Abstract
Virus-specific cytotoxic T lymphocytes (CTL) play a major role in the clearance of respiratory syncytial virus (RSV) infection. We have generated cytotoxic T-cell clones (TCC) from two infants who had just recovered from severe RSV infection. These TCC were functionally characterized and used to identify HLA class I (B57 and C12)-restricted CTL epitopes of RSV.
Respiratory syncytial virus (RSV) is a common cause of upper respiratory tract infections but may—especially in young infants, the elderly, or immunocompromised individuals—cause severe lower respiratory tract infections (12). In rodent models, CD4+, as well as CD8+, RSV-specific T lymphocytes proved to be involved in both recovery from and immune pathogenesis of the infection (1, 2, 8, 19). Therefore, a fine balance must exist in these models between protective and disease-enhancing effects of virus-specific T lymphocytes. RSV-specific CD8+ cytotoxic T lymphocytes (CTL) against virtually all RSV proteins have been demonstrated to circulate in humans after RSV infection (4, 7, 9). CTL may be expected to play a crucial role in the clearance of RSV infections, but their role in protection and immune pathology remains unclear. Therefore, the identification of CTL epitopes of human RSV may contribute to future studies concerning the role of CTL in pathogenesis and protection from RSV infection. Here we describe the functional characterization of CD8+ cytotoxic T-cell clones (TCC) generated from two infants who had just recovered from severe RSV infection. These TCC were also used to identify, for the first time, HLA class I-restricted CTL epitopes of RSV.
Peripheral blood mononuclear cells (PBMC) were collected from two infants 4 weeks after a severe, laboratory-confirmed RSV infection. At the time of infection, they were 1 and 2 months old and had both been admitted to the intensive care unit. B-lymphoblastic cell lines (B-LCL) were generated by Epstein-Barr virus transformation (22), infected with RSV A2 (ATCC VR1322), and UV irradiated (24) to serve as autologous antigen-presenting cells (APC). PBMC (3 × 104/well in 96-well round-bottom plates) were stimulated with APC (104/well) and expanded in RPMI 1640 medium supplemented with antibiotics, 10% pooled and heat-inactivated human serum, and recombinant human interleukin-2 (IL-2; 50 IU/ml). After 2 weeks, T cells were harvested and cloned by limiting dilution using phytohemagglutinin stimulation as previously described (25). TCC thus generated were expanded and tested for RSV specificity by [3H]thymidine incorporation assays as previously described (22). All of the RSV-specific TCC proved to be of the CD8+ phenotype in fluorescence-activated cell sorter analysis. TCC whose specificity for RSV was confirmed by a second proliferation assay were tested for protein specificity by a gamma interferon (IFN-γ) ELIspot assay (Mabtech AB, Stockholm, Sweden). In this test, paraformaldehyde-fixed autologous B-LCL that had been infected with recombinant vaccinia viruses (rVV) expressing different RSV proteins (F, G, N, P, M2, SH, M, 1B, or 1C) were used as APC. Briefly, TCC (5 × 103/well) were incubated with APC (104/well) for 4 h, transferred to anti-human IFN-γ-coated plates, and incubated for another 18 h. The ELIspot assay was further performed in accordance with the kit manufacturer's instructions. The results are shown as numbers of IFN-γ-producing cells (spots) per well.
