Abstract
Zoonotic Brugia pahangi parasite infections in humans have emerged over two decades in Southeast Asia (SEA), including Malaysia and Thailand. The species is commonly found in domestic cats and dogs as the natural reservoir hosts. The sporadic transmission pattern of B. pahangi zoonosis causes childhood infections in Thailand and adulthood infections in Malaysia. It is crucial to understand the vulnerability in how zoonotic B. pahangi parasite is transmitted to susceptible persons in receptive settings and the exposure to the infection under impoverished environment to which the human-vector-animal interactions are related. This acquisition of knowledge will help multiple health science professions to apply One Health approach to strengthening the capacity in diagnosis and surveillance, and hence detecting and monitoring the “lingering” zoonotic B. pahangi infections present in vulnerable populations in Thailand and elsewhere in SEA. In this review article, the authors focused on articulating the concepts of plantation-related zoonotic B. pahangi filariasis by updating current knowledge of B. pahangi life cycle, vector’s life cycle and current state of research on the epidemiology and ecology of B. pahangi zoonosis.
Keywords: Brugia pahangi, plantation-related zoonotic Brugia pahangi filariasis, sporadic transmission pattern, zoonosis
Introduction
Brugia pahangi is a mosquito-borne filarial nematode parasite, which was originally isolated from cats and dogs in Malaysia and identified as Wuchereria pahangi [1]. The species was then subsequently belonged to the genus Brugia by Buckley [2]. It is commonly found in domestic cats and dogs as the natural reservoir hosts [3–5]. In recent years, this parasite causes zoonotic infections in humans in Southeast Asia (SEA), including Malaysia [6, 7] and Thailand [8, 9]. The clinical presentations of human B. pahangi filariasis may vary with patient age due likely to host immune responses; that is, the kinetics of filarial infection and worm load may undergo the clinical, physiological, and immunological pathogenesis of lymphatic pathology as seen in cats [10] or dogs [11].
Brugia pahangi is genetically closely related to Brugia malayi [12]. Based on genomic analyses and gene annotation of B. pahangi draft genome (85.4 Mb) [13] that spans 9,687 protein-encoding genes, B. pahangi has high genomic similarity to that of zoonotic B. malayi parasite. Brugia pahangi possesses 8,681 predicted genes (89.6%) orthologous to B. malayi. Of note, 1624 genes are predicted to share exclusively similarity to B. malayi and 569 genes are unique to B. pahangi. The closely related genetic entity of B. pahangi and B. malayi implies that they share the biology of parasitic system of the interactions between these parasites and their vertebrate and/or invertebrate hosts. Nonetheless, it is evident that the physiology and vector competence of B. pahangi differ from B. malayi [8, 14–16].
In this article, the authors provide a review of the literature on B. pahangi and articulate ideas or concepts of plantation-related zoonotic B. pahangi filariasis by updating current knowledge of B. pahangi life cycle, vector’s life cycle, and current state of research on the epidemiology and ecology of B. pahangi zoonosis. Understanding the vulnerability of local people, that is, pertaining to plausible causes and consequences of human activities, relies on exploring the susceptibility of vulnerable persons in receptive settings and the exposure to the infection under impoverished environment to which the human-vector-animal interactions are related. This One Health approach would help multiple health science professions to understand veterinary public health, zoonotic disease, and the environment. More significantly, strengthening the capacity in diagnosis and surveillance is essential for detecting and monitoring the “lingering” zoonotic B. pahangi infections present in vulnerable populations in Thailand and elsewhere in SEA.
Life Cycle of Zoonotic B. pahangi Parasite
The life cycle of zoonotic B. pahangi parasite illustrated in Figure-1 involves two transmission cycles, that are, enzootic and epizootic cycles. In enzootic cycle, enzootic B. pahangi parasite circulates among domestic dogs and/or cats as natural reservoir hosts through vertical transmission (between animal and vector hosts). In epizootic cycle, epizootic B. pahangi parasite occurs in humans as an accidental host through vertical transmission (between human and vector hosts). In given foci, the numbers of enzootic B. pahangi infections present in animal reservoirs (domestic dogs and/or cats) and local vector populations infer the endemicity as the degree to which transmission of epizootic B. pahangi occurs in susceptible person(s) in an impoverished environment of urban, sub-urban, or rural areas [3–7, 17, 18].
Figure-1.
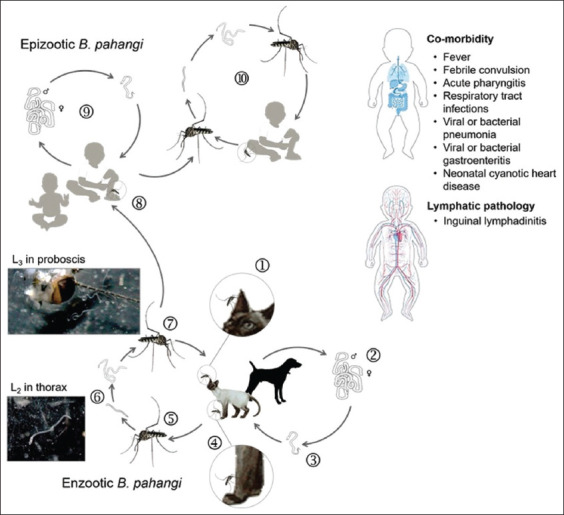
Life cycle of zoonotic Brugia pahangi. The parasite develops enzootic cycle of B. pahangi by which domestic cat or dog acquires the infection through mosquito-borne transmission ①-⑦. Transmission occurs when female adult of Armigeres subalbatus as a principal vector can transmit L3 infective stage ⑦ to susceptible cat or dog during taking blood meal ①. An infected cat or dog frequently exposed to infective bites can develop the adult worm infection ② and later microfilaremia ③. The complete cycle of transmission occurs when microfilariae are transmitted to susceptible female adult of Ar. subalbatus ⑤ that takes another blood meal from infected cat or dog ④. Microfilariae can develop further juvenile stages by exsheathment in midgut ⑤, and later L1 in hemolymph to L2 in thorax ⑥ to L3 in proboscis as infective stage ⑦. The parasite can also develop epizootic cycle of transmission ⑨ by which susceptible children acquires the accidental infection through Ar. subalbatus borne transmission ⑧. The childhood infection can undergo this epizootic B. pahangi by a single-step spillover ⑧. Meanwhile a spillback or human to human transmission of epizootic B. pahangi ⑩ remains unclear. [Source: Graphic illustration created by A. Bhumiratana].
Transmission of enzootic B. pahangi parasites occurs when a susceptible dog or cat becomes frequently exposed to bites of local vectors, including infective bites. A diverse group of local vectors (Mansonia, Aedes, Culex, Anopheles, and Armigeres subalbatus) can play a possible role in transmission [8, 16, 19, 20] if they are adapted to local environment to which the animal reservoir–vector interactions are related. The infection occurs when an exposed dog or cat is inoculated with infective third-stage larvae (L3), which are released from the proboscis (mouth parts) of an infected mosquito while taking blood meal. The kinetics of the infection with L3 inoculum undergoes the localization and molting from L3 to fourth stage (L4) to adult. Incubation period takes about 23 days, for which the infection carries male worms and about 27 days for female worms [21]. Adult worm infection then produces microfilariae present in blood about 40–60 days or up to 3 months after the infection [22–24]. Individually infected dogs or cats with microfilaremic infection can harbor a broad range of microfilarial densities [25–27], showing microfilarial periodicity (i.e., the appearance of microfilariae with a peak density in blood in infected dog or cat during daytime or nighttime). Microfilarial periodicity is clinically unimportant but likely important for the epidemiologic implication that it relates its peak density to feeding behaviors of local potent vectors. Like B. malayi causing human lymphatic filariasis [28], adult worms of B. pahangi can live in cats or dogs for 2–5 years, and can cause a spectrum of clinical manifestations. Repeated infections (or with multiple inoculations of L3) are common during pre-patent and patent periods [29] and can influence the up-regulation of host immune responses and disease, for example, the clinical, physiological, and immunological pathogenesis of the structure and function of lymphatics in infected cats [10, 24, 30–32]. Therefore, microfilarial densities can vary with the age of infected cats or dogs due likely to prolonged exposure to the enzoonotic B. pahangi infections and individual immune responses to the infections [23, 28, 30]. To complete life cycle, B. pahangi microfilarial parasites circulating in the peripheral blood of an infected cat or dog can be transmitted to local potent vectors while taking blood meals. Similar to that of B. malayi, the extrinsic period of B. pahangi can take about 7–11 days [19], during which molting of microfilariae undergoes in body parts of infected mosquito, followed by migration of infective larvae into the proboscis after the ingestion of blood containing microfilariae [8, 33]. Infected mosquitoes can then transmit L3 to other dogs or cats when taking another blood meal.
