Abstract
Background:
Cytokeratins are the largest sub-group of intermediate filaments and represent the most abundant proteins in epithelial cells. CYFRA 21-1 (human cytokeratin fragment antigen 21-1) is a soluble fragment of cytokeratin 19 known to increase in various malignancies.
Aim:
The present study is aimed to estimate salivary and serum levels of CYFRA 21-1 in oral squamous cell carcinoma (OSCC) patients and to compare them with healthy controls.
Settings and Design:
A prospective, case-control study.
Material and Methods:
This study included a total of 80 subjects, comprising 40 OSCC patients and 40 healthy controls. Saliva and blood samples were collected from the study population, and serum and salivary CYFRA 21-1 levels were measured by enzyme-linked immunosorbent assay.
Statistical Analysis Used:
The statistical tests applied were independent t-test, ANOVA test for comparison, and Post hoc test for correlation. A P value of < 0.05 was considered statistically significant.
Results:
A statistically significant increase in salivary and serum CYFRA 21-1 levels was observed between OSCC and control groups and with an increase in the pathological tumour node metastasis stage and histopathological grade of OSCC. On correlating salivary and serum CYFRA 21-1 values, there were 3-fold higher salivary levels than serum.
Conclusion:
CYFRA 21-1 can be suggested as a tumour marker that can be used for the early diagnosis of the OSCC. Further prospective studies with a larger sample size and advanced techniques recommended before CYFRA 21-1 can be recommended for routine clinical use.
Keywords: CYFRA 21-1, cytokeratin, tumour marker, OSCC, early detection
INTRODUCTION
Oral squamous cell carcinoma (OSCC) is one of the most aggressive malignancies worldwide, with a high propensity for local invasion and distant metastases.[1] Although many treatment modalities have been available, the 5-year survival mean remained as low as 30–50%. Many research studies have proposed an early detection of the lesion as a means of the prevention of the disease. Recently, there has been an ever-growing effort directed toward the immunological markers for the diagnosis of OSCC.[2]
Body fluids such as blood and saliva remain the best choice for OSCC screening and diagnosis. They are easily accessible, non-traumatic, and less time-consuming and require less and inexpensive instruments with minimal training. Samples can be taken repeatedly and can be used for mass screening of a large population.[3,4] In recent years, several exciting developments have occurred, especially in biomarkers, such as certain degradation products such as cytokeratins (CKs). These are proteins of the intermediate filament family and the main components of the cell cytoskeleton. The cytokeratin family is expressed by all epithelial cells and appear to be a useful marker of epithelial differentiation.[5,6]
Cytokeratin 19 has a low molecular weight of 40 kDa with an isoelectric pH of 5.2. It is expressed and immunohistochemically detectable in the cytoplasm of epithelial tumour cells. Although cytokeratins are part of the cytoskeleton, some fragments might be released in the body fluids because of cell lysis or tumour necrosis. For these reasons, the cytokeratin subunit 19 fragment can be used as a tumour marker for various cancers. Cytokeratin fraction 21-1 (CYFRA 21-1) is a soluble fragment of cytokeratin 19 released into the circulation. It can be quantified using various commercially available specific serological and salivary assays.[5,6]
Most frequently used CKs for the detection of carcinomas are tissue polypeptide antigen (TPA), tissue polypeptide specific antigen (TPS), and cytokeratin fragment 21-1 (CYFRA 21-1), but the latter one showed promising results in non-small-cell lung, breast, gastric, oesophagus, and pancreatic cancers.[7,8] Paucity of studies in OSCC leads us to evaluate the clinical utility of CYFRA 21-1 in OSCC.
