Abstract
Despite the seemingly important role of cytotoxic T-lymphocyte (CTL) responses in human immunodeficiency virus (HIV) disease pathogenesis, their measurement has relied on a variety of different techniques. We utilized three separate methodologies for the detection of CTLs in a cohort of HIV-infected individuals who were also human leukocyte antigen A2 (HLA-A2) positive. Among the different CTL assays, a correlation was seen only when the Gag epitope-specific HLA A*0201-restricted tetramer assay was compared with the ELISPOT assay performed after stimulation with the Gag epitope; however, this correlation was of borderline statistical significance. On average, the tetramer reagent detected a 10-fold-higher number of cells than were seen to produce gamma interferon by the ELISPOT assay. The implications of this CTL assay comparison and the possibility of phenotypic differences in HIV-specific CD8+ T lymphocytes are discussed.
Cytotoxic T lymphocytes (CTLs) are important in controlling viral replication in human immunodeficiency virus (HIV)-infected individuals. Their appearance in blood coincides with the initial decrease in plasma viral load during acute HIV infection (5, 20) and declines proportionally with viral load in patients receiving highly active antiretroviral therapy (HAART) (13, 32). Additionally, CTLs demonstrate a negative correlation with disease progression due to HIV infection (3, 19, 30, 35), and in a simian immunodeficiency virus (SIV) macaque model, the control of SIV replication correlated directly with the presence of CD8+ T lymphocytes (16, 38).
Assays for the detection of CTLs have historically relied on direct determination of cell lysis as measured by chromium release. While this assay measures an effector characteristic of CD8+ T cells, it is cumbersome and technically difficult to perform. Additionally, the standard lytic assay is qualitative and must rely on a limiting dilution analysis (LDA) for quantitative results (20). Unfortunately, LDA frequently underestimates the true level of CTL responses (22, 40).
In recent years, newer assays allowing for easier assessment of CTL responses have been developed. Assays to detect gamma interferon (IFN-γ) by use of the ELISPOT technique permit indirect visualization of antigen-specific CD8+ T cells and also have an advantage over the standard lytic method in that they are quantitative (12, 21, 22). The tetramer assay has allowed quick, simple, and reliable detection of antigen-specific HLA-restricted CTL responses (1, 32). Furthermore, this assay and other flow cytometric technologies can evaluate other cellular phenotypic characteristics in the same experiment. The tetramer assays, however, are limited in that they are specific to one HLA-restricted epitope, and both the tetramer and ELISPOT assays serve only as surrogate markers of CTL lysis. We compared the CTL responses in a group of HIV-infected individuals and uninfected controls utilizing the standard lytic method, the ELISPOT assay for the detection of IFN-γ, and the tetramer reagent for the detection of antigen-specific CD8+ T cells. Although a strong trend toward correlation between the ELISPOT and tetramer assays was observed, the latter method detected approximately a 10-fold-greater number of cells than were demonstrated to produce IFN-γ.
All volunteers had detectable CTL responses by the standard bulk lysis method.
Patients were recruited from the University of Alabama at Birmingham (UAB) AIDS clinic and were selected on the basis of low but detectable plasma viral load (<10,000 copies/ml) and a total CD4+ T-cell count above 200/μl (Table 1). Most patients (5 of 7) were taking antiretroviral therapy at the time of the study. All were in good health, and none had a history of opportunistic infection. The patient cohort included six chronically infected individuals (average duration of known infection, 6 years) and one with recent infection. HLA typing was performed by standard serologic methods for all HIV-infected patients (2). Seven healthy, uninfected individuals at low risk of acquiring HIV infection served as controls. The Institutional Review Board of the UAB approved the study.
TABLE 1.
Characteristics of the HIV-1-infected HLA-A2-positive individuals
| Patient no. | Age (yr) | Duration of infection (yr) | No. of CD4+ T cells/μl | No. of HIV RNA copies/ml | No. of ARTa medications |
|---|---|---|---|---|---|
| P1 | 53 | 4 | 546 | 574 | 4 |
| P2 | 39 | <1b | 835 | 1,291 | 3 |
| P3 | 45 | 15 | NAc | NA | 0 |
| P4 | 42 | 7 | 857 | 463 | 2 |
| P5 | 29 | 2 | 403 | <50 | 3 |
| P6 | 35 | 1 | 655 | 46,575 | 0 |
| P7 | 42 | 13 | 490 | 5,950 | 2 |
ART, antiretroviral therapy.
Infected 2 months prior to blood draw.
NA, not available (patient refused testing).
