Abstract
We investigated the immunogenicity of recombinant double-layered rotavirus-like particle (2/6-VLPs) vaccines derived from simian SA11 or human (VP6) Wa and bovine RF (VP2) rotavirus strains. The 2/6-VLPs were administered to gnotobiotic pigs intranasally (i.n.) with a mutant Escherichia coli heat-labile toxin, LT-R192G (mLT), as mucosal adjuvant. Pigs were challenged with virulent Wa (P1A[8],G1) human rotavirus at postinoculation day (PID) 21 (two-dose VLP regimen) or 28 (three-dose VLP regimen). In vivo antigen-activated antibody-secreting cells (ASC) (effector B cells) and in vitro antigen-reactivated ASC (derived from memory B cells) from intestinal and systemic lymphoid tissues (duodenum, ileum, mesenteric lymph nodes [MLN], spleen, peripheral blood lymphocytes [PBL], and bone marrow lymphocytes) collected at selected times were quantitated by enzyme-linked immunospot assays. Rotavirus-specific immunoglobulin M (IgM), IgA, and IgG ASC and memory B-cell responses were detected by PID 21 or 28 in intestinal and systemic lymphoid tissues after i.n. inoculation with two or three doses of 2/6-VLPs with or without mLT. Greater mean numbers of virus-specific ASC and memory B cells in all tissues prechallenge were induced in pigs inoculated with two doses of SA11 2/6-VLPs plus mLT compared to SA11 2/6-VLPs without mLT. After challenge, anamnestic IgA and IgG ASC and memory B-cell responses were detected in intestinal lymphoid tissues of all VLP-inoculated groups, but serum virus-neutralizing antibody titers were not significantly enhanced compared to the challenged controls. Pigs inoculated with Wa-RF 2/6-VLPs (with or without mLT) developed higher anamnestic IgA and IgG ASC responses in ileum after challenge compared to pigs inoculated with SA11 2/6-VLPs (with or without mLT). Three doses of SA 11 2/6-VLP plus mLT induced the highest mean numbers of IgG memory B cells in MLN, spleen, and PBL among all groups postchallenge. However, no significant protection against diarrhea or virus shedding was evident in any of the 2/6-VLP (with or without mLT)-inoculated pigs after challenge with virulent Wa human rotavirus. These results indicate that 2/6-VLP vaccines are immunogenic in gnotobiotic pigs when inoculated i.n. and that the adjuvant mLT enhanced their immunogenicity. However, i.n. inoculation of gnotobiotic pigs with 2/6-VLPs did not confer protection against human rotavirus challenge.
Rotaviruses are the leading cause of gastroenteritis in infants and young children worldwide (37). Rotavirus particles consist of triple-layered capsids containing a segmented double-stranded RNA genome. The rotavirus core is composed of VP2, which is the most abundant protein of the central core (15% of total virion mass) and a component of the RNA polymerase complex (26). The rotavirus major inner capsid protein is VP6, which is the most abundant structural protein of rotavirus (>50% of total virion mass) (26). VP6 is highly antigenic and contains antigenic determinants shared by all group A rotaviruses and antigenic determinants unique to the subgroup specificity. In general, most animal group A rotaviruses (including SA11) are subgroup I, whereas most human group A rotaviruses (including Wa) are subgroup II (34). The surface layer of the rotavirus capsid is composed of VP7 with VP4 spikes emanating from the outer surface (26). VP7 and VP4 independently induce virus-neutralizing (VN) antibodies (34). Diversity in VP4 and VP7 neutralizing antigenic determinants determines rotavirus P and G serotype specificity, respectively.
The viral proteins and determinants associated with protective immunity against rotavirus have not been fully delineated. Fecal or intestinal immunoglobulin A (IgA) antibodies or antibody-secreting cells (ASC) directed to rotavirus were suggested as correlates of protection in several studies of different species, including humans (15, 20, 28, 42, 64, 71). Neutralizing antibodies to VP4 and VP7 were protective against rotavirus infection in mice, using monoclonal antibodies or recombinant VP4 or VP7 rotavirus proteins (2, 6, 29, 33, 41, 52). Antibodies to VP6 do not neutralize rotaviruses in vitro, and the role of VP6 in inducing protective immunity has not been clearly defined. Several studies have indicated that antibodies to VP6 are protective against rotavirus infection in mice (7, 8, 12, 31). Burns and colleagues (7) reported that IgA monoclonal antibodies to VP6 secreted by hybridoma cell backpack tumors protected adult mice against rotavirus infection following challenge, possibly by intracellular neutralization of rotavirus. Chen et al. (8) and Herrmann et al. (31) reported that microencapsulated murine rotavirus VP6 plasmid DNA administered orally or VP6 plasmid DNA inoculated intradermally via gene gun also induced a high degree of protection against rotavirus shedding in adult mice. However, discordant results have been reported: murine rotavirus VP6 plasmid DNA was antigenic when administered intradermally to mice using a gene gun but failed to protect adult mice against infection following rotavirus challenge (11).
The success in producing different formulations of VLPs by coexpression of different combinations of rotaviral structural proteins in a baculovirus expression system (22) has facilitated studies of potentially more cost-effective and safer, noninfectious rotavirus subunit vaccines (16). The coexpression of VP2 and VP6, the major core and inner capsid proteins, results in their spontaneous assembly into double-layered 2/6-rotavirus-like particles (2/6-VLPs). VLPs are noninfectious because they lack nucleic acid, but they are morphologically and antigenically similar to the native virus (22). The advantages of VLP vaccines may include a lack of side effects seen after oral immunization with live rotavirus vaccines (diarrhea, fever, and possibly intussusception) (1, 66) and their stability during storage (27). Double-layered 2/6-VLPs administered intranasally (i.n.) to adult mice with cholera toxin or mutant Escherichia coli heat-labile toxin (mLT) conferred protection against infection upon challenge with wild-type ECwt murine rotavirus (53, 54). Similarly, double-layered inactivated rotavirus particles purified from cell culture administered i.n. with mLT elicited partial protection against EDIM rotavirus shedding in adult mice (47). In rabbits immunized intramuscularly (i.m.) with SA11 2/6-VLPs in Freund's adjuvant or QS21 (14), protection against ALA lapine rotavirus shedding was variable, with some rabbits having high levels of protection and others showing little to no protection.
Protection against infection, but not disease, was assessed in adult mice or rabbit models because these species are susceptible to rotavirus-induced diarrhea only during the first 2 weeks of life (13, 69). Because such adult mouse or rabbit models cannot be used to assess protection against rotavirus diarrhea, a neonatal gnotobiotic pig model was used in this study to determine if 2/6-VLPs could induce protection against disease. Gnotobiotic pigs present a number of important advantages as an animal model (60, 61) for investigating immune responses to human rotavirus (HRV) and for evaluating vaccine efficacy: they closely resemble humans in gastrointestinal physiology and in the development of mucosal immunity; they are susceptible up to at least 8 weeks of age to infection and disease with several HRV strains (60, 61, 62, 67, 72); they develop histopathologic lesions in the small intestine following HRV infection (67); they are born devoid of maternal antibodies but are immunocompetent, allowing assessment of true primary immune responses (38, 49, 56); and their gnotobiotic status ensures that exposures to extraneous rotaviruses or other enteric pathogens are eliminated as potential confounding variables.
To assess the immunogenicity of 2/6-VLPs, enzyme-linked immunospot (ELISPOT) assays were used to quantitate in vivo antigen-activated ASC (effector B cells) and in vitro antigen-reactivated ASC (derived from memory B cells) from intestinal and systemic lymphoid tissues of gnotobiotic pigs inoculated i.n. with 2/6-VLPs. The mLT LT-R192G was added as a mucosal adjuvant to the 2/6-VLP vaccines administered to subsets of pigs. The mLT reportedly increased immune responses and protection rates in mice vaccinated with VLPs (47, 54).
MATERIALS AND METHODS
VLPs.
VLPs containing VP2 and VP6 of simian SA11 (SA11 2/6-VLPs) and VP2 from bovine RF (39) and VP6 from virulent Wa HRV (Wa-RF 2/6-VLPs) were used to examine if VP6 of different subgroup specificities (subgroups I and II, respectively) influenced the immune responses or protection to challenge with subgroup II Wa HRV. SA11 2/6-VLPs and Wa-RF 2/6-VLPs were produced in Spodoptera frugiperda 9 insect cells. The assembled VLPs were purified using CsCl2 gradients and characterized as previously described (22). Protein concentrations of the purified 2/6-VLPs were determined by the Bio-Rad (Hercules, Calif.) protein assay. The endotoxin level in each 2/6-VLP preparation was quantitated (<0.05 U/dose) with the Limulus amebocyte assay (Associates of Cape Cod, Inc., Woods Hole, Mass.). Electron microscopy or immune electron microscopy was performed on each of the VLP preparations just prior to inoculation to confirm the integrity of the VLPs.
Adjuvant.