MATERIALS AND METHODS
The current observational study was carried out after obtaining ethical clearance and patient consent. We finalised 40 newly diagnosed OSCC subjects of both genders between the ages 30 and 78 based on Broders histopathological classification, and 40 age-matched healthy subjects were selected as controls. Staging was performed by the pathological tumour node metastasis (pTNM) classification system.[9]
After explaining the procedural details of the study, an in-depth case history was taken. Serum was procured from the blood (3 ml) that was aseptically collected and centrifuged for 10 minutes at 2000 rpm. The subjects were instructed to restrain from food intake for at least an hour before saliva procurement. The unstimulated whole saliva was taken following the diurnal rhythm between 9 am and 12 pm, and the supernatant was collected by centrifuging the saliva for 25 min at 2000 rpm and stored at -80°C till further analysis.[1,4] The serum and salivary CYFRA 21-1 were assayed using a sandwich enzyme-linked immunosorbent assay kit (XEMA Co., Ltd Moscow, Russia). The detection range was 0.5–50 ng/ml, with 620 nm micro-plate reader absorbency.
Statistical analysis
The data thus obtained were analysed using IBM SPSS ver. 23, and appropriate statistical tests were applied. Demographics, grading, and staging were subjected to descriptive statistics [Table 1]. Comparison of mean serum and saliva levels of CYFRA 21-1 between OSCC patients and the control group was performed by independent T test [Table 2]. One-way ANOVA with post hoc Tukey test were performed for inter-group and intra-group comparisons and correlations [Tables 3–6] [Figure 1]. A receiver operating characteristic (ROC) curve was used to determine sensitivity and specificity of serum and salivary CYFRA 21-1 [Figure 2].
Table 1.
Demographic groups and sub-groups among the cases
| Parameters | Cases 40 (100%) | Control 40 (100%) | ||
|---|---|---|---|---|
|
|
|
|||
| Frequency | Percentage (%) | Frequency | Percentage (%) | |
| Age Range | ||||
| <50 years | 17 | 42.5 | 16 | 40 |
| >50 years | 23 | 57.5 | 24 | 60 |
| Sex | ||||
| Men | 27 | 67.5 | 28 | 70 |
| Women | 13 | 32.5 | 12 | 30 |
| Grading | ||||
| Well-differentiated | 15 | 37.5 | - | - |
| Moderately differentiated | 16 | 40 | - | - |
| Poorly differentiated | 9 | 22.5 | - | - |
| pTNM | ||||
| pT1N0 | 8 | 20.0 | - | - |
| pT1N1 | 1 | 2.5 | - | - |
| pT1N2b | 1 | 2.5 | - | - |
| pT2N0 | 10 | 25.0 | - | - |
| pT2N1 | 8 | 20.0 | - | - |
| pT2N2 | 4 | 10.0 | - | - |
| Staging | ||||
| Stage I | 8 | 23.5 | - | - |
| Stage II | 10 | 29.4 | - | - |
| Stage III | 9 | 26.5 | - | - |
| Stage IV | 5 | 20.6 | - | - |
Table 2.
Mean serum and saliva CYFRA21-1 in the control and OSCC groups and their comparisons using independent t-test
| Group | Mean Serum | SD | Mean Saliva | SD | P-value |
|---|---|---|---|---|---|
| Control group | 1.34 | 0.79 | 4.35 | 3.60 | <0.01 |
| OSCC | 6.82 | 4.86 | 22.31 | 10.07 |
Table 3.
Intra-group comparison of mean serum and salivary CYFRA 21-1 among grades of OSCC by one-way ANOVA
| Grade | Mean Serum | SD | Mean Saliva | SD | P-Value |
|---|---|---|---|---|---|
| Well | 3.93 | 2.58 | 16.80 | 8.32 | <0.01 |
| Moderate | 6.88 | 3.78 | 22.34 | 6.65 | |
| Poor | 11.52 | 6.00 | 31.43 | 11.83 |
Table 6.
Pair-wise comparison of serum and salivary values among different stages of OSCC
| Parameter | Stage of Malignancy | ||
|---|---|---|---|
| Serum | stage I | stage II | 0.76 |
| stage III | <0.01 | ||
| stage IV | <0.01 | ||
| stage II | stage III | 0.09 | |
| stage IV | <0.01 | ||
| stage III | stage IV | <0.01 | |
| Saliva | stage I | Stage II | 0.7 |
| stage III | <0.05 | ||
| stage IV | <0.01 | ||
| stage II | stage III | 0.25 | |
| stage IV | <0.01 | ||
| stage III | stage IV | <0.05 | |
Figure 1.
