Abstract
Objective:
CD4 T cells are important regulators of atherosclerotic progression. The metabolic profile of CD4 T cells controls their signaling and function, but how atherosclerosis affects T cell metabolism is unknown. Here, we sought to determine the impact of atherosclerosis on CD4 T cell metabolism and the contribution of such alterations to atheroprogression.
Approach and Results:
Using PCR arrays, we profiled the expression of metabolism genes in CD4 T cells from atherosclerotic, apolipoprotein-E knockout (ApoE−/−) mice fed a Western diet (WD). These cells exhibited dysregulated expression of genes critically involved in glycolysis and fatty acid degradation, compared to those from animals fed a standard laboratory diet. We examined how T cell metabolism was changed in standard laboratory diet or WD-fed ApoE−/− mice or humans by measuring glucose uptake, activation, and proliferation in CD4 T cells. We found that naive CD4 T cells from WD-fed ApoE−/− mice failed to uptake glucose and thus, displayed impaired proliferation and activation, compared to CD4 T cells from standard laboratory diet-fed animals. Similarly, as in mice, we observed that naive CD4 T cell frequencies were reduced in circulation of human subjects with high CVD compared to low CVD, as assessed clinically based on medically necessary coronary angiography. Naive T cells from high CVD subjects also showed reduced proliferative capacity.
Conclusion:
These results highlight the dysfunctional changes that occur in CD4 T cell metabolism and immune responses during atherosclerosis. Targeting metabolic pathways within naive CD4 T cells could thus yield novel therapeutic approaches for improving CD4 T cell responses against atheroprogression.
Keywords: Atherosclerosis, T cells, glucose, metabolism, function
Introduction
Atherosclerosis is a chronic inflammatory disease characterized by lipid-laden plaques filled with monocytes, monocyte-derived cells, and T cells on arterial walls. CD4 T cells are themselves divided into distinct subsets, with each subpopulation displaying unique impacts on atheroprogression1–3. For example, naive CD4 T cells in the blood are inversely correlated with subclinical atherosclerosis, while memory CD4 T cells are positively correlated4. Additionally, helper-1 (Th1), IFNγ-producing CD4 T cells typically worsen atherosclerosis5, 6, whereas Foxp3+, regulatory T (Treg) cells protect against the disease7–9. CD4 T cell subsets are dynamic populations that can switch between one another, depending on their antigenic and cytokine milieu10. Indeed, IFNγ+ Treg cells have been described in atherosclerosis11 and our laboratory has shown that certain Treg cells can switch to T follicular helper cells12, which are pro-atherogenic. Still, little is known about the specific contributions of naive CD4 T cells to atherosclerosis progression.
A rapidly expanding body of evidence indicates that the metabolic state of CD4 T cells impacts their responses and contributions to disease progression13. CD4 T cells rely on oxidative phosphorylation (OXPHOS) to efficiently generate ATP14–16 but switch to aerobic glycolysis for more rapid ATP generation upon activation17, 18. Aerobic glycolysis and OXPHOS are both required for proper CD4 T cell proliferation and survival, while the former is also required for optimal IFNγ production19. Inhibition of acetyl-coA carboxylase (ACC1), a key enzyme in fatty acid synthesis, causes inflammatory T cells to switch to Treg cells20, 21. Importantly, both mouse models of obesity and obese human patients show changes in fatty acid metabolism that favor the production of inflammatory T cells over Treg cells21. In contrast, the glucose sensor AMP-activated protein kinase (AMPK) regulates the mitochondrial energetics of activated T cells and can mediate the treatment of inflammatory bowel disease via metformin by decreasing the inflammatory T cell/Treg cell ratio22, 23.
While the aforementioned studies reveal that metabolic reprogramming alters the fate, functions, and responses of CD4 T cells, little is known about the metabolic profiles of CD4 T cells in atherogenesis. In this study, we sought to examine the metabolic state of CD4 T cells during atherosclerosis progression and to assess how CD4 T cell metabolism impacts atherosclerosis.
Material and Methods
The authors declare that all supporting data are available within this article and the accompanying online supplementary files.
Human Samples:
Peripheral blood mononuclear cells (PBMCs) were isolated from blood of donors undergoing medically necessary cardiac catheterization at the Cardiac Catheterization Laboratory at the University of Virginia, Charlottesville24, 25. Enrolled participants included males and females of different ages and informed consent was obtained from all subjects. CVD subject characteristics are detailed in Table 1. Studies were performed with the approval of the Institutional Review Board of Health Science Research at the University of Virginia. PBMCs were isolated from whole blood with Ficoll-Paque™PLUS (GE Healthcare) and SepMate™ tubes (STEMCELL Technologies, Inc.). PBMCs were frozen in 10% DMSO in FCS until used for CyTOF analysis or in vitro culture assays.
Table 1 –
CVD Subject Characteristics
| Individual Characteristics | Low CVD (n=14) | High CVD (n=14) |
|---|---|---|
| General Characteristics | ||
| Male (%) | 50% (7 of 14) | 71 4% (10 of 14) |
| Age (years) | 57.57 (10.07) | 66.07 (7.81) |
| Weight (lbs) | 189.90 (20.5) | 204.90 (49.80) |
| BMI* | 30.14 (5.968) | 30.03 (4.081) |
| Statins | 71.4% (10 of 14) | 71.4% (10 of 14) |
| Diabetes | 3 of 14 (21.42%) | 4 of 14 (28.57%) |
| BP Systolic (mmHG) | 122.4 (16.90) | 137.0 (15.75) |
| BP Diastolic (mmHG) | 75.86 (11.04) | 70.71 (11.01) |
| Immune Parameters | ||
| hsCRP (mg/L)‡ | 5.159 (8.962) | 3.809 (4.156) |
| WBC (k/μL)§ | 6.70 (1.90) | 5.66 (1.12) |
| Hgb (k/μL)ǁ | 13.09 (1.216) | 13.26 (1.418) |
| Platelets (k/μL) | 230.4 (69.39) | 202.8 (52.31) |
| Lipids | ||
| Total Cholesterol (mg/dl) | 164.5 (37.36) | 173.1 (53.2) |
| Triglycerides (mg/dl) | 93.9 (57.74) | 132.5 (74.64) |
| HDL Cholesterol (mg/dl)# | 46.36 (17.44) | 40.07 (12.97) |
| LDL Cholesterol (mg/dl)** | 102.7 (27.29) | 111.1 (12.25) |
| Disease Severity | ||
| Gensini Score | 3.75 (3.58) | 42.71 (9.89) |
| Immune Compartment | ||
| Lymphocytes (k/μL) | 1.55 (0.49) | 1.67 (0.59) |
| Monocytes (k/μL) | 0.63 (0.16) | 0.51 (0.22) |
| Neutrophils (k/μL) | 4.31 (1.52) | 3.35 (0.56) |
| Basophils (k/μL) | 0.032 (0.017) | 0.03 (0.012) |
All variables are indicated as mean±SD
BMI=body mass index
BP= blood pressure
CRP= C-reacbve protein
WBC=white blood cells
Hgb = hemoglobin
HDL = high density lipoprotein.
