Abstract
Stable cell lines that express a gene of specific interest provide an advantage over transient gene expression by reducing variations in transfection efficiency between experiments, sustaining expression for long-term studies, and controlling expression levels in particular if a clonal population is selected. Transient transfection requires introduction of an exogenous gene into host cells via typically harsh chemicals or conditions that permeabilize the cell membrane, which does not normally integrate into the target cell genome. Here, we describe the method of using retroviral transduction to stably express Golgi proteins fused to a promiscuous biotin ligase (TurboID) in HeLa cells, thus creating cell lines that can be leveraged in studies of the proximome/interactome. We also demonstrate a similar protocol for stable expression of a Golgi protein fused to a fluorescent tag via lentiviral transduction. These methods can be further adapted to establish other cell lines with different sub-cellular markers or fusion tags. Viral transduction is a convenient method to create stable cell lines in cell-based studies.
Keywords: Retrovirus, Lentivirus, Stable cell line, Proximal biotinylation, TurboID, GRASP55, GRASP65, Transduction, Virus packaging
1. Introduction
Viral vectors offer stable integration of a gene of interest into a host genome, allowing for greater reproducibility when repeating experiments. They can also deliver genes into hard-to-transfect cells such as neurons and primary cells. In recent years, viral vectors have become a staple in the molecular toolbox of research laboratories to deliver nucleic acids in both tissue culture and animal models. However, the nuances of different viral vectors and important considerations to troubleshoot viral transductions are less explicitly highlighted in the literature. In this chapter, we provide a detailed guide for generating stable cell lines expressing recombinant Golgi proteins through retroviral and lentiviral transduction.
1.1. Overview
There are many types of viral transduction systems (Table 1), each with unique attributes that will determine whether the system is suitable for the desired application. Factors in consideration of viral transduction systems include packaging capacity, tropism, transduction efficiency, integration, and immunogenicity. For example, viruses such as adenoviruses, which have high transduction rates but limited space for genes and regulatory units, are useful for transient expression in vivo [1]. Of the viruses capable of integrating into the host cell genome, two members in the Retroviridae family—lentiviruses and γ-retroviruses—are most frequently used to generate stable cell lines [2, 3].
Table 1.
Summary of viral transduction systems
| Adenovirus | AAV | ?-retrovirus | Lentivirus | Baculovirus | Vaccinia virus |
Herpex simplex virus |
|
|---|---|---|---|---|---|---|---|
| Genome (kb) | dsDNA (8 kb–36 kb) | ssDNA (4.8 kb–8.5 kb) | ssRNA (7 kb–20 kb) | ssRNA (9 kb) | dsDNA (80 kb–180 kb) | dsDNA (190 kb) | dsDNA (150 kb) |
| Packaging capacity | 7.5 kb–8 kb | 4.5 kb–5 kb | 6 kb–10 kb | 8 kb–9 kb | < 38 kb | 25 kb | 30 kb–40 kb |
| Transduction | Dividing/non-dividing cells | Dividing/non-dividing cells | Dividing cells | Dividing/non-dividing cells | Dividing/non-dividing cells | Dividing cells | Dividing/non-dividing cells |
| Transduction efficiency | High | Moderate | Moderate | Moderate | Low | ||
| Expression | Transient | Transient or stable | Stable | Stable | Transient or stable | Transient | Transient |
| Integration | Non-integrating | Non-integrating | Integrating | Integrating | Integrating | Integrating | Non-integrating |
| Biosafety level | BSL-2 | BSL-1 | BSL-2 | BSL-2 | BSL-2 | BSL-2 | BSL-2 |
| Immunogenicity High | Low | Moderate–high | Moderate–high | Moderate–high | |||
| Gene therapy strategy | In vivo | In vivo | Ex vivo | Ex vivo | In vivo | In vivo & Ex vivo | Ex vivo |
| Superinduction | No | Yes | |||||
| Additional notes | Elicits strong antiviral immune response. Higher titer. Toxicity. | Requires helper virus for replication; difficult to produce pure viral stocks. Small genome limits size of foreign DNA. | All viral genes removed. Transduction requires cell division. Relatively low titer. | Insertional mutagenesis potential. | Limited mammalian host range. | Potential cytopathic effects. | No gene expression during latent infection. |
Table 1 summarizes key features of different viral transduction systems to help choose the appropriate viral vector. Transduction efficiency and packaging capacity are two important factors that dictate the successful insertion of a gene of interest. Inserts that are very large can be accommodated by adenoviruses when special serotyped helper packaging cells lines are used in conjunction with the viral vector. Some viral vectors are non-integrating and therefore only suitable for transient expression, while others can integrate genes into the host genome for stable expression. High immunogenicity can cause toxicity in some systems and may need to be optimized to achieve desired expression levels without inducing immunogenic side effects. The powerful viral transduction system can even be compatible with animal models and thus are useful tools to express a gene of interest in vivo
As retroviruses, both lentiviruses and γ-retroviruses can retro-transcribe their RNA genome into a cDNA copy that is then incorporated into the target cell genome by the integrase enzyme, making them excellent tools to deliver desired DNA fragments to target cells for heritable and stable expression of the gene of interest. In addition to exogenous gene expression, both vectors can be used to express short hairpin RNAs (shRNAs) to silence the expression of target genes [3]. However, a major difference lies between lentiviruses and γ-retroviruses in their replication cycle. γ-retroviruses can only integrate the cDNA into dividing cells since the viral dsDNA of γ-retroviruses is not able to pass through the nuclear pore complex and requires the disintegration of the nuclear membrane during mitosis. In contrast, the pre-integration complex of lentiviruses is transported into the nucleus and therefore can integrate the transgene into both dividing and non-dividing cells [4].
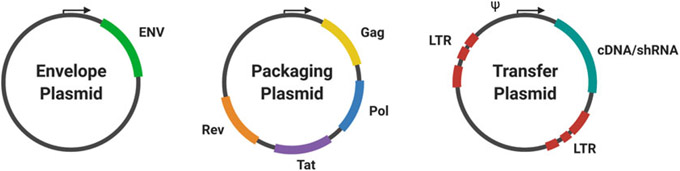
A key safety feature shared by lentiviral and retroviral gene delivery systems is that through trans-complementation, they are self-inactivating and will not continue viral replication once inside the target cells [5]. Lentiviruses and γ-retroviruses use gag, pol, and env genes to produce a fully functional virion. These retroviral components are separated into different plasmids when used in the laboratory. A packaging cell line co-transfected with helper packaging and envelope plasmids (which contain the viral capsid and envelope protein, respectively) in conjunction with the transfer plasmid (which contains the viral genome and the sequence of interest) will produce high-titer, replication-incompetent viruses that are then harvested and used to transduce the target cells (Fig. 1).
Fig. 1.
Overview of viral plasmid system. The three components, envelope, viral packaging genes, and gene of interest, are supplied by three types of plasmids, which are co-transfected into the packaging cell line. This system provides the flexibility to pseudotype viruses using different envelopes to modify tropism
1.2. Parts of the System—Plasmids and Packaging Cell Line
The viral env gene determines the viral infectivity range. Viral vectors can be pseudotyped with different coat proteins to alter their tropism [6]. While amphotropic viruses can infect most mammalian cells, ectopic pseudotyped viruses can only infect mouse or rat cells. Pantropic viruses can infect cells of any species. Most often pantropic viral vectors use G glycoprotein ofthe vesicular stomatitis virus (VSV-G) as the envelope coat protein. Other common envelope proteins are derived from rabies virus, Ebola, filovirus, measles virus, and baculovirus. In some cases, particular envelope proteins can enhance transduction of specific cell types. For example, filovirus envelope pseudotyping enhances transduction of airway epithelial and endothelial cells. It is important to appropriately pseudotype the virus for the intended target cells (Table 2).
