Abstract
Accurate control of long-range motion at the molecular scale holds great potential for the development of ground-breaking applications in energy storage and bionanotechnology. The past decade has seen tremendous development in this area, with a focus on the directional operation away from thermal equilibrium, giving rise to tailored man-made molecular motors. As light is a highly tunable, controllable, clean, and renewable source of energy, photochemical processes are appealing to activate molecular motors. Nonetheless, the successful operation of molecular motors fueled by light is a highly challenging task, which requires a judicious coupling of thermal and photoinduced reactions. In this paper, we focus on the key aspects of light-driven artificial molecular motors with the aid of recent examples. A critical assessment of the criteria for the design, operation, and technological potential of such systems is provided, along with a perspective view on future advances in this exciting research area.
Keywords: molecular machines, photochemistry, autonomous operation, directional motion, dissipative systems, nanoratchets
Introduction
Life is a non-equilibrium phenomenon where endergonic physicochemical processes are sustained by exergonic ones that are responsible for harvesting the energy needed.1−6 Naturally occurring complex molecular assemblies like motor proteins are essential to life, as they are responsible for carrying out crucial tasks, such as energy storage and conversion (ATP synthesis), cargo transport, protein synthesis, and DNA replication.1,2,7−9 Some of the most important and extensively studied motor proteins are those of the myosin and kinesin families, ATP synthase, and bacterial flagellar motor.7,10−13 These proteins are referred to as “motors” or “engines” because, like macroscopic engines, they continuously and autonomously convert the energy coming from an external source (i.e., the fuel)14 into mechanical work while performing a directional motion of the components.7,15−18 In doing so, these chemical systems are set in non-equilibrium states sustained by continuous dissipation of energy.1,3,19,20
About 30 years ago, chemists embraced the challenge of constructing synthetic systems which mimic the operation of natural molecular motors. In general, such systems exploit chemical, electrochemical, and photochemical processes to cause mechanical-like movements of the (sub)molecular components.15,17,21−23 Over the years, the field has progressed from early proof-of-principle studies to more sophisticated investigations aimed at exploring the real world applications of molecular machines. Indeed, the award of the Nobel Prize in Chemistry 2016 to Jean-Pierre Sauvage, Fraser Stoddart, and Ben Feringa “for the design and synthesis of molecular machines”24−26 highlighted the conceptual significance and the technological expectations of this research.27,28
Compared to the other energy sources, the use of light is highly desirable as it provides exquisite spatiotemporal control, can avoid the formation of waste products upon operation, and is intrinsically renewable.29 Despite these advantages,30,31 studies on artificial photochemically driven dissipative systems are highly challenging as they require a specific molecular design that involves a judicious combination of photochemical and thermal rearrangement processes.17,18,32 Moreover, as molecular motors operate by repeating cycles, reversible, clean, and fatigue-resistant reactions are needed. For this reason, only a few types of photochemical reactions have been employed so far to operate these systems, namely, photoinduced electron-transfer and photoisomerization processes.17,30,31,33−35 With regard to the second category, the most common choice involves photochemical isomerization reactions around (i) C=C (e.g., stilbenes), (ii) C=N (e.g., imines), and (iii) N=N (e.g., azobenzenes) double bonds.17,36−38
This Perspective provides an overview of the evolution of the field of photoactivated artificial molecular motors over the past 10 years, critically assessing their strengths and weaknesses as well as reviewing current applications and outlooking their future potential. For clarity, the examples described are divided into two classes depending on their molecular structure and on the type of motion produced. Thus, the first part of this review deals with motors based on covalent molecular structures that exhibit rotary motion, while the second part focuses on supramolecular host:guest complexes, which show the linear displacement of one component relative to the other.
Working Principles
In order to operate continuously, a motor (either macroscopic or molecular) must go through a closed sequence of transformations, which can be repeated indefinitely. Therefore, the basic working principle behind the majority of light-driven artificial molecular motors involves the coupling of a photoinduced reaction with a thermal rearrangement process. Since each of these processes typically interconverts two states, the result is a four-membered closed reaction network (square scheme, Figure 1a) in which the photochemical processes harvest the energy that is consumed in the thermal reactions. A few examples of “photon-only”-driven motors have been reported, whose operation will be examined in a dedicated section.
Figure 1.
General four-step reaction cycle used to describe the basic operation of most light-driven molecular motors (a). Horizontal processes, marked with “Δ”, are thermally activated, while vertical processes, marked with “hν”, are photochemical steps. Simplified energy diagrams of photochemical (b) and thermal (c) processes.
It is important to point out that in photochemical reactions (Figure 1b), the composition (i.e., the product/reactant concentration ratio) at the photostationary state (PSS) is determined by the photoreaction quantum yields (governed solely by the profile of the excited-state potential energy surface) and the molar absorption coefficients. Thus, these reactions are not bound to the constraints of microscopic reversibility, which instead regulate thermally activated processes (Figure 1c) in the electronic ground state.22,39−42
The concomitant occurrence of the two processes is, however, not a sufficient condition to obtain a motor. The second criterion that needs to be met is the unidirectional (or directionally biased) relative motion of the distinct parts/components. While this can be easily achieved in the macroscopic world, where Newtonian physics holds true, it is not the case in the nanoscopic domain. At the molecular level, in fact, thermal energy results in an endless “jiggling” of the atoms which disrupts any directionality.43,44 In order to fulfill the second law of thermodynamics, directional motion at the nanoscale requires the use of an energy source to bias Brownian motion and render the movement in a given direction more favored than those in other directions. This is achieved by employing ratchet mechanisms, which operate by breaking spatial and time-reversal symmetries along the direction of motion with the application of a time-dependent potential with repeating asymmetric features.16,17,45,46 The categories of ratchets of interest in the context of molecular motors are energy and information ratchets; their detailed description and specific characteristics are beyond the scope of this Perspective and have been extensively discussed elsewhere.16,17,21,45,47,48 Here, we will limit our explanation to saying that energy ratchets asymmetrically modulate both energy maxima (kinetics) and minima (thermodynamics), while information ratchets modulate only the energy maxima, depending on the position of the Brownian particle.
Covalent Molecular Systems: Rotary Motors
These systems mostly operate by coupling the photoisomerization of a C=C or C=N bond with thermally activated inversion of helicity (thermal helix inversion, THI). In particular, the molecule is endowed with specific stereochemical elements that break the symmetry of the potential energy surface; this results in a directional bias in the relative sense of rotation of a “rotor” with respect to a “stator” portion of the molecule.36,38 Two main classes of molecular rotary motors have been developed over the past 20 years: (i) overcrowded alkenes and (ii) imines.17,36
Overcrowded Alkenes
The first class of compounds that exhibited directionally controlled photoinduced rotation about a C=C double bond is that of overcrowded alkenes (Figure 2). Such compounds can be categorized in two distinct families sharing the alkene motif but having different substitution patterns, such as a stilbene core or a sulfur-based heterocycle.
Figure 2.
(a) Schematic representation of the four-step operation cycle of alkene-based motors, comprising photochemical reactions (horizontal processes) and thermal helix inversion (THI, vertical processes). The black arrows indicate the relative movement of the rotor (blue) with respect to the stator (red). The direction of cycling is indicated by the gray dashed arrow. (b) First, second, and third generation Feringa-type motors (refs (49−51)). The rotor (blue) and stator (red) portions of the motor molecule are highlighted where relevant.
Stilbene-containing compounds reported by Feringa and co-workers were the first molecules capable of achieving a full 360° unidirectional rotation of the rotor upon light irradiation thanks to a ratchet mechanism.49 Several generations of this motor were then developed, all of which are based on an overcrowded stilbene structure bearing a stereocenter. Thanks to the high steric hindrance around the short double bond, the molecule is forced to adopt a nonplanar conformation, which displays a specific helical chirality dictated by the configuration at the chiral carbon center(s).37,49
Moreover, they all share the same operational cycle (Figure 2a), described here for the first generation motor (Figure 2b, left), which starts with a photoinduced double bond isomerization to achieve the Z form of the stilbene unit. This step also brings the methyl group from a (pseudo)axial to a (pseudo)equatorial position, giving rise to a steric clash with the stator. The molecule relaxes from this metastable state to a more stable state by switching the chirality of the helix and bringing back the methyl group to a (pseudo)axial conformation. As in the previous step, because of steric reasons, the THI must occur directionally with the aromatic parts flipping over each other. This step is practically irreversible because of its large driving force and is responsible for the bias in the rotation direction. Thanks to the overlap in the absorption spectra of E- and Z-stilbene, a second photon of the same wavelength can cause Z–E isomerization. This leads the system again in a higher energy state due to the (pseudo)equatorial methyl group, and the energy is released by THI, closing the cycle and readying the motor for another cycle.37 Second and third generation motors operate according to a conceptually identical mechanism.
The molecular design of the motor was improved over the years, giving rise to a family of motors with enhanced performances. Particular effort was put into the optimization of the THI process by lowering its half-life, with a consequently higher rotation frequency.50 Further engineering involved the reduction of the molecular complexity with the desymmetrization of the stilbene core, introducing the concepts of rotor and stator (Figure 2b, middle)38 and ultimately leading to a meso-motor, initially achiral and desymmetrized upon photoirradiation (Figure 2b, right).51
Finally, a unidirectional rotation was also achieved in an achiral stilbene-based switch upon complexation with an optically pure phosphate anion.52 The noncovalent interactions between the motor and the anion enable the transfer of chiral information, leading to the preferential formation of one particular diastereomeric ion pair and resulting in a preferential direction of rotation of the stilbene moiety.