LDL = low density lipoprotein
Mice:
Apolipoprotein-E knockout (ApoE−/−) mice (on a C57BL/6J background) were purchased from The Jackson Laboratory (Bar Harbor, Maine; Stock number 002052). Mice were used for experiments at 6 weeks of age and were fed a Western diet (WD; 42% from fat, 0.2% from cholesterol) (Harlan, #TD 88137, Placentia, CA) for up to 20 weeks. Control (C57BL/6J) animals were fed a standard laboratory diet containing 0% cholesterol and 5% calories from fat (Pico lab, #5053, Saint Louis, MO). All mice were housed in microisolator cages in a pathogen free animal facility at La Jolla Institute for Immunology (LJI). Due to the large variation within metabolic processes, these experiments were performed by arbitrarily choosing male mice. All experiments followed the LJI Animal Care and Use Committee guidelines and all studies were approved according to criteria outlined in the Guide for the Care and Use of Laboratory Animals from the National Institutes of Health.
Mass Cytometry:
Antibodies used for mass cytometry are shown in Table 2. Directly conjugated antibodies were purchased from Fluidigm and purified antibodies were ordered from the companies listed in Table 2. Conjugations were performed with the Maxpar X8 Multi-Metal Labeling Kit (Fluidigm) according to the manufacturer’s instructions. Samples were stained for CyTOF mass cytometry, as described previously25, 26, and stained with the antibody cocktail shown in Table 2. Prior to surface staining, one low CVD sample and one high CVD sample, in addition to a staining healthy control, were barcoded with CD45 (low CVD-CD45–89Y; High CVD-CD45–147Sm; Healthy-CD45–115Ln). Cells were resuspended at 2×106 cells/mL in 0.1mLxEQ™ Four Calibration Beads (Fluidigm). Batch effects and signal drifting were minimized by tuning every 6hrs during long runs. Data was normalized using the Matlab-based NormalizerR2013a_Win64.
Table 2 –
CyTOF Antibodies
| Antigen | Clone | Metal Conjugate | Manufacturer | Conjugation |
|---|---|---|---|---|
| CD45 | HI30 | 89Y | DVS | DVS |
| CD45 | HI30 | 115ln | Biolegend | In-house |
| CCR6 | G034E3 | 141Pr | DVS | DVS |
| CD19 | H1B19 | 142Nd | DVS | DVS |
| CD127 | A019D5 | 143Nd | DVS | DVS |
| CD69 | FN50 | 144Nd | DVS | DVS |
| CD4 | RPA-T4 | 145Nd | DVS | DVS |
| CD8 | RPA-T8 | 146Nd | DVS | DVS |
| CD45 | HI30 | 147Sm | Biolegend | In-house |
| CD14 | RM052 | 148Nd | DVS | DVS |
| CD45RO | UCHL1 | 149Sm | DVS | DVS |
| CD107a | H4A3 | 151Eu | DVS | DVS |
| EOMES | WD1928 | 152Sm | DVS | DVS |
| CD45RA | 205410 | 153Eu | DVS | DVS |
| CD3 | UCHT1 | 154Sm | DVS | DVS |
| PD-1 | EH12.2H7 | 155Gd | DVS | DVS |
| CXCR3 | G025H7 | 156Gd | DVS | DVS |
| CD27 | L128 | 158Gd | DVS | DVS |
| CD39 | A1 | 160Gd | DVS | DVS |
| T-bet | 4B10 | 161 Dy | DVS | DVS |
| GITR | 621 | 162Dy | DVS | DVS |
| Bcl-6 | K11291 | 163Dy | DVS | DVS |
| CD95 | Dx2 | 164Dy | DVS | DVS |
| Foxd3 | PCH101 | 165HO | ThermoFisher | In-house |
| TIGIT | MBSA43 | 166Er | DVS | DVS |
| CCR7 | G043H7 | 167Er | DVS | DVS |
| ICOS | C398.4A | 168Er | DVS | DVS |
| CD25 | 2A3 | 169Tm | DVS | DVS |
| CTLA-4 | 14D3 | 170Er | DVS | DVS |
| CXCR5 | RFBB | 171Yb | DVS | DVS |
| Ki67 | BL168 | 172Yb | DVS | DVS |
| HLA-DR | L243 | 174Yb | DVS | DVS |
| CCR4 | 205410 | 175Lu | DVS | DVS |
| CD57 | HCD57 | 176Yb | DVS | DVS |
| CD11b | ICRF44 | 209Bi | DVS | DVS |
Flow cytometry:
Peri-aortic lymph nodes, peripheral lymph nodes, and spleens harvested from mice were passed through a 40 μm cell strainer and washed with ice cold PBS. Antibodies were purchased from Ebioscience or Biolegend (San Diego, CA). Samples were stained with LIVE/DEAD fixable dye (Life Technologies, Carlsbad, CA). Human CD4 T cells were stained with antibodies against CD4 (clone RPA-T4), CD3 (clone HIT3a), CCR7 (G01), and CD45RA. Mouse cells were stained with antibodies against CD4 (clone RM4–4), TCRβ (clone H57–597), CD44 (Clone IM-7), CD25 (clone PC61), CD69 (clone H1.2F3), and/or PD-1 (clone J43) in cold FACS buffer (2% BSA, 0.01% sodium azide in PBS). All stains were carried out for 30 minutes on ice. For 2-NBDG staining, stimulated CD4 T cells were washed and incubated in glucose free media for 1 hour. 2-NBDG (100 M; ThermoFisher) was added for 30 minutes at 37°C. Cells were then washed and stained as described above. For Glut 1 staining, stimulated CD4 T cells were stained as described above, fixed, permeabilized and stained with a rabbit anti-glut1 (Novus Biologicals), followed by a secondary goat anti-rabbit AF647. For mitochondrial potential, JC1 mitoprobe (Thermofisher) was added to stimulated CD4 T cells for 15 minutes at 37°C. Cells were then washed with PBS and stained as described above. For FCCP control wells, stimulated CD4 T cells were treated with FCCP (50 mM) for 5 minutes. For apoptosis measurements, cells were first stained for surface markers then subsequently stained for Annexin V and 7AAD, using the Apoptosis Detection Kit I (BD biosciences) according to manufacturer’s instructions. Events were acquired using LSRII (BD, Bioscience, San Diego, CA) and data were analyzed using FlowJo Ver 9.8 and 10.0.08 (Tree Star, Ashland, OR).