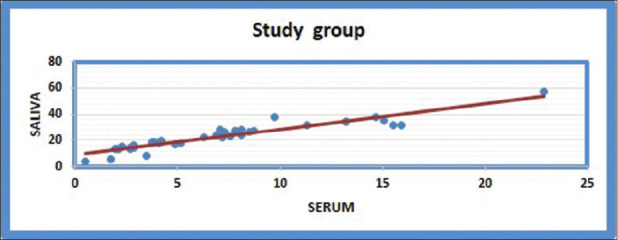
Illustration of positive correlation between serum and saliva in the study group using post hoc Tukey test. Salivary CYFRA 21-1 levels were almost 3-fold higher than those of serum
Figure 2.
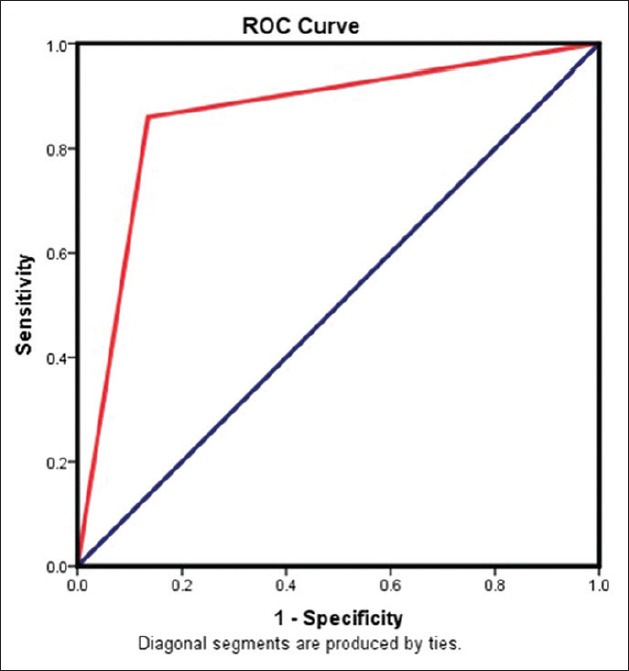
ROC curve to detect sensitivity and specificity of salivary CYFRA 21-1. AUC = 88.75. AUC was an effective and combined measure of sensitivity and specificity that describes the inherent validity of the marker
RESULTS
Demographics
Both the case and the control groups were age- and gender-matched. However, there were no subjects with the habit of smoking or chewing tobacco in the control group. The distribution of the subjects for the various grades and the stages of the OSCC are shown in [Table 1].
CYFRA 21-1 levels between the groups
A significantly higher (p < 0.01) mean serum marker was seen in the OSCC (6.82 ng/ml) than in the normal subjects (1.34 ng/ml). Similarly, significantly higher (p < 0.01) mean salivary CYFRA 21-1 (22.31 ng/ml) was seen in the OSCC than in the normal subjects (4.35 ng/ml) [Table 2].
CYFRA 21-1 levels for the various grades and the stages of the OSCC
Elevated mean serum and salivary titers were observed with grades of OSCC. The mean serum marker titer was 3.93 ng/ml in well-differentiated (WD), 6.88 ng/ml in moderately differentiated (MD), and 11.52 ng/ml poorly differentiated (PD) cases, and the mean salivary concentrations were 16.80, 22.34, and 31.43 ng/ml, respectively, in well, moderate, and poorly differentiated cases of OSCC [Table 3].
Furthermore, one-way ANOVA test was performed for pair-wise comparison, and a significant difference between all pairs of groups except between well and moderate groups was seen [Table 4].
Table 4.
