Abstract
Enzymes have firmly established themselves as bespoke catalysts for small molecule transformations in the pharmaceutical industry, from early research and development stages to large-scale production. In principle, their exquisite selectivity and rate acceleration can also be leveraged for modifying macromolecules to form bioconjugates. However, available catalysts face stiff competition from other bioorthogonal chemistries. In this Perspective, we seek to illuminate applications of enzymatic bioconjugation in the face of an expanding palette of new drug modalities. With these applications, we wish to highlight some examples of current successes and pitfalls of using enzymes for bioconjugation along the pipeline and try to illustrate opportunities for further development.
Keywords: bioconjugation, new modalities, enzymatic synthesis, biocatalysis, site-selective modification
1. Introduction
The conjugation of a chemical payload to a biomolecule and the combination of their individual therapeutic effects is a well-established concept that has found a resurgence in the last two decades. For instance, Paul Ehrlich’s proposal of a “magic bullet”, coupling a highly cytotoxic compound to an antibody for highly selective delivery, has found its way into 12 FDA approved antibody drug conjugates (ADCs) as of 2022.1,2 At the same time the market value of ADCs is already multiple billion USD and is forecasted to grow within the coming years.2 Additionally, bioconjugates are not exclusive to antibodies and their derivatives. Attaching payloads and other chemical modifiers to biopolymers is a concept that has been expanded to charging small peptide binders with radioactive payloads,3 for the formation of multimeric proteins,4 to decorate RNA molecules with targeting groups,5 and even to modify the surface of large protein complexes.6
Whereas the palette of targets, biopolymers, and payloads has expanded rapidly in the last decades, the bioconjugation chemistry of most clinical candidates has surprisingly remained largely unchanged. Typically, the bio-orthogonal reactivity of maleimides and cysteine, or N-hydroxysuccinimide esters and lysine, is being used for coupling reactions.7 This chemistry is generally robust and leads to high conversion during the process, but it comes with drawbacks. First, proteins of interest need to contain a residue on the surface that can react with the payload. Second, the reactions are often not regiospecific since proteins may contain many nucleophilic residues on their surface. This can lead to a distribution of products with different amounts of payload attached to the target. For ADCs, this is often described as the drug to antibody ratio (DAR). The DAR can be tuned based on the chemical process, but often the need to control the DAR leads to changes in the target protein sequence necessary to introduce reactive residues. Third, lysine is one of the most common surface residues of proteins which can make site-specific coupling and control of the DAR difficult. Cysteine is less abundant compared to lysine, but the thiosuccinimide formed through maleimide couplings is often unstable, potentially leading to premature linker cleavage.8,9 Fourth, since proteins are usually present in low concentrations and amino acid side chains are often sterically occluded, many equivalents of electrophilic payload are used during the coupling step to control the reaction velocity.10 Not all target proteins are stable under such harsh conditions. Some of these shortcomings have been addressed with the development of improved small molecule conjugation methods such as next-generation maleimides.11−13 However, mild and catalytic methods to access chemically defined bioconjugates remain highly desired.
Enzyme catalysis can be extremely fast, regio- and stereoselective, and generally works under mild conditions. Unsurprisingly, enzymes have already firmly established themselves as exceptional catalysts in the chemical and pharmaceutical industry.14−17 In principle, these advantages could also be harnessed for reactions involving macromolecule substrates. An early example from 1979, the production of semisynthetic insulin using carboxypeptidase A and trypsin from porcine, highlights that enzymes can be successfully employed to modify proteins for industrial purposes.18 Fast forward to 2022, the scientists at Merck reported an engineered penicillin G acylase capable of site-selective deprotection of phenylacetamide derivatives of insulin, demonstrating the advances of biocatalysis in this space.19 Since the case of semisynthetic insulin, a multitude of enzymes with a broad scope of different bioconjugation mechanisms have been described. The distinct types of bioconjugating enzymes as well as chemical bioconjugation have been extensively reviewed elsewhere.13,20−23 In this Perspective, we want to focus solely on the types of bioconjugates that have been produced using enzymes with a focus on the macromolecule type. As well as their individual challenges and opportunities.
2. Antibody Drug Conjugates
All ADCs on the market depend, at the time of writing, on chemical coupling of the payload either to lysine or cysteine side chains, apart from Moxetumomab pasudotox, which is a genetic fusion protein.1 ADCs are the most prominent example of bioconjugates in the clinic and are also the most tested for bioconjugating enzymes. Both site specificity and DAR can have a profound impact on drug efficacy, and thus, enzymes can be an impactful tool.9,24,25 Protein ligases that belong to the family of serine proteases often serve as platforms for protein chemistry. Most notably, sortase A (SrtA) of Staphylococcus aureus performs transacylation by specifically cleaving the sequence, also called sortag, LPXT/G (X being any amino acid and / denotes the site of cleavage) and the acyl intermediate can be attacked by a payload with two N-terminal glycine residues forming a regular peptide linkage (Figure 1).26 This transformation has proven to be an extremely versatile tool to rapidly functionalize and diversify biomolecules.27−29 For application to ADCs, this is particularly interesting since C-termini are distant from the antigen binding region; however, genetic modification of the antibody gene is likely needed.30
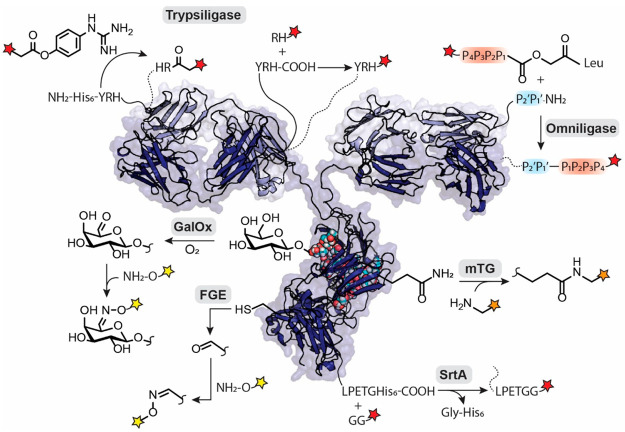
Figure 1.
Promising bioconjugation reactions for the creation of ADCs with the payloads color coded: red stars indicate payloads conjugated by peptide bond formation, orange indicates isopeptide bonds, and yellow indicates aldehyde-based conjugation strategies. COOH and NH2 denote the C- and N-terminus, respectively. Trypsiligase can modify N-termini with a simplified OGp substrate, and C-termini with a RH-dipeptide tagged payload; both cleave a short recognition sequence. Omniligase uses peptides activated with ester leaving groups that can carry a cargo to modify the N-terminus. Microbial transglutaminases (mTG) create an isopeptide bond on a glutamine side chain with an amine functionalized payload. Sortase A (SrtA) performs C-terminal transpeptidation and can cleave affinity tags, e.g., His6, while coupling Gly2-labeled payloads. Galactose oxidase (GalOx) and formylglycine generating enzyme (FGE) create aldehydes on the oligosaccharide and cysteine residues, respectively, which can selectively be labeled with a broad range of bioorthogonal chemistries.
SrtA has since found numerous applications such as labeling and immobilizing antibody single-chain variable fragments (scFvs),31−33 creating antimicrobial ADCs34 and monoclonal antibody (mAb) PEGylation,35 creating nanobody based ADCs and imaging agents,36,37 and generating artificial bispecific antibodies.38 Beerli et al. showed that SrtA can be used to generate ADCs of anti-CD30 mAb, analogous to brentuximab vedotin, and several anti-HER2 ADCs.39 In both cases, the authors inserted recognition sequences at both the C-termini of the heavy and light chain. Addition of a spacer sequence between antibody and tag allowed improvements of transpeptidation. SrtA mediated ligations typically showed high yields of >80%, with DARs of 3–3.5, corresponding to approximately 80% coupling efficiency per site. Additionally, en route to the conjugation, the affinity tag used for purification is removed, which is C-terminal to the leaving group of the sortase tag. The authors managed to show the efficacy of the ADCs with in vitro and in vivo data. The same authors, and others, showed that this technology can be utilized to introduce a highly toxic payload into full length trastuzumab40 and proteotoxins to a trastuzumab derived antigen binding fragment.41
The application of SrtA for rapid prototyping has been highlighted by the Ploegh group using SrtA to fuse immune stimulatory peptides to an anti-C-type lectin mAb αDEC205 (Figure 2a).42 By genetically introducing the sortase tag to the C-terminus of the heavy chain the group generated 49 different derivatives of αDEC205. That number of constructs would have been difficult to achieve using genetic engineering only. Importantly, it allows for the introduction of non-natural moieties and removal of the His-tag, analogous to the previous example. This enabled the characterization of the stimulation of dendritic cells in vitro and the immune response in a mouse model with a palette of epitopes as well as different linker sequences fused to αDEC205. The same group also combined SrtA with butelase 1, a plant enzyme that works analogously to SrtA that, however, uses a C-terminal N/HV recognition sequence which is transacylated with a N-terminal NH2-X1X2 sequence (X1 = not proline, X2 = Leu, Ille, Cys, or Val).43 By introducing a sortase tag on the light chain and butelase 1 tag on the heavy chain of IgG1 a dual-labeled antibody was produced in a one-pot reaction.
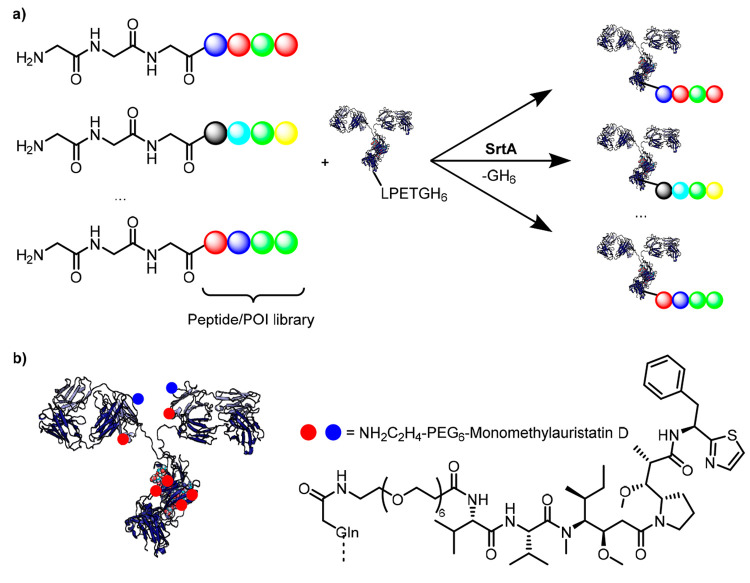
Figure 2.
(a) Sortase A enables rapid prototyping of biologics.42 A single mAb containing the LPETG motif can be functionalized with a whole library of compounds individually. SrtA is acting as an important tool when cloning, expression, and purification of a large number of fusion constructs is impossible. (b) Transglutaminase can attach monomethylauristatin D payloads to Q-tags with high specificity.74 Strop et al. showed that ADCs with high DARs can be synthesized in a controllable manner, yielding DAR 6 ADCs by targeting Q-tags in the red regions (light chain C-terminus, N297Q, and a native Gln295) and DAR 8 with the addition of Q-tags in green (LLQG inserted at position 135). Numbers are by EU numbering.
Despite the significant successes of SrtA, it has its limitations. Wild type SrtA is a very slow and highly Ca2+ dependent catalyst. Both problems have been tackled with directed evolution, yielding faster and Ca2+ independent enzyme variants.44,45 These improved variants have become the industry standard for SrtA transformations. Additionally, the more recently discovered and functionally similar enzymes from the Asx ligase family butelase 1 and OaAEP1 have significantly higher catalytic proficiencies with minimal engineering necessary.46,47 A more fundamental limitation of these enzymes is their hydrolytic mechanism, which can suffer from reversibility. Many of the previously mentioned examples circumvent this by using large excess of nucleophile, conditions unsuitable for large scale processes or for high value coupling partners. Another caveat is the production of Asx ligases, since in plants they are expressed in a zymogenic form and often are difficult to express recombinantly. However, this has already been improved in the case of OaAEP1 through expression as ubiquitin fusion proteins as well as structure guided protein engineering to boost the wild type’s ligation efficiency.46,48
Reaction reversibility can be overcome by substrate and reaction engineering, for example, running ligations in flow conditions,49 installing activated bonds in the substrate tag,50,51 self-inactivating leaving groups,52 using linker regions that form a SrtA unreactive tertiary structure upon ligation,53 and by adding Ni2+ to the reaction to complex the leaving group (requiring a sortase tag containing a His6 tag).54 Another common strategy is to engineer serine protease enzymes by replacing the active site serine with a cysteine in combination with a payload that contains an activated depsipeptide or ester bond.55−57 Cysteine is sufficiently nucleophilic to react with the payload’s ester bond and can subsequently perform the transpeptidation, but it renders the enzyme less active for amide bond cleavage, thus reducing the back reaction. Two such engineered enzymes, peptiligase and omniligase-1, have found applications in producing peptidic drugs up to gram-scale (Figure 1).58,59 Interestingly, these reactions often achieve full conversion in short reaction times (minutes) and the substrates can be used with small excesses (e.g., 1.1 equiv) of depsipeptide. With this methodology being very successful at synthesis of peptides and model substrates,60,61 similar applicability can be expected in future applications with proteins as ligation partner.62
Trypsiligase, an engineered trypsin protease, similar to SrtA recognizes a Tyr/Arg-His sequence and transacylate with a Arg-His tagged payload for C-terminal labeling (Figure 1).63 For N-terminal labeling, payloads activated with a 4-guanidinophenyl ester (OGp), acting as an arginine mimic, can be used which react with N-terminal RH.64 Importantly, this coupling can also be carried out with in situ cleavage of the purification-tag-Y/RH-protein-of-interest (POI) by trypsiligase and subsequent ligation with an OGp-ester. It has since been used to introduce click handles and cytotoxic payloads with a minimal ligation scar into the fragment antigen-binding region of an mAb,65 as well as the PEGylation of antibodies.66 The capability of N- and C-terminal tagging makes trypsiligase a very promising tool with possible downsides including: the initial cleavage reaction for N-terminal labeling being time-consuming, the need for a YRH sequence, and the requirement for zinc-(II) making it potentially unsuitable for later described radioligand approaches. However, concomitant cleavage of the purification tag during the ligation by Trypsiligase and the reduced reversibility of the OGp substrates combine the versatility of SrtA with the improved mechanism of peptiligase.
Another well-established enzyme is transglutaminase. It catalyzes the cleavage of ammonium from a glutamate side chain and the concomitant formation an isopeptide bond with a lysine residue’s side chain (Figure 1).67 In particular, microbial transglutaminases (mTGs) are interesting for their ease of production, broad payload substrate scope, and site specificity for the different “glutamine tags”, or Q-tags, for instance, LLQG or c-myc-tag,24,68−70 and in native glutamines.71−73 Strop et al.24 produced ADCs based on three mAbs (anti-EGFR, anti-HER2, and anti-M1S1) with mTG giving them tight control over the DAR and used the site specificity to examine how the attachment site influences drug properties. The authors tested 90 sites by genetic introduction of Q-tags in the constant regions of several mAbs. Only 12 positions possessed favorable biophysical characteristics. Conjugation efficiencies also varied depending on the specific mAb used. In vivo tests showed that ADCs generated by mTG coupling were similarly effective compared to ADCs prepared with maleimide chemistry. However, pharmacokinetic properties differed significantly. ADCs with payload linked to the C-terminal heavy chain were cleared faster and showed decreased stability compared to conjugates with the payload attached to the light chain.
