Abstract
Background
Post mortem human brain tissue is an essential resource to study cell types, connectivity as well as subcellular structures down to the molecular setup of the central nervous system especially with respect to the plethora of brain diseases. A key method is immunostaining with fluorescent dyes, which allows high-resolution imaging in three dimensions of multiple structures simultaneously. Although there are large collections of formalin-fixed brains, research is often limited because several conditions arise that complicate the use of human brain tissue for high-resolution fluorescence microscopy.
Results
In this study, we developed a clearing approach for immunofluorescence-based analysis of perfusion- and immersion-fixed post mortem human brain tissue, termed human Clear Lipid-exchanged Acrylamide-hybridized Rigid Imaging / Immunostaining / In situ hybridization-compatible Tissue-hYdrogel (hCLARITY). hCLARITY is optimized for specificity by reducing off-target labeling and yields very sensitive stainings in human brain sections allowing for super-resolution microscopy with unprecedented imaging of pre- and postsynaptic compartments. Moreover, hallmarks of Alzheimer’s disease were preserved with hCLARITY, and importantly classical 3,3’-diaminobenzidine (DAB) or Nissl stainings are compatible with this protocol. hCLARITY is very versatile as demonstrated by the use of more than 30 well performing antibodies and allows for de- and subsequent re-staining of the same tissue section, which is important for multi-labeling approaches, e.g., in super-resolution microscopy.
Conclusions
Taken together, hCLARITY enables research of the human brain with high sensitivity and down to sub-diffraction resolution. It therefore has enormous potential for the investigation of local morphological changes, e.g., in neurodegenerative diseases.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12915-023-01582-6.
Keywords: Alzheimer’s disease; CLARITY; Human brain archive; Immunofluorescence; Post mortem human brain tissue; Super-resolution microscopy (STED, dSTORM); Synapse
Background
Post mortem human brain tissue represents an indispensable source for the interrogation of molecules, structures, functions, and especially their changes during the course of neurodegenerative diseases. A major hindrance to quantifiable studies of the human brain is the limited access to human brain tissue, although some research institutes have brain archives, and anatomists have access to a large number of donor tissue. In contrast to immunohistochemistry (IHC) on animal tissue, fixation conditions are usually not optimal, e.g., immersion rather than perfusion is mainly used as fixation method and, thus, the tissue is fixed only by diffusion. Finally, long-term archiving in formalin can reduce the immunogenicity of the contained proteins [1]. For these reasons, beyond classical non-immuno-based staining or chromogen-based staining of neurodegenerative protein deposits, it is difficult to image brain structures specifically and, most importantly, comparably. This, however, is the prerequisite for performing quantitative assessments.
One such application is the investigation of synapse loss in neurodegenerative diseases [2–4], given the correlation between cognitive decline and synapse loss in Alzheimer’s disease (AD) [5, 6] and amyotrophic lateral sclerosis (ALS) [7]. To date, synaptic densities have mostly been quantified only by microdensitometry [6, 8–13], array tomography [14, 15], and electron microscopy (EM) [16–21]. Disadvantages of these techniques are the low information content to a single synapse with densitometry, whereas three-dimensional (3D) recordings with array tomography are laborious and only indirectly possible as serial recordings, and immunogold labeling with EM is highly demanding. Most importantly, each of these methods usually requires freshly fixed tissue. Thus, model systems are favored as surrogates under more controllable conditions, e.g., mouse models [20, 22] or induced pluripotent stem cells and their neuronal derivatives [23]. However, such models do not faithfully represent all conditions in the human organism as the ‘original’, and, in neurodegenerative diseases of the aging human brain, model systems do not reach the advanced age of brains from deceased individuals. Finally, clinical symptomatology and histopathology can only be directly matched with human brain tissue.
The clearing technique CLARITY was invented with the major goal of enabling coherent 3D labeling and microscopy of intact tissues or whole organs, thereby making laborious and error-prone serial sectioning approaches superfluous [24]. In addition, immunofluorescence staining can be performed after CLARITY, which overcomes the limitations inherent in non-immuno-based and chromogen-based techniques. In the here applied and adapted CLARITY protocol, clearing was achieved by immersing the hydrogel-stabilized tissue in a 4% sodium dodecyl sulfate (SDS) solution.
Since SDS is known as an antigen retrieval agent [25] and speculation exists about its benefits on immunostaining after CLARITY [26], we referred to the study of Brown et al. [25] and, thus, we further assessed the detergent-related effects on the accessibility of epitopes in post mortem human brain tissue. We used perfusion-fixed brains from the gross anatomy course and immersion-fixed brains from the tissue bank collection at Ulm University. First, by performing confocal microscopy, we evaluated the staining quality of a selection of neuronal and synaptic markers in direct comparison to non-cleared sections. Here, besides the reported increase in tissue translucence owing to lipid removal via the CLARITY method, we found that some antibodies stained more sensitively and specifically, i.e., that staining of target structures resulted in a stronger signal (greater sensitivity) and displayed less background (greater specificity). Second, whereas prior studies have demonstrated the applicability of CLARITY to human neural tissue using only a few antibodies or without a systematic investigation of their benefits [27, 28], we tested a large panel of neuronal, non-neuronal, and Alzheimer’s pathology markers. We established an ‘antibody toolbox’ with 30 markers as a ready-to-use panel saving time and costs for antibody testing. In addition, by stabilizing the tissue with acrylamide, CLARITY offers easy detergent-based de-staining and subsequent re-staining rounds (multiple round labeling). Finally, we show that passive CLARITY is also compatible with super-resolution microscopy (stimulated emission depletion microscopy (STED), direct stochastical optical reconstruction microscopy (dSTORM)) for imaging human cortical synapses. These techniques were mainly applied to perfusion-fixed tissue from the gross anatomy course at Ulm University, a source probably available in many anatomical institutions at universities. This fixation technique proved to be superior for immunofluorescence staining, which is in line with a recently published study [29]. Exploiting this source in combination with the here-presented tools will provide a broader basis for future studies on human tissue. We termed our adapted passive CLARITY protocol hCLARITY (‘human CLARITY’).
Methods
Human brain samples
Perfusion-fixed human post mortem brains (cases with a p (perfusion) code, main cases were p1 and p2 for most of the experiments; cases p1-p2 in Table 1) were obtained from permanent body donors of the gross anatomy course organized by the Institute for Anatomy and Cell Biology, Ulm University. All included donors provided their informed and written consent for permanent body donation. This study was approved by the ethics committee of Ulm University. After death, the cadavers were fixed with a solution according to Tutsch by perfusion via the femoral artery (per body donor: 8 l ethanol, 3 l glycerol 86% (Carl Roth, Karlsruhe, Germany), 0.6 l lysoformin (Lysoform Dr. Hans Rosemann GmbH, Berlin, Germany), 0.6 l formaldehyde 30% (Carl Roth, Karlsruhe, Germany), filled up to 15 l with water). More precisely, the femoral artery was opened longitudinally and 1.5 l of the fixans was introduced distally. Then, the distal part of the artery was closed, and 11.5 l of the fixans were infused proximally. The artery was closed with a suture. Thereafter, the cadavers were stored for a minimum of four months for post-fixation and were continuously moistened with formaldehyde-containing solution before the anatomy course; also the extracted brains few weeks before the end of this course. Afterwards, brains were collected and immersed in a 1% aqueous solution of formaldehyde. Until usage, brains were stored in a dark room.
Table 1.
Demographics and clinical-neuropathological diagnoses of the n = 6 cases studied
| Case | Age (yrs) | Sex | NFT stage | Aβ phase | PD stage | PMI (h) | Fixation year | Origin | Cause of death | |
|---|---|---|---|---|---|---|---|---|---|---|
| Anatomy course | Tissue bank | |||||||||
| p1 | 71 | M | I | 0 | 0 | 72 | 2019 | x | Cardiogenic shock | |
| p2 | 74 | M | I | 3 | 0 | 24 | 2017 | x | Decompensated heart insufficiency | |
| i1 | 49 | F | 0 | 0 | 0 | N.a. | 2016 | x | N.a. | |
| i2 | 82 | F | II | 1 | 0 | 71 | 2015 | x | Stroke | |
| i3 | 80 | M | III | 0 | 0 | 39 | 2003 | x | Malignant neoplasm | |
| i4 | 78 | M | I | 0 | 0 | 56 | 2003 | x | COPD | |
Abbreviations: yrs Years, NFT Neurofibrillary tangle, Aβ Amyloid-β, PD Parkinson’s disease, PMI Post mortem interval, h Hour, p Perfusion fixation, i Immersion fixation, F Female, M Male, N.a. Not available, COPD Chronic obstructive pulmonary disease
Centimeter-thick tissue blocks of the desired brain regions were excised and cut on a vibratome (Microm HM 650 V, Thermo Scientific/Microm, Walldorf, Germany and VT1200S vibratome, Leica, Wetzlar, Germany) into 100 µm thick sections. All cases used for evaluating the effects of CLARITY on immunostaining are described in Table 1. Cases p1 and p2 were used to assess the performance of the majority of antibodies (Table 2). If no code for the case is indicated, representative images were derived from one of ten additional perfusion-fixed cases. For comparison with the perfusion-fixed anatomy course cases, immersion-fixed brains from the tissue bank collection at Ulm University were used in selective experiments (cases i1-i4 (immersion) in Table 1), which were fixed in a 4% aqueous solution of formaldehyde [30].
Table 2.
Detailed information on the 30 primary antibodies used
| Fig. ID | Targeta | Primary antibodyb | Host | Dilutionc | RRID | Specificity (vendor) |
|---|---|---|---|---|---|---|
| Disease-related markers | ||||||
| Alzheimer’s disease (AD) tangle | Phospho-Tau Ser202, Thr205, clone AT8 | ms | 1:200 | Thermo Fisher Scientific Cat# MN1020, RRID:AB_223647 | Immunogen based on human sequence, reacts with human | |
| AD plaque | β-Amyloid, 17–24, clone 4G8 | ms | 1:1000 | BioLegend Cat# 800701, RRID:AB_2564633 | Immunogen based on human sequence, reacts with human | |
| Neuronal cells | ||||||
| A1 | Pyramidal neuron | CAMKIIA (CAMK2A) | rb | 1:300 | Abcam Cat# ab103840, RRID:AB_10900968 | Immunogen based on human sequence |
| A2 | Purkinje cell | Calbindin D28k (CALB1) | rb | 1:300 | Synaptic Systems (SySy) Cat# 214 003, RRID:AB_2619901 | Immunogen based on human sequence, reacts with human |
| A3 | Purkinje cell | CALB1 | ms | 1:300 | SySy Cat# 214 011, RRID:AB_2068201 | Immunogen based on human sequence, reacts with human |
| A4 | Interneuron | CRH | ms | 1:100 | Sigma-Aldrich Cat# WH0001392M2, RRID:AB_1840911 | Immunogen based on human sequence, reacts with human |
| A5 | Catecholaminergic neuron | TH | ms | 1:200 | Millipore Cat# MAB318, RRID:AB_2201528 | Reacts with human |
| Glial cells | ||||||
| B | Microglia | IBA1 (AIF1) | rb | 1:200 | SySy Cat# 234 003, RRID:AB_10641962 | Reacts with human, K.O. verified (SySy) |
| C1 | Astrocyte | GFAP | rb | 1:250 | Agilent Cat# Z0334, RRID:AB_10013382 | Reacts with human |
| C2 | Astrocyte | GFAP | ms | 1:200 | SySy Cat# 173 011, RRID:AB_2232308 | Immunogen based on human sequence, reacts with human, K.O. verified (SySy) |
| C3 | Astrocyte (mature) | S100B | gp | 1:200 | SySy Cat# 287 004, RRID:AB_2620025 | |
| D1 | Oligodendrocyte (myelin sheath) | MBP | rb | 1:200 | Abcam Cat# ab133620, no RRID available | Immunogen based on human sequence, reacts with human |
| D2 | Oligodendrocyte (myelin sheath) | MBP, clone SMI 94 | ms | 1:200 | BioLegend Cat# 836504, RRID:AB_2616694 | Immunogen based on human sequence, reacts with human |
| D3 | Oligodendrocyte (myelin sheath) | MBP | ms | 1:100 | Atlas Antibodies Cat# AMAb91064, RRID:AB_2665784 | Immunogen based on human sequence, reacts with human |
| Neuronal compartments | ||||||
| Neuronal processes | ||||||
| E1 | Axon | Pan-axonal neurofilament, clone SMI-312 | ms | 1:200 | BioLegend Cat# 837904, RRID:AB_2566782 | Reacts with human |
| E2 | Axon, paranodal region | Caspr/paranodin (CNTNAP1) | ms | 1:100 | UC Davis/NIH NeuroMab Facility Cat# 75–001, RRID:AB_2083496 | Reacts with human |
| F | Dendrite | MAP2 | ms | 1:200 | Millipore Cat# MAB3418, RRID:AB_94856 | Reacts with human |
| Synaptic proteins & organelles | ||||||
| G1 | Presynapse (synaptic vesicle) | Synapsin 1/2 | rb | 1:200 | SySy Cat# 106 003, RRID:AB_2619773 | Reacts with human, K.O. verified (SySy) |
| G2 | Presynapse (synaptic vesicle) | Synaptophysin 1 (SYP) | gp | 1:500 | SySy Cat# 101 004, RRID:AB_1210382 | Immunogen based on human sequence, reacts with human |
| G3 | Presynapse (synaptic vesicle) | SYP | rb | 1:100 | Abcam Cat# ab14692, RRID:AB_301417 | Immunogen based on human sequence |
| G4 | Presynapse (GABAergic synaptic vesicle) | VGAT, cytoplasmic domain (SLC32A1) | ms | 1:50 | SySy Cat# 131 011, RRID:AB_887872 | Reacts with human, K.O. verified (SySy) |
| G5 | Presynapse (glutamatergic synaptic vesicle) | VGLUT1 (SLC17A7) | gp | 1:200 | SySy Cat# 135 304, RRID:AB_887878 | Reacts with human, K.O. verified (SySy) |
| G6 | Postsynapse (inhibitory) | Gephyrin (GPHN) | ms | 1:100 | SySy Cat# 147 011, RRID:AB_887717 | Reacts with human, K.O. verified (SySy) |
| G7 | Postsynapse (excitatory) | GLUA2 (GRIA2) | ms | 1:200 | SySy Cat# 182 111, RRID:AB_10645888 | |
| G8 | Postsynapse (excitatory) | HOMER3 | rb | 1:300 | SySy Cat# 160 303, RRID:AB_10804288 | |
| G9 | Postsynapse (excitatory) | PSD-95, PDZ domain (DLG4) | ms | 1:100 | SySy Cat# 124 011, RRID:AB_10804286 | K.O. verified (SySy) |
| H | Endoplasmic reticulum | Calreticulin (CALR) | rb | 1:200 | SySy Cat# 315 003, RRID:AB_2620050 | |
| Others | ||||||
| J1 | Basal lamina (e.g., in vessels) | Collagen IV | ms | 1:200 | Agilent Cat# M0785, RRID:AB_2082944 | Immunogen based on human sequence, reacts with human |
| J2 | Smooth muscle cell (e.g., in vessels) | α-Actin (smooth muscle) | ms | 1:200 | Agilent Cat# M0851, RRID:AB_2223500 | Immunogen based on human sequence, reacts with human |
| K | Cells using glycolysis | GAPDH | rb | 1:100 | SySy Cat# 247 002, RRID:AB_10804053 | Immunogen based on human sequence, reacts with human |
Abbreviations: Fig. Figure, ID Identifier, RRID Research Resource Identifier, ms Mouse, rb Rabbit, gp Guinea pig, K.O. Knockout
a The most significant one is mentioned. b If applicable, name according to HUGO Gene Nomenclature Committee appears in brackets when different from the vendor’s antibody name. c Differing concentrations might work as well
CLARITY
CLARITY procedure and solutions were based on previously published protocols [24, 31]. Solution I (monomer solution) was prepared by dissolving 8 g (16% (wt/vol)) paraformaldehyde (Sigma-Aldrich, Steinheim, Germany) (PFA, 4% (vol/vol) final concentration) in 50 ml Dulbecco’s phosphate-buffered saline (DPBS) (Thermo Fisher Scientific, Waltham, MA). Solvation was achieved by adding several drops NaOH and by stirring at 60 °C. For solution II (cross-linking solution), 20 ml of 40% acrylamide (Bio-Rad, Munich, Germany) (4% (vol/vol) final concentration), 20 ml 10 × DPBS, and 110 ml dH2O were mixed. After combining solution I and II, the pH was adjusted to 7.35–7.45 and the 200 ml were filtered. Immediately before immersing the sections in the combined monomer and cross-linking solution, 0.25% (wt/vol) of the thermal initiator VA-044 (Wako Chemicals, Neuss, Germany) was admixed. Incubation took place for 5 days at 4 °C.
To enable polymerization, oxygen was removed by vacuumizing the samples for 15 min at 0–20 mbar and subsequently adding nitrogen in a desiccation chamber. Then, polymerization was started by placing the samples in an incubator for 3 h at 37 °C. The solution was replaced after the first 1.5 h by PBS to prevent the solidification of hydrogel on the sections’ surfaces. After polymerization, passive clearing was initiated by changing the sections to clearing solution (4% (wt/vol) sodium dodecyl sulfate (SDS) (Serva, Heidelberg, Germany), 200 mM boric acid (Merck, Darmstadt, Germany); pH 7.0–7.4). To remove any residual hydrogel, the clearing solution was exchanged on the first and second day after starting the passive clearing. Clearing of the sections took place for 7 days at 37 °C under gentle agitation on a shaking table.