Pair-wise comparison of serum and salivary values among various grades of OSCC
| Parameter | Grade of malignancy | p-value | |
|---|---|---|---|
| Serum | Moderately differentiated SCC | Poor | <0.05 |
| Well | 0.12 | ||
| Poorly differentiated SCC | Well | <0.01 | |
| Saliva | Moderately differentiated SCC | Poor | <0.05 |
| Well | 0.19 | ||
| Poorly differentiated SCC | Well | <0.01 |
The mean CYFRA 21-1 concentration ranged from 2.81 to 13.33 ng/ml from stage I to stage IV in serum, whereas in saliva, it ranged from 14.03 to 35.04 ng/ml, and both showed a close correlation with clinical staging [Table 5]. However, on pair-wise analysis, serum and the saliva marker were remarkably elevated only between stages III and IV (P < 0.01). There was no significant variation between stages II and I and between stages III and II [Table 6].
Table 5.
Comparison of mean serum and salivary CYFRA 21-1 among stages of OSCC by one-way ANOVA test
| Stage | Mean Serum | SD | P-Value | Mean Saliva | SD | P-Value |
|---|---|---|---|---|---|---|
| Stage I | 2.81 | 1.93 | <0.01 | 14.03 | 4.94 | <0.01 |
| Stage II | 4.34 | 1.74 | 17.84 | 5.34 | ||
| Stage III | 7.96 | 3.19 | 24.28 | 6.56 | ||
| Stage IV | 13.33 | 5.55 | 35.04 | 11.94 |
Correlation between serum and salivary values in the study group and control group
There was a positive correlation found between serum and salivary values between the test group and control group using post hoc Tukey test (p-value < 0.01) [Figure 1].
Analysis of specificity and sensitivity of salivary and serum CYFRA 21-1
Serum CYFRA 21-1 had a sensitivity of 82.5, and a specificity of 90 if 2.51 is considered as the cut-off value, whereas salivary CYFRA 21-1 had a sensitivity of 85 and a specificity of 92.5 if 10.88 is considered as the cut-off value. The area under curve (AUC) for the serum and the salivary marker levels was 86.25 and 88.75, respectively [Figure 2].
DISCUSSION
Cytokeratins are a type of intermediate filaments of the cell cytoskeleton found in epithelial cells. CYFRA 21-1 is a soluble fragment of cytokeratin19. Cytokeratin expression is site-specific and differentiation-dependent.[8] In the healthy oral mucosa, low-molecular-weight cytokeratins such as 18, 19, and 20 are located only in basal and para-basal layers, whereas suprabasal layers express high-molecular-weight cytokeratins 4, 5, 13, and 14.[10,11] During the normal maturation process, these low-molecular-weight cytokeratins will be replaced with high-molecular-weight keratins.[11,12]
In the present study, we found a significant rise of the CYFRA 21-1 fragment of cytokeratin 19 in the serum and saliva of OSCC patients, which is comparable with previous studies.[1,13,14,15] This might be because of disturbance in the process of differentiation during the course of malignant transformation, resulting in retention and increased expression of CK 19 even by the supra-basal cells in massive amounts.[11,12,16] It has been suggested that this increased CK19 undergoes caspase 3-mediated cleavage and facilitates the formation of apoptotic bodies during the intermediate stage of apoptosis. Apoptotic bodies further amplify the apoptotic signal, resulting in the release of excess CK19 fragments into the extra-cellular spaces which result in the presence of CYFRA 21-1 in various body fluids such as blood, saliva, cystic fluids, ascites, pleural effusions, urine, and the cerebro-spinal fluid (CSF).[17,18] Other pathways such as proteolytic degradation, tissue necrosis, abnormal mitosis, increased proliferation, and neovascularisation can cause spillover of CYFRA 21-1 into the extra-cellular space.[1,13,16]
In our study, intra-group and pair-wise comparisons between different grades showed a significant rise in mean serum and salivary values between all grades except between well-differentiated and moderate differentiated OSCC (P-value 0.12). These observations were in accordance with Doweck et al.[19] and Rewa Malhotra et al.[1] but contradicting with Zhong et al.[15] and Rafael Nagler et al.[20] The cognition for the above might be with the fact that with increasing grade, the differentiation process will be compromised, so more immature, hyper-proliferative, and atypical cells will emerge. They tend to retain and express increased amounts of CK19 and show accelerated spillage of CYFRA 21-1 into extra-cellular spaces.[8,17]
