Abstract
Background
Surveys are a widely used research method in dentistry in different specialities. The study aimed to determine the quality of survey-based research reports published in dentistry journals from 2015 to 2019.
Methods
A cross-sectional descriptive research study was conducted. The report quality assessment was carried out through the SURGE guideline modified by Turk et al. Four journals indexed in the Web of Science were selected: BMC Oral Health, American Journal of Orthodontics and Dentofacial Orthopedics, Journal of Dental Education, and Journal of Applied Oral Science. The selection of articles was made using the PubMed database considering the following search words: questionnaire OR survey, two trained reviewers applied the guideline to the selected articles, and the controversies were solved by discussion and consensus.
Results
A total of 881 articles were identified, of which 99 met the selection criteria and were included in the study. The best-reported items (n = 99) were four: the two that described the introduction of a study, the results reflecting and concerning the study objectives, and the review by an ethics committee. Five items were poorly reported: to declare the incentives to study participants (n = 93), three items on the description of statistical analyses (n = 99, 99, and 94), and information on how nonrespondents differed from respondents (n = 92).
Conclusions
There is a moderate quality of reporting of all aspects that should be considered in survey-based studies in dentistry journals. Poorly reported criteria were found mainly in the statistical analysis.
Keywords: Surveys and questionnaires, Dental health surveys, Epidemiologic studies, Health surveys, Dentistry
Background
Surveys are a widely used research method in dentistry in its different specialities, but mostly in public health, ethics, and education [1, 2]. This method can be applied in quantitative, qualitative, or mixed research; it allows for collecting information on a specific topic through low-cost questionnaires that are easy to apply [3, 4].
Research studies using surveys are as important as any other type of research; they are the beginning of exploratory studies, as well as cross-sectional axes in quantitative research. They are the basis for going on to the next levels of evidence, thus allowing for a comprehensive approach to health research, being used to address issues that are difficult to evaluate and allowing the generation of constructs in a specific topic [5, 6].
In a study conducted by Bennett et al. [5] on the evaluation of the quality of survey reports in the medical field, in 117 published studies, it was found that several criteria were poorly reported: few studies provided the survey or core questions (35%), reported the validity or reliability of the instrument (19%), defined the response rate (25%), discussed the representativeness of the sample (11%) or identified how they handled missing data (11%). Other studies evaluating the quality of survey-based study reports found similar results, e.g., Turk et al. [6] in different medical disciplines, Li et al. [7] in the area of nephrology, Pagano et al. [8] in the area of transfusion medicine, and Rybakov et al. [9] in the area of pharmacy. However, no studies were found evaluating the quality of survey reports in dentistry.
Science grows with the production of scientific knowledge informed through research articles, which should allow for evaluating the quality of the study conducted. It is necessary to know how much of the survey-based dentistry research published in high- and medium-impact journals is useful and has been clearly and completely reported. It is necessary to identify where it is failing and what needs to be improved so that these reports are useful for the profession, systematic reviews, and science. The study aimed to determine the quality of survey-based research reports published in dentistry journals from 2015 to 2019.
Methods
Type of study
A descriptive, cross-sectional investigation was carried out.
The study was approved at the institutional level, and evaluation by a research ethics committee was not considered necessary because it was a documentary evaluation that did not include human subjects.
Population and sample
Articles published from 2015 to 2019 in four dentistry journals indexed on the Web of Science. The following journals were selected: BMC Oral Health, American Journal of Orthodontics and Dentofacial Orthopedics, Journal of Dental Education, and Journal of Applied Oral Science because they are high- and medium-impact journals (Q1 and Q2) that mostly publish survey-based articles, which was determined by reviewing a pilot sample of 140 articles published in dentistry journals during 2019, found in the PubMed database and based on surveys. A 5-year time period was considered for the search for articles based on previous studies that used a variable time range, between 1 and 17 years [6, 8, 9].
Inclusion criteria
original survey studies that used a self-administered questionnaire as the primary research instrument (to answer its primary objective), cross-sectional surveys, and studies published in English.