Brugia pahangi is not only transmitted among the animal reservoirs (domestic dogs or cats) but also switching the animal reservoir hosts so-called “zoonotic spillover” [34, 35]. Zoonotic spillover requires intertwined interactions that can sustain transmission of enzootic parasites between the animal reservoir population (domestic dogs and/or cats) and the local vector population. The spillover transmission of epizootic B. pahangi parasites is generated by various factors and successive processes of multiple spillovers that enable them to establish the infection in a susceptible person through frequent exposure to multiple bites of local potent vector(s) carrying the infection in home or work locations. Thus, if accompanied by poor living conditions and the lack of preventive measure or behavior, a susceptible person, especially an infant and preschool-age child, is more likely to become frequently exposed to the infection in indoor setting in home location than an outdoor setting. As compared to other indoor-resting mosquitoes like Aedes aegypti and Culex quinquefasciatus, Ar. subalbatus is a peri-domestic species that rests outdoors but more likely it has the potential to transmit epizootic B. pahangi parasites in impoverished environment of semiurban and rural areas in Malaysia [6, 7, 19] and Thailand [8, 9, 17, 18]. Adulthood infections are evident in Malaysia [6, 7, 19]. The “lingering” childhood infections (under 2 years of age) rather than adulthood infections are sporadic in Thailand [8, 9]. Such preschool-age children who carry the epizootic B. pahangi infections may harbor a wide range of microfilarial densities in the presence or absence of lymphatic pathology [8]. More likely, they develop the co-morbidity (Figure-1) in their lifetime in pre-patent or patent period. As is compared to B. pahangi or B. malayi infected cat, the incubation period for childhood infections is estimated as early as 3 months or up to 6 months [8]. The incubation period for adulthood infections remains unclear. The extrinsic period of zoonotic B. pahangi parasite in Ar. subalbatus is estimated 7–11 days [19]. Susceptible persons acquire repeated infections with zoonotic B. pahangi parasites (or multiple spillovers of epizootic strains) through mosquito-borne transmission – nor are they protected by any preventive measures or behaviors. The establishment of B. pahangi infection in Ar. subalbatus is later mentioned in detail.
Diagnosis of Zoonotic B. pahangi Parasite
Parasitological approaches to diagnosing infections in animal reservoirs and vectors are based on several methods of the preparation, detection, and identification of diagnostic stages of B. pahangi, that is, detecting and identifying morphologic characteristics of microfilariae and adult worms in cats or dogs and L3 in mosquitoes. The adult worms are found in the lymphatic vessels and nodes in infected cat or dog. Microfilariae are found in blood vessels in infected cat or dog. L3 larvae are found in proboscis of infected mosquito. It is clear that, in experimentally animal models such as cats [36] or ferrets [37, 38], L3 larvae of both B. pahangi and B. malayi can mature into adult worms in the popliteal and inguinal lymphatics of the injected limb with L3 inoculations and later microfilariae released by the fertilized female adult worms can live in blood circulation from 3 to 8 months after infection. Jackson-Thompson et al. [38] demonstrated that in ferrets, about 90% of adult worms were found in the inguinal and femoral lymphatics draining the infected limb and relatively less amount in the draining lymphatic vessels of the contralateral side. After 3–8 months’ post-infection, all infected ferrets developed microfilaremia with a peak density between 16 and 20-weeks post-infection. However, parasitic worm burdens, that is, both adult worms and microfilariae carried by infected male and female ferrets, did not differ from each other.
The adult worms of B. pahangi are morphologically recognized, with only the males, but not females, and are distinguishable from other Brugia species. The male worms of B. pahangi are 13–17 to 20–23 μm long; the female worms are 38–43 to 55–63 μm long [2, 21]. The male worms of B. pahangi recovered from infected cats and dogs are very similar to B. malayi [2, 39] and Brugia patei [40, 41]; the difference is that they have shortest spicules. The left spicule is 200–215 μm long. The right spicule is 75–90 μm long. The male spicules of B. patei are intermediate. The left spicule of B. patei male worm is 270 μm long, and the right spicule is 116 μm long. The longest male spicules of B. malayi are also easily recognized. The left spicule of B. malayi adult male is 390 μm, whereas the right spicule is 125 μm long.
The microfilaria of B. pahangi is not easily distinguishable from other Brugia species [28, 42], but it is morphologically recognized upon the methods of preparation, staining, and identification. As is similar to that of B. malayi, the Giemsa-stained microfilaria microscopically visualized differs only in having a slightly short cephalic space [37, 43] but likely having a relatively long innenkorper [37, 44]. The body length of B. pahangi microfilaria is 270–290 μm long when examined by Knott’s concentration technique; and 180–200 μm long when examined by Giemsa-stained thick blood films [37]. When stained using the acid phosphatase histochemical method [45–47], the B. pahangi microfilaria is relatively red throughout the length of its body. By contrast, the microfilaria of B. malayi is red, mainly at the excretory and anal pores.
As is compared to B. malayi [37, 48] and Wuchereria bancrofti [49], the L3 larvae of B. pahangi can be found in some susceptible mosquitoes, that is, either experimental mosquitoes that artificially fed on microfilariae infected blood or wild-caught mosquitoes in the fields. The susceptibility of some vectors for B. pahangi is described later. It is believed that after post infective stage development of L2 in thoracic muscle of the susceptible mosquitoes, the majority of mature L3 larvae migrate into the proboscis and live several days or up to 36 days, or as long as the mosquito host dies. The morphology of infective L3 of B. pahangi is not easily distinguished from that of B. malayi. The body length of L3 larva of B. malayi is 1600–3000 μm long [48]. The morphology of L3 of B. malayi is well recognized; the tapered head bearing eight submedian papillae arranged in two circles and a pair of lateral amphids; the long cylindrical buccal capsule; and the cuticular lappets on the tail extremity. The sex of L3 larva can be identified in late L2 stage to mature L3 stage upon the position of the genital primordium. The genital primordium is located at the mid-esophagus in mature L3 female larva. It is located at or just posterior to the esophago-intestinal junction in mature L3 male larva.
Routine laboratory diagnosis of B. pahangi infections in animal reservoirs or humans relies upon standard microscopic methods of detecting and identifying the microfilaremic infection. As is compared to that of other filarial worms such as Dirofilaria spp., B. malayi, and W. bancrofti, Giemsa-stained sheathed microfilariae of B. pahangi are microscopically recognized at 100× to 400× magnification. The preferred method is the collection of paired blood samples obtained from clinical cases (cats, dogs, or humans) at time intervals (day and night). In particular, the daytime microfilaremia of B. pahangi found in a case of human B. pahangi filariasis should be followed-up by examining the nighttime microfilaremia. If the sub-periodic strains of B. pahangi are assumed, the microfilariae may appear in peripheral blood during daytime or nighttime as this microfilarial periodicity may differ the appearance in blood in infected cats or dogs.
The endemic countries implementing the national program to eliminate lymphatic filariasis do not yet establish surveillance system for detecting and monitoring the zoonotic B. pahangi infections in animal reservoirs, nor do they ignore this neglected B. pahangi. Parasitological approaches applied to or used in case surveillance for human B. pahangi filariasis rely upon standard microscopic diagnosis of B. pahangi microfilaremic infections in cats or dogs of clinical filariasis (Figure-2). The preferred methods include thick blood films (using 20–60 mL blood of the ear vein, as well as cephalic, saphenous or jugular veins) [4, 5, 17, 18, 47, 50] and other specific and sensitive methods such as Knott’s concentration technique (using 1 mL blood) and membrane filtration (using 1–2 mL blood). However, the most preferred method is the application of polymerase chain reaction (PCR) assays [6–9, 17, 18, 46, 47, 50–52], which are highly specific and sensitive for B. pahangi distinguishable from other filarial parasites present in animal or human hosts or Ar. subalbatus vector [6-8] but not many other mosquitoes [53] as described below.
Figure-2.
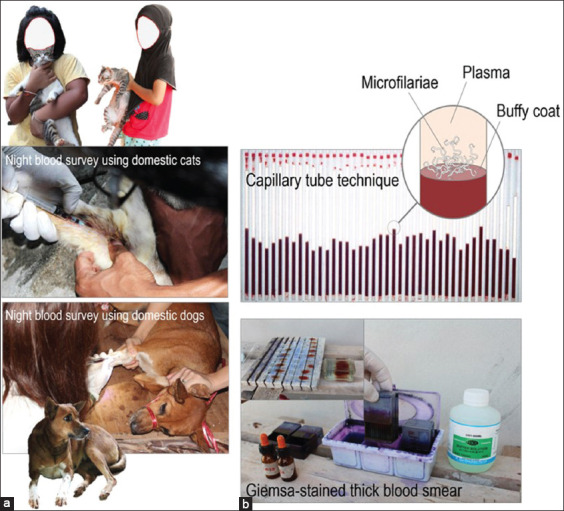
Animal reservoir survey using domestic cats and/or dogs. (a) During house-to-house visit, one collection of 1–2 mL blood volume of a domestic cat or dog is obtained by venipuncture at a time close to a peak hour of nocturnally sub-periodic microfilariae of Brugia pahangi. The cat or dog appropriately restrained by field staff is placed in either ventral or lateral recumbency with the forelimb (cephalic vein access) or hindlimb (saphenous vein access). The skin over the collection site that may or may not be clipped with an electric clipper is cleaned with 70% alcohol. (b) Standard microscopic blood examination using capillary tube technique or Giemsa’s stained thick blood smear can be applied under field conditions for screening or diagnosing Brugia spp. parasite infections. [Source: Graphic illustration created by A. Bhumiratana].