Table 2.
Viral envelope proteins
| Virus | Envelope | Receptor | Cell-type specificity | Ref. |
|---|---|---|---|---|
| gp160 (gp42 and gp120) | CD4, CD5/CXCR4 | T cells, monocytes | gp160 (gp42 and gp120) | [10] |
| Retroviruses | ||||
| Murine leukemia virus (MLV) | gp70 (SU and TM) | mCAT-1 | Hematopoietic cells, fibroblasts | [11-13] |
| Gibbon ape leukemia virus (GALV) | gp70 (SU and TM) | Pit1 | Hematopoietic cells | [14, 15] |
| Feline leukemia virus | gp70 (SU and TM) | SLC1A5, hASCT2 | Hematopoietic cells | [15, 16] |
| Amphotropic retrovirus (Ampho) | gp70 (SU and TM) | RAM1 | Hematopoietic cells | [17, 18] |
| 10A1 MLV(10A1) | gp70 (SU and TM) | Pit1, Pit2 | Hematopoietic cells | [17,18] |
| Ecotropic retrovirus (Eco) | gp70 (SU and TM) | Rec1 | Hematopoietic cells | [17,19, 20] |
| Baboon ape leukemia virus (BaEV) | gp70 (SU and TM) | hASCT2 | Hematopoietic cells | [21] |
| Paramyxoviruses | ||||
| Measles virus (MV) | H and F | CD46, SLAM | Lymphocytes, dendritic cells | [22, 23] |
| Nipah virus (NiV) | H and F | EphrinB2 | Embryonic stem cells, hematopoietic stem cells | [24, 25] |
| Rhabdoviruses | ||||
| Rabies virus (RabV) | G | P75NTR, NCAM, nAchR | Neural cells | [26-28] |
| Mokola virus (MOKV) | G | Not known | Neural cells | [29, 30] |
| Ebola Zaire virus (EboZ) | G | NPC1, TIM1 | Hematopoietic cells, lung epithelial cells | [31] |
| Lymphocytic choriomeningitis virus (LCMV) | GP1 and GP2 | α-dystroglycan | Dendritic cells, hepatocytes | [32] |
| Baculovirus | GP64 | Heparan sulfate, phospholipids | Fibroblasts, epithelial cells, hepatocytes | [33] |
| Alphaviruses | ||||
| Chikungunya virus (CHIKV) | E1and E2 | Potential involvement of heparin sulfate and integrins | Hematopoietic cells, fibroblasts, hepatocytes | [34,35] |
| Ross River virus (RRV) | E1 and E2 | Potential involvement of heparin sulfate and integrins | Fibroblasts, neuroglial cells, hepatocytes, Kupffer cells | [36] |
| Semliki Forest virus (SFV) | E1 and E2 | Potential involvement of heparin sulfate and integrins | Fibroblasts, neuroglial cells, hepatocytes, Kupffer cells | [36] |
| Sindbis virus (SV) | E1 and E2 | Potential involvement of heparin sulfate and integrins | Hematopoietic cells, fibroblasts, hepatocytes | [37] |
| Venezuelan equine encephalitis virus (VEEV) | E1 and E2 | Potential involvement of heparin sulfate and integrins | Fibroblasts, hepatocytes | [37] |
| Western equine encephalitis virus (WEEV) | E1 and E2 | Potential involvement of heparin sulfate and integrins | [37] | |
| Orthomyxoviruses | ||||
| Influenza (A-D) | HA | Sialic acid | Airway epithelia | [38] |
| Fowl Plague virus (FPV) | HA | Sialic acid | Airway epithelia | [39] |
| Vesiculoviruses | ||||
| Vesicular stomatitis virus | VSV-G | LDLR | Broad tropism | [40] |
| Chandipura virus and Piry virus | CNV-G and PRV-G | Not known | Neural cells | [41] |
Table 2 describes the various envelope proteins that can be used to pseudotype different viruses and alter their infectivity range. Some envelope proteins can render the virus more cell-specific, while others like the commonly used VSV-G have broad tropism
The packaging plasmid usually encodes the essential gag and pol viral proteins, as well as tat and rev in some systems. Gag is a polyprotein that is then cleaved into the matrix protein, capsid, and nucleocapsid of the virion. Pol is processed from the gag-pol polyprotein into the viral enzymatic proteins: reverse transcriptase, integrase, and protease. Tat and rev upregulate transcription of genomic RNA and enhance export ofthe mRNAs from the nucleus to the cytoplasm [7]. However, in newer generations of lentiviral systems, tat is removed and Rev is placed on a separate plasmid to increase safety. Table 3 describes additional features that may be found on viral vectors.
Table 3.
Common features in viral vectors
| Name | Function |
|---|---|
| 5’LTR | 5′ long terminal repeat. Cloning capacity between the LTR’s is ~8.5 kb, but inserts larger than ~3 kb are packaged less efficiently. |
| SIN/LTR | 3′ self-inactivating long terminal repeat |
| Ori | Origin of replication |
| Ampicillin or kanamycin resistance | Ampicillin- or kanamycin-resistant gene for bacterial selection |
| Psi (ψ) | RNA packaging signal |
| RRE | Rev response element |
| cPPT | Central polypurine tract |
| hPGK | Human phosphoglycerate kinase eukaryotic promoter |
| WPRE Element | Woodchuck hepatitis post-transcriptional regulatory |
| SV40 polyadenylation signal | Enables efficient termination of transcription and processing of recombinant transcripts |
| puroR | Puromycin-resistant gene for mammalian selection |
| pUC origin | Allows high copy replication and plasmid maintenance in E. coli cells |
| SV40 origin | Provides for stable propagation of the plasmid in packaging cells |
| F1 Ori | Origin of replication |
| U3 | Contains sequences necessary for activation of viral genomic RNA transcription |
| R | Repeat region found within the 5′ and 3′ LTR’s of retro/lentiviral vectors. Tat binds to this region. |
| U5 | Unique 5′; region at the 5′ end of the viral genomic RNA (but found at both the 5′ and 3′ ends of the provirus). |
| TAR | Trans-activating response element; located in the R region of the LTR and acts as a binding site for Tat. |
| Gag | Precursor structural protein of the lentiviral particle containing matrix, capsid, and nucleocapsid components |
| Pol | Precursor protein containing reverse transcriptase and integrase components |
| 3′ LTR | Terminate transcription started by 5′ LTR by the addition of a poly A just after the R sequence |
| Rev | Binds to the Rev. response element (RRE) within unspecified and partially spliced transcripts to facilitate nuclear export |
| Tat | Trans-activator; binds TAR to activate transcription from the LTR promoter |
| VSV-G | Vesicular stomatitis virus G glycoproteins, broad tropism envelope protein used to pseudotype most lentiviral vectors. |
Table 3 lists the elements found in viral vectors (on packing, transfer, and envelope plasmids). These features have specific functions that allow for the assembly ofinfectious virus and integration of recombinant DNA into the target cell genome
The promoter should be taken into consideration when choosing a transfer plasmid. A promoter that engages RNA Pol II (e.g., CMV) is more suitable for driving protein-coding RNA expression, while RNA pol III promoters are better suited for expression of shRNAs. Some examples of commonly used transfer plasmids are included in Table 4. The three plasmids (packaging, envelope, and transfer plasmids) are co-transfected into a packaging cell line to produce infectious virions for transducing target cells. Commonly used packaging cell lines are listed in Table 5. Some HEK293-derived cell lines, like Phoenix cells, carry the two helper constructs (env and gag-pol) as episomes for long-term stable production of retrovirus. With these types of cell lines, only the transfer vector is required during the transfection step to package the virus, although transfecting all three constructs may still increase the viral titer.
