Abstract
Mutations in each of three genes, myocilin (MYOC), optineurin (OPTN), and TANK binding kinase 1 (TBK1), may cause primary open-angle glaucoma (POAG) that is inherited as a Mendelian trait. MYOC mutations cause 3–4% of POAG cases with IOP > 21 mmHg, while mutations in OPTN, TBK1, and MYOC each cause ~1% of POAG with IOP ≤ 21 mmHg, i.e. normal tension glaucoma. Identification of these disease-causing genes has provided insights into glaucoma pathogenesis. Mutations in MYOC cause a cascade of abnormalities in the trabecular meshwork including intracellular retention of MYOC protein, decreased aqueous outflow, higher intraocular pressure, and glaucoma. Investigation of MYOC mutations demonstrated that abnormal retention of intracellular MYOC and stimulation of endoplasmic reticular (ER) stress may be important steps in the development of MYOC-associated glaucoma. Mutations in OPTN and TBK1 cause a dysregulation of autophagy which may directly cause retinal ganglion cell damage and normal tension glaucoma. Discovery of these Mendelian causes of glaucoma has also provided a new set of potential therapeutic targets that may ultimately lead to novel, gene-directed glaucoma treatments
1. Introduction
Glaucoma is one of the most common cause of irreversible blindness worldwide (Leske and Podgor, 1983; Quigley and Broman, 2006). Glaucoma is comprised of a group of diseases resulting in progressive optic nerve cupping with corresponding and characteristic visual field loss. Primary open-angle glaucoma (POAG) is defined as glaucoma in the absence of secondary findings (i.e. anatomically normal), and is the most common type of glaucoma worldwide. In the United States, 1.6% of subjects over the age of 40 are affected by POAG (Kahn and Milton, 1980).
Studies of the heredity of glaucoma date back to Von Graefe, who reported multigeneration families with glaucoma in 1869 (Graefe, 1869). Since then many more large pedigrees with inherited glaucoma have been described (François, 1966; Stokes, 1940). Population-based studies have also shown familial clustering of POAG cases that suggest a genetic basis (Becker et al., 1960). First degree relatives have a 9-fold increased risk, carrying a lifetime glaucoma risk of 22% (Wolfs et al., 1998). While early twins studies reported the heritability of POAG to be approximately 13% (Teikari, 1987), more recent and much larger studies have estimated POAG heritability to be 70% (Wang et al., 2017) and 93% (Polubriaginof et al., 2018). Another clue into the heritability of POAG lies with the significant variation of POAG between different races and ethnicities. POAG is five-fold more common in African Americans and in Hispanic Americans than in Caucasians (Tielsch et al., 1991; Varma et al., 2004).
The genetics of most cases of POAG are complex. As many as 95% of POAG cases are caused in part by the combined action of dozens of genetic, and possibly environmental, risk factors. (Fingert, 2011) More than 25 of these POAG risk factor genes have been discovered to date and more remain to be identified (Choquet et al., 2017; Gharahkhani et al., 2018; Shiga et al., 2018; Wiggs and Pasquale, 2017). The discovery and characterization of these genetic risk factor genes is the topic of an accompanying review article in this issue of Experiment Eye Research (Reference to other relevant article in this issue to be added by editors)
At least 5% of POAG cases are caused primarily by a mutation in one of three genes: myocilin (MYOC), optineurin (OPTN), or TANK binding kinase 1 (TBK1) (Fingert, 2011). A mutation in any one of these genes causes glaucoma with clear autosomal dominant (Mendelian) inheritance. This article aims to review the genetics of Mendelian (single gene) causes of glaucoma and will focus on the MYOC, OPTN, or TBK1 genes.
2. Myocilin (MYOC)
Perhaps the most notable genetic defects to be associated with the development of glaucoma occur within the Myocilin (MYOC) gene. MYOC was the first gene to be associated with glaucoma and remains the most common, known-cause of POAG. MYOC-associated glaucoma is inherited in an autosomal dominant pattern. One set of mutations in this gene has been associated with juvenile open angle glaucoma (JOAG) with an onset of disease before 35 years of age and markedly elevated IOP. A different set of MYOC mutations has been detected in POAG patients with an onset of disease after 40 years of age. Most cases of POAG associated with MYOC mutations have occurred with high IOP (>21 mmHg). However, MYOC mutations have also been detected in POAG patients with IOP ≤21 mmHg (i.e. normal tension glaucoma) (Alward et al., 2019; Michels-Rautenstrauss et al., 2002). The frequency of MYOC mutations in POAG patients appears to be higher with increased IOP.
In 1997, MYOC was discovered to be a glaucoma-causing gene via positional cloning studies of large JOAG pedigrees (Sheffield et al., 1993; Stone et al., 1997); studies of human trabecular meshwork cells (Polansky et al., 1997); and studies of gene expression in human retina (Kubota et al., 1997). These different research studies were conducted in parallel and referred to the same gene as GLC1A, TIGR, and MYOC. Myocilin and the gene symbol MYOC were designated as the official name for this gene by the nomenclature committee of the Human Genome Organization (HUGO).
2.1. Epidemiology of MYOC-associated glaucoma
Over 100 genetic MYOC mutations have been reported in patients with either JOAG or POAG (H.-W. Wang et al., 2018). The prevalence of MYOC mutations is much higher in cohorts of JOAG patients than in cohorts of POAG patients (described below) and myocilin mutations are rare in unexamined large general population studies (Han et al., 2018; Nag et al., 2016). The population allele frequencies of the MYOC mutations described below are catalogued in Supplemental Table 1.
MYOC and JOAG.
MYOC mutations have been detected in several studies of individual JOAG pedigrees (Kennan et al., 1998; Mansergh et al., 1998; Stoilova et al., 1997). Additionally, case-control studies have estimated that 8 to 63% of unrelated JOAG patients have MYOC mutations (Adam et al., 1997; Wiggs et al., 1998) The variability in the detected frequency of MYOC mutations is like due in part to the small cohorts of this rare form of glaucoma that have been studied.
MYOC and POAG.
The overall prevalence of MYOC mutations among POAG patients is 3 to 4% among Caucasian, Asian, and African populations worldwide (Fingert et al., 1999; 2002; Gong et al., 2004). The majority of MYOC mutations are unique to POAG patients from populations of either Caucasian, Asian, or African ancestry. However, the most commonly detected MYOC mutation overall, Gln368Stop, has been identified in POAG populations of all racial ancestries, though much more frequently among Caucasian patients. A few instances of the Gln368Stop mutation have been detected in African American POAG patients and Indian POAG patients (Bhattacharjee et al., 2007; Fingert et al., 1999). The Gln368Stop mutation has been detected in 1.6% of patients with POAG in Caucasian populations. As such, Gln368Stop is the most frequent, molecularly-defined mutation that is known to be associated with POAG (Fingert, 2011).
2.2. Genotype-phenotype correlations for MYOC-associated glaucoma
One group of MYOC mutations has been associated with early-onset disease (JOAG), while a different group of MYOC mutations has been associated with adult-onset disease (POAG).
JOAG phenotype and MYOC mutations.
Several MYOC mutations have been identified in multiple JOAG pedigrees that share key features including: high penetrance (most mutation carriers develop disease); early-onset of disease; strong family history with autosomal dominant inheritance; and markedly elevated IOP. Some examples of MYOC mutations associated with JOAG are Pro370Leu, Thr377Met, Tyr437His, and Ile477Asn (Table 1). Patients with JOAG caused by these mutations typically require surgery for adequate IOP control (Johnson et al., 1993).(Stoilova et al., 1998)
Table 1.
Features associated with MYOC mutations.
| JOAG | ||||
|---|---|---|---|---|
| Pro370Leu | Thr377Met | Tyr437His | Ile477Asn | |
| Inheritance pattern | AD | AD | AD | AD |
| Penetrance | >75% by 25 years | 88% by 30 years | 100% by 40 years | >75% by 25 years |
| Mean age at diagnosis (range) in years | 12–30 (5–35) | 37–38 (20–60) | 20 (8–41) | 21 (12–41) |
| Maximum IOP (range) in mm Hg | 45 (25–66) | 31 (20–50) | 44 (14–77) | 40 (20–52) |
| References | (Adam, 1997) | (Stone et al, 1997) | (Stone et al, 1997) | (Alward et al, 1998) |
| (Stoilova, 1998) | (Alward et al, 1998) | (Alward et al, 1998) | (Richards, 1998) | |
| (Shimizu, 2000) | (Fingert et al, 1999) | (Fingert et al, 1999) | (Fingert et al, 1999) | |
| (Shimizu, 2000) | (Shimizu, 2000) | |||
| (Mackey et al, 2003 | ||||
AD: autosomal dominant.
POAG phenotype and MYOC mutations.
Some MYOC mutations are associated with POAG that has clinical features more typical for adult-onset POAG (Table 1). The Gln368Stop mutation, for example has a mean age at diagnosis of that ranges between 36 and 59 years (Allingham et al., 1998; Alward et al., 1998; Angius et al., 2000; Shimizu et al., 2000). Mean maximum IOP for patients with the Gln368Stop has been reported as 29 to 30 mmHg with a range of 21 mmHg to 56 mmHg (Allingham et al., 1998; Alward et al., 1998; Angius et al., 2000; Shimizu et al., 2000). Patients with a Gln368Stop mutation respond to medical therapy and require glaucoma surgeries at a rate that is no different than patients without known mutations (Graul et al., 2002).
Age is major risk factor for glaucoma, even for highly heritable forms of glaucoma, such as MYOC-associated disease. The proportion of patients with a MYOC mutation that have developed glaucoma is termed the penetrance. Pedigree studies have shown that the penetrance of MYOC mutations is highly age-dependent and becomes larger with increasing age (Allingham et al., 1998; Craig et al., 2001; Hewitt et al., 2008). Two MYOC mutations that are associated with the earliest age of onset of JOAG, Ile477Asn and Pro370Leu, have >80% penetrance at 25 years of age, while the Gln368Stop mutation which has the latest onset of glaucoma (POAG) had 0% penetrance at 25 years of age. However, by 75 years of age the penetrance of all three mutations approached 100% (Hewitt et al., 2008; Mackey et al., 2003).
Although the penetrance of the Gln368Stop mutation in pedigree-based studies has been reported to be 80–100% by the seventh decade of life (Allingham et al., 1998; Hewitt et al., 2008), different results have emerged from population-based studies. More recently, huge studies of essentially unselected citizens of the United Kingdom (TwinsUK and the UKBiobank) and the Netherlands (Rotterdam Study) reported a much lower penetrance for the Gln368Stop allele, ranging from 33 to 48% by the end of the 7th decade of life. (Han et al., 2018; Nag et al., 2016). Some potential explanations for the higher penetrance of the Gln368Stop mutation observed in pedigree studies are differences in genetic background and environmental exposures, ascertainment bias, and diagnostic methods. These investigations suggest that the predictive value of detecting a Gln368Stop mutation may be substantially influenced by how subjects were enrolled for testing (Fingert, 2019).
