Abstract
Biventricular pacing (Biv) and left bundle branch area pacing (LBBAP) are methods of cardiac resynchronization therapy (CRT). Currently, little is known about how they differ in terms of ventricular activation. This study compared ventricular activation patterns in left bundle branch block (LBBB) heart failure patients using an ultra-high-frequency electrocardiography (UHF-ECG). This was a retrospective analysis including 80 CRT patients from two centres. UHF-ECG data were obtained during LBBB, LBBAP, and Biv. Left bundle branch area pacing patients were divided into non-selective left bundle branch pacing (NSLBBP) or left ventricular septal pacing (LVSP) and into groups with V6 R-wave peak times (V6RWPT) < 90 ms and ≥ 90 ms. Calculated parameters were: e-DYS (time difference between the first and last activation in V1–V8 leads) and Vdmean (average of V1–V8 local depolarization durations). In LBBB patients (n = 80) indicated for CRT, spontaneous rhythms were compared with Biv (39) and LBBAP rhythms (64). Although both Biv and LBBAP significantly reduced QRS duration (QRSd) compared with LBBB (from 172 to 148 and 152 ms, respectively, both P < 0.001), the difference between them was not significant (P = 0.2). Left bundle branch area pacing led to shorter e-DYS (24 ms) than Biv (33 ms; P = 0.008) and shorter Vdmean (53 vs. 59 ms; P = 0.003). No differences in QRSd, e-DYS, or Vdmean were found between NSLBBP, LVSP, and LBBAP with paced V6RWPTs < 90 and ≥ 90 ms. Both Biv CRT and LBBAP significantly reduce ventricular dyssynchrony in CRT patients with LBBB. Left bundle branch area pacing is associated with more physiological ventricular activation.
Keywords: Heart failure, Biv CRT, LBBAP, Ventricular synchrony, UHF-ECG
Introduction
Biventricular pacing is a standard treatment for patients with heart failure, reduced ejection fraction, and dyssynchronous ventricular activation. The reduction of ventricular dyssynchrony after biventricular cardiac resynchronization therapy (Biv CRT) improves the quality of life and mortality.1
An alternative approach to CRT is left bundle branch area pacing (LBBAP). This method provides ventricular resynchronization by direct pacing of the left bundle branch (left bundle branch pacing, LBBP).2 or by capturing myocytes in the left septal area (left ventricular septal pacing, LVSP).2 Both LBBP and LVSP reduce QRS duration (QRSd) compared with spontaneous left bundle branch block (LBBB) rhythms and are characterised by a pseudo-right bundle branch morphology in lead V1. LBBP was shown to be superior to Biv CRT in the improvement of the left ventricular ejection fraction (LVEF) in patients with non-ischaemic cardiomyopathy,3 and LVSP was associated with a similar effect as Biv CRT on LV performance when measured as dP/dT.4 However, detailed data on differences in ventricular activation between LBBAP and LBBP in LBBB patients are missing.
Ultra-high-frequency electrocardiography (UHF-ECG) is a non-invasive method that displays the sequence of ventricular activation and resultant ventricular electrical synchrony.5 In the past years, it was used to describe the differences in ventricular activation between various types of right ventricular (RV) and physiological pacing.6–8
This study aimed to better understand the differences in ventricular activation in patients with heart failure and reduced LVEF during LBBB spontaneous rhythm during Biv CRT and LBBAP.
Methods
This was a retrospective observational study from two tertiary centres experienced in LBBAP implants, i.e. the Faculty Hospital Královské Vinohrady in Prague, Czech Republic, and Catharina Hospital, Eindhoven, Netherlands. The study protocols were approved by the local hospital ethics committees and conformed with the Declaration of Helsinki.
Consecutive patients with LBBB undergoing CRT from January 2020 to September 2021 were included in the analysis. Left bundle branch block was diagnosed using Strauss criteria, i.e. QRSd ≥ 140 ms (men) or ≥ 130 ms (women), QS or rS in leads V1 and V2, and mid-QRS notching or slurring in 2 of leads V1, V2, V5, V6, I, and aVL.9 Either biventricular pacing, LBBAP, or both types of pacing were attempted, and the final pacing type was left to the physician’s discretion. For LBBAP, a Select Secure lead model 3830 and a Medtronic sheath C315HIS (both Medtronic Inc., Minneapolis, MN) were used. The LBBP technique was described previously.2 Briefly, a lead was introduced by a sheath in the right ventricle. It was positioned on the septum using fluoroscopy, and the preferred position had a normal paced QRS axis and was 1.5–2.5 cm below the level of the tricuspid valve. After that, the lead was screwed into the interventricular septum by several rotations of the whole lead, and LBBAP was confirmed by a pseudo-right bundle branch morphology in V1, and reduction of the QRSd compared with the native QRS.