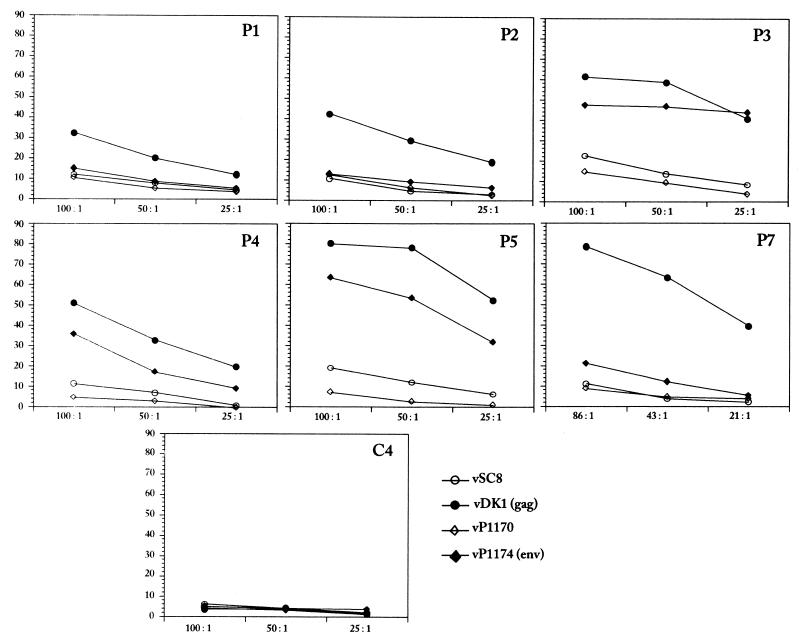
The chromium release lytic assay was performed as the current CTL “gold standard” (4, 6, 30) for comparison to the newer methods. Responses to Gag were measured after a 2-week restimulation of effector cells. The percent lysis at three separate effector-to-target (E:T) ratios is shown (Fig. 1). All patients' peripheral blood mononuclear cells (PBMCs) demonstrated a positive response, as defined by >10% HIV Gag-specific lysis at two or more E:T ratios (more than 3 standard deviations above the mean value for the negative controls). In contrast, only 3 of 6 (50%) individuals' PBMCs were able to lyse Env-expressing targets (Fig. 1; see P3, P4, and P5). Depletion of the CD8+ T cells resulted in ablation of the positive response in all patients (data not shown). Our findings are consistent with work done by others demonstrating that most HIV patients with chronic infection have detectable HIV-specific CTL precursor (CTLp) responses as measured by the chromium release assay (5, 7, 10).
FIG. 1.
CTL lytic activity for HIV-infected patients (P1 through P5 and P7) and a representative control (C4). Fresh PBMCs were stimulated for 2 weeks with vP1291 (encoding HIV Gag, Pro, and Env) and mixed with autologous B-lymphocyte cell lines (BLCL) infected with vSC8 (control for Gag), vDK1 (Gag), vP1170 (control for Env), or vP1174 (Env) at the indicated E:T ratios (x axis). The percent release of 51Cr-labeled targets relative to spontaneous release in a 5-h assay is depicted (y axis). The spontaneous release of 51Cr was <20% for all experiments.
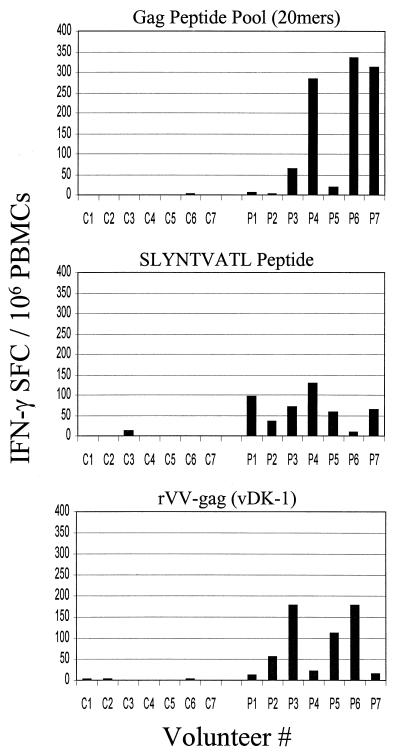
The ELISPOT assay after three separate antigenic-stimulation methods detected responses in most volunteers.
ELISPOT assays have been used increasingly due primarily to ease of performance, ability to utilize frozen cells, and quantitative results. We employed the standard ELISPOT assay (28, 37) to quantify the number of PBMCs capable of secreting IFN-γ after antigenic stimulation by one of three separate stimulation techniques: (i) a pool of overlapping 20-mer Gag peptides, (ii) an HLA A*0201-restricted Gag p17 epitope (SLYNTVATL), or (iii) a recombinant vaccinia virus (rVV) encoding Gag (vDK1). Freshly isolated or thawed PBMCs were placed into 96-well nitrocellulose plates that were coated with an anti-human IFN-γ antibody (Mabtech, Nacka, Sweden) and were stimulated with the antigen for 18 h. A biotinylated anti-human IFN-γ monoclonal antibody was then added to the plates, followed by treatment with streptavidin-alkaline phosphatase. After plates were washed with tap water and dried overnight, the spot-forming cells (SFC) were counted using a stereomicroscope. IFN-γ-secreting T lymphocytes were detected in the PBMCs of a majority of individuals irrespective of the stimulation technique utilized (Fig. 2). The highest numbers of SFC per million PBMCs were observed after Gag peptide pool stimulation (mean, 147; range, 3 to 336) compared to Gag stimulation either by SLYNTVATL (SL9) (mean, 66; range, 11 to 129) or by rVV (mean, 82; range, 11 to 178). The frequencies of IFN-γ-producing PBMCs in the negative controls were uniformly low (mean, 1.4; range, 0 to 14). By using the results from the HIV-negative controls, a positive response was defined as more than 15 SFC/106 PBMCs (more than 3 standard deviations above the mean). Due to the large number of PBMCs required to perform three separate assays, we did not repeat experiments on all of the volunteers; however, we did repeat the ELISPOT assays for patients P3 and P7, yielding findings similar to the first result (data not shown).
FIG. 2.
ELISPOT analysis for HIV-positive patients (P1 through P7) and controls (C1 through C7). Fresh or thawed PBMCs were stimulated with the indicated antigen and incubated for 18 h. After being developed, the SFC were counted under a stereomicroscope.
For all of the experiments using vaccinia virus-based stimulation, a vaccinia virus recombinant devoid of HIV antigen expression (vSC8) was utilized for the control reaction. Vaccinia virus stimulation produced a high “background” (see Table 2, patients P2, P5, and P6) in 3 of 7 (43%) patients and probably represented cellular immune responses directed toward the vaccinia virus itself. The high background response in a significant number of individuals made it difficult to determine whether responses were Gag specific.
TABLE 2.
