Abstract
Objectives
To examine the differences in durability and its determinants of humoral immunity following 2- and 3-dose COVID-19 vaccination.
Methods
Throughout the pandemic, we evaluated the anti-spike IgG antibody titers of 2- and 3-dose mRNA vaccine recipients over time among the staff of a medical and research center in Tokyo. Linear mixed models were used to estimate trajectories of antibody titers from 14 to 180 days after the last immune-conferred event (vaccination or infection) and compare antibody waning rates across prior infection and vaccination status, and across background factors in infection-naïve participants.
Results
A total of 6901 measurements from 2964 participants (median age, 35 years; 30% male) were analyzed. Antibody waning rate (percentage per 30 days [95% CI]) was slower after 3 doses (25% [23–26]) than 2 doses (36% [35–37]). Participants with hybrid immunity (vaccination and infection) had further slower waning rates: 2-dose plus infection (16% [9–22]); 3-dose plus infection (21% [17–25]). Older age, male sex, obesity, coexisting diseases, immunosuppressant use, smoking, and alcohol drinking were associated with lower antibody titers, whereas these associations disappeared after 3 doses, except for sex (lower in female participants) and immunosuppressant use. Antibody waned slightly faster in older participants, females, and alcohol drinkers after 2 doses, whereas it did not differ after 3 doses across except sex.
Discussion
The 3-dose mRNA vaccine conferred higher durable antibody titers, and previous infection modestly enhanced its durability. The antibody levels at a given time point and waning speed after 2 doses differed across background factors; however, these differences mostly diminished after 3 doses.
Keywords: Durability, Prior infection, SARS-CoV-2, Spike antibody, Vaccination
Introduction
The widespread use of primary and booster vaccinations against COVID-19 substantially decreased the risk of SARS-CoV-2 infection and hospitalization [1,2]. With the waning of vaccine-induced humoral immunity over time [3], however, vaccine effectiveness (VE) of 2- and 3-dose vaccination for infection has decreased [1,2]. Understanding the duration of vaccine-induced immunity and factors accelerating the waning is critical for formulating vaccine policy, including the recommendation regarding the timing and target population of an additional booster dose.
Epidemiological data are scarce regarding the waning pattern of humoral immunity following 2- and 3-dose COVID-19 vaccination and its related factors. Studies among 2-dose recipients showed that anti-SARS-CoV-2 spike antibody titers waned faster in those who are older [4] and female [3] and slower in those with a history of COVID-19 [5], but no such investigation has been done following 3-dose vaccination. It remains elusive for both doses whether the waning speed of antibody differs depending on obesity [6,7], comorbid conditions [7,8], immunosuppression [7,8], or behavioral factors (smoking and alcohol drinking [[9], [10], [11]]), which have been linked to lower immune response to COVID-19 vaccine.
Here, we assess the long-term humoral response and its determinants following 2- and 3-dose COVID-19 mRNA vaccines and compare the waning rates between 2- and 3-dose recipients.
Methods
We analyzed data from a repeat serological study of the staff at the National Center for Global Health and Medicine, Japan [6,7]. We conducted 6 surveys from July 2020, to June 2022. In each survey, we measured anti-SARS-CoV-2 nucleocapsid (N)- and spike- (from the second survey onward) protein antibodies using Abbott and Roche assays and collected information on histories of SARS-CoV-2 vaccination and infection, body composition, morbid status, and behavioral factors. We retrieved 6901 antibody measurements from 2964 staff who participated in at least one of the third to sixth surveys more than 7 days after receiving 2 or 3 doses of mRNA COVID-19 vaccines for the analysis of antibody waning by vaccine and infection status (Figs S1–S4). Of these, we used 6576 measurements from 2906 infection-naïve participants for the association between background factors and antibody waning rate. Linear mixed models were used to estimate antibody trajectories from 14 to 180 days after the last immune-conferred event (vaccination or infection) and compare antibody waning rates across prior infection and vaccination status, and across background factors in infection-naïve participants. Written informed consent was obtained from all participants, and the study procedure was approved by the NCGM ethics committee (approved number: NCGM-G-003598). Details of the study setting, participants, variables information, and statistical approach are described in Text S1.
