Abstract
Aim
This review aimed to estimate the level of acceptance of COVID-19 vaccine among persons with diabetes.
Methods
A systematic search was conducted on PubMed, MEDLINE, Embase, and CINAHL to identify relevant studies for this review. A random-effects meta-analysis was performed to generate an overall estimate of vaccine acceptance. The I2 statistic was used to quantify the degree of variation across studies, and subgroup analysis was conducted to identify the sources of heterogeneity. The review was conducted in line with the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA).
Results
This review included 18 studies involving 11,292 diabetes patients. The pooled prevalence of COVID-19 vaccine acceptance among persons with diabetes was 76.1% (95% CI: 66.7%–83.5%). The pooled prevalence across the continent ranged from 68.9% (95% CI: 47.8%–84.3%) in Asia to 82.1% (95% CI: 80.2%–83.8%) in Europe. Barriers to vaccine acceptance included misinformation, lack of information, mistrust, health concerns, and external influences.
Conclusion
The barriers to vaccine acceptance identified in this review, could inform the formulation of health policies and public health interventions that are specifically tailored to address the needs of persons with diabetes.
Keywords: Diabetes, COVID-19 vaccine, Vaccine acceptance, Systematic review, Meta-analysis
1. Introduction
The COVID-19 pandemic has had a devastating global impact and has led to unprecedented disruptions to healthcare systems, economies, and daily life. COVID-19 is said to have no boundaries and thus affects all individuals irrespective of their composition. However, the clinical spectrum of the disease disproportionately impacts persons with chronic disease, including diabetes [1]. Diabetes has been identified as a significant risk factor for contracting COVID-19 and is associated with higher rates of hospitalization in intensive care units [2], [3]. Additionally, individuals with diabetes are nearly two times more likely to experience COVID-19-related mortality compared to those without the condition [3].
The COVID-19 pandemic has prompted a robust response from the healthcare community, including the development and deployment of vaccines. These vaccines have demonstrated the potential to effectively curb the transmission of the virus and mitigate its severe consequences [4]. However, the success of vaccination efforts is contingent upon the willingness and preparedness of the population to accept and receive vaccines [5]. This is particularly crucial for vulnerable populations, such as persons with diabetes. Nonetheless, there have been reports of vaccine hesitancy among some diabetes patients [6], [7], despite their prioritization in early vaccine distribution programs [8]. This is partly driven by the belief that COVID-19 is not dangerous to diabetes patients’ health and that vaccination does not reduce the risk of infection [9]. Furthermore, safety and efficacy concerns associated with COVID-19 vaccines have been identified as critical predictors of vaccine acceptance [10].
Vaccine hesitancy is a complex issue that impedes efforts against vaccine-preventable diseases [11]. Considering the serious ramifications of COVID-19 on diabetes patients, vaccination is an essential aspect of diabetes management and a vital approach for mitigating the impact of the pandemic on this patient population. This underscores the importance of investigating the rate of vaccine acceptance among persons with diabetes. However, to date, there has been a lack of a comprehensive review synthesizing the acceptance of COVID-19 vaccines among individuals with diabetes. The extent of COVID-19 vaccine acceptance among diabetes patients remains unclear, as individual studies on this topic have reported varying acceptance rates. Therefore, this review aimed to estimate the level of acceptance of COVID-19 vaccines among persons with diabetes by synthesizing the results of relevant studies across the globe. The barriers to COVID-19 vaccine acceptance were also assessed.
2. Materials and methods
Prior to this study, an extensive search of electronic medical databases was done to explore the available literature and to identify evidence gaps. Subsequently, a protocol for this review was developed and registered on PROSPERO (CRD42022371963). This review adhered to the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) guidelines.
2.1. Inclusion and exclusion criteria
The present study adopted the CoCoPop framework (condition, context, and population) to establish the inclusion criteria. This framework was used given its wide recognition in systematic review assessing prevalence or incidence data [12], [13]. As such, the inclusion criteria were comprised of primary studies that quantitatively assessed COVID-19 vaccine acceptance (willingness or hesitancy) among persons with diabetes. No restrictions were placed on the study context, provided that the research was conducted in a real-world setting. In addition, only peer-reviewed articles published in the English language were considered for inclusion.
Priority was given to studies that focused primarily on diabetes patients. However, studies conducted on the general population were included if the COVID-19 vaccine acceptance rate was extractable for diabetes patients who were part of the study. On the other hand, review articles, preprints, case reports, conference abstracts, posters, and letters to the editor were excluded. In cases where duplicate studies were identified, wherein a single study was reported in multiple publications, only one study was selected based on its relevance to the outcome of interest and its high methodological quality.
