Abstract
Human immunodeficiency virus type 1 (HIV-1) bearing HLA-DR in its envelope was detected in plasma from all patients with chronic HIV-1 infection (n = 16) and was present at higher levels in patients with active tuberculosis coinfection (n = 6). Intriguingly, however, HLA-DR was not detectable in HIV-1 from patients during primary viremia (n = 6), suggesting the possibility of virus replication in less-activated cells.
Human lymphocyte antigen class II (HLA-DR) is incorporated into the envelope of human immunodeficiency virus type 1 (HIV-1) as it buds from the host cell plasma membrane. This aspect of HIV-1 maturation is seen among genotypically and phenotypically diverse strains, including laboratory-adapted strains and primary isolates (1, 2, 3, 6, 11, 15, 17). Compared to other highly expressed mononuclear cell surface proteins, HLA-DR is avidly incorporated into the viral envelope (3, 11, 20), possibly as a result of a selective process (reviewed in reference 20). Following incorporation into the HIV-1 envelope, HLA-DR retains functionality. In addition to its role in antigen presentation, HLA-DR serves as an adhesion molecule whose natural receptor is CD4. Thus, it is possible that the presence of HLA-DR in the HIV-1 envelope increases virus-cell interactions and, importantly, this may serve as the mechanism whereby the infectivity of virions bearing this molecule is enhanced (4, 5). This phenomenon, together with the ability of HIV-1-associated HLA-DR to facilitate superantigen presentation (16), may influence HIV-1 pathogenesis.
Although numerous studies have demonstrated that HLA-DR is incorporated into the envelope of HIV-1 propagated in vitro (1, 2, 3, 6, 15, 17), studies of incorporation of host protein by HIV-1 that is present in clinical plasma samples have been hindered technically by the interaction of virus with various serum proteins (11). Such phenomena may explain why, in a study by Sarloos et al. (17), virion-associated HLA-DR was detected in only three of eight plasma samples from HIV-infected persons. More recently, we have described an algorithm for the purification of HIV-1 from clinical specimens, thus facilitating the detection of virion-associated host molecules (11). By using an immunomagnetic capture technique, we found that the proportion of HLA-DR-bearing HIV-1 that is detectable in plasma decreases during treatment of tuberculosis (TB) and that this correlates with disease resolution (10). Furthermore, analysis of the HIV-1 pool replicating at an anatomic site of inflammation revealed that a greater proportion of that virus contained HLA-DR in the envelope than did virus present in the systemic circulation (12). Together, these data suggest that incorporation of HLA-DR by HIV-1 in vivo may correlate with the state of immunological activation of the cells supporting viral replication. In support of this, upregulation of surface expression of HLA-DR resulting from activation of U937 monocytoid cells in vitro enhances the incorporation of HLA-DR by HIV-1 replicating within those cells (6). This phenomenon, however, has not been studied with primary mononuclear cells.
Activation of the immune system plays a key role in the natural history of HIV-1 infection, increasing during the course of disease (18) and in the presence of opportunistic infections (13, 19, 21). The aim of this study was to determine whether the incorporation of HLA-DR by HIV-1 in plasma samples from infected persons correlates with the clinical stage of disease and whether this is also affected by the presence of opportunistic bacterial infection.
HLA-DR incorporation by both macrophage- and lymphocyte-derived HIV-1 in vitro.
We have previously shown that HLA-DR is incorporated into the envelope of dualtropic HIV-1Ba-L following propagation in either purified macrophages or lymphocytes (11). In the same studies, host cell-derived CD44 was also found to be incorporated at high levels by both macrophage- and lymphocyte-derived viral stocks in vitro (11). Furthermore, CD44 was detected in the envelope of HIV-1 derived from a panel of six chronically infected, CD44-expressing, transformed cell lines (unpublished data) as well as in the envelope of virus present in samples of blood plasma (11), cervicovaginal fluid (12), and pleural fluid (unpublished data) obtained from HIV-infected persons. We therefore selected anti-CD44 antibody capture to be used as a positive control and as a comparative index of virus capture by anti-HLA-DR antibody.