Exclusion criteria
studies for the validation of an instrument that examined only the psychometric characteristics of the instrument, surveys administered through the web (online), study designs (randomized clinical trials, cohort studies, and case-control studies) where surveys were only used for demographic data, other types of studies (reviews, letters, commentaries, etc. ), studies that were part of a larger investigation, studies that performed a secondary analysis of the survey, studies that used semistructured interviews instead of questionnaires and surveys sent by e-mail.
Article selection procedure
This study defines a survey as the research method by which information is collected by asking people written questions about a specific topic, and the data collection procedure is standardized and well-defined [3].
The PubMed database was used to select the articles for each of the selected journals using the following search words: questionnaire OR survey, filtering by publication date from January 1, 2015, to December 31, 2019.
Two reviewers independently selected all references (title and abstract) and excluded those that did not meet the established criteria. In the second stage, the full-text articles were reviewed to determine which would be included in the study. In both stages, after the independent review, the researchers compared their results, and in cases of discrepancy, these were discussed and agreed upon.
Criteria for evaluating the quality of the report
The quality of the articles was evaluated independently and in duplicate by two other reviewers. Disputes were resolved by discussion and consensus.
To evaluate the quality of the research reports, the SURGE instrument modified by Turk et al. [6] was chosen, containing 33 items, of which only one item was modified, and the telephone survey mode, which was eliminated since only self-administered questionnaires were evaluated. This instrument was tested by two researchers on a convenience sample of survey items identified by the authors. No modifications had to be made to the content or wording.
The following variables were also recorded to characterize the sample: year of publication, continent of origin and journal.
Pilot study
A pilot study was conducted to train and standardize criteria for the search and selection of the articles, according to the established selection criteria, as well as for the application of the quality criteria of the report.
Statistical analysis
Data processing and analysis were carried out using the statistical program SPSS v 26 (SPSS Inc., IL, USA). Descriptive statistics were applied to the study variables using frequency distribution tables.
Results
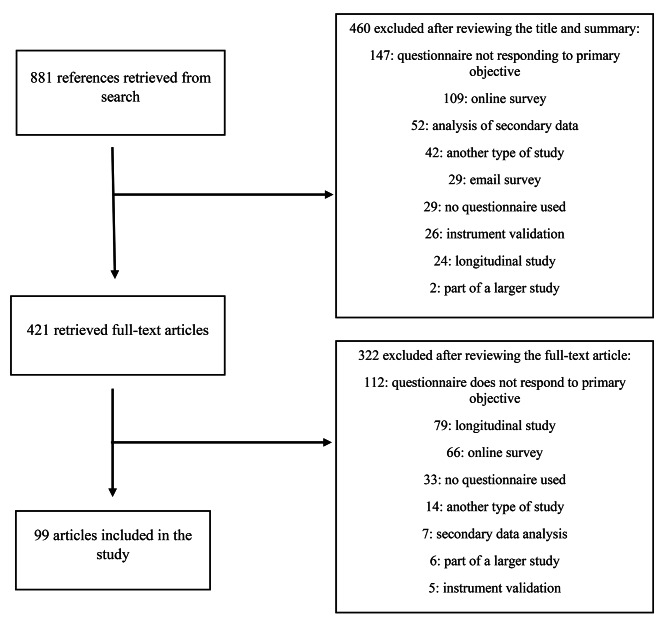
From the bibliographic search, 881 references were retrieved; then, their titles and abstracts were read, and 460 were excluded according to the established criteria. From the other references, the full text of the articles (n = 421) was obtained and evaluated to determine compliance with the selection criteria, excluding 322 articles and considering only 99 in the study to apply the SURGE guidelines and evaluate the quality of the report (Fig. 1). In the study sample, the most frequent articles were those published in 2018 (n = 29), those from the Americas (n = 41), and those published in the journal BMC Oral Health (n = 43) (Table 1). The percentage value is not mentioned in all results because it matches the absolute value.
Fig. 1.
Flowchart of selected articles for the study
Table 1.