Immunohistochemistry
Fluorescence-based immunolabeling for confocal microscopy
At the conclusion of the passive clearing process, tissue sections were rinsed three times within 24 h with PBS plus Triton X-100 (PBST; 0.1% (vol/vol) Triton X-100 (Roche, Mannheim, Germany)) under gentle agitation at room temperature. Prior to immunostaining, heat-induced epitope retrieval was performed with 0.1 M citrate buffer (pH 6.0). Briefly, sections were transferred into glass cuvettes filled with citrate buffer and boiled for 10 min. Afterwards, sections were incubated in blocking solution (3% (wt/vol) bovine serum albumin (BSA) (Bio Froxx, Einhausen, Germany) plus 0.3% (vol/vol) Triton X-100 in PBS) for 3 h under gentle agitation. Primary antibodies were diluted in blocking solution according to the protocol in Table 2 and were incubated with the sections for 48 h at 4 °C under gentle agitation. Subsequently, rinsing with PBS (1 × 1 h, 2 × 30 min) was followed by staining with Alexa Fluor-conjugated secondary antibodies (Invitrogen, Waltham, MA and Jackson ImmunoResearch Laboratories, Inc., Ely, UK, 1:200 in blocking solution) for 3 h at room temperature. Thereafter, the first 1 h rinsing step included 4’,6-diamidino-2-phenylindole (DAPI (Carl Roth, Karlsruhe, Germany), stock solution 10 mg/ml, diluted 1:10,000 in PBS), followed by 2 × 30 min washing with PBS. The aforementioned incubation steps were performed under gentle agitation at room temperature.
Finally, tissue sections were mounted in ProLong Gold antifade mountant (Invitrogen) or self-made refractive index matching solution (RIMS; according to [32]) and were cover-slipped. Sections for re-staining were mounted in aqueous mountants only (RIMS or SlowFade Gold antifade mountant, Invitrogen). For experiments with a comparison of plus and minus CLARITY staining results, ProLong Gold antifade mountant was consistently applied. In each experiment, additional tissue sections were incubated with the secondary antibodies only (negative control: omission of primary antibody) to control for unspecific binding. At least one representative staining of each antibody in Table 2 is shown in the main figures or in the supplementary files at the end of this manuscript.
Re-staining after confocal microscopy
Tissue sections mounted in aqueous mounting media (RIMS or SlowFade Gold antifade mountant) were subjected to a second staining round. By immersion of slides in PBS, coverslips were removed and sections could be carefully detached from the slide. For de-staining, sections were incubated in clearing solution at 60 °C for 24 h. Subsequently, sections were shortly rinsed in PBS, followed by a longer washing step in PBST. For re-staining, see section “Fluorescence-based immunolabeling for confocal microscopy”, starting from the heat-induced epitope retrieval step. Control sections were mounted without re-staining and imaged with a confocal microscope.
Fluorescence-based labeling for STED microscopy
Cleared 50 µm thick sections were immunostained as described above. GLUA2 and MAP2 antibodies were diluted as indicated in Table 2 (G7 & F), the CAMKIIA antibody (A1) was diluted 1:200. Synaptic staining was performed with a synaptophysin (1:300, Synaptic Systems, Göttingen, Germany, G2 in Table 2) and a GLUA1 antibody (1:200, Synaptic Systems, Cat. No. 182011, RRID: AB_2113443). Instead of Alexa Fluor-coupled secondary antibodies, ATTO dyes (Sigma-Aldrich; ATTO647N, Cat. No. 50185 and ATTO594, Cat. No. 77671 and Cat. No. 76085) were used for labeling. When commercial dye-labeled antibodies were not available, antibodies were covalently labeled in-house. Donkey anti-guinea pig secondary antibodies (Jackson ImmunoResearch Laboratories, Inc.; RRID: AB_2340442) were covalently labeled with ATTO647N succinimidyl ester or ATTO594 succinimidyl ester dye (ATTO-TEC, Siegen, Germany) using manufacturer’s protocol. Briefly, two molar excess of reactive dye was incubated with unconjugated antibodies in a 0.1 M sodium bicarbonate (Honeywell Fluka, Seelze, Germany) solution for 30 min under agitation. The unbound fluorophores were removed by gel filtration on a NAP-5 column (Sephadex-25 medium, GE Healthcare, Munich, Germany). The final antibody concentration and degree of labelling was analyzed on a spectrophotometer (NanoDrop 2000 UV–Vis; Thermo Scientific). Slides and coverslips were cleaned thoroughly and sections were finally mounted in 97% (vol/vol) 2,2’-thiodiethanol (TDE, Sigma-Aldrich) in PBS (pH 7.5) for refractive index matching.
Fluorescence-based labeling for dSTORM
Cleared 50 µm thick sections were immunostained as described above. Synaptic staining was performed with the same synaptophysin and GLUA1 primary antibodies (dilution each 1:200), as described for STED microscopy. Alexa Fluor-conjugated secondary antibodies (1:350, Invitrogen, Alexa Fluor 647, Cat. No. A21450 and Alexa Fluor 532, Cat. No. A11002) were applied 90 min for staining, followed by three rinses à 30 min with PBS. To facilitate photo switching of dyes, samples were mounted in a special imaging buffer (100 mM cysteamine, 400 U/ml of catalase, 100 U/ml glucose oxidase, and 40 mg/ml of glucose; all Sigma-Aldrich).
Chromogen-based labeling with DAB
Neuropathological stages for Alzheimer’s disease (AD) of cases from the gross anatomy course are provided in Table 1. For neurofibrillary tangle (NFT) staging, the required tissue blocks according to [33] were excised. For determination of amyloid-β (Aβ) phases, tissue blocks were assessed as described previously [34]. Despite the selection of low-stage cases, the cerebellum was additionally assessed to diagnose Aβ phase 5 [35]. Parkinson’s disease (PD) neuropathological staging was performed according to [36]. The tissue blocks were cut into 100 µm thick sections with a vibratome and on the same day, or the day after, immunolabeling was started as previously described in detail [37]. Briefly, after blocking endogenous peroxidase, sections for detection of Aβ deposits and Lewy pathology (α-synuclein) underwent antigen retrieval for 3 min with 100% formic acid (Appli Chem, Darmstadt, Germany), whereas detection of hyperphosphorylated tau was performed without retrieval. After blocking with BSA, sections were incubated overnight with an antibody directed against phospho-tau (clone AT8, 1:2000, Thermo Fisher Scientific, Cat. No. MN1020), β-amyloid (clone 4G8, 1:5000, BioLegend, Cat. No. 800701) or α-synuclein (1:2000, BD Transduction Laboratories, Cat. No. 610787, RRID: AB_398108). Following incubation with a biotinylated secondary antibody (1:200, Vector Laboratories, Burlingame, CA) and signal amplification via the ABC kit (Vectastain Elite ABC-HRP Kit, PK6100; Vector Laboratories), reactions were finally visualized with the chromogen DAB (CN75, Carl Roth). Positive (section from a case with abundant pathology) and negative (omission of primary antibody) controls were included. Sections were dehydrated in an ascending alcohol series, cleared in Histo-Clear (National Diagnostics, Atlanta, GA) or xylene, and mounted in Histomount (National Diagnostics) or Entellan New (Merck) (see also [38]). Neuropathological stages for cases from the tissue bank are also shown in Table 1.
DAB-based labeling and Nissl staining after CLARITY
The staining started after the one-day washing step (three exchanges of PBST, see above). The same protocol as described above was used for subsequent detection of phospho-tau, counterstaining with Darrow red (Sigma-Aldrich, Cat. No. 211885) was performed for detection of basophilic Nissl substance [39, 40]. For CAMKIIA staining (1:300, A1 in Table 2), no additional retrieval was performed, the goat anti-rabbit biotinylated secondary antibody was also purchased from Vector Laboratories (Cat. No. BA-1000).
Image acquisition
Confocal microscopy
Images were captured with a Leica SPE confocal microscope (Leica, Germany) using laser excitation at 635 nm, 561 nm, 488 nm, and 405 nm in that order. Samples were viewed with a 40 × oil objective (ACS APO; numerical aperture (NA) 1.15; free working distance (WD) 270 µm) or a 63 × oil objective (ACS APO; NA 1.30; WD 160 µm). Two overview scans were acquired using a 10 × dry objective (ACS APO; NA 0.3; WD 3 mm) and a 20 × multi-immersion objective (ACS APO; NA 0.60; WD 200 µm) with oil. For z-stacks, settings were kept constant in individual z-planes.
STED microscopy
Images were acquired on a custom-built dual-color STED microscope with a 100 × oil objective (HCX PL APO 100×/1.40–0.70 oil CS, Leica). A detailed description of the setup can be found in [41]. The system enables switching between confocal (excitation at 568 nm and 633 nm) and STED (additional depletion beams at 715 nm and 750 nm) mode. The excitation lasers were used at a power of 0.6–1 µW and the depletion laser power was ~ 1.8 mW. Initially, confocal images were acquired at a pixel size of 50 nm or 100 nm, followed by the corresponding STED images at a pixel size of 20 nm. The pixel dwell time was 200 µs (confocal mode) and 300 µs (STED mode).
dSTORM imaging
Imaging was performed on a custom-built widefield setup with a 60 × oil immersion objective (60 × APO TIRF, NA 1.49 Oil, Nikon, Tokyo, Japan). Samples were imaged using 405 nm activation laser and excitation lasers 647 nm or 532 nm; fluorescence was detected with an EMCCD camera (Andor Technology, Belfast, UK). The setup construction is detailed in [42, 43]. First, widefield images were captured for both channels. Then, for each channel for image reconstruction, ca. 30,000 frames were recorded consecutively at a 20 ms exposure time. Single molecule localizations were determined using custom algorithms written in MATLAB (MathWorks, Natick, MA) as described before [43] (see dSTORM reconstruction).
Conventional light microscopy
DAB-based CAMKIIA staining and the Nissl staining were imaged with the color camera of a Biorevo BZ-X810 microscope (Keyence, Neu-Isenburg, Germany) using a 100 × oil objective (CFl Plan Apo λ 100XH; NA 1.45; WD 0.13 mm) and a 20 × objective (CFl Plan Apo λ 20x; NA 0.75; WD 1 mm). Full focus of z-stacks was obtained with the BZ-X800 Analyzer (Keyence) software and exported as TIFF images. Additional DAB sections were acquired with a BX61 microscope (Olympus Optical, Tokyo, Japan). The single images from all layers of the stack were merged after the so-called EFI (extended focal imaging) algorithm extracted those features of the images with the sharpest contrast (Cell D Imaging Software (Olympus)).
Image Processing
Confocal microscopy
For comparison of plus and minus CLARITY sections, individual z-stack planes were extracted using the Leica Application Suite X (LAS-X) software, and brightness/contrast were adjusted identically with Imaris (Oxford Instruments, Abingdon, UK) software. Maximum intensity projections, 3D renderings, and video material were also created in Imaris software. The described brightness/contrast adjustments were performed on entire z-stacks; no individual adjustments of single z-planes were done. For most cases, a Gaussian filter with default filter width in Imaris was applied prior to brightness/contrast adjustment.
STED microscopy
Confocal and STED images were brightness/contrast-adjusted in Fiji (ImageJ, NIH, Bethesda, MD) and a Gaussian blur with σ = 1.0 was applied for visualization. Intensity profiles were created on raw data using the ‘plot profile’ command (also in Fig. 3d). Z-stack stabilization of confocal and STED stacks (Additional file 15) was done with the Huygens Object Stabilizer (SVI, Hilversum, The Netherlands) using identical processing steps.
Fig. 3.
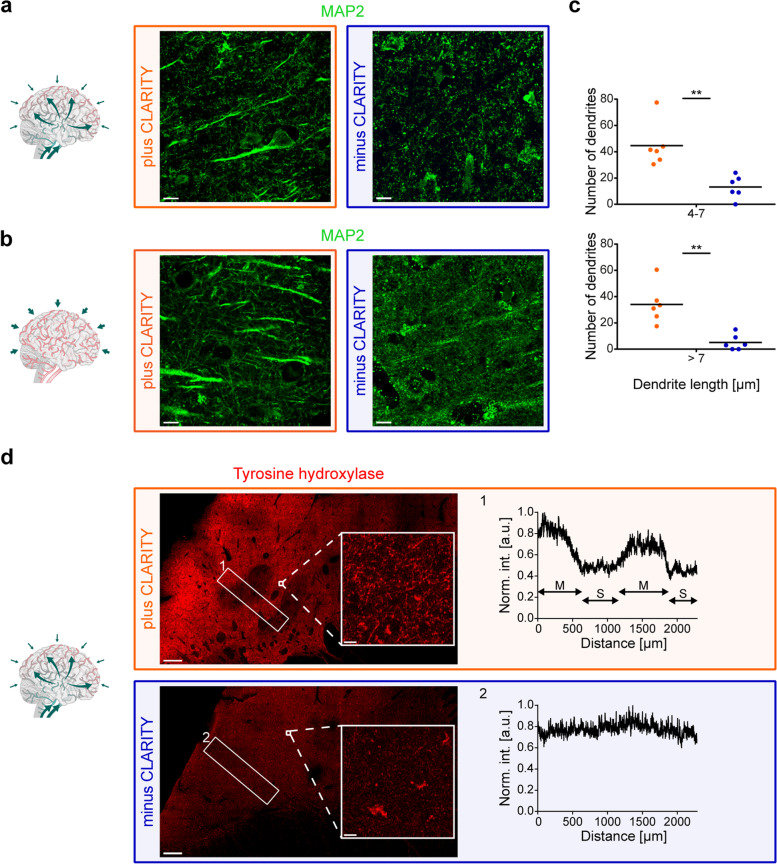
Increased specificity of antibodies applied on CLARITY-processed sections. a MAP2 (frontal cortex) labeling in a perfusion-fixed case (case p2) plus (orange) and minus (blue) CLARITY treatment. Scale bars, 10 µm. b MAP2 labeling plus (orange) and minus (blue) CLARITY treatment in an immersion (i)-fixed brain with a comparable total fixation time as (a) (frontal cortex, case i1). Scale bars, 10 µm. c Numbers of dendrites with a length of 4–7 µm and > 7 µm were determined in two 2D images per case and condition. Average numbers of the two replicates are plotted per case (n = 6) for plus and minus CLARITY in the two categories (dendrite length 4–7 µm, top and > 7 µm, bottom). Importantly, for plus and minus CLARITY, tissue sections had been cut from the same block, which excludes a bias due to the orientation of the dendrites. Mann–Whitney test, P = 0.0022 (**). d Tyrosine hydroxylase labeling (caudate nucleus, case p2) plus (orange) and minus (blue) CLARITY treatment. Specific labeling is reflected by the intensity profiles (right) of the boxed areas (left, 1 and 2): matrix (M) and striosomes (S) are only discernible in the plus CLARITY profile (1). Mean intensities (int.) were normalized (norm.) to the maximum. A.u. = arbitrary unit. Scale bars, 500 µm (overview), 10 µm (magnified insets)
dSTORM reconstruction
Centre-of-mass fitting was done on the FIRESTORM software (previously described in [43] and [42]). The localizations were filtered based on the intensity, full width at half maximum (FWHM), and symmetry distribution. A redundant cross-correlation algorithm was implemented to correct the xy-drift [44]. The chromatic aberration between channels was corrected by using a pre-determined transformation function generated from imaging Tetraspeck beads. Final images were rendered with a 10 nm pixel size, weighted by intensity of individual localizations and the two channels were combined to have two-color super-resolved images. Analysis was always conducted on raw data; the processed image (Gauss filter) was used only for visualization purposes.
Conventional light microscopy
TIFF files were opened in Fiji (ImageJ) and contrast/brightness were adjusted. Adjustments were performed equally for plus and minus CLARITY sections.
Data analysis
Stack intensity profiles and line profiles were exported from LAS-X and Fiji (ImageJ), respectively, as Excel (Microsoft Corporation, Redmond, WA) files. GLUA2- and CAMKIIA-positive surfaces were counted in Imaris software using identical parameters for plus and minus CLARITY per z-plane. Co-localization analysis of GLUA2- and SYP-positive spots in two-dimensional (2D) images was performed in Imaris. The length of dendritic, MAP2-positive structures was determined using a combination of the ‘Surface’ and ‘Filament’ creation wizard in Imaris: In detail, dendrites were traced as individual surfaces, which were used as a basis for the ‘Filament’ creation wizard (surfaces and filaments with a length < 4 µm were excluded in both plus and minus CLARITY images to exclude non-dendritic surfaces). The analysis was run completely automatically with the exception of loops generated by the ‘Filament’ creation wizard: Loops were manually deleted (if added to the beginning or end of a filament) or the shortest connection was kept (if within a filament). FWHM was calculated on raw data using the non-linear fit command (Gauss function) of OriginPro (OriginLab, Northampton, MA).
Due to the limited sample size (n = 6), normality tests were not performed and nonparametric tests were applied: Using GraphPad Prism 6 (GraphPad Software, La Jolla, CA), Mann–Whitney test was performed for pairwise comparison in Fig. 3c. Statistical significance was defined with P < 0.05 (* P < 0.05, ** P < 0.01, *** P < 0.001, **** P < 0.0001).
Graphs were created in GraphPad Prism. Final figures were set up in Adobe Illustrator (Adobe, San José, CA).
Western blot with human Protein Medley
50 µg of human cerebral cortex lysate (Protein Medley, Takara, Kusatsu, Japan, Cat. No. 635323) were diluted and prepared according to manufacturer’s instructions (protocol PT1602-1). The sample and a protein ladder (Spectra Multicolor Broad Range, Thermo Scientific) were loaded on a 4–15% precast protein gel (Bio-Rad, Cat. No. 4561085). Western blotting was performed according to standard protocols, the GLUA2 antibody (Synaptic Systems, G7 in Table 2) was diluted 1:500 and β-actin was used as loading control (1:10,000, Sigma-Aldrich, Cat. No. A5316, RRID: AB_476743). A horseradish peroxidase (HRP)-coupled rabbit anti-mouse antibody (Dako, Glostrup, Denmark, Cat. No. P0260) in combination with Clarity Western ECL Substrate (Bio-Rad) were used for signal detection according to manufacturer’s instructions.