Prior to analyzing HIV-1 in clinical plasma samples in the present study, we determined the relative efficiencies of capture of in vitro HIV-1 stocks with antibodies to CD44 and HLA-DR (Table 1) using the immunomagnetic capture technique described previously (11). Although the extent of anti-CD44 antibody capture of macrophage- and lymphocyte-derived virus stocks was similar, the proportion of the macrophage-derived virus captured using anti-HLA-DR antibody was greater than that of lymphocyte-derived virus (Table 1), possibly reflecting a higher level of expression of HLA-DR by macrophages.
TABLE 1.
Capture of in vitro HIV-1 with host protein-specific antibodiesa
| Antibody | % Input virus captured
|
|
|---|---|---|
| HIV-1Ba-L-Mφ | HIV-1Ba-L-CD4 | |
| Anti-CD44 | 37.0 ± 4.4 | 43.9 ± 6.9 |
| Anti-HLA-DR | 19.4 ± 1.6 | 12.2 ± 1.5 |
| Anti-CD19 | 1.3 ± 0.5 | 0.5 ± 0.3 |
HIV-1Ba-L was propagated in vitro in macrophages (HIV-1Ba-L-Mφ) or lymphocytes (HIV-1Ba-L-CD4) and captured by antibodies directed against the host proteins, CD44 and HLA-DR, that were present in the HIV-1 envelope; anti-CD19 antibody was used as the negative control. Data represent the mean percentages (± the standard errors) of input virus (5 × 106 virions per capture) captured by the different antibodies in three independent experiments.
Patient samples.
Next, we proceeded to analyze HIV-1 purified from anonymous plasma samples from 22 patients categorized into four groups based on clinical status (Table 2). Seroconversion panels of samples from patients with primary HIV-1 infection were obtained commercially (Boston Biomedica, Inc., West Bridgewater, Mass.), and sera obtained at the peak of viremia just prior to seroconversion (determined by Western analysis) were selected. Samples from characterized patients with chronic HIV-1 infection but no clinical or microbiological evidence of opportunistic infections were categorized into two groups according to the CD4+ T-lymphocyte count. Paired samples were also obtained from patients with smear-positive pulmonary TB at the time of diagnosis and at a later time point during treatment of TB when the patients' sputum smear stains were negative for acid-fast bacilli (described in reference 10). None of the 22 patients was receiving antiretroviral treatment. HIV-1 from these 22 plasma samples was analyzed using a purification algorithm and immunomagnetic virus capture technique as described previously in full (11).
TABLE 2.
Analysis of HIV-1 in samples from four patient groupsa
| HIV-1-infected patient groups (n) | CD4 count (106/liter) | HIV-1 load (105 copies/ml) | % HIV-1 captured by antibody to:
|
P valueb | ||
|---|---|---|---|---|---|---|
| CD44 | HLA-DR | IL-6 | ||||
| Primary infection (6) | ND | 16.0 ± 17.5 | 26.3 ± 7.7 | 0.4 ± 0.1c | 0.5 ± 0.2 | <0.01 |
| CD4 count of 200 to 500 (106/liter); no TB (5) | 411 ± 203 | 2.37 ± 2.27 | 37.5 ± 6.7 | 3.2 ± 0.7 | 0.4 ± 0.2 | |
| CD4 count of <200; no TB (5) | 124 ± 75 | 6.90 ± 5.12 | 23.4 ± 5.0 | 3.3 ± 0.8 | 0.4 ± 0.1 | |
| Active TB | ||||||
| Pretreatment (6) | 160 ± 108 | 13.1 ± 29.1 | 27.3 ± 7.0 | 10.6 ± 1.7 | 1.8 ± 0.3 | <0.01 |
| With TB treatment (6) | ND | 8.0 ± 14.1 | 39.1 ± 7.0 | 5.9 ± 0.7 | 1.8 ± 0.6 | >0.1 |
Twenty-two patients were categorized into four groups according to the stage of HIV-1 infection and presence of TB. CD4 counts and HIV-1 RNA loads are presented as mean values ± the standard deviations. Virions (6 × 104) were distributed equally between the three different antibody captures performed on each sample. The percentage of HIV-1 captured by antibodies to CD44, HLA-DR, or IL-6 (negative control) is presented as the mean ± the standard error for each group. Capture data for each patient, normalized for anti-CD44 antibody capture, are also presented in Fig. 1. n, number studied. ND, not determined.
