Abstract
Background
A critical need exists to develop remission-inducing therapies for lymphangioleiomyomatosis.
Research Question
Is the addition of resveratrol safe and more efficacious than sirolimus alone in patients with lymphangioleiomyomatosis?
Study Design and Methods
We conducted a phase 2, dose-escalating, open-label trial of resveratrol in patients with lymphangioleiomyomatosis receiving a stable regimen of sirolimus. Resveratrol was started at 250 mg/d and escalated every 8 weeks to maximum dose of 1,000 mg/d over 24 weeks. The primary outcome was ≥ 42% decline in serum vascular endothelial growth factor D (VEGF-D) levels on combined therapy compared with baseline VEGF-D levels on sirolimus. Secondary objectives included an assessment of the safety profile and the effect on lung function and health-related quality of life (HRQOL). Longitudinal change in outcome measures was assessed using linear mixed models. Adverse effects were tabulated using the National Cancer Institute’s Common Terminology Criteria for Adverse Events version 4.
Results
Twenty-five patients with lymphangioleiomyomatosis with a median age of 51 years were enrolled. Pulmonary function parameters at study inclusion were: FEV1: median absolute, 1.72 L; 64% predicted; FVC: median absolute, 2.99 L; 96% predicted; and diffusing capacity of the lungs for carbon monoxide: median absolute, 14.68 mL/mm Hg/min; 37% predicted. The median serum VEGF-D value at baseline was 617 pg/mL. Patients entered the study with a median sirolimus dose of 2 mg/d with median trough level of 6.3 ng/mL. Despite some GI side effects, the addition of resveratrol was well tolerated. Although the primary outcome was not met, a statistically significant reduction in serum VEGF-D levels and improvement in HRQOL during the study was found.
Interpretation
The addition of resveratrol was safe and well tolerated in patients with lymphangioleiomyomatosis taking sirolimus and was associated with modest improvement in HRQOL. Larger controlled trials of this combination might be warranted to assess definitively the usefulness of resveratrol as an additive therapy in lymphangioleiomyomatosis.
Trial Registry
ClinicalTrials.gov; No.: NCT03253913; URL: www.clinicaltrials.gov
Key Words: autophagy, FEV1, LAM, tuberous sclerosis complex, vascular endothelial growth factor D
Take-home Points.
Study Question: Is the addition of resveratrol safe and more efficacious in patients with lymphangioleiomyomatosis on sirolimus?
Results: Twenty-five participants with lymphangioleiomyomatosis with a median age of 51 years were enrolled in the study. Pulmonary function parameters at study inclusion were: FEV1: median, 1.72 L; 64% predicted; FVC: median, 2.99 L; 96% predicted; Dlco: median, 14.68 mL/mm Hg/min; 37% predicted. The median serum vascular endothelial growth factor D (VEGF-D) value at baseline was 617 pg/mL. Participants entered the study with a median sirolimus dose of 2 mg/d with median trough level of 6.3 ng/mL. Despite some gastrointestinal side effects, the addition of resveratrol was well tolerated. Although the primary outcome was not met, a statistically significant reduction in serum VEGF-D levels and improvement in health-related quality of life (HRQOL) was observed during the study.
Interpretation: The addition of resveratrol was safe and well tolerated in patients with lymphangioleiomyomatosis receiving sirolimus and was associated with modest improvement in HRQOL. Larger controlled trials of this combination may be warranted to assess definitively the usefulness of resveratrol as an additive therapy in lymphangioleiomyomatosis.
Lymphangioleiomyomatosis is a rare, female-predominant, diffuse cystic lung disease characterized by the infiltration of lung parenchyma with abnormal smooth muscle-like cells. Lymphangioleiomyomatosis can occur in association with tuberous sclerosis complex (TSC) or can present sporadically in patients without heritable illness. Both lymphangioleiomyomatosis in association with TSC and sporadic lymphangioleiomyomatosis occur as a result of mutations in one of the two TSC genes. Mutations in the TSC genes lead to constitutive activation of the mechanistic target of rapamycin (mTOR) pathway, which drives cell proliferation and lymphangiogenesis, at least in part, through the production of vascular endothelial growth factor C and vascular endothelial growth factor D (VEGF-D).1,2 VEGF-D, a ligand for the lymphatic growth-factor receptor VEGFR-3/Flt-4, is an angiogenic growth factor that has been shown to be a useful diagnostic, predictive, and prognostic biomarker in lymphangioleiomyomatosis.3, 4, 5, 6
Discovery of the central role of mTOR hyperactivation in lymphangioleiomyomatosis disease pathogenesis has led to the development of sirolimus (an oral mTOR inhibitor) as an effective treatment for lymphangioleiomyomatosis.7,8 However, sirolimus is a suppressive therapy that requires continuous administration for durable disease control, and long-term drug exposure can be associated with side effects. A remission-inducing treatment option for lymphangioleiomyomatosis would obviate the need for lifelong treatment. In addition, mTOR inhibition by sirolimus leads to upregulation of autophagy and paradoxically increases lymphangioleiomyomatosis cell survival.9
Resveratrol (trans-3, 5, 4′-trihydroxystilbene) is a naturally occurring polyphenol found in grapes, berries, and red wine. Resveratrol has complex mechanisms of mTOR regulation, directly by inhibiting the phosphorylation of mTORC1 in a dose- and time-dependent manner,10 and indirectly through activation of 5′ adenosine monophosphate-activated protein (AMP)-activated protein kinase11 and promoting the association between DEP domain-containing mTOR interacting protein (DEPTOR) and mTOR.12 In addition, resveratrol has proapoptotic effects postulated to be driven by the inhibition of Ak strain transforming (Akt) and Ribosomal protein S6 kinase beta-1 (S6K1) activities, which in theory would counter the prosurvival effects of sirolimus to upregulate autophagy and inhibit apoptosis.9,10 Preclinical studies demonstrated that a combination of resveratrol and sirolimus leads to downregulation of autophagy and promotes apoptosis in TSC2 null cells, decreases the metastatic capability of TSC2-deficient uterine leiomyoma-derived smooth muscle cells, and causes a significant reduction in the size and growth of TSC2-deficient xenograft tumors derived from TSC2–/– mouse renal cystadenoma cells.9,13 These data support the concept of combination therapy with resveratrol and sirolimus as a cytotoxic, remission-inducing treatment for lymphangioleiomyomatosis that would be a significant advance over the more limited suppressive and cytostatic effects of sirolimus.
To assess the potential safety and efficacy of a combination therapy approach for lymphangioleiomyomatosis further, we designed and conducted the Resveratrol and Sirolimus in LAM [lymphangioleiomyomatosis] (RESULT) trial (ClinicalTrials.gov Identifier: NCT03253913), a phase 2, open-label, dose-escalating study of combined resveratrol and sirolimus in patients with lymphangioleiomyomatosis who are already receiving a stable regimen of sirolimus. Our hypothesis was that the combination therapy induced apoptosis of VEGF-D-expressing lymphangioleiomyomatosis cells would result in a reduction of serum VEGF-D levels less than those achievable with pharmacologic suppression with sirolimus alone, and that this regimen would be well tolerated and would lead to improvement in lung function, self-reported health status, symptoms, and health-related quality of life (HRQOL) in patients with lymphangioleiomyomatosis.