Results
Among the 2964 participants, the median age was 35 (interquartile range: 27–47) and 30.2% were male (Tables S1 and S2). Those with obesity, coexisting diseases, and immunosuppression were 5.5%, 11.1%, and 1.8%, respectively, and tobacco product users and regular drinkers were 7.7% and 37.6%, respectively.
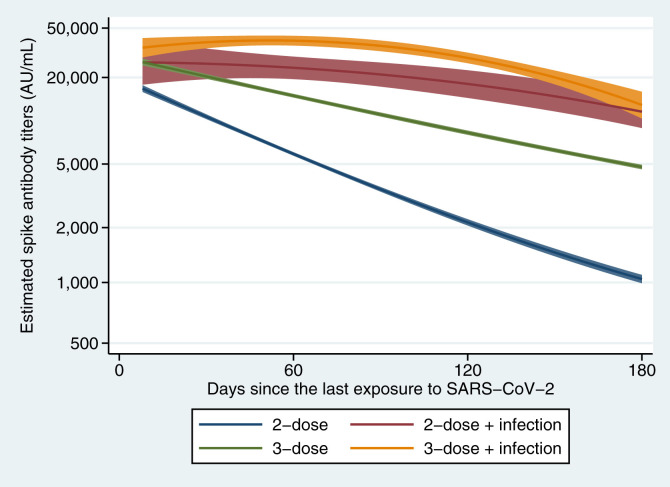
Among the infection-naïve participants, the waning rate of antibody titers was 17% slower per 30 days after the 3 doses (25% per 30 days) compared with 2 doses (36% per 30 days) (Table 1 and Fig. 1 ). The 2- and 3-dose recipients with a history of infection had slower antibody waning rates than their infection-naïve counterparts. Among the 2-dose recipients, those with a history of infection had consistently higher antibody titers during days 14–180 and a 32% slower waning rate than those without infection. Among the 3-dose recipients, those with a history of infection had higher antibody titers during days 14–180 and a 5% slower decline rate than those without infection. The 2-dose recipients with infection had higher antibody titers and a 13% slower waning rate than 3-dose recipients without infection.
Table 1.
Waning of antibody titers since the last immune-conferring event (vaccination or infection) among 2- or 3-dose vaccine recipients
| Estimated spike antibody titers (AU/mL) after the last immune-conferring event, GMT (95% CI) |
Slope of antibody waning, RoM (95% CI) |
||||||
|---|---|---|---|---|---|---|---|
| Day 14 | Day 30 | Day 90 | Day 180 | Per 30 d | Comparison of slopes | ||
| 2-dose | 14 426 (13 692–15 199) | 10 284 (9893–10 690) | 3393 (3282–3509) | 1034 (983–1089) | 0.64 (0.63–0.65) | Reference | |
| 3-dose | 24 389 (23 069–25 783) a | 20 280 (19 450–21 146)a | 10 663 (10 317–11 021)a | 4699 (4540–4864)a | 0.75 (0.74–0.76) | 1.17 (1.16–1.19) | reference |
| 2-dose + infection | 25 904 (18 164–36 942)a | 25 400 (19 323–33 388)a | 20 995 (17 000–25 928)a,b | 11 167 (8565–14 560)a,b | 0.84 (0.78–0.91) | 1.32 (1.23–1.43) | 1.13 (1.05–1.21) |
| 3-dose + infection | 35 131 (30 078–41 032)a,b | 37 543 (33 590–41 961)a,b | 35 119 (31 821–38 758)a,b | 12 476 (9986–15 586)a,b | 0.79 (0.75–0.83) | 1.24 (1.17–1.31) | 1.05 (1.01–1.11) |
Shown are the estimated GMT of anti-SARS-CoV-2 spike protein antibody titers on 14, 30, 90, and 180 d since the last immune-conferring event (vaccination or infection) and the estimated RoM of antibody waning slopes, with adjustment for age (<40 or ≥40 y), sex (male or female), body mass index (<27.5 or ≥27.5 kg/m2), coexisting diseases (yes or no), immunosuppressant use (yes or no), smoking status (current or non-smoker), and frequency of alcohol drinking (<1 or ≥1 time/wk).
