Abstract
Angiogenesis—the formation of new blood vessels from existing blood vessels—has drawn significant attention in medical research. New techniques have been developed to control proangiogenic factors to obtain desired effects. Two important research areas are 1) understanding cellular mechanisms and signaling pathways involved in angiogenesis and 2) discovering new biomaterials and nanomaterials with proangiogenic effects. This paper reviews recent developments in controlling angiogenesis in the context of regenerative medicine and wound healing. We focus on novel proangiogenic materials that will advance the field of regenerative medicine. Specifically, we mainly focus on metal nanomaterials. We also discuss novel technologies developed to carry these proangiogenic inorganic molecules efficiently to target sites. We offer a comprehensive overview by combining existing knowledge regarding metal nanomaterials with novel developments that are still being refined to identify new nanomaterials.
Keywords: Angiogenesis, tissue regeneration, nanomaterials
1. Introduction
Angiogenesis is a focus in regenerative medicine, wound healing, and cancer treatment. The formation of new blood vessels in the body delivers oxygen and nutrients to the surrounding tissues, promoting survival and growth [1]. There are mainly two categories of blood vessel formation; angiogenesis and vasculogenesis. Although vasculogenesis and angiogenesis are often used interchangeably, they are two distinct processes that result in the formation of blood vessels. Angiogenesis refers to the process when new blood vessels grow from pre-existing blood vessels, mostly from mature blood vessels [2]. Vasculogenesis refers to the process when endothelial progenitor cells, such as embryonic angioblasts, differentiate into endothelial cells and eventually form a primitive vascular network.
Angiogenesis has drawn significant attention in medical research because it is involved in numerous key functions of the body. Angiogenesis is the crucial common factor in all categories of regeneration. By extending the research on proangiogenic factors, researchers can specifically advance the field of regenerative medicine—including the regeneration of skin, bone, organs, and nerves—by reducing tissue ischemia from a lack of sufficient angiogenesis after the transplant [3]. The primary goal of angiogenesis research is to develop new techniques and materials to influence natural angiogenesis—whether it be promoting or inhibiting—in the body to obtain desired effects. Many researchers have studied novel proangiogenic drug delivery systems, for example, designing porous particles with a high surface area-to-volume ratio [4]. However, there currently exists a limited number of effective drugs to promote angiogenesis in vivo, limiting the applications of such advancements in a clinical setting. In recent years, researchers have started to explore the use of proangiogenic metals, which have been demonstrated to enhance the functions of naturally occurring biomolecules. One form of metal is a liquid metal, such as gallium, whose potential in angiogenesis-targeted cancer therapy was recently discovered [5], [6]. Gallium can serve as a drug carrier for antiangiogenic drugs, as well as inhibit critical metabolic functions surrounding its injection site [7]. Gallium also exhibited promise in promoting angiogenesis in a wound healing context [8].
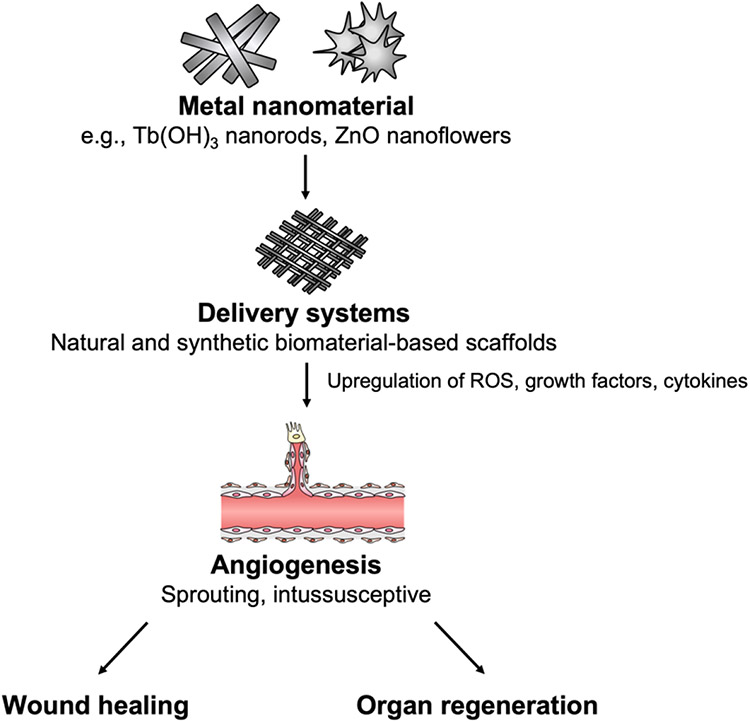
Compared with liquid metals, solid metal nanomaterials have been more extensively studied on their proangiogenic properties and will be the main focus of this review. The most notable advantages of using metal nanomaterials in regenerative medicine include their stability, limited immunogenicity, relatively cheap fabrication and storage costs, varied properties according to their oxidation state, and ability to affect multiple downstream proangiogenic signaling pathways [9]. The effects of metals, such as cerium, on promoting angiogenesis depends greatly on their oxidation state, which can be easily modified through the creation of new salts and polymers [10]. Metals in different families have different characteristics that affect the downstream mechanisms of action. For instance, lanthanides, such as europium, primarily induce angiogenesis through the production of intracellular reactive oxygen species (ROS), while many main group elements, such as nickel, promote the upregulation of proangiogenic cytokines, such as IL-8 [11], [12]. Metallic nanomaterials exhibit remarkable versatility and have been demonstrated to be a potent promoter of angiogenesis in regenerative medicine. A summary of the angiogenic effect of metal nanomaterials is shown in Figure 1.
Figure 1. Summary of the effect of metal nanomaterials on angiogenesis.
1.1. Types of angiogenesis
Angiogenesis is further organized into two categories: sprouting angiogenesis and intussusceptive angiogenesis.
Sprouting angiogenesis (SA) is marked by the branching of new endothelial cells through extensive cellular proliferation and migration. SA is induced in vivo when endothelial cells detect an angiogenic signal, such as vascular endothelial growth factor (VEGF-A) released by hypoxic, inflammatory, or tumor cells. SA requires the degradation of the extracellular matrix mediated by proteolytic enzymes, including membrane-type 1 matrix metalloproteinases (MT1-MMP) enriched in endothelial tip cells, securing a path for tip and stalk cells to migrate [13]-[15]. Tip cells initiate angiogenesis in the proper direction according to the VEGF-A gradient released from surrounding tissues. After the tip cell anchors onto a substratum in the extracellular matrix, stalk cells closely follow behind, elongating the capillary tube as angiogenesis progresses (Figure 2A). The other type of angiogenesis, or intussusceptive angiogenesis (IA), occurs when an existing blood vessel splits into two new vessels (Figure 2B). This type of angiogenesis involves the stimulation of endothelial cells by increased blood flow, forming a hollow transcapillary pillar that expands in size, causing a single blood vessel to branch into two parallel capillaries [16]. IA participates in vascular morphogenesis, remodeling, and microvascular growth. SA and IA are thought to be complementary angiogenesis processes with synergistic interaction. IA was shown to contribute to the capillary expansion following an SA phase.
Figure 2.
(A) Sprouting angiogenesis. Tip cells use their filopodia richly coated in VEGF receptors to determine the correct orientation for stalk cell proliferation. A new vascular pathway is formed when two or more filopodia meet and merge, creating a complete vascular tube. (B) Intussusceptive angiogenesis. Unlike sprouting angiogenesis, intussusceptive angiogenesis creates new vessels from one existing one. The vessel begins to split with the creation of a pillar in the middle of the vessel, composed of endothelial cells.
1.2. Regulation of angiogenesis
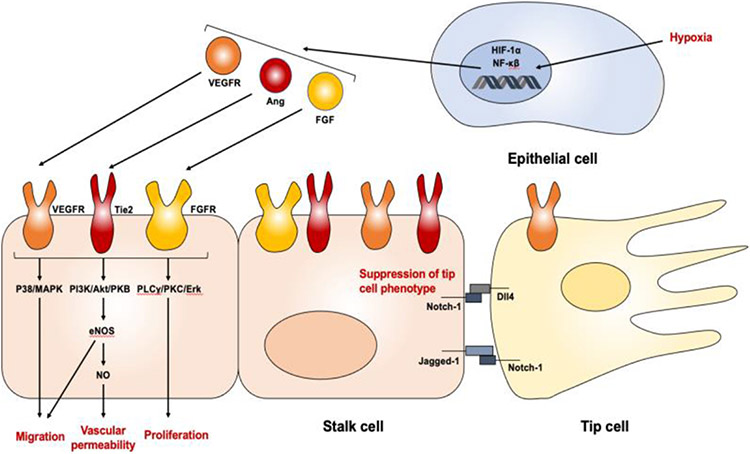
In order to effectively develop and use nanomaterials for angiogenesis, it is crucial to study the fundamental cellular mechanisms of naturally-occurring angiogenesis to identify potential targets. As outlined in an earlier section, angiogenesis occurs in systematic steps: the degradation of the capillary membrane, endothelial cell proliferation and migration, endothelial cell tube formation, endothelial cell fusion and recession, and pericyte stabilization. Natural angiogenesis involves many different signaling pathways, often induced by growth factors, transcription factors, and proteases (Figure 3). Below are a few critical molecules that initiate angiogenesis naturally in the human body.
Figure 3. A general outline of the signaling pathway to induce angiogenesis.
Although the complete picture of angiogenesis signaling pathways is much more complex than this diagram, it encompasses the concept that proangiogenic stimuli (e.g., hypoxia) lead to the expression of proangiogenic transcription and growth factors, such as VEGF, Ang and FGF, to induce angiogenesis.
1.2.1. Growth factors
The VEGF family are glycoproteins that contribute to angioblast differentiation, endothelial cell migration and proliferation, and tube formation [17], [18]. VEGF is released from stromal or endothelial cells upon the detection of hypoxia around tissues [19]. There are eight subfamilies of VEGF, namely, VEGF-A, VEGF-B, VEGF-C, VEGF-D, VEGF-E, VEGF-F, placenta growth factor (PIGF), and endocrine gland-derived VEGF [20]. Out of the eight subfamilies, VEGF-A is the most well-studied angiogenesis promoter, promoting angiogenesis by increasing vascular permeability, stimulating proliferation, and upregulating endothelial nitric oxide synthase (eNOS) expression [21], [22]. The VEGF signaling cascade causes the production of vasodilatory mediators that activate downstream receptor tyrosine kinases and phosphoinositide 3-kinase/Ak strain transforming (PI3K/Akt) pathways, leading to cell growth and survival [23], [24]. When VEGF binds to its corresponding VEGFR, the interaction results in nitric oxide (NO) production, which promotes angiogenesis through vasodilation and endothelial cell survival [25]. There are three notable VEGF receptors, VEGFR-1, VEGFR-2, and VEGFR-3. VEGFRs undergo dimerization and autophosphorylation of its tyrosine residues when bound to VEGF. The activation of VEGFR-1 is responsible for the activation of phospholipase Cγ (PLCγ) and PI3K pathways through the phosphorylation of Tyr794, Tyr1169, Tyr1213, Tyr1242, and Tyr1333 residues on the receptor kinase [26]. VEGFR-2, expressed on vascular endothelial cells, starts the signaling transduction by the VEGF-mediated phosphorylation of VEGFR-2 tyrosine residues—Tyr801, Tyr951, Tyr1008, Tyr1059, Tyr1175, Tyr1214, and Tyr1305—initiating the activation of downstream osmotic stress/ABA-activated protein kinase 2 (SAPK2) pathway, PLCγ-protein kinase C (PKC)/mitogen-activated protein kinase (MAPK), and PI3K-pathway, promoting angiogenesis [27]-[30]. VEGFR-2 is arguably the most important receptor in inducing angiogenesis, as it is a primary receptor for VEGF-A [31]. VEGFR-3 is most abundantly expressed in endothelial tip cells, activating the Notch pathways contributing to endothelial cell differentiation [32].