2.2. Outcome definition
The principal outcome of interest of this study was vaccine acceptance among persons with diabetes, operationalized as the proportion of participants who indicated their willingness to receive the COVID-19 vaccine or were not hesitant to take the vaccine. As a secondary objective, barriers to vaccine acceptance were defined as reasons for diabetes patients’ unwillingness or hesitancy to receive the COVID-19 vaccine.
2.3. Search strategy
A comprehensive systematic search was conducted on November 20, 2022 to identify relevant literature pertaining to the topic of interest. The search was executed on four major electronic databases: PubMed, MEDLINE, Embase, and Cumulative Index to Nursing and Allied Health Literature (CINAHL). To ensure exhaustiveness, the search was supplemented with additional searches on specialized databases dedicated to COVID-19 research, such as LitCovid and the World Health Organization's COVID-19 research database. A manual examination of reference lists from eligible articles was also conducted to ensure the inclusion of all relevant studies.
The search was updated on February 01, 2023 to include recently indexed studies, with the aim of ensuring the comprehensiveness of the results. The initial search was limited to articles published between the years 2020 and 2022 to reflect the most recent and up-to-date information.
The search strategy was informed by the population and outcome of interest and was developed based on a combination of key terms including “COVID-19 vaccine,” “hesitancy or uptake,” and “diabetes.” Boolean combinations (AND, OR, NOT) of these terms, along with database-specific index terms, were utilized to optimize the search results. The full details of the search strategy are presented in the Supplementary file.
2.4. Screening and study selection
Duplicates from retrieved articles were removed using EndNote 20. The remaining articles were then uploaded to Rayyan [14] for title and abstract screening based on the predefined eligibility criteria. The full text of selected studies was retrieved and assessed to ascertain if they met the requirements for inclusion in this review.
2.5. Data extraction
A standardized data extraction form in Excel format was designed to retrieve important information from the included studies. The extracted data included: first author’s name, year of publication, country, study design, type of diabetes, and data on the COVID-19 vaccination acceptance. Both reviewers independently extracted the data.
2.6. Quality assessment
This review comprised of studies that provided data on the prevalence or proportion of COVID-19 vaccine uptake or hesitancy among diabetes patients. Hence, the Joanna Briggs Institute (JBI) critical appraisal checklist for studies reporting prevalence data was used to assess the methodological quality of the included studies [15]. The appraisal tool addresses 9 critical questions and has response options such as “Yes”, “No”, “Unclear” and “Not applicable”. In the absence of a defined cut-off point for the quality score of studies, we denote studies with 50% or more “Yes” across the quality assessment parameters as low risk. A comprehensive description of the quality assessment tool is presented in the Supplementary file.
2.7. Statistical analysis
The meta-analysis was performed based on a random-effects model. This model was used given the considerable variability across the included studies. Furthermore, we computed the pooled prevalence of COVID-19 vaccine acceptance using a generalized linear mixed model with the logit transformation, as recommended by Warton and Hui [16]. The 95% confidence interval (CI) for the proportion was calculated using the Clopper-Pearson interval and results from the analysis presented in forest plots. We quantified the proportion of variability due to heterogeneity across studies using the I2 statistic, with values of 25%, 50%, and 75% indicating low, moderate, and high levels of heterogeneity, respectively [17]. Subgroup analysis was done for gender, continent, data collection method, sample size, and diabetes participants as the primary focus of the study. The presence of publication bias was evaluated using funnel plots and was statistically explored using the test proposed by Peters et al [18]. The meta-package in R statistical software was used for the statistical analysis.
3. Results
3.1. Search results
Our systematic search yielded a total of 2499 studies, which comprised of 2464 records from four databases (PubMed, MEDLINE, Embase, and CINAHL) and 35 records from others sources. After duplicate records were removed and 2351 articles had been screened for their titles and abstracts, the full text of 62 articles were assessed. Finally, 18 articles met our inclusion criteria for this study. A summary of the steps involved in the screening process and reasons for exclusion of articles after full-text review are provided in Fig. 1 .
Fig. 1.
PRISMA flow chart summarizing the article selection process.
3.2. Characteristics of included studies
All included studies consisted of cross-sectional designs. However, 1 study used a mixed-method approach to evaluate the responses of the participants [19]. Majority of the studies were published in 2022 (n = 12). The study involved 11,292 diabetes participants, the majority of which were females (54.1%) as per studies that reported both gender proportions. Apart from Osuagwu et al’s study which recruited participants from multiple countries in Sub-Saharan Africa, all the remaining studies involved a single country. Grouped under the continent of study, Asia dominated with 8 studies [6], [9], [20], [21], [22], [23], [24], [25], followed by North America (n = 5) [26], [27], [28], [29], [30], Europe (n = 2) [7], [31], Africa (n = 2) [19], [32], and Australia (n = 1) [33]. A summary of the characteristics of the included studies is provided in Table 1 .