LT-R192G was provided by J. Clements (Tulane University Medical Center, New Orleans, La.). This mLT, which has a mutation (from arginine to glycine) at amino acid position 192 of the protein, retains its adjuvant activity but with greatly reduced toxicity (24). The dose of 5 μg for gnotobiotic pigs was determined in preliminary experiments, because a higher dose (10 μg) produced diarrhea in ∼85% of the neonatal pigs.
Virus.
The attenuated (cell culture-adapted) Wa strain (P1A[8],G1) of HRV propagated in monkey kidney (MA104) cells (67, 70) was used for ELISPOT and VN assays. The virulent Wa HRV (intestinal contents from the 16th passage in gnotobiotic pigs) was used to challenge the pigs (67, 71, 72). The 50% infectious dose (ID50) of virulent Wa HRV in piglets was determined as approximately 1 focus-forming unit as previously described (67).
Gnotobiotic pigs.
Near-term pigs were derived by hysterectomy and maintained in isolation units as described previously (50). Pigs were assigned to the following groups as identified in Table 1: (i) two doses of SA11 2/6-VLPs plus mLT (SA11-2+mLT), two doses of SA11 2/6-VLPs alone (SA11-2), two doses of Wa-RF 2/6-VLPs alone (Wa-2), and mock-inoculated twice (mock control) and (ii) three doses of SA11 2/6-VLPs plus mLT (SA11-3+mLT), three doses of Wa-RF 2/6-VLPs plus mLT (Wa-3+mLT), and three doses of mLT alone (mLT control). A subset of mock-inoculated pigs were mock challenged to confirm that under the housing and feeding conditions the pigs did not develop diarrhea spontaneously.
TABLE 1.
Summary of virus shedding and diarrhea in gnotobiotic pigs after challenge with virulent Wa HRVa
| Group
|
Virus
shedding
|
Diarrhea
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Inoculum | No. of doses | mLT | n | % Sheddingb | Mean days to onsetcd | Mean duration in dayscd | Mean peak titer shed (FFU/ml)de | % with diarrheab | Mean duration in daysf | Mean cumulative scoreg |
| SA11 2/6-VLPs | 2 | + | 5 | 100 | 1.4 (0.2) | 2.0 (0.5) | 1.2 × 105 | 100 | 4.2 (0.9) | 14.2 |
| SA11 2/6-VLPs | 2 | − | 5 | 100 | 1.8 (0.2) | 2.6 (0.5) | 4.1 × 103 | 80 | 3.0 (0.8) | 11.0 |
| Wa-RF 2/6-VLPs | 2 | − | 2 | 100 | 2.0 (0.0) | 3.0 (1.0) | 7.7 × 103 | 100 | 3.0 (1.0) | 11.3 |
| Mock controls | 2 | − | 4 | 100 | 2.5 (0.5) | 3.0 (0.8) | 1.2 × 104 | 100 | 3.0 (0.8) | 9.5 |
| Mock controls, mock challenged | 2 | − | 9 | 0 | NAh | NA | NA | 11 | 0.2 (NA) | 4.8 |
| SA11 2/6-VLPs | 3 | + | 5 | 80 | 1.8 (0.2) | 3.0 (0.4) | 2.4 × 105 | 100 | 3.4 (0.8) | 11.9 |
| Wa-RF 2/6-VLPs | 3 | + | 4 | 100 | 2.0 (0.0) | 3.0 (0.6) | 2.5 × 104 | 100 | 4.0 (1.3) | 12.3 |
| mLT controls | 3 | + | 4 | 100 | 1.8 (0.3) | 3.5 (0.3) | 2.5 × 104 | 100 | 4.8 (0.6) | 11.4 |
Pigs were inoculated i.n. two or three times (10 days apart) with 2/6-VLPs with or without mLT and challenged at PID 21 (two doses) or 28 (three doses).
Fisher's exact tests were performed. There were no significant differences among groups.
Determined by ELISA; numbers in parentheses indicate standard error of the mean.
Analysis of variance (general linear models) followed by Duncan's multiple-range tests were performed. There were no significant differences among groups.
Determined by cell culture immunofluorescence infectivity assays: FFU, fluorescent focus-forming units.
Determined by number of days with fecal scores of ≥2. Feces were scored as follows: 0, normal; 1, pasty; 2, semiliquid; 3, liquid. Numbers in parentheses indicate standard error of the mean.
Calculated as (Σ daily fecal scores for 6 days postchallenge)/n.
NA, not applicable.
Inoculation. (i) Experiment I: two doses of SA11 or Wa-RF 2/6-VLPs with or without mLT.
At 3 to 5 days of age and 10 days later, pigs in the SA11-2+mLT group were inoculated i.n. with 250 μg of SA11 2/6-VLPs with 5 μg of mLT. The VLPs and mLT were diluted in 0.5 ml of sterile TNC buffer (10 mM Tris-HCl [pH 7.5], 140 mM NaCl, 10 mM CaCl2) and slowly administered by drops into the nostrils. Group SA11-2 and Wa-2 pigs were similarly inoculated i.n. with 250 μg of SA11 and with Wa-RF 2/6-VLPs without mLT, respectively; mock control pigs were mock inoculated i.n. with an equal volume of TNC buffer.
(ii) Experiment II: three doses of SA11 or Wa-RF 2/6-VLPs with mLT.
At 3 to 5 days of age, the group SA11-3+mLT pigs were inoculated i.n. with 250 μg of SA11 2/6-VLPs plus 5 μg of mLT, followed by two additional doses of SA11 2/6-VLPs plus mLT at postinoculation day (PID) 10 and PID 20. The group Wa-3+mLT pigs were given three doses of 250 μg of Wa-RF 2/6-VLPs plus 5 μg of mLT; the mLT control pigs were given three doses of 5 μg of mLT alone, in the same manner as used for groups SA11-3+mLT and Wa-3+mLT.
Assessment of protection.
At 7 (three-dose regimen) or 11 (two-dose regimen) days after the last inoculation, subsets of pigs from all groups were challenged orally with ∼106 ID50 of virulent Wa HRV (67). This high challenge dose was used previously (71, 72) and in the present study to ensure that nearly 100% of the control pigs developed diarrhea upon challenge. Rectal swabs were collected for 6 days after challenge for assessment of diarrhea and virus shedding. Daily diarrhea scores were based on fecal consistency scored as follows: 0, normal; 1, pasty; 2, semiliquid; and 3, liquid. Pigs with daily fecal consistency scores of ≥2 were considered diarrheic. The cumulative fecal score was calculated as the mean of the sum of the daily fecal scores in each group from postchallenge day (PCD) 1 to 6. Infectious Wa HRV was detected by a cell culture immunofluorescence assay (5), and Wa HRV antigen was detected by an antigen capture enzyme-linked immunosorbent assay (ELISA) from rectal swab fluids as previously described (32, 59).
Plaque reduction VN assay.
Blood samples were collected from all pigs at PID 0, PID 10, and PCD 0 and at the time of euthanasia. The VN titers of sera were determined by a plaque reduction assay as described previously (59, 71).
Isolation of MNC.
A subset of pigs from each group was euthanized, and the small intestines (duodenum and ileum), mesenteric lymph nodes (MLN), spleen, peripheral blood, and bone marrow (BM) were collected at PID 21 (pigs given two doses) or PID 28 (pigs given three doses) and at PCD 7. Mononuclear cells (MNC) from the duodenum, ileum, MLN, spleen, and peripheral blood lymphocytes (PBL) were isolated as described elsewhere (71, 72). BM lymphocytes were collected from the femurs of pigs by flushing the BM cavity with 5 ml of Ca2+-Mg2+-free Hanks balanced salt solution (Gibco BRL, Gaithersburg, Md.). Single-cell suspensions were obtained by passing the solution containing BM through stainless steel 80-mesh screens of a cell collector (Cellecter; E-C Apparatus Corp., St. Petersburg, Fla.), and the MNC were isolated from BM by Ficoll-Hypaque (Ficoll-Paque 1.077; Sigma Chemical Co., St. Louis, Mo.) density gradient centrifugation, similar to the procedure used to isolate MNC from peripheral blood (71).
ELISPOT assay for virus-specific ASC.
An isotype-specific ELISPOT assay for enumerating IgM, IgA, and IgG rotavirus-specific ASC (71, 72) was used to evaluate effector and memory B-cell responses to Wa HRV. The ELISPOT assay performed on the day of MNC extraction was used to determine the numbers of in vivo antigen-activated primary effector B cells (referred to as ASC), because plasma cells secrete antibody spontaneously. The ELISPOT assay performed after MNC were stimulated with rotavirus or mock antigen in cell culture for 5 days was used to quantitate the numbers of effector B cells derived from memory B cells (referred to as memory B cells). Memory B cells were identified on the basis of their ability to proliferate, differentiate, and secrete antibody upon stimulation by the recall Wa HRV antigen (63, 65, 72). The rotavirus antigen used in the in vitro ELISPOT assay to stimulate the in vivo-sensitized MNC for enumeration of memory B cells was prepared from attenuated Wa HRV propagated in MA104 cells and was semipurified by ultracentrifugation through a 40% (wt/vol) sucrose cushion (10). Mock antigen from uninfected MA104 cells was prepared in an identical manner. The duration of antigen stimulation (5 days) and the dose of the semipurified virus antigen added to the MNC cultures (6 μg per 3.75 × 106 MNC) were optimized according to the day and dose that resulted in maximum numbers of memory B cells detected in the ELISPOT assay. Memory B-cell responses were examined only in pigs that received SA11 2/6-VLPs with or without mLT and the mLT controls.