Comparison of CTL assays
| Volunteer | Result of:
|
||||
|---|---|---|---|---|---|
| IFN-γ ELISPOT assay (total no. of SFC/106 PBMCs [background])
|
SL9 tetramer assay (% of CD8+ T cells responding) | Gag lytic assay (% of bulk lysis specific for rVV Gaga) | |||
| rVV Gag | Gag pool | SL9 peptide | |||
| C1 | 25 (21) | 0 (0) | 0 (0) | NDb | 4 |
| C2 | 9 (5) | 0 (0) | 0 (0) | ND | 0 |
| C3 | 2 (2) | 0 (0) | 14 (0) | ND | 1 |
| C4 | 4 (4) | 1 (0) | 0 (0) | ND | 3 |
| C5 | 0 (0) | 0 (0) | 1 (0) | ND | 4 |
| C6 | 2 (0) | 6 (4) | 4 (4) | ND | ND |
| C7 | 0 (0) | 0 (7) | ND | ND | 1 |
| P1 | 16 (5) | 12 (4) | 100 (4) | 0.11 | 12 |
| P2 | 123 (66) | 6 (3) | 36 (2) | 0.45 | 25 |
| P3 | 186 (8) | 64 (1) | 72 (1) | 0.14 | 45 |
| P4 | 32 (10) | 295 (10) | 139 (10) | 0.24 | 26 |
| P5 | 193 (80) | 20 (2) | 61 (2) | 0.26 | 68 |
| P6 | 240 (62) | 337 (1) | 12 (1) | 0.01 | ND |
| P7 | 24 (7) | 314 (0) | 64 (0) | 0.13 | 60 |
Shown at the 50:1 E:T ratio.
ND, not done.
Comparison of SFC frequencies after three types of antigenic stimulation revealed no clear patterns, and these varied results are not unexpected, since none of the stimulation techniques presented identical antigens. The 9-mer SL9 peptide ELISPOT assay is limited to one epitope, while the rVV Gag presents HIV antigens in the context of vaccinia virus products. Finally, the Gag peptide pool was made up of 20-mers in contrast to the 8- to 10-mers generally believed to be optimal for major histocompatibility complex (MHC) class I-restricted antigen presentation (14, 23, 25). The possibility that the 20-mer stimulation technique was detecting a CD4+ T-lymphocyte response was not ruled out in all cases but was unlikely for two reasons. First, helper T-cell responses to HIV type 1 (HIV-1) are generally believed to be unusual during chronic infection except in a small subset of patients termed long-term nonprogressors (LTNPs) (36). None of the individuals in our cohort were LTNPs. Secondly, CD8+ T-cell depletions were performed on two patients' PBMCs (P5 and P7) using the Dynabead method (Dynal Inc., Lake Success, N.Y.) (8). In both experiments the positive responses seen with unfractionated PBMCs were absent following the depletion (data not shown). Therefore, it seems likely that the IFN-γ SFC seen after Gag 20-mer peptide pool stimulation were due to CD8+ T lymphocytes.
The HLA A*0201-restricted tetramer detects SLYNTVATL-specific CD8+ T cells in a majority of chronically infected HIV-1 patients.
We next tested MHC-peptide tetramer binding to thawed PBMCs that had been frozen from the identical blood draw used for the ELISPOT assays. The HLA A*0201-restricted Gag (p17) SL9 tetramer was chosen because it was shown to be an immunodominant CTL epitope during chronic HIV infection (11, 17, 41, 42). The tetramer reagent was produced in our facility utilizing bacterial plasmids expressing the HLA A*0201 heavy chain or β2 microglobulin molecules (kindly provided by John Altman and Beckman Coulter, Fullerton, Calif.) as previously described (1). Three-color analysis for flow cytometry (1, 29, 32, 33) used the SL9 tetramer, anti-human CD8 (Becton Dickinson, San Diego, Calif.), and anti-human CD3 (PharMingen, San Jose, Calif.) conjugated to phycoerythrin (PE), peridin chlorophyll protein (PerCP), and allophycocyanin (APC), respectively. Flow cytometry was performed on a FACSCaliber (Becton Dickinson), and data were analyzed using the WINMIDI software program, version 2.8 (Joseph Trotter, La Jolla, Calif.).