Contrary to Malaysia, which reported the zoonotic B. pahangi micorfilaremic infections in clinical adult patients between 2003 and 2004 [6, 7], Thailand has reported cumulatively clinical microfilaremic cases in recent years; four patients under 2 years of age in Eastern and Southern Thailand [8] and a 64-year-old female adult patients in Central Thailand [9]. All clinical cases are considered sporadic infections with the microfilaremic state occurring outside transmission areas of B. malayi. The investigation of these clinical cases of zoonotic B. pahangi infections contemporarily occurred in SEA relies conventionally on standard microscopic methods, and subsequently, the infections are confirmed by molecular detection methods. For instance, Giemsa-stained thick blood films examined for the presence of B. pahangi microfilariae in the patient and cat are illustrated in Figure-3. The sheathed microfilariae of zoonotic B. pahangi parasites found in two cumulative children-patients in Rayong, Eastern Thailand (Figure-3c) [8] compared well to that commonly found in microfilaremic cat (Figure-3a) and to that isolated from a B. malayi infected patient living in B. malayi endemic area of Southern Thailand (Figure-3b). In addition, laboratory-confirmed investigation of zoonotic B. pahangi infections in children-patients [8] or adult patients [6, 7] relies on molecular marker-based PCR assays specific for detection of Brugia spp. parasite infection and/or B. pahangi. There are several candidate filarial orthologous genes as molecular markers, for example, β-tubulin [8], the mitochondrial 12S ribosomal RNA (i.e., the mitochondrial small and large ribosomal subunits contain 12S and 16S rRNAs encoded by mitochondrial DNA) [9], and COX I (i.e., mitochondrial cytochrome c oxidase 1-encoding gene) [7, 19]. In Figure-4, PCR amplification of the internal transcribed spacer region 1 (ITS) I, which is the highly variable region of rRNA genes, can provide proof that the B. pahangi infection is discriminated from other filarial parasite infections present in any sources of the infection. But PCR amplification pattern of this genetic marker does not explain the zoonotic spillover of B. pahangi parasite infections present between infected persons and animal reservoirs. Analysis of sequenced amplicons authentically derived from ITS I is required for further exploring the human carrying B. pahangi infection whether or not epidemiologically linked to animal reservoirs carrying B. pahangi infection in any foci.
Figure-3.
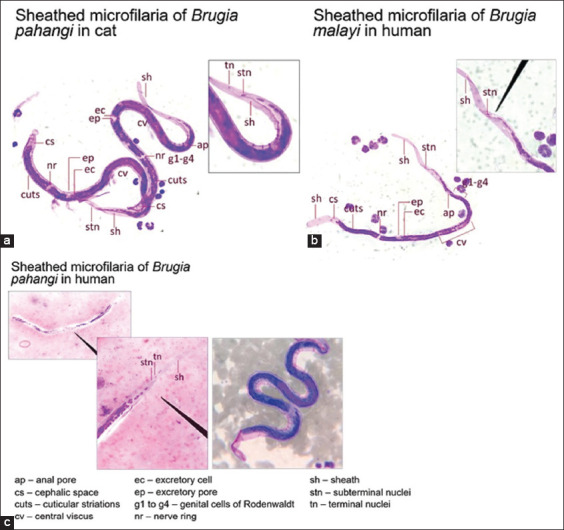
Comparison of well-defined morphologic characteristics (400× magnification) of nocturnally sub-periodic strains of Brugia pahangi and Brugia malayi. Giemsa-stained thick blood film as standard microscopic diagnosis of microfilarial parasites isolated from different sources of infection [8]: B. pahangi in cat (a) and human (c) and B. malayi in human (b). [Source: Graphic illustration created by A. Bhumiratana].
Figure-4.
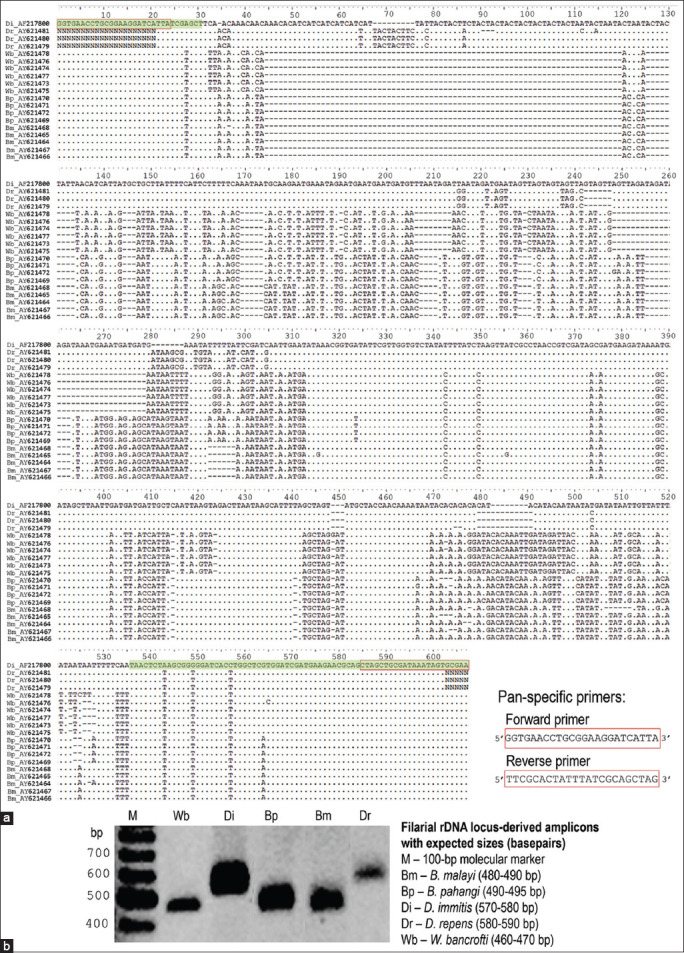
Polymerase chain reaction (PCR) using filarial ribosomal DNA internal transcribed spacer region 1 (ITS1) region-specific primers. (a) Multiple sequence alignment of amplified DNA fragments authentically derived from ribosomal genes of filarial parasites, which span partially 18S ribosomal RNA gene (green color-highlighted)-completely ITS1-partially 5.8S ribosomal RNA gene (green color-highlighted). The retrieved nucleotide sequences (accession no.) include Brugia malayi (Bm) (AY621464 to AY621468), Brugia pahangi (Bp) (AY621469 to AY621472), Wuchereria bancrofti (Wb) (AY621473 to AY621478), Dirofilaria repens (Dr) (AY621479 to AY621481), and Dirofilaria immitis (Di) (AF217800), whose DNA sequence data were derived from the GenBank database. BioEdit version 7.2 software application is used in multiple sequence alignment. The gap (insertion/deletion) is generated to maximize the homology representing conserved (•) and degenerate nucleotide residues. (b) PCR amplification using filarial ribosomal DNA ITS1 region-specific primers can yield putatively amplicons with expected sizes in reactions containing microfilarial DNA templates isolated from different sources of infections. [Source: Graphic illustration created by A. Bhumiratana].
In addition, antibody capture assays can be applied to the detection of anti-filarial immunoglobulin G4 (IgG4) antibodies present in serum or plasma samples of children patients or adult patients who are infected with zoonotic B. pahangi or other Brugia spp. parasites. Based on detection of specific IgG4 antibodies against BmR1 (Brugia Rapid) and BmSXP recombinant antigens [54–59], the commercially available antibody tests for brugian filariasis infection include the Brugia Rapid™ test (Reszon Diagnostics International Sdn. Bhd., Selangor, Malaysia) (https://reszonics.com/products/infectious-diseases-diagnosis/lateral-flow-rapid-test/Brugia-rapid-test/), and the PanLF Rapid™ test (Reszon Diagnostics International Sdn. Bhd.) (https://reszonics.com/products/infectious-diseases-diagnosis/lateral-flow-rapid-test/panlf-rapid-test/).
Vector of Zoonotic B. pahangi
Armigeres subalbatus (Coquillett, 1898) (Diptera: Culicidae) belonging to the genus Armigeres is originally a forest-associated zoophilic mosquito [8, 19, 20]. It is geographically distributed throughout South Asia, East Asia, and SEA. Two forms of Ar. subalbatus that share physiology and common characteristics are forest and plantation ecotypes. Forest ecotype is adapted to local habitats in forests at different altitudes, such as coastal forests [60], tropical forests, and deciduous forests, but unlikely swamp forests [8] and mangrove forests [53]. Plantation ecotype can breed in plantation areas [9, 53, 61], including rubber trees, oil palms, and fruit orchards, as well as rice fields [62, 63]. The species is closely associated with human settlements with poor sanitation [8, 53, 61, 62], and can thrive in rural and sub-urban areas [64–67]. As compared to other Aedes togoi and Cx. quinquefasciatus [16], it is a relatively potent vector of B. pahangi and Dirofilaria immitis [8, 17, 18], as well as other viruses [64–66], in human settlement areas. Perhaps it has the potential to transmit these zoonotic parasites temporally and spatially in receptive environments [8, 17, 19, 20]. Recently, there has been a line of evidence that the occurrence of human B. pahangi filariasis in Malaysia and Thailand has been epidemiologically linked with vector competence of Ar. subalbatus [7–9, 19]. Understanding vector competence of zoonotic B. pahangi parasite relies upon understanding its life cycle and success in adaptation of the plantation ecotype of Ar. subalbatus to human settlements.