Characteristics of the articles evaluated
| Variables | n | |
|---|---|---|
| Year of publication | ||
| 2015 | 17 | |
| 2016 | 15 | |
| 2017 | 20 | |
| 2018 | 29 | |
| 2019 | 18 | |
| Continent of origin | ||
| America | 41 | |
| Europe | 18 | |
| Africa | 6 | |
| Asia | 29 | |
| Oceania | 5 | |
| Journal | ||
| BMC Oral Health | 43 | |
| AJODO | 13 | |
| JDE | 41 | |
| JAOS | 2 | |
Table 2 shows the evaluation results of the title, abstract, introduction, and methods of the articles through 21 criteria of the SURGE instrument.
Table 2.
Evaluation of articles according to SURGE criteria: title, abstract, introduction, and methods
| Section | Description of the criterion | Categories | n |
|---|---|---|---|
| Title and abstract | It states the word ‘questionnaire’ or ‘survey’ in the title and/or abstract. | 1. In title and abstract | 19 |
| 2. In title or abstract | 78 | ||
| 3. No | 2 | ||
| Introduction | There is an explanation of why the research is necessary. | 1. Yes | 99 |
| 2. No | 0 | ||
| It indicates the purpose or objective | 1. Yes | 99 | |
| 2. No | 0 | ||
| Methods | It describes the questionnaire/provides access to the items in the questionnaire | 1. Questionnaire provided | 45 |
| Research instrument | 2. Central questions provided | 26 | |
| 3. A complete question provided | 0 | ||
| 4. Questions not provided | 28 | ||
| If an existing instrument was used, its psychometric properties were mentioned: confidence and validity | 1. Yes | 18 | |
| 2. No | 32 | ||
| 3. Not applicable | 49 | ||
| If an existing instrument was used, references to the original study are provided. | 1. Yes | 47 | |
| 2. No | 3 | ||
| 3. Not applicable | 49 | ||
| In a new instrument, the procedures used to develop it and/or the methods used for the pretest are mentioned. | 1. Yes | 26 | |
| 2. No | 23 | ||
| 3. Not applicable | 50 | ||
| In a new instrument, its validity and confidence were reported. | 1. Both | 4 | |
| 2. Only confidence | 7 | ||
| 3. Only validity | 2 | ||
| 4. None | 36 | ||
| 5. Not applicable | 50 | ||
| It describes the scoring procedures | 1. Yes | 77 | |
| 2. No | 6 | ||
| 3. Not applicable | 16 | ||
| Sample selection | It describes the study population and sampling frame. | 1. Both | 46 |
| 2. Study population | 28 | ||
| 3. Sampling frame | 1 | ||
| 4. None | 24 | ||
| It describes the representativeness of the sample. | 1. Yes | 87 | |
| 2. No | 12 | ||
| It presents a sample size calculation or justification of it. | 1. Yes | 81 | |
| 2. No | 18 | ||
| Survey administration | The mode of administration of the survey to participants is specified. | 1. In person | 75 |
| 2. Mail | 6 | ||
| 3. Mixed | 5 | ||
| 4. Not mentioned | 13 | ||
| Type and number of contacts | 1. Type and number | 33 | |
| 2. Only type | 53 | ||
| 3. No information | 13 | ||
| Financial or other incentives to study participants. | 1. Yes | 6 | |
| 2. No | 93 | ||
| Description of who approached potential participants. | 1. Yes | 38 | |
| 2. No | 61 | ||
| Analysis | It describes the method of data analysis. | 1. Adequate (complete) | 91 |
| 2. Inadequate (incomplete) | 4 | ||
| 3. Does not describe | 4 | ||
| It mentions the methods for nonresponse error analysis. | 1. Yes | 0 | |
| 2. No | 99 | ||
| It mentions the method for calculating the response rate. | 1. Yes | 0 | |
| 2. No | 99 | ||
| It mentions definitions for complete versus partial endings. | 1. Yes | 37 | |
| 2. No | 62 | ||
| It mentions methods for handling missing item data. | 1. Yes | 5 | |
| 2. No | 94 |
It was found that most articles used the term survey or questionnaire in the title or abstract (n = 97). All articles explained why the research was necessary and indicated the study objective.
