexamethasone in postextubation airway obstruction: a prospective, randomized, double-blind placebo-controlled study. Int J Pediatr Otorhinolaryngol. 2009;73(12):1639–1643. doi: 10.1016/j.ijporl.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 32.de Carvalho H.T., Fioretto J.R., Bonatto R.C., Ribeiro C.F., Martin J.G., Carpi M.F. Use of dexamethasone to prevent extubation failure in pediatric intensive care unit: a randomized controlled clinical trial. J Pediatr Intensive Care. 2020;11(1):41–47. doi: 10.1055/s-0040-1719044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harel Y., Vardi A., Quigley R., et al. Extubation failure due to post-extubation stridor is better correlated with neurologic impairment than with upper airway lesions in critically ill pediatric patients. Int J Pediatr Otorhinolaryngol. 1997;39(2):147–158. doi: 10.1016/s0165-5876(97)01488-2. [DOI] [PubMed] [Google Scholar]
- 34.Malhotra D., Gurcoo S., Qazi S., Gupta S. Randomized comparative efficacy of dexamethasone to prevent postextubation upper airway complications in children and adults in ICU. Indian J Anaesth. 2009;53(4):442–449. [PMC free article] [PubMed] [Google Scholar]
- 35.Ritu Jhamb U. Dexamethasone in prevention of postextubation stridor in ventilated children: a randomized, double-blinded, placebo-controlled trial. Indian J Crit Care Med. 2020;24(12):1230–1235. doi: 10.5005/jp-journals-10071-23679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tellez D.W., Galvis A.G., Storgion S.A., Amer H.N., Hoseyni M., Deakers T.W. Dexamethasone in the prevention of postextubation stridor in children. J Pediatr. 1991;118(2):289–294. doi: 10.1016/s0022-3476(05)80505-0. [DOI] [PubMed] [Google Scholar]
- 37.Veder L.L., Joosten K.F.M., Schlink K., et al. Post-extubation stridor after prolonged intubation in the pediatric intensive care unit (PICU): a prospective observational cohort study. Eur Arch Otorhinolaryngol. 2020;277(6):1725–1731. doi: 10.1007/s00405-020-05877-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kemper K.J., Benson M.S., Bishop M.J. Predictors of postextubation stridor in pediatric trauma patients. Crit Care Med. 1991;19(3):352–355. doi: 10.1097/00003246-199103000-00012. [DOI] [PubMed] [Google Scholar]
- 39.Wijakprasert P., Chomchoey J. High-flow nasal cannula versus conventional oxygen therapy in post-extubation pediatric patients: a randomized controlled trial. J Medical Assoc Thailand. 2018;101(10):1331–1335. [Google Scholar]
- 40.Akyildiz B., Ozturk S., Ulgen-Tekerek N., Doganay S., Gorkem S.B. Comparison between high-flow nasal oxygen cannula and conventional oxygen therapy after extubation in pediatric intensive care unit. Turk J Pediatr. 2018;60(2):126–133. doi: 10.24953/turkjped.2018.02.002. [DOI] [PubMed] [Google Scholar]
- 41.Rodriguez J.A., Von Dessauer B., Duffau G. Non-invasive continuous positive airways pressure for post-extubation laryngitis in pediatric patients [in Spanish] Arch Bronconeumol. 2002;38(10):463–467. doi: 10.1016/s0300-2896(02)75266-6. [DOI] [PubMed] [Google Scholar]
- 42.Fioretto J.R., Ribeiro C.F., Carpi M.F., et al. Comparison between noninvasive mechanical ventilation and standard oxygen therapy in children up to 3 years old with respiratory failure after extubation: a pilot prospective randomized clinical study. Pediatr Crit Care Med. 2015;16(2):124–130. doi: 10.1097/PCC.0000000000000309. [DOI] [PubMed] [Google Scholar]
- 43.Curley M.A., Wypij D., Watson R.S., et al. Protocolized sedation vs usual care in pediatric patients mechanically ventilated for acute respiratory failure: a randomized clinical trial. JAMA. 2015;313(4):379–389. doi: 10.1001/jama.2014.18399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schultheis J.M., Heath T.S., Turner D.A. Association between deep sedation from continuous intravenous sedatives and extubation failures in mechanically ventilated patients in the pediatric intensive care unit. J Pediatr Pharmacol Ther. 2017;22(2):106–111. doi: 10.5863/1551-6776-22.2.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dionisio M.T., Rebelo A., Pinto C., Carvalho L., Neves J.F. Ultrasound assessment of ventilator-induced diaphragmatic dysfunction in paediatrics [in Portuguese] Acta Med Port. 2019;32(7-8):520–528. doi: 10.20344/amp.10830. [DOI] [PubMed] [Google Scholar]