Similarly, among pTNM stages, serum and salivary CYFRA21-1 showed a significant rise from stage I to stage IV. On pair-wise comparison, a statistically significant rise was not seen between stages I and II and stages II and III. The rationale may be that with an increase in stage, the tumour size increases, which results in tumour necrosis and accelerates spillage of CYFRA21-1, and there will be an added increase with lymph node involvement.[6,8] As the stage increases, the prognosis will decrease, so CYFRA 21-1 was also providing independent prognostic information.[6] These observations were in accordance with Raj Kumar et al.,[13] Doweck et al.,[19] and Rewa Malhotra et al.[1] However, the results were contradicting with Zhong et al.[15] and Rafael Nagler et al.[14]
In this study, a positive correlation was found between serum and saliva (P < 0.01), and there was almost 3-fold higher salivary titers than serum. Nagler et al.[14] found a 4-fold increase, whereas Zhong et al.,[8] Rewa Malhotra et al.,[1] and Raj Kumar et al.[13] showed a 3-fold elevated level of this marker in saliva compared to serum. This may be because of direct contact with lesion, local cell necrosis, or active transportation across cell membranes or through tight junctions or by passive diffusion from the serum to saliva, reflecting immediately in saliva compared to serum.[1,13,15]
In the current study, the diagnostic sensitivity (85%) and specificity (92.5%) of salivary CYFRA21-1 were found to be marginally higher than the serum sensitivity (82%) and specificity (90%) when 2.51 g/ml and 10.88 ng/ml were taken as cut-off values for serum and saliva, respectively. Several researchers have reported varied sensitivity and specificity percentages with different cut-off values in both serum and saliva. This variation was often because of different biochemical techniques used to estimate the marker concentration and the difference in methods used to determine the cut-off values.[1,4,13,15,20]
Both serum (86.26) and saliva (88.75) had almost proximate AUC, suggesting the undistinguishable diagnostic potency, but saliva is easily accessible, painless, quick, and economic to both the patient and the diagnostician.[4,13] We have also found that salivary values are 3-fold higher than those of serum, proving saliva to be the best screening tool compared to serum.
The limitation of our study was a smaller sample size and that the impact of adverse habits on marker level was not correlated.
CONCLUSION
There was a significant increase in serum and salivary CYFRA 21-1 levels in OSCC patients compared to the control group, and it showed a significant association with histologic grading and staging of OSCC with positive correlation between serum and salivary levels. CYFA 21-1 is established to be an ideal tumour marker with a role in early diagnosis. Further prospective studies with a larger sample size and advanced techniques recommended before CYFRA 21-1 should be endorsed for routine diagnostic applications.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Malhotra R, Urs AB, Chakravarti A, Kumar S, Gupta VK, Mahajan B. Correlation of Cyfra 21-1 levels in saliva and serum with CK19 mRNA expression in oral squamous cell carcinoma. Tumor Biol. 2016;37:9263–71. doi: 10.1007/s13277-016-4809-4. [DOI] [PubMed] [Google Scholar]
- 2.Dahiya K, Dhankhar R. Updated overview of current biomarkers in head and neck carcinoma. World J Methodol. 2016;6:77–86. doi: 10.5662/wjm.v6.i1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rassekh CH, Johnson JT, Eibling DE. Circulating markers in squamous cell carcinoma of the head and neck: A review. Eur J Cancer B Oral Oncol. 1994;30:23–8. doi: 10.1016/0964-1955(94)90046-9. [DOI] [PubMed] [Google Scholar]