Table 4.
Common transfer vectors for lentivirus and retrovirus
| Retrovirus | Use | Backbone | Selection |
| Retroviral vector for shRNA expression | pMKO.1 | Puromycin, neomycin | |
| Retroviral expression | pBABE | Hygromycin, puromycin | |
| Mammalian retroviral gene expression with GFP | MSCV-IRES-GFP | GFP | |
| Lentivirus | Lentiviral expression | pWPXL | GFP |
| Lentiviral shRNA expression | pLKO.1 | Puromycin | |
| Tet-inducible lentiviral shRNA expression | Tet-pLKO | Puromycin | |
| cDNA expression | FUGW | Bleomycin | |
| Conditional shRNA expression under the Cre-Lox control | pSico | Puromycin | |
| cDNA expression | pLJM1-EGFP | Puromycin |
Table 4 describes popular viral transfer vectors that are compatible with retroviral and lentiviral systems for the expression of cDNA or shRNA. Some have features for doxycycline-inducible or Cre-Lox control for conditional expression. Information on the selection marker of the plasmid is also provided
Table 5.
Common packaging cell lines
| Name | Feature |
|---|---|
| Lenti-X 293 T | 6× more virus production than 293FT and 30× more virus than 293 cells |
| Lenti-Pac 293 Ta | Produces high titers |
| 293LTV | 293-derived, firm attachment to plates, faster growth rate, produces high titers |
| 293TN | Highly transfectable, produces high titers |
| Platinum Retroviral Packaging | 293 T derived, produces high titers, longer stability without drug selection, three versions: ecotropic (Plat-E), amphotropic (Plat-A), and pantropic (Plat-GP) |
| 293RTV | Retroviral expression and packaging cell line, faster cell growth, higher retrovirus titer, firmer attachment to plates |
| Phoenix-AMPHO | Amphotropic |
Table 5 contains information on commonly used packaging cell lines mostly derived from HEK293 T to achieve higher titers or for longer term virus packing. These cell lines express viral genes (gag, pol) as episomes and therefore are more efficient than other cell lines that require co-transfection of helper constructs to package virus. Some key features related to their viral packaging efficiencies are highlighted in the table for each cell line
2. Materials
This section contains the materials we used in our experiments. While we included the catalog numbers to make it clear of the materials we used, equivalent items from different companies can be used.
2.1. Cell Culture
Fetal bovine serum (FBS): Hyclone Bovine Calf Serum Supplemented (Thermo Fisher, Cat# SH30072.03).
PenStrep: Gibco Penicillin Streptomycin (Thermo Fisher, Cat# 15140).
GlutaMAX: Gibco GlutaMAXTM-I (100×) (Thermo Fisher, Cat# 35050).
Trypsin (MP Biomedicals LLC, Cat# 153571).
Accugene 0.5 M EDTA Solution (Lonza Bioscience, Cat# 51201).
DMEM: Gibco Dulbecco’s Modified Eagle Medium (Thermo Fisher, Cat# 11995).
Phoenix-AMPHO cell (ATCC, Cat# CRL-3213).
Hygromycin B (Fisher, Cat# 10687010).
HEK293 T (ATCC, Cat# CRL-3216).
- Tissue culture-treated dishes.
- 3.5 cm: BioLite 35 mm Tissue Culture Dish (Thermo Fisher, Cat# 13080)
- 6 cm: BioLite 60 mm Tissue Culture Dish (Thermo Fisher, Cat# 13081)
- 10 cm: BioLite 100 mm Tissue Culture Dish (Thermo Fisher, Cat# 13082)
- 15 cm: BioLite 150 mm Tissue Culture Dish (Thermo Fisher, Cat# 13083)
- BioLite 6-Well Multidish (Thermo Fisher, Cat# 13084).
- BioLite 12-Well Multidish (Thermo Fisher, Cat# 13085).
#1–1/2 Micro-Coverglass—12 mm Dia (Electron Microscopy Sciences, Cat# 72230-01).
2.2. Plasmids
Viral envelope plasmid: pCMV-VSV-G; ampicillin-resistant (Addgene, Cat# 8454).
gag-pol plasmid: pUMVC, kanamycin-resistant (Addgene, Cat# 8449).
- Transfer vectors (cloned in the laboratory and confirmed by DNA sequencing):
- pBabe-(puro)-V5-TurboID,
- pBabe-(puro)-GRASP55-V5-TurboID,
- pBabe-(puro)-GRASP65-V5-TurboID.
2.3. Plasmid Amplification
TOP10 Chemically Competent E. coli (Fisher, Cat# C404010).
LB Agar plates (Fisher, Cat# BP-1423-500).
LB Broth (American Bioanalytical, Cat# AB01201-01000).
Ampicillin (Fisher, Cat# BP1760-5).
Kanamycin (Fisher, Cat# BP906-5).
Cell spreader (Fisher, Cat# 07-201-930).
Erlenmeyer flask (Fisher, Cat# S63273).
Nalgene bottle (Fisher, Cat# 3141-0500).
Zymo Research “ZymoPURE™ II Plasmid Midiprep Kit” (Zymo Research, Cat#s d4200 & D4201).
NanoDropTM Lite Spectrophotometer (Fisher, Cat# BZB8917251).
Sorvall LYNX 6000 Ultracentrifuge.
2.4. Transfection and Transduction
FuGENE HD ® Transfection Reagent (Promega, Cat# E2311/2).
Polybrene Transfection Reagent (Millipore, Cat# TR-1003-G).
Puromycin, 10 mg/mL, (Invitrogen, Cat# J67236).
1 mg/mL polyethylenimine (PEI) (Polysciences, Inc. Cat# 23966-2)
5–10 mL sterile syringe (Fisher, Cat# 14-955-458)
Cytiva Whatman™ Uniflo 0.45 μm polypropylene (PES) syringe filter (Cytiva, Cat# 09-928-063).
2.5. Validation
NeutrAvidin™ Texas Red™ conjugate (Neutravidin-TxRed), 1 mg, (Invitrogen, Cat# A2665).
Streptavidin-HRP (Invitrogen, Cat# SA1001).
Bovine serum albumin: albumin, bovine fraction V (BSA) (DOT Scientific Inc., Cat# 9048-46-8).
4% paraformaldehyde (formaldehyde) aqueous solution (PFA) (EMS, Cat# 15710).
Triton X-100 (Fisher, Cat# AC215682500).
Glycerol (Fisher, Cat# PI17904).
Ammonium chloride (Fisher, Cat# A661-500).
Hydrogen peroxide (Fisher, Cat# P170-500).
Intercept® (TBS) Protein-Free Blocking Buffer (Li-Cor, Cat# 927-80001).
SuperSignal West Pico PLUS Chemiluminescent Substrate (Thermo Scientific, Cat# 34580).
Protease inhibitors: protease inhibitor cocktail (Bimake.com, Cat# B14001).
Cell scrapers (Fisher Scientific, Cat# 50-101-128).
Protein Assay Dye Reagent Concentrate (Bio-Rad Laboratories, Cat# 5000006).
Filter paper: Blotting Paper 703 (VWR, Cat# 28298-020).
Nitrocellulose: Amersham Protran 0.45 NC 300 mm × 4 m 1 roll/PK (Cytiva Life Sciences, Cat# 10600002).
Bis-acrylamide: 30% Acrylamide/Bis Solution 37.5:1 (BIO-RAD, Cat# 1610158).
Tetramethylethylenediamine: Ultrapure™ TMED (Invitrogen, Cat# 2020-02-15).