GMT, geometric mean titer; RoM, ratio of means.
p < 0.05 (reference is 2-dose).
p < 0.05 (reference is 3-dose).
Fig. 1.
Waning of anti-SARS-CoV-2 spike protein antibody titers from the last exposure to SARS-CoV-2 (infection or vaccination). Curves shown are the estimated trajectories adjusted for age (<40 or ≥40 years), sex (male or female), body mass index (<27.5 or ≥27.5 kg/m2), coexisting diseases (yes or no), immunosuppressant use (yes or no), smoking status (current or non-smoker), and frequency of alcohol drinking (<1 or ≥1 time/wk). A blue line shows participants who received 2-dose with infection-naïve (N = 2994), and a red line shows those who received 2-dose with a history of SARS-CoV-2 infection (N = 53). A green line shows those who received 3-dose with infection-naïve (N = 3582), and an orange line shows those who received 3-dose with a history of the infection (N = 272). Solid lines are the estimated geometric mean of anti-SARS-CoV-2 spike protein antibody titers, and shaded areas are the corresponding 95% CIs. N, number of measurements.
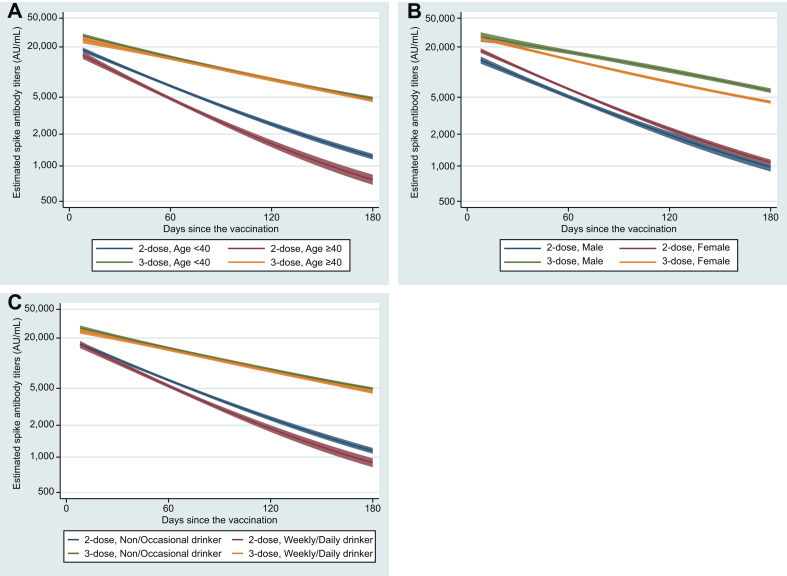
Table 2 and Fig. 2 show the patterns of antibody waning following 2- and 3-dose vaccines, respectively, according to background factors among infection-naïve participants. Older age, male sex, obesity, coexisting diseases, immunosuppressant use, smoking, and alcohol drinking were each associated with lower antibody titers after 2 doses, whereas these associations disappeared after 3 doses, except for sex (females became lower) and immunosuppressant use (still lower). Antibody waning rates were slightly but significantly faster in those who are older (4%), female (3%), and use alcohol (3%) after 2 doses, and somewhat faster in females (3%) after 3 doses.
Table 2.
