Abstract

A linker design strategy is developed to attain novel polynuclear rare-earth (RE) metal–organic frameworks (MOFs) with unprecedented topologies. We uncover the critical role of ortho-functionalized tricarboxylate ligands in directing the construction of highly connected RE MOFs. The acidity and conformation of the tricarboxylate linkers were altered by substituting with diverse functional groups at the ortho position of the carboxyl groups. For instance, the acidity difference between carboxylate moieties resulted in forming three hexanuclear RE MOFs with novel (3,3,3,10,10)-c wxl, (3,12)-c gmx, and (3,3,3,12)-c joe topologies, respectively. In addition, when a bulky methyl group was introduced, the incompatibility between the net topology and ligand conformation guided the co-appearance of hexanuclear and tetranuclear clusters, generating a novel 3-periodic MOF with a (3,3,8,10)-c kyw net. Interestingly, a fluoro-functionalized linker prompted the formation of two unusual trinuclear clusters and produced a MOF with a fascinating (3,8,10)-c lfg topology, which could be gradually replaced by a more stable tetranuclear MOF with a new (3,12)-c lee topology with extended reaction time. This work enriches the polynuclear clusters library of RE MOFs and unveils new opportunities to construct MOFs with unprecedented structural complexity and vast application potential.
Keywords: metal−organic framework, topology, rare earth, ortho effect, high connectivity
Introduction
Metal–organic frameworks (MOFs) have fueled considerable interest from researchers due to their remarkable potential for advanced applications, such as gas separation,1−4 energy storage,5−10 water harvesting,11−13 and carbon capture.14−18 As a supramolecular assembly, MOFs consist of periodically interlinked metal-containing nodes and organic linkers to give exceptional porosity and tunable topologies.19−25 Therefore, rational linker design is an effective approach to regulate MOF topologies, which can be altered by tuning the substituents, sizes, geometries, and symmetry of the organic linkers.26 For instance, the combination of a linear terephthalate (BDC) and a square paddlewheel cluster usually leads to MOF-2 as a two-dimensional (2D) sql net. Yaghi and co-workers successfully assembled the paddlewheel clusters into a three-dimensional (3D) nbo net by employing a sterically hindered o-Br-BDC linker.27 Later, Yaghi and Furukawa et al., systematically adjusted the substituent locations and linker symmetry in the 4,4′-biphenyldicarboxylate (BPDC) and attained a series of paddlewheel-based frameworks ranging from zero-dimensional (0D) to 3D.28 This design strategy was further introduced into the Zr-MOFs by our group, in which a methyl-functionalized BPDC linker was designed to construct a bcu MOF named PCN-700 with unsaturated Zr6 clusters, rather than the fcu net observed in UiO-67 (PCN = porous coordination network; UiO = University of Oslo).29−33 These practices in exploring MOF topologies significantly enriched the structural library of MOFs, empowering the functional materials with huge application potential.
Recently, rare-earth (RE) MOFs have drawn wide attention owing to their diverse structures and versatility. The highly adaptable coordination modes of RE elements allow for multiple coordination directionality of carboxylate ligands, facilitating the occurrence of polynuclear clusters with various connectivity and geometries. Yet, the observation of polynuclear RE clusters was usually viewed as synthetic serendipity at the early stage of MOF research.34,35 Beyond aforementioned, the varieties of reported RE polynuclear clusters are relatively limited in MOFs, given the fact that most reported RE MOFs are based on mono-/di-nuclear clusters or rod-shaped RE-chains.34,36,37 The scarcity of RE polynuclear clusters can be attributed to their dynamic nature, making them unattainable during MOF synthesis. Herein, compatible coordination spheres are required to stabilize the RE clusters. For instance, Rosi and co-workers constructed a series of RE4-based MOFs with an amino group-functionalized carboxylic acid.38 Notably, the ortho-amino group structurally directs the formation of the octahedral 6-c RE4 cluster.39−47 Similarly, Eddaoudi and co-workers employed ortho-fluorinated ditopic linkers to prepare fcu MOFs with fully coordinated 12-c RE6 clusters.48 They concurrently observed the occurrence of 8-c RE6 and 12-c RE9 clusters in a (3,8,12)-c pek MOF with tritopic ligands, in which the formation of RE9 clusters was attributed to the incompatibility between the net and ligand geometry.49 We also intentionally decreased the symmetry of a series of tri-/tetracarboxylate linkers and attained RE9-based MOFs with cluster connectivity numbers of 12, 14, or 18.50 Nevertheless, it remains a challenge to construct highly connected RE MOFs bearing unprecedented polynuclear clusters and new topologies through rational linker design despite recent progress.
Ortho effect is a fundamental phenomenon widely observed in organic chemistry, which significantly affects the acidity and reactivity of aromatic compounds, especially aromatic carboxylic acid51,52 (Figure S1). Although most reported MOFs are based on aromatic carboxylate linkers, the significance of ortho effect in regulating topologies of RE MOFs is grossly underestimated. Herein, we chose a tritopic linker as the prototype and deliberately introduced ortho-substituents into the central or the peripheral phenyl rings (Figure 1). The ortho-substituents will force adjacent carboxylates into a specific dihedral angle with the phenyl rings and affect the carboxylate acidity, forming unusual polynuclear RE clusters. For instance, the fluoro-functionalized linker H3L–F will prompt the formation of two unusual trigonal trinuclear clusters, 8-connected RE3 and 10-connected RE3, and produce another layered mixed-cluster MOF with a new (3,8,10)-c lfg topology (Figure 1a). More interestingly, while extending the reaction time, the RE3-based MOF can be gradually replaced by a more stable MOF with a new (3,12)-c lee topology, consisting of unusual 12-connected RE4 clusters (Figure 1b). This transformation is unprecedented in RE MOFs, attributed to the incompatibility in the linker geometry and coordination requirements of the clusters in the initial MOF. Besides, the H3L–CH3 linker with bulky methyl groups guides the co-appearance of octahedral hexanuclear RE6 clusters and rare diamondoid tetranuclear RE4 clusters (Figure 1c), generating a highly porous mixed-cluster MOF with an engaging (3,3,8,10)-c kyw topology. In addition, some other layered MOFs are discovered by using hexanuclear RE6 clusters and linkers functionalized with chloro, amino, or methoxy, demonstrating new (3,3,3,10,10)-c, (3,12)-c, or (3,3,3,12)-c topologies (Figure 1d–f).
Figure 1.
Illustration of diverse topologies in highly connected RE MOFs based on ortho-substituted tricarboxylate ligands. (a) Employment of a fluoro-functionalized linker H3L–F induced the formation of PCN-992(Eu) featuring a novel (3,8,10)-c lfg net based on 8-c and 10-c RE3 clusters. (b) PCN-992(Eu) was replaced by PCN-993(Eu) by elongated reaction time, which adopts a (3,12)-c lee net with 12-c RE4 clusters. (c) Methyl-functionalized linker H3L–CH3 resulted in the co-appearance of 10-c RE6 clusters and 8-c RE4 clusters, which were assembled into PCN-991(Eu) with a new (3,3,8,10)-c kyw net. (d) Chloro-functionalized linker H3L–Cl led to the discovery of a (3,3,3,10,10)-c wxl net with the 10-c RE6 clusters. (e) Amino-functionalized linker H3L–NH2 formed PCN-995(Eu) with a two-nodal (3,12)-c gmx net. (f) Combination of a methoxyl-functionalized linker H3L–OCH3 and 12-c RE6 clusters generated a highly connected (3,3,3,12)-c joe net. The metal clusters, C atoms, and O atoms are represented in turquoise, dark gray, and red, respectively. H atoms are omitted for clarity.