In an important contribution, the same group improved ADCs with high drug loading (DAR 6 and 8) of monomethylauristatin through site-specific conjugation (Figure 2b).74 The mAb–drug linkage proved to be more stable than corresponding maleimide-based conjugates with the same DAR, especially at high DARs. Even more importantly, through the ability of introducing the payload at sites that are spatially separated, the group managed to produce a DAR 6 ADC with similar in vivo properties to an unconjugated mAb and high efficacy against lowly expressed target compared to similar ADCs prepared with maleimide chemistry. Maleimide-based conjugation led to the payload molecules reacting with cysteines that are located close together. The resulting hydrophobic patches of the payload clustering together is thought to distort the mAb structure and impede its function. Several other examples have shown mTG mediated conjugation to mAbs with diverse payloads such as chelators,68 biotin,72 and toxins.73,75 Conversely to the previous examples, mTG can also tag lysine with a payload containing a glutamine residue.76 This affords the attachment of two orthogonal payloads to lysine and glutamines with mTG; an application of this will be discussed further in the radioligand therapy section.77
Another way of creating intrachain modifications is the use of formylglycine-generating enzyme (FGE, Figure 1).78,79 It recognizes the CXPXR motif and converts the cysteine residue to formylglycine (fGly). This can be achieved both in vitro and by in vivo coexpression of FGE. The aldehyde functionality can then be functionalized using aminooxy or hydrazide probes with >90% final conversion. The hydrolytic instability of the respective hydrazone or oxime products, as well as the need for low pH or catalysts for the aldehyde functionalization, have since been circumvented by using the trapped-Knoevenagel reaction,80,81 Pictet–Spengler bioconjugation,82 or the hydrazino-iso-Pictet–Spengler (HIPS) reaction.83,84 Additionally, increased copper concentrations boost activity toward less reactive recognition motifs with a proline to alanine mutation (e.g., CTAGR).85 Genetically introducing two distinct motifs and performing oxidations at different copper concentrations establishes orthogonal control over the sites, which has been used to create dual-labeled antibodies and DARPINs. In an important example, Drake et al. used coexpression of human FGE and genetically engineered trastuzumab, introducing an aldehyde tag either in the light and heavy chain constant regions, and the C-terminus of the heavy chain.86 Employing HIPS, the group achieved high Cys to fGly conversions between 86% and 98% as well as >90% conjugation efficiency in the heavy chain constructs and 75% for the light chains. Additionally, choosing different conjugation sites tuned the pharmacokinetic parameters while retaining target binding. The C-terminal tag proved to have longer half-life time in vivo which ultimately translated to higher efficacy in a mouse xenograft model. An approach to overcome the need for high equivalents of a payload is to first introduce an azide with the HIPS or trapped Knoevenagel reaction and subsequent strain-promoted azide–alkyne cycloaddition (SPAAC) to introduce the payload.87 Notably, the drug candidate TRPH-222, an anti-CD22-mAb maytansine conjugate drug, which is prepared using the HIPS technology, has recently passed Phase I studies for relapsed and/or refractory B-cell non-Hodgkin’s lymphoma.88
The use of sortase, FGE, and to a certain extent transglutaminase depend on specific peptide tags of the protein target. This limits the technology for native protein modification. Directed evolution can be used to change recognition sequence acceptance, however, such engineering efforts are often ambitious. For example, sortase has previously been engineered with considerable effort to recognize different sequences.89,90 For immunoglobulins, glycoengineering can offer a more universal approach to form bioconjugates.30 Chemical methods of introducing aldehydes in glycans by oxidation of vicinal alcohols exist but long exposure to oxidants may decrease the quality of the final bioconjugate.91 Oxidases are a good alternative with mild reaction conditions for such reactions which have been applied for bioconjugation (Figure 1).92 One challenge to overcome is the heterogeneity of glycans that are attached to the antibody.93 This can be circumvented by developing cell lines with specific glycan structures,91 or engineering the glycan composition using enzymes in vitro.94,95 The latter approach has been used by Angelastro et al. to introduce galactose at the nonreducing end of the dominant G0F glycoform found on trastuzumab to β-1,4-galactosyltransferase to produce four aldehydes per mAb.96 A tandem Knoevenagel reaction was used to introduce two azide-containing linkers per aldehyde, resulting in eight potential conjugation sites. The authors showed that this sequence yielded an average of 7.3 azido groups per antibody. Further SPAAC with a dibenzylcyclooctyne-tetramethylrhodamine (DBCO-TAMRA) dye resulted in the main species being DAR 8. Another possibility is to directly introduce azide-labeled glycan mimetics using a glycosyl transferase.97 The same company developed a hydrophilic linker, which in combination with glycosyl transferases are a promising platform for DAR 4 ADCs with improved stability and therapeutic index.98 Monoclonal antibodies produced with this technology have also progressed in the clinic to a Phase 1b trial (NCT05389462).
Many additional methods have been explored that we’re only going to be able to touch upon briefly. For instance, tyrosinase can form quinones from tyrosine side chains which can undergo strain promoted cycloaddition.99 Horseradish peroxidase and hydrogen peroxide can be used to modify tyrosine in a single-electron transfer reaction.100 Both methods proved to be site-selective, without depending on a specific recognition sequence, reacting with solvent accessible residues. Prenyltransferases can transfer non-natural geranyl phosphates to a CaaX motif,101 with one ADC in clinical trials (NCT03944499).102 A prenyltransferase substrate, convenient for orthogonal coupling of two payloads, has been reported.103 Similarly, phosphopantetheinyl transferases (PPTases) can transfer coenzyme-A analogues to an 11 amino acid tag (GDALSWLLRLLN).104 Subsequent work allowed shortening of the tag sequence and linker length, as well as proving in vitro efficacy and showing sufficient serum stability. Potential downsides of prenyltransferases and PPTases are the added synthetic effort for the geranyl phosphate and CoA analogues, respectively.
Mammalian enzymes have been employed for bioconjugation, often using recognition sequences native to the human proteome, potentially less prone to eliciting an immunogenic response. The Bode group, for example, reported a method to form intrachain linkages by repurposing the SUMO conjugating E2 ligase-like enzyme Ubc9.105 The enzyme was shown to be promiscuous toward linking synthetic LRLRGG peptides simply modified with a C-terminal thioesters to the Lys side chain in a LKSE motif, thus bypassing the need for a cascade reaction with E1 carrier proteins. The system proved to be flexible enough to accept a multitude of POIs with the recognition motif. In one of many proof-of-concept experiments, the group used Ubc9 in tandem with sortase A to label trastuzumab Fab with two different dyes. Purification by size-exclusion chromatography yielded a nearly quantitatively labeled and functional Fab double-conjugate. In another example, the enzyme tubulin tyrosine ligase (TTL) has been shown to be effective in conjugating clickable tyrosine analogues to the C-terminus of VDSVEGEGEEEGEE.106,107 TTL has proven effective for introducing 4-azide phenylalanine, allowing the subsequent SPAAC with DBCO-conjugated payloads. TTL requires a relatively long tag, but its ATP dependence could be an advantage to push the reaction equilibrium toward the product side.
As seen in these examples, enzymatic bioconjugation can be fast, selective, and high yielding. It has already proven useful for rapid prototyping of ADCs, creating well-defined products to probe the influence of the coupling position and DAR on stability and therapeutic efficacy of ADCs, as well as orthogonal attachment of multiple payloads. With the maturation of these methods, i.e., optimization of payload concentration, addressing reversibility issues, and removing the dependency on long recognition sequences, increasing numbers of ADCs prepared with such coupling methods can be expected to enter the clinic. Furthermore, many of the previously mentioned techniques for ADC generation are also applicable to different drug modalities, sometimes with specific caveats. These applications are highlighted in the following sections.
3. Radioligand Therapies
Radioligand therapy (RLT) designates the attachment of a radioactive moiety to a molecule that specifically binds to a target. RLTs can be used to image and effectively treat cancers. Given the transient nature of radionuclides, rapid attachment of the radioactive payload becomes critical. With faster production steps, more active compound is delivered to the biological target after administration. The loading of radionuclides into a chelator can either be done directly, where a chelator is charged with the radionuclide in isolation of the biopolymers and subsequently coupled to the biomolecule afterward, or, coupling is performed first, and the radionuclide then added to the protein-chelator conjugate (Figure 3). Directly coupling a chelator precharged with a radionuclide has the advantage of tolerating more forcing conditions during the chelation step, since the protein target is not present. Moreover, it could reduce the effort in downstream purification. However, reserving the bioconjugation step to the end is often only chosen as a second option if the protein is unstable under radiolabeling conditions.108 Other challenges for RLT include working with large excesses of radioactive material, which clearly does not scale well and is associated with increasing production volumes. Furthermore, transformations prior to the chelation step must be metal-free to prevent chelation of undesired metals. The process should also be high yielding because labeled and unlabeled drug are often difficult to separate and unlabeled drug will compete for target binding with the active drug, decreasing the therapeutic effect. Here the benefits of biocatalysis could be exploited. Enzymatic couplings can be fast, selective for a specific site in the protein, depending on the enzyme metal-free, with straightforward purification.
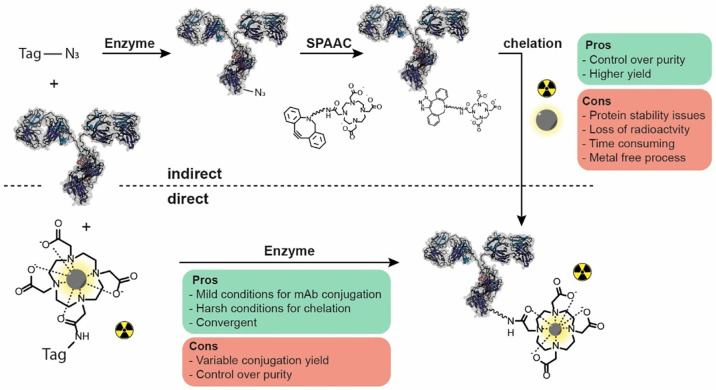
Figure 3.
Most strategies in biocatalytic conjugation of RLT payloads to date describe an indirect approach (top), where the protein is first functionalized with the chelator in multiple steps and later charged with the radioactive metal. This process is robust; however, it involves many steps, requires metal-free conditions and long incubation times, and can result in loss of radioactivity. The direct approach, where precharged chelator payload is ligated to the protein of interest, could alleviate most of these issues but requires a very efficient enzymatic step.
Transglutaminase presents an interesting case, since the enzyme can couple relatively simple organic amines. An early example of Jeger et al. showed that it was possible to modify native and engineered glutamines with several payloads, including metal chelators.68 Building on that work, the same group demonstrated the power of this approach for attaching radioligand payloads to antibodies with high site specificity.77 By introducing glutamine and lysine residues into proteins, the authors created fully functional modified antibodies, single-chain variable fragments, and antigen-binding fragments (Figure 4). Attachment of trans-cyclooctene-tetrazine-PEG4-NH3 (TCO-PEG4-NH3) using microbial transglutaminase (mTG) from Streptomyces mobaraensis to a glutamine residue in a mAb yielded a versatile handle for metal-free conjugation of a metal charged 1,4,7,10-tetrakis(carboxymethyl)-1,4,7,10-tetraazacyclododecane glutaric acid)-anhydride (DOTAGA) metal chelator using the inverse electron-demand Diels–Alder reaction (IEDDA). Additional conjugation of an azide containing peptide to a lysine demonstrated how mTG can orthogonally modify a target with two reactive handles, enabling subsequent one-pot functionalization with two payloads through metal-free click chemistry. Enzyme immobilization eliminated the need for downstream purification. Whereas attachment of a single amine payload to a glutamate usually proceeded with excellent yields, >90% the reaction of lysine with labeled peptides proceeded with significantly lower yields (<70%). The dependence on larger peptide substrates for lysine functionalization and the need for large excesses of substrate are still limitations of mTG which can likely be overcome with directed evolution.
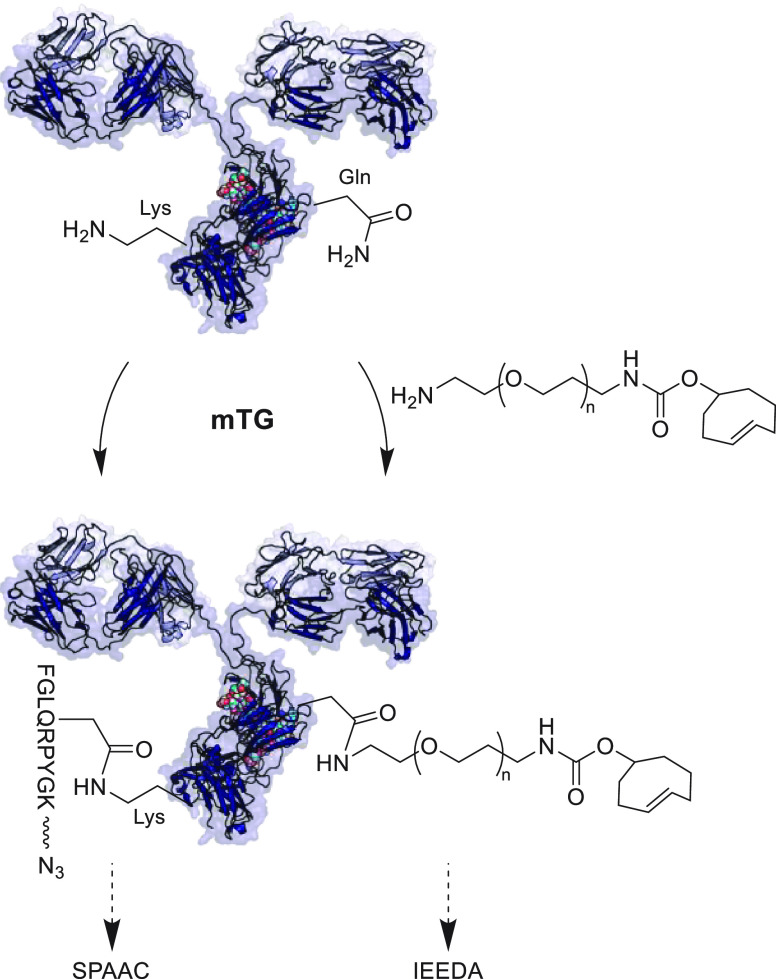
Figure 4.
Microbial transglutaminases are capable of orthogonal labeling.77 Payloads and reactive handles can be attached to glutamine-containing peptides or primary amines. Combining strain-promoted azide alkyne cycloaddition (SPAAC) and inverse electron demand Diels–Alder reactions (IEDDA) provides additional orthogonality for metal-free click chemistry.