Ammonium persulfate (Fisher Bioreagents, Cat# 7727-54-0).
Instant nonfat dry milk (Nestle Carnation).
Gibco Dulbecco’s phosphate-buffered saline (PBS) (Thermo Fisher, Cat# 21300-058).
Tween 20 (Fisher Bioreagents, Cat# 9005-64-5).
Biotin (Sigma Aldrich, Cat# B4639).
Blotting roller (Fisher, Cat# LC2100).
Trans-Blot® SD Semi-Dry Electrophoretic Transfer Cell (Bio-Rad, Cat#1703940).
FluorChem M chemi-luminescent imager (ProteinSimple, San Jose, CA).
Mowiol 4–88 (Sigma, Cat# 81381-50G).
DABCO (Sigma, Cat# D27802-25G).
β-mercaptoethanol (Fisher, Cat# 21985023),
Dithiothreitol (DTT) (Fisher, Cat# R0861).
2.6. Solutions
1× phospho-buffered saline (PBS): 137 mM NaCl, 2.7 mM KCl, 4.3 mM Na2HPO4, 1.47 mM KH2PO4. Adjust pH to 7.4
2× Laemmli buffer: 4% SDS, 5% β-mercaptoethanol or 100 mM DTT, 20% glycerol, 0.004% bromophenol blue, 0.125 MTris-HCl, pH 6.8
Mowiol: Mowiol 4–88, glycerol, Tris-Cl (0.2 M, pH 8.5), DABCO (1,4-diazabicyclo-[2,2,2]-octane). Add 2.4 g Mowiol 4–88 to 6 g glycerol, then add 6 mL H2O and 12 mL Tris-Cl, and shake to mix. Incubate in 50 °C water bath overnight. Add 2.5% (w/v) DABCO to reduce fading. When DABCO has dissolved (5–10 min), centrifuge at 5000 rpm for 15 min. Aliquot in airtight containers and store at −20 °C.
1× running buffer: 25 mM Tris base, 192 mM glycine, 0.1% SDS. Adjust pH to 8.3
1× transfer buffer: 48 mM Tris base, 39 mM glycine, 20% methanol. Adjust pH to 8.3
1× PBST: 1× PBS + 0.1% Tween-20
For primary and secondary antibody solutions: 1× PBST +1% nonfat dry milk.
For streptavidin-HRP solution/blocking: 1× PBST +1% BSA.
Lysis buffer: 20 mM Tris-HCl (pH 8.0), 150 mM NaCl, 1 mM EGTA, 1 mM EDTA, 1 mM beta-glycerolphosphate, 1% Triton X-100.
3. Methods
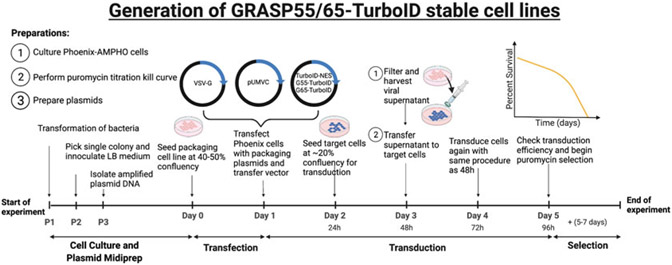
An outline of the procedure we used to generate GRASP55-TurboID and GRASP65-TurboID stable cell lines is shown in Fig. 2.
Fig. 2.
Generation of stable cell lines using retrovirus. Timeline of steps to create stable cell lines expressing GRASP proteins fused to TurboID
3.1. Safety When Handling Viral Vectors (See Note 1)
All virus-contaminated waste must be carefully collected in 100% bleach and sealed before discarding.
3.2. Plasmid Amplification and Isolation
The following steps should be done in a sterile environment or near an open flame.
Add 5 μL of each plasmid to a small microcentrifuge tube of TOP10 bacterial cells (see Note 2), and incubate on ice for 30 min.
Heat shock the tube of cells in a 37 °C water bath for 1 min, and then set back on ice for 2 min.
Add 800 μL LB media to the microcentrifuge tube, and place the tube in a shaker for 1 h at 37 °C and 200–300 rpm.
Spread the bacteria on an agar plate with ampicillin (pCMV-VSV-G) orkanamycin (pUMVC).
Incubate the plates at 37 °C overnight. Pick up colonies using a p200 pipet tip.
Eject the tip into 350 mL LB broth with 350 μL proper antibiotic [100 μg/mL ampicillin (pCMV-VSV-G) or 50 μg/mL kanamycin (pUMVC), respectively] in a large Erlenmeyer flask.
Incubate the bacteria in a shaker overnight at 37 °C and 200 rpm.
Transfer the bacterial culture to a large 450 mL Nalgene tube and spin down the bacteria at 6000 g for 10 min in a Sorvall ultracentrifuge. (From this step it is no longer necessary to be working over an open flame).
Discard the LB media supernatant and resuspend the bacterial pellet in resuspension buffer from the “ZymoPURE™ II Plasmid Midiprep Kit.”
Isolate plasmids using the “ZymoPURE™ II Plasmid Midiprep Kit” following the manufacture’s instruction (https://files.zymoresearch.com/protocols/_d4200_d4201_zymopure_ii_plasmid_midiprep.pdf).
Measure the concentration and purity of the plasmids by pipetting 1 μL of the isolated plasmid on the NanoDropTM Lite Spectrophotometer.
3.3. Phoenix Cell Transfection
All cell lines were cultured in complete growth medium: DMEM with 10% FBS, 1% penicillin/streptomycin antibiotic, and 1% GlutaMAX. All cells were maintained at 37 °C with 5% CO2. All cell lines were washed with 1× PBS. All cell lines were split and passaged using 0.25% trypsin in PBS with 0.1% EDTA (trypsin-EDTA).
Day 0. Dayprior to transfection, plate 0.5–1.0 × 106 Phoenix-AMPHO cells in 6-cm dishes with 5 mL complete growth medium, plate 3 dishes. Grow cells to 40–50% confluency.
Day 1. Change cells into fresh medium containing no antibiotics (see Note 3). Pipet 6 μL FuGENE HD Transfection Reagent into serum-free medium (see Notes 4 and 5).
- Add total of 6 μg plasmid DNA to the FuGENE/medium tube:
- 2.5 μg pUMVC plasmid
- 0.5 μg pCMV-VSV-G expression vector
- 3.0 μg respective transfer vector (see Note 6).
Incubate FuGENE/medium/DNA solution at room temperature for 15 min.
After incubation, add plasmid mixture dropwise to the Phoenix-AMPHO cells.
Incubate Phoenix-AMPHO cells overnight at 37 °C.
Day 2. After 24 h transfection, trypsinize transfected Phoenix-AMPHO cells, and transfer entire cell suspension to a 15-cm dish with 15 mL complete growth medium.
Set up the target cells (continued section below).
3.4. HeLa Cell Transduction
Day 2 Continued. Seed target cells (WT and GRASP55/GRASP65 DKO HeLa cells) [8] for transduction at roughly 20% confluency in 10-cm dishes for each respective transfer vector and one uninfected control for transduction the following day. Incubate overnight at 37 °C.
Day 3. After 48 h transfection, harvest viral supernatant from each of the three Phoenix-AMPHO dishes, and filter the supernatant through a 45 μm PES syringe filter into 15 mL conical tubes (see Notes 7 and 8).
Add polybrene transfection reagent to a concentration of 8 μg/mL to each viral supernatant (see Note 9).
Aspirate media from target HeLa cell dishes, and in a dropwise manner carefully transfer 7.5 mL of viral supernatant to target cells. Add 2.5 mL complete growth medium to completely cover the cells in the 10-cm dish. Incubate overnight at 37 °C.