- 4.Singh P, Jaiswal R. Salivary CYFRA squamous cell carcinoma an lesions. Int Med J. 2015;4:249–55. [Google Scholar]
- 5.Barak V, Goike H, Panaretakis KW, Einarsson R. Clinical utility of cytokeratins as tumor markers. Clin Biochem. 2004;37:529–40. doi: 10.1016/j.clinbiochem.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 6.Jose J, Sunil PM, Madhavan Nirmal R, Varghese SS. CYFRA 21-1: An Overview. Oral Maxillofac Pathol J. 2013;4(2) [Google Scholar]
- 7.Babu GS, Supriya AN, Kumar NG, Swetha P. Tumor markers: An overview. JOrofacial Sci. 2012;4:87. [Google Scholar]
- 8.Sawant SS, Zingde SM, Vaidya MM. Cytokeratin fragments in the serum: Their utility for the management of oral cancer. Oral Oncol. 2008;44:722–32. doi: 10.1016/j.oraloncology.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 9.Seethala RR, Weinreb I, Bullock JM. College of American Pathologists Protocol for the examination of specimens from patients with cancers of the lip and oral cavity 8th ed. Version LipOralCavity 4.0. 0.1. College of American Pathologists Protocol for the examination of specimens from patients with cancers of the lip and oral cavity. 2017 [Google Scholar]
- 10.Santoro A, Pannone G, Ninivaggi R, Petruzzi M, Santarelli A, Russo GM, et al. Relationship between CK19 expression, deregulation of normal keratinocyte differentiation pattern and high risk-human papilloma virus infection in oral and oropharyngeal squamous cell carcinoma. Infect Agent Cancer. 2015;10:1–3. doi: 10.1186/s13027-015-0041-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chu PG, Weiss LM. Keratin expression in human tissues and neoplasms. Histopathology. 2002;40:403–39. doi: 10.1046/j.1365-2559.2002.01387.x. [DOI] [PubMed] [Google Scholar]
- 12.Bragulla HH, Homberger DG. Structure and functions of keratin proteins in simple, stratified, keratinized and cornified epithelia. J Anat. 2009;214:516–59. doi: 10.1111/j.1469-7580.2009.01066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rajkumar K, Ramya R, Nandhini G, Rajashree P, Ramesh Kumar A, Nirmala Anandan S. Salivary and serum level of CYFRA 21-1 in oral precancer and oral squamous cell carcinoma. Oral Dis. 2015;21:90–6. doi: 10.1111/odi.12216. [DOI] [PubMed] [Google Scholar]
- 14.Nagler RM, Barak M, Peled M, Ben-Aryeh H, Filatov M, Laufer D. Early diagnosis and treatment monitoring roles of tumor markers Cyfra 21-1 and TPS in oral squamous cell carcinoma. Cancer. 1999;85:1018–25. [PubMed] [Google Scholar]
- 15.Zhong LP, Zhu HG, Zhang CP, Chen WT, Zhang ZY. Detection of serum Cyfra 21-1 in patients with primary oral squamous cell carcinoma. Int J Oral Maxillofac Surg. 2007;36:230–4. doi: 10.1016/j.ijom.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 16.Xu XC, Lee JS, Lippman SM, Ro JY, Hong WK, Lotan R. Increased expression of cytokeratins CK8 and CK19 is associated with head and neck carcinogenesis. Cancer Epidemiol Biomarkers Prev. 1995;4:871–6. [PubMed] [Google Scholar]
- 17.Sheard MA, Vojtesek B, Simickova M, Valik D. Release of cytokeratin-18 and-19 fragments (TPS and CYFRA 21-1) into the extracellular space during apoptosis. J Cell Biochem. 2002;85:670–7. doi: 10.1002/jcb.10173. [DOI] [PubMed] [Google Scholar]
- 18.Dohmoto K, Hojo S, Fujita J, Yang Y, Ueda Y, Bandoh S, et al. The role of caspase 3 in producing cytokeratin 19 fragment (CYFRA21-1) in human lung cancer cell lines. Int J Cancer. 2001;91:468–73. doi: 10.1002/1097-0215(200002)9999:9999<::aid-ijc1082>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 19.Doweck I, Barak M, Uri N, Greenberg E. The prognostic value of the tumour marker Cyfra 21-1 in carcinoma of head and neck and its role in early detection of recurrent disease. Br J Cancer. 2000;83:1696–701. doi: 10.1054/bjoc.2000.1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nagler R, Bahar G, Shpitzer T, Feinmesser R. Concomitant analysis of salivary tumor markers—a new diagnostic tool for oral cancer. Clin Cancer Res. 2006;12:3979–84. doi: 10.1158/1078-0432.CCR-05-2412. [DOI] [PubMed] [Google Scholar]