In contrast to transglutaminase, the selectivity of sortase A for a unique C-terminal sequence is in principle an advantage for forming homogeneous bioconjugates. This has for example, been exploited to tag nanobodies with a short peptide GGGYK where a chelator was attached to the lysine’s side chain.109 SrtA is limited by the chemical equilibrium of the reaction, which often necessitates high excesses of payload, sequestering the leaving group for instance by genetic incorporation of a C-terminal His6 and the addition of Ni-NTA during the reaction or for subsequent purification, and running reactions in flow.49 This poses a problem for RLT applications since chelators can strip Ni2+ from purification columns. Paterson et al. showed that SrtA conjugations can be used to create radiolabeled scFvs in high yields by exchanging the His-tag for a FLAG tag.110 Additionally, the authors incorporated two tryptophan residues after the sortase tag, which has been shown to slow down the reverse reaction.111 This enabled yields of up to 90% of a fully functional positron emission tomography tracer after purification with an anti-FLAG column.
A glycan engineering approach was used to make 89Zr-labeled prostate specific membrane antigen-targeting antibodies.112 First, β-1,4-galactosidase was used to cleave the terminal galactose off the oligosaccharide. Second, uracil diphosphate activated N-azidoacetylgalactosamine was coupled to the glycan using galactose transferase. The azide-labeled antibody was reacted with the chelator-cargo in a copper-free SPAAC, and the resulting antibody-chelator conjugate was subsequently radiolabeled with 89Zr. This process turned out to be high yielding and very mild. Nonetheless, the process is time-consuming with multiple 16 h incubation steps, making it potentially impractical for less stable glycoproteins. Additionally, the long incubation times make the process unsuitable for directly coupling a “hot” material to the antibody. All the described processes in this section introduce the radioactive labeling after bioconjugation with a chelator or a click handle. This necessitates metal-free click chemistry and mild chelation conditions in the former case. Immobilized biocatalysts with fast reaction kinetics, such as OaAEP1, are likely a great platform for rapid generation of radioactive therapeutics. It could reduce the need for long reaction sequences under metal-free conditions and extensive downstream purification and give rapid access to radioactive drugs.
4. Polyvalent Conjugates
So far, we focused mostly on the conjugation of small molecules and peptide payloads to proteins. Many of the previously described enzymatic transformations also lend themselves to the creation of bivalent protein conjugates, i.e., the covalent linkage of two proteins. This was recently the topic of an excellent review by the Bernardes group and will only be touched upon here for illustrative purposes for further discussion of conjugates with higher valency.10 In two publications, sortase A was used to create bispecific binders by linking a mAb with two scFVs.113,114 Here click handles for SPAAC were introduced in both proteins with SrtA and a C- to C-terminal linkage was created in a subsequent reaction. In another study, a combinatorial library of designed ankyrin repeat proteins (DARPins) was created by preparing a N-terminal glycine residue by TEV protease cleavage and direct transpeptidation with sortagged DARPins creating a C-to-N-terminal linkage.115 The direct transpeptidation enables very facile generation of libraries without the need for intermediate purification steps. Installing separate click handles adds an additional step to the synthesis, however, it enables using higher substrate loading in the enzymatic step improving overall yield. Therefore, the latter approach is often used for creating conjugates with higher valency, where reaction efficiency is critical.
Polyvalent conjugates, in this context defined as three or more protein-payload attachments, can offer several advantages over their monomeric equivalents. For example, conjugating different binders can enhance the range of targets being neutralized.116 Receptor engagement with multiple binders can induce receptor clustering and activation of signaling.117 Additionally, having multiple binders in polyvalent complexes often increases their affinity to the target.118 As an example, Moliner-Morro et al. used SrtA to introduce DBCO moieties at the C-terminus of a SARS-CoV-2 neutralizing nanobody.119 The authors created a set of nanobody dimers and tetramers and compared them to the monomer and to nanobodies fused to a IgG heavy chain Fc region, creating a dimer. The dimeric constructs and Fc-fusion were equally potent and, at around 125 pM neutralization IC50 in an in vitro assay, approximately 150-fold more efficient than the monomeric nanobody. The tetramer showed an additional 10-fold improvement with an IC50 of 13 pM. A similar conjugation strategy, albeit with copper catalyzed cycloadditions, has been used to create up to tetravalent conjugates on peptidic dendrimers.120 The same group has also reported the generation of heptameric proteins attached by click chemistry to an azide-decorated cylcodextrin.121
Targeting tyrosine for bioconjugation Minamihata et al. successfully used HRP to cross-link IgG binding peptides.122 Introducing a GGGGY sequence at the C-terminus allowed the formation of a mixture of dimer and trimer by treatment with HRP and H2O2. The group showed that the polyvalent constructs possessed increased affinity for antibodies. However, it proved difficult to control product specificity and the resulting mixture of mono-, di-, and trimer is difficult to separate. In a follow up study, the same group created branched polymers by introducing an additional N-terminal tag.123 The resulting polymer had a cross-linking degree of 70- to 150-mers.
These examples illustrate current issues with bioconjugation for multivalent complexes. Problems with low yields and specificity are exacerbated when generating polyvalent conjugates which often results in the requirement to perform multiple single conjugations rather than one convergent step. To generate well-defined polyvalent conjugates in a convergent fashion, extremely proficient conjugation reactions are required. Current enzymatic bioconjugation methods do not fulfill these criteria yet. Therefore, if using such processes during discovery phases, the approach might need to be changed when advancing to later development stages, leading to discontinuities in the development process.
5. Adeno-Associated Virus Vectors
Cell and gene therapy is a rapidly expanding area with already >20 FDA approved products.124 Adeno-associated virus vectors (AAVs) are the current delivery method of choice for gene therapies125 with hundreds of clinical trials ongoing.126 However, AAVs remain challenging to manufacture and supply, due to their limited shelf life and instability during production and downstream processing.127 Emerging research indicates that surface modificaton of the AAV capsids can help to (a) stabilize the AAVs and (b) improve their tissue specific target binding. Genetic128 or chemical129 modifications of capsids seem inevitable to further improve the therapeutic properties of this vehicle. In addition to peptide insertions, genetic code expansion with unnatural amino acids, serving as a handle for further ligand binding, has been demonstrated.128 However, introduction of non-natural amino acids to the viral surface remains technically demanding. Ideally, bioconjugation could be performed on native protein structures. Surface-exposed and reactive residues, such as lysines, arginines, and histidines, have also been applied as conjugation sites for nonselective, chemical methods.129 However, the number of ligands on the capsid surface may be highly relevant for the specific tissue targeting and the PK/PD properties of the virus. Could biocatalysis help to control the density of the ligand coating? How specific could an enzyme be in selecting a ligation (amino acid) site on the capsid surface?
Formylglycine generating enzyme (FGE) was studied for applications in AAV capsid modification.130 A 13-amino acid concensus sequence LCTPSRAALLTGR was genetically inserted into AAV2 capsid to facilitate an FGE-catalyzed aldehyde formation. The aldehyde functionality then served as a handle for conjugation of fluorescent dyes, gold nanoparticles (Au-NPs), mAbs, and peptides. Using tunnel electron microscopy, the authors showed that on average six to nine Au-NPs were conjugated with a distribution from three to 13. With a single AAV2 containing 60 CXPXR tags, this equates to roughly 12% of all sites being converted to the conjugate. Similar results were obtained for the fluorescent dye conjugation. The near identical conjugation efficiencies of the large nanoparticle and the small dye indicate that steric hindrance of the cargo on the AAV surface is not limiting the conversion, but rather the efficiency of the FGE reaction or the aldehyde conjugation. The authors used this system to couple human leukocyte antigen mAbs to AAV2, which improved transduction by up to 37% in 293T cells. These results are promising for future FGE applications in creating functional virus engineered to target leukocytes. FGE is convenient due to the of in cellulo modification of the capsid. Issues with inefficient conjugations leading to wide payload:AAV ratio distributions could be tackled by optimizing the expression of FGE in the host cell.131
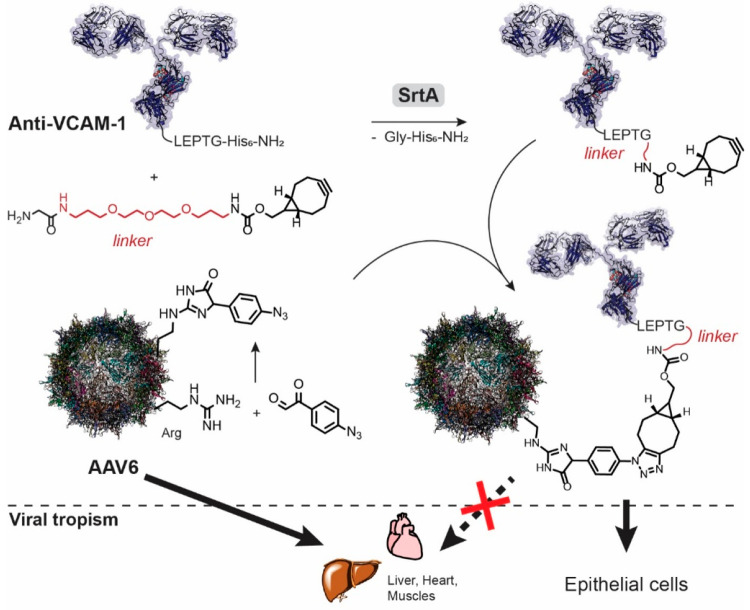
Similarly, Pearce et al. applied sortase A mediated conjugation of a single-chain antibody (ScFV) targeting vascular cell adhesion molecule (VCAM-1) to the surface of AAV6 (Figure 5).132 The conjugated antibody facilitated enhanced transduction of AAV6 to endothelial cells. The conjugation strategy included: (a) introduction of a click-handle to AAV6 via an arginine residue in the form of 4-azidophenyl glyoxal, which simultaneously removed AAV6’ native tropism, (b) sortase mediated conjugation of a PEG-linked BCN to the anti-VCAM-1 ScFV, and (c) combining the two via a click reaction. For sortase coupling, the anti-VCAM-1 was expressed as an LPETG-His8 construct. After the sortase reaction, the unreacted anti-VCAM-1, the NH2-G-His8 leaving group, and the His-tagged sortase could be removed from the conjugation product via nickel affinity chromatography. This construction elegantly removes prior tropism and introduces selectivity for epithelial cell lines, as demonstrated by in vitro experiments. Comparable to the multivalent complexes, this modular approach is more laborious compared to the previous FGE-based method, but it allows for more control over each step.
Figure 5.
Sortase mediated introduction of a click handle to an antibody anti-VCAM-1 facilitated the synthesis of an antibody-AAV6 conjugate. Whereas AAV6 shows tropism toward liver and heart, conjugation with the para-azido glyoxal removes this tropism. Conjugation with anti-VCAM-1 retargets the virus to infect epithelial cells.
In another in cellulo approach, coexpressed biotin ligase (BirA) from Escherichia coli has been used for AAV biotinylation (Figure 6).133 Additionally, BirA was used to conjugate a ketone analogue of biotin to the surface of genetically modified AAV particles.134 The attached biotin ketone analogue could serve as a site-specific handle to hydrazide or hydroxylamine probes. Both approaches require genetic introduction of the biotin acceptor peptide or also known as Avi tag (GLNDIFEAQKIEWHE) onto the capsid. Five different AAV serotypes were tolerant to the Avi tag insertion and biotinylation and the attached biotin also facilitated viral particle purification via avidin resin. The loading ratio of biotin to capsid was estimated to be 20:1 and no noticeable drop in infectivity was observed after biotinylation. This construct was efficiently transducing cells expressing scavidin, a chimeric biotin-binding membrane protein,135 whereas unmodified viruses were unable to infect this cell line, even at high virus titers. Key advantages and limitations of biocatalytic strategies for AAV modifications are highlighted in Table 1.
Figure 6.
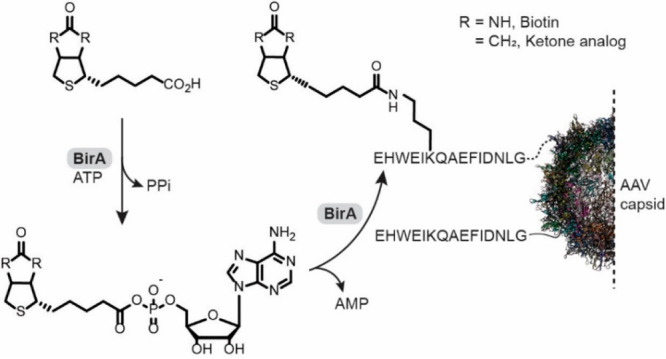
Biotin ligase (BirA) catalyzed biotinylation or biotin analogue ligation to AAV-BAP (biotin acceptor peptide) fusions. The reaction consumes an equimolar amount of ATP.
Table 1. Advantages and Limitations of Different Biocatalytic Strategies Which Have Been Used for AAV Bioconjugation.
| ligating biocatalyst | advantages | limitations |
|---|---|---|
| formylglycine generating enzyme | • aldehyde tag enables variety of chemistries | • requires a recognition tag |
| • in cellulo modification by FGE coexpression | • incomplete conversion results in heterogeneous population of nanoparticles | |
| • potential side reactions of aldehyde in complex matrices | ||
| • genetic modification necessary | ||
| sortase | • sortase has a broad nucleophile scope enabling introduction of variety of conjugate agents (e.g., for labeling and/or tissue targeting) | • requires a recognition tag |
| • modular approach | • leaves a scar, e.g., LPET | |
| • control at each step | • more laborious than labeling during expression | |
| biotin ligase | • biotinylation can be carried out in cellulo by BirA coexpression | • requires a recognition tag |
| • biotin offers a purification handle | ||
| • flexibility of conjugate agents |
6. Oligonucleotide Based Therapies
Oligonucleotides are a very versatile class of drugs used to modulate gene expression and protein function via multiple modes of action including RNA splicing modulation, gene activation, programmed gene editing, and target degradation.136 Their potential for treating currently incurable diseases is tremendous, and more than 10 oligonucleotide drugs have already gained FDA approval or are undergoing regulatory approvals.137,138 Despite their huge promise, tissue targeting represents a major translational challenge. Several strategies have been developed to enhance oligonucleotide drug delivery and have been reviewed elsewhere.139−141 Previously explored covalent conjugates to oligonucleotides include cholesterol, fatty acids, cell penetrating peptides, aptamers, antibodies, and sugars (e.g., GalNAc). The conjugation of specific molecules to oligonucleotides offers some advantages for targeted delivery over formulations with polymers or other transfecting reagents. Mostly, it provides a defined composition and simplifies analysis and characterization.
Few enzymatic bioconjugations of oligonucleotides have been disclosed. Most of them aim only to generate small quantities for cellular biology studies. For example, to study protein adduct influence on replication, GFP was azide tagged using a farnesyltransferase and subsequently coupled to DNA.142 Nanobodies have been DBCO-functionalized with SrtA to couple to azide-labeled oligos for super-resolution microscopy.143 The promiscuous activity of natural and engineered methyl transferases in combination with synthetic S-adenosyl methionine analogues to synthesize labeled nucleosides has been studied extensively at the 3′ end.144 Similar reactivity can be carried out selectively on capped mRNA, directly installing a propargyl group on the first transcribed base.145 However, the scalability of this chemistry is questionable. Often thousandfold excesses are required to drive the reactions toward completion. These limitations can potentially be overcome by clever engineering of cofactor recycling systems, as has already been shown for methylation reactions.146 The promise of enzyme catalysis has also been recognized for the synthesis of DNA-encoded libraries.147 For example, Thomas et al. chemically ligated an amine-functionalized DNA duplex to several 2-aminoethyl glycosides.148 The glycoside was subsequently modified using glycosyl transferases, trans-sialidase, and galactose oxidase. Aldehydes resulting from the latter enzymatic reaction were further used for hydrazone ligation and reductive amination to introduce non-natural functional groups. This set of reactions could in the future be leveraged to create DNA-encoded carbohydrate-based ligand libraries with by simple split-and-mix synthesis.