In one of the two centres, discrimination between LBBP and LVSP was possible by studying the QRS morphology and intracardiac signal changes using an EP system (LabsystemPro, Boston Scientific, USA). LBBP was confirmed by the presence of non-selective LBBP (NSLBBP) that transitioned to selective LBBP (SLBBP) or LVSP during decremental output pacing.10 During a transition from NSLBBP to SLBBP, the R-wave peak time in V6 (V6RWPT) remained the same; however, the distance to the R-wave peak time in V1 (V1RWPT) was prolonged.11 During the transition to LVSP, the amplitude of the late r/R in V1 decreased, and the V6RWPT was prolonged by more than 10 ms.12 If, during deep septal pacing with a late r/R in V1, none of the above-mentioned changes occurred, LVSP was confirmed. For biventricular pacing, the coronary sinus (CS) was cannulated and visualised along with its branches using a contrast injection distal to the balloon occlusion of the CS. After that, a dedicated lead was placed in one of its branches; preferably, the basal areas of the lateral, inferolateral, or anterolateral branches were targeted.
A ventricular dyssynchrony imaging (VDI) monitor (ISI Brno, Cardion, FNUSA, Czech Republic) was used to record and analyse the 5 kHz and 14-lead ECG signals with 3 nV resolution and a frequency range of 1.5 kHz. Limb and chest leads were positioned similarly to standard ECG leads. Amplitude frequency envelopes for 16 frequency bands (from 150 to 1000 Hz) are computed using the Hilbert transformation and normalised for each lead. The signal from each precordial lead was displayed as a colour map from chest leads V1–V8. Local activation times were calculated as the centre of mass of UHF-QRSs above the 50% threshold of the baseline-to-peak amplitude in each chest lead. Local depolarization durations under leads V1–V8 were computed as UHF-QRS durations at 50% of the UHF-QRS complex amplitude for each of the V1–V8 leads. e-DYS was calculated as an absolute value of the maximal difference between the first and last V1–V8 activation. Vdmean was calculated as a mean value of V1–V8 durations.5 UHF-ECG data were collected during 3–5 min of either spontaneous rhythm or pacing at 100–110 b.p.m. Biventricular pacing was delivered by simultaneous pacing from an RV apex lead and a lead in the CS. The maximally allowed delay between the RV and CS lead was 20 ms.
QRS duration was measured globally from a 12-lead ECG using an electrophysiological system (LabSystem Pro, Boston Scientific, USA). The global QRSd for paced rhythms was measured from the pacing artefact. Paced V6RWPT and V1RWPT were measured from the pacing artefact to the maximum positive QRS amplitude in lead V6 and V1, respectively. All measurements were obtained at 200 mm/s using an average value from two consecutive beats.
Statistical analysis
Exploratory data analysis was performed on all parameters. RStudio with R Version 4.2.2 was used to perform statistical analyses. Comparisons of continuous variables were made using a linear mixed effects model. The linear mixed effect model was calculated using lme4 Version 1.1-21. The results of these comparisons are given as mean differences (95% confidence interval) and P values. P < 0.05 was considered significant.
Results
Eighty consecutive patients undergoing CRT procedures were included in the analysis. One hundred eighty-three UHF-ECG maps were recorded during spontaneous rhythm (n = 80), biventricular pacing (n = 39), and LBBAP (n = 64). Baseline patient characteristics are shown in Table 1.
Table 1.
Patient characteristics
| Patients | n = 80 |
|---|---|
| Age | 73 ± 10 |
| Male sex | 51, 64% |
| Chronic coronary syndrome | 31, 39% |
| Diabetes mellitus | 31, 39% |
| Arterial hypertension | 51, 64% |
| Atrial fibrillation | 28, 35% |
| NYHA class | 2 ± 0.7 |
| LVEF (%) | 29 ± 8 |
| QRSd during spontaneous rhythm (ms) | 174 ± 18 |
| Heart failure aetiology | |
| ICM | 27, 34% |
| NICM | 53, 66% |
| CS lead position in LAO 40° (n = 39) | |
| AL | 19, 49% |
| L | 14, 36% |
| PL | 6, 15% |
| Heart failure medication | |
| ACEI/ARB/ARNI | 66, 83% |
| BB | 62, 78% |
| MRA | 40, 50% |
Data are presented as mean ± SD or n, %.
ACEI, ACE inhibitor; AL, anterolateral; ARB, angiotensin receptor blocker; ARNI, angiotensin receptor-neprilysin inhibitor; BB, beta blocker; CS, coronary sinus; ICM, ischaemic cardiomyopathy; L, lateral; LAO, left anterior oblique projection; LVEF, left ventricular ejection fraction; MRA, mineralocorticoid receptor antagonist; NICM, non-ischaemic cardiomyopathy; PL, posterolateral; QRSd, QRS duration.