Alternative motor architectures incorporating an overcrowded alkene functionality were proposed by Dube and co-workers.53,54 These systems exploit an analogous structure of the rotor unit, while the stator moiety derives from a hemithioindigo heterocyclic scaffold (Figure 3a). Innovative features with respect to stilbene-derived compounds include the chiral-at-sulfur nature of the sulfoxy moiety as well as the intrinsically red-shifted absorption band of the hemithioindigo unit. The first generation motor is able to directionally travel a closed reaction network, alternating visible-light photoisomerization, and THI steps. Due to the high efficiency, the four isomers composing the operational cycle were initially only theorized and have been fully elucidated only in later studies.55 A detailed kinetic study of the rotation efficiency confirmed the mechanism of rotation and provided inputs for further theoretical studies.56 An additional, interesting feature of these systems is the so-called hula twist, a combination of single- and double-bond rotation that expands the network of reactions interconverting the four isomers (Figure 3b, central crossing arrows).57,58 Taking advantage of the hula twist, more elaborated trajectories of relative motion can be obtained, such as the eight-shaped directional rotation (Figure 4). Importantly, the highly selective directionality is inferred by a well-defined sequence of photochemical double bond isomerizations and thermal hula twist and is preserved even at temperatures as high as 130 °C.59
Figure 3.
(a) Examples of first, second, and third generation hemithioindigo motors (refs (53), (59), and (61)). The rotor and stator portions are colored blue and red, respectively. (b) Schematic representation of the reaction network traveled by a second generation hemithioindigo motor, comprising single bond rotation (horizontal processes), double bond isomerization (vertical processes), and hula twist (central crossing processes).
Figure 4.
(a) Structure of the motor displaying “figure-of-eight” shaped motion of the methyl group marked in red (ref (59)). (b) Relative position of the methyl group with respect to the oxygen atom of the sulfoxide moiety. (c) Operational four-step cycle comprising photochemical double bond isomerizations (DBI, horizontal processes) and thermal hula twist (HT, vertical processes) highlighting the motion of the Me group relative to the stator (in plane). (d) Overall sequential motion of the Me group. Adapted with permission from ref (59). Copyright 2019 Springer Nature.
“Photon-Only” Operation of Overcrowded Alkene Motors
Generally, the frequency of rotation of rotary motors is limited by the rate of the thermal steps in the electronic ground state.36,60 “Photon-only” molecular motors, whose unidirectional rotation relies solely on photoreactions and not on thermal ratcheting, could overcome this constraint and open the possibility to achieve fast and temperature-independent rotation rates, owing to potentially barrierless light-activated pathways on the potential energy surface along the motion coordinate. As this approach is not bound to the canonical four-step cycle depicted in Figures 1a and 2b, different operational cycles can be designed. The first example of a fully photochemically driven rotary motor belongs to the class of hemithioindigo motors (Figure 3b).61 The motor operates with a three-step cycle, where all the isomerization processes (double bond isomerization, single bond rotation, and hula twist) are photochemical reactions. It is noteworthy that such a mechanism prevents any backward reaction because it is not bound to microscopic reversibility, resulting in a high directional bias (>98%) and in faster rates at low temperatures. A “photon-only”-driven motor based on a second-generation architecture (Figure 2b) was also reported by Feringa and co-workers. According to the kinetic analysis, such a system plausibly operates through a canonical four-step cycle composed only photochemical reactions.62 Olivucci and co-workers recently described a stilbene derivative that, according to theoretical calculations, could potentially act as a “photon-only” two-stroke rotary motor. Although the unidirectional rotation is only partially supported by experimental evidence, such a system could pave the way to rotary motors exploiting the simplest possible operation cycle.63
Imines
Imine-based molecular rotary motors were first theorized by Lehn in 200664 and successfully attained some years later (Figure 5). Such systems take advantage of the established isomerization process of the C=N double bond which, coupled with inversion of planar chirality, results in a four-membered closed reaction cycle.
Figure 5.

(a) Four-step operation cycle of imine-based motors, comprising photochemical reactions (horizontal processes) and thermal helix inversion (THI, vertical processes) (ref (65)). (b) Two-step operation cycle of an imine motor with a more rigid stator, which involves a photochemical out-of-plane rotation (upper pathway) and an in-plane nitrogen inversion (lower pathway). The transition states are represented in gray, while the black dashed arrows indicate the relative movement of the rotor with respect to the stator in the transition states (ref (67)). The gray dashed arrow indicates the preferred traveled direction of the network.
The first prototype of an imine-based rotary motor was based on a diaryl-N-alkylimine structure.65 The synthesis exploits a condensation of an alkylamine bearing a stereogenic α-carbon with a planar dibenzosuberenone, which results in a nonplanar axially chiral structure. Such compounds exhibit slow thermal E → Z isomerization at room temperature. Light irradiation leads to a non-equilibrium Z/E mixture of isomers attained by out-of-plane rotation about the C=N double bond.
Despite the high similarity to overcrowded alkenes, the peculiar feature of imine motors lies in the two independent reaction pathways (photochemical or thermal) that can be traveled during the Z → E back-isomerization process. While the photochemical C=N isomerization proceeds via an out-of-plane rotation, a direct inversion at the nitrogen atom occurs in the thermal process. In 2023, Martinez and Fang performed computational calculations to shed more light on the nitrogen out-of-plane rotation and showed that the origin of the unidirectional rotation is on repulsion between the nitrogen lone pair and the closest hydrogen of the stator.66 This steric hindrance, along with the nature of the excited state, which encompasses two conical intersections, ensures that in a population about 25% of the molecules rotate unidirectionally. The associated quantum yield of rotation (≈25%) is in line with the experimental value of about 29%.
Depending on the molecular design, a classical squared cycle (Figure 5a) or a simpler two-stage operational cycle (Figure 5b) can be undertaken. The four-membered cycle comprises photoisomerization reactions of the imine bond followed by thermal inversions of the axial chirality of the dibenzosuberenyl unit. In contrast, within the two-stage process the photoisomerization produces a metastable isomer by unidirectional out-of-plane rotation, which then relaxes to the starting—more stable—isomer by direct inversion at the N atom. This operational cycle was further elucidated by using a camphorimine derivative.67
The dynamic character of the imine bond endows this class of motors with unique reactivity and substantially facilitates their synthesis, which are highly desirable features for systems aiming at real world applications. Despite these clear advantages, investigations of such motors toward performing useful functions are still underdeveloped. We hypothesize that this might be due to the relatively lower stability of imine bonds compared to C=C bonds, which renders their implementation in more sophisticated systems highly challenging.
Prospective Applications
In Nature, molecular motors are exploited to build up free energy gradients which can then be consumed by coupled processes keeping the system away from thermodynamic equilibrium, that is, in an “alive” state.68,69 Artificial molecular machines have so far mostly been exploited for the first task, accumulating free energy. Recently, efforts have been directed toward using the accumulated energy in secondary tasks. In this paragraph, we showcase a selection of examples where the use of a molecular motor enabled the transmission of controlled motion to a remote part of the molecule, as well as the control of molecular topology and coupled chemical equilibria.
In light of the successful design and relatively high efficiency of rotation, stilbene-derived systems serve as the basis for the study of more complex reaction networks. In particular, Feringa and co-workers reported on the synchronous transmission of directional rotation to a covalently connected atropisomer which functions as a secondary rotor (Figure 6a). The system is based on a second-generation motor, with a pendant naphthyl substituent, resulting in an additional axial stereogenic element, which cannot freely rotate due to steric hindrance.70 Upon unidirectional rotation of the rotor, the naphthyl substituent, being locked, is forced to undergo a sliding motion around the stator portion of the motor. As a result, the same face is always exposed toward the stator. Such a system can be seen as a synchronous planetary gearset where the motion of the naphthyl unit is tidally locked to the unidirectional rotation about the C=C bond.
Figure 6.
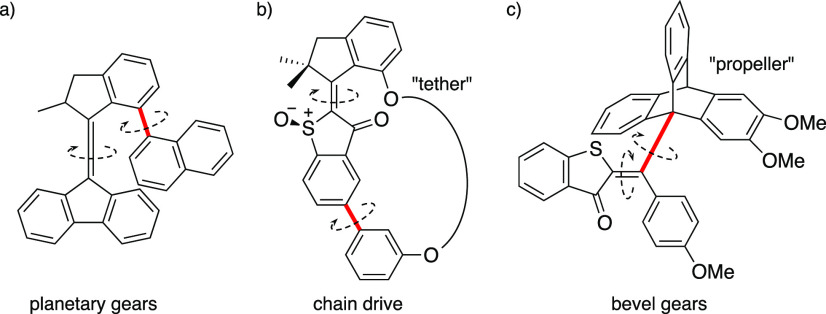
Molecular gearing with overcrowded alkene-based motors. Molecular “planetary gears” based on a Feringa-type motor (a), “chain drive” (b), and “bevel photogear” (c) developed by Dube and co-workers. The axis exhibiting synchronized (geared) motion to the motor/switch is colored red. The dashed arrows indicate the relative direction of motion of the two axes.
In a related work, Dube and co-workers tethered an atropisomeric unit to a hemithioindigo motor (Figure 6b). The unidirectional rotation of the motor is transmitted to the biaryl unit, providing a unidirectional rotation about the axially chiral carbon–carbon single bond.71 Additionally, they also demonstrated a catalytic effect on the epimerization of the axial chirality in a preferred direction due to the torque imparted by the motor through the tether.72 It is worth noting that since the biaryl unit is dragged by the motor but epimerization is rate-limiting, thermodynamic equilibrium between the two atropisomers cannot be maintained under operation. More recently, the same group also demonstrated the coupling of the photoinduced isomerization of the double bond with the rotation of a triptycene “propeller”, realizing a molecular bevel gear train (Figure 6c). Such a “photogear”, however, operates with no net directionality, because the driving gear is a switch and not a motor.73 Application of such a gearset to a motor would result in a 120° rotation of the orthogonal propeller every 180° rotation of the motor, effectively realizing a proper asynchronous driven motion.