Characteristics of antibody waning after the second and third vaccination across the background factors among infection-naïve participants
| Characteristics | Estimated spike antibody titers (AU/mL) after 2-dose, GMT (95% CI) |
Slope of antibody waning, RoM (95% CI) |
||||
|---|---|---|---|---|---|---|
| Day 14 | Day 30 | Day 90 | Day 180 | Per 30 d | Comparison of slopes | |
| Age (y) | ||||||
| <40 | 16 076 (15 103–17 113) | 11 618 (11 074–12 189) | 3967 (3803–4137) | 1210 (1140–1285) | 0.64 (0.63–0.65) | reference |
| ≥40 | 13 405 (12 187–14 744)a | 9137 (8538–9778)a | 2651 (2502–2809)a | 749 (677–829)a | 0.61 (0.60–0.63) | 0.96 (0.93–0.99)a |
| Sex | ||||||
| Male | 11 947 (10 886–13 112) | 8685 (8098–9314) | 3041 (2865–3228) | 973 (885–1070) | 0.65 (0.64–0.67) | reference |
| Female | 15 632 (14 706–16 617)a | 11 078 (10 588–11 591)a | 3585 (3446–3729)a | 1070 (1008–1135) | 0.63 (0.62–0.64) | 0.97 (0.94–0.99)a |
| Obesity | ||||||
| <27.5 (kg/m2) | 14 508 (13 765–15 291) | 10 376 (9977–10 791) | 3450 (3335–3569) | 1050 (997–1106) | 0.64 (0.63–0.65) | reference |
| ≥27.5 (kg/m2) | 15 147 (11 122–20 627) | 9956 (8081–12 266) | 2690 (2257–3206)a | 827 (622–1101) | 0.62 (0.57–0.67) | 0.97 (0.89–1.05) |
| Coexisting diseases | ||||||
| No | 14 786 (14 077–11 086) | 10 583 (10 167–11 017) | 3525 (3404–3650) | 1071 (1016–1129) | 0.64 (0.63–0.65) | reference |
| Yes | 14 077 (11 086–17 875) | 9350 (7977–109 59) | 2572 (2259–2928)a | 764 (621–941)a | 0.62 (0.58–0.65) | 0.96 (0.91–1.02) |
| Immunosuppressant use | ||||||
| No | 14 564 (13 825–15 343) | 10 388 (9993–10,798) | 3432 (3319–3549) | 1046 (993–1101) | 0.64 (0.63–0.65) | reference |
| Yes | 8074 (5221–12 486)a | 6315 (4588–8694)a | 2681 (2108–3409)a | 897 (595–1352) | 0.68 (0.61–0.76) | 1.06 (0.95–1.20) |
| Smoking | ||||||
| Non-smokers | 14 663 (13 881–15 490) | 10 463 (10 048–10 894) | 3459 (3340–3582) | 1052 (998–1110) | 0.64 (0.63–0.65) | reference |
| Current smokers | 11 911 (10 084–14 069)a | 8606 (7563–9793)a | 2957 (2642–3309)a | 930 (780–1108) | 0.65 (0.62–0.68) | 1.01 (0.97–1.06) |
| Alcohol drinking | ||||||
| Non or occasional | 14 818 (13 926–15 768) | 10 712 (10 220–11 228) | 3671 (3522–3826) | 1134 (1065–1207) | 0.64 (0.63–0.65) | reference |
| Weekly or daily |
14 269 (12 962–15 706) |
9868 (9212–10 572) |
2998 (2830–3176)a |
884 (807–968)a |
0.62 (0.61–0.64) |
0.97 (0.94–0.99)a |
| Characteristics |
Estimated spike antibody titers (AU/mL) after 3-dose, GMT (95% CI) | Slope of antibody waning, RoM (95% CI) | ||||
| Day 14 |
Day 30 |
Day 90 |
Day 180 |
Per 30 days |
Comparison of slopes |
|
| Age (y) | ||||||
| <40 | 25 788 (24 167–27 517) | 21 110 (20 077–22 196) | 10 696 (10 242–11 171) | 4756 (4543–4980) | 0.75 (0.74–0.75) | reference |
| ≥40 | 23 286 (20 993–25 829) | 19 566 (18 132–21 114) | 10 517 (9992–11 069) | 4552 (4323–4794) | 0.75 (0.74–0.76) | 1.00 (0.99–1.02) |
| Sex | ||||||
| Male | 25 563 (22 422–29 144) | 22 274 (20 255–24 494) | 13 168 (12 360–14 029) | 5827 (5472–6204) | 0.76 (0.75–0.78) | reference |