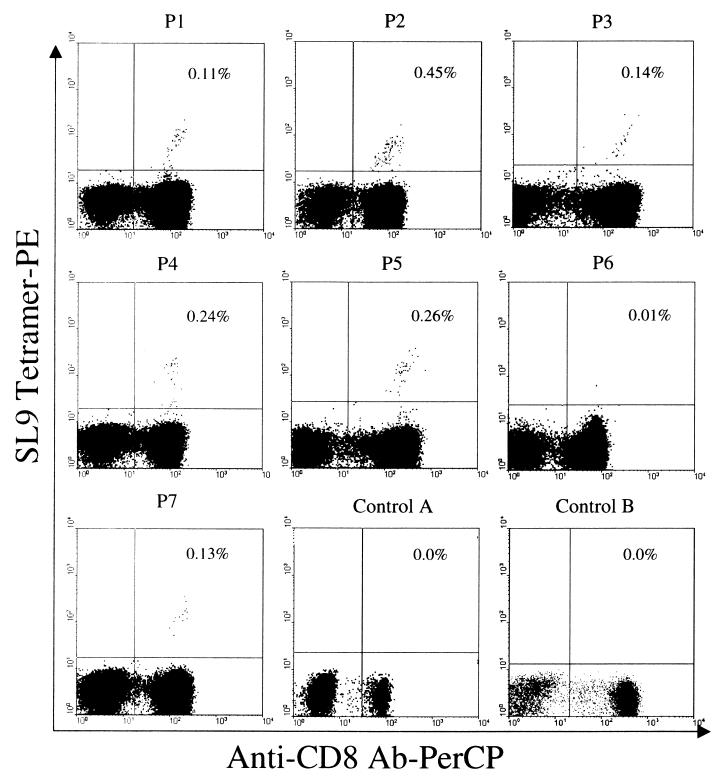
This HLA A*0201-restricted tetramer bound to >95% of cells derived from an HLA A*0201 CD8+ T-cell clone (kindly provided by Bruce Walker) specific for the SL9 epitope (data not shown). The range of results for HIV-infected subjects varied from 0.01 to 0.45% of CD8+ T cells, with a mean response of 0.19% (Fig. 3). A low background was observed, as the SL9 tetramer stained a very small number (e.g., <0.01%) of CD8+ T cells in either of the uninfected controls (Fig. 3). By calculating the SL9 tetramer response for negative controls (2 shown and 10 others not shown), a positive response was defined as more than 3 standard deviations above the mean response of the controls (>0.05% of the CD8+ T cells). By use of this definition, SL9-specific CD8+ T lymphocytes were detected in 6 of 7 (86%) HIV-infected individuals. One patient (P6) did not have SL9-specific cells as determined by either the tetramer (Fig. 3) or the ELISPOT (Fig. 2) assay. P6 was also HLA-B27 positive; therefore, it is possible that an HLA-B27-restricted Gag-specific response was immunodominant over the HLA-A2 response, as the former was also associated with a highly immunodominant epitope (31) and correlated with the lack of HIV disease progression (18, 27). However, due the fact that genotyping was not performed on our cohort, it is also possible that individual P6 did not have HLA A*0201 but another subtype of HLA-A2. Although we did not perform viral sequencing for this study, P6 could have harbored a virus not encoding the SLYNTVATL peptide sequence. Overall, the high percentage of HIV-positive patients with positive responses using the SL9 tetramer is consistent with previously published findings (11, 33).
FIG. 3.
SL9 HLA A*0201-restricted tetramer staining of thawed PBMCs from HLA-A2-positive patients. Cells were gated by using three parameters (forward and side light scatter and CD3+ T cells) in order to focus on the CD3+ T lymphocyte population. In addition to the HIV-infected cohort (P1 through P7), two types of control individuals were included (control A was HIV negative and HLA-A2 positive; control B was HIV positive and HLA-A2 negative). The percentage of CD8+ T cells that stained with the SL9 tetramer is given in the upper right quadrant of each dot plot.
The HLA A*0201-restricted tetramer detects a 10-fold-greater frequency of Gag-specific PBMCs than the IFN-γ ELISPOT assay.
The results from the three CTL assays are summarized in Table 2. As expected, no significant correlation was seen between the SL9 epitope-specific assays and the Gag-specific CTL assays, i.e., the Gag lytic assay or the ELISPOT assay using either rVV Gag or the Gag peptide pool. Additionally, no significant correlation was seen among any of the ELISPOT assays.
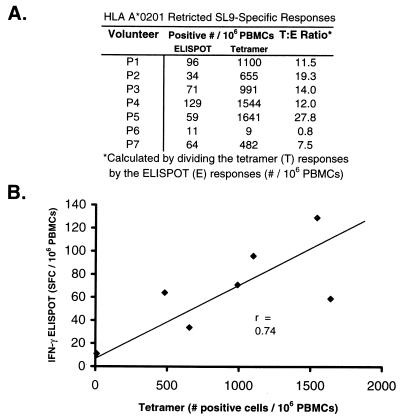
We next wanted to compare the SL9-specific ELISPOT assay to the SL9-specific tetramer assay. Since the ELISPOT assay includes PBMCs and the tetramer data was gated on CD3+ T lymphocytes, we enlarged the collecting gate on the flow cytometer to include other PBMCs in addition to lymphocytes (e.g., monocytes and neutrophils) (40). In this manner, cells that would be counted with a hemocytometer as PBMCs would more likely be included in the flow analysis. We then converted the tetramer assay data into the number of positive responses per million PBMCs (40) (Fig. 4A). A correlation was demonstrated between the SL9 ELISPOT assay and the respective tetramer assay when the number of IFN-γ-producing cells was compared to the number of cells that recognized the tetramer (Fig. 4B). This correlation was not statistically significant at the 95% confidence interval (P = 0.06); however, a larger number of patients may have given a significant correlation. Additionally, it is possible that PBMCs obtained from individuals at various stages of HIV infection may have different cytokine profiles. Although all of the volunteers had CD4 counts greater than 400 at the time of the study (Table 1), the range was 403 to 857 cells/μl, with a mean of 541 cells/μl. When the results from the patient with the lowest CD4 count (P5) were excluded from the analysis, the correlation became significant (r = 0.94; P = 0.006). A correlation between the tetramer and ELISPOT assays was also seen for Epstein-Barr virus (EBV) in healthy virus carriers (40). These findings suggest that quantitative comparison across CTL assays should be viewed with caution and that direct comparisons should be made only when the antigen specificity of the assays is identical.
FIG. 4.
Direct comparison of the ELISPOT and tetramer assays. (A) Both the percentage of CD8+ T cells and the number of positive cells per million PBMCs are shown. (B) The correlation between the numbers of PBMCs as detected by the SL9 tetramer assay (x axis) or the SL9 ELISPOT assay (y axis) is graphically represented. For the tetramer assay, the forward and side light scatter gates were enlarged to include PBMCs as counted in the hemocytometer, thereby allowing more direct comparison with the “nongated” ELISPOT assay.