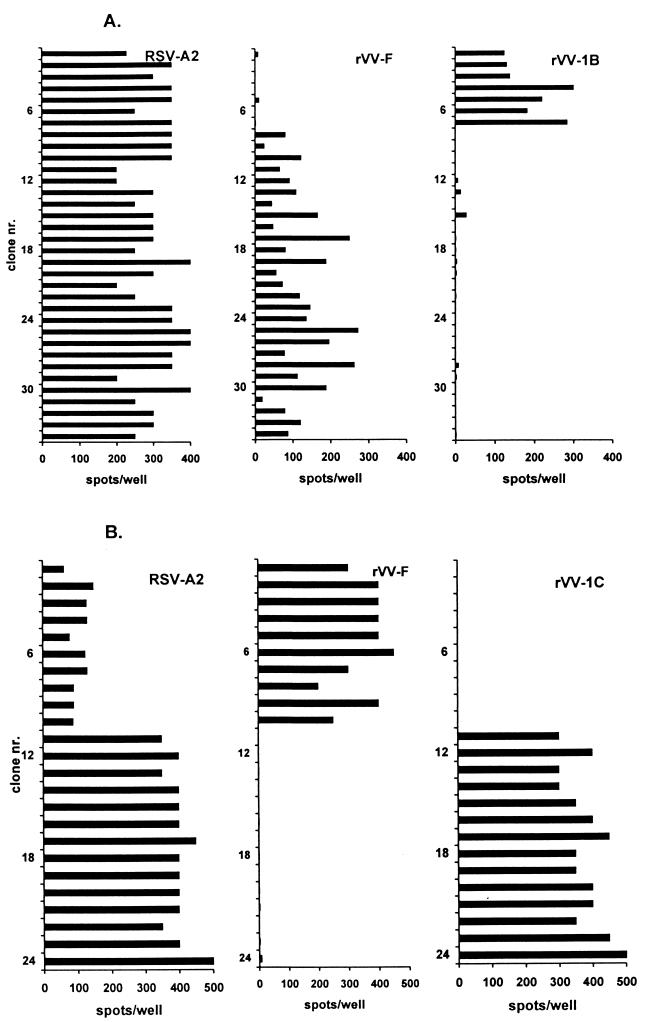
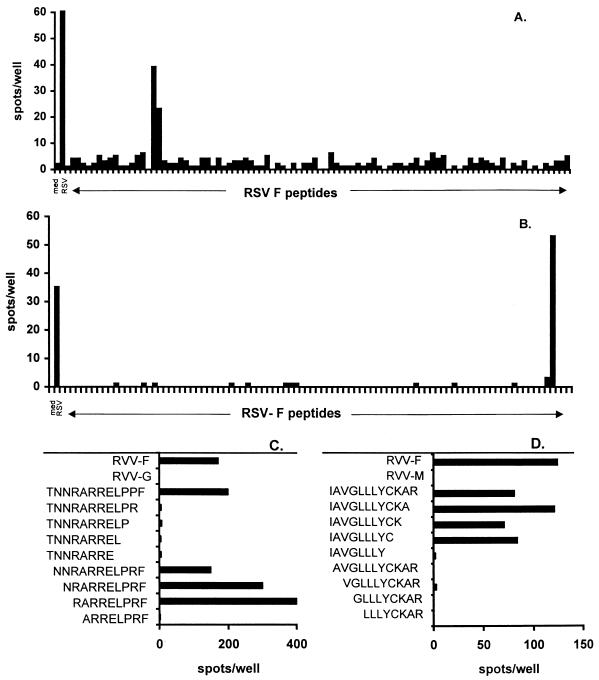
Thirty-four RSV-specific TCC were generated from the PBMC of patient 1, as detected in a [3H]thymidine incorporation assay. Of these, 27 proved to be RSV F specific and 7 were RSV 1B specific in an IFN-γ ELIspot assay (Fig. 1A). Twenty-four RSV-specific TCC were generated from the PBMC of patient 2 as detected by a [3H]thymidine incorporation assay. Of these, 10 were RSV F specific and 14 proved to be RSV 1C specific in an IFN-γ ELIspot assay (Fig. 1B). None of the clones detected by the [3H]thymidine incorporation assay was found to be negative by the IFN-γ ELIspot assay. Since the F protein is considered to be a major CTL target (4, 7, 9), further efforts were focused on the identification of CTL epitopes in the F protein. To this end, 18-mer peptide amides overlapping by 12 amino acids, together spanning the entire F protein of RSV A2 (11, 18) (n = 94), were generated in an automated multiple-peptide synthesizer as previously described (26). The purity of the peptides varied between 50 and 90%, as determined by analytical reverse-phase high-performance liquid chromatography (C8 column; gradient of 0.1% trifluoroacetic acid in water to 0.1% trifluoroacetic acid in acetonitrile). Autologous B-LCL of patients 1 and 2 were pulsed overnight with 1 and 3 μM peptide, respectively. RSV F-specific TCC of patient 1 reacted with peptides 17 and 18 (Fig. 2A). All of the RSV F TCC of patient 2 reacted with peptide 91 and marginally with peptide 90 (Fig. 2B). Subsequently, two additional sets of partially overlapping 8- to 12-mer peptides (80 to 95% purity) were generated to determine the respective minimal epitopes. Autologous B-LCL of patients 1 and 2 were pulsed for 1 h with the different peptides at 1 and 3 μM, respectively. All of the RSV F-specific TCC of patient 1 reacted with one nine-mer peptide (RARRELPRF) spanning residues 118 to 126 of the F protein (Fig. 2C). The RSV F TCC of patient 2 all reacted with one nine-mer peptide (IAVGLLLYC) spanning residues 551 to 559 of the F protein (Fig. 2D).