Results
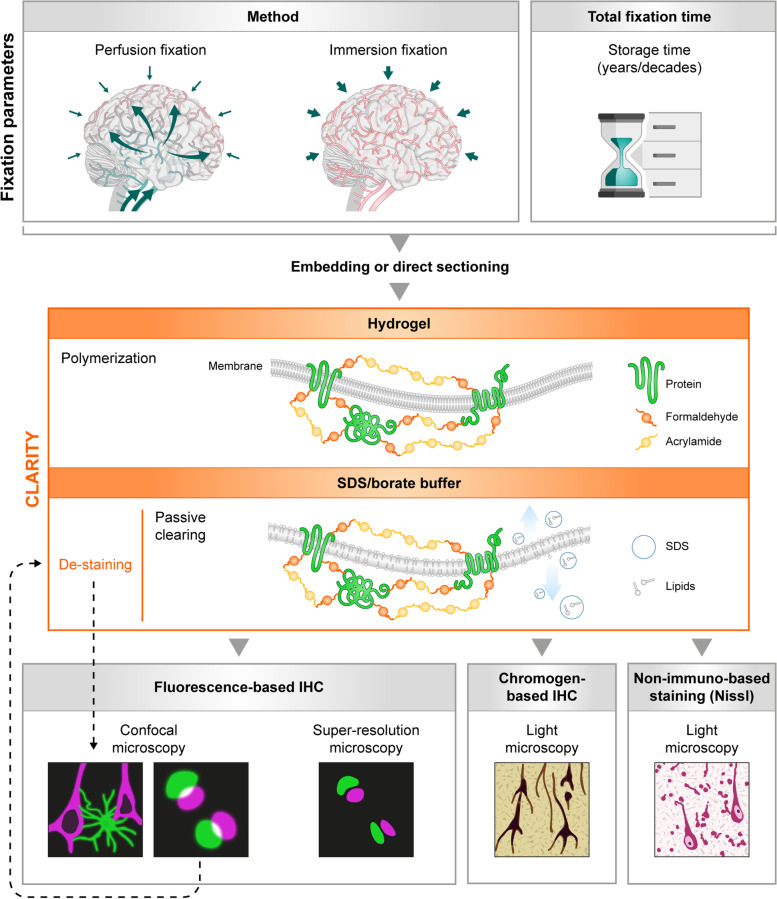
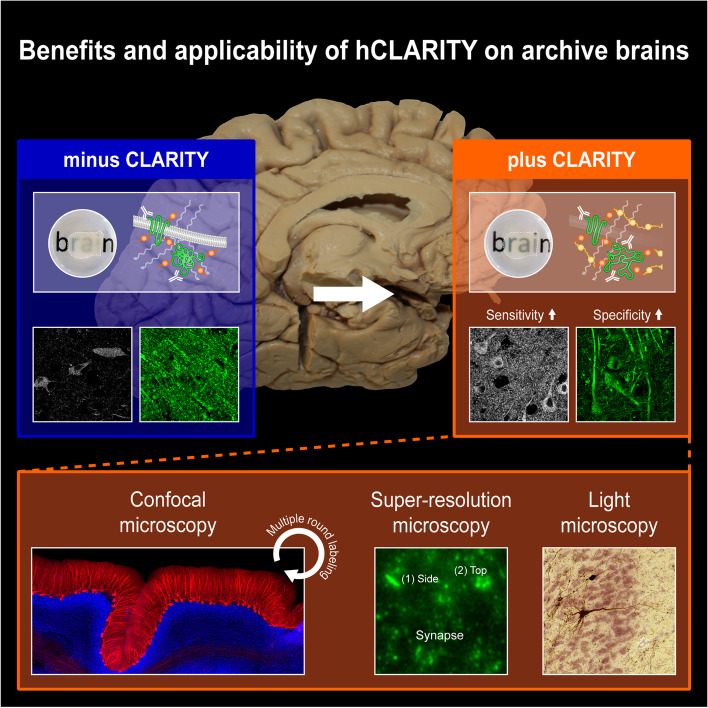
As the results of this study include a variety of methodologies, a summary of the here applied and adapted CLARITY method for long-term formalin-fixed human brain tissue, referred to as hCLARITY, and its possible applications are depicted in Fig. 1. With hCLARITY, human brain tissue fixed with different methods (immersion-fixed and perfusion-fixed, indicated with i or p in front of the case number (see Table 1 for further details)) or for different time periods can be utilized. Sections are either cut directly from the fixed tissue or from embedded tissue blocks. The sections are then stabilized by incubating them in a monomeric acrylamide-containing solution followed by polymerization introducing a hydrogel of polymeric acrylamide that binds to the protein-paraformaldehyde network. Subsequent detergent treatment on a shaking table at 37 °C, i.e., passive clearing, removes the biolipids that cause light scattering and a penetration barrier for the diffusion of macromolecules. hCLARITY is compatible with classical histological staining methods, such as Nissl, or chromogen-based IHC with DAB that is routinely used in neuropathology. In particular, hCLARITY is suitable for a broad variety of fluorescence-based imaging techniques, even with repetitive rounds of detergent based de-staining and subsequent re-staining (multiple round labeling). Below, we explain the results and benefits of our applications in greater detail.
Fig. 1.
Flowchart of compatible staining and imaging techniques for CLARITY-treated post mortem human brain tissue. Independent of type of fixation, e.g., perfusion-fixed versus exclusively immersion-fixed, or total fixation time, tissue samples can be embedded or cut directly on a vibratome. After CLARITY treatment, consisting of hydrogel polymerization with a consecutive SDS-based clearing step, fluorescence- or chromogen (e.g., 3,3’-diaminobenzidine (DAB))-based staining with a variety of cellular and synaptic markers can be performed. In addition, non-immuno-based Nissl staining is also compatible with CLARITY. Imaging can be performed with fluorescence microscopy (confocal or super-resolution microscopy) or with conventional light microscopy in case of DAB and Nissl staining. Primary and secondary antibodies as well as DAPI can be de-stained with the clearing solution, allowing for multiple staining rounds
Increased sensitivity of immunostaining after CLARITY
In two independent experimental rounds (n = two technical replicates), sections from the superior frontal gyrus (Brodmann area 9 (BA9)) underwent either CLARITY after cutting (= plus CLARITY) or they were cut shortly before immunolabeling (= minus CLARITY). For the IHC protocol, all sections were processed equally and in parallel.
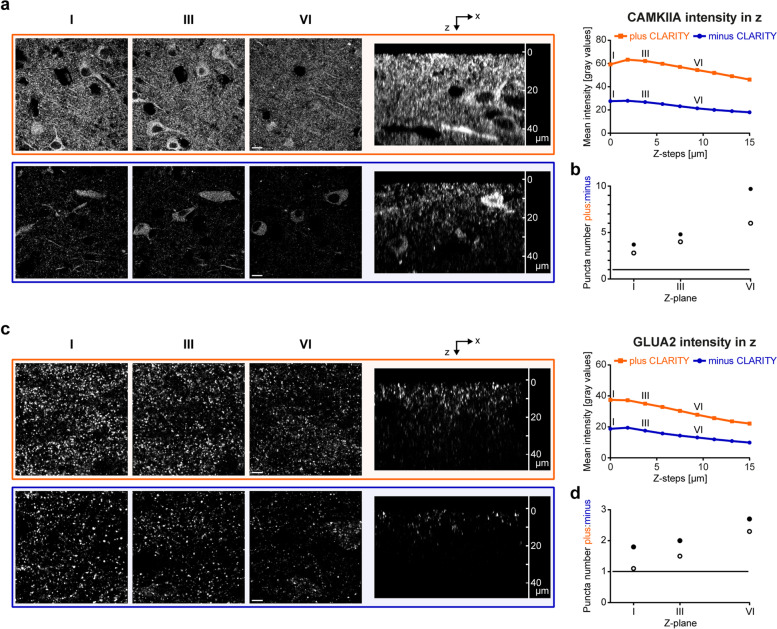
First, we aimed to compare the staining quality of antibodies directed against neuronal cells starting with a calcium/calmodulin-dependent protein kinase II alpha (CAMKIIA) antibody. CAMKIIA is not exclusively expressed in neuronal cells but is also part of the postsynaptic density (PSD) [45]. In the two perfusion-fixed cases, we found an overall higher mean intensity for plus CLARITY sections when viewed with identical parameters at a confocal microscope than minus CLARITY sections. This finding could be followed throughout a z-stack with z = 15 µm (Fig. 2a). For plane I of the z-stack, e.g., the mean intensity was increased approximately by a factor of 2 in cleared compared to non-cleared sections. Of note, while the single planes extracted from the z-stack still showed positive labeling of neurons and their processes in minus CLARITY sections, the intensity of punctate structures, presumably highlighting PSDs, was clearly enhanced in plus CLARITY sections. Similar tendencies could be observed for the immersion-fixed cases with a total fixation time of 18 years (Additional file 1a: mean intensity position I: factor 1.6 higher) and 5 years (Additional file 1b: mean intensity position I: factor 2 higher). For the remaining cases (i2, i3, and p2), CAMKIIA mean intensity in cleared compared to non-cleared tissue sections was on average twice as high (cases i2 and i3) or nearly by a factor three higher (case p2).
Fig. 2.
Increased sensitivity of antibodies applied on CLARITY-processed sections. a Cleared (orange) and non-cleared (blue) frontal cortex sections from case p1 were stained for CAMKIIA and imaged 15 µm deep in 1.8 µm steps using a confocal microscope. Z-level I (z = 0 µm), III (z = 3.6 µm), and VI (z = 9 µm) are provided as single images (left). Mean intensities of each z-step were exported with LAS-X software from two stacks (cortex layer V), and the mean was plotted in dependency of the z-level (right). A side view (xz scan) is shown in the middle. Scale bars, 10 µm. b CAMKIIA-positive surfaces in the neuropil outside dendrites of cases p1 (perfusion; filled circle) and p2 (white circle) were counted in z-levels I (z = 0 µm), III (z = 3.6 µm), and VI (z = 9 µm) of two z-stacks per case and condition (acquired with identical settings). In total, two regions of interest (ROIs) were analyzed per image in Imaris with constant parameters for plus and minus CLARITY per z-plane. The mean ratios between plus and minus CLARITY (y axis) of two technical replicates are plotted; a ratio of 1 (black line) indicates identical surface numbers for plus and minus CLARITY. c Mean intensity of GLUA2 staining on case p1 was plotted in dependency of the z-level. Acquisition strategy is the same as outlined in panel (a). Scale bars, 5 µm. d More sensitive detection of GLUA2 puncta after CLARITY, analysis as described in panel (b)
To compare plus and minus CLARITY results in a laser power-independent setting, we performed traditional DAB-based CAMKIIA staining on cleared and non-cleared sections. Not only were CLARITY-treated sections compatible with subsequent DAB staining, but again more intense labeling for cleared sections in comparison to minus CLARITY samples was observed (Additional file 1c).
To exclude the possibility that the increase in mean intensity results from an increase in non-specific signals, detailed analysis was performed to measure the effect of CLARITY (1) on specific signals (Fig. 2b) and (2) on background levels caused by autofluorescence and secondary antibodies (Additional file 1d-e). (1) For CAMKIIA, puncta analysis in cases p1 and p2 showed that after CLARITY a) the puncta density was approximately threefold increased at the most superficial z-plane and b) that these effects increased in depth (Fig. 2b), which is probably attributable not only to the effect reported here (sensitivity), but also to the previously described [24] effect of reduced light scattering and better antibody penetration. (2) In sections treated with secondary antibodies only, CLARITY did not lead to an increase in mean intensity compared to non-cleared sections (Additional file 1d-e). In summary, these findings underline that the observed increase in mean intensity after CLARITY did not derive from non-specific signals, but is most likely related to the better labeling of CAMKIIA epitopes by CLARITY.
Given the difficulty associated with staining postsynaptic structures, we then tried to see whether CLARITY improved staining quality of pre- and postsynaptic markers. In the perfusion-fixed cases, we found an overall higher mean intensity of glutamate receptor subunit 2 (GLUA2) throughout a stack with z = 15 µm, when viewed with identical parameters at a confocal microscope as compared to minus CLARITY sections. This became clearly visible in the xz scan (Fig. 2c). Puncta density analysis in cases p1 and p2 showed similar tendencies as for CAMKIIA, but less pronounced (Fig. 2d). Besides, co-localization between GLUA2 and synaptophysin (SYP) was determined in 2D images to verify whether the increase in GLUA2 puncta represents specific synaptic staining. The number of SYP-positive puncta did not change after CLARITY (see also below). In total, a higher percentage of SYP-positive puncta co-localized with GLUA2 after application of CLARITY (p1: 52% for minus CLARITY vs. 67% for plus CLARITY and p2: 57% vs. 67%, respectively; percentages are means of two technical replicates). In contrast, the proportion of GLUA2 puncta that co-localized with SYP after CLARITY treatment remained almost unchanged (p1: 42% for minus CLARITY vs. 37% for plus CLARITY and p2: 45% for minus CLARITY vs. 48% for plus CLARITY; percentages are means of two technical replicates). This suggests that the additional GLUA2 puncta detected with CLARITY are qualitatively similar to those detected with minus CLARITY.
For immersion-fixed cases, no generally applicable tendency was observed; in some cases, cleared sections revealed a finer and more homogenous punctate labeling (data not shown).
Especially in the context of AD-related synaptic decline, SYP has been a popular marker showing robust results in immunofluorescence on human post mortem tissue, see, e.g., [7, 8]. Here, we did not observe obvious differences in SYP staining quality between cleared and non-cleared sections (similar mean intensity and plus:minus puncta ratio of 1.2 for case p1 and 0.9 for p2; ratios are means of two technical replicates). These findings from the perfusion-fixed cases could not be directly compared to the immersion-fixed cases, inasmuch as we co-stained pre- and postsynaptic proteins, and the exclusively immersion-fixed brains displayed strong autofluorescence for shorter wavelengths. Related thereto, DAPI counterstaining was only successful in the perfusion-fixed cases.
Increased specificity of immunostaining after CLARITY
Besides the increased sensitivity for CAMKIIA labeling after CLARITY, we observed differences in target specificity for a microtubule-associated protein 2 (MAP2) antibody, a prominent neuronal marker. MAP2 immunofluorescence on non-cleared sections revealed strong superficial labeling of undefinable, partly globular structures (especially close to section surface) not corresponding to somata or dendrites, as shown in Fig. 3a (perfusion-fixed) and Fig. 3b (immersion-fixed). When all six study cases were viewed in a row, the MAP2 antibody provided reliable labeling of its target structures in cleared sections independent of their fixation method and total fixation time, whereas sections without CLARITY-pretreatment showed variable and inconsistent outcomes with sometimes unspecific artifact-rich or even no specific labeling (Additional file 1f). To confirm these qualitative effects, MAP2 signals were segmented as filaments in one z-plane and the filament length was measured. CLARITY resulted in a significant increase in MAP2-positive dendritic structures in both perfusion- and immersion-fixed cases (Fig. 3c). The total filament length from two images, e.g., for case p2 (Fig. 3a), is 566 µm for plus CLARITY and 122 µm for minus CLARITY and for case i1 (Fig. 3b): 655 µm and 47 µm, respectively (numbers are means of two technical replicates).
Finally, we tested CLARITY as a substitute for historical retrieval agents, e.g., detection of tyrosine hydroxylase (TH) in neurons and processes of formalin-fixed archival human tissue, which was successful after formic acid pretreatment in [46]. In the human neostriatum, TH is enriched in the matrix and has been shown to clearly delineate matrix/striosome organization [47]. In a cross-section from the caudate nucleus, TH staining showed the described mosaic pattern for cleared sections (Fig. 3d, left), and an inset shown in higher magnification unveiled neurites, most likely representing dopaminergic axons from nigral neurons [47], as the underlying structures. In the corresponding non-cleared section, only autofluorescent lipofuscin pigment was visible, and the tilescan (large overview scan) did not allow for clear distinction between matrix/striosome compartments. An intensity profile (Fig. 3d, right) confirmed the increase in specific staining after CLARITY by the clear separation of intensity peaks (matrix) and valleys (striosomes).
3D visualization of AD-related markers and concomitant synaptic decline with CLARITY
We also tested a panel of 30 antibodies for cells and subcellular compartments as well as pathology-associated markers in combination with CLARITY to provide a comprehensive toolbox intended to complement future studies on human tissue (see also Antibody toolbox for human brain interrogation with CLARITY). We used only perfusion-fixed tissue from the gross anatomy course. Only antibodies that worked in at least two different cases are listed in Table 2.
First, we aimed at assessing the performance of antibodies used for neuropathological staging on cleared sections. Neuropathological AD staging is based on the regional distribution and amount of DAB-stained intraneuronal neurofibrillary tangles (NFTs) and extraneuronal amyloid-β (Aβ) deposits [33, 48].
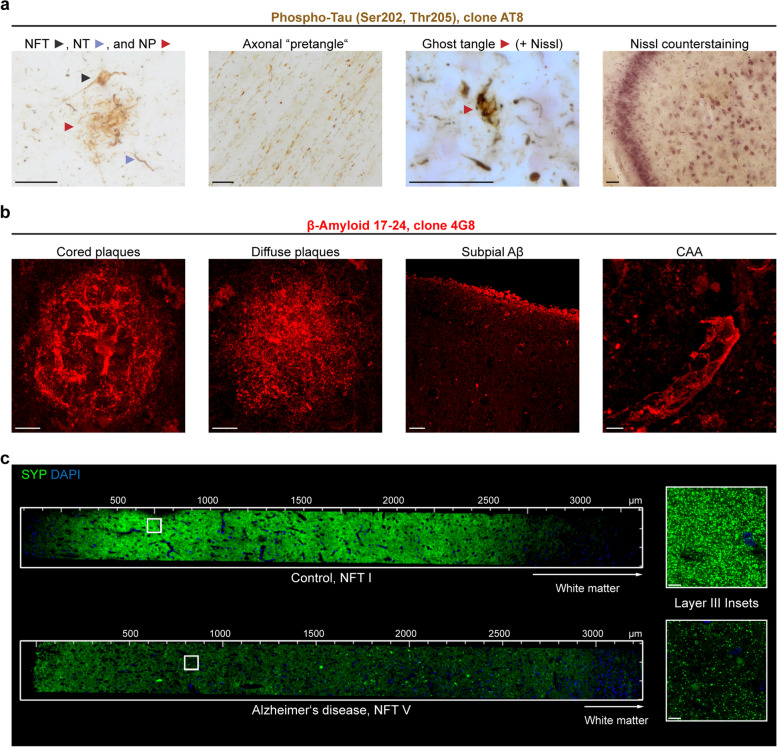
Since tau aggregates not only occur as NFTs, neuropil threads (NTs), and neuritic components of neuritic plaques (NPs) (Fig. 4a, left image), we also investigated whether other well-characterized abnormal tau aggregates are preserved after CLARITY. AT8-positive, partially soluble, material in axons of the perforant path constitutes a special form of pretangles, referred to as axonal “non-argyrophilic abnormal tau” [30]. We investigated the white matter underneath the entorhinal cortex of a NFT stage III and NFT stage V case and found AT8-positive axons in cleared sections (Fig. 4a, second image). In combination with Nissl staining, ghost tangles could be identified in cleared tissue based on the missing nucleus. Nissl counterstaining after CLARITY was successfully performed on cleared sections from immersion- and perfusion-fixed tissue (Fig. 4a, third and fourth image).