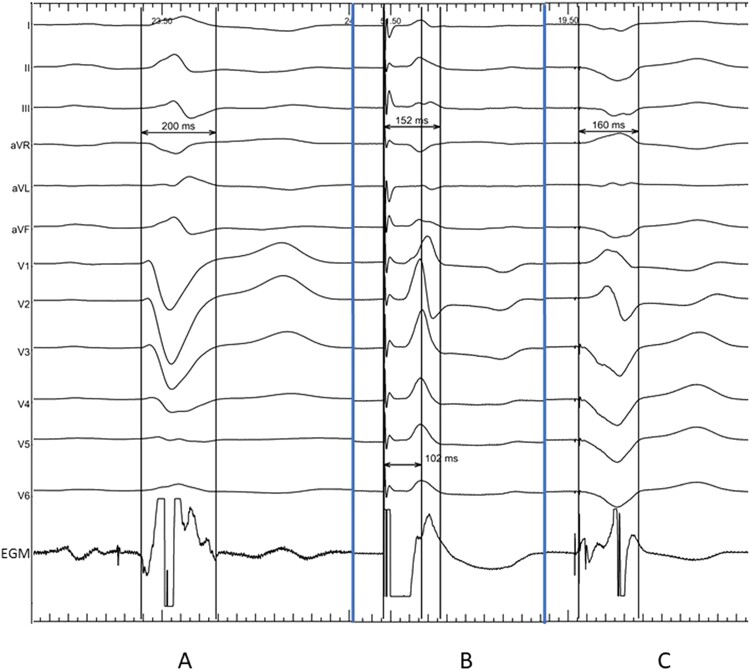
LBBAP and Biv CRT both significantly reduced QRSd from 172 to 152 ms (147; 156) and 148 ms (142; 153) during LBBAP and Biv CRT, respectively—Figure 1.
Figure 1.
QRS duration in a patient during (A) spontaneous rhythm with LBBB, (B) during non-selective left bundle branch pacing with V6RWPT of 102 ms, and (C) during biventricular pacing with VV delay 0 ms.
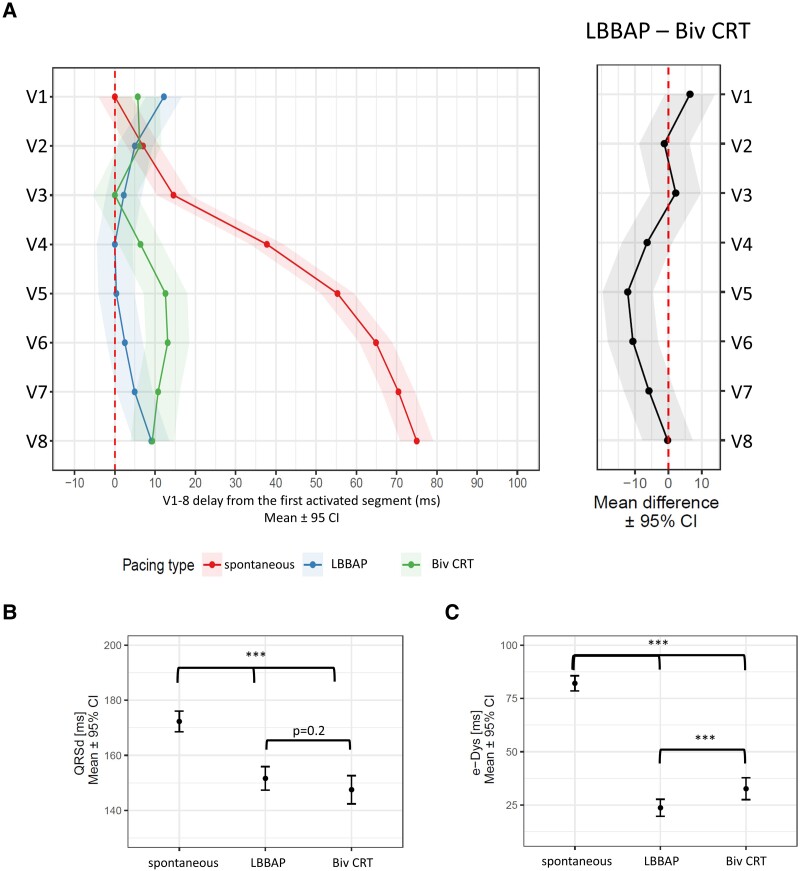
No significant difference in QRSd was observed between LBBAP and Biv CRT—Figure 2B. Both types of CRT changed the UHF-ECG ventricular activation sequence compared with the spontaneous rhythm—Figure 2A. During LBBB, the first activation occurred under V1 and was followed by slow subsequent activation of segments under V2–V8. Contrary to that, the first activation occurred under V4 during LBBAP and V3 during Biv CRT and had the same timing as the activation under V8. However, LBBAP and Biv CRT differed in the location of the last activation; during LBBAP, it was under V1, but during Biv CRT, it occurred under V6.