Fibroblast growth factors (FGFs) act as paracrine or endocrine protein ligands to form FGF-FGF receptor (FGFR) dimerization to activate downstream kinases through phosphorylation [33]. Humans have 22 members of the FGF family, that can be divided into six subfamilies [34]. Most FGF in humans stimulates angiogenesis through the activations of the rat sarcoma virus (RAS)/ mitogen-activated protein (MAP), PI3K/Akt, and PLCγ kinase pathways, which in turn promote angiogenesis through the regulation of vascular permeability and endothelial cell differentiation [35]-[37]. RAS has multiple downstream effectors, including the rapidly accelerated fibrosarcoma (RAF)/mitogen-activated protein kinase kinase (MEK)/extracellular signal-regulated kinase (ERK) pathway to regulate proangiogenic gene expression and prevent apoptosis, as well as the PI3K/Akt pathway to promote cell survival and proliferation [38]-[40]. Furthermore, studies suggest that FGF production stimulates VEGF expression in surrounding endothelial cells, indicating the interlinked nature of these proangiogenic signaling pathways [41]-[43]. There are four members of FGF receptors (FGFR), FGFR-1 through FGFR-4, whose dimerization and subsequent phosphorylation are dependent on their binding with FGF. FGF signaling has three downstream pathways, namely, PLCγ, RAS/MAP kinase, and PI3/AKT, to promote endothelial cell proliferation and differentiation.
Furthermore, angiopoietins are another family of vascular growth factors that exert its influence on the angiogenesis pathway through the tyrosine kinase receptors, tyrosine kinase with immunoglobulin-like and EGF-like domains 1 and 2 (Tie1 and Tie 2). Angiopoietins (Ang) are an interesting class of growth factors in that they can switch between proangiogenic and antiangiogenic properties depending on surrounding physiological conditions. Ang2 can dose-dependently compete with Ang1 to inhibit Tie2 phosphorylation, preventing the activation of downstream proangiogenic pathways [44], [45]. However, in the absence of Ang1, Ang2 activates Tie2 phosphorylation that promotes endothelial cell survival, migration, and growth [46], [47]. Not only do angiopoietins promote endothelial cell survival, but they also stabilize the neovasculature and regulate vessel permeability, leading the angiogenic process to a closure [48].
Finally, another notable proangiogenic signaling pathway to consider is the delta-like 4 (Dll4)-Notch signaling. Dll4-Notch regulates levels of other proangiogenic factors, such as VEGF, and controls vessel formation to prevent the hyperproliferation of tip cells and the construction of fragile, immature vessels [49]. Dll4 also regulates the postangiogenic remodeling of the newly formed blood vessels, determining the ultimate functional pattern of vessel branching in tissues [50].
1.2.2. Transcription factors
There are seven major families of transcription factors with significant impact on angiogenesis: E26 transformation-specific (ETS), cyclic adenosine monophosphate (cAMP) response element-binding (CREB), GATA binding proteins, hypoxia-inducible factors (HIF), yes-associated protein (YAP)/transcriptional co-activator with PDZ-binding motif (TAZ), mycosis transcription factor (MYC), and the nuclear factor κB (NF-κB) [51].
The most well-known proangiogenic transcription factor HIF-α is a member of the HIF transcription factor family. They contain two basic helix-loop-helix (bHLH) domains, consisting of around 60 amino acids that form two α helices intervened by a loop [52], [53]. bHLH domains from distinct proteins can form a dimer that binds to DNA to control gene expression [54]. HIF-α is a primary responder to cellular hypoxia, as well as a regulator for cellular oxygen homeostasis. HIF-α is often upregulated, except for HIF-3α, which downregulates the proangiogenic effects of other transcription factors, such as HIF-1α, by inhibiting them. HIF-α induces the transcription of hypoxia-induced genes to promote vessel remodeling and sprouting [55], [56]. HIF-1 expression induced by hypoxia induces angiogenesis through the regulation of genes involved in endothelial cell proliferation, transcriptional activation of growth factors (VEGF, ANG), as well as the regulation of proangiogenic chemokines (stromal cell-derived factor 1α, sphingosine-1-phosphate) [57]-[59].
Another notable transcription factor is NF-κB. NF-κB induces angiogenesis by upregulating the cellular inflammatory response through the expression of inflammatory cytokines such as tumor necrosis factor α (TNF-α) and interleukin 1β (IL-1β), as well as inflammatory genes such as intercellular adhesion molecule 1 (ICAM1) and IL-6. NF-κB contains a Rel homology domain (RHD), which is required for NF-κB dimerization, binding to DNA, interacting with inhibitor of nuclear factor kappa B (IκB) inhibitory proteins, and translocating to the nucleus. When inactive, NF-kB forms dimers in the cytosol, whose activity is suppressed through its interaction with IκB [60]. Upon phosphorylation, the IκBα protein releases the NF-κB dimer, which then translocates to the nucleus and regulates proangiogenic genes [61]. Once in the nucleus, the NF-κB dimer binds to the promoter region of proangiogenic genes, enhancing the transcription of proangiogenic genes such as VEGF [62].
Although HIF-α and NF-κB are the most well-studied transcription factors, there are other transcription factors that regulate angiogenesis. The ETS transcription factor family significantly influences vascular development through the expression of essential proangiogenic genes, including the ETS-related gene (ERG) and TAL BHLH Transcription Factor 1 (TAL1) [63]. CREB transcription factors regulate the expression of VEGF-A and FGF-2 when it is activated upon the phosphorylation of its Ser133 residue by protein kinases A, C, and calmodulin kinase [64]-[66]. GATA transcription factors enhance VEGF-A and VEGF-C transcription when they bind to the (A/T)GATA(A/G) promoter sequence [67], [68]. YAP/TAZ transcription factors affect angiogenesis rather indirectly by binding to the transcriptional enhanced associated domain (TEAD)/thyrotroph embryonic factor (TEF) transcription factors to activate cell division cycle 42 (Cdc42) and myosin regulatory light chain (MLC2), promoting filopodia formation and migration in endothelial tip cells [69]. Lastly, MYC transcription factors are significantly expressed in cells undergoing sprouting angiogenesis, and their activation is linked to endothelial cell proliferation and IL-1β release [70].
1.2.3. Other factors
In addition to the aforementioned proangiogenic factors, many other biomolecules participate in the complex signaling cascade to promote angiogenesis. Proteases are enzymes that can hydrolyze peptide bonds to break down proteins. The three major families of proangiogenic proteases include MMPs, serine proteases, and cathepsin cysteine proteases. These proteases, upon exposure to the extracellular environment, degrade surrounding matrix proteins to promote tip cell proliferation and stalk cell elongation [71]. Among the various proteases, MMPs are the most extensively studied in angiogenesis. MMPs are zinc-dependent endopeptidases in the metzincin superfamily that promote angiogenesis through the cleavage of protein-based growth factors upon the activation of the catalytic site by a Zn2+ ion [72]. MMPs are further categorized into subfamilies according to their substrates, including collagenases, gelatinases, and membrane-type MMPs [73]. MMPs participate in angiogenesis through the digestion of vascular basement membrane and rearrangement of the extracellular matrix (ECM) to allow endothelial cell proliferation and migration into new areas of the tissue. Downstream effects of MMP activation include VEGF and Ang2/Tie2 production, as well as an angiogenic TNF-α to induce inflammation through the MEK/ERK pathway [74].
Exosomes are extracellular vesicles used in the transportation of nucleic acids, lipids, proteins, carbohydrates, and metabolites from one cell to another. Exosomes derived from endothelial cells carry and transfer Dll4 proteins to the surrounding cells, thereby inhibiting Notch signaling and promoting capillary formation in vivo [75]. This finding communicates the importance of exosomes in the regulation of angiogenesis, as proangiogenic signals can travel from distant cells instead of being restricted between cells in direct contact. Exosomes derived from LAMA84 leukemia cells regulate angiogenesis through an IL-8-mediated activation of the vascular cell adhesion protein 1 (VCAM-1) [76]. In addition, exosomes produced by glioblastoma cells in hypoxic environments promoted endothelial cells to secrete proangiogenic factors to activate the PI3K/AKT pathway to promote cell migration.
1.3. Existing angiogenic therapies
Angiogenic therapies have traditionally been used in cancer treatments, however, there have been recent developments in proangiogenic drugs for regenerative engineering purposes. The goal of angiogenesis therapy is to manipulate angiogenesis to target surrounding tissues and organs. Existing angiogenesis therapies for regenerative engineering have focused on the enhancement of proangiogenic VEGF, FGF, and platelet-derived growth factor (PDGF) production, while on the other hand, existing cancer therapies have focused on the inhibition of such molecules or promoting the expression of antiangiogenic suppressors such as ANG. The latter aims to inhibit uncontrolled angiogenesis in tumor microenvironments, effectively depleting cancer cells of essential nutrients and molecules.
1.3.1. Proangiogenic drugs
There are currently three notable methods of approaching proangiogenic therapy, specifically, the local application of proangiogenic biomolecules and drugs (e.g., growth factors), implantation of angiogenesis precursor cells around areas of interest, and the artificial creation of environmental conditions that promote angiogenesis (e.g., hypoxia) [77].
Proangiogenic drugs that are used to treat ischemic diseases are currently based on existing diuretic drugs such as perindopril and lercanidipine. A low dose of perindopril and indapamide was administered to rats expressing ischemia in their legs, and the change in proangiogenic markers was measured. The administration of the diuretic drugs increased the expressions of VEGF and endothelial nitric-oxide synthase by 2.2-fold and 1.6-fold, respectively, promoting neovascularization around the induced ischemic region [78].
Cellular approaches to treat angiogenesis make use of bone marrow-derived mononuclear cells (BM-MNC), peripheral blood mononuclear cells (PB-MNC), endothelial progenitor cells, and pluripotent stem cells, among other differentiable cell lines to create a vascular bed that serves as a foundation for consecutive proangiogenic processes [79], [80]. When using cellular approaches, a critical consideration is how a secretome of these differentiable cells is heavily influenced by external and internal environments. To optimize the capacity of these cells to promote angiogenesis, environmental factors such as hypoxia and mechanical stress may be applied to the therapeutic cells before their implantation in vitro.
1.3.2. Antiangiogenic drugs
There are several Food and Drug Administration (FDA)-approved antiangiogenic drugs that are used primarily in cancer treatment. In 2019, axitinib was approved by the FDA for the treatment of advanced renal cell carcinoma. It functions by binding to a receptor involved in angiogenesis. Axitinib incorporates itself into the adenosine triphosphate (ATP)-binding site on VEGFR, inhibiting the initiation of angiogenic signaling pathways. The binding of axitinib inside the kinase tunnel structure confers a stabilized state of VEGFR, inactivating it [81], [82]. Axitinib is currently used to treat advanced renal cell carcinoma.