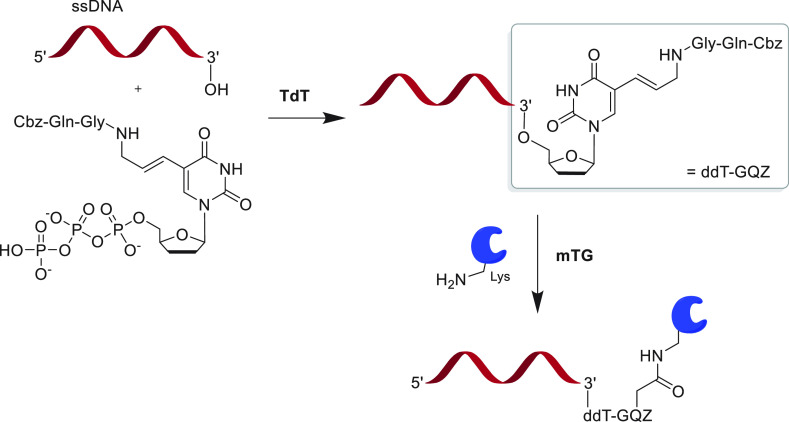
Several approaches using transglutaminase have been tested to link proteins to oligonucleotides. Huggins et al. used transglutaminase to introduce an azide into a mAb and perform SPAAC using a synthetically DBCO-tagged oligonucleotide.149 A recent applied example of transglutaminase is the conjugation reaction between a Q-tagged antitransferrin camelid antibody and 3-azido-1-propanamine which was subsequently used to link to an oligonucleotide using copper-free click chemistry.150 The resulting molecule was shown to retain transferrin binding and to be capable of carrying siRNA payloads across the blood-brain barrier in vivo. More attempts at direct coupling of proteins and oligos using mTG were made with chemical amination of the 5′ base and subsequent mTG coupling to GFP.151 Polymerases have been identified for introduction of amide-functionalized bases; however, it is hard to control the site-specificity and stoichiometry of the attachment.152 The use of template-independent incorporation of Cbz-Gln-Gly-labeled ddUTP by terminal deoxynucleotidyl transferases (TdT) allows for more controlled incorporation of a single tag (Figure 7).153 Subsequent reaction with mTG and enabled the synthesis of aptamer-protein conjugates.153,154 Whereas mTG reactions worked well with a relatively low 5-fold excess of POI,152 the TdT reaction may be limiting when scaling up with 100-fold excesses of nucleoside analogues required. This remains an emerging technology, and TdT efficiency can likely be improved using enzyme engineering.155 Additionally, TdT only acts on DNA, but alternatives to carry out similar reactions on RNA exist.156
Figure 7.
Terminal deoxynucleotidyl transferase mediated incorporation of a Q-tag (Cbz-Gln-Gly) labeled nucleotide dideoxy thymidine analogue (ddT-GQZ) into single stranded DNA oligonucleotide (ssDNA).153 The lack of a 3′ hydroxy group on ddT-GQZ prevents the polymerization reaction and leads to a selective incorporation of a single tag at the 3′ end of the oligonucleotide. The Q-tag is further used to couple a protein presenting a lysine residue with mTG.
It is worth mentioning that enzymes are already used in the synthesis of oligonucleotides, offering an alternative to traditional solid-phase synthesis using phosphoramidite building blocks. As the increased need for synthetic DNA and renewed interest into RNA- and DNA-based therapeutics puts pressure on existing solid-phase synthesis capacities, more sustainable solution-phase approaches are required. These alternatives are represented by RNA/DNA ligases,157 polymerases capable of accepting non-natural nucleotides,158 template-independent DNA synthesis dependent on TdT,159−162 and caged bases that are incorporated by polymerases and random priming.163 The same applies to synthesis of building blocks of oligonucleotide molecules where enzymatic syntheses of unnatural mono-164 and cyclic dinucleosides165 have been developed. As outlined here, enzymatic methods to manufacture therapeutic oligos and their coupling to therapeutic and targeting agents are still in their infancy. With the increased therapeutic application of synthetic oligos, we see a wealth of applications ahead for enzymatic oligo synthesis.
7. Conclusion and Outlook
As highlighted in the previous sections, every therapeutic modality poses its own challenges and opportunities for enzymatic bioconjugation. In parallel to the expanding palette of therapeutic modalities, a multitude of bioconjugation reactions using enzymatic catalysis have been established as important research tools. Sortase A, transglutaminase, and formyl-glycine generating enzyme are the most investigated enzymes and have proven their utility for almost every therapeutic modality.27,67,166 Importantly, biocatalysts offer unique advantages over chemical conjugation: Sortase A can cleave a purification tag concomitant to the conjugation reaction, transglutaminases are efficient and offer the possibility of dual-labeling, and FGE can be used co-translationally, yielding a protein with an orthogonal reaction handle after expression. More recently discovered enzymes show great improvement in their substrate scope and reaction kinetics.46,47,56,65,96
In terms of the individual therapeutic modalities, ADCs are the most explored case for enzymatic bioconjugations. Here, enzymes have proven very useful for rapid prototyping,42 site-selectivity,9,24,25 achieving high DARs,96 creating especially stable linkages,81,86 and orthogonal control over multiple payload attachments.43,77 The latter application we expect to be increasingly important with the growing complexity of newer ADC formats, e.g., with multiple payloads.167 For RLTs, developments in orthogonal and metal-free tagging with transglutaminase are most notable.77 We expect future applications exploring enzyme immobilization as well as enzymes with faster kinetics to simplify downstream purification and to preserve the final compound’s radioactivity. Oligonucleotide conjugates have great potential to improve the activity profile and scope of oligonucleotide-based therapies. For oligonucleotide conjugates, similar strategies as for ADCs and RLTs as well as DNA and RNA modifying enzymes have been explored. Additionally, novel enzymatic oligonucleotide-synthesis methods are expected to play a future role. Applications of enzymatic bioconjugation toward making multivalent conjugates and surface-modified AAVs represent a real challenge. Incomplete transformations often lead to a complex range of products being formed and are difficult to analyze or separate. Additionally, similar conjugation strategies as described in this Perspective may be amenable to other new modalities such as modified peptides, peptide nucleic acids, oligosaccharides, and other chimeric molecules (e.g., proteolysis-targeting chimeras).168
As discussed, some challenges still exist: the presented methods are still far from offering a universal and traceless system and may result in a mixture of products. Methods for native protein conjugation are required to avoid introduction of genetically encoded recognition tags, which inadvertently change the protein sequence. Further developments in this direction would decrease molecular cloning efforts and alleviate concerns regarding immunogenicity of the new sequence. Processes with higher efficiencies would be needed to limit incomplete reactions and side-product formation. Until these points are addressed, enzymatic bioconjugation still faces stiff competition from small molecule chemistry. However, in comparison, it is arguably early days for the use of enzymatic bioconjugation in drug development, but enzymes harbor a lot of synthetic potential. For example, in synthetic organic chemistry, enzymes have been increasingly applied in the recent decades and nowadays their use is commonplace, from single reactions to entire synthetic cascades.164,169 We predict a similar trajectory for the use of enzymes in bioconjugation. Additionally, many bioconjugating enzymes have not yet been subjected to and improved by directed evolution or engineering. This will likely enhance the utility of those biocatalysts further and enhance the panel of possible reactions. Illustrative examples are the large array of widely applied SrtA variants,29 the development of peptiligase,55 and the improvement of OaAEP1 for bioconjugation.46
In some cases, the above-mentioned challenges have already been overcome, as proven by the recent entrance of several biocatalytically produced ADC drug candidates into clinical trials.7 Patented processes of SrtA conjugations for ADCs report overall yields of up to 76% and reveal the reliance on binding resins to remove the affinity-tagged leaving group and retain unconjugated mAbs.170,171 Pfizer carried out a Phase 1 study with an antitrophoblast cell-surface antigen-2 ADC produced with mTG.172 Their patented processes are similar to scientific publications, with >20 equiv of the payload followed by purification with affinity chromatography and hydrophobic interaction chromatography, resulting in conjugation yields of up to 85%.173 Recently, a glycoengineered ADC has entered clinical trials (NCT05389462). The conjugation process includes partial removal of glycans and addition of an azide-labeled galactose; preparative and analytical scale reactions seem to only differ in the purification methods.97,174 Processes with >70% overall yield by in vivo coexpression of FGE and conjugation using HIPS chemistry have also been patented, and the corresponding products have entered clinical trials.88,175
These successes showcase that established biocatalytic processes can already yield sufficient product to support clinical trials. The outcomes of the ongoing trials may inform the development of best practices and protocols for the manufacturing, characterization, purification, and reporting of enzymatic bioconjugations. This progress will be vital as drug modalities increase in complexity and with more widespread application of enzymes. With several new companies founded that either specialize in enzymatic bioconjugation or base their products around a specific biocatalytic platform and more frequent off-the-shelf availability of biocatalysts and corresponding substrates, enzymatic methods are gaining momentum and are easier than ever to apply. Therefore, the broad implementation of enzymatic bioconjugation in research and subsequent development phases remains, in our opinion, merely a matter of time.
Acknowledgments
This work was supported by the Novartis Institutes for BioMedical Research Postdoctoral Program. We would like to thank Rainer Kneuer, Rainer Machauer, Cameron Lee, Christopher Brain, Susan Celliti, Tetsuo Uno, and Juerg Hunziker for fruitful discussion during the manuscript writing and Charles Moore and Rob Carroll for internally reviewing the manuscript.
Author Contributions
CRediT: Aaron Debon conceptualization, writing-original draft, writing-review & editing; Elina Siirola conceptualization, writing-original draft, writing-review & editing; Radka Snajdrova conceptualization, writing-original draft, writing-review & editing.
The authors declare no competing financial interest.
References
- Tong J. T. W.; Harris P. W. R.; Brimble M. A.; Kavianinia I. An Insight into FDA Approved Antibody-Drug Conjugates for Cancer Therapy. Molecules 2021, 26 (19), 5847. 10.3390/molecules26195847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- do Pazo C.; Nawaz K.; Webster R. M. The Oncology Market for Antibody–Drug Conjugates. Nat. Rev. Drug Discov 2021, 20 (8), 583–584. 10.1038/d41573-021-00054-2. [DOI] [PubMed] [Google Scholar]
- Strosberg J.; El-Haddad G.; Wolin E.; Hendifar A.; Yao J.; Chasen B.; Mittra E.; Kunz P. L.; Kulke M. H.; Jacene H.; Bushnell D.; O’Dorisio T. M.; Baum R. P.; Kulkarni H. R.; Caplin M.; Lebtahi R.; Hobday T.; Delpassand E.; van Cutsem E.; Benson A.; Srirajaskanthan R.; Pavel M.; Mora J.; Berlin J.; Grande E.; Reed N.; Seregni E.; Öberg K.; Lopera Sierra M.; Santoro P.; Thevenet T.; Erion J. L.; Ruszniewski P.; Kwekkeboom D.; Krenning E. Phase 3 Trial of 177 Lu-Dotatate for Midgut Neuroendocrine Tumors. New England Journal of Medicine 2017, 376 (2), 125–135. 10.1056/NEJMoa1607427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y.; Suresh M. R. Bispecific Antibodies as Novel Bioconjugates. Bioconjug Chem. 1998, 9 (6), 635–644. 10.1021/bc980044l. [DOI] [PubMed] [Google Scholar]
- Chernikov I. v.; Vlassov V. v.; Chernolovskaya E. L. Current Development of SiRNA Bioconjugates: From Research to the Clinic. Front Pharmacol 2019, 10 (APR), 444. 10.3389/fphar.2019.00444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tytgat H. L. P.; Lin C.; Levasseur M. D.; Tomek M. B.; Rutschmann C.; Mock J.; Liebscher N.; Terasaka N.; Azuma Y.; Wetter M.; Bachmann M. F.; Hilvert D.; Aebi M.; Keys T. G. Cytoplasmic Glycoengineering Enables Biosynthesis of Nanoscale Glycoprotein Assemblies. Nat. Commun. 2019, 10 (1), 5403. 10.1038/s41467-019-13283-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Berkel S. S.; van Delft F. L. Enzymatic Strategies for (near) Clinical Development of Antibody-Drug Conjugates. Drug Discov Today Technol. 2018, 30, 3–10. 10.1016/j.ddtec.2018.09.005. [DOI] [PubMed] [Google Scholar]