Study Design and Methods
Study Design
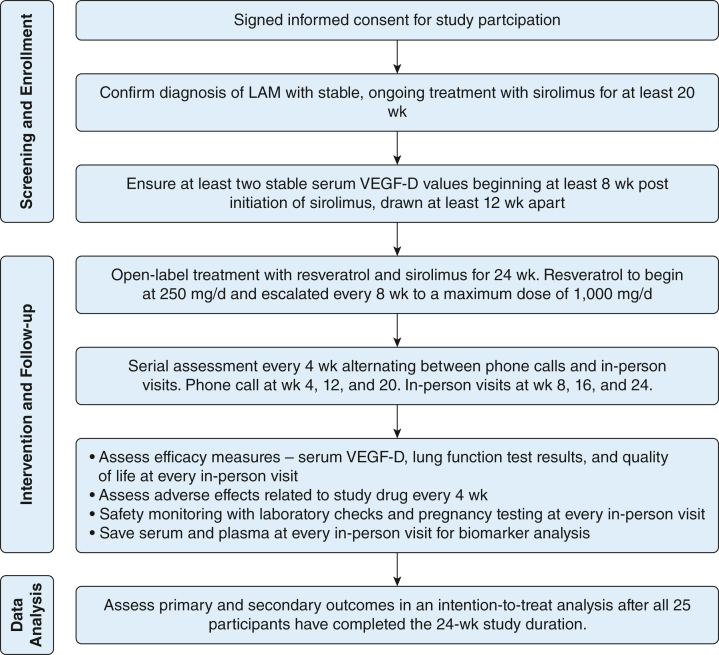
The RESULT trial was an open-label, dose-escalation, phase 2 study with longitudinal repeated measures (Fig 1). To be eligible for trial inclusion, patients had to satisfy all of the following criteria: (1) confirmed diagnosis of lymphangioleiomyomatosis per the American Thoracic Society/Japanese Respiratory Society Clinical Practice guidelines,14 (2) age ≥ 18 years with signed informed consent, (3) receiving a stable dose of sirolimus for at least 20 weeks based on clinical indications, and (4) VEGF-D stabilization as demonstrated by two stable values after initiation of sirolimus and drawn at least 12 weeks apart from each other. For the purpose of this study, a variation in serum VEGF-D of ≤ 15% was considered stable. Key exclusion criteria included inability to provide consent, active listing for lung transplantation, enrolled in another interventional clinical trial, known allergy or hypersensitivity to resveratrol, and current or planned pregnancy in the next 6 months.
Figure 1.
Overall schematic representation of the study. LAM = lymphangioleiomyomatosis; VEGF-D = vascular endothelial growth factor D.
The RESULT trial was a single-site study conducted at the University of Cincinnati. Study enrollment was open to patients with lymphangioleiomyomatosis throughout the United States with travel reimbursement provided through the study. The study was reviewed and approved by the University of Cincinnati Institutional Review Board (Identifier: 2016-4904). An independent data and safety monitoring board was convened and met every 6 months to ensure the safety of study participants. All participants provided written informed consent before the conduct of any study-related activity.
Outcome Measures
The primary objective of this study was to assess the baseline to 24-week change in serum VEGF-D value on treatment with resveratrol and sirolimus compared with sirolimus alone. Serial assessments of serum VEGF-D, spirometry after bronchodilator administration, treatment-related adverse effects (AEs), complete blood count, liver and renal function tests, and HRQOL were performed at baseline and at 8, 16, and 24 weeks. Diffusing capacity of the lungs for carbon monoxide (Dlco) was measured at baseline and at 24 weeks. Sirolimus trough levels, measured 24 ± 4 h after the last dose, were assessed at baseline and at weeks 2, 4, 8, 12, 16, 18, 20, and 24. VEGF-D quantification was performed at the Translational Trial Development and Support Laboratory based at Cincinnati Children’s Hospital Medical Center in a College of American Pathologists/Clinical Laboratory Improvement Amendments-approved manner. All other laboratory assessments were performed via Quest Diagnostics. Pulmonary function tests (PFTs) were performed and graded in accordance with the American Thoracic Society and European Respiratory Society standards.15,16 HRQOL was measured by using four validated scales: St. George’s Respiratory Questionnaire (SGRQ), University of California, San Diego, Shortness of Breath Questionnaire (SOBQ), EuroQol visual analog scale (EQVAS), and A Tool to Assess Quality of Life in LAM (ATAQ-LAM). Higher scores on the SGRQ, SOBQ, and ATAQ-LAM scales indicate worse HRQOL and improved HRQOL on the EQVAS scale. Key details regarding these scales is provided in e-Appendix 1.
Study Drug
Resveratrol used in this study was obtained from Evolva (Reinach) and manufactured by fermentation using genetically modified yeast in a process that results in transresveratrol with a purity of at least 98%, and packaged into capsules, with each capsule containing 125 mg resveratrol. The capsules were verified independently for content by mass spectrometry performed at the University of Cincinnati and for sterility by microbial testing at Q Laboratories Inc. Investigational new drug designation for studying resveratrol in this trial was obtained from the United States Food and Drug Administration (Identifier: IND 131722; sponsor: N. Gupta).
Resveratrol was administered in a stepwise dose-escalating fashion at 8-week intervals with assessment of VEGF-D, PFT results, AEs, and HRQOL before each dose escalation. The starting dose of resveratrol was 250 mg once daily for the first 8 weeks, followed by 500 mg daily from weeks 8 through 16, and 500 mg twice daily for the last 8 weeks (weeks 16-24), with provisions for dose reduction if necessitated by AEs. Treatment-related AEs were tabulated using the National Cancer Institute’s Common Terminology Criteria for Adverse Events version 4.
COVID-19 Impact
The last phase of the study coincided with the beginning of the COVID-19 pandemic and the resultant strict lockdowns. Some exceptions were made to requirements for travel to the study site and for performance of PFTs and laboratory assessments at the primary study site. In some cases, study measures were conducted closer to home or waived, and in-person visits were replaced by telehealth visits.
Sample Size Estimation
The baseline serum VEGF-D level at study inclusion in the Multicenter International Lymphangioleiomyomatosis Efficacy of Sirolimus (MILES) trial was 2,029 pg/mL, and the levels were reduced by approximately 50% in most patients after treatment with sirolimus.3,6,7 As such, we estimated that the starting serum VEGF-D level in this study would be approximately 1,000 pg/mL. The interassay variability for VEGF-D tests conducted in the Translational Trial Development and Support Laboratory ranges between 5% and 15%. In the MILES trial placebo group, the maximum change in serum VEGF-D concentration from baseline to 12 months was 42%.3 Thus, VEGF-D responders were defined conservatively as patients in whom serum VEGF-D decreased to a value of > 42% from baseline,3 and the power calculations for this study were based on the ability to show a ≥ 42% change in serum VEGF-D levels. Assuming the within-participant correlation between the baseline and subsequent VEGF-D levels was 0.7, a sample size of 20 participants was estimated to provide 80% power to detect a mean VEGF-D difference from baseline to 24 weeks of ≥ 420 pg/mL, with P ≤ .05 and SD of 800 for the difference. Allowing for a 20% dropout rate, we set our target sample size at 25 patients.
