Abstract
A unique clinical feature of Takotsubo syndrome (TTS) is the stress trigger factor. Different types of triggers exist, generally divided into emotional and physical stressor. The aim was to create long-term registry of all consecutive patients with TTS across all disciplines in our large university hospital. We enrolled patients on the basis of meeting the diagnostic criteria of the international InterTAK Registry. We aimed to determine type of triggers, clinical characteristics, and outcome of TTS patients during 10 years period. In our prospective, academic, single centre registry, we enrolled 155 consecutive patients with diagnoses of TTS between October 2013 and October 2022. The patients were divided into three groups, those having unknown (n = 32; 20.6%), emotional (n = 42; 27.1%), or physical (n = 81; 52.3%) triggers. Clinical characteristics, cardiac enzyme levels, echocardiographic findings, including ejection fraction, and TTS type did not differ among the groups. Chest pain was less common in the group of patients with a physical trigger. On the other hand, arrhythmogenic disorders such as prolonged QT intervals, cardiac arrest requiring defibrillation, and atrial fibrillation were more common among the TTS patients with unknown triggers compared with the other groups. The highest in-hospital mortality was observed between patients having physical trigger (16% vs. 3.1% in TTS with emotional trigger and 4.8% in TTS with unknown trigger; P = 0.060). Conclusion: More than half of the patients with TTS diagnosed in a large university hospital had a physical trigger as a stress factor. An essential part of caring for these types of patients is the correct identification of TTS in the context of severe other conditions and the absence of typical cardiac symptoms. Patients with physical trigger have a significantly higher risk of acute heart complications. Interdisciplinary cooperation is essential in the treatment of patients with this diagnosis.
Keywords: Takotsubo syndrome, Takotsubo Registry, Trigger mechanism, Stress, Prolonged QTc intervals
Introduction
Takotsubo syndrome (TTS) was first described 32 years ago by Sato1 in post-menopausal women after negative emotional life events and was named the broken heart syndrome. Several years after its initial description in Japan, there has been renewed interest in this curious condition, which mimics acute coronary syndrome with comparable in-hospital mortality rate (4–5% during the acute phase)2 and long term prognosis.3 TTS has been widely investigated, but its pathophysiological mechanisms remain unknown. Roles of catecholamines and sympathetic activity have been hypothesized.4 Physical and emotional stressors, including acute neurological diseases, malignancy, inflammatory diseases, and trauma, are known triggers and poor prognostic markers for TTS.5–7
Recent studies have shown that triggering factors play an important role in TTS development and outcomes.8,9 The myth that TTS is always triggered by a negative emotional experience has long been disproved. The InterTAK registry shows that such TTS cases comprise only ∼28% of TTS patients and have better outcomes.
Brain imaging has also been used to investigate the role of the brain–heart axis because neurological disabilities are frequently associated with TTS.10,11 Seizures, intracerebral haemorrhage, and ischaemic stroke are the most common neurological precursors of TTS. Such patients have a younger age, lower female pre-dominance, and worse prognosis (3.2-fold increased risk of in-hospital mortality) compared with TTS patients without neurological disorders.6
In this study, we investigated the prevalence and features of TTS in patients across all acute departments in our hospital, and we compare their characteristics and outcomes.
Methods
The ongoing prospective observational registry included consecutive TTS patients hospitalized at the University Hospital Kralovske Vinohrady, Prague who met the InterTAK diagnostic criteria developed by the Takotsubo International Registry.5 In the present study, clinical and outcome data were obtained from patients records between October 2013 and October 2022. The study protocol was approved by the local ethics committee. All research was performed in accordance with relevant guidelines/regulations, and the study has been performed in accordance with the Declaration of Helsinki.
The following data were evaluated: baseline characteristics, cardiovascular risk factors, comorbidities, electrocardiography (ECG), ejection fraction, admission and maximum high-sensitivity troponin levels (initially high-sensitivity troponin T was used but was later replaced with high-sensitivity troponin I), maximum natriuretic peptide (NT-proBNP) levels, and other standard laboratory parameters. Furthermore, TTS was classified as apical, midventricular, focal, or basal based on ventriculography findings. Medications and clinical findings (e.g. the need for mechanical ventilation and/or hemodynamic support) were also recorded.
Patient data were thoroughly analysed to identify trigger factors based on patient interviews, findings at the time of admission, and emotional events. If a trigger factor was not identifiable initially, the records were reviewed again. The patients were then divided into three groups based on TTS aetiology.
The first group (Group I) included patients with an emotional triggering factor—a death of family member, illness in the family, quarrel, anxiety disorders, etc.
Group II consisted of patients whose TTS was unknown origin.
Finally, Group III consisted of patients whose TTS was caused primarily by acute physical non-cardiac disease—trauma (fracture and fall), surgery (osteosynthesis, total hip endoprosthesis implantation, ascites puncture, and tooth extraction), neurological disability (stroke, traumatic brain injury, epilepsy, meningioma brain tumour, and migraine), and acute inflammatory disorders (urinary tract infection, bronchitis, pneumonia, pancolitis, gastroenteritis, herpes zoster, and flu), or inflammatory reaction (exacerbation of COPD/astma bronchiale, anaphylactic reaction, and pulmonary fibrosis).