Another antiangiogenic drug undergoing clinical trials is bevacizumab, a recombinant monoclonal antibody that works as an immunotherapy drug against cancers. Bevacizumab functions by binding to a ligand involved in angiogenesis. Bevacizumab selectively binds to VEGF, preventing its attachment to VEGFR [83]. It is a modified monoclonal antibody with a strong affinity for VEGF. Isoforms of bevacizumab, including ranibizumab, with higher affinities for VEGF, have also been developed [84]. Bevacizumab is approved to treat lung cancer, kidney cancer, and glioblastoma.
2. Nanomaterial design in angiogenesis
In all steps of angiogenic cellular mechanisms, signaling molecules play central roles in initiating and promoting angiogenesis for applications in regenerative medicine and wound healing. Angiogenesis can be induced by both naturally occurring molecules (e.g., growth factors and exosomes) and synthetic compounds (e.g., graphene oxides and bioactive glasses).
Existing methods to promote angiogenesis include the delivery of molecules with proangiogenic capabilities. Some have attempted to transport growth factors such as VEGF and FGF to sites with newly regenerated tissue [1]. However, clinical applications of proangiogenic molecules are still limited due to the difficulty in precisely controlling angiogenic signaling and processes in vivo. For instance, natural growth factors are ideal promoters due to their low cytotoxicity and natural abundance. However, they have short half-lives, which significantly limits their therapeutic applications [85].
The effectiveness of the proangiogenic factors depends on the dosage around the individual cells, and it is crucial for researchers to be able to accurately send the therapeutic molecules to target sites to limit undesired effects to prevent cytotoxicity. One way to deliver growth factors to target sites is the use of exosomes as selective carriers. Researchers have found that exosomes can be used to efficiently deliver antiangiogenic drugs into the retina vasculature to treat conditions such as proliferative retinopathy [86]. A significant challenge with using exosomes, however, is the specificity of the exosomes themselves; researchers must find an exosome that can biochemically and physically accommodate the drug of interest. Despite the challenges in designing specialized exosomes for proangiogenic drugs, exosomes have been identified as a promising start to accelerate angiogenic therapies in regenerative medicine.
Due to these limitations, researchers have begun looking at inorganic nanomaterials as easily accessible proangiogenic drugs. Compared to naturally found molecules such as VEGF, inorganic nanomaterials are very stable and easier to modify and deliver. Thus, inorganic nanomaterial has become a hot research topic in regenerative medicine.
One of the most important factors to consider when using inorganic and non-native nanomaterials is their biocompatibility in vivo. It is important to consider the possibility of cytotoxicity when designing novel nanomaterials. Recent studies have identified inorganic nanomaterials as effective choices for proangiogenic therapy. Nanomaterials, including metals, graphene oxides, and glasses, have a wide range of success when considering therapeutic applications and effectiveness. Researchers have designed promising nanomaterials that may promote angiogenesis more effectively and safely. Among the various inorganic nanomaterials, metal-based nanomaterials, with their superior stability, limited immunogenicity, relatively cheap fabrication and storage costs, and ability to affect multiple downstream proangiogenic signaling pathways, are particularly promising and are the focus of the discussion below. These novel nanomaterials, combined with novel scaffold and carrier techniques, may help researchers conquer the challenges of controlling angiogenesis in the most desirable way.
2.1. Lanthanides
Lanthanide metal-based nanoparticles promote angiogenesis through the production of reactive oxygen species (ROS)—including H2O2 and NO—regulated by thioredoxin and superoxide dismutase (SOD) enzymes, to promote the expression of proangiogenic growth factors such as VEGF [11], [87]. The presence of lanthanide-based nanomaterials helped to enhance the accumulation of ROS around hypoxic regions, in addition to the upregulation of natural producers of ROS, including Ang-1 and NADPH oxidase.
2.1.1. Terbium
Terbium nanomaterials can be engineered into effective nanomaterials and proangiogenic drug carriers to stimulate blood vessel formation (Figure 4A, Figure 5A). Terbium hydroxide nanoparticles (1 to 10 μg/mL) enhanced endothelial cell proliferation and differentiation through NADPH oxidase-mediated PI3K/AKT signaling pathways, as well as the induction of phosphorylation at Ser473 (Akt) and Thr180/Tyr182 (p38MAPK) [88], [89]. After the induction of angiogenesis through the accumulation of ROS, the process is then inhibited through the expression of angiostatin, halting endothelial cell growth and proliferation [90]. Terbium hydroxide nanomaterials are not only used as proangiogenic nanomaterials but also as drug carriers. Layered terbium hydroxides (TbH-Cl) were loaded with proangiogenic drugs—diclofenac, ibuprofen, and naproxen—to monitor drug release rates in vitro [91]. The terbium hydroxide carriers sustained the drug release in approximately 5 hours, which might be extended with polymeric coating on the surface of terbium hydroxide. The controlled drug release rate is a desired trait for carrier molecules, and future studies should investigate the effective containment of proangiogenic growth factors like VEGF, ANG, insulin-like growth factor (IGF), and FGF in terbium hydroxide carriers.
Figure 4. Electron microscopy (scanning and transmission) images of selected metal nanoparticles.
(A) Transmission electron microscope (TEM) image of Tb(OH)3 spheres. Reprinted with permission from Ref. [11]. Copyright 2016, John Wiley and Sons. (B) TEM image of Eu(OH)3 nanorods. Reprinted with permission from Ref. [11]. Copyright 2016, John Wiley and Sons. (C) TEM image of copper nanoparticles. Reprinted with permission from Ref. [101]. Copyright 2015, Molecular Diversity Preservation International. (D) TEM image of a ZnO nanorod. Reprinted with permission from Ref. [102]. Copyright 2021, Frontiers Media S.A. (E) TEM image of NiTi nanoparticles. Reprinted with permission from Ref. [12]. Copyright 2022, Informa. (F) Scanning electron microscope (SEM) image of MgO particles (indicated by arrows) enclosed in hydroxyapatite spheres. Reprinted with permission from Ref. [103]. Copyright 2020, Nature Portfolio.
Figure 5. Effects of nanomaterials on vessel formation in vivo and in vitro.
(A) In vivo CAM assay to determine the angiogenic response of magnesium oxide nanospheres. HAp/MgO (hydroxyapatite/magnesium oxide) nanoparticles had a significantly higher angiogenic response compared to pure HAp nanoparticles. Reprinted with permission from Ref. [103]. Copyright 2020, Nature Portfolio. (B) Nanoceria (cerium oxide nanoparticles) coated with heparin, and loaded with FGF-2 (25 ng/ml), promoted an increase in blood vessel density. Reprinted with permission from Ref. [98]. Copyright 2022, Oxford University Press. (C) An in vivo CAM assay visualizing histopathological effects of zinc oxide nanorods (ZnO-NR). 20 μg/ml ZnO-NR (c) showed significant vascularization compared to the (a) negative control (b) and 10 μg/ml ZnO-NR. Reprinted with permission from Ref. [102]. Copyright 2021, Frontiers Media S.A. (D) In vivo chick chorioallantoic membrane (CAM) assay with control (1), CuSO4 (3), and copper nanoparticles (4) measuring the number of branches and length of vessels. Reprinted with permission from Ref. [101]. Copyright 2015, Molecular Diversity Preservation International. (E) In vivo assay in zebrafish embryos to recover circulation. The green represents the blood vessels and red represents the mature blood cells. Vessel formation by blank control (i), 100 μg/mL Eu rods (ii), 100 μg/mL Eu spheres (iii), 100 μg/mL Tb rods (iv), and 100 μg/mL Tb spheres (v). Reprinted with permission from Ref. [11]. Copyright 2016, John Wiley and Sons.
2.1.2. Europium
Europium (III) hydroxide-containing nanorods have been shown to exhibit a dose-dependent increase in endothelial cell proliferation, enhancing the observed rates of angiogenesis and wound healing through the phosphorylation of MAPK (Figure 4B) [92]. Europium hydroxide nanorods mediate endothelial cell proliferation through the production of ROS, mentioned earlier, which is accelerated in the presence of superoxide dismutase-like MnTBAP (Mn(III) tetrakis(4-benzoic acid) porphyrin chloride) [93]. MnTBAP catalyzes the conversion of superoxide into hydrogen peroxide, and its presence in cells has been associated with higher levels of endothelial cell proliferation compared to controls. Furthermore, downstream to ROS-mediated endothelial cell proliferation, europium hydroxide nanorods were also found to activate endothelial nitric oxide synthase (eNOS) and induce nitric oxide (NO) formation through PI3K/AMPK/Akt signaling pathway, a notable proangiogenic signaling pathway. Europium oxide nanorod-based dressing improved wound healing and skin regeneration by regulating inflammatory factors such as TNF-α and IL-6 and by enhancing proangiogenic factors such as CD31 [94]-[96].
2.1.3. Cerium
Cerium oxides are capable of modulating angiogenesis by regulating intracellular ROS by acting as ROS scavengers. Cerium nanoparticles induce angiogenesis through the stabilization of HIF-1α and the upregulation of proangiogenic signaling molecules expression, including p38 and MAPK [97]. The reported rates are promising; a chick chorioallantoic membrane (CAM) assay with stimulated cells (treated with cerium nanoparticles) showed a 400% increase in angiogenesis when compared to an unstimulated control cell (Figure 5B) [98], [99]. Cerium oxide nanomaterials promote angiogenesis through the modulation of intracellular oxygen as well as the upregulation of HIF-1α, which led to the elevation of VEGF mRNA expression. A high surface area and increased Ce3+/Ce4+ ratio are recognized as key factors in making cerium oxide nanomaterials robust inducers of angiogenesis [100].
2.2. Other therapeutic metal nanoparticles
2.2.1. Copper
One traditional concern about using copper ions as angiogenesis activators lies in the cytotoxicity of these ions released from copper salts, such as CuSO4. To circumvent this problem, a hydrocolloid of copper nanoparticle was used to test its proangiogenic capabilities in vivo (Figure 5C). Where CuSO4 releases Cu ions into its surroundings, copper nanoparticles release particles with a bioactive surface, limiting their cytotoxicity. 50 ppm concentration of hydrocolloids of copper nanoparticles (Zeta potential, −28.1) injected into chicken embryo increased vessel density by 2-fold relative to the control and 1.6-fold relative to a 50 ppm aqueous solution of CuSO4, as well as vessel length by 1.8-fold relative to the control and 1.6-fold relative to CuSO4 [101]. An examination of the chick embryos showed enhanced transcription of mRNA for proangiogenic growth factors such as VEGF-A and FGF-2. Furthermore, the copper nanoparticles upregulated the expression of PCNA and MyoD1—signs of increased cell division and differentiation—more so than CuSO4 salt and the control, supporting the authors’ conclusions that copper nanoparticles are more effective proangiogenic agents than copper ions (Figure 5C). However, this is not to say that copper ions cannot be used to promote angiogenesis. HUVEC (human umbilical vein endothelial cells) responded well to copper ion presence at concentrations of up to 10 μM. Copper ions induce the expression of proangiogenic factors like VEGF in a dose-dependent manner. At 10 μM, copper ions promoted a 2.5-fold higher VEGF expression in HUVEC after two days [104]. Combined with copper’s antibacterial property, copper nanoparticles and ions serve as potent promoters of angiogenesis in wound healing and regenerative medicine [105].