Fig. 4.
Hallmarks of Alzheimer’s disease and synaptic decline throughout the six cortical layers after CLARITY. a 100 µm-thick polyethylene glycol (PEG) sections (tissue bank, NFT stage V, Aβ phase 4, PD stage 3) were collected in 70% ethanol after cutting and washed in water before starting with the CLARITY protocol. Following the clearing step, traditional DAB-based immunolabeling was performed against phospho-tau (clone AT8). Different kinds of tau aggregates could be identified. Ghost tangles required counterstaining for Nissl substance with Darrow red. All images were acquired with a BX61 microscope (Olympus Optical) using extended focal imaging (EFI). On the far right, a cleared hippocampal section from a perfusion-fixed case is shown after AT8 staining (DAB) and Nissl counterstaining. The 2D image was acquired with a Biorevo BZ-X810 microscope. NFT = neurofibrillary tangle, NT = neuropil thread, NP = neuritic plaque. Scale bars, 50 µm. b Different types of Aβ pathology were detected following immunolabeling (clone 4G8) after CLARITY (perfusion-fixed case). CAA = cerebral amyloid angiopathy. Scale bars, from left to right, 10 µm, 10 µm, 50 µm, 5 µm. Except the third image from left, all images represent maximum intensity projections (MIPs) of z-stacks. c Staining of comparable cortex sections (superior frontal gyrus, Brodmann area 9) with synaptophysin (SYP, G2 in Table 2) reveals the drastic decline in synaptic density between a control case (case p2, NFT stage I) and a clinically diagnosed Alzheimer’s case (NFT stage V). Both sections were derived from perfusion-fixed cases. The insets to the right show a magnification of the most heavily affected layer III. Scale bars, 8 µm
The second hallmark of AD, Aβ plaques, could be identified after CLARITY in various reported morphologies [34], e.g., cored versus diffuse plaques, subpial Aβ, and Aβ deposits along vessels (i.e., cerebral amyloid angiopathy (CAA)) (Fig. 4b).
Because AD has also been described as “synaptic failure” [49], we combined the power of coherent 3D imaging and imaged all six cortical layers in the superior frontal gyrus from a control patient (case p2, NFT stage I) and an AD patient (perfusion-fixed, NFT stage V). We observed drastically reduced SYP puncta in the AD patient, which is emphasized by higher-magnified insets (Fig. 4c) of the most heavily affected layer, layer III of the cerebral cortex [17].
Some of the here presented antibodies are shown in videos (Additional files 2, 3, and 4).
Antibody toolbox for human brain interrogation with CLARITY
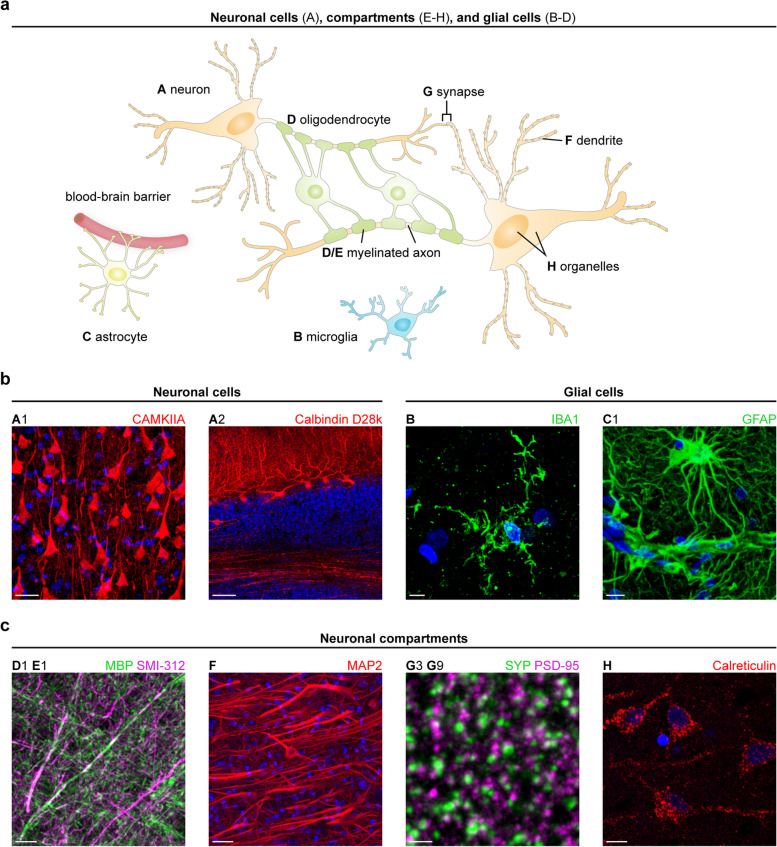
A schematic overview on the organization of neuronal cells, their compartments, and glial cells in the central nervous system is provided in Fig. 5a with examples presented in Fig. 5b-c. Stainings of human archival brain tissue with the remaining antibodies from Table 2 are shown in Additional file 5, followed by co-stainings and region-specific stainings in Additional file 6. Tilescans of cortical and subcortical structures are shown in Additional files 7 and 8. All antibodies showed expectable structures in at least two different brains.
Fig. 5.
Antibodies for specific labeling of structures in the human brain. a Schematic drawing showing the interaction between neurons (A) and glial cells, e.g., microglia (B), astrocytes (C), and oligodendrocytes (D) in the central nervous system. Neuronal compartments are highlighted (E–H). b (left) Subtypes of neurons are displayed using antibodies against CAMKIIA (pyramidal cells, cerebral cortex) and calbindin D28k (Purkinje cells, cerebellum). Scale bars, 30 µm (A1), 100 µm (A2). (right) Antibodies against IBA1 (case p2) and GFAP label morphologically distinct cell groups. Scale bars, 5 µm (B), 10 µm (C1). c Exemplary stainings of neuronal processes (axons enveloped with myelin sheaths, dendrites), synapses (pre- and postsynapse), and organelles (endoplasmic reticulum). Scale bars, from left to right, 15 µm (D1 E1), 30 µm (F), 2 µm (G), 10 µm (H). DAPI counterstaining is depicted in blue. If not otherwise indicated, immunostaining was performed on frontal cortex sections from perfusion-fixed cases (case p2 for D1 E1, case p1 for F–H). Except A2, all images represent MIPs of z-stacks. The upper-case letter at the top of each image refers to the entities outlined in panel (a) and the combination of letter and number allows for identification of the applied antibody listed in Table 2
Detection of distinct neuronal cells in cleared human sections
First, we set out to identify the different types of neurons in their specific brain region. As described above, the CAMKIIA antibody detected excitatory pyramidal cells in the cerebral cortex (A1 in Fig. 5b). Strikingly, CAMKIIA is expressed in inhibitory Purkinje cells of the cerebellum, where somata, dendrites, and axons of Purkinje cells were labeled (Additional file 7a), as reported previously for rat cerebellum [50]. Two different antibodies against calbindin D28k successfully stained somata, dendrites, and axons of Purkinje cells, as described elsewhere [51] (A2 in Fig. 5b, A3 in Additional file 5a). From the vast group of interneurons, we exemplarily tested an antibody against the rather seldom used epitope, corticotropin-releasing hormone (CRH, A4 in Additional file 5a) [52] and detected interneurons in the Ammon’s horn of the human hippocampus. The TH antibody (see Increased specificity of immunostaining after CLARITY) was also applied in the substantia nigra (data not shown) and locus coeruleus (A5 in Additional file 5a), where it highlighted the catecholaminergic principal neurons and their cellular processes.
Detection of glial cells in cleared human sections
Glial cells, including microglia, astrocytes, and oligodendrocytes, constitute a second large cell group in the central nervous system (CNS). Ependymal cells were not studied here. As an alternative to the frequently applied ionized calcium-binding adapter molecule 1 (IBA1) antibody from Wako, we identified another IBA1 antibody that unveiled specific labeling of microglia in human cerebral cortex tissue, as shown in Fig. 5b, B, and in a co-staining with astrocyte marker glial fibrillary acidic protein (GFAP) (B C2 in Additional file 6a). Astrocytes and their enormous fiber meshwork could be traced in great detail and throughout large z-stack volumes arguing for a good preservation of the 3D organization as hypothesized previously [53].
In addition to cell somata (C1 in Fig. 5b, C2 in Additional file 5b), both tested GFAP antibodies impressively unveiled the contribution of the astrocytic foot processes to the blood–brain barrier (asterisks in the tilescan of Additional file 8a, the white box is shown as higher-magnified image in Fig. 5b, C1). The second boundary layer formed by astrocytes, the glial limiting membrane (glia limitans), located between the pia mater and the cerebral cortex, is shown in Additional file 5b, C2. In a tilescan acquisition spanning 848 µm × 1.1 mm × 56 µm (in x, y, and z, respectively) of a human cerebellar section, GFAP unveiled an intricate meshwork arising from Bergmann glia with cell bodies in the Purkinje cell layer and long radial cellular protrusions in the molecular layer (Additional file 8b). Whereas GFAP is expressed more broadly, S100 calcium-binding protein B (S100B)-co-expression is restricted to more mature cells with no residual stem cell characteristics [54]. Accordingly, both antibodies showed only partial overlap in a co-staining (C2 C3 in Additional file 6a). In the CNS, oligodendrocytes form the myelin sheath around axons, and this could be clearly visualized in a co-staining with a pan-axonal marker (SMI-312, E1 in Fig. 5c) and an antibody directed against myelin basic protein (MBP, D1). Two additional MBP antibodies were successfully applied in human tissue (D2 & D3 in Additional file 5b).
Two of the here presented antibodies (GFAP and MBP) are shown in videos (Additional files 9 and 10).
Neuronal processes & organelles in cleared human sections
Besides the mere staining of axons (SMI-312, see Fig. 5c, E1), we could visualize nodes of Ranvier with an antibody directed against a protein of the paranodal region (Additional file 5c, arrows in E2). Dendrites could be traced using a MAP2 antibody (F in Fig. 5c; Additional file 11) as outlined above. We aimed to complement the list of markers for intracellular organelles, as did others before, e.g., for mitochondria [27]. For detection of nuclei, DAPI staining was applied in all cases. The endoplasmic reticulum could be represented with an antibody directed against calreticulin (H in Fig. 5c).
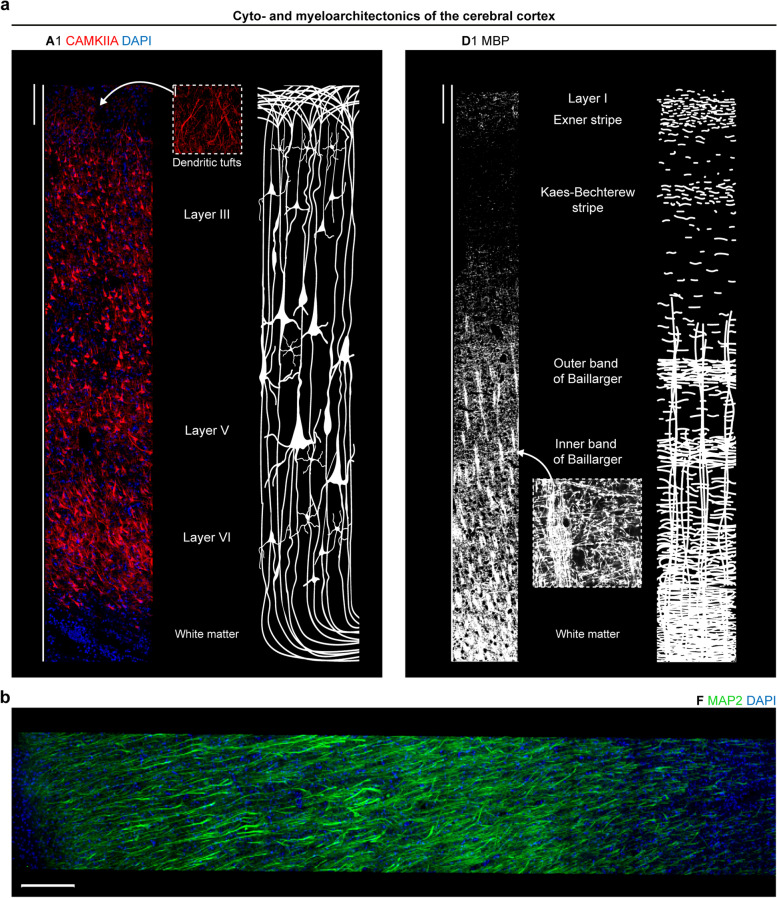
Since large overview scans (tilescans) provide best insights into the cyto- and myeloarchitectonics of the cerebral cortex, we exploited this technique to obtain xyz scans extending from the outer cortical border to the beginning of the underlying white matter. By CAMKIIA-staining, layer-characteristic neuronal morphology could be highlighted on an epitope-specific level next to a sketch based on a historical drawing published by the anatomist Henry Gray [55] (Fig. 6a). The dendritic tufts in the molecular layer were clearly visible as well as the increasing size of pyramidal neurons from layer III to layer V. Epitope-based distinction of cortical layers represents a valuable tool, which can be combined in a co-staining with a synaptic marker to specify synaptic decline in AD. The sketch showing the myeloarchitectonics is based on a non-immuno-based myelin stain found in anatomy textbooks [56]. The applied MBP antibody correlated well with the sketch, showing only little signal in the upper layers, whereas the fiber bundles in the lower layers were strongly highlighted. The transverse arrangement of fibers around layer V was reminiscent of the inner band of Baillarger. Since the cortex section (BA9) was not derived from primary sensory areas, we did not expect a clear outer band of Baillarger. The missing structural detail in the upper layers was probably due to the fact that the staining was acquired as 2D scan.
Fig. 6.
Cyto- and myeloarchitectonics in the human cerebral cortex after CLARITY. a (left) Cytoarchitectonics by 3D CAMKIIA imaging (MIP of stack with z = 25.5 µm) in the frontal cortex (perfusion-fixed) opposed to a schematic drawing based on Henry Gray. The inset (scale bar, 20 µm) shows the dendritic tufts of layer I. (right) Myeloarchitectonics as revealed by 2D MBP staining in the frontal cortex (case p1) opposed to a schematic drawing showing a non-immuno-based myelin stain found in anatomy textbooks. The inset (scale bar, 10 µm) shows the horizontal and transverse arrangement of fibers. Scale bars, 200 µm. b MAP2 immunostaining in the superior frontal gyrus of case p1, MIP of stack with z = 25.5 µm. Scale bar, 200 µm
In addition to cell and myelin organization, we captured the network of MAP2-positive dendrites and cells in a tilescan of the frontal cortex (Fig. 6b). In a z = 63 µm tilescan showing the molecular layer of the cerebellum, MAP2 allowed for a clear rendering of dendrites from Purkinje cells and somata of interneurons (Additional file 7b). Remarkably, the CAMKIIA and MAP2 antibody can be also applied for precise determination of the gray/white matter border because the immunoreactivity of both antibodies remarkably dropped where the approximate beginning of the white matter was marked with a pen on the slide, leaving only minor signal from interstitial neurons (see [57] for a review). This finding underlines the target-specificity of both antibodies.
Successful detection of synaptic subtypes in human brain sections
Since synaptic loss has been described as a molecular correlate of cognitive decline in neurodegenerative diseases like AD [5, 6] and ALS [7], excitatory and inhibitory synapse markers were tested, which is demanding in long-term formalin-fixed tissue. For overall staining of all synapses, the presynaptic protein SYP has been a prominent target as outlined above. Both SYP antibodies tested here showed the typical punctate pattern and a strong overlap in a co-staining, as seen in Additional file 6a, G2 G3. The partial overlap can be explained by targeting different epitopes of SYP (i.e., different isoforms). In the same category of antibodies, we successfully applied a synapsin 1/2 antibody (G1 in Additional file 5c).
To highlight the presynaptic site of exclusively inhibitory synapses, a cleared section from the putamen was stained for vesicular gamma-aminobutyric acid (GABA) transporter (VGAT). In line with a previous study [58], the punctate pattern could be also observed in the somata of medium spiny neurons and/or GABAergic interneurons (G4 in Additional file 5c). Robust results were obtained with a vesicular glutamate transporter 1 (VGLUT1) antibody for detection of excitatory synapses. Since VGLUT1 has been described as being enriched in the human putamen but absent in the pallidum [59], we subjected the antibody to a specificity test in both regions and found consistent expression patterns (Additional file 6d, II G5 putamen and III G5 pallidum). Moreover, in a tilescan covering the complete cortex (superior frontal gyrus), VGLUT1 (Additional file 6e) and SYP (Fig. 4c) were nearly absent in the underlying white matter.
We next tested several postsynaptic antibodies on cleared sections, a challenging endeavor in formalin-fixed archival tissue. An anti-gephyrin antibody was applied in the pallidum (G6 in Additional file 5c). To our knowledge, GLUA2 has not yet been routinely analyzed in immunolabeling studies on human brain tissue. The antibody in Table 2 not only showed a good co-localization with a presynaptic marker (G7 G2 in Additional file 6b) but also unveiled detailed expected substructure in super-resolution microscopy (see further descriptions in Super-resolution microscopy of neurons and synapses). Moreover, when applied in western blots, it detected its target protein in commercially available human tissue lysates (Additional file 6b).
From the HOMER protein family, we identified a reliably working HOMER3 antibody. While HOMER1 and 2 are widely expressed in the mouse brain, murine HOMER3 expression is mainly restricted to the cerebellum and to the hippocampus (minor levels compared to cerebellum) [60]. In line with DAB-stained human sections [61], Purkinje cell somata, dendrites, spines, and axons were labeled (G8 in Additional file 5c). The structural compartments of PSDs, the spines, were highlighted in remarkable quality along the dendrites, as seen in Additional file 7b, inset G8 and Additional file 12. The PSD-95 antibody also showed good co-localization with presynaptic SYP in cleared sections (G3 G9 in Fig. 5c).