Figure-5 illustrates a complete life cycle of the plantation ecotype of Ar. subalbatus that is constituted of adult and larval phases. It has four distinct developmental stages during its complete metamorphosis (i.e., egg, larva, pupa, and adult). Similar to other Mansonia, Aedes, Culex, and Anopheles [20, 68], female adult mosquito of Ar. subalbatus has a life span 1 month or up to 1.5 month during which its gonotrophic cycle (i.e., a fecundic life span) typically requires a blood meal by feeding on vertebrates (animals or humans) [60, 68, 69]. To obtain excess amount of blood as a source of a dietary supplement that stimulates and supports egg development, it is normally engorged. The number of its gonotophic cycle, as well as the number of laid eggs per gonotrophic cycle, is unknown. The duration of the gonotrophic cycle of Ar. subalbatus female adult mosquito delineates biting cycle (i.e., the frequency of vector–host contact) and potential transmission (i.e., the estimated number of infective L3 inoculation for transmission) of zoonotic B. pahangi parasite [8] during its life span. Two distinct circadian rhythms of female adult mosquitoes can be regulated by two different phases of the moon. Then biting activities occur when host-seeking behaviors increase during which the moon waxes as they decrease during which the moon wanes. As compared to other night-biting mosquitoes [68, 69], Ar. subalbatus elicits two biting cycles; highest peak of biting activities occurs early after the sunset or at dusk (18:00–20:00 h) [8, 68, 69] and a lesser peak at dawn (05:00–07:00 h) (Figure-6). Unlike the plantation ecotype, the forest ecotype of female adult mosquitoes actively seeks animal blood meals throughout the day in the forest.
Figure-5.
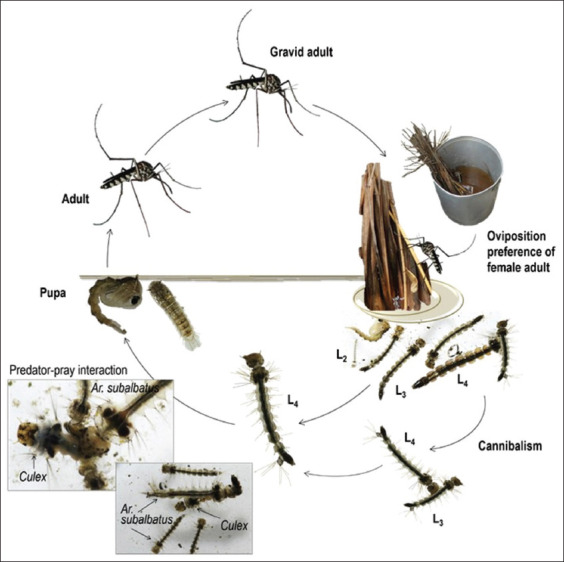
Life cycle of Armegires subalbatus for both forest and plantation ecotypes. [Source: Graphic illustration created by A. Bhumiratana].
Figure-6.
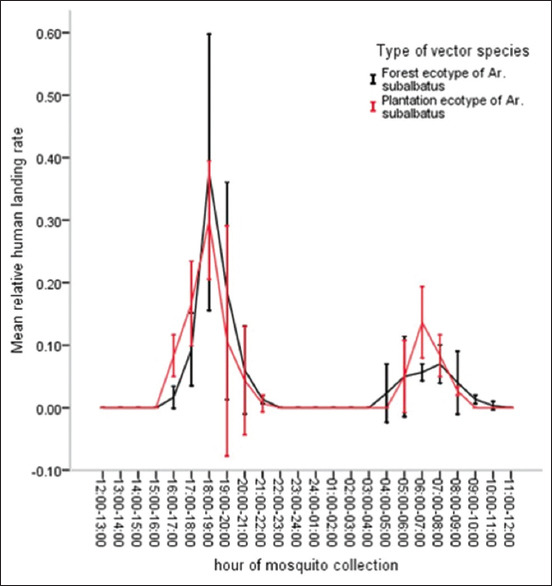
A 24 h cycle of biting activity of Armegires subalbatus. Biting activity of a given population of Ar. subalbatus represents mean relative human landing rate (HLR) (± 1 standard error denoted as bar) for each hour of mosquito collection by using 3 geographically defined population samples of each ecotype that were obtained from 3 different plantation areas connecting to forest fringes in Suratthani, Trat, and Rayong provinces. Relative HLR infers the number of human blood-seeking Ar. subalbatus female adult mosquitoes per night per person at each hour of mosquito collection (or HLR), which is divided by a sum of HLR for 24-h mosquito collection for each ecotype. Both forest and plantation ecotypes of Ar. subalbatus tend to seek human blood meals with a major peak hour (1800–1900 h) and another minor peak hour (0600–0700 h). [Source: Unpublished data by the authors].
The gravid female adult mosquito oviposits in naturally or artificially breeding sites containing stagnant foul waters (strongly polluted) with high organic contents [8, 70, 71]. The oviposition occurs during the nighttime. Most preferred natural containers include rock pools, tree holes, bamboo stumps, and banana stumps. Most preferred artificial containers include septic tanks, soakaway pits (drainages from households, rubber processing, fermented rice water, or fermented food waste), flower or plant holding baskets, fruits fermented tanks, and coagulated rubber containing cups (Figure-7). Contrary to oviposition, egg hatching, and adult eclosion occurs exclusively during the daytime. Larvae may be carnivorous [72] if particulate predator-prey interaction or cannibalism exists in food resource limited habitats. Larval molts (L1 to L4 stages) and pupation occur at night. Larval development from L1 to pupa takes about 10–14 days in suitable habitats.
Figure-7.
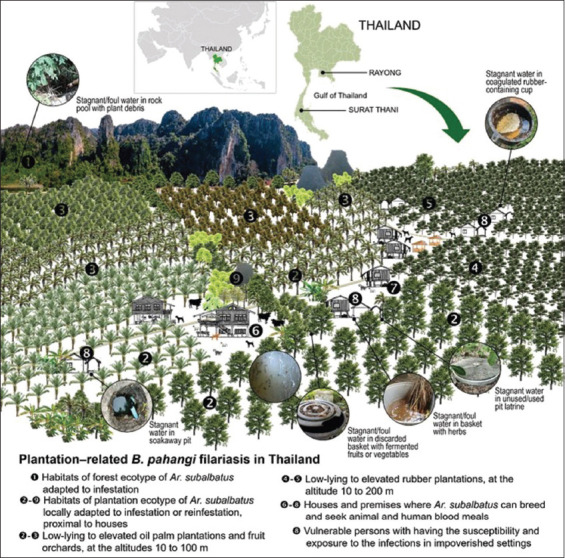
Pathogenic landscape of rural ecosystem of plantation-related Brugia pahangi filariasis in Thailand. This pathogenic landscape delineates complex eco-epidemiological settings in which vulnerable persons become close contact with animal reservoirs and Armegires subalbatus vectors under impoverished environments. [Source: Graphic illustration created by A. Bhumiratana].
Plantation-related B. pahangi Filariasis
Plantation-related B. pahangi filariasis (Figure-7) is the illness attributed to the infection of zoonotic B. pahangi parasites [8] that occurs in a susceptible person through mosquito-borne transmission in a receptive setting of the plantations mixed with human settlements. A focus of infection is a place where establishing impoverished human settlements and sustaining the infections of zoonotic B. pahangi parasites present in the animal reservoir hosts (domestic cats and/or dogs) and the local vectors. Both animal reservoir and vector hosts are the source of the zoonotic B. pahangi infections which in turn generates multiple spillovers of epizootic B. pahangi parasites over space and time. In Figure-7, a comprehensive picture of plantation-related B. pahangi filariasis can be depicted with the view of a scenic pathogenic or disease landscape [73], which integrates landscape attributes of rural ecosystem, that is, the spatial extent of, or interactions between, plantations, human settlements, animal reservoir hosts, local vectors, and local agri-environmental climatic conditions [74–76]. The study of this pathogenic landscape of zoonotic B. pahangi filariasis relies on using methods and tools used either in landscape epidemiology (the spatial and temporal variation in disease risk or incidence) [76, 77], landscape ecology (the relationships between ecological processes in the environment and particular disease ecosystems) [78–82], or the integration of landscape epidemiology and ecology.
In practical, disease landscape is a geographically defined landscape of a unique rural ecosystem of plantation-related B. pahangi filariasis, especially as the result of unique effects of spatial heterogeneity on the intertwined human-vector-animal reservoir interactions as mentioned earlier. It can be defined as small as georeferenced land unit that is the basis of the land use map legend which integrates the topological features (altitude, slope, and curvature) and the various land attribute data (land-form, soil, water body, and vegetation) as is shaped by natural and/or human alterations of land use and land cover [81–84]. The driving forces and processes of human-induced land use change are more likely to be influenced by land management strategies than natural environmental change. Disease landscape in rural ecosystem which, in turn, regulates such emerging B. pahangi infections is shaped by human alterations of land use and land cover as the result of ecological processes of such driving forces such as human settlements, crop plantations, and domestication of livestock and pet animals [77, 82, 83].
Such landscape ecology and epidemiology of zoonotic B. pahangi are crucial to better understand the vulnerability in how multiple spillovers of zoonotic B. pahangi parasites occur in a rural setting with environmental determinants in Thailand as shown in Figure-7. The environmental determinants of plantation-related B. pahangi filariasis are, therefore, all external factors and conditions that affect people’s lives, especially impoverished people and children. In rural setting, vulnerable young children experience insect or mosquito bites frequently, especially when they are exposed while sleeping inside or outside houses in the absence of preventive measures. Furthermore, many people keep dogs to guard their homes or farms; and likely keep cats to catch rats. These family-pet animals are kept or taken care of for companionship or for personal safety, but the pet owners are not aware of animal reservoirs for zoonotic B. pahangi parasites that potentially spread the parasites to people.