2.2.2. Cobalt
Cobalt has a similar mechanism of action as copper, stimulating HIF-1α expression and consequently boosting VEGF production. Cobalt ions have effectively been used in therapeutic settings when incorporated in bioactive glass (a new derivation of the SiO2─CaO─P2O5─CoO bioactive glass system) and in cobalt substituted β-tricalcium phosphate ceramics [106], [107]. Cobalt nanoparticles enhanced the production of ROS, activating the AKT/ERK1/2 signaling pathways, as well as the transcriptional activation of NF-κB, AP-1, and VEGF, enhancing rates of angiogenesis in vivo, shown by CAM assays. However, it has also been shown that cobalt at high concentrations (greater than 50 μM) inhibited human mesenchymal stem cell growth and HUVECs network formation in co-culture, though VEGF expression was upregulated. These inhibitory effects were diminished at lower Co2+ concentrations (below 50 μM), while VEGF expression remained high and even further augmented in the presence of ascorbic acid and dexamethasone [108].
2.2.3. Zinc
Zinc ions are regularly used by the body for metabolic processes to support cell growth and survival, including its use as activators of proteases that catalyze proangiogenic reactions. When elemental zinc is converted into zinc oxide nanorods, they exhibit significant proangiogenic properties through the generation of reduced oxygen species such as H2O2 [102], [109]. The production of H2O2 induces the transcription and expression of growth factors such as VEGF and FGF. Zinc ions can also serve to promote angiogenesis, specifically through the activations of the ZnR/GPR39 and the downstream Gq-PLC pathway, causing a 1.3-fold increase in endothelial adhesion in vitro (Figure 5D) [110]. In addition to the activation of proangiogenic signaling pathways, zinc oxide nanoflowers promoted angiogenesis through the increased migration of endothelial cells, the downstream production of NO, and the subsequent activation of the MAPK-Akt-eNOS pathway (Figure 4D).
Zinc also is an important cofactor of multiple enzymes—including MMP discussed earlier in this review—playing a critical regulatory function in the cell cycle and cellular respiration through the modulation of TNF, IL, and plasma glucose levels. For instance, zinc facilitates citrate secretion in prostate and osteoblast cells, which is critical for the function of the organs [111].
2.2.4. Nickel
Researchers have also begun focusing on metals that were previously overlooked, including nickel. By incorporating these metals into scaffolds and bioactive glasses, researchers have discovered their new proangiogenic properties for use in regenerative medicine. When nickel hydroxide nanoparticles were loaded onto implants (sPEEK-Ni-HA), HUVECs used the implant as a scaffold to connect to each other. The release of the Ni2+ ions from the implants facilitated the migration and angiogenic gene expressions of endothelial cells [112]. Another proangiogenic nickel-based nanoparticle is the nickel titanium (NiTi) nanoparticle (Figure 4E) [12]. They are spherical particles roughly 64 nm in size, promoting angiogenesis through stimulating the release of pro-inflammatory and proangiogenic cytokines and chemokines by the surrounding tissues in vivo, including HIF-1, IL-6, IL-8, c-inhibitor of apoptosis (c-IAP), B-cell lymphoma 2 (BCL-2), ICAM-1, IL-1β, and cyclooxygenase 2 (Cox-2).
2.2.5. Magnesium
Magnesium has long been known to promote angiogenesis, with high biocompatibility and biodegradability, making it an attractive material for use as proangiogenic nanoparticles. High levels of magnesium have been shown to stimulate endothelial cell proliferation and induce migratory signaling pathways, thereby initiating angiogenesis. Magnesium oxide nanoparticles work best in hypoxic environments, upregulating growth factors like VEGF to promote angiogenesis (Figure 4F, Figure 5E) [103], [113]. Magnesium at a concentration of 5 mM has been shown to stimulate strong HUVEC proliferation and angiogenesis in vitro through the induction of the expression and secretion of PDGF-BB [114]. Furthermore, magnesium has also been shown to promote angiogenesis by causing inflammation around the implanted area [115]. When inflammation occurs, it activates different types of cells, such as endothelial cells, macrophages, fibroblasts, and mast cells, triggering the release of angiogenic factors such as VEGF and cytokines. Thus, magnesium employs various pathways to induce angiogenesis, including growth factor production, gene expression, and inflammatory immune responses.
2.2.6. Lithium
Lithium is the smallest metal cation shown to promote angiogenesis. Treatment of brain endothelial cells with lithium chloride (LiCl) upregulated VEGF secretion through the phosphorylation of glycogen synthase kinase-3β (GSK-3β) [116]. Lithium ions can also stimulate angiogenesis through another pathway. Human microvascular endothelial cells treated with LiCl showed increased levels of cell proliferation and migration—characteristics of angiogenesis—through the augmentation of the Wnt/β-catenin signaling in the Norrin/Frizzled-4 pathway [117], [118]. Lithium can be delivered as soluble salts such as LiCl or on bioactive glasses. Lithium incorporated into bioactive glass ceramic (Li-BGC) promoted the growth of new blood vessels in vivo by elevating levels of proliferation and migration, as well as the expression of proangiogenic genes [119].
2.2.7. Cadmium
Exposure to cadmium has historically been associated with an increased risk for cancer. One possible mechanism of tumorigenesis is the extensive promotion of angiogenesis. Studies have shown that at low concentrations (1 to 10 μM), cadmium can cause an elevated secretion of VEGF and expression of VEGFR through the activation of protein kinase B (PKB)/Akt, NF-κB, and MAPK signaling cascades [120], [121]. Cadmium induced higher expressions of HIF-1 and ROS in the ERK/AKT signaling pathway as well as produced ROS, which can accelerate rates of cell proliferation, migration, and survival [122]. Although cadmium offers promising possibilities for novel angiogenesis therapies, it is important to note that high concentrations of cadmium surrounding endothelial cells initiate apoptosis, and researchers are currently attempting to identify an effective and safe in vivo dose for incorporation of cadmium into proangiogenic therapeutics[120].
2.2.8. Chromium
As with cadmium, hexavalent chromium has been identified as an environmental carcinogen. It has been shown that chromium plays a role in the induction of angiogenesis that turns a tumor malignant. In particular, the presence of chromium (VI) at low concentrations induces angiogenesis through the enhancement of inducible transcription factors such as NF-κB, p53, and c-Fos [123]. Proangiogenic molecules are activated when epidermal growth factors (EGF) bind to the epidermal growth factor receptor 1 (Her1) receptor, inducing cell migration. As with cadmium, there is a possibility that hexavalent chromium used in therapeutic contexts could cause tumorigenesis at high concentrations. Thus it is critical to determine the therapeutic dose of cadmium-containing nanomaterials. An in vitro cytotoxicity assay identified a safe range of chromium oxide nanopariticle as long as it is lower than 10ug/ml [124]. In the same study, the authors also suggested that DNA damage assay might be a useful biomarker for determining the safety of chromium in human and animal health.
2.3. Biomaterials for angiogenic nanomaterial delivery
Proangiogenic nanomaterials may be loaded onto a scaffold for efficient delivery to target tissues. However, a drug delivery scaffold is ineffective if it is unable to release therapeutic agents to target regions. Thus, to initiate the release of proangiogenic biomolecules from the scaffolds to induce angiogenesis, there are three mechanisms that researchers can utilize: degradation-dependent release, trigger specific release of biomaterials, and mechanical release. Degradation-dependent release happens when endothelial cells secrete proteolytic enzymes that allow them to easily degrade extracellular-derived scaffolds produced from natural or cultured cells [126]. Degradation-dependent release works through the attachment of drugs to carrier molecules through surface-level adhesion. However, there are risks of using degradation-dependent release to deliver proangiogenic factors to target sites, namely, burst release. Burst release happens when the scaffold decomposes in an undesired way, unloading drugs into the target region at extremely high concentrations. This could potentially lead to cytotoxicity due to high concentrations of the drugs. Trigger-specific release usually involves the release of different drugs in different layers, usually activated by enzymes (e.g., proteinases) or light of specific wavelengths (e.g., near IR). This allows for the precise and regulated release of drugs in the target site, limiting burst release and increasing biocompatibility. The drugs and carrier molecules are bound together by linkers that release drugs upon stimulation by activating factors. The primary challenge in using trigger-specific release methods is the difficulty in designing linkers that bind the drug and scaffold together and respond to the specific stimulus (e.g., an enzyme found around target sites of angiogenesis or specific wavelengths of light). Lastly, mechanical release refers to the application of mechanical stress, such as pressure, to physically release drugs from within the scaffolds. The process of mechanical release normally happens at the microscopic level and the cells apply pressure on the scaffolds.
Combined with cell surface engineering, researchers can direct scaffolds loaded with proangiogenic drugs accurately to the target site and allow cells to finish the drug release process. These scaffold systems can also work hand in hand with the nanomaterials mentioned earlier by incorporating nanomaterials into scaffolds for release inside the body. Specifically, many types of scaffolds carry nanobiomaterials to target tissue sites to promote angiogenesis. Researchers determine which scaffold system to use to enclose biomaterials by manipulating the scaffold design (e.g., materials and physical alignment). Scaffolds must be designed to be biocompatible to help facilitate wound healing processes at a faster rate; ideal scaffolds promote cell proliferation and adhesion at a rapid rate with their porosity and flexibility to allow the exchange of nutrients and essential molecules [127].
In addition to nanomaterials that can be engineered using natural materials, synthetic molecules can also effectively enhance angiogenesis in the body. Potential materials for scaffolds include synthetic and natural polymers [128]. Synthetic polymers have many benefits, such as the ease of designing scaffolds with ideal characteristics (e.g., drug release and degradation rate, affinity to drugs, size, and elasticity) and cheap cost of manufacture. However, some synthetic polymers may have high cytotoxicity and even induce inflammatory responses in vivo, limiting their therapeutic applications. Synthetic polymers like poly(lactic-co-glycolic acid) and polylactic acid are commonly used as ingredients for synthetic scaffolds due to their low cytotoxicity and high degradability in vivo [129]. These scaffolds can be used in conjunction with previously mentioned proangiogenic molecules and drugs, such as growth factors, metal oxides, and lanthanide hydroxides. Natural polymers are also effective transporters. Natural polymers are derived from organic and biological components in cells, such as collagen, proteins, and the extracellular matrix. Natural polymers have low cytotoxicity and limited inflammatory responses. However, it is difficult to design natural polymers that can effectively carry drugs and molecules of interest, unlike synthetic polymers.
All these synthetic technologies to promote angiogenesis have unique advantages, the most significant one being the ability to edit the structures and compositions of nanomaterials and carriers to best enhance their proangiogenic abilities. This is a feature unique to synthetic nanomaterials that is difficult to replicate in naturally found biomaterials. The ability to manually adjust the properties of nanomaterials opens countless possibilities to promote angiogenesis because it is easy to accommodate different combinations of nanomaterials and carrier molecules. However, it is critical that researchers evaluate the nanomaterials’ biocompatibilities in live cell assays or animals before conducting human trials. There are nanomaterials with potentially hazardous effects in regenerative medicine, such as europium’s induction of an immune response and cadmium’s carcinogenic potential. Some ways researchers have addressed this issue, as mentioned earlier in the review, is through the discovery of relatively nontoxic oxidation states of carcinogenic metals (e.g., Cr(VI)) or ultralow nanomaterial concentrations (e.g., 1–10 μM) that limit cytotoxicity while promoting angiogenesis [123],[121],[101].
3. Conclusion
In this review paper, we discussed the methods that are currently used to promote angiogenesis. Angiogenesis is critical in regenerative medicine, wound healing, and osteogenesis, as blood vessels play a crucial role in delivering oxygen and nutrients to tissues and organs to maintain sustainable conditions. The current methods have significant limitations for applications in vivo due to issues such as biocompatibility, toxicity, and efficiency. However, recent advances in nanomaterials research have opened new paths to induce angiogenesis significantly more effectively with smaller risks. We focused particularly on metal-based nanomaterials.