Results and Discussion
Design of Ortho-Functionalized Tricarboxylic Linkers
A tricarboxylate linker [1,1′:3′,1″-terphenyl]-4,4″,5′-tricarboxylic acid (H3L–H) was selected as the prototype, consisting of two peripheral phenyl rings and one central phenyl ring. The two peripheral phenyl rings represent an angle of 120°. Previous studies indicate that the H3L–H could afford MOFs with (3,3,18)-c ytw topology, in which a rare 18-connected nonanuclear RE cluster was discovered.50,53 In this work, ortho positions to H3L–H’s carboxyl were substituted with various functional groups to investigate the role of ortho effects in the MOF topology regulation. Given that there are three variables in the linker design, namely, substitution position, steric hindrance, and electronic effect, fluoro, chloro, and methyl were deliberately introduced to the 3 and 3″ positions of H3L–H, leading to H3L–F, H3L–Cl, and H3L–CH3. Moreover, the 4′ position of H3L–H can be functionalized with amino and methoxyl groups, resulting in the linkers H3L–NH2 and H3L–OCH3 (Figure 1).
Synthesis and Structural Description of a Mixed-Cluster MOF
A solvothermal reaction between H3L–CH3 and Eu(NO3)3·6H2O produced colorless crystals, named as PCN-991(Eu), in the presence of 2-fluorobenzoic acid (2-FBA). The utilization of 2-FBA can facilitate the in-situ formation of hexanuclear RE6 clusters,48,54−58 which can be interlinked and extended to afford 3-periodic networks. Interestingly, according to single-crystal X-ray diffraction (SCXRD) studies, PCN-991(Eu) consists of both 10-c hexanuclear clusters [RE6(μ3-OH)8(COO)10] and 8-c tetranuclear clusters [RE4(μ3-O)2(COO)8] (Figure 1c, Table S1). Note that the 10-c RE6 cluster can be regarded as an elongated square bipyramid, a Johnson solid labeled as J15, while the 8-c RE4 cluster can be simplified into a cube (Figure S2d,e). The two kinds of nodes are interlinked by tritopic ligands to attain a new 3-periodic (3,3,8,10)-c kyw net with a point symbol of {42·6}4{43}2{46·66·814·102}{48·620·813·104} as determined by ToposPro.59,60
Further structural analysis revealed that the mixed-cluster PCN-991(Eu) was constructed via a supramolecular building layer approach.49,61 The tritopic ligand can form a 2-c moiety, [1,1′:3′,1″-terphenyl]-4,4″-dicarboxylate (H2TDC), after eliminating the carboxyl on the central phenyl ring. The RE6 clusters were bridged by the TDC, thus leading to a 2D double cross-linked sql net (Figure 2a). Specifically, the PCN-991(Eu) features an AAA stacking of sql layers, when merely considering the TDC moieties, consisting of RE6 clusters bridged with four adjacent clusters through eight TDC moieties. The central phenyl rings of TDC moieties point the rectangular pores of sql nets inward, inducing the RE4 cluster to intercalate the adjacent two sql layers, in virtue of the incompatibility between the sql net and the RE6 clusters (Figure 2b). Herein, the (3,3,8,10)-c kyw net consists of two layers, namely, a double cross-linked sql layer and a periodic array of 8-c RE4 clusters (Figure 2c). In 2015, Eddaoudi and co-workers reported pek MOFs featuring 12-c RE9, 8-c RE6 clusters, and 3-c ligands.49 Herein, PCN-991(Eu) represents a rare MOF composed of two polynuclear clusters, which can be regarded as a complementary case for the pek net.
Figure 2.

Schematic of PCN-991(Eu) with the new (3,3,8,10)-c kyw topology. (a) Elongated square bipyramid, representing the 10-c RE6 cluster, can be interconnected by H3L–CH3 to generate a double cross-linked sql net. (b) Network incompatibility prompted the formation of the C4-symmteric 8-c RE4 cluster. (c) RE4 clusters served as pillars for the kyw net.
The powder X-ray diffraction (PXRD) patterns show that the crystallinity of PCN-991(Eu) can be maintained at a broad pH range from 4 to 10, demonstrating the framework’s chemical stability (Figures S8 and S9). The thermal stability of PCN-991(Eu) was tested through thermal gravimetric analysis (TGA) (Figure S15). Additionally, nitrogen sorption tests demonstrated that PCN-991(Eu) features a high Brunauer–Emmett–Teller (BET) surface area of 1179 m2/g and micropores at 6, 8, and 11 Å (Figures S20–S22). The application potential of PCN-991(Eu) was evaluated, which featured moderate CO2/CH4 selectivity (Figure S26).
Synthesis and Structural Description of RE6-Based MOFs
Functionalizing the peripheral phenyl rings of the tritopic linker with chloride groups can produce the linker H3L–Cl. The combination of deprotonated L-Cl and [RE6(μ3-OH)8(COO)10] leads to the formation of PCN-994(Eu) with a new (3,3,3,10,10)-c wxl net (Table S2). The RE6 clusters can be viewed as an elongated square bipyramid (J15), identical to the 10-c RE6 observed in PCN-991(Eu) (Figure S1e). One RE6 cluster is bridged with six neighboring clusters through the 3-c L-Cl (Figure 3a). Furthermore, an ABAB stacking of two layers is observed in PCN-994(Eu) (Figure 3b). Specifically, the wxl net can be separated into stacked one-dimensional (1D) supramolecular ribbons when considering the L-Cl as a TDC moiety. The supramolecular ribbon is a one-periodic array of three RE6 clusters (Figure 3c). Ribbons are closely packed along the z axis, and the matched symmetry between adjacent ribbons ensures the formation of the ABAB stacking wxl net (Figure S7).
Figure 3.
Structural illustration of PCN-994(Eu) with (3,3,3,10,10)-c wxl topology. (a) Elongated square bipyramids, representing 10-c RE6 clusters, were interlinked through the tritopic ligands to form a wxl net. (b) Layered packing of RE6 clusters was observed in PCN-994(Eu). (c) (3,3,3,10,10)-c wxl net can be viewed as a close stack of 1D supramolecular ribbons when cleaving one carboxylate of the tritopic ligand.
When the H3L–NH2 with amino group at the 4′-position serves as the ligand, a MOF named PCN-995(Eu) featuring a (3,12)-c gmx net can be attained. According to crystallographic studies, PCN-995(Eu) consists of RE6 clusters connecting with 12 neighboring clusters (Figure 4a). A disorder of ligands is observed in the crystal structure, which is originated from the C2/c space group. In addition, the RE6 clusters are interlinked through doubly cross-linked ligands to afford a sql net (Figure 4b,c). Note that the sql nets are packed through ABAB mode, leading to the 2-nodal gmx net with a point symbol of {412·638·816}{43}4.
Figure 4.

Structural illustration of PCN-995(Eu) with (3,12)-c gmx topology. (a) Cuboctahedron represents the 12-c RE6 cluster, and the triangle represents the 3-c H3L–NH2 ligand. (b) 12-c RE6 cluster, labeled in yellow, is bridged with 12 adjacent clusters, leading to a layered structure. (c) gmx net can be simplified into stacked double cross-linked sql nets.
The utilization of 4′-substituted H3L–OCH3 induced the formation of PCN-996(Eu) with [RE6(μ3-OH)8(COO)12]2– clusters, crystallized in the monoclinic space group P21/n (Figure 5a, Table S2). PCN-996(Eu) demonstrates a BET surface area of 891 m2/g with a pore size of 7.6 Å (Figures S23–S25). When viewing the carboxylates as the vertices, the 12-c RE6 cluster can be represented as a cuboctahedron, an Archimedean solid (Figure S1f). Overall, PCN-996(Eu) features a (3,3,3,12)-c joe topology with a point symbol of {416·634·816}{42·6}2{43}2. Specifically, the joe net can be converted into AAA stacking layers after removing one peripheral carboxylate group of H3L–OCH3, in which the RE6 clusters are double cross-linked to afford a sql net (Figure 5b). Compared with the (3,12)-c gmx net, one RE6 cluster connects nine adjacent clusters through one or three H3L–OCH3.
Figure 5.