P value: t-test comparison of the percentages of specific anti-HLA-DR antibody capture of HIV-1 from samples of patients with primary HIV-1 infection or TB and HIV-1 coinfection versus all those with chronic HIV-1 infection but no TB.
No significant capture over background levels for any patient.
Incorporation of HLA-DR by HIV-1 at different stages of disease.
HIV-1 was captured from plasma samples of all 22 patients using anti-CD44 antibody, and levels were broadly similar among the four patient groups (Table 2). This suggests that CD44 is incorporated at a high level in the HIV-1 envelope throughout the progression of HIV-1 infection to AIDS. Since this molecule plays a physiological role in lymphocyte homing, we have previously speculated that a high level of incorporation of CD44 into the HIV-1 envelope may assist in trafficking of virus to lymphoid tissue (11).
The efficiency of HIV-1 capture from plasma samples using anti-HLA-DR antibody was lower than that seen with anti-CD44 antibody (Table 2), as was observed on analysis of the in vitro-derived HIV-1 stocks (Table 1). Significant capture of HIV-1 from plasma using anti-HLA-DR antibody (more than threefold greater than the negative control) was detected in all patients with chronic HIV-1 infection and no opportunistic infection (n = 10) and was similar in the two groups that were stratified by CD4 lymphocyte count (Table 2; Fig. 1). Although the efficiency of anti-HLA-DR antibody capture was low, our results are an important advance from those of a previous study, in which HIV-1 from plasma samples of five out of eight HIV-infected persons could not be captured using this antibody (17). It is likely that the virus purification algorithm that we employed substantially overcame the inhibition of HIV-1 capture that is potentially attributable to the presence of acute-phase proteins, anti-HIV-1 antibodies (11), soluble HLA-DR (22), and anti-HLA-DR autoantibodies (7).
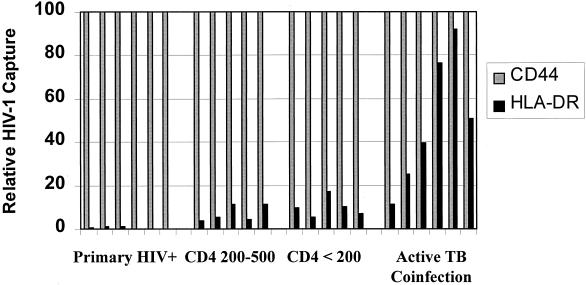
FIG. 1.
HIV-1 was analyzed in plasma samples obtained from 22 patients who were categorized into four groups as shown in Table 2. Virions (6 × 104) from each patient were distributed equally among anti-CD44, anti-HLA-DR, and negative control antibody captures. For each patient sample, specific HIV-1 capture by anti-CD44 and anti-HLA-DR antibodies was calculated by subtracting the amount of HIV-1 bound by the negative control antibody. Data for each patient are presented as a percentage of the amount of HIV-1 capture using anti-CD44 antibody. Significant amounts of HIV-1 were captured from all patients with chronic HIV-1 infection, and amounts were similar among those with CD4 counts in the ranges of 200 × 106/liter to 500 × 106/liter and <200 × 106/liter. The greatest anti-HLA-DR HIV-1 capture was from samples of patients with active TB coinfection. However, there was no significant anti-HLA-DR antibody capture of HIV-1 over background levels from plasma samples of patients with primary HIV-1 infection (Primary HIV+). Data indicating percent HIV-1 capture by each antibody are displayed in Table 2.
In marked contrast to capture of HIV-1 by anti-CD44 antibody, capture using anti-HLA-DR antibody was not uniform among samples obtained at different stages of HIV-1 infection. Most striking was the complete absence of a significant level of capture of HIV-1 from plasma samples of any of the patients with primary HIV-1 infection (n = 6), despite substantial virus capture using anti-CD44 antibody (Table 2; Fig. 1). Although it has been proposed that HIV-1 replicates predominantly in immunologically activated cells in vivo, there is now evidence that both simian immunodeficiency virus and HIV-1 also replicate substantially in inactive, HLA-DR-negative cells in vivo, most notably during acute retroviral infection (23). Our findings are consistent with and support this observation. Immediately following seroconversion, the establishment of an immune response might lead to HIV-1 replication in mononuclear cells that have a higher degree of activation, possibly leading to the ability to detect HLA-DR in the HIV-1 envelope during the transition from the acute phase to chronic HIV-1 infection. However, we were unable to analyze HIV-1 plasma samples at time points immediately following seroconversion because the amount of virus present in the plasma was insufficient for analysis.