We had several aims with our TakoTsubo Registry: (i) to assess the prevalence of TTS associated with emotional, physical, and unknown triggers, (ii) to compare the clinical characteristics, laboratory markers, mortality rates, and adverse cardiac events (e.g. acute heart failure with the need for mechanical ventilation and hemodynamic instability requiring the use of inotropes/vasopressors) among the groups, and (iii) to define the process of recognizing TTS in critically ill patients with primarily non-cardiac involvement.
Statistical analysis
Differences among the groups were analysed using Pearson’s chi-square test or Fisher’s exact test. Laboratory parameters were compared using the Mann–Whitney U test and Kruskal–Wallis test. Continuous data were analysed using unpaired t-tests and one-way analysis of variance. P-values with < 0.05 (two-sided) were considered statistically significant.
Results
Study population
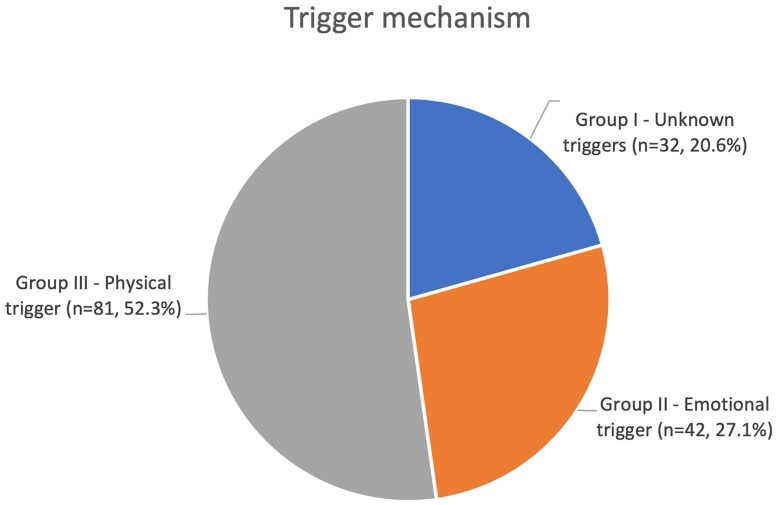
A total of 155 patients were diagnosed with TTS. Groups I, II, and III included 32 (20.6%), 42 (27.1%), and 81 (52.3%) patients, respectively (Figure 1).
Figure 1.
Trigger mechanism.
In agreement with previous studies, there was a significant female pre-dominance (92.3%) in our sample.12 The male-to-female ratio was similar in all groups. Demographic, clinical, and laboratory data are presented in Table 1.
Table 1.
Characteristics of TTS patients
| Parameter | Group I | Group II | Group III | P-value | P-value | P-value |
|---|---|---|---|---|---|---|
| Group I vs. Group II | Group I vs. Group III | Group II vs. Group III | ||||
| Characteristics | n = 32 | n = 42 | n = 81 | |||
| Demographic data | ||||||
| ȃFemales | 29 (90.9%) | 40 (95.2%) | 74 (91.4%) | 0.433 | 0.901 | 0.433 |
| ȃAge,y | 72.7 ± 10.4 | 72.7 ± 10.3 | 71.9 ± 12.9 | 0.999 | 0.769 | 0.743 |
| Cardiovascular history | ||||||
| ȃMyocardial infarction | 1 (3.2%) | 2 (4.8%) | 3 (3.7%) | 0.723 | 0.881 | 0.778 |
| ȃHypertension | 23 (71.9%) | 30 (71.4%) | 52 (64.2%) | 0.966 | 0.436 | 0.419 |
| ȃDiabetes mellitus | 5 (15.6%) | 11 (26.2%) | 17 (21%) | 0.274 | 0.517 | 0.514 |
| ȃHypercholesterolaemia | 6 (18.8%) | 12 (28.6%) | 34 (42%) | 0.329 | 0 .020 | 0.145 |
| ȃObesity | 6 (18.8%) | 5 (11.9%) | 9 (11.1%) | 0.412 | 0.281 | 0.895 |
| ȃSmoking | 15 (46.9%) | 21 (50%) | 37 (45.7%) | 0.789 | 0.906 | 0.649 |
| Comorbidities | ||||||
| ȃNeurologicaldisease | 8 (25%) | 11 (26.2%) | 27 (33.3%) | 0.907 | 0.388 | 0.416 |
| ȃPsychiatricdisease | 7 (21.9%) | 17 (40.5%) | 15 (18.5%) | 0.090 | 0.685 | 0 .008 |
| ȃPulmonarydisease | 7 (21.9%) | 4 (9.5%) | 21 (25.9%) | 0.139 | 0.653 | 0 .032 |
| ȃMenopause | 27 (84.4%) | 38 (90.5%) | 66 (81.5%) | 0.426 | 0.717 | 0.191 |
| ȃAlcohol consumption | 2 (6.3%) | 4 (9.5%) | 4 (4.9%) | 0.609 | 0.779 | 0.328 |
| Symptoms on admission | ||||||
| ȃChest pain | 22 (68.8%) | 32 (76.2%) | 30 (37%) | 0.475 | 0 .002 | 0 .000 |
| ȃDyspnoea | 19 (59.4%) | 20 (47.6%) | 44 (54.3%) | 0.316 | 0.626 | 0.480 |
| ECG on admission | ||||||
| ȃHeart rate | 85.5 ± 18.9 | 89.4 ± 20.1 | 90.2 ± 20.4 | 0.416 | 0.273 | 0.838 |
| ȃSinus rhythm | 24 (75%) | 37 (88.1%) | 65 (80.2%) | 0.142 | 0.539 | 0.273 |
| ȃAtrial fibrillation | 6 (18.7%) | 5 (11.9%) | 8 (9.9%) | 0.412 | 0.197 | 0.729 |