Statistical Analysis
The analysis was based on an intention-to-treat design. All participants who received the study drug were included in the safety and efficacy analysis set. Baseline cohort characteristics and AEs were summarized using descriptive analyses. Continuous variables are presented as median (interquartile range) or median (95% CI), computed by using the binomial distribution. Categorical variables are presented as frequencies and percentages. Linear mixed models were used to model changes in the outcome measure of interest over time while accounting for the correlation between multiple measurements within the same participant. The change of outcomes over time also were illustrated using line plots. Sample size calculations and outcome analyses were performed using SAS version 9.4 software (SAS Institute, Inc.). P values of < .05 were considered statistically significant.
Results
Baseline Characteristics
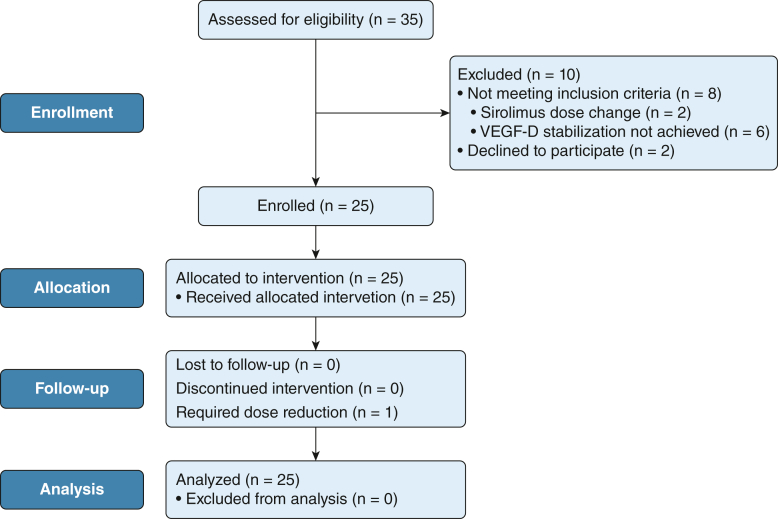
Between May 2018 and February 2020, 25 patients with lymphangioleiomyomatosis receiving a stable dose of sirolimus and with stable VEGF-D levels were enrolled in the study (Fig 2). Baseline demographic, clinical, and physiologic characteristics of the cohort are summarized in Table 1. In general, the study participants showed moderate to severe disease as demonstrated by the median FEV1 and Dlco values of 64% and 37%, respectively.
Figure 2.
Flow diagram of the study. VEGF-D = vascular endothelial growth factor D.
Table 1.
Baseline Characteristics of the Cohort (n = 25)
| Variable | Data |
|---|---|
| Age, y | 51 (47-58) |
| Race | |
| White | 23 (92) |
| Asian | 2 (8) |
| Ethnicity | |
| Not Hispanic or Latino | 25 (100) |
| Lymphangioleiomyomatosis subtype | |
| Sporadic lymphangioleiomyomatosis | 22 (88) |
| TSC lymphangioleiomyomatosis | 3 (12) |
| Menopausal status | |
| Premenopausal | 13 (52) |
| Postmenopausal | 12 (48) |
| Prior pneumothorax (n = 20) | 10 (50) |
| Dyspnea on exertion | 25 (100) |
| Supplemental oxygen use | 11 (44) |
| FEV1 | |
| L | 1.72 (1.10-2.36) |
| % Predicted | 64.5 (42-80) |
| FVC | . . . |
| L | 2.99 (2.70-3.96) |
| % Predicted | 96 (73.5-107.5) |
| Dlco | |
| mL/mm Hg/min | 14.68 (7.07-17.3) |
| % Predicted | 37 (26-64) |
| Serum VEGF-D, pg/mL | 617 (486-787) |
| Sirolimus dose, mg/d | 2 (1-2) |
| Sirolimus trough, ng/mL | 6.3 (4.2-7.7) |
| SGRQ score | 42 (39-46.5) |
| SOBQ score | 29 (19-36.5) |
| ATAQ-LAM score | 72 (51-95.5) |
| EQVAS score | 80 (65-85) |
Data are presented as No. (%) or median (interquartile range). ATAQ-LAM = A Tool to Assess Quality of Life in LAM [lymphangioleiomyomatosis]; Dlco = diffusion capacity of the lungs for carbon monoxide; EQVAS = EuroQol visual analog scale; SGRQ = St. George’s Respiratory Questionnaire; SOBQ = University of California, San Diego, Shortness of Breath Questionnaire; TSC = tuberous sclerosis complex; VEGF-D = vascular endothelial growth factor D.
VEGF-D Outcomes
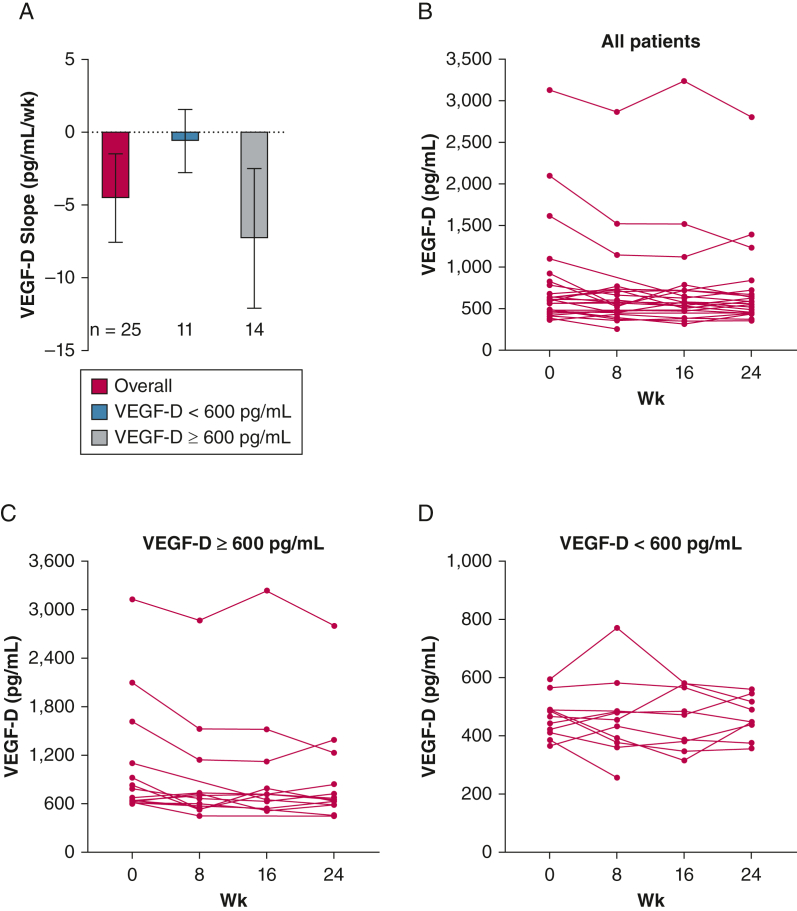