In the section on methods, almost half of the articles provided the questionnaire (n = 45) and used an existing instrument (n = 50). In this case, 32 did not mention their psychometric properties, and three did not provide references to the original work. Among the papers that used a new instrument (n = 49), 23 did not mention the procedures used to develop it or the methods used for the pretest, while 36 did not report the validity and confidence of the instrument. Among the studies that used a questionnaire that required scoring (n = 83), six did not describe the scoring procedures.
Regarding the evaluation of the sample selection, few studies did not describe the study population and the sample framework (n = 24), the representativeness of the sample (n = 12), the calculation of the sample size, or the justification thereof (n = 18).
Regarding survey administration, few articles mentioned the mode of administration or the type and number of contacts (n = 13); however, most of them did not report the incentives to respondents (n = 93) or who approached potential participants (n = 61).
In the evaluation of the statistical analysis, four articles did not describe the method of analysis, while none reported the methods for the analysis of the nonresponse error and the calculation of the response rate; most failed to mention definitions for complete versus partial endings (n = 62) and methods for handling missing data (n = 94).
Table 3 describes the evaluation of the results, discussion, and ethical aspects of the articles through 12 criteria of the SURGE instrument.
Table 3.
Article evaluation according to SURGE criteria: Results, discussion, and ethics
| Section | Description of the criterion | Categories | n |
|---|---|---|---|
| Results | It reports the response rate. | 1. Yes, defined | 57 |
| 2. Yes, not defined | 14 | ||
| 3. Partial information | 4 | ||
| 4. No information | 24 | ||
| It takes into account all respondents. | 1. Yes | 86 | |
| 2. No | 13 | ||
| Information on how nonrespondents differ from respondents. | 1. Yes | 5 | |
| 2. Subject addressed | 2 | ||
| 3. No information | 92 | ||
| Results are clearly presented. | 1. Yes, complete | 97 | |
| 2. Yes, partial | 2 | ||
| 3. No | 0 | ||
| Results reflect the objectives of the study. | 1. Yes | 99 | |
| 2. No | 0 | ||
| Discussion | Summary results in relation to the objectives of the study. | 1. Yes | 99 |
| 2. No | 0 | ||
| It mentions the strengths of the study. | 1. Yes | 53 | |
| 2. No | 46 | ||
| It indicates the limitations of the study. | 1. Yes | 90 | |
| 2. No | 9 | ||
| There is an explicit discussion of the generalization of results. | 1. Yes | 65 | |
| 2. No | 34 | ||
| Ethical quality indicators | Report on the financing of the study. | 1. Yes | 42 |
| 2. No | 57 | ||
| Review of the study by a Research Ethics Committee. | 1. Yes | 86 | |
| 2. Reported exempted from a committee | 13 | ||
| 3. No | 0 | ||
| Report on procedures for individuals’ consent | 1. Yes | 69 | |
| 2. Reported refused informed consent | 3 | ||
| 3. No | 27 |
In the results section, 24 articles did not report the response rate, 13 did not consider all respondents, and 92 did not report the difference between respondents and nonrespondents. However, in all the articles, the results were presented clearly and in relation to the study objectives.
For the Discussion section, only one criterion was correctly reported in all cases, and the results were summarized in relation to the study objectives, while 46 and six articles did not mention the strengths and limitations of the study, respectively. In addition, 34 studies discussed the generalization of results.
Finally, regarding ethical quality indicators, all articles reported on the review of the study by an ethics committee, while 57 and 27 articles did not report on the funding and procedures of respondent consent, respectively.
Discussion
In the evaluation of the quality of survey-based studies, it was found that the best-reported sections were title and abstract, introduction, sample selection, results, and discussion; specifically, there were 15 criteria very well reported (with a frequency greater than 80%), most of them within the aforementioned sections. Bennett et al. [5], Pagano et al. [8], and Rybakov et al. [9] also found the title and abstract, introduction, and discussion sections well reported. It is possible that the STROBE [10] guidelines, which are the guidelines for good reporting of observational studies required by health science journals, may have contributed to this. In addition, the recommendations given for writing the items in these sections are well-known and easy to comply with.