Replace 15 mL fresh complete growth medium into the 15-cm dishes with transfected Phoenix-AMPHO cells. Incubate overnight at 37 °C.
Day 4. Repeat steps 3.4 #2-#4, and replace the media in the target HeLa cells by adding fresh viral supernatant into each of the HeLa dishes once more (see Notes 10 and 11). Incubate overnight at 37 °C.
Day 5. Split transduced target HeLa cells. Seed at 70% confluency in 6-cm dishes for future antibiotic (puromycin) treatment. Also, seed untransduced WT HeLa and DKO HeLa cells at 70% confluency in 6-cm dishes as control dishes for antibiotic (puromycin) selection (Subheading 3.6). Optional: Seed 2 coverslips in 3.5-cm dishes at 70% confluency to check for transduction efficiency with biotinylation assay (Subheading 3.5). Incubate all dishes overnight at 37 °C.
3.5. Check Transduction Efficiency
Replace media in 3.5-cm dishes containing coverslips with 2 mL of 500 μM biotin in complete growth medium. Incubate at 37 °C for 2.5 h.
Remove coverslips from seeding plate and place in a 12-well plate. Wash coverslips 3 times with PBS at room temperature. (The following steps are done at room temperature).
Fix cells on coverslips with 1 mL of 4% PFA. Incubate at room temperature for 10 min.
Aspirate PFA, wash with PBS.
Permeabilize the cells by adding 1 mL 0.2% Triton X in PBS. Incubate at room temperature for 10 min. Wash coverslips once with PBS.
Incubate cells in 1 mL 50 mM NH4Cl for 10 min to quench the PFA. Wash coverslips once with PBS.
Block coverslips in 1 mL 1% BSA in PBS for 1 h. Discard BSA, and wash coverslips with PBS.
Set up humidity chamber with damp filter paper covered by parafilm in a light-tight container. Transfer coverslips to humidity chamber.
Incubate coverslips in 50 μL Neutravidin-TxRed 1:100 diluted in 1% BSA in PBS for 1 h in a humidity chamber.
Discard Neutravidin in BSA, and wash coverslips 3 times with 1 mL PBS in a 12-well plate.
Mount coverslips using Mowiol mounting medium to glass microscope slides and image on fluorescent microscope.
3.6. Puromycin Selection of Transduced HeLa Cells
The concentration of puromycin used in selection is determined by a kill curve established on untransduced cells. Most constructs have selection markers such as puromycin, blasticidin, hygromycin, neomycin, or bleomycin. Regardless of the selection marker that the plasmids express, you need to know the concentration of antibiotic that kills untransduced cells within a given amount of time in order to successfully select transduced cells. We recommend the following methods for obtaining a “Kill Curve.”
- Create a puromycin kill curve for your target cells by following the steps below:
- Aliquot untransduced cells in a 12-well plate at a density such that they are at 72 h from confluency.
- Add puromycin at concentrations of 0, 0.3, 0.5, 1, 2, 5, and 10 μg/mL in six different wells.
- Incubate cells at 37 °C and 5% CO2 for 72 h or longer if needed.
- Count viable cells and determine the lowest concentration of drug that kills 100% of cells in 3–5 days (see Note 12).
- Use this concentration for the selection step in your experiment.
- Selection: Discard media in each of the 6-cm dishes for each of the WT and DKO transduced HeLa cell lines and replace with appropriate antibiotic selection medium.
- Add 5 mL of 0.5 μg/mL puromycin (determined by the kill curve in Subheading 3.6, step 1) in complete growth medium to each of the 6-cm dishes of transduced WT HeLa cells to select for cells with stable expression of V5-TurboID, GRASP55-V5-TurboID, or GRASP65-V5-TurboID. DKO HeLa cells were more sensitive to puromycin and required a lower concentration (0.3 μg/mL puromycin in complete growth medium).
Incubate all cells overnight at 37 °C.
Monitor cells daily during selection. Replace with fresh medium containing the appropriate concentration of puromycin daily until all the untransduced cells in the control dish have died.
3.7. Validation by Western Blot
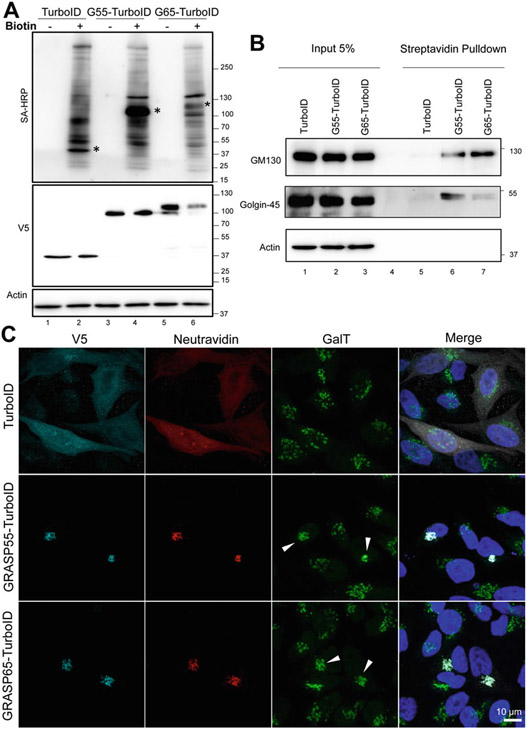
To validate the cells in experimental conditions, treat cells as desired and add biotin (500 μM for 2.5 h) with proper controls (Fig. 3).
Rinse cells with PBS 5 times to remove serum proteins and excess biotin prior to cell collection and lysis.
Add 1 mL of ice-cold PBS to dish, scrape adherent cells off the dish using a cell scraper, and transfer the cell suspension into a 1.5 mL microcentrifuge tube.
Centrifuge at 1000 rcf for 3 min at 4 °C to pellet cells.
Remove supernatant and add lysis buffer with protease inhibi-tors 3 times the estimated volume of cell pellet.
Vortex cells and incubate on ice for 30 min.
Centrifuge cell lysate mixture at 4 °C for 20 min at 14,0000 rpm in a benchtop centrifuge.
Transfer the supernatant (lysate) to a fresh tube on ice.
Determine the protein concentration of each cell lysate using Bio-Rad Protein Assay Kit following the manufacturer’s instructions.
Calculate and dilute the protein samples to the same concentration by mixing with 2× Laemmli sample buffer.
Reduce and denature the samples by boiling at 95–100 °C for 3 min. Do not overheat the samples.
Prepare or purchase a pre-made gel of appropriate polyacrylamide percentage to best resolve your protein of interest based on molecular weight.
Load samples containing equal amounts of protein prepared in sample buffer into SDS-PAGE wells. Include a molecular weight marker in one of the lanes.
Fill the electrophoresis apparatus with 1× running buffer and run the gel (see Note 13).
Following SDS-PAGE separation, remove the gel from the electrophoresis apparatus and equilibrate it by soaking in 1× transfer buffer for 10 min.
Activate 0.45 μm nitrocellulose membrane in 1× transfer buffer (see Note 14).
Soak filter paper in 1× transfer buffer prior to assembly of the transfer stack. Sequentially assemble the layers of the transfer stack: 3 layers of filter paper, membrane, gel, 3 layers of filter paper. Gently remove any air bubbles with a roller (see Note 15).
Run semi-dry transfer at 15 V for 1.5 h.
After transfer, block the membrane using protein-free blocking buffer for 1 h (see Note 16).
Agitate membrane in streptavidin-HRP at 1:10,000 in 1% BSA overnight at 4 °C.
Next day, quickly wash the membrane in PBS 3 times.
Rock the membrane in 3% BSA for 3 min to reduce back-ground signal on membrane.