FIG. 1.
Responses of RSV-specific TCC from patients 1 (A) and 2 (B) measured by an IFN-γ ELIspot assay using autologous B-LCL infected with RSV A2 or rVV expressing the RSV F, 1B, or 1C protein. Results are indicated as numbers of spots per well. No responses against the B-LCL infected with the other rVV were found (data not shown).
FIG. 2.
Fine mapping of T-cell epitopes on the RSV F protein. Responsiveness of TCC from patients 1 (A) and 2 (B) to partially overlapping 18-mer peptide amides (n = 94) spanning the F protein was measured by an IFN-γ ELIspot assay. Minimal peptide activation of TCC from patients 1 (C) and 2 (D) was measured in an IFN-γ ELIspot assay using APC pulsed with two additional sets of overlapping 8- to 12-mer peptides. med, medium.
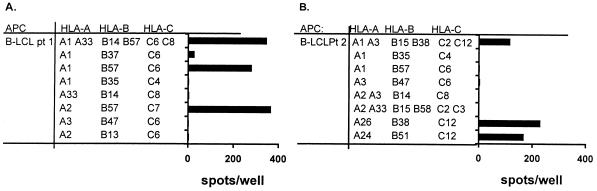
The HLA restriction of the recognition by the RSV F TCC was also determined by the IFN-γ ELIspot assay using a set of allogeneic B-LCL with partially matched HLA types loaded with the respective minimal peptides, as shown in Fig. 3. Recognition of the RSV F epitope of patient 1 proved to be HLA B57 restricted. Recognition of the epitope of patient 2 proved to be C12 restricted.
FIG. 3.
Determination of HLA restriction of RSV F protein recognition by specific TCC using a set of autologous and partially HLA class I-matched B-LCL for patients 1 (A) and 2 (B) by an IFN-γ ELIspot assay.
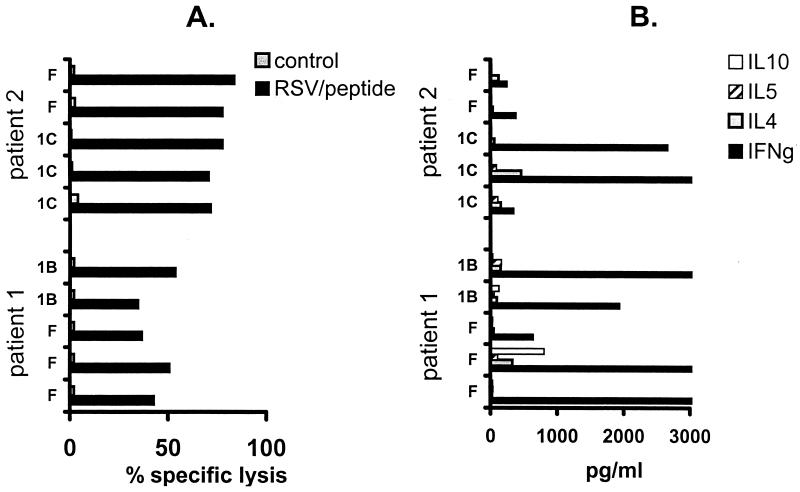
Well-growing TCC of both specificities from each of the patients were arbitrarily selected and tested for cytotoxic activity by a chromium release assay as previously described (23). Briefly, autologous 51Cr-labeled, RSV-infected B-LCL and control B-LCL or, in the case of the F-specific TCC of patient 2, minimal-peptide-loaded B-LCL and control B-LCL were incubated with TCC for 4 h at 37°C at an effector-to-target cell ratio of 10:1, which was found to be the most discriminative ratio in preliminary experiments. Spontaneous 51Cr release (target cells plus medium) and maximum 51Cr release (target cells plus Triton X-100) were determined in 12 identical wells. Supernatants were harvested and analyzed in a gamma counter. Assay results were accepted only when the spontaneous-to-maximum release ratio was <25%. All of the TCC tested showed RSV-specific lysis (Fig. 4A). Virus-specific production of cytokines in cell-free culture supernatant was measured as previously described (6). TCC were stimulated with RSV-infected B-LCL or control B-LCL or, in the case of the F-specific TCC of patient 2, with minimal-peptide-loaded B-LCL or control B-LCL for 48 h. The concentrations of the following cytokines were measured using commercially available sandwich enzyme-linked immunosorbent assay systems in accordance with the manufacturers' instructions: IL-4 (CLB, Amsterdam, The Netherlands; detection limit, 7 pg/ml), IFN-γ (Medgenix Diagnostics, Fleurs, Belgium; detection limit, 25 pg/ml), IL-10 (Pharmingen, San Diego, Calif.; detection limit, 20 pg/ml). All of the TCC produced predominantly IFN-γ, indicating a type 1 phenotype in vitro (Fig. 4B).