- 46.Xue Y., Yang C.F., Ao Y., Qi J., Jia F.Y. A prospective observational study on critically ill children with diaphragmatic dysfunction: clinical outcomes and risk factors. BMC Pediatr. 2020;20(1):422. doi: 10.1186/s12887-020-02310-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gozal D., Shoseyov D., Keens T.G. Inspiratory pressures with CO2 stimulation and weaning from mechanical ventilation in children. Am Rev Respir Dis. 1993;147(2):256–261. doi: 10.1164/ajrccm/147.2.256. [DOI] [PubMed] [Google Scholar]
- 48.Tamburro R., Bunitz M. Tracheal airleak as a predictor of post-extubation stridor in the paediatric intensive care unit. Clin Intensive Care. 1993;4(2):52–55. [Google Scholar]
- 49.Ramnarayan P., Lister P., Dominguez T., et al. FIRST-line support for Assistance in Breathing in Children (FIRST-ABC): a multicentre pilot randomised controlled trial of high-flow nasal cannula therapy versus continuous positive airway pressure in paediatric critical care. Crit Care. 2018;22(1):144. doi: 10.1186/s13054-018-2080-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Itagaki T., Nakanishi N., Okuda N., et al. Effect of high-flow nasal cannula on thoraco-abdominal synchrony in pediatric subjects after cardiac surgery. Respir Care. 2019;64(1):10–16. doi: 10.4187/respcare.06193. [DOI] [PubMed] [Google Scholar]
- 51.Vet N.J., de Wildt S.N., Verlaat C.W., et al. A randomized controlled trial of daily sedation interruption in critically ill children. Intensive Care Med. 2016;42(2):233–244. doi: 10.1007/s00134-015-4136-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Welzing L., Vierzig A., Junghaenel S., et al. Remifentanil and propofol for weaning of mechanically ventilated pediatric intensive care patients. Eur J Pediatr. 2011;170(4):477–481. doi: 10.1007/s00431-010-1312-6. [DOI] [PubMed] [Google Scholar]
- 53.Farias J.A., Retta A., Alia I., et al. A comparison of two methods to perform a breathing trial before extubation in pediatric intensive care patients. Intensive Care Med. 2001;27(10):1649–1654. doi: 10.1007/s001340101035. [DOI] [PubMed] [Google Scholar]
- 54.Tobias J.D. Tolerance, withdrawal, and physical dependency after long-term sedation and analgesia of children in the pediatric intensive care unit. Crit Care Med. 2000;28(6):2122–2132. doi: 10.1097/00003246-200006000-00079. [DOI] [PubMed] [Google Scholar]
- 55.Principi T., Fraser D.D., Morrison G.C., et al. Complications of mechanical ventilation in the pediatric population. Pediatr Pulmonol. 2011;46(5):452–457. doi: 10.1002/ppul.21389. [DOI] [PubMed] [Google Scholar]
- 56.Kneyber M.C., Zhang H., Slutsky A.S. Ventilator-induced lung injury. Similarity and differences between children and adults. Am J Respir Crit Care Med. 2014;190(3):258–265. doi: 10.1164/rccm.201401-0168CP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mourani P.M., Sontag M.K. Ventilator-associated pneumonia in critically ill children: a new paradigm. Pediatr Clin North Am. 2017;64(5):1039–1056. doi: 10.1016/j.pcl.2017.06.005. [DOI] [PubMed] [Google Scholar]
- 58.Manning J.C., Pinto N.P., Rennick J.E., Colville G., Curley M.A.Q. Conceptualizing post intensive care syndrome in children—the PICS-p Framework. Pediatr Crit Care Med. 2018;19(4):298–300. doi: 10.1097/PCC.0000000000001476. [DOI] [PubMed] [Google Scholar]
- 59.Villanueva AM, Orive JP, Cuscó MG, Blokpoel R, Ódena MP, Rimensberger P. Handbook of Paediatric and Neonatal Mechanical Ventilation, 2nd ed., Tesela Editions.