- Szijj P. A.; Bahou C.; Chudasama V. Minireview: Addressing the Retro-Michael Instability of Maleimide Bioconjugates. Drug Discov Today Technol. 2018, 30, 27. 10.1016/j.ddtec.2018.07.002. [DOI] [PubMed] [Google Scholar]
- Shen B.-Q.; Xu K.; Liu L.; Raab H.; Bhakta S.; Kenrick M.; Parsons-Reponte K. L.; Tien J.; Yu S.-F.; Mai E.; Li D.; Tibbitts J.; Baudys J.; Saad O. M.; Scales S. J.; McDonald P. J.; Hass P. E.; Eigenbrot C.; Nguyen T.; Solis W. A.; Fuji R. N.; Flagella K. M.; Patel D.; Spencer S. D.; Khawli L. A.; Ebens A.; Wong W. L.; Vandlen R.; Kaur S.; Sliwkowski M. X.; Scheller R. H.; Polakis P.; Junutula J. R. Conjugation Site Modulates the in Vivo Stability and Therapeutic Activity of Antibody-Drug Conjugates. Nat. Biotechnol. 2012, 30 (2), 184–189. 10.1038/nbt.2108. [DOI] [PubMed] [Google Scholar]
- Taylor R. J.; Geeson M. B.; Journeaux T.; Bernardes G. J. L. Chemical and Enzymatic Methods for Post-Translational Protein–Protein Conjugation. J. Am. Chem. Soc. 2022, 144 (32), 14404–14419. 10.1021/jacs.2c00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morais M.; Forte N.; Chudasama V.; Baker J. R.. Application of Next-Generation Maleimides (NGMs) to Site-Selective Antibody Conjugation. In Bioconjugation. Methods in Molecular Biology; Massa S., Devoogdt N., Eds.; Humana: New York, 2019; Vol. 2033, pp 15–24. 10.1007/978-1-4939-9654-4_2. [DOI] [PubMed] [Google Scholar]
- Walsh S. J.; Bargh J. D.; Dannheim F. M.; Hanby A. R.; Seki H.; Counsell A. J.; Ou X.; Fowler E.; Ashman N.; Takada Y.; Isidro-Llobet A.; Parker J. S.; Carroll J. S.; Spring D. R. Site-Selective Modification Strategies in Antibody–Drug Conjugates. Chem. Soc. Rev. 2021, 50 (2), 1305. 10.1039/D0CS00310G. [DOI] [PubMed] [Google Scholar]
- Hoyt E. A.; Cal P. M. S. D.; Oliveira B. L.; Bernardes G. J. L. Contemporary Approaches to Site-Selective Protein Modification. Nat. Rev. Chem. 2019, 3 (3), 147–171. 10.1038/s41570-019-0079-1. [DOI] [Google Scholar]
- Bornscheuer U. T.; Huisman G. W.; Kazlauskas R. J.; Lutz S.; Moore J. C.; Robins K. Engineering the Third Wave of Biocatalysis. Nature 2012, 485 (7397), 185–194. 10.1038/nature11117. [DOI] [PubMed] [Google Scholar]
- Turner N. J. Directed Evolution Drives the next Generation of Biocatalysts. Nat. Chem. Biol. 2009, 5 (8), 567–573. 10.1038/nchembio.203. [DOI] [PubMed] [Google Scholar]
- Nestl B. M.; Nebel B. A.; Hauer B. Recent Progress in Industrial Biocatalysis. Curr. Opin Chem. Biol. 2011, 15 (2), 187–193. 10.1016/j.cbpa.2010.11.019. [DOI] [PubMed] [Google Scholar]
- Young R. J.; Flitsch S. L.; Grigalunas M.; Leeson P. D.; Quinn R. J.; Turner N. J.; Waldmann H. The Time and Place for Nature in Drug Discovery. JACS Au 2022, 2 (11), 2400–2416. 10.1021/jacsau.2c00415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORIHARA K.; OKA T.; TSUZUKI H. Semi-Synthesis of Human Insulin by Trypsin-Catalysed Replacement of Ala-B30 by Thr in Porcine Insulin. Nature 1979, 280 (5721), 412–413. 10.1038/280412a0. [DOI] [PubMed] [Google Scholar]
- Fryszkowska A.; An C.; Alvizo O.; Banerjee G.; Canada K. A.; Cao Y.; DeMong D.; Devine P. N.; Duan D.; Elgart D. M.; Farasat I.; Gauthier D. R.; Guidry E. N.; Jia X.; Kong J.; Kruse N.; Lexa K. W.; Makarov A. A.; Mann B. F.; Milczek E. M.; Mitchell V.; Nazor J.; Neri C.; Orr R. K.; Orth P.; Phillips E. M.; Riggins J. N.; Schafer W. A.; Silverman S. M.; Strulson C. A.; Subramanian N.; Voladri R.; Yang H.; Yang J.; Yi X.; Zhang X.; Zhong W. A Chemoenzymatic Strategy for Site-Selective Functionalization of Native Peptides and Proteins. Science (1979) 2022, 376 (6599), 1321–1327. 10.1126/science.abn2009. [DOI] [PubMed] [Google Scholar]
- Zhang Y.; Park K.-Y.; Suazo K. F.; Distefano M. D. Recent Progress in Enzymatic Protein Labelling Techniques and Their Applications. Chem. Soc. Rev. 2018, 47 (24), 9106–9136. 10.1039/C8CS00537K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walper S. A.; Turner K. B.; Medintz I. L. Enzymatic Bioconjugation of Nanoparticles: Developing Specificity and Control. Curr. Opin Biotechnol 2015, 34, 232–241. 10.1016/j.copbio.2015.04.003. [DOI] [PubMed] [Google Scholar]
- Wu K.-L.; Yu C.; Lee C.; Zuo C.; Ball Z. T.; Xiao H. Precision Modification of Native Antibodies. Bioconjug Chem. 2021, 32 (9), 1947–1959. 10.1021/acs.bioconjchem.1c00342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milczek E. M. Commercial Applications for Enzyme-Mediated Protein Conjugation: New Developments in Enzymatic Processes to Deliver Functionalized Proteins on the Commercial Scale. Chem. Rev. 2018, 118 (1), 119–141. 10.1021/acs.chemrev.6b00832. [DOI] [PubMed] [Google Scholar]
- Strop P.; Liu S.-H.; Dorywalska M.; Delaria K.; Dushin R. G.; Tran T.-T.; Ho W.-H.; Farias S.; Casas M. G.; Abdiche Y.; Zhou D.; Chandrasekaran R.; Samain C.; Loo C.; Rossi A.; Rickert M.; Krimm S.; Wong T.; Chin S. M.; Yu J.; Dilley J.; Chaparro-Riggers J.; Filzen G. F.; O’Donnell C. J.; Wang F.; Myers J. S.; Pons J.; Shelton D. L.; Rajpal A. Location Matters: Site of Conjugation Modulates Stability and Pharmacokinetics of Antibody Drug Conjugates. Chem. Biol. 2013, 20 (2), 161–167. 10.1016/j.chembiol.2013.01.010. [DOI] [PubMed] [Google Scholar]
- Junutula J. R.; Raab H.; Clark S.; Bhakta S.; Leipold D. D.; Weir S.; Chen Y.; Simpson M.; Tsai S. P.; Dennis M. S.; Lu Y.; Meng Y. G.; Ng C.; Yang J.; Lee C. C.; Duenas E.; Gorrell J.; Katta V.; Kim A.; McDorman K.; Flagella K.; Venook R.; Ross S.; Spencer S. D.; Lee Wong W.; Lowman H. B.; Vandlen R.; Sliwkowski M. X.; Scheller R. H.; Polakis P.; Mallet W. Site-Specific Conjugation of a Cytotoxic Drug to an Antibody Improves the Therapeutic Index. Nat. Biotechnol. 2008, 26 (8), 925–932. 10.1038/nbt.1480. [DOI] [PubMed] [Google Scholar]
- Mao H.; Hart S. A.; Schink A.; Pollok B. A. Sortase-Mediated Protein Ligation: A New Method for Protein Engineering. J. Am. Chem. Soc. 2004, 126 (9), 2670–2671. 10.1021/ja039915e. [DOI] [PubMed] [Google Scholar]
- Antos J. M.; Truttmann M. C.; Ploegh H. L. Recent Advances in Sortase-Catalyzed Ligation Methodology. Curr. Opin Struct Biol. 2016, 38, 111–118. 10.1016/j.sbi.2016.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmohl L.; Schwarzer D. Sortase-Mediated Ligations for the Site-Specific Modification of Proteins. Curr. Opin Chem. Biol. 2014, 22, 122–128. 10.1016/j.cbpa.2014.09.020. [DOI] [PubMed] [Google Scholar]
- Freund C.; Schwarzer D. Engineered Sortases in Peptide and Protein Chemistry. ChemBioChem. 2021, 22 (8), 1347–1356. 10.1002/cbic.202000745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh S. J.; Bargh J. D.; Dannheim F. M.; Hanby A. R.; Seki H.; Counsell A. J.; Ou X.; Fowler E.; Ashman N.; Takada Y.; Isidro-Llobet A.; Parker J. S.; Carroll J. S.; Spring D. R. Site-Selective Modification Strategies in Antibody–Drug Conjugates. Chem. Soc. Rev. 2021, 50 (2), 1305–1353. 10.1039/D0CS00310G. [DOI] [PubMed] [Google Scholar]
- Ta H. T.; Prabhu S.; Leitner E.; Jia F.; von Elverfeldt D.; Jackson K. E.; Heidt T.; Nair A. K. N.; Pearce H.; von zur Muhlen C.; Wang X.; Peter K.; Hagemeyer C. E. Enzymatic Single-Chain Antibody Tagging. Circ. Res. 2011, 109 (4), 365–373. 10.1161/CIRCRESAHA.111.249375. [DOI] [PubMed] [Google Scholar]
- Madej M. P.; Coia G.; Williams C. C.; Caine J. M.; Pearce L. A.; Attwood R.; Bartone N. A.; Dolezal O.; Nisbet R. M.; Nuttall S. D.; Adams T. E. Engineering of an Anti-Epidermal Growth Factor Receptor Antibody to Single Chain Format and Labeling by Sortase A-Mediated Protein Ligation. Biotechnol. Bioeng. 2012, 109 (6), 1461–1470. 10.1002/bit.24407. [DOI] [PubMed] [Google Scholar]
- Li Z.; Theile C. S.; Chen G.; Bilate A. M.; Duarte J. N.; Avalos A. M.; Fang T.; Barberena R.; Sato S.; Ploegh H. L. Fluorophore-Conjugated Holliday Junctions for Generating Super-Bright Antibodies and Antibody Fragments. Angew. Chem., Int. Ed. 2015, 54 (40), 11706–11710. 10.1002/anie.201505277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touti F.; Lautrette G.; Johnson K. D.; Delaney J. C.; Wollacott A.; Tissire H.; Viswanathan K.; Shriver Z.; Mong S. K.; Mijalis A. J.; Plante O. J.; Pentelute B. L. Antibody-Bactericidal Macrocyclic Peptide Conjugates To Target Gram-Negative Bacteria. ChemBioChem. 2018, 19 (19), 2039–2044. 10.1002/cbic.201800295. [DOI] [PubMed] [Google Scholar]
- Chen L.; Cohen J.; Song X.; Zhao A.; Ye Z.; Feulner C. J.; Doonan P.; Somers W.; Lin L.; Chen P. R. Improved Variants of SrtA for Site-Specific Conjugation on Antibodies and Proteins with High Efficiency. Sci. Rep 2016, 6 (1), 31899. 10.1038/srep31899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang T.; Duarte J. N.; Ling J.; Li Z.; Guzman J. S.; Ploegh H. L. Structurally Defined ΑMHC-II Nanobody-Drug Conjugates: A Therapeutic and Imaging System for B-Cell Lymphoma. Angew. Chem., Int. Ed. 2016, 55 (7), 2416–2420. 10.1002/anie.201509432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massa S.; Vikani N.; Betti C.; Ballet S.; Vanderhaegen S.; Steyaert J.; Descamps B.; Vanhove C.; Bunschoten A.; van Leeuwen F. W. B.; Hernot S.; Caveliers V.; Lahoutte T.; Muyldermans S.; Xavier C.; Devoogdt N. Sortase A-Mediated Site-Specific Labeling of Camelid Single-Domain Antibody-Fragments: A Versatile Strategy for Multiple Molecular Imaging Modalities. Contrast Media Mol. Imaging 2016, 11 (5), 328–339. 10.1002/cmmi.1696. [DOI] [PubMed] [Google Scholar]
- Wagner K.; Kwakkenbos M. J.; Claassen Y. B.; Maijoor K.; Böhne M.; van der Sluijs K. F.; Witte M. D.; van Zoelen D. J.; Cornelissen L. A.; Beaumont T.; Bakker A. Q.; Ploegh H. L.; Spits H. Bispecific Antibody Generated with Sortase and Click Chemistry Has Broad Antiinfluenza Virus Activity. Proc. Natl. Acad. Sci. U. S. A. 2014, 111 (47), 16820–16825. 10.1073/pnas.1408605111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beerli R. R.; Hell T.; Merkel A. S.; Grawunder U. Sortase Enzyme-Mediated Generation of Site-Specifically Conjugated Antibody Drug Conjugates with High In Vitro and In Vivo Potency. PLoS One 2015, 10 (7), e0131177 10.1371/journal.pone.0131177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefan N.; Gébleux R.; Waldmeier L.; Hell T.; Escher M.; Wolter F. I.; Grawunder U.; Beerli R. R. Highly Potent, Anthracycline-Based Antibody–Drug Conjugates Generated by Enzymatic, Site-Specific Conjugation. Mol. Cancer Ther 2017, 16 (5), 879–892. 10.1158/1535-7163.MCT-16-0688. [DOI] [PubMed] [Google Scholar]
- Kornberger P.; Skerra A. Sortase-Catalyzed in Vitro Functionalization of a HER2-Specific Recombinant Fab for Tumor Targeting of the Plant Cytotoxin Gelonin. MAbs 2014, 6 (2), 354–366. 10.4161/mabs.27444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swee L. K.; Guimaraes C. P.; Sehrawat S.; Spooner E.; Barrasa M. I.; Ploegh H. L. Sortase-Mediated Modification of ΑDEC205 Affords Optimization of Antigen Presentation and Immunization against a Set of Viral Epitopes. Proc. Natl. Acad. Sci. U. S. A. 2013, 110 (4), 1428–1433. 10.1073/pnas.1214994110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmand T. J.; Bousbaine D.; Chan A.; Zhang X.; Liu D. R.; Tam J. P.; Ploegh H. L. One-Pot Dual Labeling of IgG 1 and Preparation of C-to-C Fusion Proteins Through a Combination of Sortase A and Butelase 1. Bioconjug Chem. 2018, 29 (10), 3245–3249. 10.1021/acs.bioconjchem.8b00563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirakawa H.; Ishikawa S.; Nagamune T. Ca 2+ -Independent Sortase-A Exhibits High Selective Protein Ligation Activity in the Cytoplasm of Escherichia Coli. Biotechnol J. 2015, 10 (9), 1487–1492. 10.1002/biot.201500012. [DOI] [PubMed] [Google Scholar]
- Chen I.; Dorr B. M.; Liu D. R. A General Strategy for the Evolution of Bond-Forming Enzymes Using Yeast Display. Proc. Natl. Acad. Sci. U. S. A. 2011, 108 (28), 11399–11404. 10.1073/pnas.1101046108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang R.; Wong Y. H.; Nguyen G. K. T.; Tam J. P.; Lescar J.; Wu B. Engineering a Catalytically Efficient Recombinant Protein Ligase. J. Am. Chem. Soc. 2017, 139 (15), 5351–5358. 10.1021/jacs.6b12637. [DOI] [PubMed] [Google Scholar]