Although we were unable to detect HLA-DR in the envelope of HIV-1 present in plasma samples that were obtained during primary HIV-1 infection (n = 6), this molecule may have been incorporated at levels below the limit of detection of the assay. Indeed, it is likely that a critical threshold density of the host molecule in the HIV-1 envelope is required to enable the virus to be captured by our technique. Increasing activation of the cells supporting HIV-1 replication may well result in increases in both the frequency of virions bearing HLA-DR and the density of the molecule in the envelope, thereby increasing the proportion of virus that can be captured using antibodies to this molecule.
Effects of active TB coinfection on incorporation of HLA-DR by HIV-1 in vivo.
The percentage of specific anti-HLA-DR antibody capture of HIV-1 present in plasma of patients with untreated smear-positive pulmonary TB (n = 6) was 3.1-fold greater than the capture of virus from patients with chronic HIV-1 infection but no opportunistic infection (n = 10) (P < 0.001) (Table 2; Fig. 1). Since active TB infection in HIV-infected persons is associated with a marked systemic immune activation (13, 21), the presence of this opportunistic infection is likely to increase mononuclear cell surface expression of HLA-DR, which is a highly inducible molecule. Our results may reflect this, with upregulation of HLA-DR on cells supporting viral replication resulting in increased incorporation of HLA-DR into progeny HIV-1 particles. In addition to this, we have previously shown that the presence of active TB coinfection leads to a substantial production of cell-free HIV-1 derived from macrophages (10). This virus may contain a higher level of HLA-DR in the envelope than lymphocyte-derived virus (Table 1), and therefore, differential cellular expression may have contributed to the findings among TB patients in this study.
Since the presence of HLA-DR within the viral envelope enhances HIV-1 infectivity (4, 5, 6), the increased incorporation of HLA-DR in the HIV-1 envelope that is associated with the presence of opportunistic infections might promote the propagation of HIV-1 in mononuclear cell pools. Such a mechanism may be important in the copathogenesis of HIV-1 and opportunistic infections, facilitating HIV-1 replication and increasing the HIV-1 load in plasma (8, 19). There is also evidence that mycobacterial superantigens are important in TB pathogenesis (14), and it is possible that HLA-DR in the HIV-1 envelope of virus from coinfected persons facilitates presentation of these superantigens (16), further contributing to mononuclear cell activation and pathogenesis.
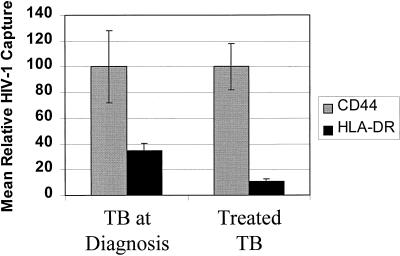
Analysis of HIV-1 in plasma samples prospectively obtained from patients with TB receiving antituberculosis treatment confirms and expands our previous findings (10). Treatment of TB resulting in sputum smear conversion was associated with both relative and absolute reductions in anti-HLA-DR antibody capture of virus, with a decline to levels similar to those observed in patients with HIV-1 infection but no opportunistic infection (Table 2; Fig. 2). This indicates that the increased incorporation of HLA-DR in the HIV-1 pool that is associated with active TB infection is reversible, and in view of the potential for enhanced viral propagation, this further highlights the importance of early diagnosis and prompt treatment of copathogens in HIV-infected persons (9).
FIG. 2.
Paired plasma samples were obtained from patients (n = 6) with HIV-1 infection and smear-positive pulmonary TB at the time of diagnosis and at a later time point following a successful response to treatment. Virus was captured as described in the legend for Fig. 1. The mean (± the standard error) percentage of input HIV-1 captured by each antibody is presented normalized for the mean anti-CD44 antibody capture at each time point. The percentage of HIV-1 captured by each antibody is presented in Table 2.
In summary, incorporation of HLA-DR into the HIV-1 envelope was detectable in the plasma of all patients with chronic HIV-1 infection but was strikingly undetectable in samples from those with primary HIV-1 infection, suggesting that viral replication prior to seroconversion occurs in less-activated cell pools. Active TB infection leads to a reversible increase in incorporation of HLA-DR by HIV-1 in vivo and may represent an important mechanism whereby TB and potentially other opportunistic infections enhance HIV-1 pathogenesis.