Statistical analysis.
Fisher's exact test (SAS Institute Inc., Cary, N.C.) was used to compare proportions of pigs with diarrhea and virus shedding among groups. One-way analysis of variance followed by Duncan's multiple-range test was used to compare mean days of onset and duration of virus shedding and mean duration and mean cumulative scores of diarrhea. The ASC numbers were compared among groups using the Kruskal-Wallis rank sum (nonparametric) test. Statistical significance was assessed at P < 0.05 for all comparisons.
RESULTS
VN antibody responses.
No pigs developed detectable Wa rotavirus-specific VN antibody responses, as expected, until after challenge. At PCD 7, the geometric mean titers of the VN antibodies were low (ranging from 11 to 22) and did not differ significantly among vaccinated or control groups (data not shown).
ASC responses pre- and postchallenge to two doses of rotavirus 2/6-VLPs and effect of mLT adjuvant.
The immunogenicity of the 2/6-VLPs and the adjuvant effect of mLT were evaluated by enumerating virus-specific ASC responses. No virus-specific ASC were detected prechallenge in the mock control pigs. Rotavirus-specific IgM, IgA, and IgG ASC responses were detected prechallenge in the duodenum and ileum of pigs given either SA11-2+mLT or SA11-2 alone. Only very low or no rotavirus-specific ASC of any isotype were detected prechallenge in MLN, spleen, PBL, or BM of these two groups of pigs except for IgM ASC in the spleen of SA11-2 pigs (Fig. 1). The IgM, IgA, and IgG ASC numbers in the duodenum and ileum of SA11-2+mLT pigs were ∼2- to 9-fold higher than those in the SA11-2 pigs and ranged from 3 to 37 ASC per 5 × 105 MNC. The mean numbers of IgM and IgG ASC in the ileum of SA11-2+mLT pigs were significantly higher than those in the SA11-2 pigs at PID 21 (Fig. 1A).
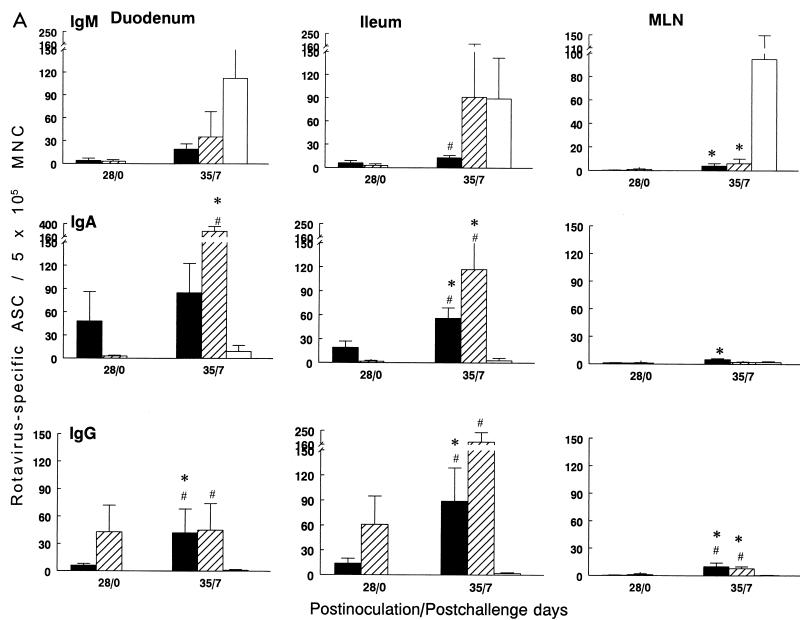
FIG. 1.
Isotype-specific ASC to Wa HRV in gnotobiotic pigs inoculated i.n. with two doses of SA11 2/6-VLPs+mLT (■), SA11 2/6-VLPs alone (▩), or Wa-RF 2/6-VLPs alone (▨) or mock inoculated (□) and challenged with virulent Wa HRV. MNC from duodenum, ileum, and MLN (A) and from spleen, PBL, and BM (B) of pigs were collected and tested by ELISPOT assay on PID 21/PCD 0 and on PID 28/PCD 7, respectively. Data represent the mean number of Wa HRV-specific ASC per 5 × 105 MNC for four to five pigs at each time point, except that only two pigs inoculated with Wa-RF 2/6-VLPs were euthanized at PID 28/PCD 7 and no pigs in this group were euthanized before challenge at PID 21/PCD 0. Error bars represent standard error of the mean. ∗, significant difference (Kruskal-Wallis rank sum test, P < 0.05) in ASC numbers between PID 21/PCD 0 and PID 28/PCD 7 for the same isotype in the same group; @, significant difference (Kruskal-Wallis rank sum test, P < 0.05) in ASC numbers between SA11 2/6-VLPs+mLT and SA11 2/6-VLPs groups for the same isotype at the same time point; #, significant difference (Kruskal-Wallis rank sum test, P < 0.05) in ASC numbers compared to mock controls for the same isotype at the same time point.
After challenge with virulent Wa HRV, significant anamnestic ASC responses were detected in both the SA11-2+mLT and SA11-2 pigs compared to the prechallenge ASC numbers (Fig. 1). In the SA11-2+mLT group, the mean numbers of IgA and IgG ASC in the duodenum and ileum increased 2.5- to 6-fold and ranged from 43 to 92 ASC per 5 × 105 MNC; the IgG ASC in MLN, spleen, and BM increased significantly and ranged from 7 to 34 ASC per 5 × 105 MNC. The numbers of IgG ASC in the duodenum and MLN and the numbers of IgA and IgG ASC in the ileum and spleen of the SA11-2+mLT pigs were significantly higher than those in challenged mock controls at PCD 7 (Fig. 1). In the SA11-2 group, the mean numbers of IgM, IgA, and IgG ASC in the duodenum and ileum increased 11- to 23-fold, ranging from 34 to 98 ASC per 5 × 105 MNC (Fig. 1A). The IgA and IgG ASC numbers in the duodenum and ileum and the IgG ASC numbers in MLN, spleen, and BM of SA11-2 pigs were also significantly higher than those in the challenged mock control pigs at PCD 7 (Fig. 1). Thus, although SA11 2/6-VLPs with mLT were more effective in inducing primary ASC responses in the intestinal lymphoid tissues than 2/6-VLPs without mLT, both regimens effectively primed for anamnestic ASC responses in intestinal and systemic lymphoid tissues after oral challenge with Wa HRV.
The immunogenicity of two doses of Wa-RF VLPs was not analyzed prior to challenge but was analyzed in two pigs following challenge with homologous Wa HRV. The mean numbers of IgM, IgA, and IgG ASC in the duodenum of Wa-2 pigs were comparable to those in the SA11-2 pigs at PCD 7. The numbers of IgA and IgG ASC in the ileum of Wa-2 pigs were higher, but not significantly, than for the SA11-2 pigs at PCD 7 (Fig. 1A).
ASC responses pre- and postchallenge to three doses of SA11 2/6-VLPs+mLT or Wa-RF 2/6-VLPs+mLT.
Three doses of SA11 2/6-VLPs+mLT or Wa-RF 2/6-VLPs+mLT were given to pigs to examine if protection could be induced and whether the number of doses (two versus three) or subgroup specificity of the VLPs and challenge virus influenced the magnitude of the immune responses. No significant protection was evident in any group given three doses of VLPs+mLT (Table 1). No virus-specific ASC were detected prechallenge in the mLT control pigs. Prechallenge, the ASC responses induced by three doses of SA11 2/6-VLPs+mLT in intestinal (Fig. 2A) and systemic lymphoid tissues (Fig. 2B) showed similar patterns and no significant differences in magnitude compared to the SA11-2+mLT group (Fig. 1). Three doses of Wa-RF 2/6-VLPs+mLT induced 4- to 7-fold-higher mean numbers of IgG ASC but 6- to 16-fold-lower mean numbers of IgA ASC in the duodenum and ileum compared to SA11-3+mLT prechallenge.