Future research may significantly improve the quality of angiogenesis in regenerative medicine by combining potent proangiogenic technologies such as stem cell-derived exosomes with the nanomaterials highlighted in this paper. However, it is important to recognize that many of the techniques to incorporate nanomaterials (e.g., exosomes and scaffolds) are highly specific; that is, proangiogenic nanoparticles must both be effective and distinct. The specificity will pose a new challenge for researchers to overcome.
Table 1.
Summaries of proangiogenic metals and their signaling pathways.
| Metal | Mechanism of action |
Nanomaterial format |
Experimental methods |
Method of fabrication |
Nanomaterial concentrations tested |
References |
|---|---|---|---|---|---|---|
| Terbium | NADPH oxidase-mediated PI3K/AKT activation, ROS accumulation | Layered terbium(III) hydroxide (Tb(OH)3) nanorods, Tb(OH)3 nanospheres | Sprouting and proangiogenesis fluorescence by an in vivo zebrafish embryo assay | Reaction of aqueous terbium(III) nitrate hydrate and NH4OH with SINEO-MAS II, reaction of TbCl3 hydrate and NaCl/NaOH at 60°C | 5–10 μg/mL | [88]-[91] |
| Europium | PI3K/AMPK/Akt activation, inhibition of TNF-α and IL-6, upregulation of CD31 | Europium(III) hydroxide (Eu(OH)3) nanorods, Eu(OH)3 nanospheres | Sprouting and proangiogenesis fluorescence by an in vivo zebrafish embryo assay | Simple microwave heating of an aqueous solution of europium(III) nitrate and NH4H2PO4 | 20–50 μg/mL | [92]-[96], [125] |
| Cerium | Upregulation of HIF-1α, enhanced VEGF mRNA translation | Ce3+/Ce4+ containing oxide | In vitro endothelial tube formation assay, branch number measured by an in vivo CAM assay | Flame spray pyrolysis of cerium-containing liquid precursor and cerium 2-ethylhexanoate in xylene | 50 μg/mL | [97]-[99] |
| Copper | VEGF-A and FGF2 upregulation, enhanced expression of PCNA, MyoD1 | Copper hydrocolloid nanospheres | Vessel length and branch number measured by an in vivo CAM assay | Copper oxide impregnation of wound dressing fibers | 0.1–10 μM | [101], [104], [105] |
| Cobalt | Activation of AKT/ERK1/2, transcriptional activation of NF-κB, AP-1 and VEGF | Tungsten carbide-cobalt nanoparticles | VEGF secretion measured in vitro, neovascularization measured in vivo with HUVEC | Sol-gel method with tetraethyl orthosilicate, triethyl phosphate, Ca(NO3)2, and CoCl2, and nitric acid | 1,000–2,000 μg/mL | [106]-[108] |
| Zinc | ROS production, VEGF and FGF upregulation, ZnR/GPR39, Gq-PLC, and MAPK-Akt-eNOS activation | Zinc oxide (ZnO) nanoflowers, ZnO nanorods | Vessel branch number measured by an in vivo CAM assay | Sol-gel method with Zn(CH3COO)2 and albumin in ultrapure water | 10–50 μg/mL | [102], [109]-[111] |
| Nickel | Release of HIF-1, IL-6, c-IAP, BCL-2, ICAM-1, IL-1β, and Cox-2 | Nickel titanium nanoparticles | Measurement of angiogenic potential in athymic (T cell-deficient) nude mice | Prepared via laser evaporation of NiTi alloy, nanoparticles heat-treated at 250°C and resuspended in LAL reagent water | 0.25–0.75 mM | [12], [112] |
| Magnesium | MC3T3-E1 expression, PDGF-BB secretion | Mg2+ ions | Angiogenic potential measured by in vivo CAM assay, cytocompatibility measured in vitro with MC3T3-E1 | Hydroxyapatite and MgO added to sodium alginate solution, and extruded to CaCl2 | 1–5 mM | [103], [113]-[115] |
| Lithium | Augmentation of Wnt/β-catenin in Norrin/Frizzled-4, VEGF secretion through the phosphorylation of glycogen synthase kinase-3β (GSK-3β) | Li+ (in the form of LiCl) | In vitro measurement of proangiogenic factors (VEGF) secretion by BMSC | LiCl in serum-free medium | 0.2–20 mM | [116]-[119] |
| Cadmium | Enhanced VEGF expression, activation of PKB/Akt, NF-κB, MAPK, and ERK/AKT | Ultralow concentrations of elemental cadmium | In vitro measurement of endothelial structure formation with HUVEC | Cadmium chloride in basal media | 1–10 μM | [120]-[122] |
| Chromium | Enhance promoters for proangiogenic transcription factors such as NF-κB, p53, and c-Fos | Hexavalent chromium (Cr(VI)) compounds | In vitro measurement of proangiogenic miR-143 and miR-145 in Cr(VI)-transformed cells | Chromium nanomaterial in the form of Cr(VI) oxidation state | 1–10 μM | [123] |
ACKNOWLEDGEMENTS
This work was supported in part by National Institutes of Health awards (AR072731, NS123433, HL158204, EB030140), Penn State College of Engineering Multidisciplinary Seed Grant.
REFERENCES
- [1].Gianni-Barrera R et al. , “Therapeutic vascularization in regenerative medicine,” Stem Cells Transl. Med, vol. 9, no. 4, pp. 433–444, Apr. 2020, doi: 10.1002/sctm.19-0319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Vailhé B, Vittet D, and Feige J-J, “In Vitro Models of Vasculogenesis and Angiogenesis,” Lab. Invest, vol. 81, no. 4, pp. 439–452, Apr. 2001, doi: 10.1038/labinvest.3780252. [DOI] [PubMed] [Google Scholar]
- [3].Novosel EC, Kleinhans C, and Kluger PJ, “Vascularization is the key challenge in tissue engineering,” Adv. Drug Deliv. Rev, vol. 63, no. 4–5, pp. 300–311, Apr. 2011, doi: 10.1016/j.addr.2011.03.004. [DOI] [PubMed] [Google Scholar]
- [4].Liu Y, Cheng Y, Zhao C, Wang H, and Zhao Y, “Nanomotor-Derived Porous Biomedical Particles from Droplet Microfluidics,” Adv. Sci, vol. 9, no. 4, p. 2104272, Feb. 2022, doi: 10.1002/advs.202104272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Kim D et al. , “Effective Delivery of Anti-Cancer Drug Molecules with Shape Transforming Liquid Metal Particles,” Cancers, vol. 11, no. 11, p. 1666, Oct. 2019, doi: 10.3390/cancers11111666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Yu Y, Guo J, Ma B, Zhang D, and Zhao Y, “Liquid metal-integrated ultra-elastic conductive microfibers from microfluidics for wearable electronics,” Sci. Bull, vol. 65, no. 20, pp. 1752–1759, Oct. 2020, doi: 10.1016/j.scib.2020.06.002. [DOI] [PubMed] [Google Scholar]
- [7].Sigel A, Sigel H, Freisinger E, and Sigel RKO, Eds., Metallo-Drugs: Development and Action of Anticancer Agents. De Gruyter, 2018. doi: 10.1515/9783110470734. [DOI] [Google Scholar]
- [8].Kurtuldu F, Mutlu N, Boccaccini AR, and Galusek D, “Gallium containing bioactive materials: A review of anticancer, antibacterial, and osteogenic properties,” Bioact. Mater, vol. 17, pp. 125–146, Nov. 2022, doi: 10.1016/j.bioactmat.2021.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Barui AK, Nethi SK, Haque S, Basuthakur P, and Patra CR, “Recent Development of Metal Nanoparticles for Angiogenesis Study and Their Therapeutic Applications,” ACS Appl. Bio Mater, vol. 2, no. 12, pp. 5492–5511, Dec. 2019, doi: 10.1021/acsabm.9b00587. [DOI] [PubMed] [Google Scholar]
- [10].Vassie JA, Whitelock JM, and Lord MS, “Endocytosis of cerium oxide nanoparticles and modulation of reactive oxygen species in human ovarian and colon cancer cells,” Acta Biomater., vol. 50, pp. 127–141, Mar. 2017, doi: 10.1016/j.actbio.2016.12.010. [DOI] [PubMed] [Google Scholar]
- [11].Zhao H et al. , “Lanthanide Hydroxide Nanoparticles Induce Angiogenesis via ROS-Sensitive Signaling,” Small, vol. 12, no. 32, pp. 4404–4411, Aug. 2016, doi: 10.1002/smll.201600291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Srivastava AK, Snapper DM, Zheng J, Yildrim BS, Srivastava S, and Wood SC, “Examining the role of nickel and NiTi nanoparticles promoting inflammation and angiogenesis,” J. Immunotoxicol, vol. 19, no. 1, pp. 61–73, Dec. 2022, doi: 10.1080/1547691X.2022.2080307. [DOI] [PubMed] [Google Scholar]
- [13].D’Amico G, Muñoz-Félix JM, Pedrosa AR, and Hodivala-Dilke KM, “‘Splitting the matrix’: intussusceptive angiogenesis meets MT 1- MMP,” EMBO Mol. Med, vol. 12, no. 2, Feb. 2020, doi: 10.15252/emmm.201911663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Norton K-A and Popel AS, “Effects of endothelial cell proliferation and migration rates in a computational model of sprouting angiogenesis,” Sci. Rep, vol. 6, no. 1, p. 36992, Nov. 2016, doi: 10.1038/srep36992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Kühn C and Checa S, “Computational Modeling to Quantify the Contributions of VEGFR1, VEGFR2, and Lateral Inhibition in Sprouting Angiogenesis,” Front. Physiol, vol. 10, p. 288, Mar. 2019, doi: 10.3389/fphys.2019.00288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Eelen G, Treps L, Li X, and Carmeliet P, “Basic and Therapeutic Aspects of Angiogenesis Updated,” Circ. Res, vol. 127, no. 2, pp. 310–329, Jul. 2020, doi: 10.1161/CIRCRESAHA.120.316851. [DOI] [PubMed] [Google Scholar]
- [17].Shinkaruk S, Bayle M, Lain G, and Deleris G, “Vascular Endothelial Cell Growth Factor (VEGF), An Emerging Target for Cancer Chemotherapy,” Curr. Med. Chem.-Anti-Cancer Agents, vol. 3, no. 2, pp. 95–117, Mar. 2003, doi: 10.2174/1568011033353452. [DOI] [PubMed] [Google Scholar]
- [18].Berendsen AD and Olsen BR, “How Vascular Endothelial Growth Factor-A (VEGF) Regulates Differentiation of Mesenchymal Stem Cells,” J. Histochem. Cytochem, vol. 62, no. 2, pp. 103–108, Feb. 2014, doi: 10.1369/0022155413516347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Ramakrishnan S, Anand V, and Roy S, “Vascular Endothelial Growth Factor Signaling in Hypoxia and Inflammation,” J. Neuroimmune Pharmacol, vol. 9, no. 2, pp. 142–160, Mar. 2014, doi: 10.1007/s11481-014-9531-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Zhou Y et al. , “The Role of the VEGF Family in Coronary Heart Disease,” Front. Cardiovasc. Med, vol. 8, p. 738325, Aug. 2021, doi: 10.3389/fcvm.2021.738325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Dvorak HF, “Vascular Permeability Factor/Vascular Endothelial Growth Factor: A Critical Cytokine in Tumor Angiogenesis and a Potential Target for Diagnosis and Therapy,” J. Clin. Oncol, vol. 20, no. 21, pp. 4368–4380, Nov. 2002, doi: 10.1200/JCO.2002.10.088. [DOI] [PubMed] [Google Scholar]