2.3. Ocular expression pattern of MYOC
MYOC encodes a secreted glycoprotein of unknown function (Fingert et al., 1998; Polansky et al., 1997). MYOC is broadly expressed at the RNA level in non-ocular and ocular tissues (Fingert et al., 1998; Swiderski et al., 2000). Production of MYOC protein, however, is more restricted and has been primarily identified within tissues of the eye (Clark et al., 2001; Karali et al., 2000; Lütjen-Drecoll et al., 1998; Polansky et al., 1997). MYOC protein has been identified in the trabecular meshwork, sclera, ciliary body, choroid, cornea, iris, lamina cribosa, retina, and optic nerve (Adam et al., 1997; Clark et al., 2001a). MYOC protein is secreted from ocular tissues into the aqueous humor where it is also detectable (Jacobson et al., 2001)
MYOC protein was first detected in primary cultures of human trabecular meshwork with studies to identify steroid-induced proteins (Nguyen et al., 1998; Polansky et al., 1997). Glucocorticoid steroids were discovered to induce MYOC protein production by approximately 47-fold in cultured trabecular meshwork cells (Clark et al., 2001b; Polansky et al., 1997). Moreover, studies of the trabecular meshwork with cell culture, organ culture, and human donor eyes have shown that glaucoma-causing MYOC mutations prevent secretion and lead to intracellular retention of MYOC protein (Jacobson et al., 2001; van der Heide et al., 2018).
2.4. Structure and function of MYOC
The MYOC gene is composed of three exons that encode a 504 amino acid polypeptide (Figure 1) that includes a signal sequence for secretion, a leucine zipper domain, and an olfactomedin domain (Adam et al., 1997; Fingert et al., 1998; Polansky et al., 1997). The structure of the olfactomedin domain has been of high interest because the majority of the disease-causing variations have been detected in this segment of MYOC. The crystal structure of the olfactomedin domain has been solved (H. Han and Kursula, 2015; Pronker et al., 2015) and demonstrated to form a five-bladed β-propeller structure that promotes protein–protein interactions and oligomerization of MYOC (Donegan et al., 2015). The leucine zipper domain of MYOC also promotes protein-protein interactions (Nguyen et al., 1998). The MYOC gene encodes a 55–57 kDa secreted protein, however, analyses of cell lysates have identified immunoreactive protein complexes with molecular weights that are 2X or 4X multiples of MYOC. These data suggest that wild-type MYOC may form homodimers or homotetramers via interactions between the leucine zipper and/or olfactomedin domains (Nguyen et al., 1998).
Figure 1. Protein Structure of MYOC, OPTN, and TBK1.
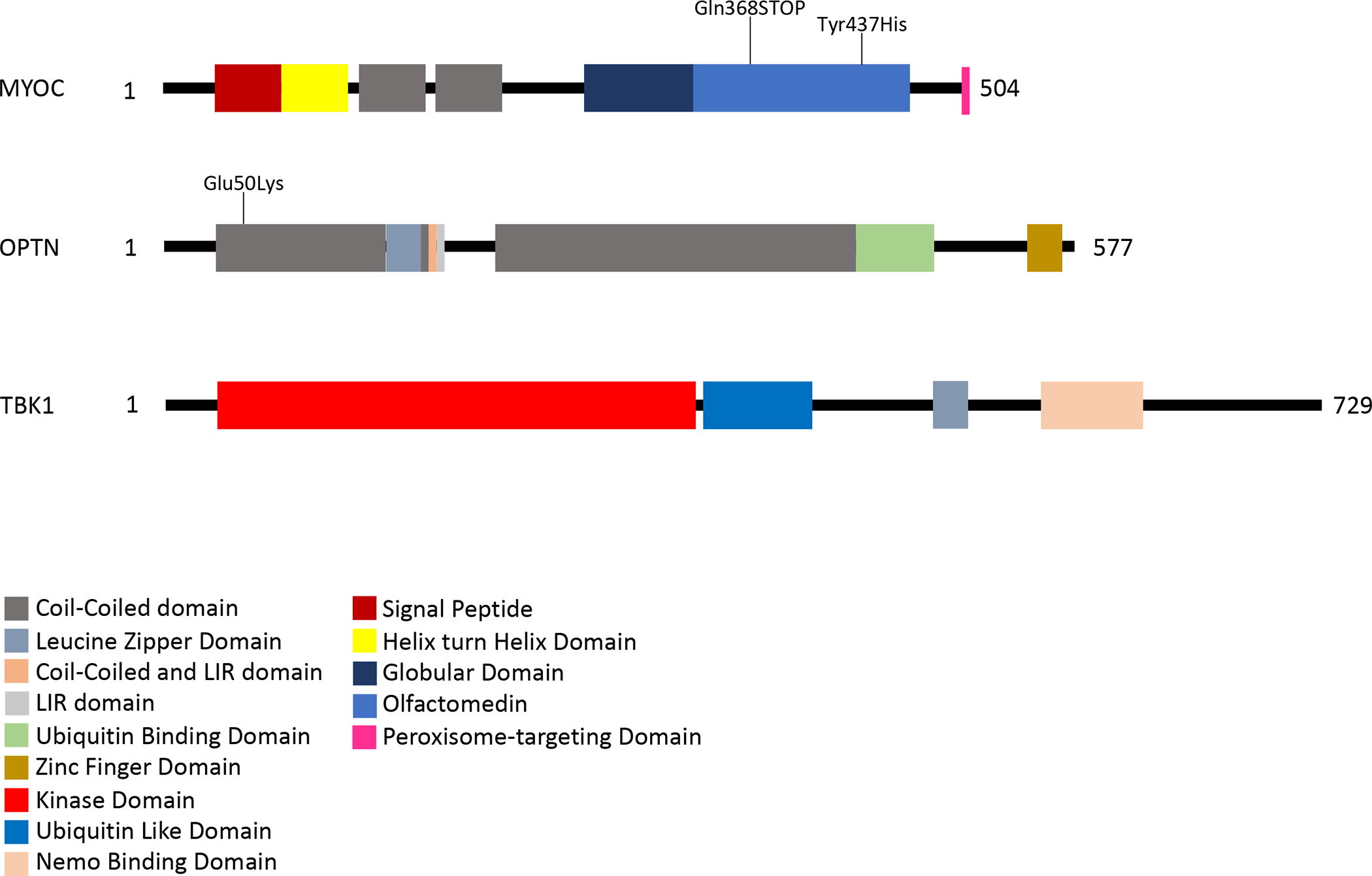
Each protein is represented by a linear diagram proportional to its length in amino acids (AA), 504 AA for MYOC, 577 AA for OPTN, and 729 AA for TBK1. Functional domains and sequence motifs are indicated by colored boxes. The location of some of the more commonly detected glaucoma-causing mutations are indicated on these diagrams.
2.5. Pathophysiology of MYOC-associated glaucoma and gene-directed therapy
The normal function of MYOC is not well characterized. In fact, there are data to suggest that MYOC may not be essential for normal ocular function. Mice with inactivated genes (Myoc−/−) have no evidence of glaucoma or any other ocular abnormalities (Kim et al., 2001). Similarly, one patient with a hemizygous deletion spanning the MYOC gene did not have glaucoma (Wiggs and Vollrath, 2001). A presumably null MYOC mutation, Arg46Stop, is not associated with glaucoma or ophthalmic disease in humans when present as either a heterozygous or homozygous mutation (Lam et al., 2000; Pang et al., 2002). Together these observations suggest that MYOC mutations cause disease via a gain-of-function rather than a loss-of-function mechanism.
An abundance of data suggests that glaucoma-causing mutations prevent normal secretion and cause intracellular accumulation of MYOC protein in trabecular meshwork cells (Kwon et al., 2009). Several MYOC mutations have been shown to cause reduced secretion of MYOC protein in human cultured trabecular meshwork cells and/or in human organ culture systems (Jacobson et al., 2001). Similarly, MYOC mutations prevent secretion of MYOC protein in transgenic mice (Zhou et al., 2008; Zode et al., 2011). Intracellular MYOC protein aggregates accumulate within the endoplasmic reticulum (ER) and increase production of ER stress molecules (Joe et al., 2003; Zode et al., 2011). These observations have led to the development of new gene-directed therapies for MYOC-associated glaucoma that are designed to eliminate intracellular MYOC protein.
Gene-directed glaucoma therapy.
A class of drugs known as chemical chaperones (Ozcan et al., 2006), such as 4-phenylbutyrate, have been evaluated for their ability to stabilize mutant MYOC protein in vitro (Burns et al., 2010) and to help prevent or disperse MYOC aggregates and ultimately promote secretion in a transgenic mouse model of MYOC glaucoma. Both oral and topical administration of 4-phenylbutyrate to transgenic MYOC mice prevented accumulation of intracellular MYOC protein; prevented elevation of IOP; and prevented glaucoma (Zode et al., 2011; 2012). These preliminary animal studies suggest therapies that promote clearance of intracellular MYOC aggregates may be an effective treatment for MYOC-associated glaucoma.
Genome-editing glaucoma therapy.
Given that loss of MYOC function does not appear to have adverse effects on the eye (Kim et al., 2001; Lam et al., 2000; Pang et al., 2002), genome editing has been investigated as a possible therapy of MYOC-associated glaucoma. The CRISPR/Cas9 endonuclease system has been used to inactivate (knock-down) expression of mutant MYOC genes in cultured trabecular meshwork cells, human organ culture (perfused anterior segments of human eyes), and in transgenic mice. Genome editing successfully reduced the abnormal intracellular accumulation of MYOC, reduced ER stress markers, and reduced IOP (Jain et al., 2017). These experiments indicate that genome editing may have utility as a gene-directed therapy for MYOC-associated glaucoma.
MYOC protein is expressed in the astrocytes and neurons of optic nerve as well as in the trabecular meshwork (Clark et al., 2001a; Karali et al., 2000; Swiderski et al., 2000). At least one study of transgenic mice has suggested that optic nerve damage may occur with MYOC mutations without high intraocular pressures (Chou et al., 2014). Consequently, MYOC mutations may also have a more direct influence on glaucoma pathophysiology at the optic head as well as via dysregulating trabecular meshwork function and IOP.