Figure 2.
Linear mixed model effects for (A) local activation times (first activated segment was placed on 0 ms), (B) QRSd, and (C) e-DYS during spontaneous rhythm, LBBAP, and Biv CRT.
Described change in the activation sequence resulted in a significant reduction of e-DYS with both pacing types. Contrary to QRSd, which was not different between the LBBAP and Biv CRT, e-DYS was reduced more during the LBBAP than during the Biv CRT—Figure 2C.
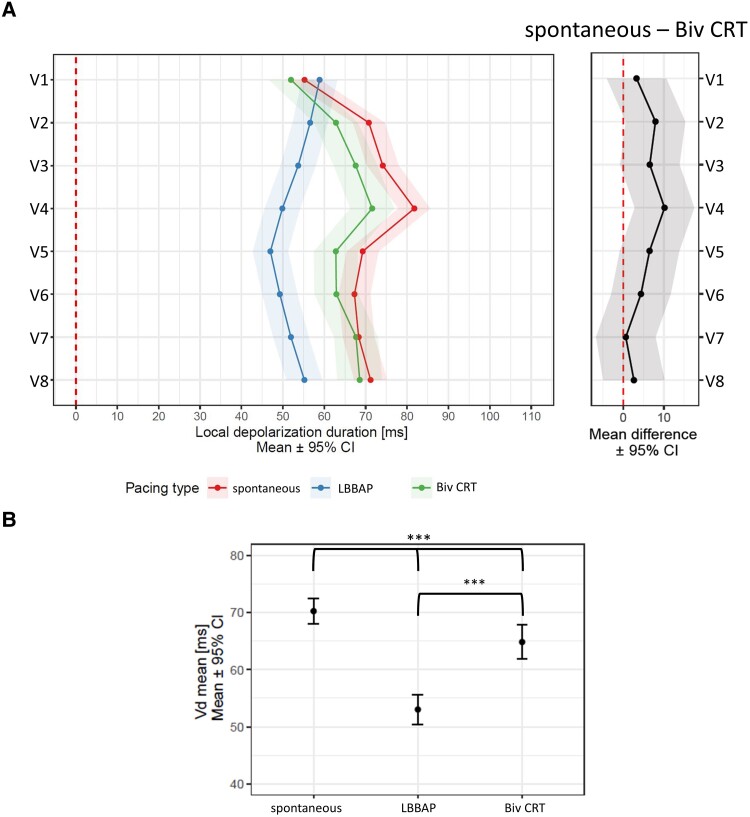
LBBAP led to the shortest local depolarization durations except for leads V1 and V2. A difference in local depolarization durations between Biv CRT and LBBB was present only in the lead V4—Figure 3A. The Vdmean was shorter during LBBAP than during Biv CRT and a spontaneous rhythm—Figure 3B.
Figure 3.
Linear mixed model effects for (A) local depolarization durations in leads V1–V8, (B) Vdmean, during spontaneous rhythm, LBBAP, and Biv CRT.
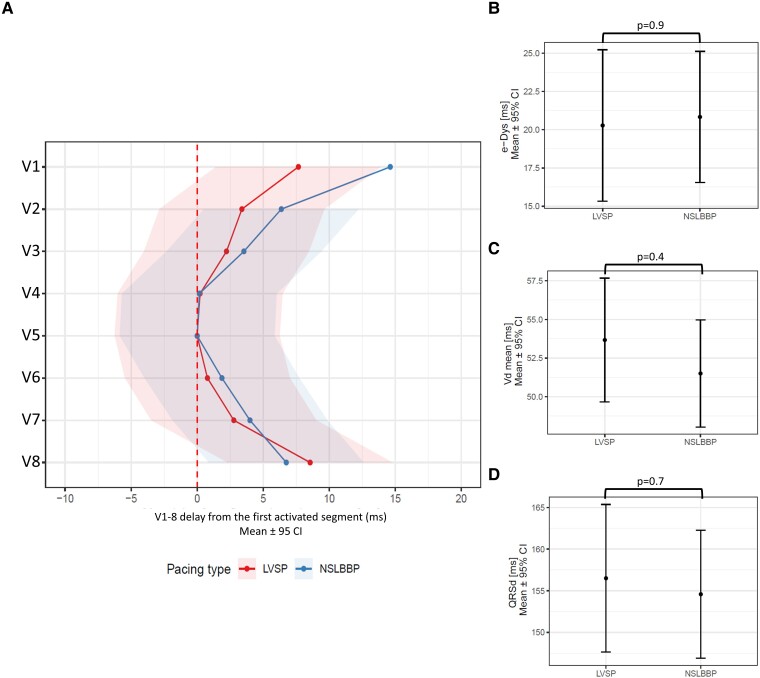
In 42 of the patients included in the study, discrimination between LVSP (n = 23) and NSLBBP (n = 19) was possible. The ventricular activation sequence did not differ between LVSP and NSLBBP, except for more delayed activation in lead V1 [7 ms (1; 13), P < 0.015] during NSLBBP, Figure 4A. e-DYS, local depolarization durations, and QRSd were the same for both pacing types—Figure 4B–D.