Table 1.
Characteristics of the included studies.
| First author (Year) | Country | Type of diabetes | Study Design | Diabetes sample | Female proportion |
Vaccine acceptance | Quality assessment score | Quality of study |
|---|---|---|---|---|---|---|---|---|
| Wang (2022) | China | Both | CS | 483 | 47.8 | 210 | 7 | High |
| Lu (2022) | China | T2DM | CS | 170 | 51.8 | 131 | 5 | High |
| Tsai (2022) | USA | T2DM | CS | 1400 | NR | 1134 | 5 | High |
| Day (2022) | Australia | NI | CS | 842 | 44.9 | 742 | 7 | High |
| Osuagwu (2022) | SSA | NI | MM | 73 | 34.2 | 48 | 5 | High |
| Asadi-Pooya (2022) |
Iran | NI | CS | 127 | NR | 108 | 5 | High |
| Kolobov (2022) | Israel | Both | CS | 308 | 61.2 | 127 | 5 | High |
| Mesele (2022) | Ethiopia | Both | CS | 386 | 30.6 | 319 | 7 | High |
| Velario (2022) | Canada | NI | CS | 193 | NR | 114 | 7 | High |
| Kanyangarara (2022) | USA | NI | CS | 1210 | NR | 1135 | 5 | High |
| Syed (2021) | Malaysia | NI | CS | 97 | NR | 73 | 8 | High |
| Czeisler (2021) | USA | NI | CS | 760 | 59.1 | 597 | 5 | High |
| Guaraldi (2021) | Italy | T2DM | CS | 1176 | 73.1 | 967 | 5 | High |
| Aldossari (2021) | Saudi Arabia | Both | CS | 709 | 59.5 | 257 | 7 | High |
| Scoccimarro (2021) |
Italy | Both | CS | 502 | 60.2 | 410 | 5 | High |
| Waite (2021) | Canada | NI | CS | 744 | NR | 589 | 6 | High |
| Abedin (2021) | Bangladesh | NI | CS | 488 | NR | 331 | 7 | High |
| Okobo (2021) | Japan | NI | CS | 1628 | NR | 1531 | 8 | High |
CS: Cross-sectional, MM: Mixed method, NI: Not indicated, NR: Not reported, T2DM: Type 2 diabetes mellitus.
3.3. Quality of included studies
The quality assessment of the included studies identified that all studies had a score of 50% and above with a mean score of 67.3%. The authors (EE and SA) involved in the quality assessment of the included studies agreed on almost 85% of the scores awarded. Disagreements were discussed and consensus was attained. Results of the quality assessment are presented in Table 1.
3.4. Meta-analysis of COVID-19 vaccine acceptance
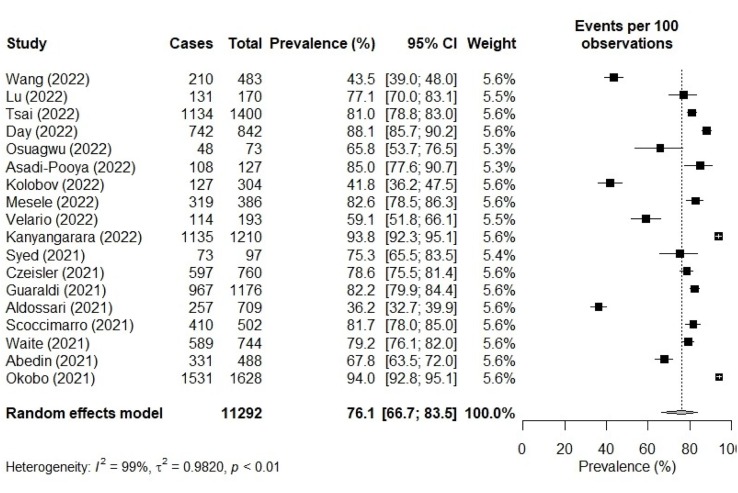
The proportion of persons with diabetes who accepted the COVID-19 vaccine ranged from 36.2% [6] to 94.0% [25]. The meta-analysis showed that the pooled prevalence of COVID-19 vaccine acceptance was 76.1% (95% CI: 66.7 – 83.5). There was a significantly high heterogeneity among the studies (I2 = 99%, p < 0.01) as shown in Fig. 2 .
Fig. 2.