Despite the correlation trend for the SL9 tetramer and SL9 ELISPOT assays, the absolute number of SL9-specific IFN-γ-producing PBMCs detected by the latter assay (mean, 66) was more than 10-fold less than the number of SL9 epitope-specific PBMCs detected by tetramer staining (mean, 917) (Fig. 4A). Since separate tetramer and ELISPOT assays were performed, we did not directly measure IFN-γ production in tetramer-positive cells; however, both assays were performed on identical blood draws. It was also possible that the ELISPOT assay did not detect all of the IFN-γ-producing PBMCs. To address this issue, we placed a dilution of cell culture medium containing approximately 100 cells that constitutively express IFN-γ (C10/MJ cells; National Institutes of Health [NIH] AIDS Research and Reference Reagent Program) onto an ELISPOT plate. An average of 83 SFC/well resulted; therefore, the IFN-γ ELISPOT assay is sensitive in detecting IFN-γ production.
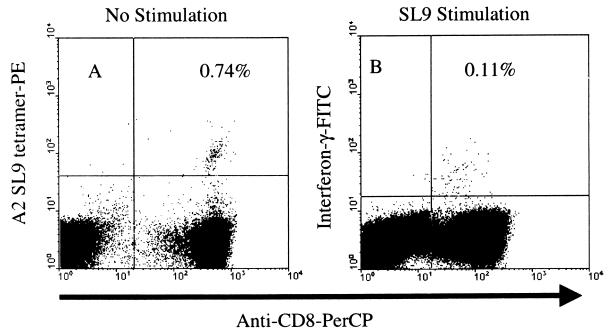
To further address the sensitivity of the IFN-γ ELISPOT assay, we also performed flow cytometric analysis of IFN-γ-producing cells as detected by intracellular cytokine staining. Fresh PBMCs were stimulated for 6 h with the SL9 peptide, and brefeldin A was added after 2 h. Cells were then stained with anti-human CD8–PerCP and anti-human CD3–APC. After fixation and permeabilization of the cells, staining was performed with anti-IFN-γ–fluorescein isothiocyanate (FITC) and analyzed by flow cytometry. Using this technique to analyze IFN-γ production in the cells from two of the individuals (P1 and P7) gave results that were below the level of detection of the flow assay (∼0.05%). This result was expected, since the ELISPOT assay detected less than 100 SFC/106 PBMCs in both individuals. We therefore performed tetramer and IFN-γ detection assays by both ELISPOT and intracellular cytokine staining with PBMCs derived from an LTNP not included in the original cohort (CD4+ T-cell count, 1,078/μl; plasma viral load, 190 copies/ml; infected for more than 12 years). A large number of PBMCs taken from this individual (P8) were previously shown to secrete IFN-γ by an ELISPOT assay after SL9 peptide stimulation (data not shown). We found that 0.74% of this individual's CD8+ T cells stained with the SL9 tetramer, a higher percentage than those seen for individuals P1 through P7 (Fig. 5). Additionally, following SL9 peptide stimulation, the ELISPOT assay detected 735 SFC/106 PBMCs, also a higher number than those seen for the other volunteers. Flow cytometric analysis of cells stained for the presence of intracellular cytokines demonstrated that 0.11% of CD8+ T cells secreted IFN-γ. Therefore, only 15% of the tetramer-positive cells produced IFN-γ in the intracellular cytokine assay. Converting the intracellular cytokine staining results into the number of cells per million PBMCs yielded numbers similar to those derived from the ELISPOT assay (834 versus 735 positive events per 106 PBMCs, respectively). The above results, with a cell line constitutively expressing IFN-γ and with PBMCs from an LTNP, indicated that the observation that the majority of tetramer-positive cells do not produce IFN-γ after antigenic stimulation was not due to a lack of sensitivity of the IFN-γ ELISPOT assay. Furthermore, our data are consistent with published findings showing that the tetramer detected a greater number of cells in mice than can be seen to produce IFN-γ (29, 40, 43).
FIG. 5.
Direct comparison of the tetramer and intracellular cytokine assays. PBMCs derived from individual P8 were either stained with the SL9 tetramer (A) or stimulated with the SL9 peptide for 6 h and stained for the presence of IFN-γ (B). Results are given in terms of the percentage of CD8+ T cells that stained with either the tetramer or IFN-γ.
Not all tetramer-positive cells may produce IFN-γ. The tetramer-positive cells could be producing other cytokines or none at all. A related possibility is that some of the tetramer-positive cells are no longer able to proliferate and are undergoing apoptosis (15, 26). Evidence for this scenario comes from studies with CD4+ T-cell-deficient mice, where tetramer-positive T cells specific for a lymphocytic choriomeningitis virus (LCMV) epitope failed to produce IFN-γ or other cytokines in response to that LCMV peptide (43). The same study also demonstrated that not all tetramer-positive cells produced IFN-γ in wild-type mice chronically infected with LCMV. The possibility that not all tetramer-positive cells may be functional has also recently been demonstrated in EBV, HIV-1, and tumor-associated antigen-specific CD8+ T lymphocytes (24, 39, 40). Finally, two recently published reports also demonstrated that not all tetramer-positive cells derived from HIV-1-infected volunteers were able to produce IFN-γ as detected by either ELISPOT (34) or intracellular cytokine staining (9) assays. Therefore, while the published data on humans remain anecdotal, it is possible that subsets of tetramer-positive cells are not fully functional. Indeed both our own data and those of others (9, 34) suggest that about 50 to 90% of SL9-specific CD8+ T cells fail to produce IFN-γ upon exposure to SL9. Whether the different SL9-specific cell frequencies observed in these two assays reflect technique or actual biology, i.e., true differences in the functionality of antigen-specific T cells, remains to be determined. Furthermore, it is not currently clear whether these differences are unique to HIV-1 infection. Studies are ongoing in our laboratory and others to more fully characterize the functional capacity of tetramer-positive human cells. The near future should provide better insights into this important question.