| ȃLBBB | 2 (6.3%) | 3 (7.1%) | 7 (8.5%) | 0.879 | 0.672 | 0.773 |
| ȃST-elevation | 9 (28.1%) | 11 (26.2%) | 24 (29.6%) | 0.853 | 0.874 | 0.689 |
| ȃST-depression | 4 (12.5%) | 4 (9.5%) | 7 (8.6%) | 0.683 | 0.533 | 0.871 |
| ȃT-wave inversion | 12 (37.5%) | 16 (38.1%) | 31 (38.3%) | 0.958 | 0.939 | 0.985 |
| ȃProlonged QTc | 16 (50%) | 10 (23.8%) | 22 (27.2%) | 0 .019 | 0 .021 | 0.688 |
| ȃQTc interval,ms | 463.3 ± 47 | 439.4 ± 41.2 | 451.9 ± 43.2 | 0 .036 | 0.273 | 0.158 |
| Echocardiography findings | ||||||
| ȃEjection fraction [%] | 36.4 ± 9.1 | 37.3 ± 8.6 | 34.6 ± 9.3 | 0.654 | 0.367 | 0.121 |
| ȃRight ventricular involvement | 4 (12.5%) | 1 (2.4%) | 14 (17.3%) | 0.079 | 0.531 | 0 .017 |
| Coronarography findings | ||||||
| ȃEjection fraction [%] | 35.7 ± 9.1 | 38.3 ± 9.0 | 34.4 ± 8.6 | 0.283 | 0.529 | 0.051 |
| ȃLVEDP [mmHg] | 22.4 ± 6.4 | 20.2 ± 5.1 | 21.6 ± 7.6 | 0.219 | 0.657 | 0.456 |
| ȃCoronary artery diseases (newly detected) | 2 (6.25%) | 10 (23.8%) | 23 (28.4%) | 0 .042 | 0 .011 | 0.586 |
| ȃPCI | 0 | 4 (9.5%) | 5 (6.2%) | 0.073 | 0.151 | 0.496 |
| TTS type | ||||||
| ȃApical | 26 (81.3%) | 32 (76.2%) | 57 (70.4%) | 0.600 | 0.238 | 0.494 |
| ȃMidventricular | 5 (15.6%) | 8 (19%) | 18 (22.2%) | 0.701 | 0.433 | 0.683 |
| ȃBasal | 0 | 0 | 3 (3.7%) | 1 | 0.269 | 0.207 |
| ȃFocal | 1 (3.1%) | 2 (4.8%) | 3 (3.7%) | 0.724 | 0.881 | 0.778 |
| Arrhythmogenic disorders | ||||||
| ȃOut-of-hospitalcardiacarrest/arrhythmia | 4 (12.5%) | 0 | 2 (2.5%) | 0 .018 | 0 .032 | 0.305 |
| ȃOut-of-hospital defibrillation | 3 (9.4%) | 0 | 1 (1.2%) | 0 .043 | 0 .035 | 0.469 |
| ȃIn-hospital atrial fibrillation | 7 (21.9%) | 5 (11.9%) | 6 (7.4%) | 0.249 | 0 .029 | 0.407 |
| Laboratory parameters | ||||||
| ȃHS-troponin T, ng/L(on admission) | 494.5 ± 494.2 | 434.7 ± 442.9 | 464 ± 396.4 | 0.674 | 0.790 | 0.778 |
| ȃHS-troponinT, ng/L (maximum) | 607.5 ± 702.0 | 486.7 ± 437.4 | 585.1 ± 494.0 | 0.493 | 0.893 | 0.406 |
| ȃNT-proBNP, ng/L (maximum) | 11756.5 ± 11793.3 | 7919.6 ± 9958.9 | 12483.9 ± 12435.3 | 0.313 | 0.846 | 0.151 |
| ȃC-reactive protein, mg/L(on admission) | 19.6 ± 32.3 | 18.6 ± 40.2 | 45.0 ± 61.5 | 0.921 | 0 .009 | 0 .021 |
| ȃC-reactive protein, mg/L (maximum) | 46.8 ± 55.7 | 26.4 ± 42.7 | 91.2 ± 87.2 | 0.115 | 0 .008 | 0 .000 |
| ȃLeukocytosis [×103μmol/L] (on admission) | 12.2 ± 4.9 | 10.4. ± 3.6 | 11.8 ± 4.9 | 0.092 | 0.677 | 0.143 |
| ȃLeukocytosis [×103μmol/L] (maximum) | 12.9 ± 6.0 | 10.8 ± 3.6 | 13.5 ± 6.3 | 0.075 | 0.749 | 0 .015 |
| ȃPlasmapotassiumlevel [mg/L] (on admission) | 4.0 ± 0.5 | 3.9 ± 0.5 | 4.0 ± 0.6 | 0.209 | 0.927 | 0.138 |
| ȃPlasmasodiumlevel [mg/L] (on admission) | 138.0 ± 2.9 | 136.3 ± 6.8 | 136 ± 7.3 | 0 .036 | 0 .013 | 0.963 |
| Adverse cardiac events | ||||||
| ȃRespiratory therapy requiring intubation | 6 (18.75%) | 4 (9.5%) | 15 (18.5%) | 0.250 | 0.977 | 0.191 |
| Hemodynamic instability requiring levosimendan/inotropes/vasopressors | 5 (15.6%) | 2 (4.8%) | 18 (22.2%) | 0.113 | 0.433 | 0 .013 |
| ȃCardiogenic shock | ||||||
| ȃDeath | 5 (15.6%) | 2 (4.8%) | 17 (21%) | 0.113 | 0.517 | 0 .018 |
| ȃ1 (3.1%) | 2 (4.8%) | 13 (16%) | 0.723 | 0.060 | 0.069 |