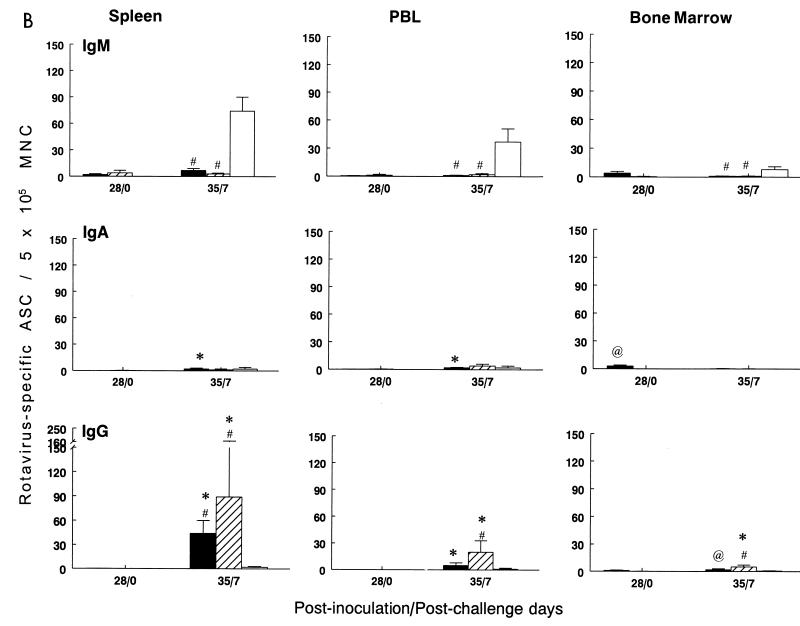
FIG. 2.
Isotype-specific ASC to Wa HRV in gnotobiotic pigs inoculated i.n. with three doses of SA11 2/6-VLPs+mLT (■), Wa-RF 2/6-VLPs+mLT (▨), or mLT control (□) and challenged with virulent Wa HRV. MNC from duodenum, ileum, and MLN (A) and from spleen, PBL, and BM (B) of pigs were collected and tested by ELISPOT assay on PID 28/PCD 0 and on PID 35/PCD 7, respectively. Data represent the mean number of Wa HRV-specific ASC per 5 × 105 MNC for four to five pigs at each time point; error bars represent standard error of the mean. ∗, significant difference (Kruskal-Wallis rank sum test, P < 0.05) in ASC numbers between PID 28/PCD 0 and PID 35/PCD 7 for the same isotype in the same group; @, significant difference (Kruskal-Wallis rank sum test, P < 0.05) in ASC numbers between SA11 2/6-VLPs+mLT and Wa-RF 2/6-VLPs+mLT groups for the same isotype at the same time point; #, significant difference (Kruskal-Wallis rank sum test, P < 0.05) in ASC numbers compared to mLT controls for the same isotype at the same time point.
After challenge, the mean numbers of IgA and IgG ASC in the SA11-3+mLT pigs increased significantly (∼2- to 5-fold for IgA and 5- to at least 44-fold for IgG) compared to prechallenge ASC numbers in most tissues, except IgG in the duodenum and IgG and IgA in BM (Fig. 2). The mean numbers of IgA ASC in the Wa-3+mLT pigs increased significantly in the duodenum and ileum (88- and 59-fold, respectively); IgG ASC increased significantly (5- to at least 89-fold) in MLN, spleen, peripheral blood, and BM postchallenge (Fig. 2). IgG ASC in the duodenum, ileum, MLN, and spleen of SA11-3+mLT pigs and in all tissues of Wa-3+mLT pigs were significantly higher than in the mLT controls postchallenge (Fig. 2). IgA ASC in the ileum of the SA11-3+mLT pigs and in the duodenum and ileum of the Wa-3+mLT pigs were significantly higher than in the corresponding tissues of the mLT controls at PCD 7 (Fig. 2A). Pigs inoculated with Wa-3+mLT and challenged with homologous Wa HRV developed higher, but not significantly, anamnestic IgA and IgG ASC responses in the ileum compared to pigs inoculated with SA11-3+mLT and challenged with Wa HRV.
Rotavirus-specific memory B-cell responses.
To confirm the immunogenicity of the 2/6-VLPs, memory B-cell responses to SA11 2/6-VLPs in the ileum, MLN, spleen, PBL, and BM of pigs inoculated i.n. with SA11 2/6-VLPs with or without mLT (groups SA11-2+mLT, SA11-2, SA11-3+mLT, and mLT controls) were quantitated by ELISPOT assay both before and after challenge with Wa HRV. Before challenge, high numbers of IgM, IgA, and IgG memory B cells (ranging from 39 to 505 ASC per 5 × 105 MNC) were detected in the ileum of pigs given either two or three doses of SA11 2/6-VLPs+mLT (Fig. 3). In contrast, memory B-cell numbers in the ileum of the SA11-2 pigs were 4- to 100-fold less at PID 21/PCD 0 (ranging from 5 to 37 ASC per 5 × 105 MNC) (Fig. 3). The memory B-cell numbers in the MLN, PBL, and BM were minimal (0 to 4 ASC per 5 × 105 MNC) in all three groups inoculated with SA11 VLPs (groups SA11-2+mLT, SA11-2, SA11-3+mLT) and zero in all tissues of the mLT controls.
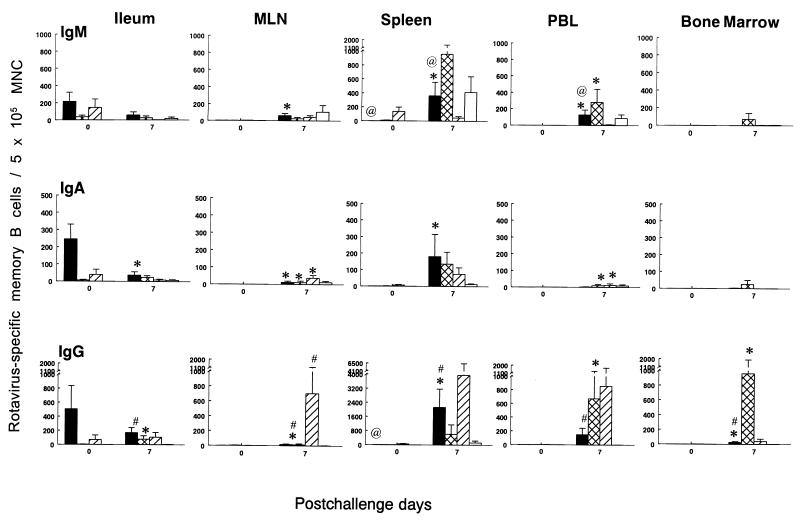
FIG. 3.
Isotype-specific memory B cells to Wa HRV in gnotobiotic pigs inoculated i.n. with two doses of SA11 2/6-VLPs+mLT (■) or SA11 2/6-VLPs alone (▩) or with three doses of SA11 2/6-VLPs+mLT (▨) or mLT control (□) and challenged with virulent Wa HRV. MNC from ileum, MLN, spleen, PBL, and BM of pigs were collected on PCD 0 and PCD 7. After the MNC were restimulated in vitro with semipurified Wa HRV antigen in cell culture for 5 days, isotype-specific ASC which derived from memory B cells were quantitated by ELISPOT assay. The viabilities of MNC from the duodenum were too low for testing after 5 days of in vitro culture. Data represent the mean number of Wa HRV-specific memory B cells per 5 × 105 viable MNC for four to five pigs at each time point; error bars represent standard error of the mean. ∗, significant difference (Kruskal-Wallis rank sum test, P < 0.05) in memory B-cell numbers between PCD 0 and PCD 7 for the same isotype in the same group; @, significant difference (Kruskal-Wallis rank sum test, P < 0.05) in memory B-cell numbers between two- and three-dose SA11 2/6-VLPs+mLT groups for the same isotype at the same time point; #, significant difference (Kruskal-Wallis rank sum test, P < 0.05) in memory B-cell numbers compared to mLT controls for the same isotype at the same time point.
After challenge, IgG ASC were the predominant isotype in the ileum of all groups of pigs inoculated with two or three doses of SA11 2/6-VLPs with or without mLT. Mean numbers of IgA and IgG memory B cells increased in MLN and all systemic lymphoid tissues in the three groups of VLP-inoculated pigs postchallenge. In SA11-2 pigs, the numbers of IgA memory B cells increased significantly postchallenge in the MLN and PBL, whereas the numbers of IgG memory B cells increased significantly in the ileum, PBL, and BM. In SA11-2+mLT pigs, the numbers of IgA memory B cells increased significantly in MLN, spleen, and PBL, whereas the numbers of IgG memory B cells increased significantly in MLN, spleen, and BM. Among the three groups (SA11-2+mLT, SA11-2, and SA11-3+mLT), the highest numbers of IgG memory B cells after challenge were in the MLN, spleen, and PBL of SA11-3+mLT pigs (Fig. 3).