Cell- and compartment specificity as well as the advantages of multiple simultaneous labeling could be demonstrated in a triple-staining of the hippocampus, a key player in neurodegenerative disorders, such as AD (A1 E1 and A1 G2 in Additional file 6c). Whereas the pan-axonal marker SMI-312 traced the fibers from the alveus and highlighted outgoing mossy fibers at the hilus, the CAMKIIA antibody highlighted pyramidal cells in the Ammon’s horn and showed punctate labeling, e.g., in stratum lucidum and stratum oriens. Co-localization with the presynaptic protein SYP was especially prominent in CA2, as shown by the white overlap in Additional file 6c (right-hand image, higher-magnified inset).
Additional targets in human brain sections
Since AD pathology can also be associated with vascular structures, e.g., CAA, we tested vessel-related markers on cleared sections. Two antibodies designed for application in human tissue gave strong signals and a penetration depth of at least 70 µm, one of which was raised against collagen IV of basal laminae, the second against α-actin of smooth muscle cells (J1 & J2 in Additional file 5d; videos in Additional files 13 and 14). From a biochemical approach, a metabolism-related antibody against glyceraldehyde-3-phosphate dehydrogenase (GAPDH), an enzyme involved in glycolysis (K in Additional file 5d), was also applied successfully.
Re-staining of fluorescently-labeled sections
Given the restricted availability of human post mortem brain tissue, the limited size of blocks through different brain regions, and especially analyses that require more than three to four different channels, multiple round labeling of already stained sections would be beneficial. As shown for mouse brain sections in the original CLARITY publication [24], de-staining in clearing solution and subsequent re-staining is feasible. We tested this option on perfusion-fixed tissue and stained a cortical section for pre- and postsynapses (SYP and GLUA2). We successfully performed a second round of staining with cellular antibodies (Fig. 7). Most important, a control section mounted and imaged after de-staining did not show any specific signal, nor was DAPI staining detectable any longer (Fig. 7). To also control for detachment of primary antibodies, the control section was subjected to the second round of staining, starting with secondary antibody incubation. Confocal microscopy did not show any signal except for re-stained nuclei (data not shown).
Fig. 7.
Multiple staining rounds with hCLARITY. (top row) Stained sections from case p1 mounted in SlowFade were de-stained in clearing solution for 24 h at 60 °C (left). A de-stained section was mounted and imaged as control (middle). Re-staining with cellular markers (right). Scale bars, 2 µm (left and middle), 10 µm (right). (bottom row) DAPI channel of the images shown in the top row before de-staining (left), after de-staining (middle), and after re-staining (right). Imaging parameters were identical. Scale bars, 10 µm
Super-resolution microscopy of neurons and synapses
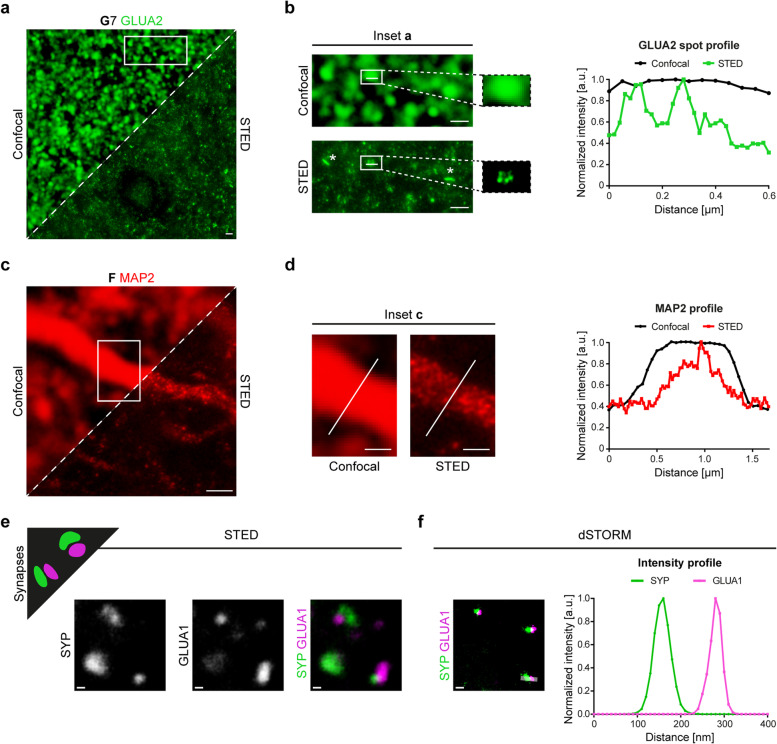
Finally, we applied tested antibodies for super-resolution microscopy to gain insights into details that are not accessible with diffraction-limited light microscopy. To this end, we employed STED [41] and dSTORM microscopy [43] with custom-built setups. First, we performed STED microscopy on GLUA2-stained sections due to the good performance of the antibody in confocal microscopy. In direct comparison to confocal images, we saw finer structures in super-resolved images (Fig. 8a). While in the confocal images all synaptic puncta had homogenous shapes, the corresponding STED images unveiled the known disk shape (in side view appearing as bars [62], full width at half maximum (FWHM) was 118 nm in STED vs. 594 nm in confocal) and further resolved the substructures within a single PSD. The latter could be reflected by the corresponding intensity profile, where a homogeneous intensity profile for confocal microscopy is split into two individual peaks in STED microscopy (Fig. 8b).
Fig. 8.
Super-resolution microscopy with STED and dSTORM imaging of synaptic and neuronal markers on human brain tissue after CLARITY. a Synaptic GLUA2 in the cerebral cortex acquired in confocal (upper left) and STED mode (lower right). Scale bar, 1 µm. b Insets (boxed area from (a)) provided in higher magnification unveil postsynaptic membrane morphologies like disk shape as seen in a side view on a synapse (asterisks, left column) and the detailed localization of GLUA2 within a single synaptic spot as seen in a top view on a synapse (right column). A line intensity profile (right part of (b)) across the spot surface demonstrates the intensity fluctuation along the spot (white line in the boxed areas of (b)). Mean intensities (gray values) were normalized on the highest value. a.u. = arbitrary unit. Scale bar, 1 µm. c MAP2 staining in a cerebral cortex section. STED and confocal modes of the same neuron are shown in comparison as in panel (a). Scale bar, 1 µm. d Insets (boxed area from (c)) provided in higher magnification show structural details in the STED mode. The intensity profile across the white lines in the images is plotted on the right. Scale bar, 0.5 µm. Sections for MAP2 and GLUA2 staining were derived from the superior frontal gyrus of perfusion-fixed cases. In (a)-(d), pixel size for confocal images is 50 nm. e Schematic drawing showing expected precision imaging of synaptic compartments in super-resolution images (upper left). SYP/GLUA1 co-staining in STED microscopy. The channels are shown individually (left and middle) and as overlay (right). Here, sections were incubated in hydrogel solution for two days, total clearing time was three days. Scale bars, 200 nm. f SYP/GLUA1 co-staining in dSTORM microscopy (left). An intensity profile across a synapse (rectangle in the left image, width of ten pixels) is shown on the right. Sections were incubated in hydrogel solution for one day, total clearing time was two days. Scale bar, 200 nm. All sections in (e)-(f) were derived from perfusion-fixed cases
Second, while the MAP2 antibody showed a rather continuous signal in confocal images (FWHM of entire dendrite = 1.21 µm), the corresponding STED image unveiled structural details with a punctate pattern of the signal (FWHM = 521 nm) (Fig. 8c), which was well comparable to STED images obtained from cultured neurons with the same antibody [63]. The intensity profile across a dendrite showed a narrower peak (on a broader background), highlighting the superior resolution of STED (Fig. 8d).
Third, to trace synaptic and neuronal structures in 3D, a series of images was acquired from a CAMKIIA-labeled section. Similar to MAP2 labeling, the detailed organization of CAMKIIA-positive structures and clusters in dendrites could be studied in the maximum intensity projection image (Additional files 15 and 16). Finally, in a proof-of-principle staining, we tested the compatibility of dSTORM imaging with a pre- and postsynaptic staining after CLARITY and opposed it to a STED image (Fig. 8e) labeled for the same compartments (SYP antibody for presynapses, GLUA1 antibody for postsynapses). Synapses were presented in high detail and we obtained better localization precision and hence increased resolution between two closely localized protein clusters with dSTORM images (Fig. 8f).
Discussion
Major advantages of hCLARITY
The main findings of this study were, first, that only with the additional application of CLARITY prior to IHC, immunostaining with some markers (MAP2, TH) was possible (i.e., more specific). With other markers (CAMKIIA, GLUA2), staining became more intense, not only in depth, as was known and expected with CLARITY, but already at the surface (i.e., more sensitive). Second, we offer a comprehensive set of 30 different well-working antibodies. Furthermore, sections can be completely de-stained (including DAPI) and re-stained. Third, super-resolution microscopy with two different techniques was possible, for the first time also at the level of synapses in the human brain. Furthermore, we could show that hCLARITY can not only be combined with modern fluorescence-based microscopy techniques but also with chromogen-based staining and Nissl staining. Perfusion-fixed tissue was derived from body donors of the gross anatomy course, which is a valuable source of post mortem tissue, for some aspects superior to solely immersion-fixed tissue (less autofluorescence in the shorter wavelength spectrum). The method is particularly attractive because it is inexpensive and easy to perform.
It should be emphasized that no loss of detectability of various AD tau and Aβ protein aggregates was observed with the CLARITY protocol applied here (hCLARITY). Already in the initial publication of the method, CLARITY was shown to be applicable to longer fixed human brain tissue for IHC staining [24]. Applicability to sections has also been previously described [64]. Taken together, in addition to the known increase in antibody penetration, we have identified further benefits of the CLARITY method in the sense of antigen retrieval by enhancing or enabling accessibility of epitopes in long-term fixed human brain tissue. Therefore, we propose that hCLARITY be applied prior to IHC.
We have presented 30 usable primary antibodies directed against different cell types, cellular structures, and neurodegenerative pathologies. Each of these antibodies proved to be robust, passing quality control only when functioning in brains of at least two individuals. For markers with no clearly identifiable localization (e.g., synaptic markers), the quality threshold was reached only when co-localizing with another synaptic marker. We compared the obtained expression patterns with available literature and found a good match, indicating the compatibility of hCLARITY with these antibodies. This is important, given the fact that staining of IBA1 was not possible with a clearing method which included OPTIClear [53], whereas in the present study using hCLARITY the staining was successful. This panel represents one of the most comprehensive ‘antibody toolboxes’ for immunofluorescence microscopy published to date for human brain research. The primary antibodies described here can be used for co-staining as an initial specificity test for antibodies applied for the first time on human brain tissue and also as a test for tissue quality. Previous studies applying methods such as array tomography or CLARITY, and additional selected clearing techniques for immunofluorescence staining of the human brain, have usually presented significantly fewer antibodies, and the focus of these publications has mostly been on other aspects (20 primary antibodies in [15]; 4 with CLARITY in [65]; 4 with CLARITY in [26]; 2 with CLARITY from [66]; 10 with CLARITY from [27]; 2 apart from several amyloid and tau antibodies in [67]; 4 in [68]; 9 with CLARITY in [28]; 3 with CLARITY in [69]).
In this regard, we consider the work of Ando et al. [65] as a forerunner of our own work; this group pioneered the use of CLARITY for 3D imaging of AD-associated neuropathological changes in tissue, hinting at its true potential. The authors were already able to show that CLARITY appeared to be partially superior to other clearing techniques for purposes of visualizing such changes [65]. Importantly, the superiority of CLARITY in comparison to other clearing methods was also demonstrated in a very recent study by Rusch et al. (2022) [69], largely in terms of physicochemical properties. Interestingly, only with CLARITY, all three tested primary antibodies (directed against β-III-tubulin, MBP, and proteolipid protein) were reported to produce specific staining in human tissue [69]. The immunostaining quality after CLARITY was investigated in detail in the present study.
In previous studies, e.g., [27], significantly longer antibody incubation times were used, but our focus was not on the depth of penetration and reduction of light scattering but, rather, on the quality of the IHC. In contrast to Ando et al. [65], we mostly used the commercial mounting reagent ProLong Gold antifade (refractive index (RI) after curing = 1.46) for cleared sections. In comparison to self-made RIMS, consistent formulation is guaranteed by the vendor. Further, we frequently observed crystal formation days after mounting in RIMS, whereas ProLong Gold antifade mountant enables long-term storage with concurrent preservation of fluorescence. Our clearing times were similar to those used in the study by Magliaro et al. who found that significantly longer detergent treatments can lead to antigen loss [70].
An additional discovery that emerged from the present work is the application of the hCLARITY procedure for manifold IHC and microscopy techniques, which is especially valuable in light of the restricted access to human post mortem tissue. Complete de-staining is already possible within one day, but has not always been reproducible with human tissue (compare [27] and [26]). Here, re-staining with the use of a completely different antibody combination (synaptic to cellular) functioned without any background or remaining residual signal from the first staining. The advantages for studying neuropathological changes are obvious: for example, neurodegenerative protein deposition and changes in synaptic integrity (first staining) and cells (second staining) can be studied on the same tissue section. To our knowledge, ours is also the first study to show that a DAPI stain is also completely de-stainable, thus, another channel can be used in a subsequent round of labeling or, if necessary, the tissue section can be re-stained with DAPI.
Besides diffraction-limited confocal microscopy, we showed human brain synapses in sub-diffraction resolution in the super-resolution microscopy techniques STED and dSTORM. Owing to the higher spatial resolution, a higher labeling density and a lower background signal is required in super-resolution optical microscopy as compared to classical fluorescence microscopy approaches. Therefore, the requirements with respect to clearing conditions of tissue are much more stringent, thus providing an important quality test of hCLARITY. Protein aggregates in neurodegenerative diseases, e.g., tau deposition in human AD samples, have already been shown in a previous work using STED [71]. Here, to our knowledge for the first time, we could show a full chemical synapse with pre- and postsynaptic markers in a human post mortem brain with super-resolution microscopy, as has been previously demonstrated only in mouse brain [62] or in rat hippocampal neurons [43] with dSTORM. Furthermore, in our hands, STED imaging with GLUA2 allowed unprecedented insights into the molecular organization of human postsynapses in the cerebral cortex, with a FWHM comparable to data obtained in mouse brain [72]. The distinct arrangement of GLUA2 puncta within one PSD might reflect the concept of nanodomains [73, 74].
The implications of these findings for neurological disease research are manifold, e.g., the still unresolved questions of pre- or postsynaptic localization of proteins that may be altered in neurodegenerative diseases, such as amyloid precursor protein (APP) [75, 76], or huntingtin [77], or the study of the mechanisms of network anterograde transsynaptic transmission of misfolded proteins [78].
Beneficial mechanisms of hCLARITY on long-term-fixed human brain tissue
Presumably, the CLARITY method has some advantageous chemical properties when using formalin-fixed archival brain tissue. In particular, CLARITY has been shown to enhance the penetration depth of immunostaining [24], including human brain tissue [28]. On the one hand, this may be due to the increase in transparency; on the other to the better penetrability of the tissue for antibodies after removal of the biolipids. When considering the benefits of CLARITY, rather too little attention was paid to the fact that SDS treatment appears to increase the immunogenicity of antigens, presumably through denaturing properties [79, 80]. Importantly, descriptions from preliminary studies have indicated that most of the tested antibodies showed either improved or unaltered labeling after SDS treatment [25, 80, 81]. While it has already been speculated about a retrieval effect of SDS in the framework of CLARITY [26], we demonstrated that hCLARITY significantly increases the sensitivity, accuracy (specificity), and comparability of tissues of different origin and fixation type (short/long fixation, fixation via perfusion or immersion). In line with the effects of SDS treatment alone, described by Brown et al. [25], we identified antibodies, that (1) did not specifically label their target proteins without hCLARITY (MAP2, TH), (2) showed stronger (more sensitive) labeling of target structures after hCLARITY (CAMKIIA, GLUA2), and (3) did not benefit from hCLARITY (SYP).
Because in laser-scanning microscopy, higher laser powers inevitably raise the proportion of tissue autofluorescence in the acquired signal (e.g., lipofuscin), more sensitive staining after hCLARITY is beneficial in this context, allowing for a decrease in laser power while keeping antibody concentrations unchanged.
The hCLARITY method has additional merits, however, inasmuch as the hydrogel stabilizes the tissue, so that modifications can be employed depending on the application, e.g., longer clearing/antibody incubation times if 3D aspects are particularly significant for the question being addressed. Multiple staining rounds with de- and re-staining are also likely to benefit from tissue stabilization. In brain tissue, there is the additional factor of its high fat content. Owing to the physicochemical removal of these lipids, the tissue probably benefits particularly strongly from the method.
We also observed that hCLARITY reduces non-specific staining of some antibodies (e.g., globular structures in MAP2 staining). It is possible that detergent treatment removes sticky particles that have been deposited. In addition, CLARITY may render individual epitopes more accessible not only by denaturation but also by mild disruption of extremely cross-linked PFA-protein networks after long fixation. The precise effect of SDS is most likely mediated on epitope level rather than on antigen level (e.g., as shown for two different caveolin-1 antibodies in [80]). We summarized the suspected chemistry behind CLARITY as well as the major applications/findings of the present study in Fig. 9.
Fig. 9.
Summary of the main advantages of hCLARITY. CLARITY was applied on human post mortem brain tissue in direct comparison to untreated sections (minus CLARITY). Owing to removal of lipids during the clearing with SDS and the resulting reduction in light scattering, cleared sections were less opaque. The denaturing effect of the detergent SDS most likely results in better accessibility of certain epitopes. The major benefits of hCLARITY were found in an increased sensitivity and specificity of antibodies for neuronal cells. The adapted hCLARITY protocol is compatible with staining techniques for confocal microscopy, super-resolution microscopy, and light microscopy
Limitations
We have not investigated in greater detail whether there are other beneficial properties in addition to those described here, e.g., deeper penetration upon increasing duration of clearing or the incubation times for antibodies. We also do not know yet how many times in succession de- and re-staining can be performed. The role of SDS treatment alone without prior hydrogel formation (Free of acrylamide SDS-based tissue clearing (FASTClear) [68]), has not been separately investigated, but two disadvantages are likely: Transparency is lower because hydrogel might also contribute to RI alignment, and tissue integrity might also suffer from prolonged SDS treatment without prior stabilization. In previous descriptions of sole SDS treatment prior to immunolabeling, much lower SDS concentrations have been used [25, 80]. Thus, it could be that higher SDS concentrations require chemical stabilization, e.g., via a hydrogel scaffold.