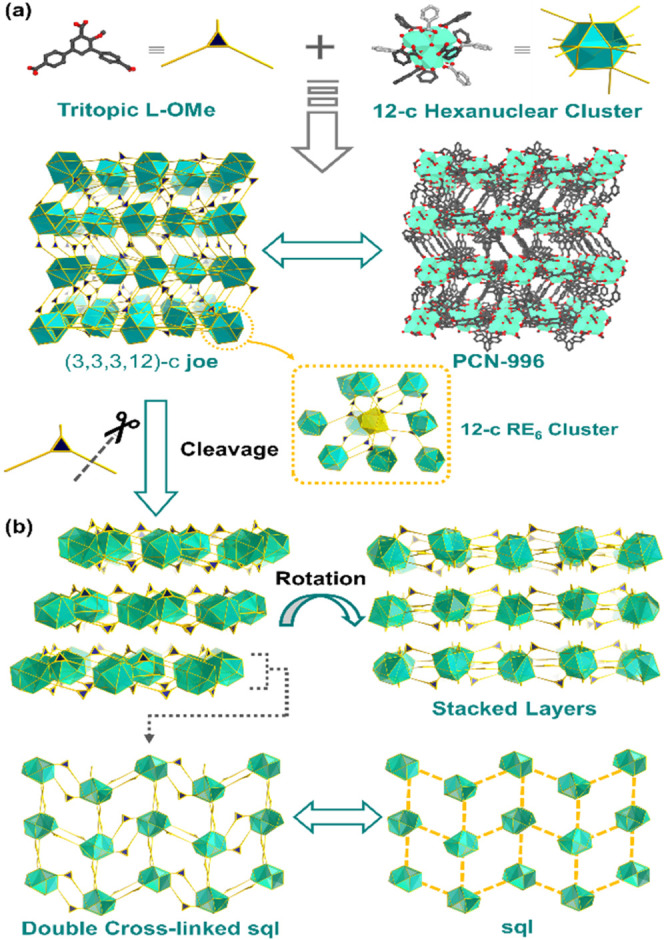
Structural illustration of PCN-996(Eu) with (3,3,3,12)-c joe topology. (a) Cuboctahedron represents the 12-c RE6 cluster, and the triangle represents the 3-c H3L–OCH3 ligand. (b) Double cross-linked RE6sql nets are closely packed with each other to give the (3,3,3,12)-c joe topology.
Phase Transformation between RE3- and RE4-Based MOFs
A unique phase transformation was observed in the solvothermal reaction between 3,3″ functionalized 3-c H3L–F and Eu(NO3)3·6H2O by varying the reaction time. Colorless rod-shaped crystals were observed after 36 h, named PCN-992(Eu). Interestingly, PCN-992(Eu) was replaced by some yellowish crystals, PCN-993(Eu), when extending the reaction time to 72 h (Figure 6). The two MOFs feature distinct RE clusters and topologies. For instance, the PCN-992(Eu) is composed of two kinds of clusters, 8-c [RE3(μ3-OH)(COO)8] and 10-c [RE3(μ3-OH)(COO)10]2–. Topologically, the 8-c RE3 cluster can be viewed as a snub-disphenoid, a Johnson solid labeled as J84 (Figure S1a). The 10-c RE3 cluster is represented as an arrowhead-tetradecahedron generated by augmenting two vertices to the middle of the snubdsphenoid (Figure S1b). 1H NMR spectrum indicates that dimethylamine cations serve as the counter cations to balance the negative charge of integral framework (Figure S46). Trinuclear clusters are common for d-block metal-based MOFs.62−71 However, only a limited number of RE3 clusters have been reported, most of which feature linear or bent geometries.72−76 To our knowledge, one rare example of trigonal prismatic RE3 clusters was observed in a MOF named JXNU-3 composed of 15-c nonanuclear and 9-c trinuclear clusters.77 Han, Gu, and co-workers reported several robust RE MOFs bearing 6-c trinuclear clusters in 2017.78 Notably, it is challenging to tailor the coordination sphere to stabilize trigonal prismatic RE3 clusters in coordination complexes, usually requiring chelating or macrocyclic auxiliary ligands.79−84 Interestingly, the chemical structures of the two [RE3(μ3-OH)(COO)8] and [RE3(μ3-OH)(COO)10]2– clusters in PCN-992(Eu) are pretty similar, in both of which the three RE ions feature coordination numbers of 8, 8, and 9, respectively, bridged by one μ3-O atom and six μ2-COO groups. Due to the high adaptability of RE-carboxylate coordination bonds, the 8-c RE3 cluster consists of eight capping carboxylate groups, while the 10-c RE3 cluster is surrounded by eight bidentate and two unidentate carboxylate groups (Figure S4). Each 10-c RE3 cluster is connected to two 10-c RE3 and six 8-c RE3 through 3-c ligands, giving a (3,8,10)-c lfg net with a point symbol of {416·64·825}{42·6}2{43}4{48·64·815·10} (Figure 7a). When viewed along the y axis, the lfg net can be divided into four different layers, in which the layers 1 and 3 and layers 2 and 4 are inverse to each other (Figure 7b,c). To further investigate the topology, one peripheral ring of the H3L–F is assumed to be cleaved, and the lfg net is converted into two sets of zigzag supramolecular chains, composed of doubly or quadruply cross-linked RE3 clusters (Figure 7c–f). The interlinked zigzag chains form the lfg net with rhomboid channels.
Figure 6.
(a) Use of fluoro-functionalized H3L–F ligand induced the formation of two RE MOFs with different reaction time. (b) PCN-992(Eu) with (3,8,10)-c lfg topology appeared after 36 h, consisting of rare 8-c and 10-c RE3 clusters. (c) After 72 h, the PCN-992(Eu) would be replaced by PCN-993(Eu) with the highly connected (3,12)-c lee topology, which was based on 12-c RE4 clusters.
Figure 7.
Structural illustration of PCN-992(Eu) with the (3,8,10)-c lfg topology. (a) Lavender snub-disphenoid represents the 8-c RE3 cluster, and the turquoise arrowhead-tetradecahedron represents the 10-c RE3 cluster. (b, d) lfg net contains four different layers along the x axis, in which the layers 1 and 3, and layers 2 and 4 are inverse to each other. (c, e, f) lfg net can be divided into two zigzag chains when cleaving one carboxylate of the H3L–F ligand.
Furthermore, a 12-c [RE4(μ3-OH)2(COO)12]2– cluster was observed in the thermodynamic product PCN-993 during the synthesis, where the integral charge of the anionic framework is balanced by dimethylamine cations (Figure S48). The 12-c RE4 cluster can be represented by the sphenomegacorona, another Johnson solid labeled as J88, with 12 vertices and 18 faces (Figure S1c). The four RE ions in the 12-c cluster are arranged in a rhombic manner, bridged by two μ3-O atoms. Notably, the [RE4(μ3-OH)2(COO)12]2– cluster can be transformed from the [RE3(μ3-OH)(COO)8] by augmenting one RE metal and four capping carboxylates (Figure S5). Although similar rhombic RE4 clusters have been reported in several coordination complexes and frameworks, the 12-c [RE4(μ3-OH)2(COO)12]2– represents one RE4 cluster with the highest connectivity number to the best of our knowledge.35,85−87
In the PCN-993(Eu) with the orthorhombic space group Fddd, each RE4 cluster is connected with eight adjacent RE4 clusters through the 3-c H3L–F ligands, affording a (3,12)-c lee net with a point symbol of {422·68·832·104}{43}4 (Figure 8a). When cleaving the carboxylate on the central phenyl ring and converting the 3-c H3L–F into the TDC moiety, the RE4 clusters will be assembled into a double cross-linked sql layer. (Figure 8c). The two neighboring sql nets can be packed closely and intercalated to give an ABAB stacking due to the compatibility between the layers (Figure 8b). Moreover, the (3,12)-c lee net can be eliminated into an ABAB stacking of hcb layers by removing one peripheral phenyl ring of the 3-c H3L–F (Figure S5). The hcb net is cross-linked by quadruple H3L–F ligands to form hexagonal pores. The adjacent hcb nets can be fused to generate hexagonal channels (Figure S6). Overall, both rhombic and hexagonal channels are present in the (3,12)-c lee net.
Figure 8.

Structural illustration of PCN-993(Eu) featuring (3,12)-c lee topology. (a) lee net is based on the sphenomegacorona, representing a rare 12-c RE4 cluster. (b) Close stack of the 12-c RE4 clusters allows for the installation of H3L–F to produce the layered structure. (c) Double cross-linked sql net is observed in the (3,12)-c lee net after the cleavage of one carboxylate of H3L–F.