TTS, Takotsubo syndrome; ECG, electrocardiography; LBBB, left bundle branch block; LVEDP, left ventricular end-diastolic pressure; PCI, percutaneous coronary intervention; HS, high sensitivity; NT-proBNP, natriuretic peptide.
However, chest pain was significantly less common in Group III than in patients of Group I and Group II.
Examination findings
The three groups had similar echocardiography findings (ejection fraction and severity of mitral regurgitation). The proportions of different TTS types were also similar among the groups (Table 1), with the apical type being the most common, followed by the midventricular, focal, and basal types.
ECG changes were significantly different among the groups. The QTc interval was prolonged in significantly more Group I patients than Group II and III patients [50% vs. 23.8% (P = 0.019) and 27.2% (P = 0.021)]. Group I also included a greater number of atrial fibrillation cases compared with Groups II and III [21.9% vs. 11.9% (P = 0.249) and 7.4% (P = 0.029)]. Furthermore, the most severe rhythm changes were observed in fibrillable and non-fibrillable out-of-hospital cardiac arrests in Group I (9.4%) than in Group II (0%, P = 0.043) and Group III (1.2%, P = 0.035). In Group I, these rhythm changes occurred in four patients (12.5%), three of whom had defibrillable rhythms on the first recorded ECG, while one had asystole. In contrast, ECG ischaemic changes, including ST segment elevation, ST segment depression, T-wave inversion, and left bundle branch block, did not differ among the groups.
Coronarography findings showed a lower prevalence of coronary artery disease in Group I (6.25%) compared with Groups II and III [23.8% (P = 0.042) and 28.4% (P = 0.011), respectively] (Table I).
Laboratory findings
There were no major differences in cardiac enzyme levels, including the admission and maximum high-sensitivity troponin levels. The maximum NT-proBNP levels were also similar among the groups.
Inflammatory parameters were statistically significantly higher in patients with a physical factor (Group III). Group I had a significantly higher plasma sodium level (138.0 ± 2.9 mg/L) compared with the other groups [Group II: 136.3 ± 6.8 mg/L (P = 0.036) and Group III: 136.3 ± 7.3 mg/L (P = 0.013)], while the potassium level did not differ significantly (Table 1).
Outcomes
Patients with a physical factor have the least favourable outcome. The rates of adverse cardiac events in terms of the use of levosimendan/inotropes/vasopressors were most common in Group III. In accordance, cardiogenic shock occurred statistically more often in Group III compared to Group II [21% vs. 4.8% (P = 0.018)]. It is also necessary to emphasize the highest in-hospital mortality rate of Group III [3.1%, 4.8% (P = 0.723) and 16% (P = 0.060) for Groups I, II, and III, respectively].
Although TTS patients with unknown triggers had a significantly higher prevalence of cardiac arrest at the time of admission, they had similar outcomes during hospitalization compared with the other TTS patients.
Discussion
We created a unique registry of patients in whom we diagnosed Takotsubo syndrome as part of the investigation of acute coronary syndrome, acute heart failure, or newly occurring hemodynamic instability in critically ill patients with primarily non-cardiac disease.