2.6. Genetic testing for MYOC mutations
When directed towards high-risk patients, genetic testing may be able to identify individuals who carry known disease-causing mutations, enabling physicians to plan closer monitoring and/or more aggressive treatment regimens to prevent or minimize vision loss.
General guidelines for genetic testing in ophthalmology have been provided by the American Academy of Ophthalmology. These guidelines suggest that testing may be appropriate if: 1) test results will impact treatment or disease surveillance; 2) tests are performed by Clinical Labs Information Act (CLIA)-certified labs; and 3) tests are conducted by experienced ophthalmic geneticists and/or genetic counselors to ensure proper interpretation of results (Stone et al., 2012). A registry of CLIA certified labs is available at www.ncbi.nlm.gov/gtr.
Because only 3–4% of unselected POAG patients will have a MYOC mutation, indiscriminate testing may have a low yield. However, testing of high-risk patients can be helpful. For example, relatives of those known to have MYOC-associated glaucoma may have up to a 50% risk of carrying a MYOC mutation. Also, testing patients who have early onset of disease, markedly high IOP, and strong family history of disease (i.e. JOAG) may have clinical utility as these patients have a higher likelihood of glaucoma caused by a MYOC mutation (8–63%) (Adam et al., 1997; Wiggs et al., 1998).
3. Optineurin (OPTN)
In 2002, a second gene associated with autosomal dominant inheritance of glaucoma was discovered. Rezaie and coworkers identified mutations in the gene optineurin (OPTN), that were co-inherited with normal tension glaucoma (NTG) in a large pedigree (Rezaie et al., 2002). OPTN mutations were subsequently detected in several case-control studies of NTG patients (Alward et al., 2003; Aung et al., 2003; Hauser et al., 2006; Rezaie et al., 2002). However, no association was detected between OPTN and POAG with elevated IOP (Alward et al., 2003; Aung et al., 2003; Wiggs et al., 2003).
3.1. Epidemiology of OPTN-associated glaucoma
Initially a large proportion of NTG cases were attributed to mutations in OPTN, including Glu50Lys, Asp128Argfs22Ter, Arg545Gln (Rezaie et al., 2002). However, analyses of larger cohorts subsequently indicated that OPTN mutations likely cause 1 to 2% of NTG cases (Alward et al., 2003; Aung et al., 2003; Hauser et al., 2006). Two OPTN mutations, Glu50Lys and Arg545Gln, have been detected in several studies of NTG patients. The Arg545Gln mutation has been detected in some control populations at nearly the same frequency as in NTG populations, arguing against a role in NTG pathogenesis. (Alward et al., 2003; Funayama et al., 2004; Tang et al., 2003; Toda et al., 2004) In contrast, the data linking the Glu50Lys mutation with NTG is strong. The Glu50Lys mutation has been detected in both large NTG pedigrees and in NTG cohorts, but has not been identified in control populations (Alward et al., 2003; Aung et al., 2003; Hauser et al., 2006; Rezaie et al., 2002). Consequently, studies of the Glu50Lys mutation have been the focus of OPTN glaucoma research.
One additional variant in the OPTN gene, Met98Lys, was commonly observed in both NTG patients and in controls. Met98Lys has been detected in as many as 16.5% of control subjects (Funayama et al., 2004). In some populations, generally Caucasian cohorts, the frequency of this mutation is similar among NTG patients and controls (Alward et al., 2003; Aung et al., 2003; Hauser et al., 2006; Weisschuh et al., 2005). However, in other populations, generally Asian cohorts, the Met98Lys mutations has been detected more frequently in NTG patients than in controls (Alward et al., 2003; Funayama et al., 2004; Fuse et al., 2004; Tang et al., 2003; Toda et al., 2004). These data have suggested that although the Met98Lys mutation doesn’t cause NTG, it may confer increased risk for developing glaucoma in some populations. The population allele frequencies of Met98Lys and the OPTN mutations described above are catalogued in Supplemental Table 1.
OPTN mutations have also been associated with amyotrophic lateral sclerosis (ALS) (Maruyama et al., 2010) and are responsible for up to 5% of familial ALS (Toth and Atkin, 2018). Of note, two OPTN mutations first detected in glaucoma patients, Asp128Argfs22Ter and Arg545Gln, have also been associated with autosomal dominant amyotrophic lateral sclerosis (ALS) (Weishaupt et al., 2013). While only limited information is available about the presence or absence of glaucoma in these patients, at least one ALS patient with an Arg545Gln mutation was noted to also have glaucoma (Weishaupt et al., 2013).
3.2. Genotype-phenotype correlations for OPTN-associated glaucoma
Glaucoma patients with a Glu50Lys mutation have been described as having an early-onset of severe optic nerve damage that occurs at low IOP (Table 2). Twelve members of the first glaucoma pedigree found to have a Glu50Lys mutation never had an IOP greater than 22 mmHg (Sarfarazi et al., 1998). The mean age at diagnosis of NTG patients with the Glu50Lys mutation was 40 years. A retrospective case-control study showed that NTG patients with the Gln50Lys mutation had a lower peak IOP, larger cup to disc ratio, more visual field loss, and a higher rate of surgery than matched NTG patients with no known mutation (Aung et al., 2005). Most Glu50Lys mutation reports have come from studies of Caucasian populations and this mutation is less commonly detected among non-Caucasian populations (Hauser et al., 2006). In Japan, where NTG is very common, the Glu50Lys mutation in OPTN has been rarely identified in NTG pedigrees (Minegishi et al., 2016) and did not account for any of the cases of NTG in some case-control studies (Tang et al., 2003; Toda et al., 2004).
Table 2.
Features associated with the OPTN Glu50Lys mutation.
| OPTN Glu50Lys Mutations in 4 cohorts | ||||
|---|---|---|---|---|
| Instances of Glu50Lys per cohort | 7/52 | 1/105 | 2/132 | 1/67 |
| Inheritance pattern | AD | NA | NA | NA |
| Penetrance | 70.40% | NA | NA | NA |
| IOP data (mm Hg) | 81.6% had IOP < 21 | NA | NA | Mean Max IOP = 16 |
| References | Rezaie et al, 2002 | Alward et al, 2003 | Aung et al, 2003 | Hauser et al 2006 |
AD: autosomal dominant, NA: Not available.
3.3. Ocular expression pattern of OPTN
OPTN is expressed early in the development of the eye, and thought to play an integral role in the formation of the optic vesicle in embryogenesis (De Marco et al., 2006; Rezaie and Sarfarazi, 2005; Ying and Yue, 2012). OPTN is also expressed in the adult eye and systemically in many tissues, including heart, liver, testes, kidney, brain, and skeletal muscle (Rezaie and Sarfarazi, 2005). In the eye, OPTN is expressed in the non-pigmented ciliary epithelium, trabecular meshwork, and most highly, in the retinal ganglion cells (Rezaie and Sarfarazi, 2005). OPTN has been further localized within the cytoplasm, perinuclear region, golgi apparatus, and trans-golgi network (De Marco et al., 2006).
3.4. Structure and function of OPTN
The OPTN gene is located at chromosome 10p13 spanning a 37kb genomic region. The gene has 16 exons: three non-coding exons and 13 coding exons (Figure 1) (Rezaie et al., 2002; Ying and Yue, 2012). Alternative splicing results in at least 3 different isoforms (Kumar et al., 2016). The resulting OPTN protein is composed of 577 amino acids and contains numerous domains, including 1) a microtubule associated protein 1 light chain 3 alpha (LC3) binding domain; 2) a ubiquitin binding domain; 3) a zinc finger domain; and 4) a leucine zipper domain (Sahlender et al., 2005; Wild et al., 2011; Ying and Yue, 2012). These domains allow the OPTN protein to be involved in multiple cellular functions, including vesicle trafficking, maintenance of the Golgi apparatus, and autophagy (Ying and Yue, 2016).
Several disparate biological functions have been attributed to OPTN. Some investigations have suggested that OPTN may influence apoptosis. In cells challenged with peroxide (oxidative stress), OPTN translocates from the Golgi apparatus to the nucleus where it may participate in a protective response to this apoptotic stimulus (De Marco et al., 2006). Moreover, OPTN is also thought to regulate intrinsic apoptosis by delaying cytochrome C release from the mitochondria. Cells that overexpress wild-type OPTN were protected against environmental stresses. By contrast, this protective effect was eliminated in cells producing OPTN with the Glu50Lys mutation, which experienced significantly higher rates of apoptosis (De Marco et al., 2006).
OPTN also has a vital role in autophagy and functions as an autophagy receptor (Wild et al., 2011). Autophagy is a process by which cells tag and selectively degrade debris and cellular components and is an important part of cellular homeostasis and cellular repair. This process prevents the accumulation of harmful cellular biproducts and is especially important in neural tissue without the capacity for cell turnover. Targets for degradation by autophagy are captured inside an endosome, also known as an autophagosome, and are delivered to the lysosome where they are eliminated. Cells mark targets for clearance via autophagy by tagging them with ubiquitin. Next, activated OPTN draws ubiquitin-tagged targets into an autphagosome via its binding domains. OPTN’s ubiquitin-binding domain attaches to targets while its LC3-binding domain attaches to the LC3 that is incorporated in the membranes of the autophagosome. Ultimately, their contents are degraded when autophagosomes fuse with lysosomes (Ying and Yue, 2016).
3.5. Pathophysiology of OPTN-associated glaucoma
Several hypotheses about the pathogenesis of OPTN-associated glaucoma have been investigated. In vivo and in vitro studies have both shown that OPTN likely is neuroprotective and that mutations may lead to disease through a downregulation or dysfunction of this protein. One effect of OPTN mutations appears to be dysregulation of oxidative stress-induced apoptosis of retinal ganglion cells (Wild et al., 2011; Ying and Yue, 2016). Due to the relatively high expression of OPTN in the retina, mutations in OPTN may preferentially damage retinal ganglion cells leading to optic nerve vulnerability and NTG. Overexpression of the Glu50Lys OPTN mutation in mice caused massive apoptosis of retinal ganglion cells (Chi et al., 2010).
Mutations within OPTN may also cause an inappropriate and abnormal activation of autophagy leading to irreparable damage to these cells (De Marco et al., 2006; Ying and Yue, 2016). Another glaucoma-causing gene, TANK binding kinase (TBK1) (Fingert et al., 2011), stimulates autophagy by phosphorylating and activating OPTN (Wild et al., 2011). Moreover, the glaucoma-causing Glu50Lys mutation increases OPTN’s affinity for TBK1 (Morton et al., 2008), which may similarly promote phosphorylation and abnormal activation of OPTN. Alternatively, OPTN mutations may cause the autophagy process to be interrupted resulting in partially digested biproducts and the OPTN protein to harmfully build up in the Golgi apparatus (Park et al., 2006). These data suggest that dysregulation of autophagy may have an important role in both OPTN-related and TBK1-related glaucomas.
