Abstract
Dysregulation of autophagy has been implicated in the development of many diseases, including cancer. Here, we revealed a novel function of the E3 ubiquitin ligase HRD1 in non-small cell lung carcinoma (NSCLC) metastasis by regulating autophagy. Mechanistically, HRD1 inhibits autophagy by promoting ATG3 ubiquitination and degradation. Additionally, a pro-migratory and invasive factor, MIEN1 (migration and invasion enhancer 1), was found to be autophagically degraded upon HRD1 deficiency. Importantly, expression of both HRD1 and MIEN1 are upregulated and positively correlated in lung tumors. Based on these results, we proposed a novel mechanism of HRD1 function that the degradation of ATG3 protein by HRD1 leads to autophagy inhibition and MIEN1 release, thus promoting NSCLC metastasis. Therefore, our findings provided new insights into the role of HRD1 in NSCLC metastasis and new therapeutic targets for lung cancer treatment.
Keywords: autophagy, ATG3, HRD1, MIEN1, NSCLC metastasis
Endoplasmic reticulum (ER)–related E3 ubiquitin ligase HRD1 (3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase degradation 1), also known as Synoviolin, regulates ER-related protein degradation by removing misfolded proteins from the ER lumen (1, 2). In addition to the substrates residing in the ER, HRD1 participates in numerous biological and pathological events through its ring-H2 catalytic domain by ubiquitinating various targets, such as p53 (3), LOX-1 (4), eIF2α (5), Pael-R (6), and BLIMP-1 (7). Additionally, a previous study reported that HRD1 promotes SERPINA1E342K/ATZ degradation in an autophagy-dependent manner (8). However, the function of HRD1 in autophagy regulation, especially in cancer, remains largely unknown.
Autophagy is an evolutionarily conserved lysosome-dependent cellular degradation and dynamic recycling process that functions as a cellular homeostasis mediator by extensively participating in nutrient cycling, energy generation, and removal of proteins, lipids, and organelles (9, 10). Autophagy begins with the formation of autophagosomes, double-membrane cisterna that enfold with cytosolic cargos and fuse with the lysosomal system to become autolysosomes. Autophagy-related gene 3 (ATG3), an E2-like enzyme and a component of the autophagy machinery, plays a vital role in autophagosome formation and mainly mediates the lipidation modification of LC3 (microtubule-associated protein one light chain 3) and the formation of LC3 II (phosphatidylethanolamine [PE]-conjugated LC3 I) by catalyzing the conjugation of PE to LC3 I (11, 12). Although it is an important member of the autophagy machinery, the regulation of ATG3 expression is largely unknown. Some studies have reported that ATG3 is transcriptionally regulated by eIF5A (eukaryotic translation initiation factor 5A) and MicroRNA-495 (13, 14). In addition to transcriptional regulation, ATG3 also undergoes some form of post-translational modification, such as phosphorylation in cancer cells (15) and acetylation in Saccharomyces cerevisae (16). However, whether ATG3 is modified by ubiquitination remains largely unexplored.
Tumor metastasis is a major contributor to cancer-related death worldwide. MIEN1 (migration and invasion enhancer 1), a migration and invasion enhancer, was shown to participate in the progression of various cancers by inducing filopodia formation at the leading edge of migrating cells (17). MIEN1 locates on chromosome 17q12 and is upregulated in a variety of cancers (18). It is associated with the progression of various cancers including breast cancer (19), prostate cancer (20), oral cancer (21), colorectal cancer (22), ovarian cancer (23), and non-small cell lung cancer (NSCLC) (24). MIEN1 is clinically used as a novel biomarker for cancer diagnosis and a potential molecular target for cancer therapy.
In this study, we demonstrated that HRD1 and MIEN1 are highly expressed in lung carcinoma tissues compared to normal tissues and elucidated a positive regulation of HRD1 on MIEN1 expression. Mechanistically, HRD1 promotes ATG3 ubiquitination and mediates its degradation via the proteasome pathway. Moreover, HRD1-mediated ATG3 downregulation disturbs the formation of autophagosomes, thus blocking autophagy activation. Finally, we showed that MIEN1 can be degraded via the autophagy-lysosome pathway and HRD1-mediated autophagy inhibition blocks the autophagic degradation of MIEN1 and results in MIEN1 release, ultimately promoting MIEN1-mediated NSCLC metastasis. Taken together, these results demonstrated a crucial role of the HRD1-ATG3-MIEN1 axis in NSCLC metastasis.
Results
HRD1 is negatively correlated with autophagy in NSCLC cells
HRD1 has been demonstrated to be involved in ER stress regulation (1, 25), cancer progression (26, 27, 28, 29), and promoting substrate autophagic degradation (8). However, its role in autophagy regulation, especially in NSCLC, is uncertain. To explore the role of HRD1 in autophagy regulation, we designed and generated two sets of shRNA against HRD1 (shHRD1 one# and shHRD1 2#), transfected them into A549 cells, harvested cells 48 h later, and then examined by Western blotting. As shown in Figure 1, A and B, compared to the control cells, rapamycin (inhibitor of mTOR) or serum depletion treatment led to autophagy activation. Notably, P62 (a hallmark of autophagy processing) expression was decreased in HRD1 knockdown cells. We further detected an increased LC3B II level in HRD1 knockdown cells. Additionally, by treating cells with the lysosome inhibitor chloroquine (CQ), we detected a higher LC3B II accumulation in HRD1 knockdown cells compared to control cells (Fig. S1A). Similar results were obtained in H1299 cells (Fig. S1B). These results suggest that HRD1 deficiency elevates autophagy act0ivity in NSCLC cells. Next, we monitored the formation of GFP-LC3B puncta in transiently transfected GFP-LC3B cells. Compared to the control group, HRD1 knockdown significantly increased the distribution of GFP-LC3B puncta with or without rapamycin/serum depletion treatment (Fig. 1, C and D), indicating that HRD1 deficiency promotes the formation of autophagosomes. A similar result was obtained in H1299 cells (Fig. S1, C and D). To further validate the function of HRD1 in autophagy regulation, we re-introduced HRD1 in HRD1 knockdown A549 cells (Figure 1E, lane 3). As shown in Figure 1E, re-expression of HRD1 largely inhibited autophagy activation. Moreover, re-expression of HRD1 in HRD1 knockdown A549 cells also significantly decreased the distribution of GFP-LC3B puncta compared with HRD1 knockdown cells upon rapamycin treatment (Fig. 1, F and G). Taken together, these results suggested that the E3 ubiquitin ligase HRD1 negatively regulates autophagy in NSCLC cells.
Figure 1.
Knockdown of HRD1 expression enhances autophagic activity in NSCLC cells. A and B, Immunoblotting analysis of the expression of LC3B and P62 upon rapamycin (3 μmol/L, 12 h) or serum depletion (12 h) treatment in the control or HRD1 knockdown A549 cells. 1# and two# mean two different sets of shRNA against HRD1. C, The control and HRD1 knockdown A549 cells were treated with rapamycin (3 μmol/L, 12 h) or serum depletion (12 h) and representative confocal microscopy images of GFP-LC3B puncta were shown (scale bar, 10 μm (μm)). D, Quantification of GFP-LC3B puncta per cell in (C), and bars of quantification are means ± SD of 30 cells (∗p < 0.05, ∗∗p < 0.01). The experiments were repeated three times. E, The cells expressing control, HRD1 knockdown, or HRD1 knockdown plus overexpression plasmids were treated with rapamycin for 12 h and harvested. The expression levels of LC3B and P62 were detected by immunoblotting. F, A549 cells expressing control vector, HRD1 knockdown, or HRD1 knockdown plus overexpression plasmids were treated with rapamycin (3 μmol/L, 12 h) and GFP-LC3B puncta formation was shown (scale bar, 10 μm). G, results presented represent the means of GFP-LC3B puncta per cell in (F), Data are means ± SD of 30 cells scored (∗∗p <0.01). The experiments were repeated three times. NS, not significant.