COVID-19 vaccine acceptance among persons with diabetes.
Results from the subgroup analysis are presented in Table 2 . Subgroup analysis per continent of the study revealed regional variability in vaccine acceptance, with the highest acceptance reported in Europe 82.1% (95% CI: 80.2 – 83.8) and the lowest in Asia 68.9% (95% CI: 47.8 – 84.3). In terms of gender-based comparisons, the acceptance of COVID-19 vaccine was higher in male diabetes patients 73.2% (95% CI: 54.3 – 86.3) compared to their female counterparts 59.1% (95% CI: 40.2 – 75.8). Moreover, studies with a sample size > 500, conducted online and primarily focused on non-diabetes participants, had a high proportion of COVID-19 vaccine acceptance. No heterogeneity was identified in studies from Europe. However, the result was insignificant (I2 = 0, p = 0.79).
Table 2.
Subgroups analysis of COVID-19 vaccine acceptance.
| Variables | No. of studies | Proportion (95% CI) |
I2 | p-value | |
|---|---|---|---|---|---|
| Sample size | <500 >500 |
9 9 |
67.7 (55.9–77.7) 82.6 (71.3–90.0) |
97% 99% |
P < 0.01 P < 0.01 |
| Gender | Male Female |
6 6 |
73.2 (54.3–86.3) 59.1 (40.2–75.8) |
96% 96% |
P < 0.01 P < 0.01 |
| Continent | Asia North America Europe Africa |
8 5 2 2 |
68.9 (47.8–84.3) 80.8 (70.8–88.0) 82.1 (80.2–83.8) 75.6 (56.0–88.3) |
99% 98% 0% 90% |
P < 0.01 P < 0.01 P = 0.79 P < 0.01 |
| Data collection method | In person Online |
6 12 |
71.7 (56.8–83.0) 78.0 (66.2–86.6) |
98% 99% |
P < 0.01 P < 0.01 |
| Diabetes as primary focus | Yes No |
9 9 |
67.5 (51.9–80.0) 83.0 (74.5–89.0) |
99% 98% |
P < 0.01 P < 0.01 |
3.5. Publication bias
The results of the funnel plot analysis, presented in Fig. 3 , revealed an asymmetrical distribution of the studies, suggesting the potential presence of publication bias. In contrast, the results from the statistical tests for publication bias, as assessed through Peters' test, indicated no evidence of publication bias (p-value = 0.3406). The divergent results between the funnel plot analysis and the statistical test may be a consequence of the high heterogeneity of the studies included in the analysis, rather than publication bias, as noted by Sterne et al [34].
Fig. 3.
Funnel plot for COVID-19 vaccine acceptance among persons with diabetes.
3.6. Barriers to vaccine acceptance
Reasons behind diabetes patients’ unwillingness to accept COVID-19 vaccine were extracted from 5 studies. In all, nineteen reasons were cited by diabetes patients as barriers for their unwillingness to accept the COVID-19 vaccine. These reasons emerged under 5 themes as summarized in Table 3 . Reasons for vaccine unwillingness stemmed predominately from health or safety concerns in relation to COVID-19 vaccination [6], [9], [19], [20], [22]. These concerns were centered on the perceived negative experience with the vaccine, such as side effects from the vaccine, adverse reactions, allergic reactions, glucose variation, and the possibility of vaccines leading to other diseases.
Table 3.
Barriers to COVID-19 vaccine acceptance.
| Themes | Reasons for vaccine hesitancy |
|---|---|
| Misinformation |
|
| Lack of Information |
|
| Mistrust |
|
| Health Concerns |
|
| External Influence |
|
4. Discussion
4.1. Summary of the results
The aim of this study was to determine the acceptance rate of the COVID-19 vaccine among individuals with diabetes. To achieve this objective, a comprehensive literature search was conducted to identify relevant studies, and a meta-analysis was performed to synthesize the prevalence data. The findings of our meta-analysis revealed that individuals with diabetes have a combined COVID-19 vaccine acceptance rate of 76.1% (95% CI: 66.7– 83.5). This high acceptance rate could be attributed to diabetes patients' heightened awareness of their susceptibility to the COVID-19 disease [35] which may have led to a better understanding of the benefits of vaccination in preventing severe illness and hospitalization. Our findings align with empirical studies that demonstrate the conscientiousness of diabetes patients regarding preventive measures against COVID-19, such as proper hand hygiene, wearing of face masks, and maintaining social distancing [36], [37]. Moreover, the earlier prioritization of individuals with diabetes in the COVID-19 vaccine rollout [8] may have played a role in increasing awareness and education about the importance of vaccination in this population, which in turn may have boosted their willingness to accept the vaccine. Compared to the findings of meta-analysis of the general population, the acceptance rate of the COVID-19 vaccine among individuals with diabetes is higher, as demonstrated in our results [11], [38], [39], [40]. It is also worth noting that the results of this study demonstrate a higher acceptance rate of COVID-19 vaccine among individuals with diabetes as compared to other high-risk populations, such as cancer patients (59%) [41] and patients with chronic disease in general (65%) [42].
