Abstract
Vasculogenic mimicry (VM) refers to the ability of malignant cells to form microvascular channels, having nature of blood vessels but are not endothelium lined. These channels contain blood cells & plasma and provide sufficient nutrient supply to the cancerous cells to meet their metabolic demands. VM can be seen in various tumors and is associated with their malignant phenotype, high tumor grade, invasion, metastasis and poor clinical outcome. In this paper, we made an attempt to explain the mechanism, visualisation and prognostic significance of vasculogenic mimicry.
Keywords: cancer, melanoma, prognosis, Vasculogenic mimicry
Carcinogenesis is a complex phenomenon where the neoplastic cells acquire a number of properties to survive, proliferate and metastasize regionally and to distant organs. One of the prerequisites for the survival of tumour cells is 'sustained angiogenesis'.[1] Although most malignant neoplasms survive by neoangiogenesis, implying non-patterned proliferation of endothelial cells to form endothelium-lined vascular spaces, derived from pre-existing microcirculation. This is applicable to all primary and metastatic tumours. Maniotis et al.,[2] in their legendary work, explained an alternative mechanism in uveal melanoma for which the term 'vasculogenic mimicry' (VM) was chosen. Vasculogenic means 'having a nature/behaviour of blood vessels but does not arise from the pre-existing vessels and are not endothelium-lined, rather they are lined by tumour cells and perform the function of blood vessels, hence the suffix 'mimicry'.
Here, we demonstrate VM in a case of amelanotic melanoma of the submental region with nodal and microsatellite metastases. Figure 1 shows a low-power view of amelanotic melanoma with dyscohesive malignant cells, septae and narrow vascular spaces. Interestingly, it is possible to demonstrate VM by simple histochemical staining employing periodic acid Schiff (PAS) staining without counterstaining with haematoxylin [Figure 2a, same case as Figure 1] and subsequent visualisation under a green filter. If VM is present, the PAS-positive (PAS+) loops appear black [Figure 2b]. It has been proposed that PAS-positive material should either form closed loops encircling the packets of malignant tumour cells or there should be at least a network of three back-to-back loops to be considered as VM.
Figure 1.

Photomicrograph of H&E-stained section demonstrating dyscohesive malignant cells, septae and narrow vascular spaces in a case of amelanotic melanoma
Figure 2.

Photomicrograph showing patterned vasculogenic mimicry demonstrated by PAS staining without counterstaining with haematoxylin (a), which appears black using green filter (b). High-power view of a PAS-stained section (without counterstaining) showing thin basal lamina-like structure lined externally by tumour cells (c)
At this point, it is essential to mention and recognise that these channels are not lined by endothelium, unlike true endothelium-lined vascular space. Antithetical to 'true' vascular spaces where the endothelial cells internally line the basal lamina (towards the luminal side), in VM, PAS + basal lamina are 'not' endothelium lined and rather delimited externally by the tumour cells [Figure 2c]. Figure 3 shows a hand-drawn picture corresponding to 2c depicting PAS + basal lamina-like structure lined externally by malignant cells. VM should not only be differentiated from endothelial cells but also fibrovascular septae traversing in between the tumour cells. The same procedure can be used to differentiate true VM from fibrovascular septae intervening in the tumour cells in addition to trichrome staining. The fibrovascular septae are variable in thickness and are multilaminar, in contrast to unilaminar thinner patterned loops.[3]
Figure 3.
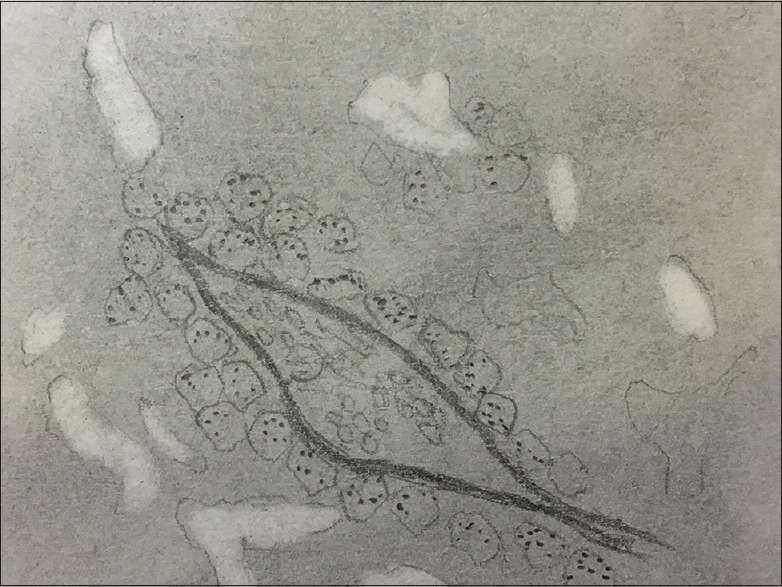
Shows a hand-drawn picture corresponding to 2c
VM is not just a captivating histological finding of academic interest but also bears a high prognostic value in various human cancers. The phenomenon was noted in highly invasive melanoma, and in contrast, these patterns were not noted in normal melanocytes and poorly invasive melanoma cells in vitro.[3] Further, significantly higher mortality rates were seen in PAS + metastatic melanoma as compared to trichrome-positive cases. Overall, poor survival has been also reported in oral and laryngeal carcinomas, breast carcinoma, gastric carcinomas, osteosarcomas and gliomas; rendering VM as an attractive target in theragnostics and prognosis of human malignancies.[4,5]
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 2.Maniotis AJ, Folberg R, Hess A, Seftor EA, Gardner LM, Pe'er J, et al. Vascular channel formation by human melanoma cells in vivo and in vitro: Vasculogenicmimicry. Am J Pathol. 1999;155:739–52. doi: 10.1016/S0002-9440(10)65173-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lin AY, Maniotis AJ, Valyi-Nagy K, Majumdar D, Setty S, Kadkol S, et al. Distinguishing fibrovascular septa from vasculogenic mimicry patterns. Arch Pathol Lab Med. 2005;129:884–92. doi: 10.5858/2005-129-884-DFSFVM. [DOI] [PubMed] [Google Scholar]
- 4.Salem A, Salo T. Vasculogenic mimicry in head and neck squamous cell carcinoma-time to take notice. Front Oral Health. 2021;2:666895. doi: 10.3389/froh.2021.666895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Luo Q, Wang J, Zhao W, Peng Z, Liu X, Li B, et al. Vasculogenic mimicry in carcinogenesis and clinical applications. J HematolOncol. 2020;13:19. doi: 10.1186/s13045-020-00858-6. [DOI] [PMC free article] [PubMed] [Google Scholar]