Acknowledgments
Stephen D. Lawn was funded by the Wellcome Trust of London, United Kingdom, and subsequently by a Research Participation Program administered by the Oak Ridge Institute for Science and Education, Oak Ridge, Tenn.
We thank R. B. Lal and C. E. Hart for supplying clinical samples. The Committee on Human Research, Publications and Ethics of the School of Medical Sciences, Kumasi, Ghana, approved collection of field samples by S. D. Lawn.
REFERENCES
- 1.Arthur L O, Bess J W, Urban R G, Strominger J L, Morton W R, Mann D L, Henderson L E, Beneviste R E. Macaques immunized with HLA-DR are protected from challenge with simian immunodeficiency virus. J Virol. 1995;69:3117–3124. doi: 10.1128/jvi.69.5.3117-3124.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bastiani L, Laal S, Kim M, Zoller-Pazner S. Host cell-dependent alterations in envelope components of human immunodeficiency virus type 1 virions. J Virol. 1997;71:3444–3450. doi: 10.1128/jvi.71.5.3444-3450.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cantin R, Fortin J-F, Tremblay M. The amount of HLA-DR proteins acquired by HIV-1 is virus strain and cell type specific. Virology. 1996;218:372–381. doi: 10.1006/viro.1996.0206. [DOI] [PubMed] [Google Scholar]
- 4.Cantin R, Fortin J-F, Lamontagne G, Tremblay M. The acquisition of host-derived major histocompatibility complex class II glycoproteins by human immunodeficiency virus type 1 accelerates the process of virus entry and infection in human T-lymphoid cells. Blood. 1997;90:1091–1100. [PubMed] [Google Scholar]
- 5.Cantin R, Fortin J-F, Lamontagne G, Tremblay M. The presence of host-derived HLA-DR1 on human immunodeficiency virus type 1 increases viral infectivity. J Virol. 1997;71:1922–1930. doi: 10.1128/jvi.71.3.1922-1930.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Castilletti C, Capobianchi M R, Fais S, Abbate I, Ficociello B, Ameglio F, Cordiali Fei P, Santini S M, Dianzani F. HIV type 1 grown on interferon γ-treated U937 cells shows selective increase in virion-associated intercellular adhesion molecule 1 and HLA-DR and enhanced infectivity for CD4-negative cells. AIDS Res Hum Retrovir. 1995;11:547–553. doi: 10.1089/aid.1995.11.547. [DOI] [PubMed] [Google Scholar]
- 7.De La Barrera S, Fainboim L, Lugo S, Pichio G R, Muchinik G R, De Bracco M M. Anti-class II antibodies in AIDS patients and AIDS-risk groups. Immunology. 1987;62:599–604. [PMC free article] [PubMed] [Google Scholar]
- 8.Goletti D, Weissman D, Jackson R W, Graham N M H, Vlahov D, Klein R S, Munsiff S S, Ortona L, Cauda R, Fauci A S. Effect of Mycobacterium tuberculosis on HIV replication. Role of immune activation. J Immunol. 1996;157:1271–1278. [PubMed] [Google Scholar]
- 9.Lawn S D, Shattock R J, Griffin G E. Delay in the diagnosis of tuberculosis: a great new cost. Int J Tuberc Lung Dis. 1997;1:485–486. [PubMed] [Google Scholar]
- 10.Lawn S D, Shattock R J, Acheampong J W, Lal R B, Folks T M, Griffin G E, Butera S T. Sustained plasma TNF-α and HIV-1 load despite resolution of other immune activation parameters during treatment of tuberculosis in Africans. AIDS. 1999;13:2231–2237. doi: 10.1097/00002030-199911120-00005. [DOI] [PubMed] [Google Scholar]
- 11.Lawn S D, Roberts B D, Griffin G E, Folks T M, Butera S T. Cellular compartments of HIV-1 replication: determination by virion-associated host proteins and the impact of opportunistic infection in vivo. J Virol. 2000;74:139–145. [PMC free article] [PubMed] [Google Scholar]