FIG. 4.
Cytotoxic responses (A) and cytokine production (B) of selected TCC with different protein specificities from patients 1 and 2. TCC were stimulated with autologous RSV-infected or control B-LCL and in the case of the F-specific TCC of patient 2, minimal-peptide- or control (measles virus) peptide-loaded B-LCL. Cytotoxic responses were measured in a chromium release assay using an effector-to-target cell ratio of 10. Cytokines in cell-free culture supernatant (IFN-γ, IL-4, IL-5, and IL-10) were measured by enzyme-linked immunosorbent assay. Levels of cytokines in the supernatant of TCC stimulated with control B-LCL were all below the detection limit and are not shown.
In the present study, we identified two nine-mer CTL epitopes on the RSV fusion protein. To our knowledge, these are the first HLA class I-restricted CTL epitopes described for RSV in humans. We found CTL epitopes with an HLA restriction with a low prevalence in the population. But using the techniques described here on samples of more children with a recent RSV infection, CTL epitopes with more prevalent HLA restrictions may be identified.
The TCC found in the children studied were all CD8+ CTL with a type 1-like cytokine profile. No RSV-specific CD4+ T cells were detected in the samples of these infants, while the use of similar stimulation protocols did result in the identification of such cells in other systems (14, 17, 20, 22). Virus-specific CTL responses play a major role in the clearance of RSV infections but may also be involved in pathogenesis (1, 3, 5, 8, 15). In mice, enhanced lung pathology induced by priming with formalin-inactivated RSV has been associated with the absence of a CTL response (13, 21). In young children, a CTL response against RSV has been described but poor responses were found in younger and more severely ill patients (10, 16). The poor CTL response in young children has been suggested to be one of the possible causes of more severe disease. In these two patients, it was possible to detect RSV-specific cytotoxic TCC, showing that also in very young children with clinically severe infections, priming of a CTL response does occur, although we cannot say anything about the quantitative responses.
In conclusion, use of the IFN-γ ELIspot assay to determine epitope specificity proved to be sensitive and convenient, since only small numbers of T cells and APC were needed. This and similar studies may be important for future studies concerning the role of CTL in the pathogenesis of RSV infection in children.
Acknowledgments
This study was financially supported by the Sophia Foundation for Medical Research, Rotterdam, The Netherlands (grant 214).
We thank Humphrey Brugghe and Bea van't Land for technical assistance and Conny Kruyssen for assistance in preparing the manuscript.