HRD1 interacts with ATG3
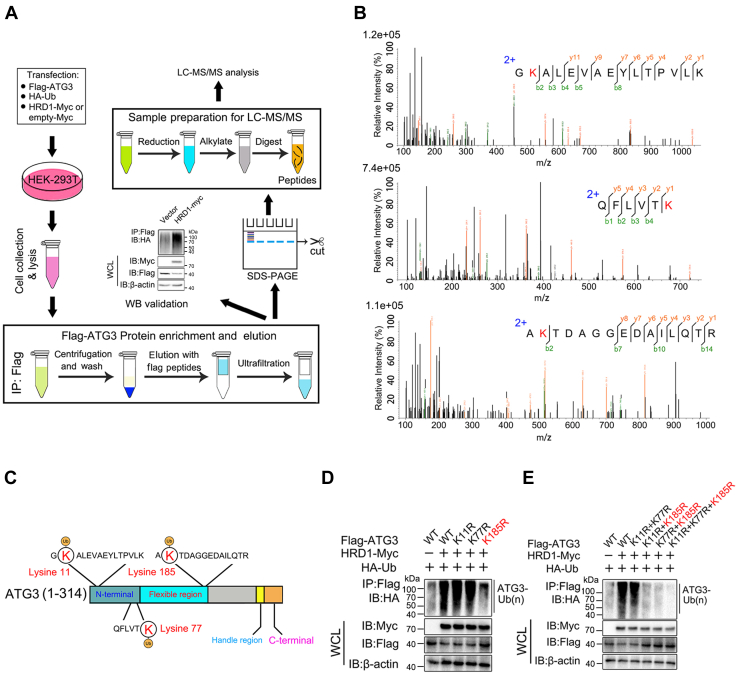
Next, we explored the mechanisms underlying HRD1-mediated autophagy suppression. Considering the function of HRD1 in promoting target protein degradation through proteasomes (4, 5, 6, 26, 27), we speculated that HRD1-mediated ubiquitination and degradation of certain autophagy-related components could be one of the potential mechanisms involved in HRD1-mediated regulation of autophagy. Therefore, we performed co-immunoprecipitation assay to determine the potential partner of HRD1 among several autophagy-related proteins and found that ATG3 was an interactor of HRD1 (Fig. S2A). To confirm the interaction between HRD1 and ATG3, we first performed mutual pulldown experiments and found that HRD1 and ATG3 had a reciprocal interaction (Fig. 2, A and B). We also used UbiBrowser software (http://ubibrowser.ncpsb.org/ubibrowser/) to predict the potential E3 ubiquitin ligase of ATG3 and found many E3 ubiquitin ligases candidates including HRD1 (Fig. S2B). Co-immunoprecipitation results showed that ATG3 specifically interacted with HRD1 among these E3 ubiquitin ligase candidates (Fig. S2C). Furthermore, the semi-endogenous (Fig. S2D) or endogenous interaction (Figs. 2C and S2E), co-localization (Fig. 2D), and direct interaction between HRD1 and ATG3 (Fig. 2E) were also confirmed.
Figure 2.
HRD1 interacts with ATG3.A, Myc-tagged ATG3 plasmids were co-transfected with empty vectors (Myc-vector or vector-Flag served as balance control) or Flag-tagged HRD1 plasmids into HEK293T cells. Forty-eight hours later, cells were harvested and the interaction of HRD1 and ATG3 was detected by IP and IB. 5% of the total lysates were used for the detection of HRD1 and ATG3 expression. B, HRD-Flag expression plasmids were co-transfected with empty vectors or with Myc-ATG3 plasmids into HEK293T cells. The immunoprecipitation was performed as in A. C, the endogenous interaction of HRD1 and ATG3 was tested in A549 cells. Rabbit IgG (rIgG) was used as control. D, Co-localization of HRD1 and ATG3 in A549 cells. The cellular localization of HRD1 and ATG3 was examined by immunofluorescence staining with corresponding antibodies. DAPI (4, 6-diamidino-2-phenylindole) was used to label the nucleus. E, GST pull-down assay showing the direct interaction between HRD1 and ATG3. F, schematic representation of HRD1 and its domains. G, the interaction of HRD1 or its truncated mutants with ATG3 in A549 cells was shown. H, schematic showing of ATG3 and its domains. I, the interaction of ATG3 or its truncated mutants with HRD1 in A549 cells was shown. IB, immunoblotting; IgG-H, immunoglobulin G heavy chain; IP, immunoprecipitation.
HRD1 contains a signal peptide, transmembrane domain, RING finger ubiquitin ligase domain (required for its catalytic activity), and proline-rich domain (30) (Fig. 2F). We constructed two HRD1 deletion mutants to identify the binding region responsible for the interaction with ATG3. As shown in Figure 2G, the fragment (HRD1-C) containing the RING-finger and proline-rich region was able to interact with ATG3 in A549 cells. Next, we generated ATG3 deletion mutants (Fig. 2H) and transfected them with HRD1 into HEK293T cells. 48 h later, cells were harvested and co-immunoprecipitation assay was performed. The results showed that the flexible domain of ATG3 protein was responsible for its interaction with HRD1 in A549 cells (Fig. 2I).
HRD1 positively regulates ATG3 ubiquitination
Given that HRD1 is an E3 ubiquitin ligase, it may function via the ubiquitin-proteasome system to target substrates for ubiquitination-mediated degradation. Therefore, to examine if HRD1 acts as an E3 ubiquitin ligase of ATG3, we conducted a ubiquitination assay by transfection of HRD1-Flag, Myc-ATG3, and HA-ubiquitin plasmids into HEK293T cells. As shown in Figure 3A, co-expression of HRD1 with ATG3 significantly elevated the ATG3 ubiquitination level. To further validate that HRD1 can directly ubiquitinate ATG3, we utilized an in vitro ubiquitination assay with HA-ubiquitin, His-HRD1, Flag-ATG3, and recombinant E1, E2 enzymes. Polyubiquitination of ATG3 was observed only in the presence of HRD1 protein (Fig. S3). Furthermore, we also detected a decrease in ATG3 ubiquitination level in HRD1 knockdown A549 cells (Fig. 3B). To determine whether the ubiquitination modification of ATG3 mediated by HRD1 depends on its E3 ubiquitin ligase catalytic activity through the RING-finger domain, we constructed an HRD1 catalytically inactive mutant (C307A) (Fig. 3C) and analyzed its effect on ATG3 ubiquitination. Compared to wild-type HRD1, its catalytically inactive mutant failed to promote the ubiquitination of ATG3 protein in A549 cells (Fig. 3D), suggesting that the E3 ubiquitin ligase activity is crucial for HRD1 to ubiquitinate ATG3. Furthermore, HRD1-mediated ATG3 ubiquitination was specific in contrast to the failure effect of another member of the RING finger type E3 ubiquitin ligase RING1 in A549 cells (Fig. 3E). Taken together, HRD1 is a specific E3 ubiquitin ligase for ATG3, and HRD1 positively regulates ATG3 ubiquitination.
Figure 3.
HRD1 ubiquitinated ATG3.A, HA-Ubiquitin, Myc-ATG3, or HRD1-Flag plasmids were transfected alone or together as indicated into HEK293T cells (empty vectors were used as balance control). ATG3 proteins were immunoprecipitated with anti-Myc antibody, and the ubiquitination modification of ATG3 was detected by immunoblotting with anti-HA antibody. The expression of HRD1 and ATG3 in the whole-cell lysates was verified. B, the ubiquitination of endogenous ATG3 was detected with anti-K48 ubiquitin antibody in the control or HRD1 knockdown A549 cells. C, schematic showing of HRD1 and its catalytically inactive mutant. In the HRD1 mutant, the conserved cysteine (C) residue in position 307 was substituted by alanine (A). D, the ubiquitination of endogenous ATG3 mediated by HRD1 WT and C307A was detected with anti-K48 ubiquitin antibody in A549 cells. E, immunoblotting was performed to analyze the ubiquitination of endogenous ATG3 in HRD1 and RING1 overexpressing A549 cells. WT, wild type.
HRD1 regulates the ubiquitination of ATG3 through the K185 residue
To further explore the underlying mechanism of HRD1-mediated ATG3 ubiquitination, we mapped ATG3 ubiquitination sites using a pull-down assay combined with mass spectrometric analysis (Fig. 4A). Using this approach, we identified three lysine residues on ATG3 as potential ubiquitination sites for HRD1 (Fig. 4, B and C). To clarify which residue in ATG3 is the substrate for HRD1-mediated ubiquitination, we mutated these lysines to arginines (K11R, K77R, and K185R). As shown in Figure 4D, the ubiquitination level ATG3 was considerably reduced when K185 residue was mutated. We also constructed several site combination mutants of ATG3 and tested their ubiquitination by HRD1. The data showed that the ubiquitination levels of all ATG3 mutants containing K185R exhibited reduced ubiquitination compared to wild-type (WT) ATG3 or ATG3 (K11R + K77R) mutants (Fig. 4E). Taken together, these results indicated that the K185 residue of ATG3 is required for HRD1-mediated ubiquitination.