- [22].Zhang B et al. , “Gene expression of vascular endothelial growth factor A and hypoxic adaptation in Tibetan pig,” J. Anim. Sci. Biotechnol, vol. 7, no. 1, p. 21, Dec. 2016, doi: 10.1186/s40104-016-0082-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Ruan G-X and Kazlauskas A, “VEGF-A engages at least three tyrosine kinases to activate PI3K/Akt,” Cell Cycle, vol. 11, no. 11, pp. 2047–2048, Jun. 2012, doi: 10.4161/cc.20535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Hemmings BA and Restuccia DF, “PI3K-PKB/Akt Pathway,” Cold Spring Harb. Perspect. Biol, vol. 4, no. 9, pp. a011189–a011189, Sep. 2012, doi: 10.1101/cshperspect.a011189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Pandey AK et al. , “Mechanisms of VEGF (Vascular Endothelial Growth Factor) Inhibitor–Associated Hypertension and Vascular Disease,” Hypertension, vol. 71, no. 2, Feb. 2018, doi: 10.1161/HYPERTENSIONAHA.117.10271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Weddell JC, Chen S, and Imoukhuede PI, “VEGFR1 promotes cell migration and proliferation through PLCγ and PI3K pathways,” Npj Syst. Biol. Appl, vol. 4, no. 1, p. 1, Dec. 2017, doi: 10.1038/s41540-017-0037-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Wang X, Bove AM, Simone G, and Ma B, “Molecular Bases of VEGFR-2-Mediated Physiological Function and Pathological Role,” Front. Cell Dev. Biol, vol. 8, p. 599281, Nov. 2020, doi: 10.3389/fcell.2020.599281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Takahashi T, “A single autophosphorylation site on KDR/Flk-1 is essential for VEGF-A-dependent activation of PLC-gamma and DNA synthesis in vascular endothelial cells,” EMBO J, vol. 20, no. 11, pp. 2768–2778, Jun. 2001, doi: 10.1093/emboj/20.11.2768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Takahashi T, Ueno H, and Shibuya M, “VEGF activates protein kinase C-dependent, but Ras-independent Raf-MEK-MAP kinase pathway for DNA synthesis in primary endothelial cells,” Oncogene, vol. 18, no. 13, pp. 2221–2230, Apr. 1999, doi: 10.1038/sj.onc.1202527. [DOI] [PubMed] [Google Scholar]
- [30].Karar J and Maity A, “PI3K/AKT/mTOR Pathway in Angiogenesis,” Front. Mol. Neurosci, vol. 4, 2011, doi: 10.3389/fnmol.2011.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Peach C et al. , “Molecular Pharmacology of VEGF-A Isoforms: Binding and Signalling at VEGFR2,” Int. J. Mol. Sci, vol. 19, no. 4, p. 1264, Apr. 2018, doi: 10.3390/ijms19041264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Zarkada G, Heinolainen K, Makinen T, Kubota Y, and Alitalo K, “VEGFR3 does not sustain retinal angiogenesis without VEGFR2,” Proc. Natl. Acad. Sci, vol. 112, no. 3, pp. 761–766, Jan. 2015, doi: 10.1073/pnas.1423278112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Goetz R and Mohammadi M, “Exploring mechanisms of FGF signalling through the lens of structural biology,” Nat. Rev. Mol. Cell Biol, vol. 14, no. 3, pp. 166–180, Mar. 2013, doi: 10.1038/nrm3528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Yun Y-R et al. , “Fibroblast Growth Factors: Biology, Function, and Application for Tissue Regeneration,” J. Tissue Eng, vol. 1, no. 1, p. 218142, Jan. 2010, doi: 10.4061/2010/218142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Katoh M and Katoh M, “FGF signaling network in the gastrointestinal tract (Review),” Int. J. Oncol, Jul. 2006, doi: 10.3892/ijo.29.1.163. [DOI] [PubMed] [Google Scholar]
- [36].Teven CM, Farina EM, Rivas J, and Reid RR, “Fibroblast growth factor (FGF) signaling in development and skeletal diseases,” Genes Dis, vol. 1, no. 2, pp. 199–213, Dec. 2014, doi: 10.1016/j.gendis.2014.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Cross MJ and Claesson-Welsh L, “FGF and VEGF function in angiogenesis: signalling pathways, biological responses and therapeutic inhibition,” Trends Pharmacol. Sci, vol. 22, no. 4, pp. 201–207, Apr. 2001, doi: 10.1016/S0165-6147(00)01676-X. [DOI] [PubMed] [Google Scholar]
- [38].Serban D, Leng J, and Cheresh D, “H-Ras Regulates Angiogenesis and Vascular Permeability by Activation of Distinct Downstream Effectors,” Circ. Res, vol. 102, no. 11, pp. 1350–1358, Jun. 2008, doi: 10.1161/CIRCRESAHA.107.169664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].McCubrey JA et al. , “Roles of the Raf/MEK/ERK pathway in cell growth, malignant transformation and drug resistance,” Biochim. Biophys. Acta BBA - Mol. Cell Res, vol. 1773, no. 8, pp. 1263–1284, Aug. 2007, doi: 10.1016/j.bbamcr.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Song M and Finley SD, “ERK and Akt exhibit distinct signaling responses following stimulation by pro-angiogenic factors,” Cell Commun. Signal, vol. 18, no. 1, p. 114, Dec. 2020, doi: 10.1186/s12964-020-00595-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Tsunoda S, Nakamura T, Sakurai H, and Saiki I, “Fibroblast growth factor-2-induced host stroma reaction during initial tumor growth promotes progression of mouse melanoma via vascular endothelial growth factor A-dependent neovascularization,” Cancer Sci, vol. 98, no. 4, pp. 541–548, Apr. 2007, doi: 10.1111/j.1349-7006.2007.00432.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Seghezzi G et al. , “Fibroblast Growth Factor-2 (FGF-2) Induces Vascular Endothelial Growth Factor (VEGF) Expression in the Endothelial Cells of Forming Capillaries: An Autocrine Mechanism Contributing to Angiogenesis,” J. Cell Biol, vol. 141, no. 7, pp. 1659–1673, Jun. 1998, doi: 10.1083/jcb.141.7.1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Claffey KP, Abrams K, Shih S-C, Brown LF, Mullen A, and Keough M, “Fibroblast Growth Factor 2 Activation of Stromal Cell Vascular Endothelial Growth Factor Expression and Angiogenesis,” Lab. Invest, vol. 81, no. 1, pp. 61–75, Jan. 2001, doi: 10.1038/labinvest.3780212. [DOI] [PubMed] [Google Scholar]
- [44].Maisonpierre PC et al. , “Angiopoietin-2, a Natural Antagonist for Tie2 That Disrupts in vivo Angiogenesis,” Science, vol. 277, no. 5322, pp. 55–60, Jul. 1997, doi: 10.1126/science.277.5322.55. [DOI] [PubMed] [Google Scholar]
- [45].Thurston G and Daly C, “The Complex Role of Angiopoietin-2 in the Angiopoietin-Tie Signaling Pathway,” Cold Spring Harb. Perspect. Med, vol. 2, no. 9, pp. a006650–a006650, Sep. 2012, doi: 10.1101/cshperspect.a006650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Yuan HT, Khankin EV, Karumanchi SA, and Parikh SM, “Angiopoietin 2 Is a Partial Agonist/Antagonist of Tie2 Signaling in the Endothelium,” Mol. Cell. Biol, vol. 29, no. 8, pp. 2011–2022, Apr. 2009, doi: 10.1128/MCB.01472-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Teichert-Kuliszewska K, “Biological action of angiopoietin-2 in a fibrin matrix model of angiogenesis is associated with activation of Tie2,” Cardiovasc. Res, vol. 49, no. 3, pp. 659–670, Feb. 2001, doi: 10.1016/S0008-6363(00)00231-5. [DOI] [PubMed] [Google Scholar]
- [48].Saharinen P, Eklund L, and Alitalo K, “Therapeutic targeting of the angiopoietin–TIE pathway,” Nat. Rev. Drug Discov, vol. 16, no. 9, pp. 635–661, Sep. 2017, doi: 10.1038/nrd.2016.278. [DOI] [PubMed] [Google Scholar]
- [49].Gurney A and Hoey T, “Anti-DLL4, a cancer therapeutic with multiple mechanisms of action,” Vasc. Cell, vol. 3, no. 1, p. 18, 2011, doi: 10.1186/2045-824X-3-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Lobov I and Mikhailova N, “The Role of Dll4/Notch Signaling in Normal and Pathological Ocular Angiogenesis: Dll4 Controls Blood Vessel Sprouting and Vessel Remodeling in Normal and Pathological Conditions,” J. Ophthalmol, vol. 2018, pp. 1–8, Jul. 2018, doi: 10.1155/2018/3565292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Hofmann M and Heineke J, “The Impact of Endothelial Transcription Factors in Sprouting Angiogenesis,” in Tumor Angiogenesis, Marmé D, Ed., Cham: Springer International Publishing, 2018, pp. 1–18. doi: 10.1007/978-3-319-31215-6_38-1. [DOI] [Google Scholar]
- [52].Ledent V and Vervoort M, “The Basic Helix-Loop-Helix Protein Family: Comparative Genomics and Phylogenetic Analysis,” Genome Res, vol. 11, no. 5, pp. 754–770, May 2001, doi: 10.1101/gr.177001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Pugh CW, O’Rourke JF, Nagao M, Gleadle JM, and Ratcliffe PJ, “Activation of Hypoxia-inducible Factor-1; Definition of Regulatory Domains within the α Subunit,” J. Biol. Chem, vol. 272, no. 17, pp. 11205–11214, Apr. 1997, doi: 10.1074/jbc.272.17.11205. [DOI] [PubMed] [Google Scholar]
- [54].Jones S, “An overview of the basic helix-loop-helix proteins,” Genome Biol., vol. 5, no. 6, p. 226, 2004, doi: 10.1186/gb-2004-5-6-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Lv X et al. , “The role of hypoxia-inducible factors in tumor angiogenesis and cell metabolism,” Genes Dis, vol. 4, no. 1, pp. 19–24, Mar. 2017, doi: 10.1016/j.gendis.2016.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Hashimoto T and Shibasaki F, “Hypoxia-Inducible Factor as an Angiogenic Master Switch,” Front. Pediatr, vol. 3, Apr. 2015, doi: 10.3389/fped.2015.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Greijer A et al. , “Up-regulation of gene expression by hypoxia is mediated predominantly by hypoxia-inducible factor 1 (HIF-1),” J. Pathol, vol. 206, no. 3, pp. 291–304, Jul. 2005, doi: 10.1002/path.1778. [DOI] [PubMed] [Google Scholar]
- [58].Manalo DJ et al. , “Transcriptional regulation of vascular endothelial cell responses to hypoxia by HIF-1,” Blood, vol. 105, no. 2, pp. 659–669, Jan. 2005, doi: 10.1182/blood-2004-07-2958. [DOI] [PubMed] [Google Scholar]