We managed to get a large number of TTS patients from other clinics due to the experience with TTS diagnostics at our workplace and the availability of cardiology specialists at any time and in any other non-cardiology department in our hospital. It was an interdisciplinary collaboration, especially with the neurology and neurosurgery clinic, as well as with the anaesthesiology and resuscitation department, and with the internal, orthopaedic, cardiosurgery, gynaecology, or burn departments.
The principal findings of the present study were as follows: first, physical mechanisms represent approximately one-half of all triggering mechanism of TTS in our cohort; second, patients with TTS caused by different trigger mechanism do not differ in terms of baseline characteristics, ECG findings, cardiac enzymes, echocardiographic results including ejection fraction, and type of TTS; and third, arrhythmogenic disorders were more common in patients with TTS of unknown origin. Finally, emotional trigger was associated with excellent in-hospital outcome. On the other hand, in-hospital mortality and rate of adverse cardiac events in TTS patients with physical trigger are not negligible.
Although TTS is known to characteristically affect post-menopausal women due to the emotional stress, a significant proportion of patients suffer from TTS due to the physical triggers. Recent studies of the pathophysiological mechanisms of TTS show the importance of the immune and inflammatory components of this disease.
Myocardial remodelling and inflammation may be mediated by T-lymphocytes which are activated by a cardiac myosin.13 Cardiac myosin occurs in inflammatory conditions, and the T cells are ready to easily activated.14 Other studies suggest an effect of alterated nitric oxide signalling. The pre-clinical rodent model of TTS established by Shao et al.15 by injection of 5 mg/kg isoproterenol intraperitoneally, and there were found decreases in apical strain and increased wall thickness of left ventrical. Several hours after dose of isoproterenol, inflammatory changes were observed within the myocardium, including increases in CD68+ macrophages and levels of inflammatory markers.16 There is growing evidence on the role of increased production of reactive oxygen species (ROS) in the pathophysiology of TTS.17 It may cause a transient impairment of myocardial contraction due to stunning (apical ballooning). Increased ROS production leads to a decreased vascular and myocardial nitric oxide bioavailability, and it causes an endothelial dysfunction.
Another studies supporting the crucial role of physical condition and inflammation in Takotsubo syndrome—acute COPD exacerbation and acute respiratory failure have been described as a common physical trigger of TTS.7 The common denominator of these diseases is risk factors that can have a synergistic effect and provoke TTS—hypoxia/hypercapnia, β2-agonist treatment, and/or inflammation. The question remains, what is the role of decompensation of lung disease in the induction of TTS and what is the role of the stress moment. A sympathetic nerve stimulation can be induced by respiratory failure (hypoxemic and/or hypercapnic). The hypoxemia alone can produce a longer-lasting sympathetic activation than hypercapnia. However, if both act simultaneously, the effect may be a significant increase in sympathetic activity with concomitant increase in catecholamine release.18
In our study the prevalence of infection, lung and neurological acute diseases were significantly high. Rates were mainly driven by a COPD, asthma, stroke, and cerebral haemorrhage. These named states have inflammatory responses that are observed during acute exacerbations.
A recent experimental study revealed the pathophysiological impact, of nitrosative stress in an animal model of TTS (also mentioned above).19 Prolonged impaired cardiac function is due to the aberrant β-adrenoceptor signalling. These findings point to potential treatment.
ii. In agreement with the previous studies, there were no significant differences in age, sex, ECG findings, and TTS types among the groups.20,21 The primary mechanism of TTS did not affect the types of TTS and cardiac enzymes including high-sensitivity troponin and NT-proBNP levels.
It is known from international registries of patients with TTS that 90% of all patients are women of age 66.8 (±13.0 years), and 90% of all patients were women older than 50 years of age.20The most common type of TTS is apical (∼80%), followed by midventricular and rare forms include basal and focal types of TTS.20 This more or less corresponds to the ventriculographic evaluation of TTS types in our patients (Table 1).
The issue of ECG remains challenging, and researchers are trying to find specific changes in TTS that would help diagnosis. However, they only confirmed ECG heterogeneity in patients with TTS who had a comparable proportion of ST elevations as normal ECG findings compared to patients with AMI.22
The key tools for diagnostic of TTS include clinical symptomatology, cardiac biomarkers, non-invasive cardiac imaging, and coronary angiography. A multiple diagnostic criteria have been established since the first description of the disease.
iii. Recent evidence suggests that patients with atrial fibrillation at admission have worse short- and long-term prognoses.23 The global prevalence of atrial fibrillation in TTS patients is 5–25%.23–25 According to the InterTak Registry data, nearly half of the patients with atrial fibrillation during a TTS event did not have a history of arrhythmia.23 In the present study, patients with unknown triggers had a greater prevalence (21.9%) of newly-detected atrial fibrillation during hospitalization compared with the other patients. Arrhythmias adversely affect hemodynamic status, which may explain the high frequency of acute complications in these patients. Management of atrial fibrillation is an indispensable part of TTS treatment.