Figure 4.
Linear mixed model effects for (A) local activation times (first activated segment was placed on 0 ms), (B) e-DYS, (C) Vdmean, and (D) QRSd during LVSP and NSLBBP.
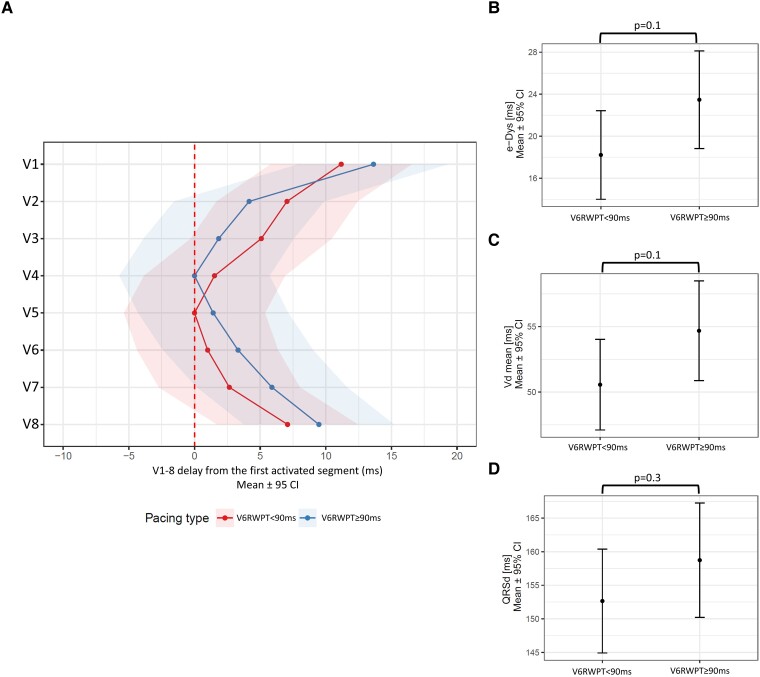
When LBBAP was divided by the distance of V6RWPT from the pacing artefact into two groups, there were 23 patients with paced V6RWPT < 90 ms and 19 patients with paced V6RWPT ≥ 90 ms. No differences were found in e-DYS [18 ms (14; 22) vs. 24 ms (19; 28), P = 0.1], Vdmean [51 ms (47; 54) vs. 55 ms (51; 59), P = 0.1], and QRSd [153 ms (145; 160) vs. 159 ms (150; 167), P = 0.3], between LBBAP with paced V6RWPT < 90 and ≥ 90 ms (Figure 5).
Figure 5.
Linear mixed model effects for (A) local activation times (first activated segment was placed on 0 ms), (B) e-DYS, (C) Vdmean, and (D) QRSd for LBBAP with V6RWPT < 90 ms and V6RWPT ≥ 90 ms.
Discussion
Both Biv CRT and LBBAP significantly reduced ventricular dyssynchrony in a population of patients with symptomatic heart failure and LBBB. Our results indicate that LBBAP reduces ventricular dyssynchrony to a greater degree than Biv CRT. No difference in ventricular dyssynchrony was found between LVSP and NSLBBP, and ventricular activation was similar during LBBAP with shorter and longer V6RWPT.