In vitro CD4 T cell stimulation:
For human samples, naive CD4 T cells were isolated from previously frozen PBMCs using the EasySep™ Human Naive T cell isolation kit II (STEMCELL Technologies, Inc., Vancouver, Canada) according to the manufacturer’s instructions. Naive CD4 T cells were then stimulated with Dynabeads™ Human T-Activator CD3/CD28 (ThermoFisher Scientific) according to the recommended concentration for 2 to 3 days. Recombinant human IL-2 (Peprotech) was added at a final concentration of 100 IU/ml. For mouse samples, naive CD4 T cells were isolated from spleens and lymph nodes of standard laboratory diet- and WD-fed ApoE−/− mice using the EasySep™ Mouse Naive CD4 T cell isolation kit (STEMCELL Technologies, Inc.). Naive CD4 T cells were stimulated in αCD3-coated plates (2 μg/ml) and soluble αCD28 (1 μg/ml) was added to these wells for 2 days. All cells were cultured in RPMI with 10% FCS, L-glutamine, penicillin and streptomycin, and β-mercaptoethanol (for mouse cells). For the proliferation assays, isolated naive CD4 T cells were labeled with CTV (ThermoFisher) according to the manufacturer’s instructions, prior to stimulation. The percentage of cells divided was calculated using FlowJo v. 10.0.08 (Tree Star, Ashland, OR).
Intracellular staining:
CD4 T cells were stimulated with PMA and Ionomycin (50 ng/ml and 1 μg/ml, respectively) (Sigma-Aldrich) for 5 hrs in the presence of GolgiPlug (BD Biosciences) at 1 μl/ml. Cells were then stained for CD4, TCRβ, CD44, and CD25 as described above. Afterwards, cells were fixed and permeabilized using the eBioscience™ Foxp3/transcription factor staining kit (ThermoFisher). Cells were then stained with antibodies against IFNγ (clone XMG-1.2) and Tbet (clone ebio4B10).
Seahorse Assay:
Equal numbers of stimulated CD4 T cells were plated in glucose free media (Seahorse Biosciences) containing glutamine (2mM), with or without glucose (11 mM), for OCR and ECAR measurements at basal conditions. 4×105 cells per well were utilized for these experiments. Oligomycin (1 μM), FCCP (1 μM), and 2-Deoxyglucose (100 nM) were added at the indicated time points. Extracellular acidification rates (ECARs) and oxygen consumption rates (OCRs) were recorded using a Seahorse XF-96 machine according to the manufacturer’s instructions. Compounds were bought from Seahorse Biosciences or Sigma. Each ECAR or OCR measurement is an average of at least three wells.
Glucose Consumption Assay:
Stimulated CD4 T cells were washed with glucose free media. Glucose (1 mg/ml; Sigma) was added and cells were incubated for 4 hours. Supernatant was taken and tested for the levels of remaining glucose using a glucose and sucrose assay kit (Sigma) according to the manufacturer’s instructions.
Fatty Acid Oxidation Assay:
Oxidation of exogenous free fatty acids was assayed utilizing the XF Palmitate-BSA FAO substrate with the XF cell mito stress kit according to the manufacturer’s protocol (Seahorse Bioscience). Negatively selected naive CD4 T cells (4×105 cells) were incubated for 30 minutes with fatty acid oxidation assay medium (111mM NaCl, 4.7mM KCl, 1.25mM CaCl2, 2.0mM MgSO4, 1.2mM Na2HPO4, 2.5mM glucose, 0.5mM carnitine, and 5mM HEPES). To block fatty acid oxidation, naive CD4 T cells were pre-treated with 40μM etomoxir for 15 minutes at 37°C. Afterwards, 34μM BSA or palmitate-BSA (200μM palmitate conjugated with 34μM BSA) was added to the medium and the OCR was assayed under basal conditions and in response to 1μM oligomycin, 1.5μM fluorocarbonyl cyanide phenylhydrazone (FCCP), and 100nM rotenone + 1μM antimycin A.
Arrays and qPCR:
Total cellular RNA from naive or stimulated CD4 T cells from standard laboratory diet- or WD-fed ApoE−/− mice was extracted by Trizol (ThermoFisher) followed by RNA purification using the Direct-zol™ RNA miniPrep kit (Zymo Research, Irvine, CA) per the manufacturer’s instructions. RNA purity and quantity were determined by a nanodrop spectrophotometer (ThermoFisher Scientific) and equal amounts of RNA was used to synthesize cDNA via either the RT2 First Strand Kit (Qiagen) or iscript cDNA synthesis kit (Bio-Rad). RT2 Profiler™ PCR Arrays (Qiagen) were used to assess the expression of genes involved in AMPK signaling and glucose metabolism in mouse cells according to the manufacturer’s instructions. mRNA expression was measured via real time quantitative PCR using TaqMan Gene Expression system in combination with pre-designed TaqMan primers for G6pc, Gapdh, Gusb, and Pfkfb3 (Applied Biosytems). Data were analyzed and presented on the basis of relative expression method. The ΔΔCT method was used with GUSB as a housekeeping gene.
Statistical Analysis:
All results are expressed as means (± s.e.m). Results were analyzed by unpaired, two-tailed Student’s t-tests. A P value of < 0.05 is considered to be significant. Statistical analyses were performed using GraphPad Prism software version 8 (GraphPad Software, Inc.). The normality and variance of our data were not examined before performing parametric tests.
Results
CD4 T cells from atherosclerotic mice have functional defects
To assess the metabolic state of CD4 T cells during atherosclerosis, we studied CD4 T cells in the well-characterized atherosclerotic apolipoprotein-E knockout (ApoE−/−) mouse. ApoE−/− mice were fed a Western diet (WD) for 20 weeks, after which we characterized the phenotypes of naive CD4 T cells. We found a 25% decrease in the percentage of naive CD4 T cells in the peri-aortic lymph nodes of WD-fed mice compared to standard laboratory diet-fed controls (Figure 1A). This was accompanied by a 5-fold increase in surface expression of the exhaustion marker, Programmed Cell Death Protein-1 (PD-1), by naive CD4 T cells (Figure 1B). When isolated from WD-fed mice and stimulated with αCD3/αCD28, naive CD4 T cells displayed a 50% reduction in IFNγ production (Figure 1C). This change was not due to decreased expression of the transcription factor T-bet, as this protein was increased 2-fold in CD4 T cells from WD-fed mice compared to those from standard laboratory diet-fed controls (Figure 1C).