Feringa and Giuseppone independently pioneered the use of rotary motors to achieve topologically complex molecules. Their systems both consist of a rotary motor in which the rotor and the stator are connected by two flexible chains in a “figure-of-eight” fashion (Figure 7a). By exploiting the unidirectional rotation of the motor, the loops wind up progressively increasing the topological complexity of the system–that is the number of crossing points between the loops–similarly to what happens with a “whirligig” toy. Giuseppone and co-workers, building on a “figure-of-eight” compound described in 2015,74 showed more recently75 that the system advances toward increasingly tensioned states, until the mechanical strain outperforms the torque inferred by the motor by significantly lowering the forward isomerization quantum yield, ultimately resulting in a suspended rotation. Therefore, this system converts light energy into potential energy in the form of mechanical strain of the loops (Figure 7b) and reaches a photostationary state dominated by the most entangled topological isomers (with two and three crossing points). Owing to the progressively disfavored isomerization reaction, the radiative dissipation of the energy accumulated upon photoexcitation (Figure 1b) becomes accessible, leading to fluorescence emission. For example, in the case of triazole-type tethers (Figure 7a, G = I), after 25 min of continuous irradiation at 365 nm, a 5-fold increase in the fluorescence emission intensity is observed (Figure 8). Measurements of fluorescence quantum yield revealed an increase from 0.08%, for the unentangled isomer, to 0.4%, at the photostationary state, while the photoisomerization quantum yield drops from 11 to 3%, confirming the competitiveness of the radiative energy relaxation pathway. However, while the accumulated energy can be stored under continuous irradiation and relaxation via fluorescence emission becomes favorable, in the dark, the system simply relaxes back to its equilibrium state by thermal isomerization of the stilbene double bond with the consequent detangling of the loops.
Figure 7.

(a) “Figure-of-eight” overcrowded alkene-based motor containing inert (G = I) and dynamic (G = II) tethers. (b) Simplified energy diagram correlating the molecular topology (tension/torque) with the photoinduced unidirectional rotation and the potential energy of the molecule, similarly to a spring. Adapted from ref (75). Copyright 2022 American Chemical Society.
Figure 8.

Maximum photoluminescence (PL) emission intensity at different irradiation times for the compound shown in Figure 5a (G = I). The initial and final (photostationary state) compositions of the topological isomer mixture are displayed on top. Adapted from ref (75). Copyright 2022 American Chemical Society.
In a related report, Kathan and co-workers developed a system that encompasses dynamic covalent bond formation along with directional rotary motion.76 Specifically, an aldimine bond is used to tie the loops around the nanoratchet (Figure 7a, G = II). Such a design enables relaxation to the ground state of the topologically entangled, high-energy structure by shifting the imine-formation chemical equilibrium. This is validated by transimination experiments carried out at different irradiation times that directly relate the equilibrium constant values to the increasing internal energy of the states. As a result, for very highly entangled states, the ring opening process becomes more favorable while its microscopic reverse is virtually inaccessible, triggering nonstepwise relaxation pathways.
These two examples highlight how it is possible to access unconventional energy dissipation pathways by driving a molecular motor away from thermodynamic equilibrium.
Analogous concepts have been applied to a noncovalent system based on a rotaxane architecture, where the unidirectional rotary motion of the motor was transmitted to the sliding motion of the ring along the axle.77 Similarly, the threading of an oligomer through a macrocycle was achieved by utilizing a motor-powered approach.78 This result discloses interesting perspectives for the preparation of mechanically interlocked architectures without the need for templated strategies and, thanks to unidirectional threading, for the preparation of molecularly interwoven structures.79
Photodriven chain entanglement was also exploited by Giuseppone and co-workers to control the macroscopic properties of a polymeric system.74,80 Polyethylene glycol-functionalized second-generation stilbene motors were used to provide cross-linked networks resulting in hydrogel formation.74,81,82 Upon light irradiation, the degree of entanglement of the polymers increases due to the unidirectional rotation, similar to the “whirligig” system. This results in the motors within the hydrogel being pulled toward each other with a consequent net stiffening of the structure (Figure 9a,b). Overall, these microscopic modifications are translated to the macroscopic scale as the hydrogel sample shrinks (Figure 9c), ultimately reaching decomposition due to excessive mechanical strain.74 This first system was then improved by introducing a modulator unit, which allows to release part of the strain via rotation about sigma bonds (Figure 9b). The modulator unit itself can be toggled by irradiation at a different wavelength with respect to the motor.81 The net result of this combination is a photostationary state in which the macroscopic size of the hydrogel sample can be reversibly controlled through light irradiation (Figure 9a–c). More recently, the authors expanded their understanding on the influence of the nature of the polymer network on hydrogel actuation.82 The photon density available per motor unit and the amount of free volume in the polymer network were found to be key parameters to improve the efficiency and applicability. Their careful optimization led to the preparation of self-standing gels working as bending actuators that display a 400-fold enhancement in the mechanical work that can be extracted from the system.
Figure 9.
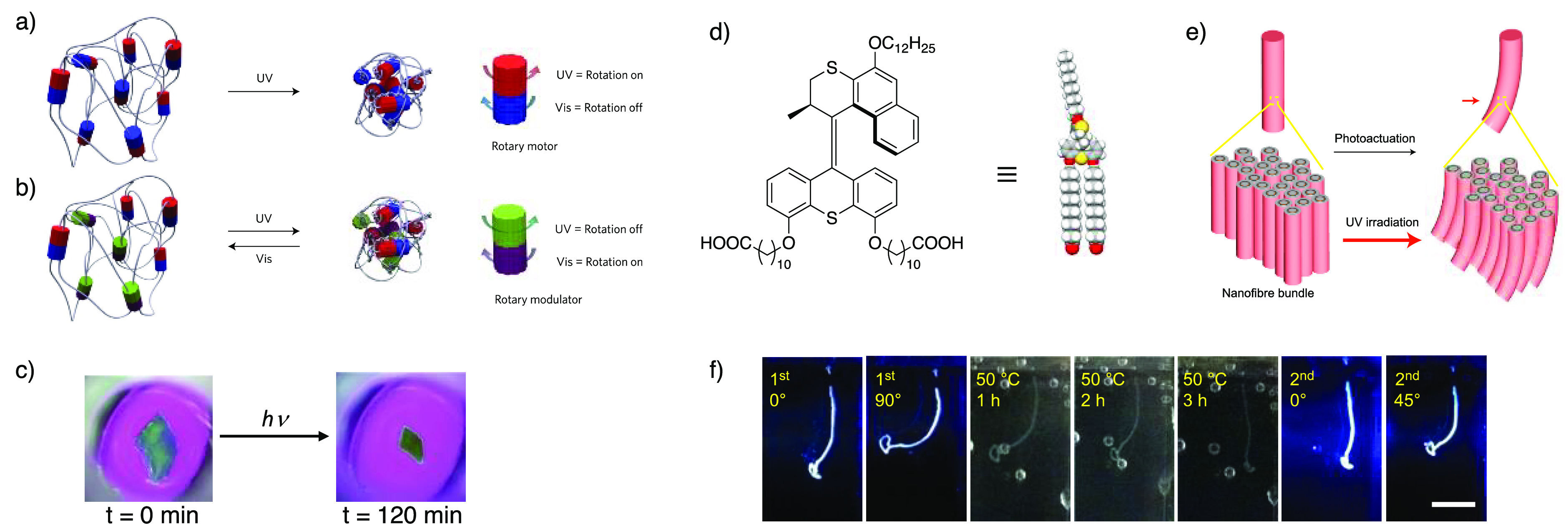
Photoactive molecular motor-based materials. Left: First- (a) and second-generation (b) photoactive hydrogel based on molecular chain entanglement to drive the shrinkage of a macroscopic sample of hydrogel (c) (refs (74) and (81)). Right: structure of the motor amphiphile(d) and hierarchical self-assembly to form fibers (e). Reversible light-driven actuation of a centimeter-sized fiber (f) (ref (83)). Scale bar in (f) is 0.5 cm. Adapted with permission from refs (74), (81), and (83). Copyright 2015, 2017, and 2018 Springer Nature.
The functionalization of rotary motors with flanking alkyl chains was exploited by Feringa and co-workers to prepare amphiphiles capable of undergoing hierarchical self-assembly in saline solution, obtaining millimeter-sized fibers. These fibers can be actuated by means of light stimulation to achieve directional bending away from the light source. The actuation and relaxation processes occur in the seconds to minutes time scale, rendering this system highly promising for the development of artificial muscles (Figure 9d–f).83 The authors also explored the use of these amphiphiles as a cell culture medium for mesenchymal stem cells, showing that they are compatible with cell viability and retain the photoactuation properties, displaying therefore high potential for the development of next generation biomimetic matrices.84 It should be pointed out, however, that, in principle, unidirectional rotation is not required to bring about such effects.
The introduction of artificial molecular machines in bilayer membranes is an interesting way to affect their behavior in terms of permeability toward different substrates.85,86 A further development in this direction would be attained by exploiting the directionality of molecular motors, which could on the long-term enable the active transport of molecular substrates across the membrane against a concentration gradient. Preliminary steps in this field span from less complicated systems up to highly articulated and tailored architectures. Standard second-generation stilbene motors were used to induce mechanical stress as a result of the rotation and increase membrane permeability in liposomal vesicles.87 Appending more elaborated motifs was seen as a strategy to achieve selective and enhanced transport of cations, fluorophores, or inhibitors across the membrane.