Our subgroup analysis, stratified according to continent, has revealed notable variations in the acceptance rates of COVID-19 vaccines. Specifically, we observed the highest acceptance rates in Europe, followed by America, Africa, and the lowest in Asia. This finding is consistent with previous meta-analyses that examined vaccine acceptance rates among individuals with chronic diseases, which similarly reported higher acceptance rates in Europe and America compared to Asia [42]. The high vaccine acceptance rate in Europe and America is attributed to the implementation of policies such as the early prioritization of persons with chronic disease which is likely to have increased awareness about the importance of vaccination [42]. It is worth nothing that other factors may also account for the variation of the vaccine willingness across continents. Additionally, our analysis of gender differences in vaccine acceptance rates revealed that male diabetes patients had a higher acceptance rate compared to their female counterparts. This finding aligns with the outcomes of previous meta-analyses conducted on the general population, which indicated that males are more inclined to accept COVID-19 vaccines [11], [38], [40]. Research has shown that males are more susceptible to contracting COVID-19, more prone to experiencing severe symptoms, and have a poorer prognosis [43], [44]. This heightened risk may serve as a motivating factor for males to actively seek vaccination. Moreover, it has been observed that males possess a greater level of confidence in the safety of the COVID-19 vaccine [22], which could also contribute to the observed higher acceptance rates. It is important to acknowledge that, apart from gender, other sociodemographic factors may also influence COVID-19 vaccine acceptance rates. However, due to limited information on these variables from the included studies, their analysis was not conducted. Future research should therefore explore the influence of these sociodemographic factors on COVID-19 vaccine acceptance rates to gain a more thorough understanding of the subject matter.
Barriers associated with COVID-19 vaccine acceptance emerged under 5 themes, namely, misinformation, lack of information, mistrust, health concerns, and external influences. Several studies on vaccine hesitancy have consistently identified these themes [45], [46]. The dissemination of false information via digital media platforms presents a major challenge, particularly in the realm of misinformation. Addressing this barrier requires a robust fact-checking and counter-messaging strategy to provide accurate information to the public [47]. It is also crucial for the scientific and healthcare communities to work in unison to dispel myths and provide reliable information, thereby building trust and encouraging vaccine uptake, particularly among high-risk populations such as individuals with diabetes. Addressing health-related concerns about COVID-19 vaccines is of utmost importance and requires healthcare providers to furnish credible and evidence-based information on vaccine safety and efficacy, in addition to closely monitoring and reporting any adverse events. Given that adverse events associated with COVID-19 vaccines are mostly mild and resolvable [48], healthcare providers must help patients to understand this concept and stay informed.
4.2. Strengths and limitations
This review provides a comprehensive analysis of the acceptance of COVID-19 vaccination among individuals diagnosed with diabetes, offering valuable insights into the current status of vaccine uptake within this population. The primary strength of this review lies in its novelty, as it establishes a benchmark for future investigations in this field. Additionally, the literature search employed specialized databases dedicated to COVID-19 research, ensuring the inclusion of all relevant studies on this topic. Despite these strengths, the review does have some limitations that must be acknowledged. Firstly, the studies included in the analysis exhibit a significant level of heterogeneity, which may challenge the validity of definitive conclusions regarding vaccine acceptance among individuals with diabetes. Another limitation lies in the fact that we did not assess the impact of different vaccine types on acceptance rates among diabetes patients, which could have influenced the outcomes. Furthermore, the exclusion of non-English language articles may introduce a potential bias by omitting perspectives from diverse cultural and linguistic groups, thereby limiting the generalizability of the findings. In light of these limitations, it is crucial to interpret the results of this review with caution.
5. Conclusion
In conclusion, the results of this study suggest that a majority of diabetes patients have accepted the COVID-19 vaccine. However, given the higher risk of severe COVID-19 among individuals with diabetes, it is important for healthcare providers to continue promoting the benefits of vaccination and addressing any concerns to increase vaccine uptake. The barriers that have been identified can be leveraged to formulate health policies and public health interventions that are specifically tailored to address the needs of persons with diabetes.
Funding
No funding was received to undertake this project.