Wash the membrane twice more in PBS.
Apply enhanced chemiluminescent reagent to the membrane for visualization of the biotinylated proteins.
To probe other proteins on the same membrane, quench the HRP signal on the membrane by incubating the membrane in 30% H2O2 for 20 min (see Note 17). Repeat blocking step and apply desired primary antibody at the recommended dilution from manufacturer.
Incubate the membrane in primary antibody solution overnight at 4 °C with gentle rocking.
Wash the membrane with 1× PBST 3 times for 10 min each with gentle rocking.
Incubate the membrane in the appropriately diluted secondary antibody for 1 h at room temperature with gentle rocking. See primary antibody information sheet for proper secondary antibody selection.
Wash the membrane in 1× PBST 3 times for 10 min each with gentle rocking.
Visualize with chemiluminescent reagent.
Fig. 3.
Validation of cell lines expressing V5-TurboID, GRASP55-V5-TurboID, and GRASP65-V5-TurboID. (a) HeLa cells expressing V5-TurboID, GRASP55-V5-TurboID, and GRASP65-V5-TurboID were treated with 50 μM biotin for 2.5 h or without biotin (control). Lysates were collected and analyzed by Western blot. Biotinylated proteins were visualized via streptavidin (SA)-HRP. (b) Same cells as in A were pulsed with 50 μM biotin for 2.5 h to induce proximity-dependent biotinylation. Biotinylated proteins were affinity purified from lysates using Streptavidin beads and analyzed by Western blot for known interactors of GRASP65 (GM130) and GRASP55 (Golgin-45). 5% of whole cell lysate was loaded as input. (c) To validate GRASP proteins fused to biotin ligase are functional, TurboID, GRASP55-TurboID, and GRASP65-TurboID were expressed in GRASP55 and GRASP65 double knockout (DKO) HeLa cells. 50 μM biotin was added to cells for 2.5 h to biotinylate proteins. Cells were fixed, stained for GalT (a Golgi marker), Neutravidin, V5, and imaged. Note that expression of GRASP proteins fused to TurboID, but not TurboID alone, rescued the fragmented Golgi in DKO cells (arrowheads)
3.8. Lentiviral Transduction of GRASP65-GFP
Day 0: One day prior to transfection, prepare the packaging cell line for transfection by seeding HEK293 T cells into a 3.5-cm dish, aiming for ~50% confluency on the next day. More dishes of cells can be pre-prepared if multiple constructs or more controls are included in parallel.
Incubate the cells at 37 °C and 5% CO2.
Day 1: Discard the old medium and add 2 mL fresh complete growth medium without antibiotics into the 3.5-cm dish of the packaging cell line (HEK293T).
- Prepare the transfection solution by pooling the following reagents:
- PsPAX2 (Addgene, Cat# 12259): 1 μg.
- PMD2.G (Addgene, Cat# 12259): 0.2 μg.
- pLenti-lox-GRASP65-GFP (gifted by Dr. Henry Paulson, University of Michigan): 1.3 μg (see Note 18).
- 10 μL of PEI.
- Add serum-free DMEM to a total of 100 μL.
Gently mix well and incubate the transfection solution at room temperature for 10 min.
Carefully add the transfection solution dropwise into the 3.5-cm dish of HEK293 T packaging cells. Gently swirl to mix well without dislodging the cells.
Change to fresh complete growth medium 6 h after transfection and further incubate at 37 °C and 5% CO2 overnight.
Day 2: At 24 h after transfection, trypsinize the transfected packaging HEK293 T cells, and transfer entire cell suspension to a 10-cm dish with 10 mL DMEM with 10% FBS.
Seed the target cells (HEK293 T or other cell lines preferred) into a 10-cm dish, aiming for 20–33% confluency on the next day.
Day 3: At 48 h after transfection, collect the virus-containing supernatant into a 15 mL tube and centrifuge at 500 g for 5 min to remove cell debris.
Use a 10 mL syringe to collect 8 mL virus-containing supernatant and filter the supernatant with a 0.45 μm PES syringe filter into a 15 mL tube containing 2 mL complete growth medium.
Add polybrene to 8 μg/mL and mix well.
Carefully add the transduction solution dropwise into the 10-cm dish of target cells (see Note 19). Incubate at 37 °C overnight.
Replace 10 mL fresh complete growth medium into the 10-cm dishes with packaging cells.
Day 4: At 72 h after transfection, discard the medium in the target cells and transduce the cells a second time by repeating the process (Subeading 3.8, steps 10–13) (see Note 20).
Day 5 and after: Validation and maintenance of transduced cells: Remove the infectious medium from transduced target cells and start antibiotic selection. Cells are submitted to microscopy, immunoblotting, and flow cytometry analysis for validation. Fluorescence-activated cell sorting (FACS) can start 48 h after transduction to collect GFP-positive cells (Fig. 4).
FACS sample preparation: Cells are trypsinized and pelleted by centrifugation at 800 × g for 3 min at 4 °C and resuspended in serum-free DMEM. Samples are kept on ice before sorting (see Note 21).
Flow cytometry analysis and sorting: Forward scatter (FSC) and side scatter (SSC) signals are collected, and gates are set for single cells. GFP signals are collected from non-transduced host cells (control) and GRASP65-GFP transduced cells. GFP signal from control cells is used to set the gate for GFP-negative events. GFP-positive events from GRASP65-GFP transduced cells are sorted into complete growth medium for further culture (see Notes 22 and 23).
Fig. 4.

Validation of cell lines stably expressing GRASP65-GFP. (a) Expression of GRASP65-GFP in transduced 293 T cells. Control and transduced 293 T cells are analyzed by SDS-PAGE and probed for GFP and actin. (b) Expressed GRASP65-GFP is correctly targeted to the Golgi in transduced 293 T cells. Transduced 293 T cells are fixed, stained for GM130, and imaged. Scale bar, 20 μm. Boxes areas are enlarged and placed underneath. (c) Flow cytometry analysis and sorting of transduced (right) 293 T cells. Un-transduced (left) and GRASP65-GFP transduced (right) 293 T cells were analyzed by flow cytometry. GFP-positive cells (peak on the right) were collected
4. Notes
The use of infectious reagents including retroviruses and lentiviruses needs to be approved by The Institutional Biosafety Committee (IBC) prior to the start of the experiment.
Gently tap to mix; cell membranes are fragile after thaw and pipetting may lyse cells.
Phoenix cells should be selected with Hygromycin B and Diphtheria toxin every 3–5 weeks to ensure maintenance of episomes.
When combining FuGENE Transfection Reagent, serum-free medium, and plasmid DNA, it is important that you add the serum-free medium to the microcentrifuge tube first. Do not use siliconized pipette tips for undiluted FuGENE and be sure to avoid pipetting FuGENE onto the sides of the tube as the FuGENE will stick to plastic and reduce your transfection efficiency.
Total transfection volume is 100 μL (serum-free medium + 6 μL FuGENE + plasmids).
Retrovirus plasmids contain LTR repeat sequences and therefore can undergo recombination after transformation. Stbl3 competent cells are recommended for plasmid amplification to minimize recombination events. It is good practice to confirm that plasmids are of the correct size before using in the transfection step.
This step is optional depending on the transduction efficiency. Virus can be harvested at 48, 72, and 96 h post-transfection, and more than 1 transduction may be performed to increase the transduction efficiency (up to 3 times). However, ecotropic and GaLV viral titers at 72 h will be ~20–40% less than at 48 h. VGV-G titers remain consistent throughout 48–96 h.
For collecting VSV-G viruses, use medium buffered with 10–25 mM HEPES (pH 7.4) to limit pH-dependent cell killing by the VSV-G protein.