Acknowledgments
We thank Kevin Perez, Tina Rogers, Marion Spell, Xiaobing Ping, and Veronica Owens for technical support, Peter Bonventre for volunteer recruitment, Yuting Zhang for statistical analysis, and Yvonne McClain for manuscript preparation. Recombinant vaccinia virus vectors and the Gag peptide pool were obtained from the NIH AIDS Research and Reference Reagent Program.
This work was supported by USPHS awards AI-45209 and AI-01380, the UAB Center for AIDS Research (AI-27767), and a Howard Hughes Medical Institute (HHMI) institutional award to UAB.
REFERENCES
- 1.Altman J D, Moss P A H, Goulder P J R, Barouch D H, McHeyzer-Williams M G, Bell J I, McMichael A J, Davis M M. Phenotypic analysis of antigen-specific T lymphocytes. Science. 1996;274:94–96. [PubMed] [Google Scholar]
- 2.Amos D B, Pool P, Grier J. Manual of clinical immunology. Washington, D.C.: American Society for Microbiology; 1980. pp. 978–986. [Google Scholar]
- 3.Bariou C, Genetet N, Ruffault A, Michelet C, Cartier F, Genetet B. Longitudinal study of HIV-specific cytotoxic lymphocytes in HIV type 1-infected patients: relative balance between host immune response and the spread of HIV type 1 infection. AIDS Res Hum Retrovir. 1997;13:1301–1312. doi: 10.1089/aid.1997.13.1301. [DOI] [PubMed] [Google Scholar]
- 4.Belshe R B, Gorse G J, Mulligan M J, Evans T G, Keefer M C, Excler J L, Duliege A M, Tartaglia J, Cox W I, McNamara J, Hwang K L, Bradney A, Montefiori D, Weinhold K J. Induction of immune responses to HIV-1 by canarypox virus (ALVAC) HIV-1 and gp120 SF-2 recombinant vaccines in uninfected volunteers. NIAID AIDS Vaccine Evaluation Group. AIDS. 1998;12:2407–2415. doi: 10.1097/00002030-199818000-00009. [DOI] [PubMed] [Google Scholar]
- 5.Carmichael A, Jin X, Sissons P, Borysiewicz L. Quantitative analysis of the human immunodeficiency virus type 1 (HIV-1)-specific cytotoxic T lymphocyte (CTL) response at different stages of HIV-1 infection: differential CTL responses to HIV-1 and Epstein-Barr virus in late disease. J Exp Med. 1993;177:249–256. doi: 10.1084/jem.177.2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Evans T G, Keefer M C, Weinhold K, Wolff M, Montefiori D, Gorse G J, Graham B S, McElrath M J, Clements-Mann M L, Mulligan M J, Fast P, Walker M C, Excler J-L, Duliege A-M, Tartaglia J the NIAID AIDS Vaccine Evaluation Group. A canarypox vaccine expressing multiple HIV-1 genes given alone or with rgp120 elicits broad and durable CD8+ CTL responses in seronegative volunteers. J Infect Dis. 1999;180:290–298. doi: 10.1086/314895. [DOI] [PubMed] [Google Scholar]
- 7.Ferrari G, Berend C, Ottinger J, Dodge R, Bartlett J, Toso J, Moody D, Tartaglia J, Cox W I, Paoletti E, Weinhold K J. Replication-defective canarypox (ALVAC) vectors effectively activate anti-human immunodeficiency virus-1 cytotoxic T lymphocytes present in infected patients: implications for antigen-specific immunotherapy. Blood. 1997;90:2406–2416. [PubMed] [Google Scholar]
- 8.Ferrari G, Humphrey W, McElrath M J, Excler J L, Duliege A M, Clements M L, Corey L C, Bolognesi D P, Weinhold K J. Clade B-based HIV-1 vaccines elicit cross-clade cytotoxic T lymphocyte reactivities in uninfected volunteers. Proc Natl Acad Sci USA. 1997;94:1396–1401. doi: 10.1073/pnas.94.4.1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gea-Banacloche J C, Migueles S A, Martino L, Shupert W L, McNeil A C, Sabbaghian M S, Ehler L, Prussin C, Stevens R, Lambert L, Altman J, Hallahan C W, de Quiros J C, Connors M. Maintenance of large numbers of virus-specific CD8+ T cells in HIV-infected progressors and long-term nonprogressors. J Immunol. 2000;165:1082–1092. doi: 10.4049/jimmunol.165.2.1082. [DOI] [PubMed] [Google Scholar]
- 10.Gotch F M, Nixon D F, Alp N, McMichael A J, Borysiewicz L K. High frequency of memory and effector gag-specific cytotoxic T lymphocytes in HIV-seropositive individuals. Int Immunol. 1990;2:707–712. doi: 10.1093/intimm/2.8.707. [DOI] [PubMed] [Google Scholar]
- 11.Goulder P J, Sewell A K, Lalloo D G, Price D A, Whelan J A, Evans J, Taylor G P, Luzzi G, Giangrande P, Phillips R E, McMichael A J. Patterns of immunodominance in HIV-1-specific cytotoxic T lymphocyte responses in two human histocompatibility leukocyte antigens (HLA)—identical siblings with HLA-A*0201 are influenced by epitope mutation. J Exp Med. 1997;185:1423–1433. doi: 10.1084/jem.185.8.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goulder P J R, Rowland-Jones A J, McMichael A J, Walker B D. Anti-HIV cellular immunity: recent advances toward vaccine design. AIDS. 1999;13(Suppl. A):S121–S136. [PubMed] [Google Scholar]