- 60.Napolitano N., Roberts T., Nickel A.J., et al. Performance evaluation of nasal prong interface for CPAP delivery on a critical care ventilator: a bench experiment. Respir Care. 2021;66(10):1514–1520. doi: 10.4187/respcare.09018. [DOI] [PubMed] [Google Scholar]
- 61.Fauroux B., Ramirez A., Amaddeo A. Springer; Noninvasive Mechanical Ventilation: 2016. Interfaces for Acute and Long-Term Noninvasive Positive Pressure Ventilation in Children: Key Technical Elements and Clinical Implications; pp. 813–825. [Google Scholar]
- 62.Dada S., Ashworth H., Sobitschka A., et al. Experiences with implementation of continuous positive airway pressure for neonates and infants in low-resource settings: a scoping review. PLoS One. 2021;16(6) doi: 10.1371/journal.pone.0252718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hassinger A.B., Breuer R.K., Nutty K., Ma C.X., Al Ibrahim O.S. Negative-pressure ventilation in pediatric acute respiratory failure. Respir Care. 2017;62(12):1540–1549. doi: 10.4187/respcare.05531. [DOI] [PubMed] [Google Scholar]
- 64.Kwon J.W. High-flow nasal cannula oxygen therapy in children: a clinical review. Clin Exp Pediatr. 2020;63(1):3–7. doi: 10.3345/kjp.2019.00626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lee J.H., Rehder K.J., Williford L., Cheifetz I.M., Turner D.A. Use of high flow nasal cannula in critically ill infants, children, and adults: a critical review of the literature. Intensive Care Med. 2013;39(2):247–257. doi: 10.1007/s00134-012-2743-5. [DOI] [PubMed] [Google Scholar]
- 66.Weiler T., Kamerkar A., Hotz J., Ross P.A., Newth C.J.L., Khemani R.G. The relationship between high flow nasal cannula flow rate and effort of breathing in children. J Pediatr. 2017;189:66–71.e3. doi: 10.1016/j.jpeds.2017.06.006. [DOI] [PubMed] [Google Scholar]
- 67.Rubin S., Ghuman A., Deakers T., Khemani R., Ross P., Newth C.J. Effort of breathing in children receiving high-flow nasal cannula. Pediatr Crit Care Med. 2014;15(1):1–6. doi: 10.1097/PCC.0000000000000011. [DOI] [PubMed] [Google Scholar]
- 68.Ramnarayan P., Richards-Belle A., Drikite L., et al. Effect of high-flow nasal cannula therapy vs continuous positive airway pressure following extubation on liberation from respiratory support in critically ill children: a randomized clinical trial. JAMA. 2022;327(16):1555–1565. doi: 10.1001/jama.2022.3367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Blackwood B., Ringrow S., Clarke M., et al. A core outcome set for critical care ventilation trials. Crit Care Med. 2019;47(10):1324–1331. doi: 10.1097/CCM.0000000000003904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ganu S.S., Gautam A., Wilkins B., Egan J. Increase in use of non-invasive ventilation for infants with severe bronchiolitis is associated with decline in intubation rates over a decade. Intensive Care Med. 2012;38(7):1177–1183. doi: 10.1007/s00134-012-2566-4. [DOI] [PubMed] [Google Scholar]
- 71.Smith A., Franca U.L., McManus M.L. Trends in the use of noninvasive and invasive ventilation for severe asthma. Pediatrics. 2020;146(4) doi: 10.1542/peds.2020-0534. [DOI] [PubMed] [Google Scholar]
- 72.Pelletier J.H., Au A.K., Fuhrman D., Clark R.S.B., Horvat C. Trends in bronchiolitis ICU admissions and ventilation practices: 2010-2019. Pediatrics. 2021;147(6) doi: 10.1542/peds.2020-039115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Krasinkiewicz J.M., Friedman M.L., Slaven J.E., Tori A.J., Lutfi R., Abu-Sultaneh S. Progression of respiratory support following pediatric extubation. Pediatr Crit Care Med. 2020;21(12):e1069–e1075. doi: 10.1097/PCC.0000000000002520. [DOI] [PubMed] [Google Scholar]
- 74.Yehya N., Harhay M.O., Curley M.A.Q., Schoenfeld D.A., Reeder R.W. Reappraisal of ventilator-free days in critical care research. Am J Respir Crit Care Med. 2019;200(7):828–836. doi: 10.1164/rccm.201810-2050CP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Richards-Belle A., Davis P., Drikite L., et al. FIRST-line support for assistance in breathing in children (FIRST-ABC): a master protocol of two randomised trials to evaluate the non-inferiority of high-flow nasal cannula (HFNC) versus continuous positive airway pressure (CPAP) for non-invasive respiratory support in paediatric critical care. BMJ Open. 2020;10(8) doi: 10.1136/bmjopen-2020-038002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.