Another theory suggests that an imbalance in the expression of the three isoforms of OPTN may contribute to glaucoma pathogenesis. A study in 2007 demonstrated that an overexpression of OPTN results in upregulation of myocilin in trabecular meshwork cells, suggesting that one of the isoforms of OPTN may have a role in stabilizing MYOC mRNA. Disruption of this tightly regulated process may result in myocilin build up and subsequent cellular damage (Park et al., 2007).
3.6. Genetic testing for OPTN mutations
Testing unselected patients with open angle glaucoma for OPTN mutations may have low yield. The prevalence of OPTN mutations is estimated to be only 1–2% among NTG patients and even lower among POAG overall (Alward et al., 2003). However, higher risk patients may benefit from testing. Family members of NTG patients with a known Glu50Lys mutation may be at up to 50% risk for inheriting this OPTN mutation and a high likelihood of developing glaucoma. Similarly, NTG patients with early-onset of disease and a strong family history of NTG may also be at higher risk of carrying an OPTN mutation. Testing such higher risk patients may facilitate earlier diagnosis, more accurate prognoses, and earlier institution of therapy which may ultimately help prevent vision loss (Fingert, 2011). Genetic testing may provide useful information to patients and their physicians, but should only be conducted by experienced ophthalmic geneticists and/or with genetic counselors to ensure appropriate tests are selected and that results are properly interpreted and explained to patients. General recommendations (Stone et al., 2012) are more completely described above in section 2.6.
3.7. Gene-targeted therapies for OPTN-associated glaucoma.
Gene-targeted therapies are not yet available for OPTN-associated glaucoma. However, the Glu50Lys mutation is associated a severe form of NTG that has an early onset of disease. Consequently, an early diagnosis may help guide clinicians to more rapidly escalate topical and surgical therapies to improve control of disease.
The efficacy of treating OPTN-associated glaucoma with gene-targeted agents has been investigated with cell culture and animal models of glaucoma. Timolol was shown to reduce aggregation of potentially toxic OPTN protein within stem cell-derived retinal ganglion cells, suggesting that timolol might have clinical utility in addition to pressure reduction in OPTN-associated glaucoma (Inagaki et al., 2018). Another drug, rapamycin, has been studied for its effects on autophagy in a rat model of OPTN glaucoma. Induction of autophagy in this rat model promoted clearance of mutant (Glu50Lys) OPTN protein (Ying et al., 2015) and might suggest future therapeutic avenues for OPTN-associated glaucoma. Finally, a TBK1 inhibitor, amlexanox has been studied in a mouse model of OPTN-associated glaucoma (Minegishi et al., 2016). These preliminary investigations may ultimately lead to new treatment paradigms for patients with glaucoma caused by the OPTN gene.
4. TANK-binding kinase 1 (TBK1)
4.1. Epidemiology of TBK1-associated glaucoma
In 2011, TANK-binding kinase 1 (TBK1) was associated with NTG via linkage analysis studies of a large pedigree with autosomal dominant inheritance of glaucoma (Fingert et al., 2011), making this gene the third and most recent to be identified as a Mendelian cause of POAG. Large duplications of a segment of chromosome 12q14 spanning the TBK1 gene were detected in all members of the NTG pedigree (Fingert et al., 2011). Subsequent reports on cohorts of unrelated NTG patients from around the world found that patients with TBK1 copy number variation (CNV) mutations account for 0.4–1.3% of NTG cases in Caucasian, Japanese, and Indian populations (Awadalla et al., 2015; Fingert et al., 2011; Kaurani et al., 2016; Kawase et al., 2012; Ritch et al., 2014). TBK1 mutations appear to be specifically associated with NTG and mutations have not been linked with additional forms of open angle glaucoma. CNVs spanning the TBK1 gene have not been identified in control subjects, POAG patients with IOP >21 mm Hg (Awadalla et al., 2015; Fingert et al., 2011; Kaurani et al., 2016; Liu et al., 2014), JOAG patients, or individuals with secondary open angle glaucoma including pigmentary glaucoma and steroid-induced glaucoma (Fingert et al., 2016). Only one out of 225 (0.44%) patients with exfoliation glaucoma was found to have a TBK1 duplication (Fingert et al., 2016), which may represent a chance co-occurrence of exfoliation syndrome and NTG. This finding is currently the only example of a TBK1 mutation detected in a patient diagnosed with a form of glaucoma other than NTG, though very rare instances of TBK1 duplications have been reported in large population databases of unexamined individuals (Supplemental Table 1).
4.2. Genotype-phenotype correlations for TBK1-associated glaucoma
The clinical phenotype of NTG patients with a TBK1 gene duplication or triplication has been described (Table 3). The association between TBK1 CNVs and NTG was discovered by genetic analyses of two pedigrees with autosomal dominant inheritance of glaucoma (Fingert et al., 2011). In a large African-American family, ten members were diagnosed with NTG, six of which had extensive medical records that demonstrated an early onset disease (mean age of diagnosis 36 ± 8.2 years). All affected members had normal IOP (mean maximum IOP 18.2 ± 4.1 mmHg, right eye; 16.7 ± 3.6 mmHg, left eye) and were noted at the time of initial examination to have profound optic nerve cupping (mean cup-to-disc ratio 0.95 ± 0.083, right eye; 0.93 ± 0.10, left eye). A duplication of the TBK1 gene was detected in members of this pedigree with NTG. The second pedigree was a Caucasian family in whom eight members were affected by NTG with autosomal dominant inheritance, six of which had extensive medical records documenting their glaucoma phenotype (Awadalla et al., 2015; Bennett et al., 1989; Fingert et al., 2011). Like the African-American pedigree, these family members showed early onset disease with a mean age of diagnosis of 29 ± 6.7 years, normal IOP (mean maximum IOP 19.0 ± 4.3 mmHg, right eye; 18.8 ± 3.8, left eye), and significant though less severe optic nerve cupping at diagnosis (mean cup-to-disc ratios of 0.85 ± 0.071). A triplication of the TBK1 gene was detected in those members of this Caucasian pedigree that were diagnosed with NTG. In both pedigrees, the glaucoma phenotype of TBK1 duplications was characterized by severe, early onset optic nerve damage occurring at normal IOP.
Table 3.
Features associated with TBK1 mutations.
| Original pedigree with a TBK1 duplication | TBK1 Copy Number Variations in 5 cohorts | |||||
|---|---|---|---|---|---|---|
| Instances of CNVs | NA | 2/152 | 4/334 | 2/158 | 1/96 | 1/252 |
| Ancestry | African American | Caucasian (USA) | Caucaisian (Austrailia) | Asian (India) | Caucasian (U.S.A.) | Asian (Japan) |
| Inheritance pattern | AD | AD | AD | NA | NA | NA |
| Prevalence | NA | 1.3% of NTG | 1.2% of NTG | 1.3% of NTG | 1% of NTG | 0.4% of NTG |
| CNV type | Duplication | Duplication (1/2) Triplication (1/2) | Duplication (3/4) Triplication (1/4) | Duplication | Duplication | Duplication |
| Mean age at diagnosis in years | 36 | 29 | 45 | 63 | 47 | 42 |
| Mean Maximum IOP in mm Hg | 18 | 19 | 14 | 16 | 16 | 18 |
| References | Fingert et al., 2011 | Fingert et al., 2011 | Awadalla et al., 2015 | Kaurani, 2016 | Ritch, 2014 | Kawase, 2011 |
AD: autosomal dominant, NA: Not applicable or not available.
4.3. Ocular expression pattern of TBK1
While TBK1 has broad expression throughout the human body and the eye, immunohistochemical analyses of human donor eyes demonstrated that TBK1 protein was most abundant in the ganglion cell layer of the retina (Fingert et al., 2011). A similar pattern of TBK1 production was observed in mouse eyes (Fingert et al., 2017).
4.4. Structure and function of TBK1
The TBK1 gene is composed of 24 exons and encodes a 729 amino acid kinase protein (Figure 1) with an estimated molecular weight of 84 kDa (Tojima et al., 2000). Four functional domains of the human TBK1 gene have been described: the kinase domain, the ubiquitin-like domain, the α-helical scaffold dimerization domain, and a carboxy-terminal domain. The crystal structure of human TBK1 has been solved and suggests that TBK1 forms dimers and is activated by phosphorylation of Ser172 and ubiquitination of the dimer (Larabi et al., 2013; Li et al., 2012; Tu et al., 2013)
TBK1 is a serine-threonine kinase best characterized for its role in antiviral responses of the innate immune system as well as its regulatory roles in humoral immunity and autophagy (Louis et al., 2018). TBK1 is ubiquitously expressed (Tojima et al., 2000) and essential for life as homozygous deletion of the gene has been shown to be lethal during embryogenesis in mice due to liver degeneration and apoptosis (Bonnard et al., 2000). TBK1 plays a key role in regulating immune responses by activating expression of factors involved in inflammation and host defense, including factors stimulated by NF-KB, IRF3, and IRF7 (Pomerantz and Baltimore, 1999; Sharma et al., 2003). Furthermore, TBK1 plays an important role in autophagy, a process essential for eukaryotic cell survival and maintenance that is responsible for the degradation and recycling of cytoplasmic damaged organelles (Heo et al., 2015; Lazarou et al., 2015), proteins (Korac et al., 2013), macromolecutes, and breakdown products. TBK1 activates autophagy proteins OPTN (Wild et al., 2011) and p62 (Pilli et al., 2012) to control autophagosome maturation into lytic bactericidal organelles. Specifically, TBK1 phosphorylates Ser177 of OPTN which activates it as an autophagy receptor. Phospho-OPTN brings cellular targets such as damaged mitochondria into autophagosomes for degradation (Wild et al., 2011).
Studies of transgenic mice have investigated the effects of TBK1 duplications in vivo (Fingert et al., 2017). Transgenic mice were engineered to have one wild-type copy of human TBK1 and its native promoter added to their genomes, resulting in a total of three copies of the gene (one human TBK1 transgene and two native mouse Tbk1 genes). Like the human TBK1 phenotype, IOP of these mice was found to be no different than measured in wild-type littermates at a range of ages (5 weeks to 18 months). Immunohistochemical assays performed on retinas of transgenic TBK1 mice demonstrated that an extra copy of the TBK1 gene increased expression of the TBK1 protein in retinal ganglion cells (Fingert et al., 2017). Moreover, transgenic TBK1 mice developed progressive loss of retinal ganglion cells as they aged. Mice with two copies of the TBK1 transgene in their genomes had even more RGC loss, suggesting a dose response between the number of TBK1 transgenes and glaucomatous damage. These data provide compelling evidence that TBK1 gene duplications or triplications are pathogenic to retinal ganglion cells in both humans and mice with normal intraocular pressure (Fingert et al., 2017).