Cardiac arrhythmias play an important role in the disease course of TTS,26 but the exact pathophysiological mechanism remains unknown. The Heart Failure Association Takotsubo Syndrome Study Group and Myocardial Function Working Group of the European Society of Cardiology released a joint scientific statement regarding the causes of TTS.4
Several pathological phenomena are simultaneously involved in TTS, including myocardial integration (receptor biology, signalling pathways, mitochondrial function, inflammation, metabolism, gene expression, and electrophysiology) and systemic (peripheral vasculature, brain, autonomic and peripheral nervous systems, limbic system, and hypothalamic–pituitary–adrenal axis) phenomena.27 We hypothesized that excessive catecholamines and sympathetic nervous system activity play crucial roles. Human cardiomyocytes develop from induced pluripotent stem cells and catecholamine excess may increase reactive oxygen species production, which may lead to QT prolongation and arrhythmias.28 A prolonged QT interval is present in 50% of TTS patients and is associated with malignant arrhythmias.26 The TTS patients with unknown triggers had a significantly higher prevalence of prolonged QTc compared with patients with a known trigger (50%, 23.8%, and 27.2% for Groups I, II, and III, respectively). In addition to atrial fibrillation, prolonged QTc may also contribute to the high rates of acute complications and arrhythmogenic disturbances. Prolonged QTc was also the most common cause of out-of-hospital cardiac arrests, with or without the need for defibrillation, which were also significantly more common in the unknown trigger group. Life-threatening ventricular arrhythmias have been reported in 3–8.6% of TTS patients.26,29 In the present study, they occurred in 12.5% of Group I patients. These arrhythmias, including ventricular fibrillation, ventricular tachycardia, and torsades de pointes, are also associated with prolonged QT intervals.30 The arrhythmogenic disorder theory also suggests that patients with unknown triggers have the lowest incidence of coronary artery disease according to coronary angiography.
iv. Prognosis of TTS patients is similar to the prognosis of patients with acute coronary syndromes (ACS).3 TTS patients had long-term outcomes comparable to age- and sex-matched ACS patients. Clinical outcome of TTS depends on triggering mechanism of TTS.20 And a severity of condition depends on the inciting stress factor.
In our study, we focused on in-hospital outcome, and there are the most significant differences in both acute complications including death and adverse cardiac events. The higher number of deaths was seen in patient with physical stressor opposite to patients with emotional stress and to patients with unknown factor. Adverse cardiac event was significantly increased in patients with acute non-cardiac disorders, too. A severe physical condition may certainly contribute to a worse prognosis in groups of patients with physical stress than in other groups. The absence of cardiac symptoms, especially chest pain, may also be involved in difficult TTS diagnosis in this cohort of patients.
Although these data suggest that patients’ physical stress have worse outcomes, it is unclear whether this is a causative association or if it is a result of the more severe underlying primarily non-cardiac physical disease.
It follows that the increase in diagnosed patients with TTS (as shown by the studies) represents a complication in patient care, which has led to an increase in the number of hospitalizations, adverse outcomes and treatment costs. Treating TTS as systemic condition is imperative marking a significant impact of the outcome. In patients in a serious medical condition, it is sometimes difficult to diagnose TTS correctly and quickly.
So far, no international guidelines have been developed for the treatment of TTS, either in the acute phase or in the chronic phase. Therapeutic procedures are therefore based on clinical experience and expert consensus (evidence level C).26
It is known from current knowledge that the administration of exogenous catecholamines should be avoided. The drug of choice as an alternative to other ionotropic is Ca2+-sensitizer levosimendan. The idea that beta-blockers would be beneficial in TTS is insufficient, they are not effective in the reduction of mortality or recurrence rate. On the other hand, use of ACEi or ARB was associated with improved survival in the long-term treatment.20 Anti-inflammatory drugs thus become the therapeutic target. Recent researches show that intensity of early inflammatory activation is directly correlated both with severity of early development of hemodynamic changes and with both short- and long-term outcome.8
Limitations
This was a single-centre study with a limited number of patients. Although there was a close cooperation between the cardiology and other departments of the hospital, some TTS cases may have been misdiagnosed. Additionally, laboratory markers were not collected at specific time intervals, and the true maximal levels may have been different. Although the data presented suggest that TTS patients with physical stressors had worse outcomes, it is unclear whether this was a causative association or a result of the underlying non-cardiac disease.
Conclusions
The present study demonstrates that TTS is related to severe physical trigger in 50% of all Takotsubo patients. These patients have worse short-term outcome than others. The serious condition of this patient cohort is complicated both by the primary non-cardiac involvement itself and extensive diagnostics also due to the absence of cardiac symptoms. Therefore, interdisciplinary cooperation plays a crucial role in improving the care of these patients.
Acknowledgements
The authors would like to thank all the health professionals who participated in this study.
Contributor Information
Karolina Polednikova, Cardiocentre of University Hospital Kralovske Vinohrady, Srobarova 1150/50, Prague 100 34, Czechia; Third Faculty of Medicine, Charles University, Ruska 2411, Prague 100 00, Czechia.
Martin Kozel, Cardiocentre of University Hospital Kralovske Vinohrady, Srobarova 1150/50, Prague 100 34, Czechia; Third Faculty of Medicine, Charles University, Ruska 2411, Prague 100 00, Czechia.