Reduction of ventricular dyssynchrony during left bundle branch area pacing and biventricular cardiac resynchronization therapy
Reduction of ventricular dyssynchrony is the reason for improved clinical outcomes in patients undergoing Biv CRT, although it utilises slow myocyte-to-myocyte conduction of the electrical impulse within the heart ventricles.13,14 In contrast, during LBBAP, when the pacing lead is placed below the level of the conduction block, the left ventricle was activated more rapidly through the native conductive system. Our results showed that both methods of CRT significantly reduce ventricular dyssynchrony in patients with an LBBB; however, LBBAP may provide more physiological ventricular activation than Biv CRT. These results are in line with the results of other authors.15–17 Nadine et al. showed that LBBAP reduces ventricular dyssynchrony more than Biv CRT using ECGi. Similarly, Elliott et al. reported the superiority of LBBAP over Biv CRT with respect to left ventricular and interventricular synchrony. The only prospective randomised comparison between LBBAP and Biv CRT in heart failure patients to date is the LEVEL-AT trial. In this trial, 70 patients were randomised to either Biv pacing or conduction system pacing (CSP). The CSP group was non-inferior to the Biv pacing group in the reduction of QRSd (−20 ± 23 ms for CSP vs. −20 ± 24 ms for Biv CRT) and in the reduction of left ventricle activation time measured by ECGi (−28 ± 26 ms for CSP vs. −21 ± 20 ms for Biv CRT). A more homogenous propagation map of both ventricles was noted in the CSP group when visualised with ECGi. All the above-mentioned findings correspond well with our data and suggest more physiological ventricular activation during LBBAP than by using Biv CRT in patients with heart failure.
The difference in ventricular activation between non-selective left bundle branch pacing and left ventricular septal pacing
The main difference between NSLBBP and LVSP is that during NSLBBP, the LV conduction system is captured directly. During LVSP, the LV is activated after a delay of 10–20 ms, which results in the prolongation of the paced V6RWPT. The question is, does this activation delay translate into worsening LV synchrony, LV performance, or clinical outcomes during the LVSP? We have already studied the difference between NSLBBP and LVSP using UHF-ECG in bradycardia patients. We showed that LVSP results in better interventricular synchrony than NSLBBP and provides more physiological LV activation.8 On the other hand, when we compared ventricular activation during LVSP in close proximity to the LBB with NSLBBP, there was no difference in LV activation between them.18 Similar observations were made by Vijayaraman in heart failure patients, who found no significant difference between NSLBBP and LVSP using an ECG belt.19 The possible explanation may be that the pacing location with LVSP in heart failure patients may differ from LVSP pacing locations in patients with bradycardia. The presence of the late r/R in V1, as a marker of LVSP, is a result of ventricular activation interplay and indicates that LV activation occurred sooner than the RV. In heart failure patients, the LV is usually dilated, and this results in its prolonged depolarization duration. This may require placing lead tips deeper in the septum, i.e. closer to the LBB, to observe the late r/R in V1, which was a marker for LVSP in our study. This may result in faster and more balanced LV activation, despite the lack of direct LBB capture, and could lead to similar LV activation as NSLBBP. Our results indicate that deep septal pacing with late r/R in V1 and the absence of proof of LBB capture, i.e. LVSP, may serve as an alternative method for reducing ventricular dyssynchrony in patients with heart failure and LBBB.
The difference in ventricular activation between left bundle branch area pacing with shorter and longer paced V6 R-wave peak times
Paced V6RWPT is used as a measure of LV activation during CSP. It reflects the time difference between the pacing artefact and the activation of the LV lateral wall under V6. Paced V6RWPT < 90 ms was suggested as a marker of LBB capture in LBBB patients.20 When we compared ventricular activation patterns during LBBAP with paced V6RWPT < 90 and ≥ 90 ms, we were unable to substantiate any differences in ventricular synchrony, local depolarization durations, and QSRd between them, although a non-significant trend towards shorter e-DYS and Vdmean was observed in the V6RWPT < 90 ms group. Although paced V6RWPT is widely used as a marker of LV activation during CSP, its shorter values were never demonstrated to be associated with better LV performance or better clinical outcomes. Moreover, a significant overlap in paced V6RWPT values was demonstrated during NSLBBP and LVSP in bradycardia patients.21 Furthermore, as we have shown previously, paced V6RWPT is also influenced by the position of the lead on the interventricular septum since it may be artificially shortened by placing the lead towards the inferior portion of the septum.8
Conclusion
Both Biv CRT and LBBAP significantly reduce ventricular dyssynchrony in heart failure patients with LBBB. LBBAP accelerates ventricular depolarization and reduces ventricular dyssynchrony to a greater extent than Biv CRT. No difference in ventricular synchrony was observed between NSLBBP and LVSP, and LBBAP with shorter and longer paced V6RWPT.
Limitations
It is possible that no difference between LVSP and NSLBBP was detected due to UHF-ECG limitations, i.e. UHF-ECGs may not be sensitive enough to detect small differences between them. It is also possible that the absence of a transition during decremental output pacing led to the occasional misclassification of NSLBBP as LVSP and consequently led to biased results in the LVSP group. This was a retrospective study on a small number of patients and no clinical follow-up was performed. This limits the interpretation of the results and translation into clinical practice.