4.5. Pathophysiology of TBK1-associated glaucoma
The key roles of TBK1 and OPTN in autophagy and specifically mitophagy (degradation of mitochondria by autophagy) have been proposed as an important pathophysiologic mechanism in NTG as well as other neurodegenerative diseases. Amyotrophic lateral sclerosis (ALS) has been linked to different mutations in these same genes. Damaged mitochondria were shown to accumulate when TBK1 is either chemically inhibited or its expression is knocked down with siRNA, indicating impairment of mitophagy in ALS-associated mutations in OPTN and TBK1 (Moore and Holzbaur, 2016).
Other TBK1 mutations that are associated with NTG, e.g. TBK1 gene duplications and triplications, have been shown to increase expression of the TBK1 protein and may also cause dysregulated autophagy. Immunohistochemical studies of human retina have shown that TBK1 protein is localized to retinal ganglion cells and the retinal nerve fiber layer, the cells affected by glaucoma (Fingert et al., 2011; 2014). Studies of patient-derived cells have also suggested that TBK1 gene duplications may alter the function of autophagy in retinal ganglion cell-like neurons. Tucker et al. has produced retinal ganglion cell-like neurons from skin biopsies of patients with NTG caused by a TBK1 gene duplication using induced pluripotent stem cell methods (Tucker et al., 2014). Compared with controls, retinal ganglion cell-like neurons with a TBK1 gene duplication have increased levels of lipidated LC3 (a component of autophagosome membranes and marker of autophagy activity). This finding suggests that TBK1 duplications may abnormally stimulate autophagy in retinal ganglion cells and subsequently contribute to their demise. However, the precise mechanism by which aberrant autophagy or mitophagy may promote retinal ganglion cell death remains unclear. Though caused by different sets of mutations and phenotypically distinct, the complex pathophysiology underlying ALS and NTG may share some common mechanisms involving TBK1 and OPTN.
4.6. Genetic testing for TBK1 mutations
Testing for TBK1 mutations associated with glaucoma is not currently available for clinical use.
4.7. Gene-targeted therapies for TBK1-associated glaucoma.
The discovery that TBK1 (or OPTN) mutations may cause glaucoma via dysregulation of autophagy has suggested new modes of therapy. Inhibition of TBK1 kinase to block phosphorylation/activation of OPTN and subsequent stimulation of autophagy has been explored as a possible therapy for glaucoma associated with TBK1 or OPTN mutations. Minegishi et al. investigated the impact of amlexanox, a TBK1 kinase inhibitor, on retinal ganglion cell loss in transgenic knock-in mice engineered to have an Optn mutation, Glu50Lys (Minegishi et al., 2016). Amlexanox was shown to prevent retinal ganglion cell loss associated with the Glu50Lys mutation, possibly via its effects on autophagy. Amlexanox or other TBK1 kinase inhibitors may have utility for treating OPTN and TBK1-associated glaucoma. While additional studies are needed to make such drugs available for clinical use, these findings serve as compelling evidence that personalized therapies for at least a subset of inherited open angle glaucoma is a possibility.
Supplementary Material
Supplemental Table 1. The population allele frequencies of MYOC, OPTN, and TBK1 variants described in this report. Allele frequencies were determined from the Exome Aggregation Consortium (ExAC) database: http://exac.broadinstitute.org. NA: Not Available. 1Indicates that a variant was not observed in the ExAC population.
Acknowledgements
This work was supported in part by an unrestricted grant from Research to Prevent Blindness and from the Hadley Carver Chair in Glaucoma
Footnotes
Declaration of interests
Nil.
References
- Adam MF, Belmouden A, Binisti P, Brézin AP, Valtot F, Béchetoille A, Dascotte JC, Copin B, Gomez L, Chaventré A, Bach JF, Garchon H-J, 1997. Recurrent mutations in a single exon encoding the evolutionarily conserved olfactomedin-homology domain of TIGR in familial open-angle glaucoma. Hum Mol Genet 6, 2091–2097. [DOI] [PubMed] [Google Scholar]
- Allingham RR, Wiggs JL, Paz La, De MA, Vollrath D, Tallett DA, Broomer B, Jones KH, Del Bono EA, Kern J, Patterson K, Haines JL, Pericak-Vance MA, 1998. Gln368STOP myocilin mutation in families with late-onset primary open-angle glaucoma. Invest Ophthalmol Vis Sci 39, 2288–2295. [PubMed] [Google Scholar]
- Alward WL, Fingert JH, Coote MA, Johnson AT, Lerner SF, Junqua D, Durcan FJ, McCartney PJ, Mackey DA, Sheffield VC, Stone EM, 1998. Clinical features associated with mutations in the chromosome 1 open-angle glaucoma gene (GLC1A). N Engl J Med 338, 1022–1027. doi: 10.1056/NEJM199804093381503 [DOI] [PubMed] [Google Scholar]
- Alward WLM, Kwon YH, Kawase K, Craig JE, Hayreh SS, Johnson AT, Khanna CL, Yamamoto T, Mackey DA, Roos BR, Affatigato LM, Sheffield VC, Stone EM, 2003. Evaluation of optineurin sequence variations in 1,048 patients with open-angle glaucoma. Am J Ophthalmol 136, 904–910. [DOI] [PubMed] [Google Scholar]
- Angius A, Spinelli P, Ghilotti G, Casu G, Sole G, Loi A, Totaro A, Zelante L, Gasparini P, Orzalesi N, Pirastu M, Bonomi L, 2000. Myocilin Gln368stop mutation and advanced age as risk factors for late-onset primary open-angle glaucoma. Arch Ophthalmol 118, 674–679. [DOI] [PubMed] [Google Scholar]
- Aung T, Ebenezer ND, Brice G, Child AH, Prescott Q, Lehmann OJ, Hitchings RA, Bhattacharya SS, 2003. Prevalence of optineurin sequence variants in adult primary open angle glaucoma: implications for diagnostic testing. J Med Genet 40, e101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aung T, Rezaie T, Okada K, Viswanathan AC, Child AH, Brice G, Bhattacharya SS, Lehmann OJ, Sarfarazi M, Hitchings RA, 2005. Clinical features and course of patients with glaucoma with the E50K mutation in the optineurin gene. Invest Ophthalmol Vis Sci 46, 2816–2822. doi: 10.1167/iovs.04-1133 [DOI] [PubMed] [Google Scholar]
- Awadalla MS, Fingert JH, Roos BE, Chen S, Holmes R, Galanopolous A, Ridge B, Souzeau E, Siggs OM, Hewitt AW, Mackey DA, Burdon KP, Craig JE, 2015. Copy Number Variations of TBK1 in Australian Patients With Primary Open-Angle Glaucoma. Am J Ophthalmol 159, 124–130.e1. doi: 10.1016/j.ajo.2014.09.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker B, Roth FD, Kolker AE, 1960. Glaucoma family study. Am J Ophthalmol 50, 557–567. [DOI] [PubMed] [Google Scholar]
- Bhattacharjee A, Acharya M, Mukhopadhyay A, Mookherjee S, Banerjee D, Bandopadhyay AK, Thakur SKD, Sen A, Ray K, 2007. Myocilin variants in Indian patients with open-angle glaucoma. Arch Ophthalmol 125, 823–829. doi: 10.1001/archopht.125.6.823 [DOI] [PubMed] [Google Scholar]
- Bonnard M, Mirtsos C, Suzuki S, Graham K, Huang J, Ng M, Itié A, Wakeham A, Shahinian A, Henzel WJ, Elia AJ, Shillinglaw W, Mak TW, Cao Z, Yeh WC, 2000. Deficiency of T2K leads to apoptotic liver degeneration and impaired NF-kappaB-dependent gene transcription. EMBO J 19, 4976–4985. doi: 10.1093/emboj/19.18.4976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns JN, Orwig SD, Harris JL, Watkins JD, Vollrath D, Lieberman RL, 2010. Rescue of glaucoma-causing mutant myocilin thermal stability by chemical chaperones. ACS Chem. Biol. 5, 477–487. doi: 10.1021/cb900282e [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi Z-L, Akahori M, Obazawa M, Minami M, Noda T, Nakaya N, Tomarev S, Kawase K, Yamamoto T, Noda S, Sasaoka M, Shimazaki A, Takada Y, Iwata T, 2010. Overexpression of optineurin E50K disrupts Rab8 interaction and leads to a progressive retinal degeneration in mice. Hum Mol Genet 19, 2606–2615. doi: 10.1093/hmg/ddq146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choquet H, Thai KK, Yin J, Hoffmann TJ, Kvale MN, Banda Y, Schaefer C, Risch N, Nair KS, Melles R, Jorgenson E, 2017. A large multi-ethnic genome-wide association study identifies novel genetic loci for intraocular pressure. Nat Commun 8, 2108. doi: 10.1038/s41467-017-01913-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou T-H, Tomarev S, Porciatti V, 2014. Transgenic mice expressing mutated Tyr437His human myocilin develop progressive loss of retinal ganglion cell electrical responsiveness and axonopathy with normal iop. Invest Ophthalmol Vis Sci 55, 5602–5609. doi: 10.1167/iovs.14-14793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark AF, Kawase K, English-Wright S, Lane D, Steely HT, Yamamoto T, Kitazawa Y, Kwon YH, Fingert JH, Swiderski RE, Mullins RF, Hageman GS, Alward WL, Sheffield VC, Stone EM, 2001a. Expression of the glaucoma gene myocilin (MYOC) in the human optic nerve head. FASEB J 15, 1251–1253. [DOI] [PubMed] [Google Scholar]