REFERENCES
- 1.Alwan W H, Kozlowska W J, Openshaw P J M. Distinct types of lung disease caused by functional subsets of antiviral T cells. J Exp Med. 1994;179:81–89. doi: 10.1084/jem.179.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alwan W H, Record F M, Openshaw P J M. CD4+ T cells clear virus but augment disease in mice infected with respiratory syncytial virus. Comparison with the effects of CD8+ T cells. Clin Exp Immunol. 1992;88:527–536. doi: 10.1111/j.1365-2249.1992.tb06482.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bangham C R, McMichael A J. Specific human cytotoxic T cells recognize B-cell lines persistently infected with respiratory syncytial virus. Proc Natl Acad Sci USA. 1986;83:9183–9187. doi: 10.1073/pnas.83.23.9183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bangham C R M, Openshaw P J M, Ball L A, King A M Q, Wertz G W, Askonas B A. Human and murine cytotoxic T cells specific to respiratory syncytial virus recognize the viral nucleoprotein (N), but not the major glycoproteins (G), expressed by vaccinia virus recombinants. J Immunol. 1986;137:3973–3977. [PubMed] [Google Scholar]
- 5.Bangham C R M, Cannon M J, Karzon D T, Askonas B A. Cytotoxic T-cell response to respiratory syncytial virus in mice. J Virol. 1985;56:55–59. doi: 10.1128/jvi.56.1.55-59.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brandenburg, A. H., A. Kleinjan, B. van't Land, H. A. Moll, H. H. Timmerman, R. L. de Swart, H. J. Neijens, W. Fokkens, and A. D. M. E. Osterhaus. A type 1 like immune response is found in children with RSV infection regardless of clinical severity. J. Med. Virol., in press. [PubMed]
- 7.Cannon M J, Bangham C R M. Recognition of respiratory syncytial virus fusion protein by mouse cytotoxic T cell clones and a human cytotoxic T cell line. J Gen Virol. 1989;70:79–87. doi: 10.1099/0022-1317-70-1-79. [DOI] [PubMed] [Google Scholar]
- 8.Cannon M J, Openshaw P J M, Askonas B A. Cytotoxic T cells clear virus but augment lung pathology in mice infected with respiratory syncytial virus. J Exp Med. 1988;168:1163–1168. doi: 10.1084/jem.168.3.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cherrie A H, Anderson K, Wertz G W, Openshaw P J M. Human cytotoxic T cells stimulated by antigen on dendritic cells recognize the N, SH, F, M, 22K, and 1b proteins of respiratory syncytial virus. J Virol. 1992;66:2102–2110. doi: 10.1128/jvi.66.4.2102-2110.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chiba Y, Higashidate Y, Suga K, Honjo K, Tsatsumi H, Ogra P L. Development of cell-mediated cytotoxic immunity to respiratory syncytial virus in human infants following naturally acquired infection. J Med Virol. 1989;28:133–139. doi: 10.1002/jmv.1890280304. [DOI] [PubMed] [Google Scholar]
- 11.Collins P L, Huang Y T, Wertz G W. Nucleotide sequence of the gene encoding the fusion (F) glycoprotein of human respiratory syncytial virus. Proc Natl Acad Sci USA. 1984;81:7683–7687. doi: 10.1073/pnas.81.24.7683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Collins P L, Mcintosh K, Chanock R M. Respiratory syncytial virus. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. 3rd ed. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 1313–1351. [Google Scholar]
- 13.Connors M, Kulkarni A B, Firestone C-Y, Holmes K L, Morse III H C, Sotnikov A V, Murphy B R. Pulmonary histopathology induced by respiratory syncytial virus (RSV) challenge of formalin-inactivated RSV-immunized BALB/c mice is abrogated by depletion of CD4+ T cells. J Virol. 1992;66:7444–7451. doi: 10.1128/jvi.66.12.7444-7451.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Evans T G, Fitzgerald T, Gibbons D C, Keefer M C, Soucier H. Th1/Th2 cytokine responses following HIV-1 immunization in seronegative volunteers. The AIDS Vaccine Evaluation Group. Clin Exp Immunol. 1998;111:243–250. doi: 10.1046/j.1365-2249.1998.00486.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fishaut M, Tubergen D, Mcintosh K. Cellular response to respiratory viruses with particular reference to children with disorders of cell-mediated immunity. J Pediatr. 1980;96:179–186. doi: 10.1016/s0022-3476(80)80799-2. [DOI] [PubMed] [Google Scholar]