Contributor Information
Ondrej Sussenbek, Cardiocenter, Third Faculty of Medicine, Charles University, University Hospital Kralovske Vinohrady, Srobarova 1150/50, Praha 10, Prague 10034, Czechia.
Leonard Rademakers, Department of Cardiology, Catharina Ziekenhuis, 5602 ZA Eindhoven, The Netherlands.
Petr Waldauf, Department of Anesthesia and Intensive Care, Charles University, University Hospital Kralovske Vinohrady, Prague 10034, Czechia.
Pavel Jurak, The Czech Academy of Sciences, Institute of Scientific Instruments, Brno 61200, Czechia.
Radovan Smisek, The Czech Academy of Sciences, Institute of Scientific Instruments, Brno 61200, Czechia.
Petr Stros, Cardiocenter, Third Faculty of Medicine, Charles University, University Hospital Kralovske Vinohrady, Srobarova 1150/50, Praha 10, Prague 10034, Czechia.
Lukas Poviser, Cardiocenter, Third Faculty of Medicine, Charles University, University Hospital Kralovske Vinohrady, Srobarova 1150/50, Praha 10, Prague 10034, Czechia.
Jana Vesela, Cardiocenter, Third Faculty of Medicine, Charles University, University Hospital Kralovske Vinohrady, Srobarova 1150/50, Praha 10, Prague 10034, Czechia.
Filip Plesinger, The Czech Academy of Sciences, Institute of Scientific Instruments, Brno 61200, Czechia.
Josef Halamek, The Czech Academy of Sciences, Institute of Scientific Instruments, Brno 61200, Czechia.
Pavel Leinveber, International Clinical Research Center, St. Anne’s University Hospital, Brno 60200, Czechia.
Dalibor Herman, Cardiocenter, Third Faculty of Medicine, Charles University, University Hospital Kralovske Vinohrady, Srobarova 1150/50, Praha 10, Prague 10034, Czechia.
Pavel Osmancik, Cardiocenter, Third Faculty of Medicine, Charles University, University Hospital Kralovske Vinohrady, Srobarova 1150/50, Praha 10, Prague 10034, Czechia.
Karol Curila, Cardiocenter, Third Faculty of Medicine, Charles University, University Hospital Kralovske Vinohrady, Srobarova 1150/50, Praha 10, Prague 10034, Czechia.
Funding
This study was supported by the Charles University Research Program “Cooperatio Cardiovascular Sciences”, by the Ministry of Health of the Czech Republic, grant number NU21-02-00584, and by the CAS project RVO: 68081731.
Data availability
The raw data supporting the conclusions of this article are available on request from the corresonding author, OS, without undue reservation.
References
- 1. Glikson M, Nielsen JC, Kronborg MB, Michowitz Y, Auricchio A, Barbash IMet al. 2021 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy developed by the Task Force on cardiac pacing and cardiac resynchronization therapy of the European Society of Cardiology (ESC) with the special contribution of the European Heart Rhythm Association (EHRA). Eur Heart J 2021;42:3427–3520.34455430 [Google Scholar]
- 2. Mafi-Rad M, Luermans JGLM, Blaauw Y, Janssen M, Crijns HJ, Prinzen FWet al. Feasibility and acute hemodynamic effect of left ventricular septal pacing by transvenous approach through the interventricular septum. Circ Arrhythm Electrophysiol 2016;9:e003344. [DOI] [PubMed] [Google Scholar]
- 3. Wang Y, Zhu H, Hou X, Wang Z, Zou F, Qian Zet al. Randomized trial of left bundle branch vs biventricular pacing for cardiac resynchronization therapy. J Am Coll Cardiol 2022;80:1205–1216. [DOI] [PubMed] [Google Scholar]
- 4. Salden FCWM, Luermans JGLM, Westra SW, Weijs B, Engels EB, Heckman LIBet al. Short-term hemodynamic and electrophysiological effects of cardiac resynchronization by left ventricular septal pacing. J Am Coll Cardiol 2020;75:347–359. [DOI] [PubMed] [Google Scholar]
- 5. Jurak P, Curila K, Leinveber P, Prinzen FW, Viscor I, Plesinger Fet al. Novel ultra-high-frequency electrocardiogram tool for the description of the ventricular depolarization pattern before and during cardiac resynchronization. J Cardiovasc Electrophysiol 2020;31:300–307. [DOI] [PubMed] [Google Scholar]
- 6. Curila K, Jurak P, Halamek J, Prinzen F, Waldauf P, Karch Jet al. Ventricular activation pattern assessment during right ventricular pacing: ultra-high-frequency ECG study. J Cardiovasc Electrophysiol 2021;32:1385–1394. [DOI] [PubMed] [Google Scholar]