Current evidence supports the fact that particularly in Malaysia [6, 7, 19] and Thailand [4, 5, 8, 9, 17, 18], local people did not know how communicable zoonotic B. pahangi parasite is, nor were they aware of the parasite being one of the most common zoonotic diseases of cats or dogs. Pet cats or dogs can pose a minimal zoonotic risk to their human companions. Although monitoring any sign of illness or disease in sick cats or dogs, the disease is not normally diagnosed and treated promptly due to the limitations of the owners’ caring. However, a person with a compromised immune system from disease or medication may render him or her slightly vulnerable to contracting zoonosis from his or her infected cat or dog. If accompanied by getting sick and of more serious illnesses or co-morbidities (Figure-1), a vulnerable person such as infants and preschool-age child has a higher chance of contracting the zoonotic B. pahangi infection [8] due likely to their immune compromise and the lack of family perception and awareness of such zoonotic disease and local vectors. Thereafter, such pathogenic landscape of plantation-related B. pahangi filariasis seen in Thailand strongly relates childhood infection or disease to the multiple spillovers of epizootic B. pahangi parasites in an infection pocket to which children-animal-vector interactions are related.
The infection pocket is explained by the establishment of “lingering” infection with, but not the propagated outbreak of, epizootic B. pahangi parasites. Such vulnerable person contracting zoonosis is likely to be epidemiologically linked with environmental determinants, for example, being frequently exposed to multiple bites of Ar. subalbatus in indoor setting in the absence of preventive measures. If accompanied by keeping close contact with infected cats or dogs and Ar. subalbabtus vectors over a period of time, such vulnerable person becomes at higher risk of, or is affected with, the infection of zoonotic B. pahangi parasites. This may be a reason why transmission pattern of zoonotic B. pahangi filariasis seems not to be the propagated outbreak as seen in transmission areas of other vector-borne diseases such as W. bancrofti filariasis [85, 86] and malaria [87, 88]. The establishment of such infection pocket in a pathogenic landscape as shown in Figure-7 relies on sustaining the prevalence and distribution of the infections with zoonotic B. pahangi parasites present in both cats and/or dogs and Ar. subalbatus vectors [8]. Taken together, this acquisition of knowledge of plantation-related B. pahangi filariasis is crucial for surveillance and case investigation, as mentioned below.
Perspective in Surveillance and Case Investigation of Epizootic B. pahangi Infection
The occurrence of sporadic infection with epizootic B. pahangi parasites can be differentiated from that observed by local transmission caused by B. malayi or W. bancrofti in endemic settings. However, the detection of anomalous infection with epizootic B. pahangi parasites among vulnerable persons residing in Ar. subalbatus infested land areas does not rely on routine background surveillance as part of lymphatic filariasis control or elimination. For instance, surveillance and case investigation of epizootic B. pahangi infection among children-patients in Thailand [8] is based on a systemic collection of data and/or information obtained by the following methods such as medical record review, household survey, animal reservoir infection survey, and entomological survey.
Medical record review
The medical record can provide information from when the patient was living, undergoing clinical symptoms and receiving differential diagnosis and empirical treatment by practitioners to their recovery. A medical record review could help the epidemiologists, infection control personnel, and public health professionals understand what caused the overt features of lymphatic pathology in the presence or absence of the co-morbidities [8], as shown for children-patients in Figure-1, or certain medical conditions. Recently the emergence of zoonotic B. pahangi infections in Thailand has revealed that the parents’ perceptions of current health problems of their children-patients may regard illnesses that are not associated with lymphatic filarial infections. More obviously, such cumulative children-patients were hospitalized with clinical presentations, which are not associated with lymphatic filarial infections [8]. On the other hand, empirical treatment of children-patients’ illnesses relies radically on epidemiological data and differential diagnosis whether clinically, parasitologically, or serologically. All microfilaremic infections were confirmed using PCR assays as mentioned earlier.
Moreover, the medical record review can provide further information of which the practitioners were diagnosing lymphatic filarial infection and the details of the eligibility criteria for treatment of children-patients with lymphatic filarial infection and treatment outcomes, that is, whether patho-physiologically, psychological, physically, or socially, that reflect favorable or adverse effects on the patients’ health and well-being. Lymphatic filarial infection often occurs in early childhood, but manifestation of clinical lymphedema typically occurs later in life. However, the detection of lymphatic obstruction in asymptomatic children has been documented using lymphoscintigraphy. Brugia pahangi in childhood infections does not seem to present overt features of lymphatic pathology. Similar to B. malayi, the infection can be treated with a single oral-dose 6 mg/kg diethylcarbamazine as anti-filarial drug, and microfilaremia clearance is observed through a course of radical treatment that can last for several months.
Household survey
A house-to-house visit can provide information about which home environments are unsafe, why parents or caregivers of children-patients are unaware of protecting local vectors’ bites, and which living conditions render children-patients frequently exposed to biting of local vectors or susceptible to contracting B. pahangi zoonosis. Both unsafe home environment and unclean household environmental cleaning observed between a patient’s house and nearby houses are among plausible factors that influence the vulnerability of zoonotic B. pahangi infection.
Animal reservoir infection survey
Children-patients contracting B. pahangi zoonosis are likely to be associated with keeping close contact with domestication of pet animals such as cats or dogs. In fact, a patient’s house may or may not keep any pet animals, but there is the preferred domestication of cats or dogs in neighboring houses. As mentioned earlier, animal reservoir infection survey using day or night blood samples of cats and/or dogs (Figure-2) can provide accurate data on the source of infection in an infection pocket, or within a 10–100-m radius of a patient’s house. The B. pahangi infection prevalence (or microfilaremia rate) determined by the preferred blood examination methods (Figure-2) is considered a proxy measure of sustaining B. pahangi zoonosis in a responsible infection pocket.
Entomological survey
An entomological survey can provide the proof that the local vector has the potential to transmit B. pahangi zoonosis or carries the zoonotic B. pahangi infection [8, 87, 88]. Soon after reporting a B. pahangi contracted case, a routine entomological survey relies on the establishment of a sentinel site suited to sampling local vector populations; this also includes a patient’s house. If the zoonotic B. pahangi infection is locally acquired through mosquito-borne transmission in certain place and time, two assumptions of the entomologic investigation of plantation-related B. pahangi filariasis in humans need to be logically analyzed. If the infection occurs in an area where B. malayi is endemic, Ar. subalbatus versus its counterparts such as Mansonia uniformis, Mansonia indiana, and Cx. quinquefasciatus are suspected to be vector-host preference of B. pahangi. If the infection occurs in an area where B. malayi is not endemic, Ar. subalbatus versus its counterparts, such as Ae. togoi and Cx. quinquefasciatus are suspected to be vectors of B. pahangi.
Such emerging B. pahangi infections occurring in non-transmission area of B. malayi in Thailand have been epidemiologically linked to be transmitted by locally adapted plantation ecotype of Ar. subalbatus vector. Based on appropriate collection methods and species identification, indoor and outdoor collections of female adult mosquitoes are needed. The availability of larval breeding sites for Ar. subalbatus is considered proximal to a patient’s house or distant within a 10–100-m radius of a patient’s house in an infection pocket. For instance, Ar. subalbatus elicits the infestation, that is, the abundance and distribution during its peak hour at night (Figure-6) for 2–3 consecutive days. Its infestation relates to adult vector abundance to the availability of larval breeding sites that elicit larval abundance surrounding a patient’s house.
Several plausible factors that influence the levels of infestation or reinfestation of Ar. subalbatus include human settlements, household sanitation, the domestication of livestock or pet animals, and local environments suited to favor larval breeding. It is possible that if accompanied by sustaining the levels of B. pahangi infection prevalence in domestic cats and/or dogs, the degrees of B. pahangi infection prevalence in Ar. subalbatus are strongly associated with a unique ecotope of plantation-related B. pahangi filariasis [8]. Nonetheless, any B. pahangi contracted patient may or may not relate the infection to keeping close contact with pet animals such as cats or dogs in impoverished patient’s house.
Conclusion
After the termination of the elimination (or interruption of transmission) of B. malayi or W. bancrofti in humans in endemic countries implementing the national program to eliminate lymphatic filariasis, the program coordinators and allied sectors need to understand the likelihood that local inhabitants residing in either transmission or non-transmission areas are at risk of, or affected with, the zoonotic B. pahangi infections if they are likely to have living or working conditions with the socio-ecological and biological vulnerability. Any local inhabitants who may carry the zoonotic B. pahangi infection in their lifetime are more likely to be infants and preschool-age children than school-age children and adults. As for case surveillance or investigation, any B. pahangi patient-cases who are diagnosed using serological and parasitological diagnostic methods are laboratory-confirmed by molecular marker-based PCR assays. However, the PCR-positive results do not differentiate between the clonal and multiple clonal PCR products that infer the spillover of zoonotic B. pahangi parasite populations circulating in any foci.
Authors’ Contributions
AB: Conceived the concepts of plantation-related zoonotic B. pahangi filariasis and original artworks or graphic illustration. AB, PN, AI, SP, and WR: Provided current knowledge of B. pahangi life cycle, vector’s life cycle and current state of research on the epidemiology and ecology of B. pahangi zoonosis. AB and AI: Conceived the diagnostic approaches to detecting and differentiating B. pahangi and leveraged the bioinformatics. AB, PN, SP, and WR: Conceived and leveraged the data/information of Ar. subalbatus. All authors have read, reviewed, and approved the final manuscript.
Acknowledgments
This study was a part of the research project financially granted by Thailand Research Fund (TRF) (grant no. MRG5680087, 2013) and China-Medical Board (CMB)-Mahidol University (grant no. PHRU/CMB 02/2558).
Competing Interests
The authors declare that they have no competing interests.