- [59].Ceradini DJ et al. , “Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1,” Nat. Med, vol. 10, no. 8, pp. 858–864, Aug. 2004, doi: 10.1038/nm1075. [DOI] [PubMed] [Google Scholar]
- [60].Oeckinghaus A and Ghosh S, “The NF- B Family of Transcription Factors and Its Regulation,” Cold Spring Harb. Perspect. Biol, vol. 1, no. 4, pp. a000034–a000034, Oct. 2009, doi: 10.1101/cshperspect.a000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Perkins ND, “Integrating cell-signalling pathways with NF-κB and IKK function,” Nat. Rev. Mol. Cell Biol, vol. 8, no. 1, pp. 49–62, Jan. 2007, doi: 10.1038/nrm2083. [DOI] [PubMed] [Google Scholar]
- [62].Huxford T and Ghosh G, “A Structural Guide to Proteins of the NF- B Signaling Module,” Cold Spring Harb. Perspect. Biol, vol. 1, no. 3, pp. a000075–a000075, Sep. 2009, doi: 10.1101/cshperspect.a000075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Morita R et al. , “ETS transcription factor ETV2 directly converts human fibroblasts into functional endothelial cells,” Proc. Natl. Acad. Sci, vol. 112, no. 1, pp. 160–165, Jan. 2015, doi: 10.1073/pnas.1413234112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Wen AY, Sakamoto KM, and Miller LS, “The Role of the Transcription Factor CREB in Immune Function,” J. Immunol, vol. 185, no. 11, pp. 6413–6419, Dec. 2010, doi: 10.4049/jimmunol.1001829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Kottakis F, Polytarchou C, Foltopoulou P, Sanidas I, Kampranis SC, and Tsichlis PN, “FGF-2 Regulates Cell Proliferation, Migration, and Angiogenesis through an NDY1/KDM2B-miR-101-EZH2 Pathway,” Mol. Cell, vol. 43, no. 2, pp. 285–298, Jul. 2011, doi: 10.1016/j.molcel.2011.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Lee H-T, Chang Y-C, Tu Y-F, and Huang C-C, “VEGF-A/VEGFR-2 Signaling Leading to cAMP Response Element-Binding Protein Phosphorylation Is a Shared Pathway Underlying the Protective Effect of Preconditioning on Neurons and Endothelial Cells,” J. Neurosci, vol. 29, no. 14, pp. 4356–4368, Apr. 2009, doi: 10.1523/JNEUROSCI.5497-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Merika M and Orkin SH, “DNA-binding specificity of GATA family transcription factors,” Mol. Cell. Biol, vol. 13, no. 7, pp. 3999–4010, Jul. 1993, doi: 10.1128/mcb.13.7.3999-4010.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Jia W et al. , “GATA4 regulates angiogenesis and persistence of inflammation in rheumatoid arthritis,” Cell Death Dis, vol. 9, no. 5, p. 503, May 2018, doi: 10.1038/s41419-018-0570-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Kim J et al. , “YAP/TAZ regulates sprouting angiogenesis and vascular barrier maturation,” J. Clin. Invest, vol. 127, no. 9, pp. 3441–3461, Aug. 2017, doi: 10.1172/JCI93825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Shchors K, Shchors E, Rostker F, Lawlor ER, Brown-Swigart L, and Evan GI, “The Myc-dependent angiogenic switch in tumors is mediated by interleukin 1β,” Genes Dev., vol. 20, no. 18, pp. 2527–2538, Sep. 2006, doi: 10.1101/gad.1455706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].van Hinsbergh VWM, Engelse MA, and Quax PHA, “Pericellular Proteases in Angiogenesis and Vasculogenesis,” Arterioscler. Thromb. Vasc. Biol, vol. 26, no. 4, pp. 716–728, Apr. 2006, doi: 10.1161/01.ATV.0000209518.58252.17. [DOI] [PubMed] [Google Scholar]
- [72].Klein T and Bischoff R, “Physiology and pathophysiology of matrix metalloproteases,” Amino Acids, vol. 41, no. 2, pp. 271–290, Jul. 2011, doi: 10.1007/s00726-010-0689-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Uria JA and Werb Z, “Matrix metalloproteinases and their expression in mammary gland,” Cell Res., vol. 8, no. 3, pp. 187–194, Sep. 1998, doi: 10.1038/cr.1998.19. [DOI] [PubMed] [Google Scholar]
- [74].Zhu T et al. , “Proangiogenic Effects of Protease-Activated Receptor 2 Are Tumor Necrosis Factor-α and Consecutively Tie2 Dependent,” Arterioscler. Thromb. Vasc. Biol, vol. 26, no. 4, pp. 744–750, Apr. 2006, doi: 10.1161/01.ATV.0000205591.88522.d4. [DOI] [PubMed] [Google Scholar]
- [75].Sheldon H et al. , “New mechanism for Notch signaling to endothelium at a distance by Delta-like 4 incorporation into exosomes,” Blood, vol. 116, no. 13, pp. 2385–2394, Sep. 2010, doi: 10.1182/blood-2009-08-239228. [DOI] [PubMed] [Google Scholar]
- [76].Mineo M et al. , “Exosomes released by K562 chronic myeloid leukemia cells promote angiogenesis in a src-dependent fashion,” Angiogenesis, vol. 15, no. 1, pp. 33–45, Mar. 2012, doi: 10.1007/s10456-011-9241-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Gaspar D, Peixoto R, De Pieri A, Striegl B, Zeugolis DI, and Raghunath M, “Local pharmacological induction of angiogenesis: Drugs for cells and cells as drugs,” Adv. Drug Deliv. Rev, vol. 146, pp. 126–154, Jun. 2019, doi: 10.1016/j.addr.2019.06.002. [DOI] [PubMed] [Google Scholar]
- [78].Silvestre J-S, Kamsu-Kom N, Clergue M, Duriez M, and Lévy BI, “Very-Low-Dose Combination of the Angiotensin-Converting Enzyme Inhibitor Perindopril and the Diuretic Indapamide Induces an Early and Sustained Increase in Neovascularization in Rat Ischemic Legs,” J. Pharmacol. Exp. Ther, vol. 303, no. 3, pp. 1038–1043, Dec. 2002, doi: 10.1124/jpet.102.040014. [DOI] [PubMed] [Google Scholar]
- [79].Hou L, Kim JJ, Woo YJ, and Huang NF, “Stem cell-based therapies to promote angiogenesis in ischemic cardiovascular disease,” Am. J. Physiol.-Heart Circ. Physiol, vol. 310, no. 4, pp. H455–H465, Feb. 2016, doi: 10.1152/ajpheart.00726.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Samura M, Hosoyama T, Takeuchi Y, Ueno K, Morikage N, and Hamano K, “Therapeutic strategies for cell-based neovascularization in critical limb ischemia,” J. Transl. Med, vol. 15, no. 1, p. 49, Dec. 2017, doi: 10.1186/s12967-017-1153-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Gross-Goupil M, Françlois L, Quivy A, and Ravaud A, “Axitinib: A Review of its Safety and Efficacy in the Treatment of Adults with Advanced Renal Cell Carcinoma,” Clin. Med. Insights Oncol, vol. 7, p. CMO.S10594, Jan. 2013, doi: 10.4137/CMO.S10594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Mittal K, Wood LS, and Rini BI, “Axitinib in Metastatic Renal Cell Carcinoma,” Biol. Ther, vol. 2, no. 1, p. 5, Jan. 2012, doi: 10.1007/s13554-012-0005-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Kazazi-Hyseni F, Beijnen JH, and Schellens JHM, “Bevacizumab,” The Oncologist, vol. 15, no. 8, pp. 819–825, Aug. 2010, doi: 10.1634/theoncologist.2009-0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Ferrara N, Hillan KJ, Gerber H-P, and Novotny W, “Discovery and development of bevacizumab, an anti-VEGF antibody for treating cancer,” Nat. Rev. Drug Discov, vol. 3, no. 5, pp. 391–400, May 2004, doi: 10.1038/nrd1381. [DOI] [PubMed] [Google Scholar]
- [85].Simón-Yarza T, Formiga FR, Tamayo E, Pelacho B, Prosper F, and Blanco-Prieto MJ, “Vascular Endothelial Growth Factor-Delivery Systems for Cardiac Repair: An Overview,” Theranostics, vol. 2, no. 6, pp. 541–552, 2012, doi: 10.7150/thno.3682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Dong X et al. , “Exosome-mediated delivery of an anti-angiogenic peptide inhibits pathological retinal angiogenesis,” Theranostics, vol. 11, no. 11, pp. 5107–5126, 2021, doi: 10.7150/thno.54755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Yamamoto N et al. , “VEGF and bFGF induction by nitric oxide is associated with hyperbaric oxygen-induced angiogenesis and muscle regeneration,” Sci. Rep, vol. 10, no. 1, p. 2744, Feb. 2020, doi: 10.1038/s41598-020-59615-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Nethi SK, Barui AK, Bollu VS, Rao BR, and Patra CR, “Pro-angiogenic Properties of Terbium Hydroxide Nanorods: Molecular Mechanisms and Therapeutic Applications in Wound Healing,” ACS Biomater. Sci. Eng, vol. 3, no. 12, pp. 3635–3645, Dec. 2017, doi: 10.1021/acsbiomaterials.7b00457. [DOI] [PubMed] [Google Scholar]
- [89].Ushio-Fukai M and Nakamura Y, “Reactive oxygen species and angiogenesis: NADPH oxidase as target for cancer therapy,” Cancer Lett., vol. 266, no. 1, pp. 37–52, Jul. 2008, doi: 10.1016/j.canlet.2008.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Matsunaga T, Weihrauch DW, Moniz MC, Tessmer J, Warltier DC, and Chilian WM, “Angiostatin Inhibits Coronary Angiogenesis During Impaired Production of Nitric Oxide,” Circulation, vol. 105, no. 18, pp. 2185–2191, May 2002, doi: 10.1161/01.CIR.0000015856.84385.E9. [DOI] [PubMed] [Google Scholar]
- [91].Strimaite M, Harman CLG, Duan H, Wang Y, Davies G-L, and Williams GR, “Layered terbium hydroxides for simultaneous drug delivery and imaging,” Dalton Trans., vol. 50, no. 29, pp. 10275–10290, 2021, doi: 10.1039/D1DT01251G. [DOI] [PubMed] [Google Scholar]
- [92].Patra CR et al. , “Pro-angiogenic Properties of Europium(III) Hydroxide Nanorods,” Adv. Mater, vol. 20, no. 4, pp. 753–756, Feb. 2008, doi: 10.1002/adma.200701611. [DOI] [Google Scholar]
- [93].Cuzzocrea S, Costantino G, Mazzon E, De Sarro A, and Caputi AP, “Beneficial effects of Mn(III)tetrakis (4-benzoic acid) porphyrin (MnTBAP), a superoxide dismutase mimetic, in zymosan-induced shock: MnTBAP and multiple organ failure,” Br. J. Pharmacol, vol. 128, no. 6, pp. 1241–1251, Nov. 1999, doi: 10.1038/sj.bjp.0702826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Shi M et al. , “Europium-doped mesoporous silica nanosphere as an immune-modulating osteogenesis/angiogenesis agent,” Biomaterials, vol. 144, pp. 176–187, Nov. 2017, doi: 10.1016/j.biomaterials.2017.08.027. [DOI] [PubMed] [Google Scholar]