Figure 1: Naive CD4 T cells are exhausted and less responsive to stimulation during atherosclerosis.
(A-B) CD4 T cells from peri-aortic lymph nodes of standard laboratory diet (SLD)- and Western diet (WD)-fed ApoE−/− mice were examined for naive CD4 T cell percentages (A), and the expression of the exhaustion marker PD-1 on naive CD4 T cells (B) using flow cytometry following 20 weeks of WD feeding. (C) Naive CD4 T cells from SLD- and WD-fed mice were stimulated with αCD3 and αCD28 and the ability to produce IFNγ and express T-bet was detected following intracellular staining. Results are expressed as mean ± s.e.m. from one of two experiments (n=4). Statistically significant differences were at * P<0.05, and ** P<0.01 (unpaired Student t-test).
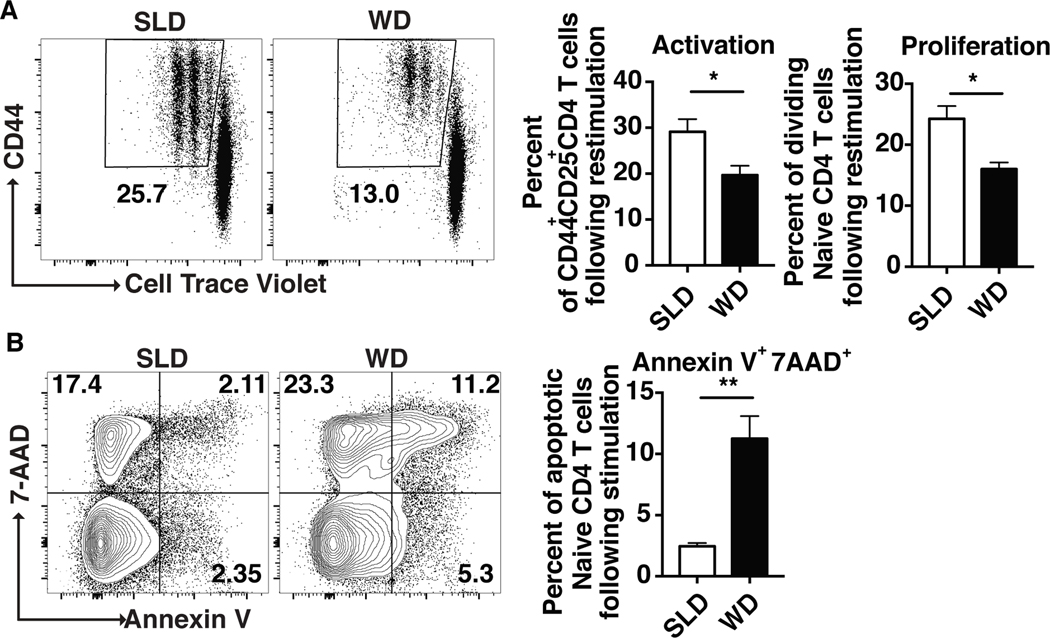
Upon examining the activation and proliferation potential of CD4 T cells from WD-fed mice, we found that T cells from WD-fed mice had a decreased capacity (30% reduction) to upregulate activation markers (CD44 and CD25) and to divide (Figure 2A), compared to CD4 T cells from control, standard laboratory diet-fed mice. This was accompanied by a 5-fold increase in the percentage of apoptotic (AnnexinV+7AAD+) CD4 T cells from WD-fed mice (Figure 2B). Taken together, these results suggest that naive CD4 T cells from WD-fed mice exhibit suboptimal responses to antigen stimulation.
Figure 2: Naive CD4 T cells from atherosclerotic mice are less proliferative and undergo more apoptosis following stimulation.
Naive CD4 T cells from standard laboratory diet (SLD) fed- and Western diet (WD)-fed mice were stimulated with αCD3 and αCD28. Flow cytometry plots and bar graphs showing the percentages of divided cells (A) and Annexin-V+7AAD+ cells following αCD3 and αCD28 stimulation of naive CD4+ T cells from SLD- or WD-fed mice (B). Results are expressed as mean ± s.e.m. from one of two experiments (n=4). Statistically significant differences were at * P<0.05, and ** P<0.01 (unpaired Student t-test).
CD4 T cells from atherosclerotic mice have impaired glucose metabolism
We next decided to compare the metabolic capacity of CD4 T cells from WD- and standard laboratory diet-fed ApoE−/− mice. We first examined the ability of stimulated CD4 T cells to catabolize glucose. CD4 T cells from WD-fed mice had a 3-fold reduction in their ability to consume glucose compared to those from standard laboratory diet-fed mice (Figure 3A). Using the Seahorse XF metabolism platform to measure glycolysis via extracellular acidification rates (ECAR), we found that CD4 T cells from WD-fed ApoE−/− mice have a lower ECAR at basal conditions (Figure 3B) and a reduced capacity to respond to treatment with oligomycin and FCCP (Figure 3B). We also observed a similar pattern with oxidative phosphorylation, when we examined the oxygen consumption rate (OCR) of these cells (Figure 3C). This was accompanied by a 50% reduction in the ability of CD4 T cells from WD-fed ApoE−/− mice to uptake 2-NBDG (Figure 3D).
Figure 3: CD4 T cells from atherosclerotic mice display reduced glucose metabolism.
Naive CD4 T cells isolated from standard laboratory diet (SLD)- and Western diet (WD)-fed mice were stimulated with αCD3 and αCD28 and the ability to consume glucose (A), undergo glycolysis (B) and oxidative phosphorylation (C), uptake 2-NBDG (D), upregulate glucose transporter Glut1 (E), CD4 T cells mitochondrial potential (F), and glycolytic reserve (G) were determined following αCD3 and αCD28 stimulation. Results are expressed as mean ± s.e.m. from one of two experiments (n=4 or 5). Statistically significant differences were at * P<0.05, *** P<0.001 and **** P<0.0001 (unpaired Student t-test).