- 13.Gray C M, Lawrence J, Schapiro J M, Altman J D, Winters M A, Crompton M, Loi M, Kundu S K, Davis M M, Merigan T C. Frequency of class I HLA-restricted anti-HIV CD8+ T cells in individuals receiving highly active antiretroviral therapy (HAART) J Immunol. 1999;162:1780–1788. [PubMed] [Google Scholar]
- 14.Guo H, Jardetzky T, Garrett T, Lane W, Strominger J, Wiley D. Different length peptides bind to HLA-Aw68 similarly at their ends but bulge out in the middle. Nature. 1992;360:364–366. doi: 10.1038/360364a0. [DOI] [PubMed] [Google Scholar]
- 15.Hamann D, Baars P A, Rep M H, Hooibrink B, Kerkhof-Garde S R, Klein M R, van Lier R A. Phenotypic and functional separation of memory and effector human CD8+ T cells. J Exp Med. 1997;186:1407–1418. doi: 10.1084/jem.186.9.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jin X, Bauer D E, Tuttleton S E, Lewin S, Gettie A, Blanchard J, Irwin C E, Safrit J T, Mittler J, Weinberger L, Kostrikis L G, Zhang L, Perelson A S, Ho D D. Dramatic rise in plasma viremia after CD8+ T cell depletion in simian immunodeficiency virus-infected macaques. J Exp Med. 1999;189:991–998. doi: 10.1084/jem.189.6.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnson R P, Trocha A, Yang L, Mazzara G P, Panicali D L, Buchanan T M, Walker B D. HIV-1 gag-specific cytotoxic T lymphocytes recognize multiple highly conserved epitopes. Fine specificity of the gag-specific response defined by using unstimulated peripheral blood mononuclear cells and cloned effector cells. J Immunol. 1991;147:1512–1521. [PubMed] [Google Scholar]
- 18.Kaslow R A, Carrington M, Apploe R, Park L, Munoz A, Saah A J, Goedert J J, Winkler C, O'Brien S J, Rinaldo C, Detels R, Blattner W, Phair J, Erlich H, Mann D L. Influence of combinations of human major histocompatibility complex genes on the course of HIV-1 infection. Nat Med. 1996;2:405–411. doi: 10.1038/nm0496-405. [DOI] [PubMed] [Google Scholar]
- 19.Klein M R, van Baalen C A, Holwerda A M, Kerkhof Garde S R, Bende R J, Keet I P, Eeftinck-Schattenkerk J K, Osterhaus A D, Schuitemaker H, Miedema F. Kinetics of Gag-specific cytotoxic T lymphocyte responses during the clinical course of HIV-1 infection: a longitudinal analysis of rapid progressors and long-term asymptomatics. J Exp Med. 1995;181:1365–1372. doi: 10.1084/jem.181.4.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koup R A, Safrit J T, Cao Y, Andrews C A, McLeod G, Borkowsky W, Farthing C, Ho D D. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J Virol. 1994;68:4650–4655. doi: 10.1128/jvi.68.7.4650-4655.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lalvani A, Brookes R, Hambleton S, Britton W J, Hill A V, McMichael A J. Rapid effector function in CD8+ memory T cells. J Exp Med. 1997;186:859–865. doi: 10.1084/jem.186.6.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Larsson M, Jin X, Ramratnam B, Ogg G S, Engelmayer J, Demoitie M A, McMichael A J, Cox W I, Steinman R M, Nixon D, Bhardwaj N. A recombinant vaccinia virus-based ELISPOT assay detects high frequencies of Pol-specific CD8 T cells in HIV-1-positive individuals. AIDS. 1999;13:767–777. doi: 10.1097/00002030-199905070-00005. [DOI] [PubMed] [Google Scholar]
- 23.Latron F, Pazmany L, Morrison J, Moots R, Saper M A, McMichael A, Strominger J L. A critical role for conserved residues in the cleft of HLA-A2 in presentation of a nonapeptide to T cells. Science. 1992;257:964–967. doi: 10.1126/science.1380181. [DOI] [PubMed] [Google Scholar]
- 24.Lee P P, Yee C, Savage P A, Fong L, Brockstedt D, Weber J S, Johnson D, Swetter S, Thompson J, Greenberg P D, Roederer M, Davis M M. Characterization of circulating T cells specific for tumor-associated antigens in melanoma patients. Nat Med. 1999;5:677–685. doi: 10.1038/9525. [DOI] [PubMed] [Google Scholar]
- 25.Madden D, Gorga J, Strominger J, Wiley D. The three-dimensional structure of HLA-B27 at 2.1 Å resolution suggests a general mechanism for tight peptide binding to MHC. Cell. 1992;70:1035–1048. doi: 10.1016/0092-8674(92)90252-8. [DOI] [PubMed] [Google Scholar]
- 26.McMichael A J, O'Callaghan C A. A new look at T cells. J Exp Med. 1998;187:1367–1371. doi: 10.1084/jem.187.9.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McNeil A J, Yap P L, Gore S M, Brettle R P, McColl M, Wyld R, Davidson S, Weightman R, Richardson A M, Robertson J R. Association of HLA types A1-B8-DR3 and B27 with rapid and slow progression of HIV disease. QJM. 1996;89:177–185. doi: 10.1093/qjmed/89.3.177. [DOI] [PubMed] [Google Scholar]
- 28.Miyahira Y, Murata K, Rodriguez D, Rodriguez J R, Esteban M, Rodrigues M M, Zavala F. Quantification of antigen-specific CD8+ T cells using an ELISPOT assay. J Immunol Methods. 1995;181:45–54. doi: 10.1016/0022-1759(94)00327-s. [DOI] [PubMed] [Google Scholar]