Formation Mechanism of Diverse Topologies
The structural diversity of RE MOFs can be attributed to the highly adaptable coordination modes of the RE ions, allowing multiple coordination directionality of carboxylate ligands. As a result, a series of polynuclear clusters with various connectivity and geometries have been attained, leading to diverse MOF topologies. In coordination complexes, structures of lanthanide–oxo clusters profoundly depend on factors such as ligand types, auxiliary ligands, metal types, and reaction conditions.40,88−91 Our work suggests that the ortho functionalization in tricarboxylate linkers contributes to the formation of diverse metal–oxo clusters, which are essential building blocks constructing the overall frameworks. Herein, we investigated how the altered ortho functionalization regulates the RE cluster structures and framework topologies.
The presence of functional groups at ortho position can significantly affect the acidity of the carboxyl group in benzoic acid, which can be attributed to the electronic effects and steric hindrance (Figure S28). Herein, introducing ortho functional groups into the prototype ligand, [1,1′:3′,1′′-terphenyl]-4,4′′,5′-tricarboxylic acid, will not only change the overall conformation but also bring acidity difference between the carboxylates. To confirm our hypothesis, density functional theory (DFT) simulations were performed to calculate the deprotonation free energies of the tricarboxylate ligands, which are negatively associated with acidity92,93 (Figure S29). According to DFT calculations, the central carboxyl groups of H3L–F and H3L–CH3 are more acidic than the peripheral ones, while the acidities of carboxyl groups are close in H3L–Cl and H3L–NH2. Moreover, the acidity of central carboxyl of H3L–OCH3 is remarkably weaker than the peripheral ones due to the strong p-π electron donation from oxygen and the intramolecular hydrogen bonding. Herein, the calculation results demonstrate that the ortho functionalization of carboxylate groups enables the tuning of the acidity of tricarboxylate ligands.
SCXRD results reveal that the linkers feature various distorted conformations when confined in the frameworks (Tables S7 and S8). Single-point calculations were performed based upon the XRD-determined ligand geometries at B3LYP level. The ligand geometries were further optimized to attain the relaxation energy in the gas phase (Figures S31–S33). The energy changes were calculated by subtracting the free energy of the confined ligand with the one after geometry optimization. The calculation results showed that the ligands H3L–Cl and H3L–NH2 feature close free energy changes around 50 kcal/mol after distortion, indicating that the two ligands have similar steric hindrances. Furthermore, the H3L–CH3 adopts three different conformations in PCN-991(Eu) with free energy changes of 62.92, 74.75, and 32.31 kcal/mol, respectively. Herein, the H3L–CH3 is distorted to accommodate the coexistence of RE4 and RE6 clusters. In addition, the fluoro-functionalized linker H3L–F adopts different conformation in PCN-992(Eu) and PCN-993(Eu). The single-point energy of H3L–F in PCN-993(Eu) is 21.9 kcal/mol lower than that in PCN-992(Eu). Such a significant energy difference demonstrates that PCN-993(Eu) is a thermodynamic product with a more stable ligand conformation.
The calculation results provide insights into the formation mechanism of RE MOFs. Likewise, the transformation from PCN-992(Eu) to PCN-993(Eu) is favored when considering the linker energy and higher connectivity number of the cluster. Herein, this MOF transformation can be dominated by the evolution of RE clusters during the synthesis, in which the RE3 clusters are generated at the early synthetic stage to induce the occurrence of PCN-992(Eu). Due to the high coordination adaptivity of RE cations, the RE3 cluster can be further extended into a 12-c RE4 cluster, resulting in a more stable MOF PCN-993(Eu) with a decreased ligand conformational energy.
Conclusions
In conclusion, we present an ortho-functionalization strategy to alter the acidities and conformations of tricarboxylate linkers, which direct the formation of unexpected RE polynuclear clusters, including 8-c RE3, 10-c RE3, and 12-c RE4. These novel building blocks led to the discovery of six MOFs named PCN-99n (n = 1–6) with unprecedented topologies. Furthermore, the utilization of H3L–CH3 functionalized with bulky methyl groups resulted in a (3,3,8,10)-c kyw net consisting of 8-c RE4 and 10-c RE6 clusters, while (3,3,3,10,10)-c wxl, (3,12)-c gmx, and (3,3,3,12)-c joe topologies were constructed based on RE6 clusters with varying connectivity numbers. Interestingly, a phase transformation from a (3,8,10)-c lfg net to a (3,12)-c lee net was discovered, involving the displacement of RE3 clusters with RE4 clusters when extending the reaction time. This work unveils the significance of ortho effects in regulating the structures of organic ligand and RE polynuclear clusters, which will provide insights into the construction of framework materials with unprecedented structural complexity and application potentials.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (NSFC, Grant No. 22275210, 22201305), the Fundamental Research Funds for the Central Universities (22CX06024A), and the Outstanding Youth Science Fund Projects of Shandong Province (2022HWYQ-070). The authors also thank the support of the Robert A. Welch Foundation through a Welch Endowed Chair to H.-C.Z. (A-0030) and Qatar National Research Fund under Award Number NPRP9-377-1-080. The authors also acknowledge the support from the Foresight Institute.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/jacsau.2c00635.
Synthetic procedures of all tricarboxylic linkers (Scheme S1–S5); crystal data and structure refinements for PCN-991(Eu) to PCN-996(Eu) (Tables S1 and S2); pKa of ortho-substituted carboxylic acid (Figure S1, Tables S3–S6); structural illustrations of RE polynuclear clusters and MOFs (Figures S2–S7); power X-ray diffraction of MOFs (Figures S8–S14); thermal gravimetric analysis of MOFs (Figures S15–S19); Gas adsorption test of PCN-991(Eu) and PCN-996(Eu) (Figures S20–S26); optical images of MOF crystals (Figure S27); dihedral angle analysis of tritopic ligands in MOFs (Figure S28, Tables S7 and S8); computational calculation about ligand’s energy and acidity (Figures S29–S33); 1H NMR spectra of synthesized compounds (Figures S34–S44); 1H NMR spectra of decomposed MOFs (Figures S45–S49) (PDF)
Accession Codes
X-ray crystallographic data for PCN-99X (X = 1-6) .The detailed crystallographic data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif (CCDC: 2122521-2122526).
Author Contributions
∥ F.L. and K.-Y.W. contributed equally. CRediT: Fugang Li data curation, methodology, project administration, resources, writing-original draft; Kunyu Wang conceptualization, data curation, formal analysis, methodology, resources, visualization, writing-review & editing; Zhengyang Liu data curation, investigation, resources; Zongsu Han data curation, investigation; Dacheng Kuai data curation, software; Weidong Fan conceptualization, funding acquisition, investigation, methodology, supervision; Liang Feng conceptualization, formal analysis, supervision; Yutong Wang data curation; Xiaokang Wang data curation, formal analysis, resources; Yue Li data curation, formal analysis, resources; Zhentao Yang formal analysis; Rongming Wang project administration, supervision; Daofeng Sun conceptualization, funding acquisition, supervision; Hong-Cai Zhou conceptualization, funding acquisition, investigation, supervision, validation.
The authors declare no competing financial interest.