Rotavirus 2/6-VLPs administered i.n. with or without mLT failed to protect pigs against rotavirus challenge. We conducted experiments to determine if two or three doses of 2/6-VLPs with or without mLT induced protection against rotavirus challenge. We also examined if subgroup specificity of the VLPs (SA11-3+mLT versus Wa-3+mLT) influenced protection against Wa HRV challenge. No statistically significant protection was conferred by any of the vaccination regimens. After challenge, mock-inoculated controls and pigs inoculated with mLT alone developed typical patterns of virus shedding and diarrhea as described previously (71, 72). All pigs shed virus except for one of five pigs inoculated with three doses of SA11 2/6-VLPs+mLT (Table 1). All pigs developed diarrhea after challenge except for one of five pigs inoculated with two doses of SA11 2/6-VLPs. There were no significant differences in percentage and mean duration of virus shedding and diarrhea, mean days to onset of shedding, mean peak titer of virus shed, and mean cumulative diarrhea scores (Table 1). Pigs inoculated with SA11 or Wa-RF 2/6-VLPs without mLT shed slightly less virus after challenge (Table 1). Because there were no statistically significant differences among any groups, the results of statistical analysis are not marked in Table 1.
DISCUSSION
The immunogenicity of rotavirus 2/6-VLPs was assessed in a gnotobiotic pig model of rotavirus disease. The VLPs derived from SA11 or Wa-RF rotaviruses and administered i.n. to gnotobiotic pigs induced rotavirus-specific IgM, IgA, and IgG ASC and memory B-cell responses in intestinal lymphoid tissues but minimal or low level ASC and memory B-cell responses in systemic lymphoid tissues before challenge. After challenge with virulent Wa HRV, anamnestic IgA and IgG ASC and memory B-cell responses were detected in intestinal lymphoid tissues of all VLP-inoculated groups. However, the intestinal ASC responses induced by the 2/6-VLP vaccines failed to confer protection against rotavirus challenge.
In pigs inoculated with live, replicating virulent or attenuated Wa HRV, the magnitude of the virus-specific IgA ASC responses in intestinal lymphoid tissues was associated with protection against challenge with virulent Wa HRV. The virulent Wa HRV-inoculated pigs were protected against infection and disease at challenge at PID 21 to 28, which was when the peak virus-specific IgA ASC responses were observed in the intestinal lymphoid tissues (71). The prechallenge peak mean number of intestinal IgA ASC induced by virulent Wa HRV was ∼2-fold higher than the peak mean IgA ASC numbers in the 2/6-VLP vaccinated groups. This lower antibody response induced by VLPs compared to live virulent rotavirus is consistent with observations in mouse studies (47). In mice inoculated orally with live EDIM, the serum IgA antibody titer was 2.8-fold higher than that in mice inoculated i.n. with purified inactivated double-layered EDIM particles plus LT as adjuvant (47). However, inoculation of mice with either live EDIM or purified inactivated double-layered EDIM particles elicited complete protection against virus shedding. The mean numbers of virus-specific IgA ASC in the intestinal lymphoid tissues of pigs inoculated with two doses of attenuated Wa HRV, which conferred partial protection (33% protection rate against diarrhea) (71), were lower, but not significantly, than in pigs inoculated i.n. with two or three doses of SA11 2/6-VLPs+mLT in the present study. However, the IgA ASC responses of pigs to the 2/6-VLPs in this study were not associated with protection against rotavirus diarrhea and shedding. Because the SA11 2/6-VLPs belong to subgroup I and the pigs were challenged with subgroup II Wa HRV, we investigated if differences in subgroup (VP6) specificity affected 2/6-VLP vaccine efficacy and protection in pigs. We found no significant differences between SA11 and Wa-RF 2/6-VLPs in inducing immune responses or protection to Wa HRV challenge.
It is unclear why 2/6-VLPs did not confer protection in neonatal pigs compared to the protection against rotavirus shedding induced by 2/6-VLPs in the adult mouse model (54). In pigs, like in mice, 2/6-VLPs did not induce VN antibodies, as expected, because of the lack of the outer capsid proteins VP4 and VP7, which induce VN responses. Also, inoculation of pigs with 2/6-VLPs did not nonspecifically enhance the VN responses by PCD 7. There are a number of possible reasons for the different results obtained for pigs and mice. First, our findings in pigs suggest that the IgA antibodies induced against VP2 or VP6 in the 2/6-VLPs are not protective against rotavirus-induced diarrhea in pigs and that IgA rotavirus-neutralizing intestinal and/or other (e.g., NSP4) (3, 36) antibodies may be necessary for protective immunity in pigs. Investigation of antibody responses of rabbits immunized with 2/6-VLPs, 2/6/7-VLPs, or 2/4/6/7-VLPs showed that the presence of neutralizing antibodies to VP4 correlated with enhanced protection rates, even though VP4 was not absolutely required to achieve protection from infection in the rabbit model when Freund's adjuvant was used with the VLPs (14).
Second, the doses of VLPs given to mice (10 μg) (53, 54) versus pigs (250 μg) may be a factor because the relative dose of VLPs used in mice (∼0.4 μg/g of body weight) was generally much higher than in pigs (∼0.2, 0.1, or 0.073 μg/g for the first, second, or third dose, respectively). Thus, the lack of protection of 2/6-VLPs in pigs may be due to the reduced relative doses of VLPs in pigs. However, dose studies in adult mice indicated that two i.n. doses of purified, inactivated double-layered rotavirus particles as low as 0.043 μg of VLPs/g of body weight plus 10 μg of mLT induced a 97.5% protection rate against rotavirus shedding (47).
Third, the difference in the doses of mLT used in pigs (5 μg) and mice (10 μg) may also have affected the level of protection obtained. The 5 μg of mLT per dose used in this study did significantly enhance the mucosal ASC responses of pigs to 2/6-VLPs, suggesting that this dose was sufficient for an adjuvant effect but may not have been sufficient to achieve optimal adjuvanticity. However, a 10-μg dose of mLT administered i.n. to neonatal gnotobiotic pigs caused diarrhea in a high percentage of pigs; therefore, doses higher than 5 μg could not be used in the piglets (data not shown).
Challenge doses of virulent rotavirus also differed between the mouse and pig studies. The challenge dose of ECwt rotavirus used in the study by O'Neal et al. (54) was 101 50% shedding dose (SD50). In subsequent studies, mice were immunized i.n. with 10 μg of 2/6-VLPs with mLT and challenged with 101, 102.3, or 104 SD50 of homologous murine ECwt rotavirus. Although protection from 104 SD50 was decreased compared to challenge with 101 SD50, protective efficacy was still high (∼70%) (C. M. O'Neal et al., unpublished data). The challenge dose of virulent Wa HRV used in our previous studies (71, 72) and in the present study was 106 ID50. The purpose of using this dose of heterologous (Wa) challenge virus was to ensure that nearly 100% of naive age-matched control pigs developed consistent infection and diarrhea. To induce consistent clinical responses in challenged pigs, generally a higher dose of heterologous virus than of homologous virus is required, similar to findings in mice (28, 35). Also, the use of outbred gnotobiotic pigs (as for humans) is likely to result in responses more variable than those seen in studies using inbred mice strains (44, 45, 46, 47). Additionally, from a clinical standpoint, children naturally infected with rotavirus shed massive amounts of rotavirus in stools, up to 1010 to 1011 particles per g of stool for 3 to 7 days, equating to 106 particles in 0.1 μg of stool (19). Because children may be commonly exposed to such large amounts of virus, to evaluate the protective efficacy of vaccines, it is highly relevant to assess protection against a dose of challenge virus more comparable to that likely to be encountered under natural conditions. Moreover, we have previously shown that prior infection of neonatal pigs with virulent Wa HRV will protect pigs to a high degree (87% to 100%) against challenge with this same dose (106 ID50) of homologous virulent Wa HRV for at least 3 to 4 weeks postinoculation (71), further confirming that infection with virulent rotavirus can confer protective immunity.
Although i.n. immunization was used in both mice and gnotobiotic pigs, there was a difference in that mice were anesthetized (54) or sedated (47) for i.n. inoculation but pigs were not. This may also have contributed to differences in results seen between mice and pigs. In mice immunized i.n. with human papillomavirus type 16 VLPs, immunization with and without anesthesia resulted in different patterns of immune responses (25). Higher titers of IgG antibodies in serum and IgA antibodies in vaginal secretions were detected in mice inoculated i.n. with VLPs while anesthetized, compared to titers in nonanesthetized mice at the time of immunization. In the present study, pigs were not anesthetized to mimic the i.n. approach for vaccinating human infants. Significant intestinal effector and memory B-cell responses were induced following this method of i.n. inoculation of pigs, indicating the efficacy of the i.n. route for stimulating mucosal immune responses.
The pathogenesis of rotavirus disease in pigs and probably in human infants differs from that in mice (19, 57, 58); therefore, the mechanisms of protective immunity may differ in these models. Rotavirus infection of young pigs and human infants induces watery diarrhea, anorexia, depression, and dehydration (19, 48, 57). Pronounced histologic changes (e.g., villous atrophy as a result of virus replication and epithelial cell lysis) occurs in gnotobiotic pigs infected with virulent Wa HRV (67), and similar intestinal villous atrophy has been reported for human infants (19, 23, 58). In mice under 15 days of age, homologous rotavirus infection causes diarrhea and lethargy but only mild intestinal lesions, consisting of vacuolization of villous tip epithelial cells with little or no villous atrophy (40). In adult mice, rotavirus infection occurs without causing disease or histopathologic changes (69). Such differences may be important to consider in extrapolating challenge results from the mouse model in which only protection against rotavirus shedding can be evaluated.