- 12.Lawn S D, Subbarao S, Wright T C, Evans-Strickfaden T, Lennox J, Ellerbrock T, Butera S, Hart C E. Correlation between HIV-1 RNA levels in the female genital tract and immune activation resulting from genital ulceration of the cervix. J Infect Dis. 2000;181:1950–1956. doi: 10.1086/315514. [DOI] [PubMed] [Google Scholar]
- 13.Lawn S D, Rudolph D, Wiktor S, Coulibaly D, Ackah A, Lal R B. Tuberculosis and HIV infection are independently associated with elevated serum concentrations of tumour necrosis factor receptor type 1 and β2-microglobulin, respectively. Clin Exp Immunol. 2000;122:79–84. doi: 10.1046/j.1365-2249.2000.01341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ohmen J D, Barnes P F, Grisso C L, Bloom B R, Modlin R L. Evidence for a superantigen in human tuberculosis. Immunity. 1994;1:35–43. doi: 10.1016/1074-7613(94)90007-8. [DOI] [PubMed] [Google Scholar]
- 15.Roberts B D, Butera S T. Host protein incorporation is conserved among diverse HIV-1 subtypes. AIDS. 1999;13:425–427. doi: 10.1097/00002030-199902250-00020. [DOI] [PubMed] [Google Scholar]
- 16.Rossio J L, Bess J, Henderson L E, Cresswell P, Arthur L O. HLA class II on HIV particles is functional in superantigen presentation to human T-cells: implications for HIV pathogenesis. AIDS Res Hum Retrovir. 1995;11:1433–1439. doi: 10.1089/aid.1995.11.1433. [DOI] [PubMed] [Google Scholar]
- 17.Saarloos M-N, Sullivan B L, Czerniewski M A, Parameswar K D, Spear G T. Detection of HLA-DR associated with monocytotropic, primary, and plasma isolates of human immunodeficiency virus type 1. J Virol. 1997;71:1640–1643. doi: 10.1128/jvi.71.2.1640-1643.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Salazar-Gonzalez J F, Martinez-Maza O, Nishanian P, Aziz N, Shen L-P, Grosser S, Taylor J, Detels R, Fahey J L. Increased immune activation precedes the inflection point of CD4 T cells and the increased serum virus load in human immunodeficiency virus infection. J Infect Dis. 1998;178:423–430. doi: 10.1086/515629. [DOI] [PubMed] [Google Scholar]
- 19.Sulkowski M S, Chaisson R E, Karp C L, Moore R D, Margolick J B, Quinn T C. The effect of acute infectious illnesses on plasma human immunodeficiency virus type I load and the expression of serologic markers of immune activation among HIV-infected adults. J Infect Dis. 1998;178:1642–1648. doi: 10.1086/314491. [DOI] [PubMed] [Google Scholar]
- 20.Tremblay M J, Fortin J-F, Cantin R. The acquisition of host-encoded proteins by nascent HIV-1. Immunol Today. 1993;19:346–351. doi: 10.1016/s0167-5699(98)01286-9. [DOI] [PubMed] [Google Scholar]
- 21.Vanham G, Edmonds K, Qing L, Hom D, Toossi Z, Jones B, Daley C L, Huebner B, Kestens L, Gigase P, Ellner J J. Generalised immune activation in pulmonary tuberculosis: coactivation with HIV infection. Clin Exp Immunol. 1996;103:30–34. doi: 10.1046/j.1365-2249.1996.907600.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weyand C M, Jendro M, Goronzy J J. Soluble HLA-DR molecules in patients with HLA class II versus class I associated disorders. Autoimmunity. 1991;8:281–287. doi: 10.3109/08916939109007635. [DOI] [PubMed] [Google Scholar]
- 23.Zhang Z, Schuler T, Zupancic M, Wietgrefe S, Staskus K A, Reimann K A, Reinhart T A, Rogan M, Cavert W, Miller C J, Veazey R S, Notermans D, Little S, Danner S A, Richman D D, Havlir D, Wong J, Jordan H L, Schacker T W, Racz P, Tenner-Racz K, Letvin N L, Wolinsky S, Haase A T. Sexual transmission and propagation of SIV and HIV in resting and activated CD4+ T cells. Science. 1999;286:1353–1357. doi: 10.1126/science.286.5443.1353. [DOI] [PubMed] [Google Scholar]