| Female | 24 200 (22 803–25 683) | 19 770 (18 889–20 692)a | 9926 (9558–10 309)a | 4336 (4166–4513)a | 0.74 (0.73–0.75) | 0.97 (0.95–0.99)a |
| Obesity (kg/m2) | ||||||
| <27.5 | 24 455 (23 117–25 870) | 20 284 (19 442–21 161) | 10 601 (10 251–10 963) | 4678 (4517–4845) | 0.75 (0.74–0.76) | reference |
| ≥27.5 | 27 046 (20 590–35 526) | 23 099 (18 859–28 291) | 12 810 (11 073–14 820)a | 5323 (4630–6120) | 0.75 (0.72–0.78) | 1.00 (0.96–1.04) |
| Coexisting diseases | ||||||
| No | 24 632 (23 250–26 096) | 20 342 (19 472–21 250) | 10 528 (10 168–10 901) | 4668 (4503–4839) | 0.75 (0.74–0.75) | reference |
| Yes | 24 091 (20 053–28 942) | 20 814 (18 166–23 850) | 11 966 (10 833–13 216) | 5133 (4619–5704) | 0.76 (0.74–0.78) | 1.01 (0.98–1.04) |
| Immunosuppressant use | ||||||
| No | 24 737 (23 391–26 160) | 20 554 (19 708–21 437) | 10 788 (10 436–11 151) | 4757 (4596–4923) | 0.75 (0.74–0.76) | reference |
| Yes | 17 168 (12 090–24 379)a | 14 067 (10 717–18 465)a | 7065 (5580–8945)a | 2987 (2331–3828)a | 0.74 (0.69–0.78) | 0.98 (0.93–1.04) |
| Smoking | ||||||
| Non-smokers | 24 621 (23 232–26 092) | 20 479 (19 606–21 392) | 10 777 (10 416–11 150) | 4750 (4584–4922) | 0.75 (0.74–0.76) | Reference |
| Current smokers | 23 542 (19 672–28 175) | 19 415 (16 893–22 313) | 9959 (8824–11 239) | 4309 (3829–4848) | 0.74 (0.72–0.77) | 0.99 (0.96–1.02) |
| Alcohol drinking | ||||||
| Non or occasional | 25 174 (23 477–26 994) | 20 807 (19 741–21 931) | 10 814 (10 372–11 275) | 4839 (4632–5056) | 0.75 (0.74–0.76) | Reference |
| Weekly or daily | 23 513 (21 483–25 735) | 19 708 (18 411–21 097) | 10 509 (9961–11 087) | 4513 (4272–4769) | 0.75 (0.74–0.76) | 1.00 (0.98–1.01) |
Shown are the estimated GMT of anti-SARS-CoV-2 spike protein antibody titers on 14, 30, 90, and 180 d since the third mRNA vaccination and the estimated RoM of antibody waning slopes, with adjustment for age (<40 or ≥40 y), sex (male or female), body mass index (<27.5 or ≥27.5 kg/m2), coexisting diseases (yes or no), immunosuppressant use (yes or no), smoking status (current or non-smoker), and frequency of alcohol drinking (<1 or ≥1 time/wk).
GMT, geometric mean titer; RoM, ratio of means.
p <0.05.
Fig. 2.
Trajectories of anti-SARS-CoV-2 spike protein antibody titers following 2- and 3-dose vaccination by age (A), sex (B), and alcohol drinking status (C) among infection-naïve participants. Curves shown are the estimated trajectories for individuals who had received 2-dose (blue and red lines) and those who had received 3-dose (green and orange lines) adjusted for age (<40 or ≥40 years), sex (male or female), body mass index (<27.5 or ≥27.5 kg/m2), coexisting diseases (yes or no), immunosuppressant use (yes or no), smoking status (current or non-smoker), and frequency of alcohol drinking (<1 or ≥1 time/wk). Solid lines are the estimated geometric mean of anti-SARS-CoV-2 spike protein antibody titers, and shaded areas are the corresponding 95% CIs.