To investigate the mechanisms underlying the diminished ability of CD4 T cells from WD-fed ApoE−/− mice to uptake glucose, we quantified surface-level expression of the glucose transporter Glut1 on these cells following stimulation. We observed a 25% reduction in the expression of Glut1 on CD4 T cells from WD-fed mice following stimulation with anti-CD3 and -CD28 (Figure 3E), which may explain the reduction in glucose uptake by these cells. We next examined the mitochondrial potential of stimulated CD4 T cells from WD-fed mice and noted lowered, albeit statistically insignificant, mitochondrial health (Figure 3F), suggesting that the observed defects in CD4 T cell metabolism result from inhibition of proper glycolysis. To further dissect how glucose metabolism is altered in CD4 T cells from WD-fed mice, we assessed the glycolytic capacity and glycolytic reserve of these cells after stimulation using a GlycoStress test from Seahorse Biosciences. Our results show that both the glycolytic capacity and reserve of CD4 T cells from WD-fed mice were 25 and 60% lower, respectively, compared to cells from standard laboratory diet-fed counterparts (Figure 3G). Taken together, these results suggest that CD4 T cells exhibit altered capacities for proper uptake and catabolism of glucose during atherosclerosis.
CD4 T cells from atherosclerotic mice show alterations in glucose and fatty acid oxidation related genes
Next, we examined the expression of metabolism-related genes in naive CD4 T cells isolated from spleens and lymph nodes of standard laboratory diet- and WD-fed ApoE−/− mice via gene-expression arrays. We observed a decrease in the enrichment of key glycolysis genes (Pfkfb1–4) and an increase in a key fatty acid β-oxidation gene (Cpt1a), encoding Carnitine palmitoyltransferase I, in naive CD4 T cells from WD-fed ApoE−/− mice compared to standard laboratory diet-fed controls (Figure 4A). These results indicate that naive CD4 T cells from WD-fed mice are biased towards fatty acid oxidation for meeting their energy demands. Upon stimulation with anti-CD3, CD28, CD4 T cells from WD-fed mice exhibited increased expression of both Gapdh and G6pc, encoding the enzyme Glucose 6 phosphatase (which reverses the first step of glycolysis), and decreased expression of the gene Pfkfb3, which encodes 6-Phosphofructo-2-Kinase, compared to standard laboratory diet-fed ApoE−/− mice (Figure 4B). This increase in Gapdh and G6pc and attenuation in Pfkfb3 mRNA accumulation was confirmed by qRT-PCR (Figure 4C). As a whole, these data illustrate profound alterations in CD4 T cell metabolism during atherosclerosis.
Figure 4: Naive CD4 T cells show changes in expression of glucose metabolism and fatty acid oxidation genes in atherosclerotic mice.
(A) Gene expression array in sorted naive CD4 T cells from standard laboratory diet (SLD)- and WD-fed mice without stimulation. (B) Gene expression array in CD4 T cells following stimulation with αCD3 and αCD28. (C) Relative mRNA expression levels of G6pc, Gapdh, and Pfkfb3 in isolated CD4 T cells from standard laboratory diet (SLD)- and WD-fed ApoE−/− mice following stimulation with αCD3 and αCD28. Data were analyzed using the 2-ΔΔCt method and it was normalized to GUSB. Results are expressed as the mean ± s.e.m from one experiment (n=3). Statistically significant differences were at * P<0.05 (unpaired Student t-test).
Naive CD4 T cells from WD-fed ApoE−/− mice display increased palmitate dependency
To determine the dependence of naive CD4 T cells on fatty acid oxidation, naive CD4 T cells from standard laboratory diet- and WD-fed ApoE−/− mice were incubated with palmitate-BSA and oxidative phosphorylation was measured via the Seahorse assay. WD-fed ApoE−/− naive CD4 T cells exhibited increased OCR in response to the free fatty acid palmitate-BSA (Fig. 5A), suggesting that WD feeding elicits increased reliance on palmitate. However, upon performing a mitochondrial stress test using oligomycin/FCCP/Antimycin A and Rotenone, our results showed no significant differences between the kinetics of OCR in naive CD4 T cells from standard laboratory diet- (Fig. 5B) and WD-fed (Fig. 5C) ApoE−/− mice. These data hint that the mitochondrial redox potential of naive CD4 T cells from both mice groups is similar, despite their differential reliance on fuel sources. Thus, T cells within the lipid-rich environment of the plaque may have adopted a better usage of lipids as a source of energy. In support of this, we examined the expression of genes involved in fatty acid and cholesterol metabolism in T cells from our published work on mouse atherosclerotic aorta using scRNA-Seq27. We found moderate to high expression of genes related to cholesterol metabolism (ABCA1, ABCG1, ACAT1, SOAT1), yet little to no expression of fatty acid metabolism genes CD36, FABP4, or Cav-1 in naive or effector CD4 T cells (Online Figure I). Collectively, our data suggest that tonic TCR signaling within T cells of the lipid-rich atherosclerotic plaque can modulate T cell metabolism and induce functional changes in T cell proliferation, activation, and exhaustion.
Figure 5: Naive CD4 T cells from WD-fed atherogenic mice display increased reliance on palmitate compared to naive CD4 T cells from standard laboratory diet-fed mice.
OCR of palmitate-BSA treated naive CD4 T cells isolated from standard laboratory diet (SLD)- and WD-fed mice (A) at baseline. Fatty acid oxidation assay of naive CD4 T cells isolated from standard laboratory diet-fed (SLD) (B) and WD-fed (C) mice using oligomycin, FCCP, and antimycin/rotenone in response to etomoxir, palmitate-BSA, and palmitate-BSA+Etomoxir. Results are expressed as mean s.e.m. from one experiment. Statistically significant differences were at **P<0.01 (unpaired Student t-test).
Naive CD4 T cell frequencies are reduced in human subjects with severe CVD
To characterize how naive CD4 T cell frequencies or phenotypes change with disease risk, we assayed naive CD4 T cells from peripheral blood mononuclear cells (PBMCs) of subjects undergoing a medically-necessary coronary angiography. CVD severity in each subject is quantified using the well-characterized clinical Gensini score28. In humans, naive T cells are phenotyped as CD45RA+CCR7+ cells that express low levels of CD95 (Fas). Examining the phenotype of naive CD4+ T cells (CD4+CD3+CD45RA+CCR7+CD95lo) revealed a significant reduction (~ 50%) in naive CD4 T cell percentages in individuals with high CVD (Gensini score >30) compared to low CVD (Gensini score<10) (Figure 6A, B). We next examined if CD4 T cells from high CVD patients were defective in their responses to stimulation compared to individuals with low CVD. Stimulation of naive CD4 T cells from high CVD patients with αCD3/αCD28 coated beads revealed a diminished proliferative capacity, as illustrated by a 10-fold less CTV dilution, in comparison to low CVD CD4 T cells (Figure 6C, D). These results suggest that similar to WD-fed ApoE−/− mice, naive CD4 T cell responses to activation are impaired in high CVD patients. Naive CD4 T cell frequencies inversely correlated with clinical Gensini score, which ranges from 0–100 (Figure 6E). The age of individuals with high CVD were significantly older than those with low CVD. Indeed, age is a well-established risk factor for CVD29. Plotting of naive CD4 T cell frequencies by age revealed no correlation. Therefore, we assert that advanced atherosclerosis severity elicits reduced naive CD4 T cell frequencies.