- Clark AF, Steely HT, Dickerson JE, English-Wright S, Stropki K, McCartney MD, Jacobson N, Shepard AR, Clark JI, Matsushima H, Peskind ER, Leverenz JB, Wilkinson CW, Swiderski RE, Fingert JH, Sheffield VC, Stone EM, 2001b. Glucocorticoid induction of the glaucoma gene MYOC in human and monkey trabecular meshwork cells and tissues. Invest Ophthalmol Vis Sci 42, 1769–1780. [PubMed] [Google Scholar]
- Craig JE, Baird PN, Healey DL, McNaught AI, McCartney PJ, Rait JL, Dickinson JL, Roe L, Fingert JH, Stone EM, Mackey DA, 2001. Evidence for genetic heterogeneity within eight glaucoma families, with the GLC1A Gln368STOP mutation being an important phenotypic modifier. Ophthalmology 108, 1607–1620. [DOI] [PubMed] [Google Scholar]
- De Marco N, Buono M, Troise F, Diez-Roux G, 2006. Optineurin increases cell survival and translocates to the nucleus in a Rab8-dependent manner upon an apoptotic stimulus. J Biol Chem 281, 16147–16156. doi: 10.1074/jbc.M601467200 [DOI] [PubMed] [Google Scholar]
- Donegan RK, Hill SE, Freeman DM, Nguyen E, Orwig SD, Turnage KC, Lieberman RL, 2015. Structural basis for misfolding in myocilin-associated glaucoma. Hum Mol Genet 24, 2111–2124. doi: 10.1093/hmg/ddu730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fingert JH, 2019. Penetrance of Myocilin Mutations-Who Gets Glaucoma? JAMA ophthalmology 137. doi: 10.1001/jamaophthalmol.2018.4470 [DOI] [PubMed] [Google Scholar]
- Fingert JH, 2011. Primary open-angle glaucoma genes. Eye 25, 587–595. doi: 10.1038/eye.2011.97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fingert JH, darbro BW, Qian Q, Van Rheeden R, Miller K, Riker M, Solivan-Timpe F, Roos BR, Robin AL, Mullins RF, 2014. TBK1 and flanking genes in human retina. Ophthalmic Genetics 35, 35–40. doi: 10.3109/13816810.2013.768674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fingert JH, Héon E, Liebmann JM, Yamamoto T, Craig JE, Rait J, Kawase K, Hoh ST, Buys YM, Dickinson J, Hockey RR, Williams-Lyn D, Trope G, Kitazawa Y, Ritch R, Mackey DA, Alward WL, Sheffield VC, Stone EM, 1999. Analysis of myocilin mutations in 1703 glaucoma patients from five different populations. Hum Mol Genet 8, 899–905. [DOI] [PubMed] [Google Scholar]
- Fingert JH, Miller K, Hedberg-Buenz A, Roos BR, Lewis CJ, Mullins RF, Anderson MG, 2017. Transgenic TBK1 mice have features of normal tension glaucoma. Hum Mol Genet 26, 124–132. doi: 10.1093/hmg/ddw372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fingert JH, Robin AL, Roos Ben R, Davis LK, Scheetz TE, Wassink TH, Kwon YH, Alward WLM, Mullins RF, Sheffield VC, Stone EM, 2011. Copy number variations on chromosome 12q14 in patients with normal tension glaucoma. Hum Mol Genet 20, 2482–2494. doi: 10.1093/hmg/ddr123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fingert JH, Robin AL, Scheetz TE, Kwon YH, Liebmann JM, Ritch R, Alward WLM, 2016. Tank-Binding Kinase 1 (TBK1) Gene and Open-Angle Glaucomas (An American Ophthalmological Society Thesis). Trans Am Ophthalmol Soc 114, T6. [PMC free article] [PubMed] [Google Scholar]
- Fingert JH, Stone EM, Sheffield VC, Alward WLM, 2002. Myocilin glaucoma. Survey of Ophthalmology 47, 547–561. [DOI] [PubMed] [Google Scholar]
- Fingert JH, Ying L, Swiderski RE, Nystuen AM, Arbour NC, Alward WL, Sheffield VC, Stone EM, 1998. Characterization and comparison of the human and mouse GLC1A glaucoma genes. Genome Res 8, 377–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- François J, 1966. Genetics and primary open-angle glaucoma. Am J Ophthalmol 61, 652–665. [DOI] [PubMed] [Google Scholar]
- Funayama T, Ishikawa K, Ohtake Y, Tanino T, Kurosaka D, Kimura I, Suzuki K, Ideta H, Nakamoto K, Yasuda N, Fujimaki T, Murakami A, Asaoka R, Hotta Y, Tanihara H, Kanamoto T, Mishima H, Fukuchi T, Abe H, Iwata T, Shimada N, Kudoh J, Shimizu N, Mashima Y, 2004. Variants in optineurin gene and their association with tumor necrosis factor-alpha polymorphisms in Japanese patients with glaucoma. Invest Ophthalmol Vis Sci 45, 4359–4367. doi: 10.1167/iovs.03-1403 [DOI] [PubMed] [Google Scholar]
- Fuse N, Takahashi K, Akiyama H, Nakazawa T, Seimiya M, Kuwahara S, Tamai M, 2004. Molecular genetic analysis of optineurin gene for primary open-angle and normal tension glaucoma in the Japanese population. J Glaucoma 13, 299–303. [DOI] [PubMed] [Google Scholar]
- Gharahkhani P, Burdon KP, Cooke Bailey JN, Hewitt AW, Law MH, Pasquale LR, Kang JH, Haines JL, Souzeau E, Zhou T, Siggs OM, Landers J, Awadalla M, Sharma S, Mills RA, Ridge B, Lynn D, Casson R, Graham SL, Goldberg I, White A, Healey PR, Grigg J, Lawlor M, Mitchell P, Ruddle J, Coote M, Walland M, Best S, Vincent A, Gale J, RadfordSmith G, Whiteman DC, Montgomery GW, Martin NG, Mackey DA, Wiggs JL, MacGregor S, Craig JE, NEIGHBORHOOD Consortium, 2018. Analysis combining correlated glaucoma traits identifies five new risk loci for open-angle glaucoma. Sci Rep 8, 3124. doi: 10.1038/s41598-018-20435-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong G, Kosoko-Lasaki O, Haynatzki GR, Wilson MR, 2004. Genetic dissection of myocilin glaucoma. Hum Mol Genet 13 Spec No 1, R91–102. doi: 10.1093/hmg/ddh074 [DOI] [PubMed] [Google Scholar]
- Graefe, von ,A, 1869. Beiträge zur Pathologie und Therapie des Glaucoms 15, 108–252. [Google Scholar]
- Graul TA, Kwon YH, Zimmerman MB, Kim C-S, Sheffield VC, Stone EM, Alward WLM, 2002. A case-control comparison of the clinical characteristics of glaucoma and ocular hypertensive patients with and without the myocilin Gln368Stop mutation. Am J Ophthalmol 134, 884–890. [DOI] [PubMed] [Google Scholar]
- Han X, Souzeau E, Ong J-S, An J, Siggs OM, Burdon KP, Best S, Goldberg I, Healey PR, Graham SL, Ruddle JB, Mills RA, Landers J, Galanopoulos A, White AJR, Casson R, Mackey DA, Hewitt AW, Gharahkhani P, Craig JE, MacGregor S, 2018. Myocilin Gene Gln368Ter Variant Penetrance and Association With Glaucoma in Population-Based and Registry-Based Studies. JAMA ophthalmology. doi: 10.1001/jamaophthalmol.2018.4477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser MA, Sena DF, Flor J, Walter J, Auguste J, LaRocque-Abramson K, Graham F, DelBono E, Haines JL, Pericak-Vance MA, Rand Allingham R, Wiggs JL, 2006. Distribution of optineurin sequence variations in an ethnically diverse population of low-tension glaucoma patients from the United States. J Glaucoma 15, 358–363. doi: 10.1097/01.ijg.0000212255.17950.42 [DOI] [PubMed] [Google Scholar]
- Heo J-M, Ordureau A, Paulo JA, Rinehart J, Harper JW, 2015. The PINK1-PARKIN Mitochondrial Ubiquitylation Pathway Drives a Program of OPTN/NDP52 Recruitment and TBK1 Activation to Promote Mitophagy. Mol. Cell 60, 7–20. doi: 10.1016/j.molcel.2015.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewitt AW, Mackey DA, Craig JE, 2008. Myocilin allele-specific glaucoma phenotype database. Hum Mutat 29, 207–211. doi: 10.1002/humu.20634 [DOI] [PubMed] [Google Scholar]
- Inagaki S, Kawase K, Funato M, Seki J, Kawase C, Ohuchi K, Kameyama T, Ando S, Sato A, Morozumi W, Nakamura S, Shimazawa M, Iejima D, Iwata T, Yamamoto T, Kaneko H, Hara H, 2018. Effect of Timolol on Optineurin Aggregation in Transformed Induced Pluripotent Stem Cells Derived From Patient With Familial Glaucoma. Invest Ophthalmol Vis Sci 59, 2293–2304. doi: 10.1167/iovs.17-22975 [DOI] [PubMed] [Google Scholar]
- Jacobson N, Andrews M, Shepard AR, Nishimura D, Searby C, Fingert JH, Hageman G, Mullins R, Davidson BL, Kwon YH, Alward WL, Stone EM, Clark AF, Sheffield VC, 2001. Non-secretion of mutant proteins of the glaucoma gene myocilin in cultured trabecular meshwork cells and in aqueous humor. Hum Mol Genet 10, 117–125. [DOI] [PubMed] [Google Scholar]
- Jain A, Zode G, Kasetti RB, Ran FA, Yan W, Sharma TP, Bugge K, Searby CC, Fingert JH, Zhang F, Clark AF, Sheffield VC, 2017. CRISPR-Cas9-based treatment of myocilin-associated glaucoma. Proceedings of the National Academy of Sciences 114, 11199–11204. doi: 10.1073/pnas.1706193114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joe MK, Sohn S, Hur W, Moon Y, Choi YR, Kee C, 2003. Accumulation of mutant myocilins in ER leads to ER stress and potential cytotoxicity in human trabecular meshwork cells. Biochem Biophys Res Commun 312, 592–600. doi: 10.1016/j.bbrc.2003.10.162 [DOI] [PubMed] [Google Scholar]
- Johnson AT, Drack AV, Kwitek AE, Cannon RL, Stone EM, Alward WL, 1993. Clinical features and linkage analysis of a family with autosomal dominant juvenile glaucoma. Ophthalmology 100, 524–529. [DOI] [PubMed] [Google Scholar]
- Kahn HA, Milton RC, 1980. Revised Framingham eye study prevalence of glaucoma and diabetic retinopathy. Am J Epidemiol 111, 769–776. [DOI] [PubMed] [Google Scholar]
- Karali A, Russell P, Stefani FH, Tamm ER, 2000. Localization of myocilin/trabecular meshwork--inducible glucocorticoid response protein in the human eye. Invest Ophthalmol Vis Sci 41, 729–740. [PubMed] [Google Scholar]
- Kaurani L, Vishal M, Ray J, Sen A, Ray K, Mukhopadhyay A, 2016. TBK1 duplication is found in normal tension and not in high tension glaucoma patients of Indian origin. J. Genet. 95, 459–461. [DOI] [PubMed] [Google Scholar]