Discussion
Among recipients of COVID-19 mRNA vaccines, we found that the durability of antibody titers was higher after 3 doses than 2 doses, consistent with a previous study of Israeli healthcare workers [3] and compatible with previous studies showing a slower waning of VE for infection over time in recipients of 3-dose vaccine relative to those who received 2-dose vaccine [12,13]. In addition, we found that hybrid immunity (vaccination and infection) has added durability over the immunity induced by vaccination alone. The novelty of this study is that 3-dose plus infection has led to more durable antibodies than 3-dose alone, albeit the difference was modest, which is compatible with a study reporting a higher VE against infection for 3-dose plus prior infection versus 3-dose alone [13]. Interestingly, we observed that those with 2-dose plus infection had more durable antibody titers than those with 3-dose alone. This result suggests that natural infection combined with vaccination conferred more durable immunity than vaccine alone, which is in line with the results of meta-analysis [13,14].
Among the infection-naïve participants, we observed that older age, male sex, obesity, coexisting diseases, immunosuppressant use, smoking, and regular alcohol use were each associated with lower antibody titers throughout 14–180 days following the second dose. These results were consistent with previous cross-sectional studies [[6], [7], [8], [9], [10]]. After the 3-dose vaccine, these associations disappeared except for sex and immunosuppressant use, suggesting that a booster shot can enhance immunity and minimize the difference between groups. Still, those with immunosuppressive status had lower antibody titers after 3 doses, a finding compatible with the VE study among 2- and 3-dose vaccine recipients [2]. Our results highlight the need for careful monitoring of infection risk and consideration of additional boosters among individuals receiving immunosuppressants.
Regarding antibody waning, we observed a slightly faster decline (3–4%) associated with older age, female sex, and regular alcohol use after 2 doses. After 3 doses, the differences by age and alcohol used disappeared, whereas the female sex remained a slight but significant driver of antibody decline (3%). This result suggests that a booster dose plays a role in eliminating gaps in antibody durability by background factors. We have no plausible explanation for the faster waning in females after both 2 and 3 doses. Interestingly, a meta-analysis of clinical trials on primary COVID-19 vaccines showed that females have a significantly lower VE than males [15]; nonetheless, subsequence studies on VE after 3 doses and waning of VE after 2 and 3 doses had not examined any sex difference. Our results highlight the need for further studies regarding sex difference in vaccine efficacy and immunogenicity. Study strengths and limitations are described in Text S2.
In summary, among the recipients of mRNA COVID-19 vaccines, the durability of anti-spike antibody titers over time was higher after 3-dose than 2-dose vaccine, and hybrid immunologic status (vaccination and infection) appears to confer additional durability of antibody titers. The pattern of waning of antibodies was modified by demographic and lifestyle factors. These results inform policymakers of the importance of consideration for personal background, the number of vaccinations received, and the history of infection in formulating vaccine recommendations.
Author contributions
Conceptualization and methodology: SY and TM. Software: SY and MK. Formal analysis: SY. Investigation: TM, SY, YO, NI, TN, KH, KO, MK, and MO. Resources: TM, MO, HS, NA, WS, and NO. Data curation: SY and MK. Writing—original draft: SY. Writing—review and editing: all authors. Visualization: SY. Supervision and project administration: TM and NO. Funding acquisition: TM.
Transparency declaration
The authors declare that they have no conflicts of interest.
Funding
This work was supported by the NCGM COVID-19 Gift Fund (grant number 19K059), the Japan Health Research Promotion Bureau Research Fund (grant number 2020-B-09), and the Grant of National Center for Global Health and Medicine (grant number 21A2013D). Abbott Japan and Roche Diagnostics provided reagents for anti-SARS-CoV-2 antibody assays.
Acknowledgements
We thank Mika Shichishima for her contribution to data collection and the staff of the Laboratory Testing Department for their contribution to measuring antibody testing.
Handling Editor: M. Cevik
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cmi.2023.05.020.
Appendix A. Supplementary data
The following is the Supplementary data to this article.