- 29.Murali-Krishna K, Altman J D, Suresh M, Sourdive D J, Zajac A J, Miller J D, Slansky J, Ahmed R. Counting antigen-specific CD8 T cells: a reevaluation of bystander activation during viral infection. Immunity. 1998;8:177–187. doi: 10.1016/s1074-7613(00)80470-7. [DOI] [PubMed] [Google Scholar]
- 30.Musey L, Hughes J, Schacker T, Shea T, Corey L, McElrath M J. Cytotoxic-T-cell responses, viral load, and disease progression in early human immunodeficiency virus type 1 infection. N Engl J Med. 1997;337:1267–1274. doi: 10.1056/NEJM199710303371803. [DOI] [PubMed] [Google Scholar]
- 31.Nixon D F, Townsend A R, Elvin J G, Rizza C R, Gallwey J, McMichael A J. HIV-1 gag-specific cytotoxic T lymphocytes defined with recombinant vaccinia virus and synthetic peptides. Nature. 1988;336:484–487. doi: 10.1038/336484a0. [DOI] [PubMed] [Google Scholar]
- 32.Ogg G S, Jin X, Bonhoeffer S, Dunbar P R, Nowak M A, Monard S, Segal J P, Cao Y, Rowland-Jones S L, Cerundolo V, Hurley A, Markowitz M, Ho D D, Nixon D F, McMichael A J. Quantitation of HIV-1-specific cytotoxic T lymphocytes and plasma load of viral RNA. Science. 1998;279:2103–2106. doi: 10.1126/science.279.5359.2103. [DOI] [PubMed] [Google Scholar]
- 33.Ogg G S, Kostense S, Klein M R, Jurriaans S, Hamann D, McMichael A J, Miedema F. Longitudinal phenotypic analysis of human immunodeficiency virus type 1-specific cytotoxic T lymphocytes: correlation with disease progression. J Virol. 1999;73:9153–9160. doi: 10.1128/jvi.73.11.9153-9160.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rinaldo C, Huang X-L, Fan Z, Margolick J, Borowski L, Hoji A, Kalinyak C, McMahon D, Riddler S, Hildebrand W, Day R, Mellors J. Anti-HIV-1 CD8+ T-lymphocyte reactivity during combination antiretroviral therapy in HIV-1-infected patients with advanced immunodeficiency. J Virol. 2000;74:4127–4138. doi: 10.1128/jvi.74.9.4127-4138.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Riviere Y, McChesney M B, Porrot F, Tanneau-Salvadori F, Sansonetti P, Lopez O, Pialoux G, Feuillie V, Mollereau M, Chamaret S. Gag-specific cytotoxic responses to HIV type 1 are associated with a decreased risk of progression to AIDS-related complex or AIDS. AIDS Res Hum Retrovir. 1995;11:903–907. doi: 10.1089/aid.1995.11.903. [DOI] [PubMed] [Google Scholar]
- 36.Rosenberg E S, Billingsley J M, Caliendo A M, Boswell S L, Sax P E, Kalams S A, Walker B D. Vigorous HIV-1 specific CD4+ T cell responses associated with control of viremia. Science. 1997;278:1447–1450. doi: 10.1126/science.278.5342.1447. [DOI] [PubMed] [Google Scholar]
- 37.Schmittel A, Keilholz U, Scheibenbogen C. Evaluation of the interferon-gamma ELISPOT-assay for quantification of peptide-specific T lymphocytes from peripheral blood. J Immunol Methods. 1997;210:167–174. doi: 10.1016/s0022-1759(97)00184-1. [DOI] [PubMed] [Google Scholar]
- 38.Schmitz J E, Kuroda M J, Santra S, Sasseville V G, Simon M A, Lifton M A, Racz P, Racz-Tanner K, Dalesandro M, Scallon B, Ghrayeb J, Forman M A, Montefiori D C, Rieber E P, Letvin N L, Reimann K A. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science. 1999;283:857–860. doi: 10.1126/science.283.5403.857. [DOI] [PubMed] [Google Scholar]
- 39.Spiegel H M, Graham S O, DeFalcon E, Sheehy M E, Monard S, Haslett P A J, Gillespie G, Donahoe S M, Pollack H, Borkowsky W, McMichael A J, Nixon D F. Human immunodeficiency virus type 1 and cytomegalovirus-specific cytotoxic T lymphocytes can persist at high frequency for prolonged periods in the absence of circulating peripheral CD4+ T cells. J Virol. 2000;74:1018–1022. doi: 10.1128/jvi.74.2.1018-1022.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tan L C, Gudgeon N, Annels N E, Hansasuta P, O'Callaghan C A, Rowland-Jones S, McMichael A J, Rickinson A B, Callan M F. A re-evaluation of the frequency of CD8+ T cells specific for EBV in healthy virus carriers. J Immunol. 1999;162:1827–1835. [PubMed] [Google Scholar]
- 41.Tsomides T J, Aldovini A, Johnson R P, Walker B D, Young R A, Eisen H N. Naturally processed viral peptides recognized by cytotoxic T lymphocytes on cells chronically infected by human immunodeficiency virus type 1. J Exp Med. 1994;180:1283–1293. doi: 10.1084/jem.180.4.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tsomides T J, Walker B D, Eisen H N. An optimal viral peptide recognized by CD8+ T cells binds very tightly to the restricting class I major histocompatibility complex protein on intact cells but not to the purified class I protein. Proc Natl Acad Sci USA. 1991;88:11276–11280. doi: 10.1073/pnas.88.24.11276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zajac A J, Blattman J N, Murali-Krishna K, Sourdive D J, Suresh M, Altman J D, Ahmed R. Viral immune evasion due to persistence of activated T cells without effector function. J Exp Med. 1998;188:2205–2213. doi: 10.1084/jem.188.12.2205. [DOI] [PMC free article] [PubMed] [Google Scholar]