Supplementary Material
References
- Li J.-R.; Kuppler R. J.; Zhou H.-C. Selective gas adsorption and separation in metal–organic frameworks. Chem. Soc. Rev. 2009, 38, 1477–1504. 10.1039/b802426j. [DOI] [PubMed] [Google Scholar]
- Li J.-R.; Sculley J.; Zhou H.-C. Metal–Organic Frameworks for Separations. Chem. Rev. 2012, 112, 869–932. 10.1021/cr200190s. [DOI] [PubMed] [Google Scholar]
- Bloch E. D.; Queen Wendy L.; Krishna R.; Zadrozny Joseph M.; Brown Craig M.; Long Jeffrey R. Hydrocarbon Separations in a Metal-Organic Framework with Open Iron(II) Coordination Sites. Science 2012, 335, 1606–1610. 10.1126/science.1217544. [DOI] [PubMed] [Google Scholar]
- Cui X.; Chen K.; Xing H.; Yang Q.; Krishna R.; Bao Z.; Wu H.; Zhou W.; Dong X.; Han Y.; Li B.; Ren Q.; Zaworotko Michael J.; Chen B. Pore chemistry and size control in hybrid porous materials for acetylene capture from ethylene. Science 2016, 353, 141–144. 10.1126/science.aaf2458. [DOI] [PubMed] [Google Scholar]
- Rosi N. L.; Eckert J.; Eddaoudi M.; Vodak David T.; Kim J.; O’Keeffe M.; Yaghi Omar M. Hydrogen Storage in Microporous Metal-Organic Frameworks. Science 2003, 300, 1127–1129. 10.1126/science.1083440. [DOI] [PubMed] [Google Scholar]
- Murray L. J.; Dincă M.; Long J. R. Hydrogen storage in metal–organic frameworks. Chem. Soc. Rev. 2009, 38, 1294–1314. 10.1039/b802256a. [DOI] [PubMed] [Google Scholar]
- Mason J. A.; Oktawiec J.; Taylor M. K.; Hudson M. R.; Rodriguez J.; Bachman J. E.; Gonzalez M. I.; Cervellino A.; Guagliardi A.; Brown C. M.; Llewellyn P. L.; Masciocchi N.; Long J. R. Methane storage in flexible metal–organic frameworks with intrinsic thermal management. Nature 2015, 527, 357–361. 10.1038/nature15732. [DOI] [PubMed] [Google Scholar]
- He Y.; Zhou W.; Qian G.; Chen B. Methane storage in metal–organic frameworks. Chem. Soc. Rev. 2014, 43, 5657–5678. 10.1039/C4CS00032C. [DOI] [PubMed] [Google Scholar]
- Chen Z.; Li P.; Anderson R.; Wang X.; Zhang X.; Robison L.; Redfern Louis R.; Moribe S.; Islamoglu T.; Gómez-Gualdrón Diego A.; Yildirim T.; Stoddart J. F.; Farha Omar K. Balancing volumetric and gravimetric uptake in highly porous materials for clean energy. Science 2020, 368, 297–303. 10.1126/science.aaz8881. [DOI] [PubMed] [Google Scholar]
- Liu J.; Xie D.; Shi W.; Cheng P. Coordination compounds in lithium storage and lithium-ion transport. Chem. Soc. Rev. 2020, 49, 1624–1642. 10.1039/C9CS00881K. [DOI] [PubMed] [Google Scholar]
- Kim H.; Yang S.; Rao Sameer R.; Narayanan S.; Kapustin Eugene A.; Furukawa H.; Umans Ari S.; Yaghi Omar M.; Wang Evelyn N. Water harvesting from air with metal-organic frameworks powered by natural sunlight. Science 2017, 356, 430–434. 10.1126/science.aam8743. [DOI] [PubMed] [Google Scholar]
- Xu W.; Yaghi O. M. Metal–Organic Frameworks for Water Harvesting from Air, Anywhere, Anytime. ACS Cent. Sci. 2020, 6, 1348–1354. 10.1021/acscentsci.0c00678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanikel N.; Pei X.; Chheda S.; Lyu H.; Jeong W.; Sauer J.; Gagliardi L.; Yaghi Omar M. Evolution of water structures in metal-organic frameworks for improved atmospheric water harvesting. Science 2021, 374, 454–459. 10.1126/science.abj0890. [DOI] [PubMed] [Google Scholar]
- Millward A. R.; Yaghi O. M. Metal–Organic Frameworks with Exceptionally High Capacity for Storage of Carbon Dioxide at Room Temperature. J. Am. Chem. Soc. 2005, 127, 17998–17999. 10.1021/ja0570032. [DOI] [PubMed] [Google Scholar]
- Banerjee R.; Phan A.; Wang B.; Knobler C.; Furukawa H.; O’Keeffe M.; Yaghi Omar M. High-Throughput Synthesis of Zeolitic Imidazolate Frameworks and Application to CO2 Capture. Science 2008, 319, 939–943. 10.1126/science.1152516. [DOI] [PubMed] [Google Scholar]
- Sumida K.; Rogow D. L.; Mason J. A.; McDonald T. M.; Bloch E. D.; Herm Z. R.; Bae T.-H.; Long J. R. Carbon Dioxide Capture in Metal–Organic Frameworks. Chem. Rev. 2012, 112, 724–781. 10.1021/cr2003272. [DOI] [PubMed] [Google Scholar]
- D’Alessandro D. M.; Smit B.; Long J. R. Carbon Dioxide Capture: Prospects for New Materials. Angew. Chem., Int. Ed. 2010, 49, 6058–6082. 10.1002/anie.201000431. [DOI] [PubMed] [Google Scholar]
- McDonald T. M.; Mason J. A.; Kong X.; Bloch E. D.; Gygi D.; Dani A.; Crocellà V.; Giordanino F.; Odoh S. O.; Drisdell W. S.; Vlaisavljevich B.; Dzubak A. L.; Poloni R.; Schnell S. K.; Planas N.; Lee K.; Pascal T.; Wan L. F.; Prendergast D.; Neaton J. B.; Smit B.; Kortright J. B.; Gagliardi L.; Bordiga S.; Reimer J. A.; Long J. R. Cooperative insertion of CO2 in diamine-appended metal-organic frameworks. Nature 2015, 519, 303–308. 10.1038/nature14327. [DOI] [PubMed] [Google Scholar]
- Yaghi O. M.; Li G.; Li H. Selective binding and removal of guests in a microporous metal–organic framework. Nature 1995, 378, 703–706. 10.1038/378703a0. [DOI] [Google Scholar]
- Li H.; Eddaoudi M.; O’Keeffe M.; Yaghi O. M. Design and synthesis of an exceptionally stable and highly porous metal-organic framework. Nature 1999, 402, 276–279. 10.1038/46248. [DOI] [Google Scholar]
- Yaghi O. M.; O’Keeffe M.; Ockwig N. W.; Chae H. K.; Eddaoudi M.; Kim J. Reticular synthesis and the design of new materials. Nature 2003, 423, 705–714. 10.1038/nature01650. [DOI] [PubMed] [Google Scholar]
- O’Keeffe M.; Yaghi O. M. Deconstructing the Crystal Structures of Metal–Organic Frameworks and Related Materials into Their Underlying Nets. Chem. Rev. 2012, 112, 675–702. 10.1021/cr200205j. [DOI] [PubMed] [Google Scholar]
- Zhou H.-C.; Long J. R.; Yaghi O. M. Introduction to Metal–Organic Frameworks. Chem. Rev. 2012, 112, 673–674. 10.1021/cr300014x. [DOI] [PubMed] [Google Scholar]
- Furukawa H.; Cordova Kyle E.; O’Keeffe M.; Yaghi Omar M. The Chemistry and Applications of Metal-Organic Frameworks. Science 2013, 341, 1230444 10.1126/science.1230444. [DOI] [PubMed] [Google Scholar]
- Zhou H.-C. J.; Kitagawa S. Metal–Organic Frameworks (MOFs). Chem. Soc. Rev. 2014, 43, 5415–5418. 10.1039/C4CS90059F. [DOI] [PubMed] [Google Scholar]