Figure 6: Human subjects with high CVD have fewer naive CD4 T cells and these cells have reduced proliferative capacity.
PBMC from human subjects with documented low CVD (Gensini<10) and high CVD (Gensini>30) were stained with a T cell-focused panel, and CyTOF mass cytometry was performed. Flow cytometry plots (A) and bar graphs showing the percentages of naive CD4 T cells (CD4+CD3+CD45RA+CCR7+CD95lo) from low (n=14) and high (n=14) CVD individuals normalized to absolute lymphocyte count (k/μL) (B). Results are expressed as mean ± s.e.m. from 14 low and 14 high CVD individuals. Naive CD4 T cells were isolated from PBMCs of low (n=7) and high (n=6) CVD individuals and stimulated with αCD3/αCD28 coated beads for 48hrs. Flow cytometry plots (C) show the percentage of proliferating CD4 T cells in the presence (red) or absence (blue) of Dynabeads using CTV proliferation dye and the percentage of cells divided (D). Results are expressed as mean ± s.e.m. from two independent experiments. Pearson correlation of Gensini score (E) and age (F) by frequencies of CD4+ TN cells of CD45+ cells, normalized to lymphocyte counts (k/μL). Statistically significant differences were at * P < 0.05 (unpaired Student’s t-test).
Discussion
In this study, we show that atherogenesis influences the phenotype, function and metabolic potential of CD4 T cells in individuals with severe CVD disease and mouse model of atherosclerosis. The association between metabolism and atherosclerosis is known. CAD patients display an abnormal fatty acid and glucose metabolism30, 31. More recently, it is becoming apparent that metabolic changes occur in macrophages present in the plaque during atherosclerosis. The hypoxic environment of the atherosclerotic plaque induces an increase in the glycolytic potential of plaque-resident macrophages32, 33. This increase in glycolysis results in attenuated cell viability in response to inflammation. Moreover, macrophages from individuals with atherosclerotic coronary artery disease display abnormal glucose utilization and hyperinflammation compared to healthy controls34. Still, the metabolic changes in CD4 T cells during atherosclerosis and their effects on CD4 T cell functions have not been characterized. Herein, we show that CD4 T cells from both humans and mice display defective glycolysis under atherosclerotic conditions, which attenuates the ability of these cells to proliferate and produce IFNγ.
Blocking glycolysis has been previously shown to result in loss of IFNγ production by CD4 T cells15, 35, 36. Additionally, Gapdh has been shown to interact with mRNA transcripts for IFNγ and block translation of the protein19. Our results are consistent with these findings and demonstrate that CD4 T cells from ApoE−/−, WD-fed mice display a reduction in IFNγ production, increased expression of Gapdh, and a reduction in glycolysis. These results suggest that atherosclerosis disrupts glycolysis in CD4 T cells and affects their ability to perform effector functions.
In the Multi-Ethnic Study of Atherosclerosis (MESA) cohort, a decrease in naive CD4 T cells is associated with subclinical atherosclerosis4. Our results show that naive CD4 T from atherosclerotic subjects display signs of exhaustion via increased expression of PD-1, which has been shown to alter the metabolic phenotype and function of CD4 T cells37. Indeed, stimulation of PD-1 results in a metabolic switch from glycolysis to lipolysis in CD4 T cells, by increasing expression of Cpt1a. Consistent with these findings, our gene array results suggest a decrease in the expression of glycolysis-related genes and an increase in fatty acid oxidation genes, including Cpt1a, by CD4 T cells. We therefore hypothesize that PD-1 ligation is at least partially responsible for this switch.
Changes in the metabolic phenotypes of T cells have been documented in other inflammatory diseases. In patients with rheumatoid arthritis, CD4 T cells exhibit reduced aerobic glycolysis, failure to generate ATP, and increased levels of apoptosis, as a result of decreased Pfkfb3 expression38. In this study, we observe a concomitant reduction in expression of Pfkfb3 in CD4 T cells from atherosclerotic mice, which could explain the increase rates of apoptosis observed in these cells. Moreover, when metabolic programs do not meet energy requirements of the cell, cells will readily undergo apoptosis. In obese patients and in mouse models of obesity, CD4 T cells show altered fatty acid and glucose metabolism, which biases the production of Th17 cells over Treg cells21, 39. This effect is driven by an upregulation of lipid kinase acetyl CoA carboxylase. Similarly, in multiple sclerosis, inhibition of carnitine palmitoyltransferase 1 (Cpt1), a mitochondrial enzyme responsible for shuttling fatty acids from the cytosol to the mitochondria for β-oxidation40, reduces disease severity41. We show here that similar metabolic shifting occurs in CD4 T cells in atherosclerosis.
Atherosclerosis is characterized by an increase in effector memory-like CD4 T cells within atherosclerotic ApoE−/− mice and humans with coronary artery disease42. Moreover, in peripheral blood, there is a positive correlation between disease severity and memory-like CD4 T cells4. Cholesterol signaling during atherosclerosis has been shown to alter T cell functioning and activation in mice43, 44, including phenotypic switching of Treg cells into T follicular helper (Tfh) or T effector cells12. While naive CD4 T cells that fail to divide or become anergic could be hypothesized to initially benefit atheroprogression, it may be more likely that this scenario indicates a disruption in the ability of the adaptive immune system to respond to stimuli. Thus, one can predict that, in individuals with severe CVD, immune responses to other insults such as viruses, bacteria, or cancer cells, would likewise be defective. Supporting this notion is a positive correlation between breast cancer and hypercholesterolemia in breast cancer patients45, 46. Whether naive CD8 T cells are similarly affected to CD4 T cells during atherosclerosis remains to be explored.
The results obtained from this work provide preclinical evidence for defects in CD4 T cell function and metabolism during atherosclerosis and may aid the design of new therapeutic targets for optimizing T cell responses against the disease.
Supplementary Material
Highlights.
Naive CD4 T cells from atherosclerotic ApoE−/− mice fed a western diet display lower capacity to proliferate and produce IFNγ following stimulation compared to cells from standard laboratory diet-fed mice.
CD4 T cells from atherosclerotic mice have a defect in glucose metabolism and show differential expression of metabolism genes.
Naive CD4 T cells in patients with high CVD have reduced capacity to respond to stimulation and uptake glucose.
The lipid-rich environment of the atherosclerotic plaque changes the metabolic potential of CD4 T cells.