Future applications of hCLARITY for neuropathology research
We view hCLARITY as a significant extension of the existing spectrum of methods for research of the human brain, especially when tissue properties are not perfectly controllable. The applicability to long and differently fixed tissues will make tissue bank resources accessible to more researchers. As an example, we point in particular to the advantages of hCLARITY as an upstream method for IHC in the study of neurodegenerative diseases. Until now, findings from classical IHC were often limited to the distribution of protein deposits in various brain areas and cell compartments [36, 48, 78, 82]. By contrast, changes in structures important for neural tissue functionality (cell densities, synaptic integrity) have often been difficult to compare or have been studied using very elaborate techniques that tend to be available only in specialized laboratories. The hCLARITY method can be combined with classical histological staining (shown here with Nissl) and with DAB chromogenic immunostaining. Importantly, the use of immunofluorescence, with the additional possibility of multiple round labeling, permits the examination of several markers simultaneously (or consecutively) on the same tissue section in a quantitative and specific manner. Thus, hCLARITY can be used to study neuropathology (protein deposition) affecting functionally important structures (cells, synapses) quantitatively on one tissue section in three dimensions. The possibility of using super-resolution microscopy also expands the arsenal of methods for investigating even at the molecular level the propagation of pathologies. Recent studies with super-resolution microscopy revealed novel insights into the composition and localization of protein aggregates in AD [83] and Parkinson’s disease (PD) [84–86].
Conclusions
In closing, the chief advantage of hCLARITY is not only the increase of antibody and light penetration depth but also restoration of the quality of long-fixed tissue. It was exemplary shown that hCLARITY has a positive effect for n = 4 of 6 antibodies. The other 24 antibodies tested were shown to be compatible with hCLARITY. This method and the provided information on applicable microscope systems could serve to study neuropathological phenomena in the human brain in much greater detail in the future with the here presented neuronal, non-neuronal, synaptic, and pathology markers (n = 30). The hCLARITY methodology can be performed with a basic confocal microscope at low time and with minimal material cost in any molecular biology laboratory ranging from basic research to neuropathological diagnostics. In particular, we recommend the use of perfusion-fixed brain tissue from permanent body donors, as it is often available in anatomical institutes and partially superior to immersion-fixed tissue.
Supplementary Information
Additional file 1. Improved antibody labeling and better comparability between different cases with CLARITY. (a) Cleared (orange) and non-cleared (blue) frontal cortex sections (superior frontal gyrus) from case i4 (total fixation time 18 years) were stained for CAMKIIA and imaged 15 µm deep in 1.8 µm steps using a confocal microscope. Mean intensities of each z-step were exported with LAS-X software from two stacks (cortex layer V) and the mean was plotted in dependency of the z-level. Z-level I (z = 0 μm), III (z = 3.6 μm), and VI (z = 9 μm) are provided as single images. A side view (xz scan) is shown on the far right. Scale bars, 10 µm. (b) Mean intensity of CAMKIIA staining on case i1 (total fixation time 5 years) was plotted in dependency of the z-level. Acquisition strategy is the same as outlined in panel (a). Scale bars, 10 µm. (c) CAMKIIA staining with DAB on cleared (orange) and non-cleared (blue) sections (case p2). Images represent full focus projections of z-stacks with identical brightness/contrast adjustments. Scale bars, 20 µm. (d) Background levels in secondary antibody only controls do not differ with CLARITY. Graphs show the mean intensities (int.) of the negative controls (secondary antibody only) of minus and plus CLARITY tissue sections; each case is represented by a different symbol (● i1, □ i2, ○ i3, ■ i4, ♦ p1, and Δ p2). The mean intensity of the entire image was determined in ImageJ. Negative control data belonging to CAMKIIA-rb, GLUA2-ms (both imaged using the 635 laser), and MAP2-ms staining (561 laser) are shown. Negative controls were always acquired with identical settings for both conditions (plus/minus CLARITY), mostly corresponding to the staining (in few cases higher laser power), which included the primary antibodies (see Figs. 2 and 3). (e) Second technical replicate. 561 laser: case p1 was excluded since negative controls acquired with identical settings were not available. (f) Comparison of MAP2 labeling on cleared (orange) and non-cleared (blue) sections from the six study cases with different fixation methods and total fixation times (see Table 1). Negative controls are part of panel (d), 561 laser. Scale bars, 10 µm. The codes A1 and F in panels (c) and (d) refer to Fig. 5.
Additional file 2: Video. 3D imaging of AD tau pathology. AT8-positive material (neurofibrillary tangles and neuropil threads) was stained in a 70 µm thick paraffin section derived from the cerebral cortex of an immersion-fixed case (LSM 710 microscope, Zeiss, 40x objective, NA 1.3, stack size 35.18 μm, step size 1.066 μm). Here, embedding in hydrogel solution took place for 11 days, clearing time was 17 days. The section was mounted in FocusClear.
Additional file 3: Video. 3D imaging of Aβ pathology (cored plaque) and VGLUT1. Co-staining of a cerebral cortex section from a perfusion-fixed case with 4G8 (red) and a presynaptic marker (VGLUT1, G5, white) (confocal microscope, 40x objective, NA 1.15, stack size 24.77 μm, step size 0.36 μm).
Additional file 4: Video. 3D imaging of Aβ pathology in vessels (CAA). Immunostaining of a cerebral cortex section from an immersion-fixed case with 4G8 (confocal microscope, 63x objective, NA 1.3, stack size 39.64 μm, step size 0.28 μm).
Additional file 5. Additional CLARITY-compatible antibodies on human brain sections. (a) Neuronal cell markers used to detect specific neurons in cerebellum (A3), hippocampus (A4), and locus coeruleus (A5). Scale bars, 20 µm (A3), 10 µm (A4), 30 µm (A5). (b) Glial cell markers used on frontal cortex sections. Scale bars, 20 µm (C2), 30 µm (D2, D3). (c) Neuronal compartments, e.g., paranodes of axons (E2, exemplarily marked with arrows, asterisks mark nodes of Ranvier), presynapses (G1, G4), and postsynapses (G6, G8) are highlighted in frontal cortex sections (E2, G1), putamen (G4), pallidum (G6), and cerebellum (G8). Scale bars, 3 µm (E2), 5 µm (G1, G4, G6), 20 µm (G8). (d) Vessel-related markers (J1, J2) and GAPDH (K) in cortical sections. Scale bars, 50 µm (J1), 20 µm (J2, K). DAPI counterstaining is depicted in blue. All images except A3 (both) and C2 (right) represent MIPs of z-stacks, K is shown in perspective view. Staining is shown for perfusion-fixed cases (mostly p1 and p2, except A3, G4, and J2). The upper-case letter at the top of each image refers to the entities outlined in Fig. 5a and the combination of letter and number allows for identification of the applied antibody listed in Table 2.
Additional file 6. Cell, compartment, and region specificity of applied antibodies with CLARITY. (a) Co-staining with glial cell markers and two presynaptic markers as proof-of-specificity. DAPI counterstaining is shown in blue. Scale bars, 15 µm (left), 10 µm (middle), 5 µm (right). Images are mainly derived from frontal cortex sections of cases p1 and p2 (C2 C3: underlying white matter). Except C2 C3, images represent MIPs of z-stacks. White color indicates overlap of markers. (b) GLUA2 (G7) was used for western blots with human cerebral cortex lysate (Protein Medley, 50 μg), exposure time was 30 min for GLUA2 and 30 s for β-actin (original blot: Additional file 17). Unit of the ladder is kilodalton. The same antibody is shown in a synaptic staining on frontal cortex sections (case p2). Scale bar, 2 µm. (c) Triple-staining with neuronal, axonal, and synaptic markers in a hippocampal section (case p2). For better visualization CAMKIIA is shown twice, in combination with SMI-312 (left) and with SYP (right). For the latter, an inset of the CA is provided in higher magnification (scale bar, 20 μm). CA = cornu ammonis, DG = dentate gyrus, alv = alveus, hil = hilus. Scale bars, 500 µm. (d) Tissue block with striatum (I, II) and pallidum (III). Immunolabeled sections from this block show matrix (TH high) and striosome (TH low) compartments in the caudate nucleus (ncl.) (I). VGLUT1 expression is strong in putamen (II) and absent in pallidum (III). G5 images represent MIPs of z-stacks. Scale bars, 400 µm (TH), 10 µm (VGLUT1). (e) VGLUT1 expression in the superior frontal gyrus and white matter (top). Intensity profile of the dashed area (raw data), normalized (norm.) to the maximum intensity (bottom). The green line marks the gray/white matter border, which was approximated by the abrupt reduction of the VGLUT1 signal. a.u. = arbitrary unit. Scale bar, 150 µm. Sections in (d) and (e) were derived from cases p1 and p2. The upper-case letter at the top of each image refers to the entities outlined in Fig. 5a and the combination of letter and number allows for identification of the applied antibody listed in Table 2.
Additional file 7. 3D tilescans of neurocellular and synaptic markers in the human cerebellum after CLARITY. (a) CAMKIIA in the cerebellum, MIP of stack with x ~3.2 mm, y ~1.5 mm, and z = 54 µm. Scale bars, tilescan 200 µm, inset 100 µm. Stainings were performed on case p1. (b) 3D tilescan showing MAP2 and HOMER3 (shown as inverted image to highlight the spines of Purkinje cells in the molecular layer) in the human cerebellum, MIP of stack with z = 63 µm (MAP2). Scale bars, tilescan 50 µm, insets 10 µm (top), 5 µm (bottom).
Additional file 8. 3D tilescans unveiling the extensive meshwork formed by GFAP-immunoreactive fibers after CLARITY. (a) Cerebral cortex and adjoining white matter stained for GFAP, MIP of stack with z = 10 µm is shown. Asterisks mark several sites where the blood-brain barrier can be recognized by GFAP-positive extensions wrapping the voids where a vessel runs along. The boxed area is shown in higher magnification in Fig. 5b. Scale bar, 70 µm. (b) Cerebellar section stained with GFAP; dimensions in xyz, x = 848 µm, y = 1.1 mm, z = 56 µm. Scale bars, 100 µm (top), 70 µm (bottom). Sections were derived from perfusion-fixed cases (for panel (b) case p1).
Additional file 9: Video. 3D imaging of GFAP-positive astrocytes and contribution to the blood-brain barrier. Immunostaining of white matter from a perfusion-fixed case with a GFAP antibody (C1, red), counterstaining with DAPI is shown in blue (confocal microscope, 40x objective, NA 1.15, stack size 98 μm, step size 1 μm).
Additional file 10: Video. 3D imaging of MBP. Immunostaining of a cerebral cortex section from case p2 with a MBP antibody (D3). The fibers were traced as surfaces with Imaris software and color-coded according to their z-position (confocal microscope, 40x objective, NA 1.15, stack size 63.53 μm, step size 0.5 μm).
Additional file 11: Video. 3D imaging of MAP2-positive neurons and dendrites. Immunostaining of a cerebral cortex section from case i4 with a MAP2 antibody (F). The video traces MAP2-positive cells and fibers from the outer cortex surface down to the beginning of the white matter (confocal microscope, 40x objective, NA 1.15, stack size 15.01 μm, step size 0.5 μm).
Additional file 12: Video. 3D imaging of HOMER3 in the cerebellum. HOMER3 (G8) is shown in red, DAPI counterstaining in blue, case p1 (confocal microscope, 40x objective, NA 1.15, stack size 47.52 μm, step size 0.22 μm).
Additional file 13: Video. 3D imaging of collagen IV in the cerebral cortex. Collagen IV (J1) is shown in red, DAPI counterstaining in blue, case p2 (confocal microscope, 40x objective, NA 1.15, stack size 46 μm, step size 1 μm).
Additional file 14: Video. 3D imaging of smooth muscle actin (SMA) in the cerebral cortex. SMA (J2) is shown in white, DAPI counterstaining in blue, perfusion-fixed case (confocal microscope, 63x objective, NA 1.3, stack size 34.27 μm, step size 0.35 μm).
Additional file 15. STED microscopy of CAMKIIA in human tissue after hCLARITY. CAMKIIA signal was acquired in confocal (left) and in STED mode (middle and right). Images are shown as MIPs of a stack with z = 3.6 µm. Z-drift was corrected with the Huygens Object Stabilizer (SVI). A higher magnification of a dendrite is shown on the right. Scale bars, 3 µm (left and middle), 1 µm (right). Pixel size for this confocal image is 100 nm, sections were incubated in hydrogel solution for seven days.
Additional file 16: Video. 3D super-resolution microscopy of CAMKIIA and VGLUT1 in the cerebral cortex. CAMKIIA (magenta, A1, 1:200) and VGLUT1-ms (1:200, Synaptic Systems, Cat. No. 135511, RRID: AB_887879) were co-stained. Zoom-in at the end of the video highlights synapses (STED microscope, stack size 2 μm, step size 250 nm). Here, embedding in hydrogel solution took place for 1 day, clearing time was 2 days. Z-planes were adjusted individually for brightness and contrast.
Additional file 17. Original blot for Additional file 6b.
Acknowledgements
We thank Ursula Pika-Hartlaub for excellent technical assistance. We also thank Sarah Mackert, Stefanie Suhm, Harun Asoglu, Helen Bauer, and Emanuel Keller for their support in the initial establishment of the CLARITY method in the laboratory. We thank Prof. Dr. Frank Kirchhoff for permission to use the Zeiss confocal microscope and Prof. Dr. Bernd Knöll for access to the Biorevo microscope. Special thanks go to the body donors of the Institute for Anatomy and Cell Biology and of the tissue bank collection at Ulm University. Without their generosity these kind of studies would not be possible.
Abbreviations
- 2D/3D
Two-/three-dimensional
- a.u.
Arbitrary unit
- AD
Alzheimer’s disease
- ALS
Amyotrophic lateral sclerosis
- alv
Alveus
- APP
Amyloid precursor protein
- Aβ
Amyloid-β
- BA
Brodmann area
- BSA
Bovine serum albumin
- CA
cornu ammonis
- CAA
Cerebral amyloid angiopathy
- CAMKIIA
Calcium/calmodulin-dependent protein kinase II alpha
- CLARITY
Clear Lipid-exchanged Acrylamide-hybridized Rigid Imaging / Immunostaining / In situ hybridization-compatible Tissue-hYdrogel
- CNS
Central nervous system
- COPD
Chronic obstructive pulmonary disease
- CRH
Corticotropin-releasing hormone
- DAB
3,3’-Diaminobenzidine
- DAPI
4’,6-Diamidino-2-phenylindole
- DG
Dentate gyrus
- DPBS
Dulbecco’s phosphate-buffered saline
- dSTORM
Direct stochastical optical reconstruction microscopy
- EFI
Extended focal imaging
- EM
Electron microscopy
- F
Female
- FASTClear
Free of acrylamide SDS-based tissue clearing
- FWHM
Full width at half maximum
- GABA
Gamma-aminobutyric acid
- GAPDH
Glyceraldehyde-3-phosphate dehydrogenase
- GFAP
Glial fibrillary acidic protein
- GLUA2
Glutamate receptor subunit 2
- gp
Guinea pig
- h
Hour
- hCLARITY
Human CLARITY
- hil
Hilus
- HRP
Horseradish peroxidase
- i
Immersion fixation
- IBA1
Ionized calcium-binding adapter molecule 1
- ID
Identifier
- IHC
Immunohistochemistry
- int.
Intensities
- K.O.
Knockout
- LAS-X
Leica Application Suite X
- M
Male
- MAP2
Microtubule-associated protein 2
- MBP
Myelin basic protein
- MIP
Maximum intensity projection
- ms
Mouse
- n.a.
Not available
- NA
Numerical aperture
- NFT
Neurofibrillary tangle
- norm.
Normalized
- NPs
Neuritic plaques
- NTs
Neuropil threads
- p
Perfusion fixation
- PBST
PBS plus Triton X-100
- PD
Parkinson’s disease
- PEG
Polyethylene glycol
- PFA
Paraformaldehyde
- PMI
Post mortem interval
- PSD
Postsynaptic density
- rb
Rabbit
- RI
Refractive index
- RIMS
Refractive index matching solution
- ROI
Region of interest
- RRID
Research Resource Identifier
- S100B
S100 calcium-binding protein B
- SDS
Sodium dodecyl sulfate
- SMA
Smooth muscle actin
- STED
Stimulated emission depletion microscopy
- SYP
Synaptophysin
- TDE
2,2’-Thiodiethanol
- TH
Tyrosine hydroxylase
- VGAT
Vesicular gamma-aminobutyric acid transporter
- VGLUT1
Vesicular glutamate transporter 1
- WD
Working distance
- yrs
Years
Authors’ contributions
TMB and MS designed the study. SW contributed to the details in the design and performed most of the experiments. SF and MS performed some of the experiments. HB and KDT helped to design and analyze the experiments and in the manuscript design. DD, SW, and MS made the STED acquisitions. DD and MS conducted the dSTORM acquisitions. JM supervised all super-resolution microscopy experiments. FR and KD contributed in discussions and ideas. KD gave MS the opportunity to learn the CLARITY method in his lab, and he and his group provided expertise for application to human brain tissue. The first draft of the manuscript was written jointly by SW and MS and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL. TMB is supported by the DFG (Project-ID 251293561 – Collaborative Research Center (CRC) 1149), the Else Kröner Foundation, the Land Baden-Württemberg and he receives funding from the Innovative Medicines Initiative 2 Joint Undertaking under grant agreement No 777394 for the project AIMS-2-TRIALS. This Joint Undertaking receives support from the European Union's Horizon 2020 research and innovation program and EFPIA and AUTISM SPEAKS, Autistica, SFARI. Moreover, funding was received from the Innovative Medicines Initiative 2 Joint Undertaking under grant agreement No 847818 - CANDY. MS receives funding from the Land Baden-Württemberg. JM is supported by the DFG (CRC 1279).
Availability of data and materials
Data not included in the manuscript may be released upon reasonable request to the corresponding author and in compliance with the ethics vote.