The median serum VEGF-D level at baseline was 617 pg/mL (range, 366-3,131 pg/mL). Although we did not meet the prespecified primary outcome of ≥ 42% reduction in serum VEGF-D levels after 24 weeks of combined sirolimus and resveratrol, a statistically significant reduction in VEGF-D levels was observed during the study (Fig 3A, Table 2). A 10% reduction in mean serum VEGF-D levels was observed during the 24-week study duration (802 pg/mL at baseline to 721 pg/mL at week 24) (e-Fig 1A). In a post hoc analysis, we segregated the cohort into two subgroups on the basis of baseline serum VEGF-D levels and found a more pronounced reduction in serum VEGF-D levels in the ≥ 600-pg/mL subgroup (n = 14; slope, –7.27 pg/mL/wk; 95% CI, –12.07 to –2.47 pg/mL/wk; P = .003) (Fig 3B) than in the < 600-pg/mL subgroup (n = 11; slope, –0.607 pg/mL/wk; 95% CI, –2.78 to 1.56 pg/mL/wk; P = .58) (Fig 3C). The mean serum VEGF-D value in the ≥ 600-pg/mL group at baseline was 1,066 pg/mL and declined to 898 pg/mL, a relative decrease of 15.75% (e-Fig 1B). The corresponding values for the < 600-pg/mL group were 466 pg/mL at baseline and 465 pg/mL at week 24, corresponding to 0.21% relative decrement (e-Fig 1C). We further examined differences in VEGF-D response after segregating the cohort on the basis of menopausal status (premenopausal vs postmenopausal), baseline lung function (FEV1 > 70% predicted vs FEV1 51%-70% predicted vs FEV1 ≤ 50% predicted), and sirolimus dose (< 2 mg/d vs ≥ 2 mg/d). We added interaction terms for these parameters in the mixed model and found no significant association between VEGF-D response and the above subgroups (data not shown), except for the subgroup comparison based on sirolimus dose. Although the baseline serum VEGF-D values seemed to be higher in the sirolimus ≥ 2-mg group (n = 13) as compared with the < 2-mg group (n = 12), the difference was not statistically significant (median VEGF-D in the sirolimus ≥ 2-mg group, 621 ng/mL [IQR, 489.5-1,361 ng/mL] vs the sirolimus < 2-mg group, 583 ng/L [IQR, 420-640.5 ng/mL]; P = .11). The interaction term was negative (β = –8.7 using a dose of < 2 mg/d as reference level) and was statistically significant (P = .003), which indicates that VEGF-D values in the sirolimus ≥ 2-mg group decreased more than in the < 2-mg group. Group effects and individual trends for these subgroups are shown in e-Figures 2-4.
Figure 3.
A-D, Graphs showing longitudinal trends in serum vascular endothelial growth factor D (VEGF-D) levels throughout the 24-wk study duration: VEGF-D slope (pg/mL/wk) in all patients and after segregating on the basis of baseline serum VEGF-D level into two subgroups (< 600 pg/mL and ≥ 600 pg/mL) (A), individual trendlines from all participants enrolled in the study (B), individual trends in patients with baseline serum VEGF-D levels of ≥ 600 pg/mL (C), and individual trends in patients with baseline serum VEGF-D of < 600 pg/mL (D). A modest decline in serum VEGF-D levels was observed during the study, and this effect was most pronounced in patients with elevated levels of ≥ 600 pg/mL at baseline.
Table 2.
Longitudinal Trends of Key Outcome Measures Throughout the Study
| Variable | Baseline | 8 Wk | 16 Wk | 24 Wk | Rate of Change (Slope)/wk | P Value |
|---|---|---|---|---|---|---|
| VEGF-D, pg/mL | 617 (489-677), n = 25 | 543 (455-704), n = 23 | 581 (485-724), n = 20 | 575 (448-673), n = 22 | –4.53 (–7.56 to –1.5) | .004 |
| FEV1, L | 1.74 (1.46-2.3), n = 25 | 1.98 (1.4-2.36), n = 23 | 2.01 (1.18-2.57), n = 17 | 1.94 (1.2-2.59), n = 18 | –0.0029 (–0.0052 to –0.0006) | .013 |
| FVC, L | 3.08 (2.84-3.94), n = 25 | 3.04 (2.83-3.81), n = 23 | 3.17 (2.6-3.87), n = 17 | 3.36 (2.64-3.93), n = 18 | –0.0019 (–0.004 to 0.0001) | .064 |
| Dlco, mL/mm Hg/min | 14.68 (10.15-16.81), n = 23 | NA | NA | 13.24 (9.25-17.07), n = 18 | –0.01 (–0.054 to 0.034) | .635 |
| SOBQ | 29 (21-35), n = 25 | 31 (16-44), n = 24 | 23 (15-42), n = 21 | 22 (12-40), n = 23 | –0.075 (–0.25 to 0.097) | .386 |