There are 12 criteria where most articles performed a bad report, and five of them were poorly reported (with a frequency greater than 80%): incentives to study participants; mentioning methods for nonresponse error analysis, calculating the response rate, and handling missing item data; and reporting how nonrespondents differed from respondents. Three of these criteria belong to the analysis section. Other investigations found a greater number of misreported criteria [5, 6, 8]; one even observed as many as 21 inadequately reported items in medical articles [5]. The items mentioned methods for nonresponse error analysis [5, 6, 8, 9] and for handling missing item data [5, 8, 9] were also poorly reported in other research studies.
This research allows warning about the aspects that should be improved in the reporting of studies based on self-administered surveys in the field of dentistry. In the Methods section, it should be emphasized that when the research is conducted using a new or existing questionnaire, the psychometric properties of the questionnaire should be mentioned. The vast majority of researchers in the studies evaluated considered it sufficient to mention only the reference to the original validation work when working with an existing questionnaire.
The SURGE guidelines [11], unlike STROBE [10], develop more precisely what needs to be reported in terms of the survey administration. This research has demonstrated that three out of the four items that should be described on this aspect, according to the SURGE criteria, were not performed correctly, which does not allow a study to be replicable or a reader or reviewer to assess its quality and the possible introduction of bias.
The statistical analysis description is a critical aspect in the communication of this research and it is necessary to train researchers in this field since statistical knowledge is an important element to prevent his or her study from lacking methodological validity. This study found that four out of the five items that SURGE recommends reporting in this area were poorly reported, which agrees with other investigations in which the quality of survey-based studies in areas such as general medicine [5, 6], transfusion medicine [8], and pharmacy [9] were evaluated. A previous study noted the poor reporting of statistical aspects in articles of different research designs in dentistry journals [12]. Although it is common for articles to report the value of response rate, no one mentioned the method for calculating it; SURGE asks to mention both in the results and methods sections, respectively.
It is also important to note that the description of how nonrespondents differ from respondents should be improved in the results section. It may be infrequent to mention this aspect because of the additional work it would take researchers to obtain this information, as it can be difficult to obtain because of the lack of access to the group of non-respondents. However, where possible, the researcher should report it, which will help to make transparent the representativeness of the sample studied in relation to the population.
Regarding the ethical aspects of a research study, improved reporting of the study’s financing is necessary. It would be advisable for journals to require authors to submit their manuscripts with this information. For example, one of the journals evaluated in this study, BMC Oral Health, provided the communication of ethical aspects since, at the end of the article, they presented sections where the authors had to declare the financing of the study, the approval of an ethics committee, and the consent of participants.
As far as the authors are aware, this is the first study that evaluates the quality of survey-based research in the area of dentistry, which warns of the aspects that should be improved for clearer, more complete, and transparent communication of this type of study, considering that the use of surveys in research in the area of health sciences is frequent [1].
One limitation of this study was that the evaluation of some quality items was not simple, and the agreement reached by the two evaluators of the article could be different from what was interpreted and evaluated in other studies that also used the SURGE criteria [5, 6, 8, 9]. For example, in some of the items evaluated, the report was considered valid even though the information was not found in the corresponding section; it was sufficient that it was present somewhere in the article. Moreover, several articles evaluated used nonprobabilistic samples, so the sampling frame was not reported since it was unnecessary. In these cases, this item was not considered misreported. There is no extended version of SURGE where the criteria for evaluating each item are explained in detail, as there is for the STROBE [13] and CONSORT [14] statements, among others.
The results of this study are not necessarily generalizable to articles published in all dentistry journals since it only evaluated four journals; however, it is the first report that provides evidence in this field.
Conclusions
It is concluded that there is a moderate quality of reporting of all the aspects to be considered for studies based on self-administered surveys in four dentistry journals. Poorly reported criteria were found mainly in the statistical analysis section.
Acknowledgements
Not aplicable.
Authors’ contributions
MAM-V: Conception and design of the study, Data acquisition, Data análisis, Discussion of the results, Drafting of the manuscript. TAE-Ch: Conception and design of the study, Data acquisition, Discussion of the results, Drafting of the manuscript. KS-V: Conception and design of the study, Data acquisition, Discussion of the results. All authors read and approved the final manuscript.