Declarations
Ethics approval and consent to participate
This study was performed in compliance with the ethics committee guidelines of Ulm University as well as German federal and state law governing human tissue usage. Informed consent was obtained for all cases.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Grillo F, Pigozzi S, Ceriolo P, Calamaro P, Fiocca R, Mastracci L. Factors affecting immunoreactivity in long-term storage of formalin-fixed paraffin-embedded tissue sections. Histochem Cell Biol. 2015;144(1):93–99. doi: 10.1007/s00418-015-1316-4. [DOI] [PubMed] [Google Scholar]
- 2.Arendt T. Synaptic degeneration in Alzheimer's disease. Acta Neuropathol. 2009;118(1):167–179. doi: 10.1007/s00401-009-0536-x. [DOI] [PubMed] [Google Scholar]
- 3.Clare R, King VG, Wirenfeldt M, Vinters HV. Synapse loss in dementias. J Neurosci Res. 2010;88(10):2083–2090. doi: 10.1002/jnr.22392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Wilde MC, Overk CR, Sijben JW, Masliah E. Meta-analysis of synaptic pathology in Alzheimer's disease reveals selective molecular vesicular machinery vulnerability. Alzheimers Dement. 2016;12(6):633–644. doi: 10.1016/j.jalz.2015.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.DeKosky ST, Scheff SW. Synapse loss in frontal cortex biopsies in Alzheimer's disease: correlation with cognitive severity. Ann Neurol. 1990;27(5):457–464. doi: 10.1002/ana.410270502. [DOI] [PubMed] [Google Scholar]
- 6.Terry RD, Masliah E, Salmon DP, Butters N, DeTeresa R, Hill R, Hansen LA, Katzman R. Physical basis of cognitive alterations in Alzheimer's disease: synapse loss is the major correlate of cognitive impairment. Ann Neurol. 1991;30(4):572–580. doi: 10.1002/ana.410300410. [DOI] [PubMed] [Google Scholar]
- 7.Henstridge CM, Sideris DI, Carroll E, Rotariu S, Salomonsson S, Tzioras M, McKenzie CA, Smith C, von Arnim CAF, Ludolph AC, et al. Synapse loss in the prefrontal cortex is associated with cognitive decline in amyotrophic lateral sclerosis. Acta Neuropathol. 2018;135(2):213–226. doi: 10.1007/s00401-017-1797-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Masliah E, Terry RD, DeTeresa RM, Hansen LA. Immunohistochemical quantification of the synapse-related protein synaptophysin in Alzheimer disease. Neurosci Lett. 1989;103(2):234–239. doi: 10.1016/0304-3940(89)90582-X. [DOI] [PubMed] [Google Scholar]
- 9.Masliah E, Mallory M, Hansen L, DeTeresa R, Alford M, Terry R. Synaptic and neuritic alterations during the progression of Alzheimer's disease. Neurosci Lett. 1994;174(1):67–72. doi: 10.1016/0304-3940(94)90121-X. [DOI] [PubMed] [Google Scholar]
- 10.Masliah E, Mallory M, Alford M, DeTeresa R, Hansen LA, McKeel DW, Jr, Morris JC. Altered expression of synaptic proteins occurs early during progression of Alzheimer's disease. Neurology. 2001;56(1):127–129. doi: 10.1212/WNL.56.1.127. [DOI] [PubMed] [Google Scholar]
- 11.Liu X, Erikson C, Brun A. Cortical synaptic changes and gliosis in normal aging, Alzheimer's disease and frontal lobe degeneration. Dementia. 1996;7(3):128–134. doi: 10.1159/000106867. [DOI] [PubMed] [Google Scholar]
- 12.Leuba G, Savioz A, Vernay A, Carnal B, Kraftsik R, Tardif E, Riederer I, Riederer BM. Differential changes in synaptic proteins in the Alzheimer frontal cortex with marked increase in PSD-95 postsynaptic protein. J Alzheimers Dis. 2008;15(1):139–151. doi: 10.3233/JAD-2008-15112. [DOI] [PubMed] [Google Scholar]
- 13.Poirel O, Mella S, Videau C, Ramet L, Davoli MA, Herzog E, Katsel P, Mechawar N, Haroutunian V, Epelbaum J, et al. Moderate decline in select synaptic markers in the prefrontal cortex (BA9) of patients with Alzheimer's disease at various cognitive stages. Sci Rep. 2018;8(1):938. doi: 10.1038/s41598-018-19154-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koffie RM, Hashimoto T, Tai HC, Kay KR, Serrano-Pozo A, Joyner D, Hou S, Kopeikina KJ, Frosch MP, Lee VM, et al. Apolipoprotein E4 effects in Alzheimer's disease are mediated by synaptotoxic oligomeric amyloid-beta. Brain. 2012;135(Pt 7):2155–2168. doi: 10.1093/brain/aws127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kay KR, Smith C, Wright AK, Serrano-Pozo A, Pooler AM, Koffie R, Bastin ME, Bak TH, Abrahams S, Kopeikina KJ, et al. Studying synapses in human brain with array tomography and electron microscopy. Nat Protoc. 2013;8(7):1366–1380. doi: 10.1038/nprot.2013.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huttenlocher PR. Synaptic density in human frontal cortex - developmental changes and effects of aging. Brain Res. 1979;163(2):195–205. doi: 10.1016/0006-8993(79)90349-4. [DOI] [PubMed] [Google Scholar]
- 17.Scheff SW, DeKosky ST, Price DA. Quantitative assessment of cortical synaptic density in Alzheimer's disease. Neurobiol Aging. 1990;11(1):29–37. doi: 10.1016/0197-4580(90)90059-9. [DOI] [PubMed] [Google Scholar]
- 18.Scheff SW, Price DA, Schmitt FA, Mufson EJ. Hippocampal synaptic loss in early Alzheimer's disease and mild cognitive impairment. Neurobiol Aging. 2006;27(10):1372–1384. doi: 10.1016/j.neurobiolaging.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 19.Scheff SW, Price DA, Schmitt FA, Scheff MA, Mufson EJ. Synaptic loss in the inferior temporal gyrus in mild cognitive impairment and Alzheimer's disease. J Alzheimers Dis. 2011;24(3):547–557. doi: 10.3233/JAD-2011-101782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Neuman KM, Molina-Campos E, Musial TF, Price AL, Oh KJ, Wolke ML, Buss EW, Scheff SW, Mufson EJ, Nicholson DA. Evidence for Alzheimer's disease-linked synapse loss and compensation in mouse and human hippocampal CA1 pyramidal neurons. Brain Struct Funct. 2015;220(6):3143–3165. doi: 10.1007/s00429-014-0848-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Domínguez-Álvaro M, Montero-Crespo M, Blazquez-Llorca L, DeFelipe J, Alonso-Nanclares L. 3D Electron Microscopy Study of Synaptic Organization of the Normal Human Transentorhinal Cortex and Its Possible Alterations in Alzheimer’s Disease. eNeuro. 2019;6(4):ENEURO.0140-19.2019. [DOI] [PMC free article] [PubMed]
- 22.Di J, Cohen LS, Corbo CP, Phillips GR, El Idrissi A, Alonso AD. Abnormal tau induces cognitive impairment through two different mechanisms: synaptic dysfunction and neuronal loss. Sci Rep. 2016;6:20833. doi: 10.1038/srep20833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Penney J, Ralvenius WT, Tsai LH. Modeling Alzheimer's disease with iPSC-derived brain cells. Mol Psychiatry. 2020;25(1):148–167. doi: 10.1038/s41380-019-0468-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chung K, Wallace J, Kim SY, Kalyanasundaram S, Andalman AS, Davidson TJ, Mirzabekov JJ, Zalocusky KA, Mattis J, Denisin AK, et al. Structural and molecular interrogation of intact biological systems. Nature. 2013;497(7449):332–337. doi: 10.1038/nature12107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brown D, Lydon J, McLaughlin M, Stuart-Tilley A, Tyszkowski R, Alper S. Antigen retrieval in cryostat tissue sections and cultured cells by treatment with sodium dodecyl sulfate (SDS) Histochem Cell Biol. 1996;105(4):261–267. doi: 10.1007/BF01463929. [DOI] [PubMed] [Google Scholar]
- 26.Liu AK, Hurry ME, Ng OT, DeFelice J, Lai HM, Pearce RK, Wong GT, Chang RC, Gentleman SM. Bringing CLARITY to the human brain: visualization of Lewy pathology in three dimensions. Neuropathol Appl Neurobiol. 2016;42(6):573–587. doi: 10.1111/nan.12293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Phillips J, Laude A, Lightowlers R, Morris CM, Turnbull DM, Lax NZ. Development of passive CLARITY and immunofluorescent labelling of multiple proteins in human cerebellum: understanding mechanisms of neurodegeneration in mitochondrial disease. Sci Rep. 2016;6:26013. doi: 10.1038/srep26013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morawski M, Kirilina E, Scherf N, Jäger C, Reimann K, Trampel R, Gavriilidis F, Geyer S, Biedermann B, Arendt T, et al. Developing 3D microscopy with CLARITY on human brain tissue: towards a tool for informing and validating MRI-based histology. Neuroimage. 2018;182:417–428. doi: 10.1016/j.neuroimage.2017.11.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McFadden WC, Walsh H, Richter F, Soudant C, Bryce CH, Hof PR, Fowkes M, Crary JF, McKenzie AT. Perfusion fixation in brain banking: a systematic review. Acta Neuropathol Commun. 2019;7(1):146. doi: 10.1186/s40478-019-0799-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Braak H, Del Tredici K. Neuroanatomy and pathology of sporadic Alzheimer's disease. Adv Anat Embryol Cell Biol. 2015;215:1–162. doi: 10.1007/978-3-319-12679-1_1. [DOI] [PubMed] [Google Scholar]
- 31.Tomer R, Ye L, Hsueh B, Deisseroth K. Advanced CLARITY for rapid and high-resolution imaging of intact tissues. Nat Protoc. 2014;9(7):1682–1697. doi: 10.1038/nprot.2014.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang B, Treweek JB, Kulkarni RP, Deverman BE, Chen CK, Lubeck E, Shah S, Cai L, Gradinaru V. Single-cell phenotyping within transparent intact tissue through whole-body clearing. Cell. 2014;158(4):945–958. doi: 10.1016/j.cell.2014.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Braak H, Alafuzoff I, Arzberger T, Kretzschmar H, Del Tredici K. Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathol. 2006;112(4):389–404. doi: 10.1007/s00401-006-0127-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thal DR, Rüb U, Schultz C, Sassin I, Ghebremedhin E, Del Tredici K, Braak E, Braak H. Sequence of Abeta-protein deposition in the human medial temporal lobe. J Neuropathol Exp Neurol. 2000;59(8):733–748. doi: 10.1093/jnen/59.8.733. [DOI] [PubMed] [Google Scholar]
- 35.Thal DR, Rüb U, Orantes M, Braak H. Phases of A beta-deposition in the human brain and its relevance for the development of AD. Neurology. 2002;58(12):1791–1800. doi: 10.1212/WNL.58.12.1791. [DOI] [PubMed] [Google Scholar]
- 36.Braak H, Del Tredici K, Rüb U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol Aging. 2003;24(2):197–211. doi: 10.1016/S0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- 37.Braak H, Thal DR, Ghebremedhin E, Del Tredici K. Stages of the pathologic process in Alzheimer disease: age categories from 1 to 100 years. J Neuropathol Exp Neurol. 2011;70(11):960–969. doi: 10.1097/NEN.0b013e318232a379. [DOI] [PubMed] [Google Scholar]
- 38.Feldengut S, Del Tredici K, Braak H. Paraffin sections of 70–100 µm: a novel technique and its benefits for studying the nervous system. J Neurosci Methods. 2013;215(2):241–4. doi: 10.1016/j.jneumeth.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 39.Braak H. Architectonics of the human telencephalic cortex. Berlin: Springer-Verlag; 1980. [Google Scholar]
- 40.Braak H, Braak E, Ohm T, Bohl J. Silver impregnation of Alzheimer's neurofibrillary changes counterstained for basophilic material and lipofuscin pigment. Stain Technol. 1988;63(4):197–200. doi: 10.3109/10520298809107184. [DOI] [PubMed] [Google Scholar]
- 41.Osseforth C, Moffitt JR, Schermelleh L, Michaelis J. Simultaneous dual-color 3D STED microscopy. Opt Express. 2014;22(6):7028–7039. doi: 10.1364/OE.22.007028. [DOI] [PubMed] [Google Scholar]
- 42.Deshpande D, Higelin J, Schoen M, Vomhof T, Boeckers TM, Demestre M, Michaelis J. Synaptic FUS Localization During Motoneuron Development and Its Accumulation in Human ALS Synapses. Front Cell Neurosci. 2019;13:256. doi: 10.3389/fncel.2019.00256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schoen M, Reichel JM, Demestre M, Putz S, Deshpande D, Proepper C, Liebau S, Schmeisser MJ, Ludolph AC, Michaelis J, et al. Super-Resolution Microscopy Reveals Presynaptic Localization of the ALS/FTD Related Protein FUS in Hippocampal Neurons. Front Cell Neurosci. 2015;9:496. doi: 10.3389/fncel.2015.00496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang Y, Schnitzbauer J, Hu Z, Li X, Cheng Y, Huang ZL, Huang B. Localization events-based sample drift correction for localization microscopy with redundant cross-correlation algorithm. Opt Express. 2014;22(13):15982–15991. doi: 10.1364/OE.22.015982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu XB, Murray KD. Neuronal excitability and calcium/calmodulin-dependent protein kinase type II: location, location, location. Epilepsia. 2012;53(Suppl 1):45–52. doi: 10.1111/j.1528-1167.2012.03474.x. [DOI] [PubMed] [Google Scholar]
- 46.Braak H, Thal DR, Matschke J, Ghebremedhin E, Del Tredici K. Age-related appearance of dendritic inclusions in catecholaminergic brainstem neurons. Neurobiol Aging. 2013;34(1):286–297. doi: 10.1016/j.neurobiolaging.2012.02.031. [DOI] [PubMed] [Google Scholar]
- 47.Morigaki R, Goto S. Putaminal Mosaic Visualized by Tyrosine Hydroxylase Immunohistochemistry in the Human Neostriatum. Front Neuroanat. 2016;10:34. doi: 10.3389/fnana.2016.00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82(4):239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 49.Selkoe DJ. Alzheimer's disease is a synaptic failure. Science. 2002;298(5594):789–791. doi: 10.1126/science.1074069. [DOI] [PubMed] [Google Scholar]
- 50.Ichikawa T, Sekihara S, Ohsako S, Hirata Y, Yamauchi T. Ca2+/calmodulin-dependent protein kinase II in the rat cerebellum: an immunohistochemical study with monoclonal antibodies specific to either alpha or beta subunit. J Chem Neuroanat. 1992;5(5):383–390. doi: 10.1016/0891-0618(92)90054-T. [DOI] [PubMed] [Google Scholar]
- 51.Flace P, Lorusso L, Laiso G, Rizzi A, Cagiano R, Nico B, Ribatti D, Ambrosi G, Benagiano V. Calbindin-D28K Immunoreactivity in the Human Cerebellar Cortex. Anat Rec. 2014;297(7):1306–1315. doi: 10.1002/ar.22921. [DOI] [PubMed] [Google Scholar]
- 52.Chen Y, Andres AL, Frotscher M, Baram TZ. Tuning synaptic transmission in the hippocampus by stress: the CRH system. Front Cell Neurosci. 2012;6:13. doi: 10.3389/fncel.2012.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lai HM, Liu AKL, Ng HHM, Goldfinger MH, Chau TW, DeFelice J, Tilley BS, Wong WM, Wu W, Gentleman SM. Next generation histology methods for three-dimensional imaging of fresh and archival human brain tissues. Nat Commun. 2018;9(1):1066. doi: 10.1038/s41467-018-03359-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Raponi E, Agenes F, Delphin C, Assard N, Baudier J, Legraverend C, Deloulme JC. S100B expression defines a state in which GFAP-expressing cells lose their neural stem cell potential and acquire a more mature developmental stage. Glia. 2007;55(2):165–177. doi: 10.1002/glia.20445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gray H, Lewis WH. Anatomy of the human body. 20. Philadelphia New York: Lea & Febiger; 1918. [Google Scholar]
- 56.Lüllmann-Rauch R. Taschenlehrbuch Histologie. Stuttgart, New York: Georg Thieme Verlag; 2009. [Google Scholar]
- 57.Suarez-Sola ML, Gonzalez-Delgado FJ, Pueyo-Morlans M, Medina-Bolivar OC, Hernandez-Acosta NC, Gonzalez-Gomez M, Meyer G. Neurons in the white matter of the adult human neocortex. Front Neuroanat. 2009;3:7. doi: 10.3389/neuro.05.007.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dunn AR, Hoffman CA, Stout KA, Ozawa M, Dhamsania RK, Miller GW. Immunochemical analysis of the expression of SV2C in mouse, macaque and human brain. Brain Res. 2019;1702:85–95. doi: 10.1016/j.brainres.2017.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vigneault E, Poirel O, Riad M, Prud'homme J, Dumas S, Turecki G, Fasano C, Mechawar N, El Mestikawy S. Distribution of vesicular glutamate transporters in the human brain. Front Neuroanat. 2015;9:23. doi: 10.3389/fnana.2015.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shiraishi-Yamaguchi Y, Furuichi T. The Homer family proteins. Genome Biol. 2007;8(2):206. doi: 10.1186/gb-2007-8-2-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jarius S, Wildemann B. 'Medusa-head ataxia': the expanding spectrum of Purkinje cell antibodies in autoimmune cerebellar ataxia. Part 1: Anti-mGluR1, anti-Homer-3, anti-Sj/ITPR1 and anti-CARP VIII. J Neuroinflammation. 2015;12:166. doi: 10.1186/s12974-015-0356-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dani A, Huang B, Bergan J, Dulac C, Zhuang X. Superresolution imaging of chemical synapses in the brain. Neuron. 2010;68(5):843–856. doi: 10.1016/j.neuron.2010.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Leschik J, Eckenstaler R, Endres T, Munsch T, Edelmann E, Richter K, Kobler O, Fischer KD, Zuschratter W, Brigadski T, et al. Prominent Postsynaptic and Dendritic Exocytosis of Endogenous BDNF Vesicles in BDNF-GFP Knock-in Mice. Mol Neurobiol. 2019;56(10):6833–6855. doi: 10.1007/s12035-019-1551-0. [DOI] [PubMed] [Google Scholar]