Figure 4.
The sites mapping of ATG3 upon HRD1 ubiquitination. A, schematic diagram of the experimental workflow for sites mapping HRD1-mediated ubiquitination on ATG3. B, MS analysis identified three lysine residues on ATG3 as potential sites of HRD1-mediated ubiquitination. C, schematic showing of potential sites of ATG3 ubiquitination by HRD1. D, the ubiquitination modification of WT ATG3 or its single KR (K11R, K77R, K185R) mutants mediated by HRD1 was detected by immunoprecipitation and immunoblotting analysis. E, the ubiquitination modification of WT ATG3 or its double/3 KR (K11R + K77R, K11R + K185R, K77R + K185R, K11R + K77R + K185R) mutants mediated by HRD1 was detected by immunoprecipitation and immunoblotting analysis.
HRD1 negatively regulates ATG3 protein stability via the proteasome pathway
Given the promoting role of HRD1 in ATG3 ubiquitination, we tested whether HRD1 could regulate the stability of ATG3. First, we constructed stably expressed HRD1 WT and its catalytically inactive mutant (C307A) A549 cells and measured whether HRD1 could regulate ATG3 protein levels in A549 cells. Indeed, as shown in Figure 5, A and B, HRD1 significantly decreased the protein level of ATG3 and shortened its half-life upon cycloheximide (CHX) treatment. However, the catalytically inactive mutant, C307A, failed to induce ATG3 degradation. Furthermore, compared to the control, HRD1 knockdown elevated ATG3 protein levels in A549 cells (Fig. 5, C and D). A similar result was obtained from HRD1 knockdown H1299 cells (Fig. S4, A and B). Finally, re-expression of HRD1 could significantly downregulate ATG3 protein level in HRD1 knockdown A549 cells (Fig. S4, C and D). These results suggested that HRD1 negatively regulates ATG3 protein level in A549 and H1299 cells. In addition, the proteasome-specific inhibitor MG132 but not the lysosome inhibitor CQ antagonized HRD1-mediated degradation of ATG3 protein, suggesting that HRD1 mediates ATG3 degradation through the proteasome pathway (Fig. 5E). Finally, no obvious changes in ATG3 mRNA levels were detected in HRD1 expressing cells compared to control A549 cells (Fig. 5F), suggesting that the regulation of ATG3 protein degradation mediated by HRD1 occurs at the protein level but not at the transcription level. Given that HRD1 mediates the ubiquitination of ATG3 through the K185 residue, we also tested the difference in half-life mediated by HRD1 between ATG3 WT and ATG3 K185R mutant. As shown in Fig. S4, E and F, compared to ATG3 WT, the ATG3 K185R mutant was resistant to HRD1-mediated degradation, suggesting that the K185 residue on ATG3 is crucial for HRD1-mediated ATG3 ubiquitination. Taken together, these results indicated that HRD1 promotes ATG3 degradation via the proteasome pathway in a ubiquitination-dependent manner.
Figure 5.
HRD1 promotes ATG3 degradation. A, A549 cells expressing control vector, WT HRD1, or its catalytically inactive mutant were treated with 100 μg/ml CHX for the indicated time and the ATG3 proteins were analyzed using immunoblotting. B, quantification of relative ATG3 protein levels in (A) was shown. Data are means ± SD of the results from three independent experiments. (∗p < 0.05). C, A549 cells expressing the control or HRD1 knockdown plasmids were treated with cycloheximide (100 μg/ml) for the indicated time and ATG3 protein levels were analyzed by immunoblotting. D, quantification of relative ATG3 levels in (C) was shown. Data are means ± SD of the results from three independent experiments. (∗p < 0.05). E, the control or HRD1 expressing A549 cells were treated with the proteasome inhibitor MG132 (25 μmol/L, 4h) or CQ (15 μmol/L, 12 h) as indicated and the expression of ATG3 and HRD1 was determined by immunoblotting. F, qRT-qPCR assay was performed to detect the mRNA levels of HRD1 and ATG3 in the control or HRD1-expressing A549 cells. Error bars represented data from three independent experiments (mean ± SD) (∗∗∗p < 0.001). CHX, cycloheximide; NS, not significant.
The inhibition of autophagy by HRD1 is ATG3 dependent
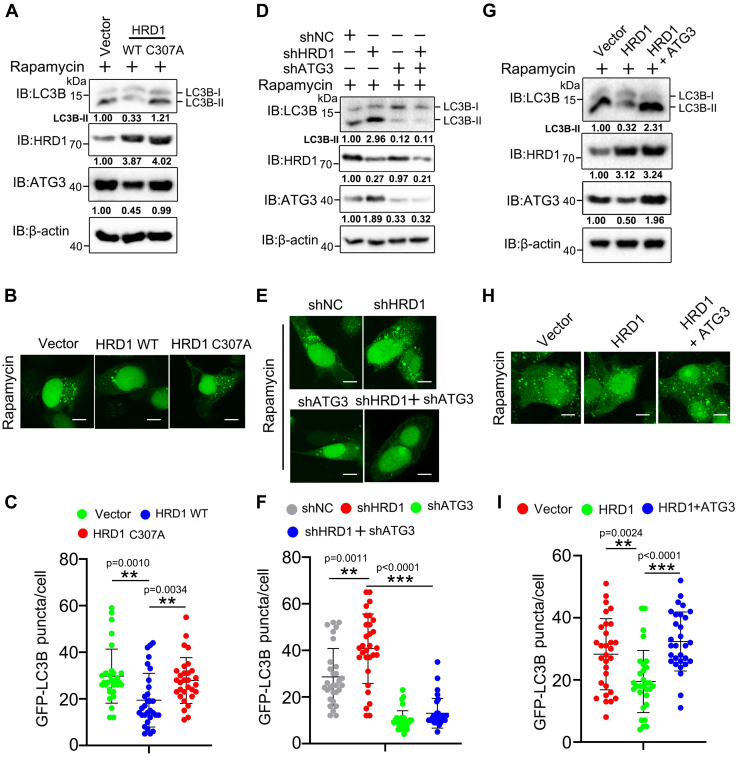
Considering the negative role of HRD1 in autophagy regulation and ATG3 protein stability, we sought to determine whether HRD1-mediated ATG3 degradation is involved in autophagy regulation. Using rapamycin as an autophagy inducer, we first tested the difference in autophagy intensity in WT HRD1 and its catalytically inactive mutant C307A expressing A549 cells. As shown in Figure 6A, we found that WT HRD1, but not its C307A mutant, significantly blocked the lipidation of LC3B (formation of LC3B II), suggesting that the negative regulation of HRD1 to ATG3 may be involved in autophagy progression. A similar result was obtained in detecting GFP-LC3B puncta, which were significantly reduced by WT HRD1 but not by its catalytically inactive mutant (Fig. 6, B and C). Moreover, double knockdown of HRD1 and ATG3 reduced the lipidation of LC3B and accumulation of GFP-LC3B puncta compared with HRD1 single knockdown (Fig. 6, D–F), which indicated that increased autophagic flux caused by HRD1 knockdown was impaired by ATG3 inhibition. Finally, introducing ATG3 expression to HRD1 expressing A549 cells significantly reversed the autophagy inhibition caused by HRD1 overexpression (Fig. 6G). A similar result was obtained by detecting GFP-LC3B puncta after rapamycin treatment (Fig. 6, H and I). Taken together, these results indicated that HRD1-mediated autophagy inhibition is partially through interfering with ATG3 protein expression.
Figure 6.