Authors’ Contributions
The study was a collaborative effort between both authors. E.E conceptualized the study and designed the methods. Both authors (S.A and E.E) performed the study selection, data extraction, quality assessment, analysis, and wrote the manuscript. Both authors also critically reviewed the manuscript. The authors read and approved the final manuscript.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.diabres.2023.110731.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Sen S., Chakraborty R., Kalita P., Pathak M.P. Diabetes mellitus and COVID-19: Understanding the association in light of current evidence. World J Clin Cases. 2021;9(28):8327–8339. doi: 10.12998/wjcc.v9.i28.8327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wolff D., Nee S., Hickey N.S., Marschollek M. Risk factors for Covid-19 severity and fatality: a structured literature review. Infection. 2021;49(1):15–28. doi: 10.1007/s15010-020-01509-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kastora S., Patel M., Carter B., Delibegovic M., Myint P.K. Impact of diabetes on COVID-19 mortality and hospital outcomes from a global perspective: An umbrella systematic review and meta-analysis. Endocrinol Diabetes Metab. 2022;5(3) doi: 10.1002/edm2.338. e00338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moghadas S.M., Vilches T.N., Zhang K., et al. The Impact of Vaccination on Coronavirus Disease 2019 (COVID-19) Outbreaks in the United States. Clin Infect Dis. 2021;73(12):2257–2264. doi: 10.1093/cid/ciab079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaine G., Wright V., Greenhalgh S. Predicting willingness to be vaccinated for Covid-19: Evidence from New Zealand. PLoS One. 2022;17(4) doi: 10.1371/journal.pone.0266485. e0266485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aldossari K.K., Alharbi M.B., Alkahtani S.M., Alrowaily T.Z., Alshaikhi A.M., Twair A.A. COVID-19 vaccine hesitancy among patients with diabetes in Saudi Arabia. Diabetes Metab Syndr. 2021;15(5) doi: 10.1016/j.dsx.2021.102271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guaraldi F., Montalti M., Di Valerio Z., et al. Rate and Predictors of Hesitancy toward SARS-CoV-2 Vaccine among Type 2 Diabetic Patients: Results from an Italian Survey. Vaccines (Basel) 2021;9(5):460. doi: 10.3390/vaccines9050460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nguyen K.H., Srivastav A., Razzaghi H., et al. COVID-19 Vaccination Intent, Perceptions, and Reasons for Not Vaccinating Among Groups Prioritized for Early Vaccination - United States, September and December 2020. MMWR Morb Mortal Wkly Rep. 2021;70(6):217–222. doi: 10.15585/mmwr.mm7006e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang Y., Duan L., Li M., et al. COVID-19 Vaccine Hesitancy and Associated Factors among Diabetes Patients: A Cross-Sectional Survey in Changzhi, Shanxi, China. Vaccines (Basel) 2022;10(1):129. doi: 10.3390/vaccines10010129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patwary M.M., Alam M.A., Bardhan M., et al. COVID-19 Vaccine Acceptance among Low- and Lower-Middle-Income Countries: A Rapid Systematic Review and Meta-Analysis. Vaccines (Basel) 2022;10(3):427. doi: 10.3390/vaccines10030427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dubé E., Vivion M., MacDonald N.E. Vaccine hesitancy, vaccine refusal and the anti-vaccine movement: influence, impact and implications. Expert Rev Vaccines. 2015;14(1):99–117. doi: 10.1586/14760584.2015.964212. [DOI] [PubMed] [Google Scholar]
- 12.Munn Z., Stern C., Aromataris E., Lockwood C., Jordan Z. What kind of systematic review should I conduct? A proposed typology and guidance for systematic reviewers in the medical and health sciences. BMC Med Res Methodol. 2018;18(1):5. doi: 10.1186/s12874-017-0468-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ekpor E., Akyirem S., Adade D.P. Prevalence and associated factors of overweight and obesity among persons with type 2 diabetes in Africa: a systematic review and meta-analysis. Ann Med. 2023;55(1):696–713. doi: 10.1080/07853890.2023.2182909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ouzzani M., Hammady H., Fedorowicz Z., Elmagarmid A. Rayyan-a web and mobile app for systematic reviews. Syst Rev. 2016;5(1):210. doi: 10.1186/s13643-016-0384-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Munn Z., Moola S., Lisy K., Riitano D., Tufanaru C. Methodological guidance for systematic reviews of observational epidemiological studies reporting prevalence and cumulative incidence data. Int J Evid Based Healthc. 2015;13(3):147–153. doi: 10.1097/XEB.0000000000000054. [DOI] [PubMed] [Google Scholar]