Funding
This work was supported by the Universidad Nacional Mayor de San Marcos under Grant A20051931.
Data Availability
The datasets generated and analysed during the current study are available in the Zenodo repository, 10.5281/zenodo.7391366.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.LaVange LM, Koch GG, Schwartz TA. Applying simple survey methods to clinical trials data. Statist Med. 2001;20:2609–23. doi: 10.1002/sim.732. [DOI] [PubMed] [Google Scholar]
- 2.Duran D, Monsalves MJ, Aubert J, Zarate V, Espinoza I. Systematic review of latin american national oral health surveys in adults. Community Dent Oral Epidemiol. 2018;46(4):328–35. doi: 10.1111/cdoe.12379. [DOI] [PubMed] [Google Scholar]
- 3.Ponto J. Understanding and evaluating survey research. J Adv Pract Oncol. 2015;6(2):168–71. [PMC free article] [PubMed] [Google Scholar]
- 4.Kelley K, Clark B, Brown V, Sitzia J. Good practice in the conduct and reporting of survey research. Int J quality in Health Core. 2003;15(3):261–6. doi: 10.1093/intqhc/mzg031. [DOI] [PubMed] [Google Scholar]
- 5.Bennett C, Khangura S, Brehaut JC, Graham ID, Moher D, Potter BK, et al. Reporting guidelines for survey research: an analysis of published guidance and reporting practices. PLoS Med. 2011;8(8):e1001069. doi: 10.1371/journal.pmed.1001069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Turk T, Elhady MT, Rashed S, Abdelkhalek M, Nasef SA, Khallaf et al. Quality of reporting web-based and non-web-based survey studies: What authors, reviewers and consumers should consider. PLoS One. 2018;13(6):e0194239. [DOI] [PMC free article] [PubMed]
- 7.Li AH, Thomas SM, Farag A, Duffett M, Garg AX, Naylor KL. Quality of survey reporting in nephrology journals: a methodologic review. Clin J Am Soc Nephrol. 2014;9(12):2089–94. doi: 10.2215/CJN.02130214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pagano MB, Dunbar NM, Tinmouth A, Apelseth TO, Lozano M, Cohn CS, et al. A methodological review of the quality of reporting of surveys in transfusion medicine. Transfusion. 2018;58(11):2720–27. doi: 10.1111/trf.14937. [DOI] [PubMed] [Google Scholar]
- 9.Rybakov KN, Beckett R, Dilley I, Sheehan AH. Reporting quality of survey research articles published in the pharmacy literature. Res Social Adm Pharm. 2020;16(10):1354–8. doi: 10.1016/j.sapharm.2020.01.005. [DOI] [PubMed] [Google Scholar]
- 10.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP, STROBE Initiative The strengthening the reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61(4):344–9. doi: 10.1016/j.jclinepi.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 11.Grimshaw J, et al. SURGE (the SUrvey Reporting GuidelinE) In: Moher D, Altman DG, Schulz KF, et al., editors. Guidelines for reporting health research: a user`s manual. Oxford: Wiley; 2014. pp. 206–13. [Google Scholar]
- 12.Vähänikkilä H, Tjäderhane L, Nieminen P. The statistical reporting quality of articles published in 2010 in five dental journals. Acta Odontol Scand. 2015;73(1):76–80. doi: 10.3109/00016357.2014.954612. [DOI] [PubMed] [Google Scholar]
- 13.Vandenbroucke JP, von Elm E, Altman DG, Gøtzsche PC, Mulrow CD, Pocock SJ, et al. STROBE Initiative. Strengthening the reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. PLoS Med. 2007;4(10):e297. doi: 10.1371/journal.pmed.0040297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moher D, Hopewell S, Schulz KF, Montori V, Gøtzsche PC, Devereaux PJ, et al. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340:c869. doi: 10.1136/bmj.c869. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and analysed during the current study are available in the Zenodo repository, 10.5281/zenodo.7391366.