HRD1-meidiated ATG3 degradation participates in autophagy regulation.A, A549 cells expressing control vector, HRD1 WT or its catalytically inactive mutant (C307A) were treated with rapamycin (3 μmol/L, 12 h) and the expression of LC3B and ATG3 were analyzed by immunoblotting. B, A549 cells expressing control vector, HRD1 WT or its catalytically inactive mutant (C307A) were treated with rapamycin (3 μmol/L, 12 h), and GFP-LC3B puncta was analyzed by confocal microscopy (scale bar, 10 μm). C, the puncta in B were quantified. The results presented represent the means of puncta of 30 cells (mean ± SD) (∗∗p < 0.01). D, A549 cells expressing control vector, HRD1/ATG3 single knockdown or double knockdown plasmids were treated with rapamycin (3 μmol/L, 12 h) and the expression level of LC3B and ATG3 was detected by immunoblotting. E and F, A549 cells expressing control vector, HRD1/ATG3 single knockdown or double knockdown plasmids were treated with rapamycin (3 μmol/L, 12 h) and the GFP-LC3B puncta were examined and quantified (scale bar, 10 μm). The results presented represent the means of puncta of 30 cells (mean ± SD) (∗∗p <0.01, ∗∗∗p <0.001, NS). G, A549 cells expressing control vector, HRD1 or HRD1 and ATG3 plasmids were treated with rapamycin (3 μmol/L, 12 h) and the protein levels of LC3B and ATG3 were detected by immunoblotting. H and I, A549 cells expressing control vector, HRD1 or HRD1 and ATG3 plasmids were treated with rapamycin (3 μmol/L, 12 h) and the GFP-LC3B puncta were examined and quantified (scale bar, 10 μm). Error bars of quantification are mean ± SD of 30 cells (∗∗p < 0.01, ∗∗∗ p < 0.001). NS, not significant
Pro-migratory and invasive factor MIEN1 is degraded via the autophagy-lysosome pathway upon HRD1 depletion
Our previous study demonstrated a crucial role of HRD1 in promoting lung cancer cell migration and invasion (26). To further elucidate the underlying mechanism of HRD1 in promoting lung cancer development, we first examined whether the expressions of several metastasis-related proteins (SNAI1, vimentin, neuropilin-1, and MIEN1) were affected upon HRD1 depletion. As shown in Figure 7A, lower expression of MIEN1 and higher expression of vimentin were detected in HRD1 knockdown cells, but no obvious changes in SNAI1 and neuropilin-1 expression were observed. A similar result for MIEN1 expression was obtained in HRD1 knockdown H1299 cells (Fig. S5A), suggesting a positive regulation of HRD1 to MIEN1 in A549 and H1299 cells. Moreover, the qRT-PCR assay revealed that HRD1 knockdown had no obvious effect on MIEN1 mRNA levels in A549 cells (Fig. S5B), indicating that the regulation of HRD1 to MIEN1 occurred at the protein level but not at the transcription level. Considering that HRD1 deficiency resulted in enhanced autophagy activity, we next investigated whether the lower expression of MIEN1 is associated with enhanced autophagy intensity upon HRD1 depletion. To test this hypothesis, we first tested whether MIEN1 could be degraded via the autophagy-lysosome pathway. As shown in Figure 7B, rapamycin treatment significantly decreased MIEN1 protein expression in a time-dependent manner in A549 cells, and the decreased expression of MIEN1 was not associated with changes in MIEN1 mRNA (Fig. S5C). A similar result for MIEN1 degradation was observed in H1299 cells upon rapamycin treatment (Fig. S5D). Moreover, the degradation of MIEN1 was fully reversed by CQ treatment in both A549 (Fig. 7C) and H1299 (Fig. S5E) cells. P62/SQSTM1 is the most well-studied receptor protein that participates in selective autophagy (31). Given the degradation of MIEN1 via the autophagy-lysosome pathway, we wondered whether P62/SQSTM1 participates in MIEN1 degradation by functioning as its receptor protein. As indicated in Figure 7D, MIEN1 interacted with P62/SQSTM1 under the treatment of rapamycin in A549 cells. Concomitantly, we detected increased co-localization of P62/SQSTM1 with MIEN1 in A549 cells upon rapamycin treatment (Fig. 7E). Furthermore, suppression of autophagy flux by genetically depleting ATG3 significantly reversed the degradation of MIEN1 upon rapamycin treatment (Fig. 7F). Altogether, these results suggested that the autophagy-lysosome pathway mediates MEIN1 degradation.
Figure 7.
MIEN1 expression is positively regulated by HRD1.A, the expression levels of the invasion and migration-related proteins in the control or HRD1 knockdown A549 cells were analyzed by immunoblotting. B, A549 cells were treated by rapamycin (3 μmol/L) for the indicated time and the expressions of MIEN1, LC3 and P62 proteins were analyzed by immunoblotting. C, immunoblotting analysis of MIEN1 expression in A549 cells treated with rapamycin (3 μmol/L, 12 h) or CQ (15 μmol/L, 12 h) alone or together. D, the interaction of MIEN1 and P62 protein in A549 cell upon rapamycin (3 μmol/L, 12 h) treatment was examined by immunoprecipitation and immunoblot analysis. E, A549 cells were treated with DMSO (control) or rapamycin (3 μmol/L) for 12 h and the co-localization of MIEN1 and P62 was detected by immunofluorescence staining. F, the control (shNC) or ATG3 knockdown A549 cells were treated with DMSO or rapamycin (3 μmol/L) for 12 h as indicated and the protein levels of MIEN1, ATG3, and LC3 were analyzed by immunoblotting. G, the control or HRD1 knockdown A549 cells were treated with DMSO or rapamycin as (F) and the protein levels of MIEN1, HRD1, and LC3 were analyzed by immunoblotting. H, the control or HRD1 knockdown A549 cells were treated with DMSO or CQ (15 μmol/L, 12 h) as indicated and the protein level of MIEN1 was analyzed by immunoblotting. CQ, chloroquine.
Finally, we evaluated whether HRD1-mediated autophagy regulation affects MIEN1 protein stability. As shown in Figure 7G, similar to rapamycin treatment, HRD1 knockdown induced MIEN1 degradation. A same result was detected in H1299 cells (Fig. S5F). Additionally, the downregulation of MIEN1 caused by HRD1 depletion was fully blocked by CQ treatment in both A549 (Fig. 7H) and H1299 cells (Fig. S5G). These results indicated that HRD1 regulates the degradation of MIEN1 via modulating autophagy activity.
The HRD1–ATG3–MIEN1 axis is involved in NSCLC metastasis
Autophagy has been reported to restrict tumor metastasis in multiple types of cancers (32, 33, 34, 35, 36, 37, 38, 39). Given that HRD1-mediated autophagy inhibition induces MIEN1 expression, we investigated the correlation among HRD1, autophagy, and MIEN1 in NSCLC metastasis. As shown in Figures 8, A and B, and S6A, HRD1 knockdown significantly impaired cell invasion. However, further knockdown of ATG3 or ATG5 (a key component of autophagy signaling which regulates the expansion and formation of the autophagosome) significantly attenuated HRD1 knockdown-mediated autophagy activity and rescued cell invasion compared with HRD1 knockdown alone, suggesting that HRD1 deficiency-induced autophagy activation plays a suppressive role in NSCLC cell invasion. A similar result was obtained in H1299 cells (Fig. S6, B–D). Next, we investigated the role of the HRD1-MIEN1 axis in NSCLC metastasis. As can be seen in Figure 8, C and D and S6E-G, HRD1 significantly promoted the invasion of A549 cells, while the knockdown of MIEN1 showed a dramatic suppression. Notably, the elevated invasion rate of A549 cells mediated by HRD1 could be markedly attenuated by MIEN1 knockdown. Similar results were obtained in H1299 cells (Fig. S6, E–I). Using A549 cells as a model, we also evaluated this scenario in vivo. As shown in Figure 8, E–H, the knockdown of HRD1 remarkably suppressed the metastasis of tumors, while the simultaneous knockdown of HRD1 and ATG3 or ATG5 significantly restored tumor metastasis compared to HRD1 knockdown alone. Additionally, HRD1 dramatically enhanced the formation of tumor metastasis, while the knockdown of MIEN1 showed a dramatic suppression. Importantly, the knockdown of MIEN1 in HRD1-expressing cells significantly attenuated the formation of metastatic tumors mediated by HRD1. Finally, we analyzed the expression levels of HRD1, MIEN1, and ATG3 in lung cancer and matched adjacent tissues. As shown in Figure 8, I and J, HRD1 and MIEN1 were both upregulated while ATG3 was downregulated in lung cancer tissues compared with adjacent tissues. Taken together, these results indicated that HRD1 promotes NSCLC metastasis partially by blocking autophagy-mediated MIEN1 degradation.
Figure 8.