- [95].Luo M et al. , “Injectable self-healing anti-inflammatory europium oxide-based dressing with high angiogenesis for improving wound healing and skin regeneration,” Chem. Eng. J, vol. 412, p. 128471, May 2021, doi: 10.1016/j.cej.2021.128471. [DOI] [Google Scholar]
- [96].Wu K, Wu X, Guo J, Jiao Y, and Zhou C, “Facile Polyphenol–Europium Assembly Enabled Functional Poly( l -Lactic Acid) Nanofiber Mats with Enhanced Antioxidation and Angiogenesis for Accelerated Wound Healing,” Adv. Healthc. Mater, vol. 10, no. 19, p. 2100793, Oct. 2021, doi: 10.1002/adhm.202100793. [DOI] [PubMed] [Google Scholar]
- [97].Nethi SK, Nanda HS, Steele TWJ, and Patra CR, “Functionalized nanoceria exhibit improved angiogenic properties,” J. Mater. Chem. B, vol. 5, no. 47, pp. 9371–9383, 2017, doi: 10.1039/C7TB01957B. [DOI] [PubMed] [Google Scholar]
- [98].Fu L, Li R, Whitelock JM, and Lord MS, “Tuning the intentional corona of cerium oxide nanoparticles to promote angiogenesis via fibroblast growth factor 2 signalling,” Regen. Biomater, vol. 9, p. rbac081, Apr. 2022, doi: 10.1093/rb/rbac081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Das S et al. , “The induction of angiogenesis by cerium oxide nanoparticles through the modulation of oxygen in intracellular environments,” Biomaterials, vol. 33, no. 31, pp. 7746–7755, Nov. 2012, doi: 10.1016/j.biomaterials.2012.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Sadidi H et al. , “Cerium Oxide Nanoparticles (Nanoceria): Hopes in Soft Tissue Engineering,” Molecules, vol. 25, no. 19, p. 4559, Oct. 2020, doi: 10.3390/molecules25194559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Mroczek-Sosnowska N et al. , “Nanoparticles of Copper Stimulate Angiogenesis at Systemic and Molecular Level,” Int. J. Mol. Sci, vol. 16, no. 3, pp. 4838–4849, Mar. 2015, doi: 10.3390/ijms16034838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Hassan A, Elebeedy D, Matar ER, Fahmy Mohamed Elsayed A, and Abd El Maksoud AI, “Investigation of Angiogenesis and Wound Healing Potential Mechanisms of Zinc Oxide Nanorods,” Front. Pharmacol, vol. 12, p. 661217, Oct. 2021, doi: 10.3389/fphar.2021.661217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Coelho CC et al. , “The antibacterial and angiogenic effect of magnesium oxide in a hydroxyapatite bone substitute,” Sci. Rep, vol. 10, no. 1, p. 19098, Nov. 2020, doi: 10.1038/s41598-020-76063-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Bosch-Rué E, Díez-Tercero L, Rodríguez-González R, Bosch-Canals BM, and Perez RA, “Assessing the potential role of copper and cobalt in stimulating angiogenesis for tissue regeneration,” PLOS ONE, vol. 16, no. 10, p. e0259125, Oct. 2021, doi: 10.1371/journal.pone.0259125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Melamed E, Kiambi P, Okoth D, Honigber I, Tamir E, and Borkow G, “Healing of Chronic Wounds by Copper Oxide-Impregnated Wound Dressings—Case Series,” Medicina (Mex.), vol. 57, no. 3, p. 296, Mar. 2021, doi: 10.3390/medicina57030296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Laia AGS et al. , “Cobalt-containing bioactive glass mimics vascular endothelial growth factor A and hypoxia inducible factor 1 function,” J. Biomed. Mater. Res. A, vol. 109, no. 7, pp. 1051–1064, Jul. 2021, doi: 10.1002/jbm.a.37095. [DOI] [PubMed] [Google Scholar]
- [107].Zhang M, Wu C, Li H, Yuen J, Chang J, and Xiao Y, “Preparation, characterization and in vitro angiogenic capacity of cobalt substituted β-tricalcium phosphate ceramics,” J. Mater. Chem, vol. 22, no. 40, p. 21686, 2012, doi: 10.1039/c2jm34395a. [DOI] [Google Scholar]
- [108].Chai YC, Mendes LF, van Gastel N, Carmeliet G, and Luyten FP, “Fine-tuning pro-angiogenic effects of cobalt for simultaneous enhancement of vascular endothelial growth factor secretion and implant neovascularization,” Acta Biomater, vol. 72, pp. 447–460, May 2018, doi: 10.1016/j.actbio.2018.03.048. [DOI] [PubMed] [Google Scholar]
- [109].Augustine R, Dominic EA, Reju I, Kaimal B, Kalarikkal N, and Thomas S, “Investigation of angiogenesis and its mechanism using zinc oxide nanoparticle-loaded electrospun tissue engineering scaffolds,” RSC Adv, vol. 4, no. 93, pp. 51528–51536, Sep. 2014, doi: 10.1039/C4RA07361D. [DOI] [Google Scholar]
- [110].Zhu D, Su Y, Zheng Y, Fu B, Tang L, and Qin Y-X, “Zinc regulates vascular endothelial cell activity through zinc-sensing receptor ZnR/GPR39,” Am. J. Physiol.-Cell Physiol, vol. 314, no. 4, pp. C404–C414, Apr. 2018, doi: 10.1152/ajpcell.00279.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Costello LC and Franklin RB, “A comprehensive review of the role of zinc in normal prostate function and metabolism; and its implications in prostate cancer,” Arch. Biochem. Biophys, vol. 611, pp. 100–112, Dec. 2016, doi: 10.1016/j.abb.2016.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Dong T et al. , “Multifunctional Surface with Enhanced Angiogenesis for Improving Long-Term Osteogenic Fixation of Poly(ether ether ketone) Implants,” ACS Appl. Mater. Interfaces, vol. 12, no. 13, pp. 14971–14982, Apr. 2020, doi: 10.1021/acsami.0c02304. [DOI] [PubMed] [Google Scholar]
- [113].Xu L, Willumeit-Römer R, and Luthringer-Feyerabend BJC, “Effect of magnesium-degradation products and hypoxia on the angiogenesis of human umbilical vein endothelial cells,” Acta Biomater, vol. 98, pp. 269–283, Oct. 2019, doi: 10.1016/j.actbio.2019.02.018. [DOI] [PubMed] [Google Scholar]
- [114].Liu W et al. , “Magnesium promotes bone formation and angiogenesis by enhancing MC3T3-E1 secretion of PDGF-BB,” Biochem. Biophys. Res. Commun, vol. 528, no. 4, pp. 664–670, Aug. 2020, doi: 10.1016/j.bbrc.2020.05.113. [DOI] [PubMed] [Google Scholar]
- [115].Bernardini D, “Magnesium and microvascular endothelial cells: a role in inflammation and angiogenesis,” Front. Biosci, vol. 10, no. 1–3, p. 1177, 2005, doi: 10.2741/1610. [DOI] [PubMed] [Google Scholar]
- [116].Guo S, Arai K, Stins MF, Chuang D-M, and Lo EH, “Lithium Upregulates Vascular Endothelial Growth Factor in Brain Endothelial Cells and Astrocytes,” Stroke, vol. 40, no. 2, pp. 652–655, Feb. 2009, doi: 10.1161/STROKEAHA.108.524504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Zeilbeck LF, Müller B, Knobloch V, Tamm ER, and Ohlmann A, “Differential Angiogenic Properties of Lithium Chloride In Vitro and In Vivo,” PLoS ONE, vol. 9, no. 4, p. e95546, Apr. 2014, doi: 10.1371/journal.pone.0095546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Li B, Lei Y, Hu Q, Li D, Zhao H, and Kang P, “Porous copper- and lithium-doped nano-hydroxyapatite composite scaffold promotes angiogenesis and bone regeneration in the repair of glucocorticoids-induced osteonecrosis of the femoral head,” Biomed. Mater, vol. 16, no. 6, p. 065012, Nov. 2021, doi: 10.1088/1748-605X/ac246e. [DOI] [PubMed] [Google Scholar]
- [119].Liu L et al. , “Lithium-containing biomaterials stimulate bone marrow stromal cell-derived exosomal miR-130a secretion to promote angiogenesis,” Biomaterials, vol. 192, pp. 523–536, Feb. 2019, doi: 10.1016/j.biomaterials.2018.11.007. [DOI] [PubMed] [Google Scholar]
- [120].Wei T, Jia J, Wada Y, Kapron CM, and Liu J, “Dose dependent effects of cadmium on tumor angiogenesis,” Oncotarget, vol. 8, no. 27, pp. 44944–44959, Jul. 2017, doi: 10.18632/oncotarget.16572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Kim J et al. , “The effects of cadmium on VEGF-mediated angiogenesis in HUVECs: The effects of angiogenesis on cadmium-treated HEVECs,” J. Appl. Toxicol, vol. 32, no. 5, pp. 342–349, May 2012, doi: 10.1002/jat.1677. [DOI] [PubMed] [Google Scholar]
- [122].Jing Y et al. , “Cadmium Increases HIF-1 and VEGF Expression through ROS, ERK, and AKT Signaling Pathways and Induces Malignant Transformation of Human Bronchial Epithelial Cells,” Toxicol. Sci, vol. 125, no. 1, pp. 10–19, Jan. 2012, doi: 10.1093/toxsci/kfr256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Saghiri MA, Asatourian A, Orangi J, Sorenson CM, and Sheibani N, “Functional role of inorganic trace elements in angiogenesis—Part II: Cr, Si, Zn, Cu, and S,” Crit. Rev. Oncol. Hematol, vol. 96, no. 1, pp. 143–155, Oct. 2015, doi: 10.1016/j.critrevonc.2015.05.011. [DOI] [PubMed] [Google Scholar]
- [124].Ali D, Alarifi S, and Alkahtani S, “Mechanistic investigation of toxicity of chromium oxide nanoparticles in murine fibrosarcoma cells,” Int. J. Nanomedicine, p. 1253, Mar. 2016, doi: 10.2147/IJN.S99995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Patra CR et al. , “Microwave approach for the synthesis of rhabdophane-type lanthanide orthophosphate (Ln = La, Ce, Nd, Sm, Eu, Gd and Tb) nanorods under solvothermal conditions,” New J. Chem, vol. 29, no. 5, p. 733, 2005, doi: 10.1039/b415693e. [DOI] [Google Scholar]
- [126].Mastrullo V, Cathery W, Velliou E, Madeddu P, and Campagnolo P, “Angiogenesis in Tissue Engineering: As Nature Intended?,” Front. Bioeng. Biotechnol, vol. 8, p. 188, Mar. 2020, doi: 10.3389/fbioe.2020.00188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Negut I, Dorcioman G, and Grumezescu V, “Scaffolds for Wound Healing Applications,” Polymers, vol. 12, no. 9, p. 2010, Sep. 2020, doi: 10.3390/polym12092010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Saberianpour S, Heidarzadeh M, Geranmayeh MH, Hosseinkhani H, Rahbarghazi R, and Nouri M, “Tissue engineering strategies for the induction of angiogenesis using biomaterials,” J. Biol. Eng, vol. 12, no. 1, p. 36, Dec. 2018, doi: 10.1186/s13036-018-0133-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Serbo JV and Gerecht S, “Vascular tissue engineering: biodegradable scaffold platforms to promote angiogenesis,” Stem Cell Res. Ther, vol. 4, no. 1, p. 8, Mar. 2013, doi: 10.1186/scrt156. [DOI] [PMC free article] [PubMed] [Google Scholar]