- 16.Warton D.I., Hui F.K. The arcsine is asinine: the analysis of proportions in ecology. Ecology. 2011;92(1):3–10. doi: 10.1890/10-0340.1. [DOI] [PubMed] [Google Scholar]
- 17.Higgins J.P., Thompson S.G. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 18.Peters J.L., Sutton A.J., Jones D.R., Abrams K.R., Rushton L. Comparison of two methods to detect publication bias in meta-analysis. JAMA. 2006;295(6):676–680. doi: 10.1001/jama.295.6.676. [DOI] [PubMed] [Google Scholar]
- 19.Osuagwu U.L., Langsi R., Ovenseri-Ogbomo G., et al. Analysis of Perception, Reasons, and Motivations for COVID-19 Vaccination in People with Diabetes across Sub-Saharan Africa: A Mixed-Method Approach. Int J Environ Res Public Health. 2022;19(13):7875. doi: 10.3390/ijerph19137875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lu D., Gao Y., Qi X., Li A., Zhang J. The COVID-19 vaccination hesitancy among Chinese individuals with diabetes and the impact on glycemic control of vaccination: a questionnaire study. BMC Endocr Disord. 2022;22(1):329. doi: 10.1186/s12902-022-01201-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Asadi-Pooya A.A., Barzegar Z., Sadeghian S., Nezafat A., Shahisavandi M., Nabavizadeh S.A. COVID-19 Vaccine Hesitancy Among Patients With Epilepsy or Other Chronic Conditions. Disaster Med Public Health Prep. 2022;16(5):1848–1850. doi: 10.1017/dmp.2021.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kolobov T., Djuraev S., Promislow S., Tamir O. Determinants of COVID-19 vaccine acceptance among adults with diabetes and in the general population in Israel: A cross-sectional study. Diabetes Res Clin Pract. 2022;189 doi: 10.1016/j.diabres.2022.109959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Syed Alwi S.A.R., Rafidah E., Zurraini A., Juslina O., Brohi I.B., Lukas S. A survey on COVID-19 vaccine acceptance and concern among Malaysians. BMC Public Health. 2021;21(1):1129. doi: 10.1186/s12889-021-11071-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abedin M., Islam M.A., Rahman F.N., et al. Willingness to vaccinate against COVID-19 among Bangladeshi adults: Understanding the strategies to optimize vaccination coverage. PLoS One. 2021;16(4) doi: 10.1371/journal.pone.0250495. e0250495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Okubo R., Yoshioka T., Ohfuji S., Matsuo T., Tabuchi T. COVID-19 Vaccine Hesitancy and Its Associated Factors in Japan. Vaccines. 2021;9(6):662. doi: 10.3390/vaccines9060662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tsai R., Hervey J., Hoffman K., et al. COVID-19 Vaccine Hesitancy and Acceptance Among Individuals With Cancer, Autoimmune Diseases, or Other Serious Comorbid Conditions: Cross-sectional, Internet-Based Survey. JMIR Public Health Surveill. 2022;8(1) doi: 10.2196/29872. e29872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Valerio V., Rampakakis E., Zanos T.P., et al. High Frequency of COVID-19 Vaccine Hesitancy among Canadians Immunized for Influenza: A Cross-Sectional Survey. Vaccines (Basel) 2022;10(9):1514. doi: 10.3390/vaccines10091514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kanyangarara M., McAbee L., Daguise V.G., Nolan M.S. Factors Associated with COVID-19 Vaccine Intentions among South Carolina Residents. Vaccines (Basel) 2022;10(6):942. doi: 10.3390/vaccines10060942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Czeisler M.É., Barrett C.E., Siegel K.R., et al. Health Care Access and Use Among Adults with Diabetes During the COVID-19 Pandemic - United States, February-March 2021. MMWR Morb Mortal Wkly Rep. 2021;70(46):1597–1602. doi: 10.15585/mmwr.mm7046a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Waite N.M., Pereira J.A., Houle S.K.D., Gilca V., Andrew M.K. COVID-19's Impact on Willingness to Be Vaccinated against Influenza and COVID-19 during the 2020/2021 Season: Results from an Online Survey of Canadian Adults 50 Years and Older. Vaccines (Basel) 2021;9(4):346. doi: 10.3390/vaccines9040346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scoccimarro D., Panichi L., Ragghianti B., Silverii A., Mannucci E., Monami M. Sars-CoV2 vaccine hesitancy in Italy: A survey on subjects with diabetes. Nutr Metab Cardiovasc Dis. 2021;31(11):3243–3246. doi: 10.1016/j.numecd.2021.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mesele H., Shiferaw M., Tunta A., Seid A., Kassahun W. Willingness to Receive COVID-19 Vaccination Among Adult Diabetes Patients in Woldia Comprehensive Specialized Hospital, North Ethiopia; A Cross-Sectional Study. Patient Prefer Adherence. 2022;16:2451–2459. doi: 10.2147/PPA.S379531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Day D., Grech L., Nguyen M., et al. Serious Underlying Medical Conditions and COVID-19 Vaccine Hesitancy: A Large Cross-Sectional Analysis from Australia. Vaccines (Basel) 2022;10(6):851. doi: 10.3390/vaccines10060851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sterne J.A., Sutton A.J., Ioannidis J.P., et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. 2011;343 doi: 10.1136/bmj.d4002. [DOI] [PubMed] [Google Scholar]