- 16.Isaacs D, Bangham C R M, McMichael A J. Cell-mediated cytotoxic response to respiratory syncytial virus in infants with bronchiolitis. Lancet. 1987;ii:769–771. doi: 10.1016/s0140-6736(87)92502-5. [DOI] [PubMed] [Google Scholar]
- 17.Kallas E G, Gibbons D C, Soucier H, Fitzgerald T, Treanor J J, Evans T G. Detection of intracellular antigen-specific cytokines in human T cell populations. J Infect Dis. 1999;179:1124–1131. doi: 10.1086/314702. [DOI] [PubMed] [Google Scholar]
- 18.Olmsted R A, Elango N, Prince G A, Murphy B R, Johnson P R, Moss B, Chanock R M, Collins P L. Expression of the F glycoprotein of respiratory syncytial virus by a recombinant vaccinia virus: comparison of the individual contributions of the F and G glycoproteins to host immunity. Proc Natl Acad Sci USA. 1986;83:7462–7466. doi: 10.1073/pnas.83.19.7462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Openshaw P J. Immunity and immunopathology to respiratory syncytial virus. The mouse model. Am J Respir Crit Care Med. 1995;152:S59–S62. doi: 10.1164/ajrccm/152.4_Pt_2.S59. [DOI] [PubMed] [Google Scholar]
- 20.Secrist H, Chelen C J, Wen Y, Marshall J D, Umetsu D T. Allergen immunotherapy decreases interleukin 4 production in CD4+ T cells from allergic individuals. J Exp Med. 1993;178:2123–2130. doi: 10.1084/jem.178.6.2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Srikiatkhachorn A, Braciale T J. Virus-specific CD8+ T lymphocytes downregulate T helper cell type 2 cytokine secretion and pulmonary eosinophilia during experimental murine respiratory syncytial virus infection. J Exp Med. 1997;186:421–432. doi: 10.1084/jem.186.3.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Van Binnendijk R S, Poelen M C, de Vries P, Voorma H O, Osterhaus A D, UytdeHaag F G. Measles virus-specific human T cell clones. Characterization of specificity and function of CD4+ helper/cytotoxic and CD8+ cytotoxic T cell clones. J Immunol. 1989;142:2847–2854. [PubMed] [Google Scholar]
- 23.Van Binnendijk R S, Poelen M C, Kuijpers K C, Osterhaus A D, UytdeHaag F G. The predominance of CD8+ T cells after infection with measles virus suggests a role for CD8+ class I MHC-restricted cytotoxic T lymphocytes (CTL) in recovery from measles. Clonal analyses of human CD8+ class I MHC-restricted CTL. J Immunol. 1990;144:2394–2399. [PubMed] [Google Scholar]
- 24.Van Binnendijk R S, Versteeg-van Oosten J P M, Poelen M C M, Brugghe H F, Hoogerhout P, Osterhaus A D M E, UytdeHaag F G C M. Human HLA class I- and HLA class II-restricted cloned cytotoxic T lymphocytes identify a cluster of epitopes on the measles virus fusion protein. J Virol. 1993;67:2276–2284. doi: 10.1128/jvi.67.4.2276-2284.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Verjans G M, Remeijer L, van Binnendijk R S, Cornelissen J G, Volker-Dieben H J, Baarsma S G, Osterhaus A D. Identification and characterization of herpes simplex virus-specific CD4+ T cells in corneas of herpetic stromal keratitis patients. J Infect Dis. 1998;177:484–488. doi: 10.1086/517382. [DOI] [PubMed] [Google Scholar]
- 26.Wiertz E J, van Gaans-van den Brink J A, Gausepohl H, Prochnicka-Chalufour A, Hoogerhout P, Poolman J T. Identification of T cell epitopes occurring in a meningococcal class 1 outer membrane protein using overlapping peptides assembled with simultaneous multiple peptide synthesis. J Exp Med. 1992;176:79–88. doi: 10.1084/jem.176.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]