Publisher’s Note
Veterinary World remains neutral with regard to jurisdictional claims in published map and institutional affiliation.
References
- 1.Buckley J.J.C. On Brugia gen. nov for Wuchereria spp. of the “malayi”group, i.e. W. malayi (Brug, 1927), W. pahangi Buckley and Edeson, 1956, W. patei Buckley, Nelson, and Heisch, 1958. Ann. Trop. Med. Parasitol. 1960;54(1):75–77. doi: 10.1080/00034983.1960.11685959. [DOI] [PubMed] [Google Scholar]
- 2.Buckley J.J, Edeson J.F. On the adult morphology of Wuchereria spp. (malayi?) from a monkey (Macaca iris) and from cats in Malaya, and on Wuchereria pahangi n. spp. from a dog and a cat. J. Helmintol. 1956;30(1):1–20. doi: 10.1017/s0022149x00032922. [DOI] [PubMed] [Google Scholar]
- 3.Mak J.W, Yen P.K, Lim K.C, Ramiah N. Zoonotic implications of cats and dogs in filarial transmission in Peninsular Malaysia. Trop. Geogr. Med. 1980;32(3):259–264. [PubMed] [Google Scholar]
- 4.Rawangchue T, Sripirom N, Sungpradit S. Surveillance of zoonotic Brugia pahangi in monastery cats, Samphran district, Nakhon Pathom, Thailand. Thai J. Vet. Med. 2022;52(1):117–125. [Google Scholar]
- 5.Jitsamai W, Piromkij P, Kamkong P, Chungpivat S, Taweethavonsawat P. Seasonal distribution and environmental parameters associated with Brugia pahangi and Dirofilaria immitis in naturally infected dogs in Bangkok and vicinity, Thailand. Sci. Rep. 2021;11(1):4594. doi: 10.1038/s41598-021-84215-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tan L.H, Fong M.Y, Mahmud R, Muslim A, Lau Y.L, Kamarulzaman A. Zoonotic Brugia pahangi filariasis in a suburbia of Kuala Lumpur City, Malaysia. Parasitol. Int. 2011;60(1):111–113. doi: 10.1016/j.parint.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 7.Muslim A, Fong M.Y, Mahmud R, Sivanandam S. Vector and reservoir host of a case of human Brugia pahangi infection in Selangor, peninsular Malaysia. Trop. Biomed. 2013;30(4):727–730. [PubMed] [Google Scholar]
- 8.Intarapuk A, Bhumiratana A. Investigation of Armigeres subalbatus, a vector of zoonotic Brugia pahangi filariasis in plantation areas in Suratthani, Southern Thailand. One Health. 2021;13:100261. doi: 10.1016/j.onehlt.2021.100261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thongpiya J, Sa-Nguanraksa D, Samarnthai N, Sarasombatha P.T. Filariasis of the breast caused by Brugia pahangi:A concomitant finding with invasive ductal carcinoma. Parasitol. Int. 2021;80:102203. doi: 10.1016/j.parint.2020.102203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rogers R, Denham D.A. Studies with Brugia pahangi. 7. Changes in lymphatics of injected cats. J. Helminthol. 1974;48(3):213–219. doi: 10.1017/s0022149x00022860. [DOI] [PubMed] [Google Scholar]
- 11.Snowden K.F, Hammerberg B. The lymphatic pathology of chronic Brugia pahangi infection in the dog. Trans. R. Soc. Trop. Med. Hyg. 1989;83(5):670–678. doi: 10.1016/0035-9203(89)90394-5. [DOI] [PubMed] [Google Scholar]
- 12.Mattick J, Libro S, Sparklin B.C, Chung M, Bromley R.E, Nadendla S, Zhao X, Ott S, Sadzewicz L, Tallon L.J, Michalski M.L, Foster J.M, Hotopp J.C.D. Nearly complete genome sequence of Brugia pahangi FR3. Microbiol. Resour. Announc. 2020;9(27):e00479–20. doi: 10.1128/MRA.00479-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lau Y.L, Lee W.C, Xia J, Zhang G.P, Razali R, Anwar A, Fong M.Y. Draft genome of Brugia pahangi:High similarity between B. pahangi and B. malayi. Parasit. Vectors. 2015;8:451. doi: 10.1186/s13071-015-1064-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beerntsen B.T, Luckhart S, Christensen B.M. Brugia malayi and Brugia pahangi:Inherent difference in immune activation in the mosquitoes Armigeres subalbatus and Aedes aegypti. J. Parasitol. 1989;75(1):76–81. [PubMed] [Google Scholar]
- 15.Aliota M.T, Fuchs J.F, Rocheleau T.A, Clark A.K, Hillyer J.F, Chen C.C, Christensen B.M. Mosquito transcriptome profiles and filarial worm susceptibility in Armigeres subalbatus. PLoS Negl. Trop. Dis. 2010;4(4):e666. doi: 10.1371/journal.pntd.0000666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vinnie-Siow W.Y, Low V.L, Tan T.K, Wong M.L, Leong C.S, Ahmad N.W, Lim Y.A.L. Identification of potential vectors of Dirofilaria immitis and Brugia pahangi (Spirurida:Filariidae):First observation of infective third-stage larva of B. pahangi in Culex quinquefasciatus (Diptera:Culicidae) Pathog. Glob. Health. 2022;116(6):356–364. doi: 10.1080/20477724.2022.2035624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaikuntod M, Arjkumpa O, Kladkempetch D, Fukumoto S, Thongkorn K, Boonyapakorn C, Punyapornwithaya V, Tiwananthagorn S. Geographic spatial distribution patterns of Dirofilaria immitis and Brugia pahangi infection in community dogs in Chiang Mai, Thailand. Animals (Basel) 2020;11(1):33. doi: 10.3390/ani11010033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Satjawongvanit H, Phumee A, Tiawsirisup S, Sungpradit S, Brownell N, Siriyasatien P, Preativatanyou K. Molecular analysis of canine filaria and its Wolbachia endosymbionts in domestic dogs collected from two animal university hospitals in Bangkok Metropolitan region, Thailand. Pathogens. 2019;8(3):114. doi: 10.3390/pathogens8030114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Muslim A, Fong M.Y, Mahmud R, Lau Y.L, Sivanandam S. Armigeres subalbatus incriminated as a vector of zoonotic Brugia pahangi filariasis in suburban Kuala Lumpur, Peninsular Malaysia. Parasit. Vectors. 2013;6(1):219. doi: 10.1186/1756-3305-6-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mulyaningsih B, Umniyati S.R, Hadisusanto S, Edyansyah E. Study on vector mosquito of zoonotic Brugia malayi in Musi Rawas, South Sumatera, Indonesia. Vet. World. 2019;12(11):1729–1734. doi: 10.14202/vetworld.2019.1729-1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schacher J.F. Developmental stages of Brugia pahangi in the final host. J. Parasitol. 1962;48(5):693–706. [PubMed] [Google Scholar]
- 22.Edeson J.F.B, Wharton R.H, Laing A.B.G. A preliminary account of the transmission, maintenance and laboratory vectors of Brugia pahangi. Trans. R. Soc. Trop. Med. Hyg. 1960;54(5):439–449. doi: 10.1016/0035-9203(60)90089-4. [DOI] [PubMed] [Google Scholar]
- 23.Ewert A, Singh M. Microfilarial levels in cats infected with Brugia pahangi by two alternative routes. Trans. R. Soc. Trop. Med. Hyg. 1969;63(5):603–607. doi: 10.1016/0035-9203(69)90178-3. [DOI] [PubMed] [Google Scholar]
- 24.Denham D.A, Rogers R. Structural and functional studies on the lymphatics of cats infected with Brugia pahangi. Trans. R. Soc. Trop. Med. Hyg. 1975;69(2):173–176. doi: 10.1016/0035-9203(75)90149-2. [DOI] [PubMed] [Google Scholar]
- 25.Sucharit S. Brugia pahangi in small laboratory animals:The microfilarial periodicity. Southeast Asian J. Trop. Med. Public Health. 1973;4(4):492–497. [PubMed] [Google Scholar]
- 26.Suswillo R.R, Denham D.A, McGreevy P. The number and distribution of Brugia pahangi in cats at different times after a primary infection. Acta Trop. 1982;39(2):151–156. [PubMed] [Google Scholar]
- 27.Chungpivat S, Sucharit S. Microfilariae in cats in Bangkok. Thai. J. Vet. Med. 1993;23(2):75–87. [Google Scholar]
- 28.Partono F, Oemijati S.H, Joesoef A, Clarke M.D, Durfee P.T, Irving G.S, Taylor J, Cross J.H. Brugia malayi in seven villages in south Kalimatnan, Indonesia. Southeast Asian J. Trop. Med. Public Health. 1977;8(3):400–407. [PubMed] [Google Scholar]
- 29.Denham D.A, Medeiros F, Baldwin C, Kumar H, Midwinter I.C.T, Vbirch D.W, Smail A. Repeated infection of cats with Brugia pahangi:Parasitological observations. Parasitology. 1992;104(pt 3):415–420. doi: 10.1017/s0031182000063666. [DOI] [PubMed] [Google Scholar]
- 30.Denham D.A, Ponnudurai T, Nelson G.S, Rogers R, Guy F. Studies with Brugia pahangi I. Parasitological observations on primary infections of cats (Felis catus) Int. J. Parasitol. 1972;2(2):239–247. doi: 10.1016/0020-7519(72)90012-4. [DOI] [PubMed] [Google Scholar]