- 35.Leong D.P., Banerjee A., Yusuf S. COVID-19 Vaccination Prioritization on the Basis of Cardiovascular Risk Factors and Number Needed to Vaccinate to Prevent Death. Can J Cardiol. 2021;37(7):1112–1116. doi: 10.1016/j.cjca.2021.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alsaleh F.M., Elzain M., Alsairafi Z.K., Naser A.Y. Perceived Knowledge, Attitude, and Practices (KAP) and Fear toward COVID-19 among Patients with Diabetes Attending Primary Healthcare Centers in Kuwait. Int J Environ Res Public Health. 2023;20(3):2369. doi: 10.3390/ijerph20032369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pal R., Yadav U., Grover S., Saboo B., Verma A., Bhadada S.K. Knowledge, attitudes and practices towards COVID-19 among young adults with Type 1 Diabetes Mellitus amid the nationwide lockdown in India: A cross-sectional survey. Diabetes Res Clin Pract. 2020;166 doi: 10.1016/j.diabres.2020.108344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Norhayati M.N., Che Yusof R., Azman Y.M. Systematic Review and Meta-Analysis of COVID-19 Vaccination Acceptance. Front Med (Lausanne) 2022;8 doi: 10.3389/fmed.2021.783982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang Q., Hu S., Du F., et al. Mapping global acceptance and uptake of COVID-19 vaccination: A systematic review and meta-analysis. Commun Med (Lond) 2022;2:113. doi: 10.1038/s43856-022-00177-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang Q., Yang L., Jin H., Lin L. Vaccination against COVID-19: A systematic review and meta-analysis of acceptability and its predictors. Prev Med. 2021;150 doi: 10.1016/j.ypmed.2021.106694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Prabani K.I.P., Weerasekara I., Damayanthi H.D.W.T. COVID-19 vaccine acceptance and hesitancy among patients with cancer: a systematic review and meta-analysis. Public Health. 2022;212:66–75. doi: 10.1016/j.puhe.2022.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhao Y, Du J, Li Z, et al. It Is Time to Improve the Acceptance of COVID-19 Vaccines Among People with Chronic Diseases: A Systematic Review and Meta-analysis [published online ahead of print, 2023 Jan 19]. J Med Virol. 2023;10.1002/jmv.28509. [DOI] [PubMed]
- 43.Jin J.M., Bai P., He W., et al. Gender Differences in Patients With COVID-19: Focus on Severity and Mortality. Front Public Health. 2020;8:152. doi: 10.3389/fpubh.2020.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ortolan A., Lorenzin M., Felicetti M., Doria A., Ramonda R. Does gender influence clinical expression and disease outcomes in COVID-19? A systematic review and meta-analysis. Int J Infect Dis. 2020;99:496–504. doi: 10.1016/j.ijid.2020.07.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fieselmann J., Annac K., Erdsiek F., Yilmaz-Aslan Y., Brzoska P. What are the reasons for refusing a COVID-19 vaccine? A qualitative analysis of social media in Germany. BMC Public Health. 2022;22(1):846. doi: 10.1186/s12889-022-13265-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shearn C., Krockow E.M. Reasons for COVID-19 vaccine hesitancy in ethnic minority groups: A systematic review and thematic synthesis of initial attitudes in qualitative research. SSM Qual Res Health. 2023;3 doi: 10.1016/j.ssmqr.2022.100210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Germani F., Pattison A.B., Reinfelde M. WHO and digital agencies: how to effectively tackle COVID-19 misinformation online. BMJ Glob Health. 2022;7(8) doi: 10.1136/bmjgh-2022-009483. e009483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Majumder M.A.A., Singh K., Johnson W.M.S., et al. Evaluation of Short-Term Side Effects Following the First Dose of COVID-19 Vaccines Among Physicians and Dentists: A Cross-Sectional Study from India. J Multidiscip Healthc. 2023;16:161–174. doi: 10.2147/JMDH.S390364. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.