Acknowledgements:
The Authors thank Deborah Yoakum for mouse colony management, and the LJI Flow Cytometry Core for assistance with cell sorting and mass cytometry. We also thank Ananda W. Goldrath (UCSD) for providing access to the Seahorse instrument.
Sources of Funding:
This work was supported in part by the American Heart Association (13POST16990031 to D.E. Gaddis and 19POST 34450020 to L.E. Padgett) and the National Institutes of Health (grants R01 HL112276, R01 HL134236, and P01 HL055798 to C.C. Hedrick, R01 HL107490 to C. McNamara, P01 HL136275 to C.C. Hedrick, C. McNamara and A.M. Taylor, T32 AI125279 and F32 HL 146069-A1 to L.E. Padgett, 1S10OD018499 for the mass cytometer, and S10 RR027366–01A1 to the LJI Flow Cytometry Core).
Abbreviations
- CVD
Cardiovascular disease
- ApoE−/−
Apolipoprotein E knockout mice
- WD
Western diet
- Treg
Regulatory T cells
Footnotes
Disclosures:
None.
References
- 1.Taleb S, Tedgui A, Mallat Z. Adaptive T cell immune responses and atherogenesis. Current opinion in pharmacology. 2010;10:197–202 [DOI] [PubMed] [Google Scholar]
- 2.Tse K, Tse H, Sidney J, Sette A, Ley K. T cells in atherosclerosis. Int Immunol. 2013;25:615–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wigren M, Nilsson J, Kolbus D. Lymphocytes in atherosclerosis. Clin Chim Acta. 2012;413:1562–1568 [DOI] [PubMed] [Google Scholar]
- 4.Olson NC, Doyle MF, Jenny NS, Huber SA, Psaty BM, Kronmal RA, Tracy RP. Decreased naive and increased memory CD4(+) T cells are associated with subclinical atherosclerosis: the multi-ethnic study of atherosclerosis. PLoS One. 2013;8:e71498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou X, Nicoletti A, Elhage R, Hansson GK. Transfer of CD4(+) T cells aggravates atherosclerosis in immunodeficient apolipoprotein E knockout mice. Circulation. 2000;102:2919–2922 [DOI] [PubMed] [Google Scholar]
- 6.Frostegard J, Ulfgren AK, Nyberg P, Hedin U, Swedenborg J, Andersson U, Hansson GK. Cytokine expression in advanced human atherosclerotic plaques: dominance of pro-inflammatory (Th1) and macrophage-stimulating cytokines. Atherosclerosis. 1999;145:33–43 [DOI] [PubMed] [Google Scholar]
- 7.Ait-Oufella H, Salomon BL, Potteaux S, et al. Natural regulatory T cells control the development of atherosclerosis in mice. Nat Med. 2006;12:178–180 [DOI] [PubMed] [Google Scholar]
- 8.Klingenberg R, Gerdes N, Badeau RM, et al. Depletion of FOXP3+ regulatory T cells promotes hypercholesterolemia and atherosclerosis. J Clin Invest. 2013;123:1323–1334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gotsman I, Grabie N, Gupta R, Dacosta R, MacConmara M, Lederer J, Sukhova G, Witztum JL, Sharpe AH, Lichtman AH. Impaired regulatory T-cell response and enhanced atherosclerosis in the absence of inducible costimulatory molecule. Circulation. 2006;114:2047–2055 [DOI] [PubMed] [Google Scholar]
- 10.Slack M, Wang T, Wang R. T cell metabolic reprogramming and plasticity. Mol Immunol. 2015;68:507–512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Butcher MJ, Filipowicz AR, Waseem TC, McGary CM, Crow KJ, Magilnick N, Boldin M, Lundberg PS, Galkina EV. Atherosclerosis-Driven Treg Plasticity Results in Formation of a Dysfunctional Subset of Plastic IFNgamma+ Th1/Tregs. Circ Res. 2016;119:1190–1203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gaddis DE, Padgett LE, Wu R, McSkimming C, Romines V, Taylor AM, McNamara CA, Kronenberg M, Crotty S, Thomas MJ, Sorci-Thomas MG, Hedrick CC. Apolipoprotein AI prevents regulatory to follicular helper T cell switching during atherosclerosis. Nat Commun. 2018;9:1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buck MD, O’Sullivan D, Pearce EL. T cell metabolism drives immunity. J Exp Med. 2015;212:1345–1360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O’Neill LA, Kishton RJ, Rathmell J. A guide to immunometabolism for immunologists. Nat Rev Immunol. 2016;16:553–565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pearce EL, Poffenberger MC, Chang CH, Jones RG. Fueling immunity: insights into metabolism and lymphocyte function. Science. 2013;342:1242454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang R, Green DR. Metabolic checkpoints in activated T cells. Nat Immunol. 2012;13:907–915 [DOI] [PubMed] [Google Scholar]
- 17.Rathmell JC, Vander Heiden MG, Harris MH, Frauwirth KA, Thompson CB. In the absence of extrinsic signals, nutrient utilization by lymphocytes is insufficient to maintain either cell size or viability. Molecular cell. 2000;6:683–692 [DOI] [PubMed] [Google Scholar]
- 18.Menk AV, Scharping NE, Moreci RS, Zeng X, Guy C, Salvatore S, Bae H, Xie J, Young HA, Wendell SG, Delgoffe GM. Early TCR Signaling Induces Rapid Aerobic Glycolysis Enabling Distinct Acute T Cell Effector Functions. Cell Rep. 2018;22:1509–1521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chang CH, Curtis JD, Maggi LB Jr., Faubert B, Villarino AV, O’Sullivan D, Huang SC, van der Windt GJ, Blagih J, Qiu J, Weber JD, Pearce EJ, Jones RG, Pearce EL. Posttranscriptional control of T cell effector function by aerobic glycolysis. Cell. 2013;153:1239–1251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berod L, Friedrich C, Nandan A, et al. De novo fatty acid synthesis controls the fate between regulatory T and T helper 17 cells. Nat Med. 2014;20:1327–1333 [DOI] [PubMed] [Google Scholar]
- 21.Endo Y, Asou HK, Matsugae N, Hirahara K, Shinoda K, Tumes DJ, Tokuyama H, Yokote K, Nakayama T. Obesity Drives Th17 Cell Differentiation by Inducing the Lipid Metabolic Kinase, ACC1. Cell Rep. 2015;12:1042–1055 [DOI] [PubMed] [Google Scholar]
- 22.Blagih J, Coulombe F, Vincent EE, et al. The energy sensor AMPK regulates T cell metabolic adaptation and effector responses in vivo. Immunity. 2015;42:41–54 [DOI] [PubMed] [Google Scholar]