- 64.Poguzhelskaya E, Artamonov D, Bolshakova A, Vlasova O, Bezprozvanny I. Simplified method to perform CLARITY imaging. Mol Neurodegener. 2014;9:19. doi: 10.1186/1750-1326-9-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ando K, Laborde Q, Lazar A, Godefroy D, Youssef I, Amar M, Pooler A, Potier MC, Delatour B, Duyckaerts C. Inside Alzheimer brain with CLARITY: senile plaques, neurofibrillary tangles and axons in 3-D. Acta Neuropathol. 2014;128(3):457–459. doi: 10.1007/s00401-014-1322-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Costantini I, Ghobril JP, Di Giovanna AP, Allegra Mascaro AL, Silvestri L, Müllenbroich MC, Onofri L, Conti V, Vanzi F, Sacconi L, et al. A versatile clearing agent for multi-modal brain imaging. Sci Rep. 2015;5:9808. doi: 10.1038/srep09808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liebmann T, Renier N, Bettayeb K, Greengard P, Tessier-Lavigne M, Flajolet M. Three-Dimensional Study of Alzheimer's Disease Hallmarks Using the iDISCO Clearing Method. Cell Rep. 2016;16(4):1138–1152. doi: 10.1016/j.celrep.2016.06.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liu AKL, Lai HM, Chang RC, Gentleman SM. Free of acrylamide sodium dodecyl sulphate (SDS)-based tissue clearing (FASTClear): a novel protocol of tissue clearing for three-dimensional visualization of human brain tissues. Neuropathol Appl Neurobiol. 2017;43(4):346–351. doi: 10.1111/nan.12361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rusch H, Brammerloh M, Stieler J, Sonntag M, Mohammadi S, Weiskopf N, Arendt T, Kirilina E, Morawski M. Finding the best clearing approach - Towards 3D wide-scale multimodal imaging of aged human brain tissue. Neuroimage. 2022;247:118832. doi: 10.1016/j.neuroimage.2021.118832. [DOI] [PubMed] [Google Scholar]
- 70.Magliaro C, Callara AL, Mattei G, Morcinelli M, Viaggi C, Vaglini F, Ahluwalia A. Clarifying CLARITY: Quantitative Optimization of the Diffusion Based Delipidation Protocol for Genetically Labeled Tissue. Front Neurosci. 2016;10:179. doi: 10.3389/fnins.2016.00179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Benda A, Aitken H, Davies DS, Whan R, Goldsbury C. STED imaging of tau filaments in Alzheimer's disease cortical grey matter. J Struct Biol. 2016;195(3):345–352. doi: 10.1016/j.jsb.2016.07.003. [DOI] [PubMed] [Google Scholar]
- 72.Masch JM, Steffens H, Fischer J, Engelhardt J, Hubrich J, Keller-Findeisen J, D'Este E, Urban NT, Grant SGN, Sahl SJ, et al. Robust nanoscopy of a synaptic protein in living mice by organic-fluorophore labeling. Proc Natl Acad Sci U S A. 2018;115(34):E8047–E8056. doi: 10.1073/pnas.1807104115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nair D, Hosy E, Petersen JD, Constals A, Giannone G, Choquet D, Sibarita JB. Super-resolution imaging reveals that AMPA receptors inside synapses are dynamically organized in nanodomains regulated by PSD95. J Neurosci. 2013;33(32):13204–13224. doi: 10.1523/JNEUROSCI.2381-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hruska M, Henderson N, Le Marchand SJ, Jafri H, Dalva MB. Synaptic nanomodules underlie the organization and plasticity of spine synapses. Nat Neurosci. 2018;21(5):671–682. doi: 10.1038/s41593-018-0138-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schubert W, Prior R, Weidemann A, Dircksen H, Multhaup G, Masters CL, Beyreuther K. Localization of Alzheimer beta A4 amyloid precursor protein at central and peripheral synaptic sites. Brain Res. 1991;563(1–2):184–194. doi: 10.1016/0006-8993(91)91532-6. [DOI] [PubMed] [Google Scholar]
- 76.Westmark CJ. What's hAPPening at synapses? The role of amyloid beta-protein precursor and beta-amyloid in neurological disorders. Mol Psychiatry. 2013;18(4):425–434. doi: 10.1038/mp.2012.122. [DOI] [PubMed] [Google Scholar]
- 77.Gutekunst CA, Levey AI, Heilman CJ, Whaley WL, Yi H, Nash NR, Rees HD, Madden JJ, Hersch SM. Identification and localization of huntingtin in brain and human lymphoblastoid cell lines with anti-fusion protein antibodies. Proc Natl Acad Sci U S A. 1995;92(19):8710–8714. doi: 10.1073/pnas.92.19.8710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Braak H, Del Tredici K. Potential Pathways of Abnormal Tau and α-Synuclein Dissemination in Sporadic Alzheimer’s and Parkinson’s Diseases. Cold Spring Harb Perspect Biol. 2016;8(11):a023630. [DOI] [PMC free article] [PubMed]
- 79.Syrbu SI, Cohen MB. An enhanced antigen-retrieval protocol for immunohistochemical staining of formalin-fixed, paraffin-embedded tissues. Methods Mol Biol. 2011;717:101–110. doi: 10.1007/978-1-61779-024-9_6. [DOI] [PubMed] [Google Scholar]
- 80.Robinson JM, Vandre DD. Antigen retrieval in cells and tissues: enhancement with sodium dodecyl sulfate. Histochem Cell Biol. 2001;116(2):119–130. doi: 10.1007/s004180100299. [DOI] [PubMed] [Google Scholar]
- 81.Salameh S, Nouel D, Flores C, Hoops D. An optimized immunohistochemistry protocol for detecting the guidance cue Netrin-1 in neural tissue. MethodsX. 2018;5:1–7. doi: 10.1016/j.mex.2017.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Brettschneider J, Del Tredici K, Toledo JB, Robinson JL, Irwin DJ, Grossman M, Suh E, Van Deerlin VM, Wood EM, Baek Y, et al. Stages of pTDP-43 pathology in amyotrophic lateral sclerosis. Ann Neurol. 2013;74(1):20–38. doi: 10.1002/ana.23937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Querol-Vilaseca M, Colom-Cadena M, Pegueroles J, Nunez-Llaves R, Luque-Cabecerans J, Munoz-Llahuna L, Andilla J, Belbin O, Spires-Jones TL, Gelpi E, et al. Nanoscale structure of amyloid-beta plaques in Alzheimer's disease. Sci Rep. 2019;9(1):5181. doi: 10.1038/s41598-019-41443-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Colom-Cadena M, Pegueroles J, Herrmann AG, Henstridge CM, Munoz L, Querol-Vilaseca M, Martin-Paniello CS, Luque-Cabecerans J, Clarimon J, Belbin O, et al. Synaptic phosphorylated alpha-synuclein in dementia with Lewy bodies. Brain. 2017;140(12):3204–3214. doi: 10.1093/brain/awx275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Shahmoradian SH, Lewis AJ, Genoud C, Hench J, Moors TE, Navarro PP, Castano-Diez D, Schweighauser G, Graff-Meyer A, Goldie KN, et al. Lewy pathology in Parkinson’s disease consists of crowded organelles and lipid membranes. Nat Neurosci. 2019;22(7):1099–1109. doi: 10.1038/s41593-019-0423-2. [DOI] [PubMed] [Google Scholar]
- 86.Moors TE, Maat CA, Niedieker D, Mona D, Petersen D, Timmermans-Huisman E, Kole J, El-Mashtoly SF, Spycher L, Zago W, et al. The subcellular arrangement of alpha-synuclein proteoforms in the Parkinson's disease brain as revealed by multicolor STED microscopy. Acta Neuropathol. 2021;142:423–448. doi: 10.1007/s00401-021-02329-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Improved antibody labeling and better comparability between different cases with CLARITY. (a) Cleared (orange) and non-cleared (blue) frontal cortex sections (superior frontal gyrus) from case i4 (total fixation time 18 years) were stained for CAMKIIA and imaged 15 µm deep in 1.8 µm steps using a confocal microscope. Mean intensities of each z-step were exported with LAS-X software from two stacks (cortex layer V) and the mean was plotted in dependency of the z-level. Z-level I (z = 0 μm), III (z = 3.6 μm), and VI (z = 9 μm) are provided as single images. A side view (xz scan) is shown on the far right. Scale bars, 10 µm. (b) Mean intensity of CAMKIIA staining on case i1 (total fixation time 5 years) was plotted in dependency of the z-level. Acquisition strategy is the same as outlined in panel (a). Scale bars, 10 µm. (c) CAMKIIA staining with DAB on cleared (orange) and non-cleared (blue) sections (case p2). Images represent full focus projections of z-stacks with identical brightness/contrast adjustments. Scale bars, 20 µm. (d) Background levels in secondary antibody only controls do not differ with CLARITY. Graphs show the mean intensities (int.) of the negative controls (secondary antibody only) of minus and plus CLARITY tissue sections; each case is represented by a different symbol (● i1, □ i2, ○ i3, ■ i4, ♦ p1, and Δ p2). The mean intensity of the entire image was determined in ImageJ. Negative control data belonging to CAMKIIA-rb, GLUA2-ms (both imaged using the 635 laser), and MAP2-ms staining (561 laser) are shown. Negative controls were always acquired with identical settings for both conditions (plus/minus CLARITY), mostly corresponding to the staining (in few cases higher laser power), which included the primary antibodies (see Figs. 2 and 3). (e) Second technical replicate. 561 laser: case p1 was excluded since negative controls acquired with identical settings were not available. (f) Comparison of MAP2 labeling on cleared (orange) and non-cleared (blue) sections from the six study cases with different fixation methods and total fixation times (see Table 1). Negative controls are part of panel (d), 561 laser. Scale bars, 10 µm. The codes A1 and F in panels (c) and (d) refer to Fig. 5.
Additional file 2: Video. 3D imaging of AD tau pathology. AT8-positive material (neurofibrillary tangles and neuropil threads) was stained in a 70 µm thick paraffin section derived from the cerebral cortex of an immersion-fixed case (LSM 710 microscope, Zeiss, 40x objective, NA 1.3, stack size 35.18 μm, step size 1.066 μm). Here, embedding in hydrogel solution took place for 11 days, clearing time was 17 days. The section was mounted in FocusClear.
Additional file 3: Video. 3D imaging of Aβ pathology (cored plaque) and VGLUT1. Co-staining of a cerebral cortex section from a perfusion-fixed case with 4G8 (red) and a presynaptic marker (VGLUT1, G5, white) (confocal microscope, 40x objective, NA 1.15, stack size 24.77 μm, step size 0.36 μm).
Additional file 4: Video. 3D imaging of Aβ pathology in vessels (CAA). Immunostaining of a cerebral cortex section from an immersion-fixed case with 4G8 (confocal microscope, 63x objective, NA 1.3, stack size 39.64 μm, step size 0.28 μm).
Additional file 5. Additional CLARITY-compatible antibodies on human brain sections. (a) Neuronal cell markers used to detect specific neurons in cerebellum (A3), hippocampus (A4), and locus coeruleus (A5). Scale bars, 20 µm (A3), 10 µm (A4), 30 µm (A5). (b) Glial cell markers used on frontal cortex sections. Scale bars, 20 µm (C2), 30 µm (D2, D3). (c) Neuronal compartments, e.g., paranodes of axons (E2, exemplarily marked with arrows, asterisks mark nodes of Ranvier), presynapses (G1, G4), and postsynapses (G6, G8) are highlighted in frontal cortex sections (E2, G1), putamen (G4), pallidum (G6), and cerebellum (G8). Scale bars, 3 µm (E2), 5 µm (G1, G4, G6), 20 µm (G8). (d) Vessel-related markers (J1, J2) and GAPDH (K) in cortical sections. Scale bars, 50 µm (J1), 20 µm (J2, K). DAPI counterstaining is depicted in blue. All images except A3 (both) and C2 (right) represent MIPs of z-stacks, K is shown in perspective view. Staining is shown for perfusion-fixed cases (mostly p1 and p2, except A3, G4, and J2). The upper-case letter at the top of each image refers to the entities outlined in Fig. 5a and the combination of letter and number allows for identification of the applied antibody listed in Table 2.
Additional file 6. Cell, compartment, and region specificity of applied antibodies with CLARITY. (a) Co-staining with glial cell markers and two presynaptic markers as proof-of-specificity. DAPI counterstaining is shown in blue. Scale bars, 15 µm (left), 10 µm (middle), 5 µm (right). Images are mainly derived from frontal cortex sections of cases p1 and p2 (C2 C3: underlying white matter). Except C2 C3, images represent MIPs of z-stacks. White color indicates overlap of markers. (b) GLUA2 (G7) was used for western blots with human cerebral cortex lysate (Protein Medley, 50 μg), exposure time was 30 min for GLUA2 and 30 s for β-actin (original blot: Additional file 17). Unit of the ladder is kilodalton. The same antibody is shown in a synaptic staining on frontal cortex sections (case p2). Scale bar, 2 µm. (c) Triple-staining with neuronal, axonal, and synaptic markers in a hippocampal section (case p2). For better visualization CAMKIIA is shown twice, in combination with SMI-312 (left) and with SYP (right). For the latter, an inset of the CA is provided in higher magnification (scale bar, 20 μm). CA = cornu ammonis, DG = dentate gyrus, alv = alveus, hil = hilus. Scale bars, 500 µm. (d) Tissue block with striatum (I, II) and pallidum (III). Immunolabeled sections from this block show matrix (TH high) and striosome (TH low) compartments in the caudate nucleus (ncl.) (I). VGLUT1 expression is strong in putamen (II) and absent in pallidum (III). G5 images represent MIPs of z-stacks. Scale bars, 400 µm (TH), 10 µm (VGLUT1). (e) VGLUT1 expression in the superior frontal gyrus and white matter (top). Intensity profile of the dashed area (raw data), normalized (norm.) to the maximum intensity (bottom). The green line marks the gray/white matter border, which was approximated by the abrupt reduction of the VGLUT1 signal. a.u. = arbitrary unit. Scale bar, 150 µm. Sections in (d) and (e) were derived from cases p1 and p2. The upper-case letter at the top of each image refers to the entities outlined in Fig. 5a and the combination of letter and number allows for identification of the applied antibody listed in Table 2.
Additional file 7. 3D tilescans of neurocellular and synaptic markers in the human cerebellum after CLARITY. (a) CAMKIIA in the cerebellum, MIP of stack with x ~3.2 mm, y ~1.5 mm, and z = 54 µm. Scale bars, tilescan 200 µm, inset 100 µm. Stainings were performed on case p1. (b) 3D tilescan showing MAP2 and HOMER3 (shown as inverted image to highlight the spines of Purkinje cells in the molecular layer) in the human cerebellum, MIP of stack with z = 63 µm (MAP2). Scale bars, tilescan 50 µm, insets 10 µm (top), 5 µm (bottom).
Additional file 8. 3D tilescans unveiling the extensive meshwork formed by GFAP-immunoreactive fibers after CLARITY. (a) Cerebral cortex and adjoining white matter stained for GFAP, MIP of stack with z = 10 µm is shown. Asterisks mark several sites where the blood-brain barrier can be recognized by GFAP-positive extensions wrapping the voids where a vessel runs along. The boxed area is shown in higher magnification in Fig. 5b. Scale bar, 70 µm. (b) Cerebellar section stained with GFAP; dimensions in xyz, x = 848 µm, y = 1.1 mm, z = 56 µm. Scale bars, 100 µm (top), 70 µm (bottom). Sections were derived from perfusion-fixed cases (for panel (b) case p1).
Additional file 9: Video. 3D imaging of GFAP-positive astrocytes and contribution to the blood-brain barrier. Immunostaining of white matter from a perfusion-fixed case with a GFAP antibody (C1, red), counterstaining with DAPI is shown in blue (confocal microscope, 40x objective, NA 1.15, stack size 98 μm, step size 1 μm).
Additional file 10: Video. 3D imaging of MBP. Immunostaining of a cerebral cortex section from case p2 with a MBP antibody (D3). The fibers were traced as surfaces with Imaris software and color-coded according to their z-position (confocal microscope, 40x objective, NA 1.15, stack size 63.53 μm, step size 0.5 μm).
Additional file 11: Video. 3D imaging of MAP2-positive neurons and dendrites. Immunostaining of a cerebral cortex section from case i4 with a MAP2 antibody (F). The video traces MAP2-positive cells and fibers from the outer cortex surface down to the beginning of the white matter (confocal microscope, 40x objective, NA 1.15, stack size 15.01 μm, step size 0.5 μm).
Additional file 12: Video. 3D imaging of HOMER3 in the cerebellum. HOMER3 (G8) is shown in red, DAPI counterstaining in blue, case p1 (confocal microscope, 40x objective, NA 1.15, stack size 47.52 μm, step size 0.22 μm).
Additional file 13: Video. 3D imaging of collagen IV in the cerebral cortex. Collagen IV (J1) is shown in red, DAPI counterstaining in blue, case p2 (confocal microscope, 40x objective, NA 1.15, stack size 46 μm, step size 1 μm).
Additional file 14: Video. 3D imaging of smooth muscle actin (SMA) in the cerebral cortex. SMA (J2) is shown in white, DAPI counterstaining in blue, perfusion-fixed case (confocal microscope, 63x objective, NA 1.3, stack size 34.27 μm, step size 0.35 μm).
Additional file 15. STED microscopy of CAMKIIA in human tissue after hCLARITY. CAMKIIA signal was acquired in confocal (left) and in STED mode (middle and right). Images are shown as MIPs of a stack with z = 3.6 µm. Z-drift was corrected with the Huygens Object Stabilizer (SVI). A higher magnification of a dendrite is shown on the right. Scale bars, 3 µm (left and middle), 1 µm (right). Pixel size for this confocal image is 100 nm, sections were incubated in hydrogel solution for seven days.
Additional file 16: Video. 3D super-resolution microscopy of CAMKIIA and VGLUT1 in the cerebral cortex. CAMKIIA (magenta, A1, 1:200) and VGLUT1-ms (1:200, Synaptic Systems, Cat. No. 135511, RRID: AB_887879) were co-stained. Zoom-in at the end of the video highlights synapses (STED microscope, stack size 2 μm, step size 250 nm). Here, embedding in hydrogel solution took place for 1 day, clearing time was 2 days. Z-planes were adjusted individually for brightness and contrast.
Additional file 17. Original blot for Additional file 6b.
Data Availability Statement
Data not included in the manuscript may be released upon reasonable request to the corresponding author and in compliance with the ethics vote.