Giuseppone and co-workers appended flanking crown ethers to selectively translocate potassium cations from the outer to the inner compartments of large unilamellar vesicles.88 A similar system was also employed by Bao, Qu, and co-workers to promote the efflux of K+ from living cells, triggering a signaling cascade that ultimately led to cell apoptosis (Figure 10a,b).89 The enhanced efflux of K+ was attributed to a local change in the membrane fluidity as a result of the mechanical motion, whose unidirectionality does not seem to be essential in this context. The selectivity for the metal ion was the result of the use of crown ether receptors of an appropriate size.
Figure 10.

Transport across cell membranes using light-operated molecular rotary motors. (a) Potassium cation transporter developed by Qu and co-workers and (b) cartoon representation of the proposed working principle (ref (89)). (c) Molecular motors equipped with receptor-targeting oligopeptides and (d) proposed mechanism of membrane rupture induced after adsorption and UV light irradiation (ref (90)). Adapted with permission from refs (89) and (90). Copyright 2022 John Wiley and Sons and 2017 Springer Nature.
Tour and co-workers investigated the ability of stilbene-based motors to induce cell death. In particular, the authors suggested that upon light irradiation active diffusion of the motor through the cell membrane takes place due to the unidirectional rotation. Such a motion was supposed to disrupt the bilayer and open pores with consequent cell necrosis (Figure 10c,d).90 The authors hypothesized that membrane rupture and pore formation are induced by a tangential mechanical force exerted by rotating nanomotors embedded in the bilayer (Figure 10d). While this possibility is highly fascinating, it should be emphasized that the ability of molecular switches or motors to enhance diffusion is a source of debate.91 Therefore, it appears that further experiments are required to collect solid evidence in support of the mechanism described above.
Supramolecular Complexes: Linear Motors
The second category of molecular motors that can be operated by light energy is based on supramolecular complexes and mechanically interlocked architectures. The major advantage of this scaffold is the wide motion amplitude of the distinct subunits relative to one another, as a direct consequence of the presence of the mechanical bond.92 In recent years, this architecture has become increasingly popular for the design of molecular motors operated not only by light but also by electrochemical and chemical stimulation.15,21,23,93 The long-range mobility of the ring(s) along an axle in rotaxane-like architectures, coupled with the implementation of a ratchet mechanism, made these the preferred scaffolds for the realization of linear motors and, in particular, supramolecular pumps.
Pioneering work on light-operated information ratchets by Leigh and co-workers demonstrated the possibility of using light as energy to rectify the motion of a ring along an axle, hence moving the system away from thermodynamic equilibrium.94
In 2013, Stoddart and co-workers described a pseudorotaxane complex in which reduction and oxidation reactions trigger the relative directional motion of the ring and the axle, thus posing the bases for the realization of artificial supramolecular pumps.95 The system consists of an asymmetric axle with an electron-rich naphthalene station flanked by an isopropylphenyl group (IPP) and a positively charged 3,5-dimethylpyridinium unit (PY), and a redox-active cyclobis(paraquat-p-phenylene) ring (CBPQT). Unidirectional sliding motion of the ring relative to the axle; i.e., pumping, is achieved by exploiting an energy ratchet scheme where sequential reduction and oxidation cycles result in the modulation of the potential energy surface. In solution a pseudorotaxane is formed where the electron-poor macrocycle encircles the electron-rich station of the axle. Threading must occur from the IPP side due to charge repulsion with the PY unit. Upon reduction of the macrocycle, the complex is destabilized and decomplexation occurs from the positively charged side of the axle, thanks to the reduced charged repulsion and structural modification of the reduced CBPQT ring. The authors proposed that the device could also be activated with light by exploiting photoinduced electron-transfer processes. Specifically, the well-known [Ru(bpy)3]2+ photosensitizer was employed to cause the photoinduced reduction of the bipyridinium units of CBPQT and operate the pump using light as the energy source (Figure 11). Irradiation of a mixture of pseudorotaxane, [Ru(bpy)3]2+, and phenothiazine with light of 450 nm results in photoinduced electron transfer (PET) from the photocatalyst to the macrocycle (“blue arrow 1” in Figure 11), which destabilizes the complex, producing slippage over the PY side. Finally, the photocatalyst is regenerated by oxidation of the phenothiazine, which acts as a sacrificial reductant (“blue arrow 2” in Figure 11).
Figure 11.

(a) Components of the redox-driven supramolecular pump described by Stoddart and co-workers: the cyclobis(paraquat-p-phenylene) (CBPQT) ring (left) and the nonsymmetric axle (right). (b) Scheme of the light-induced operation of the supramolecular pump based on photoinduced electron transfer. Blue arrows indicate the electron-transfer processes. Reprinted from ref (95). Copyright 2013 American Chemical Society.
Although the pump can be easily operated in the dark by alternating chemical reduction and oxidation processes, the photoactivated mechanism is an interesting development for at least three reasons. First, the implementation of PET is not trivial as it requires a careful evaluation of the reduction potentials and excited-state lifetimes of the various species involved; specifically, the slippage process and the oxidation of the phenothiazine must occur on a time scale faster than the backward electron transfer from the reduced ring. Second, the photoactivated mechanism allows for autonomous cycling, whereas the chemically driven operation requires an alternate supply of reductant and oxidant species. Third, the use of light as the energy source has important consequences in the potential use of these architectures for energy conversion and storage.96
The first example of a supramolecular pump (linear molecular motor) converting directly light into chemical energy in an autonomous fashion was developed in our laboratory (Figure 12).97 Conceptually, the system is analogous to the covalent rotary motors in the sense that light is the primary source of energy, and the photochemical processes are directly coupled to the thermal reactions in the network (Figure 12a). The authors developed a family of compounds based on an asymmetric axle bearing a central ammonium station flanked by a photoactive azobenzene chromophore and a nonphotoactive “pseudostopper” (Figure 12b). The complex between the axle and a crown ether macrocycle undergoes continuous and autonomous dissipative self-assembly under constant light irradiation, thanks to an energy ratchet mechanism.
Figure 12.

(a) Four-state reaction network describing the operation of Credi’s supramolecular pump. The network includes two photochemical (vertical) and two self-assembly (horizontal) reactions. The gray dashed curved arrow shows the preferred traveled direction of the cycle. (b) Components of the first- and second-generation supramolecular pumps: nonsymmetric axles (left) and diaryl-24-crown-8 ether based rings (right) (refs (97), (99), (101), and (102)). (c) Cycling rate (black dots) and quantum yield (red dots) values at different light intensities. d) Energy dissipated by the self-assembly steps for E (green bars) and Z (red bars) configurations to keep the concentrations of species away from equilibrium at different light intensities. Adapted with permission from ref (103). Copyright 2022 Springer Nature.
Specifically, in the dark, the ring threading occurs preferentially by overcoming the E-azobenzene unit, as the energy barrier for slippage over the pseudostopper unit is higher. E → Z photoconversion of azobenzene has two major consequences: (i) the energy barrier for the slippage of the macrocycle over the photochrome increases to a level above that of the pseudostopper and (ii) the stability of the complex is decreased. Therefore, fast isomerization of an equilibrated mixture generates the Z complex in a concentration that is higher than its equilibrium value and will decrease in the dark, extruding macrocycles. This step must occur preferentially via slippage over the pseudostopper unit due to the relative heights of the activation barriers. Overall, the macrocycles travel unidirectionally relative to the axle. Conversely, under constant irradiation, the detailed balance cannot be fulfilled and the reaction network traveled preferentially clockwise with all reactions proceeding at a nonzero rate.98 In this way the system composition evolves toward a non-equilibrium state which is sustained dissipating the absorbed light energy.
Several variants of the pump were developed (Figure 12b) with the dual aim of (i) optimizing the kinetics of the slippage processes at both the azobenzene and the pseudostopper side and (ii) increasing the pumping efficiency in terms of the difference in stability between the E and Z complex.99−101 Finally, a pump with an aromatic pseudostopper, amenable to further functionalization and introduction into more sophisticated architectures, was presented.102 More recently, a combined experimental and theoretical investigation of the pumping cycle was reported, which revealed that the composition of the out-of-equilibrium steady state is dependent on the intensity of the light driving the system.103 Additionally, several parameters were determined from the steady-state composition and their dependency of on the photon flow, that is, the amount of fuel, was elucidated. In particular, the rate at which the reaction network is traveled (Figure 12c, black dots), the energy dissipated—entropy production—by the self-assembly steps (Figure 12d), and the stored ΔG increases with light intensity. Conversely, the quantum and energy conversion efficiencies follow an opposite trend, decreasing at higher intensities due to kinetic factors (Figure 12c, red dots). In fact, the efficiency of the pump is dependent on the rate-limiting process of the pumping cycle. Therefore, as long as photochemical steps are rate-limiting (low intensities), the photons are employed more efficiently in the energy storage, while at higher intensities, the photoreactions are not rate-limiting anymore, and a larger fraction of light energy is dissipated in the photoisomerization steps rather than stored in the form of non-equilibrium concentrations. Importantly, this study also provided a framework for the quantitative comparison of light-operated molecular motors with their electrochemically and chemically driven counterparts.
Critical Assessment
As outlined in the previous paragraphs, in the past three decades, chemists have developed the necessary know-how to design sophisticated artificial molecular machines and motors. In particular, owing to the outstanding progress made over the past ten years, the research community has now access to a variety of molecular-based systems performing directionally controlled rotary and linear movements at the nanoscale activated by light. Along with the experimental development, our understanding of the concepts and principles governing their operation also broadly expanded, paving the way to study how they could be harnessed to perform useful functions, as happens in Nature with biomolecular motors.