- 23.Lee SY, Lee SH, Yang EJ, Kim EK, Kim JK, Shin DY, Cho ML. Metformin Ameliorates Inflammatory Bowel Disease by Suppression of the STAT3 Signaling Pathway and Regulation of the between Th17/Treg Balance. PLoS One. 2015;10:e0135858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Manichaikul A, Rich SS, Perry H, Yeboah J, Law M, Davis M, Parker M, Ragosta M, Connelly JJ, McNamara CA, Taylor AM. A functionally significant polymorphism in ID3 is associated with human coronary pathology. PLoS One. 2014;9:e90222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hamers AAJ, Dinh HQ, Thomas GD, Marcovecchio P, Blatchley A, Nakao CS, Kim C, McSkimming C, Taylor AM, Nguyen AT, McNamara CA, Hedrick CC. Human Monocyte Heterogeneity as Revealed by High-Dimensional Mass Cytometry. Arterioscler Thromb Vasc Biol. 2019;39:25–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thomas GD, Hamers AAJ, Nakao C, Marcovecchio P, Taylor AM, McSkimming C, Nguyen AT, McNamara CA, Hedrick CC. Human Blood Monocyte Subsets: A New Gating Strategy Defined Using Cell Surface Markers Identified by Mass Cytometry. Arterioscler Thromb Vasc Biol. 2017;37:1548–1558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zernecke A, Winkels H, Cochain C, et al. Meta-Analysis of Leukocyte Diversity in Atherosclerotic Mouse Aortas. Circ Res. 2020;127:402–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Neeland IJ, Patel RS, Eshtehardi P, Dhawan S, McDaniel MC, Rab ST, Vaccarino V, Zafari AM, Samady H, Quyyumi AA. Coronary angiographic scoring systems: an evaluation of their equivalence and validity. Am Heart J. 2012;164:547–552 e541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.D’Agostino RB Sr., Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM, Kannel WB. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117:743–753 [DOI] [PubMed] [Google Scholar]
- 30.Siguel EN, Lerman RH. Altered fatty acid metabolism in patients with angiographically documented coronary artery disease. Metabolism. 1994;43:982–993 [DOI] [PubMed] [Google Scholar]
- 31.Anselmino M, Wallander M, Norhammar A, Mellbin L, Ryden L. Implications of abnormal glucose metabolism in patients with coronary artery disease. Diab Vasc Dis Res. 2008;5:285–290 [DOI] [PubMed] [Google Scholar]
- 32.Tawakol A, Singh P, Mojena M, et al. HIF-1alpha and PFKFB3 Mediate a Tight Relationship Between Proinflammatory Activation and Anerobic Metabolism in Atherosclerotic Macrophages. Arterioscler Thromb Vasc Biol. 2015;35:1463–1471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yamashita A, Zhao Y, Matsuura Y, Yamasaki K, Moriguchi-Goto S, Sugita C, Iwakiri T, Okuyama N, Koshimoto C, Kawai K, Tamaki N, Zhao S, Kuge Y, Asada Y. Increased metabolite levels of glycolysis and pentose phosphate pathway in rabbit atherosclerotic arteries and hypoxic macrophage. PLoS One. 2014;9:e86426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shirai T, Nazarewicz RR, Wallis BB, Yanes RE, Watanabe R, Hilhorst M, Tian L, Harrison DG, Giacomini JC, Assimes TL, Goronzy JJ, Weyand CM. The glycolytic enzyme PKM2 bridges metabolic and inflammatory dysfunction in coronary artery disease. J Exp Med. 2016;213:337–354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Michalek RD, Gerriets VA, Jacobs SR, Macintyre AN, MacIver NJ, Mason EF, Sullivan SA, Nichols AG, Rathmell JC. Cutting edge: distinct glycolytic and lipid oxidative metabolic programs are essential for effector and regulatory CD4+ T cell subsets. J Immunol. 2011;186:3299–3303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Argus JP, Bensinger SJ. Immunology. Fueling function over expansion in T cells. Science. 2013;341:37–38 [DOI] [PubMed] [Google Scholar]
- 37.Patsoukis N, Bardhan K, Chatterjee P, Sari D, Liu B, Bell LN, Karoly ED, Freeman GJ, Petkova V, Seth P, Li L, Boussiotis VA. PD-1 alters T-cell metabolic reprogramming by inhibiting glycolysis and promoting lipolysis and fatty acid oxidation. Nat Commun. 2015;6:6692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang Z, Fujii H, Mohan SV, Goronzy JJ, Weyand CM. Phosphofructokinase deficiency impairs ATP generation, autophagy, and redox balance in rheumatoid arthritis T cells. J Exp Med. 2013;210:2119–2134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Abu-Elheiga L, Oh W, Kordari P, Wakil SJ. Acetyl-CoA carboxylase 2 mutant mice are protected against obesity and diabetes induced by high-fat/high-carbohydrate diets. Proc Natl Acad Sci U S A. 2003;100:10207–10212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee K, Kerner J, Hoppel CL. Mitochondrial carnitine palmitoyltransferase 1a (CPT1a) is part of an outer membrane fatty acid transfer complex. J Biol Chem. 2011;286:25655–25662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shriver LP, Manchester M. Inhibition of fatty acid metabolism ameliorates disease activity in an animal model of multiple sclerosis. Sci Rep. 2011;1:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ammirati E, Cianflone D, Vecchio V, et al. Effector Memory T cells Are Associated With Atherosclerosis in Humans and Animal Models. J Am Heart Assoc. 2012;1:27–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cheng HY, Gaddis DE, Wu R, McSkimming C, Haynes LD, Taylor AM, McNamara CA, Sorci-Thomas M, Hedrick CC. Loss of ABCG1 influences regulatory T cell differentiation and atherosclerosis. J Clin Invest. 2016;126:3236–3246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wojcik AJ, Skaflen MD, Srinivasan S, Hedrick CC. A critical role for ABCG1 in macrophage inflammation and lung homeostasis. J Immunol. 2008;180:4273–4282 [DOI] [PubMed] [Google Scholar]
- 45.Danilo C, Frank PG. Cholesterol and breast cancer development. Current opinion in pharmacology. 2012;12:677–682 [DOI] [PubMed] [Google Scholar]
- 46.Nelson ER, Wardell SE, Jasper JS, Park S, Suchindran S, Howe MK, Carver NJ, Pillai RV, Sullivan PM, Sondhi V, Umetani M, Geradts J, McDonnell DP. 27-Hydroxycholesterol links hypercholesterolemia and breast cancer pathophysiology. Science. 2013;342:1094–1098 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.