Hana Linkova, Cardiocentre of University Hospital Kralovske Vinohrady, Srobarova 1150/50, Prague 100 34, Czechia; Third Faculty of Medicine, Charles University, Ruska 2411, Prague 100 00, Czechia.
Marketa Novackova, Cardiocentre of University Hospital Kralovske Vinohrady, Srobarova 1150/50, Prague 100 34, Czechia; Third Faculty of Medicine, Charles University, Ruska 2411, Prague 100 00, Czechia.
Minh Duc Trinh, Cardiocentre of University Hospital Kralovske Vinohrady, Srobarova 1150/50, Prague 100 34, Czechia; Third Faculty of Medicine, Charles University, Ruska 2411, Prague 100 00, Czechia.
Petr Tousek, Cardiocentre of University Hospital Kralovske Vinohrady, Srobarova 1150/50, Prague 100 34, Czechia; Third Faculty of Medicine, Charles University, Ruska 2411, Prague 100 00, Czechia.
Funding
This study was supported by the project National Institute for Research of Metabolic and Cardiovascular Diseases (Programme EXCELES, ID Project No. LX22NPO5104), funded by the European Union—Next Generation EU. Partly the manuscript was co-funded also by the Charles University Research Programs “Cooperatio Cardiovascular Sciences” and “UNCE-MED 002”.
The funding sources had no role in the design or conduct of the study; collection, management, analysis, or interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.
Data availability
All data generated or analysed during this study are included in this manuscript.
References
- 1. Sato H, Tateishi H, Uchida T, Dote K, Ishihara M, Kodama K et al. Clinical aspect of myocardial injury: from ischemia to heart failure. Kagaku Hyoronsha 1990;2:55–64. [Google Scholar]
- 2. Redfors B, Vedad R, Angerås O, Råmunddal T, Petursson P, Haraldsson I et al. Mortality in Takotsubo syndrome is similar to mortality in myocardial infarction—a report from the SWEDEHEART registry. Int J Cardiol 2015;185:282–289. [DOI] [PubMed] [Google Scholar]
- 3. Ghadri JR, Kato K, Cammann VL, Gili S, Jurisic S, Di Vece D et al. Long-term prognosis of patients with Takotsubo syndrome. J Am Coll Cardiol 2018;72:874–882. [DOI] [PubMed] [Google Scholar]
- 4. Omerovic E, Citro R, Bossone E, Redfors B, Backs J, Bruns B et al. Pathophysiology of Takotsubo syndrome—a joint scientific statement from the Heart Failure Association Takotsubo Syndrome Study Group and Myocardial Function Working Group of the European Society of Cardiology—part 1: overview and the central role for catecholamines and sympathetic nervous system. Eur J Heart Fail 2022;24:257–273. [DOI] [PubMed] [Google Scholar]
- 5. Ghadri JR, Cammann VL, Jurisic S, Seifert B, Napp LC, Diekmann J et al. A novel clinical score (InterTAK diagnostic score) to differentiate Takotsubo syndrome from acute coronary syndrome: results from the International Takotsubo Registry. Eur J Heart Fail 2017;19:1036–1042. [DOI] [PubMed] [Google Scholar]
- 6. Cammann VL, Scheitz JF, von Rennenberg R, Jäncke L, Nolte CH, Szawan KA et al. Clinical correlates and prognostic impact of neurologic disorders in Takotsubo syndrome. Sci Rep 2021;11:23555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kato K, Cammann VL, Napp LC, Szawan KA, Micek J, Dreiding S et al. Prognostic impact of acute pulmonary triggers in patients with Takotsubo syndrome: new insights from the International Takotsubo Registry. ESC Heart Fail 2021;8:1924–1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lachmet-Thebaud L, Marchandot B, Matsushita K, Dagrenat C, Peillex M, Sato C et al. Systemic inflammatory response syndrome is a major determinant of cardiovascular outcome in Takotsubo syndrome. Circ J 2020;84:592–600. [DOI] [PubMed] [Google Scholar]
- 9. Singh S, Desai R, Gandhi Z, Fong HK, Doreswamy S, Desai V et al. Takotsubo syndrome in patients with COVID-19: a systematic review of published cases. SN Compr Clin Med 2020;2:2102–2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dichtl W, Tuovinen N, Barbieri F, Adukauskaite A, Senoner T, Rubatscher A et al. Functional neuroimaging in the acute phase of Takotsubo syndrome: volumetric and functional changes of the right insular cortex. Clin Res Cardiol 2020;109:1107–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Templin C, Hänggi J, Klein C, Topka MS, Hiestand T, Levinson RA et al. Altered limbic and autonomic processing supports brain–heart axis in Takotsubo syndrome. Eur Heart J 2019;40:1183–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ghadri J-R, Wittstein IS, Prasad A, Sharkey S, Dote K, Akashi YJ et al. International expert consensus document on Takotsubo syndrome (part I): clinical characteristics, diagnostic criteria, and pathophysiology. Eur Heart J 2018;39:2032–2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhang J, Liao Y, Cheng X, Chen J, Chen P, Gao X et al. Myosin specific-T lymphocytes mediated myocardial inflammation in adoptive transferred rats. Cell Mol Immunol 2006;3:445–451. [PubMed] [Google Scholar]