However, in order to exploit such nanoscale technologies for real-life applications, a number of critical aspects still need to be addressed. For the sake of clarity, we will first individually analyze each category to outline its strengths and weaknesses and later focus on common long-term goals.
Covalent Motors
The synthetic accessibility of these systems still represents a major challenge. In particular, overcrowded alkene motors require nontrivial multistep procedures to be prepared. On the plus side, they display a relatively efficient light-to-motion transduction and have well established functioning mechanisms. Coupled to their stability, this led to their implementation in preliminary applicative examples.
One of their major drawbacks, however, lies in the short-range motion of the rotating components, which needs to be amplified to reach larger extents. Moreover, these scaffolds selectively provide rotary motion, which in turn implies that gearing is required not only to amplify its range but also to transform it to linear motion. Due to the arene-based structure of such systems, the gearing pathways are relatively limited and often considerably increase their synthetic complexity.
Noncovalent Motors
The modular nature of noncovalent systems allows for more accessible, convergent synthetic pathways, partially alleviating the effort required for their preparation. Therefore, despite multistep synthetic pathways are still being needed, protocols are generally more user-friendly.104
On the other hand, noncovalent light-driven (but also other fuel-driven) motors reported to date suffer from a low efficiency in terms of light-to-motion transduction, energy conversion, and storage.103,105 Combined with their relatively recent establishment, this is the main cause behind their lack of practical applications. However, in such systems the high dynamicity endowed by the noncovalent interactions between the components intrinsically enables long-range, native nanoscale motion. Moreover, they provide access to both linear and rotary motion depending on the topology of the scaffold, e.g., rotaxane–linear vs catenane–rotary. It should also be recalled that the variety of new materials based on threaded and interlocked molecular architectures developed to date106−110 may be considered to integrate noncovalent motor functions with the objective of realizing stimuli-responsive dynamic materials. Appropriate combinations of the above-mentioned properties could, in principle, provide a straightforward access to articulated kinematic chains exploiting geared motion.111,112
Outlook
By taking advantage of their individual characteristic features, both covalent and noncovalent architectures display high potential for ground-breaking innovations in nanotechnology. In our opinion, two main scientific fields could benefit from their development. The first one involves the development of artificial living cells, which would incorporate highly complex molecular motors to sustain fundamental tasks (e.g., transport, growth, and replication). Toward this goal, essential requirements for the artificial motors include: (i) their effective operation in aqueous environments and/or within bilayer membranes, with a specific focus on directional transmembrane substrate transport; (ii) the development of coupling strategies to enable the utilization of the energy stored in the out-of-equilibrium state by secondary processes–either exergonic or endergonic; (iii) taking full advantage of the controlled unidirectional motion developed by the molecular motor to enable active transport across distances longer than its size, i.e., at the nano-, micro-, and mesoscales.
A crucial property to take into account is the wavelength of the light needed for the operation. It is of capital importance to shift the power source toward less energetic photons, not only to save energy but also to increase the photostability of the components and avoid interferences with the multitude of processes simultaneously occurring within the cell.
An alternative, though not less important, application would concern the area of materials chemistry. The sequential interconnection of task-specific motors within a bulk material or polymer would give rise to sophisticated systems capable of multiresponsive molecular-scale coding, accessing innovative smart materials.113 The use of photons of different wavelengths in this context could enable distinct process pathways for the same motor. In turn, this could give rise to materials with input-specific operation, such as accumulation and release of energy. In this sense, directional motion would provide selectivity toward either of the two energy conversion steps, similarly to latest reports on electrically and chemically driven pumps.114,115
Based on the above considerations, we foresee that in the next 10–20 years the field photoactivated of artificial molecular motors will experience a massive expansion, leading to unpredictable and exciting applications able to exert a direct impact on our daily lives.
Acknowledgments
Financial support from the European Research Council (H2020 Advanced Grant No. 692981) and the Italian Ministry of University and Research (FARE R16S9XXKX3 and PRIN 20173L7W8K) is gratefully acknowledged.
Glossary
Definitions
- Molecular Motor
A species traveling a network of reactions in which energy-harvesting, exergonic processes are coupled to energy-consuming, endergonic processes, driving the system away from thermodynamic equilibrium. The motor transduces the harvested energy (e.g., light) into a different form of energy such as a non-equilibrium distribution of the species and realizes the unidirectional relative motion of the components.
- Out-of-Equilibrium State
A state in the space of phases that is not located at the global minimum of the potential energy surface. If the out-of-equilibrium state is sustained by a coupled process which constantly consumes energy from an external source (with generation of entropy or “dissipation”), it is defined as a dissipative out-of-equilibrium state.
- Autonomous Operation
Continuous operation of the motor that exploits an energy source to sustain a dissipative out-of-equilibrium state without any other external intervention. In the frame of this review, the absorbed light could be viewed as the fuel;14 the fraction of its energy that is not used to sustain the dissipative state is released as heat.
Author Contributions
§ S.C. and M.C. contributed equally. CRediT: Stefano Corra conceptualization, writing-original draft, writing-review & editing; Massimiliano Curcio conceptualization, writing-original draft, writing-review & editing; Alberto Credi conceptualization, funding acquisition, writing-review & editing.
The authors declare no competing financial interest.
References
- Schliwa M.; Woehlke G. Molecular Motors. Nature 2003, 422, 759–765. 10.1038/nature01601. [DOI] [PubMed] [Google Scholar]
- Goodsell D. S.The Machinery of Life; Copernicus, 2009. [Google Scholar]
- Rao R.; Esposito M. Nonequilibrium Thermodynamics of Chemical Reaction Networks: Wisdom from Stochastic Thermodynamics. Phys. Rev. X 2016, 6, 041064 10.1103/PhysRevX.6.041064. [DOI] [Google Scholar]
- Mattia E.; Otto S. Supramolecular Systems Chemistry. Nat. Nanotechnol. 2015, 10, 111–119. 10.1038/nnano.2014.337. [DOI] [PubMed] [Google Scholar]
- Das K.; Gabrielli L.; Prins L. J. Chemically Fueled Self-Assembly in Biology and Chemistry. Angew. Chem., Int. Ed. 2021, 60, 20120–20143. 10.1002/anie.202100274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astumian R. D. Stochastic Conformational Pumping: a Mechanism for Free-energy Transduction by Molecules. Annu. Rev. Biophys. 2011, 40, 289–313. 10.1146/annurev-biophys-042910-155355. [DOI] [PubMed] [Google Scholar]
- Astumian R. D. Microscopic Reversibility as the Organizing Principle of Molecular Machines. Nat. Nanotechnol. 2012, 7, 684–688. 10.1038/nnano.2012.188. [DOI] [PubMed] [Google Scholar]
- Carter N. J.; Cross R. A. Mechanics of the kinesin step. Nature 2005, 435, 308–312. 10.1038/nature03528. [DOI] [PubMed] [Google Scholar]