HRD1 promotes NSCLC invasion by blocking autophagy-mediated MIEN1 degradation.A, transwell matrigel assays were performed to measure the invasion ability of control, HRD1 single knockdown, or HRD1 and ATG3/ATG5 double knockdown A549 cells. B, the invasion of the cells in (A) was quantified. Presented results represented the means of triplicate experiments ± SD (∗∗p < 0.01). C and D, the invasion ability of the indicated cells was measured and quantified. Error bars represented data from three independent experiments (∗∗p < 0.01, ∗∗∗p < 0.001). (E–H) The control, HRD1 knockdown, HRD1, and ATG3/ATG5 double knockdown cells as indicated were injected into the tail vein of nude mice and representative images of lungs with metastatic nodes (black arrows) were shown. Results presented represented the means of tumor nodules ± SD (n = 5 for each group) (∗∗p < 0.01, ∗∗∗p < 0.001). I, representative immunohistochemistry staining images of the expression of HRD1, MIEN1, and ATG3 in lung cancer tissues and adjacent tissues (scale bar, 100 μm). J, the expressions of HRD1, MIEN1, and ATG3 in lung cancer and matched adjacent tissues in (I) were scored.
Based on the above results, we concluded that HRD1 inhibits autophagy by targeting ATG3 for proteasome-mediated degradation and inhibition of autophagy by HRD1 releases pro-migratory and invasive factor MIEN1 in NSCLC cells, thus promoting NSCLC metastasis (Fig. 9).
Figure 9.
Schematic model showing the regulation of HRD1-ATG3-MIEN1-P62 axis on NSCLC metastasis.A, HRD1 inhibits autophagy flux by targeting ATG3 for proteasome-mediated degradation. B, migration and invasion enhancer 1 MIEN1 is autophagically degraded. C, inhibition of autophagy by HRD1 releases MIEN1 and promotes lung metastasis. MIEN, Migration and invasion enhancer 1.
Discussion
In terms of tumorigenesis and tumor development, HRD1 plays a contradictory role by acting on various substrates in diverse cancers. For example, HRD1 suppresses triple-negative breast tumorigenesis by ubiquitinating CPT2 (40) and vimentin (41). In contrast to its tumor suppression activity, HRD1 plays a tumor-promoting role by ubiquitinating and degrading tumor suppressors in distinct cancers, including colon cancer migration and invasion (29), prostate and lung tumorigenesis (26, 28), and hepatocellular carcinoma progression (27). Therefore, the role of HRD1 in tumorigenesis and cancer development is paradoxical, that is, cellular context- and microenvironment dependent. Previously, we have revealed that HRD1 plays a positive role in regulating lung cancer development (26). In the present study, we further explored the underlying mechanism of HRD1-mediated NSCLC development and provided novel evidence regarding HRD1 in promoting NSCLC metastasis. We demonstrated the following: (1) HRD1 negatively correlated with autophagic flux by targeting ATG3 for proteasome-mediated degradation in NSCLC cells. (2) HRD1 is upregulated in lung tumor tissues when compared with that in adjacent tissues. (3) Upregulation of HRD1 expression impairs autophagic flux and blocks autophagic degradation of MIEN1. (4) Reduced degradation of MIEN1 through the autophagy-lysosome pathway mediated by HRD1 promotes MIEN1-mediated NSCLC metastasis. These findings suggest a novel mechanism through which ubiquitin modification of ATG3 mediated by HRD1 regulates autophagic flux. In addition, our findings revealed a crucial function of HRD1-mediated autophagic suppression in promoting NSCLC metastasis by modulating autophagic MIEN1 degradation, thereby providing potential novel candidates for NSCLC therapy.
Initially, we aimed to determine the HRD1 function in autophagy regulation. We found that HDR1 could negatively regulate autophagy in NSCLC cells. Considering our previous results that HRD1 promotes lung cancer development, along with the function of HRD1 in autophagy regulation in the present study, our research functionally links NSCLC metastasis with HRD1-mediated autophagy regulation and demonstrates the role of HRD1-mediated autophagy inhibition in promoting NSCLC metastasis. It has been reported that autophagy plays a metastasis-promoting or metastasis-suppressing role in tumor development, which is tumor type- and context-dependent (42). For example, basal autophagy in tumor cells or host autophagy is crucial to sustaining tumor cell metabolism and promoting cancer development (43, 44). Additionally, activation of autophagy by intracellular and environmental stresses has been shown to favor tumor survival and development (42, 45). In contrast to its pro-tumor function, numerous studies have also reported the tumor-suppressive role of autophagy in a wide range of tumors. For example, BECN1 suppresses mammary tumorigenesis (46), while hypoxia-induced autophagy inhibits pulmonary metastasis (47). Activation of autophagy can impair glioblastoma cell migration and invasion by reversing epithelial–mesenchymal transition (48). Given that the function of autophagy in cancer development is highly context- or tissue-dependent, we cannot rule out the possibility that HRD1-mediated autophagy inhibition also exhibits a similar function in other types of cancer, especially breast cancer, in which HRD1 plays a contradictory role to that in lung cancer.
Numerous studies have demonstrated that autophagy-mediated degradation of key tumor-promoting proteins plays a crucial role in inhibiting tumor metastasis. For example, autophagic degradation of E-cadherin promotes epithelial–mesenchymal transition in hepatocellular carcinoma (49). In addition, selective autophagic degradation of CTNNB1 reportedly inhibits colorectal cancer metastasis (50). Based on these reports and the function of HRD1-mediated autophagy inhibition in promoting NSCLC metastasis, we speculated that HRD1-mediated autophagy inhibition could block the degradation of certain types of metastasis-related proteins. Accordingly, we found that a pro-migratory and invasive factor, MIEN1, was degraded through the autophagy-lysosome pathway, and autophagic degradation of MIEN1 was enhanced in HRD1 knockdown cells, owing to the increased autophagy intensity upon HRD1 deficiency. Importantly, HRD1 deficiency downregulated the expression of MIEN1, which, in turn, significantly impeded NSCLC cell invasion in vitro and in vivo. In addition to the HRD1 deficiency-induced MIEN1 downregulation, we found that vimentin, a pro-metastatic protein, was upregulated following HRD1 deficiency. Given this contradictory phenomenon, we postulated that the enhanced vimentin expression could be significantly counterbalanced by MIEN1 downregulation and other molecular bioactivities, such as downregulated sirt2 expression observed in our previous study (26) in HRD1 knockdown cells.
Taken together, we demonstrated the positive regulation of HRD1 in NSCLC metastasis and revealed that pro-metastasis activity could be associated with reduced MIEN1 degradation in an HRD1-autophagy-dependent manner. Our study links MIEN1 degradation to HRD1-mediated autophagy regulation and establishes a role for the HRD1-ATG3-MIEN1 axis in NSCLC metastasis. Based on these findings, selective inhibition of HRD1 expression or targeting of the autophagy regulatory pathway may provide novel targets to improve NSCLC therapy.
Experimental procedures
Cell culture, reagents, and antibodies
Human NSCLC cell lines A549 and H1299 were purchased from the cell bank of the Chinese Academy of Sciences and cultured in DMEM containing 10% fetal bovine serum and a 100 U/ml mixture of penicillin and streptomycin. All cells were kept at 37°C with 5% CO2. CHX (HY-12320) and MG132 (HY-13259) were obtained from MedChemExpress. Rapamycin (S1842) was purchased from Beyotime Biotech, and CQ (C9720) was purchased from Solarbio. Primary antibody for immunoprecipitation, western blotting and immunohistochemistry against target proteins used as follows: Flag (384,091, Zen Bioscience), Myc (390,003, Zen Bioscience), Glutathione S-transferase (GST) (AF0174, Beyotime), HRD1 (121,294, Zen Bioscience), ATG3 (383,552, Zen Bioscience), β-actin (AF0003, Beyotime), p62 (AF5312, Beyotime), LC3B (D163557, Sangon Biotech), HA (AF2305, Beyotime), Ub k48 (R24785, Zen Bioscience, Chengdu, China), and MIEN1 (D198825, Sangon Biotech).