- 31.Rogers R, Denham D.A, Nelson G.S, Guy F, Ponnudurai T. Studies with Brugia pahangi. III. Histological changes in the affected lymph nodes of infected cats. Ann. Trop. Med. Parasitol. 1975;69(1):77–84. [PubMed] [Google Scholar]
- 32.Rogers R, Denham D.A. Studies with Brugia pahangi:11. Measurements of lymph flow in infected cats. Southeast Asian J. Trop. Med. Public Health. 1975;6(2):199–205. [PubMed] [Google Scholar]
- 33.Schacher J.F. Morphology of the microfilaria of Brugia pahangi and of the larval stages in the mosquito. J. Parasitol. 1962;48(5):679–692. [PubMed] [Google Scholar]
- 34.Thompson R.C.A. Parasitic zoonoses and wildlife:One health, spillover and human activity. Int. J. Parasitol. 2013;43(12–13):1079–1088. doi: 10.1016/j.ijpara.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ellwanger J.H, Fearnside P.M, Ziliotto M, Valverde-Villegas J.M, Veiga A.B.G, Vieira G.F, Bach E, Cardoso J.C, Müller N.F.D, Lopes G, Caesar L, Kulmann-Leal B, Kaminski V.L, Silveira E.S, Spilki F.R, Weber M.N, Almeida S.E.M, Hora V.P.D, Chies J.A.B. Synthesizing the connections between environmental disturbances and zoonotic spillover. An. Acad. Bras. Cienc. 2022;94(suppl 3):e20211530. doi: 10.1590/0001-3765202220211530. [DOI] [PubMed] [Google Scholar]
- 36.Ewert A. Distribution of developing and mature Brugia malayi in cats at various times after a single inoculation. J. Parasitol. 1971;57(5):1039–1042. [PubMed] [Google Scholar]
- 37.Crandall R.B, McGreevy P.B, Connor D.H, Crandall C.A, Neilson J.T, McCall J.W. The ferret (Mustela putorius furo) as an experimental host for Brugia malayi and Brugia pahangi. Am. J. Trop. Med. Hyg. 1982;31(4):752–759. doi: 10.4269/ajtmh.1982.31.752. [DOI] [PubMed] [Google Scholar]
- 38.Jackson-Thompson B.M, Kim S.Y, Jaiswal S, Scott J.R, Jones S.R, Morris C.P, Fite J.J, Laurie K, Hoy A.R, Dardzinski B.J, Mitre E. Brugia malayi infection in ferrets-a small mammal model of lymphatic filariasis. PLoS Negl. Trop. Dis. 2018;12(3):e0006334. doi: 10.1371/journal.pntd.0006334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ahmed S.S. Location of developing and adult worms of Brugia spp. in naturally and experimentally infected animals. J. Trop. Med. Hyg. 1966;69(12):291–293. [PubMed] [Google Scholar]
- 40.Buckley J.J.C, Nelson G.S, Heisch R.B. On Wuchereria patei n.sp. from the lymphatics of cats, dogs and genet cats on Pate Island, Kenya. J. Helminthol. 1958;32(1–2):73–80. doi: 10.1017/s0022149x00019362. [DOI] [PubMed] [Google Scholar]
- 41.Laurence B.R, Pester F.R. Adaptation of a filarial worm, Brugia patei, to a new mosquito host, Aedes togoi. J. Helminthol. 1967;41(4):365–392. doi: 10.1017/s0022149x00021908. [DOI] [PubMed] [Google Scholar]
- 42.Sucharit S, Harinasuta C, Viraboonchai S, Smithanonda S. The differentiation of Brugia malayi, B. pahangi, B. tupaiae and Wuchereria bancrofti. Southeast Asian J. Trop. Med. Public Health. 1975;6(4):549–554. [PubMed] [Google Scholar]
- 43.Laurence B.R, Simpson M.G. Cephalic and pharyngeal structures in microfilariae revealed by staining. J. Helminthol. 1968;42(3):309–330. doi: 10.1017/s0022149x00017922. [DOI] [PubMed] [Google Scholar]
- 44.Sivanandam S, Fredericks H.J. The “Innenkörper”in differentiation between the microfilariae of Brugia pahangi and B. malayi (sub-periodic form) Med. J. Malaya. 1966;20(4):337–338. [PubMed] [Google Scholar]
- 45.Redington B.C, Montgomery C.A, Jervis H.R, Hockmeyer W.T. Histochemical differentiation of the microfilariae of Brugia pahangi and sub-periodic Brugia malayi. Ann. Trop. Med. Parasitol. 1975;69(4):489–492. [Google Scholar]
- 46.Ravindran R, Varghese S, Nair S.N, Balan V.M, Lakshmanan B, Ashruf R.M, Kumar S.S, Kumar A, Gopalan K, Nair A.S, Malayil A, Chandrasekhar L, Juliet S, Kopparambil D, Ramachandran R, Kunjupillai R, Kakada S.A.M. Canine filarial infections in a human Brugia malayi endemic area of India. Biomed. Res. Int. 2014;2014:630160. doi: 10.1155/2014/630160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chirayath D, Alex P.C, Pillai U.N, George S, Ajithkumar S, Panicker V.P. Identification of Brugia malayi in dogs in Kerala, India. Trop. Biomed. 2017;34(4):804–814. [PubMed] [Google Scholar]
- 48.Mutafchiev Y, Bain O, Williams Z, McCall J.W, Michalski M.L. Intraperitoneal development of the filarial nematode Brugia malayi in the Mongolian jird (Meriones unguiculatus) Parasitol. Res. 2014;113(5):1827–1835. doi: 10.1007/s00436-014-3829-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hoti S.L, Gros A.M, Paily K.P, Manonmani A.M, Mary K.A, Balaraman K. In vitro cultivation of third stage larvae of Wuchereria bancrofti to fourth stage:Influence of some physico-chemical factors. Southeast Asian J. Trop. Med. Public Health. 1994;25(2):278–283. [PubMed] [Google Scholar]
- 50.Al-Abd N.M, Nor Z.M, Kassim M, Mansor M, Al-Adhroey A.H, Ngui R.R, Sivanandam S. Prevalence of filarial parasites in domestic and stray cats in Selangor State, Malaysia. Asian Pac. J. Trop. Med. 2015;8(9):705–709. doi: 10.1016/j.apjtm.2015.07.034. [DOI] [PubMed] [Google Scholar]
- 51.Nonsaithong D, Yotmek S, Yotmek S, Nochote H, Wongkamchai S, Roytrakul S, Lek-Uthai U. High resolution melting real-time PCR detect and identify filarial parasites in domestic cats. Asian Pac. J. Trop. Med. 2018;11(12):682–687. [Google Scholar]
- 52.Loymek S, Phuakrod A, Zaelai K, Sripumkhai W, Vongjaroensanti P, Wongkamchai S. Investigation on the prevalence of canine microfilaremia in Thailand using a novel microfluidic device in combination with real-time PCR. Vet. Sci. 2021;8(3):39. doi: 10.3390/vetsci8030039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Maquart P.O, Sokha C, Boyer S. Mosquito (Diptera:Culicidae) diversity and medical importance in Koh Kong mangrove forests, Cambodia. Asian Biomed. 2022;16(3):121–129. doi: 10.2478/abm-2022-0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Omar N, Hamidon N.H, Yunus M.H, Noordin R, Choong Y.S, Lim T.S. Generation and selection of naïve Fab library for parasitic antigen:Anti-BmSXP antibodies for lymphatic filariasis. Biotechnol. Appl. Biochem. 2018;65(3):346–354. doi: 10.1002/bab.1591. [DOI] [PubMed] [Google Scholar]
- 55.World Health Organization. Diagnostic Test for Surveillance of Lymphatic Filariasis:Target Product Profile. Geneva: World Health Organization; 2021. [Google Scholar]
- 56.Pastor A.F, Silva M.R, Dos Santos W.J.T, Rego T, Brandão E, de-Melo-Neto O.P, Rocha A. Recombinant antigens used as diagnostic tools for lymphatic filariasis. Parasit. Vectors. 2021;14(1):474. doi: 10.1186/s13071-021-04980-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rahumatullah A, Lim T.S, Yunus M.H, Noordin R. Development of an antigen detection ELISA for bancroftian filariasis using BmSXP-specific recombinant monoclonal antibody. Am. J. Trop. Med. Hyg. 2019;101(2):436–440. doi: 10.4269/ajtmh.19-0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Noordin R, Zain S.N.M, Yunus M.H, Sahimin N. Seroprevalence of lymphatic filariasis among migrant workers in Peninsular Malaysia. Trans. R. Soc. Trop. Med. Hyg. 2017;111(8):370–372. doi: 10.1093/trstmh/trx062. [DOI] [PubMed] [Google Scholar]
- 59.Noordin R, Yunus M.H, Robinson K, Won K.Y, Babu S, Fischer P.U, Hisam S, Mahmud R. Laboratory evaluation of a rapid IgG4 antibody test (BLF Rapid™) for bancroftian filariasis. Am. J. Trop. Med. Hyg. 2018;99(6):1587–1590. doi: 10.4269/ajtmh.18-0566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Boonserm R, Jantorn R, Phumee A, Sor-Suwan S, Jariyapan N, Tiawsirisup S, Siriyasatien P. Identification of blood meal from field collected filarial vector mosquitoes, Armigeres subalbatus by multiplex PCR. Thai J. Vet. Med. 2019;49(2):155–160. [Google Scholar]