- Hübscher U.; Maga G.; Spadari S. Eukaryotic DNA Polymerases. Annu. Rev. Biochem. 2002, 71, 133–163. 10.1146/annurev.biochem.71.090501.150041. [DOI] [PubMed] [Google Scholar]
- Foth B. J.; Goedecke M. C.; Soldati D. New Insights into Myosin Evolution and Classification. Proc. Natl. Acad. Sci. U. S. A. 2006, 103, 3681–3686. 10.1073/pnas.0506307103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirokawa N.; Noda Y.; Tanaka Y.; Niwa S. Kinesin Superfamily Motor Proteins and Intracellular Transport. Nat. Rev. Mol. Cell Biol. 2009, 10, 682–696. 10.1038/nrm2774. [DOI] [PubMed] [Google Scholar]
- von Ballmoos C.; Cook G. M.; Dimroth P. Unique Rotary ATP Synthase and its Biological Diversity. Annu. Rev. Biophys. 2008, 37, 43–64. 10.1146/annurev.biophys.37.032807.130018. [DOI] [PubMed] [Google Scholar]
- Minamino T.; Imada K. The Bacterial Flagellar Motor and its Structural Diversity. Trends Microbiol. 2015, 23, 267–274. 10.1016/j.tim.2014.12.011. [DOI] [PubMed] [Google Scholar]
- As the operating principles of molecular-scale machines are fundamentally different from those of macroscopic machines, the use of terms such as “fuel” for molecular machines can be misleading if associated with mechanistic considerations (see, e.g., Peplow M.Chemists debate how to fuel molecular machines Chem. Eng. News 2023, 101, 22–27). In this article, we will refer to fuel as to the energy supplied to the motor to support its operation, irrespective of the way in which such an energy is converted into directed motion. 10.47287/cen-10105-cover [DOI] [Google Scholar]
- Amano S.; Borsley S.; Leigh D. A.; Sun Z. Chemical Engines: Driving Systems Away from Equilibrium Through Catalyst Reaction Cycles. Nat. Nanotechnol. 2021, 16, 1057–1067. 10.1038/s41565-021-00975-4. [DOI] [PubMed] [Google Scholar]
- Kassem S.; van Leeuwen T.; Lubbe A. S.; Wilson M. R.; Feringa B. L.; Leigh D. A. Artificial Molecular Motors. Chem. Soc. Rev. 2017, 46, 2592–2621. 10.1039/C7CS00245A. [DOI] [PubMed] [Google Scholar]
- Baroncini M.; Silvi S.; Credi A. Photo- and Redox-Driven Artificial Molecular Motors. Chem. Rev. 2020, 120, 200–268. 10.1021/acs.chemrev.9b00291. [DOI] [PubMed] [Google Scholar]
- Kathan M.; Hecht S. Photoswitchable Molecules as Key Ingredients to Drive Systems Away from the Global Thermodynamic Minimum. Chem. Soc. Rev. 2017, 46, 5536–5550. 10.1039/C7CS00112F. [DOI] [PubMed] [Google Scholar]
- Onsager L. Reciprocal Relations in Irreversible Processes. I. Phys. Rev. 1931, 37, 405–426. 10.1103/PhysRev.37.405. [DOI] [Google Scholar]
- Blackmond D. G. If Pigs Could Fly” Chemistry: A Tutorial on the Principle of Microscopic Reversibility. Angew. Chem., Int. Ed. 2009, 48, 2648–2654. 10.1002/anie.200804566. [DOI] [PubMed] [Google Scholar]
- Pezzato C.; Cheng C.; Stoddart J. F.; Astumian R. D. Mastering the Non-equilibrium Assembly and Operation of Molecular Machines. Chem. Soc. Rev. 2017, 46, 5491–5507. 10.1039/C7CS00068E. [DOI] [PubMed] [Google Scholar]
- Penocchio E.; Rao R.; Esposito M. Nonequilibrium Thermodynamics of Light Induced Reactions. J. Chem. Phys. 2021, 155, 114101. 10.1063/5.0060774. [DOI] [PubMed] [Google Scholar]
- Mondal A.; Toyoda R.; Costil R.; Feringa B. L. Chemically Driven Rotatory Molecular Machines. Angew. Chem., Int. Ed. 2022, 61, e202206631. 10.1002/anie.202206631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauvage J.-P. From Chemical Topology to Molecular Machines (Nobel Lecture). Angew. Chem., Int. Ed. 2017, 56, 11080–11093. 10.1002/anie.201702992. [DOI] [PubMed] [Google Scholar]
- Stoddart J. F. Mechanically Interlocked Molecules (MIMs) – Molecular Shuttles, Switches, and Machines (Nobel Lecture). Angew. Chem., Int. Ed. 2017, 56, 11094–11125. 10.1002/anie.201703216. [DOI] [PubMed] [Google Scholar]
- Feringa B. L. The Art of Building Small: From Molecular Switches to Motors (Nobel Lecture). Angew. Chem., Int. Ed. 2017, 56, 11060–11078. 10.1002/anie.201702979. [DOI] [PubMed] [Google Scholar]
- Leigh D. A. Genesis of the Nanomachines: the 2016 Nobel Prize in Chemistry. Angew. Chem., Int. Ed. 2016, 55, 14506–14508. 10.1002/anie.201609841. [DOI] [PubMed] [Google Scholar]
- Aprahamian I. The Future of Molecular Machines. ACS Cent. Sci. 2020, 6, 347–358. 10.1021/acscentsci.0c00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balzani V.; Armaroli N.. Energy for a Sustainable World: From the Oil Age to a Sun-Powered Future; Wiley-VCH: Weinheim, Germany, 2011. [Google Scholar]
- Corra S.; Curcio M.; Baroncini M.; Silvi S.; Credi A. Photoactivated Artificial Molecular Machines that Can Perform Tasks. Adv. Mater. 2020, 32, 1906064. 10.1002/adma.201906064. [DOI] [PubMed] [Google Scholar]
- Baroncini M.; Groppi J.; Corra S.; Silvi S.; Credi A. Light-Responsive (Supra)Molecular Architectures: Recent Advances. Adv. Optical Mater. 2019, 7, 1900392. 10.1002/adom.201900392. [DOI] [Google Scholar]
- Weißenfels M.; Gemen J.; Klajn R. Dissipative Self-Assembly: Fueling with Chemicals versus Light. Chem. 2021, 7, 23–37. 10.1016/j.chempr.2020.11.025. [DOI] [Google Scholar]
- Groppi J.; Baroncini M.; Venturi M.; Silvi S.; Credi A. Design of Photo-activated Molecular Machines: Highlights from the Past Ten Years. Chem. Commun. 2019, 55, 12595. 10.1039/C9CC06516D. [DOI] [PubMed] [Google Scholar]
- Naren G.; Hsu C.-W.; Li S.; Morimoto M.; Tang S.; Hernando J.; Guirado G.; Irie M.; Raymo F. M.; Sundén H.; Andréasson J. An All-photonic Full Color RGB System Based on Molecular Photoswitches. Nat. Commun. 2019, 10, 3996. 10.1038/s41467-019-11885-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y.; Tang S.; Thapaliya E. R.; Sansalone L.; Raymo F. M. Fluorescence Activation with Switchable Oxazines. Chem. Commun. 2018, 54, 8799–8809. 10.1039/C8CC03094D. [DOI] [PubMed] [Google Scholar]
- Pooler D. R. S.; Lubbe A. S.; Crespi S.; Feringa B. L. Designing Light-driven Rotary Molecular Motors. Chem. Sci. 2021, 12, 14964–14986. 10.1039/D1SC04781G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roke D.; Wezenberg S. J.; Feringa B. L. Molecular Rotary Motors: Unidirectional Motion Around Double Bonds. Proc. Natl. Acad. Sci. U.S.A. 2018, 115, 9423–9431. 10.1073/pnas.1712784115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kottas G. S.; Clarke L. I.; Horinek D.; Michl J. Artificial Molecular Rotors. Chem. Rev. 2005, 105, 1281–1376. 10.1021/cr0300993. [DOI] [PubMed] [Google Scholar]
- Bowen E. J.The Chemical Aspects of Light; Clarendon Press, 1946. [Google Scholar]
- Ross R. T. Some Thermodynamics of Photochemical Systems. J. Chem. Phys. 1966, 45, 1–7. 10.1063/1.1727289. [DOI] [Google Scholar]
- Porter G. Transfer and Storage of Chemical and Radiation Potential. J. Chem. Soc., Faraday Trans. 2 1983, 79, 473–482. 10.1039/f29837900473. [DOI] [Google Scholar]
- Astumian R. D. Optical vs. Chemical Driving for Molecular Machines. Faraday Discuss. 2016, 195, 583–597. 10.1039/C6FD00140H. [DOI] [PubMed] [Google Scholar]
- Feynman R.; Leighton R.; Sands M.. The Feynman Lectures on Physics; Basic Books: New York, 2011. [Google Scholar]
- Tantillo D. J. Wiggling and Jiggling. Am. Sci. 2019, 107, 22–26. 10.1511/2019.107.1.22. [DOI] [Google Scholar]
- Hoffman P. M.Life’s Ratchet. How Molecular Machines Extract Order from Chaos; Basic Books: New York, 2012. [Google Scholar]
- Chatterjee M. N.; Kay E. R.; Leigh D. A. Beyond Switches: Ratcheting a Particle Energetically Uphill with a Compartmentalized Molecular Machine. J. Am. Chem. Soc. 2006, 128, 4058–4073. 10.1021/ja057664z. [DOI] [PubMed] [Google Scholar]
- Astumian R. D. Thermodynamics and Kinetics of a Brownian Motor. Science 1997, 276, 917–922. 10.1126/science.276.5314.917. [DOI] [PubMed] [Google Scholar]
- Hwang W.; Karplus M. Structural basis for power stroke vs. Brownian Ratchet Mechanisms of Motor Proteins. Proc. Natl. Acad. Sci. U.S.A. 2019, 116, 19777–19785. 10.1073/pnas.1818589116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koumura N.; Zijlstra R. W. J.; van Delden R. A.; Harada N.; Feringa B. L. Light-driven Monodirectional Molecular Rotor. Nature 1999, 401, 152–155. 10.1038/43646. [DOI] [PubMed] [Google Scholar]