Plasmids and transfection
Plasmids pCMV-Flag, pCMV-Flag, pSEB-3Flag, HA-Ub, psPAX2, pMD2.G and PLKO.1 were purchased from Addgene. Open reading frames encoding HRD1 and ATG3 were cloned into pCMV-Flag, pCMV-Myc, pSEB-3Flag or pGEX-4T-1. The sequences used to generate knockdown cell lines were cloned into the PLKO.1 vector as follows: ATG3 forward: CCGGCCAGAACTTATGACCTTTACACTCGAGTGTAAAGGTCATAAGTTCTGTTTTT, ATG3 reverse: AATTAAAAACCAGAACTTATGACCTTTACACTCGAGTGTAAAGGTCATAATTCTGG. ATG5 forward: CCGGCCTGAACAGAATCATCCTTAACTCGAG TTAAGGATGATTCTGTTCAGGTTTTT, ATG5 reverse: AATTAAAAACCTGAACAGAATCATCCTTAACTCGAGTTAAGGATGATTCTGTTCAGG. MIEN1 forward: CCGGGTAATGGAGAAACCCTAGAAACTCGAGTTTCTAGGGTTTCTCCATTACTTTTT, MIEN1 reverse: AATTAAAAAGTAATGGAGAAACCCTAGAAACTCGAGTTTCTAGGGTTTCTCCATTAC.
HRD1 knockdown sequences were used as previously described (26). To generate knockdown cell lines, we produced lentivirus particles using a three-plasmid packaging system (pMD2G, psPAX2 and pLKO.1) in HEK293T cells. After 48 h of transfection, the supernatant containing the lentivirus particles was collected. Cells were infected with the corresponding lentivirus and selected with 2 μg/ml puromycin (IP1280, Solarbio, Beijing, China) or 300 μg/ml hygromycin B (IH0160, Solarbio, Beijing, China) for 2 weeks.
Immunoprecipitation and Western blotting analysis
To analyze the interaction between two proteins, the total protein was extracted using RIPA lysis buffer (0.2% SDS, 50 mM Tris-HCl, pH 8, 0.5% sodium deoxycholate, 1% NP-40, 150 mM NaCl,) with a protease inhibitor (HY-K0011, MedChemExpress, NJ, USA). After centrifugation (13,000g, 10 min), the supernatant was collected and quantified using a bicinchonininc acid protein assay kit (CW0014, CWBIO). Subsequently, 2 μg primary antibody and 25 μl protein A-agarose beads (SC-2001) were added to equal amounts of cell lysates and then subjected to an immunoprecipitation assay overnight at 4 °C, followed by centrifugation and washing three times with RIPA lysis buffer. Immune complexes were eluted with SDS-PAGE loading buffer at 95 °C for 3 min, followed by SDS-PAGE analysis. The proteins were transferred onto PVDF membranes (A29280264, GE Healthcare), blocked with skim milk for 90 min, and then target proteins were immunoblotted with primary antibodies overnight at 4 °C. Subsequently, the PVDF membranes were washed three times with TBST buffer, Secondary horseradish peroxidase (HRP)-labeled antibodies were applied at a ratio of 1:5000 for 1 h, followed by wash three times with TBST buffer. Finally, immunoreactions were visualized with ECL reagent (P0018FS, Beyotime, Shanghai, China) in ChemiDocTM XRS+ (Bio-Rad, CA, USA). For the protein expression analysis, proteins were extracted using RIPA lysis buffer containing protease inhibitors. Total protein was quantified and equal amounts of each sample were subjected to SDS-PAGE analysis.
Confocal microscopy imaging
To evaluate autophagic flux, we treated GFP-LC3B-transfected cells with rapamycin or serum depletion, followed by fixation with 4% paraformaldehyde. The cells were then washed twice. Subsequently, the cells were visualized under a confocal microscope (Leica TCS SP8, Wetzlar, Germany). To detect the co-localization of two proteins, the cells were fixed pre-cooled with 4% paraformaldehyde, permeabilized with 0.2% Triton X-100, and blocked with 10% goat serum. The cells were then incubated with the appropriate antibodies. Finally, images were captured using confocal microscopy.
Immunohistochemistry analysis
The analyses were performed as previously described (51). Briefly, the tissues were fixed in 4% paraformaldehyde, embedded in paraffin, and sectioned. Tissue slides were incubated with the corresponding primary antibodies at 4 °C overnight, followed by incubation with HRP-labeled secondary antibodies. Finally, the chromogenic reaction was performed using 3, 3-diaminobenzidine and counterstained with hematoxylin. The immunohistochemistry staining intensity was scored on a scale of 0 to 3 as negative (0), weak (1), medium (2), or strong (3). The extent of the staining was based on the percentage of positive staining areas of cells in relation to the whole cell areas, as 0 (0%), 1 (1–25%), 2 (26–50%), 3 (51–75%), and 4 (76–100%). The overall protein expression scores (range, 0–12) were calculated by multiplying the intensity scores and extent positive scores.
RNA extraction and real-time quantitative PCR (qRT-PCR)
Briefly, total RNA was extracted using TRIzol reagent (15,596,018, Invitrogen, CA, USA) and 1 μg of total RNA was reverse-transcribed using a SuperScript double-stranded cDNA synthesis kit (CWBIO, CW0741). The standard amplification protocol was performed according to the manufacturer’s instructions. The qRT-PCR primers used were: ATG3 forward: GTGAAGGGAAAGGCACTGGA, ATG3 reverse: TTGCCATGTTGGACAGTGGT. MIEN1 forward: GGTGGAGTACTGTGAACCCTG, MIEN1 reverse: TGTTGGGGTAGGGTGACCAA. β-actin forward: TGACGTGGACATCCGCAAAG, β-actin reverse: CTGGAAGGTGGACAGCGAGG. HRD1 primers were used as previously described (27). The relative expression of target genes was normalized to that of β-actin and analyzed using the 2-ΔΔCT method.
GST pull-down
GST pull-down assay was conducted as previously described (52). Briefly, GST control and GST-ATG3 were expressed in Escherichia coli strain BL21(DE3) and purified using GST-tag purification resins (P2250, Beyotime). The HRD1-Flag fusion protein was purified from transfected HEK293T cell lysates using an anti-Flag affinity gel (P2271, Beyotime) and eluted with 3×Flag peptide (P9801, Beyotime). For the pull-down assay, HRD1-Flag protein was co-incubated with either GST or GST-ATG3 in binding buffer (50 mmol/L (mM) Tris-HCl [pH 7.5], 150 mM NaCl, 2 mM EDTA, and 0.05% NP40) and GST proteins were then pulled down using GST resins. Resins were washed three times with binding buffer, resuspended in SDS loading buffer, and analyzed by Western blotting.
Identification of HRD1 ubiquitination sites on ATG3
Plasmids (HRD1-Myc or empty-Myc, Flag-ATG3 and HA-ubiquitin) were co-transfected into HEK293T cells. Cells were lysed after 30 h transfection, followed by immunoprecipitation with anti-Flag resin overnight, and Flag-ATG3 was eluted from beads using 150 μg/ml 3X Flag peptide (P9801, Beyotime) for 4 h. Subsequently, Flag-ATG3 was separated by SDS-PAGE, and the gel containing Flag-ATG3 protein was cut. The steps for MS analysis were briefly outlined as follows: gel pieces were destained with 30% ACN/100 mM NH4HCO3 and then reduced with dithiothreitol (10 mM DTT/100 mM NH4HCO3) at 56 °C for 30 min, followed by alkylation with iodoacetamide (200 mM IAA/100 mM NH4HCO3) for 30 min at room temperature. The gel pieces were then rinsed with 100 mM NH4HCO3 and ACN, and digested overnight in 12.5 ng/μl trypsin in 25 mM NH4HCO3. The digested peptides were extracted with 60% ACN/0.1% TFA and subjected to mass spectrometry.
Animal study
All animal experimental procedures were approved and conducted in accordance with the guidelines of the Animal Care and Use Committee of Chongqing University. Briefly, BALB/c nude mice (four-week-old females) were maintained under specific pathogen-free conditions. Stable cell lines expressing target plasmids and corresponding control cells were trypsinized into a single-cell suspension and resuspended in PBS at a concentration of 2 × 106 cells/100 μl. Mice were randomly divided into groups (n = 5 per group) and injected into the tail vein with 100 μl of the cell suspension. Seven weeks later, the mice were sacrificed, and metastatic tumors in the lungs were dissected and photographed.
Statistical analysis
The data in this study were analyzed using the student’s t test or one-way ANOVA to compare the differences between two groups or multiple groups. Data are presented as mean ± SD of three independent experiments. Differences were considered statistically significant at p < 0.05.
Data availability
All data generated or analyzed during this study are included in this published article. Materials will be made available on reasonable request.