Reagents like polybrene and protamine sulfate can enhance absorption of a virus particle to target cells and increase transduction efficiency by reducing the repulsive electrostatic forces between the two negatively charged membranes [9]. Polybrene is a linker molecule that can improve transduction efficiency but can be toxic to some cell lines. Protamine sulfate can also be used as an alternative with lower toxicity to cells. The concentration of polybrene and protamine sulfate can be increased or decreased as necessary, and removal of the viral supernatant (containing polybrene) can be replaced with fresh media within 8 h, although we usually leave the viral supernatant overnight (16–24 h). The duration that viral supernatant can remain on cells can range from 4 h to 2 days, depending on how well the target cells tolerate the conditions.
Ecotropic and amphotropic are less stable than pantropic (VSV-G pseudotyped) viruses. Therefore, ultracentrifugation to harvest/concentrate virus and freeze/thaw cycles should only be used for pantropic viruses. Additionally, one freeze/thaw cycle can reduce the titer by up to 100-fold, and thus amphotropic and ecotropic viruses should be used fresh.
A typical titer under optimized conditions can reach ~1 × 106 TU/mL. However, this value is dependent on many factors such as packaging cell line, transfection efficiency, insert size, and incubation time before harvest, which varies based on experimental setup/researcher.
Kill curve may last about a week, depending on the cell line sensitivity to the specific antibiotic. G418/neomycin selection may typically take 7–10 days, as compared to 3–5 days for puromycin/hygromycin selection. For working ranges of antibiotics in HeLa cells lines, see http://cell-lines.toku-e.com/Cell-Lines_1434.html
1–2 h at 0.2 amps per gel is standard, but time and voltage may require optimization.
Membrane pore size can be optimized for larger or smaller proteins. Do not allow the membrane to dry out. Immediately transfer from buffer onto the transfer stack.
Bubbles between the gel and the membrane will inhibit the transfer of proteins to the membrane.
Milk contains free biotin and will interfere with binding to streptavidin-HRP. Alternatively, BSA can be used a blocking agent or any other commercially available protein-free blocking buffer.
Traditional stripping methods will not work for biotin–streptavidin, as this is a strong bond that is difficult to disrupt with stripping buffer.
The amount of transfer construct may need optimization.
Depending on the titer obtained, the virus-containing supernatant collected can be used to transduce more dishes based on experimental setup and researcher’s preference.
This step is optional depending on the transduction efficiency. More than one round of transduction may be performed to increase the transduction efficiency.
Single-cell suspension is required for flow cytometry analysis.
More than one round of sorting may be required to obtain cells with stable expression.
Single clones are collected into 96-well plates if preferred.
References
- 1.Wang D, Tai P, Gao G (2019) Adeno-associated virus vector as a platform for gene therapy delivery. Nat Rev Drug Discov 18(5). 10.1038/s41573-019-0012-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Milone MC, O’Doherty U (2018) Clinical use of lentiviral vectors. Leukemia 32(7):1529–1541. 10.1038/s41375-018-0106-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bekier M, Wang L, Li J, Huang H, Tang D, Zhang X, Wang Y (2017) Knockout of the Golgi stacking proteins GRASP55 and GRASP65 impairs Golgi structure and function. Mol Biol Cell 28(21). 10.1091/mbc.E17-02-0112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hindmarsh P, Leis J (1999) Retroviral DNA integration. Microbiol Mol Biol Rev 63(4). 10.1128/MMBR.63.4.836-843.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bouard D, Alazard-Dany D, Cosset F (2009) Viral vectors: from virology to transgene expression. Br J Pharmacol 157(2). 10.1038/bjp.2008.349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Joglekar A, Sandoval S (2017) Pseudotyped lentiviral vectors: one vector, many guises. Hum Gene Ther Methods 28(6). 10.1089/hgtb.2017.084 [DOI] [PubMed] [Google Scholar]
- 7.Balvay L, Lopez LM, Sargueil B, Darlix J, Ohlmann T (2007) Translational control of retroviruses. Nat Rev Microbiol 5(2). 10.1038/nrmicro1599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bekier ME 2nd, Wang L, Li J, Huang H, Tang D, Zhang X, Wang Y (2017) Knockout of the Golgi stacking proteins GRASP55 and GRASP65 impairs Golgi structure and function. Mol Biol Cell 28(21):2833–2842. 10.1091/mbc.E17-02-0112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davis H, Morgan J, Yarmush M (2002) Polybrene increases retrovirus gene transfer efficiency by enhancing receptor-independent virus adsorption on target cell membranes. Biophys Chem 97(2–3). 10.1016/s0301-4622(02)00057-1 [DOI] [PubMed] [Google Scholar]
- 10.Moore J, Trkola A, Dragic T (1997) Co-receptors for HIV-1 entry. Curr Opin Immunol 9(4). 10.1016/s0952-7915(97)80110-0 [DOI] [PubMed] [Google Scholar]
- 11.Kafri T, Blömer U, Peterson D, Gage F, Verma I (1997) Sustained expression of genes delivered directly into liver and muscle by lentiviral vectors. Nat Genet 17(3). 10.1038/ng1197-314 [DOI] [PubMed] [Google Scholar]
- 12.Peng K, Pham L, Ye H, Zufferey R, Trono D, Cosset F, Russell S (2001) Organ distribution of gene expression after intravenous infusion of targeted and untargeted lentiviral vectors. Gene Ther 8(19). 10.1038/sj.gt.3301552 [DOI] [PubMed] [Google Scholar]
- 13.Sakuma T, De Ravin S, Tonne J, Thatava T, Ohmine S, Takeuchi Y, Malech H, Ikeda Y (2010) Characterization of retroviral and lentiviral vectors pseudotyped with xenotropic murine leukemia virus-related virus envelope glycoprotein. Hum Gene Ther 21(12). 10.1089/hum.2010.063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Diaz R, Bateman A, Emiliusen L, Fielding A, Trono D, Russell S, Vile R (2000) A lentiviral vector expressing a fusogenic glycoprotein for cancer gene therapy. Gene Ther 7(19). 10.1038/sj.gt.3301277 [DOI] [PubMed] [Google Scholar]
- 15.Jang J, Shaw K, Yu X, Petersen D, Pepper K, Lutzko C, Kohn D (2006) Specific and stable gene transfer to human embryonic stem cells using pseudotyped lentiviral vectors. Stem Cells Dev 15(1). 10.1089/scd.2006.15.109 [DOI] [PubMed] [Google Scholar]
- 16.Bell A, Fegen D, Ward M, Bank A (2010) RD114 envelope proteins provide an effective and versatile approach to pseudotype lentiviral vectors. Exp Biol Med (Maywood, NJ) 235(10). 10.1258/ebm.2010.010053 [DOI] [PubMed] [Google Scholar]
- 17.Hale C, Majumdar S, Elmore J, Pfister N, Compton M, Olson S, Resch A, Glover C, Graveley B, Terns R, Terns M (2012) Essential features and rational design of CRISPR RNAs that function with the Cas RAMP module complex to cleave RNAs. Mol Cell 45(3). 10.1016/j.molcel.2011.10.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schambach A, Galla M, Modlich U, Will E, Chandra S, Reeves L, Colbert M, Williams D, von Kalle C, Baum C (2006) Lentiviral vectors pseudotyped with murine ecotropic envelope: increased biosafety and convenience in preclinical research. Exp Hematol 34(5). 10.1016/j.exphem.2006.02.005 [DOI] [PubMed] [Google Scholar]