- Nguyen G. K. T.; Wang S.; Qiu Y.; Hemu X.; Lian Y.; Tam J. P. Butelase 1 Is an Asx-Specific Ligase Enabling Peptide Macrocyclization and Synthesis. Nat. Chem. Biol. 2014, 10 (9), 732–738. 10.1038/nchembio.1586. [DOI] [PubMed] [Google Scholar]
- Harris K. S.; Durek T.; Kaas Q.; Poth A. G.; Gilding E. K.; Conlan B. F.; Saska I.; Daly N. L.; van der Weerden N. L.; Craik D. J.; Anderson M. A. Efficient Backbone Cyclization of Linear Peptides by a Recombinant Asparaginyl Endopeptidase. Nat. Commun. 2015, 6 (1), 10199. 10.1038/ncomms10199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Policarpo R. L.; Kang H.; Liao X.; Rabideau A. E.; Simon M. D.; Pentelute B. L. Flow-Based Enzymatic Ligation by Sortase A. Angew. Chem., Int. Ed. 2014, 53 (35), 9203–9208. 10.1002/anie.201403582. [DOI] [PubMed] [Google Scholar]
- Williamson D. J.; Fascione M. A.; Webb M. E.; Turnbull W. B. Efficient N-Terminal Labeling of Proteins by Use of Sortase. Angew. Chem., Int. Ed. 2012, 51 (37), 9377–9380. 10.1002/anie.201204538. [DOI] [PubMed] [Google Scholar]
- Nguyen G. K. T.; Cao Y.; Wang W.; Liu C. F.; Tam J. P. Site-Specific N-Terminal Labeling of Peptides and Proteins Using Butelase 1 and Thiodepsipeptide. Angew. Chem., Int. Ed. 2015, 54 (52), 15694–15698. 10.1002/anie.201506810. [DOI] [PubMed] [Google Scholar]
- Liu F.; Luo E. Y.; Flora D. B.; Mezo A. R. Irreversible Sortase A-Mediated Ligation Driven by Diketopiperazine Formation. J. Org. Chem. 2014, 79 (2), 487–492. 10.1021/jo4024914. [DOI] [PubMed] [Google Scholar]
- Yamamura Y.; Hirakawa H.; Yamaguchi S.; Nagamune T. Enhancement of Sortase A-Mediated Protein Ligation by Inducing a β-Hairpin Structure around the Ligation Site. Chem. Commun. 2011, 47 (16), 4742. 10.1039/c0cc05334a. [DOI] [PubMed] [Google Scholar]
- David Row R.; Roark T. J.; Philip M. C.; Perkins L. L.; Antos J. M. Enhancing the Efficiency of Sortase–Mediated Ligations through Nickel–Peptide Complex Formation. Chem. Commun. 2015, 51 (63), 12548–12551. 10.1039/C5CC04657B. [DOI] [PubMed] [Google Scholar]
- Toplak A.; Nuijens T.; Quaedflieg P. J. L. M.; Wu B.; Janssen D. B. Peptiligase, an Enzyme for Efficient Chemoenzymatic Peptide Synthesis and Cyclization in Water. Adv. Synth Catal 2016, 358 (13), 2140–2147. 10.1002/adsc.201600017. [DOI] [Google Scholar]
- Schmidt M.; Toplak A.; Quaedflieg P. J. L. M.; Ippel H.; Richelle G. J. J.; Hackeng T. M.; van Maarseveen J. H.; Nuijens T. Omniligase-1: A Powerful Tool for Peptide Head-to-Tail Cyclization. Adv. Synth Catal 2017, 359 (12), 2050–2055. 10.1002/adsc.201700314. [DOI] [Google Scholar]
- Lewinska M.; Seitz C.; Skerra A.; Schmidtchen F. P. A Novel Method for the N-Terminal Modification of Native Proteins. Bioconjug Chem. 2004, 15 (2), 231–234. 10.1021/bc034085f. [DOI] [PubMed] [Google Scholar]
- Nuijens T.; Toplak A.; Quaedflieg P. J. L. M.; Drenth J.; Wu B.; Janssen D. B. Engineering a Diverse Ligase Toolbox for Peptide Segment Condensation. Adv. Synth Catal 2016, 358 (24), 4041–4048. 10.1002/adsc.201600774. [DOI] [Google Scholar]
- Lin N.-P.; Zhang Y.; Szabo-Fresnais N.; Chou D. H.-C.. Omniligase-1-Mediated Ligation for Insulin Analog Synthesis in Solution and on Phage Surface. ChemRxiv, November 3, 2021, ver. 2. 10.26434/chemrxiv-2021-mf3bs-v2. [DOI]
- Schmidt M.; Toplak A.; Rozeboom H. J.; Wijma H. J.; Quaedflieg P. J. L. M.; van Maarseveen J. H.; Janssen D. B.; Nuijens T. Design of a Substrate-Tailored Peptiligase Variant for the Efficient Synthesis of Thymosin-α 1. Org. Biomol Chem. 2018, 16 (4), 609–618. 10.1039/C7OB02812A. [DOI] [PubMed] [Google Scholar]
- Weeks A. M.; Wells J. A. Engineering Peptide Ligase Specificity by Proteomic Identification of Ligation Sites. Nat. Chem. Biol. 2018, 14 (1), 50–57. 10.1038/nchembio.2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuijens T.; Toplak A.; van de Meulenreek M. B. A. C.; Schmidt M.; Goldbach M.; Janssen D. B.; Quaedflieg P. J. L. M. Chemo-Enzymatic Peptide Synthesis (CEPS) Using Omniligases and Selective Peptiligases - Efficient Biocatalysts for Assembling Linear and Cyclic Peptides and Protein Conjugates. Chimica Oggi-Chemistry Today 2016, 34 (6A), 16–19. [Google Scholar]
- Thormann M.; Thust S.; Hofmann H.-J.; Bordusa F. Protease-Catalyzed Hydrolysis of Substrate Mimetics (Inverse Substrates): A New Approach Reveals a New Mechanism. Biochemistry 1999, 38 (19), 6056–6062. 10.1021/bi9828425. [DOI] [PubMed] [Google Scholar]
- Liebscher S.; Schöpfel M.; Aumüller T.; Sharkhuukhen A.; Pech A.; Höss E.; Parthier C.; Jahreis G.; Stubbs M. T.; Bordusa F. N-Terminal Protein Modification by Substrate-Activated Reverse Proteolysis. Angew. Chem., Int. Ed. 2014, 53 (11), 3024–3028. 10.1002/anie.201307736. [DOI] [PubMed] [Google Scholar]
- Meyer C.; Liebscher S.; Bordusa F. Selective Coupling of Click Anchors to Proteins via Trypsiligase. Bioconjug Chem. 2016, 27 (1), 47–53. 10.1021/acs.bioconjchem.5b00618. [DOI] [PubMed] [Google Scholar]
- Liebscher S.; Kornberger P.; Fink G.; Trost-Gross E.-M.; Höss E.; Skerra A.; Bordusa F. Derivatization of Antibody Fab Fragments: A Designer Enzyme for Native Protein Modification. ChemBioChem. 2014, 15 (8), 1096–1100. 10.1002/cbic.201400059. [DOI] [PubMed] [Google Scholar]
- Strop P. Versatility of Microbial Transglutaminase. Bioconjug Chem. 2014, 25 (5), 855–862. 10.1021/bc500099v. [DOI] [PubMed] [Google Scholar]
- Jeger S.; Zimmermann K.; Blanc A.; Grünberg J.; Honer M.; Hunziker P.; Struthers H.; Schibli R. Site-Specific and Stoichiometric Modification of Antibodies by Bacterial Transglutaminase. Angew. Chem., Int. Ed. 2010, 49 (51), 9995–9997. 10.1002/anie.201004243. [DOI] [PubMed] [Google Scholar]
- Dennler P.; Bailey L. K.; Spycher P. R.; Schibli R.; Fischer E. Microbial Transglutaminase and C-Myc-Tag: A Strong Couple for the Functionalization of Antibody-Like Protein Scaffolds from Discovery Platforms. ChemBioChem. 2015, 16 (5), 861–867. 10.1002/cbic.201500009. [DOI] [PubMed] [Google Scholar]
- Farias S. E.; Strop P.; Delaria K.; Galindo Casas M.; Dorywalska M.; Shelton D. L.; Pons J.; Rajpal A. Mass Spectrometric Characterization of Transglutaminase Based Site-Specific Antibody–Drug Conjugates. Bioconjug Chem. 2014, 25 (2), 240–250. 10.1021/bc4003794. [DOI] [PubMed] [Google Scholar]
- Strop P.; Dorywalska M. G.; Rajpal A.; Shelton D.; Liu S.-H.; Pons J.; Dushin R.. Engineered Polypeptide Conjugates and Methods for Making Thereof Using Transglutaminase. WO2012059882A2, 2012.
- Josten A.; Haalck L.; Spener F.; Meusel M. Use of Microbial Transglutaminase for the Enzymatic Biotinylation of Antibodies. J. Immunol Methods 2000, 240 (1–2), 47–54. 10.1016/S0022-1759(00)00172-1. [DOI] [PubMed] [Google Scholar]
- Dennler P.; Chiotellis A.; Fischer E.; Brégeon D.; Belmant C.; Gauthier L.; Lhospice F.; Romagne F.; Schibli R. Transglutaminase-Based Chemo-Enzymatic Conjugation Approach Yields Homogeneous Antibody–Drug Conjugates. Bioconjug Chem. 2014, 25 (3), 569–578. 10.1021/bc400574z. [DOI] [PubMed] [Google Scholar]
- Strop P.; Delaria K.; Foletti D.; Witt J. M.; Hasa-Moreno A.; Poulsen K.; Galindo Casas M.; Dorywalska M.; Farias S.; Pios A.; Lui V.; Dushin R.; Zhou D.; Navaratnam T.; Tran T.-T.; Sutton J.; Lindquist K. C.; Han B.; Liu S.-H.; Shelton D. L.; Pons J.; Rajpal A. Site-Specific Conjugation Improves Therapeutic Index of Antibody Drug Conjugates with High Drug Loading. Nat. Biotechnol. 2015, 33 (7), 694–696. 10.1038/nbt.3274. [DOI] [PubMed] [Google Scholar]
- Lhospice F.; Brégeon D.; Belmant C.; Dennler P.; Chiotellis A.; Fischer E.; Gauthier L.; Boëdec A.; Rispaud H.; Savard-Chambard S.; Represa A.; Schneider N.; Paturel C.; Sapet M.; Delcambre C.; Ingoure S.; Viaud N.; Bonnafous C.; Schibli R.; Romagné F. Site-Specific Conjugation of Monomethyl Auristatin E to Anti-CD30 Antibodies Improves Their Pharmacokinetics and Therapeutic Index in Rodent Models. Mol. Pharmaceutics 2015, 12 (6), 1863–1871. 10.1021/mp500666j. [DOI] [PubMed] [Google Scholar]
- Mindt T. L.; Jungi V.; Wyss S.; Friedli A.; Pla G.; Novak-Hofer I.; Grünberg J.; Schibli R. Modification of Different IgG1 Antibodies via Glutamine and Lysine Using Bacterial and Human Tissue Transglutaminase. Bioconjug Chem. 2008, 19 (1), 271–278. 10.1021/bc700306n. [DOI] [PubMed] [Google Scholar]
- Spycher P. R.; Amann C. A.; Wehrmüller J. E.; Hurwitz D. R.; Kreis O.; Messmer D.; Ritler A.; Küchler A.; Blanc A.; Béhé M.; Walde P.; Schibli R. Dual, Site-Specific Modification of Antibodies by Using Solid-Phase Immobilized Microbial Transglutaminase. ChemBioChem. 2017, 18 (19), 1923–1927. 10.1002/cbic.201700188. [DOI] [PubMed] [Google Scholar]
- Carrico I. S.; Carlson B. L.; Bertozzi C. R. Introducing Genetically Encoded Aldehydes into Proteins. Nat. Chem. Biol. 2007, 3 (6), 321–322. 10.1038/nchembio878. [DOI] [PubMed] [Google Scholar]
- Preusser-Kunze A.; Mariappan M.; Schmidt B.; Gande S. L.; Mutenda K.; Wenzel D.; von Figura K.; Dierks T. Molecular Characterization of the Human Cα-Formylglycine-Generating Enzyme. J. Biol. Chem. 2005, 280 (15), 14900–14910. 10.1074/jbc.M413383200. [DOI] [PubMed] [Google Scholar]
- Kudirka R.; Barfield R. M.; McFarland J.; Albers A. E.; de Hart G. W.; Drake P. M.; Holder P. G.; Banas S.; Jones L. C.; Garofalo A. W.; Rabuka D. Generating Site-Specifically Modified Proteins via a Versatile and Stable Nucleophilic Carbon Ligation. Chem. Biol. 2015, 22 (2), 293–298. 10.1016/j.chembiol.2014.11.019. [DOI] [PubMed] [Google Scholar]
- Kudirka R. A.; Barfield R. M.; McFarland J. M.; Drake P. M.; Carlson A.; Bañas S.; Zmolek W.; Garofalo A. W.; Rabuka D. Site-Specific Tandem Knoevenagel Condensation–Michael Addition to Generate Antibody–Drug Conjugates. ACS Med. Chem. Lett. 2016, 7 (11), 994–998. 10.1021/acsmedchemlett.6b00253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomplun S.; Mohamed M. Y. H.; Oelschlaegel T.; Wellner C.; Bergmann F. Efficient Pictet-Spengler Bioconjugation with N -Substituted Pyrrolyl Alanine Derivatives. Angew. Chem., Int. Ed. 2019, 58 (11), 3542–3547. 10.1002/anie.201814200. [DOI] [PubMed] [Google Scholar]
- Agarwal P.; Kudirka R.; Albers A. E.; Barfield R. M.; de Hart G. W.; Drake P. M.; Jones L. C.; Rabuka D. Hydrazino-Pictet-Spengler Ligation as a Biocompatible Method for the Generation of Stable Protein Conjugates. Bioconjug Chem. 2013, 24 (6), 846–851. 10.1021/bc400042a. [DOI] [PubMed] [Google Scholar]
- Liu J.; Barfield R. M.; Rabuka D. Site-Specific Bioconjugation Using SMARTag® Technology: A Practical and Effective Chemoenzymatic Approach to Generate Antibody–Drug Conjugates. Bioconjugation 2019, 2033, 131–147. 10.1007/978-1-4939-9654-4_10. [DOI] [PubMed] [Google Scholar]
- Boschanski M.; Krüger T.; Karsten L.; Falck G.; Alam S.; Gerlach M.; Müller B.; Müller K. M.; Sewald N.; Dierks T. Site-Specific Conjugation Strategy for Dual Antibody–Drug Conjugates Using Aerobic Formylglycine-Generating Enzymes. Bioconjug Chem. 2021, 32 (6), 1167–1174. 10.1021/acs.bioconjchem.1c00246. [DOI] [PubMed] [Google Scholar]
- Drake P. M.; Albers A. E.; Baker J.; Banas S.; Barfield R. M.; Bhat A. S.; de Hart G. W.; Garofalo A. W.; Holder P.; Jones L. C.; Kudirka R.; McFarland J.; Zmolek W.; Rabuka D. Aldehyde Tag Coupled with HIPS Chemistry Enables the Production of ADCs Conjugated Site-Specifically to Different Antibody Regions with Distinct in Vivo Efficacy and PK Outcomes. Bioconjug Chem. 2014, 25 (7), 1331–1341. 10.1021/bc500189z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janson N.; Krüger T.; Karsten L.; Boschanski M.; Dierks T.; Müller K. M.; Sewald N. Bifunctional Reagents for Formylglycine Conjugation: Pitfalls and Breakthroughs. ChemBioChem. 2020, 21 (24), 3580–3593. 10.1002/cbic.202000416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Ilizaliturri F. J.Study of TRPH-222 in Patients With Relapsed and/or Refractory B-Cell Lymphoma. https://clinicaltrials.gov/ct2/show/NCT03682796 (accessed 2022-12-20).