References
- 1.Feikin D.R., Higdon M.M., Abu-Raddad L.J., Andrews N., Araos R., Goldberg Y., et al. Duration of effectiveness of vaccines against SARS-CoV-2 infection and COVID-19 disease: results of a systematic review and meta-regression. Lancet. 2022;399:924–944. doi: 10.1016/S0140-6736(22)00152-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tseng H.F., Ackerson B.K., Luo Y., Sy L.S., Talarico C.A., Tian Y., et al. Effectiveness of mRNA-1273 against SARS-CoV-2 omicron and delta variants. Nat Med. 2022;28:1063–1071. doi: 10.1038/s41591-022-01753-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gilboa M., Regev-Yochay G., Mandelboim M., Indenbaum V., Asraf K., Fluss R., et al. Durability of immune response after COVID-19 booster vaccination and association with COVID-19 Omicron infection. JAMA Netw Open. 2022;5 doi: 10.1001/jamanetworkopen.2022.31778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim H.J., Yun H.J., Kim J., Kym S., Choi Q. Antibody response to second dose of the BNT162b2 mRNA vaccine in the first 12 weeks in South Korea: a prospective longitudinal study. Vaccine. 2022;40:437–443. doi: 10.1016/j.vaccine.2021.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhong D., Xiao S., Debes A.K., Egbert E.R., Caturegli P., Colantuoni E., et al. Durability of antibody levels after vaccination with mRNA SARS-CoV-2 vaccine in individuals with or without prior infection. JAMA. 2021;326:2524. doi: 10.1001/jama.2021.19996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yamamoto S., Mizoue T., Tanaka A., Oshiro Y., Inamura N., Konishi M., et al. Sex-associated differences between BMI and SARS-CoV-2 antibody titers following the BNT162b2 vaccine. Obesity. 2022;30:999–1003. doi: 10.1002/oby.23417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yamamoto S., Tanaka A., Oshiro Y., Inamura N., Mizoue T., Ohmagari N., et al. Antibody responses and correlates after two and three doses of BNT162b2 COVID-19 vaccine. Infection. 2023;51:523–525. doi: 10.1007/s15010-022-01898-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Levin E.G., Lustig Y., Cohen C., Fluss R., Indenbaum V., Amit S., et al. Waning immune humoral response to BNT162b2 COVID-19 vaccine over 6 months. N Engl J Med. 2021;385:e84. doi: 10.1056/NEJMoa2114583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kageyama T., Ikeda K., Tanaka S., Taniguchi T., Igari H., Onouchi Y., et al. Antibody responses to BNT162b2 mRNA COVID-19 vaccine and their predictors among healthcare workers in a tertiary referral hospital in Japan. Clin Microbiol Infect. 2021;27:1861. doi: 10.1016/j.cmi.2021.07.042. e1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nomura Y., Sawahata M., Nakamura Y., Kurihara M., Koike R., Katsube O., et al. Age and smoking predict antibody titres at 3 months after the second dose of the BNT162b2 COVID-19 vaccine. Vaccines. 2021;9:1042. doi: 10.3390/vaccines9091042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yamamoto S., Tanaka A., Ohmagari N., Yamaguchi K., Ishitsuka K., Morisaki N., et al. Use of heated tobacco products, moderate alcohol drinking, and anti-SARS-CoV-2 IgG antibody titers after BNT162b2 vaccination among Japanese healthcare workers. Prev Med. 2022;161 doi: 10.1016/j.ypmed.2022.107123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Andrews N., Stowe J., Kirsebom F., Toffa S., Rickeard T., Gallagher E., et al. COVID-19 vaccine effectiveness against the Omicron (B.1.1.529) variant. N Engl J Med. 2022;386:1532–1546. doi: 10.1056/NEJMoa2119451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Altarawneh H.N., Chemaitelly H., Ayoub H.H., Tang P., Hasan M.R., Yassine H.M., et al. Effects of previous infection and vaccination on symptomatic Omicron infections. N Engl J Med. 2022;387:21–34. doi: 10.1056/NEJMoa2203965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bobrovitz N., Ware H., Ma X., Li Z., Hosseini R., Cao C., et al. Protective effectiveness of previous SARS-CoV-2 infection and hybrid immunity against the omicron variant and severe disease: a systematic review and meta-regression. Lancet Infect Dis. 2023;23(5):556–567. doi: 10.1016/S1473-3099(22)00801-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bignucolo A., Scarabel L., Mezzalira S., Polesel J., Cecchin E., Toffoli G. Sex disparities in efficacy in COVID-19 vaccines: a systematic review and meta-analysis. Vaccines. 2021;9:825. doi: 10.3390/vaccines9080825. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.