- Kawase K, Allingham RR, Meguro A, Mizuki N, Roos B, Solivan-Timpe FM, Robin AL, Ritch R, Fingert JH, 2012. Confirmation of TBK1 duplication in normal tension glaucoma. Exp Eye Res 96, 178–180. doi: 10.1016/j.exer.2011.12.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennan AM, Mansergh FC, Fingert JH, Clark T, Ayuso C, Kenna PF, Humphries P, Farrar GJ, 1998. A novel Asp380Ala mutation in the GLC1A/myocilin gene in a family with juvenile onset primary open angle glaucoma. J Med Genet 35, 957–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim BS, Savinova OV, Reedy MV, Martin J, Lun Y, Gan L, Smith RS, Tomarev SI, John SW, Johnson RL, 2001. Targeted Disruption of the Myocilin Gene (Myoc) Suggests that Human Glaucoma-Causing Mutations Are Gain of Function. Mol Cell Biol 21, 7707–7713. doi: 10.1128/MCB.21.22.7707-7713.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korac J, Schaeffer V, Kovacevic I, Clement AM, Jungblut B, Behl C, Terzic J, Dikic I, 2013. Ubiquitin-independent function of optineurin in autophagic clearance of protein aggregates. J Cell Sci 126, 580–592. doi: 10.1242/jcs.114926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Malik MA, Goswami S, Sihota R, Kaur J, 2016. Candidate genes involved in the susceptibility of primary open angle glaucoma. Gene … 577, 119–131. doi: 10.1016/j.gene.2015.11.032 [DOI] [PubMed] [Google Scholar]
- Kwon YH, Fingert JH, Kuehn MH, Alward WLM, 2009. Primary Open-Angle Glaucoma. N Engl J Med 360, 1113–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam DS, Leung YF, Chua JK, Baum L, Fan DS, Choy KW, Pang CP, 2000. Truncations in the TIGR gene in individuals with and without primary open-angle glaucoma. Invest Ophthalmol Vis Sci 41, 1386–1391. [PubMed] [Google Scholar]
- Larabi A, Devos JM, Ng S-L, Nanao MH, Round A, Maniatis T, Panne D, 2013. Crystal structure and mechanism of activation of TANK-binding kinase 1. Cell Reports 3, 734–746. doi: 10.1016/j.celrep.2013.01.034 [DOI] [PubMed] [Google Scholar]
- Lazarou M, Sliter DA, Kane LA, Sarraf SA, Wang C, Burman JL, Sideris DP, Fogel AI, Youle RJ, 2015. The ubiquitin kinase PINK1 recruits autophagy receptors to induce mitophagy. Nature 524, 309–314. doi: 10.1038/nature14893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leske MC, Podgor MJ, 1983. Intraocular pressure, cardiovascular risk variables, and visual field defects. Am J Epidemiol 118, 280–287. [DOI] [PubMed] [Google Scholar]
- Li J, Li J, Miyahira A, Sun J, Liu Y, Cheng G, Liang H, 2012. Crystal structure of the ubiquitin-like domain of human TBK1. Protein Cell 3, 383–391. doi: 10.1007/s13238-012-2929-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Garrett ME, Yaspan BL, Bailey JC, Loomis SJ, Brilliant M, Budenz DL, Christen WG, Fingert JH, Gaasterland D, Gaasterland T, Kang JH, Lee RK, Lichter P, Moroi SE, Realini A, Richards JE, Schuman JS, Scott WK, Singh K, Sit AJ, Vollrath D, Weinreb R, Wollstein G, Zack DJ, Zhang K, Pericak-Vance MA, Haines JL, Pasquale LR, Wiggs JL, Allingham RR, Ashley-Koch AE, Hauser MA, 2014. DNA copy number variants of known glaucoma genes in relation to primary open-angle glaucoma. Invest Ophthalmol Vis Sci 55, 8251–8258. doi: 10.1167/iovs.14-15712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis C, Burns C, Wicks I, 2018. TANK-Binding Kinase 1-Dependent Responses in Health and Autoimmunity. Front Immunol 9, 434. doi: 10.3389/fimmu.2018.00434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansergh FC, Kenna PF, Ayuso C, Kiang AS, Humphries P, Farrar GJ, 1998. Novel mutations in the TIGR gene in early and late onset open angle glaucoma. Hum Mutat 11, 244–251. doi: [DOI] [PubMed] [Google Scholar]
- Maruyama H, Morino H, Ito H, Izumi Y, Kato H, Watanabe Y, Kinoshita Y, Kamada M, Nodera H, Suzuki H, Komure O, Matsuura S, Kobatake K, Morimoto N, Abe K, Suzuki N, Aoki M, Kawata A, Hirai T, Kato T, Ogasawara K, Hirano A, Takumi T, Kusaka H, Hagiwara K, Kaji R, Kawakami H, 2010. Mutations of optineurin in amyotrophic lateral sclerosis. Nature 465, 223–226. doi: 10.1038/nature08971 [DOI] [PubMed] [Google Scholar]
- Minegishi Y, Nakayama M, Iejima D, Kawase K, Iwata T, 2016. Significance of optineurin mutations in glaucoma and other diseases. Prog Retin Eye Res 55, 149–181. doi: 10.1016/j.preteyeres.2016.08.002 [DOI] [PubMed] [Google Scholar]
- Moore AS, Holzbaur ELF, 2016. Dynamic recruitment and activation of ALS-associated TBK1 with its target optineurin are required for efficient mitophagy. Proceedings of the National Academy of Sciences 113, E3349–58. doi: 10.1073/pnas.1523810113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton S, Peggie M, Cohen P, 2008. Enhanced binding of TBK1 by an optineurin mutant that causes a familial form of primary open angle glaucoma. FEBS Lett 582, 997–1002. doi: 10.1016/j.febslet.2008.02.047 [DOI] [PubMed] [Google Scholar]
- Nag A, Lu H, Arno M, Iglesias AI, Bonnemaijer P, Broer L, Uitterlinden AG, Klaver CCW, van Duijn C, Hysi PG, Hammond CJ, 2016. Evaluation of the Myocilin Mutation Gln368Stop Demonstrates Reduced Penetrance for Glaucoma in European Populations. Ophthalmology. doi: 10.1016/j.ophtha.2016.11.018 [DOI] [PubMed] [Google Scholar]
- Nguyen TD, Chen P, Huang WD, Chen H, Johnson D, Polansky JR, 1998. Gene structure and properties of TIGR, an olfactomedin-related glycoprotein cloned from glucocorticoid-induced trabecular meshwork cells. J Biol Chem 273, 6341–6350. [DOI] [PubMed] [Google Scholar]
- Ozcan U, Yilmaz E, Ozcan L, Furuhashi M, Vaillancourt E, Smith RO, Görgün CZ, Hotamisligil GS, 2006. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science 313, 1137–1140. doi: 10.1126/science.1128294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang CP, Leung YF, Fan B, Baum L, Tong WC, Lee WS, Chua JKH, Fan DSP, Liu Y, Lam DSC, 2002. TIGR/MYOC gene sequence alterations in individuals with and without primary open-angle glaucoma. Invest Ophthalmol Vis Sci 43, 3231–3235. [PubMed] [Google Scholar]
- Park B-C, Shen X, Samaraweera M, Yue BYJT, 2006. Studies of optineurin, a glaucoma gene: Golgi fragmentation and cell death from overexpression of wild-type and mutant optineurin in two ocular cell types. Am J Pathol 169, 1976–1989. doi: 10.2353/ajpath.2006.060400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park B-C, Tibudan M, Samaraweera M, Shen X, Yue BYJT, 2007. Interaction between two glaucoma genes, optineurin and myocilin. Genes Cells 12, 969–979. doi: 10.1111/j.1365-2443.2007.01102.x [DOI] [PubMed] [Google Scholar]
- Pilli M, Arko-Mensah J, Ponpuak M, Roberts E, Master S, Mandell MA, Dupont N, Ornatowski W, Jiang S, Bradfute SB, Bruun J-A, Hansen TE, Deretic V, 2012. TBK-1 promotes autophagy-mediated antimicrobial defense by controlling autophagosome maturation. Immunity 37, 223–234. doi: 10.1016/j.immuni.2012.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polansky JR, Fauss DJ, Chen P, Chen H, Lütjen-Drecoll E, Johnson D, Kurtz RM, Ma ZD, Bloom E, Nguyen TD, 1997. Cellular pharmacology and molecular biology of the trabecular meshwork inducible glucocorticoid response gene product. Ophthalmologica 211, 126–139. [DOI] [PubMed] [Google Scholar]
- Polubriaginof FCG, Vanguri R, Quinnies K, Belbin GM, Yahi A, Salmasian H, Lorberbaum T, Nwankwo V, Li L, Shervey MM, Glowe P, Ionita-Laza I, Simmerling M, Hripcsak G, Bakken S, Goldstein D, Kiryluk K, Kenny EE, Dudley J, Vawdrey DK, Tatonetti NP, 2018. Disease Heritability Inferred from Familial Relationships Reported in Medical Records. Cell 173, 1692–1704.e11. doi: 10.1016/j.cell.2018.04.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quigley HA, Broman AT, 2006. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol 90, 262–267. doi: 10.1136/bjo.2005.081224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezaie T, Child A, Hitchings R, Brice G, Miller L, Coca-Prados M, Héon E, Krupin T, Ritch R, Kreutzer D, Crick RP, Sarfarazi M, 2002. Adult-onset primary open-angle glaucoma caused by mutations in optineurin. Science 295, 1077–1079. doi: 10.1126/science.1066901 [DOI] [PubMed] [Google Scholar]
- Rezaie T, Sarfarazi M, 2005. Molecular cloning, genomic structure, and protein characterization of mouse optineurin. Genomics 85, 131–138. doi: 10.1016/j.ygeno.2004.10.011 [DOI] [PubMed] [Google Scholar]
- Ritch R, Darbro B, menon G, Khanna CL, Solivan-Timpe F, Roos BR, Sarfarzi M, Kawase K, Yamamoto T, Robin AL, Lotery AJ, Fingert JH, 2014. TBK1 Gene Duplication and Normal-Tension Glaucoma. JAMA ophthalmology 132, 544–548. doi: 10.1001/jamaophthalmol.2014.104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahlender DA, Roberts RC, Arden SD, Spudich G, Taylor MJ, Luzio JP, Kendrick-Jones J, Buss F, 2005. Optineurin links myosin VI to the Golgi complex and is involved in Golgi organization and exocytosis. J Cell Biol 169, 285–295. doi: 10.1083/jcb.200501162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarfarazi M, Child A, Stoilova D, Brice G, Desai T, Trifan OC, Poinoosawmy D, Crick RP, 1998. Localization of the fourth locus (GLC1E) for adult-onset primary open-angle glaucoma to the 10p15-p14 region. Am J Hum Genet 62, 641–652. doi: 10.1086/301767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheffield VC, Stone EM, Alward WL, Drack AV, Johnson AT, Streb LM, Nichols BE, 1993. Genetic linkage of familial open angle glaucoma to chromosome 1q21-q31. Nat Genet 4, 47–50. doi: 10.1038/ng0593-47 [DOI] [PubMed] [Google Scholar]