- Podracky C. J.; An C.; DeSousa A.; Dorr B. M.; Walsh D. M.; Liu D. R. Laboratory Evolution of a Sortase Enzyme That Modifies Amyloid-β Protein. Nat. Chem. Biol. 2021, 17 (3), 317–325. 10.1038/s41589-020-00706-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorr B. M.; Ham H. O.; An C.; Chaikof E. L.; Liu D. R. Reprogramming the Specificity of Sortase Enzymes. Proc. Natl. Acad. Sci. U. S. A. 2014, 111 (37), 13343–13348. 10.1073/pnas.1411179111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuberbühler K.; Casi G.; Bernardes G. J. L.; Neri D. Fucose-Specific Conjugation of Hydrazide Derivatives to a Vascular-Targeting Monoclonal Antibody in IgG Format. Chem. Commun. 2012, 48 (56), 7100–7102. 10.1039/c2cc32412a. [DOI] [PubMed] [Google Scholar]
- Chua M.-M.; Fan S.-T.; Karush F. Attachment of Immunoglobulin to Liposomal Membrane via Protein Carbohydrate. Biochimica et Biophysica Acta (BBA) - General Subjects 1984, 800 (3), 291–300. 10.1016/0304-4165(84)90408-2. [DOI] [PubMed] [Google Scholar]
- Raju T. S.; Briggs J. B.; Borge S. M.; Jones A. J. S. Species-Specific Variation in Glycosylation of IgG: Evidence for the Species-Specific Sialylation and Branch-Specific Galactosylation and Importance for Engineering Recombinant Glycoprotein Therapeutics. Glycobiology 2000, 10 (5), 477–486. 10.1093/glycob/10.5.477. [DOI] [PubMed] [Google Scholar]
- Rich J. R.; Withers S. G. Emerging Methods for the Production of Homogeneous Human Glycoproteins. Nat. Chem. Biol. 2009, 5 (4), 206–215. 10.1038/nchembio.148. [DOI] [PubMed] [Google Scholar]
- Zhou Q.; Stefano J. E.; Manning C.; Kyazike J.; Chen B.; Gianolio D. A.; Park A.; Busch M.; Bird J.; Zheng X.; Simonds-Mannes H.; Kim J.; Gregory R. C.; Miller R. J.; Brondyk W. H.; Dhal P. K.; Pan C. Q. Site-Specific Antibody–Drug Conjugation through Glycoengineering. Bioconjug Chem. 2014, 25 (3), 510–520. 10.1021/bc400505q. [DOI] [PubMed] [Google Scholar]
- Angelastro A.; Barkhanskiy A.; Mattey A. P.; Pallister E. G.; Spiess R.; Goundry W.; Barran P.; Flitsch S. L. Galactose Oxidase Enables Modular Assembly of Conjugates from Native Antibodies with High Drug-to-Antibody Ratios. ChemSusChem 2022, 15 (9), e202102592. 10.1002/cssc.202102592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Geel R.; Wijdeven M. A.; Heesbeen R.; Verkade J. M. M.; Wasiel A. A.; van Berkel S. S.; van Delft F. L. Chemoenzymatic Conjugation of Toxic Payloads to the Globally Conserved N-Glycan of Native MAbs Provides Homogeneous and Highly Efficacious Antibody–Drug Conjugates. Bioconjug Chem. 2015, 26 (11), 2233–2242. 10.1021/acs.bioconjchem.5b00224. [DOI] [PubMed] [Google Scholar]
- Verkade J. M. M.; Wijdeven M. A.; van Geel R.; Janssen B. M. G.; van Berkel S. S.; van Delft F. L. A Polar Sulfamide Spacer Significantly Enhances the Manufacturability, Stability, and Therapeutic Index of Antibody–Drug Conjugates. Antibodies 2018, 7 (1), 12. 10.3390/antib7010012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruins J. J.; Westphal A. H.; Albada B.; Wagner K.; Bartels L.; Spits H.; van Berkel W. J. H.; van Delft F. L. Inducible, Site-Specific Protein Labeling by Tyrosine Oxidation–Strain-Promoted (4 + 2) Cycloaddition. Bioconjug Chem. 2017, 28 (4), 1189–1193. 10.1021/acs.bioconjchem.7b00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato S.; Matsumura M.; Kadonosono T.; Abe S.; Ueno T.; Ueda H.; Nakamura H. Site-Selective Protein Chemical Modification of Exposed Tyrosine Residues Using Tyrosine Click Reaction. Bioconjug Chem. 2020, 31 (5), 1417. 10.1021/acs.bioconjchem.0c00120. [DOI] [PubMed] [Google Scholar]
- Lee J.; Choi H.-J.; Yun M.; Kang Y.; Jung J.-E.; Ryu Y.; Kim T. Y.; Cha Y.; Cho H.-S.; Min J.-J.; Chung C.-W.; Kim H.-S. Enzymatic Prenylation and Oxime Ligation for the Synthesis of Stable and Homogeneous Protein-Drug Conjugates for Targeted Therapy. Angew. Chem., Int. Ed. 2015, 54 (41), 12020–12024. 10.1002/anie.201505964. [DOI] [PubMed] [Google Scholar]
- Lee B. ill; Park M.-H.; Byeon J.-J.; Shin S.-H.; Choi J.; Park Y.; Park Y.-H.; Chae J.; Shin Y. G. Quantification of an Antibody-Conjugated Drug in Fat Plasma by an Affinity Capture LC-MS/MS Method for a Novel Prenyl Transferase-Mediated Site-Specific Antibody–Drug Conjugate. Molecules 2020, 25 (7), 1515. 10.3390/molecules25071515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashidian M.; Kumarapperuma S. C.; Gabrielse K.; Fegan A.; Wagner C. R.; Distefano M. D. Simultaneous Dual Protein Labeling Using a Triorthogonal Reagent. J. Am. Chem. Soc. 2013, 135 (44), 16388–16396. 10.1021/ja403813b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grünewald J.; Klock H. E.; Cellitti S. E.; Bursulaya B.; McMullan D.; Jones D. H.; Chiu H.-P.; Wang X.; Patterson P.; Zhou H.; Vance J.; Nigoghossian E.; Tong H.; Daniel D.; Mallet W.; Ou W.; Uno T.; Brock A.; Lesley S. A.; Geierstanger B. H. Efficient Preparation of Site-Specific Antibody–Drug Conjugates Using Phosphopantetheinyl Transferases. Bioconjug Chem. 2015, 26 (12), 2554–2562. 10.1021/acs.bioconjchem.5b00558. [DOI] [PubMed] [Google Scholar]
- Hofmann R.; Akimoto G.; Wucherpfennig T. G.; Zeymer C.; Bode J. W. Lysine Acylation Using Conjugating Enzymes for Site-Specific Modification and Ubiquitination of Recombinant Proteins. Nat. Chem. 2020, 12 (11), 1008–1015. 10.1038/s41557-020-0528-y. [DOI] [PubMed] [Google Scholar]
- Schumacher D.; Helma J.; Mann F. A.; Pichler G.; Natale F.; Krause E.; Cardoso M. C.; Hackenberger C. P. R.; Leonhardt H. Versatile and Efficient Site-Specific Protein Functionalization by Tubulin Tyrosine Ligase. Angew. Chem., Int. Ed. 2015, 54 (46), 13787–13791. 10.1002/anie.201505456. [DOI] [PubMed] [Google Scholar]
- Schumacher D.; Lemke O.; Helma J.; Gerszonowicz L.; Waller V.; Stoschek T.; Durkin P. M.; Budisa N.; Leonhardt H.; Keller B. G.; Hackenberger C. P. R. Broad Substrate Tolerance of Tubulin Tyrosine Ligase Enables One-Step Site-Specific Enzymatic Protein Labeling. Chem. Sci. 2017, 8 (5), 3471–3478. 10.1039/C7SC00574A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolzati C.; Spolaore B. Enzymatic Methods for the Site-Specific Radiolabeling of Targeting Proteins. Molecules 2021, 26 (12), 3492. 10.3390/molecules26123492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crauwels M.; Massa S.; Martin C.; Betti C.; Ballet S.; Devoogdt N.; Xavier C.; Muyldermans S. Site-Specific Radioactive Labeling of Nanobodies. Methods Mol. Biol. 2018, 505–540. 10.1007/978-1-4939-8648-4_26. [DOI] [PubMed] [Google Scholar]
- Paterson B. M.; Alt K.; Jeffery C. M.; Price R. I.; Jagdale S.; Rigby S.; Williams C. C.; Peter K.; Hagemeyer C. E.; Donnelly P. S. Enzyme-Mediated Site-Specific Bioconjugation of Metal Complexes to Proteins: Sortase-Mediated Coupling of Copper-64 to a Single-Chain Antibody. Angew. Chem., Int. Ed. 2014, 53 (24), 6115–6119. 10.1002/anie.201402613. [DOI] [PubMed] [Google Scholar]
- Pritz S.; Wolf Y.; Kraetke O.; Klose J.; Bienert M.; Beyermann M. Synthesis of Biologically Active Peptide Nucleic Acid–Peptide Conjugates by Sortase-Mediated Ligation. J. Org. Chem. 2007, 72 (10), 3909–3912. 10.1021/jo062331l. [DOI] [PubMed] [Google Scholar]
- Zeglis B. M.; Davis C. B.; Aggeler R.; Kang H. C.; Chen A.; Agnew B. J.; Lewis J. S. Enzyme-Mediated Methodology for the Site-Specific Radiolabeling of Antibodies Based on Catalyst-Free Click Chemistry. Bioconjug Chem. 2013, 24 (6), 1057–1067. 10.1021/bc400122c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartels L.; Ploegh H. L.; Spits H.; Wagner K. Preparation of Bispecific Antibody-Protein Adducts by Site-Specific Chemo-Enzymatic Conjugation. Methods 2019, 154, 93–101. 10.1016/j.ymeth.2018.07.013. [DOI] [PubMed] [Google Scholar]
- Bartels L.; de Jong G.; Gillissen M. A.; Yasuda E.; Kattler V.; Bru C.; Fatmawati C.; van Hal-van Veen S. E.; Cercel M. G.; Moiset G.; Bakker A. Q.; van Helden P. M.; Villaudy J.; Hazenberg M. D.; Spits H.; Wagner K. A Chemo-Enzymatically Linked Bispecific Antibody Retargets T Cells to a Sialylated Epitope on CD43 in Acute Myeloid Leukemia. Cancer Res. 2019, 79 (13), 3372–3382. 10.1158/0008-5472.CAN-18-0189. [DOI] [PubMed] [Google Scholar]
- Andres F.; Schwill M.; Boersma Y. L.; Plückthun A. High-Throughput Generation of Bispecific Binding Proteins by Sortase A–Mediated Coupling for Direct Functional Screening in Cell Culture. Mol. Cancer Ther 2020, 19 (4), 1080. 10.1158/1535-7163.MCT-19-0633. [DOI] [PubMed] [Google Scholar]
- Laursen N. S.; Friesen R. H. E.; Zhu X.; Jongeneelen M.; Blokland S.; Vermond J.; van Eijgen A.; Tang C.; van Diepen H.; Obmolova G.; van der Neut Kolfschoten M.; Zuijdgeest D.; Straetemans R.; Hoffman R. M. B.; Nieusma T.; Pallesen J.; Turner H. L.; Bernard S. M.; Ward A. B.; Luo J.; Poon L. L. M.; Tretiakova A. P.; Wilson J. M.; Limberis M. P.; Vogels R.; Brandenburg B.; Kolkman J. A.; Wilson I. A. Universal Protection against Influenza Infection by a Multidomain Antibody to Influenza Hemagglutinin. Science (1979) 2018, 362 (6414), 598–602. 10.1126/science.aaq0620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakase I.; Ueno N.; Katayama M.; Noguchi K.; Takatani-Nakase T.; Kobayashi N. B.; Yoshida T.; Fujii I.; Futaki S. Receptor Clustering and Activation by Multivalent Interaction through Recognition Peptides Presented on Exosomes. Chem. Commun. 2017, 53 (2), 317–320. 10.1039/C6CC06719K. [DOI] [PubMed] [Google Scholar]
- Mammen M.; Choi S.-K.; Whitesides G. M. Polyvalent Interactions in Biological Systems: Implications for Design and Use of Multivalent Ligands and Inhibitors. Angew. Chem., Int. Ed. 1998, 2 (37), 2754–2794. 10.1002/(sici)1521-3773(19981102)37:20%3C2754::aid-anie2754%3E3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Moliner-Morro A.; Sheward D. J.; Karl V.; Perez Vidakovics L.; Murrell B.; McInerney G. M.; Hanke L. Picomolar SARS-CoV-2 Neutralization Using Multi-Arm PEG Nanobody Constructs. Biomolecules 2020, 10 (12), 1661. 10.3390/biom10121661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta K.; Singh S.; Gupta K.; Khan N.; Sehgal D.; Haridas V.; Roy R. P. A Bioorthogonal Chemoenzymatic Strategy for Defined Protein Dendrimer Assembly. ChemBioChem. 2012, 13 (17), 2489–2494. 10.1002/cbic.201200559. [DOI] [PubMed] [Google Scholar]
- Singh S.; Gupta K.; Shukla S.; Sampathkumar S.-G.; Roy R. P. Sortase-Click Strategy for Defined Protein Conjugation on a Heptavalent Cyclodextrin Scaffold. PLoS One 2019, 14 (5), e0217369 10.1371/journal.pone.0217369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minamihata K.; Goto M.; Kamiya N. Site-Specific Conjugation of an Antibody-Binding Protein Catalyzed by Horseradish Peroxidase Creates a Multivalent Protein Conjugate with High Affinity to IgG. Biotechnol J. 2015, 10 (1), 222–226. 10.1002/biot.201400512. [DOI] [PubMed] [Google Scholar]
- Minamihata K.; Yamaguchi S.; Nakajima K.; Nagamune T. Tyrosine Coupling Creates a Hyperbranched Multivalent Protein Polymer Using Horseradish Peroxidase via Bipolar Conjugation Points. Bioconjug Chem. 2016, 27 (5), 1348–1359. 10.1021/acs.bioconjchem.6b00138. [DOI] [PubMed] [Google Scholar]
- Approved Cellular and Gene Therapy Products. https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products/approved-cellular-and-gene-therapy-products (accessed 2022-11-08).
- Wang D.; Tai P. W. L.; Gao G. Adeno-Associated Virus Vector as a Platform for Gene Therapy Delivery. Nat. Rev. Drug Discov 2019, 18 (5), 358–378. 10.1038/s41573-019-0012-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ongoing clinical trials with AAV. https://clinicaltrials.gov/ct2/results?cond=&term=AAV&cntry=&state=&city=&dist= (accessed 2022-11-08).