| EQVAS | 80 (65-85), n = 24 | 85 (75-90), n = 23 | 90 (75-95), n = 21 | 90 (80-90), n = 23 | 0.21 (0.016-0.41) | .035 |
| SGRQ | 42 (39-46), n = 25 | 43.5 (38-49), n = 24 | 43 (40-49), n = 22 | 42 (39-47), n = 23 | –0.024 (–0.21 to 0.16) | .797 |
| ATAQ-LAM | 72 (54-88), n = 25 | 70 (49-101), n = 24 | 60 (44-82), n = 22 | 57 (47-84), n = 23 | –0.33 (–0.57 to –0.086) | .009 |
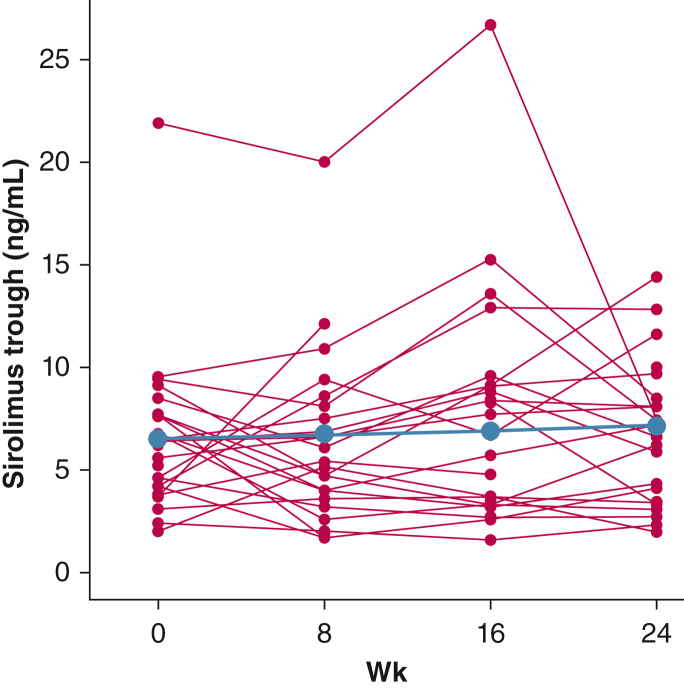
| Sirolimus troughs, ng/mL | 6.3 (4.6-7.6), n = 25 | 5.4 (4-7.5), n = 23 | 7.2 (3.4-9.1), n = 22 | 6.9 (3.4-8.5), n = 22 | 0.026 (–0.042 to 0.093) | .450 |
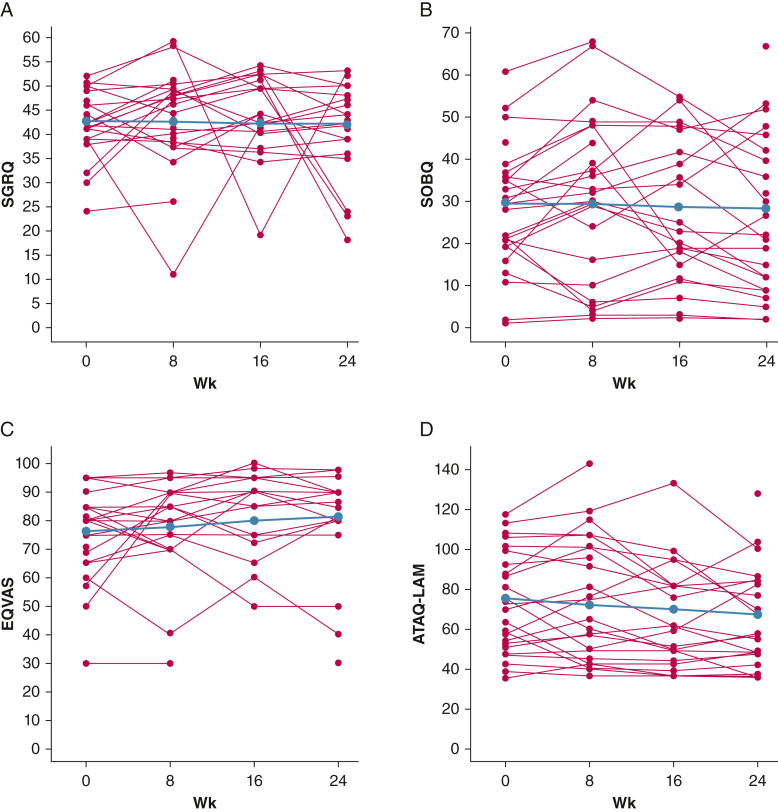
Data are presented as median (95% CI). ATAQ-LAM = A Tool to Assess Quality of Life in LAM [lymphangioleiomyomatosis]; Dlco = diffusion capacity of the lungs for carbon monoxide; EQVAS = EuroQol visual analog scale; NA = not assessed; SGRQ = St. George’s Respiratory Questionnaire; SOBQ = University of California, San Diego, Shortness of Breath Questionnaire; VEGF-D = vascular endothelial growth factor D.
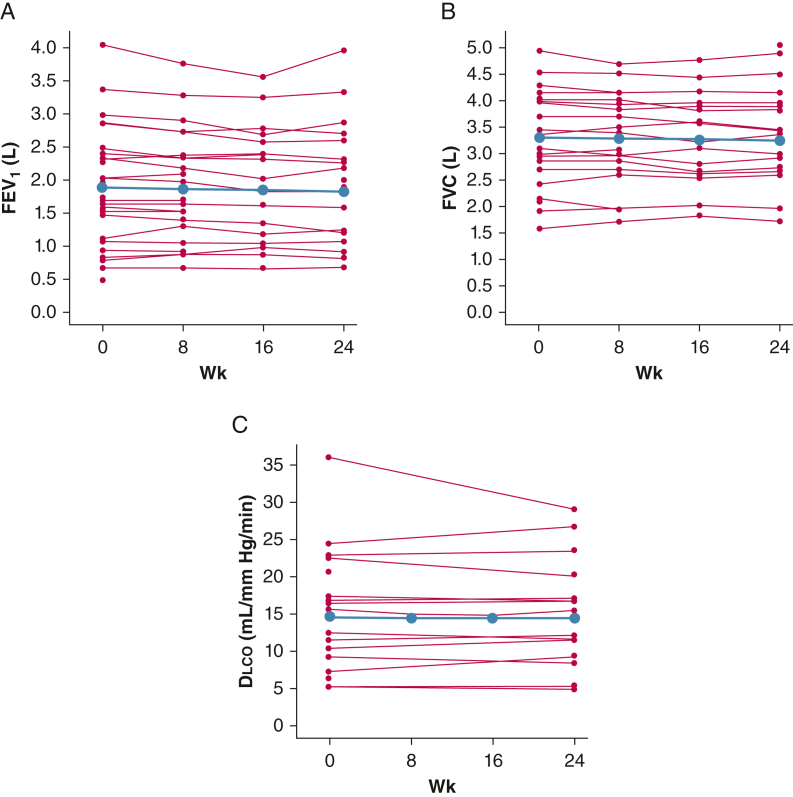
Lung Function Outcomes
The patients enrolled in the RESULT trial showed moderate to severe physiologic impairment at baseline (Table 1). During the 24-week study duration, models showed a modest but statistically significant reduction in FEV1, but no significant change in FVC or Dlco (Table 2, Fig 4). In a post hoc analysis, we examined the longitudinal trends in FEV1 after segregating the cohort on the basis of baseline serum VEGF-D values (< 600 pg/mL vs ≥ 600 pg/mL), menopausal status (premenopausal vs postmenopausal), and sirolimus dose (< 2 mg/d vs ≥ 2 mg/d). We added an interaction term in our mixed model to investigate change in FEV1 response on the basis of these segregations. The interaction terms were not significant (data not shown), suggesting no group difference in FEV1 response. Group effects and individual trends for these subgroups are shown in e-Figures 5-7.
Figure 4.
A-C, Line graphs showing longitudinal trends in pulmonary function tests throughout the 24-wk study duration: FEV1 (A), FVC (B), and Dlco (C). Group effect is depicted in blue and individual trends are depicted with red lines. A statistically significant decline in FEV1, but no significant change in FVC or Dlco, was observed. Dlco = diffusing capacity of the lungs for carbon monoxide.
Quality-of-Life and Functional Performance Outcomes
The SGRQ scores remained stable throughout the study duration; however, the SDSOB and ATAQ-LAM scores decreased and the EQVAS score increased over 24 weeks with combination therapy, suggesting an overall trend toward improved HRQOL (Table 2, Fig 5).
Figure 5.
A-D, Line graphs showing longitudinal trends in health-related quality-of-life (HRQOL) assessments throughout the 24-wk study duration: SGRQ (A), SOBQ (B), EQVAS (C), and ATAQ-LAM (D). Group effect is depicted in blue and individual trends are depicted with red lines. The SGRQ scores remained stable throughout the study duration; however, the SOBQ and ATAQ-LAM scores decreased and the EQVAS score increased over 24 wks on combination therapy, suggesting an overall trend toward improved HRQOL. ATAQ-LAM = A Tool to Assess Quality of Life in LAM [lymphangioleiomyomatosis]; Dlco = diffusing capacity of the lungs for carbon monoxide; EQVAS = EuroQol visual analog scale; SGRQ = St. George’s Respiratory Questionnaire; SOBQ = University of California, San Diego, Shortness of Breath Questionnaire.