- Geertsema E. M.; van der Molen S. J.; Martens M.; Feringa B. L. Optimizing Rotary Processes in Synthetic Molecular Motors. Proc. Natl. Acad. Sci. U.S.A. 2009, 106, 16919–16924. 10.1073/pnas.0903710106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kistemaker J. C. M.; Štacko P.; Roke D.; Wolters A. T.; Heideman G. H.; Chang M.-C.; van der Meulen P.; Visser J.; Otten E.; Feringa B. L. Third-Generation Light-Driven Symmetric Molecular Motors. J. Am. Chem. Soc. 2017, 139, 9650–9661. 10.1021/jacs.7b04412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wezenberg S. J.; Feringa B. L. Supramolecularly Directed Rotary Motion in a Photoresponsive Receptor. Nat. Commun. 2018, 9, 1984. 10.1038/s41467-018-04249-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guentner M.; Schildhauer M.; Thumser S.; Mayer P.; Stephenson D.; Mayer P. J.; Dube H. Sunlight-powered kHz Rotation of a Hemithioindigo-based Molecular Motor. Nat. Commun. 2015, 6, 8406. 10.1038/ncomms9406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petermayer C.; Dube H. Indigoid Photoswitches: Visible Light Responsive Molecular Tools. Acc. Chem. Res. 2018, 51, 1153–1163. 10.1021/acs.accounts.7b00638. [DOI] [PubMed] [Google Scholar]
- Wilcken R.; Schildhauer M.; Rott F.; Huber L. A.; Guentner M.; Thumser S.; Hoffmann K.; Oesterling S.; de Vivie-Riedle R.; Riedle E.; Dube H. Complete Mechanism of Hemithioindigo Motor Rotation. J. Am. Chem. Soc. 2018, 140, 5311–5318. 10.1021/jacs.8b02349. [DOI] [PubMed] [Google Scholar]
- Wilcken R.; Gerwien A.; Huber L. A.; Dube H.; Riedle E. Quantitative In-Situ NMR Illumination for Excitation and Kinetic Analysis of Molecular Motor Intermediates. ChemPhotoChem. 2022, 6, e202100232 10.1002/cptc.202100232. [DOI] [Google Scholar]
- Liu R. S. H. Photoisomerization by Hula-Twist: A Fundamental Supramolecular Photochemical Reaction. Acc. Chem. Res. 2001, 34, 555–562. 10.1021/ar000165c. [DOI] [PubMed] [Google Scholar]
- Gerwien A.; Schildhauer M.; Thumser S.; Mayer P.; Dube H. Direct Evidence for Hula Twist and Single-bond Rotation Photoproducts. Nat. Commun. 2018, 9, 2510. 10.1038/s41467-018-04928-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerwien A.; Mayer P.; Dube H. Green Light Powered Molecular State Motor Enabling Eight-shaped Unidirectional Rotation. Nat. Commun. 2019, 10, 4449. 10.1038/s41467-019-12463-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schildhauer M.; Rott F.; Thumser S.; Mayer P.; de Vivie-Riedle R.; Dube H. A Prospective Ultrafast Hemithioindigo Molecular Motor. ChemPhotoChem. 2019, 3, 365–371. 10.1002/cptc.201900074. [DOI] [Google Scholar]
- Gerwien A.; Mayer P.; Dube H. Photon-Only Molecular Motor with Reverse Temperature-Dependent Efficiency. J. Am. Chem. Soc. 2018, 140, 16442–16445. 10.1021/jacs.8b10660. [DOI] [PubMed] [Google Scholar]
- Boursalian G. B.; Nijboer E. R.; Dorel R.; Pfeifer L.; Markovitch O.; Blokhuis A.; Feringa B. L. All-Photochemical Rotation of Molecular Motors with a Phosphorus Stereoelement. J. Am. Chem. Soc. 2020, 142, 16868–16876. 10.1021/jacs.0c08249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filatov M.; Paolino M.; Pierron R.; Cappelli A.; Giorgi G.; Leonard J.; Huix-Rotllant M.; Ferre N.; Yang X.; Kaliakin D.; Blanco-Gonzalez A.; Olivucci M. Towards the Engineering of a Photon-only Two-stroke Rotary Molecular Motor. Nat. Commun. 2022, 13, 6433. 10.1038/s41467-022-33695-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehn J.-M. Conjecture: Imines as Unidirectional Photodriven Molecular Motors–Motional and Constitutional Dynamic Devices. Chem.—Eur. J. 2006, 12, 5910–5915. 10.1002/chem.200600489. [DOI] [PubMed] [Google Scholar]
- Greb L.; Lehn J.-M. Light-Driven Molecular Motors: Imines as Four-Step or Two-Step Unidirectional Rotors. J. Am. Chem. Soc. 2014, 136, 13114–13117. 10.1021/ja506034n. [DOI] [PubMed] [Google Scholar]
- Liu L.; Fang W.-H.; Martinez T. J. A Nitrogen Out-of-Plane (NOOP) Mechanism for Imine-Based Light-Driven Molecular Motors.. J. Am. Chem. Soc. 2023, 145, 6888. 10.1021/jacs.3c00275. [DOI] [PubMed] [Google Scholar]
- Greb L.; Eichhofer A.; Lehn J.-M. Synthetic Molecular Motors: Thermal N Inversion and Directional Photoinduced CN Bond Rotation of Camphorquinone Imines. Angew. Chem., Int. Ed. 2015, 54, 14345–14348. 10.1002/anie.201506691. [DOI] [PubMed] [Google Scholar]
- Schrödinger E.What is Life? The Physical Aspect of the Living Cell; Cambridge University Press: Cambridge, UK, 1944. [Google Scholar]
- Ornes S. How Nonequilibrium Thermodynamics Speaks to the Mystery of Life. Proc. Natl. Acad. Sci. U.S.A. 2017, 114, 423–424. 10.1073/pnas.1620001114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Štacko P.; Kistemaker J. C. M.; van Leeuwen T.; Chang M.-C.; Otten E.; Feringa B. L. Locked Synchronous Rotor Motion in a Molecular Motor. Science 2017, 356, 964–968. 10.1126/science.aam8808. [DOI] [PubMed] [Google Scholar]
- Uhl E.; Thumser S.; Mayer P.; Dube H. Transmission of Unidirectional Molecular Motor Rotation to a Remote Biaryl Axis. Angew. Chem., Int. Ed. 2018, 57, 11064–11068. 10.1002/anie.201804716. [DOI] [PubMed] [Google Scholar]
- Uhl E.; Mayer P.; Dube H. Active and Unidirectional Acceleration of Biaryl Rotation by a Molecular Motor. Angew. Chem., Int. Ed. 2020, 59, 5730–5737. 10.1002/anie.201913798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerwien A.; Gnannt F.; Mayer P.; Dube H. Photogearing as a Concept for Translation of Precise Motions at the Nanoscale. Nat. Chem. 2022, 14, 670–676. 10.1038/s41557-022-00917-0. [DOI] [PubMed] [Google Scholar]
- Li Q.; Fuks G.; Moulin E.; Maaloum M.; Rawiso M.; Kulic I.; Foy J. T.; Giuseppone N. Macroscopic Contraction of a Gel Induced by the Integrated Motion of Light-driven Molecular Motors. Nat. Nanotechnol. 2015, 10, 161–165. 10.1038/nnano.2014.315. [DOI] [PubMed] [Google Scholar]
- Gao C.; Vargas Jentzsch A.; Moulin E.; Giuseppone N. Light-Driven Molecular Whirligig. J. Am. Chem. Soc. 2022, 144, 9845–9852. 10.1021/jacs.2c02547. [DOI] [PubMed] [Google Scholar]
- Kathan M.; Crespi S.; Thiel N. O.; Stares D. L.; Morsa D.; de Boer J.; Pacella G.; van den Enk T.; Kobauri P.; Portale G.; Schalley C. A.; Feringa B. L. A Light-fuelled Nanoratchet Shifts a Coupled Chemical Equilibrium. Nat. Nanotechnol. 2022, 17, 159–165. 10.1038/s41565-021-01021-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J.-J.; Zhao L.-Y.; Shi Z.-T.; Zhang Q.; London G.; Liang W.-J.; Gao C.; Li M.-M.; Cao X.-M.; Tian H.; Feringa B. L.; Qu D.-H. Pumping a Ring-Sliding Molecular Motion by a Light-Powered Molecular Motor. J. Org. Chem. 2019, 84, 5790–5802. 10.1021/acs.joc.9b00783. [DOI] [PubMed] [Google Scholar]
- Bach N. N.; Josef V.; Maid H.; Dube H. Active Mechanical Threading by a Molecular Motor. Angew. Chem., Int. Ed. 2022, 61, e202201882. 10.1002/anie.202201882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payamyar P.; King B. T.; Ottinger H. C.; Schluter A. D. Two-dimensional Polymers: Concepts and Perspectives. Chem. Commun. 2016, 52, 18–34. 10.1039/C5CC07381B. [DOI] [PubMed] [Google Scholar]
- Doi M.; Edwards S. F. Dynamic of Concentrated Polymer System. J. Chem. Soc., Faraday Trans. 2 1978, 74, 1789–1801. 10.1039/F29787401789. [DOI] [Google Scholar]
- Foy J. T.; Li Q.; Goujon A.; Colard-Itté J.-R.; Fuks G.; Moulin E.; Schiffmann O.; Dattler D.; Funeriu D. P.; Giuseppone N. Dual-light Control of Nanomachines that Integrate Motor and Modulator Subunits. Nat. Nanotechnol. 2017, 12, 540–545. 10.1038/nnano.2017.28. [DOI] [PubMed] [Google Scholar]
- Perrot A.; Wang W.; Buhler E.; Moulin E.; Giuseppone N. Bending Actuation of Hydrogels through Rotation of Light-Driven Molecular Motors. Angew. Chem., Int. Ed. 2023, 62, e202300263 10.1002/anie.202300263. [DOI] [PubMed] [Google Scholar]
- Chen J.; Leung F.-C.; Stuart M.; Kajitani T.; Fukushima T.; van der Giessen E.; Feringa B. L. Artificial Muscle-like Function from Hierarchical Supramolecular Assembly of Photoresponsive Molecular Motors. Nat. Chem. 2018, 10, 132–138. 10.1038/nchem.2887. [DOI] [PubMed] [Google Scholar]