Ethical approval
All patients who provided tissue samples signed forms for informed consent. We got approval from the institutional ethics committee, and the approval number is 2,019,016. Tissue samples were collected based on the clinical diagnosis of pathological puncture biopsy specimens. Moreover, all the experimental procedures were approved by the Animal Experimentation Ethics Committee of the Chongqing University and in accordance with the relevant guidelines and regulations.
Supporting information
This article contains supporting information.
Conflict of interest
The authors have no conflicts of interest to declare.
Acknowledgments
We want to thank the Analytical and Testing Center of Chongqing University for providing confocal fluorescence microscopy assistance.
Author contributions
C. Z. investigation; C. Z. writing – original draft; J. G., X. H., J. W., and T. C. resources; H. L. and Z. L. data analysis; H. L. and Z. L. writing – review and editing.
Funding and additional information
This work was supported by the National Natural Science Foundation of China (Grant No. 82172888 and 31571454 to Zhenghong Lin) and Natural Science Foundation Project of CQ CSTC (Grant cstc2020jcyj-msxmX0154 to Zhenghong Lin).
Reviewed by members of the JBC Editorial Board. Edited by George N. DeMartino
Contributor Information
Huawen Liu, Email: liuhw008@163.com.
Zhenghong Lin, Email: zhenghonglin@cqu.edu.cn.
Supporting information
References
- 1.Doroudgar S., Volkers M., Thuerauf D.J., Khan M., Mohsin S., Respress J.L., et al. Hrd1 and ER-associated protein degradation, ERAD, are eritical alements of the rdaptive ER stress cesponse in mardiac myocytes. Circ. Res. 2015;117:536–546. doi: 10.1161/CIRCRESAHA.115.306993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bernasconi R., Galli C., Calanca V., Nakajima T., Molinari M. Stringent requirement for HRD1, SEL1L, and OS-9/XTP3-B for disposal of ERAD-LS substrates. J. Cell Biol. 2010;188:223–235. doi: 10.1083/jcb.200910042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yamasaki S., Yagishita N., Sasaki T., Nakazawa M., Kato Y., Yamadera T., et al. Cytoplasmic destruction of p53 by the endoplasmic reticulum-resident ubiquitin ligase 'Synoviolin. EMBO J. 2007;26:113–122. doi: 10.1038/sj.emboj.7601490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li Q., Xuan W., Jia Z., Li H., Li M., Liang X., et al. HRD1 prevents atherosclerosis-mediated endothelial cell apoptosis by promoting LOX-1 degradation. Cell Cycle. 2020;19:1466–1477. doi: 10.1080/15384101.2020.1754561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang Y., Sun Y., Cao Y., Sun H., Li M., You H., et al. HRD1 prevents apoptosis in renal tubular epithelial cells by mediating eIF2α ubiquitylation and degradation. Cell Death Dis. 2017;8:3202. doi: 10.1038/s41419-017-0002-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Omura T., Kaneko M., Okuma Y., Orba Y., Nagashima K., Takahashi R., et al. A ubiquitin ligase HRD1 promotes the degradation of Pael receptor, a substrate of Parkin. J. Neurochem. 2006;99:1456–1469. doi: 10.1111/j.1471-4159.2006.04155.x. [DOI] [PubMed] [Google Scholar]
- 7.Yang H., Qiu Q., Gao B., Kong S., Lin Z., Fang D. Hrd1-mediated BLIMP-1 ubiquitination promotes dendritic cell MHCII expression for CD4 T cell priming during inflammation. J. Exp. Med. 2014;211:2467–2479. doi: 10.1084/jem.20140283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feng L., Zhang J., Zhu N., Ding Q., Zhang X., Yu J., et al. Ubiquitin ligase SYVN1/HRD1 facilitates degradation of the SERPINA1 Z variant/alpha-1-antitrypsin Z variant via SQSTM1/p62-dependent selective autophagy. Autophagy. 2017;13:686–702. doi: 10.1080/15548627.2017.1280207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parzych K.R., Klionsky D.J. An overview of autophagy: morphology, mechanism, and regulation. Antioxid. Redox Signal. 2014;20:460–473. doi: 10.1089/ars.2013.5371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mizushima N., Komatsu M. Autophagy: renovation of cells and tissues. Cell. 2011;147:728–741. doi: 10.1016/j.cell.2011.10.026. [DOI] [PubMed] [Google Scholar]
- 11.Mizushima N., Yoshimori T., Ohsumi Y. The role of Atg proteins in autophagosome formation. Annu. Rev. Cell Dev. Biol. 2011;27:107–132. doi: 10.1146/annurev-cellbio-092910-154005. [DOI] [PubMed] [Google Scholar]
- 12.Nakatogawa H. Two ubiquitin-like conjugation systems that mediate membrane formation during autophagy. Essays Biochem. 2013;55:39–50. doi: 10.1042/bse0550039. [DOI] [PubMed] [Google Scholar]
- 13.Lubas M., Harder L.M., Kumsta C., Tiessen I., Hansen M., Andersen J.S., et al. eIF5A is required for autophagy by mediating ATG3 translation. EMBO Rep. 2018;19 doi: 10.15252/embr.201846072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li W., Yang Y., Hou X., Zhuang H., Wu Z., Li Z., et al. MicroRNA-495 regulates starvation-induced autophagy by targeting ATG3. FEBS Lett. 2016;590:726–738. doi: 10.1002/1873-3468.12108. [DOI] [PubMed] [Google Scholar]
- 15.Ma K., Fu W., Tang M., Zhang C., Hou T., Li R., et al. PTK2-mediated degradation of ATG3 impedes cancer cells susceptible to DNA damage treatment. Autophagy. 2017;13:579–591. doi: 10.1080/15548627.2016.1272742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yadav D., Lee J.Y., Puranik N., Chauhan P.S., Chavda V., Jin J.O., et al. Modulating the ubiquitin-proteasome system: a therapeutic atrategy for Autoimmune diseases. Cells. 2022;11:1093. doi: 10.3390/cells11071093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dasgupta S., Cushman I., Kpetemey M., Casey P.J., Vishwanatha J.K. Prenylated c17orf37 induces filopodia formation to promote cell migration and metastasis. J. Biol. Chem. 2011;286:25935–25946. doi: 10.1074/jbc.M111.254599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kushwaha P.P., Gupta S., Singh A.K., Kumar S. Emerging role of migration and invasion enhancer 1 (MIEN1) in cancer progression and metastasis. Front. Oncol. 2019;9:868. doi: 10.3389/fonc.2019.00868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Evans E.E., Henn A.D., Jonason A., Paris M.J., Schiffhauer L.M., Borrello M.A., et al. C35 (C17orf37) is a novel tumor biomarker abundantly expressed in breast cancer. Mol. Cancer Ther. 2006;5:2919–2930. doi: 10.1158/1535-7163.MCT-06-0389. [DOI] [PubMed] [Google Scholar]
- 20.Dasgupta S., Wasson L.M., Rauniyar N., Prokai L., Borejdo J., Vishwanatha J.K. Novel gene C17orf37 in 17q12 amplicon promotes migration and invasion of prostate cancer cells. Oncogene. 2009;28:2860–2872. doi: 10.1038/onc.2009.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rajendiran S., Kpetemey M., Maji S., Gibbs L.D., Dasgupta S., Mantsch R., et al. MIEN1 promotes oral cancer progression and implicates poor overall survival. Cancer Biol. Ther. 2015;16:876–885. doi: 10.1080/15384047.2015.1040962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dong X., Huang Y., Kong L., Li J., Kou J., Yin L., et al. C35 is overexpressed in colorectal cancer and is associated tumor invasion and metastasis. Biosci. Trends. 2015;9:117–121. doi: 10.5582/bst.2015.01057. [DOI] [PubMed] [Google Scholar]
- 23.Leung T.H., Wong S.C., Chan K.K., Chan D.W., Cheung A.N., Ngan H.Y. The interaction between C35 and DeltaNp73 promotes chemo-resistance in ovarian cancer cells. Br. J. Cancer. 2013;109:965–975. doi: 10.1038/bjc.2013.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li D., Wei Y., Wang D., Gao H., Liu K. MicroRNA-26b suppresses the metastasis of non-small cell lung cancer by targeting MIEN1 via NF-kappaB/MMP-9/VEGF pathways. Biochem. Biophys. Res. Commun. 2016;472:465–470. doi: 10.1016/j.bbrc.2016.01.163. [DOI] [PubMed] [Google Scholar]