Sirolimus Levels
The median sirolimus dose at the time of study enrollment was 2 mg/d, with median trough levels of 6.3 ng/mL (Table 1). Although some interindividual variability was found, sirolimus doses and trough levels remained stable throughout the 24-week study (Table 2, Fig 6, e-Table 1).
Figure 6.
Line graph showing longitudinal trends in sirolimus trough levels throughout the 24-wk study duration. Group effect is depicted in blue and individual trends are depicted with red lines. Overall, the sirolimus trough levels remained stable during the study, although wide interindividual variability was observed.
COVID-19 Impact
Some patients were unable to undergo protocol-specified PFTs and VEGF-D measurements because of COVID-19-related travel restrictions. Of a total of 100 VEGF-D measurements for the study (25 total participants, each with four measurements at weeks 0, 8, 16, and 24), 90 values were obtained. At least two measurements, permitting longitudinal analysis, were available from 24 of 25 participants (96%). Similarly, from a total of 100 possible PFT measurements, 82 values were obtained. At least two measurements were available from 24 of 25 participants (96%) for FEV1 and FVC and from 18 of 25 participants (72%) for Dlco.
Safety and Tolerability
No serious AEs during the study. Eighteen of the 25 participants (72%) in the study reported at least one AE for a total of 92 AEs. Most AEs were categorized as mild (83/92 [90%]), with eight AEs (9%) being moderate and one AE (1%) being severe. One participant showed worsening of pre-existing gastroesophageal reflux disease that was deemed possibly to be related to the study drug and necessitated dose reduction. No other dose reductions or interruptions occurred during the study. The most frequently reported AEs were GI in nature, with the most common ones being GI discomfort (15 instances for seven participants), diarrhea (14 instances for nine participants), and nausea (eight instances for five participants) (Table 3).
Table 3.
Adverse Effects Categorized by Body System for Each Dose of Resveratrol During the Study
| Body System | 250 mg/d | 500 mg/d | 1,000 mg/d | Total |
|---|---|---|---|---|
| Cardiac | 2 (4.8) | 1 (4) | 0 (0) | 3 (3.3) |
| Ear | 0 (0) | 1 (4) | 0 (0) | 1 (1.1) |
| Endocrine | 1 (2.4) | 0 (0) | 1 (4) | 2 (2.2) |
| GI | 17 (40.5) | 12 (48) | 14 (56) | 43 (46.7) |
| General disorders | 2 (4.8) | 1 (4) | 3 (12) | 6 (6.5) |
| Hepatobiliary | 1 (2.4) | 0 (0) | 0 (0) | 1 (1.1) |
| Infections and infestations | 7 (16.7) | 1 (4) | 3 (12) | 11 (11.9) |
| Musculoskeletal and connective tissue | 3 (7.1) | 4 (16) | 0 (0) | 7 (7.6) |
| Nervous system | 4 (9.5) | 2 (8) | 1 (4) | 7 (7.6) |
| Psychiatric | 1 (2.4) | 0 (0) | 0 (0) | 1 (1.1) |
| Renal and urinary | 1 (2.4) | 0 (0) | 0 (0) | 1 (1.1) |
| Reproductive system | 0 (0) | 0 (0) | 1 (4) | 1 (1.1) |
| Respiratory | 2 (4.8) | 1 (4) | 0 (0) | 3 (3.3) |
| Skin and subcutaneous tissue | 1 (2.4) | 1 (4) | 2 (8) | 4 (4.3) |
| Vascular | 0 (0) | 1 (4) | 0 (0) | 1 (1.1) |
| Total | 42 (100) | 25 (100) | 25 (100) | 92 (100) |
Data are presented as No. (%).
Discussion
The major findings from our analysis of the risks and benefits of resveratrol and sirolimus combination therapy over 24 weeks are: (1) although the primary outcome of ≥ 42% reduction in serum VEGF-D levels compared with the baseline VEGF-D values with sirolimus alone was not met, a statistically significant reduction in VEGF-D levels was seen; (2) an overall improvement in self-reported health status, symptoms, and HRQOL was seen; and (3) the therapy was safe and well tolerated.
The hypothesis for this study was that the combination of resveratrol and sirolimus is cytocidal for lymphangioleiomyomatosis cells, rather than cytostatic, as is the case with sirolimus alone. We chose serum VEGF-D quantification as our primary outcome based on the assumption that it reflects lymphangioleiomyomatosis cell burden. A modest reduction in VEGF-D levels was found during the study with a more pronounced effect in patients with an elevated baseline VEGF-D level of ≥ 600 pg/mL. It is noteworthy that in a post hoc analysis of the MILES trial, a baseline serum VEGF-D cutoff level of 600 pg/mL identified patients at increased risk of lung function decline in the placebo group and those more likely to respond to sirolimus treatment.6 To assess this cutoff value further and relate data from the MILES trial to those from the RESULT trial, we examined the plasticity of serum VEGF-D responses after sirolimus treatment by analyzing serum VEGF-D values from samples collected in the MILES trial after stratifying the cohort by baseline VEGF-D (≥ 600 pg/mL vs < 600 pg/mL). Indeed, those with VEGF-D levels of ≥ 600 pg/mL exhibited substantial responses to sirolimus, whereas those with VEGF-D levels of < 600 pg/mL did not show decline with treatment (e-Fig 8). Similar responses with significant reduction in VEGF-D levels limited to patients with elevated baseline levels of > 800 pg/mL also were noted in a recent early-phase trial investigating the safety of celecoxib in patients with lymphangioleiomyomatosis.17
FEV1 slope was the primary outcome measure that demonstrated the efficacy of sirolimus in lymphangioleiomyomatosis and led to the subsequent Food and Drug Administration approval of sirolimus for the treatment of lymphangioleiomyomatosis.7 Given the stability of lung function with sirolimus treatment, longitudinal assessment of lung function as an end point for future combination therapies would require untenable samples sizes. As such, a critical need exists to develop new biomarkers as surrogate end points to assess treatment response in patients with lymphangioleiomyomatosis. As an integral component of the pathogenic pathway in lymphangioleiomyomatosis, and given the almost exclusive expression of VEGF-D in lymphangioleiomyomatosis cells within the lung,18 VEGF-D is a highly promising candidate for this role. Several recently published pilot trials in lymphangioleiomyomatosis examined VEGF-D response after treatment17,19, 20, 21; however, meaningful interpretations are limited by the small sample sizes and no well-established criteria exist for the threshold of change in VEGF-D levels from baseline that should be considered significant. For our study, we chose a ≥ 42% threshold for incremental change as a measurement of treatment response beyond that achieved with sirolimus alone, a goal that might be unattainable. The serum VEGF-D levels for most of the participants in the placebo group in the MILES trial stayed within 30% of the baseline value, whereas most participants who received sirolimus achieved > 30% reduction in VEGF-D levels,6 thus suggesting that > 30% change in VEGF-D may be a good indicator of significant treatment effect. In light of the outcomes from the RESULT trial and the reanalysis of data from the MILES trial, we submit that future trials with end points based on VEGF-D outcomes should consider using a > 30% threshold of change as a marker of treatment response and restricting enrollment to participants with elevations in baseline VEGF-D.