- Chen S.; Yang L.; Leung F. K.-C.; Kajitani T.; Stuart M. C. A.; Fukushima T.; van Rijn P.; Feringa B. L. Photoactuating Artificial Muscles of Motor Amphiphiles as an Extracellular Matrix Mimetic Scaffold for Mesenchymal Stem Cells. J. Am. Chem. Soc. 2022, 144, 3543–3553. 10.1021/jacs.1c12318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S.; Wang Y.; Nie T.; Bao C.; Wang C.; Xu T.; Lin Q.; Qu D.-H.; Gong X.; Yang Y.; Zhu L.; Tian H. An Artificial Molecular Shuttle Operates in Lipid Bilayers for Ion Transport. J. Am. Chem. Soc. 2018, 140, 17992–17998. 10.1021/jacs.8b09580. [DOI] [PubMed] [Google Scholar]
- Wang C.; Wang S.; Yang H.; Xiang Y.; Wang X.; Bao C.; Zhu L.; Tian H.; Qu D.-H. A Light-Operated Molecular Cable Car for Gated Ion Transport. Angew. Chem., Int. Ed. 2021, 60, 14836–14840. 10.1002/anie.202102838. [DOI] [PubMed] [Google Scholar]
- Ribovski L.; Zhou Q.; Chen J.; Feringa B. L.; van Rijn P.; Zuhorn I. S. Light-induced Molecular Rotation Triggers On-demand Release from Liposomes. Chem. Commun. 2020, 56, 8774–8777. 10.1039/D0CC02499F. [DOI] [PubMed] [Google Scholar]
- Wang W.-Z.; Huang L.-B.; Zheng S.-P.; Moulin E.; Gavat O.; Barboiu M.; Giuseppone N. Light-Driven Molecular Motors Boost the Selective Transport of Alkali Metal Ions through Phospholipid Bilayers. J. Am. Chem. Soc. 2021, 143, 15653–15660. 10.1021/jacs.1c05750. [DOI] [PubMed] [Google Scholar]
- Yang H.; Yi J.; Pang S.; ye K.; Ye Z.; Duan Q.; Yan Z.; Lian C.; Yang Y.; Zhu L.; Qu D.-H.; Bao C. A Light-Driven Molecular Machine Controls K+ Channel Transport and Induces Cancer Cell Apoptosis. Angew. Chem., Int. Ed. 2022, 61, e202204605. 10.1002/anie.202204605. [DOI] [PubMed] [Google Scholar]
- García-López V.; Chen F.; Nilewski L. G.; Duret G.; Aliyan A.; Kolomeisky A. B.; Robinson J. T.; Wang G.; Pal R.; Tour J. Molecular Machines Open Cell Membranes. Nature 2017, 548, 567–572. 10.1038/nature23657. [DOI] [PubMed] [Google Scholar]
- MacDonald T. S. C.; Price W. S.; Astumian R. D.; Beves J. E. Enhanced Diffusion of Molecular Catalysts is Due to Convection. Angew. Chem., Int. Ed. 2019, 58, 18864–18867. 10.1002/anie.201910968. [DOI] [PubMed] [Google Scholar]
- Bruns C.; Stoddart J. F.. The Nature of the Mechanical Bond: From Molecules to Machines; Wiley: Hoboken, NJ, 2016. [Google Scholar]
- Nicoli F.; Curcio M.; Tranfić Bakić M.; Paltrinieri E.; Silvi S.; Baroncini M.; Credi A. Photoinduced Autonomous Nonequilibrium Operation of a Molecular Shuttle by Combined Isomerization and Proton Transfer Through a Catalytic Pathway. J. Am. Chem. Soc. 2022, 144, 10180–10185. 10.1021/jacs.1c13537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serreli V.; Lee C.-F.; Kay E. R.; Leigh D. A. A Molecular Information Ratchet. Nature 2007, 445, 523–527. 10.1038/nature05452. [DOI] [PubMed] [Google Scholar]
- Li H.; Cheng C.; McGonigal P. R.; Fahrenbach A. C.; Frasconi M.; Liu W.-G.; Zhu Z.; Zhao Y.; Ke C.; Lei J.; Young R. M.; Dyar S. M.; Co D. T.; Yang Y.-W.; Botros Y. Y.; Goddard W. A. III; Wasielewski M. R.; Astumian R. D.; Stoddart J. F. Relative Unidirectional Translation in an Artificial Molecular Assembly Fueled by Light. J. Am. Chem. Soc. 2013, 135, 18609–18620. 10.1021/ja4094204. [DOI] [PubMed] [Google Scholar]
- Andreoni L.; Baroncini M.; Groppi J.; Silvi S.; Taticchi C.; Credi A. Photochemical Energy Conversion with Artificial Molecular Machines. ACS Energy & Fuels 2021, 35 (23), 18900–18914. 10.1021/acs.energyfuels.1c02921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragazzon G.; Baroncini M.; Silvi S.; Venturi M.; Credi A. Light-powered Autonomous and Directional Molecular Motion of a Dissipative Self-assembling System. Nat. Nanotechnol. 2015, 10, 70–75. 10.1038/nnano.2014.260. [DOI] [PubMed] [Google Scholar]
- Sabatino A.; Penocchio E.; Ragazzon G.; Credi A.; Frezzato D. Individual-Molecule Perspective Analysis of Chemical Reaction Networks: The Case of a Light-Driven Supramolecular Pump. Angew. Chem., Int. Ed. 2019, 58, 14341–14348. 10.1002/anie.201908026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casimiro L.; Groppi J.; Baroncini M.; La Rosa M.; Credi A.; Silvi S. Photochemical Investigation of Cyanoazobenzene Derivatives as Components of Artificial Supramolecular Pumps. Photochem. Photobiol. Sci. 2018, 17, 734–740. 10.1039/c8pp00062j. [DOI] [PubMed] [Google Scholar]
- Groppi J.; Casimiro L.; Canton M.; Corra S.; Jafari-Nasab M.; Tabacchi G.; Cavallo L.; Baroncini M.; Silvi S.; Fois E.; Credi A. Precision Molecular Threading/Dethreading. Angew. Chem., Int. Ed. 2020, 59, 14825–14834. 10.1002/anie.202003064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corra S.; Casimiro L.; Baroncini M.; Groppi J.; La Rosa M.; Tranfić Bakić M.; Silvi S.; Credi A. Artificial Supramolecular Pumps Powered by Light. Chem.—Eur. J. 2021, 27, 11076–11083. 10.1002/chem.202101163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canton M.; Groppi J.; Casimiro L.; Corra S.; Baroncini M.; Silvi S.; Credi A. Second-Generation Light-Fueled Supramolecular Pump. J. Am. Chem. Soc. 2021, 143, 10890–10894. 10.1021/jacs.1c06027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corra S.; Tranfić Bakić M.; Groppi J.; Baroncini M.; Silvi S.; Penocchio E.; Esposito M.; Credi A. Kinetic and Energetic Insights into the Dissipative Non-equilibrium Operation of an Autonomous Light-powered Supramolecular Pump. Nat. Nanotechnol. 2022, 17, 746–751. 10.1038/s41565-022-01151-y. [DOI] [PubMed] [Google Scholar]
- Waelès P.; Gauthier M.; Coutrot F. Challenges and Opportunities in the Post-Synthetic Modification of Interlocked Molecules. Angew. Chem., Int. Ed. 2021, 60, 16778–16799. 10.1002/anie.202007496. [DOI] [PubMed] [Google Scholar]
- Amano S.; Esposito M.; Kreidt E.; Leigh D. A.; Penocchio E.; Roberts B. M. W. Insights from an Information Thermodynamics Analysis of a Synthetic Molecular Motor. Nat. Chem. 2022, 14, 530–537. 10.1038/s41557-022-00899-z. [DOI] [PubMed] [Google Scholar]
- Chen K.-J.; Tsai Y.-C.; Suzaki Y.; Osakada K.; Miura A.; Horie M. Rapid and Reversible Photoinduced Switching of a Rotaxane Crystal. Nat. Commun. 2016, 7, 13321. 10.1038/ncomms13321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vukotic V. N.; O’Keefe C. A.; Zhu K.; Harris K. J.; To C.; Schurko R. W.; Loeb S. J. Mechanically Interlocked Linkers inside Metal-Organic Frameworks: Effect of Ring Size on Rotational Dynamics. J. Am. Chem. Soc. 2015, 137 (30), 9643–9651. 10.1021/jacs.5b04674. [DOI] [PubMed] [Google Scholar]
- Baggi G.; Wilson B. H.; Dhara A.; O’Keefe C. A.; Schurko R. W.; Loeb S. J. Dynamics of a [2]Rotaxane Wheel in a Crystalline Molecular Solid. Chem. Commun. 2021, 57, 8210–8213. 10.1039/D1CC03009D. [DOI] [PubMed] [Google Scholar]
- Zhu K.; O’Keefe C. A.; Vukotic V. N.; Schurko R. W.; Loeb S. J. A molecular Shuttle that Operates Inside a Metal-organic Framework. Nat. Chem. 2015, 7, 514–519. 10.1038/nchem.2258. [DOI] [PubMed] [Google Scholar]
- Takata T. Switchable Polymer Materials Controlled by Rotaxane Macromolecular Switches. ACS Cent. Sci. 2020, 6, 129–143. 10.1021/acscentsci.0c00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu K.; Baggi G.; Loeb S. J. Ring-through-ring Molecular Shuttling in a Saturated [3]Rotaxane. Nat. Chem. 2018, 10, 625–630. 10.1038/s41557-018-0040-9. [DOI] [PubMed] [Google Scholar]
- Corra S.; de Vet C.; Baroncini M.; Credi A.; Silvi S. Stereodynamics of E/Z Isomerization in Rotaxanes Through Mechanical Shuttling and Covalent Bond Rotation. Chem. 2021, 7, 2137–2150. 10.1016/j.chempr.2021.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dattler D.; Fuks G.; Heiser J.; Moulin E.; Perrot A.; Yao X.; Giuseppone N. Design of Collective Motions from Synthetic Molecular Switches, Rotors, and Motors. Chem. Rev. 2020, 120, 310–433. 10.1021/acs.chemrev.9b00288. [DOI] [PubMed] [Google Scholar]
- Thomas D.; Tetlow D. J.; Ren Y.; Kassem S.; Karaca U.; Leigh D. A. Pumping Between Phases with a Pulsed-fuel Molecular Ratchet. Nat. Nanotechnol. 2022, 17, 701–707. 10.1038/s41565-022-01097-1. [DOI] [PubMed] [Google Scholar]
- Feng L.; Qiu Y.; Guo Q.-H.; Chen Z.; Seale J. S. W.; He K.; Wu H.; Feng Y.; Farha O. K.; Astumian R. D.; Stoddart J. F. Active Mechanisorption Driven by Pumping Sassettes. Science 2021, 374, 1215–1221. 10.1126/science.abk1391. [DOI] [PubMed] [Google Scholar]