- 14. Sattler S, Couch LS, Harding SE. Takotsubo syndrome: latest addition to the expanding family of immune-mediated diseases? JACC Basic Transl Sci 2018;3:779–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shao Y, Redfors B, Täng MS, Möllmann H, Troidl C, Szardien S et al. Novel rat model reveals important roles of beta-adrenoreceptors in stress-induced cardiomyopathy. Int J Cardiol 2013;168:1943–1950. [DOI] [PubMed] [Google Scholar]
- 16. Couch LS, Harding SE. Takotsubo syndrome: stress or NO stress? JACC Basic Transl Sci 2018;3:227–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Munzel T, Templin C, Cammann VL, Hahad O.. Takotsubo syndrome: impact of endothelial dysfunction and oxidative stress. Free Radic Biol Med 2021;169:216–223. [DOI] [PubMed] [Google Scholar]
- 18. Morgan BJ, Crabtree DC, Palta M, Skatrud JB.. Combined hypoxia and hypercapnia evokes long-lasting sympathetic activation in humans. J Appl Physiol 1995;79:205–213. [DOI] [PubMed] [Google Scholar]
- 19. Surikow SY, Nguyen TH, Stafford I, Chapman M, Chacko S, Singh K et al. Nitrosative stress as a modulator of inflammatory change in a model of Takotsubo syndrome. JACC Basic Transl Sci 2018;3:213–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Templin C, Ghadri JR, Diekmann J, Napp LC, Bataiosu DR, Jaguszewski M et al. Clinical features and outcomes of Takotsubo (stress) cardiomyopathy. N Engl J Med 2015;373:929–938. [DOI] [PubMed] [Google Scholar]
- 21. Looi J-L, Verryt T, McLeod P, Chan C, Pemberton J, Webster M et al. Type of stressor and medium-term outcomes after Takotsubo syndrome: what becomes of the broken hearted? (ANZACS-QI 59). Heart Lung Circ 2022;31:499–507. [DOI] [PubMed] [Google Scholar]
- 22. Frangieh AH, Obeid S, Ghadri J-R, Imori Y, D'Ascenzo F, Kovac M et al. ECG criteria to differentiate between Takotsubo (stress) cardiomyopathy and myocardial infarction. J Am Heart Assoc 2016;5:e003418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. El-Battrawy I, Cammann VL, Kato K, Szawan KA, Di Vece D, Rossi A et al. Impact of atrial fibrillation on outcome in Takotsubo syndrome: data from the International Takotsubo Registry. J Am Heart Assoc 2021;10:e014059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Stiermaier T, Santoro F, Eitel C, Graf T, Möller C, Tarantino N et al. Prevalence and prognostic relevance of atrial fibrillation in patients with Takotsubo syndrome. Int J Cardiol 2017;245:156–161. [DOI] [PubMed] [Google Scholar]
- 25. Jesel L, Berthon C, Messas N, Lim HS, Girardey M, Marzak H et al. Atrial arrhythmias in Takotsubo cardiomyopathy: incidence, predictive factors, and prognosis. Europace 2019;21:298–305. [DOI] [PubMed] [Google Scholar]
- 26. Ghadri J-R, Wittstein IS, Prasad A, Sharkey S, Dote K, Akashi YJ et al. International expert consensus document on Takotsubo syndrome (part II): diagnostic workup, outcome, and management. Eur Heart J 2018;39:2047–2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Omerovic E, Citro R, Bossone E, Redfors B, Backs J, Bruns B et al. Pathophysiology of Takotsubo syndrome—a joint scientific statement from the Heart Failure Association Takotsubo Syndrome Study Group and Myocardial Function Working Group of the European Society of Cardiology—part 2: vascular pathophysiology, gender and sex hormones, genetics, chronic cardiovascular problems and clinical implications. Eur J Heart Fail 2022;24:274–286. [DOI] [PubMed] [Google Scholar]
- 28. El-Battrawy I, Zhao Z, Lan H, Schünemann J-D, Sattler K, Buljubasic F et al. Estradiol protection against toxic effects of catecholamine on electrical properties in human-induced pluripotent stem cell derived cardiomyocytes. Int J Cardiol 2018;254:195–202. [DOI] [PubMed] [Google Scholar]
- 29. El-Battrawy I, Lang S, Ansari U, Tülümen E, Schramm K, Fastner C et al. Prevalence of malignant arrhythmia and sudden cardiac death in Takotsubo syndrome and its management. Europace 2018;20:843–850. [DOI] [PubMed] [Google Scholar]
- 30. Brown KH, Trohman RG, Madias C. Arrhythmias in Takotsubo cardiomyopathy. Card Electrophysiol Clin 2015;7:331–340. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analysed during this study are included in this manuscript.