- Guillerm V.; Maspoch D. Geometry Mismatch and Reticular Chemistry: Strategies To Assemble Metal–Organic Frameworks with Non-default Topologies. J. Am. Chem. Soc. 2019, 141, 16517–16538. 10.1021/jacs.9b08754. [DOI] [PubMed] [Google Scholar]
- Eddaoudi M.; Kim J.; O’Keeffe M.; Yaghi O. M. Cu2[o-Br-C6H3(CO2)2]2(H2O)2·(DMF)8(H2O)2: A Framework Deliberately Designed To Have the NbO Structure Type. J. Am. Chem. Soc. 2002, 124, 376–377. 10.1021/ja017154e. [DOI] [PubMed] [Google Scholar]
- Furukawa H.; Kim J.; Ockwig N. W.; O’Keeffe M.; Yaghi O. M. Control of Vertex Geometry, Structure Dimensionality, Functionality, and Pore Metrics in the Reticular Synthesis of Crystalline Metal–Organic Frameworks and Polyhedra. J. Am. Chem. Soc. 2008, 130, 11650–11661. 10.1021/ja803783c. [DOI] [PubMed] [Google Scholar]
- Yuan S.; Chen Y.-P.; Qin J.-S.; Lu W.; Zou L.; Zhang Q.; Wang X.; Sun X.; Zhou H.-C. Linker Installation: Engineering Pore Environment with Precisely Placed Functionalities in Zirconium MOFs. J. Am. Chem. Soc. 2016, 138, 8912–8919. 10.1021/jacs.6b04501. [DOI] [PubMed] [Google Scholar]
- Yuan S.; Lu W.; Chen Y.-P.; Zhang Q.; Liu T.-F.; Feng D.; Wang X.; Qin J.; Zhou H.-C. Sequential Linker Installation: Precise Placement of Functional Groups in Multivariate Metal–Organic Frameworks. J. Am. Chem. Soc. 2015, 137, 3177–3180. 10.1021/ja512762r. [DOI] [PubMed] [Google Scholar]
- Cavka J. H.; Jakobsen S.; Olsbye U.; Guillou N.; Lamberti C.; Bordiga S.; Lillerud K. P.; New A. A New Zirconium Inorganic Building Brick Forming Metal Organic Frameworks with Exceptional Stability. J. Am. Chem. Soc. 2008, 130, 13850–13851. 10.1021/ja8057953. [DOI] [PubMed] [Google Scholar]
- Feng L.; Day G. S.; Wang K.-Y.; Yuan S.; Zhou H.-C. Strategies for Pore Engineering in Zirconium Metal-Organic Frameworks. Chem 2020, 6, 2902–2923. 10.1016/j.chempr.2020.09.010. [DOI] [Google Scholar]
- Ejegbavwo O. A.; Martin C. R.; Olorunfemi O. A.; Leith G. A.; Ly R. T.; Rice A. M.; Dolgopolova E. A.; Smith M. D.; Karakalos S. G.; Birkner N.; Powell B. A.; Pandey S.; Koch R. J.; Misture S. T.; Loye H.-C. z.; Phillpot S. R.; Brinkman K. S.; Shustova N. B. Thermodynamics and Electronic Properties of Heterometallic Multinuclear Actinide-Containing Metal–Organic Frameworks with “Structural Memory. J. Am. Chem. Soc. 2019, 141, 11628–11640. 10.1021/jacs.9b04737. [DOI] [PubMed] [Google Scholar]
- Saraci F.; Quezada-Novoa V.; Donnarumma P. R.; Howarth A. J. Rare-earth metal–organic frameworks: from structure to applications. Chem. Soc. Rev. 2020, 49, 7949–7977. 10.1039/D0CS00292E. [DOI] [PubMed] [Google Scholar]
- Zheng Z. Ligand-controlled self-assembly of polynuclear lanthanide–oxo/hydroxo complexes: from synthetic serendipity to rational supramolecular design. Chem. Commun. 2001, 24, 2521–2529. 10.1039/b107971a. [DOI] [Google Scholar]
- Feng L.; Pang J.; She P.; Li J. L.; Qin J. S.; Du D. Y.; Zhou H. C. Metal-Organic Frameworks Based on Group 3 and 4 Metals. Adv. Mater. 2020, 32, e2004414 10.1002/adma.202004414. [DOI] [PubMed] [Google Scholar]
- Devic T.; Serre C.; Audebrand N.; Marrot J.; Férey G. MIL-103, A 3-D Lanthanide-Based Metal Organic Framework with Large One-Dimensional Tunnels and A High Surface Area. J. Am. Chem. Soc. 2005, 127, 12788–12789. 10.1021/ja053992n. [DOI] [PubMed] [Google Scholar]
- Luo T.-Y.; Liu C.; Eliseeva S. V.; Muldoon P. F.; Petoud S.; Rosi N. L. Rare Earth pcu Metal–Organic Framework Platform Based on RE4(μ3-OH)4(COO)62+ Clusters: Rational Design, Directed Synthesis, and Deliberate Tuning of Excitation Wavelengths. J. Am. Chem. Soc. 2017, 139, 9333–9340. 10.1021/jacs.7b04532. [DOI] [PubMed] [Google Scholar]
- Ma B.-Q.; Zhang D.-S.; Gao S.; Jin T.-Z.; Yan C.-H.; Xu G.-X. From Cubane to Supercubane: The Design, Synthesis, and Structure of a Three-Dimensional Open Framework Based on a Ln4O4 Cluster. Angew. Chem., Int. Ed. 2000, 39, 3644–3646. . [DOI] [PubMed] [Google Scholar]
- Wang R.; Liu H.; Carducci M. D.; Jin T.; Zheng C.; Zheng Z. Lanthanide Coordination with α-Amino Acids under Near Physiological pH Conditions: Polymetallic Complexes Containing the Cubane-Like [Ln4(μ3-OH)4]8+ Cluster Core. Inorg. Chem. 2001, 40, 2743–2750. 10.1021/ic001469y. [DOI] [PubMed] [Google Scholar]
- Gao Y.; Xu G.-F.; Zhao L.; Tang J.; Liu Z. Observation of Slow Magnetic Relaxation in Discrete Dysprosium Cubane. Inorg. Chem. 2009, 48, 11495–11497. 10.1021/ic901806g. [DOI] [PubMed] [Google Scholar]
- Lin P.-H.; Korobkov I.; Wernsdorfer W.; Ungur L.; Chibotaru L. F.; Murugesu M. A Rare μ4-O Centred Dy4 Tetrahedron with Coordination-Induced Local Chirality and Single-Molecule Magnet Behaviour. Eur. J. Inorg. Chem. 2011, 2011, 1535–1539. 10.1002/ejic.201100038. [DOI] [Google Scholar]
- Yi X.; Bernot K.; Calvez G.; Daiguebonne C.; Guillou O. 3D Organization of Dysprosium Cubanes. Eur. J. Inorg. Chem. 2013, 2013, 5879–5885. 10.1002/ejic.201300937. [DOI] [Google Scholar]
- Zhou J.-M.; Shi W.; Li H.-M.; Li H.; Cheng P. Experimental Studies and Mechanism Analysis of High-Sensitivity Luminescent Sensing of Pollutional Small Molecules and Ions in Ln4O4 Cluster Based Microporous Metal–Organic Frameworks. J. Phys. Chem. C 2014, 118, 416–426. 10.1021/jp4097502. [DOI] [Google Scholar]
- Zou D.; Zhang J.; Cui Y.; Qian G. Near-infrared-emissive metal–organic frameworks. Dalton Trans. 2019, 48, 6669–6675. 10.1039/C9DT01197H. [DOI] [PubMed] [Google Scholar]
- Maruyama T.; Kawabata H.; Kikukawa Y.; Hayashi Y. Yttrium-Containing Sandwich-, Ring-, and Cage-Type Polyoxovanadates: Synthesis and Characterization. Eur. J. Inorg. Chem. 2019, 2019, 529–533. 10.1002/ejic.201800540. [DOI] [Google Scholar]
- Litvinova Y. M.; Gayfulin Y. M.; van Leusen J.; Samsonenko D. G.; Lazarenko V. A.; Zubavichus Y. V.; Kögerler P.; Mironov Y. V. Metal–organic frameworks based on polynuclear lanthanide complexes and octahedral rhenium clusters. Inorg. Chem. Front. 2019, 6, 1518–1526. 10.1039/C9QI00339H. [DOI] [Google Scholar]