Finally, differences in the protective efficacy of 2/6-VLPs in pigs and mice may also have been influenced by the differences in timing of the immunizations and rotavirus challenge. Mice received three doses i.n. of 2/6-VLPs with mLT at 0, 14, and 45 PID and were challenged 1 month after the last vaccine dose (54). This vaccination and challenge schedule may have maximized the immune responses by the time of challenge, resulting in protection from rotavirus shedding in mice. In pigs infected with Wa HRV, or mice and rabbits infected with homologous live rotavirus, optimal antibody titers are achieved at PID 21 to 28 (17, 18, 71). However, the kinetics of the immune response to nonreplicating vaccines is likely much different. Typically, only low levels of antibody responses are observed after the first dose of nonreplicating rotavirus immunogens, and antibody responses increase following the second or subsequent doses administered i.m. or i.n. to rabbits, mice, or pigs (17, 21, 64; M. Ciarlet et al., unpublished data). Therefore, timing of vaccine doses relative to each other and to the rotavirus challenge are likely to be critical for nonreplicating vaccines. Although in the present study and previously, we used an immunization and challenge schedule shorter than that used for mice with inactivated rotavirus or VLP vaccines, this shorter two- or three-dose schedule evoked at least moderate to high antibody responses to nonreplicating rotavirus vaccines but failed to induce significant protection in gnotobiotic pigs (64, 72). An inactivated rotavirus vaccine administered i.m. to pigs by the same schedule as the current VLP vaccines induced extremely high VN and ELISA IgG rotavirus antibody titers in serum and high numbers of IgG ASC in systemic lymphoid tissues but little or no protection (64, 72). Similarly, in the present study, two doses of SA11 2/6-VLPs without mLT and in the previous study (71) two doses of attenuated Wa HRV induced similar mean numbers of IgA ASC in the intestines of pigs, but only the attenuated Wa HRV induced partial (33%) protection against disease (71). Two or three doses of SA11 2/6-VLPs+mLT induced even higher mean numbers of IgA ASC in the intestines of pigs compared to pigs inoculated with three doses of attenuated Wa HRV, but again, only the latter induced partial (62%) protection against diarrhea in pigs (Yuan et al., unpublished data), suggesting that the difference in VLP immunization and challenge schedule alone may not entirely explain the failure of 2/6-VLP to confer protection in pigs. Thus, whether simply increasing the intervals between inoculation and challenge using nonreplicating vaccines in the pig model would induce antibody responses higher than those induced previously and greater protection is unclear.
There are numerous reports of protective immunity against rotavirus infection in mice induced by various routes of inoculation using different forms of rotavirus antigen (e.g., live or inactivated, homologous or heterologous rotavirus [16, 28, 44, 45, 46, 51]); recombinant rotaviral proteins or VLPs [12, 53, 54]; and DNA plasmids [8, 9, 31]). To date, the protective efficacy of the inactivated or 2/6-VLP rotavirus vaccines in the adult mouse model (protection against infection) did not predict the protective efficacy against rotavirus disease in the neonatal pig model. Protection of neonatal pigs against rotavirus diarrhea may require much higher levels of intestinal IgA antibodies or IgA antibodies with neutralizing specificity against VP4 or VP7 than protection of adult mice against rotavirus infection. However, both virulent Wa and live EDIM rotaviruses induced in the intestinal lamina propria IgA antibodies which were correlated with protection of mice and gnotobiotic pigs, respectively (15, 28, 71). The association between local (fecal) IgA antibody responses to HRV and protection against rotavirus disease has also been reported after natural rotavirus infection of children (20, 42, 43, 55, 68) and from some oral rotavirus vaccine trials in humans (4, 30).
Protection induced by the i.n. route with live or inactivated rotavirus, either triple- or double-layered particles, was not included as a control in this study in pigs. Therefore, it is difficult to know whether the lack of protection in pigs compared to mice is due to differences in the ability of pigs and mice to respond to immunogens administered by this route with mLT or due to the differences discussed earlier. Future studies will examine the immunogenicity and protective efficacy of Wa 2/4/6/7-VLPs and will include a few pigs inoculated with purified inactivated triple- or double-layered Wa HRV as controls. Because 2/4/6/7-VLPs induce VN antibodies, this will allow assessment of their role in conferring protection. Also, by determining protein-specific ASC responses to 2/4/6/7-VLPs, the correlation between antibodies against individual rotavirus proteins and protection can be assessed.
In conclusion, 2/6-VLPs administered i.n. to pigs were immunogenic and induced ASC and memory B-cell responses but did not induce protection. Our findings indicate that the levels of protection induced by 2/6-VLPs differ between the adult mouse model of rotavirus shedding and neonatal pig model of rotavirus-induced diarrhea.
ACKNOWLEDGMENTS
We thank J. Clements (Tulane University Medical Center, New Orleans, La.) for providing the LT-R192G used in this study. We thank Kelly Warfield and Julie Thompson (Baylor College of Medicine, Houston, Tex.) for preparing the SA11 VLPs. We also thank Kathy Gadfield, Peggy Lewis, and Paul Nielsen for technical assistance.
This work was supported by grants from the National Institutes of Health (RO1AI33561 and RO1AI37111). Salaries and research support were provided by state and federal funds appropriated to the Ohio Agricultural Research and Development Center, The Ohio State University.
REFERENCES
- 1.Advisory of Committee on Immunization Practices (ACIP) Withdrawal of rotavirus vaccine recommendation. Morbid Mortal Wkly Rep. 1999;48:1007. [PubMed] [Google Scholar]
- 2.Andrew M E, Boyle D B, Coupar B E H, Reddy D, Bellamy A R, Both G W. Vaccinia-rotavirus VP7 recombinants protect mice against rotavirus-induced diarrhea. Vaccine. 1992;10:185–191. doi: 10.1016/0264-410x(92)90010-h. [DOI] [PubMed] [Google Scholar]
- 3.Ball J M, Tian P, Zeng C Q, Morris A P, Estes M K. Age-dependent diarrhea induced by a rotaviral nonstructural glycoprotein. Science. 1996;272:101–104. doi: 10.1126/science.272.5258.101. [DOI] [PubMed] [Google Scholar]
- 4.Bernstein D I, Smith V E, Sander D S, Pax K A, Schiff G M, Ward R L. Evaluation of WC3 rotavirus vaccine and correlates of protection in healthy infants. J Infect Dis. 1990;162:1055–1062. doi: 10.1093/infdis/162.5.1055. [DOI] [PubMed] [Google Scholar]
- 5.Bohl E H, Saif L J, Theil K W, Agnes A G, Cross R F. Porcine pararotavirus: detection, differentiation from rotavirus, and pathogenesis in gnotobiotic pigs. J Clin Microbiol. 1982;15:312–319. doi: 10.1128/jcm.15.2.312-319.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Both G W, Lockett L J, Janardhana V, Edwards S J, Bellamy A R, Graham F L, Prevec L, Andrew M E. Protective immunity to rotavirus-induced diarrhea is passively transferred to newborn mice from naïve dams vaccinated with a single dose of a recombinant adenovirus expressing rotavirus VP7sc. Virology. 1993;193:940–950. doi: 10.1006/viro.1993.1203. [DOI] [PubMed] [Google Scholar]
- 7.Burns J W, Siadat-Pajouh M, Krishnaney A A, Greenberg H B. Protective effect of rotavirus VP6-specific IgA monoclonal antibodies that lack neutralizing activity. Science. 1996;272:104–107. doi: 10.1126/science.272.5258.104. [DOI] [PubMed] [Google Scholar]
- 8.Chen S C, Jones D H, Fynan E F, Farrar G H, Clegg J C S, Greenberg H B, Herrmann J E. Protective immunity induced by oral immunization with a rotavirus DNA vaccine encapsulated in microparticles. J Virol. 1998;72:5757–5761. doi: 10.1128/jvi.72.7.5757-5761.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen S C, Fynan E F, Robinson H L, Lu S, Greenberg H B, Santoro J C, Herrmann J E. Protective immunity induced by rotavirus DNA vaccines. Vaccine. 1997;15:899–902. doi: 10.1016/s0264-410x(96)00272-1. [DOI] [PubMed] [Google Scholar]