- 7. Curila K, Prochazkova R, Jurak P, Jastrzebski M, Halamek J, Moskal Pet al. Both selective and nonselective His bundle, but not myocardial, pacing preserve ventricular electrical synchrony assessed by ultra-high-frequency ECG. Heart Rhythm 2020;17:607–614. [DOI] [PubMed] [Google Scholar]
- 8. Curila K, Jurak P, Jastrzebski M, Prinzen F, Waldauf P, Halamek Jet al. Left bundle branch pacing compared to left ventricular septal myocardial pacing increases interventricular dyssynchrony but accelerates left ventricular lateral wall depolarization. Heart Rhythm 2021;18:1281–1289. [DOI] [PubMed] [Google Scholar]
- 9. Strauss DG, Selvester RH, Wagner GS. Defining left bundle branch block in the era of cardiac resynchronization therapy. Am J Cardiol 2011;107:927–934. [DOI] [PubMed] [Google Scholar]
- 10. Jastrzebski M. ECG and pacing criteria for differentiating conduction system pacing from myocardial pacing. Arrhythm Electrophysiol Rev 2021;10:172–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jastrzȩbski M, Burri H, Kiełbasa G, Curila K, Moskal P, Bednarek Aet al. The V6-V1 interpeak interval: a novel criterion for the diagnosis of left bundle branch capture. Europace 2022;24:40–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Su L, Wang S, Wu S, Xu L, Huang Z, Chen Xet al. Long-term safety and feasibility of left bundle branch pacing in a large single-center study. Circ Arrhythm Electrophysiol 2021;14:e009261. [DOI] [PubMed] [Google Scholar]
- 13. Lecoq G, Leclercq C, Leray E, Crocq C, Alonso C, de Place Cet al. Clinical and electrocardiographic predictors of a positive response to cardiac resynchronization therapy in advanced heart failure. Eur Heart J 2005;26:1094–1100. [DOI] [PubMed] [Google Scholar]
- 14. Pujol-López M, Jiménez Arjona R, Guasch E, Doltra A, Borràs R, Roca Luque Iet al. Septal flash correction with His-Purkinje pacing predicts echocardiographic response in resynchronization therapy. Pacing Clin Electrophysiol 2022;45:374–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ali N, Arnold A, Miyazawa AA, Keene D, Peters NS, Kanagaratnam Pet al. PO-673-06 cardiac resynchronization with left bundle area pacing compared to His bundle and biventricular pacing; an acute electrical and haemodynamic within patient comparison. Heart Rhythm 2022;19:S334. [Google Scholar]
- 16. Elliott MK, Strocchi M, Sieniewicz BJ, Sidhu B, Mehta V, Wijesuriya Net al. Biventricular endocardial pacing and left bundle branch area pacing for cardiac resynchronization: mechanistic insights from electrocardiographic imaging, acute hemodynamic response, and magnetic resonance imaging. Heart Rhythm 2022;20:207–216. [DOI] [PubMed] [Google Scholar]
- 17. Pujol-Lopez M, Jiménez-Arjona R, Garre P, Guasch E, Borràs R, Doltra Aet al. Conduction system pacing vs biventricular pacing in heart failure and wide QRS patients: LEVEL-AT trial. JACC Clin Electrophysiol 2022;8:1431–1445. [DOI] [PubMed] [Google Scholar]
- 18. Curila K, Jurak P, Vernooy K, Jastrzebski M, Waldauf P, Prinzen Fet al. Left ventricular myocardial septal pacing in close proximity to LBB does not prolong the duration of the left ventricular lateral wall depolarization compared to LBB pacing. Front Cardiovasc Med 2021;8:787414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Vijayaraman P, Hughes G, Manganiello M, Johns A, Ghosh S. Non-invasive assessment of ventricular electrical heterogeneity to optimize left bundle branch area pacing. J Interv Card Electrophysiol 2022. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 20. Vijayaraman P, Ponnusamy SS, Cano Ó, Sharma PS, Naperkowski A, Subsposh FAet al. Left bundle branch area pacing for cardiac resynchronization therapy: results from the international LBBAP collaborative study group. JACC Clin Electrophysiol 2021;7:135–147. [DOI] [PubMed] [Google Scholar]
- 21. Jastrzębski M, Kiełbasa G, Curila K, Moskal P, Bednarek A, Rajzer Met al. Physiology-based electrocardiographic criteria for left bundle branch capture. Heart Rhythm 2021;18:935–943. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw data supporting the conclusions of this article are available on request from the corresonding author, OS, without undue reservation.