Patient-reported outcome measures (PROMs) that assess symptoms or HRQOL arguably are the most relevant and meaningful clinical outcome assessments to use as end points in lymphangioleiomyomatosis trials. To capture a range of constructs from the patients’ perspective, we used four PROMs as secondary end points in the RESULT trial, including a general measure of health status (EQVAS), a dyspnea index (SOBQ), a respiratory-specific tool (SGRQ), and a lymphangioleiomyomatosis-specific instrument that assesses symptoms and quality of life (ATAQ-LAM) (e-Appendix 1). Although the minimal important change for scores from some of these measures has been established in other patient populations, none of their minimal important changes are known for lymphangioleiomyomatosis.22,23 Although SGRQ scores have longitudinal validity for assessing symptoms and their impacts in patients with lymphangioleiomyomatosis,24 the SGRQ has some items that tap constructs known not to be major concerns for patients with lymphangioleiomyomatosis (eg, cough and wheezing), thus detracting from its face validity (as an outcome measure in lymphangioleiomyomatosis) and responsiveness, and likely partially explaining why only 7 of 25 participants (28%) had SGRQ total scores improve by ≥ 4 points over the course of the trial. The SOBQ questionnaire is a 24-item measure that assesses dyspnea,25 the most common symptom endorsed by patients with lymphangioleiomyomatosis.26 SOBQ scores decreased by ≥ 5 points in 40% of participants (10/25), EQVAS scores improved by ≥ 10 points in 8 of the 25 participants (32%), and a statistically significant improvement occurred in the ATAQ-LAM scores during our study. Although encouraging, without knowing all the psychometric properties of these PROMs in patients with lymphangioleiomyomatosis, including minimal important changes for their scores, the significance of these results is unclear. Conducting a comprehensive psychometric evaluation of ATAQ-LAM is an unmet need and future goal for our group.
Multiple trials have shown that resveratrol has a favorable safety profile, with occasional dose-dependent GI intolerance being the major AE.27, 28, 29 In the current trial, resveratrol showed excellent tolerability, with only one instance of an AE requiring dose reduction. Given the unknown potential for interaction between sirolimus and resveratrol, we closely monitored sirolimus trough levels throughout the study. Although wide interindividual variability was observed, no statistically significant difference in sirolimus troughs were observed during the study, suggesting the lack of significant interaction between sirolimus and resveratrol, at least with doses of up to 1,000 mg/d.
The major limitations of our study arises from the missed assessments in a subset of patients because of the COVID-19 pandemic, potential adverse impact on HRQOL resulting from COVID-19-related emotional stress, and the open-label design that makes it difficult to ascertain whether the trends toward response seen in this study could be attributed to the addition of resveratrol. Future trials of combination therapies in lymphangioleiomyomatosis should consider strongly a sirolimus-only control group and adaptive designs (eg, crossover studies so all participants receive the investigational agent within the trial). Such strategies could help to maximize enrollment and the likelihood of assessing treatment response while reducing sample size requirements to levels that are feasible for a rare disease. The major strengths of our study include the measurement of serum VEGF-D levels in a College of American Pathologists/Clinical Laboratory Improvement Amendments-approved fashion and the sample size when compared with other recent pilot trials in lymphangioleiomyomatosis.17,19, 20, 21 In addition, our study provided useful information regarding the use of outcome measures beyond FEV1, such as longitudinal assessment of serum VEGF-D levels and PROMs, that could inform the design of future trials in lymphangioleiomyomatosis.
Interpretation
In patients with lymphangioleiomyomatosis receiving sirolimus, the addition of resveratrol was safe and well tolerated. Although the primary efficacy outcome was not met, some patients reported improvement in various PROMs assessing outcomes that were meaningful to patients, and we believe larger, controlled trials of this combination are warranted to assess better the usefulness of resveratrol as an additive therapy in lymphangioleiomyomatosis. Serum VEGF-D quantification, especially in patients with elevated baseline values, may be a useful efficacy end point, stratification, or cohort-enrichment variable for future lymphangioleiomyomatosis clinical trials.
Funding/Support
This study was funded by the National Heart, Lung, and Blood Institute, National Institutes of Health [Grant R34HL138235].
Financial/Nonfinancial Disclosures
The authors have reported to CHEST the following: F. X. M. holds the patent for serum VEGF-D testing. All royalties from this patent are waived to the parent institution, the University of Cincinnati. None declared (N. G., B. Z., Y. Z., R. I., N. R., E. J. K., S. M., A. S., J. S., A. G. C., M. K. H.).
Acknowledgments
Author contributions: N. G., F. X. M., and M. K. H. conceived the study design. N. G. and F. X. M. were the site investigators and helped with patient enrollment and data collection. A. G. C. and A. S. provided oversight and quality control for pulmonary function tests. Y. Z., N. G., and B. Z. performed the data analysis. R. I., N. R., and S. M. were the study coordinators and helped in all aspects of study conduct. J. S. created the ATAQ-LAM questionnaire and helped in the interpretation of results. All authors contributed substantially in writing and editing the manuscript. N. G. had full access to all the data in this study and had final responsibility for the decision to submit for publication.
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Other contributions: The authors thank the providers in the LAM Clinic Network for referring participants for inclusion in this study, the LAM Foundation for their assistance in study recruitment, the members of the data and safety monitoring board—Drs MeiLan Han, Darcy Krueger, Jingyang Zhang, and Brenna Carey—for helping to ensure safe trial conduct, and the patients with lymphangioleiomyomatosis for taking part in this study despite the travel ordeals and numerous other unexpected challenges posed by the COVID-19 pandemic and for their constant support and commitment to advancing scientific knowledge in lymphangioleiomyomatosis.
Additional information: The e-Appendix, e-Figures, and e-Table are available online under “Supplementary Data.”