- 61.Maquart P.O, Fontenille D, Nil Rahola N, Yean S, Boyer S. Checklist of the mosquito fauna (Diptera, Culicidae) of Cambodia. Parasite. 2021;28:60. doi: 10.1051/parasite/2021056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pilagolla S.A.S, Amarasinghe L.D. Assessing the filariasis causing parasites in adult mosquitoes and the vector mosquito larval breeding in selected medical officer of health areas in Gampaha District, Sri Lanka. J. Trop. Med. 2021;2021:6643226. doi: 10.1155/2021/6643226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Garjito T.A, Widiarti Anggraeni Y.M, Alfiah S, Satoto T.B.T, Farchanny A, Samaan G, Afelt A, Manguin S, Frutos R, Aditama T.Y. Japanese encephalitis in Indonesia:An update on epidemiology and transmission ecology. Acta Trop. 2018;187:240–247. doi: 10.1016/j.actatropica.2018.08.017. [DOI] [PubMed] [Google Scholar]
- 64.Xu X, Wang J, Liu H, Wang Q, Fu S, Zhang J, Wang B, He Y, Li F, Nie K, Xu S, Wang H, Lu X, Shi M, Liang G. Two rhabdoviruses, one novel, isolated from Armigeres subalbatus in China. Pathogens. 2022;11(6):624. doi: 10.3390/pathogens11060624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yang W, Zhao S, Xie Y, Liu T, Kong L, Guo Y, Xie Z, Liu P, Chen X.G. Armigeres subalbatus is a potential vector for Zika virus but not dengue virus. Infect. Dis. Poverty. 2022;11(1):62. doi: 10.1186/s40249-022-00990-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liu W.T, Chen Y.J, Chen C.C, Liao K.M, Tzeng H.Y, Tu W.C. Impact of temperature on infection with Japanese encephalitis virus of three potential urban vectors in Taiwan;Aedes albopictus, Armigeres subalbatus, and Culex quinquefasciatus. Acta Trop. 2023;237:106726. doi: 10.1016/j.actatropica.2022.106726. [DOI] [PubMed] [Google Scholar]
- 67.Li S, Jiang F, Lu H, Kang X, Wang Y, Zou Z, Wen D, Zheng A, Liu C, Liu Q, Kang L, Xia Q, Cui F. Mosquito diversity and population genetic structure of six mosquito species from Hainan island. Front. Genet. 2020;11:602863. doi: 10.3389/fgene.2020.602863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ridha M.R, Rahayu N, Hairani B, Perwitasari D, Kusumaningtyas H. Biodiversity of mosquitoes and Mansonia uniformis as a potential vector of Wuchereria bancrofti in Hulu Sungai Utara District, South Kalimantan, Indonesia. Vet. World. 2020;13(12):2815–2821. doi: 10.14202/vetworld.2020.2815-2821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chen C.D, Wan-Norafikah O, Nurin-Zulkifli I.M, Lee H.L, Faezah K, Izzul A.A, Abdullah A.G, Lau K.W, Norma-Rashid Y, Sofian-Azirun M. Biting behaviour of medically important mosquitoes (Diptera:Culicidae) in Peninsular Malaysia. Trop. Biomed. 2017;34(1):199–211. [PubMed] [Google Scholar]
- 70.Singh B, Baruah C, Saikia D, Gurung J. Species composition of mosquito breeding in bamboo stumps in Sikkim, India. J. Vector Borne Dis. 2020;57(1):96–100. doi: 10.4103/0972-9062.308808. [DOI] [PubMed] [Google Scholar]
- 71.Wang Y, Cheng P, Jiao B, Song X, Wang H, Wang H, Wang H, Huang X, Liu H, Gong M. Investigation of mosquito larval habitats and insecticide resistance in an area with a high incidence of mosquito-borne diseases in Jining, Shandong Province. PLoS One. 2020;15(3):e0229764. doi: 10.1371/journal.pone.0229764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chathurang W.G.D, Karunaratne S.H.P.P, Priyanka W.A, De Silvaa P. Predator-prey interactions and the cannibalism of larvae of Armigeres subalbatus (Diptera:Culicidae) J. Asia Pac. Entomol. 2020;23(1):124–131. [Google Scholar]
- 73.Chaves L.F. Mosquito species (Diptera:Culicidae) persistence and synchrony across an urban altitudinal gradient. J. Med. Entomol. 2017;54(2):329–339. doi: 10.1093/jme/tjw184. [DOI] [PubMed] [Google Scholar]
- 74.Lambin E.F, Tran A, Vanwambeke S.O, Linard C.C, Soti V. Pathogenic landscapes:Interactions between land, people, disease vectors, and their animal hosts. Int. J. Health Geogr. 2010;9:54. doi: 10.1186/1476-072X-9-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Weterings R, Umponstira C, Buckley H.L. Landscape variation influences trophic cascades in dengue vector food webs. Sci. Adv. 2018;4(2):eaap9534. doi: 10.1126/sciadv.aap9534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.De Thoisy B, Duron O, Epelboin L, Musset L, Quénel P, Roche B, Binetruy F, Briolant S, Carvalho L, Chavy A, Couppié P, Demar M, Douine M, Dusfour I, Epelboin Y, Flamand C, Franc A, Ginouvès M, Gourbière S, Houël E, Kocher A, Lavergne A, Le Turnier P, Mathieu L, Murienne J, Nacher M, Pelleau S, Prévot G, Rousset D, Roux E, Schaub R, Talaga S, Thill P, Tirera S, Guégan J.F. Ecology, evolution, and epidemiology of zoonotic and vector-borne infectious diseases in French Guiana:Transdisciplinarity does matter to tackle new emerging threats. Infect. Genet. Evol. 2021;93:104916. doi: 10.1016/j.meegid.2021.104916. [DOI] [PubMed] [Google Scholar]
- 77.Montoya-Alonso J.A, Morchón R, Costa-Rodríguez N, Matos J.I, Falcón-Cordón Y, Carretón E. Current distribution of selected vector-borne diseases in dogs in Spain. Front. Vet. Sci. 2020;7:564429. doi: 10.3389/fvets.2020.564429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wu J.J. Landscape ecology. In: Leemans R, editor. Ecological Systems. New York: Springer; 2013. [Google Scholar]
- 79.Kaewwaen W, Bhumiratana A. Landscape ecology and epidemiology of malaria associated rubber plantations in Thailand:Integrated approaches to malaria ecotoping. Interdiscip. Perspect. Infect. Dis. 2015;2015:909106. doi: 10.1155/2015/909106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kache P.A, Santos-Vega M, Stewart-Ibarra A.M, Cook E.M, Seto K.C, Diuk-Wasser M.A. Bridging landscape ecology and urban science to respond to the rising threat of mosquito-borne diseases. Nat. Ecol. Evol. 6(11):1601–1616. doi: 10.1038/s41559-022-01876-y. [DOI] [PubMed] [Google Scholar]
- 81.Kozakiewicz C.P, Burridge C.P, Funk W.C, Van de Woude S, Craft M.E, Crooks K.R, Ernest H.B, Fountain-Jones N.M, Carver S. Pathogens in space:Advancing understanding of pathogen dynamics and disease ecology through landscape genetics. Evol. Appl. 2018;11(10):1763–1778. doi: 10.1111/eva.12678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ahmad H.I, Ahmad M.J, Jabbir F, Ahmar S, Ahmad N, Elokil A.A, Chen J. The domestication makeup:Evolution, survival, and challenges. Front. Ecol. Evol. 2020;8:103. [Google Scholar]
- 83.Diuk-Wasser M.A, VanAcker M.C, Fernandez M.P. Impact of land use changes and habitat fragmentation on the eco-epidemiology of tick-borne diseases. J. Med. Entomol. 2021;58(4):1546–1564. doi: 10.1093/jme/tjaa209. [DOI] [PubMed] [Google Scholar]
- 84.Beaulieu M.R.S, Hopperstad K, Dunn R.R, Reiskind M.H. Simplification of vector communities during suburban succession. PLoS One. 2019;14(5):e0215485. doi: 10.1371/journal.pone.0215485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bhumiratana A, Koyadun S, Suvannadabba S, Kanjanopas K, Rojanapremsuk J, Buddhirakkul P, Tantiwattanasup W. Field trial of the ICT filariasis for diagnosis of Wuchereria bancrofti infections in an endemic population of Thailand. Southeast Asian J. Trop. Med. Public Health. 1999;30(3):562–568. [PubMed] [Google Scholar]
- 86.Bhumiratana A, Wattanakull B, Koyadun S, Suvannadabba S, Rojanapremsuk J, Tantiwattanasup W. Relationship between male hydrocele and infection prevalences in clustered communities with uncertain transmission of Wuchereria bancrofti on the Thailand-Myanmar border. Southeast Asian J. Trop. Med. Public Health. 2002;33(1):7–17. [PubMed] [Google Scholar]
- 87.Pimnon S, Bhumiratana A. Adaptation of Anopheles vectors to anthropogenic malaria-associated rubber plantations and indoor residual spraying:Establishing population dynamics and insecticide susceptibility. Can. J. Infect. Dis. Med. Microbiol. 2018;2018:9853409. doi: 10.1155/2018/9853409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Markwardt R, Sorosjinda-Nunthawarasilp P. Innovations in Entomological Surveillance. Cambridge Scholars Publishing, United Kingdom. 2021. Available at: https://www.cambridgescholars.com/product/978-1-5275-7024-5 . Retrieved on 19-07-2021.