- 10.Chen W K, Campbell T, VanCott J, Saif L J. Enumeration of isotype-specific antibody-secreting cells derived from gnotobiotic piglets inoculated with porcine rotaviruses. Vet Immunol Immunopathol. 1995;45:265–284. doi: 10.1016/0165-2427(94)05343-Q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Choi A H-C, Knowlton D R, McNeal M M, Ward R L. Particle bombardment-mediated DNA vaccination with rotavirus VP6 induces high levels of serum rotavirus IgG but fails to protect mice against challenge. Virology. 1997;232:129–138. doi: 10.1006/viro.1997.8552. [DOI] [PubMed] [Google Scholar]
- 12.Choi A H-C, Basu M, McNeal M M, Clements J D, Ward R L. Antibody-independent protection against rotavirus infection of mice is stimulated by intranasal immunization with chimeric VP4 or VP6 proteins. J Virol. 1999;73:7574–7581. doi: 10.1128/jvi.73.9.7574-7581.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ciarlet M, Gilger M A, Barone C, McArthur M, Estes M K, Conner M E. Rotavirus disease, but not infection and development of intestinal histopathological lesions, is age restricted in rabbits. Virology. 1998;251:343–360. doi: 10.1006/viro.1998.9406. [DOI] [PubMed] [Google Scholar]
- 14.Ciarlet M, Crawford S E, Barone C, Bertolotti-Ciarlet A, Ramig R F, Estes M K, Conner M E. Subunit rotavirus vaccine administered parenterally to rabbits induces active protective immunity. J Virol. 1998;72:9233–9246. doi: 10.1128/jvi.72.11.9233-9246.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coffin S E, Moser C A, Cohen S, Clark H F, Offit P A. Immunologic correlates of protection against rotavirus challenge after intramuscular immunization of mice. J Virol. 1997;71:7851–7856. doi: 10.1128/jvi.71.10.7851-7856.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Conner M E, Zarley C D, Hu B, Parsons S, Drabinski D, Greiner S, Smith R, Jiang B, Corsaro B, Barniak V, Madore H P, Crawford S, Estes M K. Virus-like particles as a rotavirus subunit vaccine. J Infect Dis. 1996;174(Suppl. 1):S88–S92. doi: 10.1093/infdis/174.supplement_1.s88. [DOI] [PubMed] [Google Scholar]
- 17.Conner M E, Gilger M A, Estes M K, Graham D Y. Serologic and mucosal immune response to rotavirus infection in the rabbit model. J Virol. 1991;65:2562–2571. doi: 10.1128/jvi.65.5.2562-2571.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Conner M E, Estes M, Offit P, Clark H F, Franco M, Feng N, Greenberg H B. Development of a mucosal rotavirus vaccine. In: Kiyono H, McGhee J R, Ogra P L, editors. Mucosal vaccines. San Diego, Calif: Academic Press; 1996. pp. 325–344. [Google Scholar]
- 19.Conner M E, Ramig R F. Viral enteric diseases. In: Nathanson N, et al., editors. Viral pathogenesis. Philadelphia, Pa: Lippincott-Raven; 1997. pp. 713–742. [Google Scholar]
- 20.Coulson B S, Grimwood K, Hudson I L, Barnes G L, Bishop R F. Role of coproantibody in clinical protection of children during reinfection with rotavirus. J Clin Microbiol. 1992;30:1678–1684. doi: 10.1128/jcm.30.7.1678-1684.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Crawford S E, Estes M K, Ciarlet M, Barone C, O'Neal M C, Cohen J, Conner M E. Heterotypic production and induction of a broad heterotypic neutralization response by rotavirus-like particles. J Virol. 1999;73:4813–4822. doi: 10.1128/jvi.73.6.4813-4822.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Crawford S E, Labble M, Cohen J, Burroughs M H, Zhou Y J, Estes M K. Characterization of virus-like particles produced by the expression of rotavirus capsid proteins in insect cells. J Virol. 1994;68:5945–5922. doi: 10.1128/jvi.68.9.5945-5952.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Davidson G P, Barnes G I. Structural and functional abnormalities of the small intestine in infants and young children with rotaviral enteritis. Acta Paediatr Scand. 1979;68:181–186. doi: 10.1111/j.1651-2227.1979.tb04986.x. [DOI] [PubMed] [Google Scholar]
- 24.Dickinson B L, Clements J D. Dissociation of Escherichia coliheat-labile enterotoxin adjuvanticity from ADP-ribosyltransferase activity. Infect Immun. 1995;63:1617–1623. doi: 10.1128/iai.63.5.1617-1623.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dupuy C, Buzoni-Gatel D, Touze A, Bout D, Coursaget P. Nasal immunization of mice with human papillomavirus type 16 (HRV-16) virus-like particles or with HPV-16 L1 gene elicits specific cytotoxic T lymphocytes in vaginal draining lymph nodes. J Virol. 1999;73:9063–9071. doi: 10.1128/jvi.73.11.9063-9071.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Estes, M. K. Rotaviruses and their replication, p. 1625–1655. In B. N. Fields et al. (ed.), Virology. Lippincott-Raven, Philadelphia, Pa.
- 27.Estes M K, Ball J M, Crawford S E, O'Neal C, Opekun A A, Graham D Y, Conner M E. Virus-like particle vaccines for mucosal immunization. Adv Exp Med Biol. 1997;412:387–395. doi: 10.1007/978-1-4899-1828-4_61. [DOI] [PubMed] [Google Scholar]
- 28.Feng N, Burns J, Bracy L, Greenberg H. Comparison of mucosal and systemic humoral immune responses and subsequent protection in mice orally inoculated with a homologous or a heterologous rotavirus. J Virol. 1994;68:7766–7773. doi: 10.1128/jvi.68.12.7766-7773.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fiore L, Dunn S J, Ridolfi B, Ruggeri F M, Mackow E R, Greenberg H B. Antigenicity, immunogenicity and passive protection induced by immunization of mice with baculovirus-expressed VP7 protein from rhesus rotavirus. J Gen Virol. 1995;76:1981–1988. doi: 10.1099/0022-1317-76-8-1981. [DOI] [PubMed] [Google Scholar]
- 30.Flores J, Perez-Schael I, Blanco M, Rojas A M, Alfonzo E, Crespo I, Cunto W, Pittman A L, Kapikian A Z. Reactogenicity and immunogenicity of a high-titer rhesus rotavirus-based quadrivalent rotavirus vaccine. J Clin Microbiol. 1993;31:2439–2445. doi: 10.1128/jcm.31.9.2439-2445.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Herrmann J E, Chen S C, Fynan E F, Santoro J C, Greenberg H B, Wang S, Robinson H L. Protection against rotavirus infections by DNA vaccination. J Infect Dis. 1996;174(Suppl.):S93–S97. doi: 10.1093/infdis/174.supplement_1.s93. [DOI] [PubMed] [Google Scholar]
- 32.Hoblet K H, Saif L J, Kohler E M, Theil K W, Bech-Nielsen S, Stitzlein G A. Efficacy of an orally administered modified-live porcine origin rotavirus vaccine against postweaning diarrhea inpigs. Am J Vet Res. 1986;47:169–1703. [PubMed] [Google Scholar]
- 33.Hoshino Y, Saif L J, Sereno M M, Chanock R M, Kapikian A Z. Infection immunity of piglets to either VP3 or VP7 outer capsid protein confers resistance to challenge with a virulent rotavirus bearing the corresponding antigen. J Virol. 1988;62:744–748. doi: 10.1128/jvi.62.3.744-748.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hoshino Y, Kapikian A Z. Rotavirus antigens. In: Ramig R F, editor. Rotavirus. New York, N.Y: Springer-Verlag; 1994. pp. 179–227. [DOI] [PubMed] [Google Scholar]
- 35.Ishida S I, Feng N, Gilbert J M, Tang B, Greenberg H B. Immune responses to individual rotavirus proteins following heterologous and homologous rotavirus infection in mice. J Infect Dis. 1997;175:1317–1323. doi: 10.1086/516462. [DOI] [PubMed] [Google Scholar]
- 36.Johansen K, Hinkula J, Espinoza F, Levi M, Zeng C, Ruden U, Vesikari T, Estes M, Svensson L. Humoral and cell-mediated immune responses in humans to the NSP4 enterotoxin of rotavirus. J Med Virol. 1999;59:369–377. [PubMed] [Google Scholar]
- 37.Kapikian A Z, Chanock R M. Rotaviruses. In: Fields B N, et al., editors. Virology. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 1657–1708. [Google Scholar]
- 38.Kim Y M. Development immunity in the piglet. Birth Defects. 1980;11:549–557. [PubMed] [Google Scholar]