- Xue D.-X.; Cairns A. J.; Belmabkhout Y.; Wojtas L.; Liu Y.; Alkordi M. H.; Eddaoudi M. Tunable Rare-Earth fcu-MOFs: A Platform for Systematic Enhancement of CO2 Adsorption Energetics and Uptake. J. Am. Chem. Soc. 2013, 135, 7660–7667. 10.1021/ja401429x. [DOI] [PubMed] [Google Scholar]
- Alezi D.; Peedikakkal A. M. P.; Weseliński ŁJ.; Guillerm V.; Belmabkhout Y.; Cairns A. J.; Chen Z.; Wojtas Ł.; Eddaoudi M. Quest for Highly Connected Metal–Organic Framework Platforms: Rare-Earth Polynuclear Clusters Versatility Meets Net Topology Needs. J. Am. Chem. Soc. 2015, 137, 5421–5430. 10.1021/jacs.5b00450. [DOI] [PubMed] [Google Scholar]
- Wang Y.; Feng L.; Fan W.; Wang K.-Y.; Wang X.; Wang X.; Zhang K.; Zhang X.; Dai F.; Sun D.; Zhou H.-C. Topology Exploration in Highly Connected Rare-Earth Metal–Organic Frameworks via Continuous Hindrance Control. J. Am. Chem. Soc. 2019, 141, 6967–6975. 10.1021/jacs.9b00122. [DOI] [PubMed] [Google Scholar]
- Lin S.-k.; March J. March’s Advanced Organic Chemistry: Reactions, Mechanisms, and Structure, 5th Edition. Molecules 2001, 6, 1064 10.3390/61201064. [DOI] [Google Scholar]
- Böhm S.; Fiedler P.; Exner O. Analysis of the ortho effect: acidity of 2-substituted benzoic acids. New J. Chem. 2004, 28, 67–74. 10.1039/B305986C. [DOI] [Google Scholar]
- Chen L.; Hu H.-J.; Wang Y.-L.; Zhang X.-F.; Xu L.-P.; Liu Q.-Y. Metal–Organic Frameworks Featuring 18-Connected Nonanuclear Rare-Earth Oxygen Clusters and Cavities for Efficient C2H2/CO2 Separation. Inorg. Chem. 2021, 60, 13471–13478. 10.1021/acs.inorgchem.1c01827. [DOI] [PubMed] [Google Scholar]
- Guillerm V.; Weseliński ŁJ.; Belmabkhout Y.; Cairns A. J.; D’Elia V.; Wojtas Ł.; Adil K.; Eddaoudi M. Discovery and introduction of a (3,18)-connected net as an ideal blueprint for the design of metal–organic frameworks. Nat. Chem. 2014, 6, 673–680. 10.1038/nchem.1982. [DOI] [PubMed] [Google Scholar]
- Lin W.; Ning E.; Yang L.; Rao Y.; Peng S.; Li Q. Snapshots of Postsynthetic Modification in a Layered Metal–Organic Framework: Isometric Linker Exchange and Adaptive Linker Installation. Inorg. Chem. 2021, 60, 11756–11763. 10.1021/acs.inorgchem.1c01341. [DOI] [PubMed] [Google Scholar]
- Mahé N.; Guillou O.; Daiguebonne C.; Gérault Y.; Caneschi A.; Sangregorio C.; Chane-Ching J. Y.; Car P. E.; Roisnel T. Polynuclear Lanthanide Hydroxo Complexes: New Chemical Precursors for Coordination Polymers. Inorg. Chem. 2005, 44, 7743–7750. 10.1021/ic0401086. [DOI] [PubMed] [Google Scholar]
- Savard D.; Lin P.-H.; Burchell T. J.; Korobkov I.; Wernsdorfer W.; Clérac R.; Murugesu M. Two-Dimensional Networks of Lanthanide Cubane-Shaped Dumbbells. Inorg. Chem. 2009, 48, 11748–11754. 10.1021/ic901807k. [DOI] [PubMed] [Google Scholar]
- Wang R.; Carducci M. D.; Zheng Z. Direct Hydrolytic Route to Molecular Oxo–Hydroxo Lanthanide Clusters. Inorg. Chem. 2000, 39, 1836–1837. 10.1021/ic991391p. [DOI] [PubMed] [Google Scholar]
- Blatov V. A.; Shevchenko A. P.; Proserpio D. M. Applied Topological Analysis of Crystal Structures with the Program Package ToposPro. Cryst. Growth Des. 2014, 14, 3576–3586. 10.1021/cg500498k. [DOI] [Google Scholar]
- Blatov V. A.Multipurpose Crystallochemical Analysis with the Program Package TOPOS, IUCr CompComm Newsletter, 2006; pp 4–38.
- Guillerm V.; Eddaoudi M. The Importance of Highly Connected Building Units in Reticular Chemistry: Thoughtful Design of Metal–Organic Frameworks. Acc. Chem. Res. 2021, 54, 3298–3312. 10.1021/acs.accounts.1c00214. [DOI] [PubMed] [Google Scholar]
- Férey G.; Serre C.; Mellot-Draznieks C.; Millange F.; Surblé S.; Dutour J.; Margiolaki I. A Hybrid Solid with Giant Pores Prepared by a Combination of Targeted Chemistry, Simulation, and Powder Diffraction. Angew. Chem., Int. Ed. 2004, 43, 6296–6301. 10.1002/anie.200460592. [DOI] [PubMed] [Google Scholar]
- Férey G.; Mellot-Draznieks C.; Serre C.; Millange F.; Dutour J.; Surblé S.; Margiolaki I. A Chromium Terephthalate-Based Solid with Unusually Large Pore Volumes and Surface Area. Science 2005, 309, 2040–2042. 10.1126/science.1116275. [DOI] [PubMed] [Google Scholar]
- Serre C.; Mellot-Draznieks C.; Surblé S.; Audebrand N.; Filinchuk Y.; Férey G. Role of Solvent-Host Interactions That Lead to Very Large Swelling of Hybrid Frameworks. Science 2007, 315, 1828–1831. 10.1126/science.1137975. [DOI] [PubMed] [Google Scholar]
- Horcajada P.; Surblé S.; Serre C.; Hong D.-Y.; Seo Y.-K.; Chang J.-S.; Grenèche J.-M.; Margiolaki I.; Férey G. Synthesis and catalytic properties of MIL-100(Fe), an iron(iii) carboxylate with large pores. Chem. Commun. 2007, 27, 2820–2822. 10.1039/B704325B. [DOI] [PubMed] [Google Scholar]
- Chen Z.; Li P.; Zhang X.; Li P.; Wasson M. C.; Islamoglu T.; Stoddart J. F.; Farha O. K. Reticular Access to Highly Porous acs-MOFs with Rigid Trigonal Prismatic Linkers for Water Sorption. J. Am. Chem. Soc. 2019, 141, 2900–2905. 10.1021/jacs.8b13710. [DOI] [PubMed] [Google Scholar]
- Feng D.; Wang K.; Wei Z.; Chen Y.-P.; Simon C. M.; Arvapally R. K.; Martin R. L.; Bosch M.; Liu T.-F.; Fordham S.; Yuan D.; Omary M. A.; Haranczyk M.; Smit B.; Zhou H.-C. Kinetically tuned dimensional augmentation as a versatile synthetic route towards robust metal–organic frameworks. Nat. Commun. 2014, 5, 5723 10.1038/ncomms6723. [DOI] [PubMed] [Google Scholar]
- Bhattacharjee S.; Chen C.; Ahn W.-S. Chromium terephthalate metal–organic framework MIL-101: synthesis, functionalization, and applications for adsorption and catalysis. RSC Adv. 2014, 4, 52500–52525. 10.1039/C4RA11259H. [DOI] [Google Scholar]
- Lian X.; Chen Y.-P.; Liu T.-F.; Zhou H.-C. Coupling two enzymes into a tandem nanoreactor utilizing a hierarchically structured MOF. Chem. Sci. 2016, 7, 6969–6973. 10.1039/C6SC01438K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dezotti Y.; Ribeiro M. A.; Pirota K. R.; Barros W. P. Influence of the Metal Ion on the Topology and Interpenetration of Pyridylvinyl(benzoate) Based Metal–Organic Frameworks. Cryst. Growth Des. 2019, 19, 5592–5603. 10.1021/acs.cgd.9b00552. [DOI] [Google Scholar]