Supplementary Data
References
- 1.McCarthy C., Gupta N., Johnson S.R., Yu J.J., McCormack F.X. Lymphangioleiomyomatosis: pathogenesis, clinical features, diagnosis, and management. Lancet Respir Med. 2021;9(11):1313–1327. doi: 10.1016/S2213-2600(21)00228-9. [DOI] [PubMed] [Google Scholar]
- 2.Gupta N., Hagner M., Wu H., et al. Serum vascular endothelial growth factor C as a marker of therapeutic response to sirolimus in lymphangioleiomyomatosis. Ann Am Thorac Soc. 2021;18(1):174–177. doi: 10.1513/AnnalsATS.202006-702RL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Young L., Lee H.S., Inoue Y., et al. Serum VEGF-D a concentration as a biomarker of lymphangioleiomyomatosis severity and treatment response: a prospective analysis of the Multicenter International Lymphangioleiomyomatosis Efficacy of Sirolimus (MILES) trial. Lancet Respir Med. 2013;1(6):445–452. doi: 10.1016/S2213-2600(13)70090-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Young L.R., Inoue Y., McCormack F.X. Diagnostic potential of serum VEGF-D for lymphangioleiomyomatosis. N Engl J Med. 2008;358(2):199–200. doi: 10.1056/NEJMc0707517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Young L.R., Vandyke R., Gulleman P.M., et al. Serum vascular endothelial growth factor-D prospectively distinguishes lymphangioleiomyomatosis from other diseases. Chest. 2010;138(3):674–681. doi: 10.1378/chest.10-0573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gupta N., Lee H.S., Young L.R., et al. Analysis of the MILES cohort reveals determinants of disease progression and treatment response in lymphangioleiomyomatosis. Eur Respir J. 2019;53(4) doi: 10.1183/13993003.02066-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McCormack F.X., Inoue Y., Moss J., et al. Efficacy and safety of sirolimus in lymphangioleiomyomatosis. N Engl J Med. 2011;364(17):1595–1606. doi: 10.1056/NEJMoa1100391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McCormack F.X., Gupta N., Finlay G.R., et al. Official American Thoracic Society/Japanese Respiratory Society clinical practice guidelines: lymphangioleiomyomatosis diagnosis and management. Am J Respir Crit Care Med. 2016;194(6):748–761. doi: 10.1164/rccm.201607-1384ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alayev A., Sun Y., Snyder R.B., Berger S.M., Yu J.J., Holz M.K. Resveratrol prevents rapamycin-induced upregulation of autophagy and selectively induces apoptosis in TSC2-deficient cells. Cell Cycle. 2014;13(3):371–382. doi: 10.4161/cc.27355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alayev A., Berger S.M., Holz M.K. Resveratrol as a novel treatment for diseases with mTOR pathway hyperactivation. Ann N Y Acad Sci. 2015;1348(1):116–123. doi: 10.1111/nyas.12829. [DOI] [PubMed] [Google Scholar]
- 11.Puissant A., Robert G., Fenouille N., et al. Resveratrol promotes autophagic cell death in chronic myelogenous leukemia cells via JNK-mediated p62/SQSTM1 expression and AMPK activation. Cancer Res. 2010;70(3):1042–1052. doi: 10.1158/0008-5472.CAN-09-3537. [DOI] [PubMed] [Google Scholar]
- 12.Liu M., Wilk S.A., Wang A., et al. Resveratrol inhibits mTOR signaling by promoting the interaction between mTOR and DEPTOR. J Biol Chem. 2010;285(47):36387–36394. doi: 10.1074/jbc.M110.169284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alayev A., Salamon R.S., Sun Y., et al. Effects of combining rapamycin and resveratrol on apoptosis and growth of TSC2-deficient xenograft tumors. Am J Respir Cell Mol Biol. 2015;53(5):637–646. doi: 10.1165/rcmb.2015-0022OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gupta N., Finlay G.A., Kotloff R.M., et al. Lymphangioleiomyomatosis diagnosis and management: high-resolution chest computed tomography, transbronchial lung biopsy, and pleural disease management. An official American Thoracic Society/Japanese Respiratory Society clinical practice guideline. Am J Respir Crit Care Med. 2017;196(10):1337–1348. doi: 10.1164/rccm.201709-1965ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Graham B.L., Steenbruggen I., Miller M.R., et al. Standardization of spirometry 2019 update. An official American Thoracic Society and European Respiratory Society technical statement. Am J Respir Crit Care Med. 2019;200(8):e70–e88. doi: 10.1164/rccm.201908-1590ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Culver B.H., Graham B.L., Coates A.L., et al. Recommendations for a standardized pulmonary function report. An official American Thoracic Society technical statement. Am J Respir Crit Care Med. 2017;196(11):1463–1472. doi: 10.1164/rccm.201710-1981ST. [DOI] [PubMed] [Google Scholar]
- 17.El-Chemaly S., Taveira-DaSilva A., Bagwe S., et al. Celecoxib in lymphangioleiomyomatosis: results of a phase I clinical trial. Eur Respir J. 2020;55(5) doi: 10.1183/13993003.02370-2019. [DOI] [PubMed] [Google Scholar]
- 18.Guo M., Yu J.J., Perl A.K., et al. Single-cell transcriptomic analysis identifies a unique pulmonary lymphangioleiomyomatosis cell. Am J Respir Crit Care Med. 2020;202(10):1373–1387. doi: 10.1164/rccm.201912-2445OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lu C., Lee H.S., Pappas G.P., et al. A phase II clinical trial of an aromatase inhibitor for postmenopausal women with lymphangioleiomyomatosis. Ann Am Thorac Soc. 2017;14(6):919–928. doi: 10.1513/AnnalsATS.201610-824OC. [DOI] [PubMed] [Google Scholar]
- 20.Krymskaya V.P., Courtwright A.M., Fleck V., et al. A phase II clinical trial of the Safety Of Simvastatin (SOS) in patients with pulmonary lymphangioleiomyomatosis and with tuberous sclerosis complex. Respir Med. 2020;163 doi: 10.1016/j.rmed.2020.105898. [DOI] [PubMed] [Google Scholar]
- 21.El-Chemaly S., Taveira-Dasilva A., Goldberg H.J., et al. Sirolimus and autophagy inhibition in lymphangioleiomyomatosis: results of a phase I clinical trial. Chest. 2017;151(6):1302–1310. doi: 10.1016/j.chest.2017.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ries A.L. Minimally clinically important difference for the UCSD Shortness of Breath Questionnaire, Borg scale, and visual analog scale. COPD. 2005;2(1):105–110. doi: 10.1081/copd-200050655. [DOI] [PubMed] [Google Scholar]
- 23.Jones P.W. St. George’s Respiratory Questionnaire: MCID. COPD. 2005;2(1):75–79. doi: 10.1081/copd-200050513. [DOI] [PubMed] [Google Scholar]
- 24.Swigris J.J., Lee H.S., Cohen M., et al. St. George’s Respiratory Questionnaire has longitudinal construct validity in lymphangioleiomyomatosis. Chest. 2013;143(6):1671–1678. doi: 10.1378/chest.12-0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eakin E.G., Resnikoff P.M., Prewitt L.M., Ries A.L., Kaplan R.M. Validation of a new dyspnea measure: the UCSD Shortness of Breath Questionnaire. University of California, San Diego. Chest. 1998;113(3):619–624. doi: 10.1378/chest.113.3.619. [DOI] [PubMed] [Google Scholar]
- 26.Ryu J.H., Moss J., Beck G.J., et al. The NHLBI lymphangioleiomyomatosis registry: characteristics of 230 patients at enrollment. Am J Respir Crit Care Med. 2006;173(1):105–111. doi: 10.1164/rccm.200409-1298OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cottart C.H., Nivet-Antoine V., Beaudeux J.L. Review of recent data on the metabolism, biological effects, and toxicity of resveratrol in humans. Mol Nutr Food Res. 2014;58(1):7–21. doi: 10.1002/mnfr.201200589. [DOI] [PubMed] [Google Scholar]
- 28.Brown V.A., Patel K.R., Viskaduraki M., et al. Repeat dose study of the cancer chemopreventive agent resveratrol in healthy volunteers: safety, pharmacokinetics, and effect on the insulin-like growth factor axis. Cancer Res. 2010;70(22):9003–9011. doi: 10.1158/0008-5472.CAN-10-2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.la Porte C., Voduc N., Zhang G., et al. Steady-state pharmacokinetics and tolerability of trans-resveratrol 2000 mg twice daily with food, quercetin and alcohol (ethanol) in healthy human participants. Clin Pharmacokinet. 2010;49(7):449–454. doi: 10.2165/11531820-000000000-00000. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.