- Shiga Y, Akiyama M, Nishiguchi KM, Sato K, Shimozawa N, Takahashi A, Momozawa Y, Hirata M, Matsuda K, Yamaji T, Iwasaki M, Tsugane S, Oze I, Mikami H, Naito M, Wakai K, Yoshikawa M, Miyake M, Yamashiro K, Japan Glaucoma Society Omics Group (JGS-OG), Kashiwagi K, Iwata T, Mabuchi F, Takamoto M, Ozaki M, Kawase K, Aihara M, Araie M, Yamamoto T, Kiuchi Y, Nakamura M, Ikeda Y, Sonoda K-H, Ishibashi T, Nitta K, Iwase A, Shirato S, Oka Y, Satoh M, Sasaki M, Fuse N, Suzuki Y, Cheng C-Y, Khor CC, Baskaran M, Perera S, Aung T, Vithana EN, Cooke Bailey JN, Kang JH, Pasquale LR, Haines JL, NEIGHBORHOOD Consortium, Wiggs JL, Burdon KP, Gharahkhani P, Hewitt AW, Mackey DA, MacGregor S, Craig JE, Allingham RR, Hauser M, Ashaye A, Budenz DL, Akafo S, Williams SEI, Kamatani Y, Nakazawa T, Kubo M, 2018. Genome-wide association study identifies seven novel susceptibility loci for primary open-angle glaucoma. Hum Mol Genet 27, 1486–1496. doi: 10.1093/hmg/ddy053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu S, Lichter PR, Johnson AT, Zhou Z, Higashi M, Gottfredsdottir M, Othman M, Moroi SE, Rozsa FW, Schertzer RM, Clarke MS, Schwartz AL, Downs CA, Vollrath D, Richards JE, 2000. Age-dependent prevalence of mutations at the GLC1A locus in primary open-angle glaucoma. Am J Ophthalmol 130, 165–177. [DOI] [PubMed] [Google Scholar]
- Stoilova D, Child A, Brice G, Crick RP, Fleck BW, Sarfarazi M, 1997. Identification of a new “TIGR” mutation in a family with juvenile-onset primary open angle glaucoma. Ophthalmic Genetics 18, 109–118. [DOI] [PubMed] [Google Scholar]
- Stoilova D, Child A, Brice G, Desai T, Barsoum-Homsy M, Ozdemir N, Chevrette L, Adam MF, Garchon H-J, Pitts Crick R, Sarfarazi M, 1998. Novel TIGR/MYOC mutations in families with juvenile onset primary open angle glaucoma. J Med Genet 35, 989–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokes WH, 1940. Hereditary primary glaucoma: a pedigree with five generations. Arch Ophthalmol 24, 885–909. [Google Scholar]
- Stone EM, Aldave AJ, Drack AV, Maccumber MW, Sheffield VC, Traboulsi E, Weleber RG, 2012. Recommendations for genetic testing of inherited eye diseases: report of the American Academy of Ophthalmology task force on genetic testing. Ophthalmology. doi: 10.1016/j.ophtha.2012.05.047 [DOI] [PubMed] [Google Scholar]
- Stone EM, Fingert JH, Alward WLM, Nguyen TD, Polansky JR, Sunden SLF, Nishimura D, Clark AF, Nystuen A, Nichols BE, Mackey DA, Ritch R, Kalenak JW, Craven ER, Sheffield VC, 1997. Identification of a Gene That Causes Primary Open Angle Glaucoma. Science 275, 668–670. doi: 10.1126/science.275.5300.668 [DOI] [PubMed] [Google Scholar]
- Swiderski RE, Ross JL, Fingert JH, Clark AF, Alward WL, Stone EM, Sheffield VC, 2000. Localization of MYOC transcripts in human eye and optic nerve by in situ hybridization. Invest Ophthalmol Vis Sci 41, 3420–3428. [PubMed] [Google Scholar]
- Tang S, Toda Y, Kashiwagi K, Mabuchi F, Iijima H, Tsukahara S, Yamagata Z, 2003. The association between Japanese primary open-angle glaucoma and normal tension glaucoma patients and the optineurin gene. Hum Genet 113, 276–279. doi: 10.1007/s00439-003-0964-y [DOI] [PubMed] [Google Scholar]
- Teikari JM, 1987. Genetic factors in open-angle (simple and capsular) glaucoma. A population-based twin study. Acta ophthalmologica 65, 715–720. [DOI] [PubMed] [Google Scholar]
- Toda Y, Tang S, Kashiwagi K, Mabuchi F, Iijima H, Tsukahara S, Yamagata Z, 2004. Mutations in the optineurin gene in Japanese patients with primary open-angle glaucoma and normal tension glaucoma. Am J Med Genet 125A, 1–4. doi: 10.1002/ajmg.a.20439 [DOI] [PubMed] [Google Scholar]
- Tojima Y, Fujimoto A, Delhase M, Chen Y, Hatakeyama S, Nakayama K, Kaneko Y, Nimura Y, Motoyama N, Ikeda K, Karin M, Nakanishi M, 2000. NAK is an IkappaB kinase-activating kinase. Nature 404, 778–782. doi: 10.1038/35008109 [DOI] [PubMed] [Google Scholar]
- Tu D, Zhu Z, Zhou AY, Yun C-H, Lee K-E, Toms AV, Li Y, Dunn GP, Chan E, Thai T, Yang S, Ficarro SB, Marto JA, Jeon H, Hahn WC, Barbie DA, Eck MJ, 2013. Structure and ubiquitination-dependent activation of TANK-binding kinase 1. Cell Reports 3, 747–758. doi: 10.1016/j.celrep.2013.01.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker BA, Solivan-Timpe F, Roos BR, Anfinson KR, Robin AL, Wiley LA, Mullins RF, Fingert JH, 2014. Duplication of TBK1 Stimulates Autophagy in iPSC-derived Retinal Cells from a Patient with Normal Tension Glaucoma. Journal of Stem cell research & therapy 3, 161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K, Gaitsch H, Poon H, Cox NJ, Rzhetsky A, 2017. Classification of common human diseases derived from shared genetic and environmental determinants. Nat Genet 49, 1319–1325. doi: 10.1038/ng.3931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weishaupt JH, Waibel S, Birve A, Volk AE, Mayer B, Meyer T, Ludolph AC, Andersen PM, 2013. A novel optineurin truncating mutation and three glaucoma-associated missense variants in patients with familial amyotrophic lateral sclerosis in Germany. Neurobiol. Aging 34, 1516.e9–15. doi: 10.1016/j.neurobiolaging.2012.09.007 [DOI] [PubMed] [Google Scholar]
- Weisschuh N, Neumann D, Wolf C, Wissinger B, Gramer E, 2005. Prevalence of myocilin and optineurin sequence variants in German normal tension glaucoma patients. Mol Vis 11, 284–287. [PubMed] [Google Scholar]
- Wiggs JL, Allingham RR, Vollrath D, Jones KH, Paz La, De M, Kern J, Patterson K, Babb VL, Del Bono EA, Broomer BW, Pericak-Vance MA, Haines JL, 1998. Prevalence of mutations in TIGR/Myocilin in patients with adult and juvenile primary open-angle glaucoma. Am J Hum Genet 63, 1549–1552. doi: 10.1086/302098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiggs JL, Pasquale LR, 2017. Genetics of glaucoma. Hum Mol Genet 26, R21–R27. doi: 10.1093/hmg/ddx184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wild P, Farhan H, McEwan DG, Wagner S, Rogov VV, Brady NR, Richter B, Korac J, Waidmann O, Choudhary C, Dötsch V, Bumann D, Dikic I, 2011. Phosphorylation of the autophagy receptor optineurin restricts Salmonella growth. Science 333, 228–233. doi: 10.1126/science.1205405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfs RC, Klaver CC, Ramrattan RS, van Duijn CM, Hofman A, de Jong PT, 1998. Genetic risk of primary open-angle glaucoma. Population-based familial aggregation study. Arch Ophthalmol 116, 1640–1645. [DOI] [PubMed] [Google Scholar]
- Ying H, Turturro S, Nguyen T, Shen X, Zelkha R, Johnson EC, Morrison JC, Yue BY, 2015. Induction of autophagy in rats upon overexpression of wild-type and mutant optineurin gene. BMC Cell Biol. 16, 14. doi: 10.1186/s12860-015-0060-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying H, Yue BYJT, 2016. Optineurin: The autophagy connection. Exp Eye Res 144, 73–80. doi: 10.1016/j.exer.2015.06.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying H, Yue BYJT, 2012. Cellular and molecular biology of optineurin. Int Rev Cell Mol Biol 294, 223–258. doi: 10.1016/B978-0-12-394305-7.00005-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Grinchuk O, Tomarev SI, 2008. Transgenic mice expressing the Tyr437His mutant of human myocilin protein develop glaucoma. Invest Ophthalmol Vis Sci 49, 1932–1939. doi: 10.1167/iovs.07-1339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zode GS, Bugge KE, Mohan K, Grozdanic SD, Peters JC, Koehn DR, Anderson MG, Kardon RH, Stone EM, Sheffield VC, 2012. Topical ocular sodium 4-phenylbutyrate rescues glaucoma in a myocilin mouse model of primary open angle glaucoma. Invest Ophthalmol Vis Sci 53, 1557–1565. doi: 10.1167/iovs.11-8837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zode GS, Kuehn MH, Nishimura DY, Searby CC, Mohan K, Grozdanic SD, Bugge K, Anderson MG, Clark AF, Stone EM, Sheffield VC, 2011. Reduction of ER stress via a chemical chaperone prevents disease phenotypes in a mouse model of primary open angle glaucoma. J Clin Invest 121, 3542–3553. doi: 10.1172/JCI58183 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1. The population allele frequencies of MYOC, OPTN, and TBK1 variants described in this report. Allele frequencies were determined from the Exome Aggregation Consortium (ExAC) database: http://exac.broadinstitute.org. NA: Not Available. 1Indicates that a variant was not observed in the ExAC population.