- 19.Hanawa H, Kelly P, Nathwani A, Persons D, Vandergriff J, Hargrove P, Vanin E, Nienhuis A (2002) Comparison of various envelope proteins for their ability to pseudotype lentiviral vectors and transduce primitive hematopoietic cells from human blood. Mol Ther 5(3). 10.1006/mthe.2002.0549 [DOI] [PubMed] [Google Scholar]
- 20.Janssens W, Chuah M, Naldini L, Follenzi A, Collen D, Saint-Remy J, VandenDriessche T (2003) Efficiency of onco-retroviral and lentiviral gene transfer into primary mouse and human B-lymphocytes is pseudotype dependent. Hum Gene Ther 14(3). 10.1089/10430340360535814 [DOI] [PubMed] [Google Scholar]
- 21.Levy C, Fusil F, Amirache F, Costa C, Girard-Gagnepain A, Negre D, Bernadin O, Garaulet G, Rodriguez A, Nair N, Vandendriessche T, Chuah M, Cosset F, Verhoeyen E (2016) Baboon envelope pseudotyped lentiviral vectors efficiently transduce human B cells and allow active factor IX B cell secretion in vivo in NOD/SCIDγc −/− mice. J Thromb Haemost 14(12). 10.1111/jth.13520 [DOI] [PubMed] [Google Scholar]
- 22.Uhlig KM, Schülke S, Scheuplein VAM, Malczyk AH, Reusch J, Kugelmann S, Muth A, Koch V, Hutzler S, Bodmer BS, Schambach A, Buchholz CJ, Waibler Z, Scheurer S, Mühlebach MD, Kirchhoff F (2015) Lentiviral protein transfer vectors are an efficient vaccine platform and induce a strong antigen-specific xytotoxic T cell response. J Virol 89(17):9044–9060. 10.1128/JVI.00844-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou Q, Schneider I, Gallet M, Kneissl S, Buchholz C (2011) Resting lymphocyte transduction with measles virus glycoprotein pseudotyped lentiviral vectors relies on CD46 and SLAM. Virology 413(2). 10.1016/j.virol.2011.02.010 [DOI] [PubMed] [Google Scholar]
- 24.Witting SR, Vallanda P, Gamble AL (2013) Characterization ofa third generation lentiviral vector pseudotyped with Nipah virus envelope proteins for endothelial cell transduction. Gene Therapy 20(10):997–1005. 10.1038/gt.2013.23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Palomares K, Vigant F, Handel BV, Pernet O, Chikere K, Hong P, Sherman SP, Patterson M, An DS, Lowry WE, Mikkola HKA, Morizono K, Pyle AD, Lee B (2013) Nipah virus envelope-pseudotyped lentiviruses efficiently target ephrinB2-positive stem cell populations in vitro and bypass the liver sink when administered in vivo. J Virol 87(4):2094–2108. 10.1128/JVI.02032-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carpentier DCJ, Vevis K, Trabalza A, Georgiadis C, Ellison SM, Asfahani RI, Mazarakis ND (2011) Enhanced pseudotyping efficiency of HIV-1 lentiviral vectors by a rabies/vesicular stomatitis virus chimeric envelope glycoprotein. Gene Therapy 19(7):761–774. 10.1038/gt.2011.124 [DOI] [PubMed] [Google Scholar]
- 27.Cronin J, Zhang X, Reiser J (2005) Altering the tropism of lentiviral vectors through pseudotyping. Curr Gene Ther 5(4). 10.2174/1566523054546224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kato S, Kobayashi K, Kobayashi K (2014) Improved transduction efficiency of a lentiviral vector for neuron-specific retrograde gene transfer by optimizing the junction of fusion envelope glycoprotein, J Neurosci Methods 227. 10.1016/j.jneumeth.2014.02.015 [DOI] [PubMed] [Google Scholar]
- 29.Hachiya A, Sriwiriyanont P, Patel A, Saito N, Ohuchi A, Kitahara T, Takema Y, Tsuboi R, Boissy RE, Visscher MO, James WM, Kobinger GP (2007) Gene transfer in human skin with different pseudotyped HIV-based vectors. Gene Ther 14(8):648–656. 10.1038/sj.gt.3302915 [DOI] [PubMed] [Google Scholar]
- 30.Colin A, Faideau M, Dufour N, Auregan G, Hassig R, Andrieu T, Brouillet E, Hantraye P, Bonvento G, Déglon N (2009) Engineered lentiviral vector targeting astrocytes in vivo. Glia 57(6). 10.1002/glia.20795 [DOI] [PubMed] [Google Scholar]
- 31.Sinn P, Coffin J, Ayithan N, Holt K, Maury W (2017) Lentiviral Vectors Pseudotyped with Filoviral Glycoproteins. Methods in molecular biology (Clifton, NJ) 1628. 10.1007/978-1-4939-7116-9_5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dylla DE, Xie L, Michele DE, Kunz S, McCray PB (2011) Altering α-dystroglycan receptor affinity of LCMV pseudotyped lentivirus yields unique cell and tissue tropism. Genet Vaccines Ther 9(1):1–13. 10.1186/1479-0556-9-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kumar M, Bradow BP, Zimmerberg J (2004) Large-scale production of pseudotyped lentiviral vectors using baculovirus GP64. https://home.liebertpub.com/hum. 10.1089/10430340360464723 [DOI] [PubMed] [Google Scholar]
- 34.Kahl CA, Marsh J, Fyffe J, Sanders DA, Cornetta K (2004) Human immunodeficiency virus type 1-derived lentivirus vectors pseudotyped with envelope glycoproteins derived from Ross River virus and Semliki Forest virus. J Virol 78(3):1421–1430. 10.1128/JVI.78.3.1421-1430.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Salvador B, Zhou Y, Michault A, Muench M, Simmons G (2009) Characterization of Chikungunya pseudotyped viruses: identification of refractory cell lines and demonstration of cellular tropism differences mediated by mutations in E1 glycoprotein. Virology 393(1). 10.1016/j.virol.2009.07.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kang Y, Stein C, Heth J, Sinn P, Penisten A, Staber P, Ratliff K, Shen H, Barker C, Martins I, Sharkey C, Sanders D, McCray P, Davidson B (2002) In vivo gene transfer using a nonprimate lentiviral vector pseudotyped with Ross River Virus glycoproteins. J Virol 76(18). 10.1128/jvi.76.18.9378-9388.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Poluri A, Ainsworth R, Weaver SC, Sutton RE (2008) Functional pseudotyping of human immunodeficiency virus type 1 vectors by western equine encephalitis virus envelope glycoprotein. 10.1128/JVI.01503-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McKay T, Patel M, Pickles RJ, Johnson LG, Olsen JC (2006) Influenza M2 envelope protein augments avian influenza hemagglutinin pseudotyping of lentiviral vectors. Gene Ther 13(8):715–724. 10.1038/sj.gt.3302715 [DOI] [PubMed] [Google Scholar]
- 39.Patel M, Giddings A, Sechelski J, Olsen J (2013) High efficiency gene transfer to airways of mice using influenza hemagglutinin pseudotyped lentiviral vectors. J Gene Med 15(1). 10.1002/jgm.2695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Burns JC, Friedmann T, Driever W, Burrascano M, Yee JK (1993) Vesicular stomatitis virus G glycoprotein pseudotyped retroviral vectors: concentration to very high titer and efficient gene transfer into mammalian and nonmammalian cells. Proc Natl Acad Sci U S A 90(17):8033–8037. 10.1073/pnas.90.17.8033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wong L, Azzouz M, Walmsley L, Askham Z, Wilkes F, Mitrophanous K, Kingsman S, Mazarakis N (2004) Transduction patterns of pseudotyped lentiviral vectors in the nervous system. Mol Ther 9(1). 10.1016/j.ymthe.2003.09.017 [DOI] [PubMed] [Google Scholar]