- 39.Labbe M, Charpilienne A, Crawford S E, Estes M K, Cohen J. Expression of rotavirus VP2 produces empty corelike particles. J Virol. 1991;65:2946–2952. doi: 10.1128/jvi.65.6.2946-2952.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Little L M, Shadduck J A. Pathogenesis of rotavirus infection in mice. Infect Immun. 1982;38:755–763. doi: 10.1128/iai.38.2.755-763.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mackow E R, Vo P T, Broome R, Bass D, Greenberg H B. Immunization with baculovirus-expressed VP4 protein passively protects against simian and murine rotavirus challenge. J Virol. 1990;64:1698–1703. doi: 10.1128/jvi.64.4.1698-1703.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Matson D O, O'Ryan M, Herrera I, Pickering L, Estes M K. Fecal antibody responses to symptomatic and asymptomatic rotavirus infections. J Infect Dis. 1993;167:577–583. doi: 10.1093/infdis/167.3.577. [DOI] [PubMed] [Google Scholar]
- 43.Matson D O, Velazquez F R, Calva J J, Morrow A L, Lourdes Guerrero L, Shults J N, Estes M K, Carter-Campbell S, Glass R I, Pickering L K, Ruiz-Palacios G M. Protective immunity against group A rotavirus infection and illness in infants. Arch Virol. 1996;12(Suppl.):129–139. doi: 10.1007/978-3-7091-6553-9_15. [DOI] [PubMed] [Google Scholar]
- 44.McNeal M M, Sheridan J, Ward R L. Active protection against rotavirus infection of mice following intraperitoneal immunization. Virology. 1992;191:150–157. doi: 10.1016/0042-6822(92)90176-p. [DOI] [PubMed] [Google Scholar]
- 45.McNeal M M, Rae M N, Conner M E, Ward R L. Stimulation of local immunity and protection in mice by intramuscular immunization with triple- or double-layered rotavirus particles and QS-21. Virology. 1998;243:158–166. doi: 10.1006/viro.1998.9060. [DOI] [PubMed] [Google Scholar]
- 46.McNeal M M, Rae M N, Ward R L. Effects of different adjuvants on rotavirus antibody responses and protection in mice following intramuscular immunization with inactivated rotavirus. Vaccine. 1999;17:1573–1580. doi: 10.1016/s0264-410x(98)00359-4. [DOI] [PubMed] [Google Scholar]
- 47.McNeal M M, Rae M N, Bean J A, Ward R L. Antibody-dependent and -independent protection following intranasal immunization of mice. J Virol. 1999;73:7565–7563. doi: 10.1128/jvi.73.9.7565-7573.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McNulty M S, Pearson G R, McFerran J B, Collins D S, Allan G M. A reovirus-like agent (rotavirus) associated with diarrhoea in neonatal pigs. Vet Microbiol. 1976;1:55–63. [Google Scholar]
- 49.Mehrazar K, Kim Y B. Total parenteral nutrition in germfree colostrum-deprived neonatal miniature piglets: a unique model to study the ontogeny of the immune response. J Parenter Enter Nutr. 1988;12:563–568. doi: 10.1177/0148607188012006563. [DOI] [PubMed] [Google Scholar]
- 50.Meyer R C, Bohl E H, Kohler E M. Procurement and maintenance of germfree swine for microbiological investigation. Appl Microbiol. 1964;12:295–300. doi: 10.1128/am.12.4.295-300.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Offit P A, Dudzik K I. Noninfectious rotavirus (strain RRV) induces an immune response in mice which protects against rotavirus challenge. J Clin Microbiol. 1989;27:885–888. doi: 10.1128/jcm.27.5.885-888.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Offit P A, Shaw R, Greenberg H B. Protection against rotavirus-induced gastroenteritis in newborn mice by monoclonal antibodies to surface proteins VP3 and VP7. J Virol. 1986;58:700–703. doi: 10.1128/jvi.58.2.700-703.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.O'Neal C M, Crawford S E, Estes M K, Conner M E. Rotavirus virus-like particles administered mucosally induce protective immunity. J Virol. 1997;71:8707–8717. doi: 10.1128/jvi.71.11.8707-8717.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.O'Neal C M, Clements J D, Estes M K, Conner M E. Rotavirus 2/6 viruslike particles administered intranasally with cholera toxin, Escherichia coliheat-labile toxin (LT), and LT-R192G induce protection from rotavirus challenge. J Virol. 1998;72:3390–3393. doi: 10.1128/jvi.72.4.3390-3393.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.O'Ryan M, Matson D O, Estes M K, Pickering L. Anti-rotavirus G type-specific and isotype-specific antibodies in children with natural rotavirus infection. J Infect Dis. 1994;169:504–511. doi: 10.1093/infdis/169.3.504. [DOI] [PubMed] [Google Scholar]
- 56.Phillips R W, Tumbleson M E. Models. In: Tumbleson M E, editor. Swine in biomedical research—1986. New York, N.Y: Plenum Press; 1986. pp. 437–440. [Google Scholar]
- 57.Saif L J, Rosen B I, Parwani A V. Animal rotaviruses. In: Kapikian A L, editor. Viral infections of the gastrointestinal tract. New York, N.Y: Marcel Dekker, Inc.; 1994. pp. 279–367. [Google Scholar]
- 58.Saif L J, Greenberg H G. Rotavirus gastroenteritis. In: Conner D H, et al., editors. Pathology of infectious diseases. Stamford, Conn: Appleton & Lange; 1997. pp. 297–302. [Google Scholar]
- 59.Saif L J, Redman D R, Smith K L, Theil K W. Passive immunity to bovine rotavirus in newborn calves fed colostrum supplements from immunized or nonimmunized cows. Infect Immun. 1983;41:1118–1131. doi: 10.1128/iai.41.3.1118-1131.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Saif L J, Ward L A, Yuan L, Rosen B I, To T L. The gnotobiotic pig as a model for studies of disease pathogenesis and immunity to rotavirus. Arch Virol Suppl. 1996;12:153–161. doi: 10.1007/978-3-7091-6553-9_17. [DOI] [PubMed] [Google Scholar]
- 61.Saif L J, Yuan L, Ward L A, To T. Comparative studies of the pathogenesis, antibody immune responses, and homologous protection to porcine and human rotaviruses in gnotobiotic piglets. In: Paul P S, et al., editors. Mechanisms in the pathogenesis of enteric diseases. New York, N.Y: Plenum Press; 1997. pp. 397–403. [DOI] [PubMed] [Google Scholar]
- 62.Schaller J P, Saif L J, Cordle C T, et al. Prevention of human rotavirus-induced diarrheal in gnotobiotic piglets using bovine antibody. J Infect Dis. 1992;165:623–630. doi: 10.1093/infdis/165.4.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Slifka M K, Ahmed R. Limiting dilution analysis of virus-specific memory B cells by an ELISPOT assay. J Immunol Methods. 1996;199:37–46. doi: 10.1016/s0022-1759(96)00146-9. [DOI] [PubMed] [Google Scholar]
- 64.To L T, Ward L A, Yuan L, Saif L J. Serum and intestinal isotype antibody responses and correlates of protective immunity to human rotavirus in a gnotobiotic pig model of disease. J Gen Virol. 1998;79:2662–2672. doi: 10.1099/0022-1317-79-11-2661. [DOI] [PubMed] [Google Scholar]
- 65.VanCott J, Brim T A, Simkins R A, Saif L J. Isotype-specific antibody-secreting cells to transmissible gastroenteritis virus and porcine respiratory coronavirus in gut- and bronchus-associated lymphoid tissues of sucking pigs. J Immunol. 1993;150:3980–3990. [PubMed] [Google Scholar]
- 66.Vesikari T, Joensuu J. Review of rotavirus vaccine trials in Finland. J Infect Dis. 1996;174:S81–S87. doi: 10.1093/infdis/174.supplement_1.s81. [DOI] [PubMed] [Google Scholar]
- 67.Ward L A, Rosen B I, Yuan L, Saif L J. Pathogenesis of an attenuated and a virulent strain of group A human rotavirus in neonatal gnotobiotic pigs. J Gen Virol. 1996;77:1431–1441. doi: 10.1099/0022-1317-77-7-1431. [DOI] [PubMed] [Google Scholar]
- 68.Ward R L. Mechanisms of protection against rotavirus in humans and mice. J Infect Dis. 1996;174(Suppl. 1):S51–S58. doi: 10.1093/infdis/174.supplement_1.s51. [DOI] [PubMed] [Google Scholar]
- 69.Ward R L, McNeal M M, Sheridan J F. Development of an adult mouse model for studies on protection against rotavirus. J Virol. 1990;64:5070–5075. doi: 10.1128/jvi.64.10.5070-5075.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wyatt R G, James W D, Bohl E H, Theil K W, Saif L J, Kalica A R, Greenberg H B, Kapikian A Z, Chanock R M. Human rotavirus type 2: cultivation in vitro. Science. 1980;207:189–191. doi: 10.1126/science.6243190. [DOI] [PubMed] [Google Scholar]
- 71.Yuan L, Ward L A, Rosen B I, To T L, Saif L J. Systemic and intestinal antibody-secreting cell responses and correlates of protective immunity to human rotavirus in a gnotobiotic pig model of disease. J Virol. 1996;70:3075–3083. doi: 10.1128/jvi.70.5.3075-3083.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yuan L, Kang S-Y, Ward L A, To T L, Saif L J. Antibody-secreting cell responses and protective immunity assessed in gnotobiotic pigs inoculated orally or intramuscularly with inactivated human rotavirus. J Virol. 1998;72:330–338. doi: 10.1128/jvi.72.1.330-338.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]