- Srivastava A.; Mallela K. M. G.; Deorkar N.; Brophy G. Manufacturing Challenges and Rational Formulation Development for AAV Viral Vectors. J. Pharm. Sci. 2021, 110 (7), 2609–2624. 10.1016/j.xphs.2021.03.024. [DOI] [PubMed] [Google Scholar]
- Büning H.; Srivastava A. Capsid Modifications for Targeting and Improving the Efficacy of AAV Vectors. Mol. Ther Methods Clin Dev 2019, 12, 248–265. 10.1016/j.omtm.2019.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam A. K.; Frabutt D.; Li L.; Xiao W. Chemical Modifications of the Capsid for Redirecting and Improving the Efficacy of Adeno-Associated Virus Vectors. Hum. Gene Ther. 2021, 32 (23–24), 1433–1438. 10.1089/hum.2021.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y.; Fang Y.; Zhou Y.; Zandi E.; Lee C.-L.; Joo K.-I.; Wang P. Site-Specific Modification of Adeno-Associated Viruses via a Genetically Engineered Aldehyde Tag. Small 2013, 9 (3), 421–429. 10.1002/smll.201201661. [DOI] [PubMed] [Google Scholar]
- Rabuka D.; Rush J. S.; deHart G. W.; Wu P.; Bertozzi C. R. Site-Specific Chemical Protein Conjugation Using Genetically Encoded Aldehyde Tags. Nat. Protoc 2012, 7 (6), 1052–1067. 10.1038/nprot.2012.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce H. A.; Qian H.; Connell T. U.; Huang D.; Gottstein C.; Donnelly P. S.; Peter K.; Gregorevic P.; Hagemeyer C. E. Site-Specific Glycation and Chemo-Enzymatic Antibody Sortagging for the Retargeting of RAAV6 to Inflamed Endothelium. Mol. Ther Methods Clin Dev 2019, 14, 261–269. 10.1016/j.omtm.2019.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold G. S.; Sasser A. K.; Stachler M. D.; Bartlett J. S. Metabolic Biotinylation Provides a Unique Platform for the Purification and Targeting of Multiple AAV Vector Serotypes. Molecular Therapy 2006, 14 (1), 97–106. 10.1016/j.ymthe.2006.02.014. [DOI] [PubMed] [Google Scholar]
- Stachler M. D.; Chen I.; Ting A. Y.; Bartlett J. S. Site-Specific Modification of AAV Vector Particles With Biophysical Probes and Targeting Ligands Using Biotin Ligase. Molecular Therapy 2008, 16 (8), 1467–1473. 10.1038/mt.2008.129. [DOI] [PubMed] [Google Scholar]
- Lehtolainen P.; Taskinen A.; Laukkanen J.; Airenne K. J.; Heino S.; Lappalainen M.; Ojala K.; Marjomäki V.; Martin J. F.; Kulomaa M. S.; Ylä-Herttuala S. Cloning and Characterization of Scavidin, a Fusion Protein for the Targeted Delivery of Biotinylated Molecules. J. Biol. Chem. 2002, 277 (10), 8545–8550. 10.1074/jbc.M109431200. [DOI] [PubMed] [Google Scholar]
- Kulkarni J. A.; Witzigmann D.; Thomson S. B.; Chen S.; Leavitt B. R.; Cullis P. R.; van der Meel R. The Current Landscape of Nucleic Acid Therapeutics. Nat. Nanotechnol 2021, 16 (6), 630–643. 10.1038/s41565-021-00898-0. [DOI] [PubMed] [Google Scholar]
- Rossi J. J.; Rossi D. J. SiRNA Drugs: Here to Stay. Molecular Therapy 2021, 29 (2), 431–432. 10.1016/j.ymthe.2021.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zogg H.; Singh R.; Ro S. Current Advances in RNA Therapeutics for Human Diseases. Int. J. Mol. Sci. 2022, 23 (5), 2736. 10.3390/ijms23052736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geary R. S.; Norris D.; Yu R.; Bennett C. F. Pharmacokinetics, Biodistribution and Cell Uptake of Antisense Oligonucleotides. Adv. Drug Deliv Rev. 2015, 87, 46–51. 10.1016/j.addr.2015.01.008. [DOI] [PubMed] [Google Scholar]
- Wan W. B.; Seth P. P. The Medicinal Chemistry of Therapeutic Oligonucleotides. J. Med. Chem. 2016, 59 (21), 9645–9667. 10.1021/acs.jmedchem.6b00551. [DOI] [PubMed] [Google Scholar]
- Dovgan I.; Koniev O.; Kolodych S.; Wagner A. Antibody–Oligonucleotide Conjugates as Therapeutic, Imaging, and Detection Agents. Bioconjug Chem. 2019, 30 (10), 2483–2501. 10.1021/acs.bioconjchem.9b00306. [DOI] [PubMed] [Google Scholar]
- Yeo J. E.; Wickramaratne S.; Khatwani S.; Wang Y.-C.; Vervacke J.; Distefano M. D.; Tretyakova N. Y. Synthesis of Site-Specific DNA–Protein Conjugates and Their Effects on DNA Replication. ACS Chem. Biol. 2014, 9 (8), 1860–1868. 10.1021/cb5001795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabricius V.; Lefèbre J.; Geertsema H.; Marino S. F.; Ewers H. Rapid and Efficient C-Terminal Labeling of Nanobodies for DNA-PAINT. J. Phys. D Appl. Phys. 2018, 51 (47), 474005. 10.1088/1361-6463/aae0e2. [DOI] [Google Scholar]
- Anhäuser L.; Hüwel S.; Zobel T.; Rentmeister A. Multiple Covalent Fluorescence Labeling of Eukaryotic MRNA at the Poly(A) Tail Enhances Translation and Can. Be Performed in Living Cells. Nucleic Acids Res. 2019, 47 (7), e42–e42. 10.1093/nar/gkz084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dülmen M.; Muthmann N.; Rentmeister A. Chemo-Enzymatic Modification of the 5′ Cap Maintains Translation and Increases Immunogenic Properties of MRNA. Angew. Chem., Int. Ed. 2021, 60 (24), 13280–13286. 10.1002/anie.202100352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao C.; Seebeck F. P. S-Adenosylhomocysteine as a Methyl Transfer Catalyst in Biocatalytic Methylation Reactions. Nat. Catal 2019, 2 (8), 696–701. 10.1038/s41929-019-0300-0. [DOI] [Google Scholar]
- Shi Y.; Wu Y.; Yu J.; Zhang W.; Zhuang C. DNA-Encoded Libraries (DELs): A Review of on-DNA Chemistries and Their Output. RSC Adv. 2021, 11 (4), 2359–2376. 10.1039/D0RA09889B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas B.; Lu X.; Birmingham W. R.; Huang K.; Both P.; Reyes Martinez J. E.; Young R. J.; Davie C. P.; Flitsch S. L. Application of Biocatalysis to On-DNA Carbohydrate Library Synthesis. ChemBioChem. 2017, 18 (9), 858–863. 10.1002/cbic.201600678. [DOI] [PubMed] [Google Scholar]
- Huggins I. J.; Medina C. A.; Springer A. D.; van den Berg A.; Jadhav S.; Cui X.; Dowdy S. F. Site Selective Antibody-Oligonucleotide Conjugation via Microbial Transglutaminase. Molecules 2019, 24 (18), 3287. 10.3390/molecules24183287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen R.; David M.; Khrestchatisky M.. Transferrin Receptor-Binding Molecules, Conjugates Thereof and Their Uses. WO2020144233A1, 2020.
- Tominaga J.; Kemori Y.; Tanaka Y.; Maruyama T.; Kamiya N.; Goto M. An Enzymatic Method for Site-Specific Labeling of Recombinant Proteins with Oligonucleotides. Chem. Commun. 2007, (No. 4), 401. 10.1039/B613793H. [DOI] [PubMed] [Google Scholar]
- Kitaoka M.; Tsuruda Y.; Tanaka Y.; Goto M.; Mitsumori M.; Hayashi K.; Hiraishi Y.; Miyawaki K.; Noji S.; Kamiya N. Transglutaminase-Mediated Synthesis of a DNA–(Enzyme) n Probe for Highly Sensitive DNA Detection. Chemistry – A European Journal 2011, 17 (19), 5387–5392. 10.1002/chem.201003744. [DOI] [PubMed] [Google Scholar]
- Takahara M.; Hayashi K.; Goto M.; Kamiya N. Tailing DNA Aptamers with a Functional Protein by Two-Step Enzymatic Reaction. J. Biosci Bioeng 2013, 116 (6), 660–665. 10.1016/j.jbiosc.2013.05.025. [DOI] [PubMed] [Google Scholar]
- Takahara M.; Wakabayashi R.; Minamihata K.; Goto M.; Kamiya N. Primary Amine-Clustered DNA Aptamer for DNA–Protein Conjugation Catalyzed by Microbial Transglutaminase. Bioconjug Chem. 2017, 28 (12), 2954–2961. 10.1021/acs.bioconjchem.7b00594. [DOI] [PubMed] [Google Scholar]
- Chua J. P. S.; Go M. K.; Osothprarop T.; Mcdonald S.; Karabadzhak A. G.; Yew W. S.; Peisajovich S.; Nirantar S. Evolving a Thermostable Terminal Deoxynucleotidyl Transferase. ACS Synth. Biol. 2020, 9 (7), 1725–1735. 10.1021/acssynbio.0c00078. [DOI] [PubMed] [Google Scholar]
- Thomas C.; Rusanov T.; Hoang T.; Augustin T.; Kent T.; Gaspar I.; Pomerantz R. T. One-Step Enzymatic Modification of RNA 3′ Termini Using Polymerase θ. Nucleic Acids Res. 2019, 47 (7), 3272–3283. 10.1093/nar/gkz029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crameri A.; Hill M. L.; Lovelock S. L.; Schober M.; Tew D. G.; Thomas P. J.. Novel Processes for the Production of Oligonucleotides. WO2018011067A3, 2018.
- Wang X.; Zhang J.; Li Y.; Chen G.; Wang X. Enzymatic Synthesis of Modified Oligonucleotides by PEAR Using Phusion and KOD DNA Polymerases. Nucleic Acid Ther 2015, 25 (1), 27–34. 10.1089/nat.2014.0513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen M. A.; Davis R. W. Template-Independent Enzymatic Oligonucleotide Synthesis (TiEOS): Its History, Prospects, and Challenges. Biochemistry 2018, 57 (12), 1821–1832. 10.1021/acs.biochem.7b00937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palluk S.; Arlow D. H.; de Rond T.; Barthel S.; Kang J. S.; Bector R.; Baghdassarian H. M.; Truong A. N.; Kim P. W.; Singh A. K.; Hillson N. J.; Keasling J. D. De Novo DNA Synthesis Using Polymerase-Nucleotide Conjugates. Nat. Biotechnol. 2018, 36 (7), 645–650. 10.1038/nbt.4173. [DOI] [PubMed] [Google Scholar]
- Mathews A. S.; Yang H.; Montemagno C. 3′- O -Caged 2′-Deoxynucleoside Triphosphates for Light-Mediated, Enzyme-Catalyzed, Template-Independent DNA Synthesis. Curr. Protoc Nucleic Acid Chem. 2017, 71 (1), 13.17.1. 10.1002/cpnc.41. [DOI] [PubMed] [Google Scholar]
- Mathews A. S.; Yang H.; Montemagno C. Photo-Cleavable Nucleotides for Primer Free Enzyme Mediated DNA Synthesis. Org. Biomol Chem. 2016, 14 (35), 8278–8288. 10.1039/C6OB01371F. [DOI] [PubMed] [Google Scholar]
- Hoff K.; Halpain M.; Garbagnati G.; Edwards J.; Zhou W.. Rapid and Dynamic Nucleic Acid Hybridization Enables Enzymatic Oligonucleotide Synthesis by Cyclic Reversible Termination: A Novel Mechanism for Enzymatic DNA Synthesis. bioRxiv, April 15, 2019, ver. 1. 10.1101/561092. [DOI]
- Huffman M. A.; Fryszkowska A.; Alvizo O.; Borra-Garske M.; Campos K. R.; Canada K. A.; Devine P. N.; Duan D.; Forstater J. H.; Grosser S. T.; Halsey H. M.; Hughes G. J.; Jo J.; Joyce L. A.; Kolev J. N.; Liang J.; Maloney K. M.; Mann B. F.; Marshall N. M.; McLaughlin M.; Moore J. C.; Murphy G. S.; Nawrat C. C.; Nazor J.; Novick S.; Patel N. R.; Rodriguez-Granillo A.; Robaire S. A.; Sherer E. C.; Truppo M. D.; Whittaker A. M.; Verma D.; Xiao L.; Xu Y.; Yang H. Design of an in Vitro Biocatalytic Cascade for the Manufacture of Islatravir. Science (1979) 2019, 366 (6470), 1255–1259. 10.1126/science.aay8484. [DOI] [PubMed] [Google Scholar]
- McIntosh J. A.; Liu Z.; Andresen B. M.; Marzijarani N. S.; Moore J. C.; Marshall N. M.; Borra-Garske M.; Obligacion J. v.; Fier P. S.; Peng F.; Forstater J. H.; Winston M. S.; An C.; Chang W.; Lim J.; Huffman M. A.; Miller S. P.; Tsay F.-R.; Altman M. D.; Lesburg C. A.; Steinhuebel D.; Trotter B. W.; Cumming J. N.; Northrup A.; Bu X.; Mann B. F.; Biba M.; Hiraga K.; Murphy G. S.; Kolev J. N.; Makarewicz A.; Pan W.; Farasat I.; Bade R. S.; Stone K.; Duan D.; Alvizo O.; Adpressa D.; Guetschow E.; Hoyt E.; Regalado E. L.; Castro S.; Rivera N.; Smith J. P.; Wang F.; Crespo A.; Verma D.; Axnanda S.; Dance Z. E. X.; Devine P. N.; Tschaen D.; Canada K. A.; Bulger P. G.; Sherry B. D.; Truppo M. D.; Ruck R. T.; Campeau L.-C.; Bennett D. J.; Humphrey G. R.; Campos K. R.; Maddess M. L.. A Kinase-CGAS Cascade to Synthesize a Therapeutic STING Activator. Nature 2022, 603 ( (7901), ). 439. 10.1038/s41586-022-04422-9. [DOI] [PubMed] [Google Scholar]
- Appel M. J.; Bertozzi C. R. Formylglycine, a Post-Translationally Generated Residue with Unique Catalytic Capabilities and Biotechnology Applications. ACS Chem. Biol. 2015, 10 (1), 72–84. 10.1021/cb500897w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki C. M.; Yamaguchi A.; Anami Y.; Xiong W.; Otani Y.; Lee J.; Ueno N. T.; Zhang N.; An Z.; Tsuchikama K. Antibody-Drug Conjugates with Dual Payloads for Combating Breast Tumor Heterogeneity and Drug Resistance. Nat. Commun. 2021, 12 (1), 3528. 10.1038/s41467-021-23793-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valeur E.; Guéret S. M.; Adihou H.; Gopalakrishnan R.; Lemurell M.; Waldmann H.; Grossmann T. N.; Plowright A. T. New Modalities for Challenging Targets in Drug Discovery. Angew. Chem., Int. Ed. 2017, 56 (35), 10294–10323. 10.1002/anie.201611914. [DOI] [PubMed] [Google Scholar]
- Savile C. K.; Janey J. M.; Mundorff E. C.; Moore J. C.; Tam S.; Jarvis W. R.; Colbeck J. C.; Krebber A.; Fleitz F. J.; Brands J.; Devine P. N.; Huisman G. W.; Hughes G. J. Biocatalytic Asymmetric Synthesis of Chiral Amines from Ketones Applied to Sitagliptin Manufacture. Science (1979) 2010, 329 (5989), 305–309. 10.1126/science.1188934. [DOI] [PubMed] [Google Scholar]
- Grawunder U.; Beerli R. R.. Binding Protein Drug Conjugates Comprising Anthracycline Derivatives. US10517959B2, 2019.
- Grawunder U.; Beerli R. R.. Method of Producing an Immunoligand/Payload Conjugate. US11364301B2, 2022.
- King G. T.; Eaton K. D.; Beagle B. R.; Zopf C. J.; Wong G. Y.; Krupka H. I.; Hua S. Y.; Messersmith W. A.; El-Khoueiry A. B. A Phase 1, Dose-Escalation Study of PF-06664178, an Anti-Trop-2/Aur0101 Antibody-Drug Conjugate in Patients with Advanced or Metastatic Solid Tumors. Invest New Drugs 2018, 36 (5), 836–847. 10.1007/s10637-018-0560-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strop P.; Dorywalska M. G.; Rajpal A.; Shelton D.; Liu S.-H.; Pons J.; Dushin R.. Engineered Polypeptide Conjugates and Methods for Making Thereof Using Transglutaminase. US20210115159A1, 2017.
- van Delft F. L.; van Geel R.; Wijdeven M. A.. Modified Antibody, Antibody-Conjugate and Process for the Preparation Thereof. US9504758B2, 2015.
- Maclaren A.Anti-Cd22 Antibody-Maytansine Conjugates, Combinations, and Methods of Use Thereof. US20190201541A1, 2019.