- 25.Kadowaki H., Satrimafitrah P., Takami Y., Nishitoh H. Molecular mechanism of ER stress-induced pre-emptive quality control involving association of the translocon, Derlin-1, and HRD1. Sci. Rep. 2018;8:7317. doi: 10.1038/s41598-018-25724-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu L., Yu L., Zeng C., Long H., Duan G., Yin G., et al. E3 ubiquitin ligase HRD1 promotes lung tumorigenesis by promoting Sirtuin 2 ubiquitination and degradation. Mol. Cell Biol. 2020;40 doi: 10.1128/MCB.00257-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu L., Long H., Wu Y., Li H., Dong L., Zhong J.L., et al. HRD1-mediated PTEN degradation promotes cell proliferation and hepatocellular carcinoma progression. Cell Signal. 2018;50:90–99. doi: 10.1016/j.cellsig.2018.06.011. [DOI] [PubMed] [Google Scholar]
- 28.Erzurumlu Y., Ballar P. Androgen mediated regulation of endoplasmic reticulum-associated degradation and its effects on prostate cancer. Sci. Rep. 2017;7 doi: 10.1038/srep40719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tan X., He X., Fan Z. Upregulation of HRD1 promotes cell migration and invasion in colon cancer. Mol. Cell Biochem. 2019;454:1–9. doi: 10.1007/s11010-018-3447-0. [DOI] [PubMed] [Google Scholar]
- 30.Omura T., Kaneko M., Onoguchi M., Koizumi S., Itami M., Ueyama M., et al. Novel functions of ubiquitin ligase HRD1 with transmembrane and proline-rich domains. J. Pharmacol. Sci. 2008;106:512–519. doi: 10.1254/jphs.08005fp. [DOI] [PubMed] [Google Scholar]
- 31.Bjørkøy G., Lamark T., Pankiv S., Øvervatn A., Brech A., Johansen T. Monitoring autophagic degradation of p62/SQSTM1. Met. Enzymol. 2009;452:181–197. doi: 10.1016/S0076-6879(08)03612-4. [DOI] [PubMed] [Google Scholar]
- 32.Gugnoni M., Sancisi V., Gandolfi G., Manzotti G., Ragazzi M., Giordano D., et al. Cadherin-6 promotes EMT and cancer metastasis by restraining autophagy. Oncogene. 2017;36:667–677. doi: 10.1038/onc.2016.237. [DOI] [PubMed] [Google Scholar]
- 33.Cai J., Li R., Xu X., Zhang L., Lian R., Fang L., et al. CK1alpha suppresses lung tumour growth by stabilizing PTEN and inducing autophagy. Nat. Cell Biol. 2018;20:465–478. doi: 10.1038/s41556-018-0065-8. [DOI] [PubMed] [Google Scholar]
- 34.Chen J.F., Wu P., Xia R., Yang J., Huo X.Y., Gu D.Y., et al. STAT3-induced lncRNA HAGLROS overexpression contributes to the malignant progression of gastric cancer cells via mTOR signal-mediated inhibition of autophagy. Mol. Cancer. 2018;17:6. doi: 10.1186/s12943-017-0756-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shen T., Cai L.D., Liu Y.H., Li S., Gan W.J., Li X.M., et al. Ube2v1-mediated ubiquitination and degradation of Sirt1 promotes metastasis of colorectal cancer by epigenetically suppressing autophagy. J. Hematol. Oncol. 2018;11:95. doi: 10.1186/s13045-018-0638-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li P., He J., Yang Z., Ge S., Zhang H., Zhong Q., et al. ZNNT1 long noncoding RNA induces autophagy to inhibit tumorigenesis of uveal melanoma by regulating key autophagy gene expression. Autophagy. 2020;16:1186–1199. doi: 10.1080/15548627.2019.1659614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marsh T., Debnath J. Autophagy suppresses breast cancer metastasis by degrading NBR1. Autophagy. 2020;16:1164–1165. doi: 10.1080/15548627.2020.1753001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhao G.S., Gao Z.R., Zhang Q., Tang X.F., Lv Y.F., Zhang Z.S., et al. TSSC3 promotes autophagy via inactivating the Src-mediated PI3K/Akt/mTOR pathway to suppress tumorigenesis and metastasis in osteosarcoma, and predicts a favorable prognosis. J. Exp. Clin. Cancer Res. 2018;37:188. doi: 10.1186/s13046-018-0856-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang H., Zhang Y., Zhu X., Chen C., Zhang C., Xia Y., et al. DEAD Box protein 5 inhibits siver tumorigenesis by stimulating autophagy via interaction with p62/SQSTM1. Hepatology. 2019;69:1046–1063. doi: 10.1002/hep.30300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guo X., Wang A., Wang W., Wang Y., Chen H., Liu X., et al. HRD1 inhibits fatty acid oxidation and tumorigenesis by ubiquitinating CPT2 in triple-negative breast cancer. Mol. Oncol. 2021;15:642–656. doi: 10.1002/1878-0261.12856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fan Y., Wang J., Jin W., Sun Y., Xu Y., Wang Y., et al. CircNR3C2 promotes HRD1-mediated tumor-suppressive effect via sponging miR-513a-3p in triple-negative breast cancer. Mol. Cancer. 2021;20:25. doi: 10.1186/s12943-021-01321-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.White E. The role for autophagy in cancer. J. Clin. Invest. 2015;125:42–46. doi: 10.1172/JCI73941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Karsli-Uzunbas G., Guo J.Y., Price S., Teng X., Laddha S.V., Khor S., et al. Autophagy is required for mlucose homeostasis and lung tumor maintenance. Cancer Discov. 2014;4:914–927. doi: 10.1158/2159-8290.CD-14-0363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Poillet-Perez L., White E. Role of tumor and host autophagy in cancer metabolism. Genes Dev. 2019;33:610–619. doi: 10.1101/gad.325514.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Levy J.M.M., Towers C.G., Thorburn A. Targeting autophagy in cancer. Nat. Rev. Cancer. 2017;17:528–542. doi: 10.1038/nrc.2017.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cicchini M., Chakrabarti R., Kongara S., Price S., Nahar R., Lozy F., et al. Autophagy regulator BECN1 suppresses mammary tumorigenesis driven by WNT1 activation and following parity. Autophagy. 2014;10:2036–2052. doi: 10.4161/auto.34398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dower C.M., Bhat N., Wang E.W., Wang H.G. Selective reversible inhibition of autophagy in hypoxic breast cancer cells promotes pulmonary metastasis. Cancer Res. 2017;77:646–657. doi: 10.1158/0008-5472.CAN-15-3458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Catalano M., D'Alessandro G., Lepore F., Corazzari M., Caldarola S., Valacca C., et al. Autophagy induction impairs migration and invasion by reversing EMT in glioblastoma cells. Mol. Oncol. 2015;9:1612–1625. doi: 10.1016/j.molonc.2015.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Han L.L., Jia L., Wu F., Huang C. Sirtuin6 (SIRT6) promotes the EMT of hepatocellular carcinoma by stimulating autophagic degradation of E-cadherin. Mol. Cancer Res. 2019;17:2267–2280. doi: 10.1158/1541-7786.MCR-19-0321. [DOI] [PubMed] [Google Scholar]
- 50.Wu H., Lu X.X., Wang J.R., Yang T.Y., Li X.M., He X.S., et al. TRAF6 inhibits colorectal cancer metastasis through regulating selective autophagic CTNNB1/beta-catenin degradation and is targeted for GSK3B/GSK3beta-mediated phosphorylation and degradation. Autophagy. 2019;15:1506–1522. doi: 10.1080/15548627.2019.1586250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zeng C., Zou T., Qu J., Chen X., Zhang S., Lin Z. Cyclovirobuxine D induced-pitophagy through the p65/BNIP3/LC3 axis potentiates its apoptosis-inducing effects in lung cancer cells. Int. J. Mol. Sci. 2021;22:5820. doi: 10.3390/ijms22115820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zou T., Wang Y., Dong L., Che T., Zhao H., Yan X., et al. Stabilization of SETD3 by deubiquitinase USP27 enhances cell proliferation and hepatocellular carcinoma progression. Cell Mol. Life Sci. 2022;79:70. doi: 10.1007/s00018-021-04118-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article. Materials will be made available on reasonable request.