- Ma G.; Zhang J.-J.; Sun L.; Xu Y.-X.; Gao H.-L.; Zhang H.-Y.; He X.-W. A New Cluster-Based MOF for Selective Gas Sorption and Treatment Effect on Acute glomerulonephritis by Reducing NF-κb Pathway Activation and Cytokines Release. J. Cluster Sci. 2020, 31, 1061–1069. 10.1007/s10876-019-01711-8. [DOI] [Google Scholar]
- Chen X.-Y.; Zhao B.; Shi W.; Xia J.; Cheng P.; Liao D.-Z.; Yan S.-P.; Jiang Z.-H. Microporous Metal–Organic Frameworks Built on a Ln3 Cluster as a Six-Connecting Node. Chem. Mater. 2005, 17, 2866–2874. 10.1021/cm050526o. [DOI] [Google Scholar]
- Karmakar A.; Hazra S.; Guedes da Silva M. F. C.; Paul A.; Pombeiro A. J. L. Nanoporous lanthanide metal–organic frameworks as efficient heterogeneous catalysts for the Henry reaction. CrystEngComm 2016, 18, 1337–1349. 10.1039/C5CE01456E. [DOI] [Google Scholar]
- Liu B.; Wu W.-P.; Hou L.; Wang Y.-Y. Four uncommon nanocage-based Ln-MOFs: highly selective luminescent sensing for Cu2+ ions and selective CO2 capture. Chem. Commun. 2014, 50, 8731–8734. 10.1039/C4CC03049D. [DOI] [PubMed] [Google Scholar]
- Zhang L.; Song T.; Xu J.; Sun J.; Zeng S.; Wu Y.; Fan Y.; Wang L. Polymorphic Ln(iii) and BPTC-based porous metal–organic frameworks with visible, NIR photoluminescent and magnetic properties. CrystEngComm 2014, 16, 2440–2451. 10.1039/c3ce42181c. [DOI] [Google Scholar]
- Xu H.; Fang M.; Cao C.-S.; Qiao W.-Z.; Zhao B. Unique (3,4,10)-Connected Lanthanide–Organic Framework as a Recyclable Chemical Sensor for Detecting Al3. Inorg. Chem. 2016, 55, 4790–4794. 10.1021/acs.inorgchem.6b00190. [DOI] [PubMed] [Google Scholar]
- Li Y.-J.; Wang Y.-L.; Liu Q.-Y. The Highly Connected MOFs Constructed from Nonanuclear and Trinuclear Lanthanide-Carboxylate Clusters: Selective Gas Adsorption and Luminescent pH Sensing. Inorg. Chem. 2017, 56, 2159–2164. 10.1021/acs.inorgchem.6b02811. [DOI] [PubMed] [Google Scholar]
- Wei N.; Zuo R.-X.; Zhang Y.-Y.; Han Z.-B.; Gu X.-J. Robust high-connected rare-earth MOFs as efficient heterogeneous catalysts for CO2 conversion. Chem. Commun. 2017, 53, 3224–3227. 10.1039/C7CC00363C. [DOI] [PubMed] [Google Scholar]
- Evans W. J.; Sollberger M. S. Synthetic and structural studies on the formation of a tetradecametallic yttrium oxide alkoxide chloride complex: an example of how molecular yttrium oxygen frameworks form extended arrays. Inorg. Chem. 1988, 27, 4417–4423. 10.1021/ic00297a017. [DOI] [Google Scholar]
- Wong K.-L.; Zhu Y.-M.; Yang Y.-Y.; Law G.-L.; Fan H.-H.; Tanner P. A.; Wong W.-T. Structure and photophysical properties of new trinuclear lanthanide complexes (Ln=Eu and Tb) with 1,10-phenanthroline. Inorg. Chem. Commun. 2009, 12, 52–54. 10.1016/j.inoche.2008.11.002. [DOI] [Google Scholar]
- Paluch M.; Ślepokura K.; Lis T.; Lisowski J. Enantiopure trinuclear lanthanide(III) complexes: Cooperative formation of Ln3(μ3-OH)2 core within the macrocycle. Inorg. Chem. Commun. 2011, 14, 92–95. 10.1016/j.inoche.2010.09.039. [DOI] [Google Scholar]
- Costes J.-P.; Dahan F.; Nicodème F.; Trinuclear A. Gadolinium Complex: Structure and Magnetic Properties. Inorg. Chem. 2001, 40, 5285–5287. 10.1021/ic0103704. [DOI] [PubMed] [Google Scholar]
- Xue S.; Chen X.-H.; Zhao L.; Guo Y.-N.; Tang J. Two Bulky-Decorated Triangular Dysprosium Aggregates Conserving Vortex-Spin Structure. Inorg. Chem. 2012, 51, 13264–13270. 10.1021/ic301785v. [DOI] [PubMed] [Google Scholar]
- Kobyłka M. J.; Ślepokura K.; Acebrón Rodicio M.; Paluch M.; Lisowski J. Incorporation of Trinuclear Lanthanide(III) Hydroxo Bridged Clusters in Macrocyclic Frameworks. Inorg. Chem. 2013, 52, 12893–12903. 10.1021/ic400508y. [DOI] [PubMed] [Google Scholar]
- Barash E. H.; Coan P. S.; Lobkovsky E. B.; Streib W. E.; Caulton K. G. Anhydrous yttrium acetylacetonate and the course of thermal ″dehydration″ of Y(acac)3.3H2O. Inorg. Chem. 1993, 32, 497–501. 10.1021/ic00057a003. [DOI] [Google Scholar]
- Ma S.; Yuan D.; Wang X.-S.; Zhou H.-C. Microporous Lanthanide Metal-Organic Frameworks Containing Coordinatively Linked Interpenetration: Syntheses, Gas Adsorption Studies, Thermal Stability Analysis, and Photoluminescence Investigation. Inorg. Chem. 2009, 48, 2072–2077. 10.1021/ic801948z. [DOI] [PubMed] [Google Scholar]
- Han L.; Zhang S.; Wang Y.; Yan X.; Lu X. A Strategy for Synthesis of Ionic Metal-Organic Frameworks. Inorg. Chem. 2009, 48, 786–788. 10.1021/ic800632r. [DOI] [PubMed] [Google Scholar]
- Wang R.; Selby H. D.; Liu H.; Carducci M. D.; Jin T.; Zheng Z.; Anthis J. W.; Staples R. J. Halide-Templated Assembly of Polynuclear Lanthanide-Hydroxo Complexes. Inorg. Chem. 2002, 41, 278–286. 10.1021/ic010859x. [DOI] [PubMed] [Google Scholar]
- Kong X.-J.; Long L.-S.; Zheng L.-S.; Wang R.; Zheng Z. Hydrolytic Synthesis and Structural Characterization of Lanthanide Hydroxide Clusters Supported by Nicotinic Acid. Inorg. Chem. 2009, 48, 3268–3273. 10.1021/ic802357m. [DOI] [PubMed] [Google Scholar]
- Wu Y.; Morton S.; Kong X.; Nichol G. S.; Zheng Z. Hydrolytic synthesis and structural characterization of lanthanide-acetylacetonato/hydroxo cluster complexes – A systematic study. Dalton Trans. 2011, 40, 1041–1046. 10.1039/C0DT01218A. [DOI] [PubMed] [Google Scholar]
- Zheng X.-Y.; Kong X.-J.; Zheng Z.; Long L.-S.; Zheng L.-S. High-Nuclearity Lanthanide-Containing Clusters as Potential Molecular Magnetic Coolers. Acc. Chem. Res. 2018, 51, 517–525. 10.1021/acs.accounts.7b00579. [DOI] [PubMed] [Google Scholar]
- Bodnarchuk M. S.; Heyes D. M.; Dini D.; Chahine S.; Edwards S. Role of Deprotonation Free Energies in pKa Prediction and Molecule Ranking. J. Chem. Theory Comput. 2014, 10, 2537–2545. 10.1021/ct400914w. [DOI] [PubMed] [Google Scholar]
- Williams S. L.; de Oliveira C. A. F.; McCammon J. A. Coupling Constant pH Molecular Dynamics with Accelerated Molecular Dynamics. J. Chem. Theory Comput. 2010, 6, 560–568. 10.1021/ct9005294. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.






